Adsorption Structure and Mechanism of Styryl Phosphoric Acid at the Rutile–Water Interface
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Methods
2.2.1. Zeta Potential Measurements
2.2.2. Adsorption Experiments
2.2.3. FT-IR Spectroscopy of Rutile–SPA Complexes
2.2.4. XPS Measurements
3. Results and Discussion
3.1. Zeta Potentials of the TiO2 Surface
3.2. Adsorption Isotherms
3.3. FT-IR
3.4. XPS Analysis
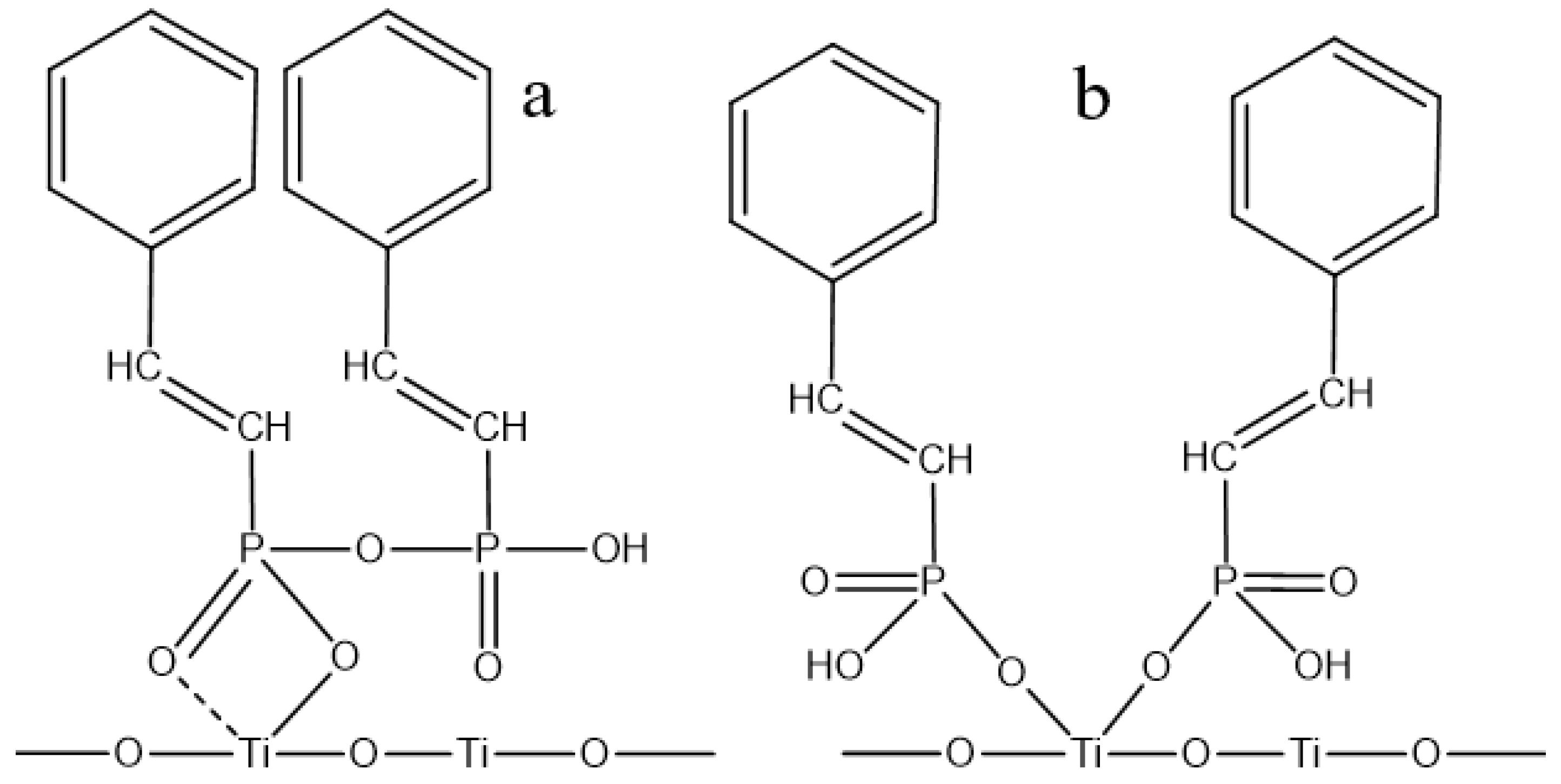
3.5. Establishment and Discussion of Adsorption Models
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Monich, P.R.; Berti, F.V.; Porto, L.M.; Henriques, B.; de Oliveira, A.P.N.; Fredel, M.C.; Souza, J.C.M. Physicochemical and biological assessment of PEEK composites embedding natural amorphous silica fibers for biomedical applications. Mater. Sci. Eng. C 2017, 79, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Zhang, S.Y.; Gao, Y.G. A high-temperature stable antenna array for the satellite navigation system. IEEE Antennas Wirel. Propag. Lett. 2017, 16, 1397–1400. [Google Scholar] [CrossRef]
- Song, J.; Liu, T.; Shi, H.; Yan, S.; Liao, Z.; Liu, Y.; Liu, W.; Peng, Z. Time-frequency analysis of the tribological behaviors of Ti6Al4V alloy under a dry sliding condition. J. Alloys Compd. 2017, 724, 752–762. [Google Scholar] [CrossRef]
- Ye, Z.; Tai, H.; Guo, R.; Yuan, Z.; Liu, C.; Su, Y.; Chen, Z.; Jiang, Y. Excellent ammonia sensing performance of gas sensor based on graphene/titanium dioxide hybrid with improved morphology. Appl. Surf. Sci. 2017, 419, 84–90. [Google Scholar]
- Kumara, N.T.R.N.; Lim, A.; Lim, C.M.; Petra, M.I.; Ekanayake, P. Recent progress and utilization of natural pigments in dye sensitized solar cells: A review. Renew. Sust. Energ. Rev. 2017, 78, 301–317. [Google Scholar] [CrossRef]
- Fontelles-Carceller, O.; Munoz-Batista, M.J.; Conesa, J.C.; Fernandez-Garcia, M.; Kubacka, A. UV and visible hydrogen photo-production using Pt promoted Nb-doped TiO2 photo-catalysts: Interpreting quantum efficiency. Appl. Catal. B Environ. 2017, 216, 133–145. [Google Scholar] [CrossRef]
- Xiao, W.; Cao, P.; Liang, Q.; Peng, H.; Zhao, H.; Qin, W.; Qiu, G.; Wang, J. The activation mechanism of Bi3+ ions to rutile flotation in a strong acidic environment. Minerals 2017, 7, 113. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, H.; Zhao, H.; Qin, W.; Qiu, G. Flotation behavior and mechanism of rutile with nonyl hydroxamic acid. Rare Met. 2016, 35, 419–424. [Google Scholar] [CrossRef]
- Li, H.; Mu, S.; Weng, X.; Zhao, Y.; Song, S. Rutile flotation with Pb2+ ions as activator: Adsorption of Pb2+ at rutile/water interface. Colloid Surf. A Physicochem. Eng. Asp. 2016, 506, 431–437. [Google Scholar] [CrossRef]
- Terzi, M.; Kursun, I. Investigation of recovery possibilities of rutile minerals from the feldspar tailings with gravity separation methods. Russ. J. Non-Ferrous Metals 2015, 56, 235–245. [Google Scholar] [CrossRef]
- Chachula, F.; Liu, Q. Upgrading a rutile concentrate produced from Athabasca oil sands tailings. Fuel 2003, 82, 929–942. [Google Scholar] [CrossRef]
- Liu, Q.; Peng, Y. The development of a composite collector for the flotation of rutile. Miner. Eng. 1999, 12, 1419–1430. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Y.; Xu, S. Effect of interactions between styryl phosphoric acid and aliphatic alcohol on rutile flotation. Chin. J. Nonferrous Met. 1999, 9, 358–362. (In Chinese) [Google Scholar]
- Madeley, J.D.; Graham, K. Flotation of rutile with anionic and cationic collectors. J. Appl. Chem. 1966, 16, 169–170. [Google Scholar] [CrossRef]
- Xiao, W.; Cao, P.; Liang, Q.; Huang, X.; Li, K.; Zhang, Y.; Qin, W.; Qiu, G.; Wang, J. Adsorption behavior and mechanism of Bi(III) ions on rutile–water interface in the presence of nonyl hydroxamic acid. Trans. Nonferrous Met. Soc. China 2018, 28, 348–355. [Google Scholar] [CrossRef]
- Xiao, W.; Fang, C.; Wang, J.; Liang, Q.; Cao, P.; Wang, X.; Zhang, L.; Qiu, G.; Hu, J. The role of EDTA on rutile flotation using Al3+ ions as an activator. RSC Adv. 2018, 8, 4872–4880. [Google Scholar] [CrossRef]
- Xiao, W.; Ke, S.; Quan, N.; Zhou, L.; Wang, J.; Zhang, L.; Dong, Y.; Qin, W.; Qiu, G.; Hu, J. The role of nanobubbles in the precipitation and recovery of organic-phosphine-containing beneficiation wastewater. Langmuir 2018, 34, 6217–6224. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Zhao, H.; Qin, W.; Qiu, G.; Wang, J. Adsorption mechanism of Pb2+ activator for the flotation of rutile. Minerals 2018, 8, 266. [Google Scholar] [CrossRef]
- Kang, J.; Hu, Y.; Sun, W.; Liu, R.; Yin, Z.; Tang, H.; Meng, X.; Zhang, Q.; Liu, H. A significant improvement of scheelite flotation efficiency with etidronic acid. J. Clean Prod. 2018, 180, 858–865. [Google Scholar] [CrossRef]
- Jin, S.; Shi, Q.; Li, Q.; Ou, L.; Ouyang, K. Effect of calcium ionic concentrations on the adsorption of carboxymethyl cellulose onto talc surface: Flotation, adsorption and AFM imaging study. Powder Technol. 2018, 331, 155–161. [Google Scholar] [CrossRef]
- Yin, Z.; Hu, Y.; Sun, W.; Zhang, C.; He, J.; Xu, Z.; Zou, J.; Guan, C.; Zhang, C.; Guan, Q.; et al. Adsorption mechanism of 4-amino-5-mercapto-1,2,4-triazole as flotation reagent on chalcopyrite. Langmuir 2018, 34, 4071–4083. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Sun, Z.; Xu, L.; Hu, Y.; Huang, K.; Zhu, S. A comparison study of the flotation and adsorption behaviors of diaspore and kaolinite with quaternary ammonium collectors. Miner. Eng. 2014, 65, 124–129. [Google Scholar] [CrossRef]
- Yang, F.; Sun, W.; Hu, Y.; Long, S. Cationic flotation of scheelite from calcite using quaternary ammonium salts as collector: Adsorption behavior and mechanism. Miner. Eng. 2015, 81, 18–28. [Google Scholar] [CrossRef]
- Gao, Z.; Bai, D.; Sun, W.; Cao, X.; Hu, Y. Selective flotation of scheelite from calcite and fluorite using a collector mixture. Miner. Eng. 2015, 72, 23–26. [Google Scholar] [CrossRef]
- Deng, L.; Wang, S.; Zhong, H.; Liu, G. N–(6-(hydroxyamino)-6-oxohexyl) decanamide collector: Flotation performance and adsorption mechanism to diaspore. Appl. Surf. Sci. 2015, 347, 79–87. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, X.; Liu, H.; Zhang, H.; Miller, J.D. Some physicochemical aspects of water-soluble mineral flotation. Adv. Colloid Interface Sci. 2016, 235, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Somasundaran, P. Advances in adsorption of surfactants and their mixtures at solid/solution interfaces. Adv. Colloid Interface Sci. 2006, 123–126, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Li, C.; Sun, W.; Hu, Y. Anisotropic surface properties of calcite: A consideration of surface broken bonds. Colloid Surf. A Physicochem. Eng. Asp. 2017, 520, 53–61. [Google Scholar] [CrossRef]
- Li, C.; Gao, Z. Effect of grinding media on the surface property and flotation behavior of scheelite particles. Powder Technol. 2017, 322, 386–392. [Google Scholar] [CrossRef]
- Fuerstenau, D.W.; Jang, H.M. On the nature of alkylsulfonate adsorption at the rutile/water interface. Langmuir 1991, 7, 3138–3143. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Gong, X.Q. Unique adsorption behaviors of carboxylic acids at rutile TiO2 (110). Surf. Sci. 2015, 641, 82–90. [Google Scholar] [CrossRef]
- Han, J.; Jiao, F.; Liu, W.; Qin, W.; Xu, T.; Xue, K.; Zhang, T. Innovative methodology for comprehensive utilization of spent MgO–Cr2O3 bricks: Copper flotation. ACS Sustain. Chem. Eng. 2016, 4, 5503–5510. [Google Scholar] [CrossRef]
- Park, S.J.; Yoon, T.I. Effects of iron species and inert minerals on coagulation and direct filtration for humic acid removal. Desalination 2009, 239, 146–158. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, L.; Zhang, S. Adsorption of phenanthrene and 1,3-dinitrobenzene on cation-modified clay minerals. Colloid Surf. A Physicochem. Eng. Asp. 2011, 377, 278–283. [Google Scholar] [CrossRef]
- Guégan, R.; Giovanela, M.; Warmont, F.; Motelica-Heino, M. Nonionic organoclay: A “Swiss Army knife” for the adsorption of organic micro-pollutants? Adv. Colloid Interface Sci. 2015, 437, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gao, Z.; Sun, W.; Yin, Z.; Wang, J.; Hu, Y. Adsorption of a novel reagent scheme on scheelite and calcite causing an effective flotation separation. J. Colloid Interface Sci. 2017, 512, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Gao, Z.; Khoso, S.A.; Gao, J.; Sun, W.; Pu, W.; Hu, Y. Selective adsorption of benzhydroxamic acid on fluorite rendering selective separation of fluorite/calcite. Appl. Surf. Sci. 2018, 435, 752–758. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, W.; Zhao, H.; Cao, P.; Hu, Q.; Qin, W.; Zhang, Y.; Qiu, G.; Wang, J. Hydrophobic flocculation flotation of rutile fines in presence of styryl phosphonic acid. Trans. Nonferrous Met. Soc. China 2018, 28, 1424–1432. [Google Scholar] [CrossRef]
- Xu, L.; Tian, J.; Wu, H.; Lu, Z.; Yang, Y.; Sun, W.; Hu, Y. Effect of Pb2+ ions on ilmenite flotation and adsorption of benzohydroxamic acid as a collector. Appl. Surf. Sci. 2017, 425, 796–802. [Google Scholar] [CrossRef]
- Singh, B.K.; Mercier-Bion, F.; Lefevre, G.; Simoni, E. Effect of short chain aliphatic carboxylic acids for sorption of uranyl on rutile Zeta potential and in situ ATR-FTIR studies. J. Ind. Eng. Chem. 2016, 35, 325–331. [Google Scholar] [CrossRef]
- Gregory, J. Review of chemistry of the solid-water interface. Processes at the mineral–water and particle–water interface in natural systems, by Werner Stumm. J. Colloid Interface Sci. 1993, 159, 520. [Google Scholar] [CrossRef]
- Chen, P.; Zhai, J.; Sun, W.; Hu, Y.; Yin, Z.; Lai, X. Adsorption mechanism of lead ions at ilmenite/water interface and its influence on ilmenite flotability. J. Ind. Eng. Chem. 2017, 53, 285–293. [Google Scholar] [CrossRef]
- Acharya, S.; Nayak, A. Separtion of D2EHPA and M3EHPA. Hydrometallurgy 1988, 19, 309–320. [Google Scholar] [CrossRef]
- Koopal, L.K.; Lee, E.M.; Böhmer, M.R. Adsorption of cationic and anionic surfactants on charged metal oxide surfaces. J. Colloid Interface Sci. 1995, 170, 85–97. [Google Scholar] [CrossRef]
- Socrates, G. IR Characteristic Group Frequencies; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Olu-Owolabi, B.I.; Unuabonah, E.I. Kinetic and thermodynamics of the removal of Zn2+ and Cu2+ from aqueous solution by sulphate and phosphate-modified bentonite clay. J. Hazard. Mater. 2010, 184, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Brzezinski, B.; Wojciechowski, G.; Urjasz, H.; Zundel, G. FT-IR study of the proton polarizability of hydrogen bonds and of the hydrogen-bonded systems in a di-Mannich base of 5,5′-dimethoxy-2,2′-biphenol. J. Mol. Struct. 1998, 470, 335–339. [Google Scholar] [CrossRef]
- Deval, V.; Kumar, A.; Gupta, V.; Sharma, A.; Gupta, A.; Tandon, P.; Kunimoto, K.K. Molecular structure (monomeric and dimeric) and hydrogen bonds in 5-benzyl 2-thiohydantoin studied by FT-IR and FT-Raman spectroscopy and DFT calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Academic Press: London, UK, 1991; Chapter 1; pp. 1–7. [Google Scholar]
- Meng, X.; Li, L.; Zou, K.; Liu, J. The effect of SiO2 on TiO2 up-conversion photoluminescence film. Opt. Mater. 2014, 37, 367–370. [Google Scholar] [CrossRef]
- Aksakal, B.; Koç, K.; Yargı, Ö.; Tsobkallo, K. Effect of UV-light on the uniaxial tensile properties and structure of uncoated and TiO2 coated Bombyx mori silk fibers. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 152, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhang, W.; Jin, L.; Yang, X.; Xu, F.; Zhu, J.; Huang, X. Direct XPS evidence for charge transfer from a reduced rutile TiO2(110) surface to Au clusters. J. Phys. Chem. C 2007, 111, 12434–12439. [Google Scholar] [CrossRef]
- Lee, C.Y.; Gong, P.; Harbers, G.M.; Grainger, D.W.; Castner, D.G.; Gamble, L.J. Surface coverage and structure of mixed DNA/alkylthiol monolayers on gold: Characterization by XPS, NEXAFS, and fluorescence intensity measurements. Anal. Chem. 2006, 78, 3316–3325. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.T.; Diebold, U.; Madey, T.E.; Garfunkel, E. Titanium and reduced titania overlayers on titanium dioxide (110). J. Electron Spectrosc. Relat. Phenom. 1995, 7, 1–11. [Google Scholar] [CrossRef]
- Kurtz, R.L.; Henrich, V.E. Comparison of Ti 2p core-level peaks from TiO2, Ti2O3, and Ti metal, by XPS. Surf. Sci. Spectra 1998, 5, 179–181. [Google Scholar] [CrossRef]
- Atla, S.B.; Chen, C.C.; Chen, C.Y.; Lin, P.Y.; Pan, W.; Cheng, K.C.; Huang, Y.M.; Chang, Y.F.; Jean, J.S. Visible light response of Ag+/TiO2–Ti2O3 prepared by photodeposition under foam fractionation. J. Photochem. Photobiol. A Chem. 2012, 236, 1–8. [Google Scholar] [CrossRef]
- He, G.; Pan, G.; Zhang, M. Studies on the reaction pathway of arsenate adsorption at water–TiO2 interfaces using density functional theory. J. Colloid Interface Sci. 2011, 364, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Pena, M.; Meng, X.; Korfiatis, G.P.; Jing, C. Adsorption mechanism of arsenic on nanocrystalline titanium dioxide. Int. J. Environ. Sci. Technol. 2006, 40, 1257–1262. [Google Scholar] [CrossRef]
- Leppinen, J.O. FT-IR and flotation investigation of the adsorption of ethyl xanthate on activated and non-activated sulfide minerals. Int. J. Miner. Process. 1990, 30, 245–263. [Google Scholar] [CrossRef]
- Gu, J.; Yuan, C.; Xiao, Z.; Xu, M.; Huang, D.; Lu, Z. Atomic force microscopic observation of local supramolecular structure in phosphatidic acid langmuir blodgett films. J. Chin. Electron. Microsc. Soc. 1997, 5, 791–794. (In Chinese) [Google Scholar]
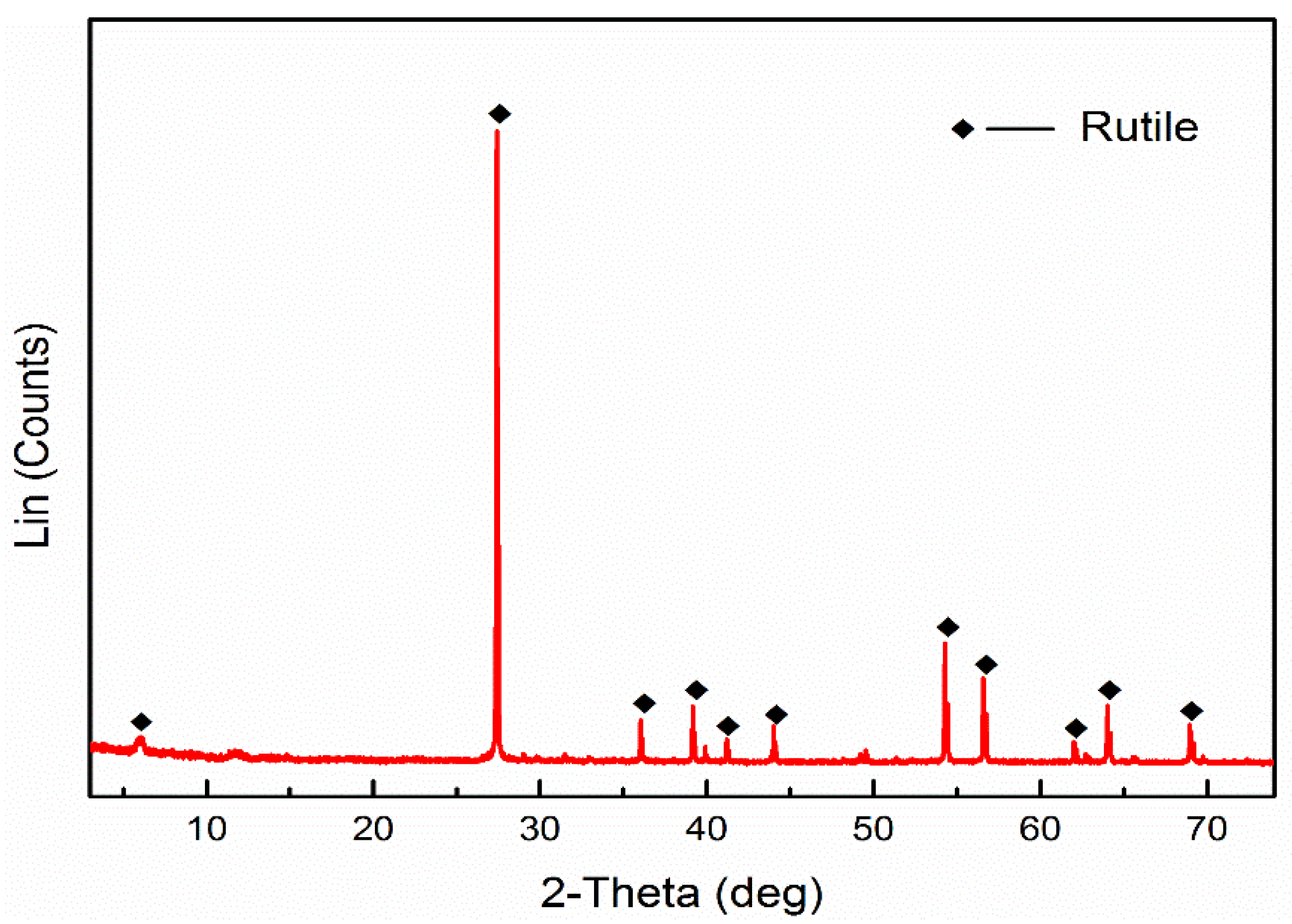
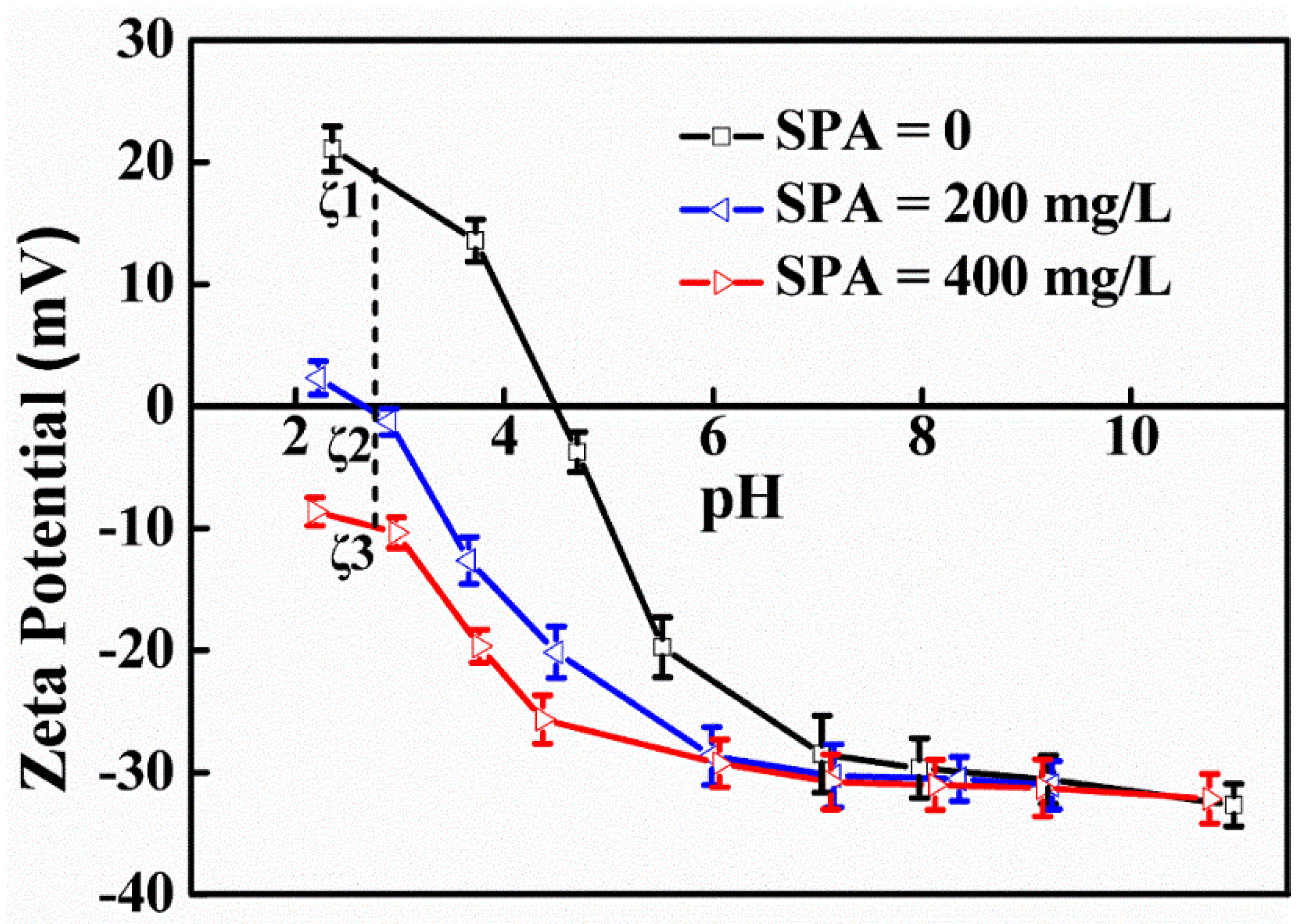
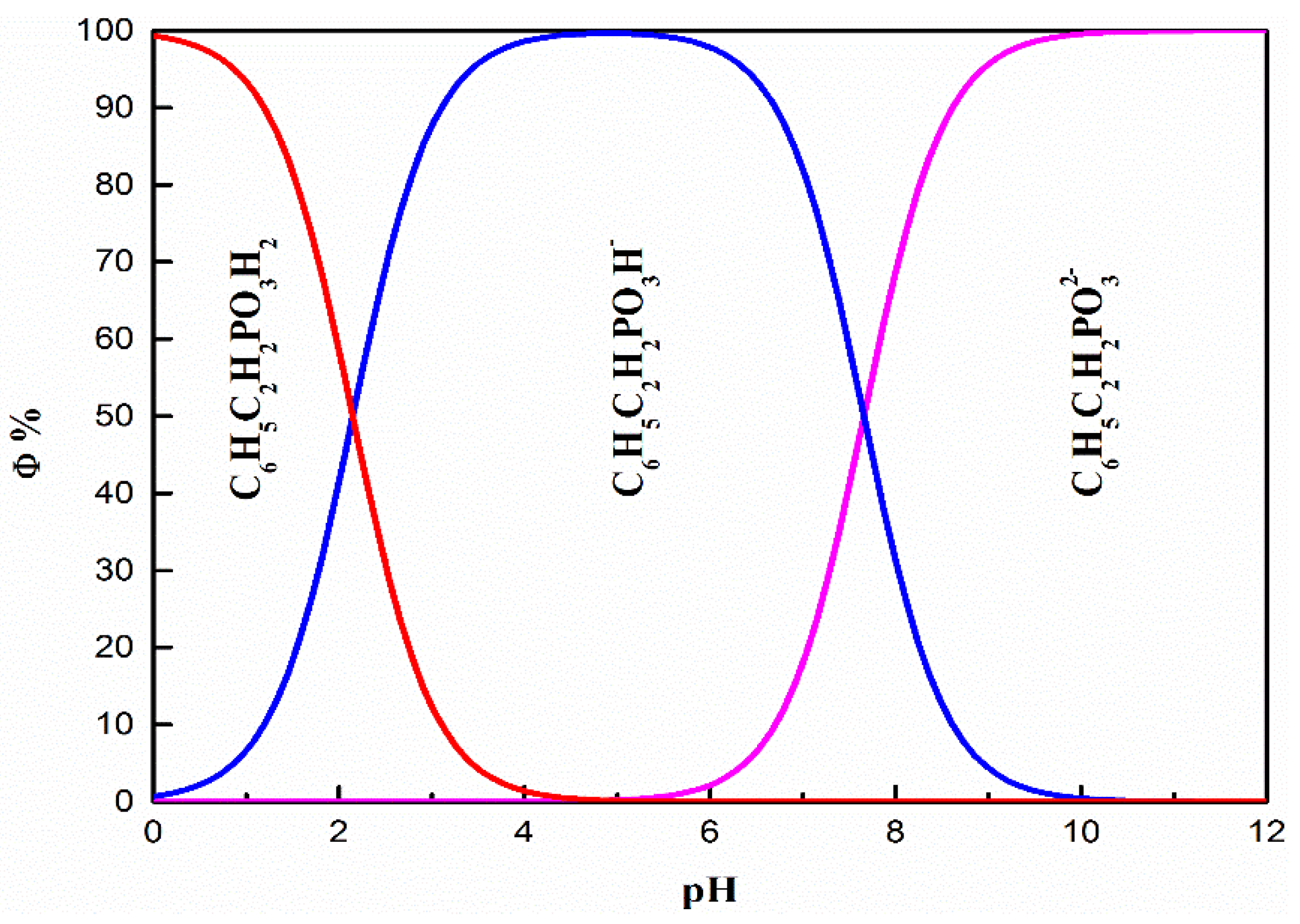
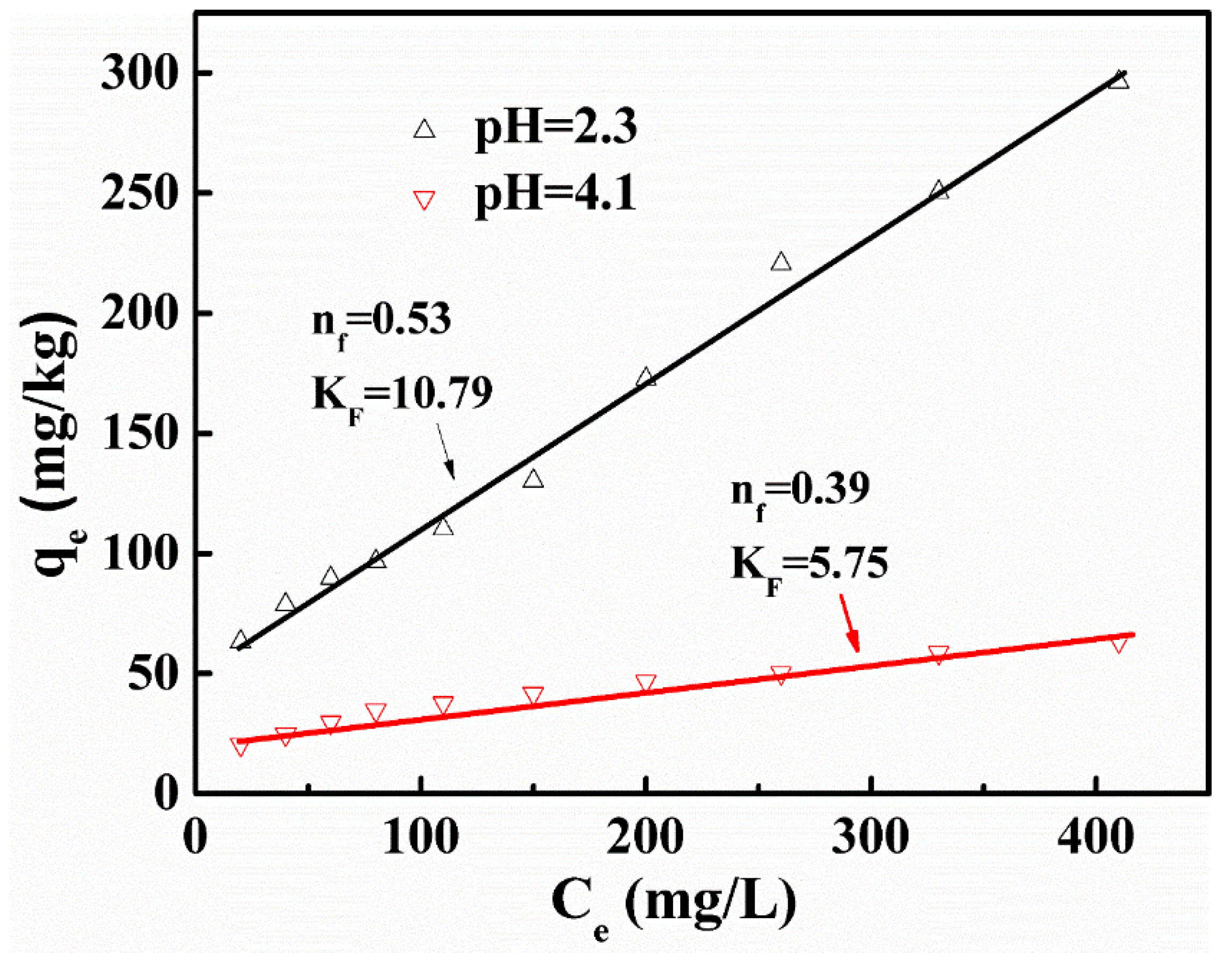
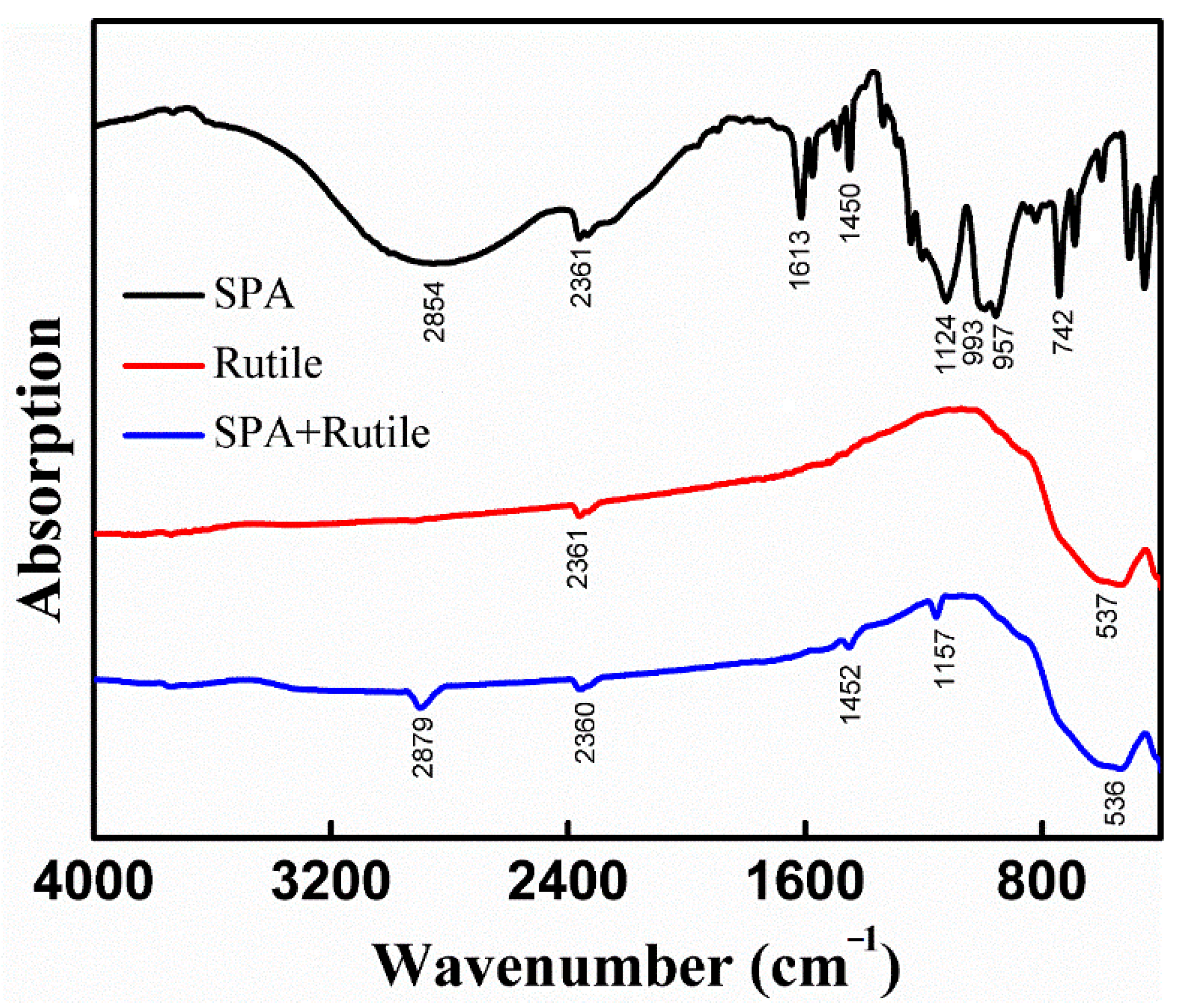
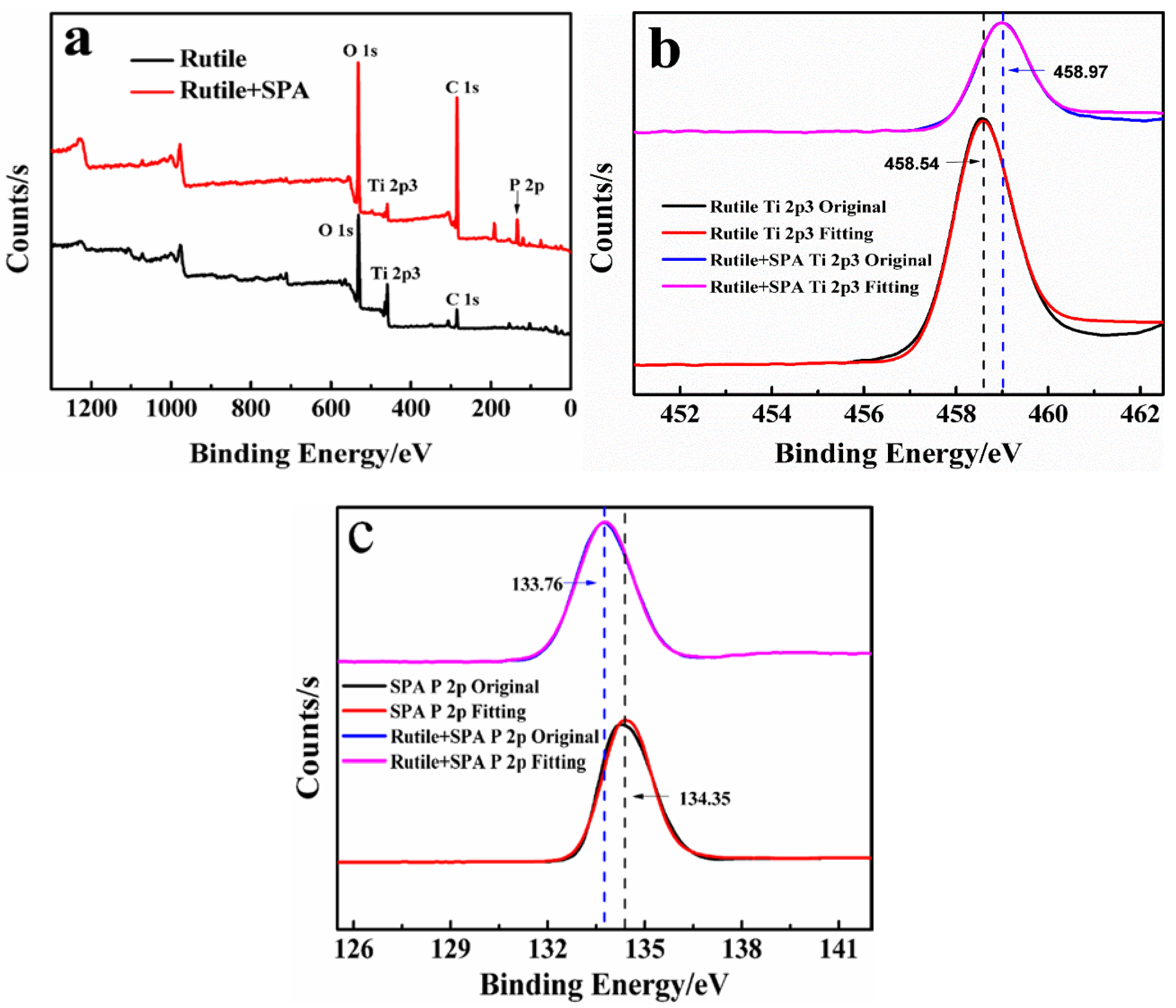
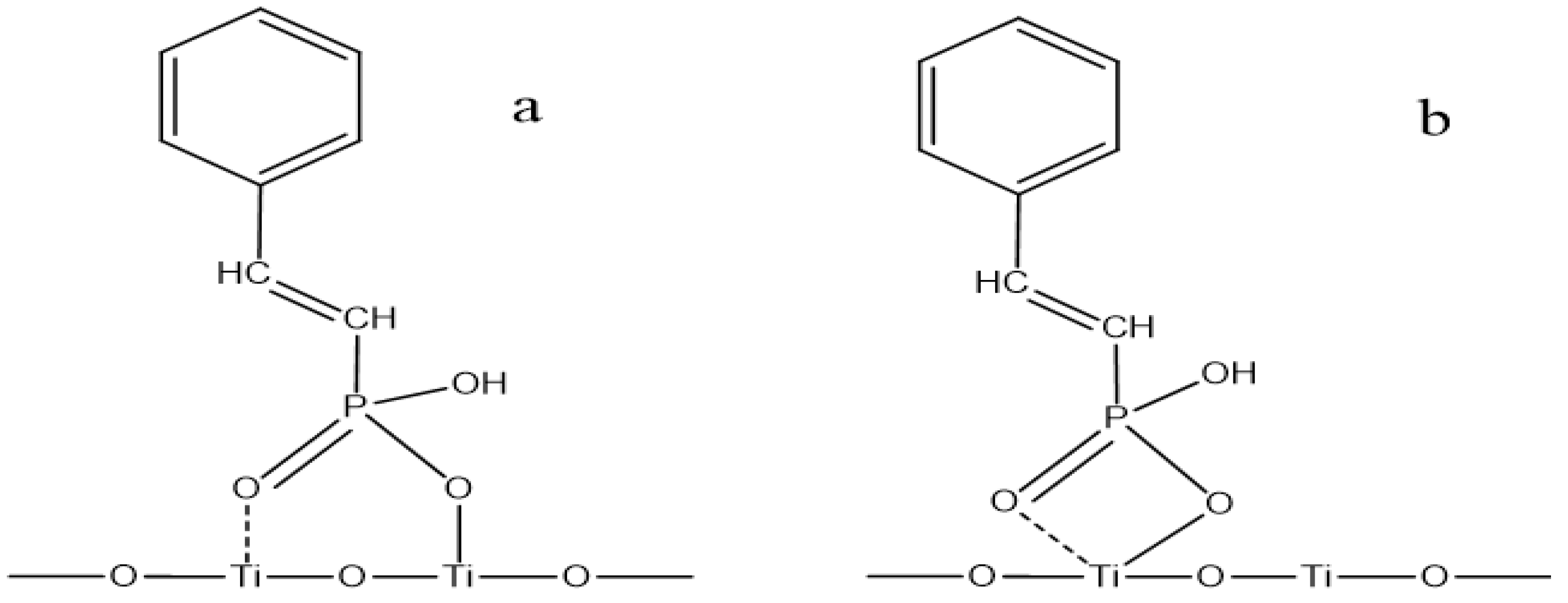
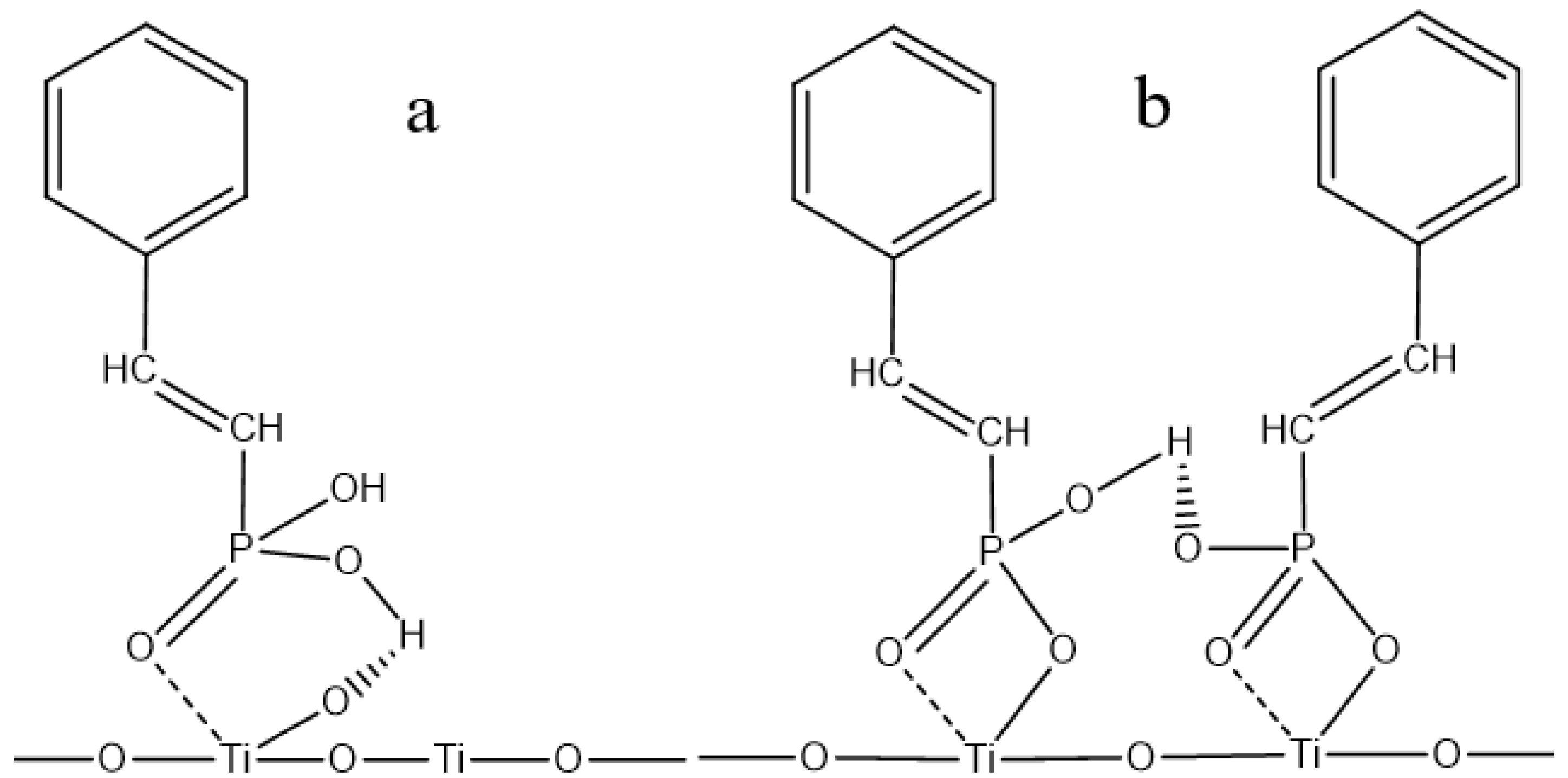
| Element | TiO2 | FeO | Fe2O3 | SiO2 | CaO | MgO | Al2O3 |
| Content | 93.80 | 1.53 | 1.33 | 2.17 | 0.17 | 0.31 | 0.99 |
| Condition * | Δζ (mV) | Г (mol/L) | Δ |
|---|---|---|---|
| Δ1 | 20.62 | kC exp (0.000803) | −1.99 |
| Δ2 | 29.25 | kC exp (0.00114) | −2.82 |
| Δ3 | 8.63 | kC exp (0.000336) | −0.83 |
| Sample | Binging Energy (eV) | Chemical Shift (eV) | ||||||
|---|---|---|---|---|---|---|---|---|
| C(1s) | O(1s) | Ti(2p) | P(2p) | C(1s) | O(1s) | Ti(2p) | P(2p) | |
| Rutile | 284.8 | 531.10 | 458.54 | - | - | - | - | - |
| SPA | - | - | - | 34.35 | - | - | - | - |
| Rutile + SPA | 284.9 | 531.49 | 458.97 | 133.76 | +0.1 | +0.39 | +0.43 | −0.59 |
| Sample | Relative Contents (%) | |||
|---|---|---|---|---|
| C(1s) | O(1s) | Ti(2p) | P(2p) | |
| Rutile | 25.01 | 62.50 | 12.49 | - |
| Rutile + SPA | 51.61 | 36.06 | 4.67 | 6.77 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, W.; Jiao, F.; Zhao, H.; Qin, W.; Qiu, G.; Wang, J. Adsorption Structure and Mechanism of Styryl Phosphoric Acid at the Rutile–Water Interface. Minerals 2018, 8, 360. https://doi.org/10.3390/min8080360
Xiao W, Jiao F, Zhao H, Qin W, Qiu G, Wang J. Adsorption Structure and Mechanism of Styryl Phosphoric Acid at the Rutile–Water Interface. Minerals. 2018; 8(8):360. https://doi.org/10.3390/min8080360
Chicago/Turabian StyleXiao, Wei, Fen Jiao, Hongbo Zhao, Wenqing Qin, Guanzhou Qiu, and Jun Wang. 2018. "Adsorption Structure and Mechanism of Styryl Phosphoric Acid at the Rutile–Water Interface" Minerals 8, no. 8: 360. https://doi.org/10.3390/min8080360
APA StyleXiao, W., Jiao, F., Zhao, H., Qin, W., Qiu, G., & Wang, J. (2018). Adsorption Structure and Mechanism of Styryl Phosphoric Acid at the Rutile–Water Interface. Minerals, 8(8), 360. https://doi.org/10.3390/min8080360








