Influence of Geological Origin on the Physicochemical Characteristics of Sepiolites
Abstract
1. Introduction
2. Geological Setting
2.1. Tolsa Quarry
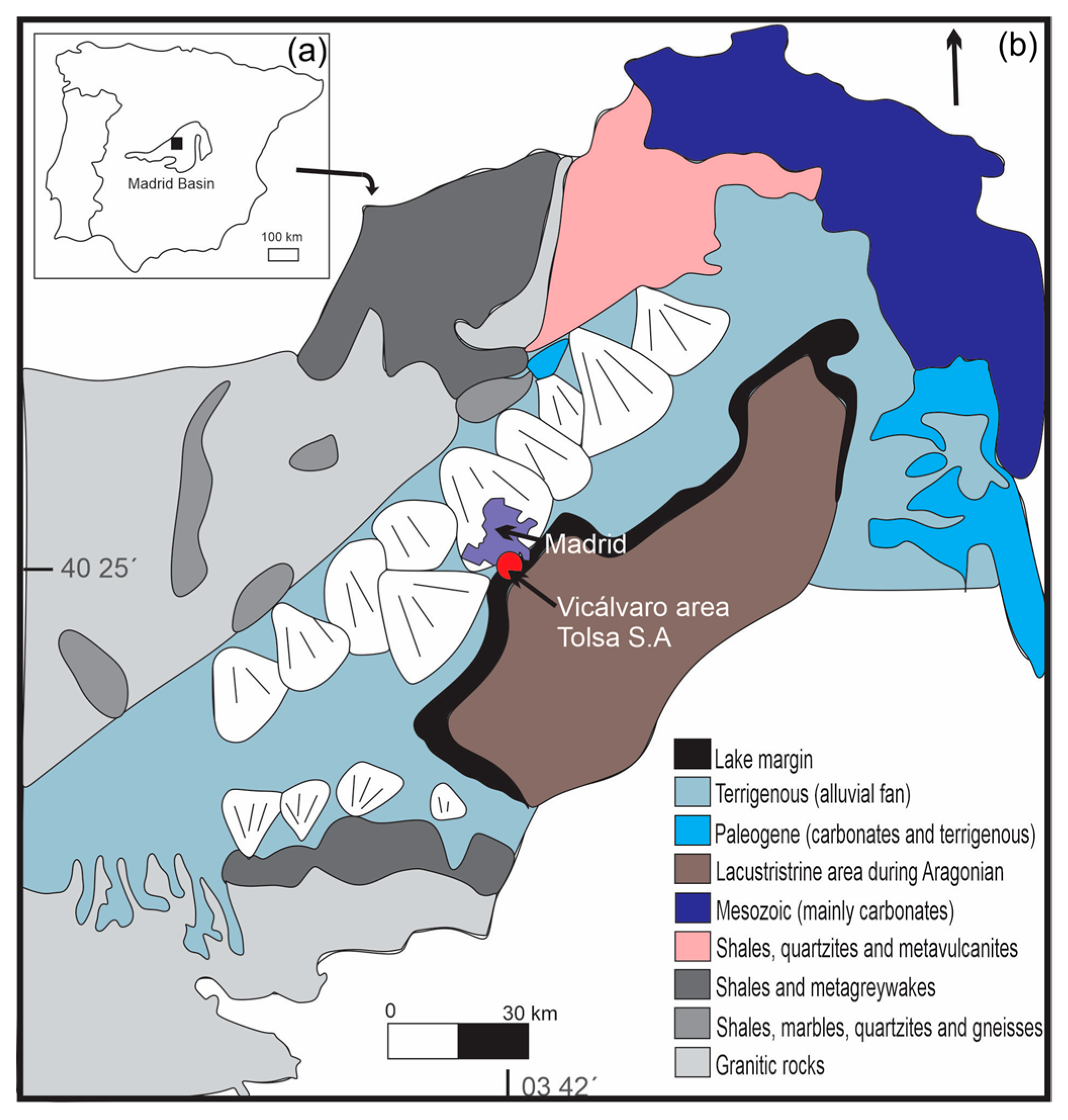
2.2. La Adela Quarry
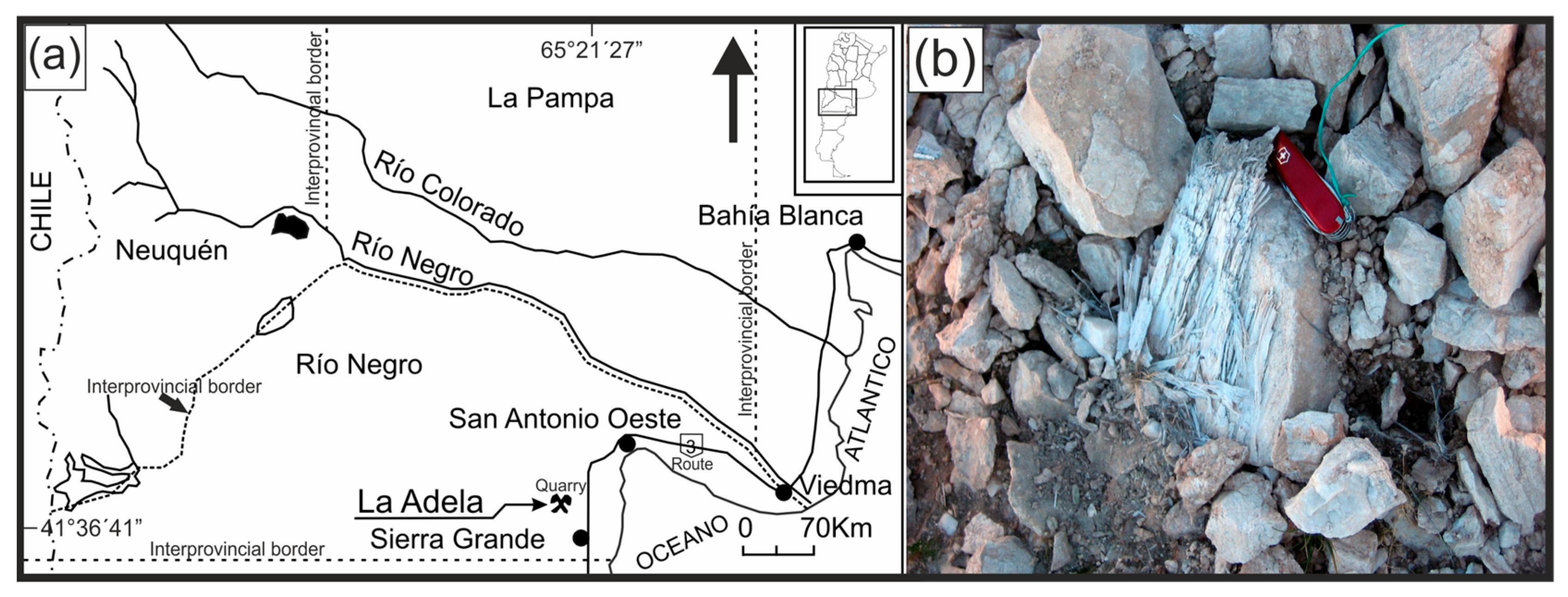
3. Materials and Methods
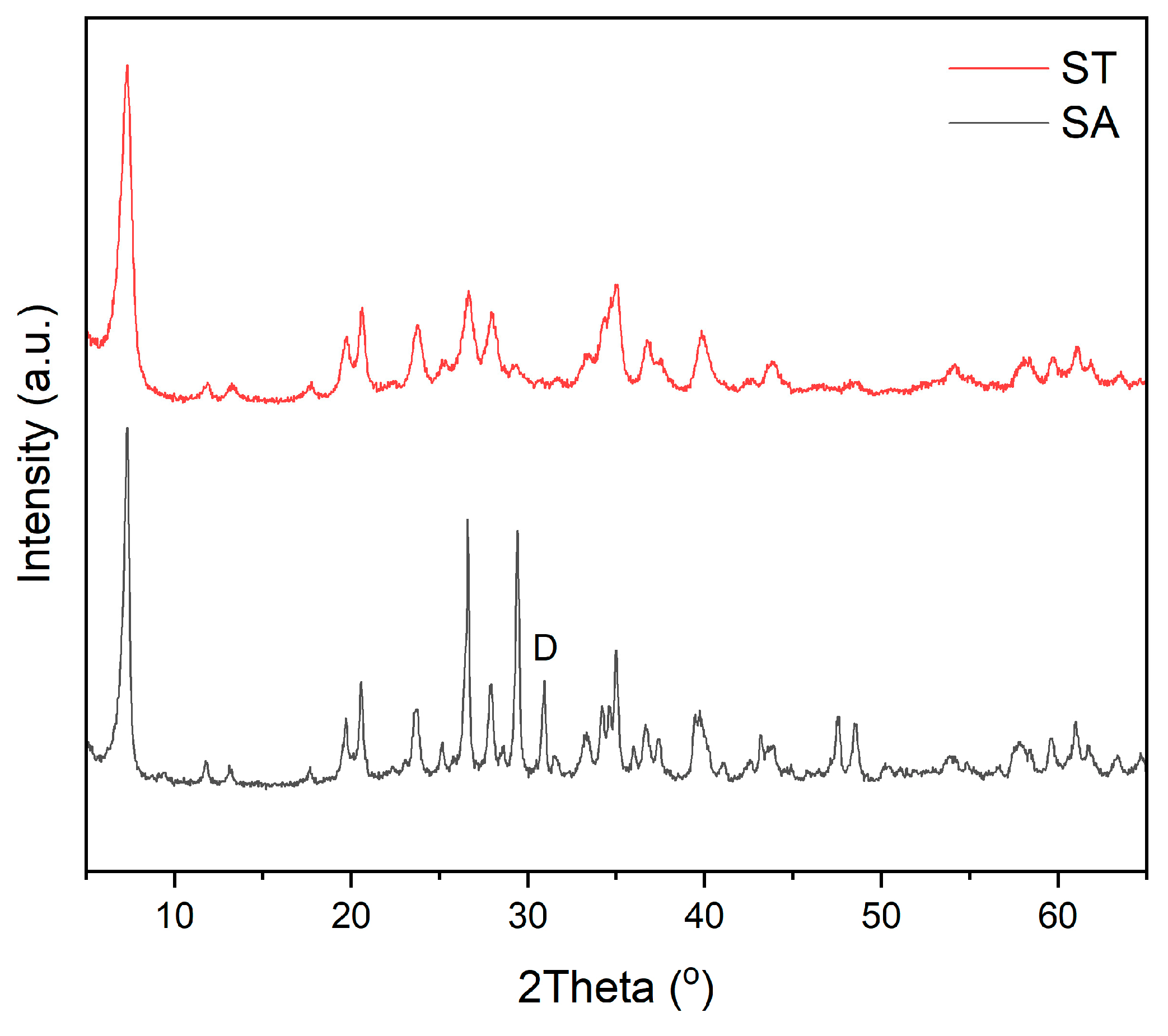
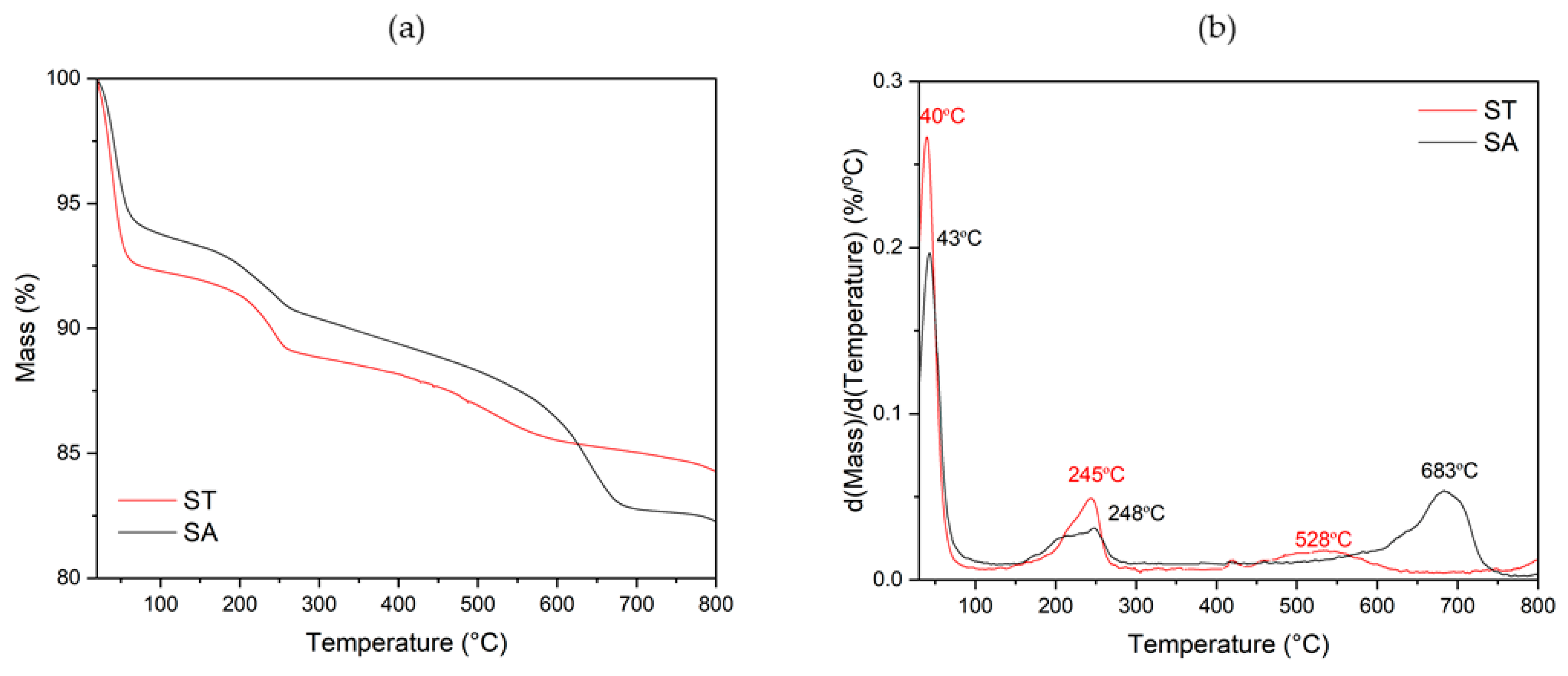
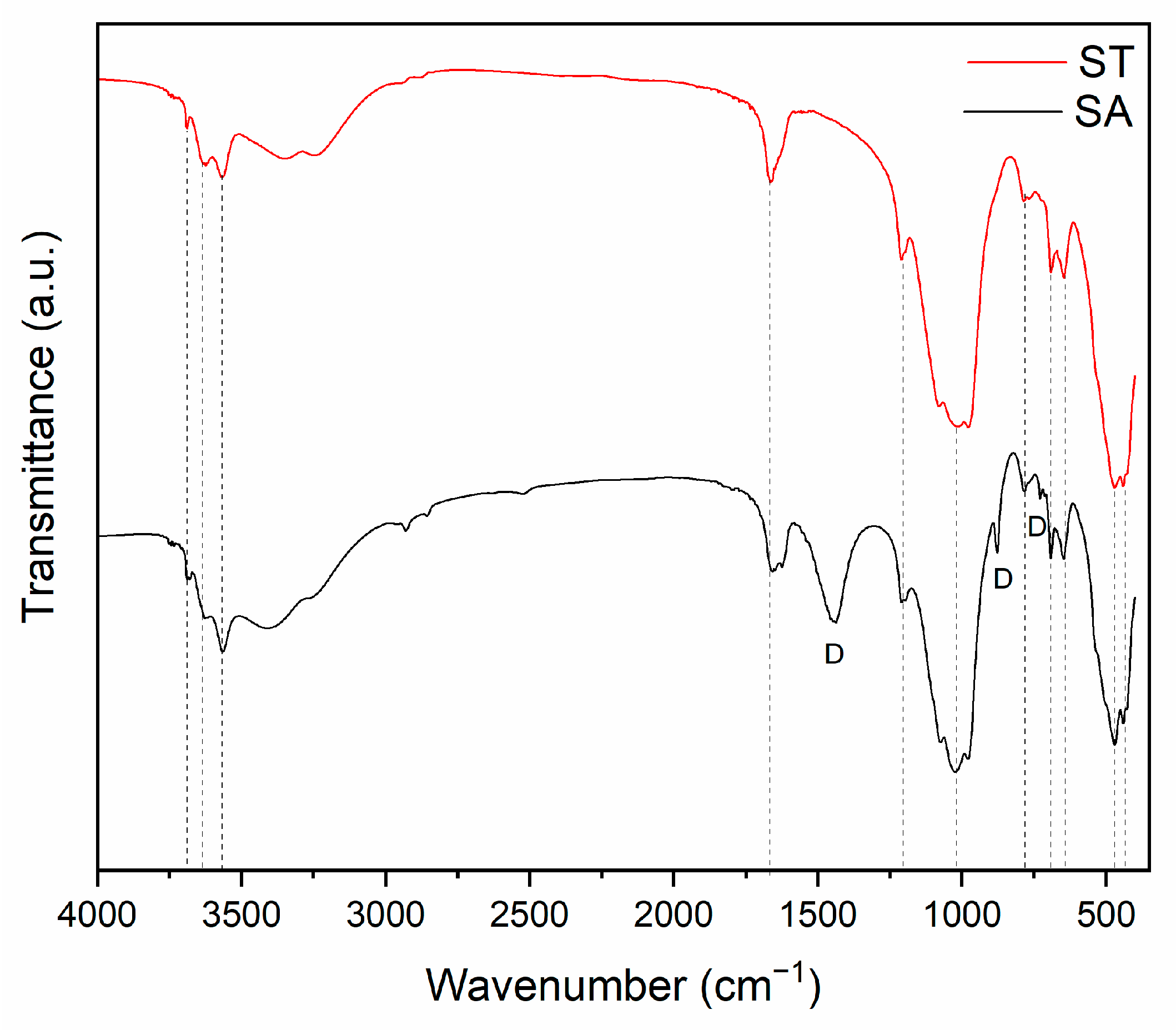
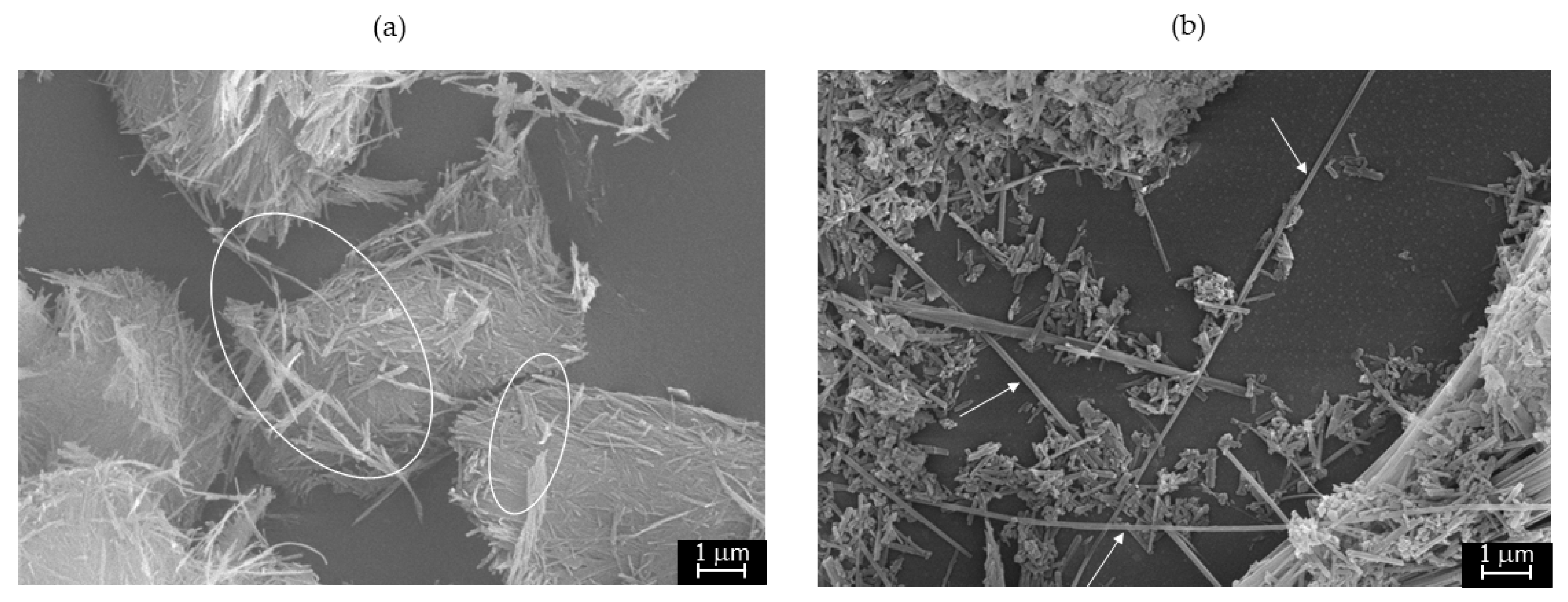
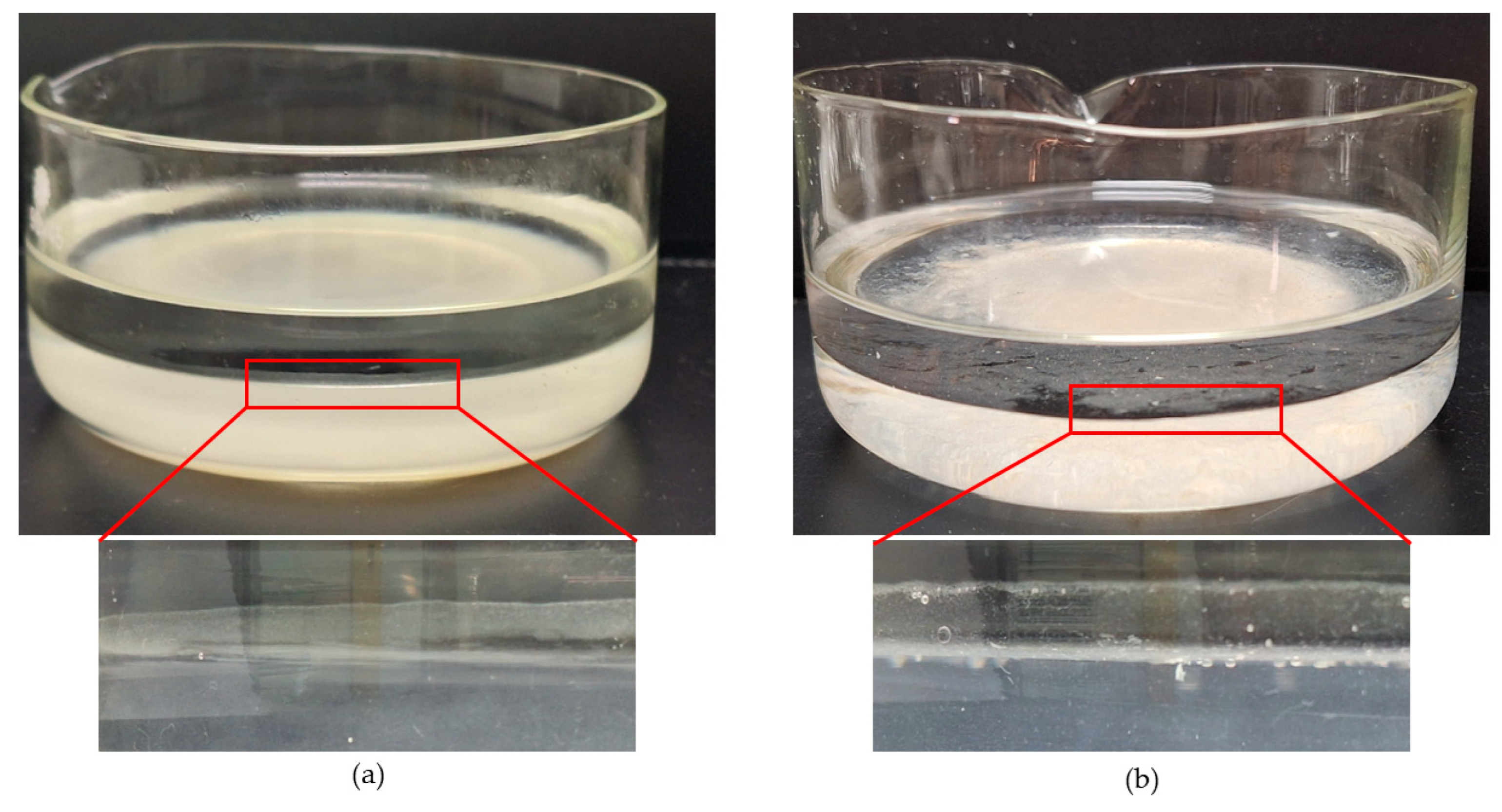
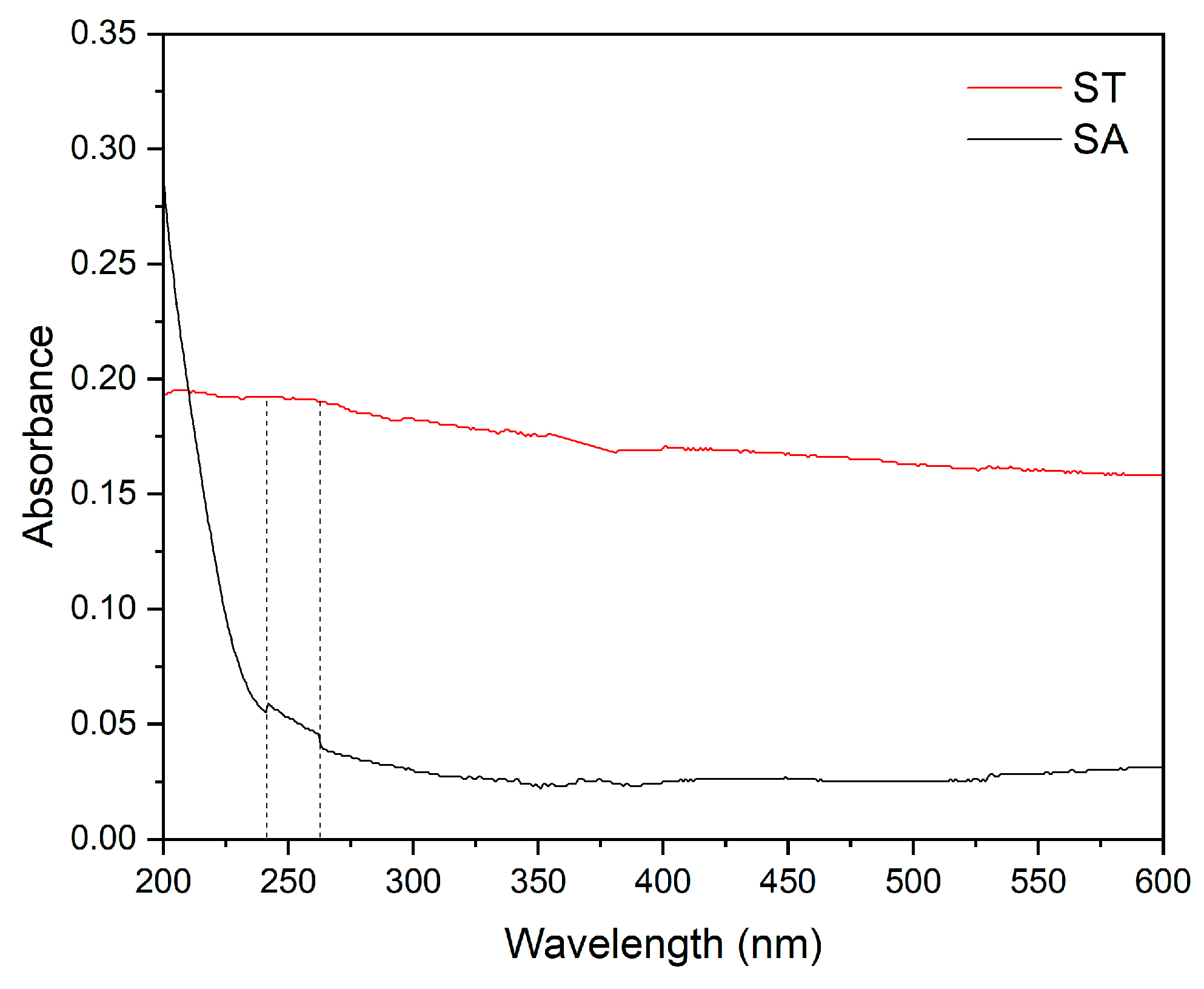
4. Results and Discussion
5. Industrial and Environmental Applications
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giustetto, R.; Macaluso, L.; Berlier, G.; Ganjkhanlou, Y.; Barale, L. Characterisation and possible hazard of an atypical asbestiform sepiolite associated with aliphatic hydrocarbons from Sassello, Ligurian Apennines, Italy. Mineral. Mag. 2019, 83, 209–222. [Google Scholar] [CrossRef]
- Jones, B.F.; Galán, E. Sepiolite and palygorskite. Rev. Mineral. Geochem. 1988, 19, 631–674. [Google Scholar]
- Ruiz, A.I.; Ruiz-García, C.; Ruiz-Hitzky, E. From old to new inorganic materials for advanced applications: The paradigmatic example of the sepiolite clay mineral. Appl. Clay Sci. 2023, 235, 106874. [Google Scholar] [CrossRef]
- Stoessell, R.K.; Hay, R.L. The geochemical origin of sepiolite and kerolite at Amboseli, Kenya. Contrib. Mineral. Petrol. 1978, 65, 255–267. [Google Scholar] [CrossRef]
- Herranz, J.E.; Pozo, M. Sepiolite and other authigenic Mg-clay minerals formation in different Palustrine environments (Madrid Basin, Spain). Minerals 2022, 12, 987. [Google Scholar] [CrossRef]
- Tateo, F.; Sabbadini, R.; Morandi, N. Palygorskite and sepiolite occurrence in Pliocene lake deposits along the River Nile: Evidence of an arid climate. J. Afr. Earth Sci. 2000, 31, 633–645. [Google Scholar] [CrossRef]
- Mokatse, T.; Prud’Homme, C.; Vainer, S.; Adatte, T.; Shemang, E.; Verrecchia, E.P. Sepiolite as a multifactorial indicator of paleoenvironments in the Chobe Enclave (northern Botswana). Sediment. Geol. 2023, 454, 106459. [Google Scholar] [CrossRef]
- Kadir, S.; Erkoyun, H.; Eren, M.; Huggett, J.; Önalgil, N. Mineralogy, geochemistry, and genesis of sepiolite and palygorskite in Neogene lacustrine sediments, Eskişehir Province, west central Anatolia, Turkey. Clays Clay Miner. 2016, 64, 145–166. [Google Scholar] [CrossRef]
- Calvo, J.P.; Pozo, M. Geology of magnesian clays in sedimentary and non-sedimentary environments. In Magnesian Clays: Characterization, Origin and Applications; Pozo, M., Galán, E., Eds.; Digilabs: Bari, Italy, 2015; pp. 123–174. [Google Scholar]
- Draidia, S.; Ouahabi, M.E.; Daoudi, L.; Havenith, H.B.; Fagel, N. Occurrences and genesis of palygorskite/sepiolite and associated minerals in the Barzaman formation, United Arab Emirates. Clay Miner. 2016, 51, 763–779. [Google Scholar] [CrossRef]
- Baldermann, A.; Mavromatis, V.; Frick, P.M.; Dietzel, M. Effect of aqueous Si/Mg ratio and pH on the nucleation and growth of sepiolite at 25 °C. Geochim. Cosmochim. Acta 2018, 227, 211–226. [Google Scholar] [CrossRef]
- Cañaveras, J.C.; Calvo, J.P.; Ordóñez, S.; Muñoz-Cervera, M.C.; Sánchez-Moral, S. Tectono-Sedimentary Evolution of the Madrid Basin (Spain) during the Late Miocene: Data from Paleokarst Profiles in Diagenetically-Complex Continental Carbonates. Geosciences 2020, 10, 433. [Google Scholar] [CrossRef]
- Reijmer, J.J.; Blok, C.N.; El-Husseiny, A.; Kleipool, L.M.; Hogendorp, Y.C.; Alonso-Zarza, A.M. Petrophysics and sediment variability in a mixed alluvial to lacustrine carbonate system (Miocene, Madrid Basin, Central Spain). Depos. Rec. 2022, 8, 317–339. [Google Scholar] [CrossRef]
- Herranz, J.E.; Pozo, M. Authigenic Mg-clay minerals formation in lake margin deposits (the Cerro de los Batallones, Madrid Basin, Spain). Minerals 2018, 8, 418. [Google Scholar] [CrossRef]
- Pozo, M.; Galán, E.; González, J.L. Sepiolite and palygorskite from the Madrid Basin, Spain: Mineralogy and genetic relations. Appl. Clay Sci. 2014, 95, 31–45. [Google Scholar]
- Ordóñez, S.; Calvo, J.P.; García del Cura, M.A.; Alonso Zarza, A.M.; Hoyos, M. Sedimentology of sodium sulphate deposits and special clays from the Tertiary Madrid Basin (Spain). In Lacustrine Facies Analysis; Anadón, P., Cabrera, L., Kelts, K., Eds.; Blackwell Scientific Publications: Oxford, UK, 1991; pp. 39–55. [Google Scholar]
- Cortelezzi, C.R.; Marfil, S.A.; Maiza, P.J. A sepiolite of large crystalline growth from “La Adela” mine province of Río Negro, Argentina. N. Jb. Miner. Mh. 1994, 4, 157–166. [Google Scholar] [CrossRef]
- Caminos, R.; Llambías, E.J. El basamento cristalino. In Proceedings of the Geología y Recursos Naturales de la Provincia de Río Negro, IX Congreso Geológico Argentino, Bariloche, Argentina, 5–9 November 1984; Ramos, V., Ed.; pp. 37–63. [Google Scholar]
- Maiza, P.; Marfil, S.A. Diaclasas mineralizadas con sepiolita de cantera La Adela, provincia de Río Negro, Argentina. In Proceedings of the XII Congreso Geológico Argentino y II Congreso de Exploración de Hidrocarburos, Mendoza, Argentina, 10–15 October 1993; pp. 82–86. [Google Scholar]
- Dominguez, E.A.; Maiza, P.J. Yacimientos no metalíferos y rocas de aplicación. In Proceedings of the Geología y Recursos Naturales de la Provincia de Río Negro, IX Congreso Geológico Argentino, Bariloche, Argentina, 5–9 November 1984; Ramos, V., Ed.; pp. 611–628. [Google Scholar]
- Cordenons, P.D.; Remesal, M.B.; Salani, F.M.; Cerredo, M.E. Temporal and spatial evolution of the Somún Curá magmatic province, northern extra-andean Patagonia, Argentina. J. S. Am. Earth Sci. 2020, 104, 102881. [Google Scholar] [CrossRef]
- International Mineralogical Association (IMA). 2025. Available online: https://cnmnc.units.it/files/editor/IMA_Master_List_(2025-07).pdf (accessed on 15 August 2025).
- Velde, B. Introduction to Clay Minerals: Chemistry, Origins, Uses and Environmental Significance; Chapman and Hall Ltd.: London, UK, 1992; Volume 198, 198p. [Google Scholar]
- JCPDS, International Centre for Diffraction Data (ICDD). Mineral Powder Diffraction File Databook; ICDD: Swarthmore, PA, USA, 1993; pp. 614–615. [Google Scholar]
- Pozo, M.; Calvo, J.P.; Pozo, E.; Moreno, A. Genetic constraints on crystallinity, thermal behavior and surface area of sepiolite from the Cerro de los Batallones deposits (Madrid Basin, Spain). Appl. Clay Sci. 2014, 91–92, 30–45. [Google Scholar] [CrossRef]
- Dikmen, S. Zeta potential study of natural- and acid-activated sepiolites in electrolyte solutions. Can. J. Chem. Eng. 2012, 90, 421–427. [Google Scholar] [CrossRef]
- Locatelli, D.; Pavlovic, N.; Barbera, V.; Giannini, L.; Galimberti, M. Sepiolite as reinforcing filler for rubber composites: From the chemical compatibilization to the commercial exploitation. Kautsch. Gummi Kunststoffe 2020, 73, 34–41. [Google Scholar]
- Zhou, F.; Ye, G.; Gao, Y.; Wang, H.; Zhou, S.; Liu, Y.; Yan, C. Cadmium adsorption by thermal-activated sepiolite: Application to in-situ remediation of artificially contaminated soil. J. Hazard. Mater. 2022, 423A, 127104. [Google Scholar] [CrossRef]
- Shahraki, B.; Mehrabi, B.; Dabiri, R. Thermal behavior of Zefreh dolomite mine (Central Iran). J. Min. Metall. 2009, 45B, 35–44. [Google Scholar] [CrossRef]
- Suárez, M.; García-Rivas, J.; García-Romero, E.; Jara, N. Mineralogical characterisation and surface properties of sepiolite from Polatli (Turkey). Appl. Clay Sci. 2016, 131, 124–130. [Google Scholar] [CrossRef]
- Largo, F.; Haounati, R.; Ouachtak, H.; Hafid, N.; Jada, A.; Addi, A.A. Design of organically modified sepiolite and its use as adsorbent for hazardous Malachite Green dye removal from water. Water Air Soil Pollut. 2023, 234, 183. [Google Scholar] [CrossRef]
- Wei, S.; Wang, L.; Wu, Y.; Liu, H. Study on removal of copper ions from aqueous phase by modified sepiolite flocs method. Environ. Sci. Pollut. Res. 2022, 29, 73492–73503. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, S.; Ge, L.; Lin, F.; Lu, Q.; Wang, T.; Lu, B. Development of organic–inorganic hybrid beads from sepiolite and cellulose for effective adsorption of malachite green. RSC Adv. 2017, 7, 38965–38972. [Google Scholar] [CrossRef]
- Zhou, X.; Li, H.; Liu, Y.; Hao, J.; Liu, H.; Lu, X. Improvement of stability of insecticidal proteins from Bacillus thuringiensis against UV-irradiation by adsorption on sepiolite. Adsorpt. Sci. Technol. 2018, 36, 1233–1245. [Google Scholar] [CrossRef]
- Yang, L.; Deng, Y.; Gong, D.; Luo, H.; Zhou, X.; Jiang, F. Effects of low molecular weight organic acids on adsorption of quinclorac by sepiolite. Environ. Sci. Pollut. Res. 2021, 28, 9582–9597. [Google Scholar] [CrossRef]
- Alves, L.; Ferraz, E.; Santarén, J.; Rasteiro, M.G.; Gamelas, J.A. Improving colloidal stability of sepiolite suspensions: Effect of the mechanical disperser and chemical dispersant. Minerals 2020, 10, 779. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, L.; Shiko, E.; Cheng, Y.; Zhang, R.; Zeng, Z.; Zhao, T.; Zhou, Y.; Chen, H.; Liu, Y.; et al. Low-price MnO2 loaded sepiolite for Cd2+ capture. Adsorption 2019, 25, 1271–1283. [Google Scholar] [CrossRef]
- Caicedo-Pineda, G.A.; Prada-Fonseca, M.C.; Casas-Botero, A.E.; Martínez-Tejada, H.V. Effect of the tryptone concentration on the calcium carbonate biomineralization mediated by Bacillus cereus. Dyna 2018, 85, 69–75. [Google Scholar] [CrossRef]
- Nguyen, M.B.; Le, G.H.; Pham, T.T.; Pham, G.T.; Quan, T.T.; Nguyen, T.D.; Vu, T.A. Novel Nano-Fe2O3-Co3O4 Modified Dolomite and Its use as highly efficient catalyst in the ozonation of ammonium solution. J. Nanomater. 2020, 2020, 4593054. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, A.; Cerdán, M.; Jordá, J.D.; Amat, B.; Cortina, J. Characterization of soil mineralogy by FTIR: Application to the analysis of mineralogical changes in soils affected by vegetation patches. Plant Soil 2019, 439, 447–458. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Ruiz-García, C.; Fernandes, F.M.; Lo Dico, G.; Lisuzzo, L.; Prevot, V.; Darder, M.; Aranda, P. Sepiolite-hydrogels: Synthesis by ultrasound irradiation and their use for the preparation of functional clay-based nanoarchitectured materials. Front. Chem. 2021, 9, 733105. [Google Scholar] [CrossRef] [PubMed]
- Lescano, L.; Castillo, L.; Marfil, S.; Barbosa, S.; Maiza, P. Separation and purification of sepiolite fibers. Contribution to special processing for industrial use. Appl. Clay Sci. Intern. J. Appl. Technol. Clays Clay Miner. 2014, 93, 378–382. [Google Scholar]
- Castillo, L.; Lescano, L.; Sirvent, L.; Barbosa, S.; Marfil, S.; Maiza, P. Separación y purificación de fibras de sepiolita: Contribución al procesamiento de arcillas especiales para uso industrial. Geoacta 2011, 36, 113–127. [Google Scholar]
- Fajdek-Bieda, A.; Wróblewska, A.; Miądlicki, P.; Szymańska, A.; Dzięcioł, M.; Booth, A.M.; Michalkiewicz, B. Influence of technological parameters on the isomerization of geraniol using sepiolite. Catal. Lett. 2020, 150, 901–911. [Google Scholar] [CrossRef]
- Tian, G.; Han, G.; Wang, F.; Liang, J. Sepiolite nanomaterials: Structure, properties and functional applications. In Nanomaterials from Clay Minerals; Wang, A., Wang, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–201. [Google Scholar]
- Tolsa. Available online: https://www.tolsa.com/es/ (accessed on 15 August 2025).
| Samples | SiO2 (%) | Al2O3 (%) | Fe2O3 (%) | MgO (%) | CaO (%) | Na2O (%) | K2O (%) | LOI (%) |
|---|---|---|---|---|---|---|---|---|
| ST | 63.10 | 1.08 | 0.27 | 23.80 | 0.49 | 0.09 | 0.21 | 10.96 |
| SA | 53.15 | 1.83 | 1.31 | 24.08 | 0.90 | 0.10 | 0.08 | 18.54 |
| S | 56.26 | - | - | 25.27 | - | - | - | 18.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lescano, L.; Marfil, S.A.; Castillo, L.A.; Barbosa, S.E. Influence of Geological Origin on the Physicochemical Characteristics of Sepiolites. Minerals 2025, 15, 950. https://doi.org/10.3390/min15090950
Lescano L, Marfil SA, Castillo LA, Barbosa SE. Influence of Geological Origin on the Physicochemical Characteristics of Sepiolites. Minerals. 2025; 15(9):950. https://doi.org/10.3390/min15090950
Chicago/Turabian StyleLescano, Leticia, Silvina A. Marfil, Luciana A. Castillo, and Silvia E. Barbosa. 2025. "Influence of Geological Origin on the Physicochemical Characteristics of Sepiolites" Minerals 15, no. 9: 950. https://doi.org/10.3390/min15090950
APA StyleLescano, L., Marfil, S. A., Castillo, L. A., & Barbosa, S. E. (2025). Influence of Geological Origin on the Physicochemical Characteristics of Sepiolites. Minerals, 15(9), 950. https://doi.org/10.3390/min15090950







