Adsorption Behavior of Methyl Laurate and Dodecane on the Sub-Bituminous Coal Surface: Molecular Dynamics Simulation and Experimental Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
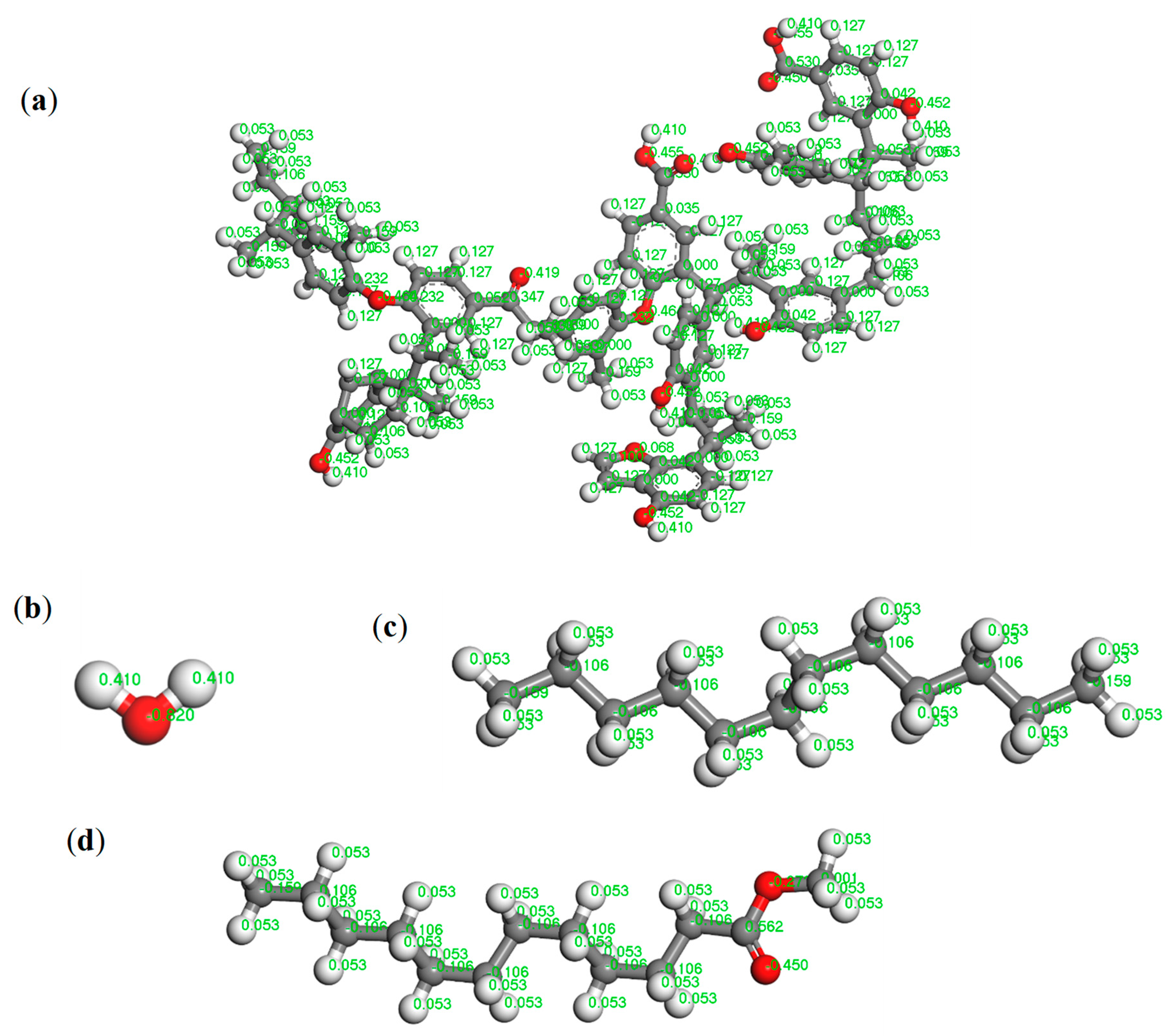
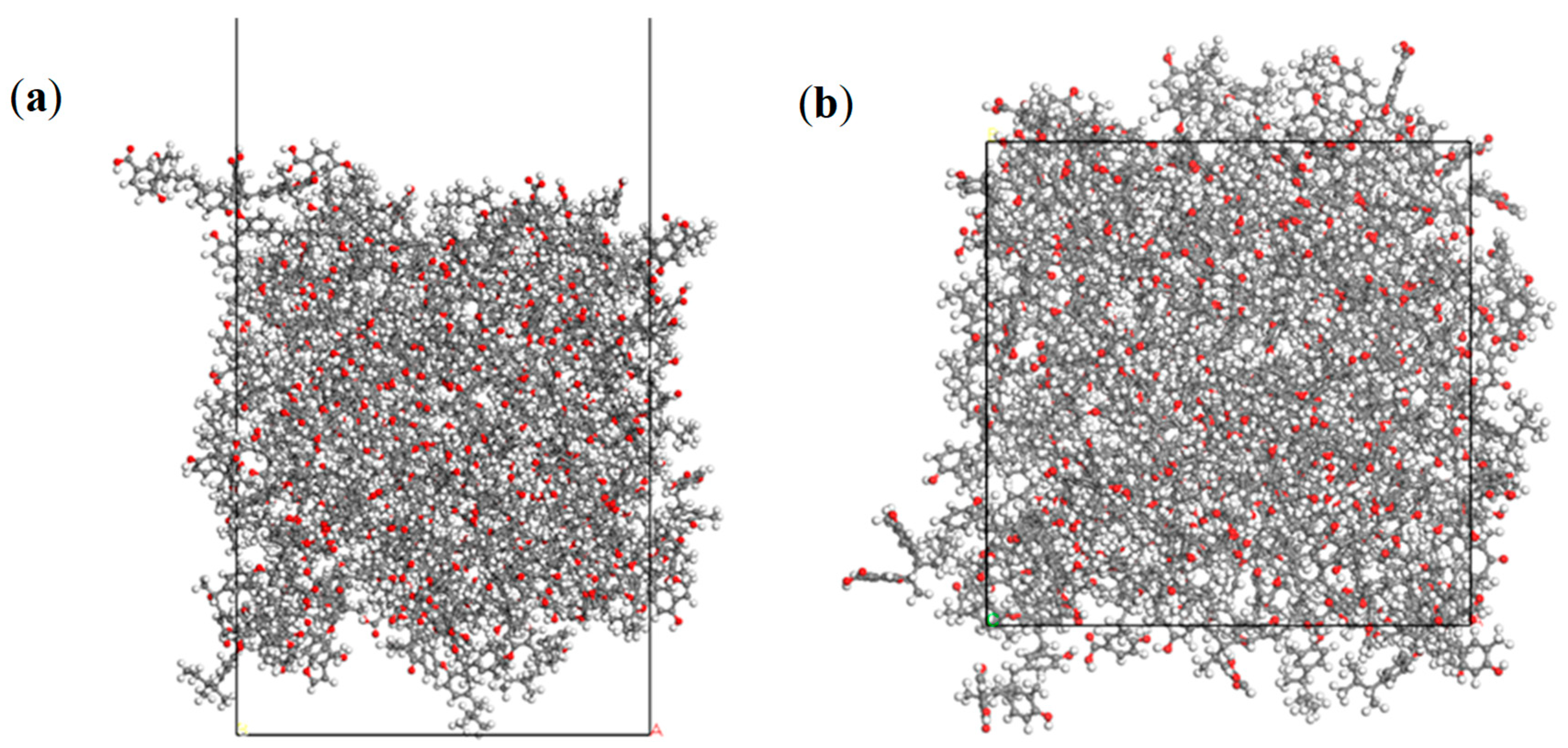
2.2. Molecular Simulation Details
2.3. XPS
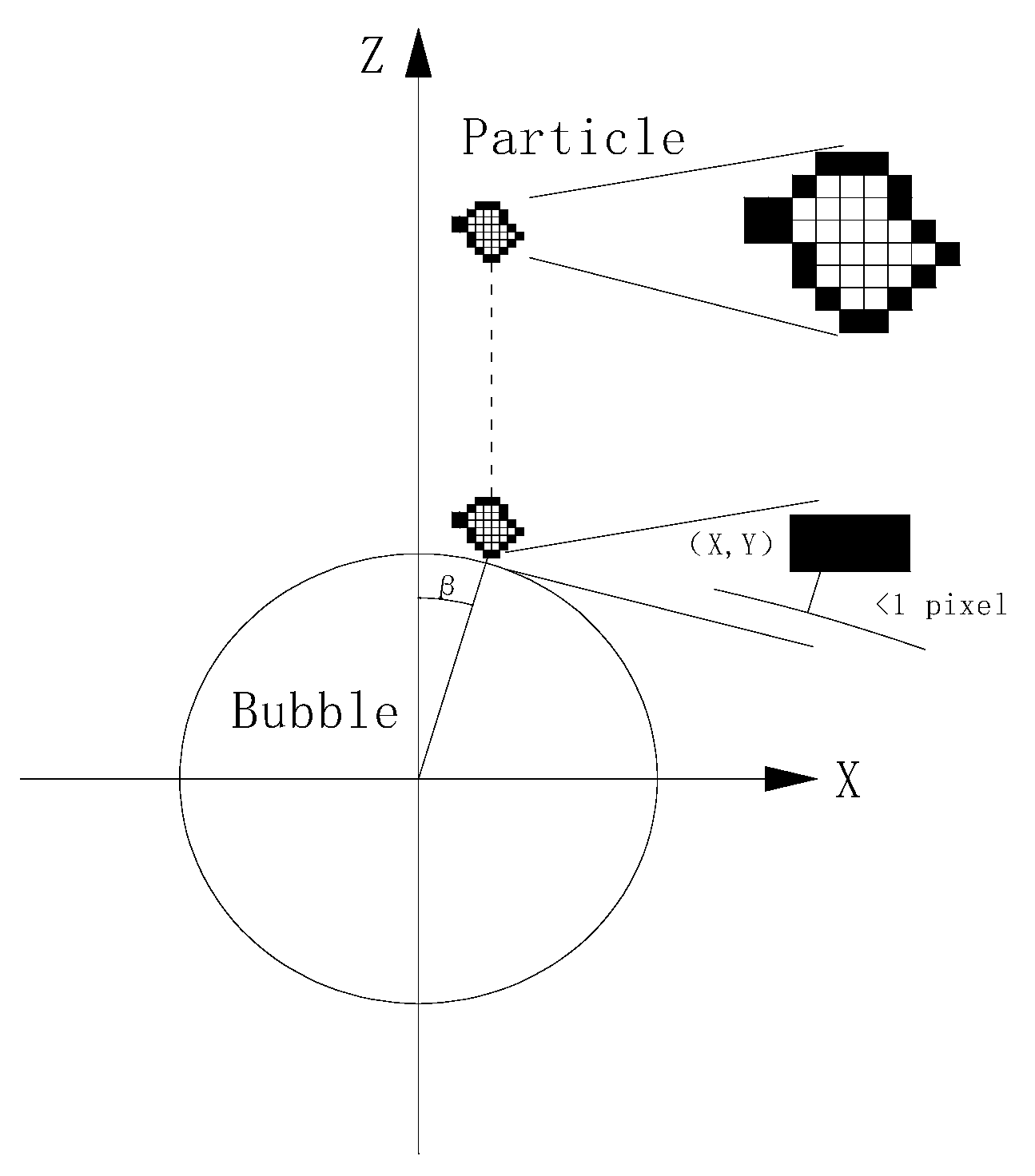
2.4. Attachment Efficiency Measurement
3. Results and Discussion
3.1. Molecular Dynamics Simulation
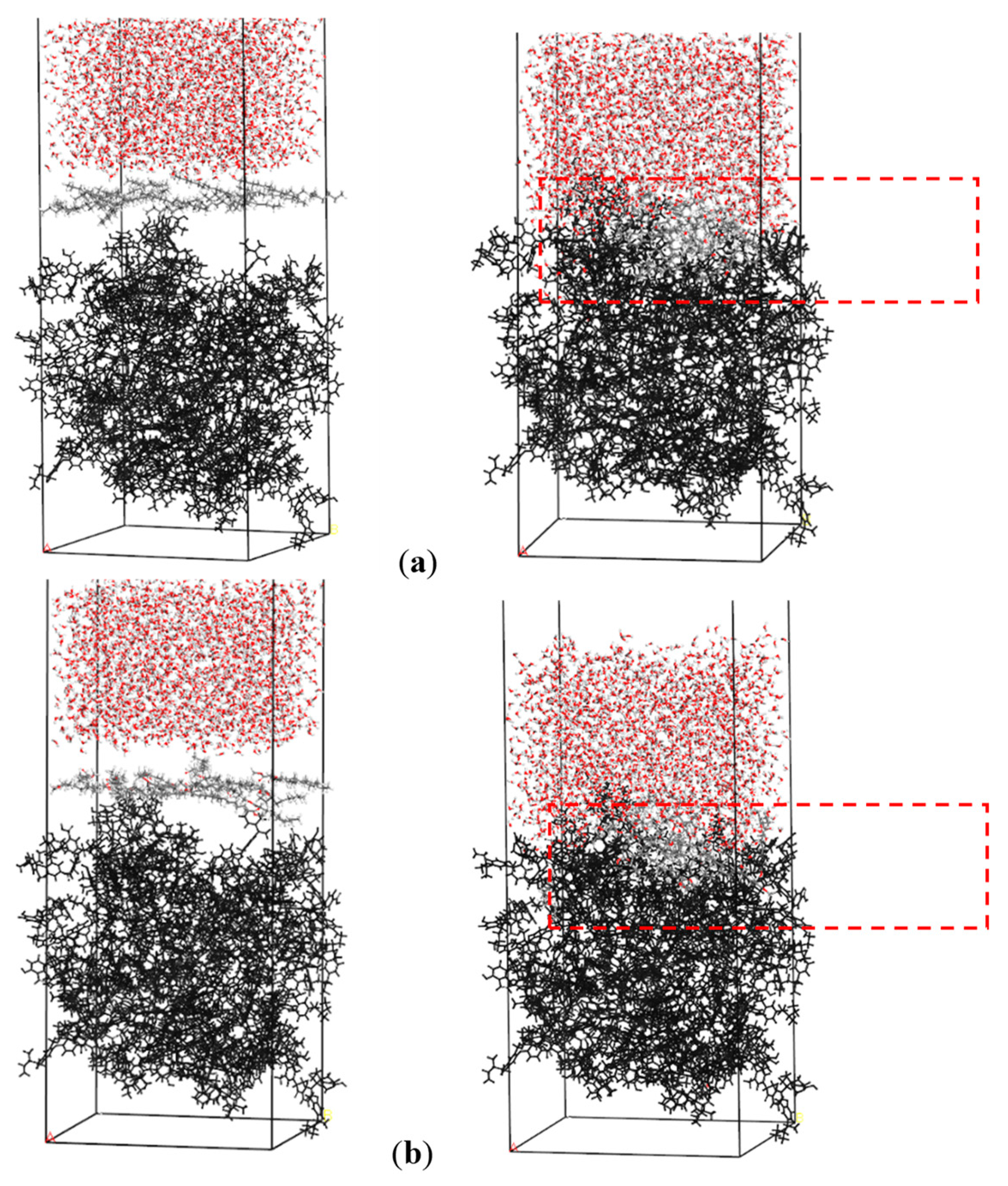
3.1.1. Adsorption Structure of Collectors on the Coal/Water Interface
3.1.2. Interaction Energies between Collectors and Coal
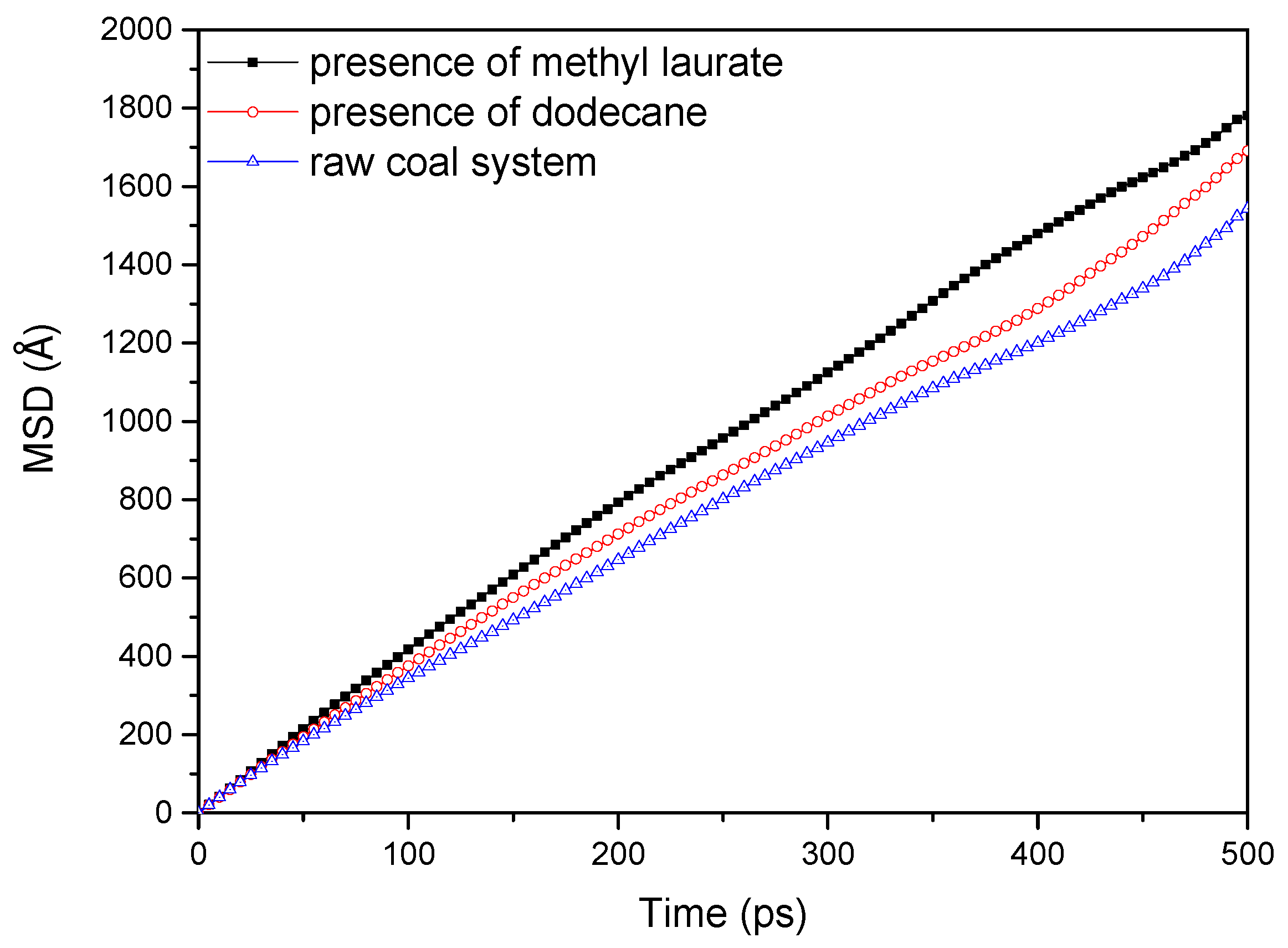
3.1.3. Mobility of Water Molecules before and after the Adsorption of Collectors
3.2. Analysis of Experimental Results
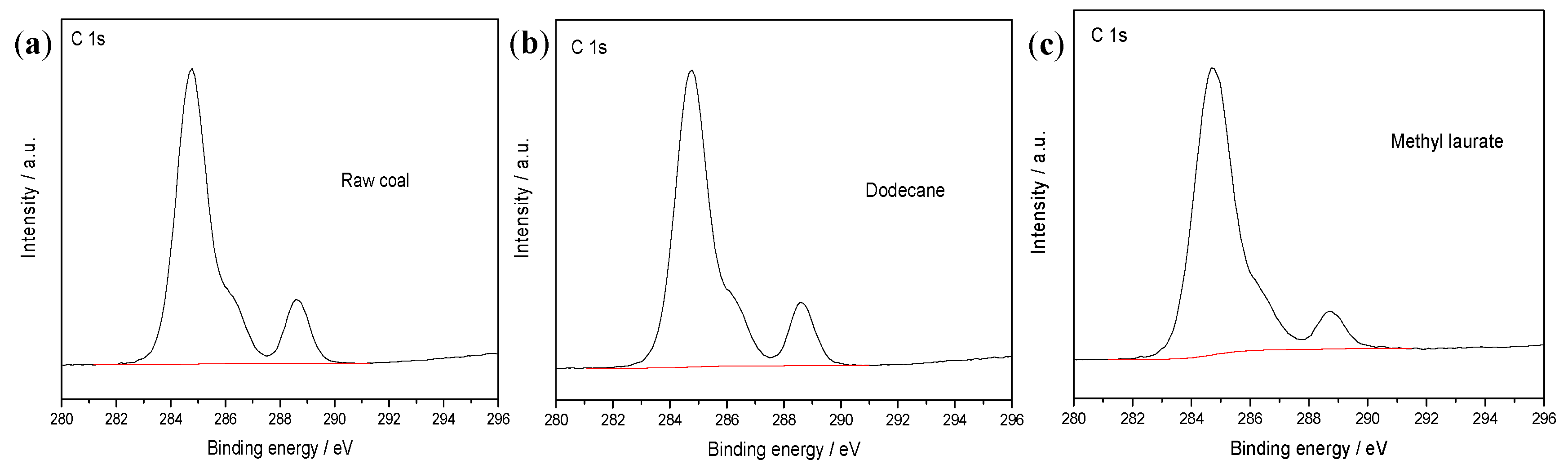
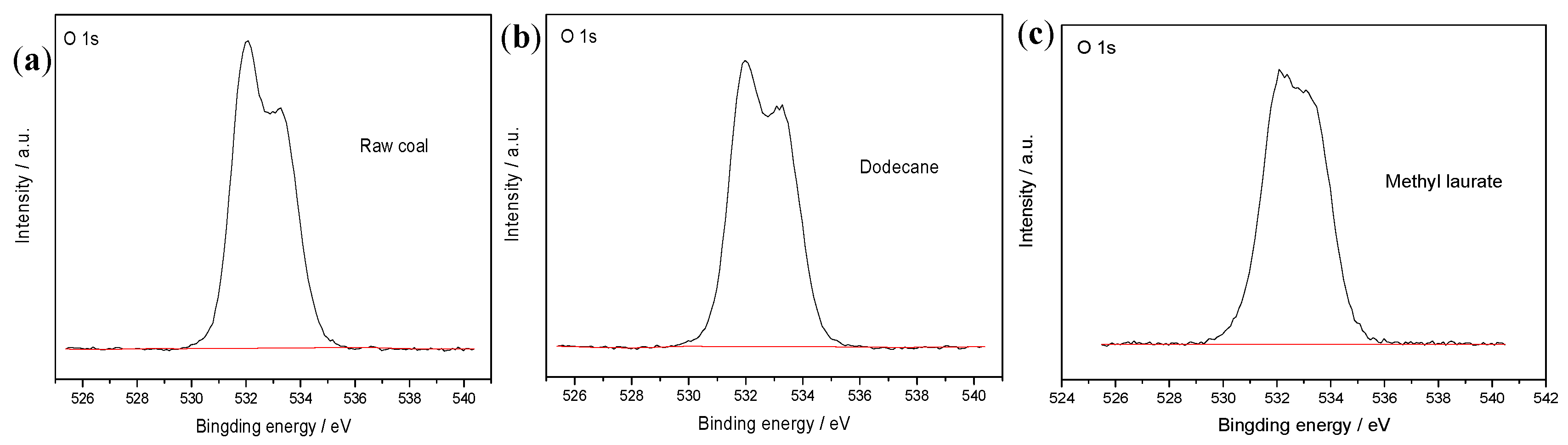
3.2.1. XPS Analysis
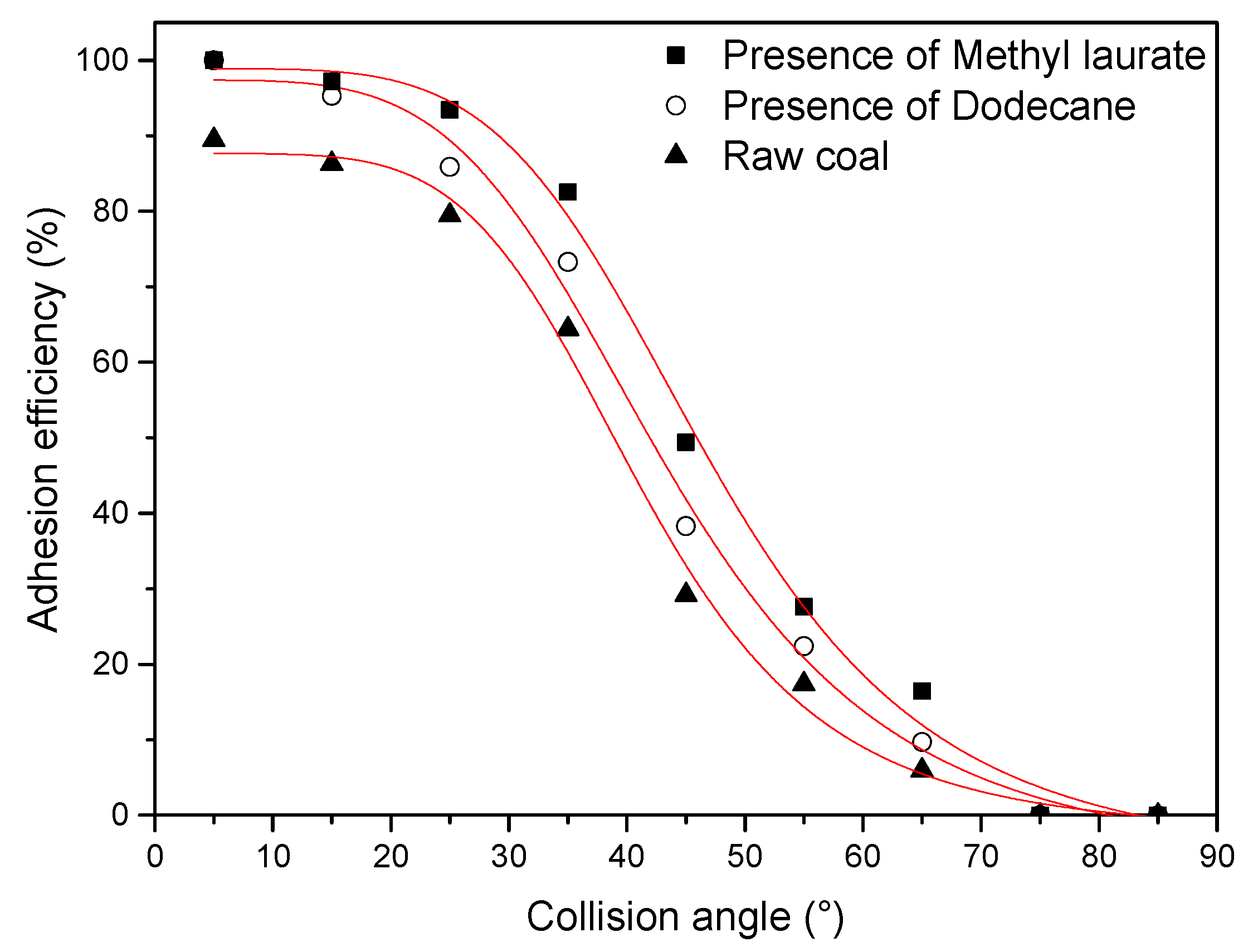
3.2.2. Attachment Efficiency Measurement
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Willson, W.G.; Dan, W.; Irwinc, W. Overview of Low-Rank Coal (LRC) Drying. Coal Prep. 1997, 18, 1–15. [Google Scholar] [CrossRef]
- Osman, H.; Jangam, S.V.; Lease, J.D.; Mujumdar, A.S. Drying of Low-Rank Coal (LRC)—A Review of Recent Patents and Innovations. Dry Technol. 2011, 29, 1763–1783. [Google Scholar] [CrossRef]
- Xia, W.; Yang, J.; Liang, C. A short review of improvement in flotation of low rank/oxidized coals by pretreatments. Powder Technol. 2013, 237, 1–8. [Google Scholar] [CrossRef]
- Jia, R.; Harris, G.H.; Fuerstenau, D.W. An improved class of universal collectors for the flotation of oxidized and/or low-rank coal. Int. J. Miner. Process. 2000, 58, 99–118. [Google Scholar] [CrossRef]
- Wen, B.; Xia, W.; Sokolovic, J.M. Recent advances in effective collectors for enhancing the flotation of low rank/oxidized coals. Powder Technol. 2017, 319, 1–11. [Google Scholar] [CrossRef]
- Dey, S. Enhancement in hydrophobicity of low rank coal by surfactants—A critical overview. Fuel Process Technol. 2012, 94, 151–158. [Google Scholar] [CrossRef]
- Harris, G.H.; Diao, J.; Fuerstenau, D.W. Coal Flotation with Nonionic Surfactants. Coal Prep. 1995, 16, 135–147. [Google Scholar] [CrossRef]
- Jena, M.S.; Biswal, S.K.; Rudramuniyappa, M.V. Study on flotation characteristics of oxidised Indian high ash sub-bituminous coal. Int. J. Miner. Process. 2008, 87, 42–50. [Google Scholar] [CrossRef]
- Chen, S.; Tang, L.; Tao, X.; He, H.; Chen, L.; Yang, Z. Enhancing flotation performance of low rank coal by improving its hydrophobicity and the property of oily bubbles using 2-ethylhexanol. Int. J. Miner. Process. 2017, 167, 61–67. [Google Scholar] [CrossRef]
- Vyas, A.P.; Verma, J.L.; Subrahmanyam, N. A review on FAME production processes. Fuel 2010, 89, 1–9. [Google Scholar] [CrossRef]
- Xia, W. Biodiesel as a renewable collector for coal flotation in the future. Energy Sources 2016, 38, 1938–1943. [Google Scholar] [CrossRef]
- Xia, W.; Yang, J.; Liang, C. Improving Oxidized Coal Flotation Using Biodiesel as a Collector. Coal Prep. 2013, 33, 181–187. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Y.L.; He, D.D.; Liu, G.S. Adsorption of cationic collectors and water on muscovite (001) surface: A molecular dynamics simulation study. Miner. Eng. 2013, 53, 101–107. [Google Scholar] [CrossRef]
- Rai, B.; Sathish, P.; Tanwar, J.; Pradip; Moon, K.S.; Fuerstenau, D.W. A molecular dynamics study of the interaction of oleate and dodecylammonium chloride surfactants with complex aluminosilicate minerals. J. Colloid Interface Sci. 2011, 362, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhang, R.; Xing, Y.; Gui, X. Improving the adsorption of oily collector on the surface of low-rank coal during flotation using a cationic surfactant: An experimental and molecular dynamics simulation study. Fuel 2019, 235, 687–695. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, Z.; Zhang, R.; Xing, Y.; Gui, X. Enhancement of the surface hydrophobicity of low-rank coal by adsorbing DTAB: An experimental and molecular dynamics simulation study. Fuel 2019, 239, 145–152. [Google Scholar] [CrossRef]
- Li, L.; Hao, H.; Yuan, Z.; Liu, J. Molecular dynamics simulation of siderite-hematite-quartz flotation with sodium oleate. Appl. Surf. Sci. 2017, 419, 557–563. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, W.; Han, C.; Wei, D. Intensify dodecylamine adsorption on magnesite and dolomite surfaces by monohydric alcohols. Appl. Surf. Sci. 2018, 444, 729–738. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.; Yan, K. Adsorption of collectors on model surface of Wiser bituminous coal: A molecular dynamics simulation study. Miner. Eng. 2015, 79, 31–39. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, L.; Liu, S.; Li, B. Effects of hydrophilic groups of nonionic surfactants on the wettability of lignite surface: Molecular dynamics simulation and experimental study. Fuel 2018, 231, 449–457. [Google Scholar] [CrossRef]
- Sun, H.; Jin, Z.; Yang, C.; Akkermans, R.L.; Robertson, S.H.; Spenley, N.A.; Miller, S.; Todd, S.M. COMPASS II: Extended coverage for polymer and drug-like molecule databases. J. Mol. Model. 2016, 22, 47. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, P.G. Chemical structural models for coalified wood (vitrinite) in low rank coal. Org. Geochem. 1990, 16, 959–968. [Google Scholar] [CrossRef]
- Nose, S.A. A Unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Li, P.D. Investigation of Bituminous Coal Hydrophobicity and its Influence on Flotation. Energ. Fuel 2009, 23, 5536–5543. [Google Scholar]
- Kozłowski, M. XPS study of reductively and non-reductively modified coals. Fuel 2004, 83, 259–265. [Google Scholar] [CrossRef]
- Zhuo, Q.; Liu, W.; Liu, W. Experimental study on the attachment behavior of coal particles and bubbles. J. Chin. Coal Soc. 2018, 43, 2029–2035. [Google Scholar]
- You, X.; He, M.; Zhang, W.; Wei, H.; He, Q.; Lyu, X. Molecular dynamics simulations and contact angle of surfactant at the coal–water interface. Mol. Simul. 2018, 44, 1–6. [Google Scholar] [CrossRef]
- He, M.; Zhang, W.; Cao, X.; You, X.; Li, L. Adsorption Behavior of Surfactant on Lignite Surface: A Comparative Experimental and Molecular Dynamics Simulation Study. Int. J. Mol. Sci. 2018, 19, 437. [Google Scholar] [CrossRef]
- Tao, C.G.; Feng, H.J.; Zhou, J.; Lv, L.H.; Lu, X.H. Molecular Simulation of Oxygen Adsorption and Diffusion in Polypropylene. Acta Phys. -Chim. Sin. 2009, 25, 1373–1378. [Google Scholar]
- Núñez-Rojas, E.; Domínguez, H. Computational studies on the behavior of sodium dodecyl sulfate (SDS) at TiO2 (rutile)/water interfaces. J. Colloid Interface Sci. 2011, 364, 417–427. [Google Scholar] [CrossRef]
- Verrelli, D.I.; Koh, P.T.L.; Nguyen, A.V. Particle–bubble interaction and attachment in flotation. Chem. Eng. Sci. 2011, 66, 5910–5921. [Google Scholar] [CrossRef]
- Lecrivain, G.; Petrucci, G.; Rudolph, M.; Hampel, U.; Yamamoto, R. Attachment of solid elongated particles on the surface of a stationary gas bubble. Int. J. Multiph. Flow 2015, 71, 83–93. [Google Scholar] [CrossRef]
- Zhuo, Q.; Liu, W.; Xu, H.; Sun, X.; Zhang, H.; Liu, W. The Effect of Collision Angle on the Collision and Adhesion Behavior of Coal Particles and Bubbles. Processes 2018, 6, 218. [Google Scholar] [CrossRef]

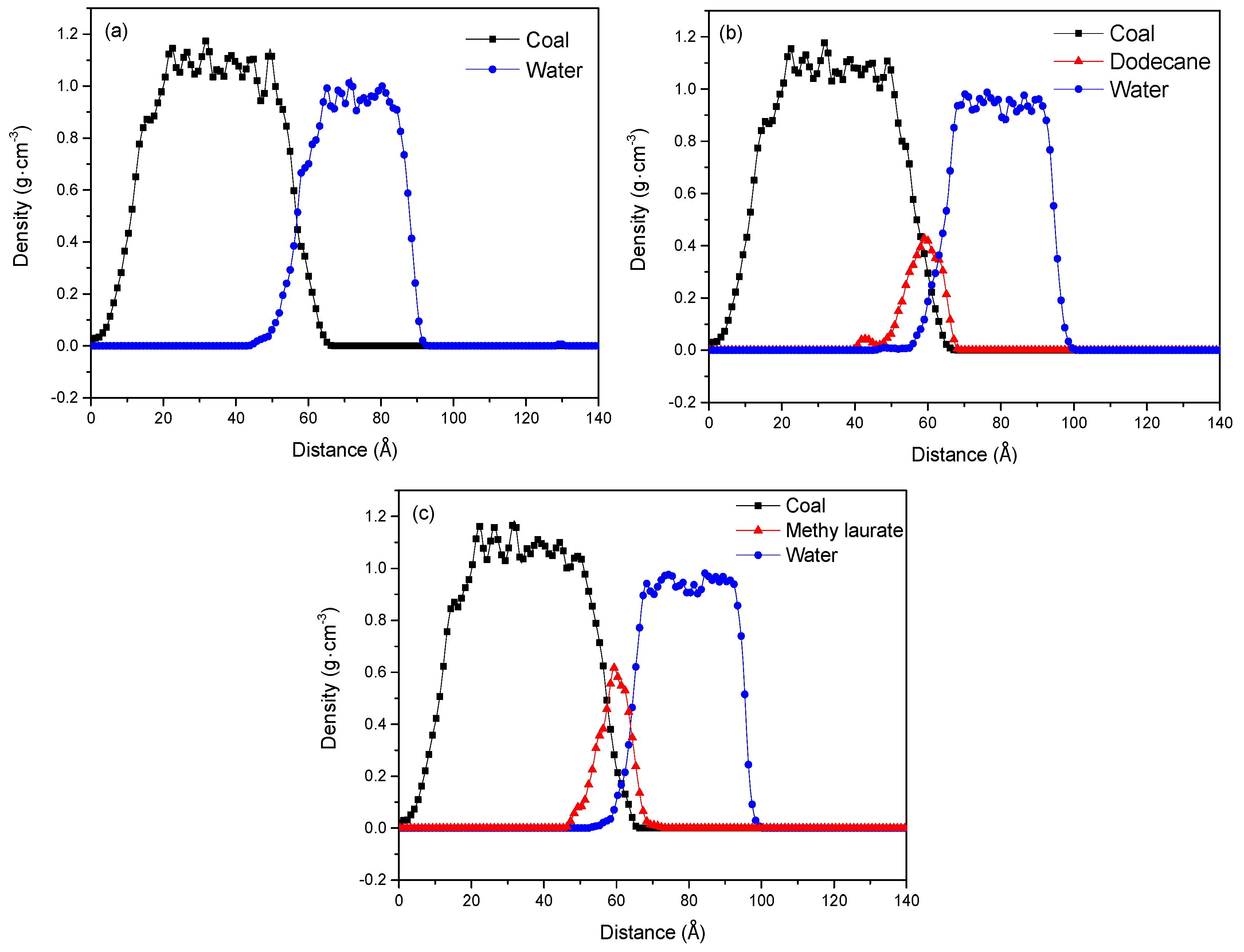
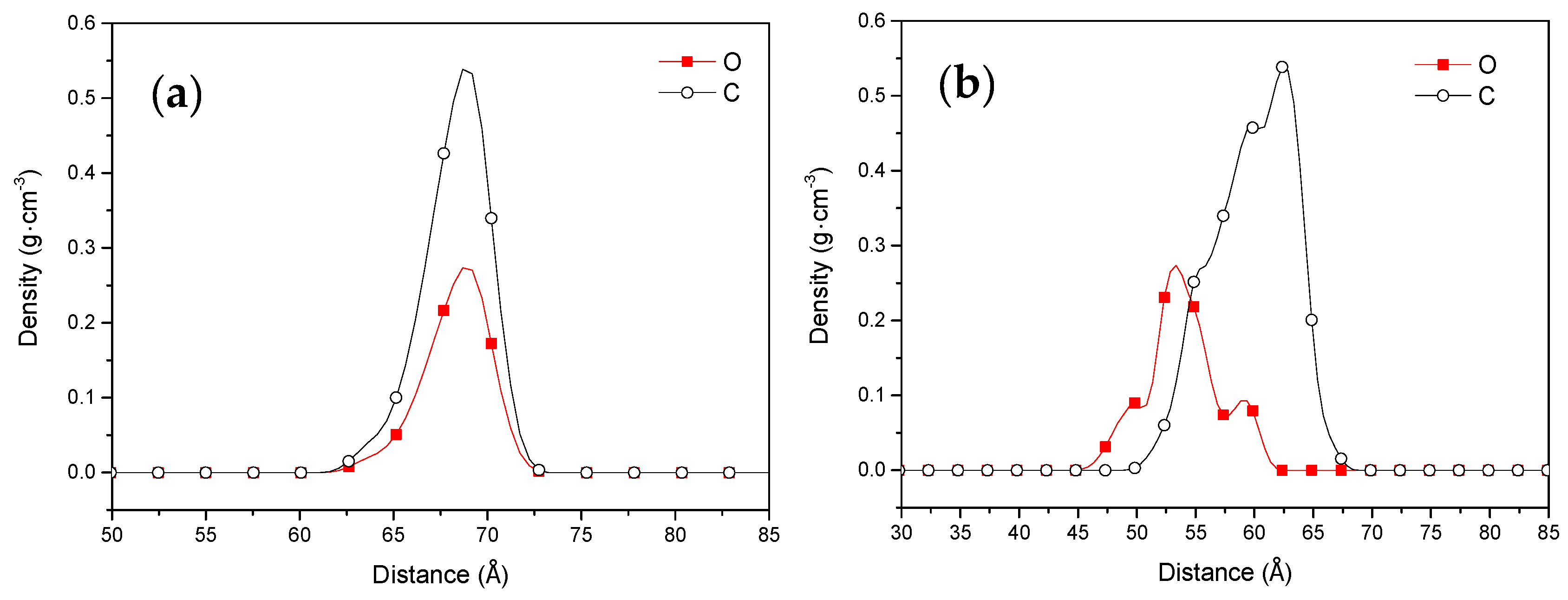
| Proximate Analysis (wt %) | Ultimate Analysis (wtdaf %) | ||||||
|---|---|---|---|---|---|---|---|
| Mad | Aad | Vdaf | C | H | O 2 | N | S |
| 5.84 | 10.46 | 31.26 | 74.33 | 5.23 | 18.97 | 1.03 | 0.44 |
| System | Coal | Collectors | Water |
|---|---|---|---|
| water/coal | 0–66.66 | - | 43.34–93.02 |
| water/dodecane/coal | 0–66.62 | 39.32–70.16 | 45.34–100.56 |
| water/methyl laurate/coal | 0–66.59 | 45.31–70.44 | 52.34–100.42 |
| System | D (10−9 m2/s) |
|---|---|
| water/coal | 4.90 |
| water/dodecane/coal | 5.32 |
| water/methyl laurate/coal | 5.88 |
| Element | Raw Coal | Dodecane | Methyl Laurate |
|---|---|---|---|
| C | 77.13 | 79.11 | 84.16 |
| O | 22.87 | 20.89 | 15.84 |
| O/C | 29.65 | 26.41 | 18.82 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Liu, W.; Xu, H.; Zhuo, Q.; Sun, X. Adsorption Behavior of Methyl Laurate and Dodecane on the Sub-Bituminous Coal Surface: Molecular Dynamics Simulation and Experimental Study. Minerals 2019, 9, 30. https://doi.org/10.3390/min9010030
Zhang H, Liu W, Xu H, Zhuo Q, Sun X. Adsorption Behavior of Methyl Laurate and Dodecane on the Sub-Bituminous Coal Surface: Molecular Dynamics Simulation and Experimental Study. Minerals. 2019; 9(1):30. https://doi.org/10.3390/min9010030
Chicago/Turabian StyleZhang, He, Wenli Liu, Hongxiang Xu, Qiming Zhuo, and Xiaopeng Sun. 2019. "Adsorption Behavior of Methyl Laurate and Dodecane on the Sub-Bituminous Coal Surface: Molecular Dynamics Simulation and Experimental Study" Minerals 9, no. 1: 30. https://doi.org/10.3390/min9010030
APA StyleZhang, H., Liu, W., Xu, H., Zhuo, Q., & Sun, X. (2019). Adsorption Behavior of Methyl Laurate and Dodecane on the Sub-Bituminous Coal Surface: Molecular Dynamics Simulation and Experimental Study. Minerals, 9(1), 30. https://doi.org/10.3390/min9010030






