Dry Concentration of Phosphate Ore by Using a Triboelectrostatic Belt Separator in Pilot Scale
Abstract
1. Background
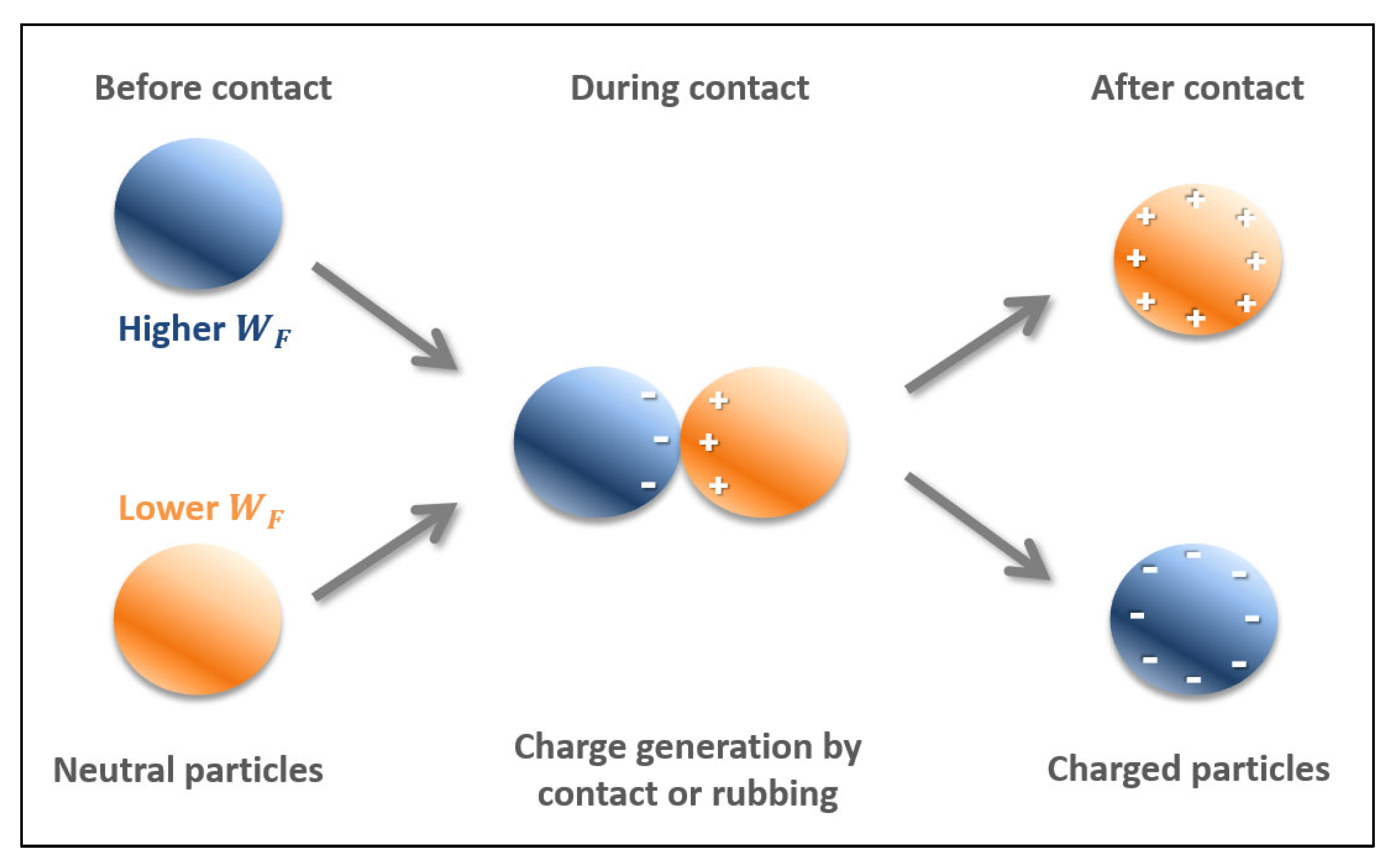
1.1. Triboelectrostatic Separation
1.2. Factors Influencing the Separation Process
- Temperature of the processed material: The charging behavior of phosphate minerals is temperature-dependent, which directly affects their separation from silicate and carbonate gangue minerals [36].
- Electric field geometry: Asymmetries in electrode configuration may produce low-intensity regions, insufficient to deflect denser particles, thereby reducing selectivity [11].
- Turbulence in the inter-electrode gap: Aerodynamic instabilities between electrodes can alter particle trajectories, leading to drag-related losses and cross-contamination of product streams [11].
- Residence time: Prolonged residence times may increase particle accumulation on electrodes, causing overloading, while turbulence induced by the counter-current belt motion may dislodge weakly adhered particles. Both phenomena reduce recovery efficiency and separation selectivity [11].
- Residual particle moisture: Surface moisture interferes with triboelectric charging mechanisms, diminishing their effectiveness [11].
- Particle size and shape distribution: Morphology and granulometry affect the mass-to-surface ratio, thereby influencing particle responses to both aerodynamic drag and electrostatic forces [11].
- Charging via belt contact: Heavier particles exert greater pressure on the belt surface and on adjacent particles, enhancing triboelectric charging through intensified contact. The belt’s material properties and surface conditions strongly affect particle polarization and, consequently, separation efficiency [11].
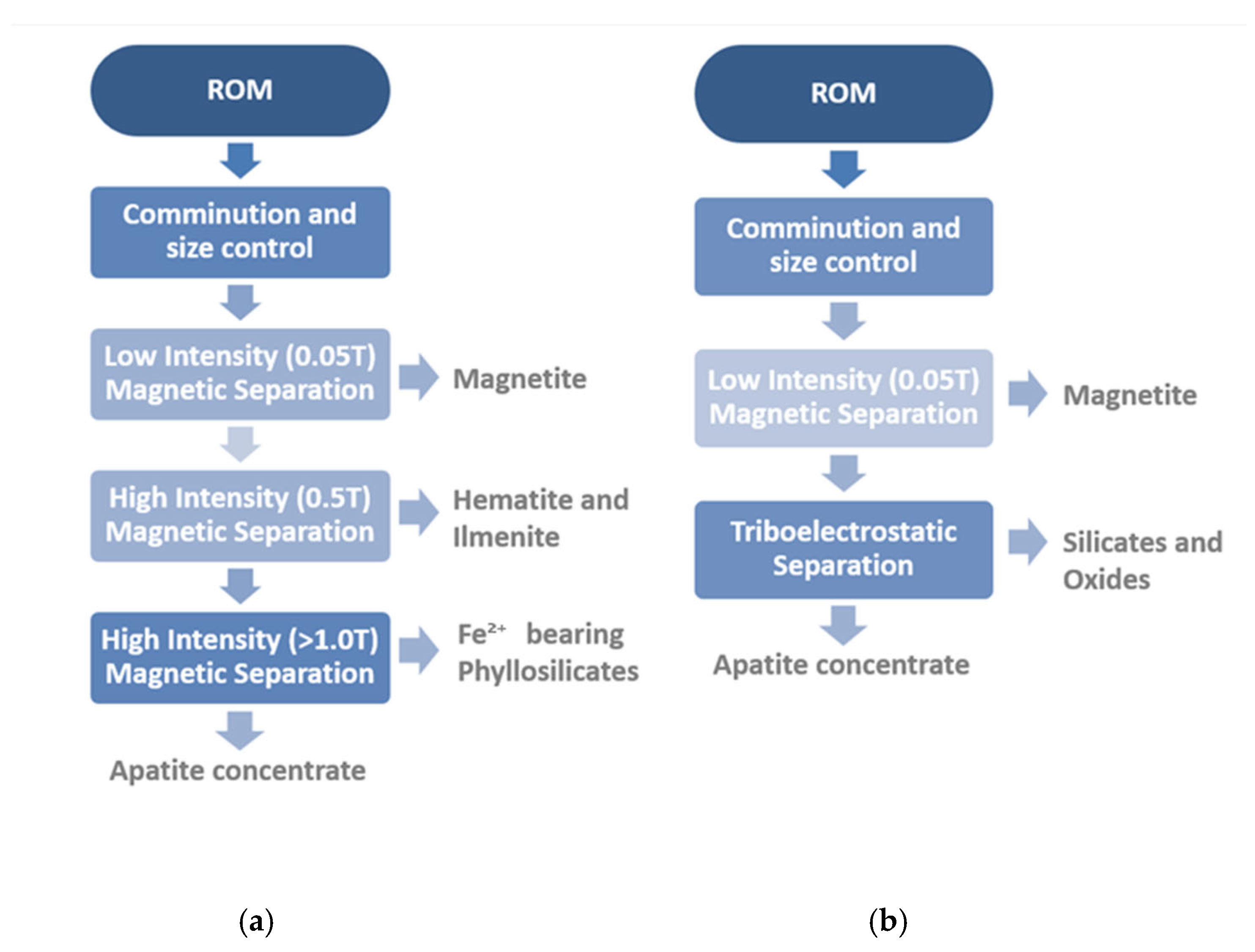
2. Materials and Methods
2.1. Sample Preparation
2.2. Sample and Test Products Characterization
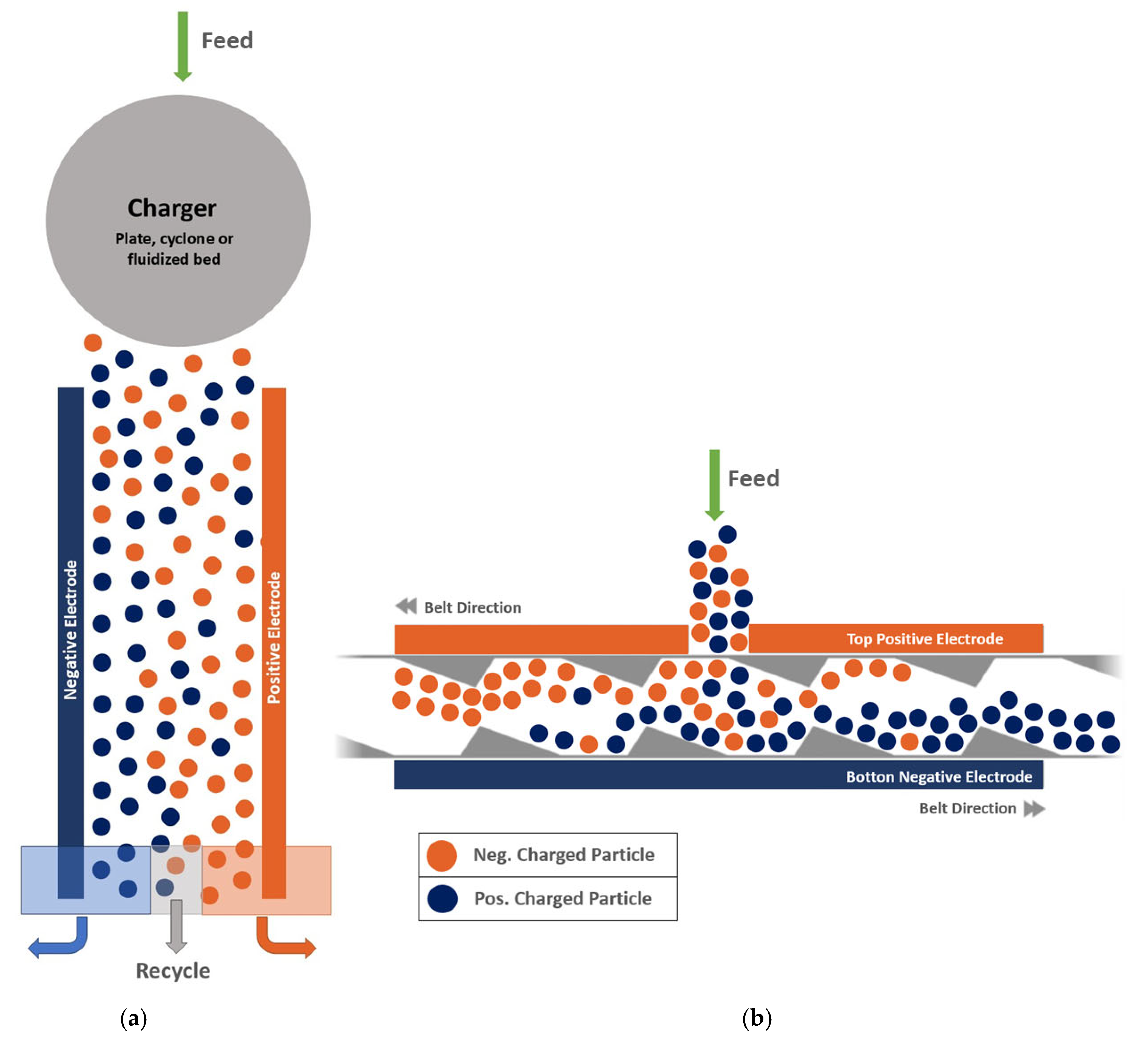
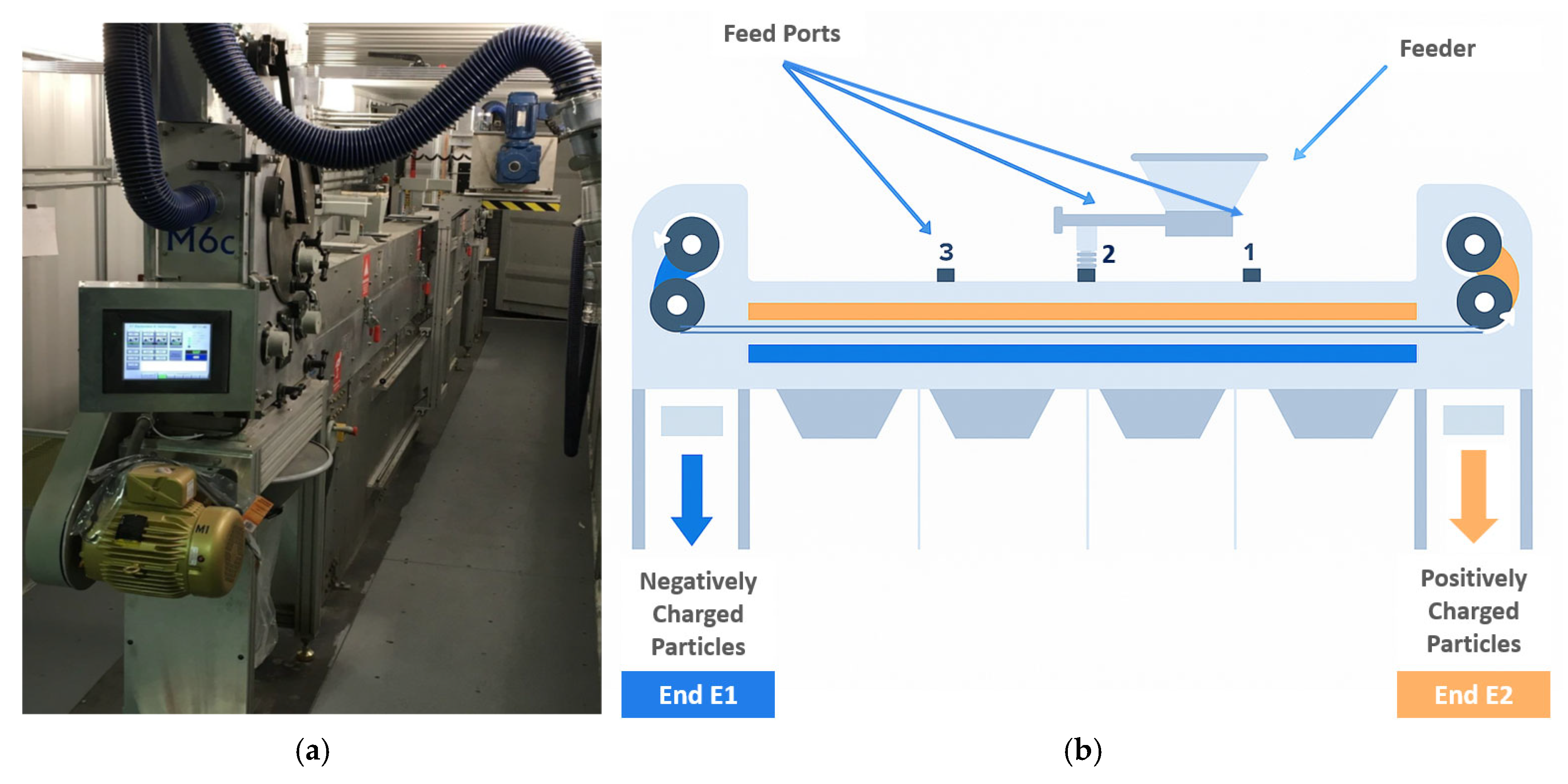
2.3. Triboelectrostatic Belt Separator (TBS): Description and Mode of Operation
| Feed Port | LE1 (m) | LE2 (m) |
|---|---|---|
| 1 | 4.58 | 1.53 |
| 2 | 3.05 | 3.05 |
| 3 | 1.53 | 4.58 |
2.4. Pilot Tests
3. Results and Discussion
3.1. Sample Characterization
3.2. Triboelectrostatic Separation Test Results
3.2.1. Rougher Stage
3.2.2. Cleaner Stage
3.2.3. Full Circuit Configuration Rougher/Cleaner
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TBS | Triboelectrostatic Belt Separator |
| TES | Triboelectrostatic Separation |
| UMA | Unidade de Mineração de Angico |
| FFSs | Free-Fall Separators |
| TM | Testing Material |
| STET | ST Equipment & Technology LLC |
| XRD | X-ray Diffraction |
| ICDD | International Center for Diffraction Data |
| ICSD | Inorganic Crystal Structure Database |
| EDS | Energy Dispersion X-ray Spectrometer |
| SEM | Scanning Electron Microscope |
| MLA | Mineral Liberation Analyzer |
References
- Notholt, A.J.G.; Sheldon, R.P.; Davidson, D.F. Phosphate Deposits of the World—Phosphate Rock Resources; Cambridge University Press: Cambridge, UK, 1969; Volume 1, p. 565. [Google Scholar]
- Boujlel, H.; Daldoul, G.; Tlil, H.; Souissi, R.; Chebbi, N.; Fattah, N.; Souissi, F. The Beneficiation Processes of Low-Grade Sedimentary Phosphates of Tozeur-Nefta Deposit (Gafsa-Metlaoui Basin: South of Tunisia). Minerals 2019, 9, 2. [Google Scholar] [CrossRef]
- Leal Filho, L.S.; Assis, S.M.; Araujo, A.C.; Chaves, A.P. Process mineralogy studies for optimizing the flotation performance of two refractory phosphate ores. Miner. Eng. 1993, 6, 907–917. [Google Scholar] [CrossRef]
- Lynch, A.J.; Harbort, G.J.; Nelson, M.G. History of Flotation; AusIMM: Carlton, Australia, 2010; Volume 1, p. 348. [Google Scholar]
- Steiner, G.; Geissler, B.; Watson, I.; Mew, M.C. Efficiency developments in phosphate rock mining over the last three decades. Resour. Conserv. Recycl. 2015, 105, 235–245. [Google Scholar] [CrossRef]
- Abouzeid, A.M. Physical and thermal treatment of phosphate ores—An overview. Int. J. Miner. Process. 2008, 85, 59–84. [Google Scholar] [CrossRef]
- Northey, S.A.; Mudd, G.M.; Wener, T.T.; Jowitt, S.M.; Haque, N.; Yellishety, M.; Weng, Z. The exposure of global base metal resources to water criticality, scarcity and climate change. Glob. Environ. Change 2017, 44, 109–124. [Google Scholar] [CrossRef]
- Bittner, J.D.; Gasiorowski, S.A.; Hrach, F.J.; Guicherd, H. Electrostatic beneficiation of phosphate ores: Review of past work and discussion of an improvised separation system. Procedia Eng. 2015, 1, 1–11. [Google Scholar]
- Luciano, R.L.; Godoy, A.M. Geologia do complexo metacarbonatítico de Angico dos Dias. Geociências 2017, 1891, 301–314. [Google Scholar] [CrossRef]
- Mata, C.E.D.; Sousa, P.L.R.; Pereira, C.A. Technological characterization of phosphate ore blended with the micacea and mafic typologies of the Angico dos Dias-BA alkaline-carbonatitic complex. In Proceedings of the 28. ENTMME: Brazilian National Meeting on Ore Treatment and Extractive Metallurgy, Belo Horizonte, Brazil, 4–8 November 2019; pp. 4–8. Available online: http://www.entmme2019.entmme.org/trabalhos/084.pdf (accessed on 4 October 2022).
- Mirkowska, M.; Kratzer, M.; Teichert, C.; Flachberger, H. Principal factors of contact charging of minerals for a successful triboelectrostatic separation process—A review. Berg Hüttenmännische Monatshefte (BHM) 2016, 161, 359–382. Available online: https://link.springer.com/article/10.1007/s00501-016-0515-1 (accessed on 30 March 2025). [CrossRef]
- Leja, J. Surface Chemistry of Froth Flotation; Plenum Press: New York, NY, USA, 1982; Volume 1, p. 758. [Google Scholar]
- Manouchehri, H.R.; Hanumantha Rao, K.; Forssberg, K.S.E. Review of electrical separation methods—Part 1: Fundamental aspects. Min. Metall. Explor. 2000, 17, 23–36. [Google Scholar] [CrossRef]
- Gupta, R.; Gidaspow, D.; Wasan, D.T. Electrostatic separation of powder mixture based on the work function of its constituents. Powder Technol. 1993, 75, 79–87. [Google Scholar] [CrossRef]
- Turcaniova, L.; Soong, Y.; Lovas, M.; Mockcciakova, A.; Orinak, A.; Justinova, M.; Znamenackova, I.; Bezovska, M.; Marchant, S. The effect of microwave radiation on the triboelectrostatic separation of coal. Fuel 2004, 83, 2075–2079. [Google Scholar] [CrossRef]
- Horn, G.D.; Smith, D.T.; Grabbe, A. Contact electrification induced by monolayer modification of a surface and relation to acid-base interactions. Nature 1993, 366, 442–443. [Google Scholar] [CrossRef]
- Wills, B.A.; Hopkins, D.W. Mineral Processing Technology—An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery; Pergamon Press: Oxford, UK, 2013; p. 646. [Google Scholar]
- Shen, Y.; Tao, D.; Zhang, L.; Shao, H.; Bai, X.; Yu, X. An experimental study of triboelectrostatic particle charging behavior and its associated fundamentals. Powder Technol. 2023, 429, 118880. [Google Scholar] [CrossRef]
- Bittner, J.D.; Flynn, K.P.; Hrach, F.J. Expanding applications in dry triboelectric separation of minerals. In Proceedings of the XXVII International Mineral Processing Congress—IMPC 2014, Santiago, Chile, 20–24 October 2014; pp. 216–230. Available online: https://steqtech.com/wp-content/uploads/2015/11/IMPC-2014-Bittner-et-al-revised-140808.pdf (accessed on 19 March 2025).
- Kelly, E.G.; Spottiswood, D.J. The theory of electrostatic separations: A review—Part I. Fundamentals. Miner. Eng. 1989, 2, 33–46. [Google Scholar] [CrossRef]
- Zhou, H.; Yao, J.; Chen, S.; Li, H.; Chen, Y.; Wu, X. Effect of moisture content on charging and triboelectrostatic separation of coal gasification fine ash. Sep. Purif. Technol. 2024, 333, 125976. [Google Scholar] [CrossRef]
- Lindley, K.S.; Rowson, N.A. Feed preparation factors affecting the efficiency of the electrostatic separation. Magn. Electr. Sep. 1997, 8, 161–169. [Google Scholar] [CrossRef]
- Galembeck, F.; Burgo, T.A.L.; Balestrin, B.S.; Gouveia, R.F.; Silva, C.A.; Galembeck, A. Friction, tribochemistry and triboelectricity: Recent progress and perspectives. R. Soc. Chem. Adv. 2014, 4, 64280–64298. [Google Scholar] [CrossRef]
- Ferguson, D.N. A basic triboelectric series for heavy minerals from inductive electrostatic separation behavior. J. South. Afr. Inst. Min. Metall. 2010, 110, 75–78. Available online: http://www.scielo.org.za/scielo.php?script=sci_arttext&pid=S2225-62532010000200005&lng=en&nrm=iso (accessed on 25 February 2025).
- Fraas, F. Electrostatic Separation of Granular Materials; US Bureau of Mines: Washington, DC, USA, 1962; Volume B603, p. 26. [Google Scholar]
- Inculet, I. Electrostatic Mineral Separation, Electrostatics and Electrostatic Application Series; Research Studies Press, John Wiley & Sons: New York, NY, USA, 1994; 215p. [Google Scholar]
- He, J.; Huang, S.; Chen, H.; Zhu, L.; Guo, C.; He, X.; Yang, B. Recent advances in the intensification of triboelectric separation and its application in resource recovery: A review. Chem. Eng. Process. Process Intensif. 2023, 185, 109308. [Google Scholar] [CrossRef]
- Rankin, W.J. Minerals, Metals and Sustainability; CRC Press/Balkema: Leiden, The Netherlands, 2011; 419p. [Google Scholar]
- Souza Pinto, T.C.; Lima, O.A.; Leal Filho, L.S. Sphericity of apatite particles determined by gas permeability through packed beds. Miner. Metall. Process. 2009, 26, 105–108. [Google Scholar] [CrossRef]
- Küppers, H.; Knauer, H. Electrostatic Free Fall Separator. United States Patent 4,797,201, 10 January 1989. Available online: https://patents.google.com/patent/US4797201A/en (accessed on 8 March 2025).
- Bittner, J.D.; Hrach, F.J.; Gasiorowskia, S.A.; Canellopoulus, L.A.; Guicherd, H. Triboelectric belt separator for beneficiation of fine minerals. Procedia Eng. 2014, 83, 122–129. [Google Scholar] [CrossRef]
- Chelgani, S.C.; Neisiani, A.S. Dry Mineral Processing; Springer Verlag: Berlim, Germany, 2022; Volume 1, p. 156. [Google Scholar]
- Ban, H.; Li, T.X.; Hower, J.C.; Schaefer, J.L.; Stencel, J.M. Dry triboelectrostatic beneficiation of fly ash. Fuel 1997, 76, 801–805. [Google Scholar] [CrossRef]
- Hrach, F.; Flynn, K.; Miranda, P.J. Beneficiation of Industrial Minerals Using a Triboelectrostatic Belt Separator; Canadian Institute of Mining Metallurgy and Petroleum: Westmount, QC, Canada, 2016; Available online: https://onetunnel.org/documents/beneficiation-of-industrial-minerals-using-a-tribo-electric-belt-separator (accessed on 8 March 2025).
- Bada, S.O.; Falcon, L.M.; Falcon, R.M.S.; Bergmann, C.P. Feasibility study on triboelectrostatic concentration of <105 μm phosphate ore. J. South Afr. Inst. Min. Metall. 2012, 112, 2–6. Available online: https://scielo.org.za/scielo.php?script=sci_arttext&pid=S2225-62532012000500004 (accessed on 25 February 2025).
- Ciccu, R.; Ghiani, M.M.; Ferrara, G. Selective tribocharging of particles for separation. Kona 1993, 11, 5–16. Available online: https://www.jstage.jst.go.jp/article/kona/11/0/11_1993006/_pdf (accessed on 26 February 2025). [CrossRef]
- Shockley, W. Currents to conductors induced by a moving point charge. J. Appl. Phys. 1938, 9, 635–636. [Google Scholar] [CrossRef]
- Ramo, S. Currents induced by electron motion. Proc. IRE 1939, 27, 584–585. [Google Scholar] [CrossRef]
- Parker, K.R. Applied Electrostatic Precipitation; Blackie Academic & Professional: London, UK, 1997; Volume 1, p. 521. [Google Scholar]
- Keller, G.V. Section 26: Electrical Properties of Rocks and Minerals; Handbook of Physical Constants; Geological Society of America: Baltimore, MD, USA, 1966; Volume 1, p. 553. [Google Scholar] [CrossRef]
- ST Equipment & Technology LLC. M6c Manual. In Installation and Operation Manual; ST Equipment & Technology LLC: Needham, MA, USA, 2020; 50p. [Google Scholar]
- Whitlock, D.R. Separating Constituents of a Mixture of Particles. United States Patent 4,839,032, 13 June 1989. Available online: https://patents.google.com/patent/US4839032A/en (accessed on 24 February 2025).
- Whitlock, D.R. Separating Constituents of a Mixture of Particles. United States Patent 4,874,507, 17 October 1989. Available online: https://patents.google.com/patent/US4874507A/en (accessed on 24 February 2025).
- Schulz, N.F. Separation efficiency. Trans. Am. Inst. Min. Metall. Pet. Eng. 1970, 247, 81–87. [Google Scholar]


| Attributes | Froth Flotation [10] | TES [11,12,13,14] |
|---|---|---|
| Separating medium | Water | Air |
| Differentiating property | Wettability by water | Fermi level/work function |
| Variable that controls the differentiating property | Contact angle | Density and sign of the acquired surface charge. |
| Surface modification previous to separation | Conditioning with chemical reagents (collectors, frothers, modifiers) | Contact/friction between mineral/mineral, mineral/polymers, mineral/walls of equipment, assisted or not by gas adsorption and radiation. |
| Modus operandi of the mineral separation | Hydrophobic particles collide and adhere to air bubbles and float; Hydrophilic particles do not adhere to air bubbles and sink | Negatively charged particles move to the positively charged electrode, vice versa. |
| Intensity and Relative Signs of the Acquired Charge | Triboelectrostatic Series | |
|---|---|---|
| Fraas [25] | Ferguson [24] | |
| ++++++++++++++ | Siderite | |
| ++++++++++++ | Olivine | |
| +++++++++++ | Andracite | |
| ++++++++++ | Apatite | Apatite |
| +++++++++ | Nepheline | Carbonates |
| ++++++++ | Magnesite | Monazite |
| +++++++ | Allanite | Titanomagnetite |
| +++++ | Staurolite | Ilmenite |
| ++++ | Beryl | Rutile |
| +++ | Grossularite | Leucoxene |
| ++ | Eudialyte | Magnetite/Hematite |
| + | Sphene | Spinel |
| − | Stilbite | Garnet |
| −− | Netafite | Staurolite |
| −−− | Diopside | Altered Ilmenite |
| −−−− | Cryolite | Goethite |
| −−−−−− | Hornblende | Zircon |
| −−−−−−− | Monazite | Epidote |
| −−−−−−−− | Chromite (Spinel) | Tremolite |
| −−−−−−−−− | Euxenite | Hydrous Silicates |
| −−−−−−−−−−− | Scheelite | Aluminosilicates |
| −−−−−−−−−−−−− | Microcline | Tourmaline |
| −−−−−−−−−−−−−− | Albite | Actinolite |
| −−−−−−−−−−−−−−− | Quartz | Pyroxene |
| −−−−−−−−−−−−−−−−− | Rinodolite | Titanite |
| −−−−−−−−−−−−−−−−−−− | Actinolite | Feldspar |
| −−−−−−−−−−−−−−−−−−−−− | Hexagonite | Quartz |
| −−−−−−−−−−−−−−−−−−−−−−− | Glauconite | |
| Forces | Cause | Effect | Contribution to the Selectivity |
|---|---|---|---|
| Electrostatic | Magnitude, density, and sign of mineral surface charge prior to separation [26]; Magnitude and polarity of the static field generated by the electrodes positioned within the separators [26,27]. | Attractive/repulsive forces between mineral particles and the equipment’s electrodes [26,27]. | Different position of apatite versus silicates in the triboelectric series maintained by either Ferguson [24] or Fraas [25]. |
| Gravity | Volume (size) and specific gravity () of the particles involved in the separation [26,27]. | Larger, heavier particles settle more quickly than smaller, lighter ones despite their buoyancy [26,27]. | Apatite particles (p > 3000 kg/m3) are denser than many silicates (p < 2800 kg/m3) [28]. |
| Drag | Particles’ shape and air temperature [26,27]. | Particles of lower sphericity factor () settle at a lower rate than rounded particles [26,27,29]. | Apatite particles settle as rough tetrahedrons (0.62 < Ψ < 0.64), whereas mica and clay particles show a platy-shaped habit (Ψ < 0.3) [29]. |
| Variables | Units | Typical Range |
|---|---|---|
| Top electrode polarity | - | Positive/negative |
| Electrode voltage | 6 (*) | |
| Belt speed (S) | 4.6–19.8 | |
| Feed port | - | 1, 2, and 3 |
| Electrode gap (G) | 1.0–1.5 (×10−2) | |
| Mass feed rate of solids (F) | 0.13–1.25 |
| Secondary Variables | Units | Assessment |
|---|---|---|
| Cross-sectional area (A) | Based on electrode gap (G) | |
| Solids volumetric flowrate (Q) | Equation (2) | |
| Solids flux velocity (V) | Equation (3) | |
| Total flux velocity () | Equation (4) | |
| Residence time (T) | s | Equation (5) |
| Electric current on the electrodes (I) | mA | Current meter (*) |
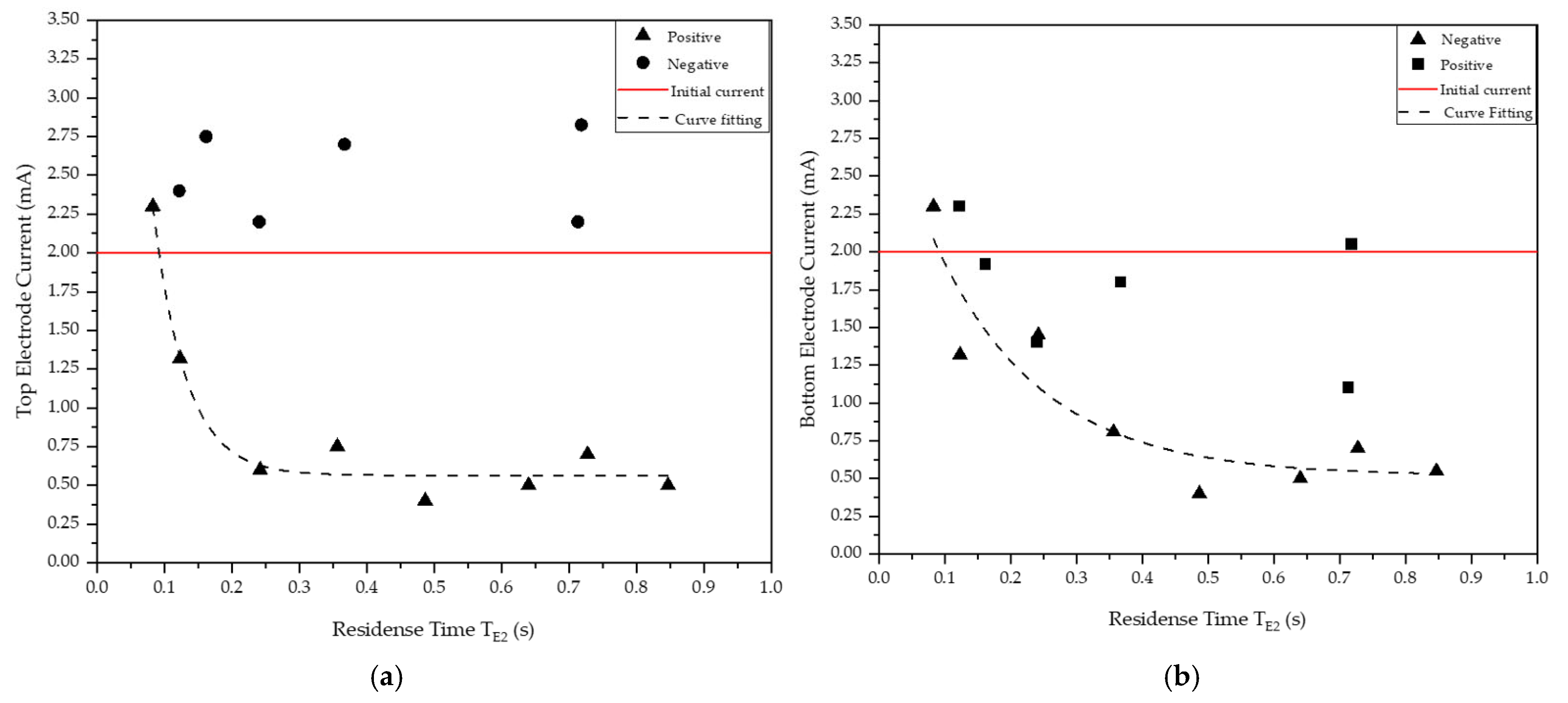
| Run | Duty | Independent Variables | Secondary Variables | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Feed Port | Top Electrode Polarity | Feed Rate (kg/s) | Gap (×10−2 m) | Belt Speed (m/s) | Solids Flowrate (×10−4 m3/s) | Total Flux Velocity (m/s) | Time to Reach E2 (s) (*) | Time to Reach E1 (s) (**) | Top Electrode Current (mA) | Bottom Electrode Current (mA) | ||
| #1 | Rougher | 1 | Positive | 0.56 | 1.52 | 18.3 | 1.9 | 18.4 | 0.08 | 0.25 | 2.3 | 2.3 |
| 2 | Rougher | 1 | Positive | 0.56 | 1.32 | 12.2 | 1.9 | 12.4 | 0.12 | 0.37 | 1.3 | 1.3 |
| 3 | Rougher | 1 | Positive | 0.47 | 1.21 | 6.1 | 1.6 | 6.3 | 0.24 | 0.73 | 0.6 | 1.5 |
| 4 | Rougher | 2 | Positive | 0.44 | 1.25 | 8.4 | 1.5 | 8.6 | 0.36 | 0.36 | 0.8 | 0.8 |
| 5 | Rougher | 2 | Positive | 0.44 | 1.27 | 6.1 | 1.5 | 6.3 | 0.49 | 0.49 | 0.4 | 0.4 |
| 6 | Rougher | 2 | Positive | 0.39 | 1.14 | 4.6 | 1.3 | 4.8 | 0.64 | 0.64 | 0.5 | 0.5 |
| 7 | Rougher | 3 | Positive | 0.39 | 1.14 | 6.1 | 1.3 | 6.3 | 0.73 | 0.24 | 0.7 | 0.7 |
| 8 | Rougher | 3 | Positive | 0.44 | 1.14 | 5.2 | 1.5 | 5.4 | 0.85 | 0.28 | 0.5 | 0.6 |
| 9 | Rougher | 1 | Negative | 0.56 | 1.08 | 12.2 | 1.9 | 12.5 | 0.12 | 0.37 | 2.4 | 2.3 |
| 10 | Rougher | 1 | Negative | 0.56 | 1.13 | 9.1 | 1.9 | 9.4 | 0.16 | 0.49 | 2.8 | 1.9 |
| 11 | Rougher | 1 | Negative | 0.56 | 1.20 | 6.1 | 1.9 | 6.3 | 0.24 | 0.72 | 2.2 | 1.4 |
| 12 | Rougher | 3 | Negative | 0.56 | 1.14 | 12.2 | 1.9 | 12.5 | 0.37 | 0.12 | 2.7 | 1.8 |
| 13 | Rougher | 3 | Negative | 0.56 | 1.08 | 6.1 | 1.9 | 6.4 | 0.71 | 0.24 | 2.2 | 1.1 |
| 14 | Rougher | 3 | Negative | 0.56 | 1.14 | 6.1 | 1.9 | 6.4 | 0.72 | 0.24 | 2.8 | 2.1 |
| 15 | Cleaner | 1 | Positive | 0.22 | 1.33 | 15.2 | 0.7 | 15.3 | 0.10 | 0.30 | 0.6 | 0.6 |
| 16 | Cleaner | 1 | Positive | 0.56 | 1.27 | 12.2 | 1.9 | 12.4 | 0.12 | 0.37 | 0.5 | 0.5 |
| 17 | Cleaner | 1 | Positive | 0.56 | 1.27 | 6.1 | 1.9 | 6.3 | 0.24 | 0.73 | 1.7 | 1.8 |
| 18 | Cleaner | 2 | Positive | 0.56 | 1.27 | 12.2 | 1.9 | 12.4 | 0.25 | 0.25 | 0.6 | 0.6 |
| 19 | Cleaner | 2 | Positive | 0.31 | 1.27 | 9.1 | 1.0 | 9.3 | 0.33 | 0.33 | 0.8 | 0.7 |
| 20 | Cleaner | 2 | Positive | 0.48 | 1.23 | 6.1 | 1.6 | 8.3 | 0.48 | 0.48 | 1.5 | 1.5 |
| Size Fractions (mm) | Mass (%) | |
|---|---|---|
| Retained | Accumulated | |
| +0.600 | 9.8 | 9.8 |
| −0.600 + 0.500 | 10.3 | 20.1 |
| −0.500 + 0.300 | 14.6 | 34.7 |
| −0.300 + 0.210 | 17.3 | 52.0 |
| −0.210 + 0.150 | 14.5 | 66.5 |
| −0.150 + 0.074 | 16.7 | 83.2 |
| −0.074 | 16.8 | 100.0 |
| Total | 100.0 | - |
| Size Fractions (mm) | Mass (%) | Content of Analytes (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retained | Accum. (*) | P2O5 | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | TiO2 | K2O | LOI | CaO:P2O5 | |
| +0.30 | 34.7 | 34.7 | 25.1 | 33.8 | 15.4 | 4.35 | 4.79 | 6.76 | 0.45 | 1.18 | 4.08 | 1.35 |
| −0.30 + 0.21 | 17.3 | 52.0 | 26.5 | 35.5 | 14.1 | 3.81 | 5.01 | 5.56 | 0.49 | 1.03 | 3.73 | 1.34 |
| −0.21 + 0.15 | 14.5 | 66.5 | 27.3 | 36.6 | 12.9 | 3.40 | 5.29 | 4.95 | 0.65 | 0.93 | 3.51 | 1.34 |
| −0.15 + 0.074 | 16.7 | 83.2 | 25.0 | 33.9 | 15.1 | 4.01 | 7.09 | 5.26 | 0.51 | 0.99 | 4.12 | 1.36 |
| −0.74 | 16.8 | 100.0 | 11.4 | 16.3 | 27.4 | 8.51 | 14.6 | 7.39 | 0.71 | 1.34 | 7.67 | 1.43 |
| Head (TM) | 100.0 | - | 22.9 | 31.0 | 17.4 | 4.99 | 6.81 | 6.50 | 0.48 | 1.16 | 4.62 | 1.35 |
| Total Calc. | 100.0 | - | 23.3 | 31.6 | 16.8 | 4.76 | 6.94 | 6.15 | 0.54 | 1.11 | 4.55 | 1.35 |
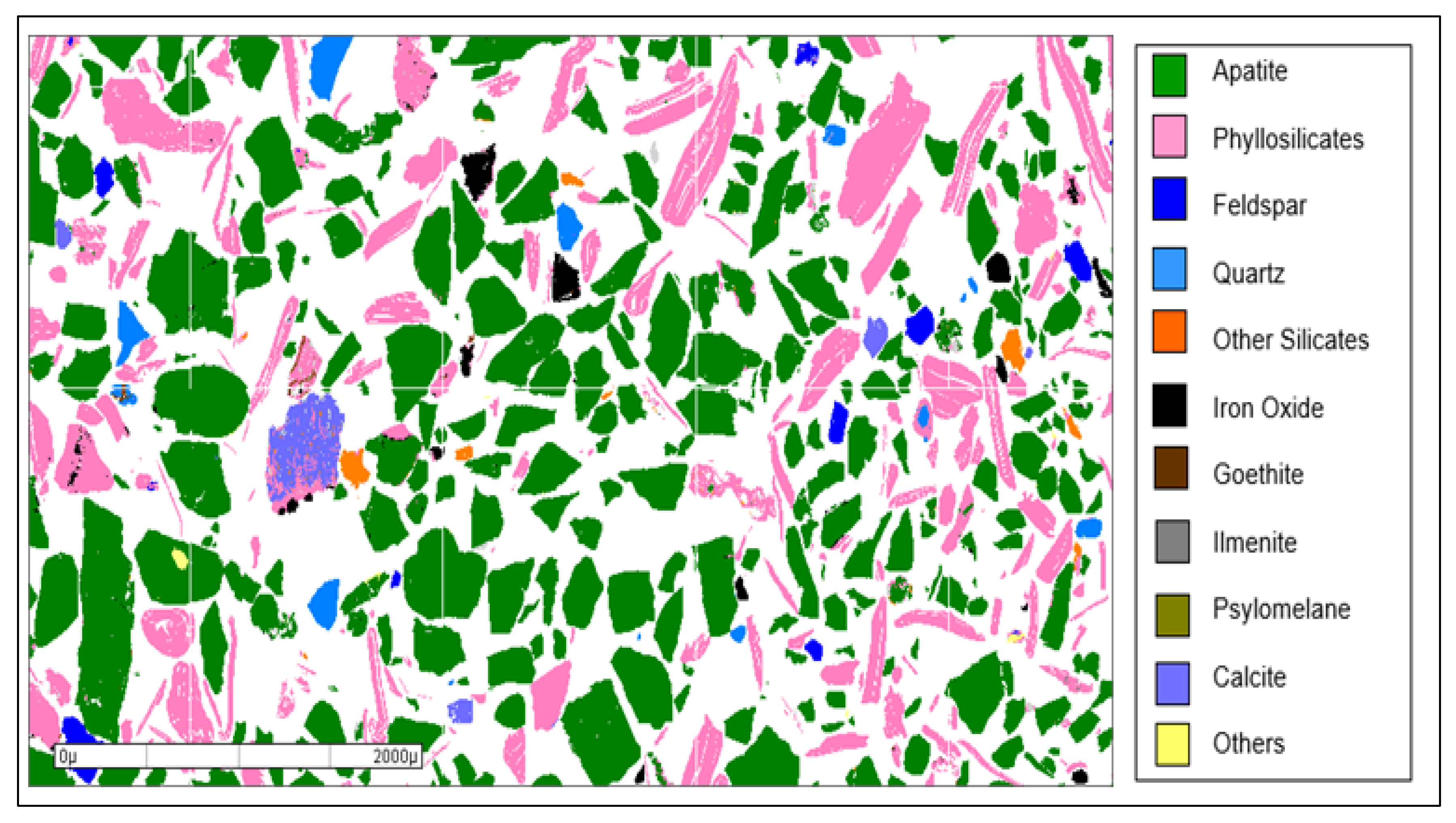
| Mineral Species | Content (%) | |
|---|---|---|
| Fraction +0.600 mm | Fraction −0.600 + 0.074 mm | |
| Apatite | 61 | 60 |
| Phyllosilicates | 32 | 30 |
| Feldspar | 2.5 | 2.5 |
| Quartz | 0.8 | 1.2 |
| Pyroxene + amphibole | 1.2 | 2.0 |
| Iron oxides (*) | 1.1 | 1.7 |
| Carbonates (**) | 1.1 | 1.1 |
| Titanium oxides (***) | 0.1 | 0.5 |
| Psilomelane | 0.1 | 0.2 |
| Others | 0.1 | 0.8 |
| Minerals | P2O5 | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | TiO2 | K2O |
|---|---|---|---|---|---|---|---|---|
| Apatite | 100 | 97 | ||||||
| Phyllosilicates | <1 | 1 | 78 | 85 | 68 | 95 | 56 | 86 |
| Feldspar | 9 | 13 | 13 | |||||
| Quartz | 7 | |||||||
| Other Silicates | 1 | 5 | 2 | 4 | 4 | <1 | ||
| Hematite/magnetite | <1 | 23 | <1 | 8 | ||||
| Goethite | 3 | |||||||
| Ilmenite | 3 | 31 | ||||||
| Psilomelane | ||||||||
| Others | <1 | <1 | 1 | 1 | <1 | <1 | 5 |
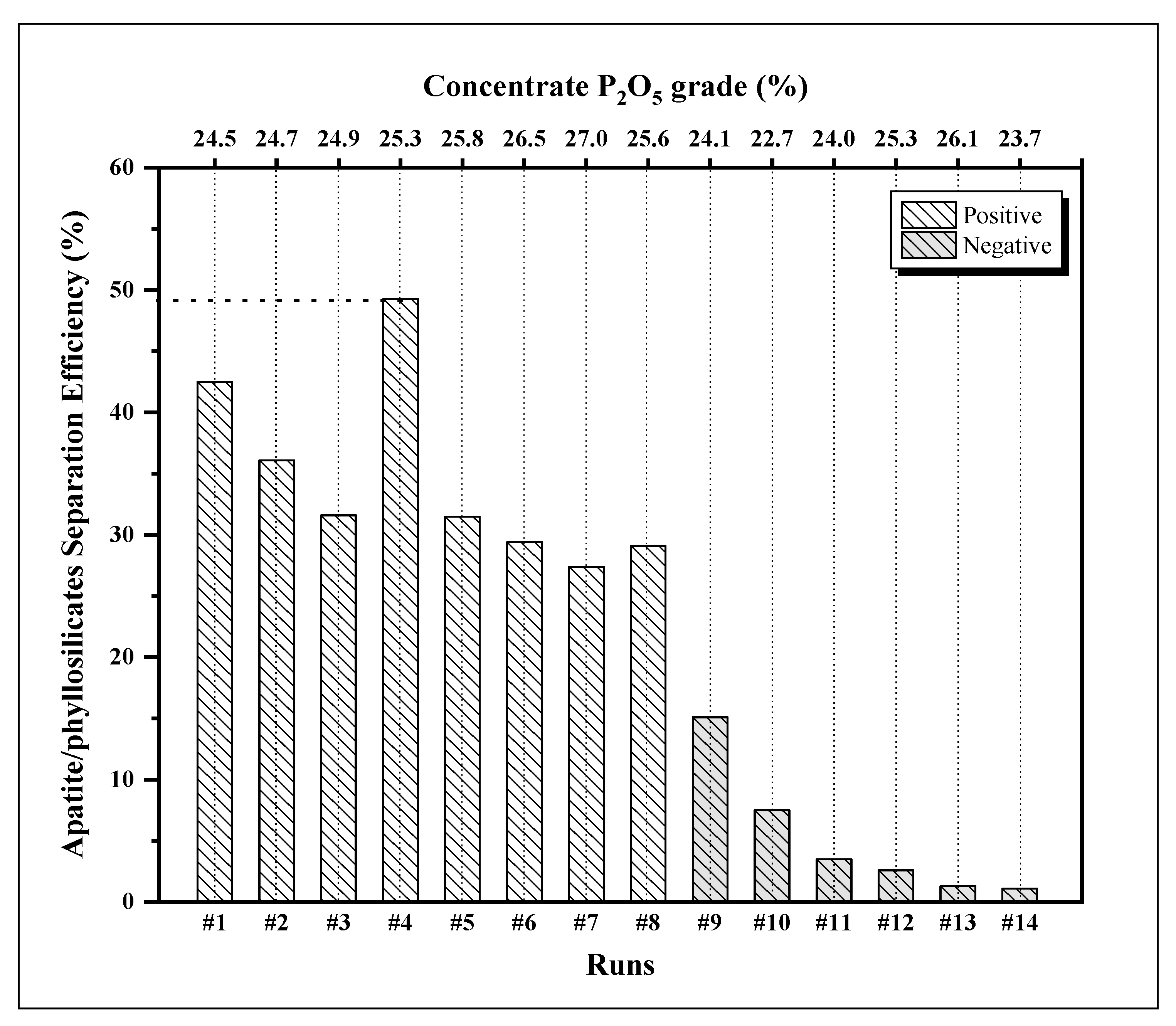
| Run | Concentrate Composition (%) | Recovery (%) | ||||||
|---|---|---|---|---|---|---|---|---|
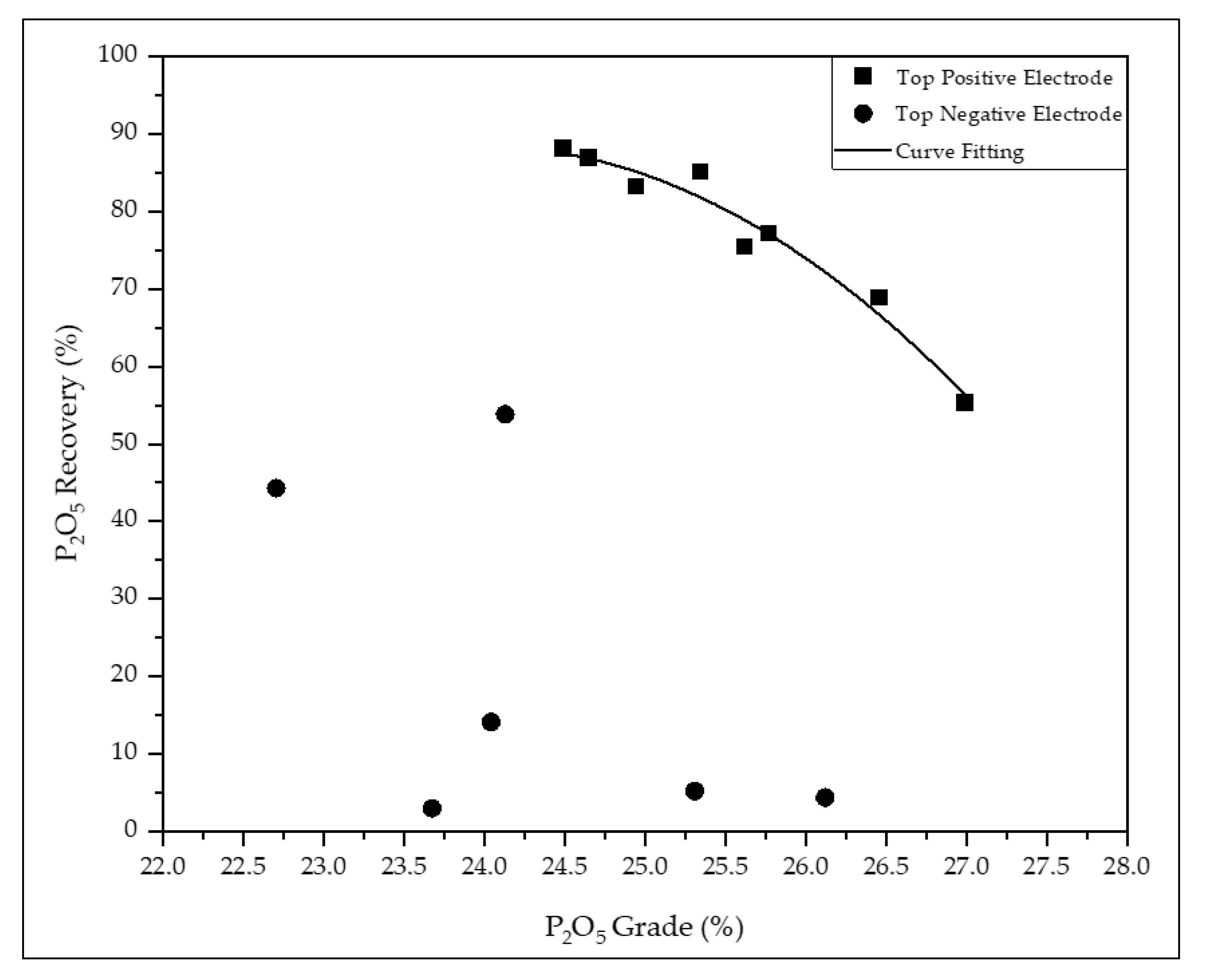
| P2O5 | SiO2 | Fe2O3 | Al2O3 | MgO | Mass | P2O5 | MgO | |
| 1 | 24.5 | 11.0 | 6.18 | 3.78 | 3.75 | 81.7 | 88.1 | 45.6 |
| 2 | 24.7 | 11.4 | 6.20 | 3.86 | 4.16 | 80.2 | 86.9 | 50.8 |
| 3 | 24.9 | 10.9 | 7.33 | 3.84 | 3.51 | 76.9 | 83.2 | 51.6 |
| 4 | 25.3 | 10.9 | 5.85 | 3.52 | 3.37 | 75.3 | 85.1 | 35.8 |
| 5 | 25.8 | 11.2 | 5.82 | 3.60 | 3.33 | 68.7 | 77.1 | 45.8 |
| 6 | 26.5 | 10.6 | 5.61 | 3.41 | 4.22 | 59.3 | 68.9 | 39.5 |
| 7 | 27.0 | 11.0 | 5.58 | 3.70 | 3.83 | 46.9 | 55.3 | 27.9 |
| 8 | 25.6 | 11.0 | 5.92 | 3.54 | 3.56 | 65.1 | 75.4 | 46.3 |
| 9 | 24.1 | 9.8 | 8.08 | 3.89 | 3.14 | 48.0 | 53.8 | 38.7 |
| 10 | 22.7 | 10.5 | 9.41 | 4.13 | 4.04 | 41.3 | 44.3 | 36.8 |
| 11 | 24.0 | 10.1 | 8.85 | 3.76 | 3.36 | 12.9 | 14.1 | 10.6 |
| 12 | 25.3 | 9.0 | 7.62 | 3.08 | 2.42 | 4.5 | 5.2 | 2.6 |
| 13 | 26.1 | 10.4 | 6.70 | 3.76 | 3.67 | 3.7 | 4.4 | 3.1 |
| 14 | 23.7 | 10.4 | 8.61 | 4.11 | 3.66 | 2.6 | 3.0 | 1.9 |
| Analytes | (%) | s | (*) |
|---|---|---|---|
| P2O5 | 25.8 | 0.4 | 1.6 |
| SiO2 | 11.4 | 0.5 | 4.8 |
| Al2O3 | 4.0 | 0.8 | 5.0 |
| MgO | 3.8 | 0.3 | 8.8 |
| Runs | Concentrate Composition (%) | Recovery (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| P2O5 | SiO2 | Fe2O3 | Al2O3 | MgO | Mass | P2O5 | MgO | |
| 15 | 29.0 | 8.62 | 4.62 | 2.96 | 2.84 | 66.5 | 81.4 | 55.3 |
| 16 | 27.9 | 10.16 | 5.43 | 3.28 | 2.42 | 67.3 | 76.7 | 41.8 |
| 17 | 27.7 | 10.34 | 5.19 | 3.14 | 3.06 | 62.3 | 71.0 | 50.2 |
| 18 | 28.9 | 9.78 | 4.81 | 2.99 | 2.89 | 72.8 | 87.6 | 59.6 |
| 19 | 27.2 | 9.51 | 5.10 | 3.14 | 2.99 | 63.5 | 71.6 | 55.1 |
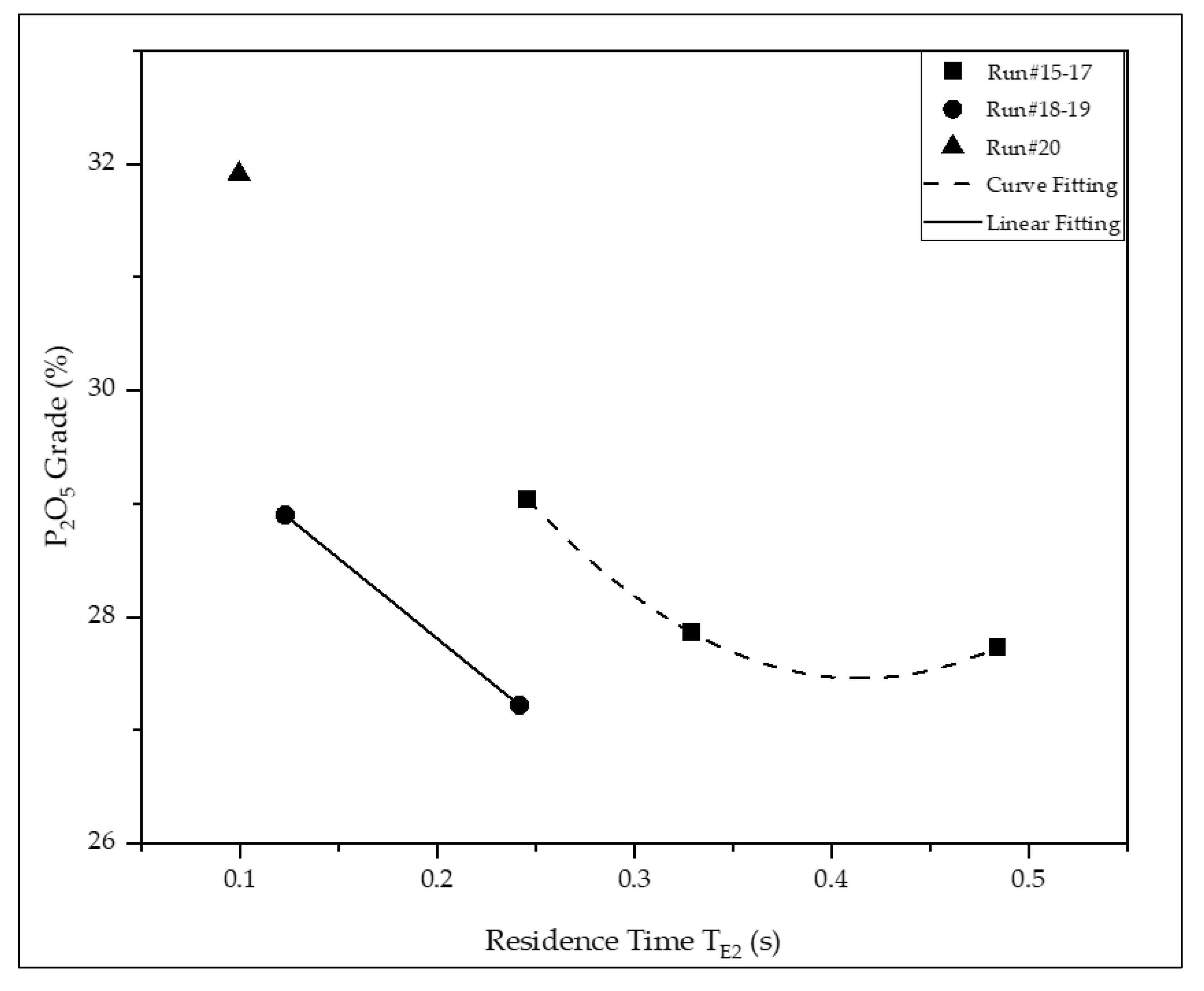
| 20 | 31.9 | 8.89 | 3.94 | 2.29 | 1.64 | 20.8 | 33.2 | 12.9 |
| Item | Chemical Composition (%) | Overall Recovery (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| P2O5 | SiO2 | Fe2O3 | Al2O3 | MgO | Mass | P2O5 | MgO | |
| Feed | 22.9 | 17.4 | 6.81 | 4.99 | 6.50 | - | - | - |
| Run 4 (*) | 25.3 | 10.9 | 5.85 | 3.52 | 3.37 | 75.3 | 85.1 | 35.8 |
| Run 15 (*) | 29.0 | 8.62 | 4.62 | 2.96 | 2.84 | 66.5 | 81.4 | 55.3 |
| Run 18 (*) | 28.9 | 9.78 | 4.81 | 2.99 | 2.89 | 72.8 | 87.6 | 59.6 |
| Total Option 1(**) | 29.0 | 8.62 | 4.62 | 2.96 | 2.84 | 50.0 | 69.3 | 19.8 |
| Total Option 2 (***) | 28.9 | 9.78 | 4.81 | 2.99 | 2.89 | 54.8 | 74.5 | 21.3 |
| Fosnor (****) | 29.5 | 9.51 | 4.94 | 3.13 | 1.98 | 36.3 | 55.7 | 27.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedlmaier Costa Coelho, B.; Neves de Oliveira, R.; da Silva, G.E.; de Salles Leal Filho, L. Dry Concentration of Phosphate Ore by Using a Triboelectrostatic Belt Separator in Pilot Scale. Minerals 2025, 15, 994. https://doi.org/10.3390/min15090994
Sedlmaier Costa Coelho B, Neves de Oliveira R, da Silva GE, de Salles Leal Filho L. Dry Concentration of Phosphate Ore by Using a Triboelectrostatic Belt Separator in Pilot Scale. Minerals. 2025; 15(9):994. https://doi.org/10.3390/min15090994
Chicago/Turabian StyleSedlmaier Costa Coelho, Brenda, Ricardo Neves de Oliveira, Gleison Elias da Silva, and Laurindo de Salles Leal Filho. 2025. "Dry Concentration of Phosphate Ore by Using a Triboelectrostatic Belt Separator in Pilot Scale" Minerals 15, no. 9: 994. https://doi.org/10.3390/min15090994
APA StyleSedlmaier Costa Coelho, B., Neves de Oliveira, R., da Silva, G. E., & de Salles Leal Filho, L. (2025). Dry Concentration of Phosphate Ore by Using a Triboelectrostatic Belt Separator in Pilot Scale. Minerals, 15(9), 994. https://doi.org/10.3390/min15090994






