Fe–Si–O Isotope Characteristics and Ore Formation Mechanisms of the Hugushan Area BIF-Type Iron Deposits in the Central North China Craton
Abstract
1. Introduction
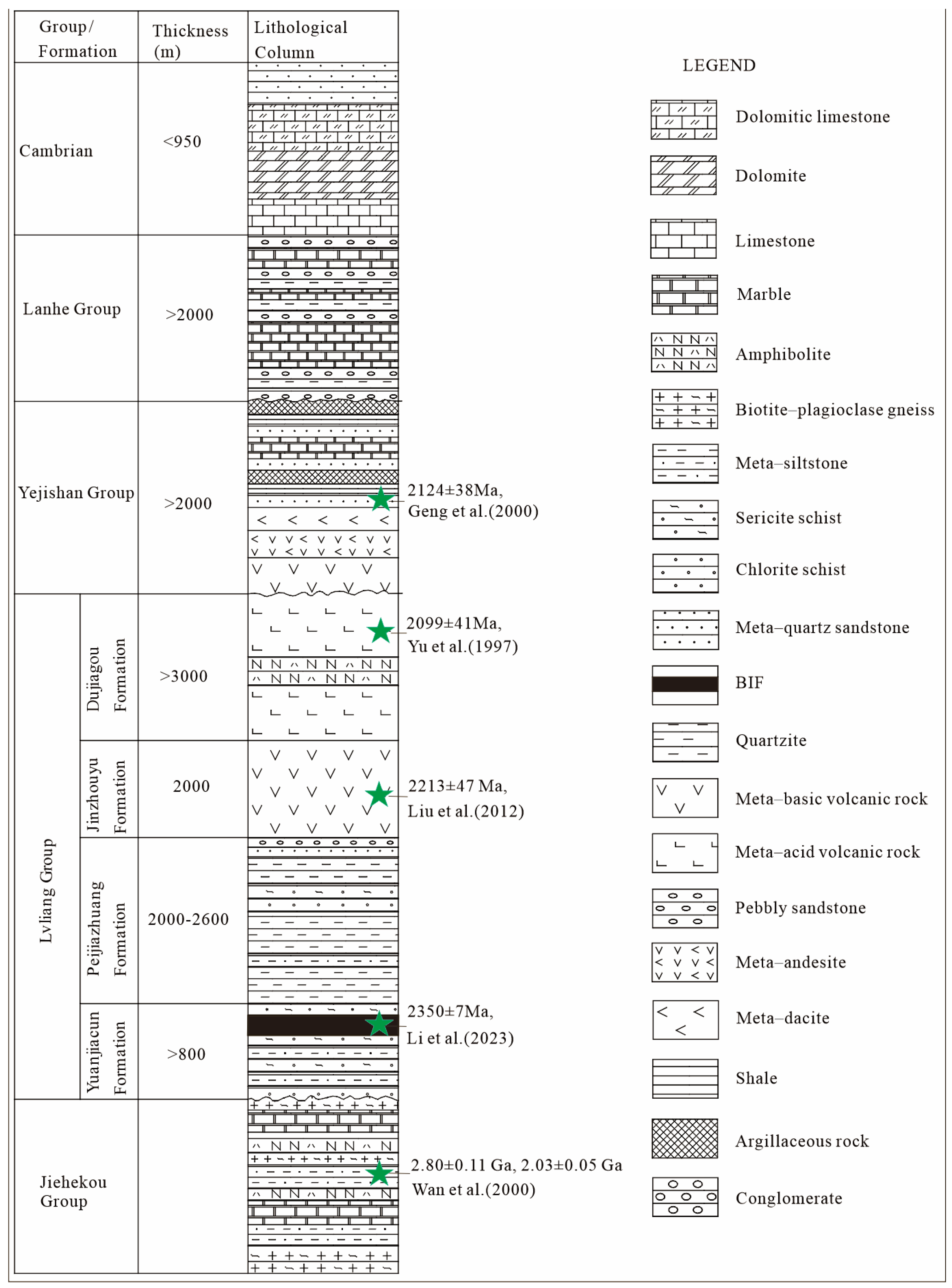
2. Geological Setting
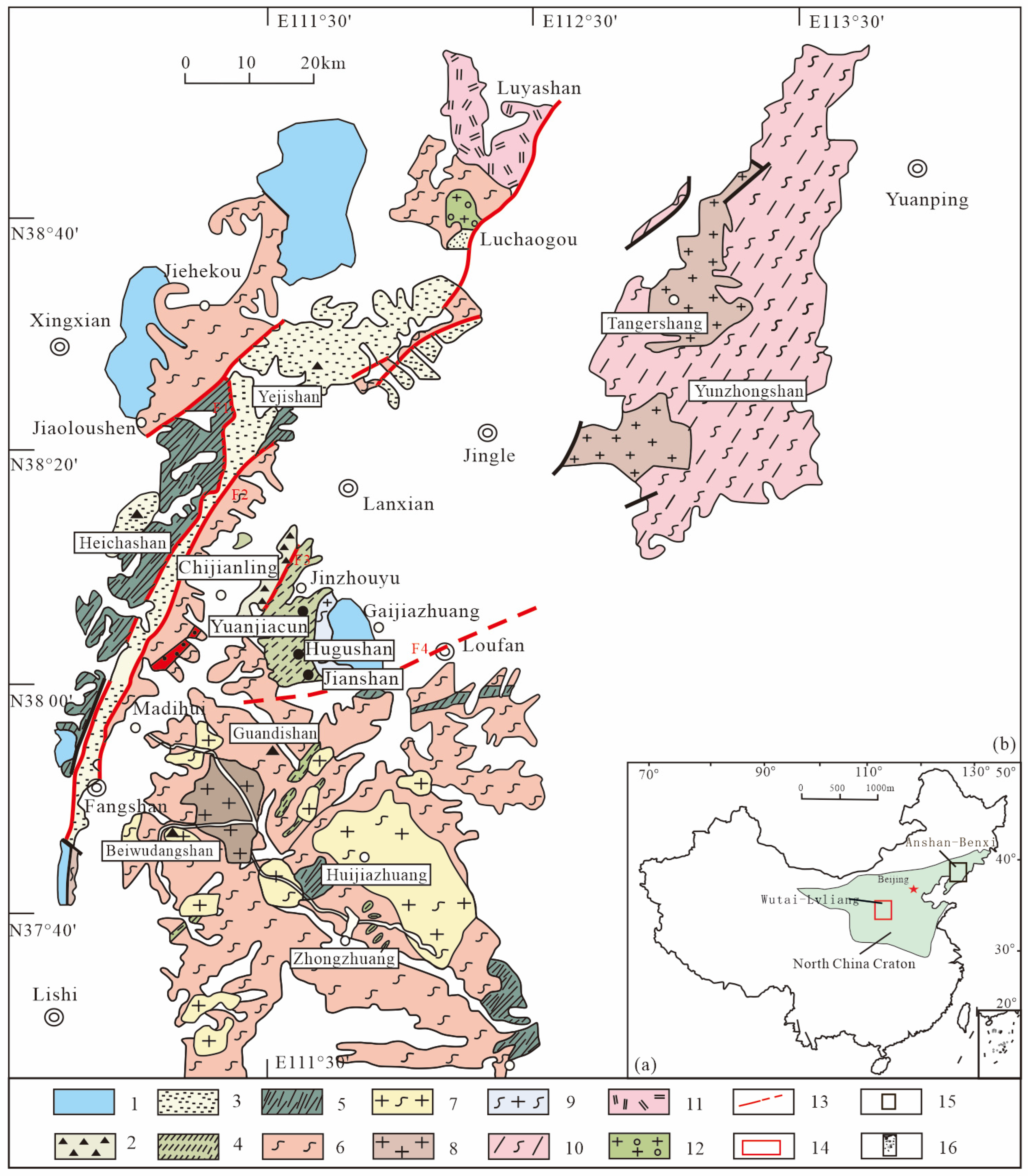
2.1. Regional Geology
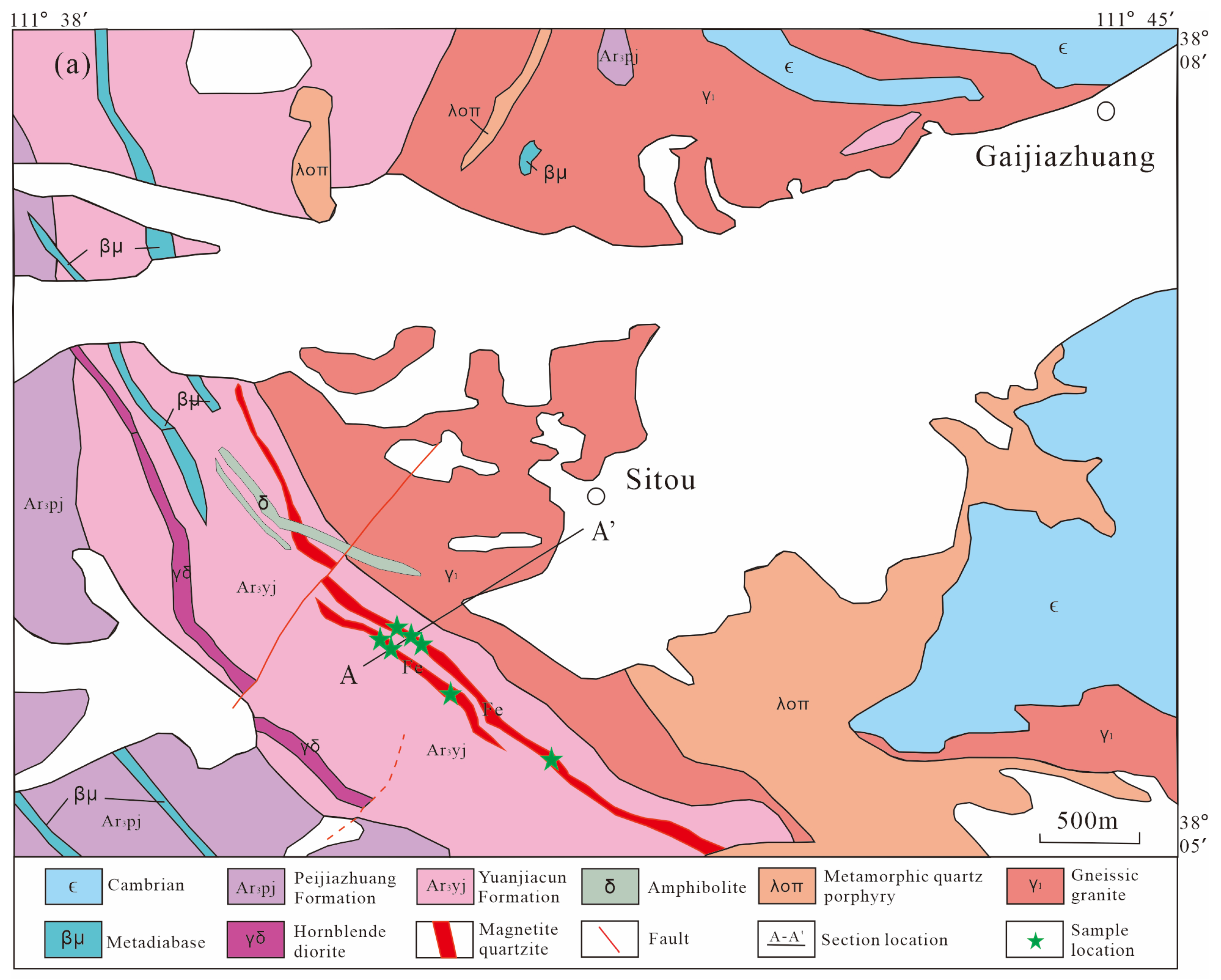
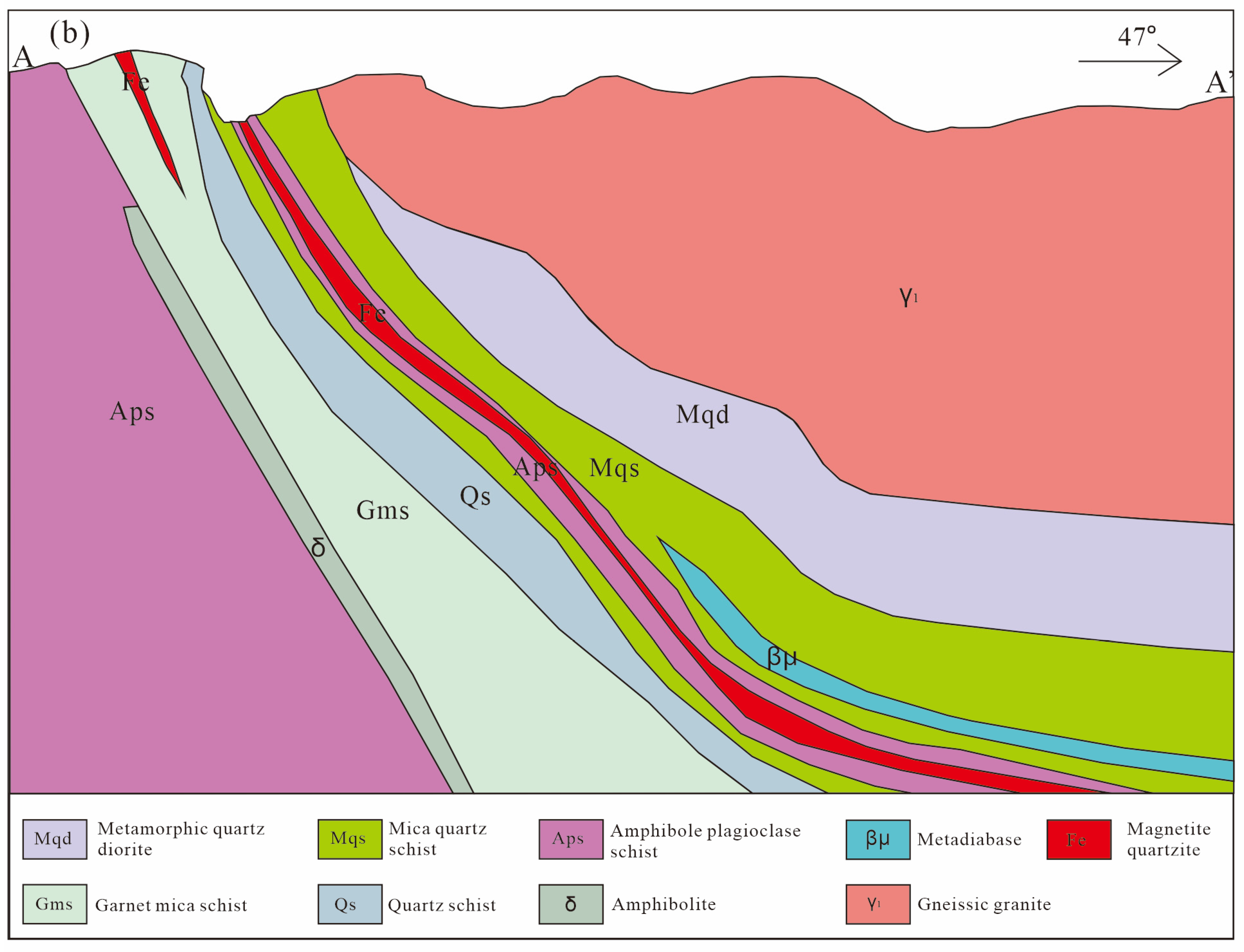
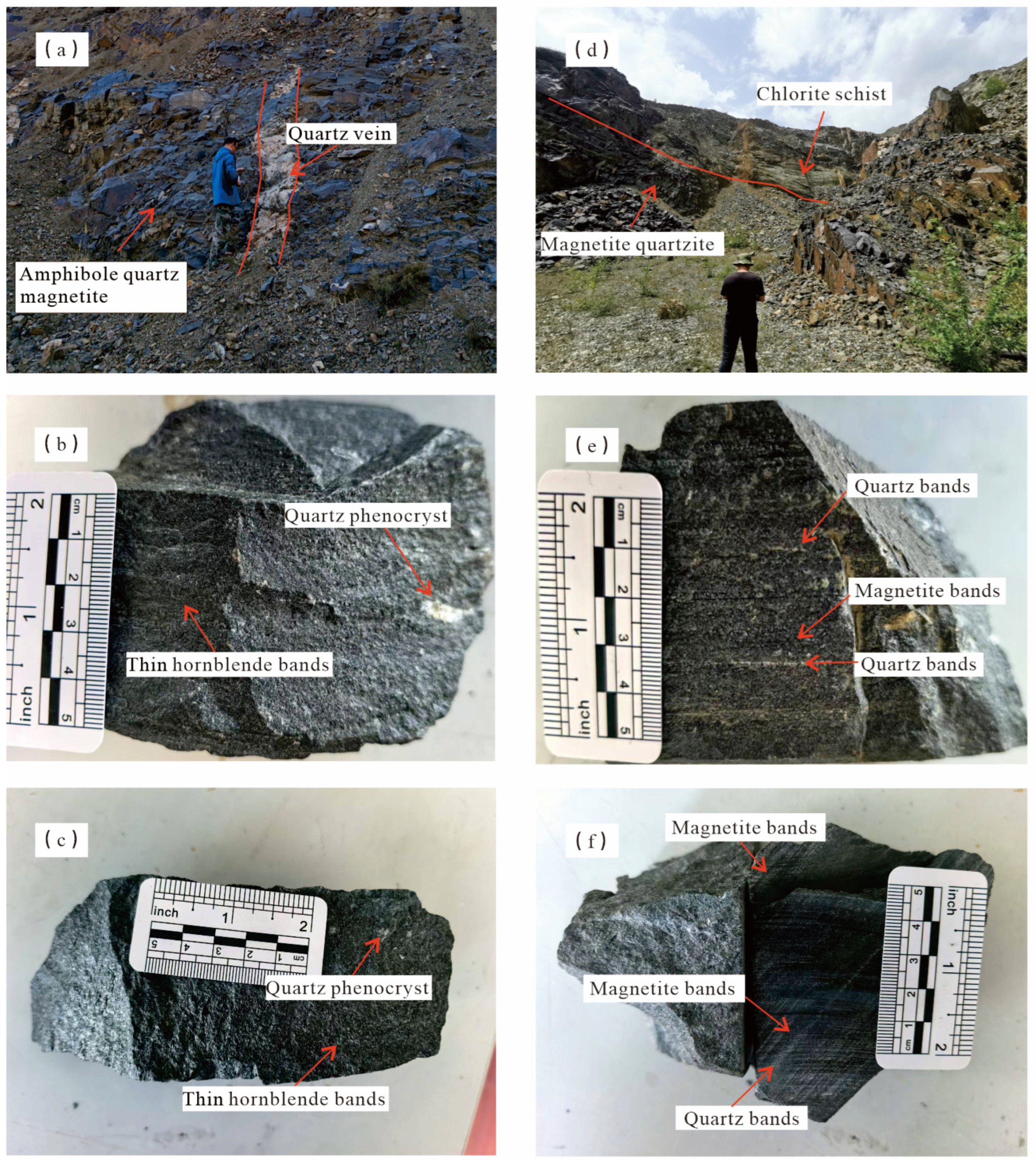
2.2. Mine Geology
3. Sample Description and Analytical Methods
3.1. Major and Trace Element Analyses
3.2. Fe Isotope Analyses
3.3. Quartz O–Si Isotopic Compositions
4. Analytical Results
4.1. Whole-Rock Geochemistry
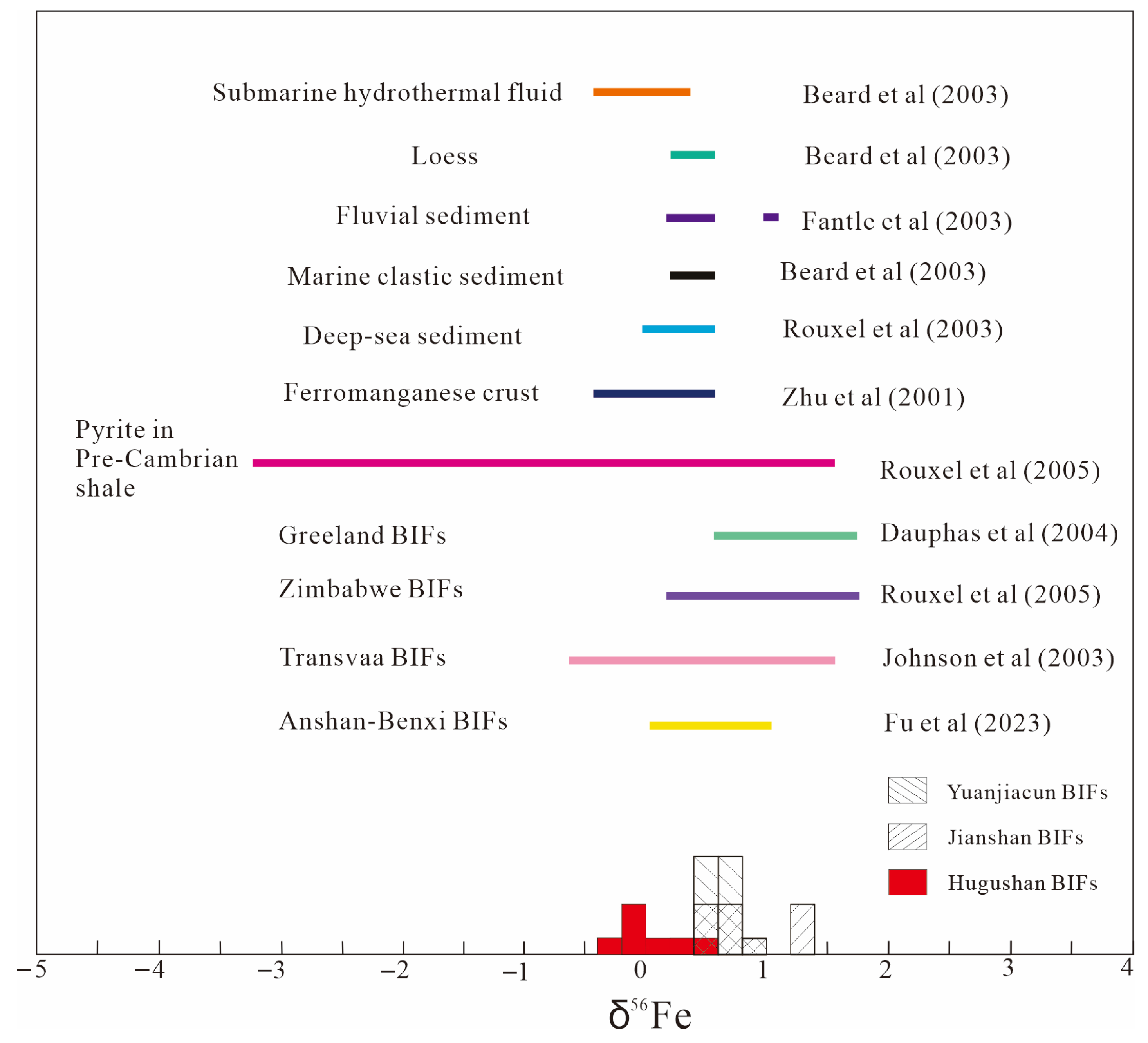
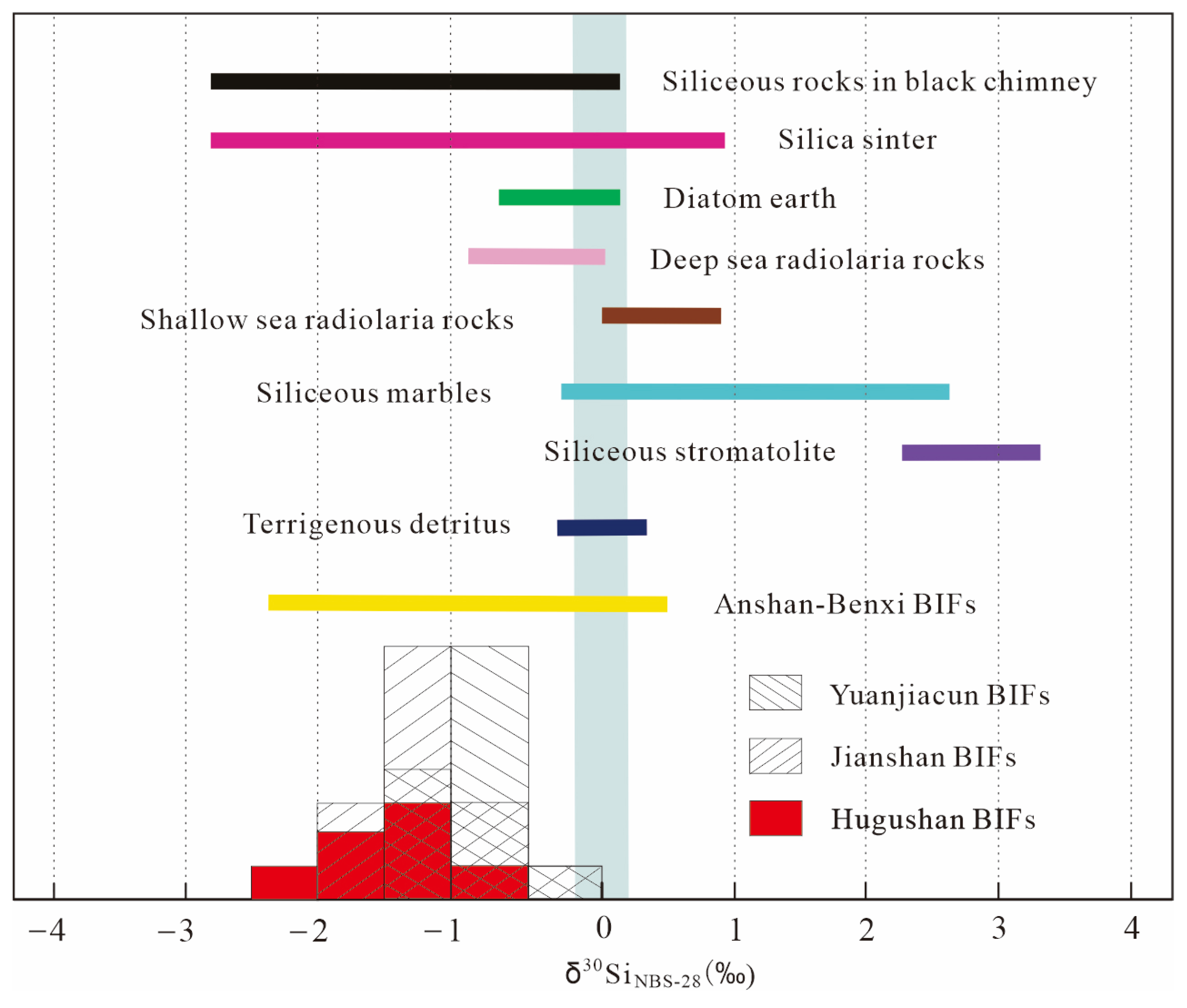
4.2. Fe, Si and O Isotopic Compositions
5. Discussion
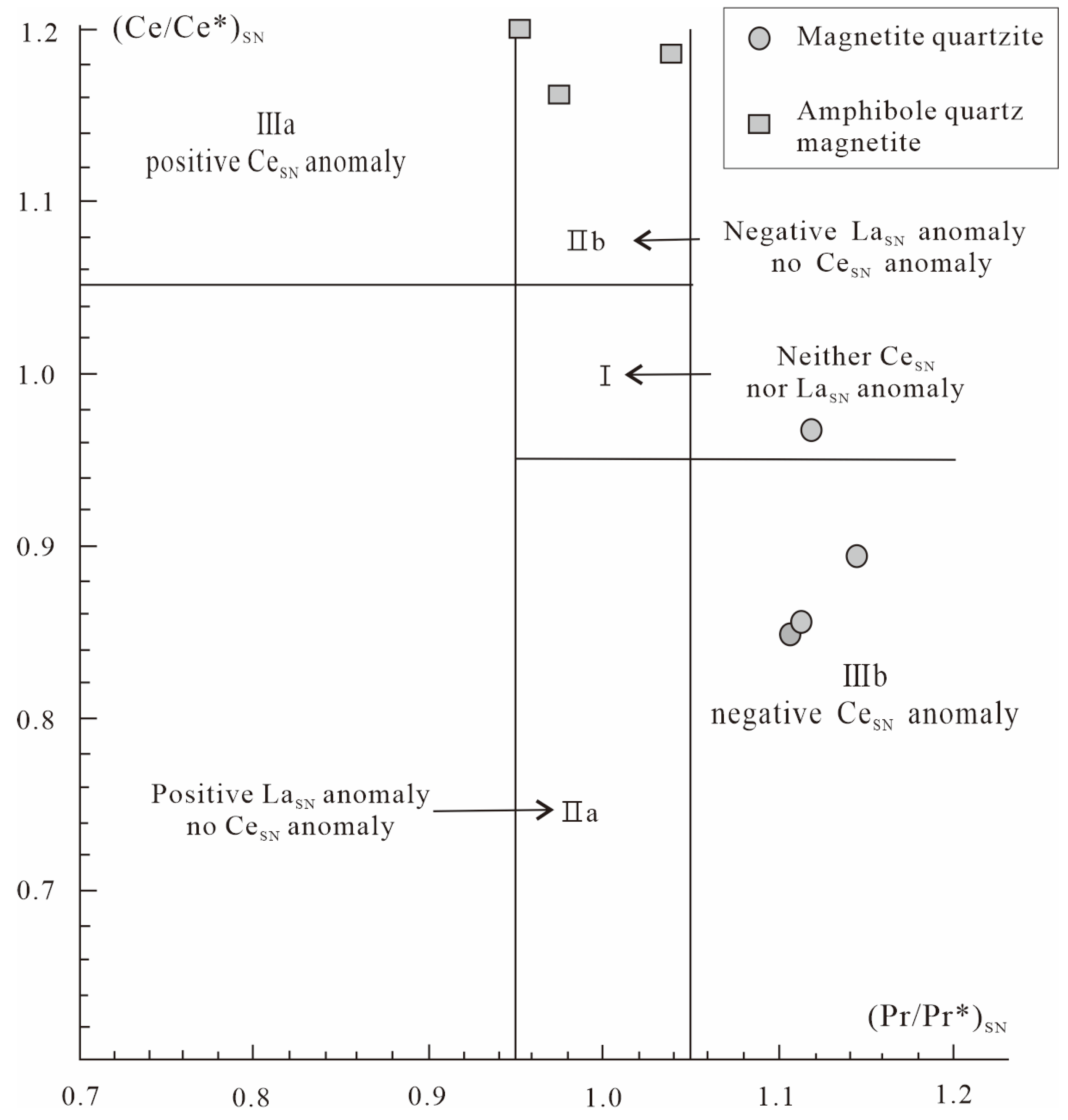
5.1. Depositional Environment
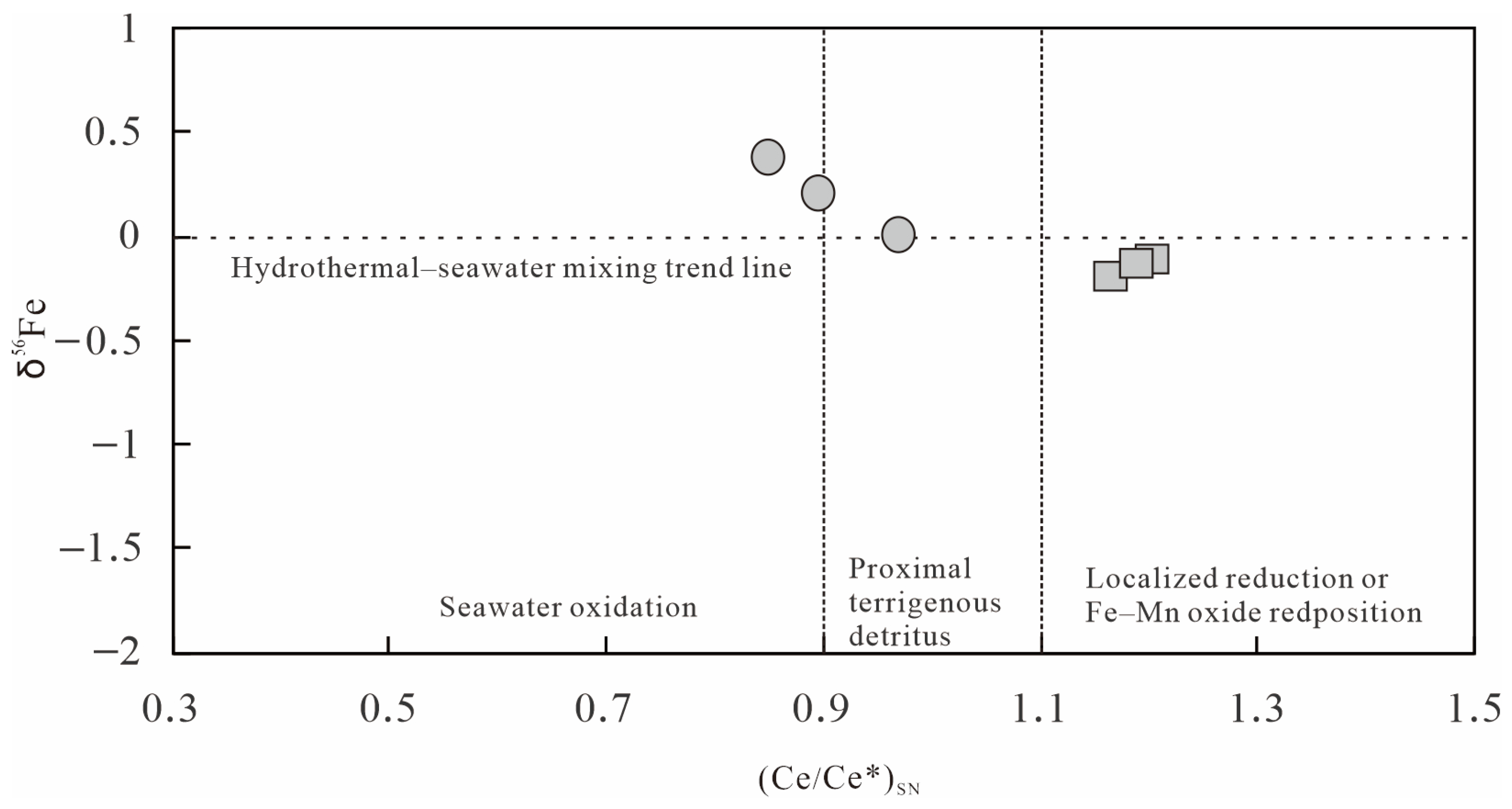
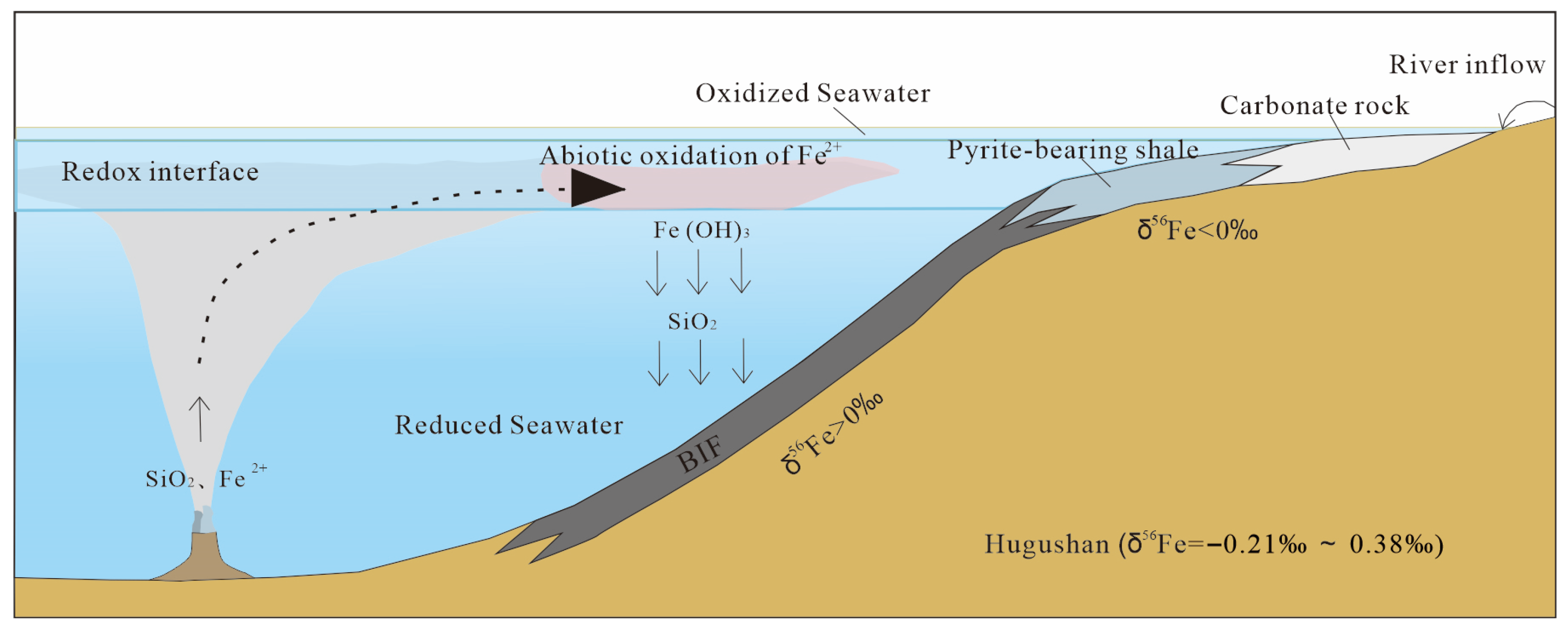
5.2. Origin of the Major Components (Iron and Silica) of BIFs
5.3. Genesis of BIFs
6. Conclusions
- (1).
- The shallow seawater was in a partially oxidized state, whereas the deep seawater remained in a reductive environment during the depositional period.
- (2).
- The primary mineralizing materials of the Hugushan BIF-type iron ore deposit mainly originated from the interaction between high-temperature hydrothermal fluids discharged from the seafloor and seawater, with a minor contribution from terrigenous materials.
- (3).
- The alternating siliceous and ferruginous layers of the Hugushan BIFs are primary sedimentary products, which might be attributed to the cyclic precipitation of iron and silicon in seawater.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sample | 23W2-1 | 23W2-2 | 23W2-3 | 23W2-10 | 23W3-4 | 23W3-5 | 23W3-7 |
|---|---|---|---|---|---|---|---|
| SiO2 | 45.50 | 43.72 | 44.00 | 51.08 | 15.52 | 19.12 | 17.24 |
| Al2O3 | 0.80 | 0.72 | 0.71 | 0.86 | 1.39 | 1.24 | 1.34 |
| Fe2O3T | 50.64 | 52.73 | 52.62 | 44.34 | 79.68 | 76.31 | 77.64 |
| CaO | 0.78 | 1.28 | 0.78 | 2.06 | 2.14 | 1.97 | 1.85 |
| MgO | 1.99 | 1.24 | 1.44 | 1.94 | 2.00 | 1.84 | 1.73 |
| K2O | 0.14 | 0.11 | 0.11 | 0.11 | 0.22 | 0.18 | 0.17 |
| Na2O | 0.68 | 0.51 | 0.42 | 0.04 | 0.09 | 0.06 | 0.08 |
| TiO2 | 0.02 | 0.02 | 0.02 | 0.02 | 0.72 | 0.68 | 0.70 |
| P2O5 | 0.11 | 0.16 | 0.06 | 0.35 | 0.11 | 0.15 | 0.13 |
| MnO | 0.05 | 0.03 | 0.03 | 0.03 | 0.13 | 0.17 | 0.14 |
| LOI | −1.05 | −0.74 | −0.61 | −1.14 | −2.26 | −2.01 | −1.14 |
| Total | 99.64 | 99.77 | 99.59 | 99.69 | 99.75 | 99.71 | 99.88 |
| Li | 28.32 | 27.16 | 29.72 | 15.11 | 14.51 | 14.32 | 15.02 |
| Be | 1.66 | 1.11 | 1.21 | 1.85 | 3.76 | 3.12 | 3.71 |
| Sc | 0.70 | 0.74 | 1.54 | 2.02 | 7.70 | 7.36 | 7.64 |
| V | 5.32 | 3.75 | 3.57 | 7.70 | 214.70 | 224.52 | 235.14 |
| Cr | 16.05 | 21.61 | 42.83 | 35.16 | 40.12 | 39.19 | 41.51 |
| Co | 0.78 | 0.56 | 0.78 | 1.32 | 21.85 | 19.78 | 24.72 |
| Ni | 7.69 | 9.16 | 13.77 | 13.55 | 28.25 | 24.45 | 27.77 |
| Ga | 0.57 | 0.41 | 1.60 | 1.79 | 7.83 | 7.14 | 8.41 |
| Rb | 2.27 | 1.02 | 1.07 | 2.90 | 20.83 | 22.72 | 19.45 |
| Sr | 15.43 | 44.38 | 20.72 | 32.45 | 71.24 | 68.13 | 72.15 |
| Y | 4.52 | 5.69 | 5.86 | 10.89 | 8.25 | 7.98 | 8.01 |
| Zr | 4.63 | 4.16 | 3.12 | 5.31 | 3.58 | 3.64 | 3.24 |
| Nb | 0.15 | 0.09 | 0.09 | 0.23 | 0.77 | 0.53 | 0.81 |
| Cs | 0.20 | 0.10 | 0.08 | 1.03 | 6.96 | 6.87 | 6.91 |
| Ba | 6.75 | 8.87 | 20.94 | 20.18 | 64.56 | 57.41 | 68.42 |
| La | 1.68 | 1.86 | 2.55 | 3.53 | 2.66 | 2.34 | 2.74 |
| Ce | 2.98 | 3.18 | 4.96 | 8.37 | 8.04 | 7.81 | 8.35 |
| Pr | 0.38 | 0.40 | 0.63 | 1.09 | 0.86 | 0.91 | 0.95 |
| Nd | 1.36 | 1.42 | 2.16 | 3.95 | 3.55 | 3.42 | 3.95 |
| Sm | 0.29 | 0.31 | 0.51 | 0.88 | 0.98 | 0.87 | 0.94 |
| Eu | 0.20 | 0.21 | 0.26 | 0.52 | 0.41 | 0.37 | 0.44 |
| Gd | 0.49 | 0.47 | 0.66 | 1.19 | 1.27 | 1.13 | 1.37 |
| Tb | 0.10 | 0.10 | 0.13 | 0.24 | 0.26 | 0.25 | 0.25 |
| Dy | 0.61 | 0.68 | 0.89 | 1.56 | 1.62 | 1.57 | 1.66 |
| Ho | 0.16 | 0.17 | 0.21 | 0.38 | 0.37 | 0.37 | 0.39 |
| Er | 0.51 | 0.61 | 0.71 | 1.23 | 1.08 | 1.04 | 1.03 |
| Tm | 0.09 | 0.10 | 0.11 | 0.20 | 0.17 | 0.14 | 0.11 |
| Yb | 0.52 | 0.63 | 0.67 | 1.26 | 1.07 | 1.03 | 1.22 |
| Lu | 0.11 | 0.11 | 0.11 | 0.20 | 0.17 | 0.16 | 0.17 |
| Hf | 0.17 | 0.15 | 0.11 | 0.18 | 0.22 | 0.21 | 0.19 |
| Ta | 0.08 | 0.06 | 0.06 | 0.06 | 0.13 | 0.11 | 0.14 |
| Tl | 0.04 | 0.02 | 0.02 | 0.04 | 0.12 | 0.13 | 0.11 |
| Pb | 1.06 | 1.27 | 0.92 | 1.50 | 2.70 | 2.34 | 2.47 |
| Th | 0.15 | 0.15 | 0.61 | 1.34 | 0.88 | 0.77 | 0.97 |
| U | 0.05 | 0.03 | 0.03 | 0.08 | 0.33 | 0.27 | 0.29 |
| δEu | 1.60 | 1.68 | 1.38 | 1.54 | 1.12 | 1.14 | 1.18 |
| ΣREE | 9.48 | 10.24 | 14.57 | 24.59 | 22.50 | 21.41 | 23.57 |
| LREE/HREE | 2.66 | 2.58 | 3.17 | 2.94 | 2.75 | 2.76 | 2.80 |
| (La/Yb)SN | 0.24 | 0.22 | 0.28 | 0.21 | 0.18 | 0.17 | 0.17 |
| (Sm/Yb)SN | 0.29 | 0.25 | 0.39 | 0.36 | 0.46 | 0.43 | 0.39 |
| (δEu)SN | 2.63 | 2.75 | 2.24 | 2.51 | 1.81 | 1.85 | 1.94 |
| (Ce/Ce*)SN | 0.86 | 0.85 | 0.89 | 0.97 | 1.20 | 1.19 | 1.16 |
| (Nd/Yb)SN | 0.22 | 0.19 | 0.27 | 0.26 | 0.28 | 0.28 | 0.27 |
| (Gd/Gd*)SN | 0.72 | 0.67 | 0.72 | 0.69 | 0.78 | 0.73 | 0.84 |
| La/La* | 0.91 | 0.94 | 0.76 | 0.67 | 0.84 | 0.57 | 0.80 |
| Y/Ho | 28.02 | 33.94 | 28.02 | 28.80 | 22.30 | 21.57 | 20.54 |
| Eu/Sm | 0.68 | 0.68 | 0.51 | 0.59 | 0.42 | 0.43 | 0.47 |
| Sm/Yb | 0.56 | 0.49 | 0.76 | 0.70 | 0.91 | 0.84 | 0.77 |
| Sample | Description | Subject | δ56Fe (‰) | 2σ | δ57Fe (‰) | 2σ |
|---|---|---|---|---|---|---|
| 23W2-2 | Magnetite quartzite | Magnetite | 0.38 | 0.01 | 0.55 | 0.05 |
| 23W2-3 | Magnetite quartzite | Magnetite | 0.21 | 0.03 | 0.31 | 0.01 |
| 23W2-10 | Magnetite quartzite | Magnetite | 0.01 | 0.02 | 0.02 | 0.02 |
| 23W3-5 | Amphibole quartz magnetite | Magnetite | −0.11 | 0.06 | −0.16 | 0.03 |
| 23W3-6 | Amphibole quartz magnetite | Magnetite | −0.13 | 0.03 | −0.19 | 0.02 |
| 23W3-7 | Amphibole quartz magnetite | Magnetite | −0.19 | 0.05 | −0.26 | 0.03 |
| 23W3-8 | Amphibole quartz magnetite | Magnetite | −0.21 | 0.05 | −0.31 | 0.03 |
| Sample | Description | Subject | δ18OV-SMOW (‰) | δ30SiNBS-28 (‰) |
|---|---|---|---|---|
| 23W2-2 | Magnetite quartzite | Quartz | 15.50 | −1.1 |
| 23W2-3 | Magnetite quartzite | Quartz | 15.83 | −1.3 |
| 23W2-10 | Magnetite quartzite | Quartz | 13.16 | −1.1 |
| 23W3-5 | Amphibole quartz magnetite | Quartz | 13.79 | −0.9 |
| 23W3-6 | Amphibole quartz magnetite | Quartz | 12.62 | −1.5 |
| 23W3-7 | Amphibole quartz magnetite | Quartz | 11.95 | −1.7 |
| 23W3-8 | Amphibole quartz magnetite | Quartz | 11.43 | −2.1 |
References
- Hagemann, S.G.; Angerer, T.; Duuring, P.; Rosière, C.A.; Figueiredoe, S.R.C.; Lobato, L.; Hensler, A.S.; Walde, D.H.G. BIF-hosted iron mineral system: A review. Ore Geol. Rev. 2016, 76, 317–359. [Google Scholar] [CrossRef]
- Bekker, A.; Slack, J.F.; Planavsky, N.; Krapez, B.; Hofmann, A.; Konhauser, K.O.; Rouxel, O.J. Iron Formation: The Sedimentary Product of a Complex Interplay among Mantle, Tectonic, Oceanic, and Biospheric Processes. Econ. Geol. 2010, 105, 467–508. [Google Scholar] [CrossRef]
- James, R.H.; Elderfield, H.; Palmer, M.R. The chemistry of hydrothermal fluids from the broken spur site, 29-degrees-n mid-atlantic ridge. Geochim. Cosmochim. Acta 1995, 59, 651–659. [Google Scholar] [CrossRef]
- Shen, B.F.; Luo, H.; Jin, W.-S.; Li, S.B.; Chen, Y.H. Metallogenic model of the Archean BIF iron deposits in the North China Craton. Ore Geol. Rev. 2009, 35, 40–51. [Google Scholar]
- Fu, J.F.; Luan, J.P.; Jia, S.S.; Luo, Y.H.; Chen, J.D.; Zhao, F.Q. Fe-Si-C isotope constraints on the genesis of iron ores in Gongchangling iron deposit in northeastern China. J. Geochem. Explor. 2023, 250, 107233. [Google Scholar] [CrossRef]
- Tong, X.X.; Mänd, K.; Li, Y.H.; Zhang, L.C.; Peng, Z.D.; Wu, Q.; Li, P.B.; Zhai, M.G.; Robbins, L.J.; Wang, C.L. Iron and Carbon Isotope Constraints on the Formation Pathway of Iron-Rich Carbonates within the Dagushan Iron Formation, North China Craton. Minerals 2021, 11, 94. [Google Scholar] [CrossRef]
- Li, X.P.; Chen, Y.R. A brief discussion on the depositional and metamorphic mineralization of Precambrian banded iron formations. Acta Petrol. Sin. 2021, 37, 253–268. [Google Scholar]
- Wang, C.L.; Zhang, L.C.; Lan, C.Y.; Dai, Y.P. Rare earth element and yttrium compositions of the Paleoproterozoic Yuanjiacun BIF in the Luliang area and their implications for the Great Oxidation Event (GOE). Sci. China Earth Sci. 2014, 57, 2469–2485. [Google Scholar] [CrossRef]
- Tong, X.X.; Zhang, L.C.; Wang, C.L.; Peng, Z.D.; Zhai, M.G. Depositional and environmental constraints on the late Neoarchean Dagushan–Hugushan banded iron formation, North China Craton: An Algoma-type BIF with controversial genesis. Precambrian Res. 2021, 359, 106178. [Google Scholar]
- Zhai, M.G.; Guo, J.H.; Liu, W.J. Neoarchean to Paleoproterozoic continental evolution and tectonic history of the North China Craton. J. Asian Earth Sci. 2005, 24, 547–561. [Google Scholar] [CrossRef]
- Wang, H.C.; Miao, P.S.; Kang, J.L.; Ren, Y.W.; Zhang, J.H.; Li, J.R.; Xian, Z.Q.; Xiao, Z.B. New Evidence for the formation age of the Luliang Group. Acta Petrol. Sin. 2020, 36, 2313–2330. [Google Scholar]
- Zhao, G.C.; Wilde, S.A.; Sun, M.; Li, S.Z.; Li, X.P.; Zhang, J. SHRIMP U-Pb zircon ages of granitoid rocks in the Lüliang Complex: Implications for the accretion and evolution of the Trans-North China Orogen. Precambrian Res. 2008, 160, 213–226. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Dai, Y.; Lan, C.Y. Geochronological and geochemical constraints on the origin of clastic meta-sedimentary rocks associated with the Yuanjiacun BIF from the Lvliang Complex, North China. Lithos 2015, 212, 231–246. [Google Scholar] [CrossRef]
- Geng, Y.S.; Wan, Y.S.; Shen, Q.H.; Li, H.M.; Zhang, R.X. Chronologic framework of the Early Precambrain imoportant events in the Luliang area, Shanxi Province. Acta Geol. Sin. 2000, 74, 216–223. [Google Scholar]
- Yu, J.H.; Wang, D.Z.; Wang, C.Y.; Li, H.M. Ages of the Lüliang Group and its main metamorphism in the Lüliang Mountains, Shanxi: Evidence from single-grain zircon U-Pb ages. Geol. Rev. 1997, 43, 403–408. [Google Scholar]
- Liu, S.W.; Zhang, J.; Li, Q.G.; Zhang, L.F.; Wang, W.; Yang, P.T. Geochemistry and U-Pb zircon ages of metamorphic volcanic rocks of the Paleoproterozoic Lüliang Complex and constraints on the evolution of the Trans-North China Orogen, North China Craton. Precambrian Res. 2012, 222–223, 173–190. [Google Scholar] [CrossRef]
- Wan, Y.S.; Geng, Y.S.; Shen, Q.H.; Zhang, R.X. Khondalite series- geochronology and geochemistry of the Jiehekou Group in Luliang area, Shanxi province. Acta Petrol. Sin. 2000, 16, 49–58. [Google Scholar]
- Li, J.X.; Hu, T.Y.; Liu, L. Metallogenie Age and Metallogenic Environment of Yuanjiacun lron Deposit in Shanxi Province. Earth Sci. 2023, 48, 12. [Google Scholar]
- Geng, Y.S.; Yang, C.H.; Wan, Y.S. Paleoproterozoic granitic magmatism in the Lüliang area, North China Craton: Constraint from isotopic geochronology. Acta Petrol. Sin. 2006, 22, 305–314, (In Chinese with English Abstract). [Google Scholar]
- Liu, Y.G. 3D Geological Modeling and Metallogenic Prediction of Hugushan Iron Deposit in Shanxi Province; Central South University: Changsha, China, 2023; pp. 1–102. [Google Scholar]
- Chen, J.Z.; Zhao, J.L.; Wang, C. Rock and Mineral Analysis, 4th ed.; Geological Publishing House: Beijing, China, 2011; pp. 1–904. [Google Scholar]
- Hou, K.J.; Li, Y.H.; Gao, J.F.; Liu, F.; Qin, Y. Geochemistry and Si-O-Fe isotope constraints on the origin of banded iron formations of the Yuanjiacun Formation, Lvliang Group, Shanxi, China. Ore Geol. Rev. 2014, 57, 288–298. [Google Scholar] [CrossRef]
- Clayton, R.N.; Mayeda, T.K. The use of bromine pentafluoride in the extraction of oxygen from oxides and silicates for isotopic analysis. Geochim. Cosmochim. Acta 1963, 27, 43–52. [Google Scholar] [CrossRef]
- Ding, T.P.; Jiang, S.Y.; Wan, D.F.; Li, Y.H.; Li, J.C.; Song, H.B.; Liu, Z.J.; Yao, X.M. Silicon Isotope Geochemistry; Geological Publishing House: Beijing, China, 1996; pp. 1–122. [Google Scholar]
- Sun, S.S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- McLennan, S.M. Rare earth elements in sedimentary rocks: Influence of provenance and sedimentary processes. Rev. Mineral. 1989, 21, 169–200. [Google Scholar]
- Beard, B.L.; Johnson, C.M.; Von Damm, K.L.; Poulson, R.L. Iron isotope constraints on Fe cycling and mass balance in oxygenated Earth oceans. Geol. Soc. Am. 2003, 31, 629–632. [Google Scholar] [CrossRef]
- Fantle, M.S.; DePaolo, D.J. Iron isotopic fractionation during continental weathing. Earth Planet. Sci. Lett. 2004, 228, 547–562. [Google Scholar] [CrossRef]
- Rouxel, O.; Dobbek, N.; Ludden, J.; Fouquet, Y. Iron isotope fractionation during oceanic crust alteration. Chem. Geol. 2003, 202, 155–182. [Google Scholar] [CrossRef]
- Zhu, X.K.; Guo, Y.L.; O’Nions, R.K.; Yound, E.D.; Ash, R.D. Isotopic homogeneity of iron in the early solar nebula. Nature 2001, 412, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Rouxel, O.; Bekker, A.; Edwards, K.J. Iron isotope constraints on the Archean and Paleoproterozoic ocean redox state. Science 2005, 307, 1088–1091. [Google Scholar] [CrossRef]
- Dauphas, N.; Janney, P.E.; Mendybaev, R.A.; Wadhwa, M.; Richter, F.M.; Davis, A.M.; Zuilen, M.; Hines, R.; Foley, C.N. Chromatographic separation and multicollection-ICPMS analysis of iron. Investigating mass dependent and -independent isotope effects. Anal. Chem. 2004, 76, 5855–5863. [Google Scholar] [CrossRef]
- Johnson, C.M.; Beard, B.L.; Beukes, N.; Klein, C.; O’Leary, J.M. Ancient geochemical cycling in the Earthas inferred from Fe isotope studies of banded iron formations from the Transvaal Craton, Contrib. Mineral. Petrol. 2003, 144, 523–547. [Google Scholar] [CrossRef]
- Men, Y.K.; Mi, Z.J.; Wang, E.D.; Han, L.D.; Wang, Y.J.; Jia, S.S.; Xia, J.M.; Song, K. Geology and Geochemistry of the Jianshan Banded Iron Formation in Shanxi Province, China: Constraints on the Genesis. J. Geol. 2022, 130, 499–518. [Google Scholar] [CrossRef]
- Douthitt, C.B. The geochemistry of the stable isotopes of silicon. Geochim. Cosmochim. Acta 1982, 46, 1449–1458. [Google Scholar] [CrossRef]
- Taylor, H.P., Jr.; Sheppard, S.M.F. Igneous rocks: II. The processes of isotopic fractionation and isotope systematics. Rev. Mineral. 1986, 16, 227–271. [Google Scholar]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef]
- Zhang, L.; Planavsky, N.J.; Wang, X.; Wang, G.; Reinhard, C.T. Silicon isotope evidence for widespread anoxic and iron-rich surface waters in Mesoproterozoic oceans. Earth Planet. Sci. Lett. 2020, 531, 115964. [Google Scholar]
- Ehlert, C.; Grasse, P.; Frank, M. Variations of silicic acid isotope composition (δ30Si) in the ocean: Reconstruction of marine weathering and reverse weathering. Geochim. Cosmochim. Acta 2016, 177, 1–18. [Google Scholar]
- Sun, J.; Zhu, X.K.; Li, S.Z. Iron Isotope Geochemical Behavior and Applications in Biological Processes. Acta Geol. Sin. 2015, 34, 777–784. [Google Scholar]
- Bullen, T.D.; White, A.F.; Childs, C.W.; Vivit, D.V.; Schulz, M.S. Demonstration of significant abiotic iron isotope fractionation in nature. Geology 2001, 29, 699–702. [Google Scholar] [CrossRef]
- Balci, N.; Bullen, T.D.; Witte-Lien, K.; Shanks, W.C.; Motelica, M.; Mandernack, K.W. Iron isotope fractionation during microbially stimulated Fe(II) oxidation and Fe(III) precipitation. Geochim. Cosmochim. Acta 2006, 70, 622–639. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, W.H.; Zhang, W.; Bai, H.F.; Li, Z.Y.; Wang, X.F.; Zhang, D.D.; Chen, X.Y.; Li, W.H. Formation conditions of Jixian System cherts in the Qishan area, Ordos Basin: Implications for marine redox conditions and paleoecology. Sediment. Geol. 2024, 467, 106651. [Google Scholar] [CrossRef]
- Kumar, R.; Hameed, A.; Tiwari, P.; Kumar, N.; Srivastava, P. Major, trace and rare earth element geochemistry of Archaean carbonate sediments of Tanwan group rocks of the Bhilwara supergroup, India: Implications for seawater geochemistry and depositional environment. Carbonates Evaporites 2024, 39, 14. [Google Scholar] [CrossRef]
- Shimizu, H.; Umemoto, N.; Masuda, A. Sources of iron-fommations in the Archean lsua and Malene supracrustals West Greenland Evidence from La-Ce and Sm-Nd isotopic data and REE abundances. Geochim. Cosmochim. Acta 1990, 54, 1147–1154. [Google Scholar] [CrossRef]
- Wheat, C.G.; Mottl, M.J.; Rudnicki, M. Trace element and REE composition of a low-Temperature ridge-flank hydrothermal spring. Geochim. Cosmochim. Acta 2002, 66, 3693–3705. [Google Scholar] [CrossRef]
- Bau, M. Effects of syn- and post-depositional processes on the rare-earth element distribution in Precambrian iron-formations. Eur. Miner. 1993, 5, 257–267. [Google Scholar] [CrossRef]
- Bau, M.; Dulski, P. Distribution of yttrium and rare-earth elements in the Penge and Kuruman iron-formations, Transvaal Supergroup, South Africa. Precambrian Res. 1996, 79, 37–55. [Google Scholar] [CrossRef]
- Alibo, D.S.; Nozaki, Y. Rare earth elements in seawater: Particle association, shale-normalization, and Ce oxidation. Geochim. Cosmochim. Acta 1999, 63, 363–372. [Google Scholar] [CrossRef]
- Tanaka, K.; Tani, Y.; Takahashi, Y.; Tanimizu, M.; Suzuki, Y.; Kozai, N.; Ohnuki, T. A specific Ce oxidation process during sorption of rare earth elements on biogenic Mn oxide produced by Acremonium sp. strain KR21-2. Geochim. Cosmochim. Acta 2010, 74, 5463–5477. [Google Scholar] [CrossRef]
- Ohta, A.; Kawabe, I. REE(III) adsorption onto Mn dioxide (δ-MnO2) and Fe oxyhydroxide: Ce(III) oxidation by δ-MnO2. Geochim. Cosmochim. Acta 2001, 65, 695–703. [Google Scholar] [CrossRef]
- Acadi, T. Rare earth element (REE)–silicic acid complexes in seawater to explain the incorporation of REEs in opal and the “leftover” REEs in surface water: New interpretation of dissolved REE distribution profiles. Geochim. Cosmochim. Acta 2013, 113, 174–192. [Google Scholar]
- Hathorne, E.C.; Stichel, N.; Brück, B.; Frank, M. Rare earth element distribution in the Atlantic sector of the Southern Ocean: The balance between particle scavenging and vertical supply. Mar. Chem. 2015, 177, 157–171. [Google Scholar] [CrossRef]
- Sholkovitz, E.R.; Landing, W.M.; Lewis, B.L. Ocean particle chemistry: The fractionation of rare earth elements between suspended particles and seawater. Geochim. Cosmochim. Acta 1994, 58, 1567–1579. [Google Scholar] [CrossRef]
- Hatje, V.; Schijf, J.; Johannesson, K.H.; Andrade, R.; Caetano, M.; Brito, P.; Haley, B.A.; Lagarde, M.; Jeandel, C. The global biogeochemical cycle of the rare earth elements. Glob. Biogeochem. Cycles 2024, 38, e2024GB008125. [Google Scholar] [CrossRef]
- Planavsky, N.; Bekker, A.; Rouxel, O.J.; Kamber, B.; Hofmann, A.; Knudsen, A.; Lyons, T.W. Rare earth element and yttrium compositions of Archean and Paleoproterozoic Fe formations revisited: New perspectives on the significance and mechanisms of deposition. Geochim. Cosmochim. Acta 2010, 74, 6387–6405. [Google Scholar] [CrossRef]
- Basta, F.F.; Maurice, A.E.; Fontboté, L.; Favarger, P.Y. Petrology and geochemistry of the banded iron formation (BIF) of Wadi Karim and Um Anab, Eastern Desert Egypt: Implications for the origin of Neoproterozoic BIF. Precambrian Res. 2011, 187, 277–292. [Google Scholar] [CrossRef]
- Hu, J.; Wang, H.; Wang, M. Geochemistry and origin of the Neoproterozoic Dahongliutan banded iron formation (BIF) in the Western Kunlun orogenic belt, Xinjiang (NW China). Ore Geol. Rev. 2017, 89, 836–857. [Google Scholar] [CrossRef]
- Iderbayar, B.; Oyungerel, S.; Kim, Y. Geochemistry and depositional environment of the Neoproterozoic Ereen and Dartsagt banded iron formation (BIF) deposits in the Idermeg terrane, eastern Mongolia. Geosci. J. 2024, 28, 179–192. [Google Scholar] [CrossRef]
- Sun, X.H.; Zhu, X.Q.; Tang, H.S.; Zhang, Q.; Luo, T.Y. The Gongchangling BIFs from the Anshan-Benxi area, NE China: Petrological-geochemical characteristics and genesis of high-grade iron ores. Ore Geol. Rev. 2014, 60, 112–125. [Google Scholar] [CrossRef]
- Belevtsev, Y.N.; Belevtsev, R.Y.; Siroshtan, R.I. The Krivoy Rog Basin. In Iron-Formation: Facts and Problems; Trendall, A.F., Morris, R.C., Eds.; Elsevier: Amsterdam, The Netherlands, 1982; pp. 211–252. [Google Scholar]
- Yin, T.T.; Jia, R.F.; Xiong, Y.Q.; Zhao, C.C. Determination of the banded iron formation sources in the Lanling area of Western Shandong of the North China Craton through rare earth element testing. Carbonates Evaporites 2024, 39, 87. [Google Scholar] [CrossRef]
- Chen, Y.J.; Yang, J.Q.; Deng, J.; Ji, H.Z.; Fu, S.G.; Zhou, X.P.; Lin, Q. An important change in Earth’s evolution: An environmental catastrophe at 2300 Ma and its implications. Geol. Geochem. 1996, 3, 106–128. [Google Scholar]
- Kato, Y.; Kawakami, T.; Kano, T.; Kunugiza, K.; Swamy, N.S. Rare-earth element geochemistry of banded iron formations and associated amphibolite from the Sargur belts, south India. J. S. Asian Earth Sci. 1996, 14, 161–164. [Google Scholar] [CrossRef]
- Tang, H.S.; Chen, Y.J.; Santosh, M.; Zhong, H.; Yang, T. REE geochemistry of carbonates from the Guanmenshan Formation, Liaohe Group NE Sino-Korean Craton: Implications for seawater compositional change during the Great Oxidation Event. Precambrian Res. 2013, 227, 316–336. [Google Scholar] [CrossRef]
- Nan, J.B.; Huang, H.; Wang, C.L.; Peng, Z.D.; Tong, X.X.; Zhang, L.C. Geochemistry and depositional setting of Banded Iron Formations in Guyang greenstone belt, Inner Mongolia. Geol. China 2017, 44, 331–345. [Google Scholar]
- Calvert, S.E.; Pedersen, T.F. Geochemistry of recent oxic and anoxic marine sediments: Implications for the geological record. Mar. Geol. 1993, 113, 67–88. [Google Scholar] [CrossRef]
- Troll, V.R.; Weis, F.A.; Jonsson, E.; Andersson, U.B.; Majidi, S.A.; Hogdahl, K.; Harris, C.; Millet, M.A.; Chinnasamy, S.S.; Kooiiman, E.; et al. Global Fe-O isotope correlation reveals magmatic origin of Kiruna-type apatite-iron-oxide ores. Nature 2019, 10, 1712. [Google Scholar] [CrossRef]
- Hildebrand, R.S. Kiruna-type deposits: Their origin and relationship to intermediate subvolcanic plutons in the Great Bear magmatic zone, northwest Canada. Econ. Geol. 1986, 81, 640–659. [Google Scholar] [CrossRef]
- González, P.D.; Sato, A.M.; Llambias, E.J. Petrology and geochemistry of the banded iron formation in the Eastern Sierras Pampeanas of San Luis (Argentina): Implications for the evolution of the Nogoli Metamorphic Complex. J. S. Am. Earth Sci. 2009, 28, 89–112. [Google Scholar] [CrossRef]
- Klinkhammer, G.P.; Elderfield, H.; Edmond, J.M.; Mitra, A. Geochemical implications of rare earth element patterns in hydrothermal fluids from mid-ocean ridges. Geochim. Cosmochim. Acta 1994, 58, 5105–5113. [Google Scholar] [CrossRef]
- German, C.R.; Klinkhammer, G.P.; Edmond, J.M.; Mura, A.; Elderfield, H. Hydrothermal scavenging of rare–earth elements in the ocean. Nature 1990, 345, 516–518. [Google Scholar] [CrossRef]
- Douville, E.; Charlou, J.L.; Oelkers, E.H.; Bienvenu, P.; Colon, C.F.J.; Donval, J.P.; Fouquet, Y.; Prieur, D.; Appriou, P. The rainbow vent fluids (36°14′ N, MAR): The influence of ultramafic rocks and phase separation on trace metal content in Mid-Atlantic Ridge hydrothermal fluids. Chem. Geol. 2002, 184, 37–48. [Google Scholar] [CrossRef]
- Danielson, A.; Moller, P.; Dulski, P. The europium anomalies in banded iron formations and the thermal history of the oceanic crust. Chem. Geol. 1992, 97, 89–100. [Google Scholar] [CrossRef]
- Dai, Y.P.; Zhang, L.C.; Zhu, M.T.; Wang, C.L.; Liu, L.; Xiang, P. The composition and genesis of the Mesoarchean Dagushan banded iron formation (BIF) in the Anshan area of the North China Craton. Ore Geol. Rev. 2014, 63, 353–373. [Google Scholar] [CrossRef]
- Klein, C. Some Precambrian banded iron-formations (BIFs) from around the world: Their age, geologic setting, mineralogy, metamorphism, geochemistry, and orgin. Am. Mineral. 2005, 90, 1473–1499. [Google Scholar] [CrossRef]
- Keyser, W.; Ciobanu, C.L.; Cook, N.J. Petrography and trace element signatures of iron-oxides in deposits from the Middleback Ranges, South Australia: From banded iron formation to ore. Ore Geol. Rev. 2018, 93, 337–360. [Google Scholar] [CrossRef]
- Wonder, J.D.; Spry, P.G.; Windom, K.E. Geochemistry and origin of manganese-rich rocks related to iron-formation and sulfide deposits, western Georgia. Econ. Geol. 1988, 83, 1070–1081. [Google Scholar] [CrossRef]
- Nozaki, Y.; Zhang, J.; Amakawa, H. The fractionation between Y and Ho in the marine environment. Earth Planet. Sci. Lett. 1997, 148, 329–340. [Google Scholar] [CrossRef]
- Bau, M.; Dulski, P. Comparing yttrium and rare earths in hydrothermal fluids from the Mid-Atlantic Ridge: Implications for Y and REE behaviour during near-vent mixing and for the Y/Ho ratio of Proterozoic seawater. Chem. Geol. 1999, 155, 77–90. [Google Scholar] [CrossRef]
- Alexander, B.W.; Bau, M.; Andersson, P.; Dulski, P. Continentally-derived solutes in shallow Archean seawater: Rare earth element and Nd isotope evidence in iron formation from the 2.9 Ga Pongola Supergroup, South Africa. Geochim. Cosmochim. Acta 2008, 72, 378–394. [Google Scholar] [CrossRef]
- Gao, X.Y.; Wang, D.H.; Huang, F.; Wang, Y.; Wang, C.H. Chronolgy and Geochemistry of the Sijiaying Iron Deposit in Eastern Hebei Province, North China Craton: Implications for the Genesis of High-Grade Iron Ores. Minerals 2023, 13, 775. [Google Scholar] [CrossRef]
- Han, X.; Liu, J.L. Genesis of the Neoarchean Algoma-type banded iron formation: Constraints from Fe isotope and element geochemistry of the Qian’an iron deposit, eastern North China craton. Precambrian Res. 2024, 410, 107480. [Google Scholar] [CrossRef]
- Holland, H.D. The Chemical Evolution of the Atmosphere and Oceans; Princeton University Press: New York, NY, USA, 1984; pp. 1–582. [Google Scholar]
- Bekker, A.; Holland, H.D.; Wang, P.L.; Rumble, D.; Stein, H.J.; Hannah, J.L.; Coetzee, L.L.; Beukes, N.J. Dating the rise of atmospheric oxygen. Nature 2004, 427, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Wilde, S.A.; Zhao, G.C.; Sun, M. Development of the North China Craton during the Late Archaean and its final amalgamation at 1.8 Ga: Some speculations on its position within a global Palaeoproterozoic supercontinent. Gondwana Res. 2002, 5, 85–94. [Google Scholar] [CrossRef]
- Sujith, P.P.; Gonsalves, M.J.B.D. Ferromanganese oxide deposits: Geochemical and microbiological perspectives of interactions of cobalt and nickel. Ore Geol. Rev. 2021, 139, 104458. [Google Scholar] [CrossRef]
- Trendall, A.F.; Blockley, J.G. The iron formations of the Precambrian Hamersley Group, Western Australia with special reference to the cmcidolite. Geol. Surv. West. Aust. Bull. 1970, 119, 366. [Google Scholar]
- Morris, R.C. Genetic modelling for banded iron formation of the Hamersley Group, Pilbara Craton, Western Australia. Precambrian Res. 1993, 60, 243–286. [Google Scholar] [CrossRef]
- Webb, A.D.; Dickens, G.R.; Oliver, N.H.S. From banded iron-formation to iron ore: Geochemical and mineralogical constraints fromacrss the Hamersley Province, Western Australia. Chem. Geol. 2003, 197, 215–251. [Google Scholar] [CrossRef]
- Bolhar, R.; Kamber, B.S.; Moorbah, S. Characterisation of early Archaean chemical sediments by trace element signatures. Earth Planet. Sci. Lett. 2004, 222, 43–60. [Google Scholar] [CrossRef]
- Steinhoefel, G.; Horn, I.; Blanckenburg, F. Micro-scale tracing of Fe and Si isotope signatures in banded iron formation using femtosecond laser ablation. Geochim. Cosmochim. Acta 2009, 73, 5343–5360. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, E.; Zhang, D.; Luan, J.; Men, Y.; Wang, R.; Xia, J.; Zhang, S. Fe–Si–O Isotope Characteristics and Ore Formation Mechanisms of the Hugushan Area BIF-Type Iron Deposits in the Central North China Craton. Minerals 2025, 15, 996. https://doi.org/10.3390/min15090996
Wang E, Zhang D, Luan J, Men Y, Wang R, Xia J, Zhang S. Fe–Si–O Isotope Characteristics and Ore Formation Mechanisms of the Hugushan Area BIF-Type Iron Deposits in the Central North China Craton. Minerals. 2025; 15(9):996. https://doi.org/10.3390/min15090996
Chicago/Turabian StyleWang, Ende, Deqing Zhang, Jinpeng Luan, Yekai Men, Ran Wang, Jianming Xia, and Suibo Zhang. 2025. "Fe–Si–O Isotope Characteristics and Ore Formation Mechanisms of the Hugushan Area BIF-Type Iron Deposits in the Central North China Craton" Minerals 15, no. 9: 996. https://doi.org/10.3390/min15090996
APA StyleWang, E., Zhang, D., Luan, J., Men, Y., Wang, R., Xia, J., & Zhang, S. (2025). Fe–Si–O Isotope Characteristics and Ore Formation Mechanisms of the Hugushan Area BIF-Type Iron Deposits in the Central North China Craton. Minerals, 15(9), 996. https://doi.org/10.3390/min15090996