Niobium Mineralogy of Pliocene A1-Type Granite of the Carpathian Back-Arc Basin, Central Europe
Abstract
:1. Introduction
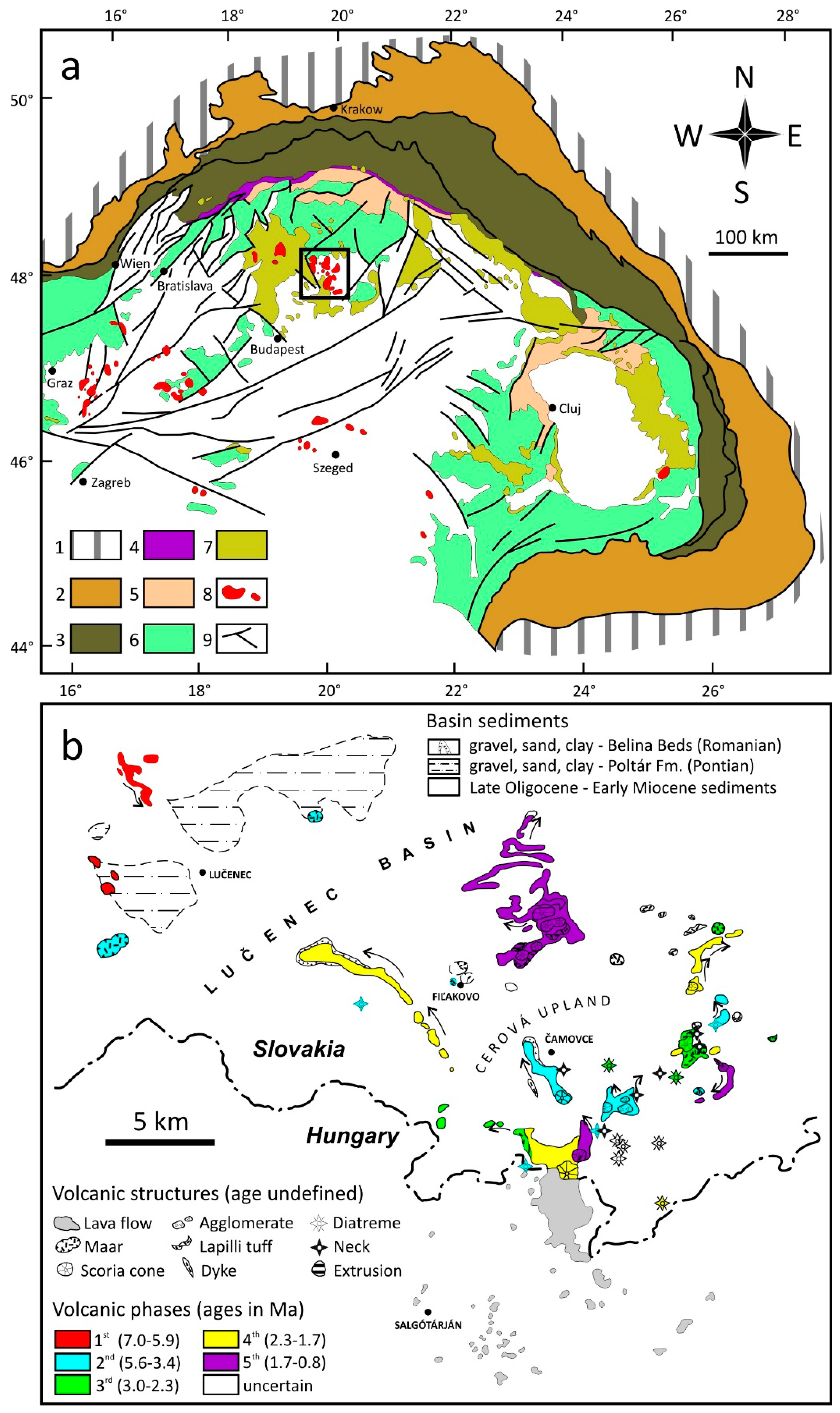
2. Geological Setting
3. Methods
4. Results
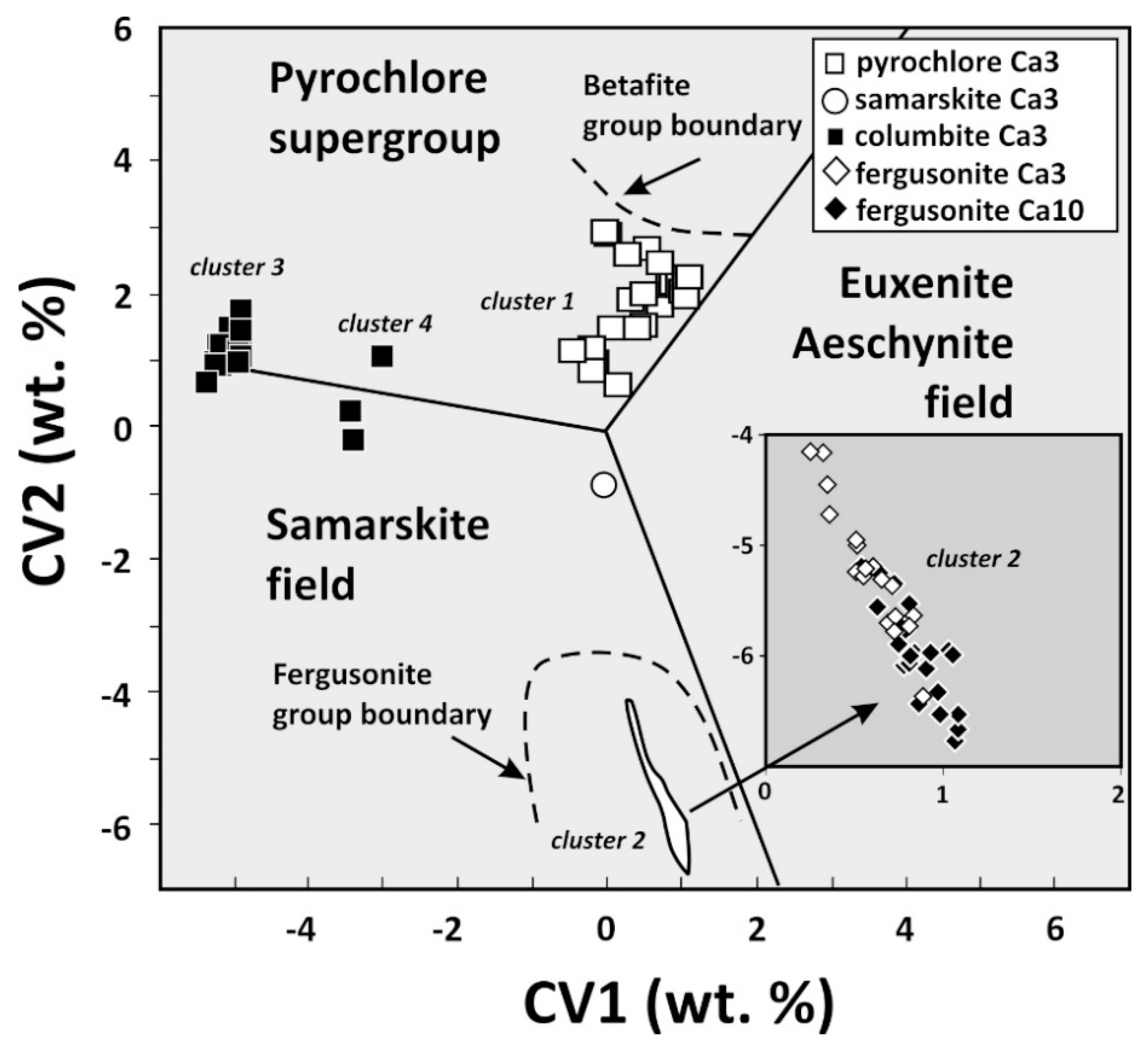
4.1. Characterization of Nb-Bearing Phases
4.2. Crystal Chemistry of Nb-Bearing Phases
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Simandl, G.J.; Burt, R.O.; Trueman, D.L.; Paradis, S. Economic Geology Models 2. Tantalum and Niobium: Deposits, resources, exploration methods and Market—A primer for geoscientists. Geosci. Can. 2018, 45, 85–96. [Google Scholar] [CrossRef]
- Bonin, B. A-type granites and related rocks: Evolution of a concept, problems and prospects. Lithos 2007, 97, 1–29. [Google Scholar] [CrossRef]
- Dall’Agnol, R.; Frost, C.D.; Tapani Rämö, O. IGCP Project 510 “A-type granites and related rocks through time” Project vita, results, and contribution to granite research. Lithos 2012, 151, 1–16. [Google Scholar] [CrossRef]
- Loiselle, M.C.; Wones, D.R. Characteristics and origin of anorogenic granites. In Geological Society of America Abstracts with Programs; Geological Society of America: Boulder, CO, USA, 1979; Volume 11, p. 468. [Google Scholar]
- Pearce, J.A.; Harris, N.B.W.; Tindle, A.G. Trace element discrimination diagrams for the tectonic interpretation of granitic rocks. J. Petrol. 1984, 25, 956–983. [Google Scholar] [CrossRef]
- Whalen, J.B.; Currie, K.L.; Chappell, B.W. A-type granites: Geochemical characteristics, discrimination and petrogenesis. Contrib. Mineral. Petrol. 1987, 95, 407–419. [Google Scholar] [CrossRef]
- Eby, G.N. The A-type granitoids: A review of their occurrence and chemical characteristics and speculations on their petrogenesis. Lithos 1990, 26, 115–134. [Google Scholar] [CrossRef]
- Eby, G.N. Chemical subdivision of the A-type granitoids: Petrogenetic and tectonic implications. Geology 1992, 20, 641–644. [Google Scholar] [CrossRef]
- Shellnutt, J.G.; Zhou, M.-F. Permian peralkaline, peraluminous and metaluminous A-type granites in the Panxi district, SW China: Their relationship to the Emeishan mantle plume. Chem. Geol. 2007, 243, 286–316. [Google Scholar] [CrossRef]
- Frost, C.D.; Frost, B.R. On ferroan (A-type) granitoids: Their compositional variability and modes of origin. J. Petrol. 2011, 52, 39–55. [Google Scholar] [CrossRef]
- King, P.L.; White, A.J.R.; Chappell, B.W.; Allen, C.M. Characterization and origin of aluminous A-type granites from the Lachlan Fold Belt, Southeastern Australia. J. Petrol. 1997, 38, 371–391. [Google Scholar] [CrossRef]
- Shao, F.; Niu, Y.; Regelous, M.; Zhu, D.-C. Petrogenesis of peralkaline rhyolites in an intra-plate setting: Glass House Mountains, southeast Queensland, Australia. Lithos 2015, 216–217, 196–210. [Google Scholar] [CrossRef]
- Wang, C.; Chen, L.; Bagas, L.; Lu, Y.; He, X.; Lai, X. Characterization and origin of the Taishanmiao aluminous A-type granites: Implications for early Cretaceous lithospheric thinning at the southern margin of the North China Craton. Int. J. Earth Sci. 2016, 105, 1563–1589. [Google Scholar] [CrossRef]
- Jiang, X.-Y.; Ling, M.-X.; Wu, K.; Zhang, Z.-K.; Sun, W.-D.; Sui, Q.-L.; Xia, X.-P. Insights into the origin of coexisting A1- and A2-type granites: Implications from zircon Hf-O isotopes of the Huayuangong intrusion in the Lower Yiangtze River Belt, eastern China. Lithos 2018, 318–319, 230–243. [Google Scholar] [CrossRef]
- Martin, R.F. A-type granites of crustal origin ultimately result from open-system fenitization-type reactions in an extensional environment. Lithos 2006, 91, 125–135. [Google Scholar] [CrossRef]
- Huraiová, M.; Hurai, V.; Paquette, J.-L. Petrogenesis of Miocene-Pliocene A-type granitoids of southern Slovakia. Acta Geol. Slovaca 2015, 7, 37–50, (In Slovak with English Resume). [Google Scholar]
- Huraiová, M.; Paquette, J.-L.; Konečný, P.; Gannoun, A.-M.; Hurai, V. Geochemistry, mineralogy, and zircon U-Pb-Hf isotopes in peraluminous A-type granite xenoliths in Pliocene-Pleistocene basalts of northern Pannonian Basin (Slovakia). Contrib. Mineral. Petrol. 2017, 172, 59. [Google Scholar] [CrossRef]
- Nemcok, M.; Pospisil, L.; Lexa, J.; Donelick, R.A. Tertiary subduction and slab break-off model of the Carpathian-Pannonian region. Tectonophys 1998, 295, 307–340. [Google Scholar] [CrossRef]
- Konečný, V.; Kováč, M.; Lexa, J.; Šefara, J. Neogene evolution of the Carpatho-Pannonian region: An interplay of subduction and back-arc diapiric uprise in the mantle. EGU Stephan Mueller Spec. Publ. Ser. 2002, 1, 165–194. [Google Scholar] [CrossRef]
- Lexa, J.; Seghedi, I.; Németh, K.; Szakács, A.; Konečný, V.; Pécskay, Z.; Fülöp, A.; Kovacs, M. Neogene-Quaternary volcanic forms in the Carpathian-Pannonian Region: A review. Cent. Eur. J. Geosci. 2010, 2, 207–270. [Google Scholar] [CrossRef]
- Konečný, V.; Lexa, J.; Balogh, K.; Konečný, P. Alkali basalt volcanism in Southern Slovakia: Volcanic forms and time evolution. Acta Vulcanol. 1995, 7, 167–172. [Google Scholar]
- Pécskay, Z.; Lexa, J.; Szakács, A.; Seghedi, I.; Balogh, K.; Konečný, V.; Zelenka, T.; Kovacs, M.; Póka, T.; Fülöp, A.; et al. Geochronology of Neogene-Quaternary magmatism in the Carpathian arc and intra-Carpathian area. Geol. Carpath. 2006, 57, 511–530. [Google Scholar]
- Huraiová, M.; Konečný, P.; Konečný, V.; Simon, K.; Hurai, V. Mafic and salic igneous xenoliths in late Tertiary alkaline basalts: Fluid inclusion and mineralogical evidence for a deep crustal magmatic reservoir in the Western Carpathians. Eur. J. Mineral. 1996, 8, 901–9016. [Google Scholar] [CrossRef]
- Hurai, V.; Simon, K.; Wiechert, U.; Hoefs, J.; Konečný, P.; Huraiová, M.; Pironon, J.; Lipka, J. Immiscible separation of metalliferous Fe/Ti oxide melt from fractionating alkali basalt: P-T-fO2 conditions and two-liquid elemental partitioning. Contrib. Mineral. Petrol. 1998, 133, 12–29. [Google Scholar] [CrossRef]
- Konečný, P.; Konečný, V.; Lexa, J.; Huraiová, M. Mantle xenoliths in alkali basalts of Southern Slovakia. Acta Vulcanol. 1995, 7, 241–247. [Google Scholar]
- Dérerová, J.; Zeyen, H.; Bielik, M.; Salman, K. Application of integrated geophysical modeling for determination of the continental lithospheric thermal structure in the eastern Carpathians. Tectonics 2006, 25. [Google Scholar] [CrossRef]
- Tašárová, A.; Afonso, J.C.; Bielik, M.; Götze, H.-J.; Hók, J. The lithospheric structure of the Western Carpathian-Pannonian Basin region based on the CELEBRATION 2000 seismic experiment and gravity modeling. Tectonophys 2009, 475, 454–469. [Google Scholar] [CrossRef]
- Bielik, M.; Alasonati-Tašárová, Z.; Zeyen, H.; Dérerová, J.; Afonso, J.C.; Csicsay, K. Improved geophysical image of the Carpathian-Pannonian basin region. Acta Geol. Geophys. Hung. 2010, 45, 284–298. [Google Scholar] [CrossRef]
- Konečný, V. Palaeogeographical reconstruction, volcanology and time evolution of the Cerova basalt formation. In Geológia Lučenskej Kotliny a Cerovej Vrchoviny; Vass, D., Elečko, M., Konečný, V., Eds.; Štátny Geologický ústav D. Štúra: Bratislava, Slovakia, 2007; pp. 196–202. (In Slovak) [Google Scholar]
- Vass, D.; Elečko, M.; Konečný, V. Geology of Lučenská Kotlina Depression and Cerová Vrchovina Upland; GÚDŠ Publishers: Bratislava, Slovakia, 2007; pp. 1–284. [Google Scholar]
- Hurai, V.; Paquette, J.-L.; Huraiová, M.; Konečný, P. U-Th-Pb geochronology of zircon and monazite from syenite and pincinite xenoliths in Pliocene alkali basalts of the intra-Carpathian back-arc basin. J. Volcanol. Geotherm. Res. 2010, 198, 275–287. [Google Scholar] [CrossRef]
- Hurai, V.; Paquette, J.-L.; Huraiová, M.; Sabol, M. U-Pb geochronology of zircons from fossiliferous sediments of the Hajnáčka I maar (Slovakia)—Type locality of the MN16a biostratigraphic subzone. Geol. Mag. 2012, 149, 989–1000. [Google Scholar] [CrossRef]
- Hurai, V.; Danišík, M.; Huraiová, M.; Paquette, J.-L.; Ádám, A. Combined U/Pb and (U-Th)/He geochronometry of basalt maars in Western Carpathians: Implications for age of intraplate volcanism and origin of zircon metasomatism. Contrib. Mineral. Petrol. 2013, 166, 1235–1251. [Google Scholar] [CrossRef]
- Merlet, C. An accurate computer correction program for quantitative electron probe microanalysis. Microchim. Acta 1994, 114, 363–376. [Google Scholar] [CrossRef]
- Åmli, R.; Griffin, W.L. Standards and correction factors for microprobe analysis of REE minerals. Am. Mineral. 1975, 60, 599–606. [Google Scholar]
- Konečný, P.; Siman, P.; Holický, I.; Janák, M.; Kollárová, V. Method of monazite dating by means of the microprobe. Miner. Slovaca 2004, 36, 225–235. (In Slovak) [Google Scholar]
- Atencio, D.; Andrade, M.B.; Christy, A.G.; Gieré, R.; Kartashov, P.M. The pyrochlore supergroup of minerals: Nomenclature. Can. Mineral. 2010, 48, 673–698. [Google Scholar] [CrossRef]
- Ercit, T.S. Identification and alteration trends of granitic-pegmatite-hosted (Y,REE,U,Th)-(Nb,Ta,Ti) oxide minerals: A statistical approach. Can. Mineral. 2005, 43, 1291–1303. [Google Scholar] [CrossRef]
- Cámara, F.; Willimas, C.T.; Della Ventura, G.; Oberti, R.; Caprilli, E. Non-metamict betafite from Le Carcarelle (Vico volcanic complex, Italy): Occurrence and crystal structure. Mineral. Mag. 2004, 68, 939–950. [Google Scholar] [CrossRef]
- Caprilli, E.; Della Ventura, G.; Williams, T.C.; Parodi, G.C.; Tuccimei, P. The crystal chemistry of non-metamict pyrochlore group minerals from Latium, Italy. Can. Mineral. 2006, 44, 1367–1378. [Google Scholar] [CrossRef]
- Lumpkin, G.R.; Gieré, R.; Williams, C.T.; McGlinn, P.J.; Payne, T.E. Petrography and chemistry of tungsten-rich oxycalciobetafite in hydrothermal veins of the Adamello contact aureole, northern Italy. Mineral. Petrol. 2017, 111, 499–509. [Google Scholar] [CrossRef]
- Hatert, F.; Burke, E.A.J. The IMA–CNMNC dominant-constituent rule revisited and extended. Can. Mineral. 2008, 46, 717–728. [Google Scholar] [CrossRef]
- Hanson, S.L.; Simmons, W.B.; Falster, A.U.; Foord, E.E.; Lichte, F.E. Proposed nomenclature for samarskite-group minerals: New data on ishikawaite and calciosamarskite. Mineral. Mag. 1999, 63, 27–36. [Google Scholar] [CrossRef]
- Le Maitre, R.W.; Streckeisen, A.; Zanettin, B.; Le Bas, M.J.; Bonin, B.; Bateman, P.; Bellieni, G.; Dudek, A.; Efremova, S.; Keller, J.; et al. Igneous Rocks: A Classification and Glossary of Terms, Recommendations of the International Union of Geological Sciences, Subcommision of the Systematics of Igneous Rocks; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Frost, B.R.; Arculus, R.J.; Barnes, C.G.; Collins, W.J.; Ellis, D.J.; Frost, C.D. A geochemical classification of granitic rocks. J. Petrol. 2001, 42, 2033–2048. [Google Scholar] [CrossRef]
- Graeser, S.; Schwander, H.; Hänni, H. Vigezzite, (Ca, Ce)(Nb,Ta,Ti)2O6, a new aeschynite-type mineral from the Alps. Mineral. Mag. 1979, 43, 459–492. [Google Scholar] [CrossRef]
- Černý, P.; Ercit, T.S. Mineralogy of niobium and tantalum: Crystal chemical relationships, paragenetic aspects and their economic implications. In Lanthanides, Tantalum and Niobium; Möller, P., Černý, P., Saupé, F., Eds.; Springer: Berlin/Heidelberg, Germany, 1989; pp. 27–79. [Google Scholar]
- Zhuravleva, L.N.; Ryabeva, Y.G.; Yurkina, K.V.; Solntseva, L.S. Vigezzite—First finding in USSR, Kola Peninsula. Izv. Akad. Nauk SSSR 1989, 8, 134–136. (In Russian) [Google Scholar]
- Bohnstedt-Kupletskaya, E.M.; Burova, T.A. Fersmite, a new calcium niobate from the pegmatites of the Vishnevye Mts.; the Central Urals. Dokl. Akad. Nauk SSSR 1946, 52, 69–71. (In Russian) [Google Scholar]
- Černý, P.; Novák, M.; Chapman, R. Effects of sillimanite-grade metamorphism and shearing on Nb-Ta oxide minerals in granitic pegmatites: Maršíkov, northern Moravia, Czechoslovakia. Can. Mineral. 1992, 30, 699–718. [Google Scholar]
- Aurisicchio, C.; De Vito, C.; Ferrini, V.; Orlandi, P. Nb-Ta oxide minerals from miarolitic pegmatites of the Baveno pink granite, NW Italy. Mineral. Mag. 2001, 65, 509–522. [Google Scholar] [CrossRef]
- Beurlen, H.; Soares, D.R.; Thomas, R.; Prado-Borges, L.E.; De Castro, C. Mineral chemistry of tantalate species in the Borborema Pegmatitic Province, Northeast Brazil. Anais da Academia Brasileira de Ciências 2005, 77, 169–182. [Google Scholar] [CrossRef]
- Guastoni, A.; Diella, V.; Pezzotta, F. Vigezzite and associated oxides of Nb-Ta from emerald-bearing pegmatites of the Vigezzo Valley, Western Alps, Italy. Can. Mineral. 2008, 46, 619–633. [Google Scholar] [CrossRef]
- Hess, H.D.; Trumpour, H.J. Second occurrence of fersmite. Am. Mineral. 1959, 44, 1–8. [Google Scholar]
- Săbău, G. Lanthanide-free fersmite in the lithian pegmatites at Conţu, Lotru Mts., and its relationship with other niobo-tantalates. In Proceedings of the Annual Conference of the Geological Society of Romania, Bucharest, Romania, 5–6 November 2010. [Google Scholar]
- Černý, P.; Chapman, R.; Simmons, W.B.; Chackowsky, L.E. Niobian rutile from the McGuire granitic pegmatite, Park County, Colorado: Solid solution, exsolution, and oxidation. Am. Mineral. 1999, 84, 754–763. [Google Scholar] [CrossRef]
- Yang, Z.; Smith, M.; Henderson, P.; LeBas, M.J.; Tao, K.; Zhang, P. Compositional variation of aeschynite-group minerals in the Bayan Obo Nb-REE-Fe ore deposit, Inner Mongolia, China. Eur. J. Mineral. 2001, 13, 1207–1214. [Google Scholar] [CrossRef]
- Hirtopanu, P.; Fairhurst, R.J.; Jakab, G. Niobian rutile and its associations at Jolotca, Ditrau alkaline intrusive massif, east Carpathians, Romania. Proc. Rom. Acad. Ser. B 2015, 17, 39–55. [Google Scholar]
- Gronen, L.H.; Sindern, S.; Katzmarzyk, J.L.; Bormann, U.; Hellman, A.; Wotruba, H.; Meyer, F.M. Mineralogical and chemical characterization of Zr-REE-Nb ores from Khalzan Buregtei (Mongolia)–Approaches to more efficient extraction of rare metals from alkaline granitoids. Minerals 2019, 9, 217. [Google Scholar] [CrossRef]
- Bea, F. Residence of REE, Y, Th and U in granites and crustal protoliths; implications for the chemistry of crustal melts. J. Petrol. 1996, 37, 521–552. [Google Scholar] [CrossRef]
- Sharygin, V.V.; Sobolev, N.V.; Channer, D.M. Oscillatory-zoned crystals of pyrochlore-group minerals from the Guaniamo kimberlites, Venezuela. Lithos 2009, 112, 976–985. [Google Scholar] [CrossRef]
- Ohnenstetter, D.; Piantone, P. Pyrochlore group minerals in the Beauvoir peraluminous leucogranite, Massif Central, France. Can. Mineral. 1992, 30, 771–784. [Google Scholar]
- Ogunleye, P.O.; Garba, I.; Ike, E.C. Factors contributing to enrichment and crystallization of niobium in pyrochlore in the Kaffo albite arfvedsonite granite, Ririwai Complex, Younger Granite province of Nigeria. J. Afr. Earth Sci. 2006, 44, 372–382. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, Z.; Santosh, M.; Zhang, D. Geochronology, geochemistry and metallogenic implications of the Boziguo’er rare metal-bearing peralkaline granitic intrusion in South Tianshan, NW China. Ore Geol. Rev. 2014, 61, 157–174. [Google Scholar] [CrossRef]
- Novák, M.; Černý, P.; Uher, P. Extreme variation and apparent reversal Nb-Ta fractionation in columbite-group minerals from Scheibengraben beryl-columbite granitic pegmatite, Maršíkov, Czech Republic. Eur. J. Mineral. 2003, 15, 565–574. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Reguir, E.P.; Kressall, R.D.; Crozier, J.; Pisiak, L.K.; Sidhu, R.; Yang, P. Carbonatite-hosted niobium deposit at Aley, northern British Columbia (Canada): Mineralogy, geochemistry and petrogenesis. Ore Geol. Rev. 2015, 64, 642–666. [Google Scholar] [CrossRef]
- Paquette, J.-L.; Mergoil-Daniel, J. Origin and U-Pb dating of zircon-bearing nepheline syenite xenoliths preserved in basaltic tephra (Massif Central, France). Contrib. Mineral. Petrol. 2009, 158, 245–262. [Google Scholar] [CrossRef]
- Mokhov, A.V.; Kartashov, P.M.; Bogatikov, O.A.; Ashikhmina, N.A.; Magazina, L.O.; Koporulina, E.V. Fluorite, hatchettolite, calcium sulfate, and bastnäsite-(Ce) in the lunar regolith from Mare Crisium. Dokl. Earth Sci. 2008, 422, 1178–1180. [Google Scholar] [CrossRef]
- Hogarth, D.D. A study of pyrochlore and betafite. Can. Mineral. 1961, 6, 610–633. [Google Scholar]
- Černý, P.; Hawthorne, F.C.; Laflamme, J.H.G.; Hinthorne, J.R. Stibiobetafite, a new member of the pyrochlore group from Vežná, Czechoslovakia. Can. Mineral. 1979, 17, 583–588. [Google Scholar]
- Williams, C.T. The occurrence of niobian zirconolite, pyrochlore and baddeleyite in the Kovdor carbonatite complex, Kola Peninsula, Russia. Mineral. Mag. 1996, 60, 639–646. [Google Scholar] [CrossRef]
- Matsubara, S.; Miyawaki, R.; Yokoyama, K.; Momma, K.; Shigeoka, M.; Hashimoto, E. Pyrochlore and microlite in a pegmatite at Atagoyama, Koriyama City, Fukushima Prefecture, Japan. Bull. Nat. Mus. Nat. Sci. Ser. C 2013, 39, 1–6. [Google Scholar]
- Chukanov, N.V.; Skrigitil, A.M.; Kuzmina, O.V.; Zadov, A.E. Bismuthopyrochlore (Bi, U, Ca, Pb)1+x(Nb,Ta)2O6(OH)·nH2O—A new mineral from the Mika pegmatite vein (eastern Pamirs). Zap. Vses. Mineral. Obsh. 1999, 128, 36–41. (In Russian) [Google Scholar]
- Meyer, C.; Yang, S.V. Tungsten-bearing yttrobetafite in lunar granophyre. Am. Mineral. 1988, 73, 1420–1425. [Google Scholar]
- Mackay, D.A.R.; Simandl, G.J. Pyrochlore and columbite-tantalite as indicator minerals for specialty metal deposits. Geochem. Explor. Environ. Anal. 2015, 15, 167–178. [Google Scholar] [CrossRef]
- Uher, P.; Ondrejka, M.; Konečný, P. Magmatic and post-magmatic Y-REE-Th phosphate, silicate and Nb-Ta-Y-REE oxide minerals in A-type metagranite: An example from the Turčok massif, the Western Carpathians, Slovakia. Mineral. Mag. 2009, 73, 1009–1025. [Google Scholar] [CrossRef]
- Chudík, P.; Uher, P. Pyrochlore-group minerals from granite pegmatites of Western Carpathians: Compositional variations and substitution mechanisms. Miner. Slovaca 2009, 41, 159–168. (In Slovak) [Google Scholar]
- Chudík, P.; Uher, P.; Gadas, P.; Skoda, R.; Pršek, J. Niobium-tantalum oxide minerals in the Jezuitske Lesy granitic pegmatite, Bratislava Massif, Slovakia: Ta to Nb and Fe to Mn evolutionary trends in a narrow Be,Cs-rich and Li,B-poor dike. Mineral. Petrol. 2011, 102, 15–27. [Google Scholar] [CrossRef]
- Dianiška, I.; Uher, P.; Hurai, V.; Huraiová, M.; Frank, W.; Konečný, P.; Kráľ, J. Mineralization of rare-metal granites. In Source of Fluids and Origin of Mineralizations of the Gemeric Unit. Geological Project 0503: Source of Fluids and metallogenesis of Western Carpathians; Hurai, V., Ed.; State Geological Institute of D. Štúr and Ministry of Environment of the Slovak Republic: Bratislava, Slovakia, 2007. [Google Scholar]
- Liptai, N.; Patkó, L.; Kovács, I.J.; Hidas, K.; Pintér, Z.; Jeffries, T.; Zajacz, Z.; O’Reilly, S.Y.; Griffin, W.L.; Pearson, N.J.; et al. Multiple metasomatism beneath the Nógrád-Gömör Volcanic Field (northern Pannonian Basin) revealed by upper mantle peridotite xenoliths. J. Petrol. 2017, 58, 1107–1144. [Google Scholar] [CrossRef]
- Woolley, A.R. Lithosphere metasomatism and the petrogenesis of the Chilwa Province of alkaline igneous rocks and carbonatites, Malawi. J. Afr. Earth Sci. 1987, 6, 891–898. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, R.; Marignac, C.; Cuney, M.; Mercadier, J.; Che, X.; Lespinasse, M.-Y. A new style of rare metal granite with Nb-rich mica: The Early Cretaceous Huangshan rare-metal granite suite, northern Jiangxi Province, southeast China. Am. Mineral. 2018, 103, 1530–1544. [Google Scholar] [CrossRef]




| Composition (wt. %) | Bulk | Interstitial | Interstitial | Interstitial | MI in frg |
|---|---|---|---|---|---|
| SiO2 | 75.54 | 68.82 | 70.52 | 69.21 | 73.17 |
| TiO2 | 0.04 | 0.01 | 0.01 | bdl | 0.03 |
| Al2O3 | 14.07 | 15.68 | 15.21 | 17.26 | 12.66 |
| FeO | 0.69 | 0.09 | 0.15 | 0.17 | 0.40 |
| MnO | 0.03 | 0.00 | 0.08 | 0.01 | 0.16 |
| MgO | 0.03 | 0.02 | 0.01 | bdl | bdl |
| CaO | 0.78 | 0.21 | 0.10 | 0.19 | 0.60 |
| Na2O | 4.64 | 2.95 | 3.97 | 4.64 | 2.79 |
| K2O | 3.44 | 7.32 | 6.94 | 8.39 | 3.22 |
| Cl | na | 0.04 | 0.04 | 0.02 | na |
| O=Cl | −0.01 | −0.01 | −0.01 | ||
| Total | 99.26 | 95.13 | 97.02 | 99.88 | 93.03 |
| Normative minerals (wt. %) | |||||
| quartz | 33.34 | 24.50 | 21.30 | 9.67 | 46.10 |
| albite | 39.55 | 25.94 | 34.33 | 39.16 | 25.38 |
| anorthite | 3.9 | 1.10 | 0.51 | 0.94 | 3.20 |
| K-feldspar | 20.48 | 45.49 | 42.28 | 49.65 | 20.45 |
| corundum | 1.3 | 2.71 | 1.07 | 0.23 | 3.76 |
| hypersthene | 1.34 | 0.21 | 0.45 | 0.33 | 1.06 |
| Total-alkali-silica (TAS) Scheme [44] | |||||
| A/NKC | 1.10 | 1.19 | 1.07 | 1.01 | 1.38 |
| NK/A | 0.81 | 0.81 | 0.92 | 0.97 | 0.64 |
| prefix 1 | potassic | potassic | potassic | potassic | potassic |
| prefix 2 | peraluminous | peraluminous | peraluminous | peraluminous | peraluminous |
| prefix 3 | subalkalic | alkalic | alkalic | alkalic | subalkalic |
| TAS name | rhyolite | rhyolite | rhyolite | rhyolite | rhyolite |
| Frost et al. Scheme [45] | |||||
| Fe * | 0.96 | 0.82 | 0.96 | 1.00 | 1.00 |
| MALI | 7.35 | 10.57 | 11.14 | 12.85 | 5.82 |
| prefix 4 | ferroan | magnesian | ferroan | ferroan | ferroan |
| prefix 5 | calc-alkalic | alkalic | alkalic | alkalic | calcic |
| Ca | Ti | Th | Nb | |
|---|---|---|---|---|
| W | −0.50 | −0.39 | −0.57 | 0.18 |
| Nb | −0.25 | −0.62 | −0.28 | 1.00 |
| Ta | 0.02 | 0.10 | 0.03 | −0.25 |
| Ti | 0.52 | 1.00 | 0.54 | −0.62 |
| Th | 0.90 | 0.59 | 1.00 | −0.28 |
| U | 0.00 | 0.01 | 0.00 | −0.13 |
| Y | −0.87 | −0.66 | −0.96 | 0.27 |
| Ce | 0.55 | 0.50 | 0.72 | −0.16 |
| Pr | 0.53 | 0.41 | 0.68 | −0.11 |
| Nd | 0.36 | 0.33 | 0.54 | −0.07 |
| Sm | 0.04 | 0.02 | 0.11 | −0.01 |
| Gd | −0.18 | −0.07 | −0.07 | 0.05 |
| Tb | −0.30 | −0.15 | −0.23 | 0.08 |
| Dy | −0.60 | −0.29 | −0.42 | 0.26 |
| Ho | −0.41 | −0.15 | −0.29 | 0.22 |
| Er | −0.88 | −0.47 | −0.81 | 0.35 |
| Tm | −0.61 | −0.42 | −0.59 | 0.21 |
| Yb | −0.53 | −0.31 | −0.62 | 0.11 |
| Lu | −0.25 | −0.32 | −0.38 | 0.15 |
| Mn | 0.28 | 0.04 | 0.45 | −0.06 |
| Ca | 1.00 | 0.37 | 0.90 | −0.25 |
| LREE | 0.39 | 0.27 | 0.59 | −0.03 |
| HREE | −0.92 | −0.46 | −0.78 | 0.34 |
| Y + HREE | −0.94 | −0.62 | −0.97 | 0.32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huraiová, M.; Konečný, P.; Hurai, V. Niobium Mineralogy of Pliocene A1-Type Granite of the Carpathian Back-Arc Basin, Central Europe. Minerals 2019, 9, 488. https://doi.org/10.3390/min9080488
Huraiová M, Konečný P, Hurai V. Niobium Mineralogy of Pliocene A1-Type Granite of the Carpathian Back-Arc Basin, Central Europe. Minerals. 2019; 9(8):488. https://doi.org/10.3390/min9080488
Chicago/Turabian StyleHuraiová, Monika, Patrik Konečný, and Vratislav Hurai. 2019. "Niobium Mineralogy of Pliocene A1-Type Granite of the Carpathian Back-Arc Basin, Central Europe" Minerals 9, no. 8: 488. https://doi.org/10.3390/min9080488
APA StyleHuraiová, M., Konečný, P., & Hurai, V. (2019). Niobium Mineralogy of Pliocene A1-Type Granite of the Carpathian Back-Arc Basin, Central Europe. Minerals, 9(8), 488. https://doi.org/10.3390/min9080488






