3.1. X-ray Diffraction
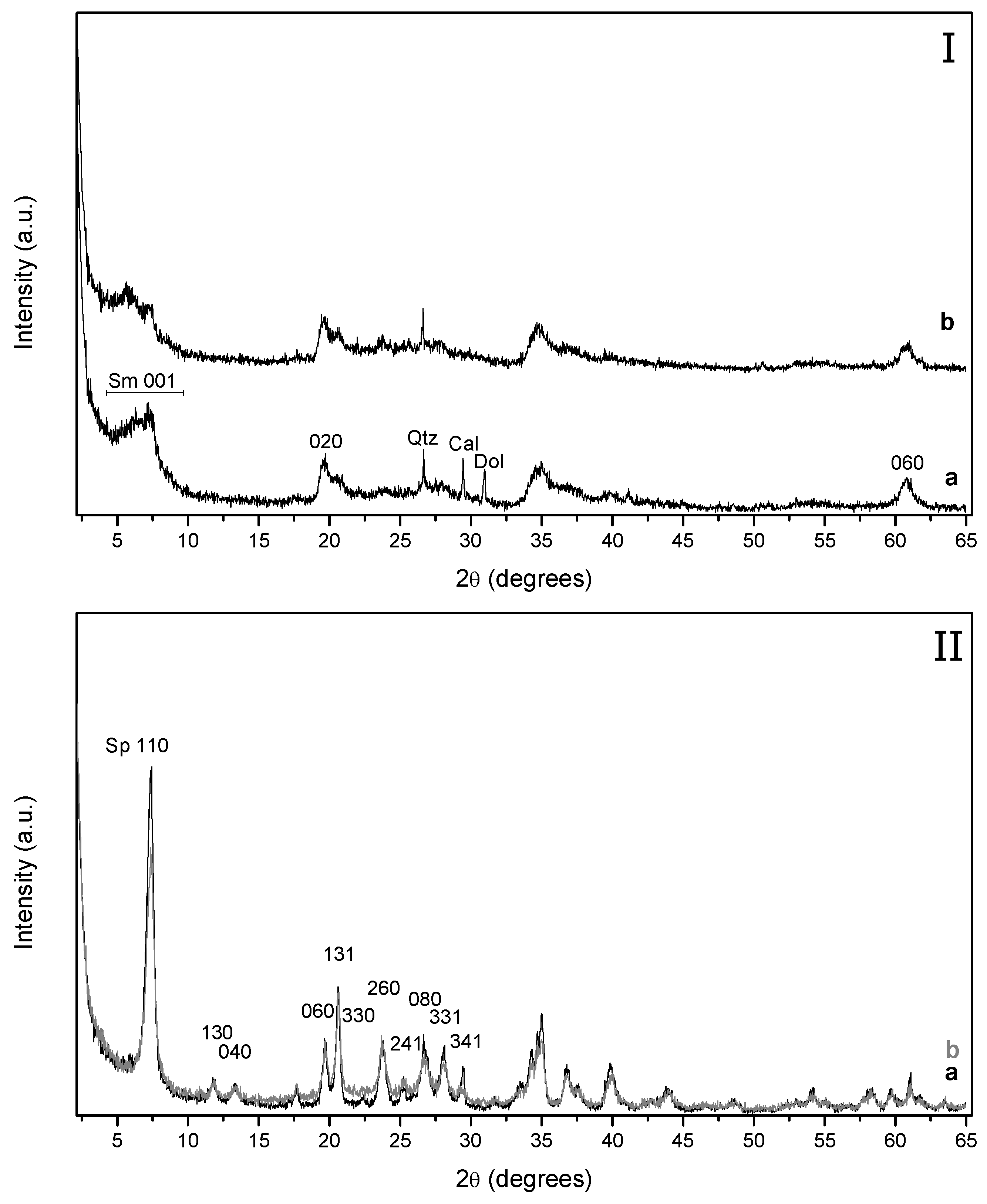
The X-ray diffraction patterns of the Mg-bentonite and sepiolite are shown in
Figure 1. Mg-bentonite sample (Sm) (
Figure 1a) shows the presence of minor proportions of quartz, calcite, and dolomite, whereas only traces of calcite are identified in sepiolite (Sp) (
Figure 1b). Mg-bentonite shows a wide 001 reflection band with two maxima suggesting the presence of at least two types of interlayer cations and/or a great variability in the hydration degree in interlayer space. The XRD reflections of sepiolite are sharp and well-defined in comparison to those displayed by Mg-bentonite, indicating a higher ordered structure for the fibrous clay mineral. The study of the clay fraction of the Mg-bentonite (oriented aggregates method) indicates the predominance of Mg-smectite and traces of sepiolite.
XRD profile of the Mg-bentonite after the microwave-assisted acid treatment (Sm–H) reveals the disappearance of the diffraction reflections (
Figure 2(Ia,Ib)) attributed to calcite and dolomite due to the dissolution of these carbonates after 8 min of acid treatment. Moreover, the intensity of the (001) and (020) diffraction reflections notably decreases suggesting the partial alteration of the Mg-smectite structure. On the other hand, the crystallinity of the quartz seems to be maintained after the microwave-assisted acid treatment.
The comparison between the XRD patterns of the raw sepiolite (Sp) and the material obtained after the microwave acid treatment also shows modifications (Sp–H) (
Figure 2(IIa,IIb)). Generally, a decrease in the intensity of the diffraction lines is noticeable, especially the (110) reflection. In addition, the occurrence of a broad band in the range of 15 to 33° (2θ) can be also observed. Both facts suggest that sepiolite structure is sensibly affected with the acid treatment and that the formation of an amorphous silica phase can take place [
17].
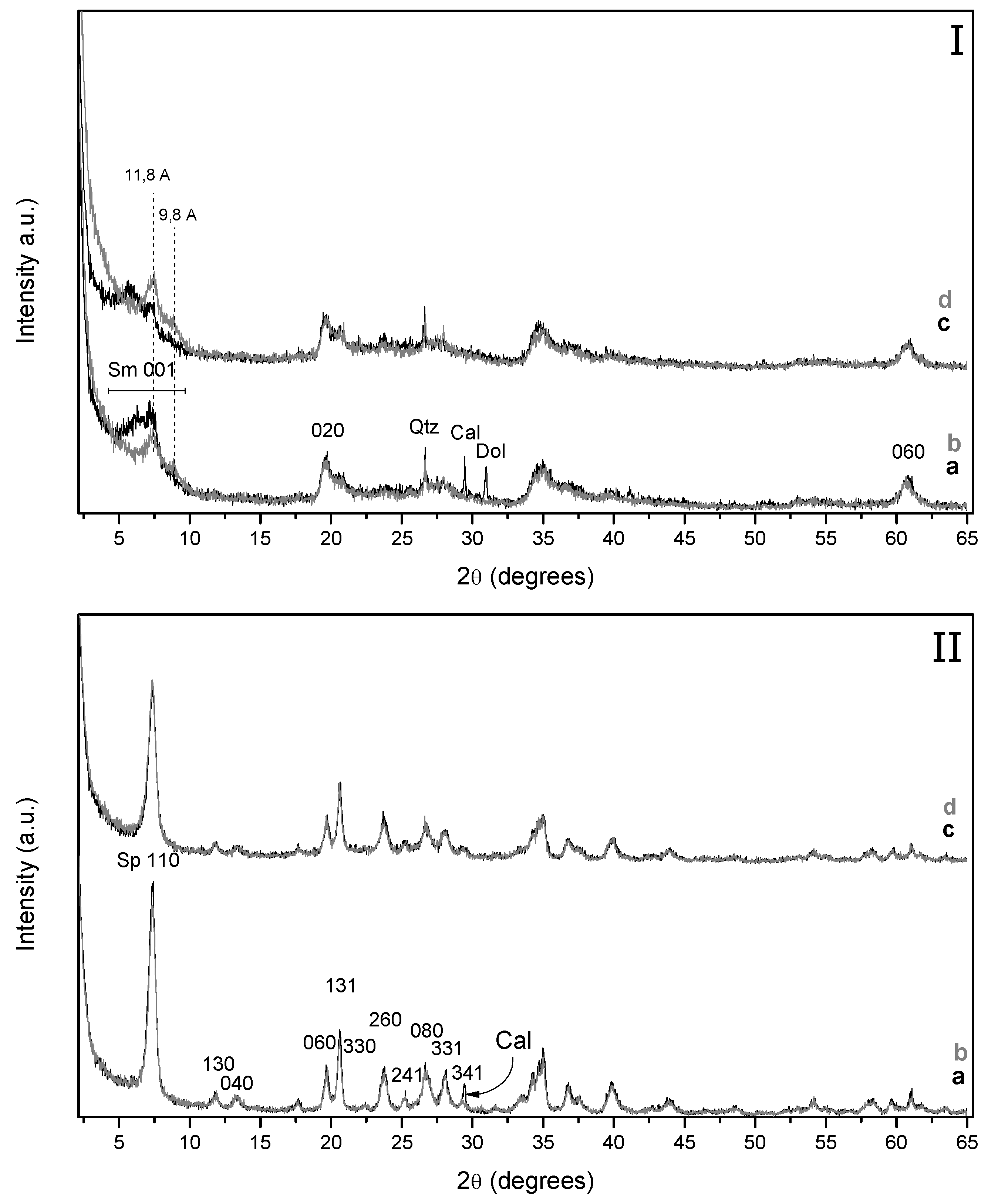
The incorporation of iron species into the starting Mg-smectite (Fe–Sm) and that modified by acid treatment (Fe–Sm–H) also causes changes in the XRD profile (
Figure 3I). As it took place for the sample modified by the microwave-assisted acid treatment, the diffraction peaks attributed to carbonate minerals disappears from the XRD diffraction patterns after the treatment with Fe
3+ (
Figure 3(Ia)), since the FeCl
3 solution is enough acidic (pH ~ 1) to dissolve the carbonate species. In addition, the (001) diffraction displays two peaks located at 9.8 and 11.8 Å, which suggests the coexistence of a different hydration state and/or interlayer cations [
18]. Moreover, we must take into account that the intensity of this last reflection is affected by the presence of minor amounts of sepiolite. On the contrary, the comparison of the XRD diffraction patterns of the Fe-treated sepiolites suggests that the incorporation of Fe
3+ within the raw sepiolite (Fe–Sp) and in the acid activated sepiolite (Fe–Sp–H) has no noticeably effect on their structure (
Figure 3II).
3.2. Chemical Analysis (XRF)
The chemical composition of the studied materials was determined by XRF. From data reported in
Table 1, it can be observed the predominance of magnesium which is coherent with the trioctahedral character of the clay minerals. The content of K, Ca, and Na, must be related with the presence of exchangeable cations although the content of Ca in Mg-smectite is also due to carbonates. Chemical analysis data (
Table 1) also reveal that microwave-assisted acid treatment produces a remarkable decrease of the magnesium content in both Mg-smectite and sepiolite, while the Si, Al, and Fe content are maintained after the acid treatment. This implies a partial dissolution of the octahedral sheet, releasing amorphous and insoluble silica, as was indicated for the XRD profile. In the same way, it is also noticeable the decrease of Ca content, which confirms the dissolution of the calcite phase as well as the Ca
2+ species located in the interlayer space, which must be replaced by H
+ species. With regard to potassium, the chemical data show similar values after the acid treatment, indicating that this cation remain in the interlayer space after the treatment.
The chemical analysis of the sample treated with FeCl3 solution shows little effect how the acid treatment does not seem to affect to the Fe3+ species incorporated, being in the same range for the raw Mg-smectite (4.56 wt % Fe2O3) and the Mg-smectite treated by acid treatment (4.18 wt % Fe2O3). In the case of sepiolite, the treatment of the raw sepiolite with the FeCl3 solution display higher Fe2O3 content (3.09 wt %) than that modified by acid treatment (1.58 wt %). The lower iron values could be ascribed to the formation of amorphous silica, which can cause a decrease in the adsorption capacity.
3.4. Nitrogen Adsorption
The specific surface area was determined from the S
BET equation [
23] (
Table 2). The natural sepiolite displays a S
BET value of 308 m
2·g
−1, while the starting Mg-smectite displays a S
BET of 182 m
2·g
−1. Sepiolite displays microporosity ascribed to the channels formed by the reversal of the tetrahedral layer [
10]. The microporosity of Mg-smectite can be related with its low structural ordering, which can generate small cavities leading to this microporosity. In all cases, it can be observed an increase of the N
2 adsorbed at high relative pressure, indicating the presence of macroporosity ascribed to the presence of interparticle voids as was shown from the SEM images.
The acid treatment causes a clear increase of the S
BET values, microporosity and mesoporosity, reaching a S
BET value of 400 m
2·g
−1 for Sp–H and 327 m
2·g
−1 for Sm–H, respectively. This increase is directly related with the partial loss of the Mg
2+ cations located in the octahedral sheet and the formation of amorphous silica along the microwave-assisted acid treatment, as indicated by the XRF data (
Table 1).
The incorporation of iron species from Fe3+ solution and subsequent calcination displays different behavior in Mg-smectite and sepiolite. Thus, both sepiolites (Sp and Sp–H) suffer a decrease in the SBET value attributed to a slight decrease of its microporosity, probably since iron species (in Fe–Sp and Fe–Sp–H) can be located within the channels of the sepiolite. In the case of the Mg-smectite, the incorporation of the iron species causes an increase of the surface attributed to the microporosity. This fact suggests that iron species are not blocking the porosity of the structure but rather favors the formation of higher proportion of micropores by the formation of a pillared structure, as was suggested by XRD data. The incorporation of iron species after the acid treatment in the Mg-smectite leads to a slightly higher microporosity and a slightly lower external surface probably due to the formation of larger aggregates, as was shown in the SEM images.
3.5. DRIFT Analysis
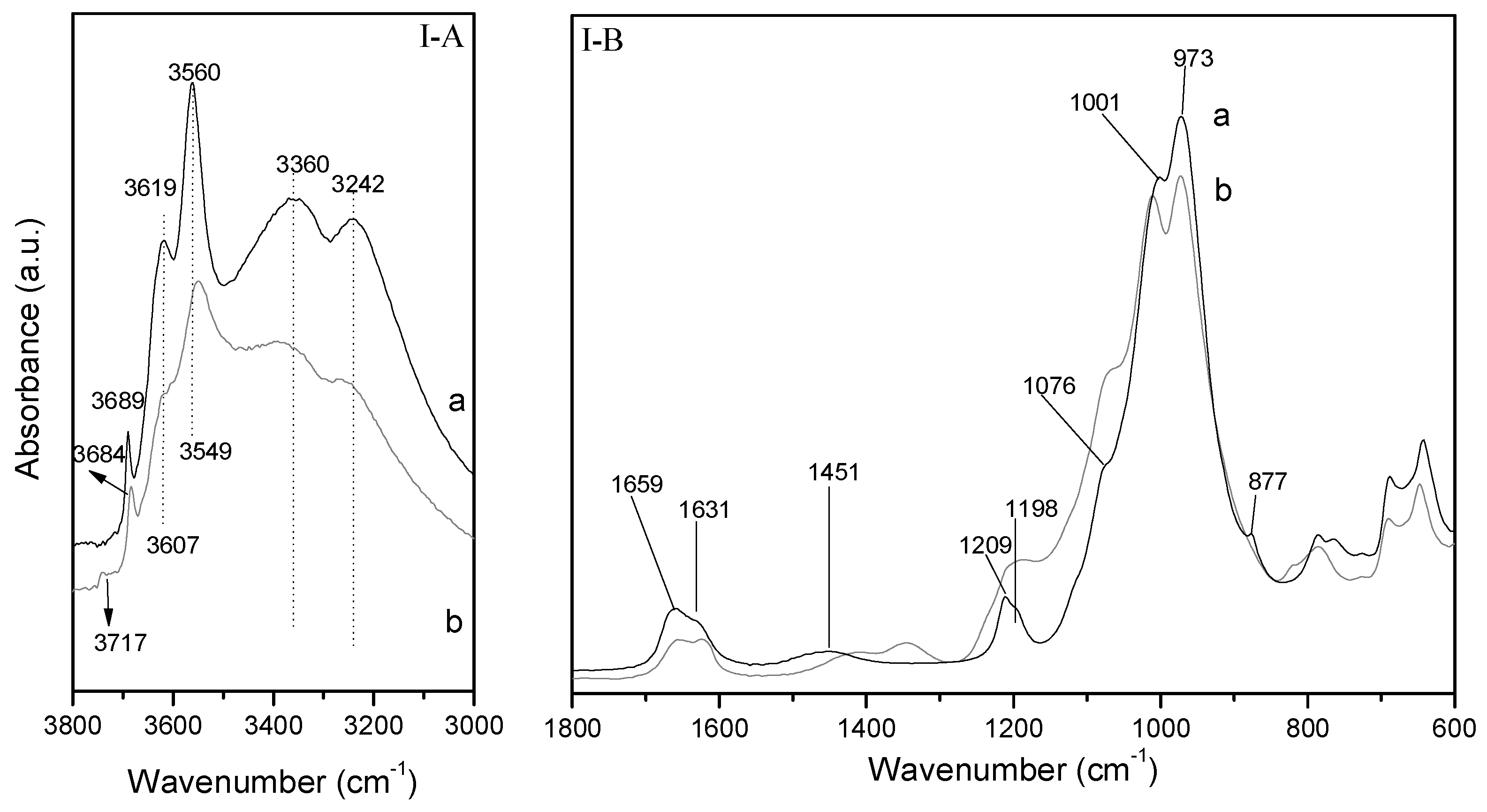
Figure 6I-A shows the 3750–3000 cm
−1 region of the DRIFT spectra of the starting Mg-smectite (Sm). The position of the O–H stretching bands is notably influenced by the chemical compositions of the octahedral sheet [
25]. For this reason, there are many differences related with both the number and the positions of the O–H stretching band in the DRIFT spectra of the natural di- and trioctahedral smectites. Regarding Sm (
Figure 6I-A), the O–H stretching bands appear at higher wavenumbers compared to those of dioctahedral smectites. In this case, the O–H stretching region is characterized by the presence three overlapping bands centered at 3681, 3619, and 3554 cm
−1.
The band at 3681 cm
−1 is classically assigned to the O–H stretching mode of structural Mg(OH)
2 groups in the octahedral sheet of Sm structure [
26,
27,
28]. Regarding the band at 3619 cm
−1, it can be assigned to the O–H stretching modes of AlOHSi groups formed by isomorphous substitutions of Si(IV) by Al(III) in the tetrahedral sheet of Sm [
18]. Moreover, DRIFT the band at 3554 cm
−1 can be assigned to the AlOHMg and MgOHFe stretching vibrations [
29]. The DRIFT spectrum of the starting Sm also shows water-stretching vibrations bands at 3379 and 3242 cm
−1 [
26,
30].
The Si–O stretching region of DRIFT spectra of the untreated Sm is shown in
Figure 6II-B(a). The di- or trioctahedral nature of the starting mineral apparently influences the position of the Si–O stretching band in the DRIFT spectrum [
26]. The Si–O stretching band appears centered at 963 cm
−1 in the starting Mg-smectite. This band is classically assigned to the in-plane, ν(Si–O) mode [
31]. Moreover, in both starting smectites the in-plane Si–O stretching band is accompanied by a small band at 1090 cm
−1 which is ascribed to the out-of-plane Si–O stretching modes [
29]. On the other hand, the band at 1632 cm
−1 is related with the adsorbed water bending modes and the bands at 1644 and 1410 cm
−1 to carbonates [
30], while the band at 881 cm
−1 is related with the deformation modes of OH-Mg [
29]. The band located about 655 cm
−1 is attributed to Mg
3OH bending vibrations [
26]. Moreover, the absence of bands located about 916 and 844 cm
−1 assigned to (Al
2OH) and (AlOHMg) [
26], respectively, confirms the low content of Al-species in the clay mineral, which is in agreement with the chemical analysis, shown in
Table 1.
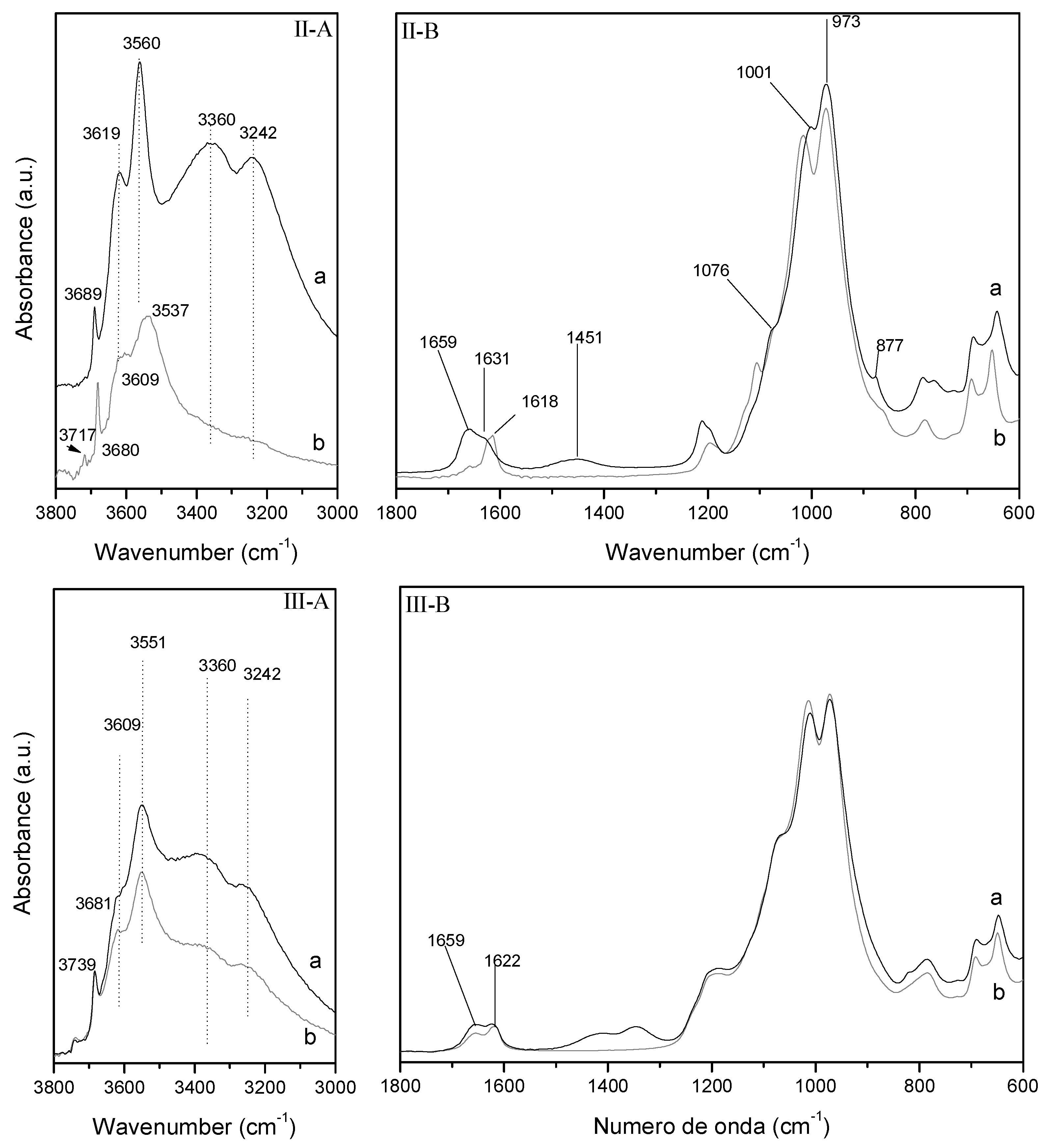
The O–H stretching region of acid activated Mg-smectite (Sm–H) is shown in
Figure 6I-A(b). A general decrease in the intensity of the O–H bands and water stretching bands (3379, 3242) is observed in the first 8 min of treatment and a new band appears at 3710 cm
−1. This band is commonly observed in the DRIFT spectra of the acid activated smectites and related with the O–H stretching mode of Si-OH groups [
32]. Moreover, the OH-Mg deformation band and carbonate bands disappear after 8 min of microwave-assisted acid treatment (
Figure 6I-B(b)). The decrease of the bands attributed to Mg-species in less pronounced in the region 1800–600 cm
−1, although it is noteworthy the slight shift of the Si–O stretching band at higher wavenumber, which confirms the formation of amorphous silica formed after the partial solution of the tetrahedral sheet [
26].
Similar observations can be made in the O–H stretching region after the treatment of Mg-smectite with Fe
3+ solutions (Fe–Sm) (
Figure 6II-A,II-B). The unique difference is the displacements of the Si–O stretching band from 963 to 994 cm
−1. This result suggests that the presence of oxohydroxides of Fe in the interlayer of Mg-smectite affect the Si–O stretching out-of-plane vibrational modes.
In the case of the sample Fe–Sm–H, the O–H stretching region of DRIFT spectra shown the appearance of a wide band from 3700 to 2700 cm−1 overlapping the small bands at 3681 and 3614 cm−1 observed in the DRIFT spectra of Sm–H. Moreover, and intense water bending band at 1633 cm−1 and the higher intensity of the spectra from 900 to 700 cm−1 suggest of a great amount of water molecules coordinated to oxohydroxides of Fe supported at the external surfaces of the lamellar particles.
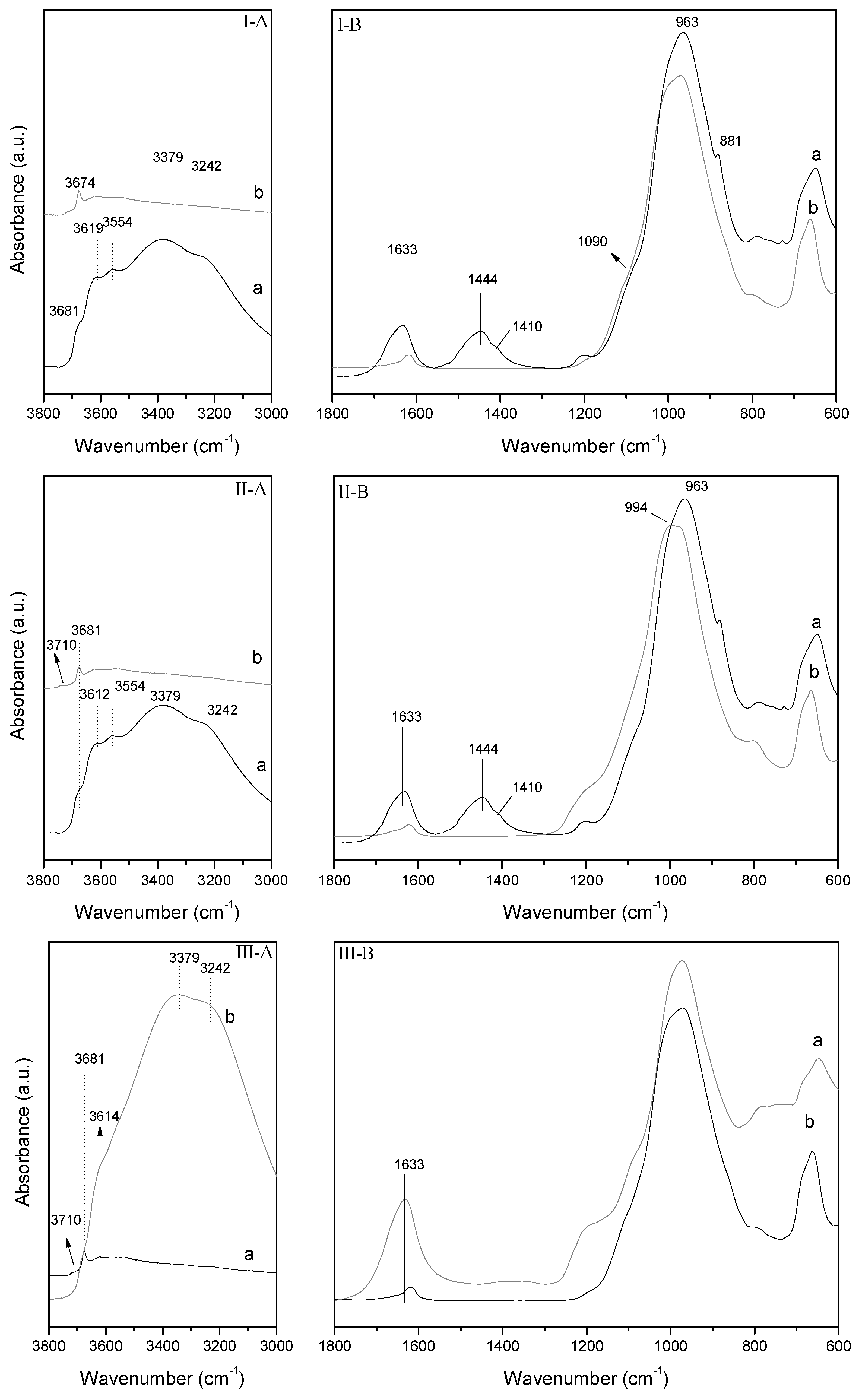
The hydroxyl-stretching region of the DRIFT spectra of the starting sepiolite sample (Sp) is shown in
Figure 7I-A. In this region, sepiolite shows two bands at 3689 and 3619 cm
−1 which are associated with the stretching vibration of the hydroxyls coordinated with magnesium; a band at 3560 cm
−1 related with the stretching modes of molecular water coordinated with magnesium, and two bands at 3360 and 3242 cm
−1 that have been attributed to zeolitic and coordinated water [
33,
34].
Figure 7I-B shows a comparison between the raw sepiolite and the sepiolite modified by microwave assisted acid treatment. The Si–O stretching region of the DRIFT spectra of natural sepiolite. According to Frost et al., bands at 1209, 1076, 1001, and 973 cm
−1 are assigned to Si–O stretching modes, whereas band at 877 cm
−1 are attributed to Mg–OH deformation and translation modes [
32]. In other hand bands at 1659 and 1631 are attributed to these bands can be attributed to the zeolitic and adsorbed water, respectively [
30], and band at 1451 cm
−1 to the presence of carbonates [
30].
The microwave assisted acid treatment (Sp–H) causes a drastic decreasing of the water stretching bands and O–H stretching bands (
Figure 7I-B). These later are also broadened and shifted to lower wavenumbers. At the same time the Si–O bands are progressively widened to higher wavenumber causing a significant change in the profile of the DRIFT spectrum. In addition, it is noticeable the presence of a new band about 800 cm
−1 as well as the shift of the Si–O stretching bands at higher wavenumber values, which confirms the formation of amorphous silica after the acid treatment, as other authors have previously indicated [
26,
35].
Similar observations can be made in the O–H stretching region after the treatment of sepiolite with Fe
3+ solutions (
Figure 7II-A,II-B). Nevertheless, in this case, despite the acidity of the Fe solution and the decrease in intensity of the O–H bands suggesting the partial dissolution of the octahedral sheets, a minor amount of amorphous silica is observed.
In the case of the sample Fe–Sp–H, the O–H stretching region of DRIFT spectra shows scarce modifications when compared with the DRIFT of Sp–H. The only observed modifications are those related with a new decreasing of O–H stretching and water stretching bands.
3.6. Batch Adsorption Results
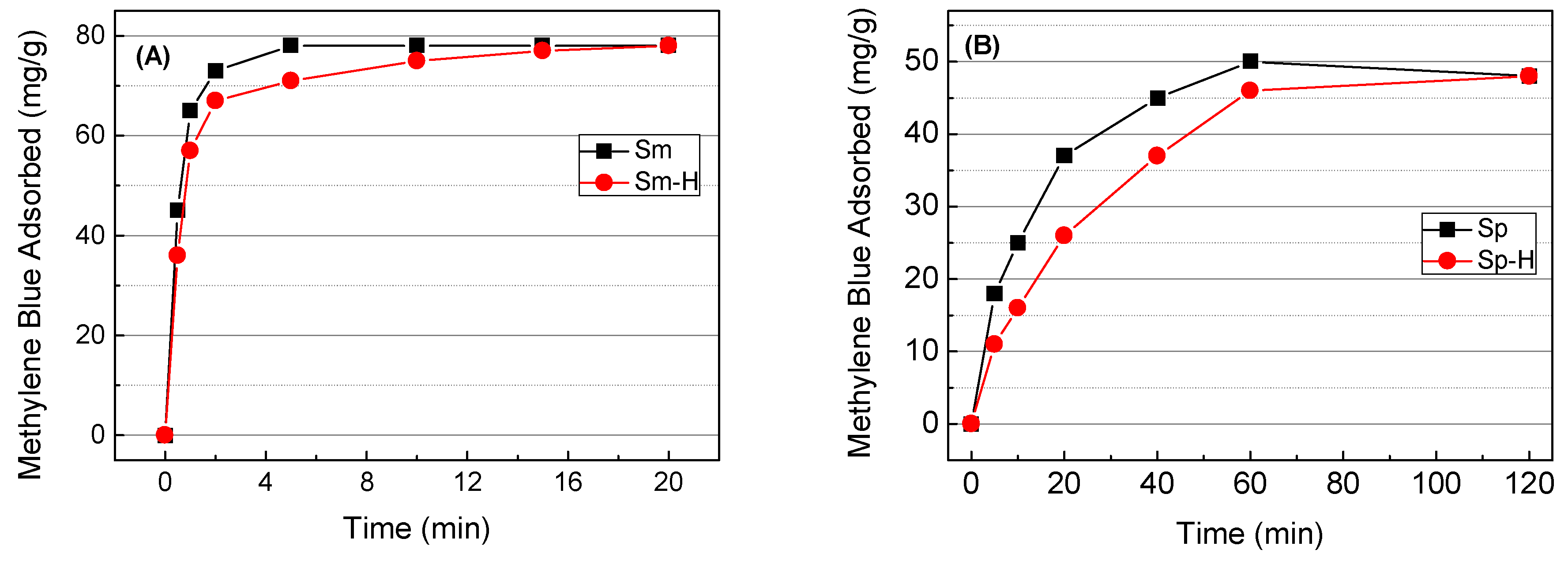
The adsorption capacity of the clay mineral-based materials as a function of time is shown in
Figure 8. These data reveals that the methylene blue adsorption on Mg-smectite-based materials is extremely fast, reaching equilibrium conditions after the first minutes. This result indicates the existence of a fast cationic exchange between the cations and methylene blue molecules. Although the adsorption on the external surface of the smectite particles plays an important role, the higher adsorption capacity of smectites compared with sepiolite suggests that a great proportion of methylene blue molecules are adsorbed between adjacent 2:1 layers of Mg-bentonite. These adsorption sites are very accessible since adjacent sheets are easily expanded in contact with the water molecules, favoring the introduction of the methylene blue in the interlayer space. Once the active sites are saturated, it has not been observed an increase of the methylene blue adsorption along the time.
In the case of the sepiolite-based materials (
Figure 8), about 60 minutes are required to reach the equilibrium conditions. The absence on linearity in the adsorption process suggests the existence of several adsorption sites. Thus, methylene blue molecules are easily adsorbed on the external surface of the fibers, while the adsorption in fibers aggregates takes place slowly by the existence of lower porosity. The methylene blue adsorption in the structural microchannels must be discarded due to the dimensions of the methylene blue (17 Å × 7,6 Å × 3.25 Å), which are higher than the dimensions of the structural channels in the sepiolite (10.6 Å × 3.6 Å) [
36].
The use of microwave-assisted acid treatment modifies the adsorption kinetics. Thus, Mg-smectite reveals that the acid treatment slightly slows the adsorption rate in the first minutes (
Figure 9). This change in the adsorption kinetics suggests that some of the new adsorption sites, generated after the acid treatment, display H
+ species adsorbed on the active sites, which slows the adsorption of methylene blue molecules, since H
+ species have higher affinity by the active centers than methylene blue cations.
The microwave-assisted acid treatment of the raw sepiolite shows a similar effect in the adsorption kinetics to those observed in Mg-smectite (
Figure 8) since the methylene adsorption is low at short and medium times, although after 120 min both materials reach similar values.
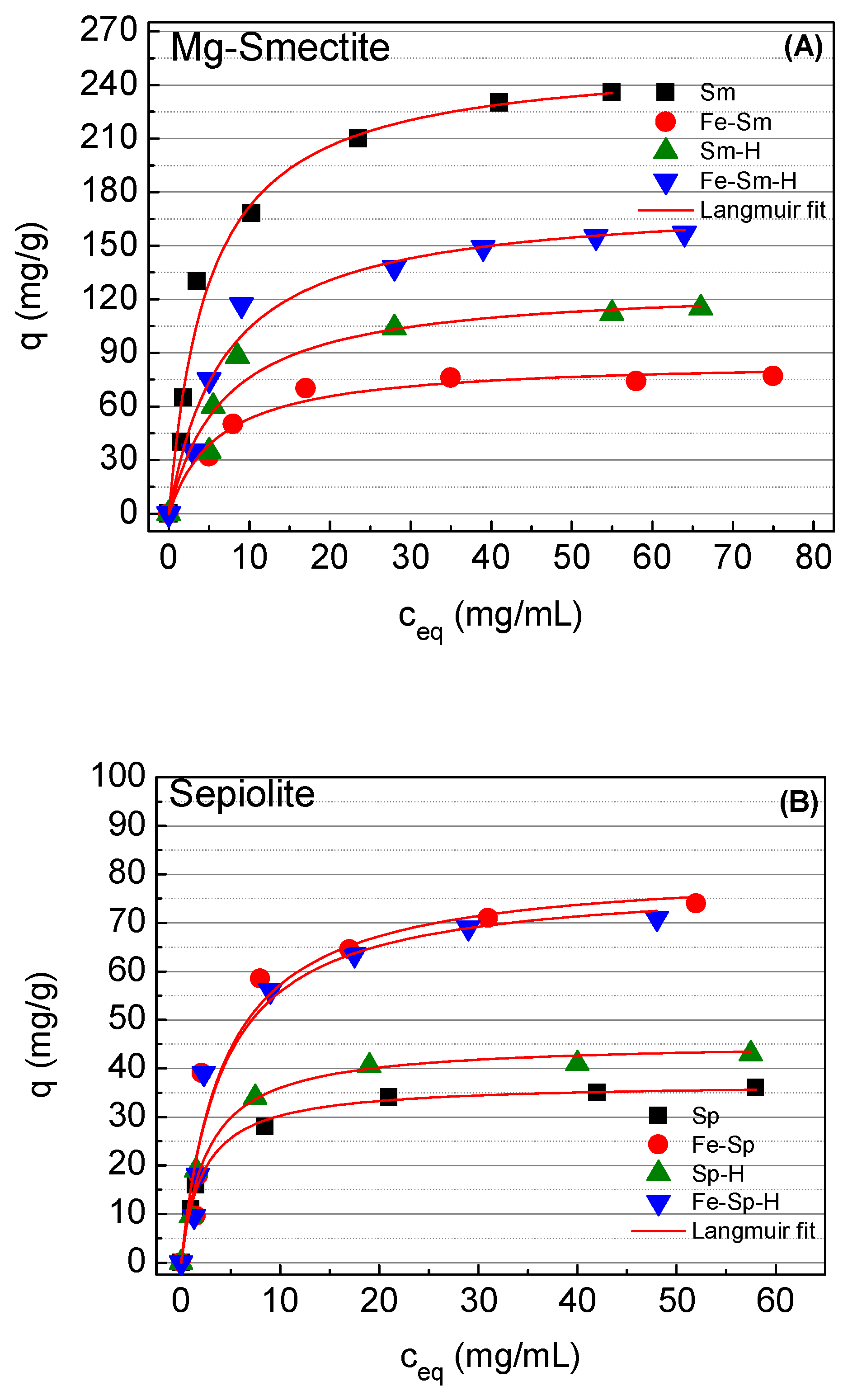
Considering the adsorption kinetics, an adsorption time of 20 min was chosen for the adsorption isotherms of Sm, while the adsorption isotherms were carried out after 120 min for the sepiolite-based materials.
In all cases, the adsorption isotherms were well-fitted to the Langmuir model [
37]. This elemental adsorption model assumes that occurs up to the formation of a monolayer, where a methylene blue molecule is adsorbed per each active site. In addition, these sites have the same binding energy, which supposes a homogeneous surface with no interactions between neighboring molecules. The Langmuir model is defined by the following equation:
where
q is the amount of methylene blue adsorbed per unit weight (mg·g
−1),
ceq is the concentration of methylene blue in the liquid phase at adsorption equilibrium (mg·mL
−1),
KL is the dissociation constant of Langmuir (mL·mg
−1) that is associated with the energy of adsorption, and
qm is the saturation capacity per unit weight (mg·g
−1).
Although the Langmuir model [
37] supposes that all adsorption sites have the same binding energy we must to take into account that this is a simplification of the reality since the starting minerals have different adsorption sites with different environments and, moreover, these differences are accentuated with the different treatments tested. However, we assume this simplification since it allows an adequate comparison of adsorbent properties. The adsorption data of the Sm-based material are compiled in
Figure 9A. From this figure, it can be observed how the raw Sm displays the highest adsorption capacity, reaching a
qm value of 256 mg methylene blue g
−1 (0.80 mmol·g
−1). Considering that the Mg-smectite displays a cationic exchange capacity (CEC) of 0.87 m
eq·g
−1, the obtained values are slightly smaller to the theoretical one. The modification of the Sm by microwave-assisted acid treatment (Sm–H) cause a clear decrease in the adsorption capacity, obtaining a
qm value of only 128 mg·g
−1. This fact can be attributed to the acid treatment leads to loss of the active sites, by the disappearance of –OH groups coordinated with Mg species, which are leached in the acid treatment and the formation of amorphous silica. The decrease of the adsorption capacity after the acid treatment could also assigned to a proportion of cations located in the interlayer spacing can be replaced by H
+ species, which have higher affinity by the active sites. In this sense, the
KL data, that defines the interaction adsorbate–adsorbent, reveals that the K
L data of the raw Mg-smectite is higher in comparison to the material treated with acid, which can confirm the presence of H
+ that diminish the affinity with the methylene blue molecules.
The methylene blue adsorption of the Mg-smectite decreases even more when iron oxohydroxides species are incorporated in its interlayer spacing (
Figure 9A), which led to the formation a pillared and rigid structure, as was suggested from the 001 reflection shown in the XRD data (
Figure 3I). This fact limits the expansion of the interlayer spacing to retain methylene blue in aqueous medium, obtaining a
qm value of 86 and 176 mg·g
−1 for Sm–Fe and Fe–Sm–H, respectively (
Table 3). With regard to the affinity defined by
KL value, in both cases the affinity is lower to that observer for the raw material, probably due to the increase of rigidity of the pillared structure that avoids the expansion of the interlayer spacing, limiting the amount of available active sites.
The adsorption isotherms of the sepiolite-based adsorbents are shown in
Figure 9B. In the case of the raw sepiolite (Sp) the
qm adsorption is only of 37 mg of methylene blue g
−1 (0.12 mmol·g
−1) (
Table 3). These values are well below the CEC (37 m
eq·g
−1), which can be expected since the methylene blue molecular is too bulky to access the small zeolitic channels of sepiolite. From these data it can be inferred that the adsorption capacity is directly related with the structure of both materials since the adsorption capacity of the septiolite is five times lower to that observed for the raw Mg-smectite. On the one hand, Mg-smectite (Sm) can adsorb methylene blue on its external surface as well as in the interlayer space since the interlayer space can expand to accommodate the methylene blue cations. On the other hand, the rigid structure of sepiolite (Sp) and the cross-section of its channels, smaller than the dimension of the methylene blue cation. This limits the adsorption to the external surface of sepiolite fibers exclusively. The modification of the raw sepiolite by acid treatment causes a slight increase of the adsorption capacity, reaching a
qm value of 45 mg·g
−1, although the S
BET value increases in higher proportion (from 308 to 400 m
2·g
−1) (
Table 3). This increasing of the S
BET value is related to both the dispersion of fibers making accessible new active centers for adsorption and the formation of amorphous silica phase without active centers. Thus, only a part of the new generated S
BET has the ability to adsorb methylene blue cations. For this reason, the increasing of adsorption capacity is notably lower than the increasing of S
BET.
Unlike to that observed in Mg-smectite, the incorporation of iron oxohydroxides species into both Sp and Sp–H clearly improves the methylene blue adsorption capacity, reaching a
qm values of 82 and 79 mg·g
−1 for Fe–Sp and Fe–Sp–H, respectively. Nonetheless, the isotherms report that the affinity of these sites are lower to those shown in the case of the starting sepiolite, as indicates the
KL value (
Table 3). This remarkable increasing in the adsorption capacities are not in line with the decreasing in S
BET, mainly decreasing of micropore surface, observed with the Fe-treatment (
Table 2). This decreasing the micropore surface is related to the steric impediment generated by iron species within the channels of Sepiolite. However, taking into account that the methylene blue adsorption in Sepiolite occurs exclusively at the external surface of fibers, the formation of new adsorption centers due to the presence of iron oxohydroxides species can explain the increasing of adsorption capacities.