Crystal Structure of Kristiansenite from Szklarska Poręba, Southwestern Poland
Abstract
1. Introduction
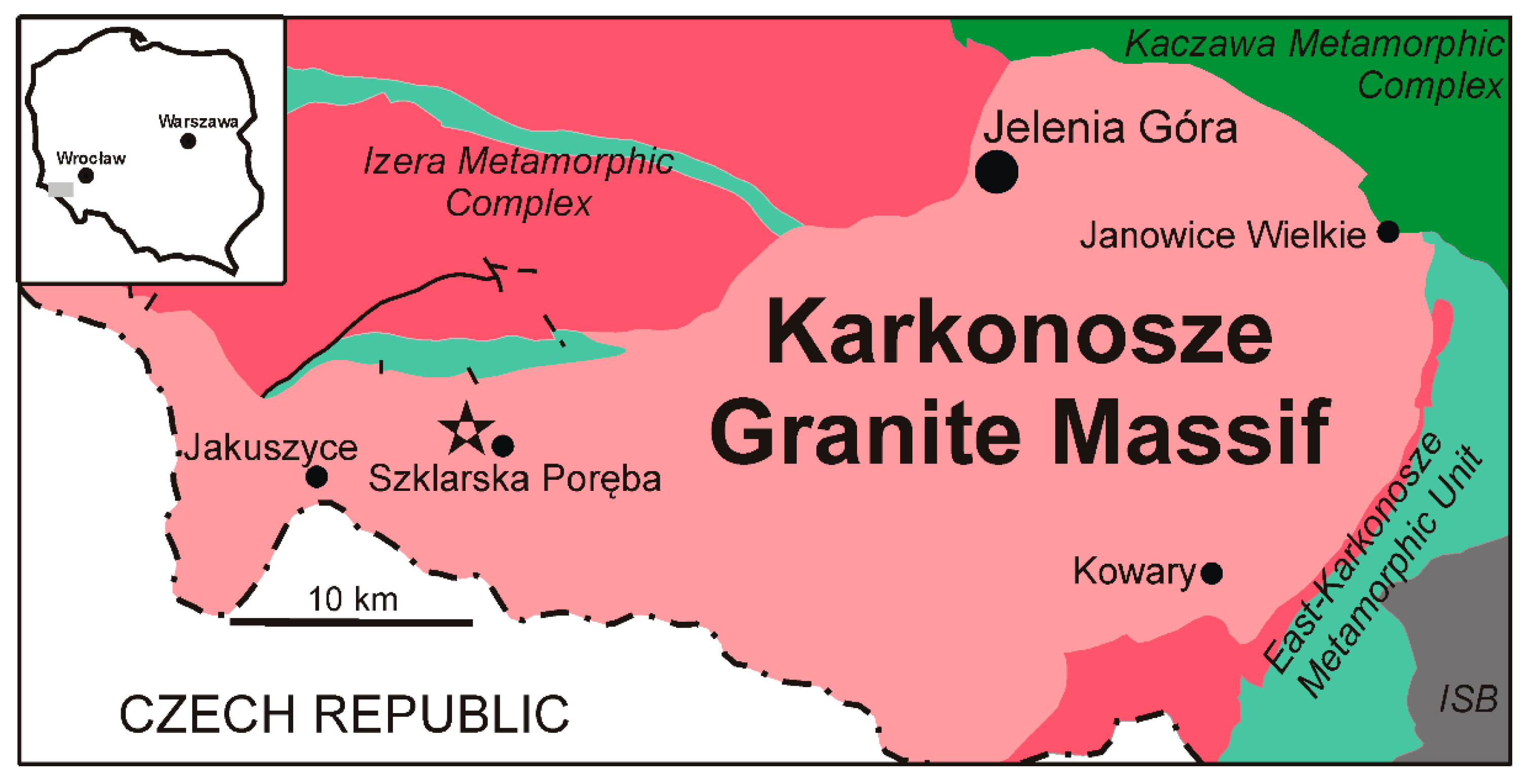
2. Geological Setting
3. Occurrence
4. Materials and Methods
4.1. Electron-Microprobe Analysis
4.2. Crystal Structure Refinement
5. Results
6. Discussion
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Raade, G.; Ferraris, G.; Gula, A.; Ivaldi, G.; Bernhard, F. Kristiansenite, a new calcium–scandium–tin sorosilicate from granite pegmatite in Tørdal, Telemark, Norway. Miner. Petrol. 2002, 75, 89–99. [Google Scholar] [CrossRef]
- Guastoni, A.; Pezzotta, F. Kristiansenite a Baveno, secondo ritrovamento mondiale della specie. Riv. Mineral. Ital. 2004, 4, 247–251. [Google Scholar]
- Nestola, F.; Guastoni, A.; Camara, F.; Secco, L.; Dal Negro, A.; Pedron, D.; Beran, A. Aluminocerite-(Ce): A new species from Baveno, Italy: Description and crystal-structure determination. Am. Mineral. 2009, 94, 487–493. [Google Scholar] [CrossRef]
- Prado-Herrero, P.; Garcia-Guinea, J.; Crespo-Feo, E.; Correcher, V.; Menor, C. First find of Kristiansenite in Spain: Comparison with the type specimen by non-descriptive techniques. Estudos Geológicos 2009, 19, 135–139. [Google Scholar]
- Výravsky, J.; Škoda, R.; Novák, M. Kristiansenite, thortveitite and ScNbO4: Products of Ca-metasomatism of Sc-enriched columbite-(Mn) from NYF pegmatite Kožichovice II, Czech Republic. In Proceedings of the PEG2017 8th International Symposium on Granitic Pegmatites, Kristiansand, Norway, 13–15 June 2017; Müller, A., Rosing-Schow, N., Eds.; Norwegian Geological Society: Trondheim, Norway, 2017; Volume 2, pp. 169–172. [Google Scholar]
- Ferraris, G.; Gula, A.; Ivaldi, G.; Nespolo, M.; Raade, G. Crystal structure of kristiansenite: A case of class IIB twinning by metric merohedry. Z. Kristallogr. 2001, 216, 442–448. [Google Scholar] [CrossRef]
- Berg, G. Der Granit des Riesengebirges und seine Ganggesteine (Petrographische Studien). Abh. Preuss. Geol. Land. 1923, 94. [Google Scholar]
- Borkowska, M. Petrography of Karkonosze granite. Geol. Sudet. 1966, 2, 7–119. [Google Scholar]
- Duthou, J.-L.; Couturie, J.P.; Mierzejewski, M.P.; Pin, C. Age determination of the Karkonosze granite using the Rb-Sr isochrone whole-rock method. Prz. Geol. 1991, 36, 75–79. (In Polish) [Google Scholar]
- Oberc-Dziedzic, T.; Żelaźniewicz, A.; Cwojdziński, S. Granitoids of the Odra fault zone: Late to post-orogenic Variscan intrusions in the Saxothuringian zone, SW Poland. Geol. Sudetica 1999, 32, 55–71. [Google Scholar]
- Mazur, S.; Aleksandrowski, P.; Turniak, K.; Awdankiewicz, M. Geology, tectonic evolution and Late Palaeozoic magmatism of Sudetes—An overview. Granitoids Poland. AM Monogr. 2007, 1, 59–87. [Google Scholar]
- Mikulski, S.Z. Metal ore potential of the parent magma of granite—The Karkonosze massif example. Granitoids Poland. AM Monogr. 2007, 1, 123–145. [Google Scholar]
- Słaby, E.; Martin, H. Mafic and felsic magma interaction in granites: The Hercynian Karkonosze pluton (Sudetes, Bohemian Massif). J. Petrol. 2008, 49, 353–391. [Google Scholar] [CrossRef]
- Kryza, R.; Pin, C.; Oberc-Dziedzic, T.; Crowley, Q.G.; Larionov, A. Deciphering the geochronology of a large granitoid pluton (Karkonosze Granite, SW Poland): An assessment of U-Pb zircon SIMS and Rb-Sr whole-rock dates relative to U-Pb zircon CA-ID-TIMS. Int. Geol. Rev. 2014, 56, 756–782. [Google Scholar] [CrossRef]
- Kryza, R.; Schaltegger, U.; Oberc-Dziedzic, T.; Pin, C.; Ovtcharova, M. Geochronology of a composite granitoid pluton: A high-precision ID-TIMS U-Pb zircon study of the Variscan Karkonosze Granite (SW Poland). Int. J. Earth Sci. 2014, 103, 683–696. [Google Scholar] [CrossRef]
- Kusiak, M.A.; Williams, I.S.; Dunkley, D.J.; Konečny, P.; Słaby, E.; Martin, H. Monazite to the rescue: U-Th-Pb dating of the intrusive history of the composite Karkonosze pluton, Bohemian Massif. Chem. Geol. 2014, 364, 76–92. [Google Scholar] [CrossRef]
- Pieczka, A.; Szuszkiewicz, A.; Szełęg, E.; Janeczek, J.; Nejbert, K. Granitic pegmatites of the Polish part of the Sudetes (NE Bohemian massif, SW Poland). In Proceedings of the PEG2015 7th International Symposium on Granitic Pegmatites, Książ, Poland, 17–19 June 2015; Gadas, P., Novák, M., Szuszkiewicz, A., Cempírek, J., Eds.; Tigris: Zlín, Czech Republic, 2015. [Google Scholar]
- Černý, P.; Ercit, T.S. The classification of granitic pegmatites revisited. Can. Mineral. 2005, 43, 2005–2026. [Google Scholar] [CrossRef]
- Pieczka, A.; Ma, C.; Rossman, G.R.; Evans, R.J.; Groat, L.A.; Gołębiowska, B. Silesiaite, IMA 2017-064. CNMNC Newsletter No. 40, December 2017, page 1578. Mineral. Mag. 2017, 81, 1577–1581. [Google Scholar] [CrossRef]
- Karwowski, Ł.; Olszyński, W.; Kozłowski, A. Wolframite mineralization from the vicinity of Szklarska Poręba Huta. Prz. Geol. 1973, 21, 633–637. (In Polish) [Google Scholar]
- Kozłowski, A.; Karwowski, Ł.; Olszyński, W. Tungsten-tin-molybdenum mineralization in the Karkonosze massif. Acta Geol. Pol. 1975, 25, 415–430. [Google Scholar]
- Olszyński, W.; Kozłowski, A.; Karwowski, Ł. Bismuth minerals from the Karkonosze massif. Acta Geol. Pol. 1976, 26, 443–449. [Google Scholar]
- Pieczka, A.; Gołębiowska, B. Pegmatites of the Szklarska Poręba Huta granite quarry: Preliminary data on REE mineralization. Mineral. Spec. Pap. 2002, 20, 175–177. [Google Scholar]
- Pieczka, A.; Gołębiowska, B.; Ilnicki, S.; Dzierżanowski, P.; Jeżak, L. Gadolinite group minerals from Szklarska Poręba (SW Poland, Lover Silesia, Karkonosze Mts). In Proceedings of the International Symposium on Light Elements in Rock-forming Minerals, Nové Město na Moravě, Czech Republic, 20–25 June 2003; pp. 61–62. [Google Scholar]
- Pieczka, A.; Gołębiowska, B. Cuprobismutite homologues in granitic pegmatites from Szklarska Poręba, Karkonosze Massif, Southwestern Poland. Can. Mineral. 2012, 50, 313–324. [Google Scholar] [CrossRef]
- Pieczka, A.; (AGH UST, Cracow, Poland); Gołębiowska, B.; (AGH UST, Cracow, Poland). Personal communication, 2012.
- Pouchou, I.L.; Pichoir, F. “PAP” (phi-rho-z) procedure for improved quantitativemicroanalysis. In Microbeam Analysis; Armstrong, I.T., Ed.; San Francisco Press: San Francisco, CA, USA, 1985; pp. 104–106. [Google Scholar]
- Bruker. SAINT; Bruker AXS Inc.: Madison, WI, USA, 2007. [Google Scholar]
- Sheldrick, G.M. SADABS; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Cromer, D.T.; Waber, J.T. International Tables for X-ray Crystallography, Volume IV; The Kynoch Press: Birmingham, UK, 1974. [Google Scholar]
- Ibers, J.A.; Hamilton, W.C. Dispersion corrections and crystal structure refinements. Acta Cryst. 1964, 17, 781–782. [Google Scholar] [CrossRef]
- Creagh, D.C.; McAuley, W.J. X-ray dispersion corrections. In International Tables for Crystallography, Volume C; Kluwer Academic Publishers: Boston, MA, USA, 1992; pp. 219–222. [Google Scholar]
- Creagh, D.C.; Hubbell, J.H. X-ray absorption (or attenuation) coefficients. In International Tables for Crystallography, Volume C; Kluwer Academic Publishers: Boston, MA, USA, 1992; pp. 200–206. [Google Scholar]
- Aroyo, M.I.; Perez-Mato, J.M.; Capillas, C.; Kroumova, E.; Ivantchev, S.; Madariaga, G.; Kirov, A.; Wondratschek, H. Bilbao Crystallographic Server I: Databases and crystallographic computing programs. Z. Krist. 2006, 221, 15–27. [Google Scholar] [CrossRef]
- Brese, N.E.; O’Keefe, M. Bond-valence parameters for solids. Acta Cryst. 1991, B47, 192–197. [Google Scholar] [CrossRef]

| Constituent | wt. % | apfu |
|---|---|---|
| Ta2O5 | 0.47 | 0.01 |
| Nb2O5 | 0.49 | 0.02 |
| SiO2 | 40.78 | 3.98 |
| TiO2 | 0.50 | 0.04 |
| ZrO2 | 0.56 | 0.03 |
| SnO2 | 24.79 | 0.97 |
| Al2O3 | 0.29 | 0.03 |
| Sc2O3 | 8.16 | 0.69 |
| Fe2O3 * | 2.34 | 0.17 |
| MnO | 0.64 | 0.05 |
| CaO | 19.11 | 2.00 |
| H2O(calc.) | 1.49 | 0.97 |
| Total | 99.61 |
| Crystal Data | |
| Crystal size (mm) | 0.045 × 0.060 × 0.040 |
| Space group | C1 |
| a (Å) | 10.0304(5) |
| b (Å) | 8.4056(4) |
| c (Å) | 13.3228(6) |
| α | 90.001(3) |
| β | 109.105(3) |
| γ | 89.997(3) |
| V (Å3) | 1061.40(9) |
| Z | 2 |
| Data Collection and Refinement | |
| Radiation | MoKα |
| Monochromator | graphite |
| Temperature | 100 K |
| Total Fo | 29,210 |
| Unique Fo | 6213 |
| Fo > 4σ Fo | 4503 |
| Range of h, k, l | −14 ≤ h ≤ 14, −11 ≤ k ≤ 11, −18 ≤ l ≤ 18 |
| Max. 2θ (°) | 60.17 |
| Inversion twin components | 0.956:0.044 |
| Rint | 0.0936 |
| L.s. parameters | 339 |
| R1 for Fo > 4σ Fo | 0.0496 |
| R1, all unique Fo | 0.0836 |
| wR2 | 0.1141 |
| a | 0.0497 |
| b | 0.00 |
| GooF | 1.052 |
| x | y | z | Ueq | Occupancy | |
|---|---|---|---|---|---|
| Ca1 | 0 | 0 | 0 | 0.0177(6) | Ca |
| Ca2 | 0.0010(4) | 0.7003(4) | −0.4984(3) | 0.0171(6) | Ca |
| Ca3 | 0.2017(4) | −0.0030(4) | −0.2699(3) | 0.0177(7) | Ca |
| Ca4 | 0.2019(2) | 0.7035(2) | −0.7699(2) | 0.0166(6) | Ca |
| M1 | −0.2522(4) | 0.0879(4) | −0.2458(3) | 0.0084(6) | 0.214(5) Sn + 0.787(5) Sc |
| M2 | −0.2530(3) | 0.6135(4) | −0.7469(3) | 0.0104(5) | 0.339(5) Sn + 0.661(5) Sc |
| M3 | 0.4605(3) | 0.0856(3) | −0.0262(3) | 0.0073(4) | 0.785(6) Sn + 0.215(6) Sc |
| M4 | 0.4607(3) | 0.6146(3) | −0.5255(3) | 0.0089(4) | 0.798(6) Sn + 0.202(6) Sc |
| Si1 | −0.2317(5) | 0.2728(5) | −0.0195(4) | 0.0089(7) | Si |
| Si2 | −0.2319(5) | 0.4280(5) | −0.5194(4) | 0.0093(7) | Si |
| Si3 | 0.4362(5) | 0.2748(5) | −0.2527(4) | 0.0086(8) | Si |
| Si4 | 0.4359(5) | 0.4260(5) | −0.7531(4) | 0.0088(7) | Si |
| Si5 | −0.0276(5) | 0.3829(5) | −0.8161(4) | 0.0086(7) | Si |
| Si6 | −0.0276(5) | 0.3182(5) | −0.3164(4) | 0.0093(7) | Si |
| Si7 | 0.2286(5) | 0.3824(5) | −0.4567(4) | 0.0094(7) | Si |
| Si8 | 0.2281(5) | 0.3185(5) | 0.0433(4) | 0.0092(7) | Si |
| O1 | 0.575(1) | 0.255(1) | −0.2877(9) | 0.015(2) * | O |
| O2 | 0.5808(10) | 0.446(1) | −0.7846(8) | 0.009(2) * | O |
| O3 | −0.371(1) | 0.256(1) | 0.0136(9) | 0.015(2) | O |
| O4 | −0.3756(10) | 0.444(1) | −0.4871(8) | 0.007(2) | O |
| O5 | −0.071(1) | −0.0512(10) | −0.2004(8) | 0.012(2) | O |
| O6 | −0.0688(10) | 0.7518(10) | −0.7013(7) | 0.010(2) * | O |
| O7 | 0.279(1) | −0.053(1) | −0.0687(8) | 0.014(2) | O |
| O8 | 0.276(1) | 0.753(1) | −0.5689(8) | 0.013(2) | O |
| O9 | −0.211(1) | 0.128(1) | −0.0914(8) | 0.012(2) | O |
| O10 | −0.211(1) | 0.573(1) | −0.5911(8) | 0.010(2) * | O |
| O11 | 0.413(1) | 0.128(1) | −0.1813(8) | 0.011(2) | O |
| O12 | 0.414(1) | 0.573(1) | −0.6817(8) | 0.010(2) * | O |
| O13 | −0.0941(10) | 0.252(1) | 0.0908(7) | 0.012(2) | O |
| O14 | −0.0940(9) | 0.4488(10) | −0.4087(7) | 0.010(2) * | O |
| O15 | 0.2999(9) | 0.251(1) | −0.3601(7) | 0.014(2) | O |
| O16 | 0.3004(10) | 0.450(1) | −0.8614(8) | 0.013(2) * | O |
| O17 | −0.139(1) | 0.284(1) | −0.2584(8) | 0.009(2) * | O |
| O18 | −0.147(1) | 0.418(1) | −0.7629(8) | 0.009(2) * | O |
| O19 | 0.344(1) | 0.284(1) | −0.0150(8) | 0.012(2) | O |
| O20 | 0.353(1) | 0.416(1) | −0.5079(8) | 0.014(2) | O |
| O21 | −0.3117(10) | 0.0388(10) | −0.4038(7) | 0.010(2) | O |
| O22 | −0.3117(10) | 0.659(1) | 0.0966(7) | 0.012(2) | O |
| O23 | 0.5155(10) | 0.039(1) | −0.8674(8) | 0.012(2) * | O |
| O24 | 0.516(1) | 0.659(1) | −0.3675(8) | 0.013(2) | O |
| O25 | −0.3834(10) | −0.107(1) | −0.2289(8) | 0.015(2) | O |
| O26 | −0.3852(9) | 0.807(1) | −0.7313(7) | 0.012(2) | O |
| O27 | 0.5868(10) | −0.0979(10) | −0.0419(8) | 0.012(2) * | O |
| O28 | 0.5908(10) | 0.7993(10) | −0.5393(7) | 0.007(2) * | O |
| Ca1-O22 | 2.327(9) | Ca2-O20 | 2.319(10) |
| Ca1-O9 | 2.328(10) | Ca2-O21 | 2.328(9) |
| Ca1-O19 | 2.362(10) | Ca2-O10 | 2.344(9) |
| Ca1-O3 | 2.398(10) | Ca2-O4 | 2.375(9) |
| Ca1-O5 | 2.564(10) | Ca2-O6 | 2.599(9) |
| Ca1-O2 | 2.753(10) | Ca2-O1 | 2.699(11) |
| Ca1-O13 | 2.757(8) | Ca2-O14 | 2.747(8) |
| <Ca1-O> | 2.498 | <Ca2-O> | 2.487 |
| O2-Ca1-O3 | 79.3(3) | O1-Ca2-O4 | 80.0(3) |
| O2-Ca1-O13 | 72.3(3) | O1-Ca2-O14 | 72.3(3) |
| O2-Ca1-O19 | 85.6(3) | O1-Ca2-O20 | 82.7(3) |
| O2-Ca1-O22 | 66.9(3) | O1-Ca2-O21 | 68.8(3) |
| O3-Ca1-O5 | 83.9(3) | O4-Ca2-O6 | 83.1(3) |
| O3-Ca1-O19 | 71.0(3) | O4-Ca2-O20 | 68.8(3) |
| O3-Ca1-O22 | 97.4(3) | O4-Ca2-O21 | 99.1(3) |
| O5-Ca1-O9 | 68.7(3) | O6-Ca2-O10 | 68.9(3) |
| O5-Ca1-O19 | 80.4(3) | O6-Ca2-O20 | 82.3(3) |
| O5-Ca1-O22 | 124.8(3) | O6-Ca2-O21 | 123.7(3) |
| O9-Ca1-O13 | 59.5(3) | O10-Ca2-O14 | 59.5(3) |
| O9-Ca1-O19 | 81.3(4) | O10-Ca2-O20 | 82.7(3) |
| O9-Ca1-O22 | 117.3(3) | O10-Ca2-O21 | 117.0(3) |
| O13-Ca1-O19 | 108.5(3) | O14-Ca2-O20 | 108.5(3) |
| O13-Ca1-O22 | 69.4(3) | O14-Ca2-O21 | 69.5(3) |
| Ca3-O24 | 2.325(10) | Ca4-O18 | 2.340(9) |
| Ca3-O11 | 2.335(10) | Ca4-O12 | 2.342(10) |
| Ca3-O1 | 2.367(10) | Ca4-O23 | 2.347(9) |
| Ca3-O17 | 2.370(9) | Ca4-O2 | 2.348(9) |
| Ca3-O7 | 2.568(10) | Ca4-O8 | 2.569(10) |
| Ca3-O4 | 2.775(10) | Ca4-O3 | 2.772(12) |
| Ca3-O15 | 2.785(9) | Ca4-O16 | 2.794(9) |
| <Ca3-O> | 2.504 | <Ca4-O> | 2.501 |
| O4-Ca3-O1 | 78.6(3) | O3-Ca4-O2 | 79.8(3) |
| O4-Ca3-O15 | 72.0(3) | O3-Ca4-O16 | 71.2(3) |
| O4-Ca3-O17 | 84.4(3) | O3-Ca4-O18 | 82.3(3) |
| O4-Ca3-O24 | 67.1(3) | O3-Ca4-O23 | 68.2(3) |
| O1-Ca3-O7 | 86.2(3) | O2-Ca4-O8 | 84.9(3) |
| O1-Ca3-O17 | 71.7(3) | O2-Ca4-O18 | 69.3(3) |
| O1-Ca3-O24 | 98.0(4) | O2-Ca4-O23 | 99.8(3) |
| O7-Ca3-O11 | 68.4(3) | O8-Ca4-O12 | 69.3(3) |
| O7-Ca3-O17 | 80.7(3) | O8-Ca4-O18 | 82.5(3) |
| O7-Ca3-O24 | 126.4(3) | O8-Ca4-O23 | 125.4(3) |
| O11-Ca3-O15 | 57.8(3) | O12-Ca4-O16 | 57.8(3) |
| O11-Ca3-O17 | 81.0(3) | O12-Ca4-O18 | 82.4(3) |
| O11-Ca3-O24 | 115.8(3) | O12-Ca4-O23 | 115.5(3) |
| O15-Ca3-O17 | 106.5(3) | O16-Ca4-O18 | 106.7(3) |
| O15-Ca3-O24 | 69.0(3) | O16-Ca4-O23 | 68.9(3) |
| M1-O9 | 1.989(9) | M2-O22 | 2.008(8) |
| M1-O17 | 2.035(9) | M2-O18 | 2.009(9) |
| M1-O21 | 2.037(9) | M2-O10 | 2.011(9) |
| M1-O5 | 2.078(9) | M2-O6 | 2.099(9) |
| M1-O25 | 2.159(10) | M2-O2 | 2.113(9) |
| M1-O1 | 2.163(10) | M2-O26 | 2.153(9) |
| <M1-O> | 2.077 | <M2-O> | 2.065 |
| O9-M1-O17 | 90.7(4) | O22-M2-O18 | 91.8(4) |
| O9-M1-O5 | 85.8(4) | O22-M2-O6 | 97.2(3) |
| O9-M1-O25 | 87.1(4) | O22-M2-O2 | 86.5(3) |
| O9-M1-O1 | 92.0(4) | O22-M2-O26 | 88.6(3) |
| O17-M1-O21 | 93.6(4) | O18-M2-O10 | 92.0(4) |
| O17-M1-O5 | 91.2(4) | O18-M2-O6 | 92.1(4) |
| O17-M1-O1 | 82.7(4) | O18-M2-O2 | 80.5(4) |
| O21-M1-O5 | 96.8(4) | O10-M2-O6 | 86.1(4) |
| O21-M1-O25 | 88.4(4) | O10-M2-O2 | 90.7(4) |
| O21-M1-O1 | 85.8(4) | O10-M2-O26 | 87.4(3) |
| O5-M1-O25 | 92.9(4) | O6-M2-O26 | 93.7(3) |
| O25-M1-O1 | 93.1(4) | O2-M2-O26 | 93.7(3) |
| M3-O11 | 1.991(9) | M4-O12 | 2.010(9) |
| M3-O23 | 2.044(9) | M4-O24 | 2.024(9) |
| M3-O27 | 2.050(9) | M4-O20 | 2.042(9) |
| M3-O19 | 2.067(9) | M4-O28 | 2.074(8) |
| M3-O7 | 2.073(9) | M4-O8 | 2.102(9) |
| M3-O3 | 2.145(9) | M4-O4 | 2.110(9) |
| <M3-O> | 2.061 | <M4-O> | 2.06 |
| O11-M3-O27 | 88.5(4) | O12-M4-O20 | 91.6(4) |
| O11-M3-O19 | 89.0(4) | O12-M4-O28 | 88.6(4) |
| O11-M3-O7 | 85.6(4) | O12-M4-O8 | 85.8(4) |
| O11-M3-O3 | 92.2(4) | O12-M4-O4 | 91.8(4) |
| O23-M3-O27 | 89.8(3) | O24-M4-O20 | 90.2(4) |
| O23-M3-O19 | 92.7(4) | O24-M4-O28 | 89.4(4) |
| O23-M3-O7 | 95.2(4) | O24-M4-O8 | 95.9(4) |
| O23-M3-O3 | 87.2(4) | O24-M4-O4 | 86.8(4) |
| O27-M3-O7 | 93.8(4) | O20-M4-O8 | 92.0(4) |
| O27-M3-O3 | 93.7(4) | O20-M4-O4 | 79.4(4) |
| O19-M3-O7 | 90.3(4) | O28-M4-O8 | 94.7(3) |
| O19-M3-O3 | 82.0(4) | O28-M4-O4 | 93.9(3) |
| Si1-O3 | 1.603(10) | Si2-O10 | 1.600(10) |
| Si1-O9 | 1.606(10) | Si2-O8 | 1.624(10) |
| Si1-O7 | 1.627(10) | Si2-O4 | 1.640(9) |
| Si1-O13 | 1.663(9) | Si2-O14 | 1.667(9) |
| <Si1-O> | 1.625 | <Si2-O> | 1.633 |
| O3-Si1-O9 | 113.1(5) | O4-Si2-O14 | 107.8(5) |
| O3-Si1-O7 | 111.7(6) | O8-Si2-O4 | 110.3(5) |
| O3-Si1-O13 | 107.2(5) | O8-Si2-O14 | 108.5(5) |
| O9-Si1-O7 | 113.8(5) | O10-Si2-O8 | 114.4(5) |
| O9-Si1-O13 | 102.5(5) | O10-Si2-O4 | 112.7(5) |
| O7-Si1-O13 | 107.7(5) | O10-Si2-O14 | 102.5(5) |
| Si3-O1 | 1.612(11) | Si4-O12 | 1.617(10) |
| Si3-O11 | 1.626(10) | Si4-O6 | 1.624(9) |
| Si3-O5 | 1.634(9) | Si4-O16 | 1.639(10) |
| Si3-O15 | 1.636(9) | Si4-O2 | 1.649(10) |
| <Si3-O> | 1.627 | <Si4-O> | 1.632 |
| O1-Si3-O11 | 113.4(5) | O6-Si4-O16 | 110.5(5) |
| O1-Si3-O5 | 112.1(5) | O6-Si4-O2 | 110.5(5) |
| O1-Si3-O15 | 106.7(5) | O12-Si4-O6 | 114.5(5) |
| O5-Si3-O15 | 109.9(5) | O12-Si4-O16 | 101.1(5) |
| O11-Si3-O5 | 113.4(5) | O12-Si4-O2 | 111.7(5) |
| O11-Si3-O15 | 100.4(5) | O16-Si4-O2 | 108.0(5) |
| Si5-O23 | 1.598(9) | Si6-O17 | 1.586(10) |
| Si5-O18 | 1.602(10) | Si6-O14 | 1.623(9) |
| Si5-O13 | 1.626(9) | Si6-O24 | 1.631(10) |
| Si5-O26 | 1.633(9) | Si6-O25 | 1.655(10) |
| <Si5-O> | 1.615 | <Si6-O> | 1.624 |
| O13-Si5-O26 | 108.2(5) | O14-Si6-O24 | 109.9(5) |
| O18-Si5-O13 | 106.4(5) | O14-Si6-O25 | 108.8(5) |
| O18-Si5-O26 | 112.1(5) | O17-Si6-O14 | 108.4(5) |
| O23-Si5-O18 | 112.3(5) | O17-Si6-O24 | 112.7(5) |
| O23-Si5-O13 | 109.3(5) | O17-Si6-O25 | 108.5(5) |
| O23-Si5-O26 | 108.5(5) | O24-Si6-O25 | 108.4(5) |
| Si7-O21 | 1.602(9) | Si8-O19 | 1.625(10) |
| Si7-O28 | 1.619(9) | Si8-O22 | 1.632(9) |
| Si7-O20 | 1.630(10) | Si8-O27 | 1.655(10) |
| Si7-O15 | 1.666(9) | Si8-O16 | 1.659(9) |
| <Si7-O> | 1.629 | <Si8-O> | 1.643 |
| O20-Si7-O15 | 104.0(5) | O19-Si8-O22 | 112.2(5) |
| O21-Si7-O28 | 111.8(5) | O19-Si8-O27 | 109.6(5) |
| O21-Si7-O20 | 112.2(5) | O19-Si8-O16 | 106.5(5) |
| O21-Si7-O15 | 108.0(5) | O22-Si8-O27 | 111.8(5) |
| O28-Si7-O20 | 113.2(5) | O22-Si8-O16 | 108.7(5) |
| O28-Si7-O15 | 107.2(5) | O27-Si8-O16 | 107.8(5) |
| Cations | Anions | ||
|---|---|---|---|
| Ca1:Ca2+ | 1.85 | O1:O2− | 1.93 |
| Ca2:Ca2+ | 1.90 | O2:O2− | 1.89 |
| Ca3:Ca2+ | 1.84 | O3:O2− | 1.98 |
| Ca4:Ca2+ | 1.86 | O4:O2− | 1.95 |
| M1:Sn4+ | 3.83 | O5:O2−, (OH)− | 1.70 |
| M1:Sc3+ | 2.58 | O6:O2−, (OH)− | 1.68 |
| M1:Fe3+ | 3.29 | O7:O2−, (OH)− | 1.80 |
| M2:Sn4+ | 3.94 | O8:O2−, (OH)− | 1.76 |
| M2:Sc3+ | 2.65 | O9:O2− | 2.09 |
| M2:Fe3+ | 3.39 | O10:O2− | 2.06 |
| M3:Sn4+ | 3.96 | O11:O2− | 2.12 |
| M3:Sc3+ | 2.67 | O12:O2− | 2.11 |
| M3:Fe3+ | 3.40 | O13:O2− | 2.01 |
| M4:Sn4+ | 3.96 | O14:O2− | 2.01 |
| M4:Sc3+ | 2.67 | O15:O2− | 1.97 |
| M4:Fe3+ | 3.41 | O16:O2− | 1.98 |
| Si1:Si4+ | 4.00 | O17:O2− | 2.04 |
| Si2:Si4+ | 3.92 | O18:O2− | 2.07 |
| Si3:Si4+ | 3.97 | O19:O2− | 1.96 |
| Si4:Si4+ | 3.91 | O20:O2− | 2.03 |
| Si5:Si4+ | 4.10 | O21:O2− | 2.03 |
| Si6:Si4+ | 4.01 | O22:O2− | 2.00 |
| Si7:Si4+ | 3.95 | O23:O2− | 2.09 |
| Si8:Si4+ | 3.81 | O24:O2− | 2.06 |
| O25:(OH)−, O2− | 1.34 | ||
| O26:(OH)−, O2− | 1.41 | ||
| O27:O2−, (OH)− | 1.57 | ||
| O28:O2−, (OH)− | 1.62 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans, R.J.; Gołębiowska, B.; Groat, L.A.; Pieczka, A. Crystal Structure of Kristiansenite from Szklarska Poręba, Southwestern Poland. Minerals 2018, 8, 584. https://doi.org/10.3390/min8120584
Evans RJ, Gołębiowska B, Groat LA, Pieczka A. Crystal Structure of Kristiansenite from Szklarska Poręba, Southwestern Poland. Minerals. 2018; 8(12):584. https://doi.org/10.3390/min8120584
Chicago/Turabian StyleEvans, R. James, Bożena Gołębiowska, Lee A. Groat, and Adam Pieczka. 2018. "Crystal Structure of Kristiansenite from Szklarska Poręba, Southwestern Poland" Minerals 8, no. 12: 584. https://doi.org/10.3390/min8120584
APA StyleEvans, R. J., Gołębiowska, B., Groat, L. A., & Pieczka, A. (2018). Crystal Structure of Kristiansenite from Szklarska Poręba, Southwestern Poland. Minerals, 8(12), 584. https://doi.org/10.3390/min8120584





