The Nature of Laponite: Pure Hectorite or a Mixture of Different Trioctahedral Phases?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
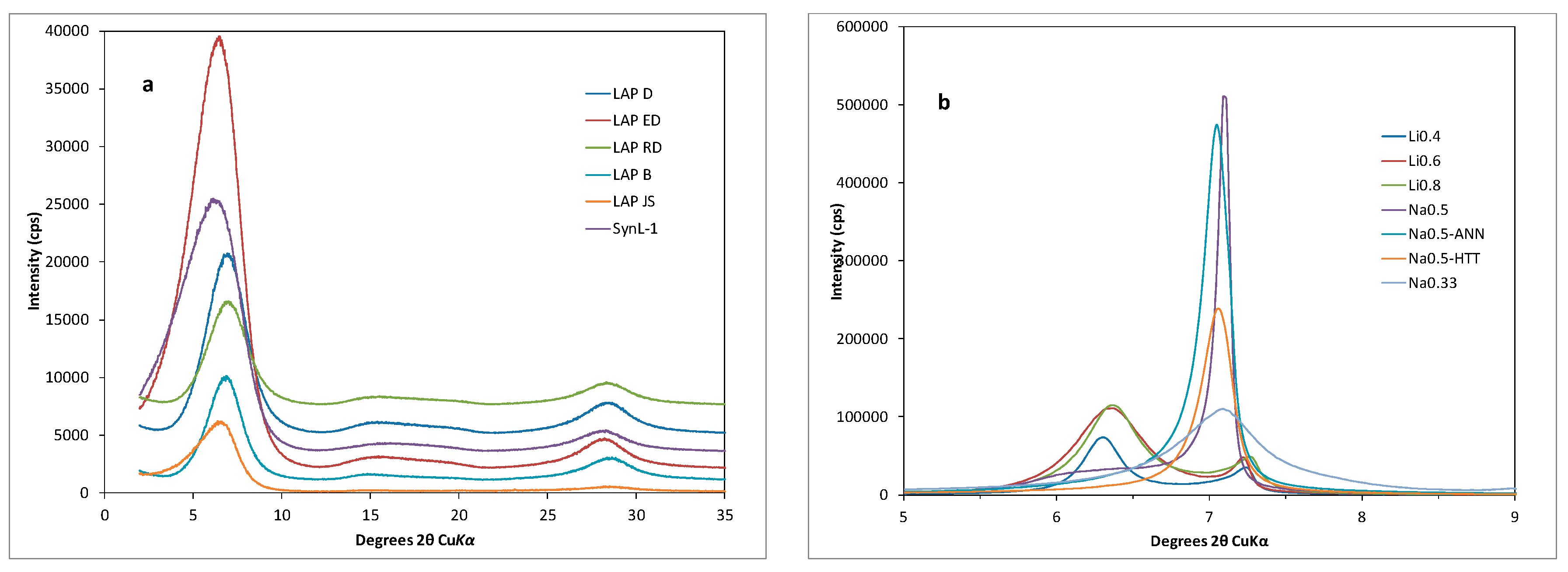
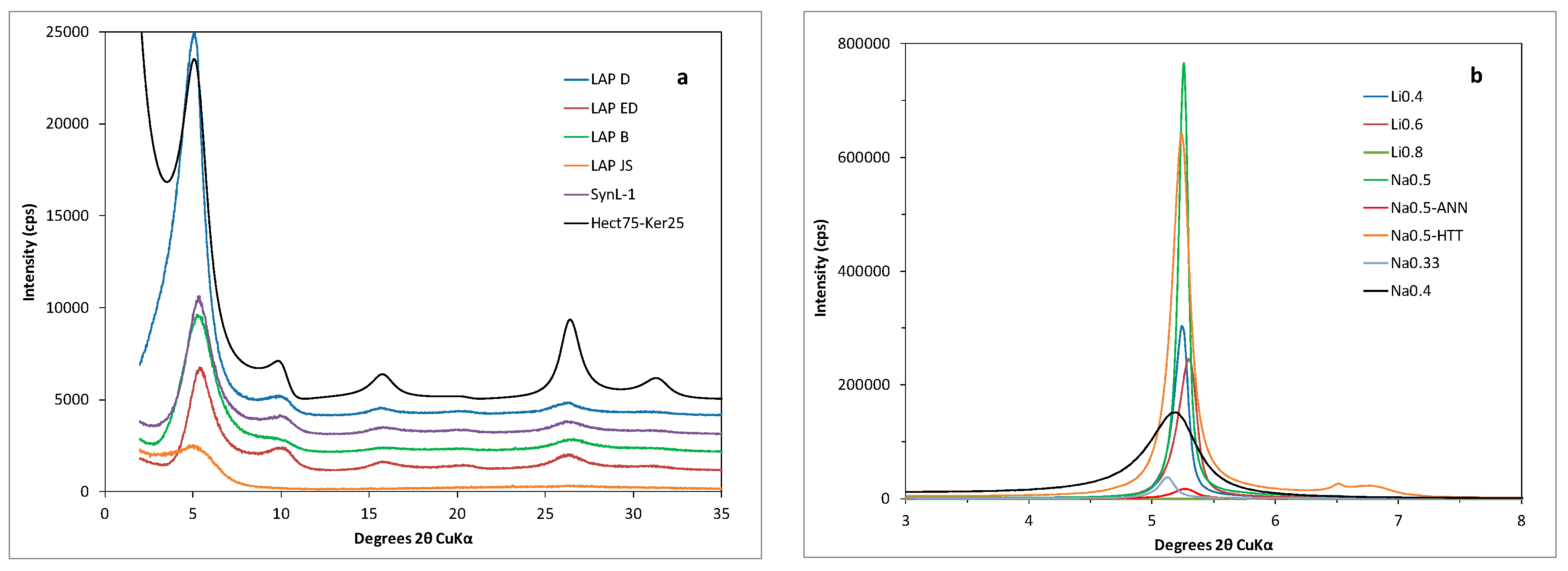
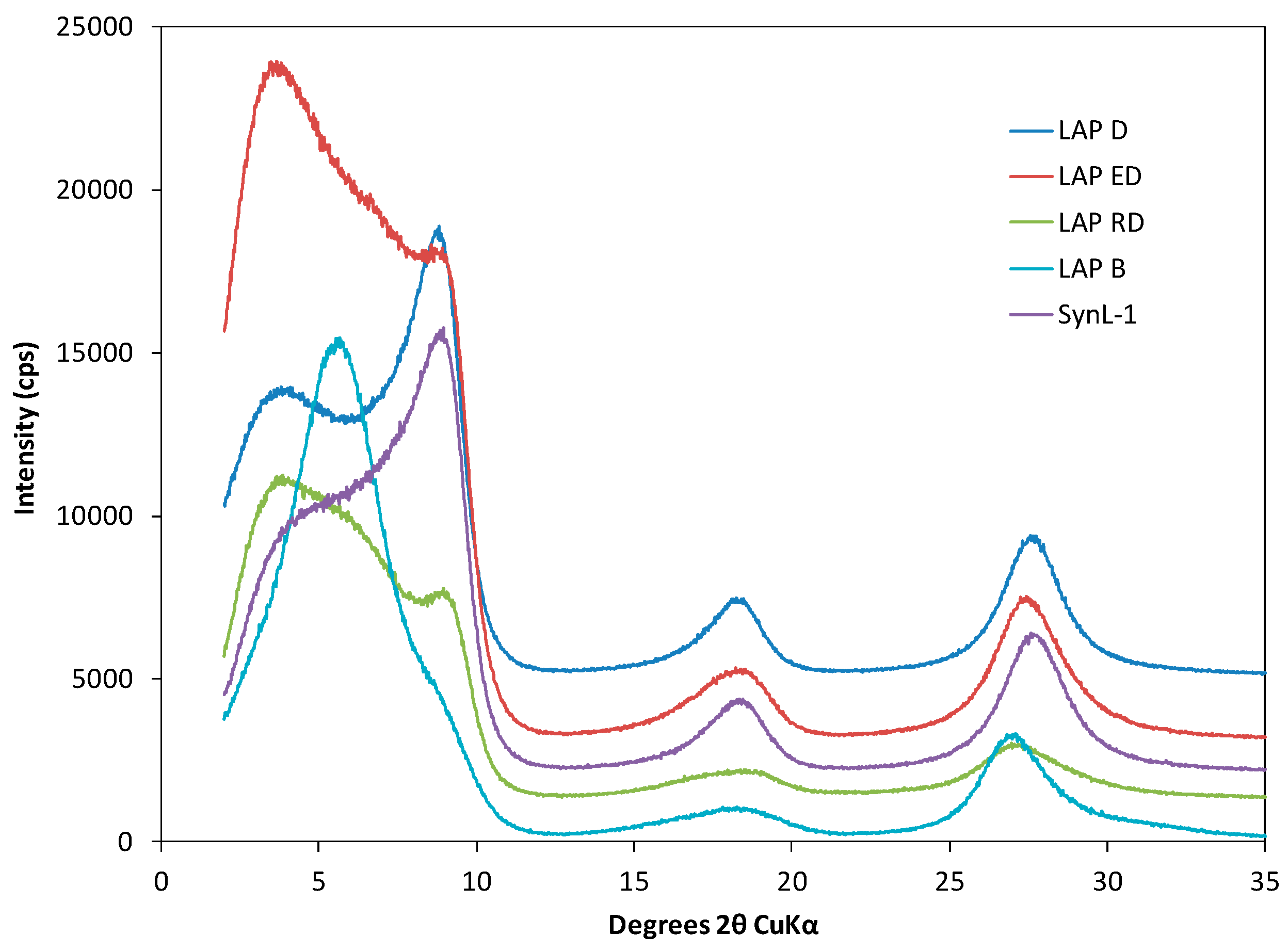
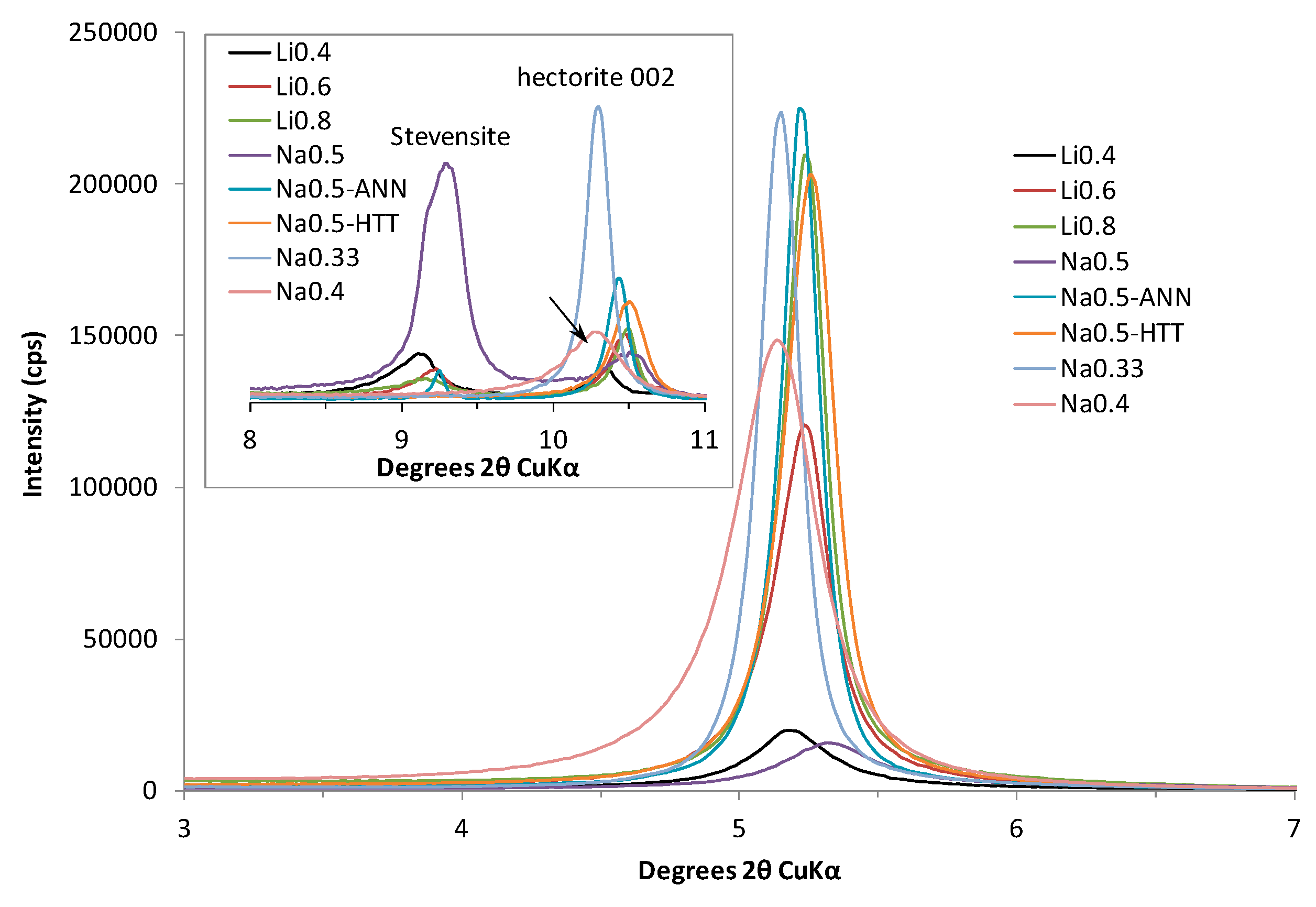
3.1. XRD Results
3.2. Structural Formula of the BYK-Chemie OH-Laponites
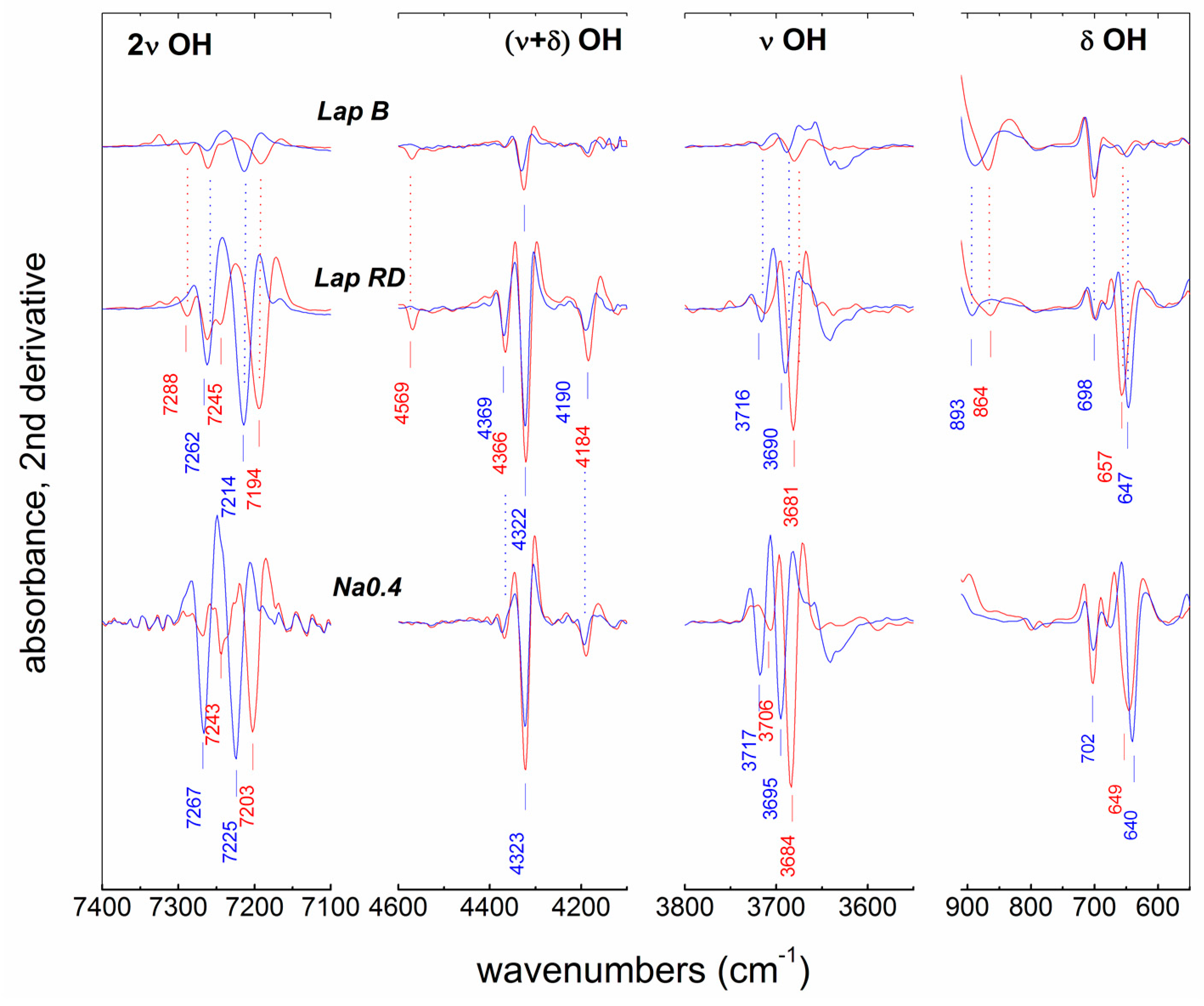
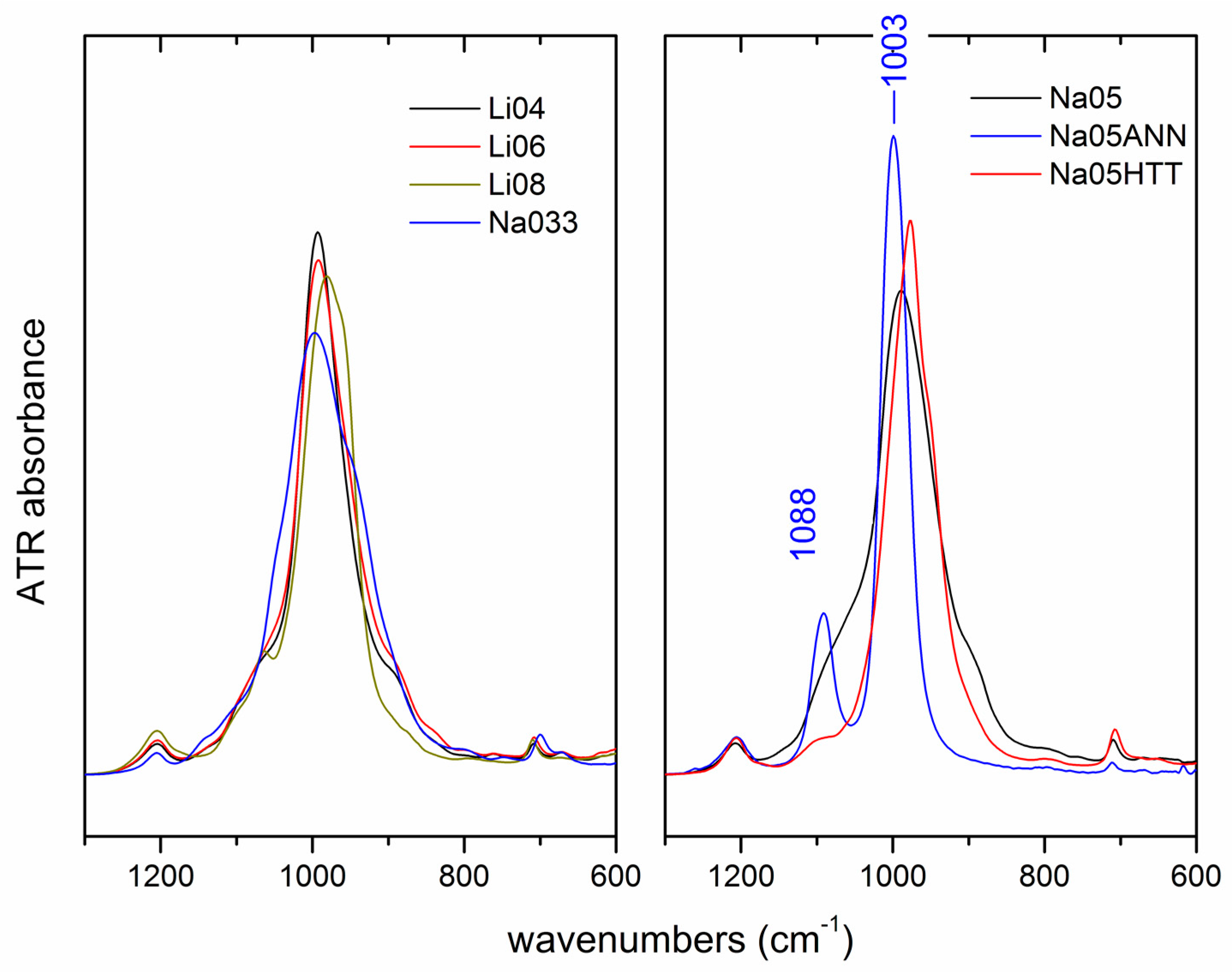
3.3. IR Results
3.3.1. Structural OH Groups
3.3.2. Fluorinated Hectorite
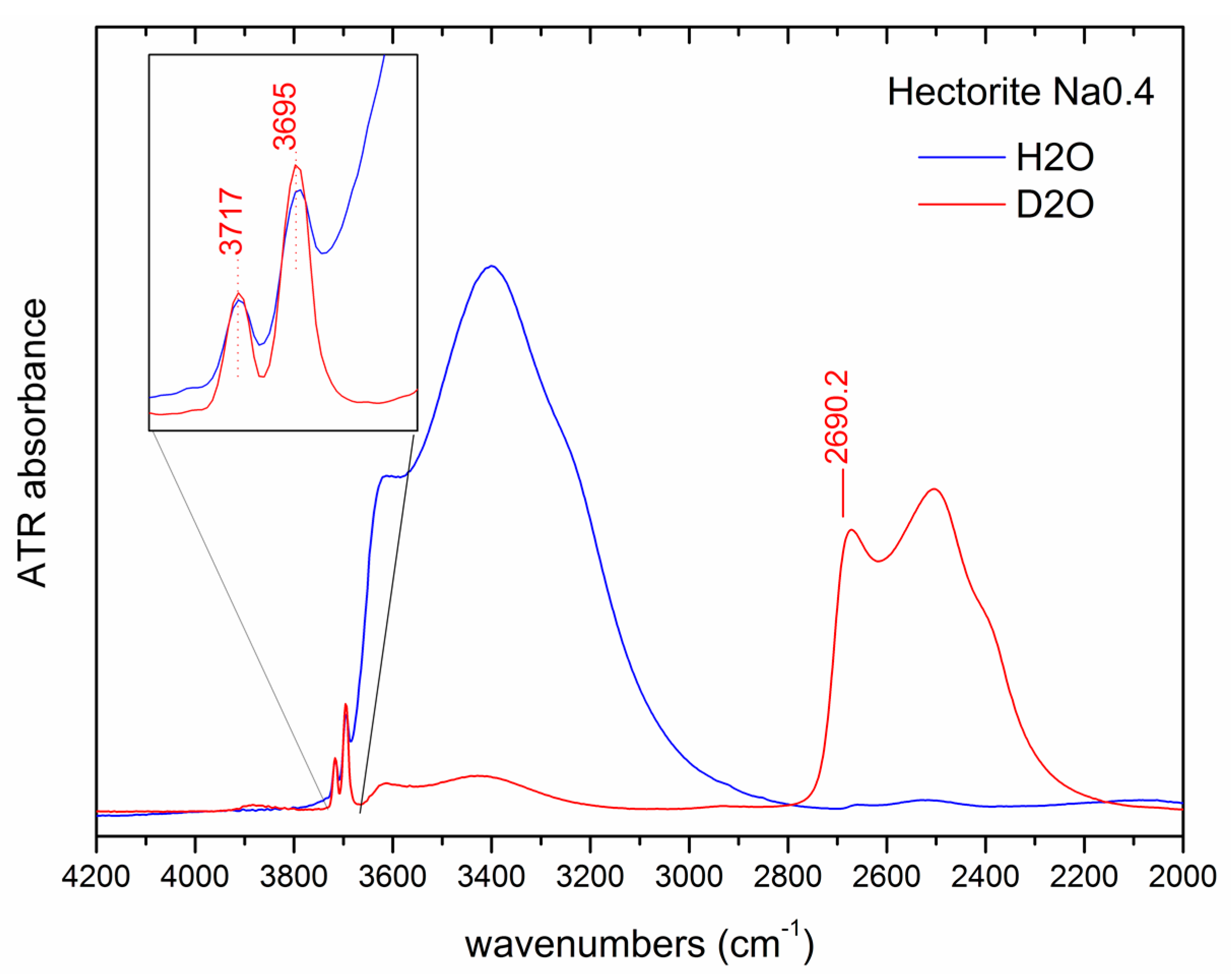
3.3.3. D2O Exchange and Layer Charge Determination
4. Discussion
4.1. Characteristics of the Synthetic Mg-Li Smectites
4.2. Implications for Natural Systems
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neumann, B.S. Behaviour of a Synthetic Clay in Pigment Dispersions. Rheol. Acta 1965, 4, 250–255. [Google Scholar] [CrossRef]
- CMS Nomenclature Committee. The Clay Minerals Society Glossary of Clay Science; Part 1; The Clay Minerals Society: Chantilly, VA, USA, 2018. [Google Scholar]
- Jaber, M.; Komarneni, S.; Zhou, C.-H. Synthesis of clay minerals. In Handbook of Clay Science, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 223–240. [Google Scholar]
- Strese, H.; Hofmann, U. Synthesis of magnesium silicate gels with two-dimensional regular structure. Zeit. Anorg. Allg. Chem. 1941, 247, 65–95. [Google Scholar] [CrossRef]
- Caillere, S.; Oberlin, A.; Henin, S. Etude au microscope electronique de quelques silicates phylliteux obtenus par syntheses a basse temperature. Clay Miner. Bull. 1954, 2, 146–156. [Google Scholar] [CrossRef]
- Henin, S. Synthesis of clay minerals at low temperatures. Clays Clay Miner. 1956, 4, 54–60. [Google Scholar] [CrossRef]
- Granquist, W.T.; Pollack, S.S. A study of the synthesis of hectorite. Clays Clay Miner. 1960, 8, 150–169. [Google Scholar] [CrossRef]
- Harder, H. Clay mineral formation under lateritic weathering conditions. Clay Miner. 1977, 12, 281–288. [Google Scholar] [CrossRef]
- Decarreau, A. Cristallogenese expdrimentale des smectites magnesiennes: Hectorite, stevensite. Bull. Mineral. 1980, 103, 579–590. [Google Scholar]
- Saunders, J.M.; Goodwin, J.W.; Richardson, R.M.; Vincent, B. A small-angle X-ray scattering study of the structure of aqueous laponite dispersions. J. Phys. Chem. B 1999, 103, 9211–9218. [Google Scholar] [CrossRef]
- Breu, J.; Seidl, W.; Stoll, A.J.; Lange, K.G.; Probst, T.U. Charge homogeneity in synthetic fluorohectorite. Chem. Mater. 2001, 13, 4213–4220. [Google Scholar] [CrossRef]
- Zhou, C.H.; Du, Z.X.; Li, X.N.; Lu, C.S.; Ge, Z.H. Structure development of hectorite in hydrothermal crystallization synthesis process. Chin. J. Inorg. Chem. 2005, 21, 1327–1332. [Google Scholar]
- Malikova, N.; Cadene, A.; Dubois, E.; Marry, V.; Durand-Vidal, S.; Turq, P.; Breu, J.; Longeville, S.; Zanotti, J.-M. Water diffusion in a synthetic hectorite clay studied by quasielastic neutron scattering. J. Phys. Chem. C 2007, 111, 17603–17611. [Google Scholar] [CrossRef]
- Kalo, H.; Moller, M.W.; Ziadeh, M.; Dolejs, D.; Breu, J. Large scale melt synthesis in an open crucible of Na-fluorohectorite with superb charge homogeneity and particle size. Appl. Clay Sci. 2010, 48, 39–45. [Google Scholar] [CrossRef]
- Kalo, H.; Möller, M.W.; Kunz, D.A.; Breu, J. How to Maximize the Aspect Ratio of Clay Nanoplatelets. Nanoscale 2012, 4, 5633–5639. [Google Scholar] [CrossRef] [PubMed]
- Stöter, M.; Kunz, D.A.; Schmidt, M.; Hirsemann, D.; Kalo, H.; Putz, B.; Senker, J.; Breu, J. Nanoplatelets of Sodium Hectorite Showing Aspect Ratios of ≈20,000 and Superior Purity. Langmuir 2013, 29, 1280–1285. [Google Scholar] [CrossRef] [PubMed]
- Kloprogge, J.T.; Komarneni, S.; Amonette, J.E. Synthesis of smectite clay minerals: A critical review. Clays Clay Miner. 1999, 47, 529–554. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, C.-H.; Lin, C.-X.; Tong, D.-S.; Yu, W.-H. The synthesis of clay minerals. Appl. Clay Sci. 2010, 50, 1–11. [Google Scholar] [CrossRef]
- Carrado, K.A. Synthetic organo- and polymer–clays: Preparation, characterization, and materials applications. Appl. Clay Sci. 2000, 17, 1–23. [Google Scholar] [CrossRef]
- Christidis, G.E. Industrial Minerals: Significance and Important Characteristics. In Advances in the characterization of Industrial Minerals; Christidis, G.E., Ed.; EMU Notes in Mineralogy; Mineralogical Society: London, UK, 2011; Volume 9, pp. 1–12. [Google Scholar]
- Thomas, F.; Michot, L.J.; Vantelon, D.; Montarges, E.; Prelot, B.; Cruchaudet, M.; Delon, J.F. Layer charge and electrophoretic mobility of smectites. Colloids Surf. A 1999, 159, 351–358. [Google Scholar] [CrossRef]
- Thompson, D.W.; Butterworth, J.T. The nature of laponite and its aqueous dispersions. J. Colloid Interface Sci. 1992, 151, 236–243. [Google Scholar] [CrossRef]
- Kroon, M.; Vos, W.L.; Wedgam, G.H. Structure and formation of a gel of colloidal disks. Phys. Rev. E 1998, 57, 1962–1970. [Google Scholar] [CrossRef] [Green Version]
- Nikolai, T.; Cocard, S. Light Scattering Study of the Dispersion of Laponite. Langmuir 2000, 16, 8189–8193. [Google Scholar] [CrossRef]
- Vantelon, D.; Belkhou, R.; Bihannic, I.; Michot, L.J.; Montargès-Pelletier, E.; Robert, J.-L. An XPEEM study of structural cation distribution in swelling clays. I. Synthetic trioctahedral smectites. Phys. Chem. Miner. 2009, 36, 593–602. [Google Scholar] [CrossRef]
- Christidis, G.E.; Koutsopoulou, E. A simple approach to the identification of trioctahedral smectites by X-ray diffraction. Clay Miner. 2013, 48, 687–696. [Google Scholar] [CrossRef]
- Neumann, B.S.; Sansom, K.G. The formation of stable sols from laponite, a synthetic hectorite-like clay. Clay Miner. 1965, 8, 389–404. [Google Scholar] [CrossRef]
- Bärwinkel, K.; Markus, M.M.; Rieß, M.; Sato, H.; Li, L.; Avadhut, Y.S.; Kemnitzer, T.W.; Hussein Kalo, H.; Senker, J.; Matsuda, R.; et al. Constant Volume Gate-Opening by Freezing Rotational Dynamics in Microporous Organically Pillared Layered Silicates. J. Am. Chem. Soc. 2017, 139, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Karmous, M.S.; Samira, J.; Robert, J.-L.; Ben Haj Amara, A. Nature of disorder in synthetic hectorite. Appl. Clay Sci. 2009, 51, 23–32. [Google Scholar] [CrossRef]
- Bukas, V.J.; Tsampodimou, M.; Gionis, V.; Chryssikos, G.D. Synchronous ATR infrared and NIR-spectroscopy investigation of sepiolite upon drying. Vibr. Spectr. 2013, 68, 51–60. [Google Scholar] [CrossRef]
- Kuligiewicz, A.; Derkowski, A.; Emmerich, K.; Christidis, G.E.; Tsiantos, C.; Gionis, V.; Chryssikos, G.D. Measuring the layer charge of dioctahedral smectite by O-D vibrational spectroscopy. Clays Clay Miner. 2015, 63, 443–456. [Google Scholar] [CrossRef]
- MacEwan, D.M.C.; Wilson, M.J. Interlayer and intercalation complexes of clay minerals. In Crystal Structures of Clay Minerals and Their X-Ray Identification; Bindley, G.W., Brown, G., Eds.; Mineralogical Society: London, UK, 1984; pp. 187–248. [Google Scholar]
- Chipera, S.J.; Bish, D.L. Thermal evolution of fluorine from smectite and kaolinite. Clays Clay Miner. 2002, 50, 38–46. [Google Scholar] [CrossRef]
- Reynolds, R.C., Jr.; Reynolds, R.C., III. Newmod-for-Windows. The Calculation of One-Dimensional X-ray Diffraction Patterns of Mixed-Layered Clay Minerals; Computer Program: Hanover, NH, USA, 1996. [Google Scholar]
- Brindley, G.W.; Bish, D.L.; Wan, H.-M. The nature of kerolite and its relation to talc and stevensite. Mineral. Mag. 1977, 41, 443–452. [Google Scholar] [CrossRef]
- Stathopoulou, E.T.; Suárez, M.; García-Romero, E.; Sánchez del Río, M.; Kacandes, G.H.; Gionis, V.; Chryssikos, G.D. Trioctahedral entities in palygorskite: Near-infrared evidence for sepiolite/palygorskite polysomatism. Eur. J. Mineral. 2011, 23, 567–576. [Google Scholar] [CrossRef]
- Prost, R. Etude de l’ hydratation des argiles: Interactions eau-minéral et mécanisme de la rétention de l’ eau. Ann. Agron. 1975, 26, 401–461. [Google Scholar]
- Pálková, H.; Madejová, J.; Zimowska, M.; Serwicka, E.W. Laponite-derived porous clay heterostructures: II. FTIR study of the structure evolution. Microporous Mesoporous Mater. 2010, 127, 237–244. [Google Scholar] [CrossRef]
- Ras, R.H.A.; Johnston, C.T.; Franses, E.I.; Ramaekers, R.; Maes, G.; Foubert, P.; De Schryver, F.C.; Schoonheydt, R.A. Polarized infrared study of hybrid Langmuir-Blodgett monolayers containing clay mineral nanoparticles. Langmuir 2003, 19, 4295–4302. [Google Scholar] [CrossRef]
- Russell, J.D.; Fraser, A.R. Infrared methods. In Clay Minerals, Spectroscopic and Chemical Determinative Methods; Wilson, M.J., Ed.; Springer: Heidelberg, Germany, 1994; pp. 11–67. [Google Scholar]
- Pelletier, M.; Michot, L.J.; Barrès, O.; Humbert, B.; Petit, S.; Robert, J.-L. Influence of KBr conditioning on the infrared hydroxyl-stretching region of saponites. Clay Miner. 1999, 34, 439–445. [Google Scholar] [CrossRef]
- Pelletier, M.; Michot, L.J.; Humbert, B.; Barrès, O.; D’ Espinose de la Caillerie, J.-B.; Robert, J.-L. Influence of layer charge on the hydroxyl stretching of trioctahedral clay minerals: A vibrational study of synthetic Na- and K-saponites. Amer. Mineral. 2003, 88, 1801–1808. [Google Scholar] [CrossRef]
- Theng, B.K.G. Polymer–Clay Nanocomposites. In Formation and Properties of Clay-Polymer Complexes; Developments in Clay Science; Theng, B.K.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 201–241. [Google Scholar]
- Carrado, K.A.; Komadel, P. Acid activation of bentonites and polymer-clay nanocomposites. Elements 2009, 5, 111–116. [Google Scholar] [CrossRef]
- Skoubris, E.N.; Chryssikos, G.D.; Christidis, G.E.; Gionis, V. Structural characterization of reduced charge montmorillonites. Evidence based on FTIR spectroscopy, thermal behavior and layer charge systematics. Clays Clay Miner. 2013, 61, 83–97. [Google Scholar] [CrossRef]
- Giese, R.F., Jr. The effect of F/OH substitution on some layer-silicate minerals. Zeitschrift für Kristallographie 1975, 138, 138–144. [Google Scholar] [CrossRef]
- Christidis, G.E.; Huff, W.D. Geological Aspects and Genesis of Bentonites. Elements 2009, 5, 93–98. [Google Scholar] [CrossRef]
- Pozo, M.; Casas, J. Origin of kerolite and associated Mg clays in palustrine-lacustrine environments; the Esquivias Deposit (Neogene Madrid Basin, Spain). Clay Miner. 1999, 34, 395–418. [Google Scholar] [CrossRef]
- Galan, E.; Palygorskite, P.M.; Sepiolite Deposits in Continental Environments. Description, genetic patterns and sedimentary settings. In Developments in Palygorskite-Sepiolite Research; Developments in Clay Science; Galan, E., Singer, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 125–173. [Google Scholar]
- Tosca, N.J.; Masterson, A.L. Chemical controls on incipient Mg-silicate crystallization at 25 °C: Implications for early and late diagenesis. Clay Miner. 2014, 49, 165–194. [Google Scholar] [CrossRef] [Green Version]
| First Sample Set (BYK-Chemie Ltd.) | Second Sample Set (CMS Source Clay Project) | |||
|---|---|---|---|---|
| LAP D, ED and RD | LAP B | LAP JS | SynL-1 | |
| SiO2 | 59.5 | 55.0 | 50.2 | 57–61% |
| MgO | 27.5 | 27.0 | 22.2 | 25–29% |
| Li2O | 0.8 | 1.4 | 1.2 | 0.5–0.9% |
| Na2O | 2.8 | 3.8 | 7.5 | 2.5–3.5% |
| LOI | 8.2 | - | 8.7 | <10% |
| P2O5 | - | - | 4.8 | |
| F | - | 5.6 | 5.4 | |
| Third Sample Set (University of Bayreuth, Germany) | ||||
| First group | Li0.4: Li0.4[Mg2.6Li0.4]Si4O10F2 Li0.6: Li0.6[Mg2.4Li0.6]Si4O10F2 Li0.8: Li0.8[Mg2.2Li0.8]Si4O10F2 | |||
| Second group | Na0.5, Na0.5-ANN, Na0.5-HTT: Na0.5[Mg2.6Li0.5]Si4O10F2 | |||
| Third group | Na0.33: Na0.33[Mg2.67Li0.33]Si4O10F2 | |||
| Fourth Sample Set IMPMC, CNRS, Paris, France | ||||
| Na0.4: Na0.4(Mg2.6Li0.4)Si4O10(OH)2 | ||||
| LAP D, -ED, -RD, LAP B, -JS, SynL-1 | Na0.4 | Talc | SepSp-1 | ||||
|---|---|---|---|---|---|---|---|
| Wet | Dry | Wet | Dry | Wet | Dry | ||
| 2ν (X) | 7262 (85) | 7245(84) | 7267 (84) | 7243 (85) | - | - | - |
| 7214 (83) | 7194 (84) | 7225 (83) | 7203 (83) | 7184 (84) | 7214 (83) | 7194 (83) | |
| ν + δ | 4322 | 4322 | 4323 | 4323 | 4324 | 4324 | 4327 |
| ν | 3716 | 3706 | 3717 | 3706 | - | - | - |
| 3690 | 3681 | 3695 | 3684 | 3676 | 3690 | 3680 | |
| δ | 647 | 657 | 640 | 649 | 669 | 644 | 654 |
| νO-D, cm−1 | Qapp, e/huc | |
|---|---|---|
| LAP D | 2693.6 | 0.37 |
| LAP ED | 2692.9 | 0.39 |
| LAP RD | 2692.5 | 0.40 |
| LAP B | 2686.6 | 0.58 |
| LAP JS | 2686.5 | 0.58 |
| SynL-1 | 2693.6 | 0.37 |
| Na0.5 | 2684.2 | 0.65 |
| Na0.5-ANN | 2690.9 | 0.45 |
| Na0.5-HTT | 2687.0 | 0.57 |
| Li0.4 | 2688.8 | 0.52 |
| Li0.6 | 2688.7 | 0.52 |
| Li0.8 | 2685.8 | 0.61 |
| Na0.33 | 2694.3 | 0.35 |
| Na0.4 | 2690.2 | 0.47 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christidis, G.E.; Aldana, C.; Chryssikos, G.D.; Gionis, V.; Kalo, H.; Stöter, M.; Breu, J.; Robert, J.-L. The Nature of Laponite: Pure Hectorite or a Mixture of Different Trioctahedral Phases? Minerals 2018, 8, 314. https://doi.org/10.3390/min8080314
Christidis GE, Aldana C, Chryssikos GD, Gionis V, Kalo H, Stöter M, Breu J, Robert J-L. The Nature of Laponite: Pure Hectorite or a Mixture of Different Trioctahedral Phases? Minerals. 2018; 8(8):314. https://doi.org/10.3390/min8080314
Chicago/Turabian StyleChristidis, George E., Carlos Aldana, Georgios D. Chryssikos, Vassilis Gionis, Hussein Kalo, Matthias Stöter, Josef Breu, and Jean-Louis Robert. 2018. "The Nature of Laponite: Pure Hectorite or a Mixture of Different Trioctahedral Phases?" Minerals 8, no. 8: 314. https://doi.org/10.3390/min8080314
APA StyleChristidis, G. E., Aldana, C., Chryssikos, G. D., Gionis, V., Kalo, H., Stöter, M., Breu, J., & Robert, J.-L. (2018). The Nature of Laponite: Pure Hectorite or a Mixture of Different Trioctahedral Phases? Minerals, 8(8), 314. https://doi.org/10.3390/min8080314





