CO2 Adsorption of Materials Synthesized from Clay Minerals: A Review
Abstract
:1. Introduction
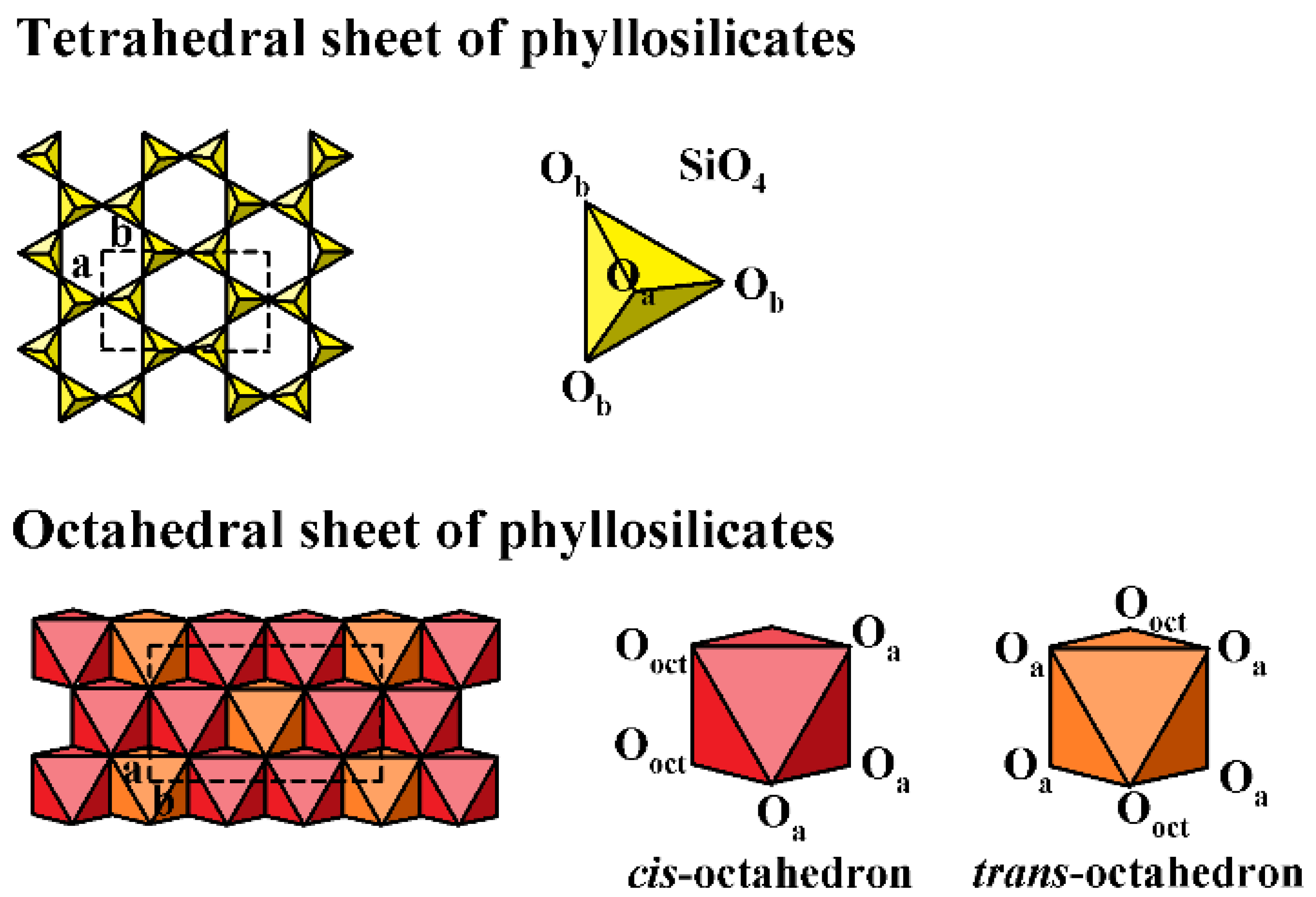
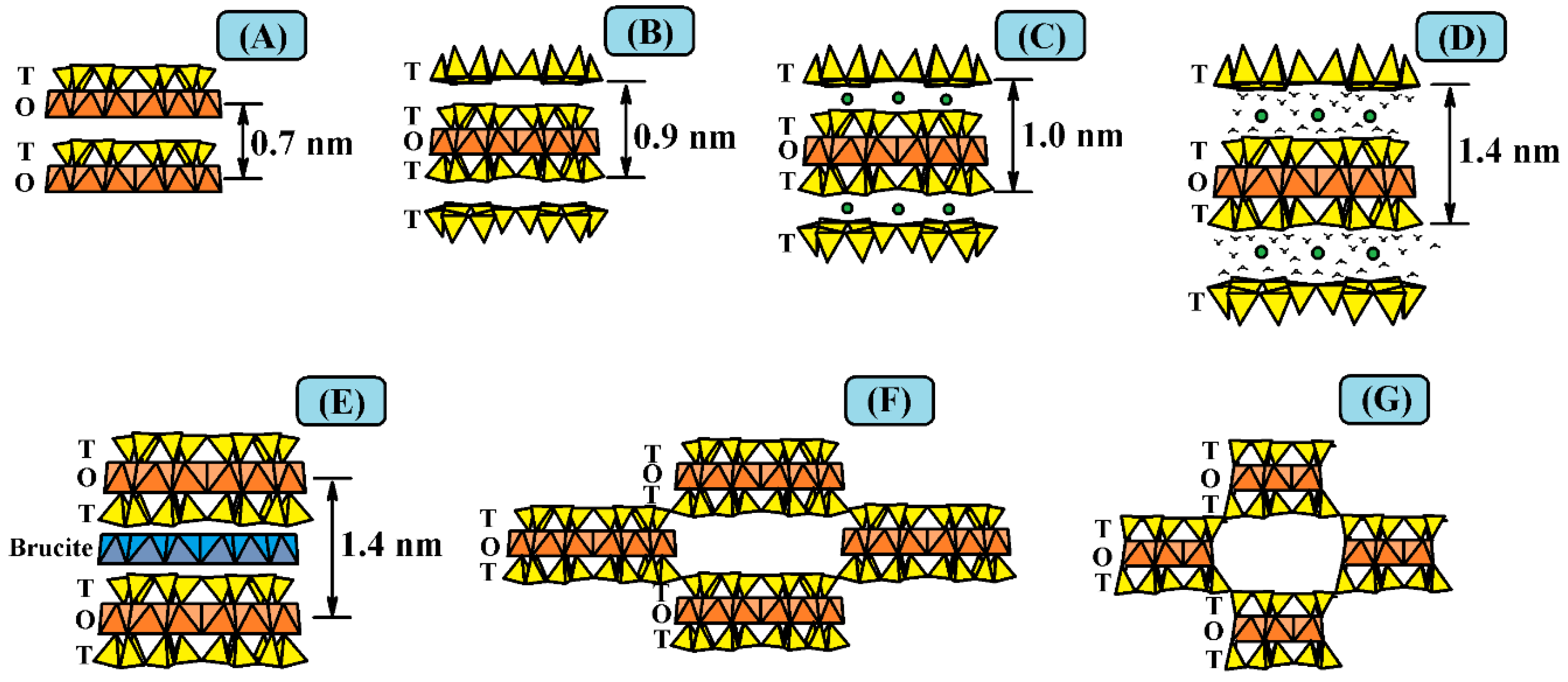
2. Structure of the Clay Minerals
3. CO2 Adsorption
3.1. CO2 Adsorption in Raw Clay Minerals
3.2. CO2 Adsorption in Clay Minerals Activated by Acid Treatment
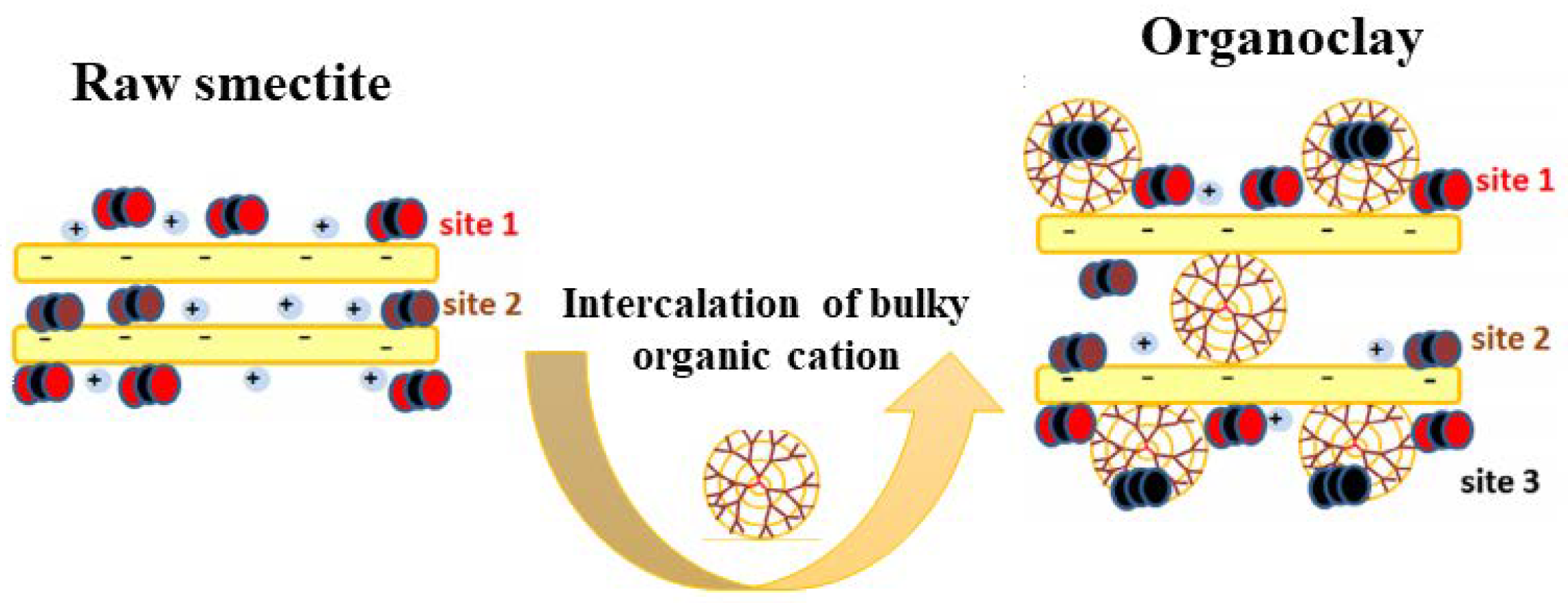
3.3. CO2 Adsorption in Organoclays
3.4. CO2 Adsorption of Materials Synthesized from Clay Minerals
3.5. Clay Minerals Modified with Amino Groups Applied in CO2 Adsorption Processes
3.5.1. Functionalization of Clay Minerals by Grafting
3.5.2. Functionalization of Clay Minerals by Impregnation
3.5.3. Double Functionalization of Clay Minerals (Grafting + Impregnation)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, H.; Xu, Z.; Fan, M.; Guptaa, R.; Slimanec, R.; Bland, A. Progress in carbon dioxide separation and capture: A review. Environ. Sci. 2008, 20, 14–27. [Google Scholar] [CrossRef]
- Pera-Titus, M. Porous inorganic membranes for CO2 capture: Present and prospects. Chem. Rev. 2014, 114, 1413–1492. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Drese, J.D.; Jones, C.W. An efficient and effective convolutional auto-encoder extreme learning machine network for 3d feature learning. Neurocomputing 2018, 174, 49–61. [Google Scholar]
- Yamasaki, A. An overview of CO2 mitigation options for global warming-emphasizing CO2 sequestration options. J. Chem. Eng. Jpn. 2003, 36, 361–375. [Google Scholar] [CrossRef]
- Gray, M.L.; Champagne, K.J.; Fauth, D.; Baltrus, J.P.; Pennline, H. Performance of immobilized tertiary amine solid sorbents for the capture of carbon dioxide. Int. J. Greenh. Gas Control 2008, 2, 3–8. [Google Scholar] [CrossRef]
- Cinke, M.; Li, J.; Bauschlicher, C.W., Jr.; Ricca, A.; Meyyappan, M. CO2 adsorption in single-walled carbon nanotubes. Chem. Phys. Lett. 2003, 376, 761–766. [Google Scholar] [CrossRef]
- Su, F.; Lu, C.; Cnen, W.; Bai, H.; Hang, J.F. Capture of CO2 from flue gas via multiwalled carbon nanotubes. Sci. Total Environ. 2009, 407, 3017–3023. [Google Scholar] [CrossRef] [PubMed]
- Pevida, C.; Plaza, M.G.; Arias, B.; Fermoso, J.; Rubiera, F.; Pis, J.J. Surface modification of activated carbons for CO2 capture. Appl. Surf. Sci. 2008, 254, 7165–7172. [Google Scholar] [CrossRef]
- Sayari, A.; Belmabkhout, Y.; Serna-Guerrero, R. Flue gas treatment via CO2 adsorption. Chem. Eng. J. 2011, 171, 760–774. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.H.; Long, J.R. Carbon dioxide capture in metal-organic frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- Haque, E.; Islam, M.; Pourazadi, E.; Sarkar, S.; Harris, A.T.; Minett, A.I.; Yanmaz, E.; Alshehri, S.M.; Ide, Y.; Wu, K.C.-W.; et al. Boron functionalized graphene oxide-organic frameworks for highly efficient CO2 capture. Chem Asian J. 2017, 12, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Hiyoshi, N.; Yogo, Y.; Yashima, T. Adsorption characteristics of carbon dioxide on organically functionalized SBA-15. Microporous Mesoporous Mater. 2005, 84, 357–365. [Google Scholar] [CrossRef]
- Vilarrasa-García, E.; Cecilia, J.A.; Ortigosa-Moya, E.M.; Cavalcante, C.L., Jr.; Azevedo, D.C.S.; Rodríguez-Castellón, E. “Low-cost” pore expanded SBA-15 functionalized with amine groups applied to CO2 adsorption. Materials 2015, 8, 2495–2513. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Vilarrasa-García, E.; García-Sancho, C.; Saboya, R.M.A.; Azevedo, D.C.S.; Cavalcante, C.L., Jr.; Rodríuez-Castellón, E. Functionalization of hollow silica microspheres by impregnation or grafted of amine groups for the CO2 capture. Int. J. Greenh. Gas Control 2016, 52, 344–356. [Google Scholar] [CrossRef]
- Sanz-Pérez, E.S.; Arencibia, A.; Calleja, G.; San, R. Tuning the textural properties of HMS mesoporous silica. Functionalization towards CO2 adsorption. Microporous Mesoporous Mater. 2018, 260, 235–244. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, L.; Zhang, Y.; Qiao, K.; Yan, Z.; Komarnemi, S. Amine-modified mesocellular silica foams for CO2 capture. Chem. Eng. J. 2011, 168, 918–924. [Google Scholar] [CrossRef]
- Li, G.; Xiao, P.; Webley, P.; Zhang, J.; Singh, R.; Marshall, M. Capture of CO2 from high humidity flue gas by vacuum swing adsorption with zeolite 13X. Adsorption 2008, 14, 415–422. [Google Scholar] [CrossRef]
- Merel, J.; Clausse, M.; Meurier, F. Experimental investigation on CO2 post-combustion capture by indirect thermal swing adsorption using 13X and 5A zeolites. Ind. Eng. Chem. Res. 2008, 47, 209–215. [Google Scholar] [CrossRef]
- Plaza, M.G.; Pevida, C.; Arenillas, A.; Rubiera, F.; Pis, J.J. CO2 capture by adsorption with nitrogen enriched carbons. Fuel 2007, 86, 2204–2212. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G. Some Other Materials Related to Clay Minerals. Dev. Clay Sci. 2013, 5, 743. [Google Scholar]
- Brigatti, M.F.; Galan, E.; Theng, B.K.G. Structure and Mineralogy of Clay Minerals. Dev. Clay Sci. 2006, 1, 19–86. [Google Scholar]
- Chen, Y.H.; Lu, D.L. Amine modification on kaolinites to enhance CO2 adsorption. J. Colloid Interface Sci. 2014, 436, 47–51. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lu, D.L. CO2 capture by kaolinite and its adsorption mechanism. Appl. Clay Sci. 2015, 104, 221–228. [Google Scholar] [CrossRef]
- Chen, C.; Park, D.W.; Ahn, W.S. Surface modification of a low cost bentonite for post-combustion CO2 capture. Appl. Surf. Sci. 2013, 283, 699–704. [Google Scholar] [CrossRef]
- Vilarrasa-García, E.; Cecilia, J.A.; Azevedo, D.C.S.; Cavalcante, C.L., Jr.; Rodríguez-Castellón, E. Evaluation of porous clay heterostructures modified with amine species as adsorbent for the CO2 capture. Microporous Mesoporous Mater. 2017, 249, 25–33. [Google Scholar] [CrossRef]
- Gómez-Pozuelo, G.; Sanz-Pérez, E.S.; Arencibia, A.; Pizarro, P.; Sanz, R.; Serrano, D.P. CO2 adsorption on amine-functionalized clays. Microporous Mesoporous Mater. 2019, 282, 38–47. [Google Scholar] [CrossRef]
- Stevens, L.; Williams, K.; Han, W.Y.; Drage, T.; Snape, C.; Wood, J.; Wang, J. Preparation and CO2 adsorption of diamine modified montmorillonite via exfoliation grafting route. Chem. Eng. J. 2013, 215, 699–708. [Google Scholar] [CrossRef]
- Vilarrasa-García, E.; Cecilia, J.A.; Aguado, E.R.; Jiménez-Jiménez, J.; Cavalcante, C.L., Jr.; Azevedo, D.C.S.; Rodríguez-Castellón, E. Amino-modified pillared adsorbent from water-treatment solid wastes applied to CO2/N2 separation. Adsorption 2017, 23, 405–421. [Google Scholar] [CrossRef]
- Romanov, V.N. Evidence of irreversible CO2 intercalation in montmorillonite. Int. J. Greenh. Gas Control 2013, 14, 220–226. [Google Scholar] [CrossRef]
- Loring, S.; Schaef, H.T.; Turcu, R.V.F.; Thompson, C.J.; Miller, Q.R.S.; Martin, P.F.; Hu, J.; Hoyt, D.W.; Qafoku, O.; Ilton, E.S.; et al. In situ molecular spectroscopic evidence for CO2 intercalation into montmorillonite in supercritical carbon dioxide. Langmuir 2012, 28, 7125–7128. [Google Scholar] [CrossRef]
- Giesting, P.; Guggenheim, S.; van Groos, A.F.K.; Busch, A. Interaction of carbon dioxide with Na-exchanged montmorillonite at pressures to 640 bars: Implications for CO2 sequestration. Int. J. Greenh. Gas Control 2012, 8, 73–81. [Google Scholar] [CrossRef]
- Makaremi, M.; Jordan, K.D.; Guthrie, G.D.; Myshakin, E.M. Multiphase Monte Carlo and molecular dynamics simulations of water and CO2 intercalation in montmorillonite and beidellite. J. Phys. Chem. C 2015, 119, 15112–15124. [Google Scholar] [CrossRef]
- Michels, L.; Fossum, J.O.; Rozynek, Z.; Hemmen, H.; Rustenberg, K.; Sobas, P.A.; Kalantzopoulos, G.N.; Knudsen, K.D.; Janek, M.; Plivelic, T.S.; et al. Intercalation and retention of carbon dioxide in a smectite clay promoted by interlayer cations. Sci. Rep. 2015, 5, 8775. [Google Scholar] [CrossRef]
- Bowers, G.M.; Schaef, H.T.; Loring, J.S.; Hoyt, D.W.; Burton, S.D.; Walter, E.D.; Kirkpatrick, R.J. Swelling stress development in confined smectite clays through exposure to CO2. Int. J. Greenh. Gas Control 2018, 74, 49–61. [Google Scholar]
- Yang, N.; Liu, S.; Yang, X. Molecular simulation of preferential adsorption of CO2 over CH4 in Na-montmorillonite clay material. Appl. Surf. Sci. 2015, 356, 1262–1271. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, L. Molecular insight into competitive adsorption of methane and carbon dioxide in montmorillonite: Effect of clay structure and water content. Fuel 2019, 239, 32–43. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G. Adsorption of carbon dioxide on mica surfaces. Langmuir 1989, 5, 1155–1162. [Google Scholar] [CrossRef]
- Christenson, H.K. Adhesion and surface energy of mica in air and water. J. Phys. Chem. 1997, 97, 12034–12041. [Google Scholar] [CrossRef]
- Cole, D.R.; Chialvo, A.A.; Rother, G.; Vlcek, L.; Cumming, P.T. Supercritical fluid behavior at nanoscale interfaces: Implications for CO2 sequestration in geologic formations. Philos. Mag. 2010, 90, 2339–2363. [Google Scholar] [CrossRef]
- Súarez, M.; García-Romero, E. Advances in the crystal chemistry of sepiolite and palygorskite. Dev. Clay Sci. 2011, 3, 33–65. [Google Scholar]
- Jones, B.F.; Galán, E. Sepiolite and palygorskite. Rev. Miner. 1988, 19, 631–674. [Google Scholar]
- Cecilia, J.; Vilarrasa-García, E.; Cavalcante, C.C., Jr.; Azevedo, D.; Franco, F.; Rodríguez-Castellón, E. Evaluation of two fibrous clay minerals (sepiolite and palygorskite) for CO2 Capture. J. Environ. Chem. Eng. 2018, 6, 4573–4587. [Google Scholar] [CrossRef]
- Jeon, P.R.; Choi, J.; Yun, T.S.; Lee, C.H. Sorption equilibrium and kinetics of CO2 on clay minerals from subcritical to supercritical conditions: CO2 sequestration at nanoscale interfaces. Chem. Eng. J. 2014, 255, 705–715. [Google Scholar] [CrossRef]
- Komadel, P.; Madejová, J. Acid activation of clay minerals. Dev. Clay Sci. 2013, 5, 385–409. [Google Scholar]
- Osthaus, B.B. Chemical determination of tetrahedral ions in nontronite and montmorillonite. Clays Clay Miner. 1953, 2, 404–417. [Google Scholar] [CrossRef]
- Osthaus, B.B. Kinetic studies on montmorillonites and nontronite by the acid-dissolution technique. Clays Clay Miner. 1955, 4, 301–321. [Google Scholar] [CrossRef]
- Franco, F.; Pozo, M.; Cecilia, J.A.; Benítez-Guerrero, M.; Pozo, E.; Martín-Rubí, J.A. Microwave assisted acid treatment of sepiolite: The role of composition and “crystallinity”. Appl. Clay Sci. 2014, 102, 15–27. [Google Scholar] [CrossRef]
- Franco, F.; Pozo, M.; Cecilia, J.A.; Benítez-Guerrero, M.; Lorente, M. Effectiveness of microwave assisted acid treatment on dioctahedral and trioctahedral smectites. The influence of octahedral composition. Appl. Clay Sci. 2016, 120, 70–80. [Google Scholar] [CrossRef]
- Venaruzzo, J.L.; Volzone, C.; Rueda, M.L.; Ortiga, J. Modified bentonitic clay minerals as adsorbents of CO, CO2 and SO2 gases. Microporous Mesoporous Mater. 2002, 56, 73–80. [Google Scholar] [CrossRef]
- He, H.; Ma, L.; Zhu, J.; Frost, R.L.; Theng, B.K.G.; Bergaya, F. Synthesis of organoclays: A critical review and some unresolved issues. Appl. Clay Sci. 2014, 100, 22–28. [Google Scholar] [CrossRef]
- Azzouz, A.; Ursub, A.V.; Nistor, D.; Sajin, T.; Assaad, E.; Roy, R. TPD study of the reversible retention of carbon dioxide over montmorillonite intercalated with polyol dendrimers. Thermochim. Acta 2009, 496, 45–49. [Google Scholar] [CrossRef]
- Azzouz, A.; Assaad, E.; Ursu, A.V.; Sajin, T.; Nistor, D.; Roy, R. Carbon dioxide retention over montmorillonite–dendrimer materials. Appl. Clay Sci. 2010, 48, 133–137. [Google Scholar] [CrossRef]
- Azzouz, A.; Platon, N.; Nousir, S.; Ghomari, K.; Nistor, D.; Shiao, T.C.; Roy, R. OH-enriched organo-montmorillonites for potential applications in carbon dioxide separation and concentration. Sep. Purif. Technol. 2013, 108, 181–188. [Google Scholar] [CrossRef]
- Sahah, K.J.; Imae, T. Analytical investigation of specific adsorption kinetics of CO2 gas on dendrimer loaded in organoclays. Chem. Eng. J. 2016, 283, 1366–1373. [Google Scholar] [CrossRef]
- Shah, K.J.; Imae, T.; Ujihara, M.; Huang, S.J.; Wu, P.H.; Liu, S.B. Poly(amido amine) dendrimer-incorporated organoclays as efficient adsorbents for capture of NH3 and CO2. Chem. Eng. J. 2017, 312, 118–125. [Google Scholar] [CrossRef]
- Sahah, K.J.; Imae, T.; Shukla, A. Selective capture of CO2 by poly (amido amine) dendrimer-loaded organoclays. RSC Adv. 2015, 5, 35985–35992. [Google Scholar] [CrossRef]
- Drag, E.B.; Miecznikowski, A.; Abo-Lemon, F.; Rutkowski, M. Synthesis of A, X and Y zeolites from clay minerals. Stud. Surf. Sci. Catal. 1985, 24, 147–154. [Google Scholar]
- Chen, C.; Park, D.W.; Ahn, W.S. CO2 capture using zeolite 13X prepared from bentonite. Appl. Surf. Sci. 2014, 294, 63–67. [Google Scholar] [CrossRef]
- Garshasbi, V.; Jahangiri, M.; Anbia, M. Equilibrium CO2 adsorption on zeolite 13X prepared from natural clays. Appl. Surf. Sci. 2017, 393, 225–233. [Google Scholar] [CrossRef]
- Vieira, L.O.; Madeira, A.C.; Merlini, A.; Melo, C.R.; Mendes, E.; Santos, M.G.; da Rocha, M.R.; Angioletto, E. Synthesis of 4A-zeolite for adsorption of CO2. Mater. Sci. Forum 2015, 805, 632–637. [Google Scholar] [CrossRef]
- Pour, A.A.; Sharifnia, S.; Salehi, R.N.; Ghodrati, M. Adsorption separation of CO2/CH4 on the synthesized NaA zeolite shaped with montmorillonite clay in natural gas purification process. J. Nat. Gas Sci. Eng. 2016, 36, 630–643. [Google Scholar] [CrossRef]
- Djeffal, N.; Benbouzid, M.; Boukoussa, B.; Sekkiou, H.; Bengueddach, A. CO2 adsorption properties of ion-exchanged zeolite Y prepared from natural clays. Mater. Res. Express 2017, 4, 35504. [Google Scholar] [CrossRef]
- Thakkar, H.; Issa, A.; Rownaghi, A.A.; Rezaei, F. CO2 capture from air using amine-functionalized kaolin-based zeolites. Chem. Eng. Technol. 2017, 40, 1999–2007. [Google Scholar] [CrossRef]
- Bkour, Q.; Faqir, N.; Shawabkeh, R.; Ul-Hamid, A.; Bart, H.J. Synthesis of a Ca/Na-aluminosilicate from kaolin and limestone and its use for adsorption of CO2. J. Environ. Chem. Eng. 2016, 4, 973–983. [Google Scholar] [CrossRef]
- Barrer, R.M.; MacLeod, D.M. Activation of montmorillonite by ion exchange and sorption complexes of tetra-alkyl ammonium montmorillonites. Trans. Faraday Soc. 1955, 51, 1290–1300. [Google Scholar] [CrossRef]
- Gil, A.; Trujillano, R.; Vicente, M.A.; Korili, S.A. Adsorption of nitrogen, hydrogen and carbon dioxide on alumina pillared clays. Stud. Surf. Sci. Catal. 2007, 160, 327–334. [Google Scholar]
- Wang, K.; Yan, X.; Komarneni, S. CO2 adsorption by several types of pillared montmorillonite clays. Appl. Petrochem. Res. 2018, 8, 173–177. [Google Scholar] [CrossRef]
- Galarneau, A.; Barodawalla, A.; Pinnavaia, T. Porous clay heterostructures formed by gallery-templated synthesis. Nature 1955, 374, 529–531. [Google Scholar] [CrossRef]
- Caplow, M. Kinetics of carbamate formation and breakdown. J. Am. Chem. Soc. 1968, 90, 6795–6803. [Google Scholar] [CrossRef]
- Danckwerts, P.V. The reaction of CO2 with ethanolamines. Chem. Eng. Soc. 1979, 34, 443–446. [Google Scholar] [CrossRef]
- Pinto, M.L.; Mafra, L.; Guil, J.M.; Pires, J.; Rocha, J. Adsorption and activation of CO2 by amine-modified nanoporous materials studied by solid-state NMR and 13CO2 adsorption. Chem. Mater. 2011, 23, 1387–1395. [Google Scholar] [CrossRef]
- Donaldson, T.L.; Nguyen, N.Y. Carbon dioxide reaction kinetics and transport in aqueous amine membranes. Ind. Eng. Chem. Fundam. 1980, 19, 260–266. [Google Scholar] [CrossRef]
- Mebane, D.S.; Kress, J.D.; Storlie, C.B.; Fauth, D.J.; Gray, M.L.; Li, K. Transport, zwitterions, and the role of water for CO2 adsorption in mesoporous silica-supported amine sorbents. J. Phys. Chem. C 2013, 117, 26617–26627. [Google Scholar] [CrossRef]
- Didas, S.A.; Sakwa-Novak, M.A.; Foo, G.S.; Sievers, C.; Jones, C.W. Effect of amine surface coverage on the co-adsorption of CO2 and water: Spectral deconvolution of adsorbed species. J. Phys. Chem. Lett. 2014, 5, 4194–4200. [Google Scholar] [CrossRef]
- Bishnoi, S.; Rochelle, G.T. Absorption of carbon dioxide into aqueous piperazine: Reaction kinetics, mass transfer and solubility. Chem. Eng. Sci. 2000, 55, 5531–5543. [Google Scholar] [CrossRef]
- Xiao, J.; Li, C.W.; Li, M.H. Kinetics of absorption of carbon dioxide into aqueous solutions of 2-amino-2-methyl-1-propanol+monoethanolamine. Chem. Eng. Sci. 2000, 55, 161–175. [Google Scholar] [CrossRef]
- Ouyang, J.; Zheng, C.; Gu, W.; Zhang, Y.; Yang, H.; Yang, H.; Suib, S.L. Textural properties determined CO2 capture of tetraethylenepentamine loaded SiO2 nanowires from sepiolite. Chem. Eng. J. 2018, 337, 342–350. [Google Scholar] [CrossRef]
- Jana, S.; Das, S.; Ghosh, C.; Maity, A.; Pradhan, M. Halloysite nanotubes capturing isotope selective atmospheric CO2. Sci. Rep. 2015, 5, 8711. [Google Scholar] [CrossRef]
- Elkhalifah, A.E.I.; Bustam, M.A.; Shariff, A.M.; Murugesan, T. Carbon dioxide retention on bentonite clay adsorbents modified by mono-, di- and triethanolamine compounds. Adv. Mater. Res. 2014, 917, 115–122. [Google Scholar] [CrossRef]
- Elkhalifah, A.E.I.; Bustam, M.A.; Shariff, A.M.; Murugesan, T. Selective adsorption of CO2 on a regenerable amine-bentonite hybrid adsorbent. Appl. Clay Sci. 2015, 107, 213–219. [Google Scholar] [CrossRef]
- Atilhan, M.; Atilhan, S.; Ullah, R.; Anaya, B.; Cagin, T.; Yavuz, C.T.; Aparicio, S. High-pressure methane, carbon dioxide, and nitrogen adsorption on amine-impregnated porous montmorillonite nanoclays. J. Chem. Eng. Data 2016, 61, 2749–2760. [Google Scholar] [CrossRef]
- Wang, W.; Xiao, J.; Wei, X.; Ding, J.; Wang, X.; Song, C. Development of a new clay supported polyethylenimine composite for CO2 capture. Appl. Energy 2014, 113, 334–341. [Google Scholar] [CrossRef]
- Irani, M.; Fan, M.; Ismail, H.; Tuwatia, A.; Dutcher, B.; Russell, A.G. Modified nanosepiolite as an inexpensive support of tetraethylenepentamine for CO2 sorption. Nano Energy 2015, 11, 235–246. [Google Scholar] [CrossRef]
- Ouyang, J.; Gu, W.; Zheng, C.; Yang, H.; Zhang, X.; Jin, Y.; Chen, J.; Jiang, J. Polyethyleneimine (PEI) loaded MgO-SiO2 nanofibers from sepiolite minerals for reusable CO2 capture/release applications. Appl. Clay Sci. 2018, 152, 267–275. [Google Scholar] [CrossRef]
- Yuan, M.; Gao, G.; Hu, X.; Luo, X.; Huang, Y.; Jin, B.; Liang, Z. Premodified sepiolite functionalized with triethylenetetramine as an effective and inexpensive adsorbent for CO2 capture. Ind. Eng. Chem. Res. 2018, 57, 6189–6200. [Google Scholar] [CrossRef]
- Liu, L.; Chena, H.; Shiko, E.; Fan, X.; Zhou, Y.; Zhang, G.; Luo, X.; Hu, X. Low-cost DETA impregnation of acid-activated sepiolite for CO2 capture. Chem. Eng. J. 2018, 353, 940–948. [Google Scholar] [CrossRef]
- Ouyang, J.; Gu, W.; Zhang, Y.; Yang, H.; Jin, Y.; Chen, J.; Jiang, J. CO2 capturing performances of millimeter scale beads made by tetraethylenepentamine loaded ultra-fine palygorskite powders from jet pulverization. Chem. Eng. J. 2018, 341, 432–440. [Google Scholar] [CrossRef]
- Niu, M.; Yang, H.; Zhang, X.; Wang, Y.; Tang, A. Amine-impregnated mesoporous silica nanotube as an emerging nanocomposite for CO2 capture. ACS Appl. Mater. Interfaces 2016, 8, 17312–17320. [Google Scholar] [CrossRef]
- Sanz, R.; Calleja, G.; Arencibia, A.; Sanz-Pérez, E.S. Development of high efficiency adsorbents for CO2 capture based on a double-functionalization method of grafting and impregnation. J. Mater. Chem. A 2013, 1, 1956–1962. [Google Scholar] [CrossRef]




| Clay Mineral | CO2 Adsorption Capacity (mg CO2/g) | Adsorption Conditions | Ref. |
|---|---|---|---|
| Kaolinite | 3 | 25 °C, 1 bar | [22] |
| Kaolinite | 0 | 25 °C, 1 bar | [23] |
| Bentonite | 6 | 25 °C, 1 bar | [24] |
| Bentonite | 5 | 25 °C, 1 bar | [25] |
| Bentonite | 14 | 45 °C, 1 bar | [26] |
| Montmorillonite | 10 | 45 °C, 1 bar | [26] |
| Montmorillonite | 7 | 25 °C, 1 bar | [28] |
| Montmorillonite | 22 | 10 °C, 1 bar, 90 min | [27] |
| Saponite | 15 | 45 °C, 1 bar | [26] |
| Sepiolite | 41 | 45 °C, 1 bar | [26] |
| Sepiolite | 65 | 25 °C, 1 bar | [42] |
| Sepiolite | 137 | 25 °C, 120 bar | [43] |
| Palygorskite | 12 | 45 °C, 1 bar | [26] |
| Palygorskite | 18 | 25 °C, 1 bar | [42] |
| Clay Mineral | CO2 Adsorption Capacity (mg CO2/g) | Adsorption Conditions | Ref. |
|---|---|---|---|
| Kaolinite | 3 | 25 °C, 1 bar | [23] |
| Bentonite | 9 | 25 °C, 1 bar | [47] |
| Bentonite | 24 | 25 °C, 1 bar | [49] |
| Sepiolite | 41 | 25 °C, 1 bar | [42] |
| Palygorskite | 43 | 25 °C, 1 bar | [42] |
| Clay Mineral | Organic Compound Intercalated | CO2 Adsorption Capacity (mg CO2/g) | Adsorption Conditions | Ref. |
|---|---|---|---|---|
| Montmorillonite | Polyols | 110 | 25 °C, 1 bar | [51] |
| Laponite | PAMAN | 36 | 25 °C, 1 bar | [54] |
| Montmorillonite | Polyamido amine | 20 | 40 °C, 1 bar | [56] |
| Clay Mineral | Porous Material Synthesized | CO2 Adsorption Capacity (mg CO2/g) | Adsorption Conditions | Ref. |
|---|---|---|---|---|
| Bentonite | Zeolite 13X | 211 | 25 °C, 1 bar | [58] |
| Kaolinite | Zeolite A | 0.46 | 25 °C, 1 bar, 77 s | [60] |
| Kaolinite | ZSM-5 | 13 | 25 °C, 1 bar | [63] |
| Kaolinite | Zeolite A | 40 | 25 °C, 1 bar | [63] |
| Kaolinite | SAPO-34 | 26 | 25 °C, 1 bar | [63] |
| Kaolinite | Zeolite Y | 130 | 0 °C, 1 bar | [62] |
| Kaolinite/limestone | Gehlenite/Stilbite | 295 | 100 °C, 45 bar | [64] |
| Montmorillonite | Zeolite A | 220 | 25 °C, 1 bar | [61] |
| Montmorillonite | Pillared clays (PILCs) | 57 | 0 °C, 1 bar | [66] |
| Saponite | Pillared clays (PILCs) | 48 | 0 °C, 1 bar | [66] |
| Montmorillonite | Pillared clays (PILCs) | 52 | 25 °C, 1 bar | [67] |
| Montmorillonite | Porous clay heterostructure (PCH) | 28 | 25 °C, 1 bar | [25] |
| Montmorillonite | Porous clay heterostructure (PCH) | 22 | 25 °C, 1 bar | [28] |
| Clay Mineral | Amine Grafted | CO2 Adsorption Capacity (mg CO2/g) | Adsorption Conditions | Ref. |
|---|---|---|---|---|
| Bentonite | APTES | 43 | 45 °C, 1 bar | [26] |
| Bentonite | TMSPDEA | 32 | 45 °C, 1 bar | [26] |
| Montmorillonite | APTES | 33 | 45 °C, 1 bar | [64] |
| Montmorillonite | TMSPDEA | 50 | 45 °C, 1 bar | [26] |
| Montmorillonite | AEAPTS | 77 | 100 °C, 1 bar, 90 min | [27] |
| Exfoliated-montmorillonite | AEAPTS | 105 | 100 °C, 1 bar, 90 min | [27] |
| Saponite | APTES | 35 | 45 °C, 1 bar | [26] |
| Saponite | TMSPDEA | 39 | 45 °C, 1 bar | [26] |
| Porous clay heterostructure from montmorillonite | APTES | 50 | 25 °C, 1 bar | [25] |
| Porous clay heterostructure from montmorillonite | APTES | 48 | 25 °C, 1 bar | [28] |
| Sepiolite | APTES | 44 | 25 °C, 1 bar | [42] |
| Sepiolite | APTES | 121 | 70 °C, 1 bar, 120 min | [77] |
| Sepiolite | APTES | 44 | 45 °C, 1 bar | [26] |
| Sepiolite | TMSPDEA | 61 | 45 °C, 1 bar | [26] |
| Palygorskite | APTES | 33 | 25 °C, 1 bar | [42] |
| Palygorskite | APTES | 38 | 45 °C, 1 bar | [26] |
| Palygorskite | TMSPDEA | 57 | 45 °C, 1 bar | [26] |
| Halloysite | APTES | 5 | 85 °C, 1 bar | [33] |
| Clay Mineral | Amine-Rich Polymer | CO2 Adsorption Capacity (mg CO2/g) | Adsorption Conditions | Ref. |
|---|---|---|---|---|
| Kaolinite | MEA + DEA | 144 | 25 °C, 1 bar | [22] |
| ZSM-5 from kaolinite | TEPA | 7 | 25 °C, 1 bar | [63] |
| Zeolite-Y from kaolinite | TEPA | 50 | 25 °C, 1 bar | [63] |
| SAPO-34 from kaolinite | TEPA | 23 | 25 °C, 1 bar | [63] |
| Bentonite | MEA | 129 | 25 °C, 1 bar | [79] |
| Bentonite | DEA | 125 | 25 °C, 1 bar | [79] |
| Bentonite | TEA | 114 | 25 °C, 1 bar | [79] |
| Bentonite | PEI | 46 | 45 °C, 1 bar | [26] |
| Bentonite modified by acid treatment | PEI | 47 | 75 °C, 1 bar | [24] |
| Montmorillonite | PEI | 60 | 45 °C, 1 bar | [26] |
| Montmorillonite | DMDAA | 211 | 25 °C, 50 bar | [81] |
| Montmorillonite modified by acid treatment | PEI | 112 | 70 °C, 1 bar | [67] |
| Montmorillonite modified by acid treatment | TEPA | 136 | 70 °C, 1 bar | [67] |
| Montmorillonite modified by acid treatment | TEPA | 190 | 70 °C, 1 bar, 18% moisture | [67] |
| Saponite | PEI | 67 | 45 °C, 1 bar | [26] |
| Porous clay heterostructure from montmorillonite | PEI | 64 | 25 °C, 1 bar | [25] |
| Porous clay heterostructure from montmorillonite | TEPA | 72 | 25 °C, 1 bar | [25] |
| Porous clay heterostructure from montmorillonite | PEI | 110 | 25 °C, 1 bar | [28] |
| Sepiolite | TEPA | 165 | 60 °C, 1 bar | [83] |
| Sepiolite | PEI | 56 | 45 °C, 1 bar | [26] |
| Sepiolite | PEI | 45 | 25 °C, 1 bar | [42] |
| Sepiolite modified by acid treatment | DETA | 73 | 70 °C, 1 bar | [86] |
| Sepiolite modified by acid treatment | TEPA | 160 | 70 °C, 1 bar | [77] |
| Sepiolite modified by acid treatment | PEI | 109 | 75 °C, 1 bar | [84] |
| Sepiolite modified by acid treatment | TETA | 85 | 50 °C, 1 bar | [85] |
| Palygorskite | PEI | 67 | 45 °C, 1 bar | [26] |
| Palygorskite | PEI | 32 | 25 °C, 1 bar | [42] |
| Palygorskite modified by acid treatment | PEI | 34 | 25 °C, 1 bar | [42] |
| Palygorskite modified by acid treatment | TEPA | 110 | 25 °C, 1 bar | [87] |
| Halloysite | PEI | 121 | 85 °C, 1 bar, 2 h | [88] |
| Clay Mineral | Grafted Amine/Amine-Rich Polymer | CO2 Adsorption Capacity (mg CO2/g) | Adsorption Conditions | Ref. |
|---|---|---|---|---|
| Montmorillonite | APTES/PEI | 18 | 45 °C, 1 bar | [26] |
| Montmorillonite | TMSPDEA/PEI | 11 | 45 °C, 1 bar | [26] |
| Sepiolite | APTES/PEI | 37 | 45 °C, 1 bar | [26] |
| Sepiolite | TMSPDEA/PEI | 33 | 45 °C, 1 bar | [26] |
| Sepiolite | APTES/PEI | 62 | 25 °C, 1 bar | [42] |
| Sepiolite | APTES/PEI | 91 | 65 °C, 1 bar | [42] |
| Sepiolite | APTES/Octadecylamine | 185 | 25 °C, 50 bar | [81] |
| Sepiolite modified by acid treatment | APTES/PEI | 48 | 25 °C, 1 bar | [42] |
| Palygorskite | APTES/PEI | 46 | 25 °C, 1 bar | [42] |
| Paygorskite modified by acid treatment | APTES/PEI | 34 | 25 °C, 1 bar | [42] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chouikhi, N.; Cecilia, J.A.; Vilarrasa-García, E.; Besghaier, S.; Chlendi, M.; Franco Duro, F.I.; Rodriguez Castellon, E.; Bagane, M. CO2 Adsorption of Materials Synthesized from Clay Minerals: A Review. Minerals 2019, 9, 514. https://doi.org/10.3390/min9090514
Chouikhi N, Cecilia JA, Vilarrasa-García E, Besghaier S, Chlendi M, Franco Duro FI, Rodriguez Castellon E, Bagane M. CO2 Adsorption of Materials Synthesized from Clay Minerals: A Review. Minerals. 2019; 9(9):514. https://doi.org/10.3390/min9090514
Chicago/Turabian StyleChouikhi, Nesrine, Juan Antonio Cecilia, Enrique Vilarrasa-García, Sabrine Besghaier, Mohamed Chlendi, Francisco Ignacio Franco Duro, Enrique Rodriguez Castellon, and Mohamed Bagane. 2019. "CO2 Adsorption of Materials Synthesized from Clay Minerals: A Review" Minerals 9, no. 9: 514. https://doi.org/10.3390/min9090514
APA StyleChouikhi, N., Cecilia, J. A., Vilarrasa-García, E., Besghaier, S., Chlendi, M., Franco Duro, F. I., Rodriguez Castellon, E., & Bagane, M. (2019). CO2 Adsorption of Materials Synthesized from Clay Minerals: A Review. Minerals, 9(9), 514. https://doi.org/10.3390/min9090514









