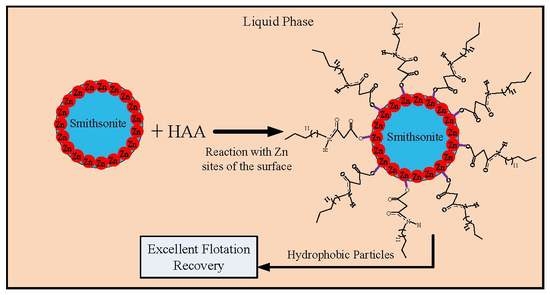
A Mechanism for the Adsorption of 2-(Hexadecanoylamino)Acetic Acid by Smithsonite: Surface Spectroscopy and Microflotation Experiments
Abstract
:1. Introduction
2. Experiments
2.1. Materials and Reagents
2.2. Microflotation Experiments
2.3. TOC Content Study
2.4. Zeta Potential Measurements
2.5. FTIR Spectrum
2.6. XPS Analysis
3. Results and Discussion
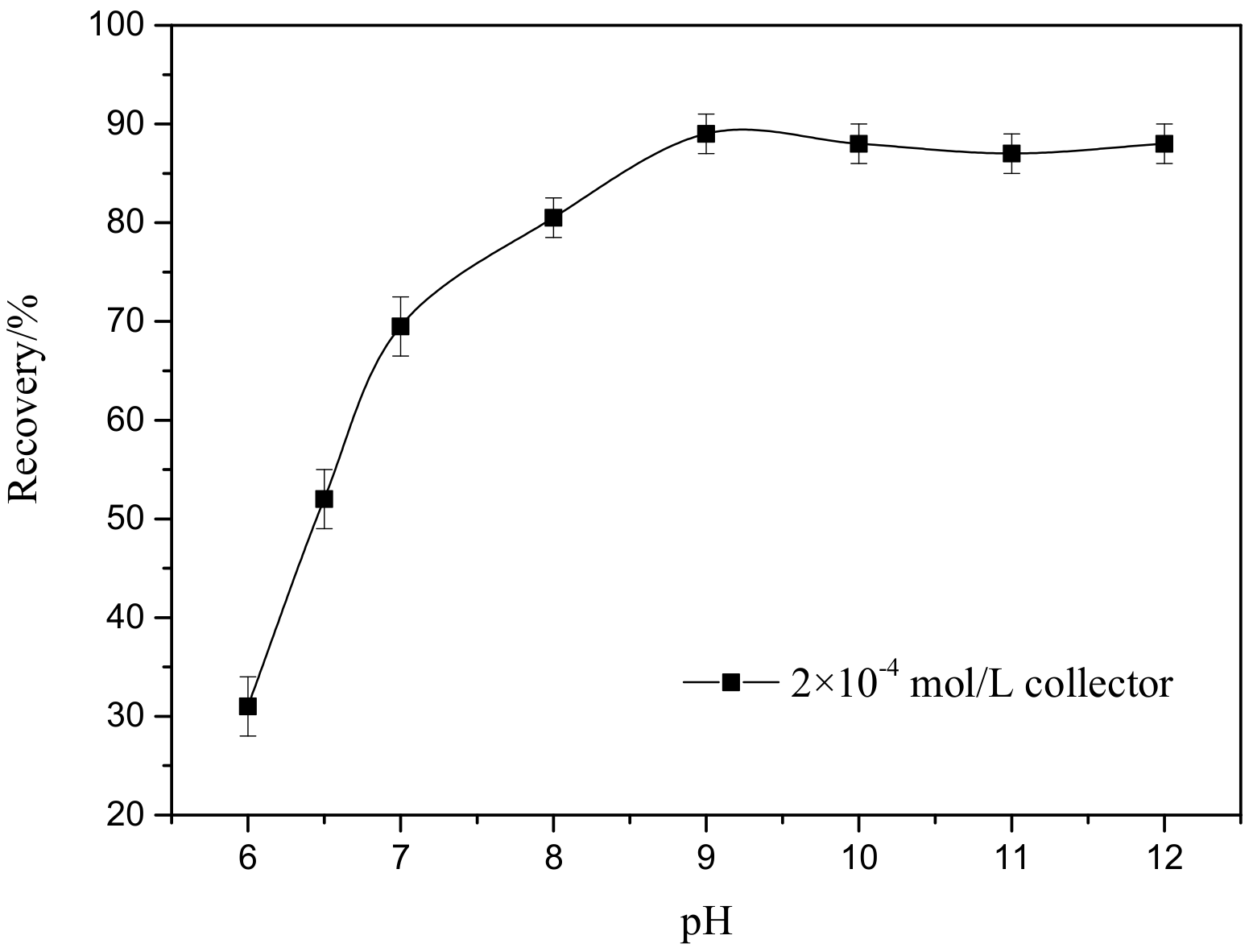
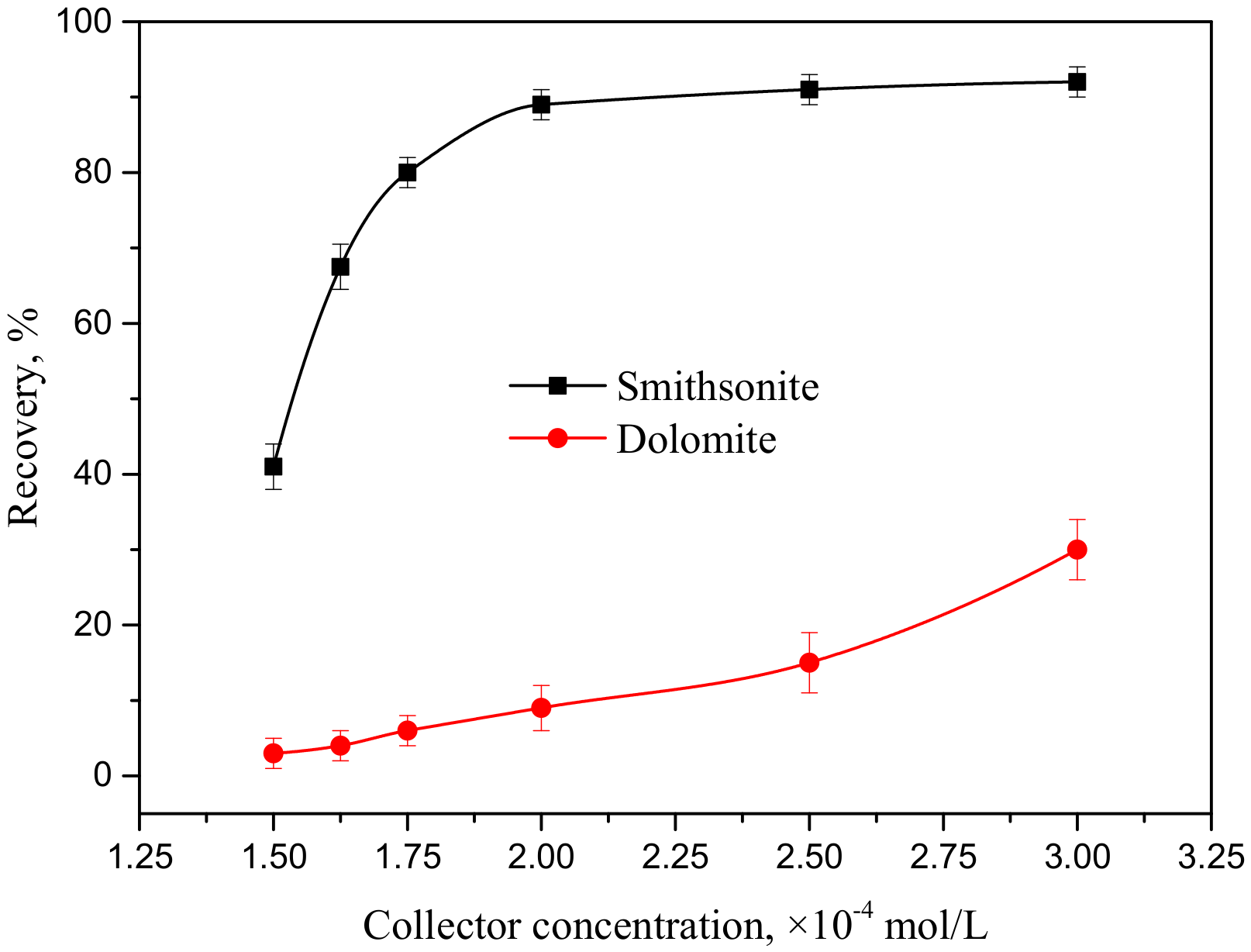
3.1. Flotation Study
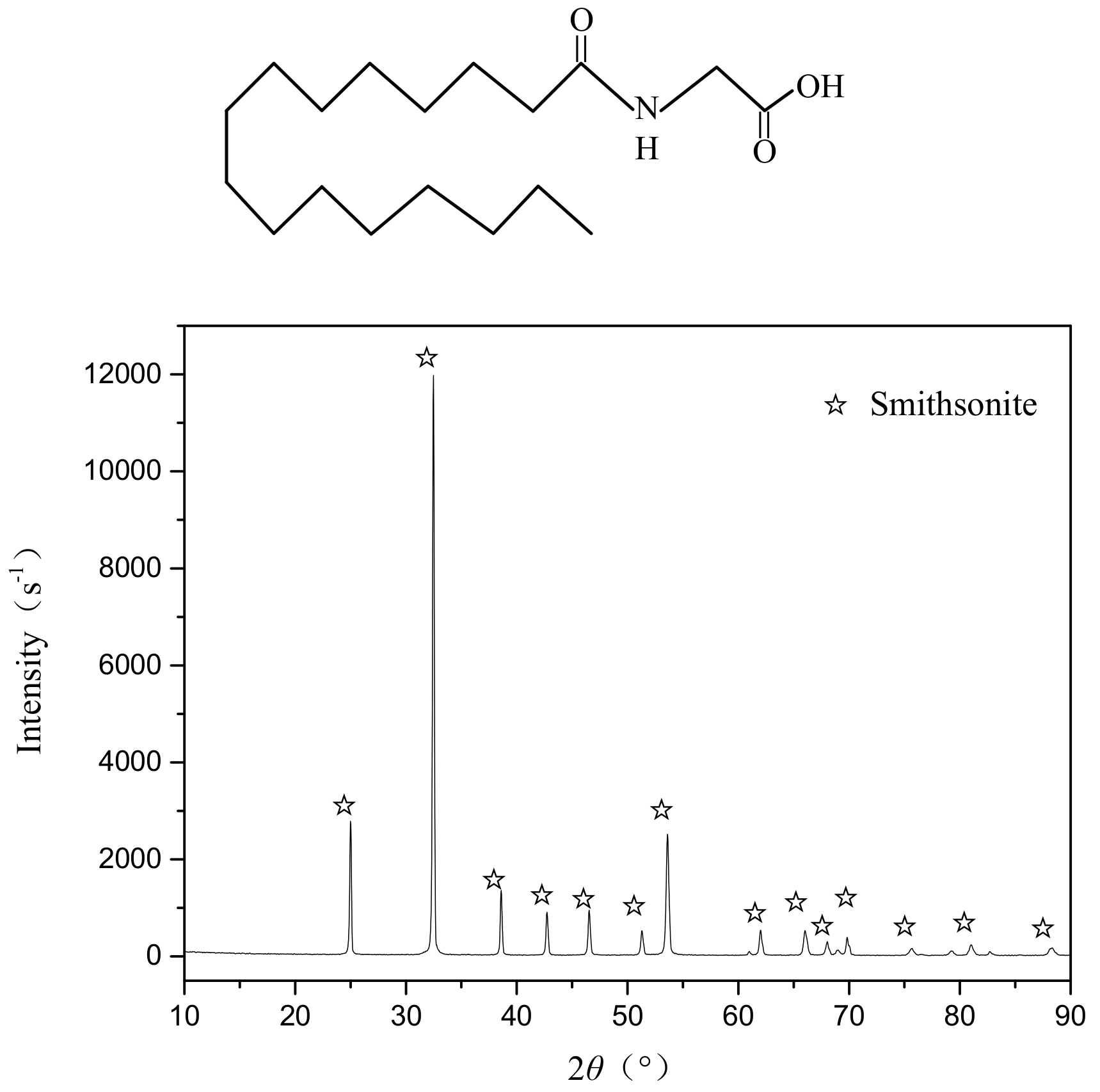
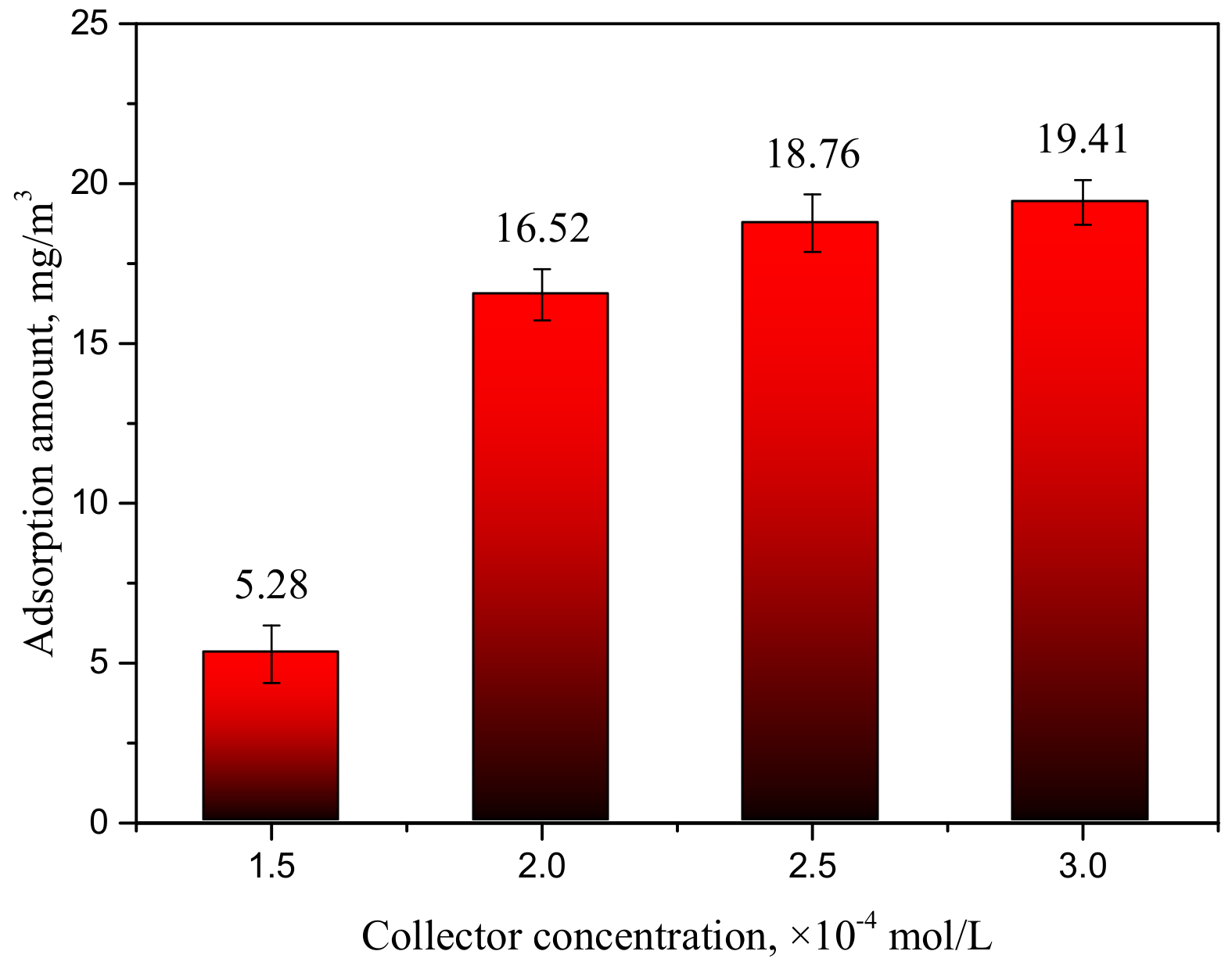
3.2. HAA Adsorbance on the Smithsonite Surface
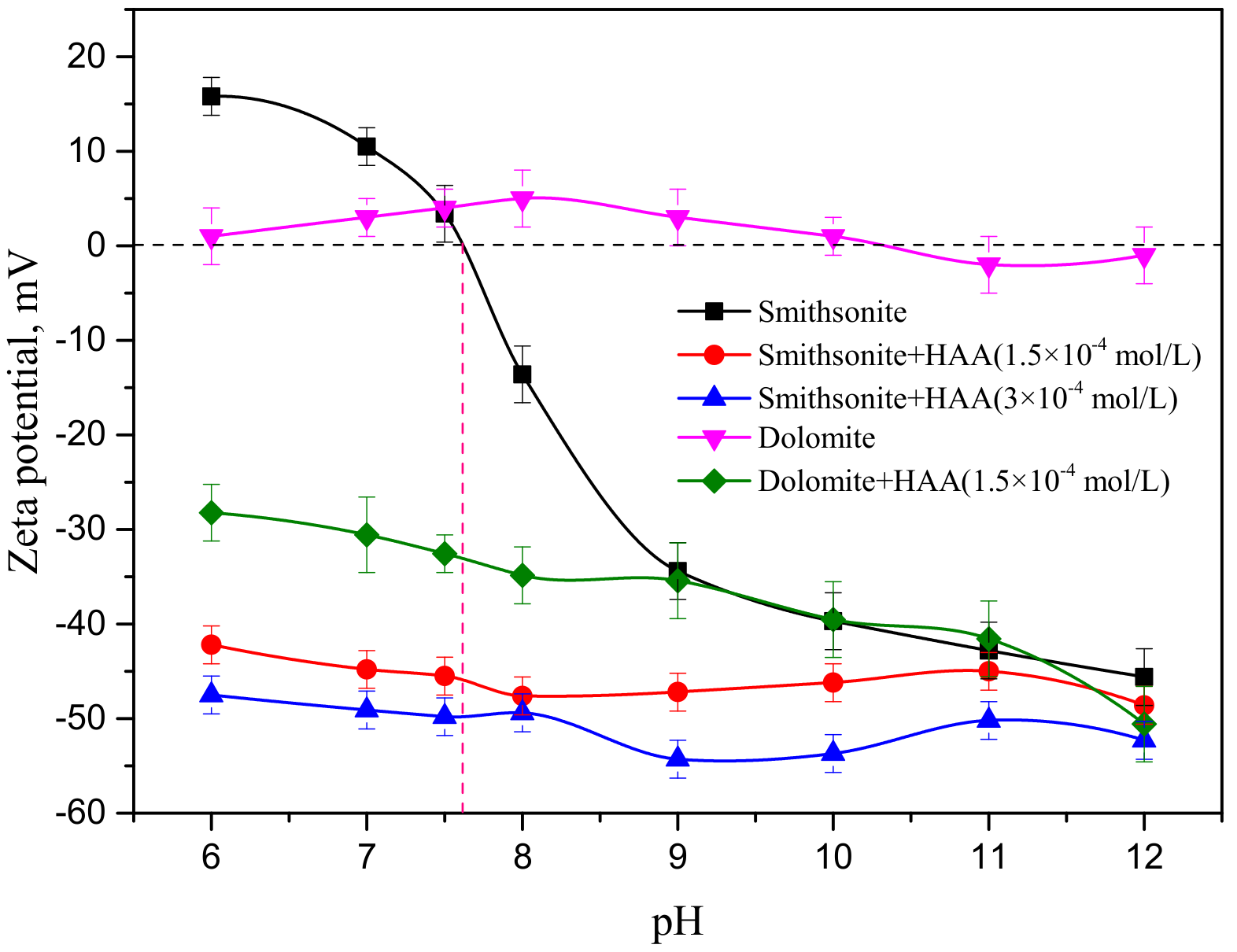
3.3. Effect of HAA on the Zeta Potential of Smithsonite
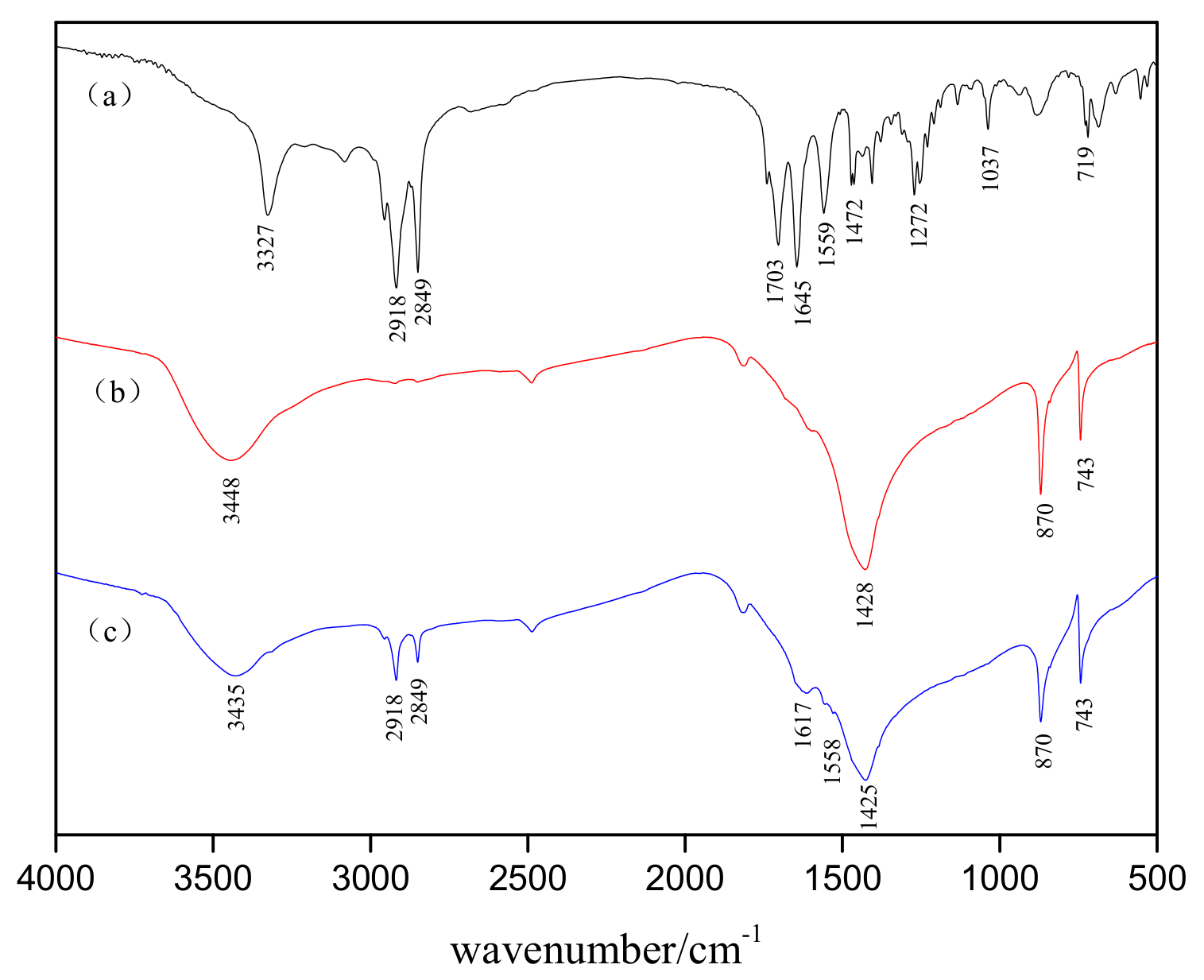
3.4. FTIR Spectroscopy
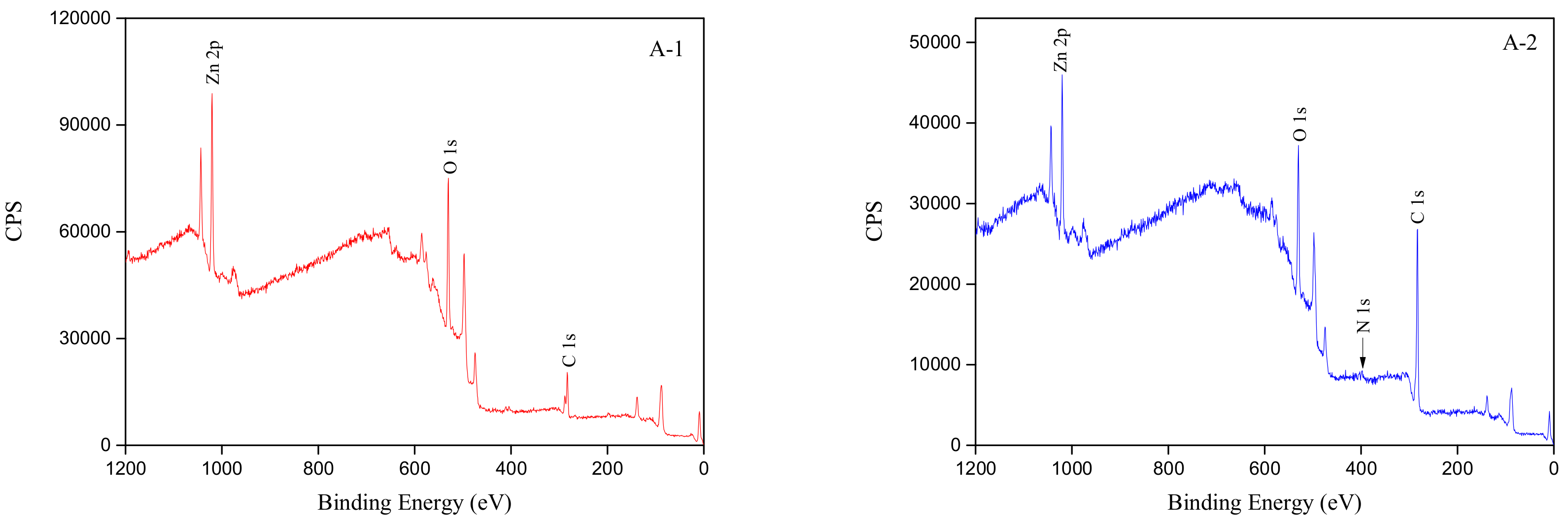
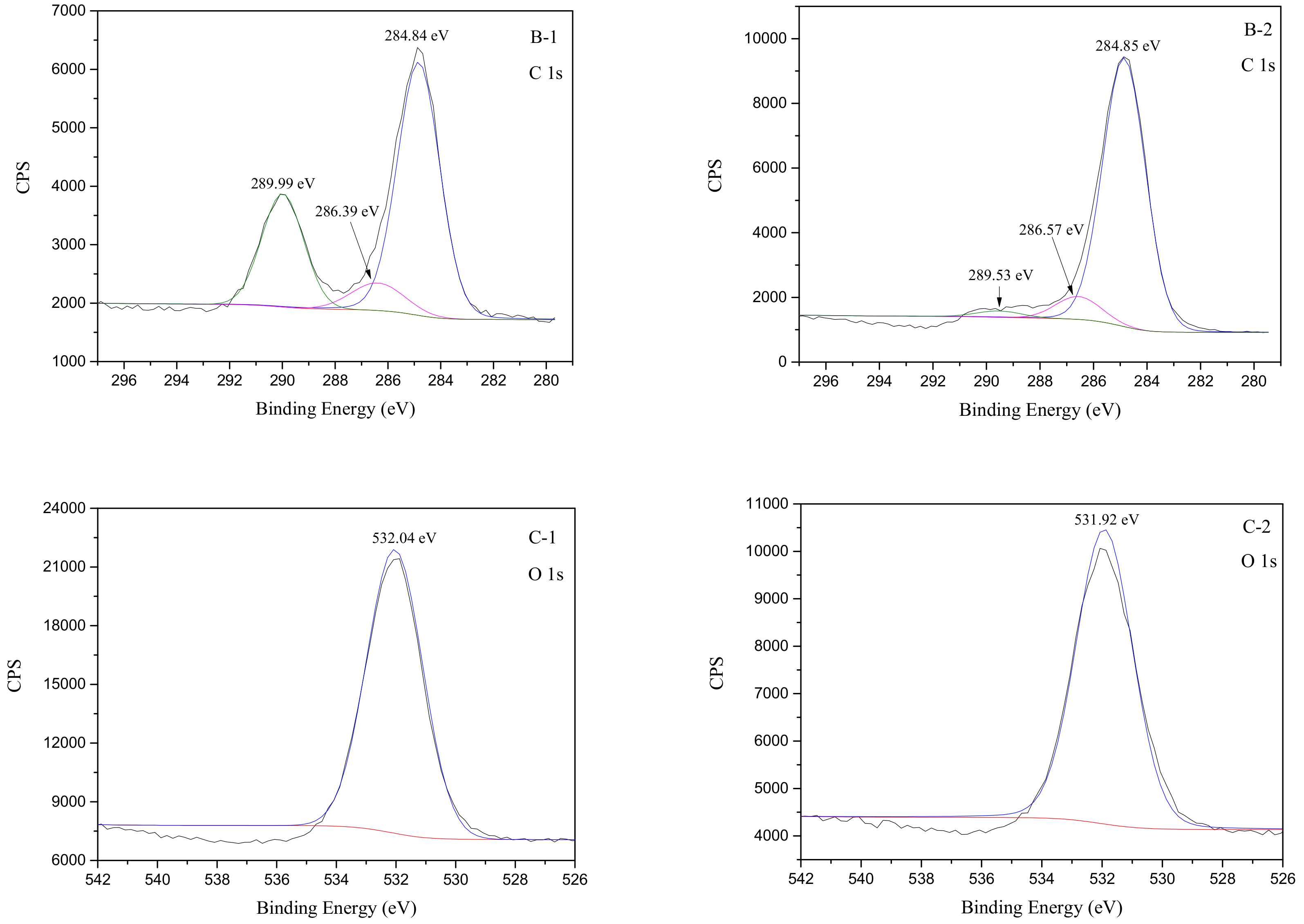
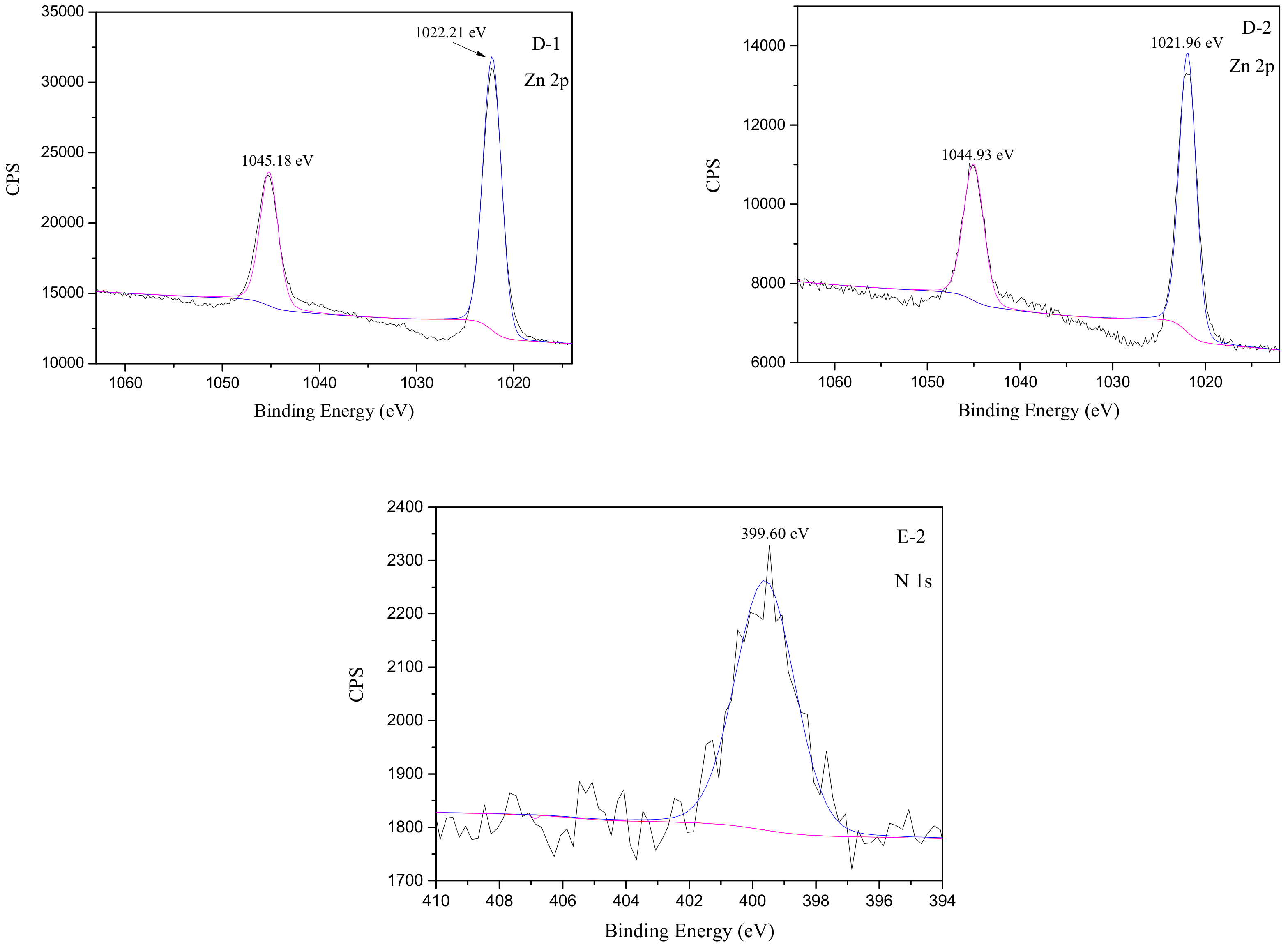
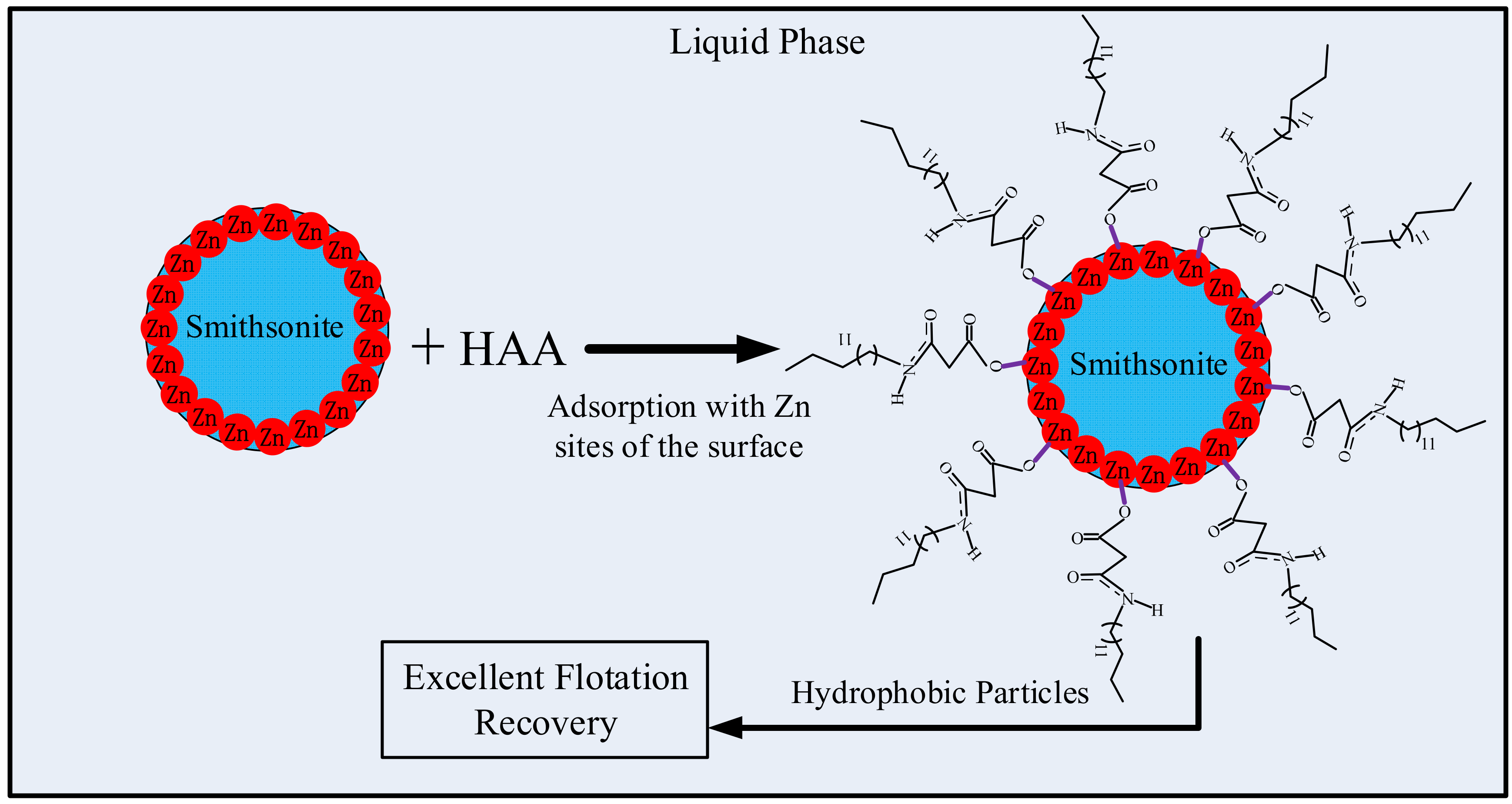
3.5. XPS Analyses
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wu, D.D.; Ma, W.H.; Wen, S.M.; Bai, S.J.; Deng, J.S.; Yin, Q. Contribution of ammonium ions to sulfidation-flotation of smithsonite. J. Taiwan Inst. Chem. 2017, 78, 20–26. [Google Scholar] [CrossRef]
- Amelunxen, P.; LaDouceur, R.; Amelunxen, R.; Young, C. A phenomenological model of entrainment and froth recovery for interpreting laboratory flotation kinetics tests. Miner. Eng. 2018, 125, 60–65. [Google Scholar] [CrossRef]
- Feng, Q.C.; Wen, S.M. Formation of zinc sulfide species on smithsonite surfaces and its response to flotation performance. J. Alloys Compd. 2017, 709, 602–608. [Google Scholar] [CrossRef]
- Feng, Q.C.; Wen, S.M.; Zhao, W.J.; Bai, X.; Chen, Y. Dissolution regularities of smithsonite in methane sulfonic acid. Russ. J. Non-Ferr. Met. 2015, 56, 365–371. [Google Scholar] [CrossRef]
- Wu, D.D.; Ma, W.H.; Wen, S.M.; Deng, J.S.; Bai, S.J. Enhancing the sulfidation of smithsonite by superficial dissolution with a novel complexing agent. Miner. Eng. 2017, 114, 1–7. [Google Scholar] [CrossRef]
- Irannajad, M.; Ejtemaei, M.; Gharabaghi, M. The effect of reagents on selective flotation of smithsonite–calcite–quartz. Miner. Eng. 2009, 22, 766–771. [Google Scholar]
- Ejtemaei, M.; Gharabaghi, M.; Irannajad, M. A review of zinc oxide mineral beneficiation using flotation method. Adv. Colloid Interface Sci. 2014, 206, 68–78. [Google Scholar] [CrossRef]
- Wu, D.D.; Wen, S.M.; Deng, J.S.; Liu, J.; Mao, Y.B. Study on the sulfidation behavior of smithsonite. Appl. Surf. Sci. 2015, 329, 315–320. [Google Scholar] [CrossRef]
- Feng, Q.C.; Wen, S.M.; Deng, J.S.; Zhao, W.J. Combined DFT and XPS investigation of enhanced adsorption of sulfide species onto cerussite by surface modification with chloride. Appl. Surf. Sci. 2017, 425, 8–15. [Google Scholar] [CrossRef]
- Bai, S.J.; Li, C.L.; Fu, X.Y.; Liu, J.; Wen, S.M. Characterization of zinc sulfide species on smithsonite surfaces during sulfidation processing: Effect of ammonia liquor. J. Ind. Eng. Chem. 2018, 61, 19–27. [Google Scholar] [CrossRef]
- Chai, L.Y.; Liang, Y.J.; Ke, Y.; Min, X.B.; Tang, C.J.; Zhang, H.J.; Xie, X.D.; Yuan, C.Y. Mechano-chemical sulfidization of zinc oxide by grinding with sulfur and reductive additives. Trans. Nonferr. Met. Soc. 2013, 23, 1129–1138. [Google Scholar] [CrossRef]
- Karlkvist, T.; Patra, A.; Rao, K.H.; Bordes, R.; Holmberg, K. Flotation selectivity of novel alkyl dicarboxylate reagents for apatite-calcite separation. J. Colloid Interface Sci. 2015, 445, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Weast, R.C. Handbook of Chemistry and Physics; CRC Press Inc.: Boca Raton, FL, USA, 1974. [Google Scholar]
- Glembotskii, V. Flotation; Primary Sources: New York, NY, USA, 1963. [Google Scholar]
- Pokrovsky, O.S.; Schott, J. Surface Chemistry and Dissolution Kinetics of Divalent Metal Carbonates. Environ. Sci. Technol. 2002, 36, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Zhang, G.F.; Feng, Q.M.; Deng, H. Effect of solution chemistry on the flotation system of smithsonite and calcite. Int. J. Miner. Process. 2013, 119, 34–39. [Google Scholar] [CrossRef]
- Rimmerman, N.; Bradshaw, H.B.; Hughes, H.V.; Chen, J.S.; Hu, S.S.; McHugh, D.; Vefring, E.; Jahnsen, J.A.; Thompson, E.L.; Masuda, K.; et al. N-palmitoyl glycine, a novel endogenous lipid that acts as a modulator of calcium influx and nitric oxide production in sensory. Mol. Phamracol. 2008, 74, 213–224. [Google Scholar] [CrossRef]
- Sabrina, D.; Mika, F.; Akie, O.; Chihiro, Y.; Hiroaki, T.; Kenji, S. Combined Use of N-Palmitoyl-Glycine-Histidine Gel and Several Penetration Enhancers on the Skin Permeation and Concentration of Metronidazole. Pharmaceutics 2018, 10, 163. [Google Scholar]
- Vucinic, D.R.; Radulovic, D.S.; Deusic, S.D. Electrokinetic properties of hydroxyapatite under flotation conditions. J. Colloid Interface Sci. 2010, 343, 239–245. [Google Scholar] [CrossRef]
- Shi, Q.; Feng, Q.M.; Zhang, G.; Deng, H. Electrokinetic properties of smithsonite and its floatability with anionic collector. Colloids Surf. A 2012, 410, 178–183. [Google Scholar] [CrossRef]
- Nunes, A.P.; Peres, A.E.; Araujo, A.C.; Valadao, G.E. Electrokinetic properties of wavellite and its floatability with cationic and anionic collectors. J. Colloid Interface Sci. 2011, 361, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.D.; Hu, Y.; Ku, J.G.; Zuo, W.R.; Yang, Z.G. Adsorption of Fe(III) on smithsonite surfaces and implications for flotation. Colloids Surf. A 2017, 533, 308–315. [Google Scholar] [CrossRef]
- Araujo, A.C.A.; Lima, R.M.F. Influence of cations Ca2+, Mg2+ and Zn2+ on the flotation and surface charge of smithsonite and dolomite with sodium oleate and sodium silicate. Int. J. Miner. Process. 2017, 167, 35–41. [Google Scholar] [CrossRef]
- Hu, Y.H.; Luo, L.; Qiu, G.Z.; Wang, D.Z. Solution chemistry of electrokinetic behavior of carbonate minerals. Trans. Nonferr. Met. Soc. 2016, 95, 185–196. [Google Scholar]
- Zachara, J.M.; Kittrick, J.A.; Harsh, J.B. The mechanism of Zn2+ adsorption on calcite. Geochim. Cosmochim. Acta 1988, 52, 2281–2291. [Google Scholar] [CrossRef]
- Zachara, J.M.; Kittrick, J.A.; Dake, L.S.; Harsh, J.B. Solubility and surface spectroscopy of zinc precipitates on calcite. Geochim. Cosmochim. Acta 1989, 53, 9–19. [Google Scholar] [CrossRef]
- Siciliano, T.; Siciliano, M.; Malitesta, C.; Proto, A.; Cucciniello, R.; Giove, A.; Iacobellis, S.; Genga, A. Carbonaceous PM10 and PM2.5 and secondary organic aerosol in a coastal rural site near Brindisi (Southern Italy). Environ. Sci. Pollut. Res. 2018, 25, 23929–23945. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.H.; Thomas, P.; Hume, P.; Jin, J. Effective Conversion of Amide to Carboxylic Acid on Polymers of Intrinsic Microporosity (PIM-1) with Nitrous Acid. Membranes 2018, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Hamedan, N.A.; Razali, A.A.A.; Uyup, N.H.; Zaki, H.M. Synthesis and characterization of p-dimethylaminobenzaldehyde benzoylthiourea and study towards selective and sensitive fluorescent sensor for detection of iron (III) cation in aqueous solution. IOP Conf. Ser. Mater. Sci. 2017, 172, 12–50. [Google Scholar] [CrossRef]
- Siwatch, M.; Yadav, R.B.; Yadav, B.S. X-ray diffraction, rheological and FT-IR spectra studies of processed amaranth (Amaranthus hypochondriacus). J. Food Meas. Charact. 2017, 11, 1717–1724. [Google Scholar] [CrossRef]
- Grigoruţă, M.; Vargas-Caraveo, A.; Vázquez-Mayorga, E.; Castillo-Michel, H.A.; Díaz-Sánchez, Á.G.; Reyes-Herrera, J.; Martínez-Martínez, A. Blood mononuclear cells as speculum of emotional stress analyzed by synchrotron infrared spectroscopy and a nootropic drug. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 204, 475–483. [Google Scholar] [CrossRef]
- Iwaki, M.; Takeshita, K.; Kondo, H.X.; Kinoshita, K.; Okamura, Y.; Takano, Y.; Nakagawa, A.; Kandori, H. Zn2+-Binding to the Voltage-Gated Proton Channel Hv1/VSOP. J. Phys. Chem. B 2018, 122, 9076–9080. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Forssberg, E. Physicochemical studies of smithsonite flotation using mixed anionic/cationic collector. Miner. Eng. 2007, 20, 621–624. [Google Scholar] [CrossRef]
- Zhu, H.L.; Qin, W.Q.; Chen, C.; Chai, L.Y.; Li, L.S.; Liu, S.J.; Zhang, T. Selective flotation of smithsonite, quartz and calcite using alkyl diamine ether as collector. Trans. Nonferr. Met. Soc. 2018, 28, 163–168. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, L.; Wang, J.; Wang, L.; Xiao, J. A comparison study of adsorption of benzohydroxamic acid and amyl xanthate on smithsonite with dodecylamine as co-collector. Appl. Surf. Sci. 2017, 426, 1141–1147. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Forssberg, E. Adsorption studies of smithsonite flotation using dodecylamine and oleic acid. Miner. Metall. Proc. 2006, 23, 87–96. [Google Scholar] [CrossRef]
- Hamid, H.S.; Eric, F. XPS & FTIR Study of Adsorption Characteristics Using Cationic and Anionic Collectors on Smithsonite. J. Miner. Mater. Charact. Eng. 2006, 5, 21–45. [Google Scholar]
- Hajji, L.; Boukir, A.; Assouik, J.; Pessanha, S.; Figueirinhas, J.L.; Carvalho, M.L. Artificial aging paper to assess long-term effects of conservative treatment. Monitoring by infrared spectroscopy (ATR-FTIR), X-ray diffraction (XRD), and energy dispersive X-ray fluorescence (EDXRF). Microchem. J. 2016, 124, 646–656. [Google Scholar] [CrossRef]
- Garbassi, F.; Marabini, A.M. Spectroscopic Investigation of Sulphidation of Zinc and Lead Carbonates. J. Chem. Soc. Pak. 1986, 82, 2043–2055. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray photoelectron spectroscopy a reference book of standard spectra for identification and interpretation of XPS data. Chem. Phys. Lett. 1999, 220, 7–10. [Google Scholar]
- Dake, L.S.; Baer, D.R.; Zachara, J.M. Auger parameter measurements of zinc compounds relevant tozinc transport in the environment. Surf. Interface Anal. 2010, 14, 71–75. [Google Scholar] [CrossRef]
- Charlton, M.R.; Suhr, K.J.; Holliday, B.J.; Stevenson, K.J. Electrochemical modification of indium tin oxide using di(4-nitrophenyl) iodonium tetrafluoroborate. Langmuir 2015, 31, 695–702. [Google Scholar] [CrossRef]
- Jong, K.; Han, Y.; Ryom, S. Flotation mechanism of oleic acid amide on apatite. Colloids Surf. A 2017, 523, 127–131. [Google Scholar] [CrossRef]
| Pertinent Reaction and Constant | Pertinent Reaction and Constant | ||
|---|---|---|---|
| (a) CO32− + H+ ⇔ HCO3− | 10.33 | (h) Zn(CO3)0.4(OH)1.2 ⇔ Zn2+ + 0.4CO32− + 1.2OH− | 14.85 |
| (b) CO2(g) + OH− ⇔ HCO3− | 6.18 | (i) Zn2+ + 4OH− ⇔ Zn(OH)42− | 14.80 |
| (c) OH− + H+ ⇔ H2O | 14.00 | (j) Zn2+ + HCO3− ⇔ ZnHCO3+ | 2.10 |
| (d) HCO3− + H+ ⇔ H2CO3 | 6.35 | (k) Zn2+ + CO32− ⇔ ZnCO3 | 5.30 |
| (e) Zn2+ + OH− ⇔ ZnOH+ | 5.00 | (l) Zn2+ + 2CO32− ⇔ Zn(CO3)22− | 9.63 |
| (f) Zn2+ + 2OH− ⇔ Zn(OH)2(aq) | 11.10 | (n) Zn2+ + CO32− ⇔ ZnCO3(s) | 10.00 |
| (g) Zn2+ + 3OH− ⇔ Zn(OH)3− | 13.60 | ||
| Samples | Atomic Concentration, % | |||
|---|---|---|---|---|
| C1s | O1s | Zn2p | N1s | |
| Raw material | 42.38 | 39.08 | 15.53 | - |
| Collector adsorption | 69.59 | 24.40 | 7.38 | 2.97 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, B.; Liu, J.; Liu, Q.; Song, C.; Yu, L.; Li, S.; Lai, H. A Mechanism for the Adsorption of 2-(Hexadecanoylamino)Acetic Acid by Smithsonite: Surface Spectroscopy and Microflotation Experiments. Minerals 2019, 9, 15. https://doi.org/10.3390/min9010015
Luo B, Liu J, Liu Q, Song C, Yu L, Li S, Lai H. A Mechanism for the Adsorption of 2-(Hexadecanoylamino)Acetic Acid by Smithsonite: Surface Spectroscopy and Microflotation Experiments. Minerals. 2019; 9(1):15. https://doi.org/10.3390/min9010015
Chicago/Turabian StyleLuo, Bin, Junbo Liu, Quanjun Liu, Chao Song, Li Yu, Shimei Li, and Hao Lai. 2019. "A Mechanism for the Adsorption of 2-(Hexadecanoylamino)Acetic Acid by Smithsonite: Surface Spectroscopy and Microflotation Experiments" Minerals 9, no. 1: 15. https://doi.org/10.3390/min9010015
APA StyleLuo, B., Liu, J., Liu, Q., Song, C., Yu, L., Li, S., & Lai, H. (2019). A Mechanism for the Adsorption of 2-(Hexadecanoylamino)Acetic Acid by Smithsonite: Surface Spectroscopy and Microflotation Experiments. Minerals, 9(1), 15. https://doi.org/10.3390/min9010015