Mineralogical and Geochemical Characterization of the Benavila (Portugal) Bentonites
Abstract
1. Introduction
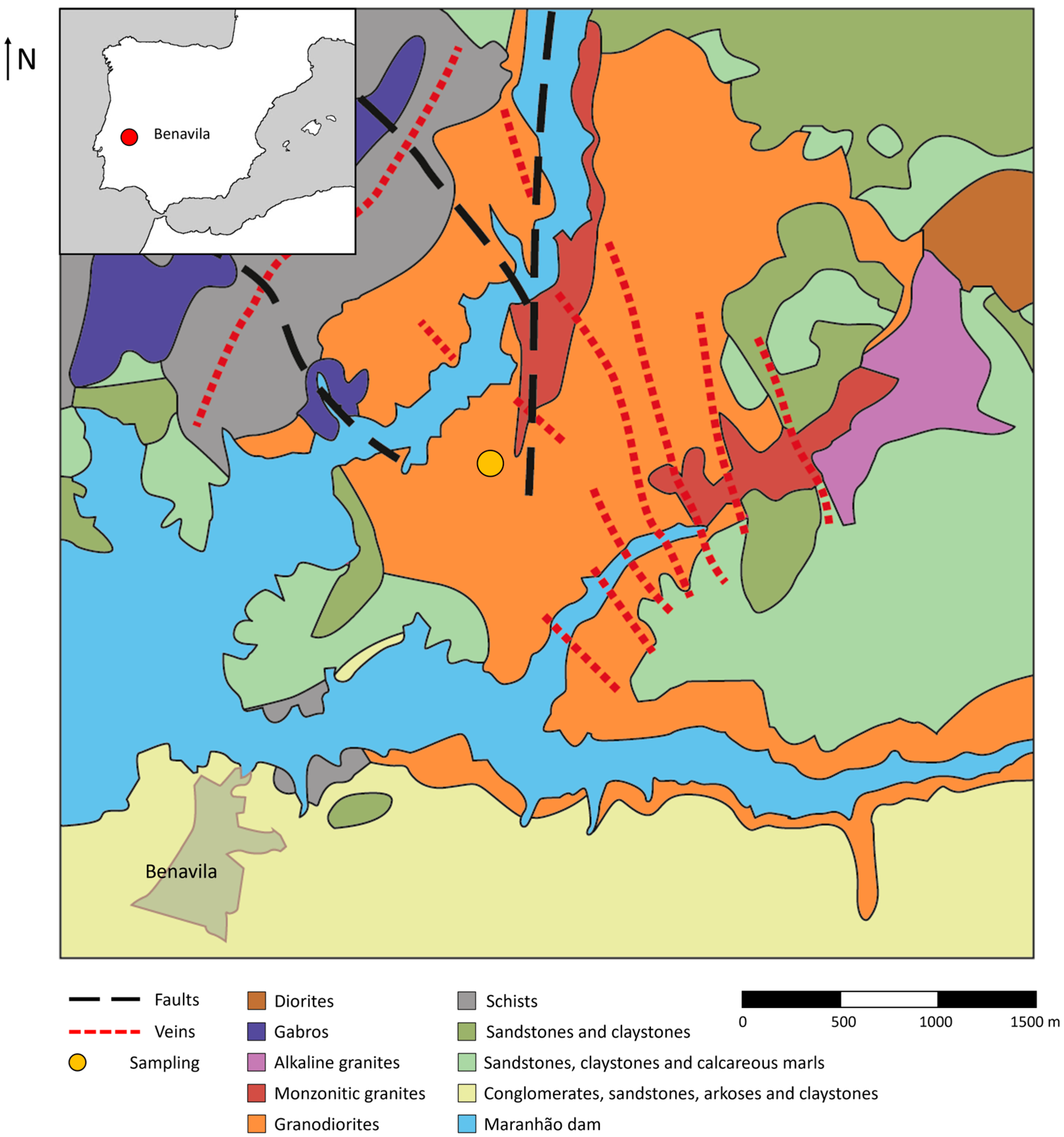
2. Geological Setting
3. Materials and Methods
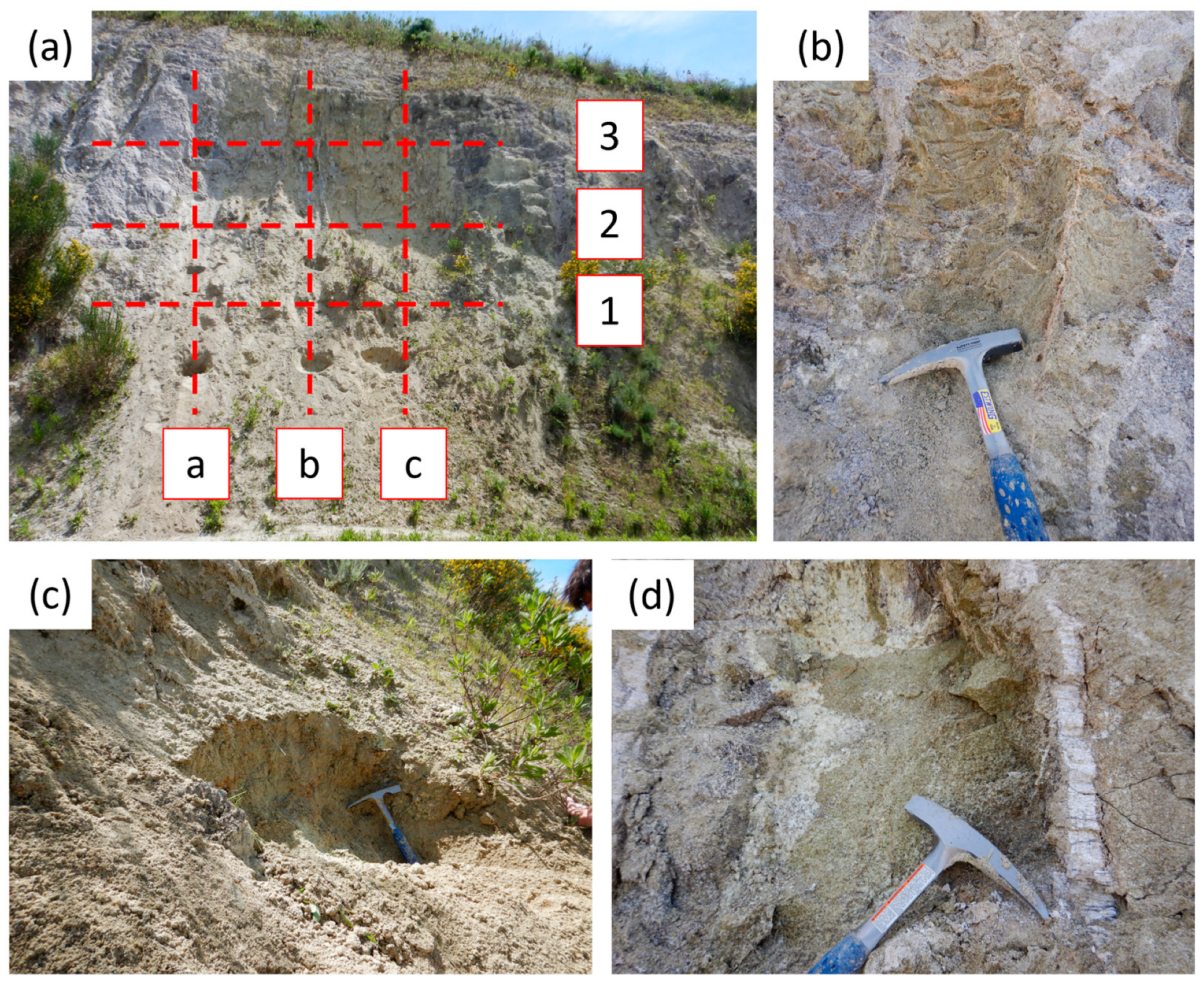
3.1. Samples
3.2. Methodology
4. Results
4.1. Mineralogical Characterization
4.1.1. XRD
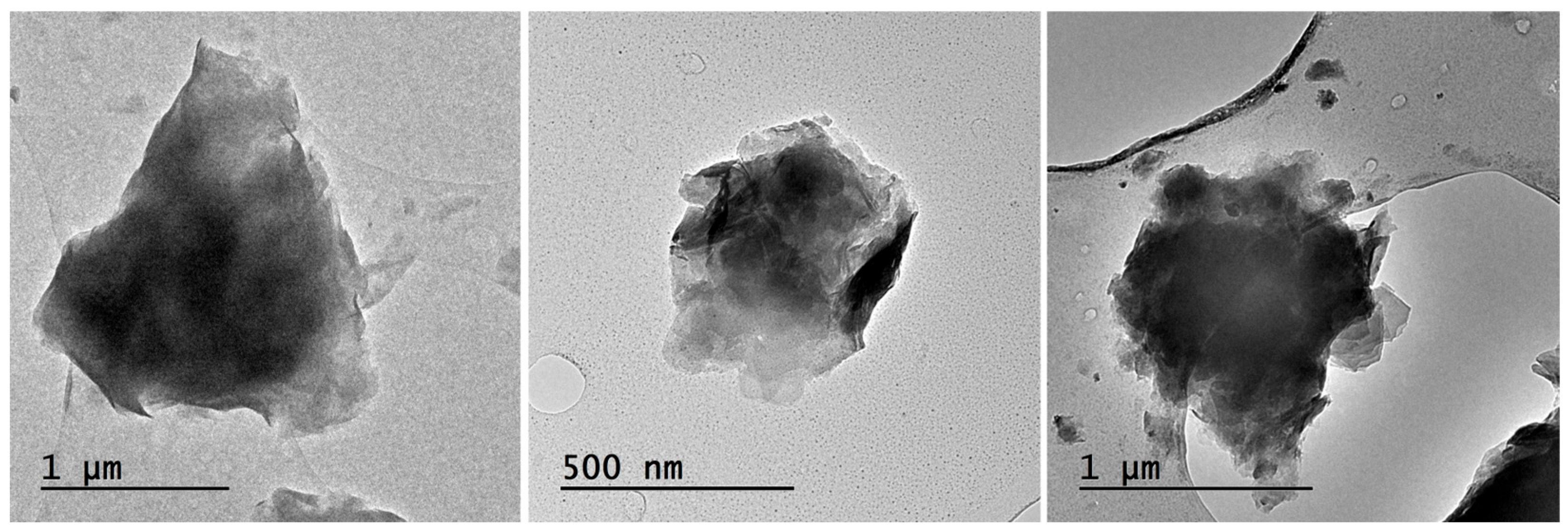
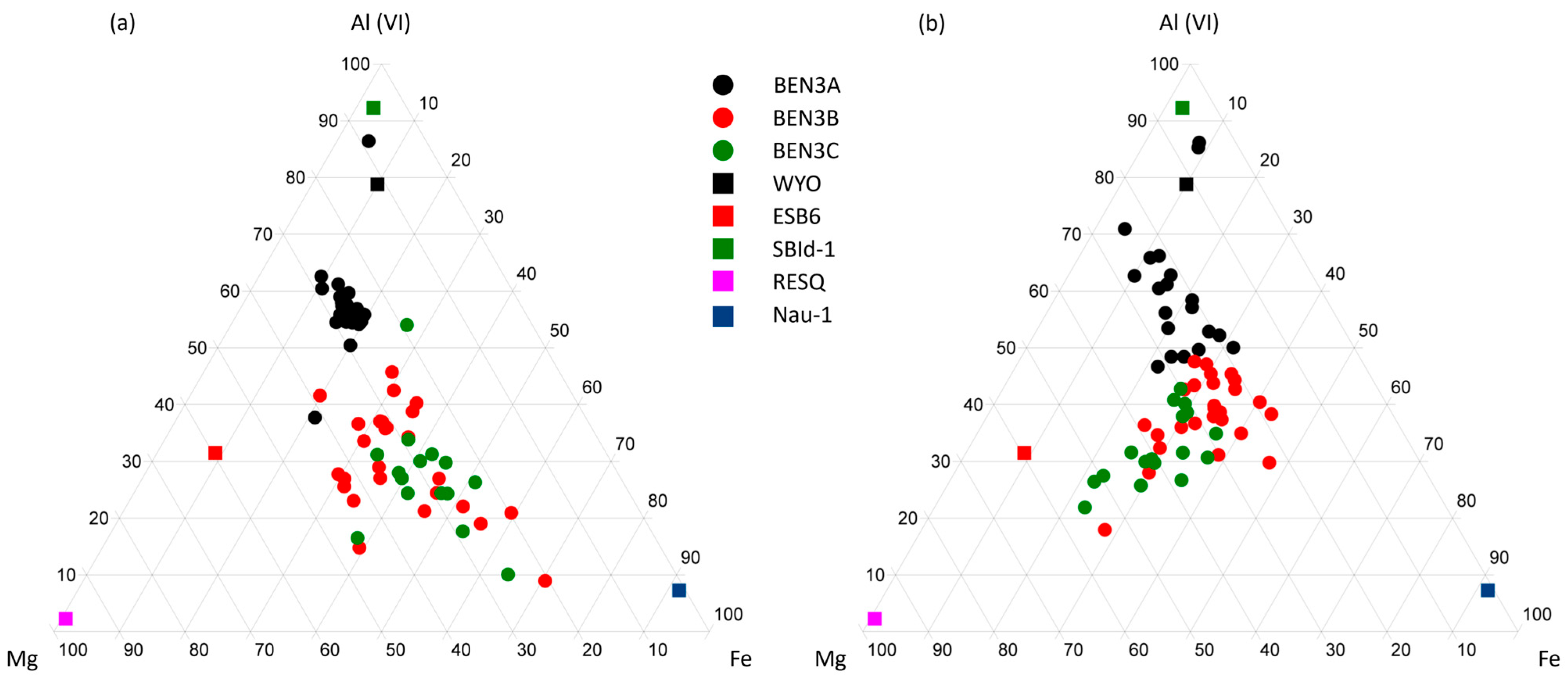
4.1.2. TEM-AEM
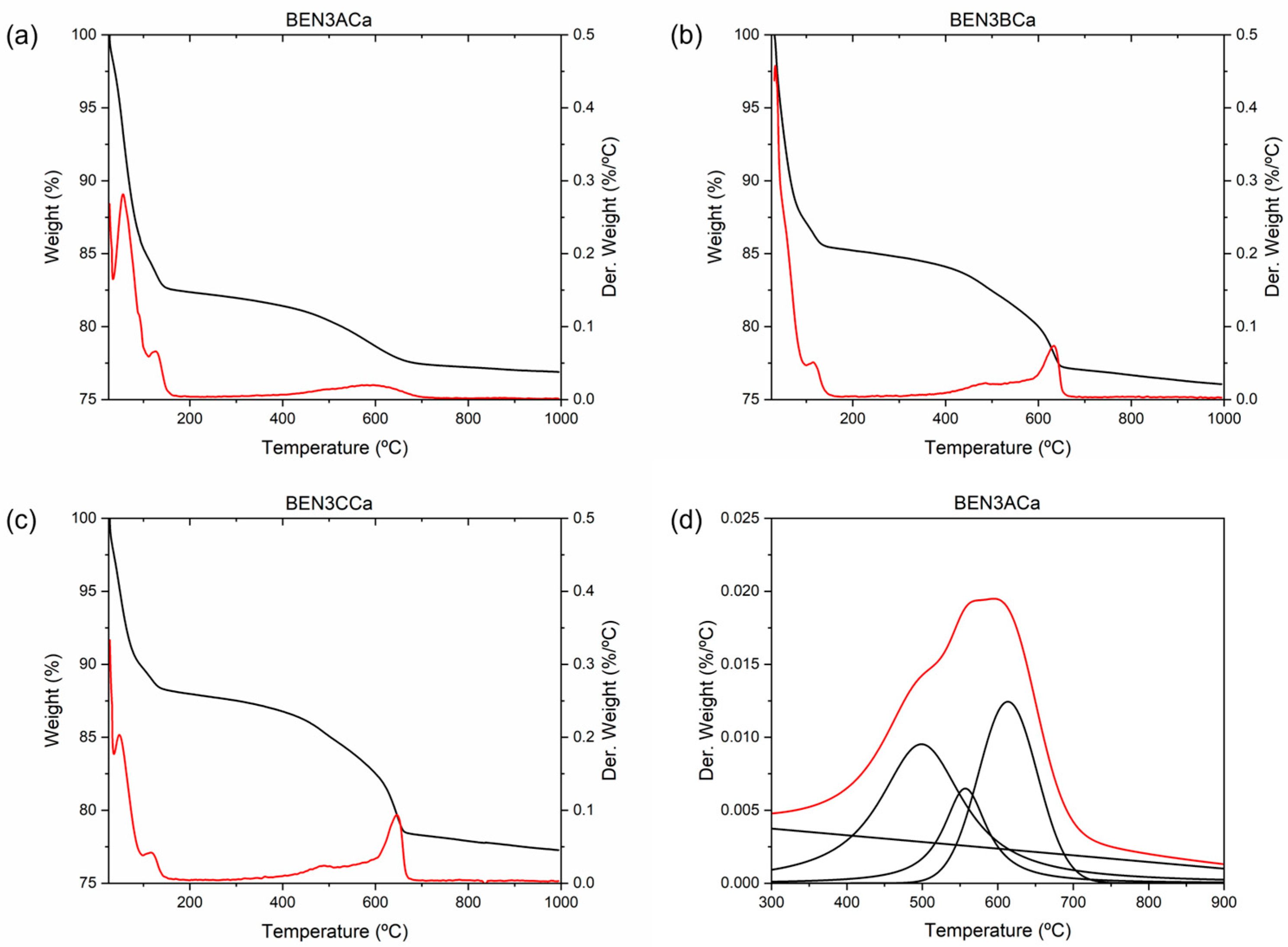
4.1.3. TGA
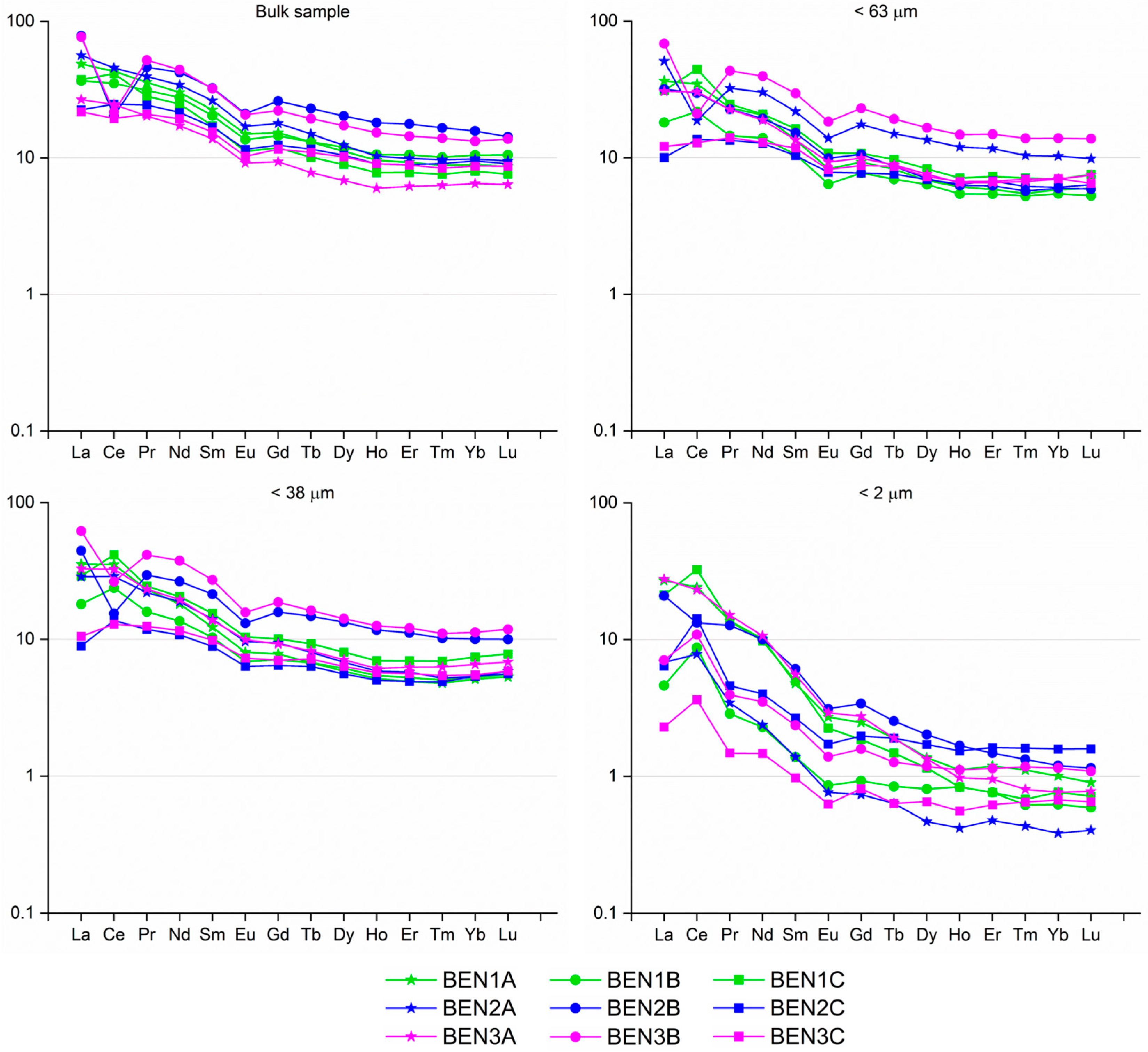
4.2. Geochemistry
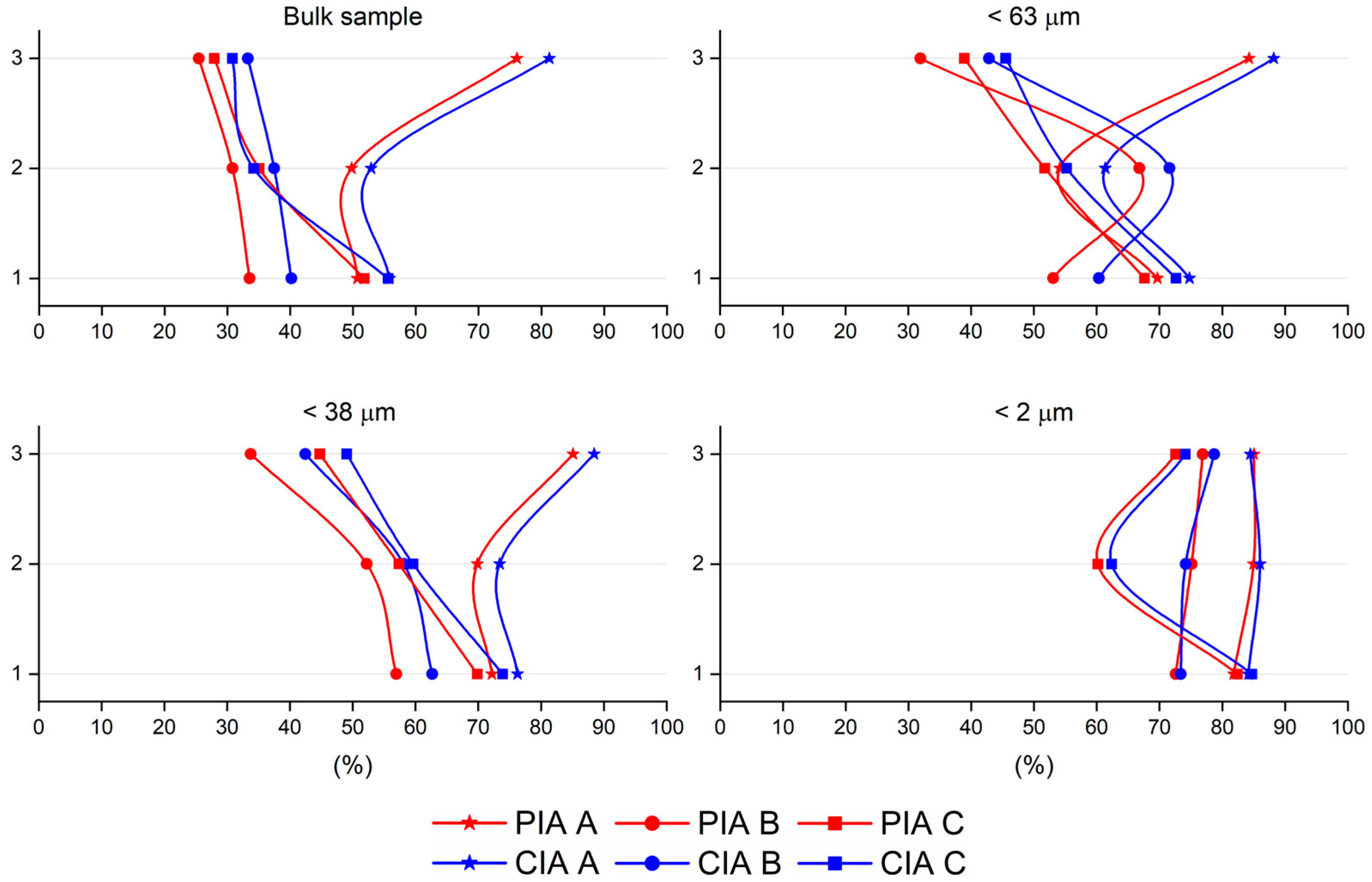
5. Discussion
6. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Newman, A.C.D. The Specific Surface of Soils Determined by Water Sorption. J. Soil Sci. 1983, 34, 23–32. [Google Scholar] [CrossRef]
- Środoń, J.; MaCarty, D.K. Surface Area and Layer Charge of Smectite from CEC and EGME/H2O-Retention Measurements. Clays Clay Miner. 2008, 56, 155–174. [Google Scholar] [CrossRef]
- Woodruff, W.F.; Revil, A. CEC-Normalized Clay-Water Sorption Isotherm. Water Resour. Res. 2011, 47, W11502. [Google Scholar] [CrossRef]
- Suárez, M.; Lorenzo, A.; García-Vicente, A.; Morales, J.; García-Rivas, J.; García-Romero, E. New Data on the Microporosity of Bentonites. Eng. Geol. 2022, 296, 106439. [Google Scholar] [CrossRef]
- Murray, H.H. Bentonite Applications. In Applied Clay Mineralogy Occurrences, Processing and Application of Kaolins, Bentonites, Palygorskite-Sepiolite, and Common Clays. Developments in Clay Science; Murray, H.H., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 2, pp. 111–130. ISBN 978-0-444-51701-2. [Google Scholar]
- Harvey, C.C.; Lagaly, G. Industrial Applications. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 451–490. ISBN 978-0-08-099364-5. [Google Scholar]
- Eisenhour, D.D.; Brown, R.K. Bentonite and Its Impact on Modern Life. Elements 2009, 5, 83–88. [Google Scholar] [CrossRef]
- Babu Valapa, R.; Loganathan, S.; Pugazhenthi, G.; Thomas, S.; Varghese, T.O. An Overview of Polymer–Clay Nanocomposites. In Clay-Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 29–81. ISBN 978-0-323-46153-5. [Google Scholar]
- Ishimaru, S.; Yamauchi, M.; Ikeda, R. A New Semiconducting Organic–Inorganic Nanocomposite, 1,5-Diaminonphthalene–Saponite Intercalation Compound. Solid State Commun. 2003, 127, 57–59. [Google Scholar] [CrossRef]
- Yang, Q.; Saito, T.; Isogai, A. Transparent, Flexible, and High-Strength Regenerated Cellulose/Saponite Nanocomposite Films with High Gas Barrier Properties. J. Appl. Polym. Sci. 2013, 130, 3168–3174. [Google Scholar] [CrossRef]
- Carretero, M.I.; Pozo, M. Clay and Non-Clay Minerals in the Pharmaceutical Industry. Appl. Clay Sci. 2009, 46, 73–80. [Google Scholar] [CrossRef]
- Khurana, I.S.; Kaur, S.; Kaur, H.; Khurana, R.K. Multifaceted Role of Clay Minerals in Pharmaceuticals. Future Sci. OA 2015, 1. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, S.; Sugunan, S. Enzymes Immobilized on Montmorillonite K 10: Effect of Adsorption and Grafting on the Surface Properties and the Enzyme Activity. Appl. Clay Sci. 2007, 35, 67–75. [Google Scholar] [CrossRef]
- Long, L.-H.; Zhang, Y.-T.; Wang, X.-F.; Cao, Y.-X. Montmorillonite Adsorbs Urea and Accelerates Urea Excretion from the Intestine. Appl. Clay Sci. 2009, 46, 57–62. [Google Scholar] [CrossRef]
- Müller, H.-J.; Dobler, D.; Schmidts, T.; Rusch, V. Smectite for Medical Use and Their Toxin Binding Capacity. J. Food Nutr. Popul. Health 2019, 03. [Google Scholar] [CrossRef]
- Ali Khan, S.; Riaz-ur-Rehman; Ali Khan, M. Sorption of Cesium on Bentonite. Waste Manag. 1994, 14, 629–642. [Google Scholar] [CrossRef]
- Bradbury, M.H.; Baeyens, B. A Generalised Sorption Model for the Concentration Dependent Uptake of Caesium by Argillaceous Rocks. J. Contam. Hydrol. 2000, 42, 141–163. [Google Scholar] [CrossRef]
- Durrant, C.B.; Begg, J.D.; Kersting, A.B.; Zavarin, M. Cesium Sorption Reversibility and Kinetics on Illite, Montmorillonite, and Kaolinite. Sci. Total Environ. 2018, 610–611, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Galamboš, M.; Magula, M.; Daňo, M.; Osacký, M.; Rosskopfová, O.; Rajec, P. Comparative Study of Cesium Adsorption on Dioctahedral and Trioctahedral Smectites. J. Radioanal. Nucl. Chem. 2012, 293, 829–837. [Google Scholar] [CrossRef]
- Missana, T.; Benedicto, A.; García-Gutiérrez, M.; Alonso, U. Modeling Cesium Retention onto Na-, K- and Ca-Smectite: Effects of Ionic Strength, Exchange and Competing Cations on the Determination of Selectivity Coefficients. Geochim. Cosmochim. Acta 2014, 128, 266–277. [Google Scholar] [CrossRef]
- Cuevas, J.; Ruiz, A.I.; Fernández, R.; Torres, E.; Escribano, A.; Regadío, M.; Turrero, M.J. Lime Mortar-Compacted Bentonite–Magnetite Interfaces: An Experimental Study Focused on the Understanding of the EBS Long-Term Performance for High-Level Nuclear Waste Isolation DGR Concept. Appl. Clay Sci. 2016, 124–125, 79–93. [Google Scholar] [CrossRef]
- Park, S.-M.; Alessi, D.S.; Baek, K. Selective Adsorption and Irreversible Fixation Behavior of Cesium onto 2:1 Layered Clay Mineral: A Mini Review. J. Hazard Mater. 2019, 369, 569–576. [Google Scholar] [CrossRef]
- Galopim de Carvalho, A.M.; Carvalhosa, A.B. Carta Geológica de Portugal Na Escala de 1 / 50.000. Notícia Explicativa Da Folha 32-A. Ponte de Sor; Serviços Geológicos de Portugal: Lisboa, Portugal, 1982. [Google Scholar]
- Piçarra, J.M.; Dias, R.P.; Ribeiro, M.L.; Solá, R.; Barbosa, B.; Pais, J.; Vivar, V.; Baltuille Martín, J.M. Carta Geológica de Portugal Na Escala de 1 / 50.000. Notícia Explicativa Da Folha 32-C. Avis; LNEG: Lisboa, Portugal, 2009. [Google Scholar]
- Pinto Henriques, S. Ensaios de Beneficiação de “Bentonite” de Avis (Portugal) Para Aplicação Em Geologia Médica. Master’s Thesis, Universidade de Aveiro, Aveiro, Portugal, 2014. [Google Scholar]
- Galopim de Carvalho, A.M.; Alegria, M.F.; Mira Azevedo, M.T. Aspectos de Alteração Em Rochas Dioríticas de Benavila (Avis). Comun. Geológicas 1980, 66, 67–70. [Google Scholar]
- Canilho, M.H. Contribuição Para o Conhecimento Petrográfico e Geoquímico Do Maciço Ígneo de Benavila (Avis). Ciênc. Terra 1992, 11, 255–273. [Google Scholar]
- de Gomes, C.S.F. Industrial minerals: Present situation in Portugal of the commercial clays. In A Geologia de Engenharia e os Recursos Geológicos. Volume 2: Recursos Geológicos e Formação; Imprensa da Universidade de Coimbra: Coimbra, Portugal, 2003; pp. 349–366. ISBN 978-989-26-0322-3. [Google Scholar]
- Dias, M.I.; Suárez Barrios, M.; Prates, S. Las Bentonitas de Benavila (Portugal). Caracterización Mineralógica y Propiedades. [Bentonites from Benavila (Portugal). Mineralogical Characterisation and Properties]. Geogactea 2004, 35, 99–102. [Google Scholar]
- Dias, M.I. Caracterização Mineralógica e Tecnológica de Argilas Especiais de Bacias Terciárias Portuguesas. Ph.D. Thesis, University of Lisbon, Lisbon, Portugal, 1998. [Google Scholar]
- Rebelo, M.; Viseras, C.; López-Galindo, A.; Rocha, F.; da Silva, E.F. Characterization of Portuguese Geological Materials to Be Used in Medical Hydrology. Appl. Clay Sci. 2011, 51, 258–266. [Google Scholar] [CrossRef]
- Guimarães, V.; Bobos, I.; Rocha, F. Acid-Base Properties of Ca-Montmorillonite from Benavila Region (Portugal). Comun. Geológicas 2014, 101, 791–794. [Google Scholar]
- Dziadkowiec, J.; Mansa, R.; Quintela, A.; Rocha, F.; Detellier, C. Preparation, Characterization and Application in Controlled Release of Ibuprofen-Loaded Guar Gum/Montmorillonite Bionanocomposites. Appl. Clay Sci. 2017, 135, 52–63. [Google Scholar] [CrossRef]
- Bastos, C.M.; Rocha, F.; Patinha, C.; Marinho-Reis, P. Bioaccessibility by Perspiration Uptake of Minerals from Two Different Sulfurous Peloids. Environ. Geochem. Health 2023, 45, 6621–6641. [Google Scholar] [CrossRef]
- Gonçalves, F. Estado Actual Do Conhecimento Geológico Do Nordeste Alentejano. In Proceedings of the IV Curso de Extensão Universitária de Ciências Geológicas; Faculdade Ciências de Lisboa: Lisbon, Portugal, 1978; pp. 193–213. [Google Scholar]
- Pereira, V.B. Prospecção de Bentonites. Referência Aos Trabalhos Realizados e Resultados Obtidos. Estud. Notas E Trab. 1993, 35, 35–54. [Google Scholar]
- Martín Pozas, J.M. Analisis Cuantitativo de Fases Cristalinas Por DRX. In Método de Debye-Scherrer; Saja, J., Ed.; ICE Universidad de Valladolid: Valladolid, Spain, 1975. [Google Scholar]
- García-Romero, E.; Lorenzo, A.; García-Vicente, A.; García-Rivas, J.; Morales, J.; Suárez, M. On the Structural Formula of Smectites: A Review and New Data on the Influence of the Exchangeable Cations. J. Appl. Crystallogr. 2021, 54, 251–262. [Google Scholar] [CrossRef]
- Bahlburg, H.; Dobrzinski, N. A Review of the Chemical Index of Alteration (CIA) and Its Application to the Study of Neoproterozoic Glacial Deposits and Climate Transitions. Geol. Soc. Lond. Mem. 2011, 36, 81–92. [Google Scholar] [CrossRef]
- Buggle, B.; Glaser, B.; Hambach, U.; Gerasimenko, N.; Marković, S. An Evaluation of Geochemical Weathering Indices in Loess–Paleosol Studies. Quat. Int. 2011, 240, 12–21. [Google Scholar] [CrossRef]
- Fedo, C.M.; Nesbitt, H.W.; Young, G.M. Unraveling the Effects of Potassium Metasomatism in Sedimentary Rocks and Paleosols, with Implications for Paleoweathering Conditions and Provenance. Geology 1995, 23, 921–924. [Google Scholar] [CrossRef]
- Meunier, A.; Caner, L.; Hubert, F.; El Albani, A.; Pret, D. The Weathering Intensity Scale (WIS): An Alternative Approach of the Chemical Index of Alteration (CIA). Am. J. Sci. 2013, 313, 113–143. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Young, G.M. Early Proterozoic Climates and Plate Motions Inferred from Major Element Chemistry of Lutites. Nature 1982, 299, 715–717. [Google Scholar] [CrossRef]
- Price, J.R.; Velbel, M.A. Chemical Weathering Indices Applied to Weathering Profiles Developed on Heterogeneous Felsic Metamorphic Parent Rocks. Chem. Geol. 2003, 202, 397–416. [Google Scholar] [CrossRef]
- Newman, A.C.D.; Brown, G. The Chemical Constitution of Clays. In Chemistry of Clays and Clay Minerals; Newman, A.C.D., Ed.; Monograph No. 6; Mineralogical Society and Longman: London, UK, 1987; pp. 1–128. [Google Scholar]
- Fesharaki, O.; García-Romero, E.; Cuevas-González, J.; López-Martínez, N. Clay Mineral Genesis and Chemical Evolution in the Miocene Sediments of Somosaguas, Madrid Basin, Spain. Clay Miner. 2007, 42, 187–201. [Google Scholar] [CrossRef]
- de Santiago Buey, C.; Suárez Barrios, M.; García-Romero, E.; Domínguez Diaz, M.C.; Doval Montoya, M. Electron Microscopic Study of the Illite-Smectite Transformation in the Bentonites from Cerro Del Aguila (Toledo, Spain). Clay Miner. 1998, 33, 501–510. [Google Scholar] [CrossRef]
- Pelayo, M.; Schmid, T.; Díaz-Puente, F.J.; López-Martínez, J. Characterization and Distribution of Clay Minerals in the Soils of Fildes Peninsula and Ardley Island (King George Island, Maritime Antarctica). Clay Miner. 2022, 57, 264–284. [Google Scholar] [CrossRef]
- García-Romero, E.; Suárez, M. A Structure-Based Argument for Non-Classical Crystal Growth in Natural Clay Minerals. Mineral. Mag. 2018, 82, 171–180. [Google Scholar] [CrossRef]
- Post, J.L.; Cupp, B.L.; Madsen, F.T. Beidellite and Associated Clays from the Delamar Mine and Florida Mountain Area, Idaho. Clays Clay Miner. 1997, 45, 240–250. [Google Scholar] [CrossRef]
- Keeling, J.L.; Raven, M.D.; Gates, W.P. Geology and Characterization of Two Hydrothermal Nontronites from Weathered Metamorphic Rocks at the Uley Graphite Mine, South Australia. Clays Clay Miner. 2000, 48, 537–548. [Google Scholar] [CrossRef]
- Emmerich, K. Full Characterization of Smectites. In Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 381–404. ISBN 978-0-08-099364-5. [Google Scholar]
- Emmerich, K.; Wolters, F.; Kahr, G.; Lagaly, G. Clay Profiling: The Classification of Montmorillonites. Clays Clay Miner. 2009, 57, 104–114. [Google Scholar] [CrossRef]
- El-Barawy, K.A.; Girgis, B.S.; Felix, N.S. Thermal Treatment of Some Pure Smectites. Thermochim. Acta 1986, 98, 181–189. [Google Scholar] [CrossRef]
- Drits, V.A.; Lindgreen, H.; Salyn, A.L.; Ylagan, R.; McCarty, D.K. Semiquantitative Determination of Trans-Vacant and Cis-Vacant 2:1 Layers in Illites and Illite-Smectites by Thermal Analysis and X-Ray Diffraction. Am. Miner. 1998, 83, 1188–1198. [Google Scholar] [CrossRef]
- Drits, V.A. An Improved Model for Structural Transformations of Heat-Treated Aluminous Dioctahedral 2:1 Layer Silicates. Clays Clay Miner. 1995, 43, 718–731. [Google Scholar] [CrossRef]
- Wolters, F.; Emmerich, K. Thermal Reactions of Smectites—Relation of Dehydroxylation Temperature to Octahedral Structure. Thermochim. Acta 2007, 462, 80–88. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A General-Purpose Peak Fitting Program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Wang, H. Structural Characterization of Octahedral Sheet in Dioctahedral Smectites by Thermal Analysis. Minerals 2020, 10, 347. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock-Forming Minerals; Longman: London, UK, 1966. [Google Scholar]
- González López, J.M.; Bauluz, B.; Fernández-Nieto, C.; Oliete, A.Y. Factors Controlling the Trace-Element Distribution in Fine-Grained Rocks: The Albian Kaolinite-Rich Deposits of the Oliete Basin (NE Spain). Chem. Geol. 2005, 214, 1–19. [Google Scholar] [CrossRef]
- Middelburg, J.; Vanderweijden, C.; Woittiez, J. Chemical Processes Affecting the Mobility of Major, Minor and Trace Elements during Weathering of Granitic Rocks. Chem. Geol. 1988, 68, 253–273. [Google Scholar] [CrossRef]
- Mosser, C.; Brillanceau, A.; Besnus, Y. Relationship between Sediments and Their Igneous Source Rocks Using Clay Mineral Multi-Element Chemistry: The Cenozoic Lacustrine Anloua Basin (Adamaoua, Cameroon). Chem. Geol. 1991, 90, 319–342. [Google Scholar] [CrossRef]
- Smith, J.V. Feldspar Minerals 2: Chemical and Textural Properties; Springer: Berlin/Heidelberg, Germany, 1974. [Google Scholar]
- Baker, P.A.; Gieskes, J.M.; Elderfield, H. Diagenesis of Carbonates in Deep-Sea Sediments—Evidence from Sr/Ca Ratios and Interstitial Dissolved Sr2+ Data. J. Sediment. Petrol. 1982, 52, 71–82. [Google Scholar]
- Gabitov, R.I.; Watson, E.B. Partitioning of Strontium between Calcite and Fluid. Geochem. Geophys. Geosystems 2006, 7, 2005GC001216. [Google Scholar] [CrossRef]
- García-Rivas, J.; Suárez, M.; Torres, T.; Sánchez-Palencia, Y.; García-Romero, E.; Ortiz, J.E. Geochemistry and Biomarker Analysis of the Bentonites from Esquivias (Toledo, Spain). Minerals 2018, 8, 291. [Google Scholar] [CrossRef]
- McLennan, S.M.; Nance, W.B.; Taylor, S.R. Rare Earth Element-Thorium Correlations in Sedimentary Rocks, and the Composition of the Continental Crust. Geochim. Cosmochim. Acta 1980, 44, 1833–1839. [Google Scholar] [CrossRef]
- Louvel, M.; Etschmann, B.; Guan, Q.; Testemale, D.; Brugger, J. Carbonate Complexation Enhances Hydrothermal Transport of Rare Earth Elements in Alkaline Fluids. Nat. Commun. 2022, 13, 1456. [Google Scholar] [CrossRef] [PubMed]
- Reece, M.E.; Migdisov, A.A.; Williams-Jones, A.E.; Strzelecki, A.C.; Waters, L.; Boukhalfa, H.; Guo, X. Stability of Aqueous Neodymium Complexes in Carbonate-Bearing Solutions from 100–600 °C. Commun. Earth Environ. 2025, 6, 353. [Google Scholar] [CrossRef]
- Di, J.; Ding, X. Complexation of REE in Hydrothermal Fluids and Its Significance on REE Mineralization. Minerals 2024, 14, 531. [Google Scholar] [CrossRef]
- Boynton, W.V. Cosmochemistry of the Rare Earth Elements: Meteorite Studies. In Developments in Geochemistry; Henderson, P., Ed.; Elsevier: Amsterdam, The Netherlands, 1984; Volume 2, pp. 63–114. ISBN 978-0-444-42148-7. [Google Scholar]
- Foley, N.K.; Jaskula, B.W.; Kimball, B.E.; Schulte, R.F. Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply. Chapter H: Gallium.; Professional Paper; U.S. Geological Survey: Reston, VA, USA, 2017; p. 862.
- Eliopoulos, I.-P.D.; Eliopoulos, G.D. Factors Controlling the Gallium Preference in High-Al Chromitites. Minerals 2019, 9, 623. [Google Scholar] [CrossRef]
- Lorenzo, A.; Sánchez-Santos, J.M.; Rivas, M.J.; García-Romero, E.; Suárez, M. Geochemistry of Bentonites: A Statistical Analysis of Trace Element Distribution in Smectites. Appl. Clay Sci. 2024, 257, 107449. [Google Scholar] [CrossRef]
- Shields, G.; Stille, P. Diagenetic Constraints on the Use of Cerium Anomalies as Palaeoseawater Redox Proxies: An Isotopic and REE Study of Cambrian Phosphorites. Chem. Geol. 2001, 175, 29–48. [Google Scholar] [CrossRef]
- Lewis, A.J.; Palmer, M.R.; Sturchio, N.C.; Kemp, A.J. The Rare Earth Element Geochemistry of Acid-Sulphate and Acid-Sulphate-Chloride Geothermal Systems from Yellowstone National Park, Wyoming, USA. Geochim. Cosmochim. Acta 1997, 61, 695–706. [Google Scholar] [CrossRef]
- Karakaya, M.Ç.; Karakaya, N.; Kupeli, S. Mineralogical and Geochemical Properties of the Na- And Ca-Bentonites of Ordu (Ne Turkey). Clays Clay Miner. 2011, 59, 75–94. [Google Scholar] [CrossRef]
- Class, C.; le Roex, A.P. Ce Anomalies in Gough Island Lavas—Trace Element Characteristics of a Recycled Sediment Component. Earth Planet. Sci. Lett. 2008, 265, 475–486. [Google Scholar] [CrossRef]
- Braun, J.-J.; Pagel, M.; Muller, J.-P.; Bilong, P.; Michard, A.; Guillet, B. Cerium Anomalies in Lateritic Profiles. Geochim. Cosmochim. Acta 1990, 54, 781–795. [Google Scholar] [CrossRef]



| Sample | Amp | Cal | Dol | Kfs | Pl | Qz | Chl | Ilt | Sme | C/S |
|---|---|---|---|---|---|---|---|---|---|---|
| BEN1A | 12 | 2 | 4 | - | 6 | - | - | 74 | - | |
| BEN1A < 63 µm | 5 | - | - | - | 2 | - | - | 90 | - | |
| BEN1A < 38 µm | 2 | - | - | - | - | - | - | 96 | - | |
| BEN1A < 2 µm | 2 | - | - | 97 | - | |||||
| BEN1B | 25 | 2 | - | - | - | - | - | 70 | - | |
| BEN1B < 63 µm | 11 | - | - | - | - | - | 1 | 86 | - | |
| BEN1B <3 8 µm | 9 | - | - | - | - | 1 | 89 | - | ||
| BEN1B < 2 µm | 4 | - | - | 95 | - | |||||
| BEN1C | - | 16 | - | - | - | - | - | 82 | - | |
| BEN1C < 63 µm | - | 4 | - | - | - | - | - | 94 | - | |
| BEN1C < 38 µm | - | 5 | - | - | - | - | - | 92 | - | |
| BEN1C < 2 µm | 2 | - | - | 97 | - | |||||
| BEN2A | 17 | - | - | - | - | - | - | 81 | ||
| BEN2A < 63 µm | 12 | 4 | - | - | - | - | 83 | |||
| BEN2A < 38 µm | 5 | - | - | - | - | - | 93 | |||
| BEN2A < 2 µm | - | - | - | 99 | ||||||
| BEN2B | 27 | - | - | - | - | - | 71 | - | ||
| BEN2B < 63 µm | 9 | 3 | - | - | - | - | 86 | - | ||
| BEN2B < 38 µm | 15 | 4 | - | - | - | - | 80 | - | ||
| BEN2B < 2 µm | 2 | - | - | 97 | - | |||||
| BEN2C | 9 | 26 | 2 | 7 | - | - | - | 55 | ||
| BEN2C < 63 µm | 4 | 13 | - | - | 82 | |||||
| BEN2C < 38 µm | 3 | 5 | - | - | - | - | - | 90 | ||
| BEN2C < 2 µm | 2 | - | - | 96 | ||||||
| BEN3A | - | - | - | 2 | - | 95 | 1 | |||
| BEN3A < 63 µm | - | - | - | 97 | 1 | |||||
| BEN3A < 38 µm | - | - | - | - | 96 | 1 | ||||
| BEN3A < 2 µm | - | 98 | 1 | |||||||
| BEN3B | 34 | - | - | 64 | 1 | |||||
| BEN3B < 63 µm | 27 | - | - | - | 71 | 1 | ||||
| BEN3B < 38 µm | 28 | - | - | 70 | 1 | |||||
| BEN3B < 2 µm | 1 | - | - | 96 | 2 | |||||
| BEN3C | 4 | 38 | 4 | - | 2 | - | - | 51 | ||
| BEN3C < 63 µm | - | 16 | - | - | - | - | - | 82 | ||
| BEN3C < 38 µm | 2 | 11 | - | - | - | - | - | 86 | ||
| BEN3C < 2 µm | 2 | - | - | 97 |
| Samples | Chemical analysis of major oxides | Mean structural formulae | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | MgO | CaO | NaO | K2O | TiO2 | Tetrahedral | Octaheral | Interlayer | |||||||||||||
| Si | Al | ΣTC | Al | Mg | Fe | Ti | ΣOC | Ca | Na | K | TCh | OCh | LC | ||||||||||
| BEN3A | Max | 66.33 | 32.64 | 11.02 | 8.21 | 9.15 | 0.10 | 6.87 | 0.26 | ||||||||||||||
| Min | 54.81 | 18.33 | 0.00 | 2.57 | 0.25 | 0.00 | 0.00 | 0.00 | |||||||||||||||
| Mean | 63.98 | 19.90 | 7.89 | 6.84 | 0.92 | 0.00 | 0.40 | 0.06 | 7.67 | 0.33 | 8.00 | 2.48 | 1.22 | 0.71 | 0.01 | 4.42 | 0.12 | 0.06 | −0.33 | 0.05 | 0.28 | ||
| n = 26 | SD | 1.97 | 2.58 | 2.28 | 0.90 | 1.60 | 0.02 | 1.26 | 0.09 | ||||||||||||||
| BEN3B | Max | 62.46 | 19.56 | 33.00 | 11.94 | 2.75 | 0.00 | 1.43 | 1.14 | ||||||||||||||
| Min | 48.07 | 11.58 | 10.40 | 4.60 | 0.46 | 0.00 | 0.00 | 0.00 | |||||||||||||||
| Mean | 56.49 | 15.20 | 18.43 | 8.03 | 1.20 | 0.00 | 0.54 | 0.11 | 7.12 | 0.88 | 8.00 | 1.39 | 1.51 | 1.75 | 0.01 | 4.66 | 0.16 | 0.09 | −0.88 | 0.48 | 0.40 | ||
| n = 29 | SD | 3.85 | 1.72 | 5.04 | 2.06 | 0.60 | 0.00 | 0.40 | 0.29 | ||||||||||||||
| BEN3C | Max | 63.18 | 16.95 | 29.57 | 11.95 | 2.93 | 1.83 | 0.70 | 0.74 | ||||||||||||||
| Min | 49.74 | 11.85 | 11.77 | 4.20 | 1.41 | 0.00 | 0.00 | 0.00 | |||||||||||||||
| Mean | 56.80 | 13.35 | 20.07 | 7.08 | 1.89 | 0.12 | 0.38 | 0.12 | 7.22 | 0.78 | 8.00 | 1.23 | 1.34 | 1.92 | 0.01 | 4.50 | 0.26 | 0.03 | 0.06 | −0.78 | 0.17 | 0..61 | |
| n = 18 | SD | 3.37 | 1.24 | 4.10 | 1.79 | 0.41 | 0.47 | 0.24 | 0.23 | ||||||||||||||
| BEN3ACa | Max | 66.65 | 29.57 | 13.24 | 6.85 | 16.03 | 0.00 | 9.76 | 0.57 | ||||||||||||||
| Min | 54.10 | 15.68 | 2.04 | 1.13 | 2.22 | 0.00 | 0.04 | 0.00 | |||||||||||||||
| Mean | 61.53 | 19.19 | 7.64 | 4.71 | 5.49 | 0.00 | 1.40 | 0.06 | 7.54 | 0.46 | 8.00 | 2.31 | 0.86 | 0.70 | 0.01 | 3.88 | 0.72 | 0.22 | −0.46 | −1.20 | 1.66 | ||
| n = 20 | SD | 3.42 | 3.74 | 3.17 | 1.41 | 2.68 | 0.00 | 2.88 | 0.20 | ||||||||||||||
| BEN3BCa | Max | 63.19 | 17.80 | 19.73 | 13.66 | 6.48 | 0.00 | 2.77 | 1.38 | ||||||||||||||
| Min | 49.28 | 12.84 | 11.67 | 4.03 | 1.77 | 0.00 | 0.00 | 0.00 | |||||||||||||||
| Mean | 58.80 | 15.13 | 14.75 | 6.54 | 3.67 | 0.00 | 0.70 | 0.42 | 7.36 | 0.64 | 8.00 | 1.59 | 1.22 | 1.39 | 0.04 | 4.24 | 0.49 | 0.11 | −0.64 | −0.46 | 1.10 | ||
| n = 26 | SD | 3.36 | 1.05 | 1.98 | 2.17 | 1.05 | 0.00 | 0.81 | 0.33 | ||||||||||||||
| BEN3CCa | Max | 62.60 | 16.75 | 16.39 | 13.30 | 8.25 | 0.00 | 7.10 | 1.03 | ||||||||||||||
| Min | 53.74 | 12.86 | 10.97 | 6.04 | 2.18 | 0.00 | 0.31 | 0.02 | |||||||||||||||
| Mean | 57.10 | 14.73 | 13.27 | 8.99 | 3.74 | 0.00 | 1.67 | 0.50 | 7.20 | 0.80 | 8.00 | 1.38 | 1.69 | 1.26 | 0.05 | 4.38 | 0.48 | 0.34 | −0.80 | −0.50 | 1.30 | ||
| n = 17 | SD | 2.49 | 1.11 | 1.77 | 2.30 | 1.39 | 0.00 | 1.94 | 0.25 | ||||||||||||||
| Bulk Sample | <63 µm | <38 µm | <2 µm | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Max | Min | Mean | SD | Max | Min | Mean | SD | Max | Min | Mean | SD | Max | Min | Mean | SD | |
| SiO2 | 51.86 | 29.76 | 39.27 | 6.96 | 48.76 | 32.96 | 42.36 | 4.57 | 49.33 | 34.20 | 43.31 | 4.22 | 50.23 | 45.27 | 48.14 | 1.50 |
| Al2O3 | 13.58 | 8.26 | 11.37 | 2.00 | 14.39 | 8.72 | 12.02 | 2.24 | 14.48 | 8.55 | 12.14 | 2.32 | 16.08 | 10.38 | 13.47 | 1.89 |
| Fe2O3 | 7.81 | 6.10 | 6.96 | 0.62 | 10.60 | 5.27 | 7.56 | 1.50 | 11.52 | 5.30 | 8.10 | 1.81 | 10.53 | 4.85 | 7.63 | 2.02 |
| MnO | 0.13 | 0.06 | 0.10 | 0.02 | 0.09 | 0.04 | 0.06 | 0.02 | 0.09 | 0.04 | 0.07 | 0.02 | 0.07 | 0.02 | 0.05 | 0.02 |
| MgO | 6.18 | 4.30 | 4.93 | 0.54 | 5.42 | 3.99 | 4.61 | 0.43 | 5.44 | 3.96 | 4.67 | 0.47 | 5.57 | 4.66 | 5.14 | 0.28 |
| CaO | 21.65 | 1.67 | 13.80 | 6.32 | 17.32 | 1.32 | 8.15 | 4.42 | 15.84 | 1.38 | 7.31 | 3.94 | 4.47 | 1.27 | 2.64 | 0.93 |
| Na2O | 1.20 | 0.04 | 0.34 | 0.38 | 0.27 | 0.05 | 0.12 | 0.08 | 0.24 | 0.02 | 0.08 | 0.07 | 1.08 | 0.22 | 0.55 | 0.28 |
| K2O | 1.49 | 0.50 | 0.87 | 0.31 | 0.56 | 0.29 | 0.43 | 0.10 | 0.52 | 0.23 | 0.39 | 0.09 | 0.59 | 0.25 | 0.35 | 0.10 |
| TiO2 | 1.02 | 0.40 | 0.65 | 0.15 | 0.75 | 0.39 | 0.61 | 0.12 | 0.77 | 0.35 | 0.61 | 0.13 | 0.34 | 0.12 | 0.19 | 0.07 |
| P2O5 | 0.21 | 0.09 | 0.15 | 0.04 | 0.23 | 0.03 | 0.13 | 0.06 | 0.26 | 0.03 | 0.13 | 0.07 | 0.02 | 0.01 | 0.01 | 0.00 |
| LOI | 26.20 | 16.19 | 20.88 | 3.32 | 28.34 | 22.11 | 24.20 | 1.79 | 26.72 | 20.87 | 22.94 | 1.62 | 23.78 | 19.00 | 21.35 | 1.26 |
| Total | 100.00 | 98.43 | 99.31 | 0.60 | 100.80 | 99.18 | 100.24 | 0.52 | 100.80 | 98.59 | 99.75 | 0.72 | 100.90 | 98.70 | 99.53 | 0.81 |
| Bulk Sample | <63 µm | <38 µm | <2 µm | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Max | Min | Mean | SD | Max | Min | Mean | SD | Max | Min | Mean | SD | Max | Min | Mean | SD | |
| Zr | 119.00 | 35.00 | 72.44 | 23.22 | 122.00 | 49.00 | 69.67 | 22.23 | 127.00 | 44.00 | 65.00 | 24.09 | 56.00 | 31.00 | 40.33 | 8.16 |
| Nb | 6.60 | 3.40 | 4.76 | 0.96 | 6.10 | 2.00 | 4.21 | 1.26 | 6.10 | 2.10 | 4.24 | 1.21 | 1.30 | 0.20 | 0.76 | 0.28 |
| Hf | 2.70 | 1.30 | 1.89 | 0.40 | 3.20 | 1.70 | 2.19 | 0.50 | 3.40 | 1.60 | 2.12 | 0.55 | 2.20 | 0.90 | 1.49 | 0.36 |
| Ta | 0.74 | 0.23 | 0.35 | 0.15 | 0.71 | 0.09 | 0.30 | 0.17 | 1.25 | 0.52 | 0.78 | 0.20 | 0.15 | 0.01 | 0.08 | 0.04 |
| Tl | 0.38 | 0.16 | 0.26 | 0.08 | 0.21 | 0.08 | 0.11 | 0.04 | 0.14 | 0.05 | 0.09 | 0.03 | 0.07 | 0.05 | 0.05 | 0.01 |
| Th | 5.22 | 0.79 | 2.86 | 1.83 | 4.78 | 0.77 | 2.54 | 1.59 | 5.21 | 0.87 | 2.70 | 1.70 | 3.27 | 0.40 | 1.59 | 1.17 |
| U | 1.22 | 0.43 | 0.80 | 0.30 | 1.21 | 0.55 | 0.71 | 0.21 | 1.19 | 0.50 | 0.71 | 0.22 | 0.24 | 0.09 | 0.15 | 0.04 |
| Sc | 33.00 | 18.00 | 27.33 | 4.52 | 40.00 | 20.00 | 30.89 | 5.26 | 40.00 | 21.00 | 31.22 | 5.65 | 42.00 | 20.00 | 31.00 | 5.93 |
| V | 265.00 | 53.00 | 111.67 | 61.02 | 194.00 | 45.00 | 89.22 | 47.79 | 208.00 | 46.00 | 93.89 | 52.20 | 171.00 | 27.00 | 81.44 | 48.44 |
| Cr | 810.00 | 20.00 | 400.00 | 256.95 | 850.00 | 30.00 | 443.33 | 282.13 | 1080.00 | 40.00 | 528.89 | 372.48 | 890.00 | 60.00 | 442.22 | 302.65 |
| Co | 34.00 | 10.00 | 21.89 | 6.35 | 17.00 | 11.00 | 14.33 | 1.89 | 22.00 | 10.00 | 17.44 | 3.72 | 17.00 | 6.00 | 11.44 | 3.59 |
| Ni | 120.00 | 30.00 | 68.89 | 34.78 | 100.00 | 20.00 | 51.25 | 30.18 | 130.00 | 20.00 | 61.25 | 40.45 | 120.00 | 30.00 | 55.71 | 32.89 |
| Cu | 40.00 | 20.00 | 25.00 | 7.07 | 30.00 | 10.00 | 17.50 | 6.61 | 30.00 | 10.00 | 20.00 | 5.00 | 20.00 | 10.00 | 15.00 | 5.00 |
| Zn | 80.00 | 50.00 | 64.44 | 8.31 | 80.00 | 40.00 | 48.89 | 12.86 | 90.00 | 40.00 | 55.56 | 14.99 | 60.00 | 30.00 | 43.33 | 10.54 |
| Rb | 68.00 | 19.00 | 43.44 | 17.68 | 47.00 | 15.00 | 24.78 | 9.59 | 44.00 | 13.00 | 23.67 | 8.73 | 16.00 | 7.00 | 10.89 | 2.51 |
| Sr | 175.00 | 81.00 | 122.56 | 26.58 | 98.00 | 80.00 | 90.44 | 5.89 | 96.00 | 80.00 | 88.56 | 6.45 | 133.00 | 93.00 | 108.78 | 12.73 |
| Cs | 3.70 | 0.70 | 2.42 | 0.95 | 2.30 | 0.80 | 1.51 | 0.48 | 2.20 | 0.60 | 1.43 | 0.48 | 1.50 | 0.60 | 1.08 | 0.27 |
| Ba | 604.00 | 176.00 | 348.56 | 145.07 | 363.00 | 106.00 | 225.33 | 71.50 | 327.00 | 98.00 | 208.67 | 67.81 | 556.00 | 111.00 | 277.89 | 153.05 |
| Pb | 11.00 | 5.00 | 8.25 | 2.17 | 27.00 | 6.00 | 13.67 | 9.46 | 8.00 | 5.00 | 6.00 | 1.22 | 12.00 | 5.00 | 8.50 | 3.50 |
| La | 24.30 | 6.73 | 13.97 | 6.35 | 21.20 | 3.11 | 9.98 | 5.45 | 19.20 | 2.77 | 9.30 | 4.89 | 8.57 | 0.71 | 4.27 | 2.99 |
| Ce | 36.80 | 15.70 | 24.77 | 8.06 | 35.90 | 10.40 | 20.39 | 7.91 | 33.60 | 10.40 | 20.69 | 7.70 | 26.20 | 2.93 | 12.40 | 7.09 |
| Pr | 6.34 | 2.47 | 4.05 | 1.28 | 5.27 | 1.64 | 2.85 | 1.11 | 5.06 | 1.44 | 2.77 | 1.05 | 1.84 | 0.18 | 0.97 | 0.65 |
| Nd | 26.50 | 10.30 | 17.42 | 5.46 | 23.70 | 7.65 | 12.59 | 4.97 | 22.60 | 6.46 | 11.81 | 4.72 | 6.39 | 0.88 | 3.61 | 2.25 |
| Sm | 6.34 | 2.69 | 4.27 | 1.29 | 5.77 | 2.02 | 3.09 | 1.14 | 5.30 | 1.73 | 2.88 | 1.10 | 1.19 | 0.19 | 0.65 | 0.37 |
| Eu | 1.55 | 0.67 | 1.06 | 0.30 | 1.35 | 0.47 | 0.76 | 0.26 | 1.16 | 0.47 | 0.71 | 0.22 | 0.23 | 0.05 | 0.13 | 0.07 |
| Gd | 6.75 | 2.43 | 4.06 | 1.33 | 5.96 | 2.00 | 3.03 | 1.26 | 4.84 | 1.67 | 2.64 | 1.04 | 0.88 | 0.19 | 0.47 | 0.23 |
| Tb | 1.09 | 0.37 | 0.65 | 0.21 | 0.91 | 0.33 | 0.49 | 0.18 | 0.77 | 0.30 | 0.44 | 0.16 | 0.12 | 0.03 | 0.07 | 0.03 |
| Dy | 6.52 | 2.20 | 3.92 | 1.26 | 5.34 | 2.05 | 2.87 | 1.09 | 4.56 | 1.80 | 2.62 | 0.99 | 0.65 | 0.15 | 0.38 | 0.15 |
| Ho | 1.30 | 0.43 | 0.76 | 0.26 | 1.06 | 0.39 | 0.57 | 0.22 | 0.90 | 0.36 | 0.51 | 0.19 | 0.12 | 0.03 | 0.07 | 0.03 |
| Er | 3.72 | 1.30 | 2.18 | 0.70 | 3.12 | 1.14 | 1.67 | 0.63 | 2.54 | 1.03 | 1.47 | 0.54 | 0.34 | 0.10 | 0.21 | 0.08 |
| Tm | 0.54 | 0.20 | 0.33 | 0.10 | 0.45 | 0.17 | 0.24 | 0.09 | 0.36 | 0.16 | 0.21 | 0.07 | 0.05 | 0.01 | 0.03 | 0.01 |
| Yb | 3.29 | 1.36 | 2.11 | 0.55 | 2.90 | 1.14 | 1.59 | 0.54 | 2.35 | 1.07 | 1.44 | 0.45 | 0.33 | 0.08 | 0.19 | 0.07 |
| Lu | 0.46 | 0.21 | 0.32 | 0.08 | 0.44 | 0.17 | 0.25 | 0.08 | 0.38 | 0.17 | 0.23 | 0.07 | 0.05 | 0.01 | 0.03 | 0.01 |
| Be | 3.00 | 1.00 | 1.89 | 0.57 | 3.00 | 1.00 | 1.67 | 0.67 | 2.00 | 1.00 | 1.33 | 0.47 | 2.00 | 1.00 | 1.50 | 0.50 |
| Ga | 15.00 | 10.00 | 12.44 | 1.77 | 15.00 | 11.00 | 12.78 | 1.47 | 15.00 | 12.00 | 13.11 | 1.10 | 14.00 | 12.00 | 13.22 | 0.63 |
| Ge | 1.40 | 0.70 | 0.94 | 0.23 | 0.90 | 0.50 | 0.70 | 0.14 | 0.90 | 0.50 | 0.69 | 0.14 | 0.70 | 0.60 | 0.65 | 0.05 |
| Y | 39.50 | 12.30 | 21.91 | 7.86 | 32.90 | 11.80 | 16.09 | 6.94 | 26.60 | 10.00 | 14.46 | 5.71 | 3.00 | 0.80 | 1.82 | 0.69 |
| Sn | 5.00 | 1.00 | 2.33 | 1.05 | 6.00 | 2.00 | 2.89 | 1.37 | 6.00 | 2.00 | 3.22 | 1.40 | 5.00 | 2.00 | 2.78 | 1.13 |
| Sb | 0.30 | 0.20 | 0.23 | 0.04 | 3.50 | 0.20 | 1.54 | 1.02 | 0.40 | 0.20 | 0.27 | 0.09 | 135.00 | 0.50 | 38.03 | 46.11 |
| W | 4.00 | 0.60 | 1.43 | 1.03 | 2.70 | 0.50 | 1.26 | 0.68 | 8.00 | 0.90 | 2.38 | 2.22 | 1.90 | 0.60 | 1.05 | 0.42 |
| Whole Rock | <63 µm | <38 µm | <2 µm | TEM-AEM | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PIA | CIA | PIA | CIA | PIA | CIA | PIA | CIA | PIA | CIA | |
| BEN1A | 50.68 | 55.80 | 69.69 | 74.79 | 72.16 | 76.25 | 81.81 | 84.14 | ||
| BEN1B | 33.53 | 40.18 | 53.07 | 60.36 | 56.95 | 62.68 | 72.57 | 73.37 | ||
| BEN1C | 51.81 | 55.59 | 67.61 | 72.63 | 69.83 | 73.87 | 82.40 | 84.69 | ||
| BEN2A | 49.78 | 52.89 | 54.13 | 61.38 | 69.85 | 73.42 | 84.99 | 86.00 | ||
| BEN2B | 30.80 | 37.44 | 66.81 | 71.61 | 52.23 | 58.13 | 75.09 | 74.16 | ||
| BEN2C | 35.03 | 34.18 | 51.74 | 55.23 | 57.36 | 59.62 | 60.17 | 62.38 | ||
| BEN3A | 76.09 | 81.27 | 84.27 | 88.20 | 85.09 | 88.46 | 85.02 | 84.47 | 91.90 | 90.41 |
| BEN3B | 25.46 | 33.27 | 31.93 | 42.86 | 33.77 | 42.47 | 76.89 | 78.73 | 86.57 | 84.61 |
| BEN3C | 27.89 | 30.79 | 38.92 | 45.51 | 44.75 | 49.05 | 72.56 | 74.11 | 88.73 | 76.77 |
| Whole Rock | <63 µm | <38 µm | <2 µm | |||||
|---|---|---|---|---|---|---|---|---|
| Ce/Ce* | Eu/Eu* | Ce/Ce* | Eu/Eu* | Ce/Ce* | Eu/Eu* | Ce/Ce* | Eu/Eu* | |
| BEN1A | 1.01 | 0.81 | 1.11 | 0.74 | 1.19 | 0.82 | 1.13 | 0.79 |
| BEN1B | 1.04 | 0.79 | 1.30 | 0.71 | 1.43 | 0.81 | 2.27 | 0.76 |
| BEN1C | 1.24 | 0.78 | 1.60 | 0.81 | 1.59 | 0.83 | 1.87 | 0.74 |
| BEN2A | 0.93 | 0.78 | 0.42 | 0.71 | 1.14 | 0.84 | 1.46 | 0.76 |
| BEN2B | 0.31 | 0.72 | 1.07 | 0.78 | 0.40 | 0.71 | 0.77 | 0.68 |
| BEN2C | 1.12 | 0.80 | 1.24 | 0.87 | 1.44 | 0.84 | 2.55 | 0.75 |
| BEN3A | 1.05 | 0.81 | 1.14 | 0.80 | 1.14 | 0.88 | 1.05 | 0.75 |
| BEN3B | 0.33 | 0.77 | 0.36 | 0.70 | 0.49 | 0.70 | 1.85 | 0.72 |
| BEN3C | 0.93 | 0.77 | 1.04 | 0.81 | 1.18 | 0.88 | 1.80 | 0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Rivas, J.; Dias, M.I.; Paiva, I.; Fernandes, P.G.; Marques, R.; García-Romero, E.; Suárez, M. Mineralogical and Geochemical Characterization of the Benavila (Portugal) Bentonites. Minerals 2025, 15, 836. https://doi.org/10.3390/min15080836
García-Rivas J, Dias MI, Paiva I, Fernandes PG, Marques R, García-Romero E, Suárez M. Mineralogical and Geochemical Characterization of the Benavila (Portugal) Bentonites. Minerals. 2025; 15(8):836. https://doi.org/10.3390/min15080836
Chicago/Turabian StyleGarcía-Rivas, Javier, Maria Isabel Dias, Isabel Paiva, Paula G. Fernandes, Rosa Marques, Emilia García-Romero, and Mercedes Suárez. 2025. "Mineralogical and Geochemical Characterization of the Benavila (Portugal) Bentonites" Minerals 15, no. 8: 836. https://doi.org/10.3390/min15080836
APA StyleGarcía-Rivas, J., Dias, M. I., Paiva, I., Fernandes, P. G., Marques, R., García-Romero, E., & Suárez, M. (2025). Mineralogical and Geochemical Characterization of the Benavila (Portugal) Bentonites. Minerals, 15(8), 836. https://doi.org/10.3390/min15080836







