Study of K-Feldspar and Lime Hydrothermal Reaction: Phase and Mechanism with Reaction Temperature and Increasing Ca/Si Ratio
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrothermal Method
2.2.1. Reaction Temperature
2.2.2. Reactant Ratio
2.3. Leaching Experiment
2.4. Analytical Methods
2.4.1. SEM and EDS
2.4.2. XRPD
2.4.3. ICP-OES
3. Results and Discussion
3.1. Phase
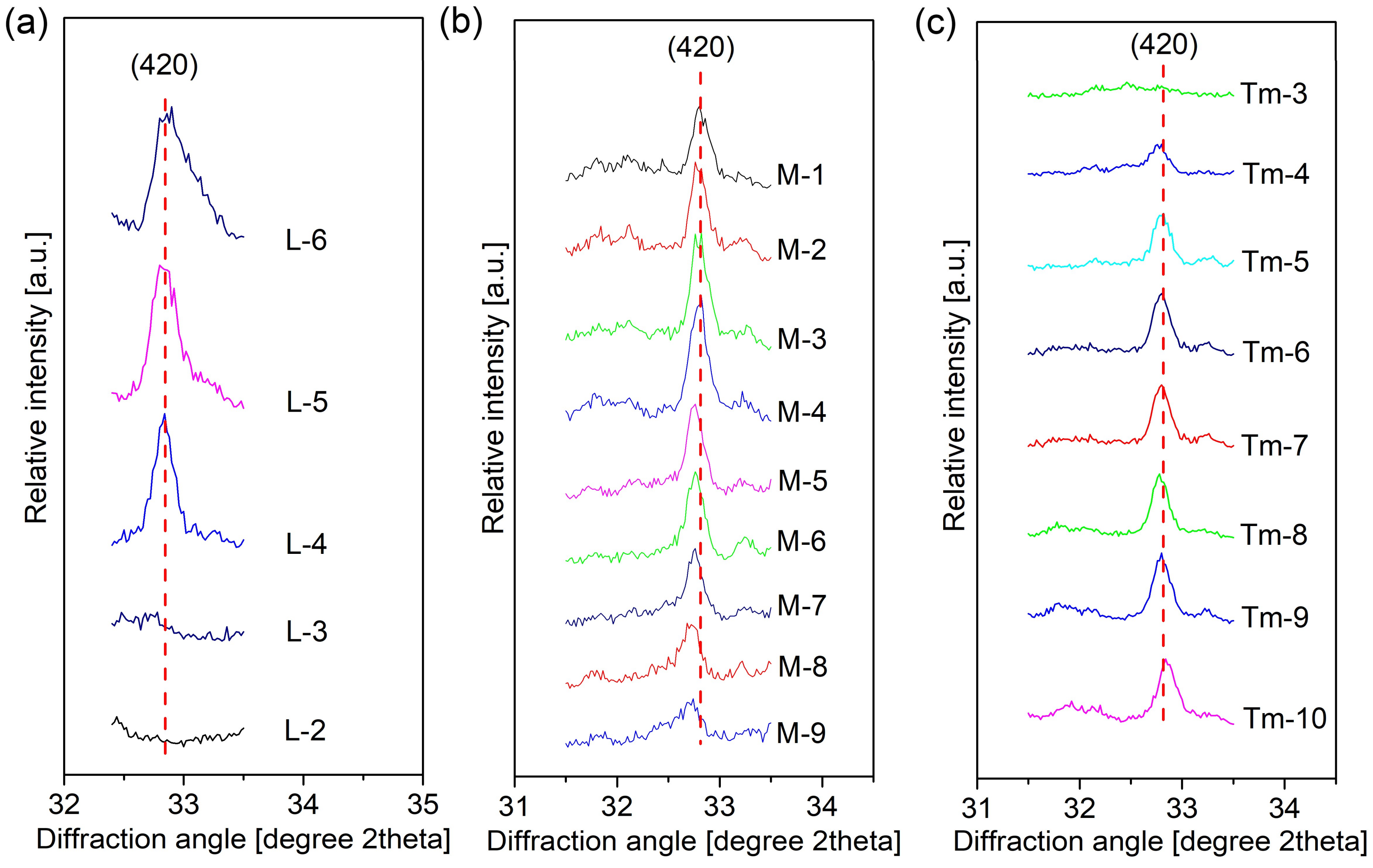
3.1.1. Crystal
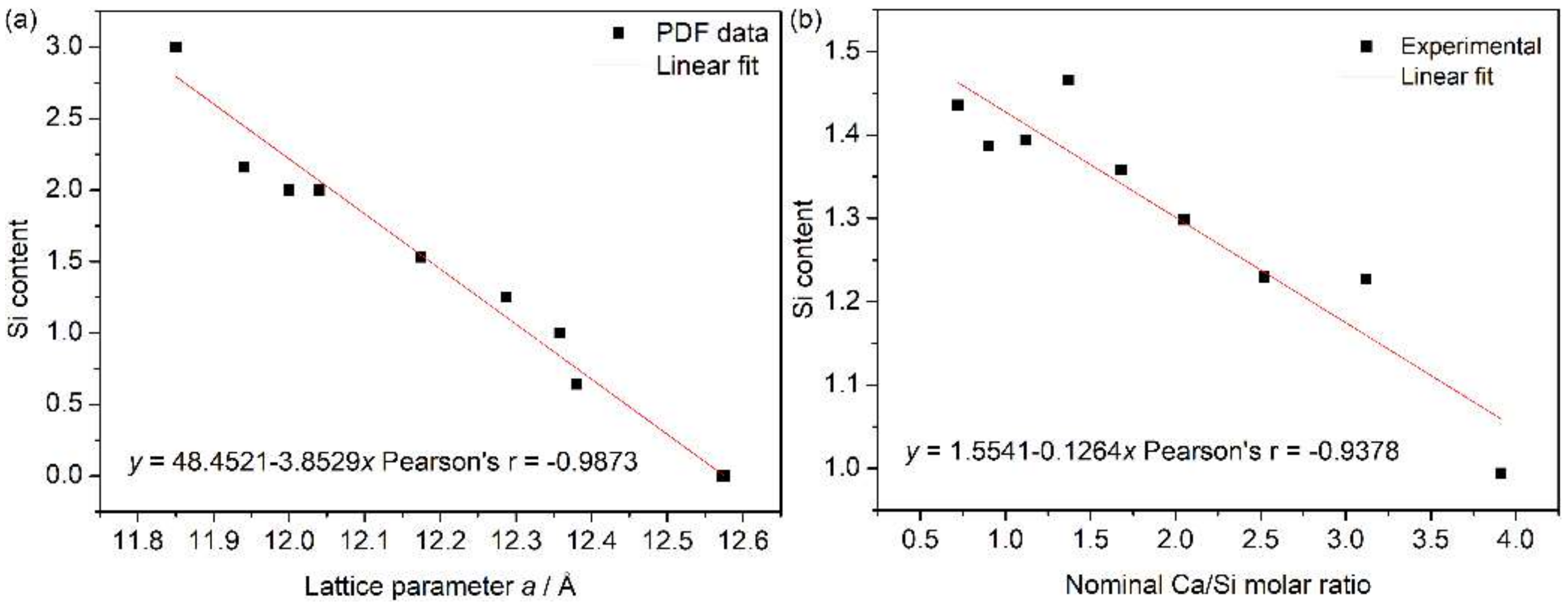
3.1.2. Amorphous
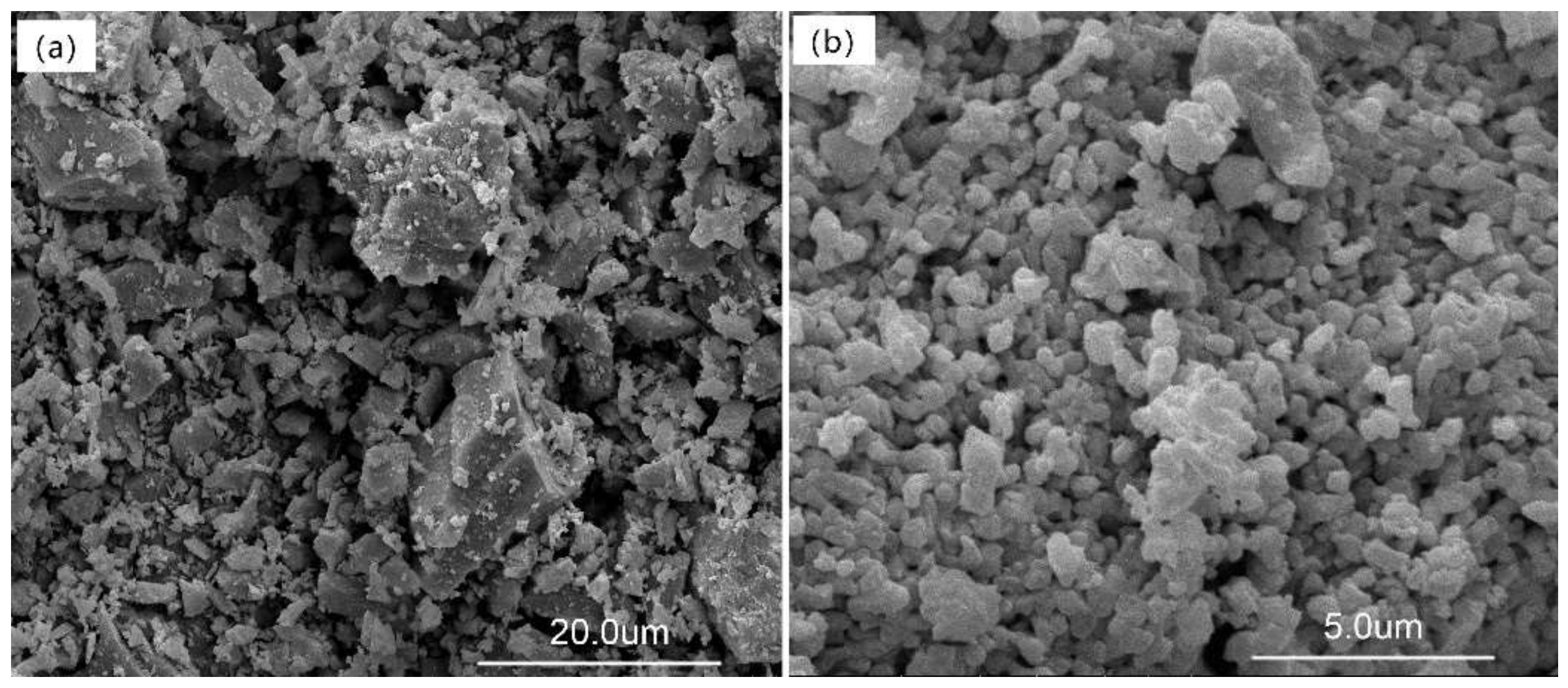
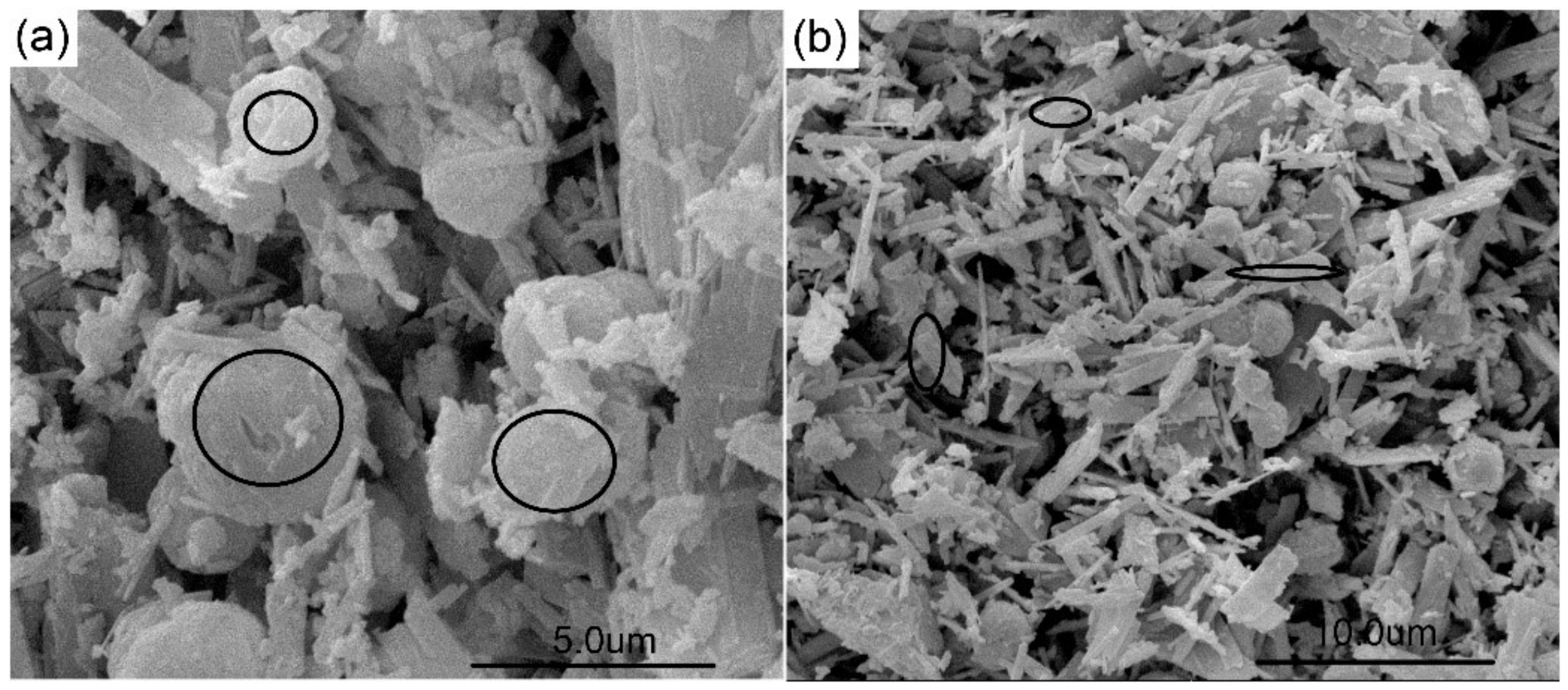
3.2. Morphology
3.3. Dissolution Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klein, C.; Philpotts, A.R. Chapter 7 Igneous rock-forming minerals. In Earth Materials: Introduction to Mineralogy and Petrology, 2nd ed.; Klein, C., Philpotts, A.R., Eds.; Cambridge University Press: New York, NY, USA, 2017; p. 161. [Google Scholar]
- Xu, Y.; Liang, X.; Xu, Y.; Qin, X.; Huang, Q.; Wang, L.; Sun, Y. Remediation of heavy metal-polluted agricultural soils using clay minerals: A review. Pedosphere 2017, 27, 193–204. [Google Scholar] [CrossRef]
- Guo, B.; Liu, B.; Yang, J.; Zhang, S. The mechanisms of heavy metal immobilization by cementitious material treatments and thermal treatments: A review. J. Environ. Manag. 2017, 193, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Misaelides, P. Application of natural zeolites in environmental remediation: A short review. Microporous Mesoporous Mater. 2011, 144, 15–18. [Google Scholar] [CrossRef]
- Liu, J.M.; Liu, S.K.; Han, C.; Sheng, X.B.; Qi, X.; Zhang, Z.L. Mineral technology for soil remediation and improvement: A new applied research direction from mineralogy, petrology and geochemistry. Bull. Mineral. Petrol. Geochem. 2014, 33, 556–560. [Google Scholar]
- Han, C.; Liu, J.M. Method for Preparing Microporous Mineral Fertilizer from Silicates Rock Using Hydrothermal Chemical Reaction. Patent WO2009070953A1, 5 December 2007. [Google Scholar]
- Han, C. Method for Producing Micropore Silicon-Potassium-Calcium Mineral Fertilizer. Patent CN101054313B, 26 April 2007. [Google Scholar]
- Liu, S.K.; Qi, X.; Han, C.; Liu, J.M.; Sheng, X.B.; Li, H.; Luo, A.M.; Li, J.L. Novel nano-submicron mineral-based soil conditioner for sustainable agricultural development. J. Clean. Prod. 2017, 149, 896–903. [Google Scholar] [CrossRef]
- Liu, S.K.; Li, H.; Han, C.; Sheng, X.B.; Liu, J.M. Cd inhibition and pH improvement via a nano-submicron mineral-based soil conditioner. Environ. Sci. Pollut. Res. 2017, 24, 4942–4949. [Google Scholar] [CrossRef] [PubMed]
- Richardson, I.G. The calcium silicate hydrates. Cem. Concr. Res. 2008, 38, 137–158. [Google Scholar] [CrossRef]
- Komarneni, S.; Roy, D.M. Tobermorites: A new family of cation exchangers. Science 1983, 221, 647–648. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.J.; Guo, C.B.; Zhou, X.Q.; Sun, Y.J.; Yang, Z. Sorption capacity and mechanism of Cr3+ on tobermorite derived from fly ash acid residue and carbide slag. Colloids Surf. A 2018, 538, 825–833. [Google Scholar] [CrossRef]
- Guo, X.L.; Shi, H.S. Microstructure and heavy metal adsorption mechanisms of hydrothermally synthesized Al-substituted tobermorite. Mater. Struct. 2017, 50, 10. [Google Scholar] [CrossRef]
- Yao, Z.D.; Tamura, C.; Matsuda, M.; Miyake, M. Resource recovery of waste incineration fly ash: Synthesis of tobermorite as ion exchanger. J. Mater. Res. 1999, 14, 4437–4442. [Google Scholar] [CrossRef]
- Rios, C.A.; Williams, C.D.; Fullen, M.A. Hydrothermal synthesis of hydrogarnet and tobermorite at 175 °C from kaolinite and metakaolinite in the CaO-Al2O3-SiO2-H2O system: A comparative study. Appl. Clay Sci. 2009, 43, 228–237. [Google Scholar] [CrossRef]
- Meller, N.; Kyritsis, K.; Hall, C. The mineralogy of the CaO-Al2O3-SiO2-H2O (CASH) hydroceramic system from 200 to 350 °C. Cem. Concr. Res. 2009, 39, 45–53. [Google Scholar] [CrossRef]
- Meller, N.; Hall, C.; Kyritsis, K.; Giriat, G. Synthesis of cement based CaO-Al2O3-SiO2-H2O (CASH) hydroceramics at 200 and 250 °C: Ex-situ and in-situ diffraction. Cem. Concr. Res. 2007, 37, 823–833. [Google Scholar] [CrossRef]
- Liu, S.K.; Han, C.; Liu, J.M.; Li, H. Research of extracting potassium, silica and aluminum from potassium feldspar by hydrothermal chemical reaction. Acta Mineral. Sin. 2009, 29, 320–326. [Google Scholar]
- Liu, S.K.; Han, C.; Liu, J.M.; Li, H. Hydrothermal decomposition of potassium feldspar under alkaline conditions. RSC Adv. 2015, 5, 93301–93309. [Google Scholar] [CrossRef]
- Ciceri, D.; de Oliveira, M.; Allanore, A. Potassium fertilizer via hydrothermal alteration of K-feldspar ore. Green Chem. 2017, 19, 5187–5202. [Google Scholar] [CrossRef]
- Skorina, T.; Allanore, A. Alkali Metal Ion Source with Moderate Rate of Ion Release and Methods of Forming. U.S. Patent US9340465B2, 6 May 2013. [Google Scholar]
- Liu, S.; Han, C.; Liu, J. Study of K-feldspar and lime hydrothermal reaction at 190 °c: Phase, kinetics and mechanism with reaction time. ChemistrySelect 2018, 3, 13010–13016. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS); Los Alamos National Laboratory Report LAUR; Los Alamos National Laboratory: New Mexico, NM, USA, 2004; pp. 86–748. [Google Scholar]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Reardon, E.J.; Fagan, R. The calcite/portlandite phase boundary: Enhanced calcite solubility at high pH. Appl. Geochem. 2000, 15, 327–335. [Google Scholar] [CrossRef]
- Pabst, A. Synthesis, properties, and structure of K2Ca(CO3)2, Bütschliite. Am. Mineral. 1974, 59, 353–358. [Google Scholar]
- Al-Wakeel, E.I.; El-Korashy, S.A. Reaction mechanism of the hydrothermally treated CaO-SiO2-Al2O3 and CaO-SiO2-Al2O3-CaSO4 systems. J. Mater. Sci. 1996, 31, 1909–1913. [Google Scholar] [CrossRef]
- Klimesch, D.S.; Ray, A. DTA-TG study of the CaO-SiO2-H2O and CaO-Al2O3-SiO2-H2O systems under hydrothermal conditions. J. Therm. Anal. Calorim. 1999, 56, 27–34. [Google Scholar] [CrossRef]
- Klimesch, D.S.; Ray, A. DTA-TGA evaluations of the CaO-Al2O3-SiO2-H2O system treated hydrothermally. Thermochim. Acta 1999, 334, 115–122. [Google Scholar] [CrossRef]
- Watanabe, O.; Kitamura, K.; Maenami, H.; Ishida, H. Hydrothermal treatment of a silica sand complex with lime. J. Am. Ceram. Soc. 2001, 84, 2318–2322. [Google Scholar] [CrossRef]
- Kyritsis, K.; Meller, N.; Hall, C. Chemistry and morphology of hydrogarnets formed in cement-based CASH hydroceramics cured at 200 to 350 °C. J. Am. Ceram. Soc. 2009, 92, 1105–1111. [Google Scholar] [CrossRef]
- Guo, X.L.; Meng, F.J.; Shi, H.S. Microstructure and characterization of hydrothermal synthesis of Al-substituted tobermorite. Constr. Build. Mater. 2017, 133, 253–260. [Google Scholar] [CrossRef]
- Organova, N.I.; Koporulina, E.V.; Ivanova, A.G.; Trubkini, N.V.; Zadov, A.E.; Khomyakov, A.P.; Marcille, I.M.; Chukanov, N.V.; Shmakov, A.N. Structure model of Al,K-substituted tobermorite and structural changes upon heating. Crystallogr. Rep. 2002, 47, 950–956. [Google Scholar] [CrossRef]
- Hu, X.L.; Yanagisawa, K.; Onda, A.; Kajiyoshi, K. Stability and phase relations of dicalcium silicate hydrates under hydrothermal conditions. J. Ceram. Soc. Jpn. 2006, 114, 174–179. [Google Scholar] [CrossRef]
- Pena, R.; Guerrero, A.; Goni, S. Hydrothermal treatment of bottom ash from the incineration of municipal solid waste: Retention of Cs(I), Cd(II), Pb(II) and Cr(III). J. Hazard. Mater. 2006, 129, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Coleman, N.J. Interactions of Cd(II) with waste-derived 11 angstrom tobermorites. Sep. Purif. Technol. 2006, 48, 62–70. [Google Scholar] [CrossRef]
- Hinsinger, P. Potassium. In Encyclopedia of Soil Science; Lal, R., Ed.; Marcel Dekker Inc.: New York, NY, USA, 2002; pp. 1354–1358. [Google Scholar]
- Xu, R.K.; Zhao, A.Z.; Yuan, J.H.; Jiang, J. pH buffering capacity of acid soils from tropical and subtropical regions of China as influenced by incorporation of crop straw biochars. J. Soils Sediments 2012, 12, 494–502. [Google Scholar] [CrossRef]
- Weaver, A.R.; Kissel, D.E.; Chen, F.; West, L.T.; Adkins, W.; Rickman, D.; Luvall, J.C. Mapping soil pH buffering capacity of selected fields in the coastal plain. Soil Sci. Soc. Am. J. 2004, 68, 662–668. [Google Scholar] [CrossRef]
- Biagioni, C.; Merlino, S.; Bonaccorsi, E. The tobermorite supergroup: A new nomenclature. Mineral. Mag. 2015, 79, 485–495. [Google Scholar] [CrossRef]
- Wang, Z.H.; Ma, S.H.; Zheng, S.L.; Wang, X.H. Incorporation of Al and Na in Hydrothermally Synthesized Tobermorite. J. Am. Ceram. Soc. 2017, 100, 792–799. [Google Scholar] [CrossRef]
- Matsui, K.; Kikuma, J.; Tsunashima, M.; Ishikawa, T.; Matsuno, S.-Y.; Ogawa, A.; Sato, M. In situ time-resolved X-ray diffraction of tobermorite formation in autoclaved aerated concrete: Influence of silica source reactivity and Al addition. Cem. Concr. Res. 2011, 41, 510–519. [Google Scholar] [CrossRef]
- Maeda, H.; Abe, K.; Ishida, E.H. Hydrothermal synthesis of aluminum substituted tobermorite by using various crystal phases of alumina. J. Ceram. Soc. Jpn. 2011, 119, 375–377. [Google Scholar] [CrossRef]
- Grew, E.S.; Locock, A.J.; Mills, S.J.; Galuskina, I.O.; Galuskin, E.V.; Halenius, U. Nomenclature of the garnet supergroup. Am. Mineral. 2013, 98, 785–810. [Google Scholar] [CrossRef]
- Cohenaddad, C.; Ducros, P.; Bertaut, E.F. Etude de la substitution du groupement SiO4 par (OH)4 dans les composes Al2Ca3(OH)12 et Al2Ca3(SiO4)2,16(OH)3,36 de type grenat. Acta Crystallogr. 1967, 23, 220–230. [Google Scholar] [CrossRef]
- Oneill, B.; Bass, J.D.; Rossman, G.R. Elastic properties of hydrogrossular garnet and implications for water in the upper-mantle. J. Geophys. Res. Solid Earth 1993, 98, 20031–20037. [Google Scholar] [CrossRef]
- Okoronkwo, M.U.; Glasser, F.P. Compatibility of hydrogarnet, Ca3Al2(SiO4)(x)(OH)(4(3-x)), with sulfate and carbonate-bearing cement phases: 5–85 °C. Cem. Concr. Res. 2016, 83, 86–96. [Google Scholar] [CrossRef]
- Adhikari, P.; Dharmawardhana, C.C.; Ching, W.Y. Structure and properties of hydrogrossular mineral series. J. Am. Ceram. Soc. 2017, 100, 4317–4330. [Google Scholar] [CrossRef]
- Galvankova, L.; Masilko, J.; Solny, T.; Stepankova, E. Tobermorite synthesis under hydrothermal conditions. In Ecology and New Building Materials and Products 2016; Drdlova, M., Kubatova, D., Bohac, M., Eds.; Elsevier Science Bv: Amsterdam, The Netherlands, 2016; Volume 151, pp. 100–107. [Google Scholar]
- Maeda, H.; Kurosaki, Y.; Nakamura, T.; Nakayama, M.; Ishida, E.H.; Kasuga, T. Control of chemical composition of hydrogrossular prepared by hydrothermal reaction. Mater. Lett. 2014, 131, 132–134. [Google Scholar] [CrossRef]
- Grangeon, S.; Fernandez-Martinez, A.; Baronnet, A.; Marty, N.; Poulain, A.; Elkaim, E.; Roosz, C.; Gaboreau, S.; Henocq, P.; Claret, F. Quantitative X-ray pair distribution function analysis of nanocrystalline calcium silicate hydrates: A contribution to the understanding of cement chemistry. J. Appl. Crystallogr. 2017, 50, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.S.; Ma, H.Y.; Li, Z.J. Morphology of calcium silicate hydrate (C-S-H) gel: A molecular dynamic study. Adv. Cem. Res. 2015, 27, 135–146. [Google Scholar] [CrossRef]
- Grangeon, S.; Claret, F.; Lerouge, C.; Warmont, F.; Sato, T.; Anraku, S.; Numako, C.; Linard, Y.; Lanson, B. On the nature of structural disorder in calcium silicate hydrates with a calcium/silicon ratio similar to tobermorite. Cem. Concr. Res. 2013, 52, 31–37. [Google Scholar] [CrossRef]
- Houston, J.R.; Maxwell, R.S.; Carroll, S.A. Transformation of meta-stable calcium silicate hydrates to tobermorite: Reaction kinetics and molecular structure from XRD and NMR spectroscopy. Geochem. Trans. 2009, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Shaw, S.; Clark, S.M.; Henderson, C.M.B. Hydrothermal formation of the calcium silicate hydrates, tobermorite (Ca5Si6O16(OH)(2) · 4H(2)O) and xonotlite (Ca6Si6O17(OH)(2)): An in situ synchrotron study. Chem. Geol. 2000, 167, 129–140. [Google Scholar] [CrossRef]
- Mitsuda, T.; Sasaki, K.; Ishida, H. Phase evolution during autoclaving process of aerated concrete. J. Am. Ceram. Soc. 1992, 75, 1858–1863. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, H.W. The synthesis of tobermorite from potassium feldspar powder: An experimental study. Acta Petrol. Et Mineral. 2005, 24, 333–338. [Google Scholar]
- Qiu, M.Y.; Ma, H.W.; Nie, Y.M.; Zhang, P.; Liu, H. Experimental study on synthesis of tobermorite by decomposing potassium feldspar. Geoscience 2005, 19, 348–354. [Google Scholar]
- Klimesch, D.S.; Ray, A. Effects of quartz particle size and kaolin on hydrogarnet formation during autoclaving. Cem. Concr. Res. 1998, 28, 1317–1323. [Google Scholar] [CrossRef]
- Klimesch, D.S.; Ray, A. Hydrogarnet formation during autoclaving at 180 °C in unstirred metakaolin-lime-quartz slurries. Cem. Concr. Res. 1998, 28, 1109–1117. [Google Scholar] [CrossRef]
- Klimesch, D.S.; Ray, A. Autoclaved cement-quartz pastes with metakaolin additions. Adv. Cem. Based Mater. 1998, 7, 109–118. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, H.; Ma, H. Kinetics of hydrothermal decomposition of potassium feldspar with calcium hydroxide. Adv. Mater. Res. 2012, 549, 65–69. [Google Scholar] [CrossRef]
- Skorina, T.; Allanore, A. Aqueous alteration of potassium-bearing aluminosilicate minerals: From mechanism to processing. Green Chem. 2015, 17, 2123–2136. [Google Scholar] [CrossRef]
- Eckert, J.O.; HungHouston, C.C.; Gersten, B.L.; Lencka, M.M.; Riman, R.E. Kinetics and mechanisms of hydrothermal synthesis of barium titanate. J. Am. Ceram. Soc. 1996, 79, 2929–2939. [Google Scholar] [CrossRef]
- Berger, G.; Beaufort, D.; Lacharpagne, J.C. Experimental dissolution of sanidine under hydrothermal conditions: Mechanism and rate. Am. J. Sci. 2002, 302, 663–685. [Google Scholar] [CrossRef]
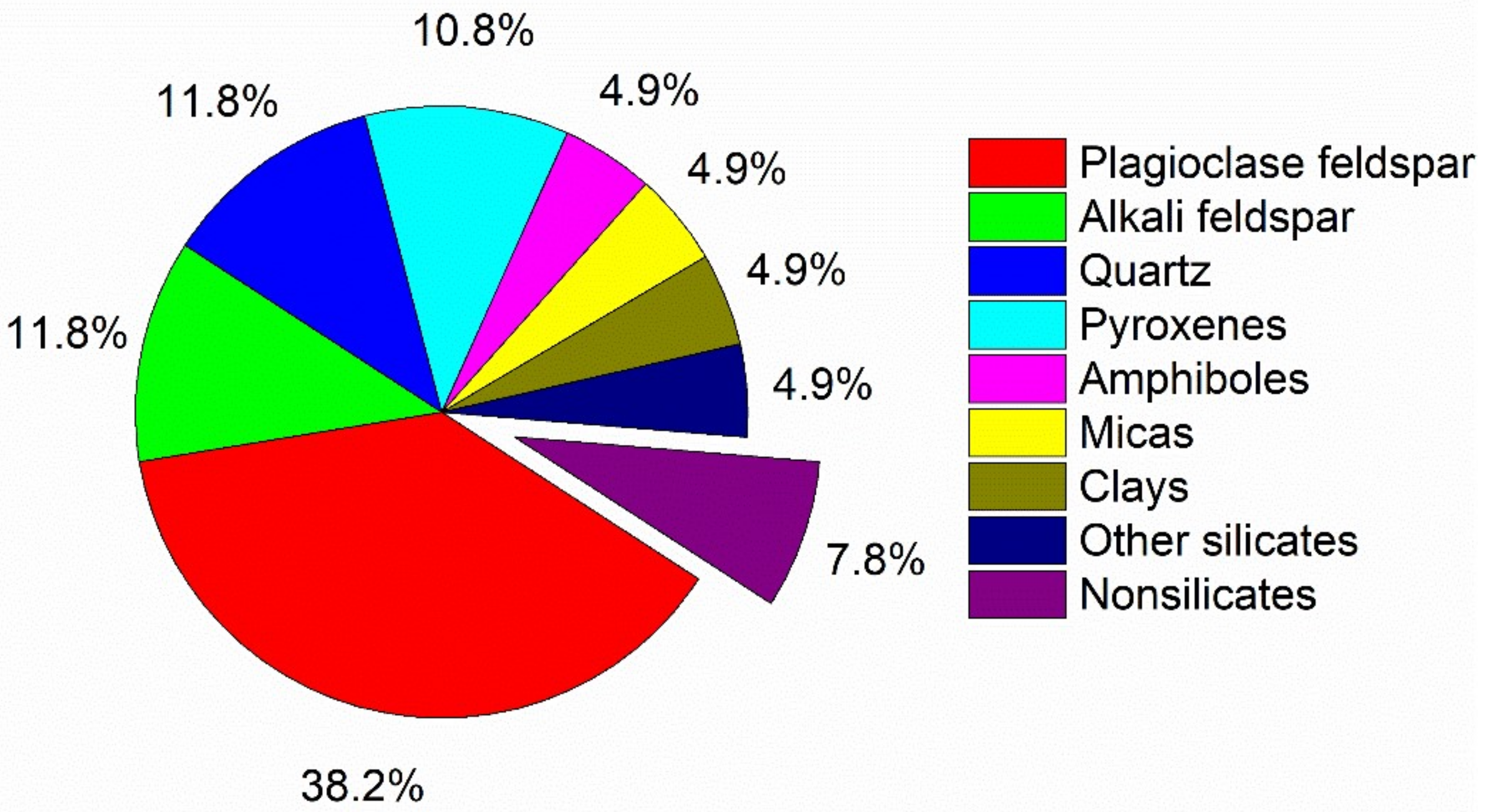
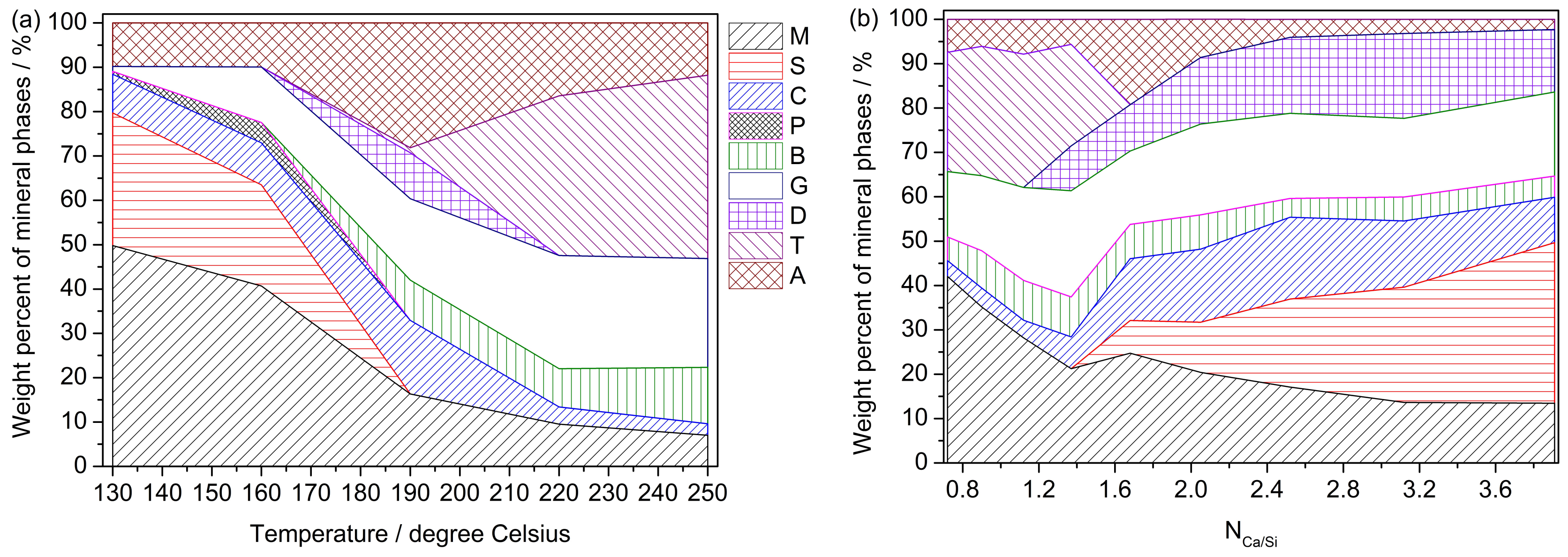
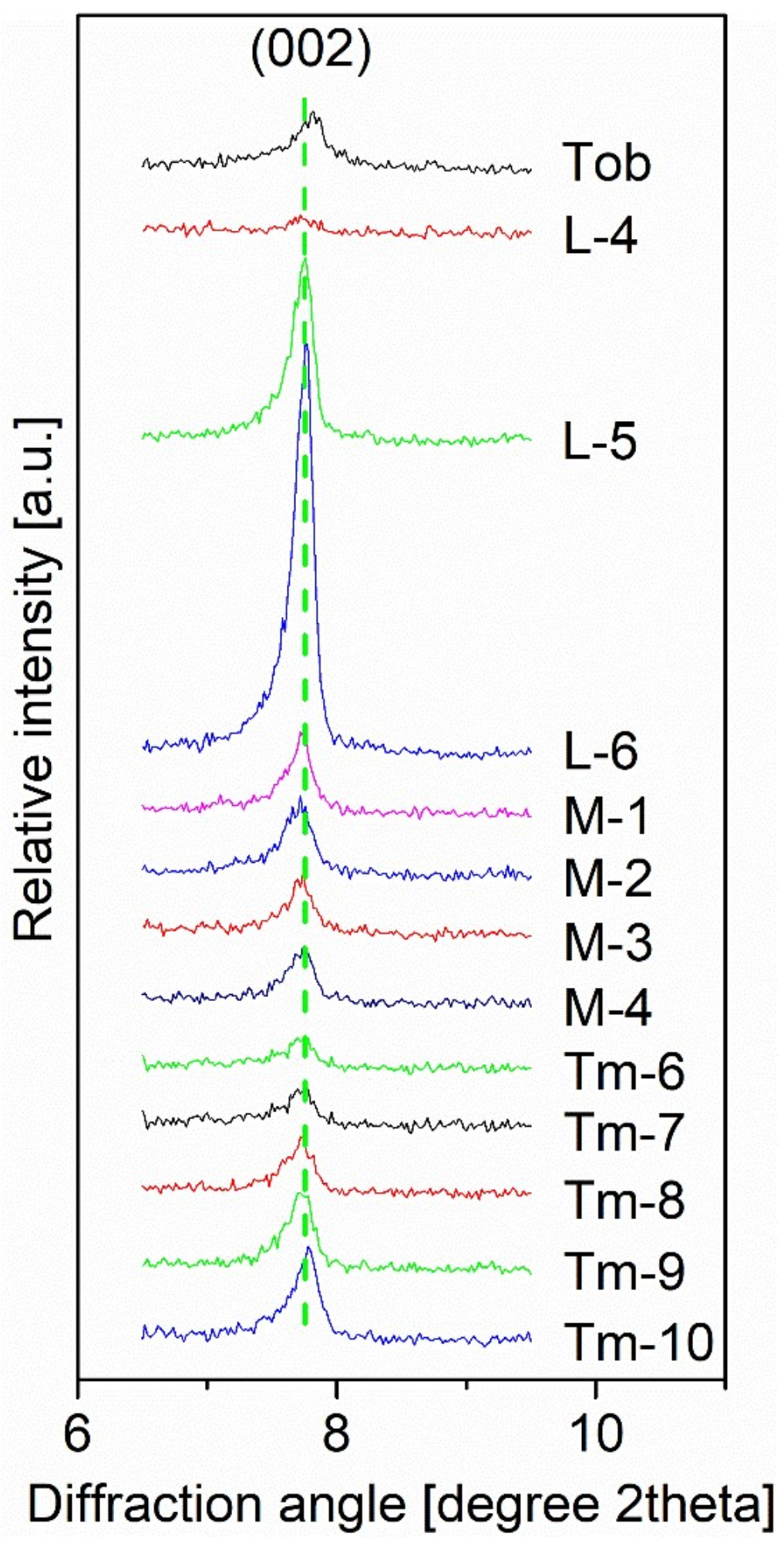

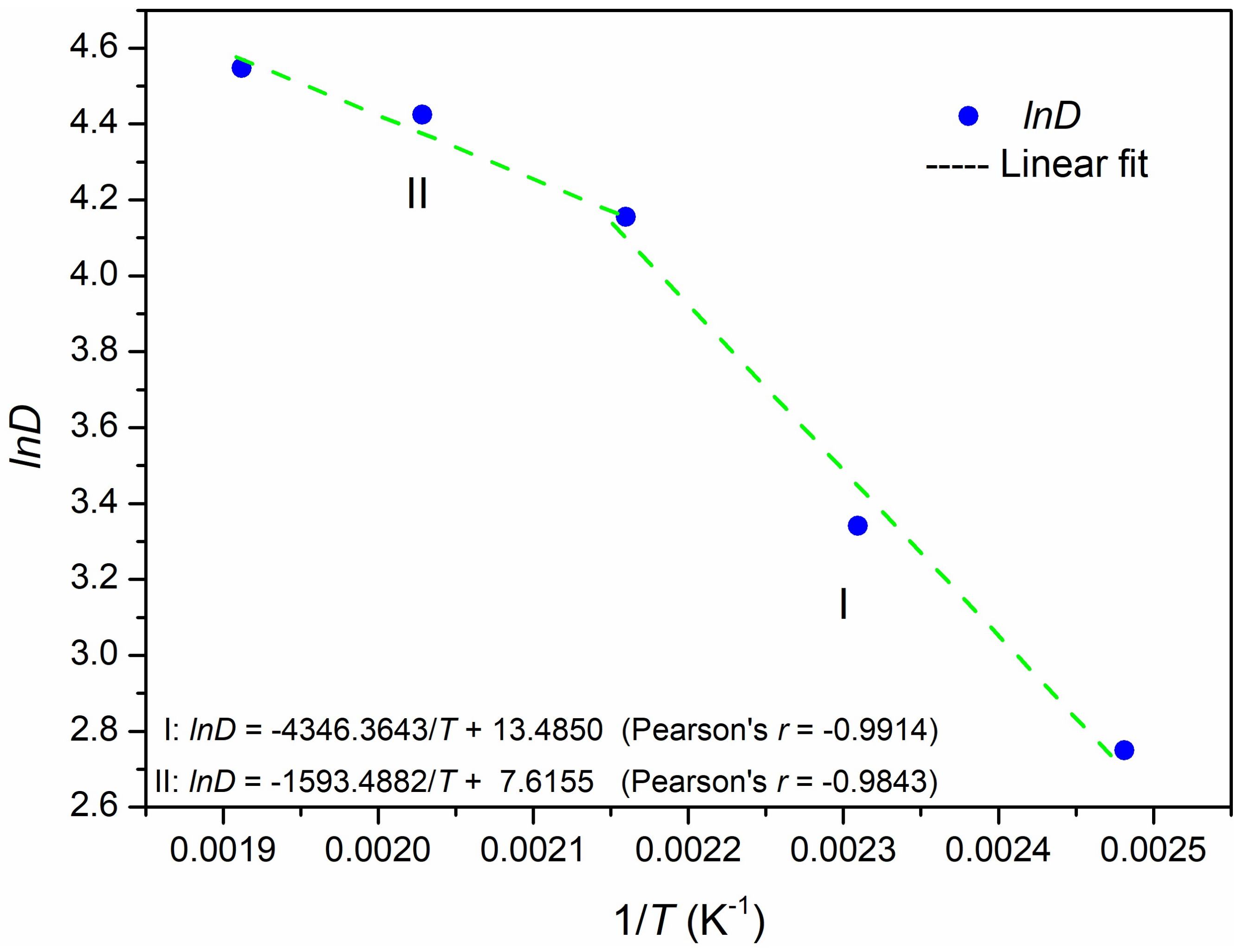
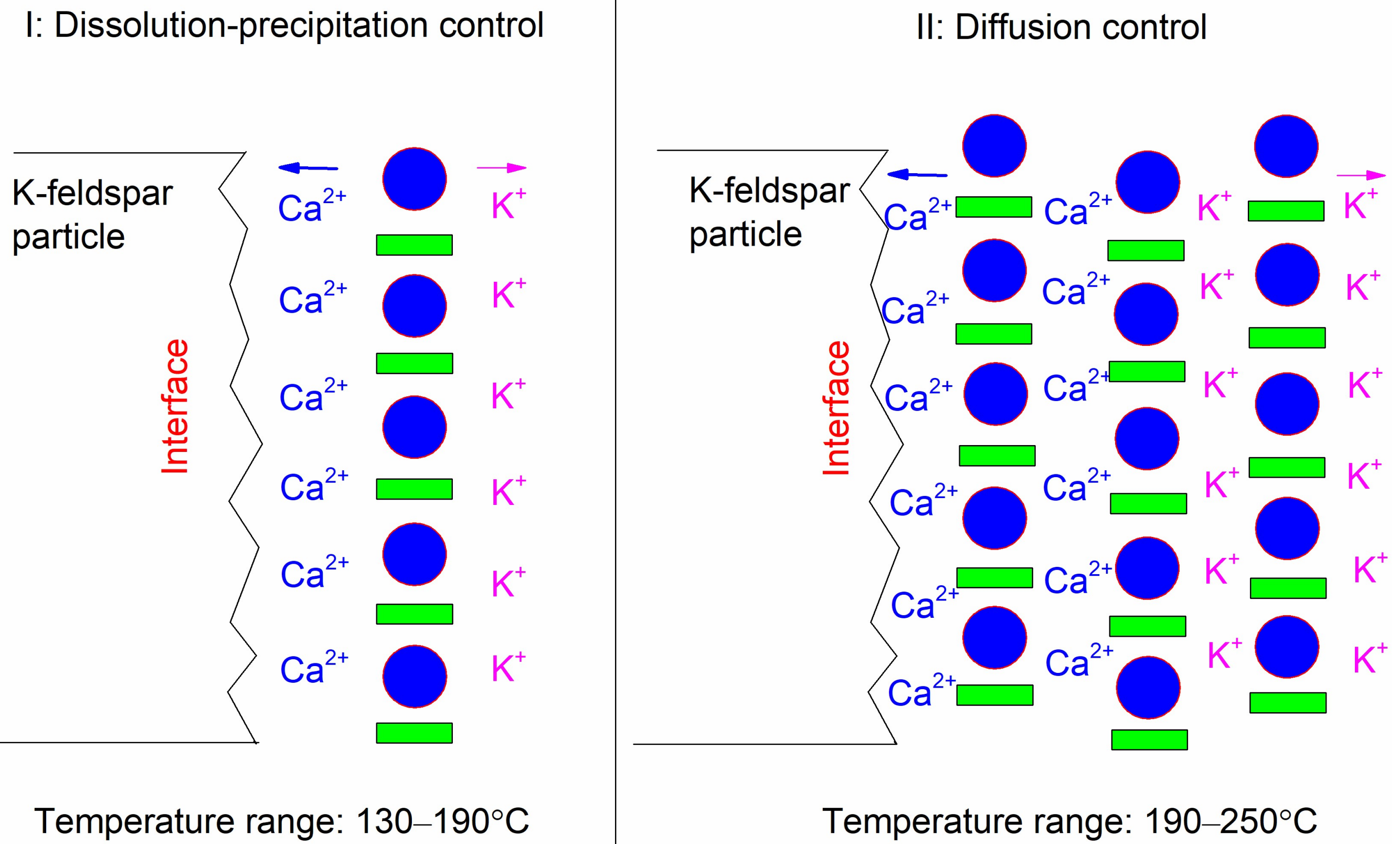
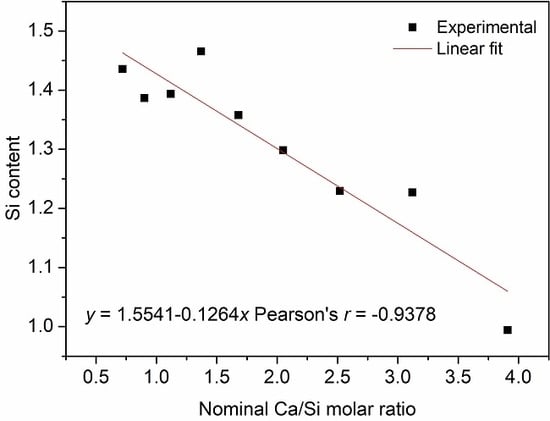
| Single Factor | NCa/Si | Temperature | Time (b) |
|---|---|---|---|
| Experimental condition | Temp = 190 °C | 130 °C ≤ Temp ≤ 250 °C | Temp = 190 °C |
| Time = 13.6 h | Time = 20 h | 0 h ≤ Time ≤ 36 h | |
| 0.72 ≤ NCa/Si ≤ 3.91 | NCa/Si = 1.37 | NCa/Si = 1.37 | |
| M (a) | all | all | all |
| S | NCa/Si ˃ 1.37 | 130 °C ≤ Temp < 190 °C | Time < 16 h |
| C | all | all | all |
| P | / | 130 °C ≤ Temp < 190 °C | 4 h < Time < 16 h |
| B | all | Temp ˃ 160 °C | Time ˃ 8 h |
| G | all | all | Time ˃ 4 h |
| D | NCa/Si ˃ 1.12 | 16 °C < Temp < 220 °C | 8 h < Time < 32 h |
| T | NCa/Si < 1.68 | Temp ˃ 160 °C | Time ˃ 16 h |
| A | all | all | all |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Han, C.; Liu, J. Study of K-Feldspar and Lime Hydrothermal Reaction: Phase and Mechanism with Reaction Temperature and Increasing Ca/Si Ratio. Minerals 2019, 9, 46. https://doi.org/10.3390/min9010046
Liu S, Han C, Liu J. Study of K-Feldspar and Lime Hydrothermal Reaction: Phase and Mechanism with Reaction Temperature and Increasing Ca/Si Ratio. Minerals. 2019; 9(1):46. https://doi.org/10.3390/min9010046
Chicago/Turabian StyleLiu, Shanke, Cheng Han, and Jianming Liu. 2019. "Study of K-Feldspar and Lime Hydrothermal Reaction: Phase and Mechanism with Reaction Temperature and Increasing Ca/Si Ratio" Minerals 9, no. 1: 46. https://doi.org/10.3390/min9010046
APA StyleLiu, S., Han, C., & Liu, J. (2019). Study of K-Feldspar and Lime Hydrothermal Reaction: Phase and Mechanism with Reaction Temperature and Increasing Ca/Si Ratio. Minerals, 9(1), 46. https://doi.org/10.3390/min9010046