Mechanochemical Preparation of Slow Release Fertilizer Based on Glauconite–Urea Complexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mechanochemical Process
2.3. Characterization of SRFs
3. Results
3.1. Mineral Composition of SRFs per XRD
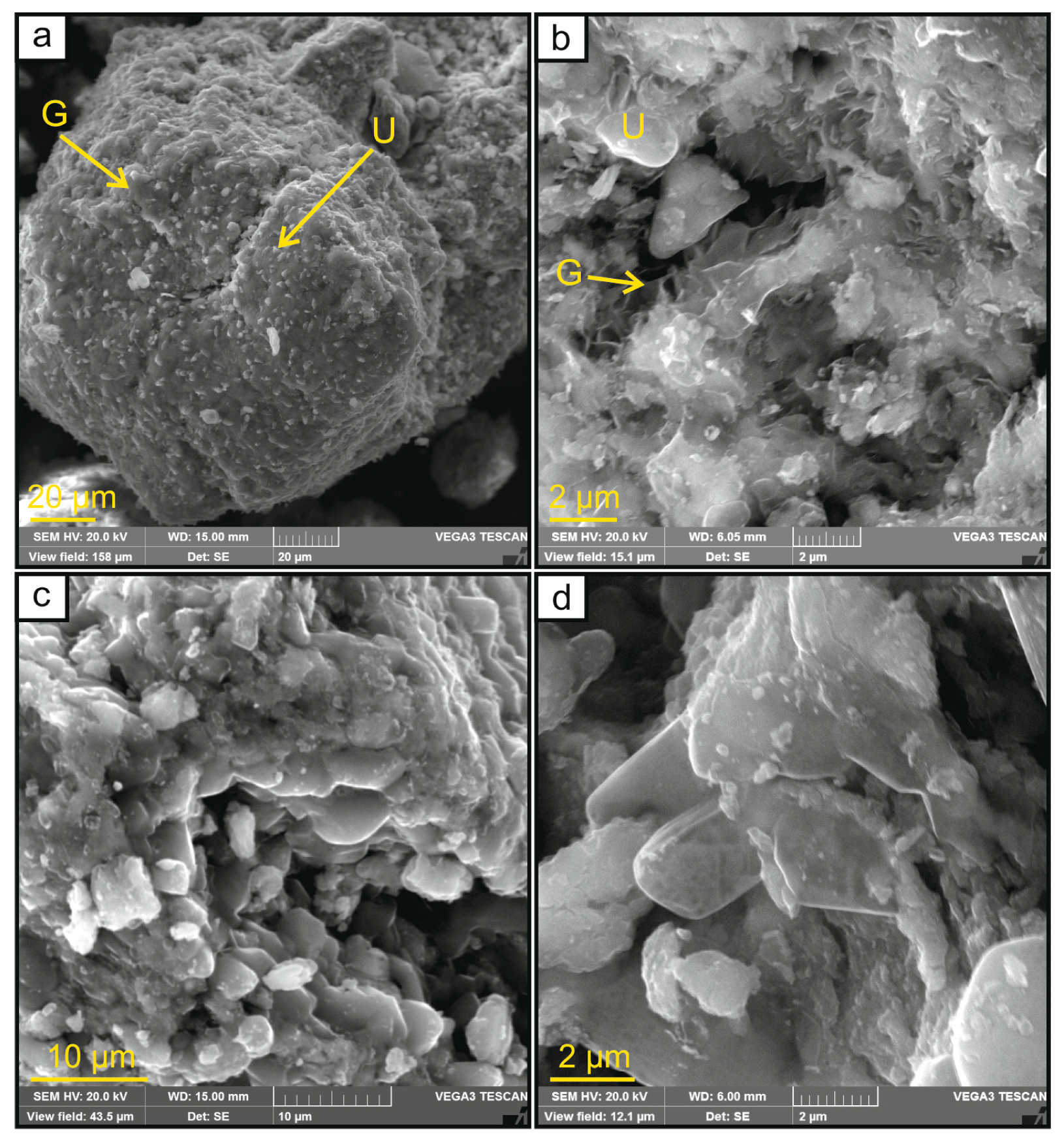
3.2. Morphology and Chemical Composition of SRFs per SEM-EDS and XRF
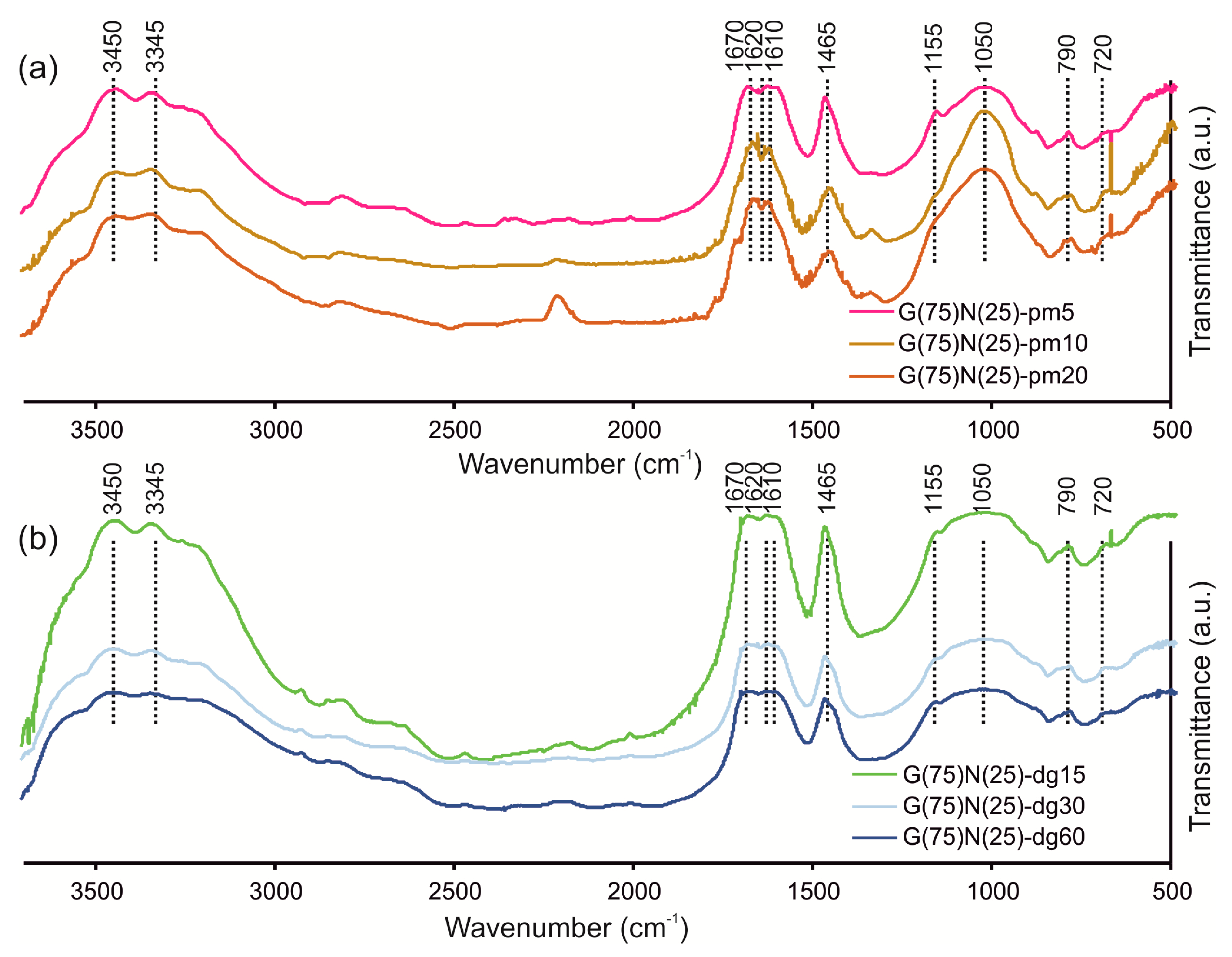
3.3. Chemical Structure of SRFs Determined Using FTIR
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- FAO. Current World Fertilizer Trends and Outlook to 2015; FAO: Rome, Italy, 2015. [Google Scholar]
- Matson, P.A.; Parton, W.J.; Power, A.G.; Swift, M.J. Agricultural intensification and ecosystem properties. Science 1997, 277, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.T.; Xing, G.X.; Chen, X.P.; Zhang, S.L.; Zhang, L.J.; Liu, X.J.; Cui, Z.L.; Yin, B.; Christie, P.; Zhu, Z.L.; et al. Reducing environmental risk by improving N management in intensive Chinese agricultural systems. Proc. Natl. Acad. Sci. United States Am. 2009, 106, 3041–3046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.S.; Chen, J.; Liu, T.Q.; Cao, C.G.; Li, C.F. Effects of nitrogen fertilizer sources and tillage practices on greenhouse gas emissions in paddy fields of central China. Atmos. Environ. 2016, 144, 274–281. [Google Scholar] [CrossRef]
- Akiyama, H.; Yan, X.; Yagi, K. Evaluation of effectiveness of enhanced-efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: Meta-analysis. Glob. Chang. Biol. 2010, 16, 1837–1846. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Naylor, R.; Crews, T.; David, M.B.; Drinkwater, L.E.; Holland, E.; Johnes, P.J.; Katzenberger, J.; Martinelli, L.A.; Matson, P.A.; et al. Nutrient imbalances in agricultural development. Science 2009, 324, 1519–1520. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.C. Controlled-release fertilizers and horticultural applications. Sci. Hortic. 1979, 11, 107–129. [Google Scholar] [CrossRef]
- Oertli, J.J. Controlled-release fertilizers. Fertil. Res. 1980, 1, 103–123. [Google Scholar] [CrossRef]
- Ni, B.; Liu, M.; Lu, S.; Xie, L.; Wang, Y. Environmentally Friendly Slow-Release Nitrogen Fertilizer. J. Agric. Food Chem. 2011, 59, 10169–10175. [Google Scholar] [CrossRef]
- Borges, R.; Brunatto, S.F.; Leitão, A.A.; De Carvalho, G.S.G.; Wypych, F. Solid-state mechanochemical activation of clay minerals and soluble phosphate mixtures to obtain slow-release fertilizers. Clay Miner. 2015, 50, 153–162. [Google Scholar] [CrossRef]
- Trenkel, M.E. Controlled-Release and Stabilized Fertilizers in Agriculture; International Fertilizer Industry Association: Paris, France, 1997. [Google Scholar]
- Teodorescu, M.; Lungu, A.; Stanescu, P.O.; Neamţu, C. Preparation and properties of novel slow-release NPK agrochemical formulations based on poly (acrylic acid) hydrogels and liquid fertilizers. Ind. Eng. Chem. Res. 2009, 48, 6527–6534. [Google Scholar] [CrossRef]
- Liang, R.; Liu, M.; Wu, L. Controlled release NPK compound fertilizer with the function of water retention. React. Funct. Polym. 2007, 67, 769–779. [Google Scholar] [CrossRef]
- Liang, R.; Liu, M. Preparation and properties of coated nitrogen fertilizer with slow release and water retention. Ind. Eng. Chem. Res. 2006, 45, 8610–8616. [Google Scholar] [CrossRef]
- Ni, B.; Liu, M.; Lü, S. Multifunctional slow-release urea fertilizer from ethylcellulose and superabsorbent coated formulations. Chem. Eng. J. 2009, 155, 892–898. [Google Scholar] [CrossRef]
- González, M.E.; Cea, M.; Medina, J.; González, A.; Diez, M.C.; Cartes, P.; Monreal, C.; Navia, R. Evaluation of biodegradable polymers as encapsulating agents for the development of a urea controlled-release fertilizer using biochar as support material. Sci. Total. Environ. 2015, 505, 446–453. [Google Scholar] [CrossRef]
- Yamamoto, C.F.; Pereira, E.I.; Mattoso, L.H.C.; Matsunaka, T.; Ribeiro, C. Slow release fertilizers based on urea/urea–formaldehyde polymer nanocomposites. Chem. Eng. J. 2016, 287, 390–397. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; Gao, B.; Li, Y.C.; Xie, J. Bio-based elastic polyurethane for controlled-release urea fertilizer: Fabrication, properties, swelling and nitrogen release characteristics. J. Clean. Prod. 2019, 209, 528–537. [Google Scholar] [CrossRef]
- Pang, W.; Hou, D.; Wang, H.; Sai, S.; Wang, B.; Ke, J.; Wu, G.; Li, Q.; Holtzapple, M.T. Preparation of microcapsules of slow-release NPK compound fertilizer and the release characteristics. J. Braz. Chem. Soc. 2018, 29, 2397–2404. [Google Scholar] [CrossRef]
- Baldanza, V.A.R.; Souza, F.G.; Filho, S.T.; Franco, H.A.; Oliveira, G.E.; Caetano, R.M.J.; Hernandez, J.A.R.; Ferreira Leite, S.G.; Furtado Sousa, A.M.; Nazareth Silva, A.L. Controlled-release fertilizer based on poly(butylene succinate)/urea/clay and its effect on lettuce growth. J. Appl. Polym. Sci. 2018, 47, 46858. [Google Scholar] [CrossRef]
- Hermida, L.; Agustian, J. Slow release urea fertilizer synthesized through recrystallization of urea incorporating natural bentonite using various binders. Environ. Technol. Innov. 2019, 13, 113–121. [Google Scholar] [CrossRef]
- Golbashy, M.; Sabahi, H.; Allahdadi, I.; Nazokdast, H.; Hosseini, M. Synthesis of highly intercalated urea-clay nanocomposite via domestic montmorillonite as eco-friendly slow-release fertilizer. Arch. Agron. Soil Sci. 2017, 63, 84–95. [Google Scholar] [CrossRef]
- Rutkai, G.; Makó, É.; Kristóf, T. Simulation and experimental study of intercalation of urea in kaolinite. J. Colloid Interface Sci. 2009, 334, 65–69. [Google Scholar] [CrossRef]
- Makó, É.; Kristóf, J.; Horváth, E.; Vágvölgyi, V. Kaolinite–urea complexes obtained by mechanochemical and aqueous suspension techniques—A comparative study. J. Colloid Interface Sci. 2009, 330, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.; Baika, L.M.; Grassi, M.T.; Wypych, F. Mechanochemical conversion of chrysotile/K2HPO4 mixtures into potential sustainable and environmentally friendly slow-release fertilizers. J. Environ. Manag. 2018, 206, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Fatimah, I.; Yudha, S.P.; Rubiyanto, D.; Widodo, I.D. Methenamine-smectite clay as slow release fertiliser: Physicochemical and kinetics study. Chem. Eng. Trans. 2017, 56, 1639–1644. [Google Scholar]
- Borges, R.; Prevot, V.; Forano, C.; Wypych, F. Design and kinetic study of sustainable potential slow-release fertilizer obtained by mechanochemical activation of clay minerals and potassium monohydrogen phosphate. Ind. Eng. Chem. Res. 2017, 56, 708–716. [Google Scholar] [CrossRef]
- Drits, V.A. Isomorphous cation distribution in celadonites, glauconites and Fe-illites determined by infrared, Mössbauer and EXAFS spectroscopies. Clay Miner. 1997, 32, 153–179. [Google Scholar] [CrossRef]
- Meunier, A.; El Albani, A. The glauconite-Fe-illite-Fe-smectite problem: A critical review. Terra Nova 2007, 19, 95–104. [Google Scholar] [CrossRef]
- McRae, S.G. Glauconite. Earth-Sci. Rev. 1972, 8, 397–440. [Google Scholar] [CrossRef]
- Odin, G.S.; Matter, A. De glauconiarum origine. Sedimentology 1981, 28, 611–641. [Google Scholar] [CrossRef]
- Amorosi, A.; Sammartino, I.; Tateo, F. Evolution patterns of glaucony maturity: A mineralogical and geochemical approach. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 1364–1374. [Google Scholar] [CrossRef]
- Amorosi, A. Glaucony and sequence stratigraphy: A conceptual framework of distribution in siliciclastic sequences. J. Sediment. Res. B Stratigr. Glob. Stud. 1995, B65, 419–425. [Google Scholar]
- Baldermann, A.; Dietzel, M.; Mavromatis, V.; Mittermayr, F.; Warr, L.N.; Wemmer, K. The role of Fe on the formation and diagenesis of interstratified glauconite-smectite and illite-smectite: A case study of Upper Cretaceous shallow-water carbonates. Chem. Geol. 2017, 453, 21–34. [Google Scholar] [CrossRef]
- Banerjee, S.; Bansal, U.; Vilas Thorat, A. A review on palaeogeographic implications and temporal variation in glaucony composition. J. Palaeogeogr. 2016, 5, 43–71. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Farouk, S.; Nagm, E.; Choudhury, T.R.; Meena, S.S. High Mg-glauconite in Campanian Duwi Formation of Abu Tartur Plateau, Egypt and its implications. J. Afr. Earth Sci. 2019. [Google Scholar] [CrossRef]
- Rudmin, M.; Banerjee, S.; Mazurov, A. Compositional variation of glauconites in Upper Cretaceous-Paleogene sedimentary iron-ore deposits in South-eastern Western Siberia. Sediment. Geol. 2017, 355, 20–30. [Google Scholar] [CrossRef]
- Merchant, R.J. Glauconite-the future potash for fertilisers in New Zealand. AusIMM Bull. 2012, 1, 78–81. [Google Scholar]
- Prakash, S.; Verma, J.P. Global perspective of potash for fertilizer production. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V., Maurya, B., Eds.; Springer: New Delhi, India, 2016; pp. 327–331. [Google Scholar]
- Franzosi, C.; Castro, L.N.; Celeda, A.M. Technical evaluation of glauconies as alternative potassium fertilizer from the Salamanca Formation, Patagonia, Southwest Argentina. Nat. Resour. Res. 2014, 23, 311–320. [Google Scholar] [CrossRef]
- Castro, L.; Tourn, S. Direct application of phosphate rocks and glauconite as alternative sources of fertilizer in Argentina. Explor. Min. Geol. 2003, 12, 71–78. [Google Scholar] [CrossRef]
- Karimi, E.; Abdolzadeh, A.; Sadeghipour, H.R.; Aminei, A. The potential of glauconitic sandstone as a potassium fertilizer for olive plants. Arch. Agron. Soil Sci. 2012, 58, 983–993. [Google Scholar] [CrossRef]
- Rudmin, M.; Banerjee, S.; Mazurov, A.; Makarov, B.; Martemyanov, D. Economic potential of glauconitic rocks in Bakchar deposit (S-E Western Siberia) for alternate potash fertilizer. Appl. Clay Sci. 2017, 150, 225–233. [Google Scholar] [CrossRef]
- Rudmin, M.; Oskina, Y.; Banerjee, S.; Mazurov, A.; Soktoev, B.; Shaldybin, M. Roasting-leaching experiments on glauconitic rocks of Bakchar ironstone deposit (Western Siberia) for evaluation their fertilizer potential. Appl. Clay Sci. 2018, 162, 121–128. [Google Scholar] [CrossRef]
- Rudmin, M.; Banerjee, S.; Makarov, B.; Mazurov, A.; Ruban, A.; Oskina, Y.; Tolkachev, O.; Buyakov, A.; Shaldybin, M. An investigation of plant growth by the addition of glauconitic fertilizer. Appl. Clay Sci. 2019, 180, 105178. [Google Scholar] [CrossRef]
- Drits, V.A.; Ivanovskaya, T.A.; Sakharov, B.A.; Zvyagina, B.B.; Derkowski, A.; Gor’kova, N.V.; Pokrovskaya, E.V.; Savichev, A.T.; Zaitseva, T.S. Nature of the structural and crystal-chemical heterogeneity of the Mg-rich glauconite (Riphean, Anabar Uplift). Lithol. Miner. Resour. 2010, 45, 555–576. [Google Scholar] [CrossRef]
- Zviagina, B.B.; Drits, V.A.; Sakharov, B.A.; Ivanovskaya, T.A.; Dorzhieva, O.V.; McCarty, D.K. Crystal-chemical regularities and identification criteria in fe-bearing k-dioctahedral micas 1 m from X-Ray diffraction and infrared spectroscopy data. Clays Clay Miner. 2017, 65, 234–251. [Google Scholar] [CrossRef]
- Rashidzadeh, A.; Olad, A. Slow-released NPK fertilizer encapsulated by NaAlg-g-poly(AA-co-AAm)/MMT superabsorbent nanocomposite. Carbohydr. Polym. 2014, 114, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, X.L.; Zhou, C.H.; Yang, H.M.; Ji, S.F.; Tong, D.S.; Zhong, Z.K.; Yu, W.H.; Chu, M.Q. Environmental-friendly montmorillonite-biochar composites: Facile production and tunable adsorption-release of ammonium and phosphate. J. Clean. Prod. 2017, 156, 648–659. [Google Scholar] [CrossRef]
- Liang, D.; Zhang, Q.; Zhang, W.; Liu, L.; Liang, H.; Quirino, R.L.; Chen, J.; Liu, M.; Lu, Q.; Zhang, C. Tunable thermo-physical performance of castor oil-based polyurethanes with tailored release of coated fertilizers. J. Clean. Prod. 2019, 210, 1207–1215. [Google Scholar] [CrossRef]


| Composites | N2O5 | MgO | Al2O3 | SiO2 | P2O5 | SO3 | K2O | CaO | TiO2 | Fe2O3(total) |
|---|---|---|---|---|---|---|---|---|---|---|
| GN-pm5 | 15.7 | 2.8 | 12.7 | 44.8 | 0.5 | 0.3 | 3.2 | 0.7 | 0.4 | 19.4 |
| GN-pm10 | 11.8 | 2.7 | 8.2 | 43.9 | 0.4 | 0.4 | 4.2 | 0.6 | 0.3 | 27.9 |
| GN-pm20 | 12.3 | 3.0 | 9.8 | 45.4 | 0.5 | 0.3 | 4.2 | 0.7 | 0.3 | 25.4 |
| GN-dg15 | 17.2 | 2.7 | 10.1 | 45.3 | 0.3 | 0.2 | 4.1 | 0.6 | 0.2 | 19.8 |
| GN-dg30 | 13.8 | 1.5 | 6.4 | 47.5 | 0.3 | 0.3 | 4.0 | 0.6 | 0.3 | 26.1 |
| GN-dg60 | 16.7 | 2.0 | 7.5 | 44.0 | 0.3 | 0.3 | 3.9 | 0.6 | 0.2 | 25.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudmin, M.; Abdullayev, E.; Ruban, A.; Buyakov, A.; Soktoev, B. Mechanochemical Preparation of Slow Release Fertilizer Based on Glauconite–Urea Complexes. Minerals 2019, 9, 507. https://doi.org/10.3390/min9090507
Rudmin M, Abdullayev E, Ruban A, Buyakov A, Soktoev B. Mechanochemical Preparation of Slow Release Fertilizer Based on Glauconite–Urea Complexes. Minerals. 2019; 9(9):507. https://doi.org/10.3390/min9090507
Chicago/Turabian StyleRudmin, Maxim, Elshan Abdullayev, Alexey Ruban, Ales Buyakov, and Bulat Soktoev. 2019. "Mechanochemical Preparation of Slow Release Fertilizer Based on Glauconite–Urea Complexes" Minerals 9, no. 9: 507. https://doi.org/10.3390/min9090507





