Extracellular and Intracellular Biomineralization Induced by Bacillus licheniformis DB1-9 at Different Mg/Ca Molar Ratios
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification and Cultivation of DB1-9 Bacteria
2.2. Biological and Chemical Measurements
2.3. Amino Acid Composition in EPS
2.4. Precipitation Experiments
2.5. Characterization of Precipitates
2.6. Analysis of the Ultrathin Slices of B. licheniformis
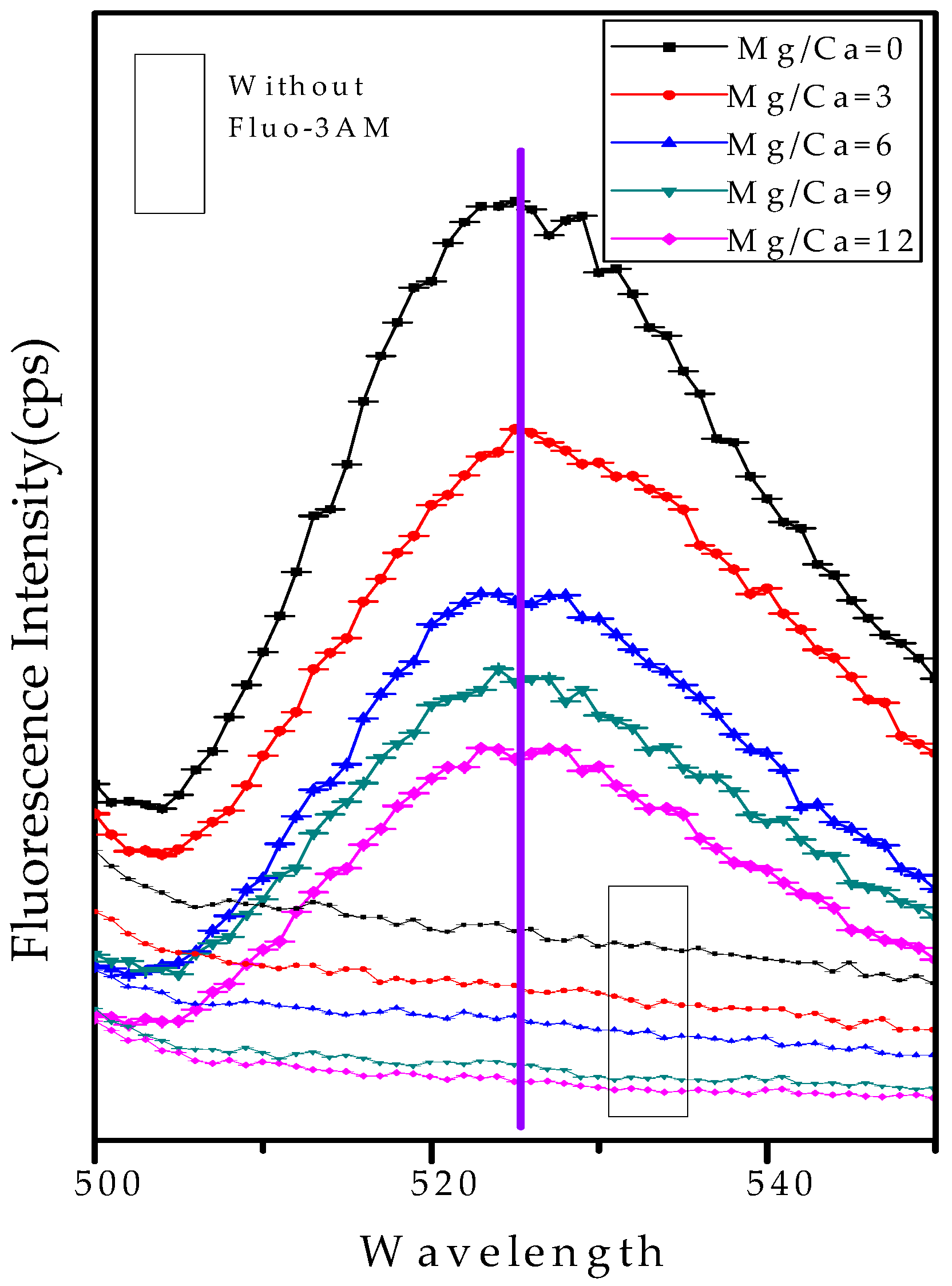
2.7. Analysis of the Intracellular Ca2+ Ions by Fluorescence Intensity
3. Results
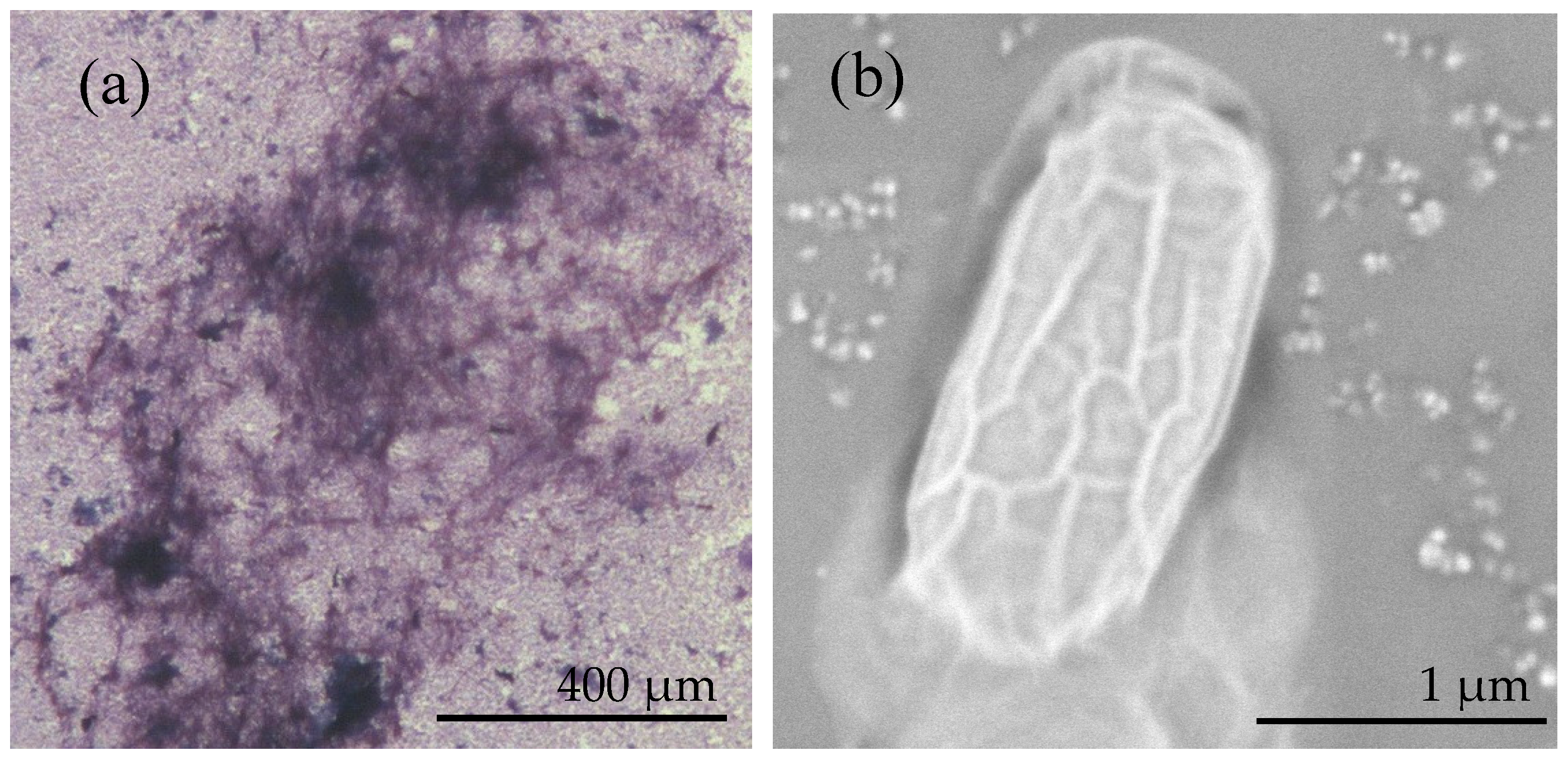
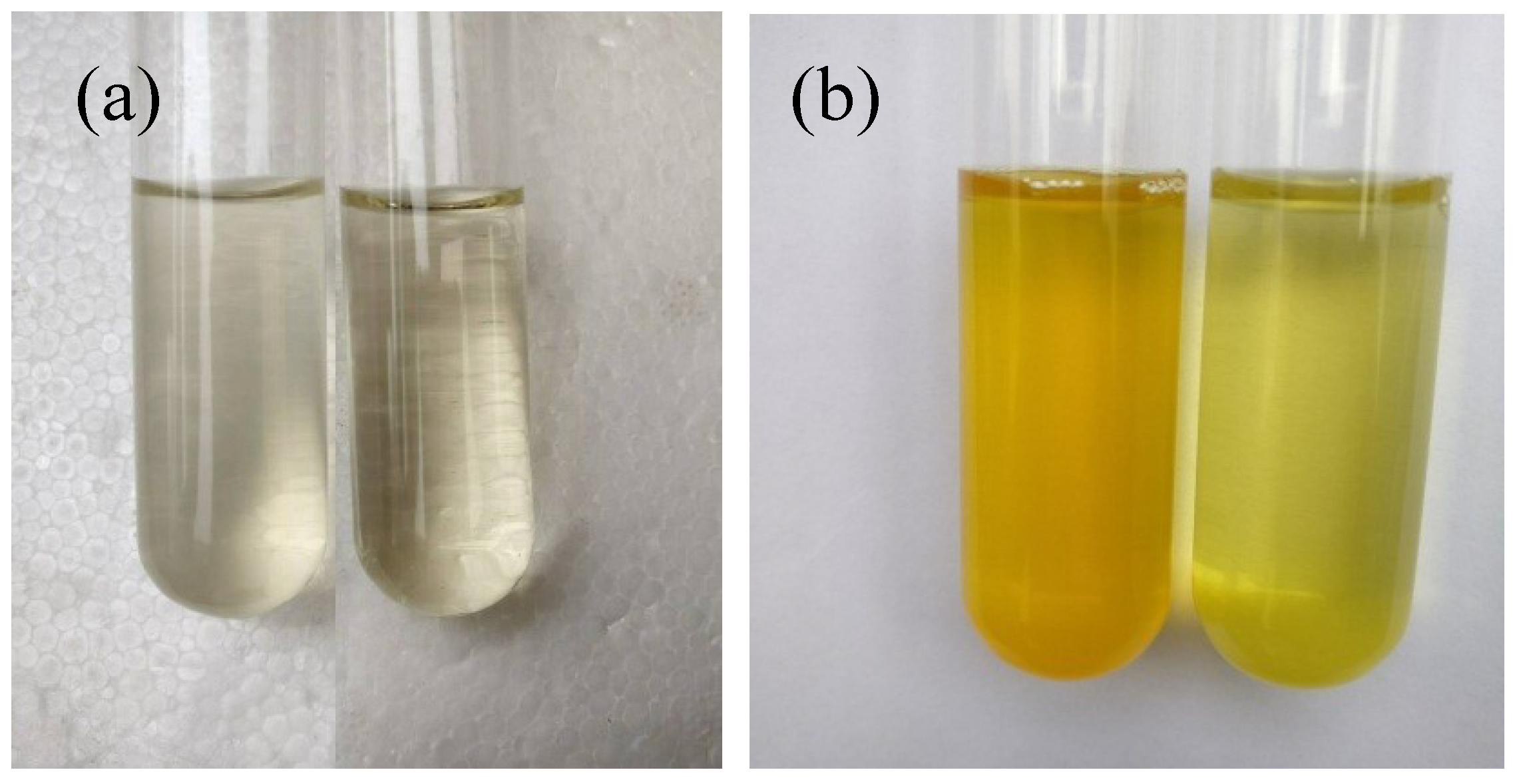
3.1. Identification and Characterization of B. licheniformis DB1-9 Bacteria
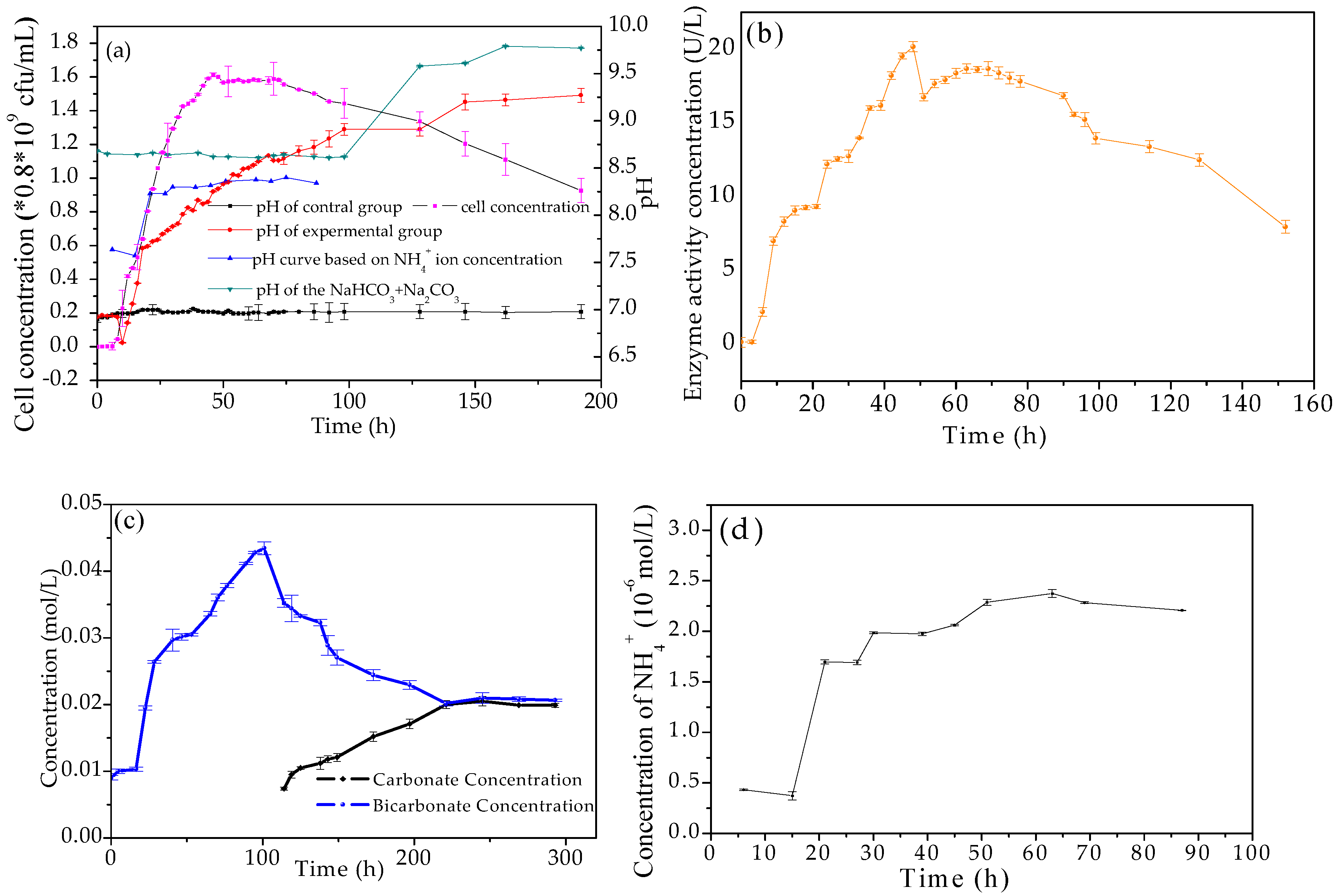
3.2. Evolution of Biological and Chemical Parameters
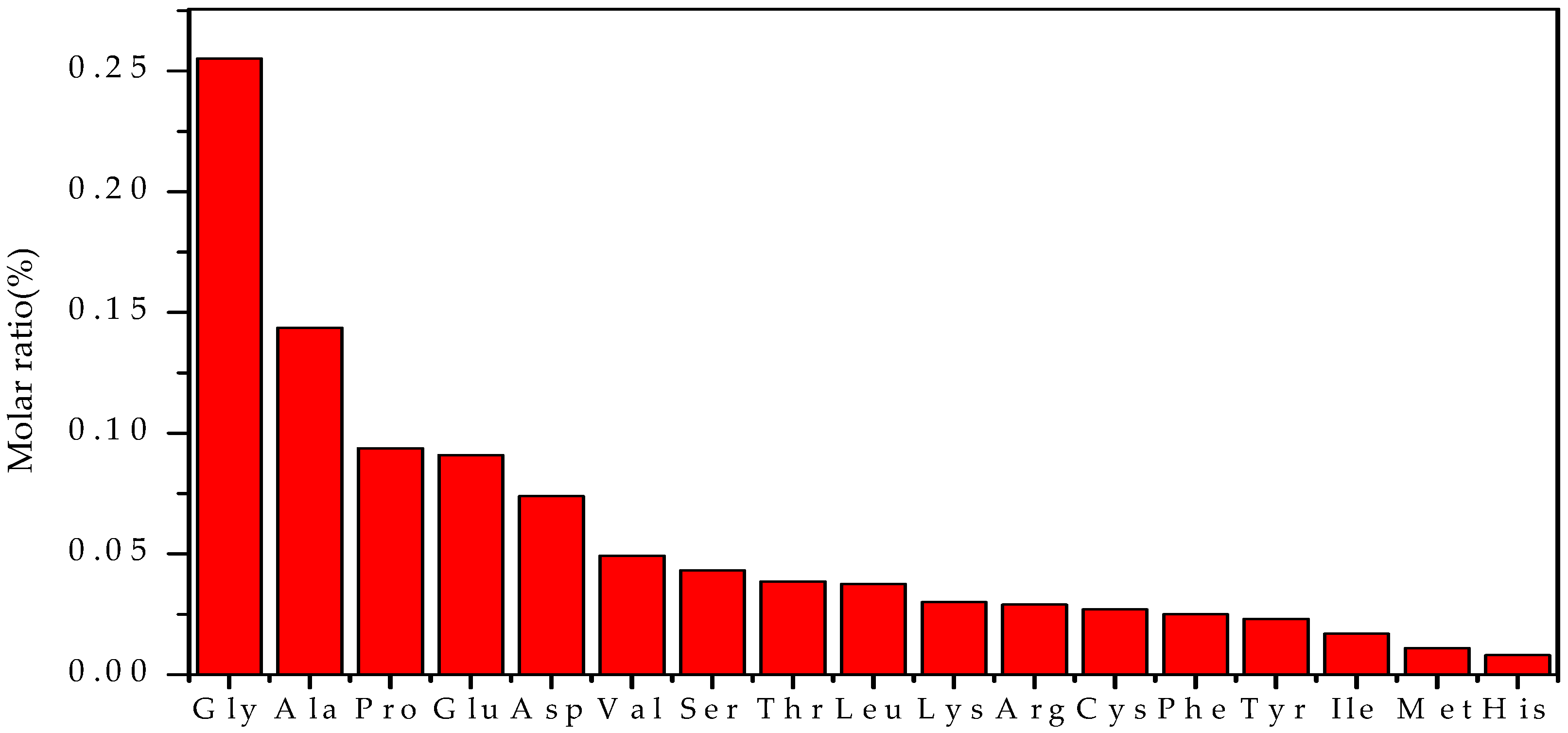
3.3. The Amino Acid Composition of EPS
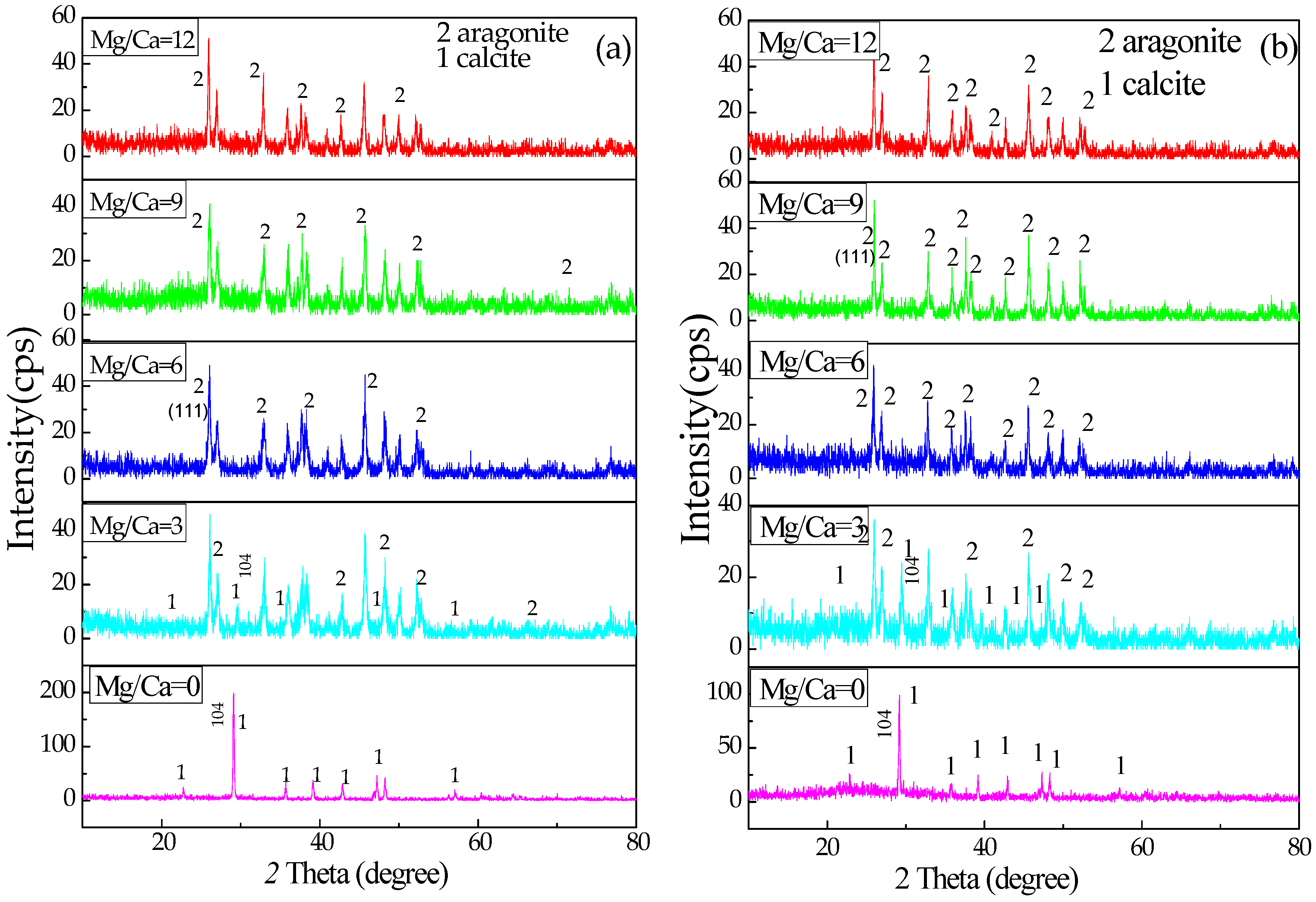
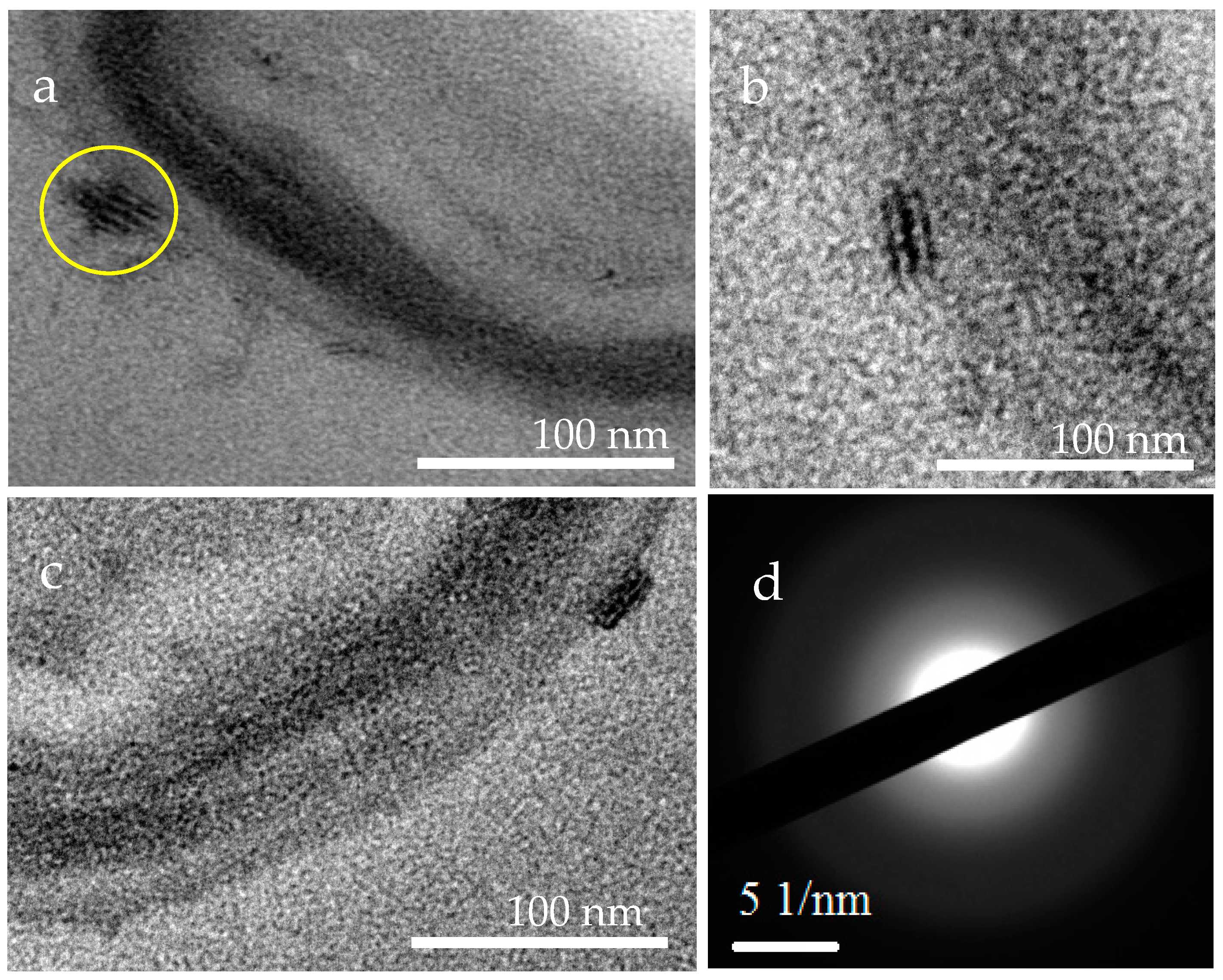
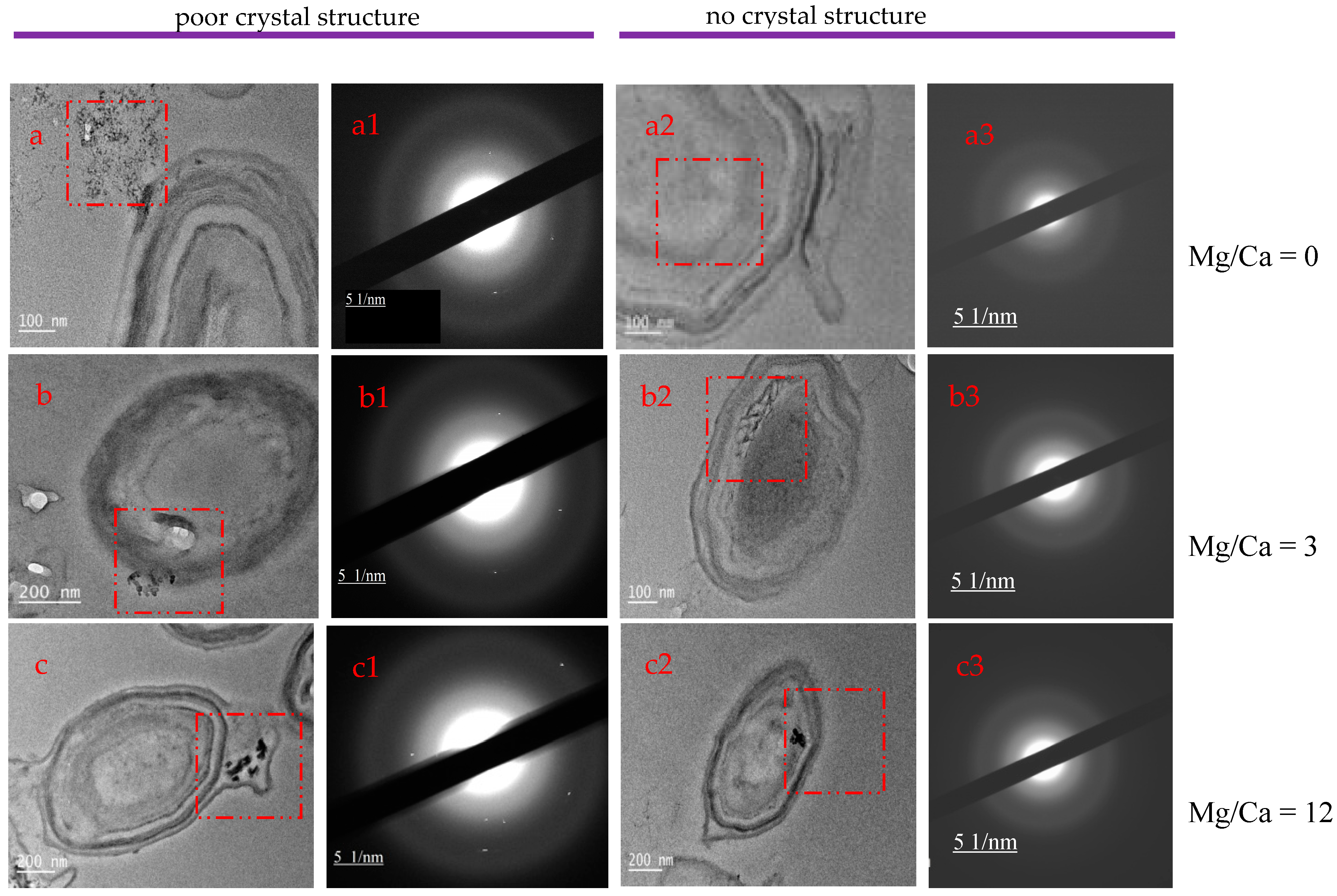
3.4. XRD Analysis of the Minerals Induced by B. licheniformis
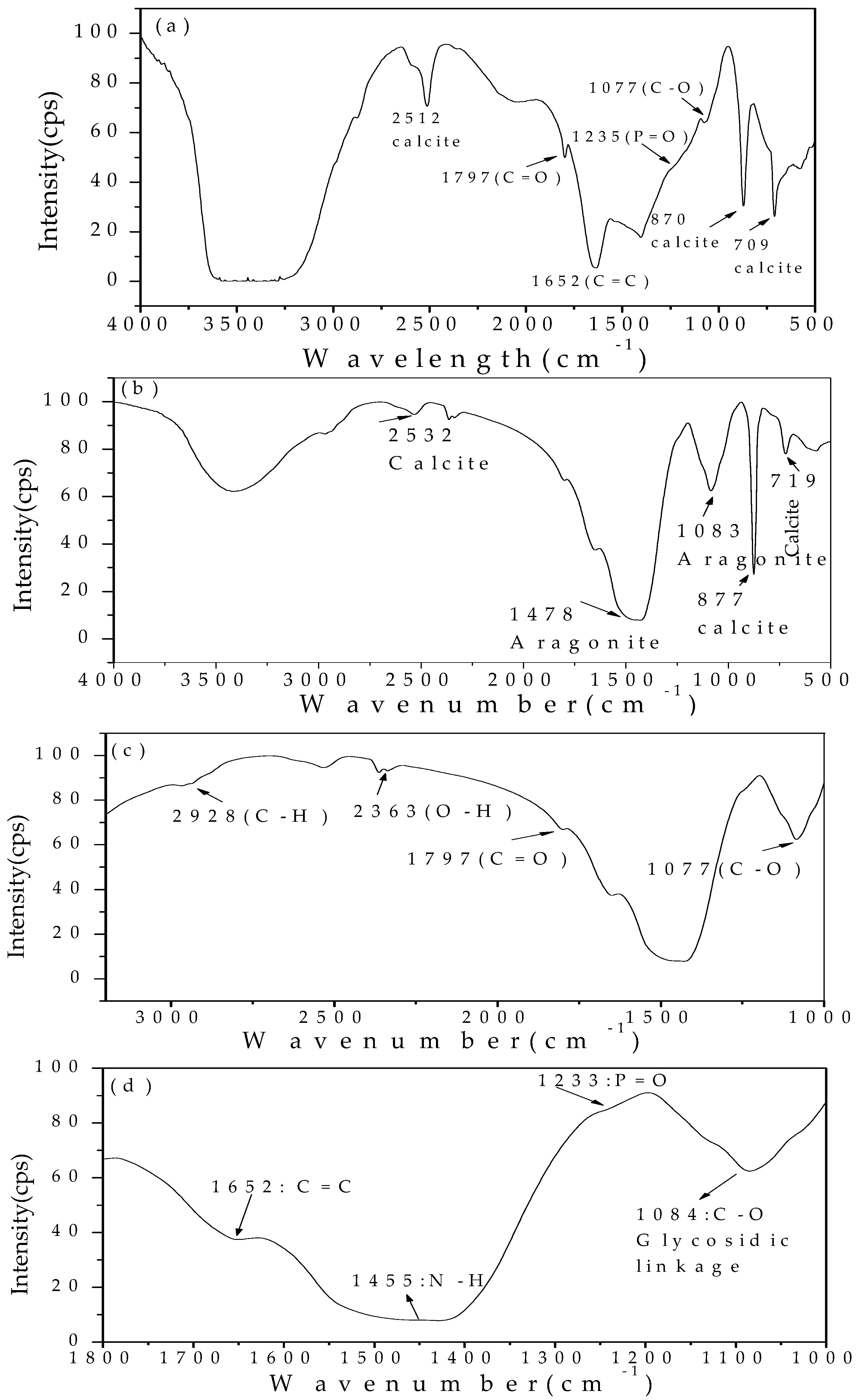
3.5. FTIR Analysis of the Minerals at a Mg/Ca Molar Ratio of 0 and 3 Induced by Bacteria
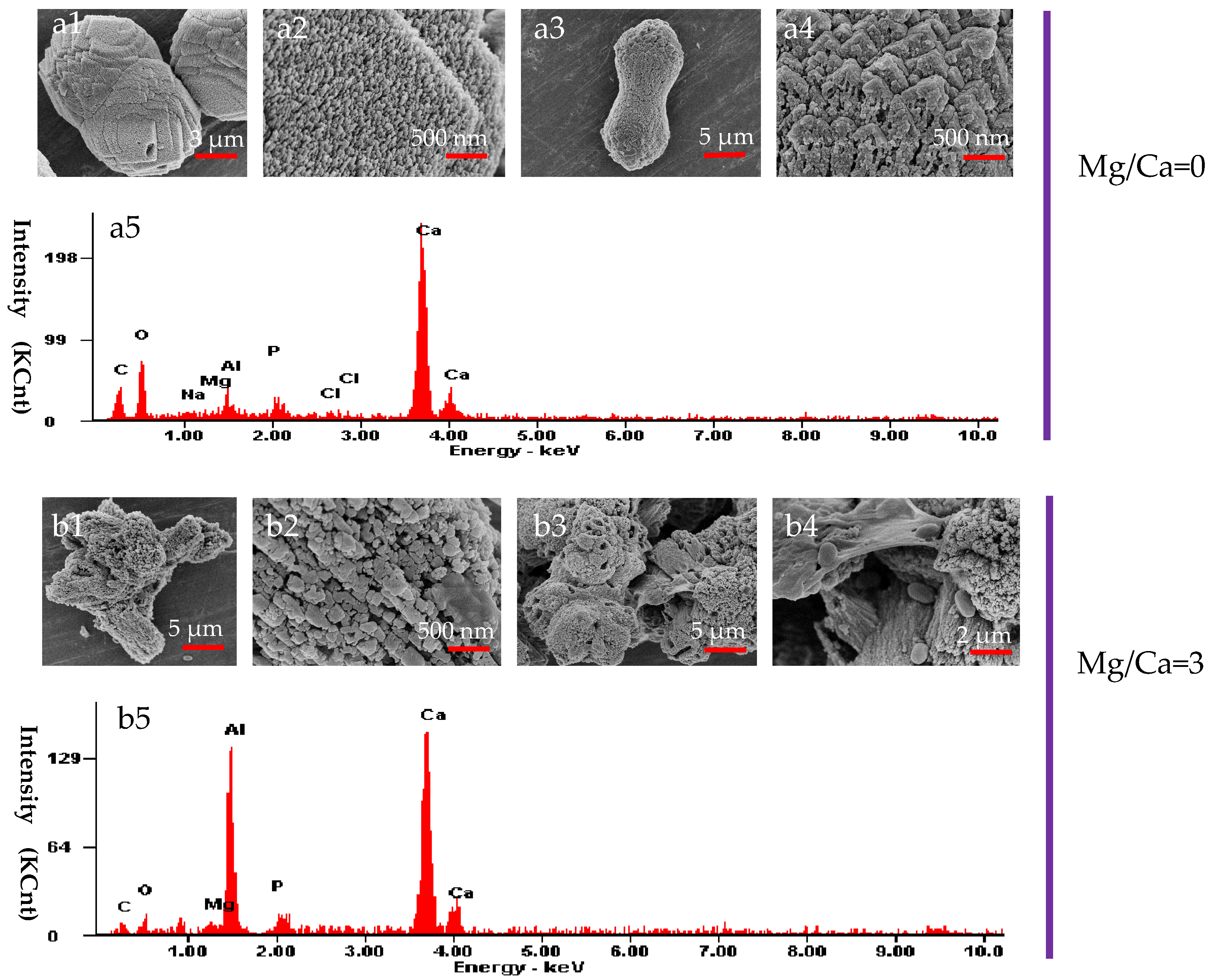
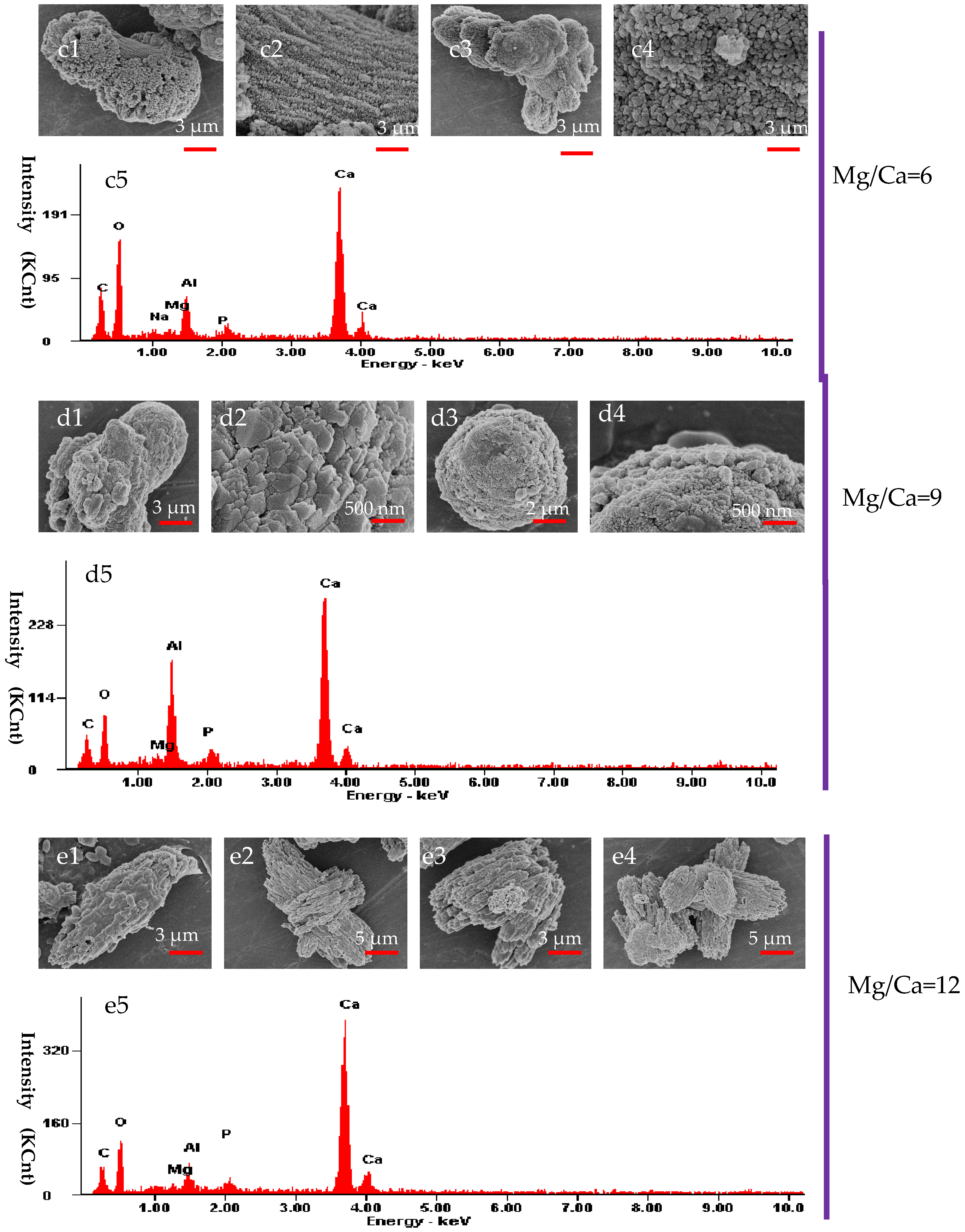
3.6. The Morphology and Elemental Composition of the Minerals Analyzed by SEM and EDX
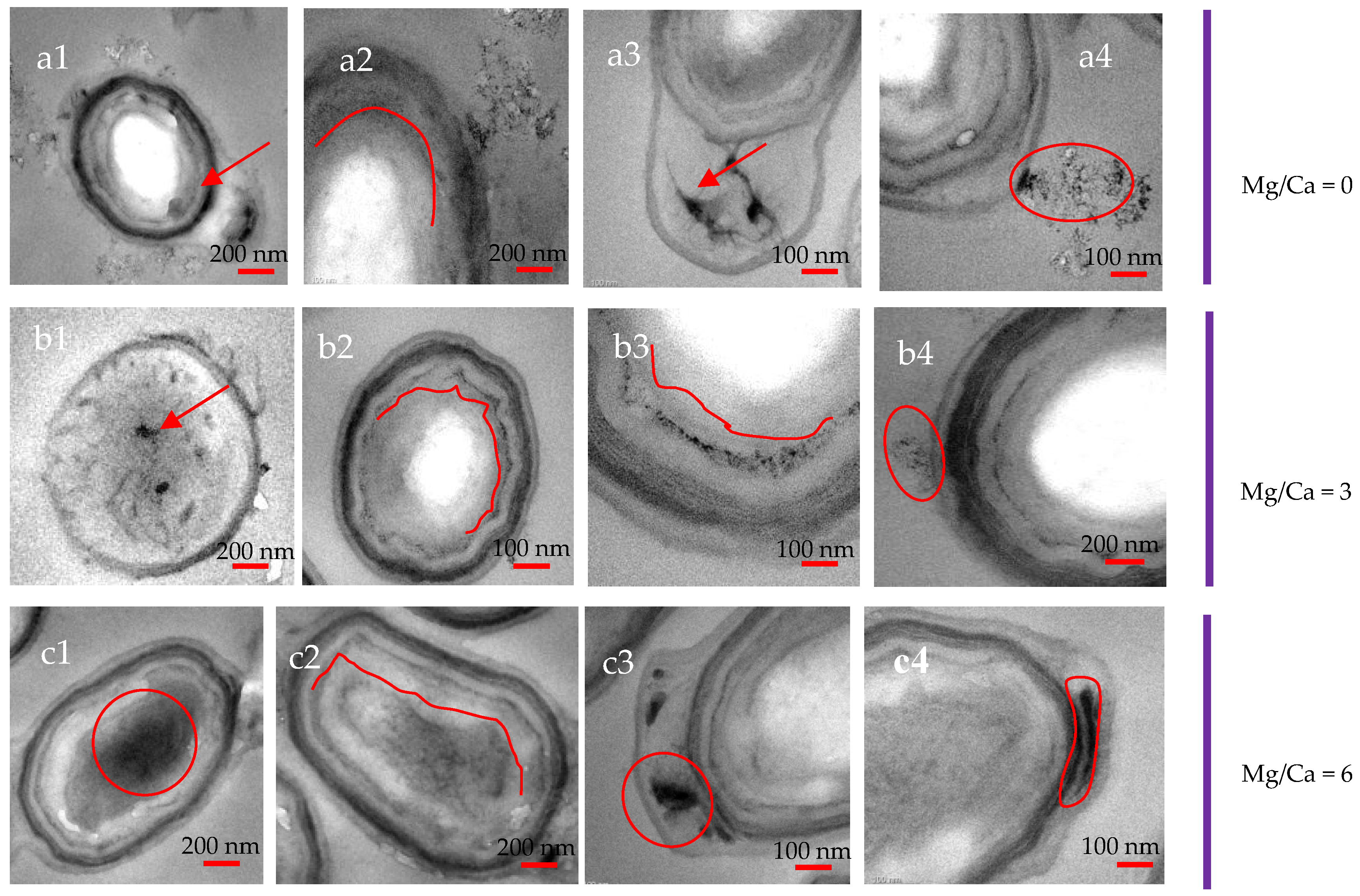
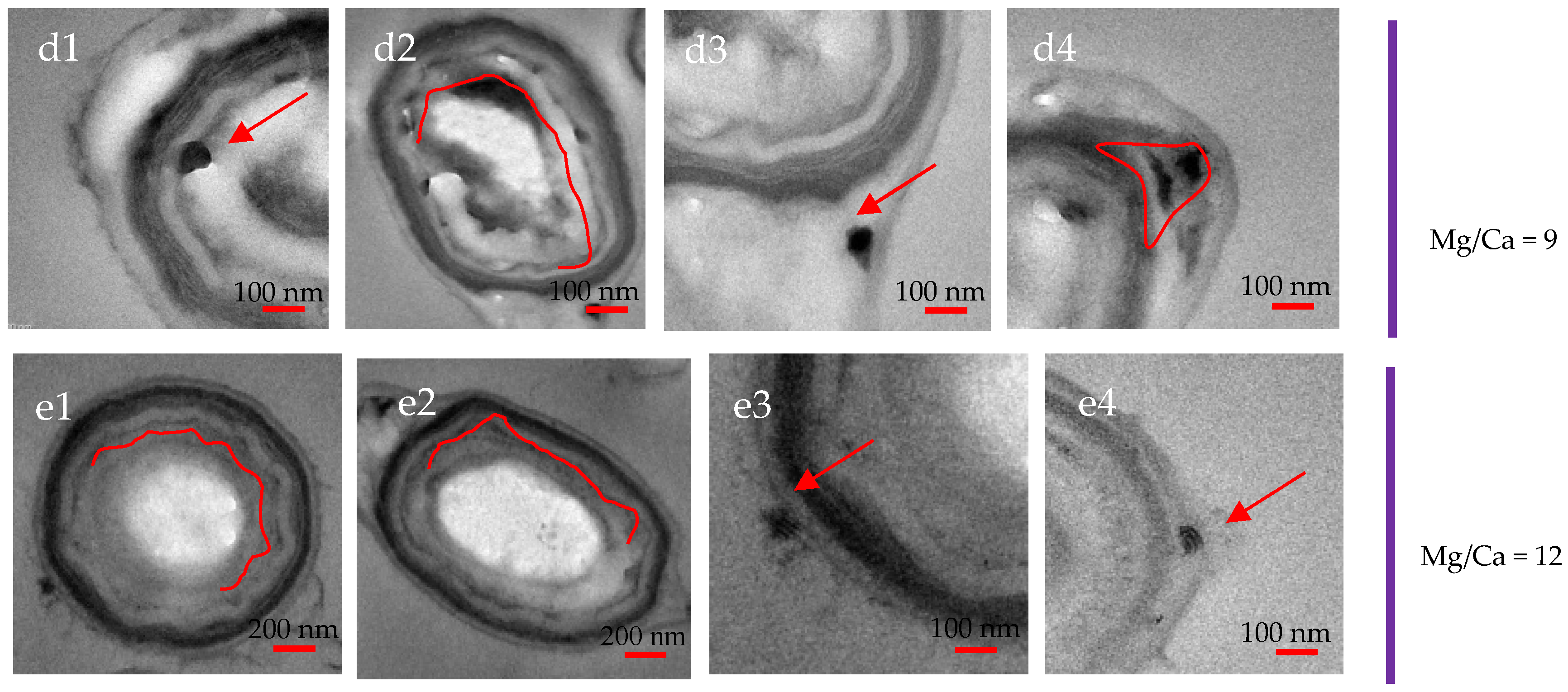
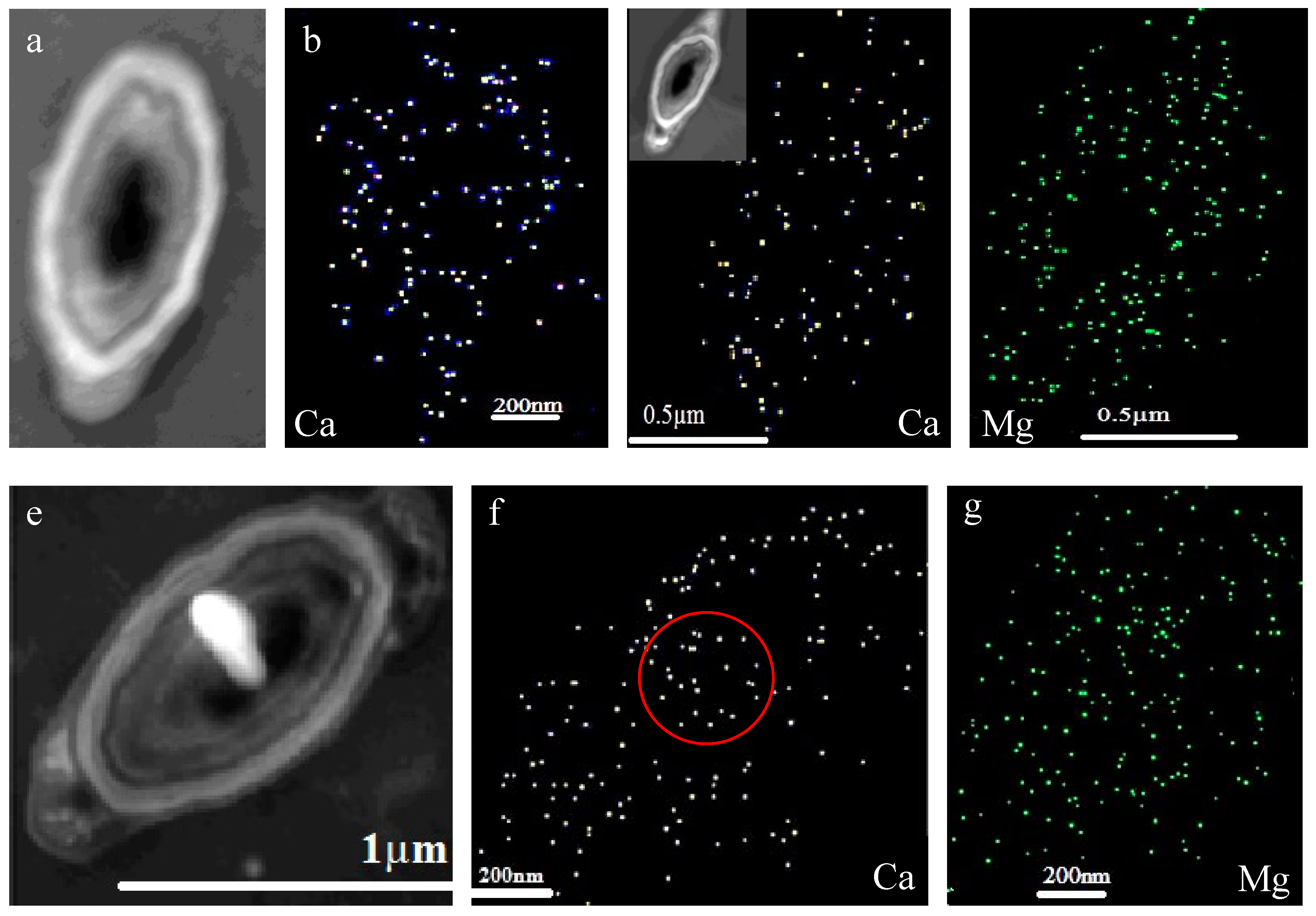
3.7. Intracellular and Extracellular Biomineralization of B. licheniformis
3.8. Ca and Mg Elemental Analysis of B. licheniformis
4. Discussion
4.1. The Mechanism of Extracellular Biomineralization Induced by B. licheniformis
4.2. The Organic Functional Groups
4.3. The Relationship between Mg/Ca Molar Ratio and Calcium Carbonate Minerals
4.4. The Transport Mode of Ca2+ and Mg2+ Ions and the Nucleation Site
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Burne, R.V.; Moore, L.S. Microbialites: Organosedimentary deposits of benthic microbial communities. Palaios 1987, 2, 241–254. [Google Scholar] [CrossRef]
- Riding, R. Microbial carbonates: The geological record of calcified bacterial–algal mats and biofilms. Sedimentology 2010, 47, 179–214. [Google Scholar] [CrossRef]
- Chen, J.T.; VanLoon, A.J.; Han, Z.; Chough, S.K. Funnel-shaped, breccia-filled clastic dykes in the late Cambrian Chaomidian formation (Shandong Province, China). Sediment. Geol. 2009, 221, 1–6. [Google Scholar] [CrossRef]
- Chen, J.T.; Chough, S.K.; Chun, S.S. Limestone pseudoconglomerates in the Late Cambrian Gushan and Chaomidian Formations (Shandong Province, China): Soft-sediment deformation induced by storm-wave loading. Sedimentology 2009, 56, 1174–1195. [Google Scholar] [CrossRef]
- Grotzinger, J.P.; Knoll, A.H. Stromatolites in Precambrian carbonates: evolutionary mileposts or environmental dipsticks? Annu. Rev. Earth Planet. Sci. 1999, 27, 313–358. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, H.J.; Grey, K.; Hickman, A.H.; Thorpe, R.I. Origin of 3.45 Ga coniform stromatolites in Warrawoona Group, Western Australia. Geol. Soc. Am. Bull. 1999, 111, 1256–1262. [Google Scholar] [CrossRef]
- Nutman, A.P.; Bennett, V.C.; Friend, C.R.L.; Kranendonk, M.J.V.; Chivas, A.R. Rapid emergence of life shown by discovery of 3,700-million-year-old microbial structures. Nature 2016, 537, 535. [Google Scholar] [CrossRef]
- Chen, J.T.; Han, Z.Z.; Zhang, X.L.; Fan, A.P.; Yang, R.C. Early diagenetic deformation structures of the Furongianribbon rocks in Shandong Province of China—A new perspective of the genesis of limestone conglomerates. Sci. China Earth Sci. 2010, 53, 241–252. [Google Scholar] [CrossRef]
- Yang, R.C.; Fan, A.P.; Han, Z.Z. Characteristics and genesis of microbial lumps in the Maozhuang Stage (Cambrian Series 2), Shandong Province, China. Sci. China Earth Sci. 2013, 56, 494–503. [Google Scholar] [CrossRef]
- Chen, J.T.; Chough, S.K.; Han, Z.; Lee, J.H. An extensive erosion surface of a strongly deformed limestone bed in theGushan and Chaomidian formations (late middle Cambrian to Furongian), Shandong Province, China: Sequence-stratigraphic implications. Sediment. Geol. 2011, 233, 129–149. [Google Scholar] [CrossRef]
- Brasier, M.D.; Jonathan, A.; Martin, S.; David, W. Changing the picture of Earth’s earliest fossils (3.5-1.9 Ga) with new approaches and new discoveries. Proc. Natl. Acad. Sci. USA 2015, 112, 4859–4864. [Google Scholar] [CrossRef] [PubMed]
- Kranendonk, M.J.V.; Philippot, P.; Lepot, K.; Bodorkos, S.; Pirajno, F. Geological setting of Earth’s oldest fossils in the ca. 3.5 Ga Dresser Formation, Pilbara Craton, Western Australia. Precambrian Res. 2008, 167, 93–124. [Google Scholar] [CrossRef]
- Braissant, O.; Decho, A.W.; Dupraz, C.; Glunk, C.; Przekop, K.M.; Visscher, P.T. Exopolymeric Substances of Sulfate-Reducing Bacteria: Interactions with Calcium at Alkaline pH and Implication for Formation of Carbonate Minerals. Geobiology 2010, 5, 401–411. [Google Scholar] [CrossRef]
- Decho, A.W. Overview of biopolymer-induced mineralization: What goes on in biofilms? Ecol. Eng. 2010, 36, 137–144. [Google Scholar] [CrossRef]
- Dupraz, C.; Reid, R.P.; Braissant, O.; Decho, A.W.; Norman, R.S.; Visscher, P.T. Processes of carbonate precipitation in modern microbialmats. Earth Sci. Rev. 2009, 96, 141–162. [Google Scholar] [CrossRef]
- Vasconcelos, C.; Warthmann, R.; Mckenzie, J.A.; Visscher, P.T.; Bittermann, A.G.; Lith, Y.V. Lithifying microbial mats in Lagoa Vermelha, Brazil: Modern Precambrian relics? Sediment. Geol. 2006, 185, 175–183. [Google Scholar] [CrossRef]
- Han, C.; Jiang, Z.; Han, M. The lithofacies and reservoir characteristics of the Upper Ordovician and Lower Silurian black shale in the Southern Sichuan Basin and its periphery, China. Mar. Pet. Geol. 2016, 75, 181–191. [Google Scholar] [CrossRef]
- Liu, X.H.; Luo, J.L. Diagenesis and diagenetic facies of gas reservoirs in the Upper Paleozoic 8th member of Shihezi Fm and 2nd member of Shanxi Fm in Yulin-Shenmu area, Ordos basin. Oil Gas Geol. 2006, 2, 010. [Google Scholar]
- Pacton, M.; Wacey, D.; Corinaldesi, C.; Tangherlini, M.; Kilburn, M.R.; Gorin, G.E.; Danovaro, R.; Vasconcelos, C. Viruses as new agents of organomineralization in the geological record. Nat. Commun. 2014, 5, 4298. [Google Scholar] [CrossRef]
- Perri, E.; Tucker, M.E.; Słowakiewicz, M.; Whitaker, F.; Bowen, L.; Perrotta, I.D. Carbonate and silicate biomineralization in a hypersaline microbial mat (Mesaieed sabkha, Qatar): Roles of bacteria, extracellular polymeric substances and viruses. Sedimentology 2018, 65, 1213–1245. [Google Scholar] [CrossRef]
- Planavsky, N.; Reid, R.P.; Lyons, T.W.; Myshrall, K.L.; Visscher, P.T. Formation and diagenesis of modern marine calcified cyanobacteria. Geobiology 2010, 7, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Georgelin, T.; Jaber, M.; Lambert, J.F.; Benzerara, K. In vitro synthesis of amorphous Mg-, Ca-, Sr- and Ba-carbonates: What do we learn about intracellular calcification by cyanobacteria? Geochim. Cosmochim. Acta 2015, 161, 36–49. [Google Scholar] [CrossRef]
- Han, Z.; Meng, R.; Yan, H.; Zhao, H.; Han, M.; Zhao, Y.; Sun, B.; Sun, Y.; Wang, J.; Zhuang, D. Calcium carbonate precipitation by Synechocystis sp. PCC6803 at different Mg/Ca molar ratios under the laboratory condition. Carbonates Evaporites 2017, 32, 561–575. [Google Scholar] [CrossRef]
- Sánchez-Román, M.; Romanek, C.S.; Fernández-Remolar, D.C.; Sánchez-Navas, A.; Mckenzie, J.A.; Pibernat, R.A.; Vasconcelos, C. Aerobic biomineralization of Mg-rich carbonates: Implications for natural environments. Chem. Geol. 2011, 281, 143–150. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, H.; Yao, Y.; Duan, Y. High salinity facilitates dolomite precipitation mediated by Haloferax volcanii DS52. Earth Planet. Sci. Lett. 2017, 472, 197–205. [Google Scholar] [CrossRef]
- Han, Z.; Li, D.; Zhao, H.; Yan, H.; Li, P. Precipitation of Carbonate Minerals Induced by the Halophilic Chromohalobacter Israelensis under High Salt Concentrations: Implications for Natural Environments. Minerals 2017, 7, 95. [Google Scholar] [CrossRef]
- Rivadeneyra, M.A.; Párraga, J.; Delgado, R.; Ramos-Cormenzana, A.; Delgado, G. Biomineralization of carbonates by Halobacillus trueperi in solid and liquid media with different salinities. FEMS Microbiol. Ecol. 2004, 48, 39–46. [Google Scholar] [CrossRef]
- Deng, S.; Dong, H.; Lv, G.; Jiang, H.; Yu, B.; Bishop, M.E. Microbial dolomite precipitation using sulfate reducing and halophilic bacteria: Results from Qinghai Lake, Tibetan Plateau, NW China. Chem. Geol. 2010, 278, 151–159. [Google Scholar] [CrossRef]
- Han, Z.; Zhao, Y.; Yan, H.; Zhao, H.; Han, M.; Sun, B.; Sun, X.; Hou, F.; Sun, H.; Han, L. Struvite Precipitation Induced by a Novel Sulfate-Reducing Bacterium Acinetobacter calcoaceticus SRB4 Isolated from River Sediment. Geomicrobiol. J. 2015, 32, 868–877. [Google Scholar] [CrossRef]
- Bontognali, T.R.R.; Vasconcelos, C.; Warthmann, R.J.; Bernasconi, S.M.; Dupraz, C.; Strohmenger, C.J.; Mckenzie, J.A. Dolomite formation within microbial mats in the coastal sabkha of Abu Dhabi (United Arab Emirates). Sedimentology 2010, 57, 824–844. [Google Scholar] [CrossRef]
- Kenward, P.A.; Goldstein, R.H.; González, L.A.; Roberts, J.A. Precipitation of low-temperature dolomite from an anaerobic microbial consortium: The role of methanogenic Archaea. Geobiology 2010, 7, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Román, M.; Vasconcelos, C.; Warthmann, R.; Rivadeneyra, M.; Mckenzie, J.A. Microbial Dolomite Precipitation under Aerobic Conditions: Results from Brejo do Espinho Lagoon (Brazil) and Culture Experiments; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 167–178. [Google Scholar]
- Lv, J.J.; Ma, F.; Li, F.C.; Zhang, C.H.; Chen, J.N. Vaterite induced by Lysinibacillus sp. GW-2 strain and its stability. J. Struct. Biol. 2017, 200, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Gonzalezmunoz, M.T.; Rodrigueznavarro, C.; Martinezruiz, F.; Arias, J.M.; Merroun, M.L.; Rodriguezgallego, M. Bacterial biomineralization: New insights from Myxococcus-induced mineral precipitation. Geol. Soc. Lond. Spec. Publ. 2010, 336, 31–50. [Google Scholar] [CrossRef]
- Buczynski, C.; Chafetz, H.S. Habit of bacterially induced precipitates of calcium carbonate and the influence of medium viscosity on mineralogy. J. Sediment. Res. 1991, 61, 226–233. [Google Scholar] [CrossRef]
- Zhuang, D.; Yan, H.; Tucker, M.E.; Zhao, H.; Han, Z.; Zhao, Y.; Sun, B.; Li, D.; Pan, J.; Zhao, Y. Calcite precipitation induced by Bacillus cereus MRR2 cultured at different Ca2+ concentrations: Further insights into biotic and abiotic calcite. Chem. Geol. 2018, 500, 64–87. [Google Scholar] [CrossRef]
- Grasby, S.E. Naturally precipitating vaterite (μ-CaCO3) spheres: Unusual carbonates formed in an extreme environment. Geochim. Cosmochim. Acta 2003, 67, 1659–1666. [Google Scholar] [CrossRef]
- Teng, H.H.; Dove, P.M.; Deyoreo, J.J. Reversed calcite morphologies induced by microscopic growth kinetics: Insight into biomineralization. Geochim. Cosmochim. Acta 1999, 63, 2507–2512. [Google Scholar] [CrossRef]
- Addadi, L.; Weiner, S. Interactions between Acidic Macromolecules and Structured Crystal Surfaces. Stereochemistry and Biomineralization. Mol. Cryst. 1986, 134, 305–322. [Google Scholar] [CrossRef]
- Kalmar, L.; Homola, D.; Varga, G. Structural disorder in proteins brings order to crystal growth in biomineralization. Bone 2012, 51, 528–534. [Google Scholar] [CrossRef]
- Marin, F.; Luquet, G. Molluscan biomineralization: The proteinaceous shell constituents of Pinna nobilis L. Mater. Sci. Eng. C 2005, 25, 105–111. [Google Scholar] [CrossRef]
- Couradeau, E.; Benzerara, K.; Gérard, E.; Moreira, D.; Bernard, S. An early-branching microbialite cyanobacterium forms intracellular carbonates. Science 2012, 336, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Benzerara, K.; Skouripanet, F.; Li, J.; Férard, C.; Gugger, M.; Laurent, T.; Couradeau, E.; Ragon, M.; Cosmidis, J.; Menguy, N. Intracellular Ca-carbonate biomineralization is widespread in cyanobacteria. Proc. Natl. Acad. Sci. USA 2014, 111, 10933–10938. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Niu, M.; Yuan, S.; Teng, H. Durable antimicrobial finishing of cellulose with QSA silicone by supercritical adsorption. Appl. Surf. Sci. 2013, 264, 171–175. [Google Scholar] [CrossRef]
- Martignier, A.; Pacton, M.; Filella, M.; Jaquet, J.M.; Barja, F.; Pollok, K.; Langenhorst, F.; Lavigne, S. Intracellular amorphous carbonates uncover a new biomineralization process in eukaryotes. Geobiology 2016, 15, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhu, J.; Sun, T.; Zhou, H.; Shao, Q.; Li, G.; Liu, X.; Yin, Y. Porphyrin nanotubes composed of highly ordered molecular arrays prepared by anodic aluminum template method. RSC Adv. 2013, 3, 2765–2769. [Google Scholar] [CrossRef]
- Martignier, A.; Filella, M.; Pollok, K.; Melkonian, M.; Bensimon, M.; Barja, F.; Langenhorst, F.; Jaquet, J.M.; Ariztegui, D. Marine and freshwater micropearls: Biomineralization producing strontium-rich amorphous calcium carbonate inclusions is widespread in the genus Tetraselmis (Chlorophyta). Biogeosciences 2018, 15, 6591–6605. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, X.; Chi, N.; Han, M.; Woo, J.; Lee, H.S.; Chen, J. Cambrian oncoids and other microbial-related grains on the North China Platform. Carbonates Evaporites 2015, 30, 373–386. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, H.; Ding, C.; Niu, H.; Zhang, T.; Wang, N.; Zhang, Q.; Liu, D.; Han, S.; Yu, H. Effectiveness of crystallitic carbon from coal as milling aid and for hydrogen storage during milling with magnesium. Fuel 2013, 109, 68–75. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, H.; Ran, W.; Wang, N.; Han, Z.; Zhang, Q.; Zhang, X.; Niu, H.; Yu, H.; Liu, D. Effect of carbon from anthracite coal on decomposition kinetics of magnesium hydride. J. Alloys Compd. 2014, 592, 231–237. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, W.; Wang, J.; Lu, J.; Yang, J. Hollow mesoporous silica spheres synthesized with cationic and anionic mixed surfactant as templates. Mater. Lett. 2015, 142, 269–272. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Zhao, Q.; Wang, Y.H.; Ge, S.S.; Shao, Q. A New Layered Six-Connected Network Based on Tetranuclear Copper(II) Cores. J. Chem. Crystallogr. 2012, 42, 706–710. [Google Scholar] [CrossRef]
- Zhao, B.; Shao, Q.; Hao, L.; Zhang, L.; Liu, Z.; Zhang, B.; Ge, S.; Guo, Z. Yeast-template synthesized Fe-doped cerium oxide hollow microspheres for visible photodegradation of acid orange 7. J. Colloid Interface Sci. 2017, 511, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Dong, S.; Zhang, Y.; Sun, B.; Zhang, C. Thermogravimetry study of the pyrolytic characteristics and kinetics of macro-algae Macrocystis pyrifera residue. J. Therm. Anal. Calorim. 2013, 111, 1685–1690. [Google Scholar] [CrossRef]
- Hu, X.; Cheng, W.; Nie, W.; Shao, Z. Synthesis and characterization of a temperature-sensitive hydrogel based on sodium alginate and N-isopropylacrylamide. Polym. Adv. Technol. 2015, 26, 1340–1345. [Google Scholar] [CrossRef]
- Hu, X.; Cheng, W.; Shao, Z. Novel authigenic gas foaming hydrogels for preventing coal spontaneous combustion. e-Polymers 2015, 15, 361–368. [Google Scholar] [CrossRef]
- Wu, T.; Shao, Q.; Ge, S.; Bao, L.; Liu, Q. The facile preparation of novel magnetic zirconia composites with the aid of carboxymethyl chitosan and their efficient removal of dye. RSC Adv. 2016, 6, 58020–58027. [Google Scholar] [CrossRef]
- Huang, J.; Cao, Y.; Shao, Q.; Peng, X.; Guo, Z. Magnetic Nanocarbon Adsorbents with Enhanced Hexavalent Chromium Removal: Morphology Dependence of Fibrillar vs Particulate Structures. Ind. Eng. Chem. Res. 2017, 56, 10689–10701. [Google Scholar] [CrossRef]
- Wu, Z.; Gao, S.; Chen, L.; Jiang, D.; Shao, Q.; Zhang, B.; Zhai, Z.; Wang, C.; Zhao, M.; Ma, Y. Electrically Insulated Epoxy Nanocomposites Reinforced with Synergistic Core–Shell SiO2@MWCNTs and Montmorillonite Bifillers. Macromol. Chem. Phys. 2017, 218, 1700357. [Google Scholar] [CrossRef]
- Zhu, W.; Ge, S.; Shao, Q. Adsorption properties of ZrO2 hollow microboxes prepared using CaCO3 cubes as templates. RSC Adv. 2016, 6, 81736–81743. [Google Scholar] [CrossRef]
- Zhao, J.; Ge, S.; Liu, L.; Shao, Q.; Mai, X.; Zhao, C.X.; Hao, L.; Wu, T.; Yu, Z.; Guo, Z. Microwave Solvothermal Fabrication of Zirconia Hollow Microspheres with Different Morphologies Using Pollen Templates and Their Dye Adsorption Removal. Ind. Eng. Chem. Res. 2017, 57, 231–241. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Jia, Q.Y.; Zhu, J.Q.; Shao, Q.; Fan, J.F.; Wang, D.M.; Yin, Y.S. Highly ordered arrangement of meso-tetrakis (4-aminophenyl) porphyrin in self-assembled nanoaggregates via hydrogen bonding. Chin. Chem. Lett. 2014, 25, 752–756. [Google Scholar] [CrossRef]
- Ge, S.; Zhu, W.; Shao, Q. Fabrication and characterization of hollow zirconia microspheres using calcium carbonate as template. Zeitschrift für Physikalische Chemie 2016, 230, 1617–1628. [Google Scholar] [CrossRef]
- Liu, X.; Shao, X.Y.; Fang, G.B. Preparation and properties of chemically reduced graphene oxide/copolymer-polyamide nanocomposites. e-Polymers 2017, 17, 3–14. [Google Scholar] [CrossRef]
- Tian, J.; Shao, Q.; Dong, X.; Zheng, J.; Pan, D.; Zhang, X.; Cao, H.; Hao, L.; Liu, J.; Mai, X. Bio-template synthesized NiO/C hollow microspheres with enhanced Li-ion battery electrochemical performance. Electrochim. Acta 2018, 261, 236–245. [Google Scholar] [CrossRef]
- Ge, S.; Yang, X.; Shao, Q.; Liu, Q.; Wang, T.; Wang, L.; Wang, X. Self-assembled flower-like antimony trioxide microstructures with high infrared reflectance performance. J. Solid State Chem. 2013, 200, 136–142. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, Q.; Li, Y. CdCl2·H2O nanorods oriented parallel on the langmuir film of (Phthalocyaninato) [Tetrakis(4-pyridyl)porphyrinato] cerium complex. CrystEngComm 2012, 14, 1105–1110. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, Y.; Hui, L.; Zhu, R.; Qian, S.; Yang, S.; Xu, J. NiO nanoparticles modified with 5,10,15,20-tetrakis(4-carboxyl pheyl)-porphyrin: Promising peroxidase mimetics for H2O2 and glucose detection. Biosens. Bioelectron. 2015, 64, 147–153. [Google Scholar] [CrossRef]
- He, M.; Jin, H.; Zhang, L.; Jiang, H.; Yang, T.; Cui, H.; Fossard, F.; Wagner, J.B. Environmental transmission electron microscopy investigations of Pt-Fe2O3 nanoparticles for nucleating carbon nanotubes. Carbon 2016, 110, 243–248. [Google Scholar] [CrossRef]
- Zhao, H.; Yan, H.; Zhang, C.; Sun, B.; Zhang, Y.; Dong, S.; Xue, Y.; Qin, S. Thermogravimetry study of pyrolytic characteristics and kinetics of the giant wetland plant Phragmites australis. J. Therm. Anal. Calorim. 2012, 110, 611–617. [Google Scholar] [CrossRef]
- Tian, B.; Qiao, Y.Y.; Tian, Y.Y.; Xie, K.C.; Liu, Q.; Zhou, H.F. FTIR study on structural changes of different–rank coals caused by single/multiple extraction with cyclohexanone and NMP/CS 2 mixed solvent. Fuel Process. Technol. 2016, 154, 210–218. [Google Scholar] [CrossRef]
- Wang, J.; Chen, D.; Xu, Y. Influence of the crystal texture on Raman spectroscopy of the AlN films prepared by pulse laser deposition. J. Spectrosc. 2012. [Google Scholar] [CrossRef]
- Scozzafava, A.; Supuran, C.T. Hydroxyurea is a carbonic anhydrase inhibitor. Bioorg. Med. Chem. 2003, 11, 2241–2246. [Google Scholar] [CrossRef]
- Lindskog, S. Structure and mechanism of carbonic anhydrase. Pharmacol. Ther. 1997, 74, 1–20. [Google Scholar] [CrossRef]
- Hermann, G.J.; Thatcher, J.W.; Mills, J.P. Mitochondrial fusion in yeast requires the transmembrane GTPase Fzo1p. J. Cell Biol. 1998, 143, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Aizenberg, J.; Lambert, G.; Weiner, S.; Addadi, L. Factors involved in the formation of amorphous and crystalline calcium carbonate: A study of an ascidian skeleton. J. Am. Chem. Soc. 2002, 124, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Albeck, S.; Addadi, L.; Weiner, S. Regulation of calcite crystal morphology by intracrystalline acidic proteins and glycoproteins. Connect. Tissue Res. 1996, 35, 419–424. [Google Scholar] [CrossRef]
- Yan, H.; Han, Z.; Zhao, H. Characterization of calcium deposition induced by Synechocystis sp. PCC6803 in BG11 culture medium. Chin. J. Oceanol. Limnol. 2014, 32, 503–510. [Google Scholar] [CrossRef]
- Tourney, J.; Ngwenya, B.T. Bacterial extracellular polymeric substances (EPS) mediate CaCO3 morphology and polymorphism. Chem. Geol. 2009, 262, 138–146. [Google Scholar] [CrossRef]
- Wei, J.; Anuradha, S.; Bongkeun, S.; Ward, B.B.; Beveridge, T.J.; Myneni, S.C.B. Elucidation of functional groups on gram-positive and gram-negative bacterial surfaces using infrared spectroscopy. Langmuir 2004, 20, 11433–11442. [Google Scholar] [CrossRef]
- Lev, S.; Moreno, H.; Martinez, R.; Canoll, P.; Peles, E.; Musacchio, J.M.; Plowman, G.D. Protein tyrosine kinase PYK2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature 1995, 376, 737. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Zhao, Y.; Yan, H. The Characterization of Intracellular and Extracellular Biomineralization Induced by Synechocystis sp. PCC6803 Cultured under Low Mg/Ca Ratios Conditions. Geomicrobiology 2017, 34, 362–373. [Google Scholar] [CrossRef]
- Lowenstein, T.K.; Timofeeff, M.N.; Brennan, S.T.; Hardie, L.A.; Demicco, R.V. Oscillations in Phanerozoic seawater chemistry: Evidence from fluid inclusions. Science 2001, 294, 1086–1088. [Google Scholar] [CrossRef] [PubMed]
- Ries, J.B. Review: Geological and experimental evidence for secular variation in seawater Mg/Ca (calcite-aragonite seas) and its effects on marine biological calcification. Biogeosciences 2010, 7, 2795–2849. [Google Scholar] [CrossRef]
- Ries, J.B.; Anderson, M.A.; Hill, R.T. Seawater Mg/Ca controls polymorph mineralogy of microbial CaCO3: A potential proxy for calcite-aragonite seas in Precambrian time. Geobiology 2008, 6, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yan, C.; Zhang, F.; Konishi, H.; Xu, H.; Teng, H.H. Testing the cation-hydration effect on the crystallization of Ca-Mg-CO3 systems. Proc. Natl. Acad. Sci. USA 2013, 110, 17750–17755. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 2000, 16, 521–555. [Google Scholar] [CrossRef] [PubMed]
- Miedema, H.; Meter-Arkema, A.; Wierenga, J.; Tang, J.; Eisenberg, B.; Nonner, W.; Hektor, H.; Gillespie, D.; Meijberg, W. Permeation Properties of an Engineered Bacterial OmpF Porin Containing the EEEE-Locus of Ca2+ Channels. Biophys. J. 2004, 87, 3137–3147. [Google Scholar] [CrossRef] [PubMed]
- Moomaw, A.S.; Maguire, M.E. Cation Selectivity by the CorA Mg2+ Channel Requires a Fully Hydrated Cation. Biochemistry 2010, 49, 5998–6008. [Google Scholar] [CrossRef] [PubMed]
- Dudev, T.; Lim, C. Competition among Ca2+, Mg2+, and Na+ for Model Ion Channel Selectivity Filters: Determinants of Ion Selectivity. J. Phys. Chem. B 2012, 116, 10703–10714. [Google Scholar] [CrossRef]

| Time (h) | CO32− (mol/L) | HCO3− (mol/L) | Na2CO3 (g/L) | NaHO3 (g/L) | pH |
|---|---|---|---|---|---|
| 0 | 0 | 0.0280 | 0 | 1.7080 | 8.68 ± 0.0033 |
| 4.5 | 0 | 0.0268 | 0 | 1.7446 | 8.56 ± 0.0032 |
| 16.5 | 0 | 0.0288 | 0 | 1.7568 | 8.64 ± 0.0001 |
| 22.5 | 0 | 0.0344 | 0 | 2.0984 | 8.66 ± 0.0033 |
| 28.5 | 0 | 0.0386 | 0 | 2.3546 | 8.64 ± 0.0030 |
| 40.5 | 0 | 0.0406 | 0 | 2.4766 | 8.66 ± 0.0031 |
| 46.5 | 0 | 0.0409 | 0 | 2.4949 | 8.62 ± 0.0033 |
| 53 | 0 | 0.0411 | 0 | 2.5071 | 8.62 ± 0.0035 |
| 65.5 | 0 | 0.0430 | 0 | 2.6230 | 8.61 ± 0.0057 |
| 70.5 | 0 | 0.0445 | 0 | 2.7145 | 8.63 ± 0.0032 |
| 76.5 | 0 | 0.0456 | 0 | 2.7816 | 8.64 ± 0.0036 |
| 89 | 0 | 0.0476 | 0 | 2.9036 | 8.62 ± 0.0008 |
| 95 | 0 | 0.0486 | 0 | 2.9646 | 8.61 ± 0.0034 |
| 101 | 0 | 0.0490 | 0 | 2.9890 | 8.62 ± 0.0058 |
| 125 | 0.0105 | 0.0428 | 0.6300 | 2.6108 | 9.58 ± 0.0059 |
| 143 | 0.0118 | 0.0401 | 0.7080 | 2.4461 | 9.61 ± 0.0062 |
| 173 | 0.0152 | 0.0374 | 0.9120 | 2.2814 | 9.79 ± 0.0037 |
| 197 | 0.0171 | 0.0365 | 1.0260 | 2.2265 | 9.77 ± 0.0033 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Z.; Gao, X.; Zhao, H.; Tucker, M.E.; Zhao, Y.; Bi, Z.; Pan, J.; Wu, G.; Yan, H. Extracellular and Intracellular Biomineralization Induced by Bacillus licheniformis DB1-9 at Different Mg/Ca Molar Ratios. Minerals 2018, 8, 585. https://doi.org/10.3390/min8120585
Han Z, Gao X, Zhao H, Tucker ME, Zhao Y, Bi Z, Pan J, Wu G, Yan H. Extracellular and Intracellular Biomineralization Induced by Bacillus licheniformis DB1-9 at Different Mg/Ca Molar Ratios. Minerals. 2018; 8(12):585. https://doi.org/10.3390/min8120585
Chicago/Turabian StyleHan, Zuozhen, Xiao Gao, Hui Zhao, Maurice E. Tucker, Yanhong Zhao, Zhenpeng Bi, Juntong Pan, Guangzhen Wu, and Huaxiao Yan. 2018. "Extracellular and Intracellular Biomineralization Induced by Bacillus licheniformis DB1-9 at Different Mg/Ca Molar Ratios" Minerals 8, no. 12: 585. https://doi.org/10.3390/min8120585
APA StyleHan, Z., Gao, X., Zhao, H., Tucker, M. E., Zhao, Y., Bi, Z., Pan, J., Wu, G., & Yan, H. (2018). Extracellular and Intracellular Biomineralization Induced by Bacillus licheniformis DB1-9 at Different Mg/Ca Molar Ratios. Minerals, 8(12), 585. https://doi.org/10.3390/min8120585





