Distribution, Source Identification, and Assessment of Potentially Toxic Elements in the Sediment Core from the Estuarine Region of the Golmud River to the Qarhan Salt Lake, Qinghai, China
Abstract
1. Introduction
2. Materials and Methods
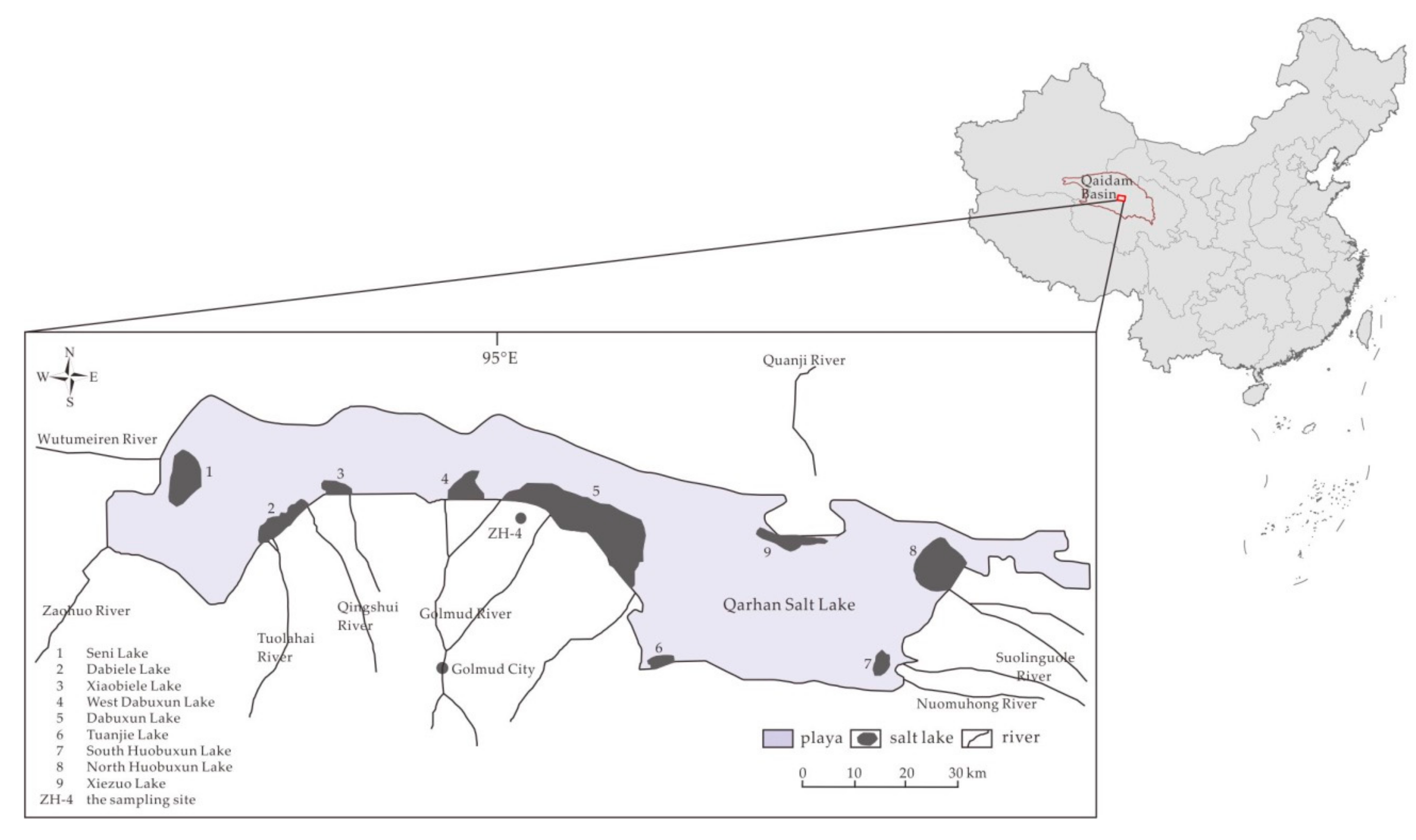
2.1. Sample Collection
2.2. Analyses
2.2.1. Elements and 210Pb
2.2.2. Multivariate Statistical Analysis
2.2.3. Methods for Estimating Pollutant Impact and Ecological Risks
3. Results and Discussion
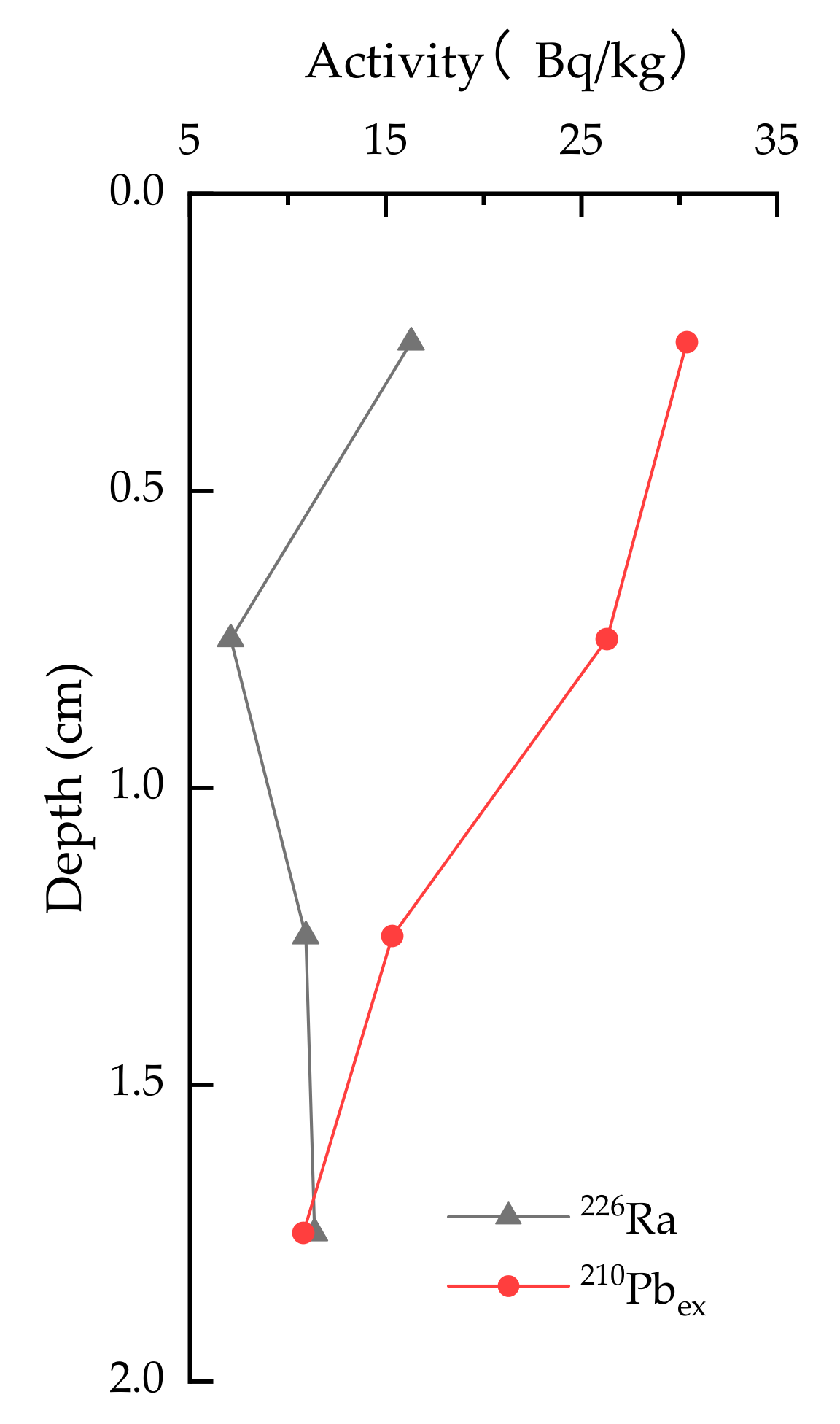
3.1. Sedimentation Rate
3.2. General Features of Elements
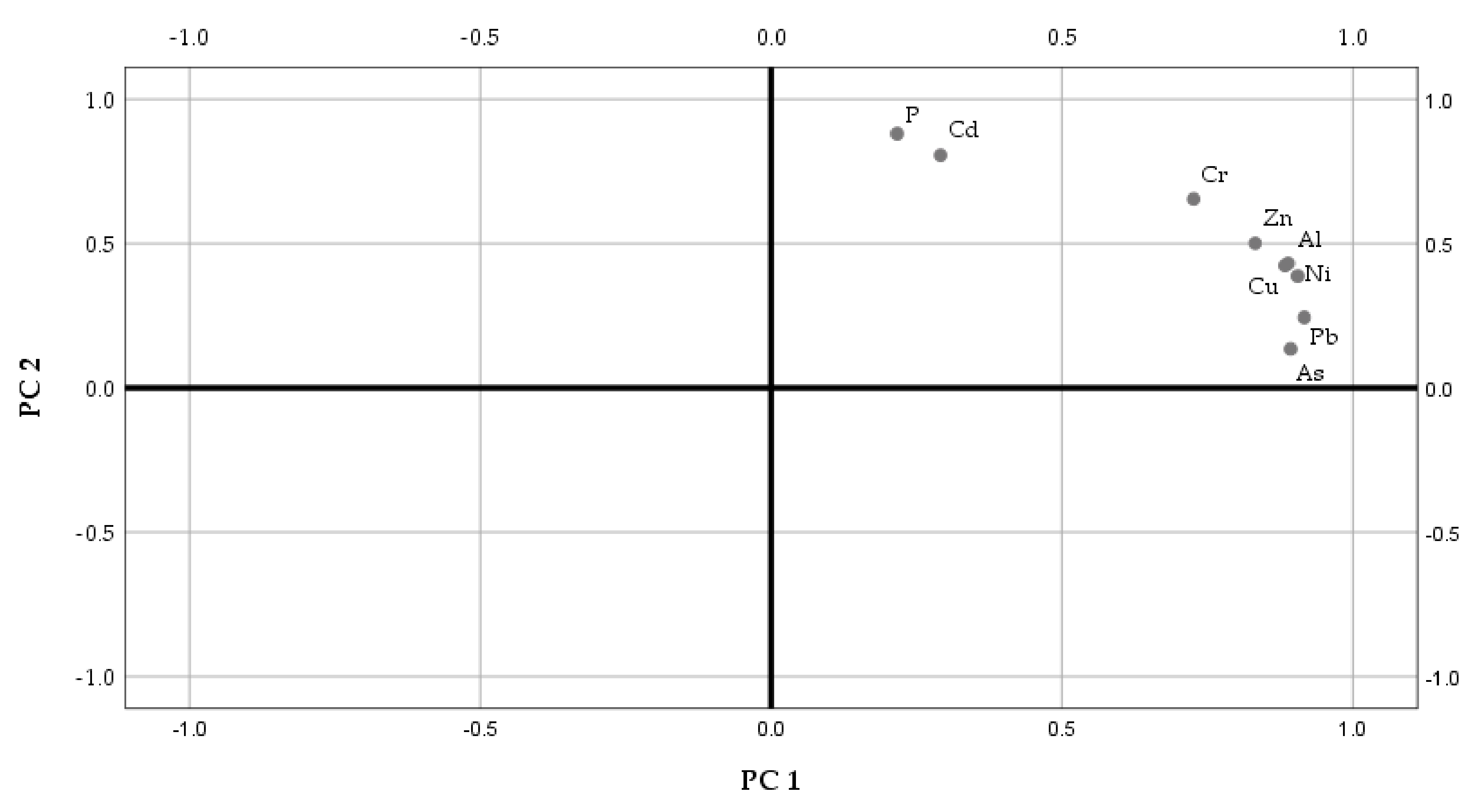
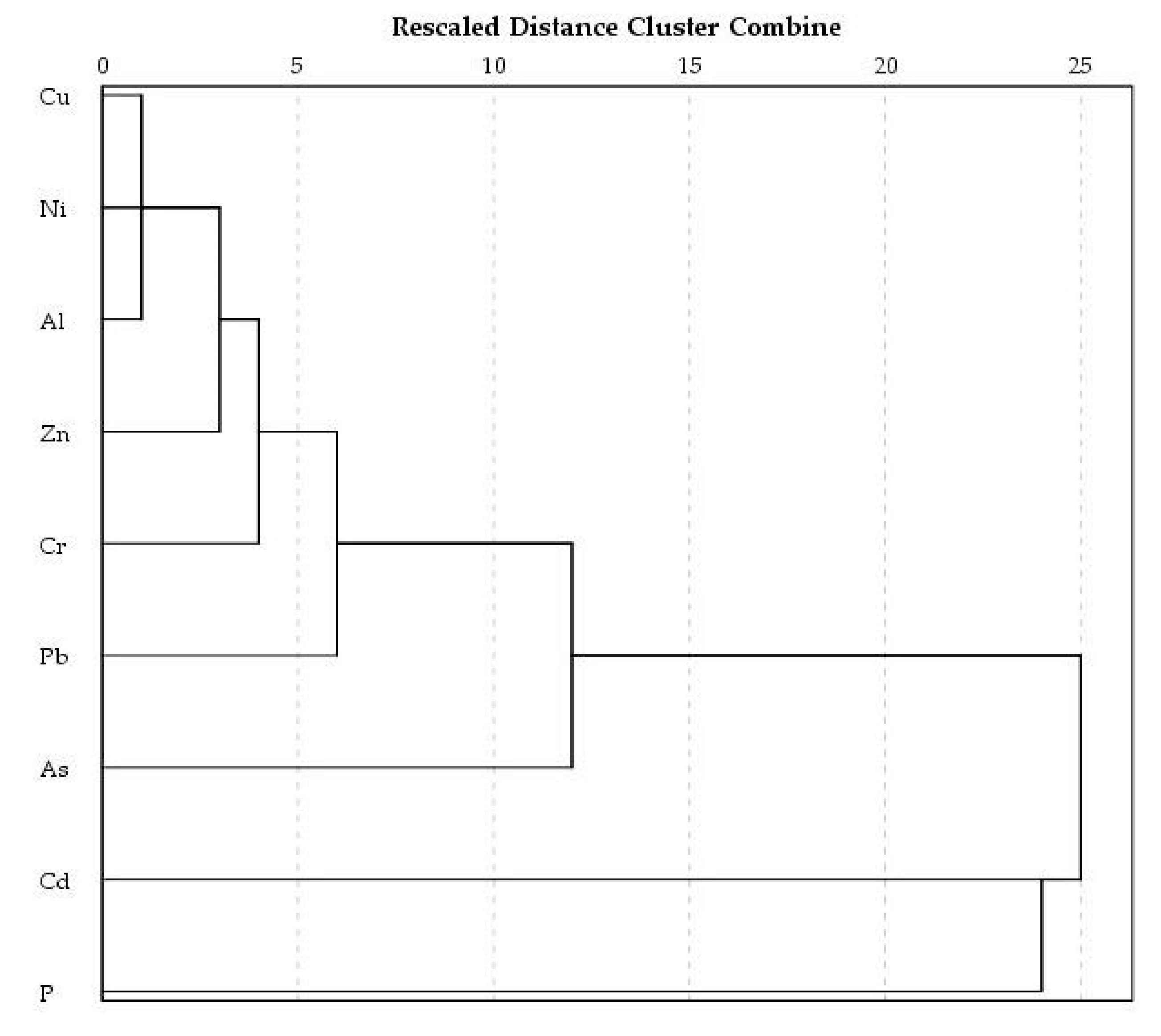
3.3. Multivariate Statistical Analysis
3.4. Pollution and Ecological Assessment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, K.; Hur, S.D.; Hou, S.; Burn-Nunes, L.J.; Hong, S.; Barbante, C.; Boutron, C.F.; Rosman, K.J.R. Isotopic signatures for natural versus anthropogenic Pb in high-altitude Mt. Everest ice cores during the past 800 years. Sci. Total. Environ. 2011, 412–413, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Nriagu, J.O. A history of global metal pollution. Science 1996, 272, 223–224. [Google Scholar] [CrossRef]
- Bennion, H.; Battarbee, R. The European Union water framework directive: Opportunities for palaeolimnology. J. Paleolimnol. 2007, 38, 285–295. [Google Scholar] [CrossRef]
- Wu, J.; Duan, D.; Jian, L.; Luo, Y.; Wen, X.; Guo, X.; Boman, B.J. Inorganic pollution around the Qinghai-Tibet Plateau: An overview of the current observations. Sci. Total. Environ. 2016, 550, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Sha, Z.J.; Wang, Q.; Wang, J.; Du, J.; Hu, J.; Ma, Y.; Kong, F.; Wang, Z. Regional environmental change and human activity over the past hundred years recorded in the sedimentary record of Lake Qinghai, China. Environ. Sci. Pollut. Res. Int. 2017, 24, 9662. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Mao, X.; Jin, Z.; Song, L.; Yang, J.; Yang, H. Sedimentary biogeochemical record in Lake Gonghai: Implications for recent lake changes in relatively remote areas of China. Sci. Total. Environ. 2019, 649, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Kuzyk, Z.Z.A.; McDonald, R.W.; Johannessen, S.C. Calculating rates and dates and interpreting contaminant profiles in biomixed sediments. In Environmental Contaminants; Jules, M.B., Michael, R.R., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 61–87. [Google Scholar]
- Marchand, C.; Allenbach, M. Redox conditions and heavy metals distribution in mangrove forests receiving effluents from shrimp farms (Teremba Bay, New Caledonia). J. Soils Sediments 2011, 11, 529–541. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Li, B.; Feng, C.; Chen, Y.; Shen, Z. Distribution and speciation of heavy metals in surface sediments from the Yangtze estuary and coastal areas. Environ. Earth Sci. 2013, 69, 1537–1547. [Google Scholar] [CrossRef]
- Costa-Böddeker, S.; Hoelzmann, P.; de Stigter, H.C.; van Gaever, P.; Huy, H.; Schwalb, A. The hidden threat of heavy metal pollution in high sedimentation and highly dynamic environment: Assessment of metal accumulation rates in the Thi Vai Estuary, Southern Vietnam. Environ. Pollut. 2018, 242, 348–356. [Google Scholar] [CrossRef]
- Sanchez-Cabeza, J.A.; Ruiz-Fernández, A.C. 210Pb sediment radiochronology: An integrated formulation and classification of dating models. Geochim. Et Cosmochim. Acta 2012, 82, 183–200. [Google Scholar] [CrossRef]
- Robbins, J.A. Geochemical and geophysical applications of radioactive lead isotopes. Biochem. Lead Environ. 1978, 1978, 285–393. [Google Scholar]
- Robbins, J.A.; Edgington, D.N. Determination of recent sedimentation rates in Lake Michigan using Pb-210 and Cs-137. Geochim. Et Cosmochim. Acta 1975, 39, 285–304. [Google Scholar] [CrossRef]
- Crozaz, G.; Picciotto, E.; de Breuck, W. Antarctic snow chronology with Pb210. Geophys 1964, 69, 2597–2604. [Google Scholar] [CrossRef]
- Muller, G. Index of geoaccumulation in sediments of the Rhine River. Geojournal 1969, 2, 108–118. [Google Scholar]
- Hakanson, L. An ecological risk index for aquatic pollution control; a sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Salomons, W.; Forstner, U. Metals in the Hydrocycle; Springer: Berlin/Heidelberg, Germany, 1984; pp. 79–84. [Google Scholar]
- Ramachandra, T.V.; Sudarshan, P.B.; Mahesh, M.K.; Vinay, S. Spatial patterns of heavy metal accumulation in sediments and macrophytes of Bellandur wetland, Bangalore. J. Environ. Manag. 2018, 206, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Reimann, C.; Filzmoser, P.; Fabian, K.; Hron, K.; Birke, M.; Demetriades, A.; Dinelli, E.; Ladenberger, A.; The GEMAS Project Team. The concept of compositional data analysis in practice—Total major element concentrations in agricultural and grazing land soils of Europe. Sci. Total. Environ. 2012, 426, 166–210. [Google Scholar] [CrossRef] [PubMed]
- de Caritat, P.; Grunsky, E.C. Defining element associations and inferring geological processes from total element concentrations in Australian catchment outlet sediments: Multivariate analysis of continental-scale geochemical data. Appl. Geochem. 2013, 33, 104–126. [Google Scholar] [CrossRef]
- Mueller, U.A.; Grunsky, E.C. Multivariate spatial analysis of lake sediment geochemical data; Melville Peninsula, Nunavut, Canada. Appl. Geochem. 2016, 75, 247–262. [Google Scholar] [CrossRef]
- Yongming, H.; Peixuan, D.; Junji, C.; Posmentier, E.S. Multivariate analysis of heavy metal contamination in urban dusts of Xi’an, Central China. Sci. Total. Environ. 2006, 355, 176–186. [Google Scholar] [CrossRef]
- Wang, J.; Liu, R.; Zhang, P.; Yu, W.; Shen, Z.; Feng, C. Spatial variation, environmental assessment and source identification of heavy metals in sediments of the Yangtze River Estuary. Mar. Pollut. Bull. 2014, 1–2, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Ni, Z.X.; Diao, Z.H.; Hu, Y.X.; Xu, X.R. Contamination level, chemical fraction and ecological risk of heavy metals in sediments from Daya Bay, South Chixiuna Sea. Mar. Pollut. Bull. 2018, 128, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Tepanosyan, G.; Sahakyan, L.; Belyaeva, O.; Maghakyan, N.; Saghatelyan, A. Human health risk assessment and riskiest heavy metal origin identification in urban soils of Yerevan, Armenia. Chemosphere 2017, 184, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lu, J.; Li, L.; Min, X.; Luo, Y. Pollution, ecological-health risks, and sources of heavy metals in soil of the northeastern Qinghai-Tibet Plateau. Chemosphere 2018, 20, 12–34. [Google Scholar] [CrossRef] [PubMed]
- Rubio, B.; Nombela, M.A.; Vilas, F. Geochemistry of major and trace elements in sediments of the Ria de Vigo (NW Spain): An assessment of metal pollution. Mar. Pollut. Bull. 2000, 40, 968–980. [Google Scholar] [CrossRef]
- Xia, F.; Qu, L.; Wang, T.; Luo, L.; Chen, H.; Dahlgren, R.A.; Zhang, M.; Mei, K.; Huang, H. Distribution and source analysis of heavy metal pollutants in sediments of a rapid developing urban river system. Chemosphere 2018, 207, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Tainosho, Y. Characterization of heavy metal particles embedded in tire dust. Environ. Int. 2004, 30, 1009–1017. [Google Scholar] [CrossRef]
- Hou, S.; Zheng, N.; Tang, L.; Ji, X.; Li, Y.; Hua, X. Pollution characteristics, sources, and health risk assessment of human exposure to Cu, Zn, Cd and Pb pollution in urban street dust across China between 2009 and 2018. Environ. Int. 2019, 128, 430–437. [Google Scholar] [CrossRef]
- Kar, D.; Sur, P.; Mandai, S.K.; Saha, T.; Kole, R.K. Assessment of heavy metal pollution in surface water. Int. J. Environ. Sci. Technol. 2008, 1, 119–124. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. Assessment of heavy metal contamination and Hg-resistant bacteria in surface water from different regions of Delhi, India. Saudi J. Biol. Sci. 2018, 25, 1687–1695. [Google Scholar] [CrossRef]
- Dahmani-Muller, H.; van Oort, F.; Gélie, B.; Balabane, M. Strategies of heavy metal uptake by three plant species growing near a metal smelter. Environ. Pollut. 2000, 109, 231–238. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Fan, Q.; Gao, D.; Wei, H.; Shan, F.; Li, B.; Zhang, X.; Yuan, Q.; Qin, Z.; Ren, Q. Evaluation of boron isotopes in halite as an indicator of the salinity of Qarhan paleolake water in the eastern Qaidam Basin, western China. Geosci. Front. 2019, 1, 253–262. [Google Scholar] [CrossRef]
- Wang, P.; Cao, J.; Han, Y.; Jin, Z.; Wu, F.; Zhang, F. Elemental distribution in the topsoil of the Lake Qinghai catchment, NE Tibetan Plateau, and the implications for weathering in semi-arid areas. J. Geochem. Explor. 2015, 152, 1–9. [Google Scholar] [CrossRef]
- Li, L.; Wu, J.; Lu, J.; Min, X.; Xu, J.; Yang, L. Distribution, pollution, bioaccumulation, and ecological risks of trace elements in soils of the northeastern Qinghai-Tibet Plateau. Ecotoxicol. Environ. Saf. 2018, 166, 345–353. [Google Scholar] [CrossRef]
- Wang, J.L. Using Multi-Tracers Technique to Understand the Transport of Sediment in the East China Sea. Ph.D. Thesis, East China Normal Univerisity, Shanghai, China, 2016. [Google Scholar]
- Liu, W.X.; Li, X.D.; Shen, Z.G.; Wang, D.C.; Wai, O.W.H.; Li, Y.S. Multivariate statistical study of heavy metal enrichment in sediments of the Pearl River Estuary. Environ. Pollut. 2003, 121, 377–388. [Google Scholar] [CrossRef]
- Loska, K.; Wiechula, D. Application of principal component analysis for the estimation of source of heavy metal contamination in surface sediments from the Rybnik Reservoir. Chemosphere 2003, 51, 723–733. [Google Scholar] [CrossRef]
- Kaiser, H.F. The application of electronic computers to factor analysis. Educ. Psychol. Meas. 1960, 1, 141–151. [Google Scholar] [CrossRef]
- Facchinelli, A.; Sacchi, E.; Mallen, L. Multivariate statistical and GIS-based approach to identify heavy metal sources in soils. Environ. Pollut. 2001, 114, 313–324. [Google Scholar] [CrossRef]
- Abrahim, G.M.S.; Parker, R.J. Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Environ. Monit. Assess. 2008, 136, 227–238. [Google Scholar] [CrossRef]
- Turekian, K.K.; Wedepohl, K.H. Distribution of the elements in some major units of the earth’s crust. Geol. Soc. Am. Bull. 1961, 72, 175. [Google Scholar] [CrossRef]
- Taylor, S.R. Abundance of chemical elements in the continental crust: A new table. Geochim. Et Cosmochim. Acta 1964, 28, 1273–1285. [Google Scholar] [CrossRef]
- Chester, R.; Stoner, J.H. Pb in Particulates from the lower atmosphere of the Eastern Atlantic. Nature 1973, 245, 27–28. [Google Scholar] [CrossRef]
- Sakan, S.M.; Djordjevic, D.S.; Manojlovic, D.D.; Polic, P.S. Assessment of heavy metal pollutants accumulation in the Tisza river sediments. J. of Environmental Management 2009, 90, 3382–3390. [Google Scholar] [CrossRef] [PubMed]
- Abrahim, G.M.S. Holocene Sediments of Tamaki Estuary: Characterisation and Impact of Recent Human Activity on an Urban Estuary in Auckland, New Zealand. Ph.D. Thesis, The University of Auckland, Auckland, New Zealand, 2005. [Google Scholar]
- Shukla, B.S.; Joshi, S.R. An evaluation of the CIC model of 210Pb dating of sediments. Environ. Geol. Water Sci. 1989, 14, 73–76. [Google Scholar] [CrossRef]
- Hernández-Crespo, C.; Martin, M. Determination of background levels and pollution assessment for seven metals (Cd, Cu, Ni, Pb, Zn, Fe, Mn) in sediments of a Mediterranean coastal lagoon. Catena 2015, 133, 206–214. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Abuduwaili, J.; Jinag, F.Q. Sources, pollution statue and potential ecological risk of heavy metals in surface sediments of Aibi Lake. Northwest China Env. 2015, 2, 490–496. [Google Scholar]
- Rodríguez-Seijo, A.; Arenas-Lago, D.; Andrade, M.L.; Vega, F.A. Identifying sources of Pb pollution in urban soils by means of MC-ICP-MS and TOF-SIMS. Environ. Sci. Pollut. Res. 2015, 22, 7859–7872. [Google Scholar] [CrossRef]
- Sinha, S.; Singh, K.P.; Malik, A.; Mohan, D. Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of Gomti River (India): A case study. Water Res. J. Int. Water Assoc. 2004, 38, 3980–3992. [Google Scholar]
- Varol, M. Assessment of heavy metal contamination in sediments of the Tigris River (Turkey) using pollution indices and multivariate statistical techniques. J. Hazard. Mater. 2011, 195, 355–364. [Google Scholar] [CrossRef]
- Li, C.; Yu, H.; Tabassum, S.; Li, L.; Wu, D.; Zhang, Z.; Kong, H.; Xu, P. Effect of calcium silicate hydrates (CSH) on phosphorus immobilization and speciation in shallow lake sediment. Chem. Eng. J. 2017, 317, 844–853. [Google Scholar] [CrossRef]
- Costa, M. Potential hazards of hexavalent chromate in our drinking water. Toxicol. Appl. Pharmacol. 2003, 188, 1–5. [Google Scholar] [CrossRef]
- Baudo, R.; Barbero, P.; Beltrami, M.; Rossi, D. Chemical composition of the sediment from Lake 20 (Antarctica). J. Limnol. 2000, 59, 55–60. [Google Scholar] [CrossRef]
- Swarazenski, P.W.; Baskaran, M.; Rosenbauer, R.J.; Orem, W.H. Histrorical trace element distribution in sediments from the Mississippi River delta. Estuaries Coasts 2006, 29, 1094–1107. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Sha, Z.J.; Wang, J.J.; Du, J.Z.; Hu, J.F.; Ma, Y.J. Historical changes in the major and trace elements in the sedimentary records of Lake Qinghai, Qinghai-Tibet Plateau: Implications for anthropogenic activities. Environ. Geochem. Health 2009, 2009, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Iglesias, P.; Rubio, B.; Millos, J. Isotopic identification of natural vs. anthropogenic lead sources in marine sediments from the inner Ría de Vigo (NW Spain). Sci. Total. Environ. 2012, 437, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Yu, S.; Li, G.L.; Deng, H. Lead contanmination and source in Shanghai in the past century using dated sediment cores from urban park lakes. Chemosphere 2012, 88, 1161–1169. [Google Scholar] [CrossRef]
- Moulis, J.M.; Frank, T. New perspectives in cadmium toxicity: An introduction. Biometals 2010, 23, 763–768. [Google Scholar] [CrossRef]
- Varol, M. Temporal and spatial dynamics of nitrogen and phosphorus in surface water and sediments of a transboundary river located in the semi-arid region of Turkey. Catena 2013, 100, 1–9. [Google Scholar] [CrossRef]
- Carmen, H.C.; Miguel, M. Mid-term variation of vertical distribution of acid volatile sulphide and simultaneously extracted metals in sediment cores from Lake Albufera (Valencia, Spain). Arch. Environ. Contam. Toxicol. 2013, 65, 654–664. [Google Scholar]
- Maanan, M.; Zourarah, B.; Carruesco, C.; Aajjane, A.; Naud, J. The distribution of heavy metals in the Sidi Moussa lagoon sediments (Atlantic Moroccan Coast). J. Afr. Earth Sci. 2004, 39, 473–483. [Google Scholar] [CrossRef]
- Muller, J.; Wüst, R.A.J.; Weiss, D.; Hu, Y. Geochemical and stratigraphic evidence of environmental change at Lynch’s Crater, Queensland, Australia. Glob. Planet. Chang. 2006, 53, 269–277. [Google Scholar] [CrossRef]
- Haixi Statistical Bureau. The Statistical Yearbook of Haibei State of Qinghai Province 1975–2017; Haixi Statistical Bureau: Haixi, China. (In Chinese)
- Wang, Q.Q. Sedimentary Environmental Record over Past Hundred Years and Effected by the Anthropogenic Activity of Lake Qinghai, China. Ph.D. Thesis, Qinghai Institute of Salt Lakes, Chinese Academy of Science, Xining, China, 2019. [Google Scholar]

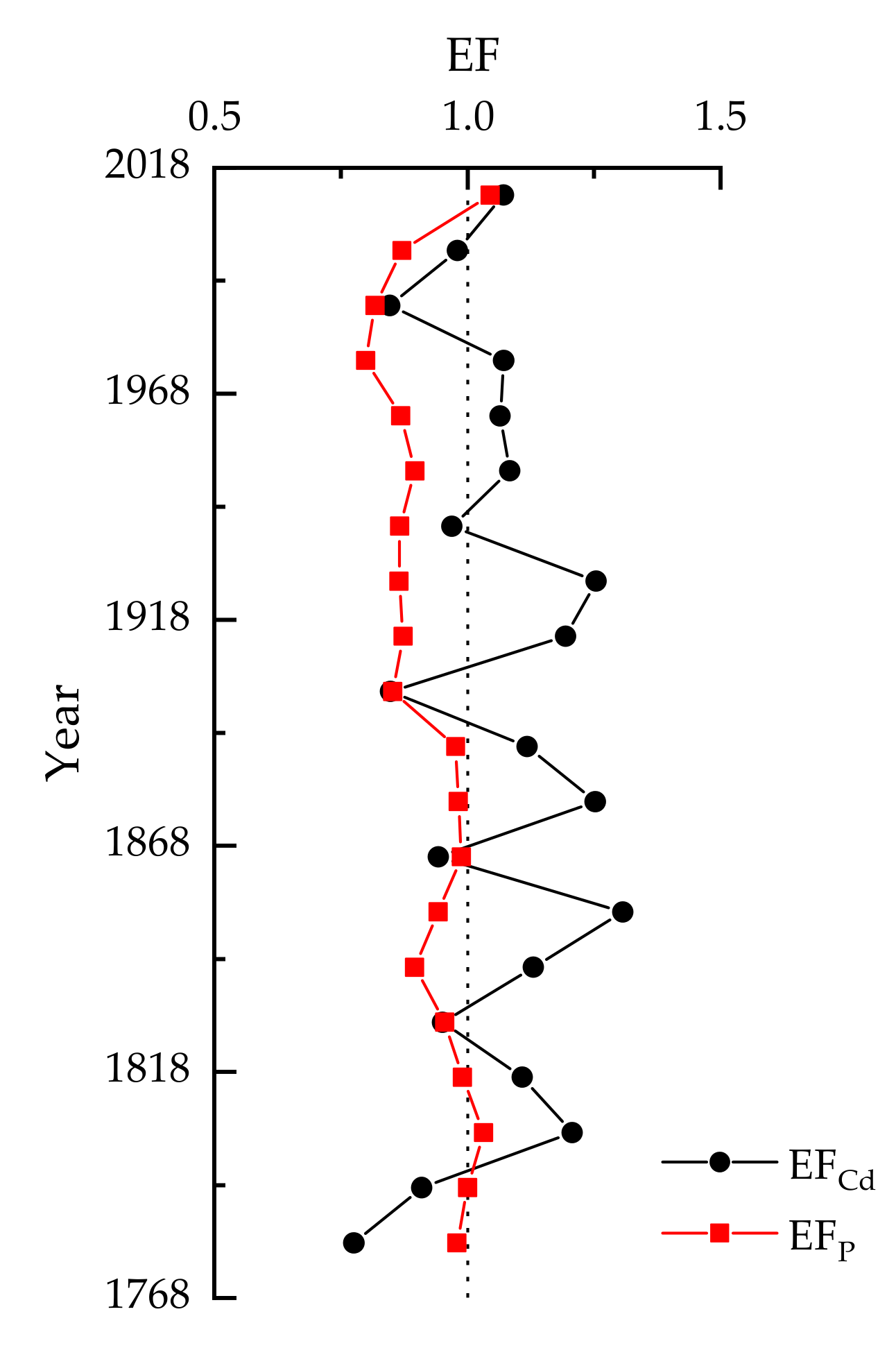
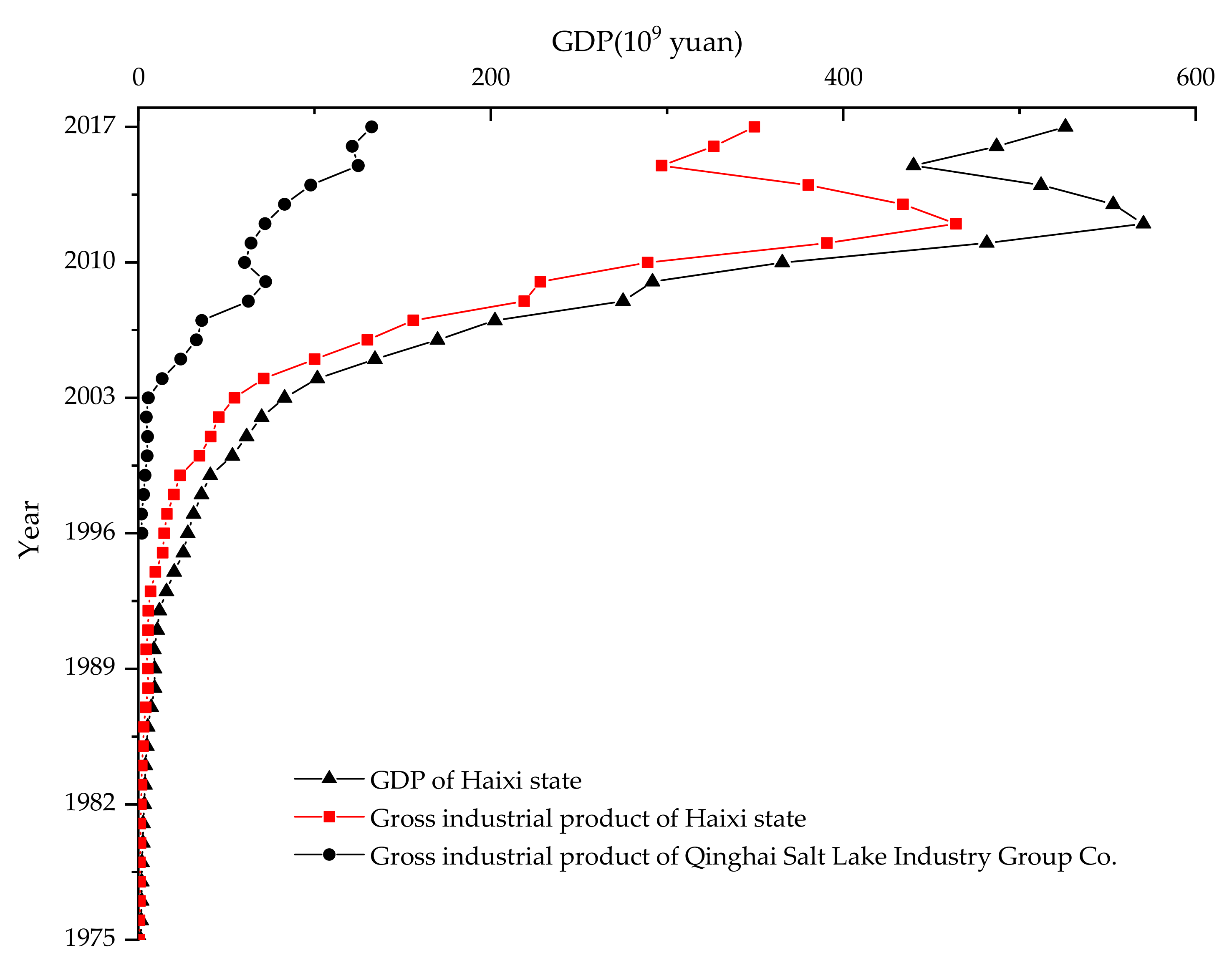
| Element | Mean (µg/g) | Range (µg/g) | CV (%) |
|---|---|---|---|
| Al | 58.7 | 50.5–70.0 | 8.69 |
| As | 16.73 | 13.10–21.80 | 12.01 |
| Cd | 0.09 | 0.06–0.12 | 17.37 |
| Cr | 41.80 | 34.00–52.00 | 12.85 |
| Cu | 21.61 | 15.90–27.60 | 13.51 |
| Ni | 23.82 | 18.90–30.40 | 12.48 |
| P | 684.00 | 580.00–770.00 | 6.41 |
| Pb | 16.85 | 14.20–19.70 | 6.96 |
| Zn | 56.85 | 43.00–73.00 | 12.95 |
| Element | Component Matrix | Rotated Component Matrix | ||
|---|---|---|---|---|
| PC1 | PC2 | PC1 | PC2 | |
| Al | 0.983 | −0.106 | 0.889 | 0.432 |
| As | 0.828 | −0.360 | 0.893 | 0.135 |
| Cd | 0.675 | 0.528 | 0.291 | 0.806 |
| Cr | 0.963 | 0.169 | 0.726 | 0.655 |
| Cu | 0.974 | −0.110 | 0.884 | 0.424 |
| Ni | 0.973 | −0.153 | 0.905 | 0.387 |
| P | 0.652 | 0.631 | 0.216 | 0.881 |
| Pb | 0.906 | −0.280 | 0.917 | 0.244 |
| Zn | 0.971 | −0.018 | 0.832 | 0.501 |
| Element | As | Cd | Cr | Cu | Ni | P | Pb | Zn |
|---|---|---|---|---|---|---|---|---|
| Al | 0.858 ** | 0.646 ** | 0.925 ** | 0.965 ** | 0.965 ** | 0.542 * | 0.911 ** | 0.947 ** |
| As | 0.425 | 0.709 ** | 0.791 ** | 0.844 ** | 0.329 | 0.772 ** | 0.774 ** | |
| Cd | 0.694 ** | 0.576 ** | 0.561 * | 0.579 ** | 0.433 | 0.617 ** | ||
| Cr | 0.935 ** | 0.918 ** | 0.740 ** | 0.807 ** | 0.941 ** | |||
| Cu | 0.982 ** | 0.562 ** | 0.920 ** | 0.940 ** | ||||
| Ni | 0.541 * | 0.910 ** | 0.937 ** | |||||
| P | 0.439 | 0.626 ** | ||||||
| Pb | 0.901 ** |
| Index | Min. | Max. | Median | CV (%) |
|---|---|---|---|---|
| mCd | 0.96 | 1.40 | 1.13 | 10.27 |
| RI | 51.40 | 83.38 | 67.63 | 12.72 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, J.; Wang, J.; Chen, L.; Ling, Z.; Han, J.; Li, Q.; Wang, Q. Distribution, Source Identification, and Assessment of Potentially Toxic Elements in the Sediment Core from the Estuarine Region of the Golmud River to the Qarhan Salt Lake, Qinghai, China. Minerals 2019, 9, 506. https://doi.org/10.3390/min9090506
Xiang J, Wang J, Chen L, Ling Z, Han J, Li Q, Wang Q. Distribution, Source Identification, and Assessment of Potentially Toxic Elements in the Sediment Core from the Estuarine Region of the Golmud River to the Qarhan Salt Lake, Qinghai, China. Minerals. 2019; 9(9):506. https://doi.org/10.3390/min9090506
Chicago/Turabian StyleXiang, Jiangyun, Jianping Wang, Liang Chen, Zhiyong Ling, Jinjun Han, Qingkuan Li, and Qiugui Wang. 2019. "Distribution, Source Identification, and Assessment of Potentially Toxic Elements in the Sediment Core from the Estuarine Region of the Golmud River to the Qarhan Salt Lake, Qinghai, China" Minerals 9, no. 9: 506. https://doi.org/10.3390/min9090506
APA StyleXiang, J., Wang, J., Chen, L., Ling, Z., Han, J., Li, Q., & Wang, Q. (2019). Distribution, Source Identification, and Assessment of Potentially Toxic Elements in the Sediment Core from the Estuarine Region of the Golmud River to the Qarhan Salt Lake, Qinghai, China. Minerals, 9(9), 506. https://doi.org/10.3390/min9090506






