Abstract
The environmental problems generated by waste from the mining industry in the mineral extraction for business purposes are known worldwide. The aim of this work is to evaluate the microalga Muriellopsis sp. as a potential remover of metallic ions such as copper (Cu2+), zinc (Zn2+) and iron (Fe2+), pollutants of acid mine drainage (AMD) type waters. For this, the removal of these ions was verified in artificial acid waters with high concentrations of the ions under examination. Furthermore, the removal was evaluated in waters obtained from areas contaminated by mining waste. The results showed that Muriellopsis sp. removed metals in waters with high concentrations after 4–12 h and showed tolerance to pH between 3 and 5. These results allow proposing this species as a potential bioremediator for areas contaminated by mining activity. In this work, some potential alternatives for application in damaged areas are proposed as a decontamination plan and future prevention.
1. Introduction
The Atacama Desert is located in Chile, and it is also one of the aridest in the world due to its low rainfall and scarce superficial and underground water resources, and also is one of the most important mining reserves of copper, gold, silver, molybdenum and lithium in the world [1]. These geographical conditions have driven the main economic development of our country to be based on mining production. Nevertheless, as a consequence of the mining waste, there is a production of acid mine drainages (AMD) which is a leachate that results from the oxidation of sulfides exposed to water, air, bacterial activity and heavy metal compounds, that are harmful to the environment and human health.
The AMD main characteristics are [2]: (a) low pH values (between 2 and 5); (b) high sulfate levels (several thousand mg/L), iron (between 50 and 1000 mg/L), zinc (up to 200 mg/L), manganese (between 1 and 100 mg/L), aluminum, lead, copper, nickel, mercury, cadmium, chromium and other toxic elements such as arsenic, and (c) high calcium and magnesium concentrations. The AMD formation begins when sulfide minerals present in coal or mine waste (such as pyrite) are exposed to air and water in mining operations [3]: Pyrite is chemically oxidized, creating a slightly acid environment suitable for the growth of some acidophilic chemoatropic microorganisms such as bacteria Acidithiobacillus ferrooxidans (formerly Thiobacillus thiooxidans). The resulting ferrous iron is regenerated to ferric by the action of these bacteria. The ferric ion becomes available again to oxidize more pyrite and the cycle continues once it has started. The acid solution loaded with iron goes from a sulfide-rich environment and encounter rocks, soils and waters with a higher pH (>2.5); in this way, the ferric iron produced is hydrolyzed and generates greater acidity [4]. This ferric ion is responsible for dissolving many heavy metal sulfide minerals such as lead, copper, zinc, and cadmium.
In Chile, the discharge of these industrial waste is regulated by Decree 90/2000 [5] which establishes the emission standard for pollutants associated with discharges of liquid waste in marine and continental waters superficial. In order to comply with this regulation, have been used for water remediation methods such as chemical precipitation, ion exchange, adsorption, membrane purification [6], passive treatments, alkalinity production systems and in the last decades biosorption processes [7,8,9].
Biosorption processes use plants, including algae, which have the ability to bind metallic ions in negatively charged sites [10]. Several mechanisms have been proposed to explain metal tolerance in plants. These mechanisms can be divided into two broad categories: those that involve detoxification of metallic ions within the cell and those that prevent the metal from crossing the plasma membrane [11]. From these data, it has been proposed that the ability of metals to accumulate in microalgal cells by continuous exposures of the metal contaminant would lead to mechanisms of resistance through physiological adaptive processes [12]. In some microalgae, there is a case of cross-resistance, which is when a species or population is resistant to more than one metal at the same time [13].
Microorganisms such as microalgae have demonstrated the ability to remove inorganic nutrients from wastewater such as nitrogen and phosphorus, which are assimilated for their growth [14]. Scientific support indicates the advantage of the use of microalgae in metal biosorption [15], its affinity to different metals has been recognized [16] and has been used in the remediation of metal ions [17]. For example, the use of marine algae and freshwater has been reported for the adsorption and elution of gold, silver and cobalt [18,19]. Based on these data, the aim of this work is to evaluate the viability of the microalgal biomass of Muriellopsis sp. to reduce the concentrations of metal ions (Cu2+, Zn2+ and Fe2+) from acid artificial water matrixes and with high metal concentrations, and from natural waters from acid drainages obtained from areas contaminated by local mining processes. This removal could be considered as a potential alternative to mitigate the contamination of areas with mining waste.
2. Materials and Methods
2.1. Obtaining Microalgal Strains
The microalga Muriellopsis sp. was obtained from the strain collections of the Unidad de Microbiología Aplicada (UMA) at the Universidad de Antofagasta. It was cultivated in commercial liquid culture medium F/2 [20] modified and incubated at 20 ± 1 °C. It was cultivated in a 25-L Photobioreactor at 20 °C with a continuous photoperiod of 70 µE m−2 s−1 continuous exposure (24 h light) for 30 days.
2.2. Metal Removal by Muriellopsis sp. from Artificial Acid Drainage (AAD)
The AAD consisted in the simulation of water with similar characteristics to acid drainage obtained from mining waste. For this, 2 acid matrixes were established at pH 5 and 3, standardized with HCl 0.1 N in 100 mL Erlenmeyer flasks with 50 mL of 35% sterilized Marine Saline Solution (7 mg/L MgSO4·7H2O; 0.8 mg/L KCl; 24 mg/L NaCl). Then, Cu2+, Zn2+ and Fe2+ ions (Trizol of 1000 mg/L, Merck) [21] were inoculated to the solutions with the aim of obtaining concentrations of 20, 50 and 100 mg/L. As control 35% marine saline solution (SSM) at pH 5 and 3 without metallic ions were used. Once the solutions were prepared, a 1.1 × 107 cells/mL concentration of Muriellopsis sp. was added. The treatments and controls were incubated at room temperature with constant shaking to keep the sample homogenized in Shaking (JSSI-100T, JSR Corporation, Tokyo, Japan). The microalgal count was recorded at 4, 8 and 12 h through the Neubauer chamber with an OLYMPUS BX microscope (Olympus Corporation, Tokyo, Japan). The pH was measured through pH-meter (PHS-W-LIDA, Bante Instruments, Shanghai, China) and the metal removal was recorded with the Cu2+, Zn2+ and Fe2+ kits (Spectroqant®, Merck & Company, Inc., Kenilworth, NJ, USA) using a spectrophotometer (Pharo 300, Merck & Company, Inc., Kenilworth, NJ, USA).
2.3. Removal of Fe2+ Ion by Muriellopsis sp. from Natural Acid Drainage (NAD)
The NAD sample from mining waste was obtained 45 km northeast from Antofagasta, an area affected by mining activity (coordinates U.T.M 7,406,500–7,409,000 N and 389,000–494,500 E). At the laboratory, the sample was recorded, Fe2+ concentration with Spectroqant® Kit through a Pharo 300 spectrophotometer, pH (pHmeter PHS-W-LIDA) and salinity (ATAGO-ATC-S/MILL-E, ATAGO CO LTD., Tokyo, Japan). The sample was kept at room temperature.
Based on the natural parameters of metal concentration, pH, and salinity of NAD sample from the contaminated area, 3 artificial waters were prepared as controls. For this, 100 mL Erlenmeyer flasks were inoculated with 30 mL of SSM (35%) sterilized and acidified to pH 4 with HCl 0.1 N. In order to obtain concentrations of 50, 100, 800 mg/L, Fe2+ (Trisol 1000 mg/L, Merck) was added. Likewise, a negative control was prepared with 35% SSM and pH 4 without inoculating metallic ions. In parallel, 100 mL flasks were used with 30 mL of NAD as a treatment. Then, a 1.0 × 107 cells/mL concentration of Muriellopsis sp. was added to treatments and controls. Controls and treatments were incubated at room temperature with constant shaking to keep the sample homogenized (Shaking JSR JSSI-100C/JSSI-100T). The microalgal count was recorded at 6 and 12 h through the Neubauer chamber with an OLYMPUS BX microscope. The pH was measured by pH-meter (PHS-W-LIDA) and the metal removal was recorded with the Iron kit (Spectroqant®, Merck) through a spectrophotometer (Pharo 300, Merck).
2.4. Data Analysis
Tests of each treatment and control were carried out in triplicate. The relation in the microalga Muriellopsis sp., of the variables of density, metal removal, and pH variations were evaluated through analysis of variance (ANOVA) and differences of means by multiple comparisons Tukey’s, previous verification of normality and homocedasticity of data. The analysis was performed using the GraphPad PRISM 5.0 statistical software (GraphPad Software Inc., San Diego, CA, USA).
3. Results
3.1. Density and Metal Removal by Muriellopsis sp. in AAD
At the end of the treatment after 12 h., the lowest density of the microalga Muriellopsis sp., was observed in treatments of 100 mg/L in pH 5 and pH 3. Considering as initial inoculum 1.0 × 107 cells/mL, final values were: Cu2+ 7.9 × 106 cells/mL in pH 5 and 3, in Zn2+ 8.8 × 106 cells/mL in pH 5 and 8.5 × 106 cells/mL in pH 3 and in Fe2+ 8.9 × 106 cells/mL in pH 5 and 7.9 × 106 cells/mL in pH 3. In addition to observing a tolerance of the microalga to survive acid pH, an increase of pH in the medium was recorded at the end of the experiment. For example, the pH maximums observed in treatments were: in Cu2+ pH 7.0 (pH 5) and 6.3 (pH 3); in Zn2+ pH 7.1 (pH 5) and 6.7 (pH 3), and in Fe2+ pH 7.0 (pH 5) and 6.3 (pH 3). Unlike the other ions, in Fe2+ a decrease in pH was registered in the 100 mg/L concentration at the end of the experiment as 2.4 (pH 5) and 1.7 (pH 3). In controls (without metals) in pH 5 and 3 a maximum pH of 7.6 was registered. Finally, the microalgal survival (%) fluctuated among the different concentrations of metals between 72–99% in pH 5 and 65–95% in pH 3 (Figure 1, Figure 2 and Figure 3).
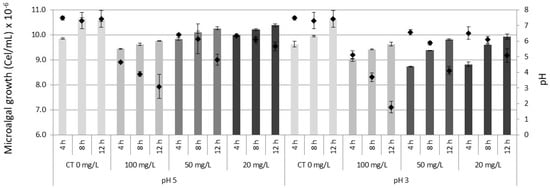
Figure 1.
Growth of the microalga Muriellopsis sp., In artificial acid drainage (AAD) cultivated at different pH and copper concentrations. CT (Control). The bars represent ± standard error of the mean. Diamond symbol microalgal growth.
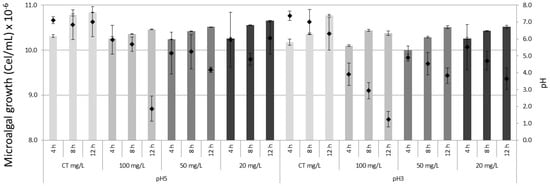
Figure 2.
Growth of the microalga Muriellopsis sp. in artificial acid drainage (AAD) cultivated at different pH and zinc concentrations. CT (Control). The bars represent ± standard error of the mean. Diamond symbol microalgal growth.
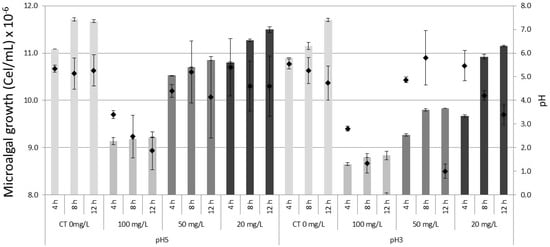
Figure 3.
Growth of the microalga Muriellopsis sp. in artificial acid drainage (AAD) cultivates different pH and iron concentrations. CT (Control). The bars represent ± standard error of the mean. Diamond symbol microalgal growth.
The results showed that from 4 to 12 h of treatment, an increase in metal removal was observed (Cu2+, Zn2+ and Fe2+). At 12 h of treatment, it was observed that the best removal in all metals was obtained in 20 mg/L, in a range from minimum to maximum of 63–99.6% in pH 3 and 84 to 99.9% in pH 5. Followed by 50 mg/L with 37.6 to 85.5% in pH 3 and between 71.5 to 99.7% in pH 5. Finally, in 100 mg/L with 18.6–80.9% in pH 3 and 41–93.6% in pH 5 (Table 1). The highest percentage of removal was obtained in Fe2+ in pH 5 and 3, this result encouraged that in samples of natural acid drainage we focused only on measuring Fe+2 removal at different concentrations.

Table 1.
Copper, zinc and iron removal from AAD by the microalga Muriellopsis sp., cultivated in SSM (35%), different pHs and metal concentrations. Values in parentheses are in mg/L.
The ANOVA statistical analysis performed to compare effects between the measured variables (pH variation, density, and metal removal) indicated statistically significant differences between the variables in Cu2+ (F-statistic or F = 662.4, p value or p < 0.0001), Zn2+ (F = 1235, p < 0.0001) and Fe2+ (F = 666, p < 0.0001). The analysis of means differences by Tukey’s multiple comparisons revealed that in metals, the microalga density was influenced by the pH of the culture medium, observing significant differences (p < 0.001). In addition, the pH variation recorded at the end of the experiment was not significant (p < 0.001) between pH 5 and pH 3 in all treatments with metals. Furthermore, no significant differences were observed in the microalgae density obtained at the end of the experiment at both pH in all treatments with metals. Regarding removal, the statistical analysis indicated that it is related to the microalgae density in the samples at pH 5 and pH 3 when significant differences were observed between these variables (p < 0.001). However, metal removal from the microalgae is not related to the pH of the culture medium, as there are no significant differences between these variables.
3.2. Density and Metal Removal by Muriellopsis sp. in NAD
The collected NAD from a contaminated area presented an orange color, with pH 4, salinity of 35‰ and a concentration of Fe2+ of 80 mg/L ± 0.1 mg/L. At the end of the treatment after 12 h and considering as initial inoculum 1.0 × 107 cells/mL, the lowest density of the microalga Muriellopsis sp. in AAD was observed in 800 mg/L with 2.7 × 106 cells/mL and in NAD 8.7 × 106 cells/mL was registered. Considering pH 4 as an initial value, it was observed that the microalga presented a tendency to increase the pH of the medium, registering 8.7 in control, pH 4.7 in AAD 50 mg/L, and pH 4.6 in NAD 80 mg/L. However, there was a tendency to lower the pH in AAD of 100 mg/L (pH 3.1) and 800 mg/L (pH 1). Finally, the microalgal survival percentage fluctuated between 28% (AAD 800 mg/L) and 127% (control) (Figure 4). The results of the removal showed that from 6 h of sampling, Fe2+ removal by the microalga was recorded in controls and treatment. The greatest removal was 71.6 mg/L (71.6%) in AAD 100 mg/L, followed by NAD 80 mg/L with 64.5 mg/L (80.6%). A similar trend was recorded at the end of the treatment (12 h) with 91.3 mg/L (91.3%) in AAD 100 mg/L and 74 mg/L (92.5%) in NAD 80 mg/L. Although in treatment of 800 mg/L, the Fe2+ concentration was exaggerated, the recorded removal was 63.3 mg/L (7.9%), the result was important since the high resistance of the microalga and the effective removal could be verified (Table 2).
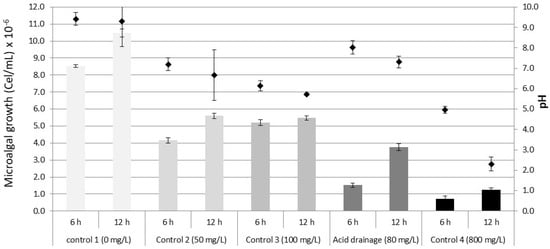
Figure 4.
Growth of the microalga Muriellopsis sp. in NAD (80 mg/L of iron, pH 4 and salinity 35‰). Diamond symbol microalgal growth. Diamond symbol microalgal growth.

Table 2.
Iron removal from AAD by the microalga Muriellopsis sp.
The ANOVA statistical analysis of the variables (pH variation, density, and metal removal) indicated significant differences between the evaluated variables (F = 82 p < 0.0001). The analysis of means differences by Tukey’s multiple comparisons revealed that the microalgae density was influenced by the pH from the culture medium when significant differences were observed (p < 0.001). Regarding the removal, the analysis indicates that the microalgae density is related to metal removal from the samples when significant differences were observed (p < 0.001). However, metal removal of the microalga is not related to the pH of the culture medium as no significant differences were observed.
4. Discussion
Acid mine drainages contain dissolved metals, being iron one of the main compounds of AMD [22], an important aspect to be considered is the impact caused by these discharges, since it strongly affects biodiversity (flora and fauna), both in the soil and in the water, since the acidity condition of the AMD alters the natural cycle of the affected ecosystems [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23]. Considering the toxicity and the duration of the AMD, it is essential to prevent its formation or apply the most appropriate treatment for its mitigation and control, which must comply with the maximum acceptable limits [24], which in the case of Chile is regulated by Decree 90/2000 [5].
The aim of AAD with NAD parameters was due to the fact that we previously needed to verify if the microalga Muriellopsis sp. had the capacity to tolerate acid pH and remove metals from samples with high concentrations, without affecting its viability. After this analysis, this behavior was compared in NAD samples naturally contaminated by mining processes.
Regarding metal removal in AAD and NAD tests, based on Decree 90/2000 which establishes that the maximum discharge limit is 4.8 mg/L in Cu2+, 1 mg/L in Zn2+ and Fe2+. In our tests, it was observed that Muriellopsis sp., at 12 h of treatment, managed to remove high concentrations of these metals. These results are preliminarily interesting to be used as potential bioremediators, since microalga Muriellopsis sp. removes metals from liquid samples in a short time, survives in high metal concentrations, and acid pH. Tolerance tests have been carried out in other microalgae at high metal concentrations, whose results have demonstrated that they are below the tolerated concentrations by Muriellopsis sp. in our work. For example, with respect to copper, studies by Cordero (2005) [25] demonstrated that the microalga Tetraselmis chuii in LC50 tests tolerated a maximum of 6.4 ± 3.2 mg/L of copper. In toxicity tests with Zinc, it was found that the tolerance of Selenastrum capricornut and Nannochloropsis oculata microalgae were around 0.76 and 3.22 mg/L respectively at 24 h [26]. In iron, Estupiñan (2015) [27] observed that in acid drainage samples (36.9 mg/L) from a coal mine the Chlorella Vulgaris and Scenedesmus Quadricauda microalgae managed to absorb 86.75% and 92.77%. The potential of bioremediation of heavy metals, of the microalgae have been studied extensively, establishing that they are very efficient in this task. Below is a review elaborated by Zeraatkar et al., 2016 [28] (Table 3) with modifications and new references that were incorporated in this paper.

Table 3.
Heavy metal absorption capacity from different microalgal species (Modified from [28]).
Regarding pH in the test with AAD, it was observed that in most cases it tended to rise in the culture medium. This can be explained because due to photosynthesis, the microalgae produce bicarbonate (HCO3−) and carbonates (CO32−) [58] which could be basifying the culture medium. With the exception of the iron controls (NAD test) in treatments of 100 and 800 mg/L, the pH decreased coinciding with the decrease in the microalgal density that was probably affected by the high copper concentrations and as a consequence prevented that these regulate pH. This fall could also have occurred because the ion’s standard solution is dissolved in sulfuric acid, which provides the solution with Fe2+, SO42−, H+ that upon exposure to water and oxygen generates oxidation, producing an increase in acidity [24]. In the NAD treatment (80 mg/L) the tendency to raise the pH ranged from 4.0 to 4.5. Likewise, since it is a natural sample, it is probable that other dissolved solids or its components interfere in the development of the microalga to regulate pH, although the absorption of the microalga was not affected.
5. Conclusions
Our results allow us to conclude that the microalga Muriellopsis sp. can survive 12 h exposed to acid pH (between 3 and 5), to high concentrations of metallic ions up to 100 mg/L in Cu2+, Zn2+ and 800 mg/L in Fe2+. This is the first work that reports the tolerance of the microalga Muriellopsis sp. to parameters similar of acid drainages in mining. Based on these results, we propose the microalga Muriellopsis sp. as a potential bioremediator of waters contaminated by mining processes. As a biotechnological application, reactors could be used which allow the entry of contaminated water that will be inoculated with microalgae for a period of 12 h, then the treated water will be separated by precipitation (in our tests we have been able to observe qualitatively that the microalga without agitation has the capacity to precipitate in a short time). Another treatment alternative is the use of raceway pools with contaminated waters which could be inoculated with the microalgae Muriellopsis sp. as a treatment. Parallel to field work, it is important to conduct a more specific studies that identify the feasibility of applying this treatment system at an industrial scale.
Author Contributions
M.M. and Y.L. performed the experiments; M.M., Y.L., L.A.C. and C.R. formulated the hypotheses, reviewed and analyzed the results, and formulated the conclusions; M.M., Y.L., and L.A.C. wrote the paper.
Funding
This research was funded by CONICYT through PAI program grant number ACM 170005.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Verdugo Gallegos, L.A. Remoción de Iones Sulfato y Metales Pesados Desde Soluciones Acuosas que Simulan Aguas de Mina Usando Mezcla de Cal, Silicatos Nano-Estructurados y Policloruro de Aluminio en una Celda DAF. Bachelor’s Thesis, Universidad De Chile, Santiago, Chile, 2013. (In Spanish). [Google Scholar]
- Murcia, E.; Trillos, C. Estudio Cinético para la Predicción de la Formación de Drenajes Ácidos en Minas de Carbon; Technical Report; Universidad Industrial de Santander, Escuela de Ingeniería Química: Bucaramanga, Colombia, 2000; pp. 5–20. (In Spanish) [Google Scholar]
- Leusmary, D.; Villafrades, R. Remoción de Fe y Mn Provenientes de Drenajes Ácidos de Minas de Carbón Utilizando Algas y Plantas Acuáticas; Technical Report; Universidad Industrial de Santander, Escuela de Ingeniería Química: Bucaramanga, Colombia, 2001; pp. 74–76. (In Spanish) [Google Scholar]
- Laverde, D. Prevención de la Contaminación por Drenajes Ácidos de Minas de Carbon; Technical Report; Informe final presentado a Colciencias-Minercol: Bucaramanga, Colombia, 2001; pp. 18–28. (In Spanish) [Google Scholar]
- Presidencia, S.G.D.L. Normas de Emisión para la Regulación de Contaminantes Asociados a las descargas de Residuos Líquidos a Aguas Marinas y Continentales Superficiales. Available online: http://www.siss.gob.cl/586/w3-article-4127.html (accessed on 1 August 2018).
- Chen, J.P.; Hong, L.; Wu, S.N.; Wang, L. Elucidation of interactions between metal ions and Ca alginate-based ion-exchange resin by spectroscopic analysis and modeling simulation. Langmuir 2002, 18, 9413–9421. [Google Scholar] [CrossRef]
- Hedin, R.S.; Nairn, R.W.; Kleinmann, R.L.P. Passive Treatment of Coal Mine Drainage; Technical Report; US Dept of the Interior, Bureau of Mines: Washington, DC, USA, 1994.
- Díaz, A.; Arias, J.; Gelves, G.; Maldonado, A.; Laverde, D.; Pedraza, J.; Escalante, H. Biosorción de Fe, Al y Mn de Drenajes Ácidos de Mina de Carbón Empleando Algas Marinas Sargassum sp. en Procesos Continuos; Technical Report; Revista Facultad de Ingeniería Universidad de Antioquia: Medellín, Colombia, 2003. (In Spanish) [Google Scholar]
- Devia Torres, D.; Cáceres Sepúlveda, S.; Roa, A.L.; Suárez Gelvez, J.H.; Urbina Suárez, N.A. Use of microalgae of Chlorophyta division in the biological treatment of acid drains of coal mines. Rev. Colomb. Biotecnol. 2017, 19, 95–104. (In Spanish) [Google Scholar]
- Macfie, S.M.; Welbourn, P.M. The cell wall as a barrier to uptake of metal ions in the unicellular green alga Chlamydomonas reinhardtii (Chlorophyceae). Arch. Environ. Contam. Toxicol. 2000, 39, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Cumming, J.R.; Taylor, G.J. Mechanisms of metal tolerance in plants: Physiological adaptations for exclusion of metal ions from the cytoplasm. In Stress Responses in Plants: Adaptation and Acclimation; Alscher, R.G., Cumming, J.R., Eds.; Wiley-Liss: New York, NY, USA, 1990. [Google Scholar]
- Belfore, N.M.; Anderson, S.L. Effects of contaminants on genetic patterns in aquatic organisms: A review. Mutat. Res. 2001, 489, 97–122. [Google Scholar] [CrossRef]
- Gupta, D.K.; Sandalio, L.M. Metal Toxicity in Plants: Perception, Signaling and Remediation; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Alvarez, H.M. Biorremediación de ambientes contaminados con hidrocarburos: Un proceso complejo que involucra múltiples variables. Rev. Quím. Viva 2015, 14, 18–25. (In Spanish) [Google Scholar]
- Kumar-Gupta, S.; Ahmad-Ansari, F.; Shriwastav, A.; Kumar-Sahoo, N.; Rawat, I.; Bux, F. Dual Role of Chlorella sorokiniana and Scenedesmus obliquus for Comprehensive Wastewater Treatment and Biomass Production for Bio-fuels. J. Clean. Prod. 2015, 115, 255–264. [Google Scholar] [CrossRef]
- Doshi, H.; Seth, C.; Ray, A.; Kothari, I.L. Bioaccumulation of heavy metals by green algae. Curr. Microbiol. 2008, 56, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Volesky, B. Biosorption of Heavy Metals; CRC Press: Boca Raton, FL, USA, 1990; pp. 7–14. [Google Scholar]
- Hamdy, A.A. Biosorption of heavy metals by marine algae. Curr. Microbiol. 2000, 41, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Kuzuno, E.; Mamiya, M. Adsorption of metal ions briver algae. Bunseki Kagaku 1992, 108, 123–128. [Google Scholar]
- Guillard, R.R.L.; Ryther, J.H. Studies of marine planktonic diatoms: I. Cyclotella Nana Hustedt, and Detonula Confervacea (CLEVE) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Toral, M.; Lara, N.; Gomez, J.; Richter, P. Determinación de hierro en fase sólida por espectrofotometría derivada de segundo orden. Bol. Soc. Chil. Quím. 2001, 46, 51–60. (In Spanish) [Google Scholar] [CrossRef]
- De la Cruz, C.E. Mitigación de drenaje ácido en minas subterráneas aplicando fangos artificiales. Caso: Mina Orcopampa. Revista del Instituto de Investigación de la Facultad de Ingeniería Geológica, Minera, Metalúrgica y Geográfica 2006, 9, 69–74. (In Spanish) [Google Scholar]
- Leal, L.T.C. Drenajes Ácidos de Mina Formación y Manejo. Rev. ESAICA 2015, 1, 53–57. (In Spanish) [Google Scholar] [CrossRef]
- Montesinos León, M.I. Caracterización de Afluentes de Mina para Elección de la Alternativa Óptima de Tratamiento. Ph.D. Thesis, Pontificia Universidad Católica del Perú, Lima, Peru, 2017. (In Spanish). [Google Scholar]
- Cordero, J.; Guevara, M.; Morales, E.; Lodeiros, C. Efecto de metales pesados en el crecimiento de la microalga tropical Tetraselmis chuii (Prasinophyceae). Rev. Biol. Trop. 2005, 53, 325–330. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Santo, G.E. Efectos Agudos y Crónicos de Diversos Metales en una Batería de Organismos Dulceacuícolas. Ph.D. Thesis, Universidad Autónoma de Aguas Calientes, Aguascalientes, México, 2013. (In Spanish). [Google Scholar]
- Estupiñan, J.C. Evaluación de un Tratamiento para Drenaje Ácido Proveniente de una Mina de Carbon. Bachelor’s Thesis, Universidad de La Sabana, Chía, Colombia, 2015. (In Spanish). [Google Scholar]
- Zeraatkar, A.K.; Ahmadzadeh, H.; Talebi, A.F.; Moheimani, N.R.; McHenry, M.P. Potential use of algae for heavy metal bioremediation, a critical review. J. Environ. Manag. 2016, 181, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Silva, K.R.; Vega-Bolaños, A.M.; Hernández-Rodríguez, L.C.; Parra-Ospina, D.A.; Ballen-Segura, M.Á. Uso de scenedesmus para la remoción de metales pesados y nutrientes de aguas residuales de la industria textil. Ingeniería Solidaria 2016, 12, 95–105. (In Spanish) [Google Scholar] [CrossRef]
- Jaramillo Ramos, R.A.; Romero Jara, H.M. Tratamiento de las Aguas del Sector las Katyas del Estero el Macho en Machala Mediante la Thalassiosira y Tetraselmis. Bachelor’s Thesis, Universidad Técnica de Machala, Machala, Ecuador, 2018. (In Spanish). [Google Scholar]
- Aksu, Z. Equilibrium and kinetic modelling of cadmiun (II) biosorption by C. Vulgaris in a batch system: Effect of temperatura. Sep. Purif. Technol. 2001, 21, 285–294. [Google Scholar] [CrossRef]
- Ortega, P.; Yomaira, B.; Valdez Álvarez, C.A. Análisis de Remoción de Cadmio por Acción de la Microalga Chlorella sp. Inmovilizada en Perlas de Alginate. Bachelor’s Thesis, Universidad Politécni Ca Salesiana, Quito, Ecuador, 2017. (In Spanish). [Google Scholar]
- Aksu, Z.; Dönmez, G. Binary biosorption of cadmiun (II) and nickel (II) onto dried Chlorella vulgaris: Co-ion effect on mono-component isotherm parameters. Process Biochem. 2006, 41, 860–868. [Google Scholar] [CrossRef]
- Gaber, E.; Yahia, A.; Abdulrahim, A. Biosorption of Cadmium and Lead from Aqueous Solutions by Chlorella vulgaris Biomass: Equilibrium and Kinetic Study. Arab. J. Sci. Eng. 2014, 39, 87–93. [Google Scholar]
- Tüzün, I.; Bayramoglu, G.; Yalcin, E.; Basaran, G.; Anca, M.Y. Equilibrium and kinetic studies on biosorption of Hg (II), Cd (II) and Pb (II) ion sonto microalgae Chlamydomonas reinhardtii. J. Environ. Manag. 2005, 77, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Adhiya, J.; Cai, X.; Sayre, R.T.; Traina, S.J. Binding of aqueour cadmium by the lyophilized biomass of Chlamydomonas reinhardtii. Colloid Surf. A-Physicochem. Eng. Asp. 2002, 210, 1–11. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chang, H.W.; Kao, P.C.; Pan, J.L.; Chan, J.S. Biosorption of cadmium by CO2-fixing microalga Scenedesmus obliquus CNW-N. Bioresour. Technol. 2012, 105, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Amézquita Imata, E.E. Remoción de Cadmio Bivalente (Cd+2) Mediante Bioadsorción en un Sistema de Flujo Continuo Empacado con Biomasa Muerta e Inmovilizada de Scenedesmus Obliquus (Turpin) Kützing 1833 a Escala de Laboratorio. Bachelor’s Thesis, Universidad Nacional De San Agustin De Arequipa, Arequipa, Peru, 2018. (In Spanish). [Google Scholar]
- Han, X.; Wong, Y.S.; Tam, N.F.Y. Surface complexation mechanism and modeling in Cr (III) biosorption by a microalgal isolate, Chlorella miniata. J. Colloid Interface Sci. 2006, 303, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Akthar, N.; Iqbal, M.; Zafar, S.I.; Iqbal, J. Biosorption characterisitics of unicelular Green alga Chlorella sorokinian immobilized in loofa sponge of removal of Cr (III). J. Environ. Sci. 2008, 20, 231–239. [Google Scholar]
- Gokhale, S.V.; Jyoti, K.K.; Lele, S.S. Kinetic and equilibrium modeling of chromium (VI) biosorption on fresh and spent Spirulina platensis/Chlorella vulgaris biomass. Bioresour. Technol. 2008, 99, 3600–3608. [Google Scholar] [CrossRef] [PubMed]
- Arica, M.Y.; Tüzün, I.; Yalcin, E.; Ince, O.; Bayramoglu, G. Utilisation of native, heat and acid-treated microalge Chlamydomonas reinhardtii preparations for biosorption of Cr (VI) ions. Process Biochem. 2005, 40, 2351–2358. [Google Scholar] [CrossRef]
- Dönmez, G.; Aksu, Z. Removal of chromium (VI) from saline wastewaters by Dunaliella species. Process Biochem. 2002, 38, 751–762. [Google Scholar] [CrossRef]
- Jácome-Pilco, C.R.; Cristiani-Urbina, E.; Flores-Cotera, L.B.; Velasco-García, R.; Ponce-Noyola, T.; Cañizares-Villanueva, R.O. Continuous Cr (VI) removal by Scenedesmus incrassatulus in an airlift photobioreactor. Bioresour. Technol. 2009, 100, 2388–2391. [Google Scholar] [CrossRef] [PubMed]
- Quezada, R.; Varela, E.; Rosa, M.A. Remediación natural para completar la depuración del cromo (VI) en efluentes de curtiembres. In Proceedings of the Quinto Congreso Deficiencia y Tecnología para Alumnos, Simposio Llevado a cabo en el Congreso de la Facultad Regional de Villa María, Córdoba, Argentina, 15–16 de Agosto 2012. (In Spanish). [Google Scholar]
- Bayramoğlu, G.; Yakup Arıca, M. Construction a hybrid biosorbent using Scenedesmus quadricauda and Ca-alginate for biosorption of Cu (II), Zn (II) and Ni (II): Kinetics and equilibrium studies. Bioresour. Technol. 2009, 100, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K.; Gaur, J.P. Removal of Ni and Cu from single and binary metal solutions by free and immobilized Chlorella vulgaris. Eur. J. Protistol. 2001, 37, 261–271. [Google Scholar] [CrossRef]
- Mehta, S.K.; Tripathi, B.N.; Gaur, J.P. Enhanced sorption of Cu2+ and Ni2+ by acid-pretreated Chlorella vulgaris from single and binary metal solutions. J. Appl. Phycol. 2002, 14, 267–273. [Google Scholar] [CrossRef]
- Vela García, F.N. Remoción de Mercurio en Aguas Residuales de la Actividad Minera con el Uso de Microalgas. Bachelor’s Thesis, Universidad de las Américas, Quito, Ecuador, 2016. (In Spanish). [Google Scholar]
- Wong, J.P.K.; Wong, Y.S.; Tam, N.F.Y. Nickel biosorption by two Chlorella species, C. vulgaris (a comercial species) and C. Miniata (a local isolate). Bioresour. Technol. 2000, 73, 133–137. [Google Scholar] [CrossRef]
- Akthar, N.; Iqbal, J.; Iqbal, M. Removal and recovery of nickel (II) from aqueous solution by loofa sponge-immobilized biomass of Chlorella sorokiniana: Characterization studies. J. Hazard. Mater. 2004, 108, 85–94. [Google Scholar]
- Al-Rub Abu, F.A.; El-Naas H, M.; Benyahia, F.; Ashour, I. Biosorption of nickel on blank alginate beads, free and immobilized algal cells. Process Biochem. 2004, 39, 1767–1773. [Google Scholar] [CrossRef]
- Aneja, R.K.; Chaudhary, G.; Ahluwalia, S.S.; Goyal, D. Biosorption of Pb2+ and Zn2+ by Non-Living Biomass of Spirulina sp. Indian J. Microbiol. 2010, 50, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Villanueva Vega, J.A. Determinación de la Biorremocion de Plomo (pb+2) Mediante Hongos y Microalgas Nativas Aisladas de Efluentes Industriales Empacadas en un Sistema en Serie de Agitación Continua. Bachelor’s Thesis, Universidad Nacional de San Agustin de Arequipa, Arequipa, Peru, 2015. (In Spanish). [Google Scholar]
- Mendoza Espinoza, S. Efecto de las Concentraciones de Plomo en el Crecimiento de la Microalga Marina Tetraselmis Suecica. Bachelor’s Thesis, Universidad Nacional del Santa, Nuevo Chimbote, Peru, 2017. (In Spanish). [Google Scholar]
- Vogel, M.; Gunther, A.; Rossberg, A.; Li, B.; Bernhard, G.; Raff, J. Biosorption of U (VI) by the Green algae Chlorella vulgaris in dependence of pH value and cell activity. Sci. Total Environ. 2010, 409, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.; Castro, P.L.; Xavier Malcata, F. Biosorption of zinc ions from aqueous solution by the microalga Scenedesmus obliquus. Environ. Chem. Lett. 2011, 9, 169–176. [Google Scholar] [CrossRef]
- Sobczuk, T.M. Influencia de las Condiciones Hidrodinámicas y de la Fracción Molar de CO2 en la Fase Gaseosa Sobre el Crecimiento Celular en Cultivos de Microalgas. Ph.D. Thesis, Universidad De Almería, Almería, Spain, 2005. (In Spanish). [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).