Nanoscale Microstructure and Microbially Mediated Mineralization Mechanisms of Deep-Sea Cobalt-Rich Crusts
Abstract
1. Introduction
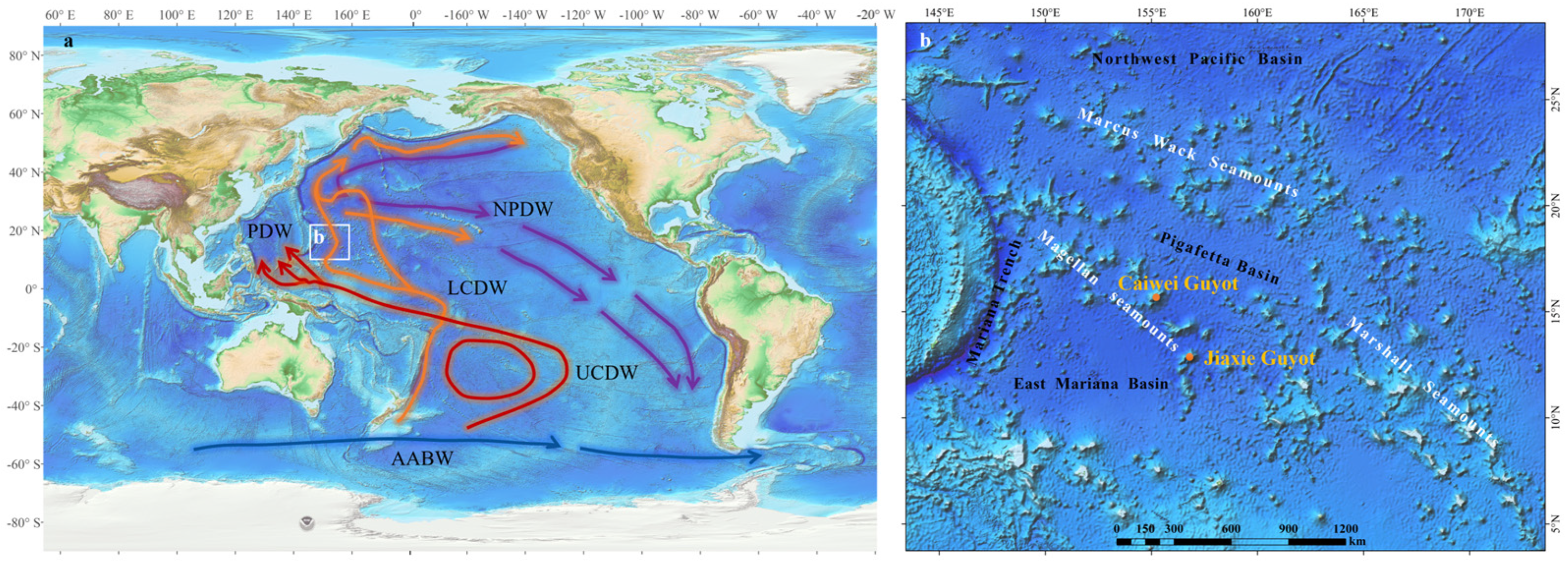
2. Regional Context of the Western Pacific Region
3. Mineralogy and Occurrence of Ferromanganese Crusts
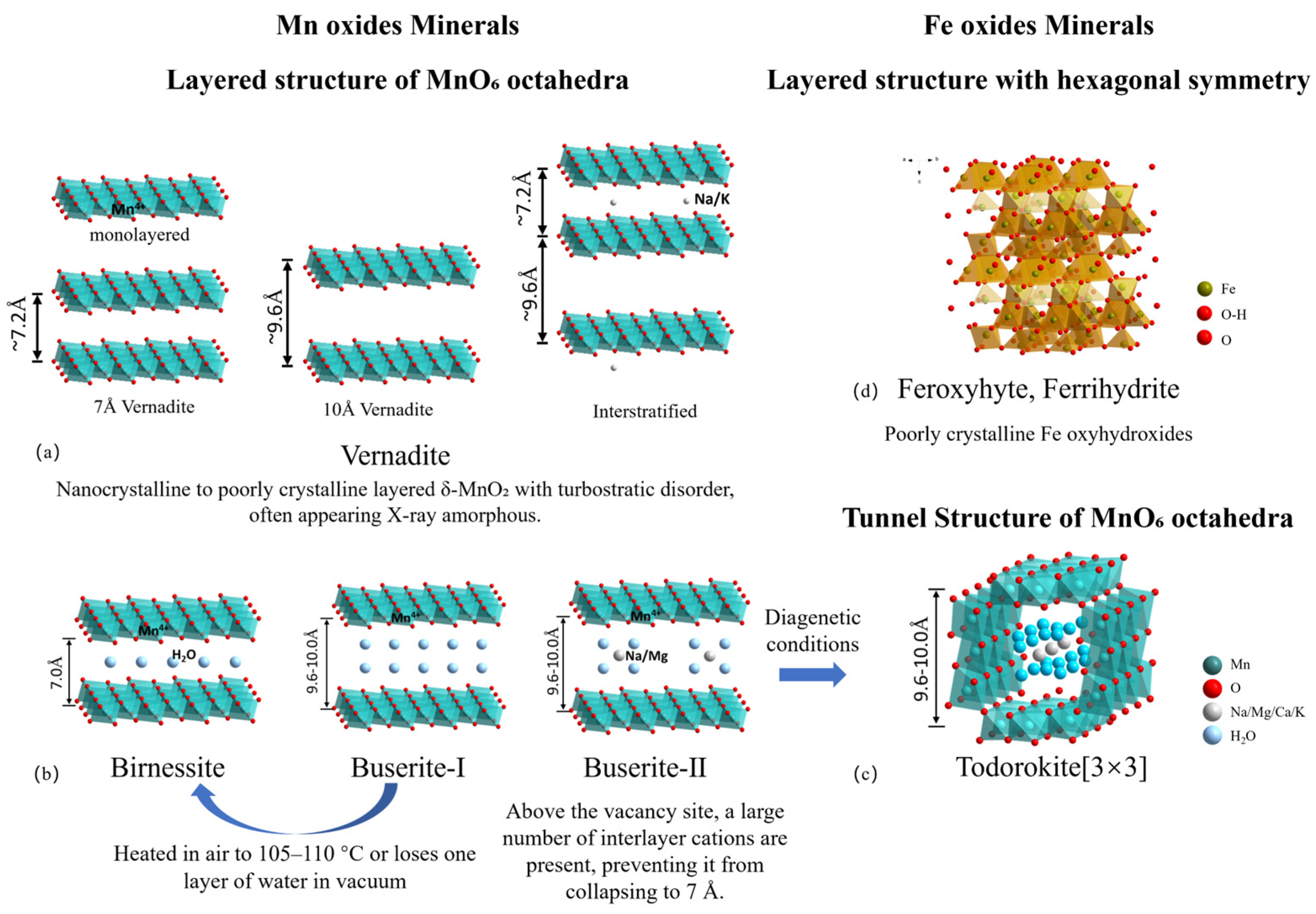
3.1. Manganese Oxide Phases
3.2. Iron Oxide Phases
3.3. Other Mineral Phases
4. Mechanisms of Cobalt-Rich Crust Mineralization
4.1. Chemical Formation, Stable Aggregation, and Precipitation of Manganese/Iron Hydroxide Colloids
4.1.1. Initial Formation of Fe–Mn Nanocolloids by Chemical and Microbial Oxidation
4.1.2. Colloidal Stabilization and Metal Adsorption Stage
4.1.3. Colloidal Deposition and Authigenic Mineralization Stage
4.2. Enrichment Characteristics of Critical Metals and the Structural Adsorption Mechanism of Co
4.2.1. Enrichment Characteristics of Critical Metals
- The metal enrichment of cobalt-rich crusts is primarily controlled by the adsorption capacity of Fe–Mn oxides and variations in marine redox conditions. Manganese and iron are the principal ore-forming elements in cobalt-rich crusts, occurring in multiple oxidation states and undergoing migration and fractionation within the marine environment [21]. Recent high-resolution observations reveal that hydrogenetic ferromanganese crusts and nodules exhibit submicron-scale alternating porous and compact laminae, which record short-term fluctuations in bottom-water redox conditions [71]. Variations in the Mn3+/Mn4+ ratio and the crystallinity of δ-MnO2 indicate periodic redox oscillations during crust growth, affecting the partitioning and fixation of transition metals such as Co, Ni, and Cu within different micro-layers [5,11,72]. Under relatively oxic conditions, the oxidation of Mn2+ to Mn4+ generates negatively charged Mn oxide surfaces that preferentially adsorb divalent cations, while mildly reducing microenvironments promote the partial reduction of Mn4+ to Mn3+, altering the layer charge balance and enhancing the selective uptake of Co3+ and Ni2+ [62]. Conversely, Fe oxyhydroxides dominate under less oxidizing or slightly reducing conditions, favoring the incorporation of Fe3+-associated anions such as and . These redox-driven transformations dynamically regulate the physicochemical reactivity of Fe–Mn oxides, thereby determining their metal adsorption selectivity and enrichment efficiency. Overall, the interplay between redox fluctuations, mineral structural evolution, and surface complexation processes governs the multi-scale enrichment mechanism of cobalt-rich crusts in deep-sea environments [62,71,73].
- Marine microorganisms also play a significant role in the metal enrichment process. Many microorganisms can promote the transformation of Fe and Mn via redox reactions and influence the speciation of metallic elements. Under oxic conditions, microorganisms catalyze the oxidation of Mn2+, causing Mn4+ to precipitate as Mn oxides, thereby enhancing their adsorption capacity for metal ions such as Co, Ni, and Cu. Furthermore, microorganisms can affect metal solubility and mobility by complexing with metal ions through extracellularly secreted organic ligands [74]. Biogeochemical cycling further regulates the distribution of metallic elements in the water column, leading to a trend where Mn and Co are enriched in surface waters and depleted in deep waters [75]. Fe exhibits dual characteristics of scavenging and cycling; it can be removed by adsorption onto colloidal oxides or re-enter the water column through the microbial reduction of Fe3+ [76]. Co is primarily sequestered by Mn oxides, whereas Cu and Zn have lower regeneration rates in the water column due to their strong binding affinity with organic matter [77]. The combined effect of these biogeochemical processes determines the speciation and distribution patterns of metals in deep-sea cobalt-rich crusts.
- Metallic elements and their adsorption mechanisms. Metallic elements are enriched in ferromanganese oxide colloids via surface complexation, redox reactions, and structural incorporation: ① Selective adsorption by surface charge: Manganese oxides (negative charge) preferentially adsorb positively charged cations (e.g., Co2+, Ni2+, Zn2+) and high-valence metals (e.g., Co3+, Ce4+). Iron oxyhydroxides (weak positive charge) adsorb negatively charged complexes (e.g., , ) and neutral molecules (e.g., ). ② Adsorption via oxidative substitution mechanism: Cobalt (Co): Co2+ is oxidized to Co3+ on the manganese oxide surface, forming edge-sharing complexes and becoming embedded in the layered structure. Cerium (Ce): Ce3+ is oxidized to Ce4+ and immobilized in the Mn phase as CeO2, forming significant positive Ce anomalies. Platinum (Pt): Pt2+ is oxidized to Pt4+ and enriched via surface complexation or as discrete phases. For Co, Ce, and Tl, enrichment via oxidative adsorption is primarily related to the oxidative substitution mechanism associated with δ-MnO2. Redox-sensitive elements in seawater remain at lower valences and are oxidized by MnO2 in oxic environments. Co, Ce, and Tl are preferentially oxidized to insoluble, inner-sphere, high-valence species within the Mn oxides. These insoluble oxidized species participate less in exchange reactions, gradually accumulating over time and eventually becoming exceptionally abundant, thus showing a high correlation with Mn [69]. ③ Direct substitution via surface complexation: Nickel (Ni2+) and Copper (Cu2+) are stably incorporated by occupying vacancies in the manganese oxide layers (e.g., the hexagonal structure of vernadite). For elements like Ni, Cu, Zn, and Li, this primarily occurs on the δ-MnO2 surface. For these elements, direct surface complexation occurs on the Fe-Mn oxides. Ni, Cu, Zn, and Li form abundant inner-sphere complexes on the mineral surfaces (Li also exhibits outer-sphere complexation in carbonate phases and at tunnel wall sites), and Mn oxides generally make a larger contribution to the enrichment of this group [68].
4.2.2. Structural Adsorption Characteristics and Mechanism of Co Element
5. Metallogenic and Ore-Controlling Factors and Mechanisms
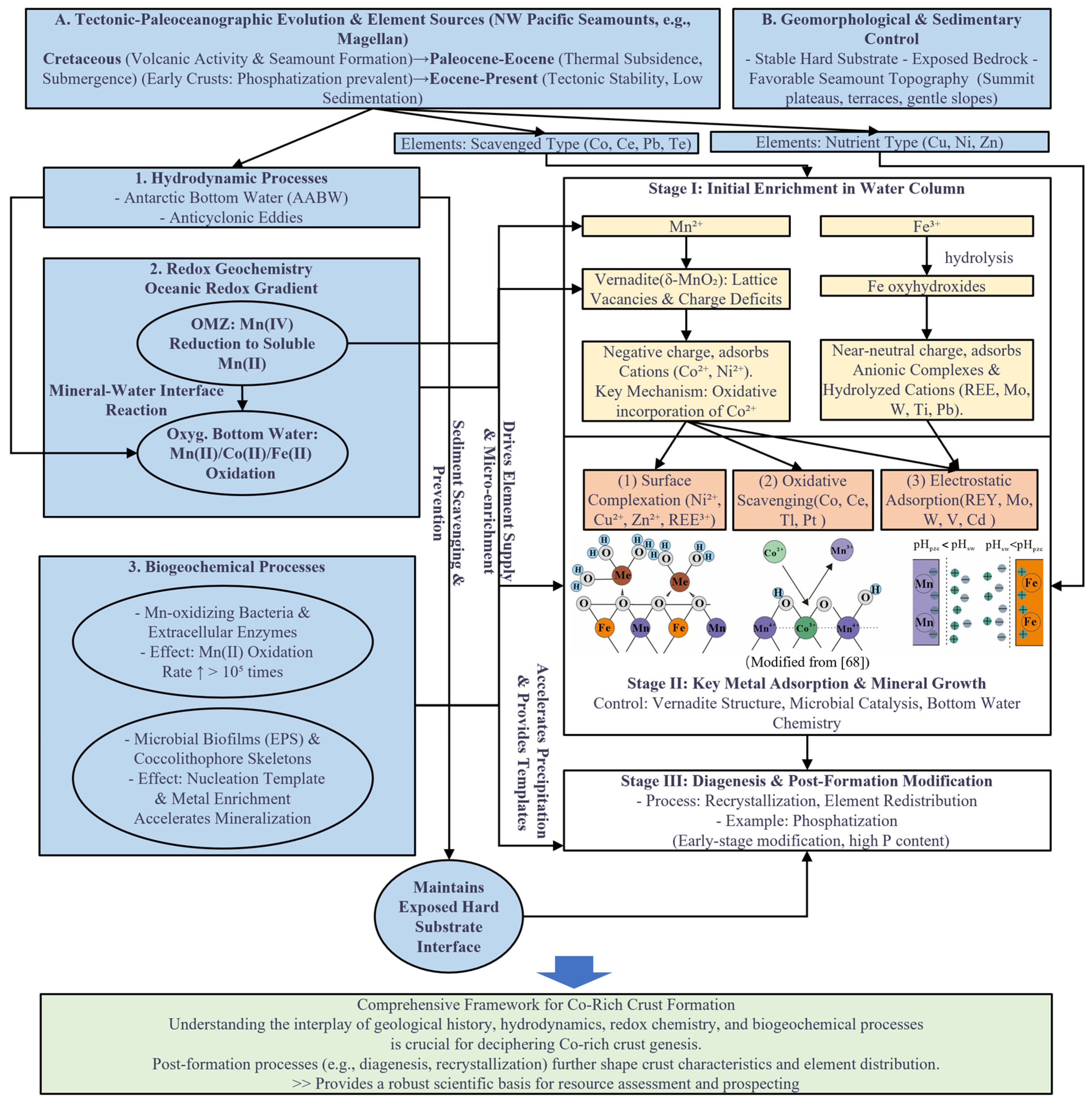
5.1. Control of Tectonics and Paleoceanographic Evolution on the Metallogenesis of Cobalt-Rich Crusts
5.1.1. Influence of Tectonics and Paleoceanographic Evolution
5.1.2. Constraints of Topography, Micro-Geomorphology, and Seamount Stability
5.2. Microscopic Mineral–Water Interface Drivers of Mineralization: Microstructural and Microbial Coupling Mechanisms
6. Conclusions
- (1)
- Based on cobalt-rich crusts from the Magellan Seamount region in the northwestern Pacific, the metallogenic process can be divided into three stages: ① Formation of Mn/Fe colloids—At the chemical interface between oxygen-rich bottom water and the oxygen minimum zone (OMZ), Mn2+ and Fe2+ are oxidized to produce hydrated oxide colloids such as δ-MnO2 and Fe(OH)3 and suspended in water. ② Metal adsorption stage—The formed colloidal particles adsorb key metal ions such as Co2+, Ni2+, and Cu2+ through surface complexation and oxidation–substitution reactions. Among them, Co2+ can be oxidized to Co3+ and stabilized within the octahedral layer vacancies of MnO6. Metal adsorption is continuous in the process of colloid formation, suspension aggregation and precipitation. ③ Colloid deposition and mineralization stage—Mn–Fe colloidal particles aggregate, dehydrate, and cement on the exposed seamount bedrock surface, forming layered cobalt-rich crusts. The entire process is governed by the Fe/Mn redox cycle, reflecting a continuous evolution from colloidal reactions to solid-phase mineral formation.
- (2)
- Biological processes act as crucial catalytic mechanisms promoting the microstructural evolution of crusts. Mn-oxidizing bacteria oxidize Mn2+ to MnO2 nanoparticles via extracellular oxidase systems, providing precursors for the initial nucleation of crusts. Meanwhile, extracellular polymeric substances (EPS) chelate metal ions at the interface, control crystal orientation, and enhance interparticle binding, leading to the formation of dense, laminated Mn–Fe oxide composites. This bio–mineral coupling mechanism significantly enhances both the oxidation rate and adsorption efficiency of metal ions, representing one of the key microscopic driving forces for crustal growth.
- (3)
- The metallogenesis of cobalt-rich crusts in the Magellan Seamount region is jointly driven by macro-scale geological backgrounds and microscale physicochemical mechanisms. Tectonic and paleoceanographic evolution determine the formation, drift, and stability of seamounts, providing long-term exposed hard substrates and stable depositional environments for mineralization. The inflow of Antarctic Bottom Water (AABW), the spatiotemporal variability of the OMZ, and the evolution of the carbonate compensation depth (CCD) collectively control redox conditions and metal sources. At the microscale, the crystal defects, interlayer vacancies of Mn–Fe oxides, and biological activities together constitute the interfacial driving forces for metal enrichment. The macro-tectonic framework defines the metallogenic site, while microchemical and biological processes determine the metallogenic mechanism—their coupling jointly governs the spatiotemporal distribution and elemental enrichment intensity of cobalt-rich crusts.
- (4)
- This study establishes a regionally constrained, multi-level metallogenic model for cobalt-rich crusts in the Magellan Seamount region, linking tectonic–paleoceanographic evolution with micro- to nanoscale mineralogical and geochemical processes. By synthesizing existing mineralogical and geochemical evidence, this review highlights that crystal defects, lattice vacancies, and redox-active surface sites of Mn–Fe oxides play a key role in controlling the selective enrichment and stabilization of cobalt and other critical metals. To rigorously evaluate the relative importance of these nanoscale crystal-chemical features, future studies should integrate conventional bulk and microanalytical approaches (e.g., SEM, XRD, XRF, ICP-OES, ICP-MS, EPMA) with advanced nanoscale imaging and spectroscopic techniques, including (S)TEM, synchrotron-based XANES/EXAFS, XPS, NanoSIMS, and PDF analysis, to directly constrain metal coordination environments, oxidation states, and lattice incorporation mechanisms, in combination with in situ observations of redox gradients, ocean circulation, and substrate characteristics. Such integrated, multi-technique and multi-scale approaches will allow quantitative assessment of the coupled roles of crystal chemistry, redox conditions, microbial mediation, and oceanographic setting, thereby refining metallogenic models and providing a robust scientific framework for predicting cobalt-rich crusts development and identifying economically significant cobalt-rich crust provinces in the western Pacific.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hein, J.R.; Koschinsky, A.; Bau, M.; Manheim, F.T.; Kang, J.-K.; Roberts, L. Cobalt-rich ferromanganese crusts in the Pacific. In Handbook of Marine Mineral Deposits; Cronan, D.S., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. 239–279. [Google Scholar]
- Koschinsky, A.; Hein, J.R. Marine Ferromanganese Encrustations: Archives of Changing Oceans. Elements 2017, 13, 177–182. [Google Scholar] [CrossRef]
- Hein, J.R.; Mizell, K.; Koschinsky, A.; Conrad, T.A. Deep-Ocean Mineral Deposits as a Source of Critical Metals for High- and Green-Technology Applications: Comparison with Land-Based Resources. Ore Geol. Rev. 2013, 51, 1–14. [Google Scholar] [CrossRef]
- Hein, J.R.; Koschinsky, A.; Halliday, A.N. Global Occurrence of Tellurium-Rich Ferromanganese Crusts and a Model for the Enrichment of Tellurium. Geochim. Cosmochim. Acta 2003, 67, 1117–1127. [Google Scholar] [CrossRef]
- Hein, J.R.; Koschinsky, A. Deep-Ocean Ferromanganese Crusts and Nodules. In Treatise on Geochemistry; Elsevier: Amsterdam, The Netherlands, 2014; pp. 273–291. ISBN 978-0-08-098300-4. [Google Scholar]
- Halbach, P.E.; Jahn, A.; Cherkashov, G. Marine Co-Rich Ferromanganese Crust Deposits: Description and Formation, Occurrences and Distribution, Estimated World-Wide Resources. In Deep-Sea Mining: Resource Potential, Technical and Environmental Considerations; Sharma, R., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 65–141. ISBN 978-3-319-52557-0. [Google Scholar]
- Verlaan, P.A.; Cronan, D.S. Origin and Variability of Resource-Grade Marine Ferromanganese Nodules and Crusts in the Pacific Ocean: A Review of Biogeochemical and Physical Controls. Geochemistry 2022, 82, 125741. [Google Scholar] [CrossRef]
- Maciąg, Ł.; Zawadzki, D.; Kozub-Budzyń, G.A.; Piestrzyński, A.; Kotliński, R.A.; Wróbel, R.J. Mineralogy of Cobalt-Rich Ferromanganese Crusts from the Perth Abyssal Plain (E Indian Ocean). Minerals 2019, 9, 84. [Google Scholar] [CrossRef]
- Deng, X.; Ren, J.; Deng, X.; Tu, J.; Hong, S.; He, G.; Zhang, L.; Yang, Y. Occurrence and Enrichment of Critical Metals in Ferromanganese Deposits in the Western Pacific. Ore Geol. Rev. 2024, 174, 106314. [Google Scholar] [CrossRef]
- Koschinsky, A.; Stascheit, A.; Bau, M.; Halbach, P. Effects of Phosphatization on the Geochemical and Mineralogical Composition of Marine Ferromanganese Crusts. Geochim. Cosmochim. Acta 1997, 61, 4079–4094. [Google Scholar] [CrossRef]
- Kuhn, T.; Wegorzewski, A.; Ruehlemann, C.; Vink, A. Composition, Formation, and Occurrence of Polymetallic Nodules; Springer: Cham, Switzerland, 2017; pp. 23–63. [Google Scholar]
- Jiang, X.-D.; Sun, X.-M.; Guan, Y.; Gong, J.-L.; Lu, Y.; Lu, R.-F.; Wang, C. Biomineralisation of the Ferromanganese Crusts in the Western Pacific Ocean. J. Asian Earth Sci. 2017, 136, 58–67. [Google Scholar] [CrossRef]
- Sujith, P.P.; Ramanan, D.; Gonsalves, M.J.B.D.; LokaBharathi, P.A. Microbial Activity Promotes the Enrichment of Cobalt over Nickel on Hydrogenetic Ferromanganese Crusts. Mar. Georesour. Geotechnol. 2017, 35, 1158–1167. [Google Scholar] [CrossRef]
- Sujith, P.P.; Gonsalves, M.J.B.D. Ferromanganese Oxide Deposits: Geochemical and Microbiological Perspectives of Interactions of Cobalt and Nickel. Ore Geol. Rev. 2021, 139, 104458. [Google Scholar] [CrossRef]
- Yang, K.; Park, H.; Son, S.-K.; Baik, H.; Park, K.; Kim, J.; Yoon, J.; Park, C.H.; Kim, J. Electron Microscopy Study on the Formation of Ferromanganese Crusts, Western Pacific Magellan Seamounts. Mar. Geol. 2019, 410, 32–41. [Google Scholar] [CrossRef]
- Park, K.; Jung, J.; Park, J.; Ko, Y.; Lee, Y.; Yang, K. Geochemical-Mineralogical Analysis of Ferromanganese Oxide Precipitated on Porifera in the Magellan Seamount, Western Pacific. Front. Mar. Sci. 2023, 9, 1086610. [Google Scholar] [CrossRef]
- Wang, X.; Müller, W.E.G. Marine biominerals: Perspectives and challenges for polymetallic nodules and crusts. Trends Biotechnol. 2009, 27, 375–383. [Google Scholar] [CrossRef]
- Santelli, C.M.; Webb, S.M.; Dohnalkova, A.C.; Hansel, C.M. Diversity of Mn Oxides Produced by Mn(II)-Oxidizing Fungi. Geochim. Cosmochim. Acta 2011, 75, 2762–2776. [Google Scholar] [CrossRef]
- Usui, A.; Hino, H.; Suzushima, D.; Tomioka, N.; Suzuki, Y.; Sunamura, M.; Kato, S.; Kashiwabara, T.; Kikuchi, S.; Uramoto, G.-I.; et al. Modern precipitation of hydrogenetic ferromanganese minerals during on-site 15-year exposure tests. Sci. Rep. 2020, 10, 3558. [Google Scholar] [CrossRef] [PubMed]
- Josso, P.; Lusty, P.; Chenery, S.; Murton, B. Controls on metal enrichment in ferromanganese crusts: Temporal changes in oceanic metal flux or phosphatisation? Geochim. Cosmochim. Acta 2021, 308, 60–74. [Google Scholar] [CrossRef]
- Glasby, G.P. Manganese: Predominant Role of Nodules and Crusts. In Marine Geochemistry; Schulz, H.D., Zabel, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 371–427. ISBN 978-3-540-32144-6. [Google Scholar]
- Yamazaki, T.; Sharma, R. Morphological Features of Co-Rich Manganese Deposits and Their Relation to Seabed Slopes. Mar. Georesour. Geotechnol. 2000, 18, 43–76. [Google Scholar] [CrossRef]
- Cottrell, R.D.; Tarduno, J.A. Late Cretaceous true polar wander: Not so fast. Science 2000, 288, 2283. [Google Scholar] [CrossRef]
- Natland, J.H. Capture of Helium and Other Volatiles during the Growth of Olivine Phenocrysts in Picritic Basalts from the Juan Fernandez Islands. J. Petrol. 2003, 44, 421–456. [Google Scholar] [CrossRef]
- Halbach, P. Processes Controlling the Heavy Metal Distribution in Pacific Ferromanganese Nodules and Crusts. Geol. Rundsch. 1986, 75, 235–247. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, Z. The Formation of Ferromanganese Crusts from the Western Mariana Ridge and Implications for Deep-water Environment since the Late Pliocene. Geochem. Geophys. Geosyst. 2025, 26, e2024GC011964. [Google Scholar] [CrossRef]
- Yang, K.; Ma, W.; Zhang, W.; Li, Z.; He, G.; Li, X.; Qiu, Z.; Wang, H.; Zhao, B.; Yang, Y.; et al. Geological and geochemical characteristics of shallow-buried ferromanganese crusts from Weijia Guyot and their resource potential. Mar. Geol. 2023, 464, 107119. [Google Scholar] [CrossRef]
- Peretyazhko, I.S.; Savina, E.A.; Pulyaeva, I.A. Cobalt-Rich Fe-Mn Crusts in the Western Pacific Magellan Seamount Trail: Geochemistry and Chronostratigraphy. Geosciences 2025, 15, 411. [Google Scholar] [CrossRef]
- Johnson, G.C. Quantifying Antarctic Bottom Water and North Atlantic Deep Water Volumes. J. Geophys. Res. Ocean. 2008, 113, C05027. [Google Scholar] [CrossRef]
- Elderfield, H.; Greaves, M.J. The Rare Earth Elements in Seawater. Nature 1982, 296, 214–219. [Google Scholar] [CrossRef]
- Zhu, M.; Farrow, C.L.; Post, J.E.; Livi, K.J.T.; Billinge, S.J.L.; Ginder-Vogel, M.; Sparks, D.L. Structural Study of Biotic and Abiotic Poorly-Crystalline Manganese Oxides Using Atomic Pair Distribution Function Analysis. Geochim. Cosmochim. Acta 2012, 81, 39–55. [Google Scholar] [CrossRef]
- Manceau, A.; Marcus, M.A.; Grangeon, S.; Lanson, M.; Lanson, B.; Gaillot, A.-C.; Skanthakumar, S.; Soderholm, L. Short-Range and Long-Range Order of Phyllomanganate Nanoparticles Determined Using High-Energy X-Ray Scattering. J. Appl. Crystallogr. 2013, 46, 193–209. [Google Scholar] [CrossRef]
- Lee, S.; Xu, H.; Xu, W.; Sun, X. The Structure and Crystal Chemistry of Vernadite in Ferromanganese Crusts. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2019, 75, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Manceau, A.; Kersten, M.; Marcus, M.A.; Geoffroy, N.; Granina, L. Ba and Ni Speciation in a Nodule of Binary Mn Oxide Phase Composition from Lake Baikal. Geochim. Cosmochim. Acta 2007, 71, 1967–1981. [Google Scholar] [CrossRef]
- Giovanoli, R. Vernadite Is Random-Stacked Birnessite: A Discussion of the Paper by F.V. Chukhrov et al.: Contributions to the Mineralogy of Authigenic Manganese Phases from Marine Manganese Deposits? [Mineralium Deposita 14, 249–261 (1979)]. Miner. Depos. 1980, 15, 251–253. [Google Scholar] [CrossRef]
- Grangeon, S.; Manceau, A.; Guilhermet, J.; Gaillot, A.-C.; Lanson, M.; Lanson, B. Zn Sorption Modifies Dynamically the Layer and Interlayer Structure of Vernadite. Geochim. Cosmochim. Acta 2012, 85, 302–313. [Google Scholar] [CrossRef]
- Manceau, A.; Steinmann, S.N. Density functional theory modeling of the oxidation mechanism of Co (II) by birnessite. ACS Earth Space Chem. 2022, 6, 2063–2075. [Google Scholar] [CrossRef]
- Post, J.E. Manganese Oxide Minerals: Crystal Structures and Economic and Environmental Significance. Proc. Natl. Acad. Sci. USA 1999, 96, 3447–3454. [Google Scholar] [CrossRef]
- Bodeï, S.; Manceau, A.; Geoffroy, N.; Baronnet, A.; Buatier, M. Formation of todorokite from vernadite in Ni-rich hemipelagic sediments. Geochim. Cosmochim. Acta 2007, 71, 5698–5716. [Google Scholar] [CrossRef]
- Golden, D.C.; Dixon, J.B.; Chen, C.C. Ion exchange, thermal transformations, and oxidizing properties of birnessite. Clays Clay Miner. 1986, 34, 511–520. [Google Scholar] [CrossRef]
- Manceau, A.; Lanson, M.; Takahashi, Y. Mineralogy and Crystal Chemistry of Mn, Fe, Co, Ni, and Cu in a Deep-Sea Pacific Polymetallic Nodule. Am. Mineral. 2014, 99, 2068–2083. [Google Scholar] [CrossRef]
- Chukhrov, F.V.; Zvyagin, B.B.; Gorshkov, A.I.; Yermilova, L.P.; Korovushkin, V.V.; Rudnitskaya, Y.S.; Yakubovskaya, N.Y. Feroxyhyte, a New Modification of FeOOH. Int. Geol. Rev. 1977, 19, 873–890. [Google Scholar] [CrossRef]
- Drits, V.A.; Sakharov, B.A.; Manceau, A. Structure of Feroxyhite as Determined by Simulation of X-Ray Diffraction Curves. Clay Miner. 1993, 28, 209–222. [Google Scholar] [CrossRef]
- Manceau, A.; Drits, V.A. Local Structure of Ferrihydrite and Feroxyhite by Exafs Spectroscopy. Clay Miner. 1993, 28, 165–184. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Chen, H.Y. Formation of Oceanic Cobalt-rich Crust: Progress and Perspectives. Geotectonica Metallogenia 2021, 47, 80–97. [Google Scholar]
- Wang, X.; Wiens, M.; Schröder, H.C.; Schloßmacher, U.; Müller, W.E.G. Molecular Biomineralization: Toward an Understanding of the Biogenic Origin of Polymetallic Nodules, Seamount Crusts, and Hydrothermal Vents. In Molecular Biomineralization: Aquatic Organisms Forming Extraordinary Materials; Müller, W.E.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 77–110. ISBN 978-3-642-21230-7. [Google Scholar]
- Hiemstra, T. Ferrihydrite Interaction with Silicate and Competing Oxyanions: Geometry and Hydrogen Bonding of Surface Species. Geochim. Cosmochim. Acta 2018, 238, 453–476. [Google Scholar] [CrossRef]
- Jambor, J.L.; Dutrizac, J.E. Occurrence and Constitution of Natural and Synthetic Ferrihydrite, a Widespread Iron Oxyhydroxide. Chem. Rev. 1998, 98, 2549–2586. [Google Scholar] [CrossRef]
- Hein, J.R.; Morgan, C.L. Influence of Substrate Rocks on Fe–Mn Crust Composition. Deep Sea Res. Part Oceanogr. Res. Pap. 1999, 46, 855–875. [Google Scholar] [CrossRef]
- Bruland, K.W.; Franks, R.P. Mn, Ni, Cu, Zn and Cd in the Western North Atlantic. In Trace Metals in Sea Water; Wong, C.S., Boyle, E., Bruland, K.W., Burton, J.D., Goldberg, E.D., Eds.; Springer US: Boston, MA, USA, 1983; pp. 395–414. ISBN 978-1-4757-6866-4. [Google Scholar]
- Liu, X.; Millero, F.J. The Solubility of Iron in Seawater. Mar. Chem. 2002, 77, 43–54. [Google Scholar] [CrossRef]
- Kato, S.; Hirai, M.; Ohkuma, M.; Suzuki, K. Microbial Metabolisms in an Abyssal Ferromanganese Crust from the Takuyo-Daigo Seamount as Revealed by Metagenomics. PLoS ONE 2019, 14, e0224888. [Google Scholar] [CrossRef] [PubMed]
- Tebo, B.M.; Bargar, J.R.; Clement, B.G.; Dick, G.J.; Murray, K.J.; Parker, D.; Verity, R.; Webb, S.M. Biogenic Manganese Oxides: Properties and Mechanisms of Formation. Annu. Rev. Earth Planet. Sci. 2004, 32, 287–328. [Google Scholar] [CrossRef]
- Bargar, J.R.; Tebo, B.M.; Villinski, J.E. In Situ Characterization of Mn(II) Oxidation by Spores of the Marine Bacillus Sp. Strain SG-1. Geochim. Cosmochim. Acta 2000, 64, 2775–2778. [Google Scholar] [CrossRef]
- Villalobos, M.; Toner, B.; Bargar, J.; Sposito, G. Characterization of the Manganese Oxide Produced by Pseudomonas Putida Strain MnB1. Geochim. Cosmochim. Acta 2003, 67, 2649–2662. [Google Scholar] [CrossRef]
- Learman, D.R.; Wankel, S.D.; Webb, S.M.; Martinez, N.; Madden, A.S.; Hansel, C.M. Coupled Biotic–Abiotic Mn(II) Oxidation Pathway Mediates the Formation and Structural Evolution of Biogenic Mn Oxides. Geochim. Cosmochim. Acta 2011, 75, 6048–6063. [Google Scholar] [CrossRef]
- Nedkov, I.; Slavov, L.; Angelova, R.; Blagoev, B.; Kovacheva, D.; Abrashev, M.V.; Iliev, M.; Groudeva, V. Biogenic Nanosized Iron Oxides Obtained from Cultivation of Iron Bacteria from the Genus Leptothrix. J. Biol. Phys. 2016, 42, 587–600. [Google Scholar] [CrossRef]
- Lyu, J.; Yu, X.; Jiang, M.; Cao, W.; Saren, G.; Chang, F. The Mechanism of Microbial-Ferromanganese Nodule Interaction and the Contribution of Biomineralization to the Formation of Oceanic Ferromanganese Nodules. Microorganisms 2021, 9, 1247. [Google Scholar] [CrossRef] [PubMed]
- Dzombak, D.A.; Morel, F. Surface Complexation Modeling: Hydrous Ferric Oxide; A Wiley-Interscience publication; Wiley: New York, NY, USA, 1990; ISBN 978-0-471-63731-8. [Google Scholar]
- Murray, J.W.; Dillard, J.G.; Giovanoli, R.; Moers, H.; Stumm, W. Oxidation of Mn(II): Initial Mineralogy, Oxidation State and Ageing. Geochim. Cosmochim. Acta 1985, 49, 463–470. [Google Scholar] [CrossRef]
- Aplin, A.C.; Cronan, D.S. Ferromanganese Oxide Deposits from the Central Pacific Ocean, II. Nodules and Associated Sediments. Geochim. Cosmochim. Acta 1985, 49, 437–451. [Google Scholar] [CrossRef]
- Ren, J.; He, G.; Yang, Y.; Yu, M.; Deng, Y.; Pang, Y.; Zhao, B.; Yao, H. Ultraselective Enrichment of Trace Elements in Seawater by Co-Rich Ferromanganese Nodules. Glob. Planet. Change 2024, 239, 104498. [Google Scholar] [CrossRef]
- Moffett, J.W.; Ho, J. Oxidation of Cobalt and Manganese in Seawater via a Common Microbially Catalyzed Pathway. Geochim. Cosmochim. Acta 1996, 60, 3415–3424. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Wang, X.; Gan, L.; Müller, W.E.G. Contribution of Biomineralization during Growth of Polymetallic Nodules and Ferromanganese Crusts from the Pacific Ocean. Front. Mater. Sci. China 2009, 3, 109–123. [Google Scholar] [CrossRef]
- Koschinsky, A.; Halbach, P. Sequential Leaching of Marine Ferromanganese Precipitates: Genetic Implications. Geochim. Cosmochim. Acta 1995, 59, 5113–5132. [Google Scholar] [CrossRef]
- Wang, X.; Schloßmacher, U.; Natalio, F.; Wolf, S.E.; Tremel, W. Evidence for Biogenic Processes during Formation of Ferromanganese Crusts from the Pacific Ocean: Implications of Biologically Induced Mineralization. Micron 2009, 40, 526–535. [Google Scholar] [CrossRef]
- Guan, Y.; Sun, X.; Jiang, X.; Sa, R.; Zhou, L.; Huang, Y.; Liu, Y.; Li, X.; Lu, R.; Wang, C. The Effect of Fe-Mn Minerals and Seawater Interface and Enrichment Mechanism of Ore-Forming Elements of Polymetallic Crusts and Nodules from the South China Sea. Acta Oceanol. Sin. 2017, 36, 34–46. [Google Scholar] [CrossRef]
- Huang, S.; Fu, Y. Enrichment Characteristics and Mechanisms of Critical Metals in Marine Fe-Mn Crusts and Nodules: A Review. Minerals 2023, 13, 1532. [Google Scholar] [CrossRef]
- Peng, J.; Li, D.; Poulton, S.W.; O’Sullivan, G.J.; Chew, D.; Fu, Y.; Sun, X. Episodic Intensification of Marine Phosphorus Burial over the Last 80 Million Years. Nat. Commun. 2024, 15, 7446. [Google Scholar] [CrossRef]
- Zhou, J.; Kogure, T.; Okumura, T.; Takahashi, Y.; Liu, J.; Yang, S.; Yuan, P. Characterization of Submicron-thick Layered Structure in Hydrogenetic Ferromanganese Nodule Suggests Short-term Redox Fluctuation of Paleo-ocean. J. Geophys. Res. Ocean. 2024, 129, e2023JC020240. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Q.; Yang, Y.; Wei, H.; Laipan, M.; Zhu, R.; He, H.; Hochella, M.F. Coupled Redox Cycling of Fe and Mn in the Environment: The Complex Interplay of Solution Species with Fe- and Mn-(Oxyhydr)Oxide Crystallization and Transformation. Earth-Sci. Rev. 2022, 232, 104105. [Google Scholar] [CrossRef]
- Su, R.; Sun, F.; Li, X.; Chu, F.; Sun, G.; Li, J.; Wang, H.; Li, Z.; Zhang, C.; Zhang, W.; et al. Diverse Early Diagenetic Processes of Ferromanganese Nodules from the Eastern Pacific Ocean: Evidence from Mineralogy and in-Situ Geochemistry. Int. Geol. Rev. 2023, 65, 2131–2147. [Google Scholar] [CrossRef]
- Sunda, W.G.; Kieber, D.J. Oxidation of Humic Substances by Manganese Oxides Yields Low-Molecular-Weight Organic Substrates. Nature 1994, 367, 62–64. [Google Scholar] [CrossRef]
- Martin, J.H.; Knauer, G.A. Lateral Transport of Mn in the North-East Pacific Gyre Oxygen Minimum. Nature 1985, 314, 524–526. [Google Scholar] [CrossRef]
- Rue, E.L.; Bruland, K.W. The Role of Organic Complexation on Ambient Iron Chemistry in the Equatorial Pacific Ocean and the Response of a Mesoscale Iron Addition Experiment. Limnol. Oceanogr. 1997, 42, 901–910. [Google Scholar] [CrossRef]
- Coale, K.H.; Bruland, K.W. Copper Complexation in the Northeast Pacific. Limnol. Oceanogr. 1988, 33, 1084–1101. [Google Scholar] [CrossRef]
- Li, F.; Yin, H.; Zhu, T.; Zhuang, W. Understanding the role of manganese oxides in retaining harmful metals: Insights into oxidation and adsorption mechanisms at microstructure level. Eco-Environ. Health 2024, 3, 89–106. [Google Scholar] [CrossRef]
- Wu, Z.; Lanson, B.; Feng, X.; Yin, H.; Tan, W.; He, F.; Liu, F. Transformation of the Phyllomanganate Vernadite to Tectomanganates with Small Tunnel Sizes: Favorable Geochemical Conditions and Fate of Associated Co. Geochim. Cosmochim. Acta 2021, 295, 224–236. [Google Scholar] [CrossRef]
- Wu, Z.; Peacock, C.L.; Lanson, B.; Yin, H.; Zheng, L.; Chen, Z.; Tan, W.; Qiu, G.; Liu, F.; Feng, X. Transformation of Co-Containing Birnessite to Todorokite: Effect of Co on the Transformation and Implications for Co Mobility. Geochim. Cosmochim. Acta 2019, 246, 21–40. [Google Scholar] [CrossRef]
- Manceau, A.; Silvester, E.; Bartoli, C.; Lanson, B.; Drits, V.A. Structural Mechanism of Co2+ Oxidation by the Phyllomanganate Buserite. Am. Mineral. 1997, 82, 1150–1175. [Google Scholar] [CrossRef]
- Zhao, H.; Feng, X.; Lee, S.; Reinhart, B.; Elzinga, E.J. Sorption and Oxidation of Co(II) at the Surface of Birnessite: Impacts of Aqueous Mn(II). Chem. Geol. 2023, 618, 121281. [Google Scholar] [CrossRef]
- Ren, X. The Metallogenic System of Co-Rich Manganese Crusts in Western Pacific. Doctoral Dissertation, Institute of Oceanology, Chinese Academy of Sciences, Beijing, China, 2005. [Google Scholar]
- Wang, L.; Zeng, Z. The Geochemical Features and Genesis of Ferromanganese Deposits from Caiwei Guyot, Northwestern Pacific Ocean. J. Mar. Sci. Eng. 2022, 10, 1275. [Google Scholar] [CrossRef]
- Clouard, V.; Bonneville, A. How Many Pacific Hotspots Are Fed by Deep-Mantle Plumes? Geology 2001, 29, 695–698. [Google Scholar] [CrossRef]
- Kennett, J.P. Marine Geology; Englewood Cliffs, N.J., Ed.; Prentice-Hall: Hoboken, NJ, USA, 1982; ISBN 978-0-13-556936-8. [Google Scholar]
- Lawver, L.A.; Gahagan, L.M. Evolution of Cenozoic Seaways in the Circum-Antarctic Region. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2003, 198, 11–37. [Google Scholar] [CrossRef]
- Kawabe, M.; Fujio, S. Pacific Ocean Circulation Based on Observation. J. Oceanogr. 2010, 66, 389–403. [Google Scholar] [CrossRef]
- Haq, B. Paleogene Paleoceanography: Early Cenozoic Oceans Revisited. Oceanol. Acta Spec. Issue 1981, 4, 71–82. [Google Scholar]
- Xu, D. Paleo–Ocean Events and Mineralization in the Pacific Ocean. In Marine Geology and Palaeoceanography; CRC Press: Boca Raton, FL, USA, 1997; Volume 13, pp. 129–144. [Google Scholar]
- Roden, G.I. Effects of the Fieberling Seamount Group upon Flow and Thermohaline Structure in the Spring of 1991. J. Geophys. Res. Ocean. 1994, 99, 9941–9961. [Google Scholar] [CrossRef]
- Halbach, P.; Kriete, C.; Prause, B.; Puteanus, D. Mechanisms to Explain the Platinum Concentration in Ferromanganese Seamount Crusts. Chem. Geol. 1989, 76, 95–106. [Google Scholar] [CrossRef]
- Usui, A.; Nishi, K.; Sato, H.; Nakasato, Y.; Thornton, B.; Kashiwabara, T.; Tokumaru, A.; Sakaguchi, A.; Yamaoka, K.; Kato, S.; et al. Continuous Growth of Hydrogenetic Ferromanganese Crusts since 17 Myr Ago on Takuyo-Daigo Seamount, NW Pacific, at Water Depths of 800–5500 m. Ore Geol. Rev. 2017, 87, 71–87. [Google Scholar] [CrossRef]
- Bulmer, M.H.; Wilson, J.B. Comparison of Flat-Topped Stellate Seamounts on Earth’s Seafloor with Stellate Domes on Venus Using Side-Scan Sonar and Magellan Synthetic Aperture Radar. Earth Planet. Sci. Lett. 1999, 171, 277–287. [Google Scholar] [CrossRef]
- Koppers, A.A.P.; Staudigel, H.; Wijbrans, J.R.; Pringle, M.S. The Magellan Seamount Trail: Implications for Cretaceous Hotspot Volcanism and Absolute Pacific Plate Motion. Earth Planet. Sci. Lett. 1998, 163, 53–68. [Google Scholar] [CrossRef]
- Mitchell, N.C. Characterising the Irregular Coastlines of Volcanic Ocean Islands. Geomorphology 1998, 23, 1–14. [Google Scholar] [CrossRef]
- Bogdanov, Y.A.; Bogdanova, O.Y.; Dubinin, A.V.; Gorand, A.; Gorshkov, A.I.; Gurvich, E.G.; Isaeva, A.B.; Ivanov, G.V.; Jansa, L.F.; Monaco, A. Composition of Ferromanganese Crusts and Nodules at Northwest Pacific Guyots and Geologic and Paleoceanographic Considerations. Proc. Ocean Drill. Program Sci. Results 1995, 144, 745–768. [Google Scholar]
- Nealson, K.H.; Saffarini, D. Iron and Manganese in Anaerobic Respiration: Environmental Significance, Physiology, and Regulation. Annu. Rev. Microbiol. 1994, 48, 311–343. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Zhang, K.; You, X.; Li, C.; Wang, H.; Wu, J.; Dang, Y.; Guan, Q.; Huang, X. Nanoscale Microstructure and Microbially Mediated Mineralization Mechanisms of Deep-Sea Cobalt-Rich Crusts. Minerals 2026, 16, 91. https://doi.org/10.3390/min16010091
Zhang K, You X, Li C, Wang H, Wu J, Dang Y, Guan Q, Huang X. Nanoscale Microstructure and Microbially Mediated Mineralization Mechanisms of Deep-Sea Cobalt-Rich Crusts. Minerals. 2026; 16(1):91. https://doi.org/10.3390/min16010091
Chicago/Turabian StyleZhang, Kehui, Xuelian You, Chao Li, Haojia Wang, Jingwei Wu, Yuan Dang, Qing Guan, and Xiaowei Huang. 2026. "Nanoscale Microstructure and Microbially Mediated Mineralization Mechanisms of Deep-Sea Cobalt-Rich Crusts" Minerals 16, no. 1: 91. https://doi.org/10.3390/min16010091
APA StyleZhang, K., You, X., Li, C., Wang, H., Wu, J., Dang, Y., Guan, Q., & Huang, X. (2026). Nanoscale Microstructure and Microbially Mediated Mineralization Mechanisms of Deep-Sea Cobalt-Rich Crusts. Minerals, 16(1), 91. https://doi.org/10.3390/min16010091





