Mineralogical, Petrological, 3D Modeling Study and Geostatistical Mineral Resources Estimation of the Zone C Gold Prospect, Kofi (Mali)
Abstract
1. Introduction
- (i)
- Macroscopic rock descriptions and thin-section analysis using optical microscopy, scanning electron microscopy (SEM), and electron microprobe to establish paragenesis (Interested reader can find details on instrumentation and procedures at https://georessources.univ-lorraine.fr/en/content/equipment accessed on 15 May 2025);
- (ii)
- Using univariate and multivariate statistical analyses of geochemical data to characterize element distributions, identify correlations, and assess lithological controls;
- (iii)
- Three-dimensional grade modeling to delineate anomalous zones. The objectives are to characterize mineralization and geological controls at Zone C and to identify previously overlooked but potentially mineralized areas. Ultimately, this methodology supports exploration in underrecognized prospective zones.
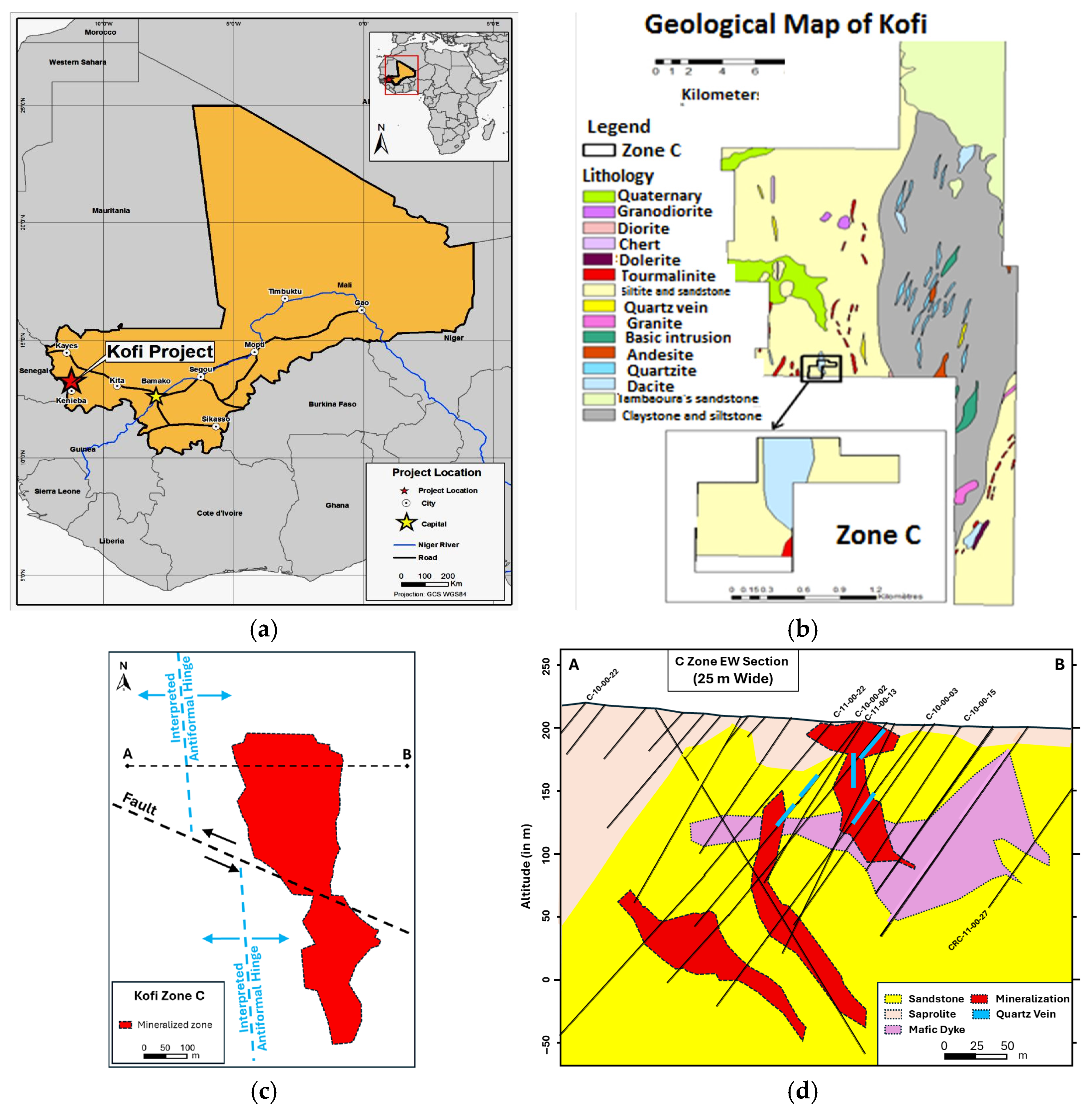
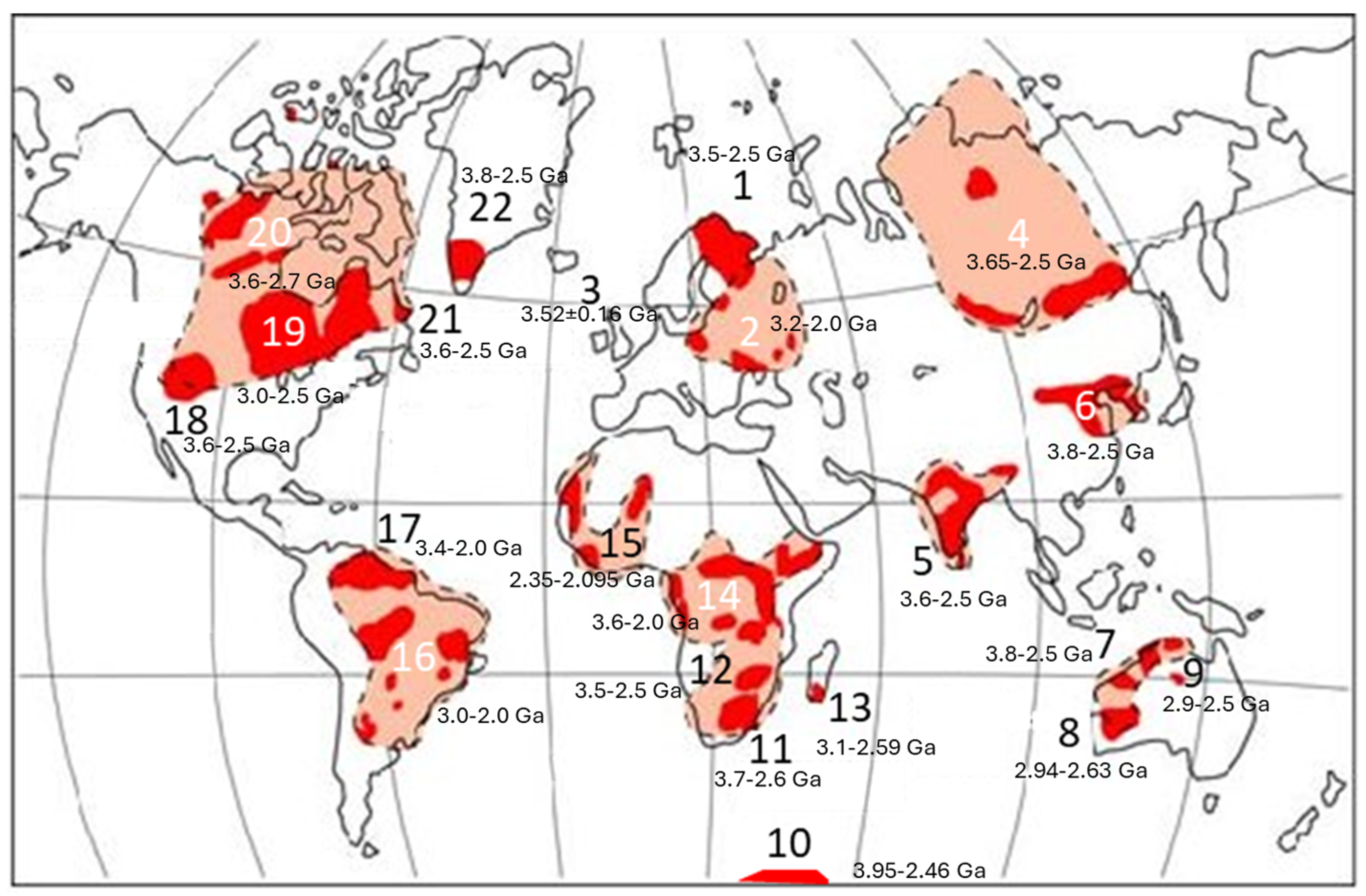
2. Regional Geology of the Kofi Area
2.1. Regional Geological Settings of the Kofi Area
2.2. Mineralization of the Kofi Zone C
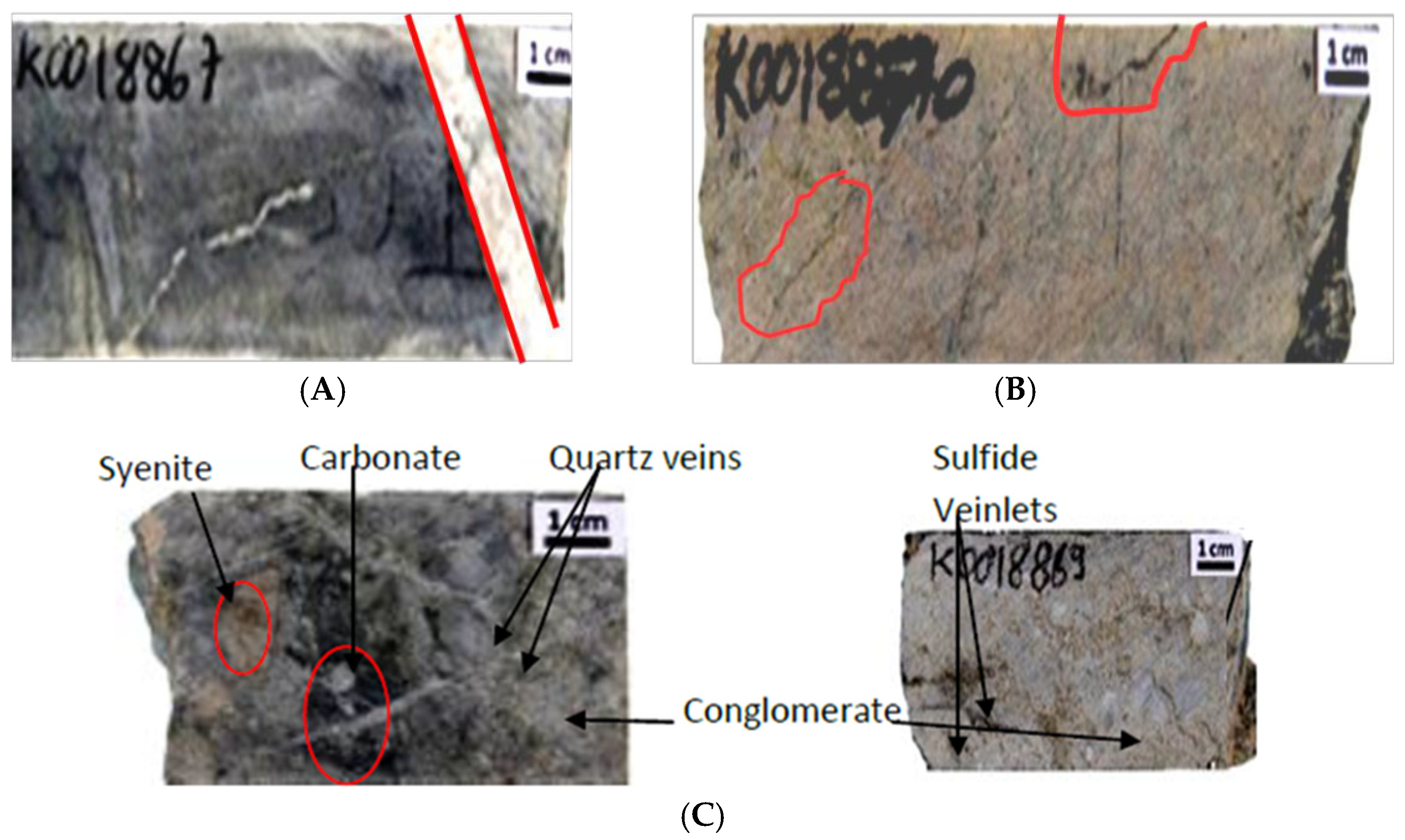
2.2.1. Macroscopic Description
2.2.2. Microscopic Description
2.2.3. Chlorite Petrography and Geothermobarometers
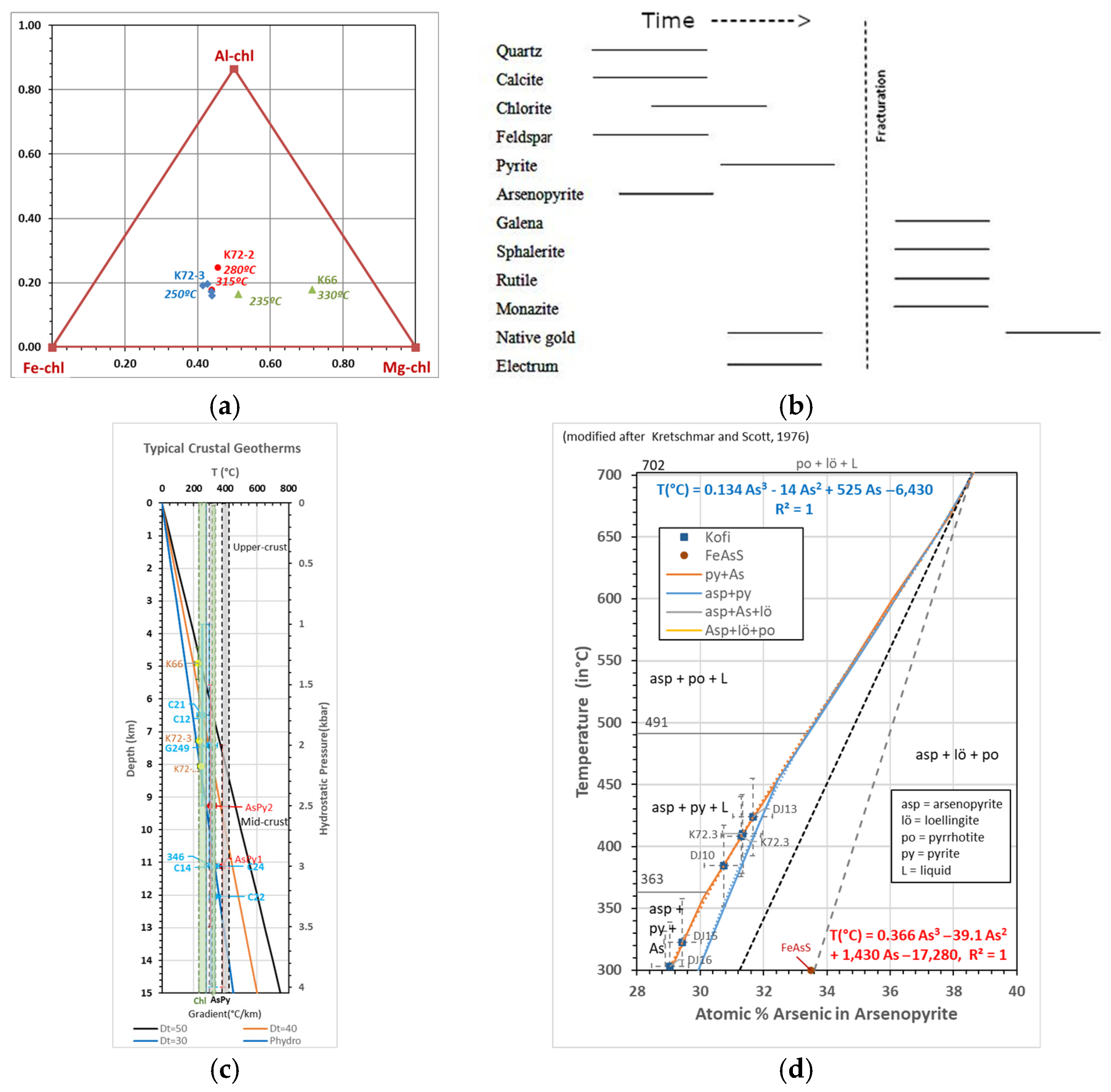
2.2.4. Concluding Overview
3. Spatial Analysis
3.1. Available Data
3.2. Univariable and Multivariable Statistical Analysis
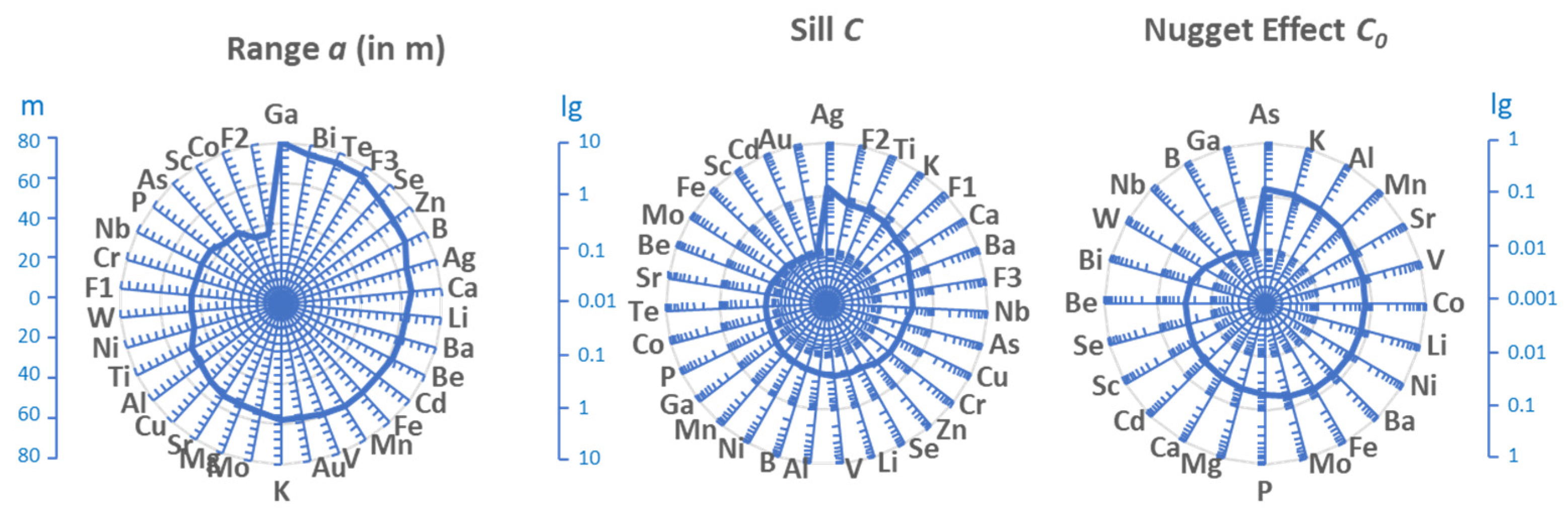
3.3. Variography
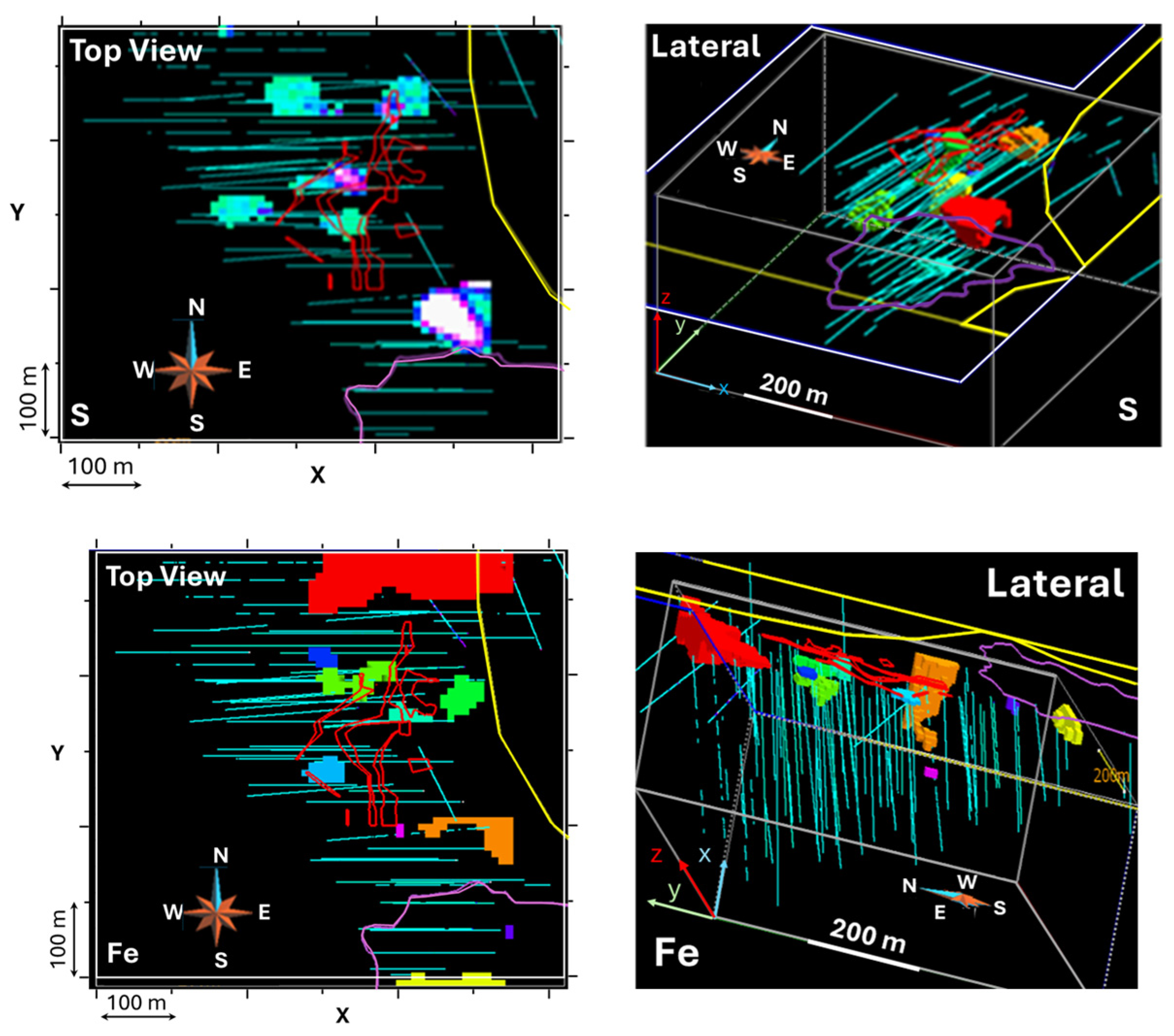
3.4. Three-Dimensional Block Model
4. Geostatistical Resource Estimation
5. Conclusions
- Gold Mineralization: Petrographic analysis reveals two phases of gold mineralization: (a) native gold and electrum as inclusions in pyrite (40–50 μm), and (b) disseminated native gold in pyrite-hosted fractures (~100 μm). As pyrite consistently hosts arsenopyrite, the latter is interpreted as the earlier phase. No gold was observed in quartz veins under SEM.
- Hydrothermal Alteration: Four alteration types were identified: epidotization, chloritization, carbonation, and dominant albitization, often linked with regional tourmalinization. The matrix comprises carbonates and silicates, with variable sulfide mineralization.
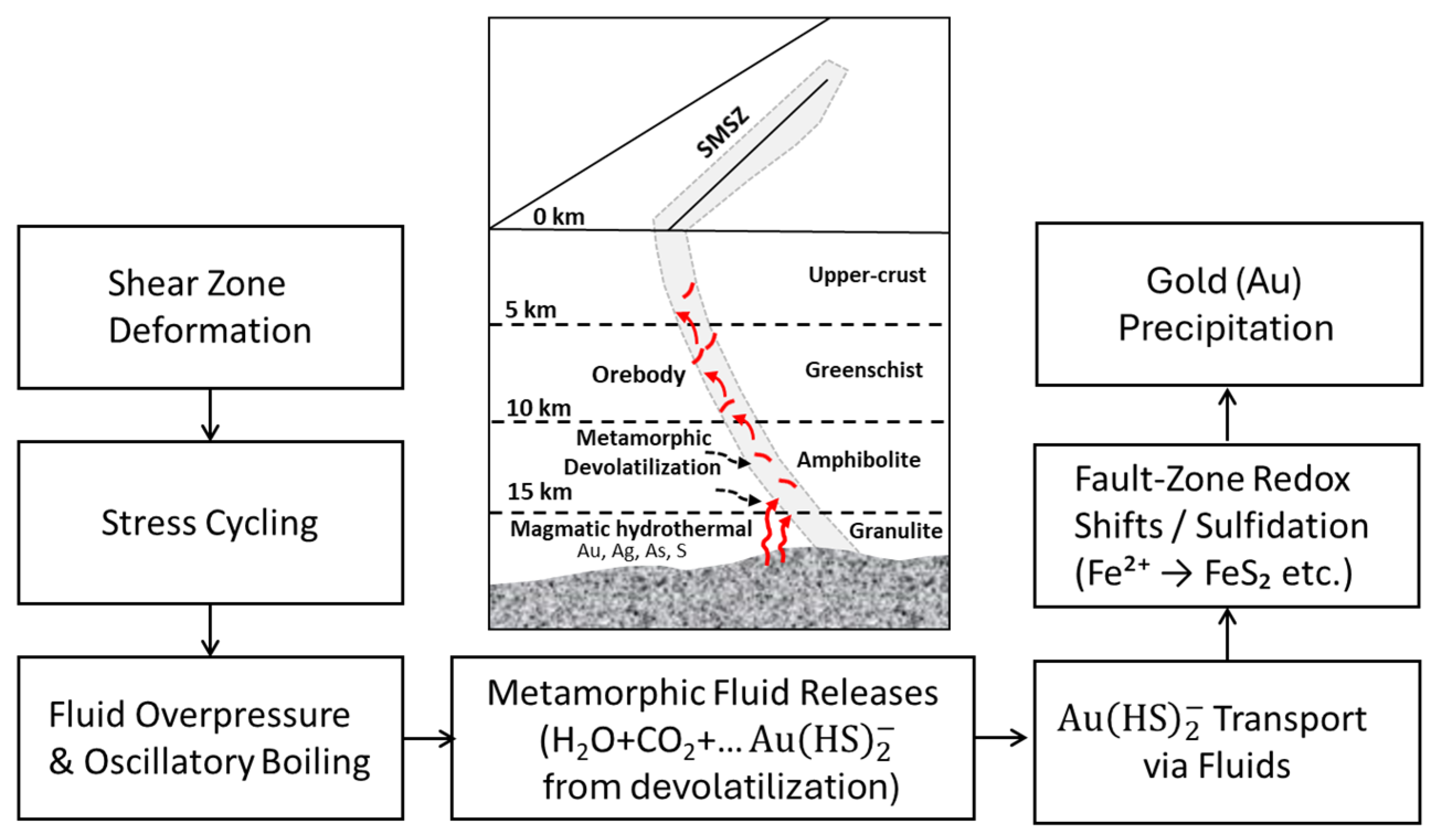
- Thermobarometry: Data from chlorite, arsenopyrite, biotite, feldspar, and carbonates indicate greenschist to amphibolite facies conditions (250–550 °C, 3–8 kbar), suggesting mid- to deep-crustal fluid origins with varying metamorphic overprints.
- Hydrothermal Evolution: Geothermometric and mineralogical evidence confirms a complex, multi-phase hydrothermal evolution typical of orogenic gold systems. Gold was transported by reduced, CO2-rich fluids and precipitated through cooling, pressure drops, and redox changes. Pathfinder minerals such as Mg-rich chlorite and arsenian pyrite help direct the gold towards mineralized zones.
- Multivariate Analysis: PCA identified three key factors: F1 lithology (mafic/ultra-mafic rocks and quartz vein breccias), F2 hydrothermal alteration (mainly carbonatization), and F3 mineralization. F2 shows the strongest correlation with gold content.
- Spatial Modeling: A 3D block model delineated potential mineralized zones. Variogram analysis of log-transformed gold values indicates a stationary, isotropic spatial structure with no nugget effect and a 60 m range.
- Resource Estimation: In situ gold resources were estimated using ordinary kriging on a regular structured grid. Gold grades follow a log-normal distribution. Mineralization is associated with mafic intrusions and occurs in dolomitized and albitized zones. Despite the presence of electrum, no significant gold-silver correlation was observed across 780 mineralized samples.
- Structural Controls: Subsurface mineralization aligns with surface structural trends. In situ resource total 1.295 Mt of ore, with an average of 6.36 g/t for Au (at a 4 g/t cut-off), yielding ~8.2 t Au, confirming strong exploration potential in Zone C, Kofi, Mali.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Borehole | Sample ID | From (m) | To (m) | Comments |
|---|---|---|---|---|
| C-11-14 | K66 | 127.9 | 128 | Poly conglomerate with disseminated Py |
| C-11-14 | K67 | 146.1 | 146.25 | Dark rock. Few Py + Qz carbonate veins with big Py |
| C-10-05 | K70 | 96.75 | 96.9 | Strongly altered silt with veinlets filled by Chlr and Py |
| C-10-14 | K72 | 112.5 | 112.6 | Strongly altered fault breccia zone Qz stockwork |
| Oxide | K72-3a | K72-3b | K72-3c | K72-3d | K66a | K66b | K72-2a | K72-2b |
|---|---|---|---|---|---|---|---|---|
| Na2O | 0.04 | 0.03 | 0.01 | 0.07 | 0.04 | 0.06 | 0.05 | 0.05 |
| MgO | 11.55 | 12.73 | 12.46 | 10.98 | 24.2 | 15.61 | 11.27 | 12.24 |
| Al2O3 | 20.11 | 15.88 | 17.58 | 17.72 | 20.5 | 16.3 | 21.62 | 18.51 |
| SiO2 | 26.05 | 28.63 | 27.51 | 28.23 | 27.36 | 29.55 | 28.31 | 26.28 |
| P2O5 | 0 | 0.04 | 0 | 0 | 0 | 0.01 | 0.02 | 0 |
| K2O | 0.04 | 0.01 | 0.05 | 0.09 | 0.02 | 0.06 | 0.07 | 0 |
| CaO | 0.06 | 0.18 | 0.2 | 0.32 | 0 | 0.2 | 0.17 | 0.08 |
| TiO2 | 0.06 | 0.03 | 0 | 0.02 | 0.07 | 0 | 0.27 | 0.02 |
| MnO | 0 | 0.14 | 0.1 | 0.02 | 0 | 0.08 | 0 | 0.07 |
| FeO | 30.23 | 30.41 | 30.24 | 30.48 | 12.79 | 26.18 | 25.88 | 29.85 |
| K72-3 | K72-3 | K72-3 | K72-3 | K66 | K66 | K72-2 | K72-2 | |
|---|---|---|---|---|---|---|---|---|
| Fe/(Fe + Mg) | ||||||||
| Fe/(Fe + Mg) | 0.59 | 0.57 | 0.58 | 0.61 | 0.23 | 0.48 | 0.56 | 0.58 |
| Structural formula (half-cell) 1 | ||||||||
| 2.80 | 3.08 | 2.96 | 3.04 | 2.78 | 3.10 | 2.96 | 2.86 | |
| 1.20 | 0.92 | 1.04 | 0.96 | 1.22 | 0.90 | 1.04 | 1.14 | |
| 1.34 | 1.09 | 1.18 | 1.28 | 1.23 | 1.11 | 1.62 | 1.23 | |
| 2.71 | 2.73 | 2.72 | 2.74 | 1.09 | 2.30 | 2.26 | 2.72 | |
| 1.85 | 2.04 | 2.00 | 1.76 | 3.66 | 2.44 | 1.75 | 1.98 | |
| 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | |
| 5.91 | 5.88 | 5.90 | 5.79 | 5.98 | 5.86 | 5.64 | 5.94 | |
| End-member proportions | ||||||||
| Al-chl | 0.23 | 0.19 | 0.20 | 0.22 | 0.21 | 0.19 | 0.29 | 0.21 |
| Fe-chl | 0.46 | 0.47 | 0.46 | 0.47 | 0.18 | 0.39 | 0.40 | 0.46 |
| Mg-chl | 0.31 | 0.35 | 0.34 | 0.30 | 0.61 | 0.42 | 0.31 | 0.33 |
| Alciv = Aliv + 0.1 [Fe/(Fe + Mg)] 2 | ||||||||
| Alivc | 1.26 | 0.98 | 1.10 | 1.02 | 1.24 | 0.95 | 1.10 | 1.20 |
| Geothermometers 3 | ||||||||
| MC88 T (°C) | 325 | 235 | 275 | 250 | 330 | 230 | 275 | 305 |
| WJ91 T (°C) | 335 | 245 | 280 | 260 | 330 | 235 | 280 | 315 |
| Geobarometer 4 | ||||||||
| P (kbar) | 3.1–3.2 | 2.2–2.3 | 2.6–2.6 | 2.3–2.4 | 3.2 | 2.1–2.2 | 2.6–2.7 | 2.9–3 |
| Depth (km) | 8.3–8.6 | 5.7–6.0 | 6.9–7.0 | 6.1–6.4 | 8.4 | 5.6–5.7 | 6.9–7.0 | 7.7–8.0 |
| Chlorites | Si | Ti | Al/AlIV | AlVI | Cr | Fe2+ | Mg2+ | Mg | Ca | Na | K | Ni | OH | Sum Cat# | XMg | Altot apfu |
| 267-C1.4 | 6.04 | 0.03 | 1.96 | 2.84 | 0.00 | 3.30 | 0.00 | 5.28 | 0.01 | 0.00 | 0.13 | 0.01 | 16 | 35.59 | 0.62 | 4.80 |
| 267-C2.1 | 5.28 | 0.00 | 2.72 | 2.90 | 0.01 | 4.11 | 0.01 | 4.88 | 0.00 | 0.00 | 0.00 | 0.00 | 16 | 35.91 | 0.54 | 5.62 |
| 267-C2.3 | 5.29 | 0.02 | 2.71 | 2.79 | 0.00 | 3.98 | 0.00 | 5.16 | 0.00 | 0.00 | 0.01 | 0.00 | 16 | 35.95 | 0.57 | 5.50 |
| 284-C1.1 | 5.48 | 0.00 | 2.53 | 2.60 | 0.00 | 5.77 | 0.02 | 3.54 | 0.01 | 0.02 | 0.02 | 0.00 | 16 | 35.98 | 0.38 | 5.12 |
| 346-C3.2 | 5.59 | 0.03 | 2.41 | 2.46 | 0.00 | 4.04 | 0.01 | 5.38 | 0.01 | 0.00 | 0.00 | 0.01 | 16 | 35.95 | 0.57 | 4.87 |
| 346-C1.3 | 5.56 | 0.01 | 2.44 | 2.78 | 0.01 | 4.23 | 0.00 | 4.75 | 0.03 | 0.00 | 0.03 | 0.01 | 16 | 35.84 | 0.53 | 5.22 |
| 249-C2.1 | 5.31 | 0.00 | 2.69 | 2.87 | 0.01 | 4.75 | 0.00 | 4.22 | 0.03 | 0.00 | 0.00 | 0.02 | 16 | 35.90 | 0.47 | 5.56 |
| 249-C2.3 | 5.30 | 0.01 | 2.71 | 2.80 | 0.02 | 4.55 | 0.01 | 4.48 | 0.01 | 0.00 | 0.00 | 0.05 | 16 | 35.93 | 0.50 | 5.51 |
| T (°C) 2 | P 3 (kbar) | Depth 4 (km) | P 5 (kbar) (σ = ± 0.1) | Depth 4 (km) (σ = ± 0.2) | ∆log fO2 6 | |||||||||||
| 267-C1.4 | 245–255 | 2.25–2.35 | 6.0–6.2 | 4.2 | 11 | ~FMQ | ||||||||||
| 267-C2.1 | 365–375 | 3.55–3.67 | 9.4–9.7 | 4.8 | 13 | ~FMQ−1 | ||||||||||
| 267-C2.3 | 365–375 | 3.54–3.66 | 9.4–9.7 | 4.7 | 13 | ~FMQ | ||||||||||
| 284-C1.1 | 335–345 | 3.22–3.34 | 8.5–8.8 | 4.4 | 12 | ~FMQ + 1 | ||||||||||
| 346-C3.2 | 315–325 | 3.02–3.13 | 8.0–8.3 | 4.1 | 11 | ~FMQ | ||||||||||
| 346-C1.3 | 320–330 | 3.07–3.18 | 8.1–8.4 | 4.5 | 12 | ~FMQ−0.5 | ||||||||||
| 249-C2.3 | 360–370 | 3.49–3.61 | 9.3–9.6 | 4.8 | 13 | ~FMQ | ||||||||||
| 284-C1–3 | 360–370 | 3.51–3.63 | 9.3–9.6 | 4.7 | 13 | ~FMQ | ||||||||||
| AsPy | K72-3-2 | K72-3-4 | DJ-9 | DJ-12 | DJ-13 | DJ-14 | DJ-15 |
|---|---|---|---|---|---|---|---|
| S | 35.79 | 35.23 | 36.29 | 34.73 | 37.36 | 37.37 | 37.57 |
| Fe | 32.7 | 33.27 | 32.73 | 33.49 | 33.59 | 33.11 | 33.21 |
| As | 31.29 | 31.34 | 30.75 | 31.66 | 28.9 | 29.44 | 29.05 |
| Os | 0.11 | 0.13 | 0.16 | 0.08 | 0.04 | 0.03 | 0.1 |
| Geothermometers 2 | |||||||
| asp + py + As T (°C) | 410 ± 30 | 410 ± 30 | 385 ± 30 | 425 ± 30 | 295 ± 35 | 325 ± 35 | 300 ± 35 |
| py + asp T (°C) | 390 ± 45 | 390 ± 40 | 355 ± 45 | 410 ± 40 | 215 ± 60 | 260 ± 55 | 230 ± 60 |
| Biotites | Si | Ti | Al/AlIV | AlVI | Fe2+ | Mg | Mg | Ca | Na | F | OH | Sum Cat# | XMg |
| 267-C2-4 | 6.58 | 0.01 | 1.42 | 3.46 | 0.32 | 6.58 | 0.28 | 0.19 | 1.72 | 0.10 | 3.90 | 17.95 | 0.47 |
| 346-C1-2 | 6.50 | 0.05 | 1.50 | 3.41 | 0.33 | 6.50 | 0.27 | 0.08 | 1.77 | 0.01 | 3.99 | 17.92 | 0.46 |
| Hematite | |||||||||||||
| 284-C1-3 | 1.99 | 2.00 | |||||||||||
| T (°C) 2 | P (kbar) 3 | Depth (km) | ∆log fO2 | ||||||||||
| 267-C2-4 | ~400–500 | ~5–10 | ~5–10 | FMQ Buffer | |||||||||
| 346-C1-2 | ~500–550 | ||||||||||||
| 284-C1-3 | ≥FMQ + 2 | ||||||||||||
| Feldspars | Si | Al/AlIV | Ca | Na | K | Sum Cat# | Ab% | An% | Or% |
| 267-C1.1 | 2.93 | 1.08 | 0.03 | 0.95 | 0.04 | 5.03 | 92.72 | 3.35 | 3.93 |
| 267-C1.3 | 2.99 | 0.98 | 0.01 | 1.03 | 0.00 | 5.03 | 98.85 | 0.97 | 0.17 |
| G249-C2 | 3.01 | 0.98 | 0.00 | 1.02 | 0.00 | 5.01 | 99.83 | 0.08 | 0.10 |
| T (°C) 2 | P (kbar) 3 | Depth (km) | ∆log fO2 4 | ||||||
| ~300–450 | ~5–8 | ~15–25 | FMQ Buffer | ||||||
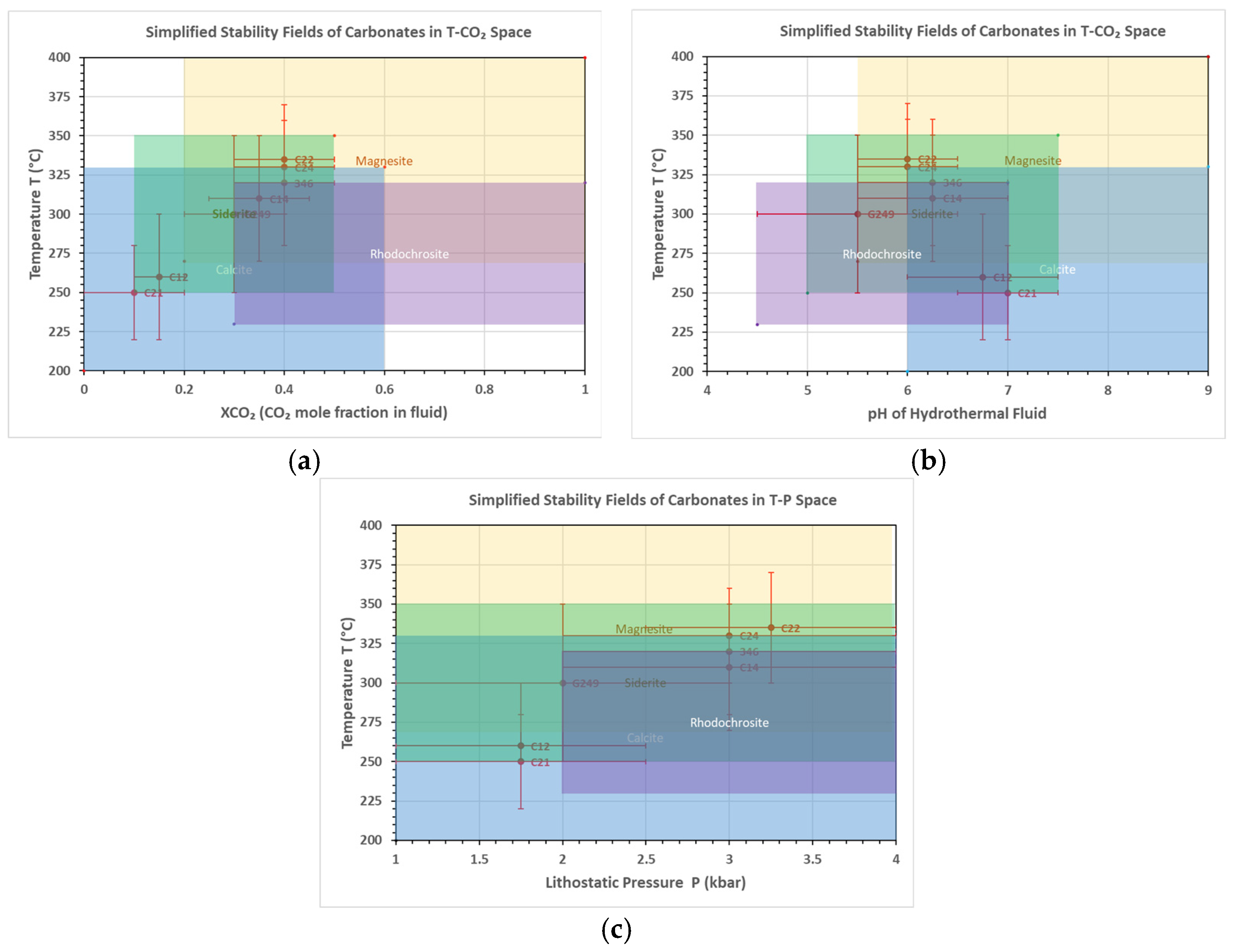
| Carbonate | 346- | G249- | 284- | 346- | |||
|---|---|---|---|---|---|---|---|
| C2-1 | C1 | C1-2 | C1-4 | C2-1 | C2-2 | C1-4a | |
| Rhodochrosite | 0.6 | 0.1 | 1.9 | 1.1 | 1.1 | 1.5 | 0.1 |
| Magnesite | 36.0 | 35.9 | 0.7 | 25.9 | 0.6 | 21.1 | 34.4 |
| Siderite | 13.0 | 15.6 | 1.4 | 17.9 | 1.1 | 27.4 | 15.2 |
| Calcite | 50.3 | 48.4 | 95.4 | 54.8 | 97.0 | 50.0 | 50.2 |
| Geothermometers 1 | |||||||
| T (°C) | 320 ± 40 | 300 ± 50 | 260 ± 40 | 310 ± 40 | 250 ± 30 | 335 ± 35 | 330 ± 30 |
| P (kbar) | 3.0 ± 1.0 | 2.0 ± 1.0 | 1.75 ± 0.75 | 3.0 ± 1.0 | 1.75 ± 0.75 | 3.25 ± 0.75 | 3.0 ± 1.0 |
| pH | 6.3 ± 0.75 | 5.5 ± 1.0 | 6.8 ± 0.75 | 6.3 ± 0.75 | 7.0 ± 0.5 | 6.0 ± 0.5 | 6.0 ± 0.5 |
| XCO2 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.05 | 0.4 ± 0.1 | 0.1 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 |
| N° | Mineral 1 Composition (in %) | T (°C) | P (kbar) | pH | fO2 | XCO2 | Key Observations | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Rho. | Mag. | Sid. | Cal. | |||||||
| C1 | 0.1 | 35.9 | 15.6 | 48.4 | 280–340 | 2–4 | 5.5–7 | PMB 2 | 0.2–0.4 | High Mg and Fe content; late stage calcite formation under reducing conditions |
| C1.4 | 1.1 | 25.9 | 17.9 | 54.8 | 270–350 | 2–4 | 5.5–67 | reducing | 0.25–0.45 | High Mg and Fe content; late-stage calcite formation under reducing conditions with H2O-CO2 dominant fluids. |
| C1.4a | 0.1 | 34.4 | 15.2 | 50.2 | 300–360 | 2–4 | 5.5–6.5 | PPMB 3 | 0.3–0.5 | High magnesite and siderite content, indicating Fe-Mg-rich fluid interaction with CO2. |
| C2.2 | 1.5 | 21.1 | 27.4 | 50.0 | 300–370 | 2.5–4 | 5.5–6.5 | PPMB | 0.3–0.5 | Siderite and magnesite suggest Mg-Fe-rich fluids under moderate to high CO2 activity. |
| C2.1 | 0.6 | 36.0 | 13.0 | 50.3 | 280–360 | 2–4 | 5.5–7 | QFMB 4 | 0.3–0.5 | High magnesite and siderite content, indicating interaction with CO2 rich, Fe-Mg-bearing fluids. |
| C1.2 | 1.9 | 0.75 | 1.4 | 95.4 | 220–300 | 1–2.5 | 6–7.5 | PMB | 0.1–0.2 | Calcite-dominated assemblage suggests fluid cooling; high CO2 activity is required for siderite and magnetite formation. |
| C2.1 | 1.1 | 0.6 | 1.1 | 97.0 | 220–280 | 1–2.5 | 6.5–7.5 | mildly reducing | 0.1–0.2 | Predominantly calcite with trace Fe and Mn carbonates, indicating late-stage fluid neutralization. |
| N° | Carbonate 1 (in %) | AsPy 2 | Alteration Minerals | Dominant Features | T (°C) | Gold Association | Interpretation | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Rod. (MnCO3) | Mag. (MgCO3) | Sid. (FeCO3) | Cal. (CaCO3) | |||||||
| C1 | 0.1 | 35.9 | 15.6 | 48.4 | Arsenopyrite present | Chlorite + minor sericite | Fe-Mg dominant | 280–350 | Mid-stage/Gold | Strong interaction with ultramafic/mafic rocks |
| C1.4 | 1.9 | 25.9 | 17.9 | 54.8 | Common pyrite | Moderate chlorite | Mg-Fe rich | 250–340 | Mid-stage | Moderate fluid-rock interaction in a proximal zone |
| C1.4a | 0.1 | 34.4 | 15.2 | 50.2 | Pyrite + arsenopyrite | Strong chlorite | Mg-Fe rich | 260–350 | Mid-stage | Mafic host interaction in a proximal setting |
| C2.2 | 1.5 | 21.1 | 27.4 | 50.0 | Pyrite + arsenopyrite | Chlorite-dominant | Mg-Fe rich | 300–370 | Mid-stage | Fluid-rock interaction in a proximal zone |
| C2.1 | 0.6 | 36.0 | 13.0 | 50.3 | Abundant arsenopyrite | Chlorite-dominant | Mg-Fe dominant | 280–360 | Likely coeval | Shear-hosted proximal alteration with gold potential |
| C1.2 | 1.9 | 0.75 | 1.4 | 95.4 | Trace sulfides | Weak sericite | Ca-dominant | 200–280 | Late/Post | Weak fluid-rock interaction in a distal zone |
| C2.1 | 1.1 | 0.6 | 1.1 | 97.0 | Trace sulfides | None to weak | Ca-dominant | 180–260 | Late/Post | Distal alteration halo with minimal Mg-Fe input |
| Elements | Units | n | med | m | σ | IQR | σ2 | 25th | 75th |
|---|---|---|---|---|---|---|---|---|---|
| LgAg | ppm | 9612 | 1 | 1.07 | 0.2 | 0 | 0.03 | 1 | 1 |
| LgAl | % | 11,835 | 1 | 0.7 | 0.5 | 0 | 0.2 | 1 | 1 |
| LgAs | ppm | 11,965 | 24 | 24 | 0.7 | 55 | 0.5 | 8 | 63 |
| LgB | ppm | 202 | 10 | 1.4 | 0.6 | 0 | 0.4 | 10 | 10 |
| LgBa | ppm | 11,968 | 28 | 26 | 0.7 | 98 | 0.5 | 7 | 105 |
| LgBe | ppm | 11,968 | 1 | 0.3 | 0.4 | 0 | 0.2 | 1 | 1 |
| LgBi | ppm | 11,968 | 2.5 | 2 | 0.4 | 0.5 | 0.1 | 2 | 2.5 |
| LgCa | % | 11,916 | 3 | 1 | 0.9 | 3 | 0.7 | 1 | 4 |
| LgCd | ppm | 11,968 | 1 | 0.6 | 0.3 | 0 | 0.08 | 1 | 1 |
| LgCo | ppm | 11,968 | 19 | 11 | 0.5 | 17 | 0.2 | 6 | 23 |
| LgCr | ppm | 11,968 | 42 | 1.6 | 0.7 | 98 | 0.4 | 13 | 111 |
| LgCu | ppm | 11,835 | 6 | 5.5 | 0.7 | 15 | 0.4 | 2 | 17 |
| LgFe | % | 11,841 | 2.4 | 2.4 | 0.3 | 1.6 | 0.07 | 2 | 3.6 |
| LgGa | ppm | 202 | 10 | 0.2 | 1 | 0 | 1.6 | 10 | 10 |
| LgHg | ppm | 7560 | 1 | 0.58 | 0.2 | −9 | 0.6 | 10 | 1 |
| LgK | % | 11,968 | 1 | 0.09 | 0.9 | 0 | 0.8 | 1 | 1 |
| LgLi | ppm | 11,968 | 7 | 6.42 | 0.6 | 14 | 0.3 | 3 | 17 |
| LgMg | % | 11,968 | 2.2 | 1.25 | 0.7 | 1.6 | 0.5 | 1.4 | 3 |
| LgMn | ppm | 11,966 | 349 | 295 | 0.5 | 243 | 0.3 | 235 | 478 |
| LgMo | ppm | 11,838 | 1 | 1 | 0.4 | 0 | 0.2 | 1 | 1 |
| LgNa | % | 11,968 | 1 | 0.04 | 0.5 | 0 | 0.2 | 1 | 1 |
| LgNb | ppm | 568 | 10 | 4.9 | 0.3 | 0 | 0.09 | 10 | 10 |
| LgNi | ppm | 11,968 | 30 | 27 | 0.5 | 32 | 0.3 | 19 | 51 |
| LgP | % | 11,884 | 1 | 0.04 | 0.5 | 0 | 0.2 | 1 | 1 |
| LgPb | ppm | 11,968 | 2 | 2 | 0.5 | 0 | 0.6 | 1 | 1 |
| LgS | % | 9386 | 1 | 0.07 | 0.8 | −0.5 | 0.6 | 3 | 2.5 |
| LgSb | ppm | 11,835 | 2.5 | 3 | 0.3 | 7.7 | 0.06 | 2.5 | 10.2 |
| LgSc | ppm | 11,968 | 6.4 | 6.4 | 0.3 | 5.7 | 0.1 | 4.3 | 10 |
| LgSe | ppm | 202 | 10 | 5.5 | 0.2 | 0 | 0.1 | 10 | 10 |
| LgSn | ppm | 11,835 | 27.5 | 17 | 0.7 | 0 | 0.01 | 5 | 5 |
| LgSr | ppm | 11,835 | 5 | 5 | 0.1 | 36.4 | 0.1 | 9.6 | 46 |
| LgTe | ppm | 202 | 10 | 4 | 1.7 | 0 | 1.8 | 10 | 10 |
| LgTi | % | 11,835 | 1 | 0.02 | 0.8 | 0 | 0.8 | 1 | 1 |
| LgTl | ppm | 202 | 10 | 4 | 0.8 | 0 | 0.6 | 10 | 10 |
| LgU | ppm | 133 | 10 | 7.5 | 0.3 | 0 | 0.1 | 10 | 10 |
| LgV | ppm | 11,968 | 32 | 1.5 | 0.5 | 70 | 0.3 | 10 | 80 |
| LgW | ppm | 11,968 | 5 | 6 | 0.2 | 0 | 0.06 | 5 | 5 |
| LgY | ppm | 11,965 | 5 | 5 | 0.2 | 3.4 | 0.06 | 4 | 7.4 |
| LgZn | ppm | 11,835 | 6 | 6 | 0.6 | 12 | 0.3 | 3 | 15 |
| LgZr | ppm | 11,598 | 5 | 5 | 0.2 | 4 | 0.08 | 3 | 7 |
| Volume (in m3) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elements 1 | Cut-Off | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Comments |
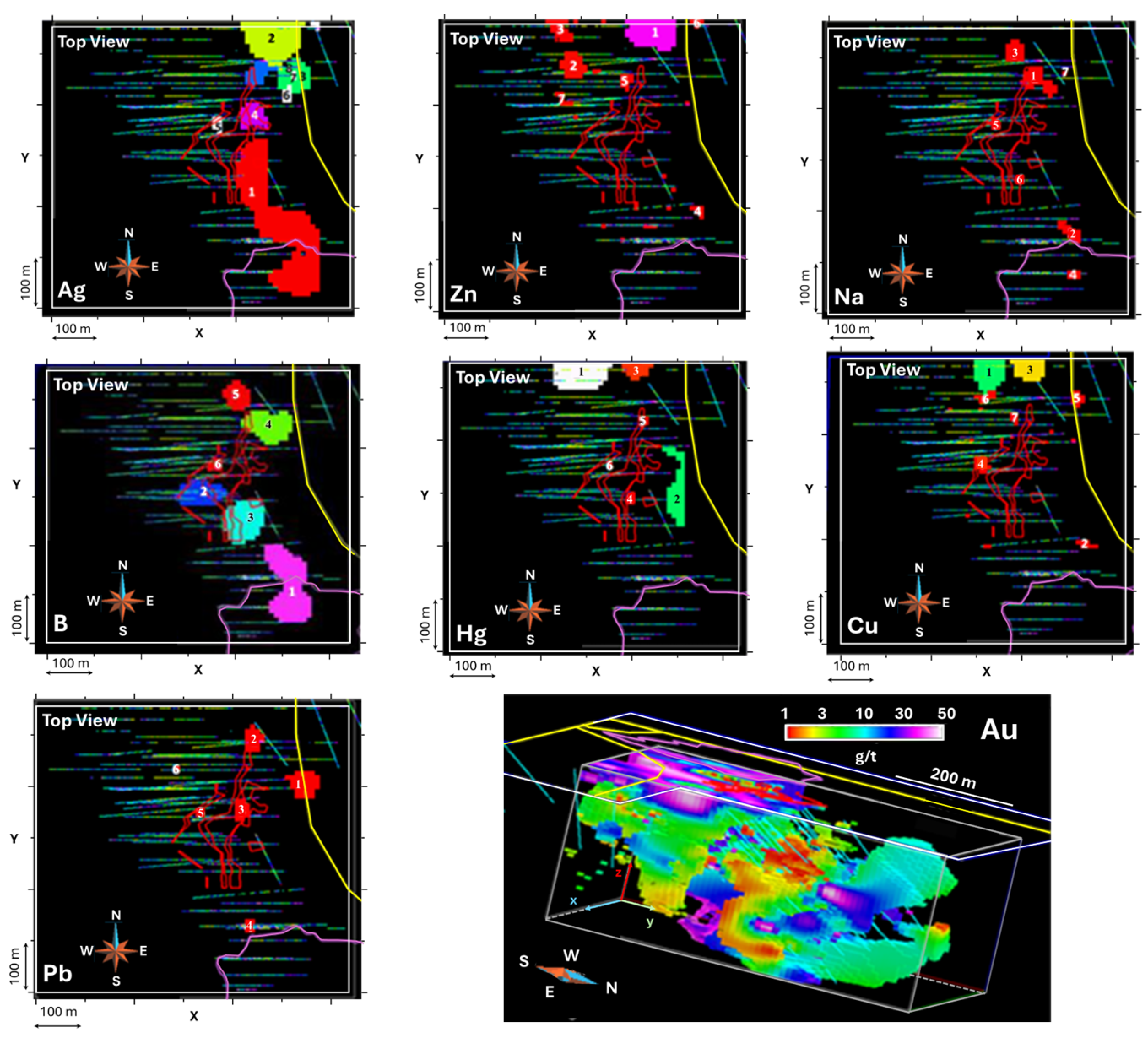
| B | >0.003 | 220,500 | 171,000 | 139,500 | 92,000 | 24,000 | 4500 | Tourmalinization | |||||
| Cu | >100 | 121,500 | 12,000 | 66,500 | 7000 | 4500 | 4000 | 2500 | 2000 | Cu Anomaly | |||
| Na(%) | >0.3% | 35,500 | 24,000 | 12,500 | 6500 | 5500 | 3500 | Albitization | |||||
| Hg | >5 | 403,000 | 121,000 | 42,000 | 2500 | 2500 | Sulfides | ||||||
| Pb | >35 | 21,000 | 17,000 | 8000 | 6000 | 2000 | Galena | ||||||
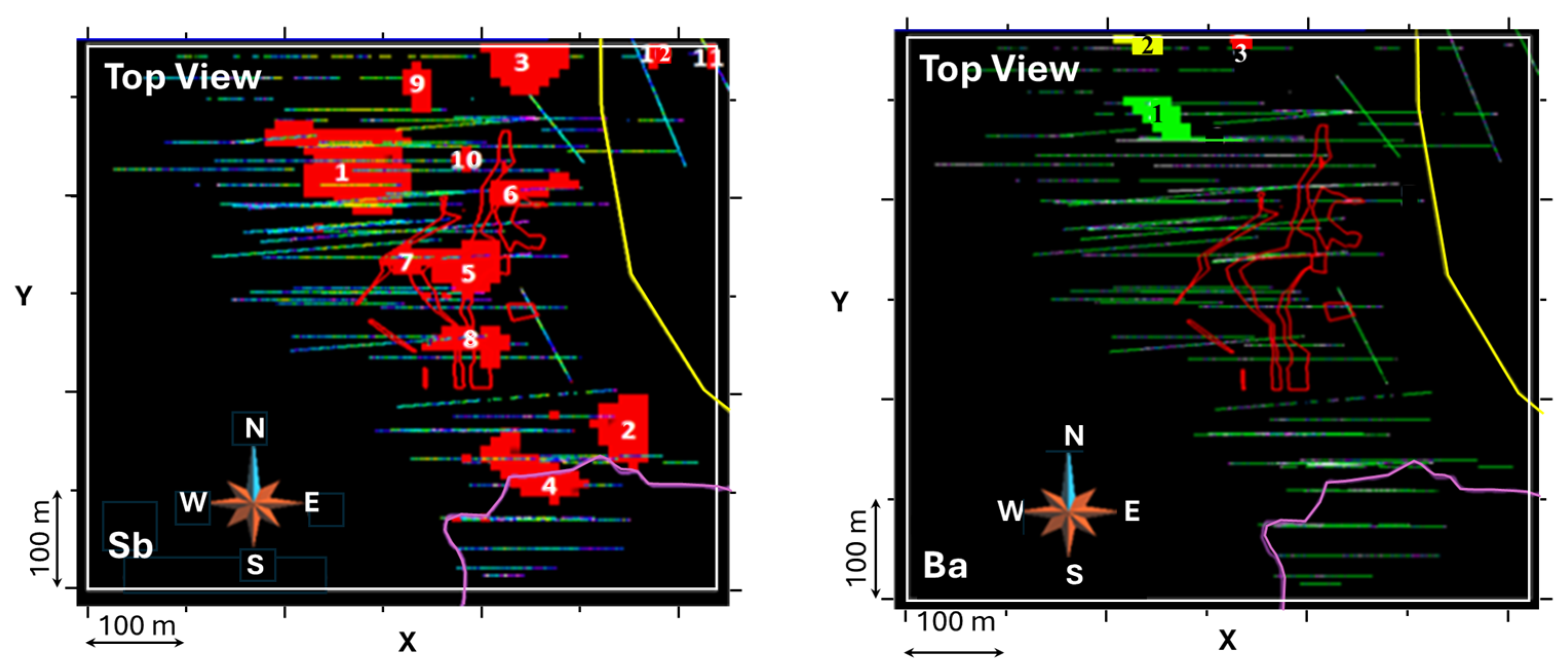
| Sb | >10 | 415,500 | 120,000 | 109,500 | 78,000 | 58,000 | 23,000 | 18,000 | 16,500 | 7000 | 5000 | 5000 | Antimony |
| Zn | >25 | 214,500 | 37,000 | 34,000 | 9500 | 4500 | 4000 | 3000 | 2000 | Sphalerite | |||
| Ag | >2 | 938,000 | 125,000 | 51,000 | 21,000 | 14,500 | 12,500 | 12,500 | 12,500 | 9500 | 9000 | Silver zone | |
| Ba | >500 | 31,000 | 17,000 | 2000 | Barium | ||||||||
| Nb | Vol | Qore | Qmet | Qmet | Grade | Grade | |
|---|---|---|---|---|---|---|---|
| (g/t) | blocs | (Mm3) | (Mt) | (t) | (Moz) | (Au g/t) | (Au oz/t) |
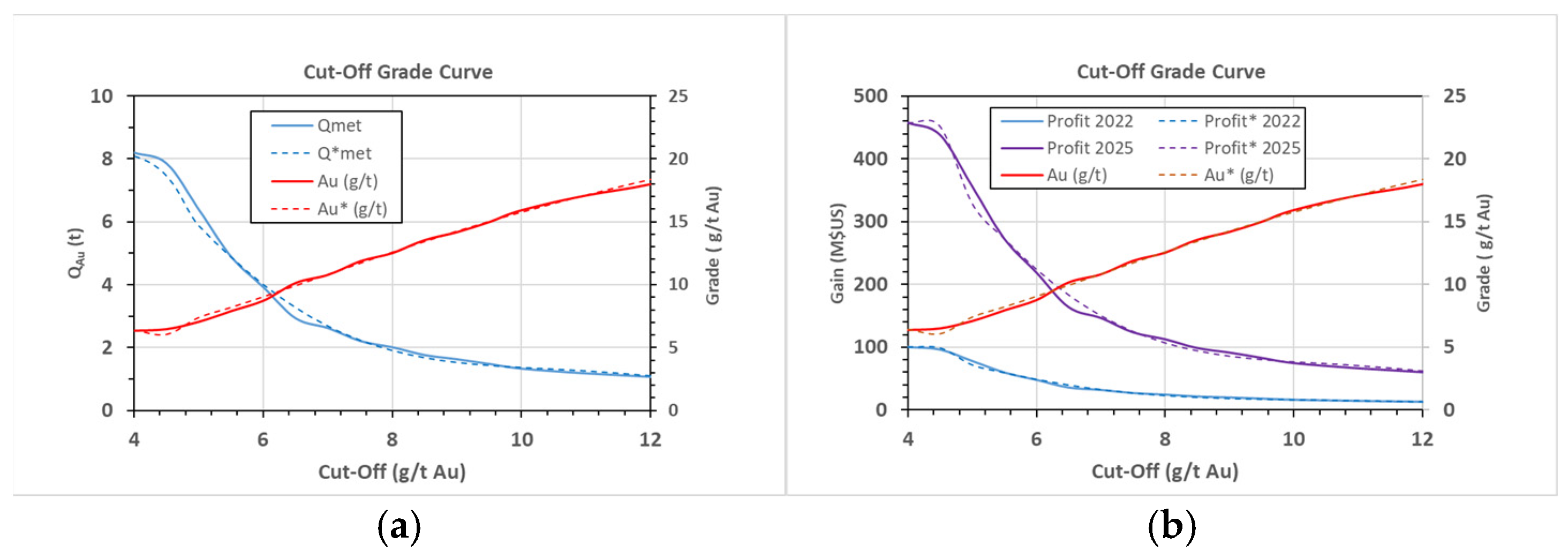
| 4 | 956 | 0.48 | 1.29 | 8.20 | 0.289 | 6.36 | 0.22 |
| 4.5 | 897 | 0.45 | 1.21 | 7.86 | 0.277 | 6.49 | 0.23 |
| 5 | 670 | 0.34 | 0.90 | 6.39 | 0.225 | 7.07 | 0.25 |
| 5.5 | 457 | 0.23 | 0.62 | 4.89 | 0.172 | 7.92 | 0.28 |
| 6 | 332 | 0.17 | 0.45 | 3.92 | 0.138 | 8.75 | 0.31 |
| 6.5 | 214 | 0.11 | 0.29 | 2.93 | 0.103 | 10.16 | 0.36 |
| 7 | 180 | 0.09 | 0.24 | 2.62 | 0.092 | 10.80 | 0.38 |
| 7.5 | 138 | 0.07 | 0.19 | 2.21 | 0.078 | 11.88 | 0.42 |
| 8 | 119 | 0.06 | 0.16 | 2.01 | 0.071 | 12.54 | 0.44 |
| 8.5 | 96 | 0.05 | 0.13 | 1.76 | 0.062 | 13.57 | 0.48 |
| 9 | 85 | 0.04 | 0.11 | 1.63 | 0.057 | 14.19 | 0.50 |
| 9.5 | 73 | 0.04 | 0.10 | 1.48 | 0.052 | 15.00 | 0.53 |
| 10 | 62 | 0.03 | 0.08 | 1.33 | 0.047 | 15.94 | 0.56 |
| 11 | 51 | 0.03 | 0.07 | 1.18 | 0.042 | 17.11 | 0.60 |
| 12 | 44 | 0.02 | 0.06 | 1.07 | 0.038 | 18.00 | 0.63 |
| 13 | 35 | 0.02 | 0.05 | 0.92 | 0.032 | 19.39 | 0.68 |
| 14 | 26 | 0.01 | 0.04 | 0.75 | 0.026 | 21.41 | 0.76 |
| 15 | 23 | 0.01 | 0.03 | 0.69 | 0.024 | 22.34 | 0.79 |
| Cut-Off | Nb | Vol | Qore | Qmet | Qmet | Grade | Grade |
|---|---|---|---|---|---|---|---|
| (g/t) | blocs | (Mm3) | (Mt) | (t) | (Moz) | (Au g/t) | (Au oz) |
| 4 | 526 | 0.26 | 0.71 | 5.24 | 0.185 | 7.38 | 0.26 |
| 4.5 | 507 | 0.25 | 0.68 | 5.13 | 0.181 | 7.50 | 0.26 |
| 5 | 406 | 0.20 | 0.55 | 4.48 | 0.158 | 8.16 | 0.29 |
| 5.5 | 372 | 0.19 | 0.50 | 4.24 | 0.150 | 8.44 | 0.30 |
| 6 | 353 | 0.18 | 0.48 | 4.09 | 0.144 | 8.59 | 0.30 |
| 6.5 | 173 | 0.09 | 0.23 | 2.60 | 0.092 | 11.13 | 0.39 |
| 7 | 171 | 0.09 | 0.23 | 2.58 | 0.091 | 11.18 | 0.39 |
| 7.5 | 149 | 0.07 | 0.20 | 2.36 | 0.083 | 11.74 | 0.41 |
| 8 | 134 | 0.07 | 0.18 | 2.21 | 0.078 | 12.21 | 0.43 |
| 8.5 | 75 | 0.04 | 0.10 | 1.55 | 0.055 | 15.28 | 0.54 |
| 9 | 61 | 0.03 | 0.08 | 1.38 | 0.049 | 16.75 | 0.59 |
| 9.5 | 24 | 0.01 | 0.03 | 0.92 | 0.032 | 28.43 | 1.00 |
| 10 | 22 | 0.01 | 0.03 | 0.89 | 0.031 | 30.13 | 1.06 |
| 11 | 12 | 0.01 | 0.02 | 0.75 | 0.026 | 46.46 | 1.64 |
| 12 | 11 | 0.01 | 0.01 | 0.74 | 0.026 | 49.60 | 1.75 |
| 13 | 11 | 0.01 | 0.01 | 0.74 | 0.026 | 49.60 | 1.75 |
| 14 | 11 | 0.01 | 0.01 | 0.74 | 0.026 | 49.60 | 1.75 |
| 15 | 11 | 0.01 | 0.01 | 0.74 | 0.026 | 49.60 | 1.75 |
| Orebody N° | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vol (m3) | 266,000 | 71,500 | 44,500 | 26,000 | 24,500 | 22,000 | 9000 | 7000 | 2500 | 473,000 | |
| Qmet (t) | 2.873 | 0.772 | 0.481 | 0.281 | 0.265 | 0.238 | 0.097 | 0.076 | 0.027 | 5.110 | |
| Qmet (oz) | 101,344 | 27,232 | 16,967 | 9912 | 9348 | 8395 | 3422 | 2681 | 952 | 180,253 | |
| Qore (t) | 718,200 | 193,050 | 120,150 | 70,200 | 66,150 | 59,400 | 24,300 | 18,900 | 6750 | 1,277,100 | |
| 2022 | Vmet (MUSD) | 177.352 | 47.656 | 29.692 | 17.346 | 16.359 | 14.692 | 5.988 | 4.692 | 1.519 | 315.296 |
| PC (MUSD) | 138.740 | 37.281 | 23.228 | 13.570 | 12.797 | 11.493 | 4.684 | 3.670 | 1.304 | 246.767 | |
| Gain (MUSD) | 38.612 | 10.375 | 6.464 | 3.777 | 3.562 | 3.199 | 1.304 | 1.021 | 0.215 | 68.529 | |
| 2025 | Vmet (MUSD) | 336.413 | 90.397 | 56.323 | 32.904 | 31.030 | 27.869 | 11.358 | 8.899 | 3.162 | 598.354 |
| PC (MUSD) | 160.609 | 43.157 | 26.889 | 15.709 | 14.814 | 13.305 | 5.423 | 4.249 | 1.509 | 285.663 | |
| Gain (MUSD) | 175.805 | 47.240 | 29.433 | 17.195 | 16.216 | 14.564 | 5.936 | 4.651 | 1.652 | 312.691 |
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | ||
|---|---|---|---|---|---|---|---|---|
| QAu (t) | −0.0002 | 0.0142 | −0.337 | 4.065 | −26.039 | 81.476 | −88.426 | 0.996 |
| Au (g/t) | 0.0026 | −0.1002 | 1.333 | −5.667 | 13.188 | - | - | 0.998 |
| Gain 2022 (MUSD) | −0.0035 | 0.2048 | −4.877 | 59.36 | −384.76 | 1229 | −1404 | 0.995 |
| Gain 2025 (MUSD) | −0.0158 | 0.9326 | −22.206 | 270.3 | −1752 | 5594 | −6392 | 0.995 |
Appendix B

Appendix C
Appendix C.1. Regional Geological Settings
Appendix C.1.1. Paleoproterozoic Domains

Appendix C.1.2. Geodynamic Context
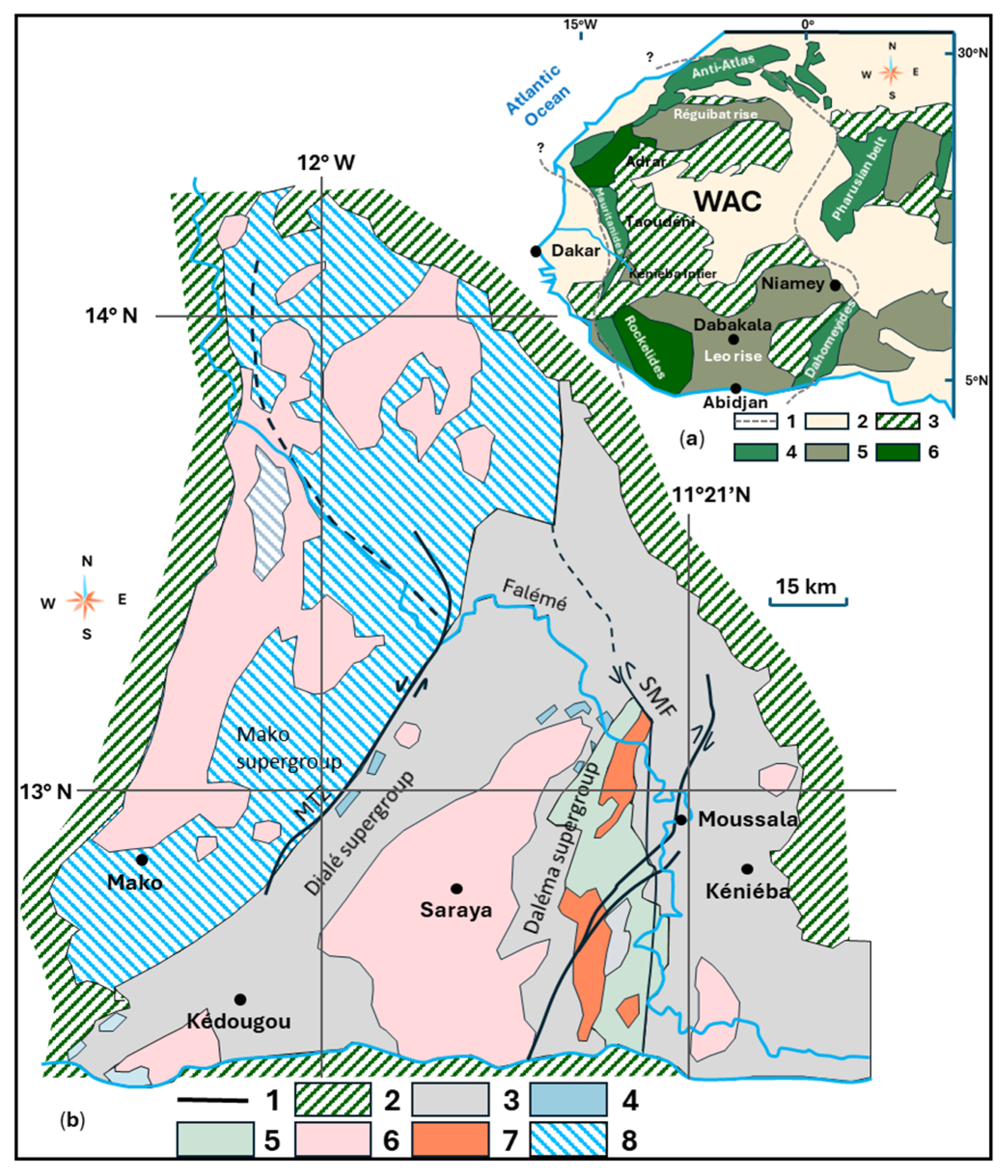
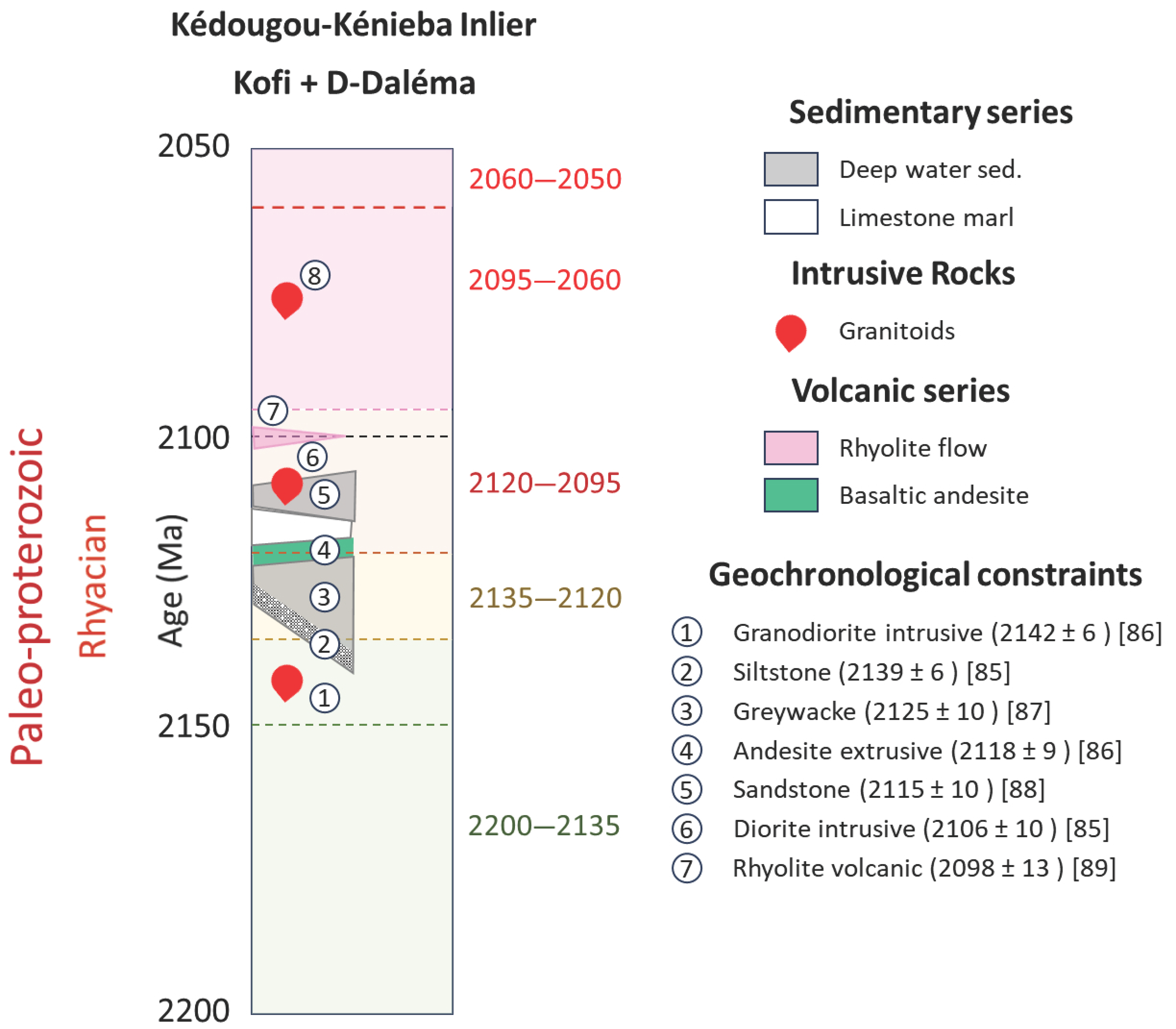
Appendix C.1.3. Lithostratigraphy
Appendix C.1.4. Gold Mineralization of the West African Craton
Mineralization Related to Magmatic Accretion
Mineralization Related to Tectonic Accretion
Appendix C.1.5. Classification of Gold Deposits in West Africa
References
- Goldfarb, R.J.; Groves, D.I.; Gardoll, S. Orogenic gold and geologic time: A global synthesis. Ore Geol. Rev. 2001, 18, 1–75. [Google Scholar] [CrossRef]
- Kohanpour, F.; Lindsay, M.D.; Occhipinti, S.; Gorczyk, W. Structural controls on Proterozoic nickel and gold mineral systems identified from geodynamic modelling and geophysical interpretation, east Kimberley, Western Australia. Ore Geol. Rev. 2018, 95, 552–568. [Google Scholar] [CrossRef]
- Camara, N.; Royer, J.J. 3D Modeling and Mineral Resources Estimation of the Kofi Birimian Gold deposit, Zone C, Mali. In Proceedings of the 13th Biennial SGA Meeting, Nancy, France, 24–27 August 2015; pp. 1–4. [Google Scholar]
- Hervé, M.; Moyen, J.F. Secular changes in TTG composition as markers of the progressive cooling of the Earth. Geology 2002, 30, 319–322. [Google Scholar]
- Condie, T.C.; Des Marais, D.J.; Abbott, D. Precambrian superplumes and super-continents: A record black shales, carbon isotopes and paleoclimates. Precambrian Res. 2001, 106, 239–260. [Google Scholar] [CrossRef]
- Peck, W.H.; Valley, J.W. Archean environment. In Encyclopedia of Paleoclimatology and Ancient Environments; Gornitz, V., Ed.; Encyclopedia of Earth Sciences Series; Springer: Dordrecht, The Netherlands, 2009; pp. 34–38. [Google Scholar] [CrossRef]
- Groves, D.I.; Condie, K.C.; Goldfarb, R.J.; Hronsky, J.M.M.; Vielreicher, R.M. Secular changes in gold tectonic processes and their influence on the temporal distribution of gold-bearing mineral deposits. Econ. Geol. 2005, 100, 203–224. [Google Scholar] [CrossRef]
- Prokofiev, V.Y.; Naumov, V.B. Physicochemical parameters and geochemical features of ore-forming fluids for orogenic gold deposits throughout geological time. Minerals 2020, 10, 50. [Google Scholar] [CrossRef]
- Evans, K.A.; Tomkins, A.G. Metamorphic fluids in orogenic settings. Elements 2020, 16, 381–388. [Google Scholar] [CrossRef]
- Goldfarb, R.J.; Pitcairn, I. Orogenic gold: Is a genetic association with magmatism realistic? Miner. Depos. 2023, 58, 5–35. [Google Scholar] [CrossRef]
- Hrstka, T.; Dubessy, J.; Zachariáš, J. Bicarbonate-rich fluid inclusions and hydrogen diffusion in quartz from the Libčice orogenic gold deposit, Bohemian Massif. Chem. Geol. 2011, 281, 317–332. [Google Scholar] [CrossRef]
- Cepedal, A.; Fuertes-Fuente, M.; Martin-Izard, A.; Aragon, D.; Martinez, N.; Arias, D. Constrains on gold metallogeny in NW Iberia: Insights from LA-ICP-MS analysis of trace elements, fluid inclusions, and stable isotopes in the unexplored Portas del Sil gold deposit. Ore Depos. Rev. 2024, 169, 106077. [Google Scholar] [CrossRef]
- Lawrence, D.M.; Treloar, P.J.; Rankin, A.H.; Harbidge, P.; Holliday, J. The geology and mineralogy of the Loulo Mining District, Mali, West Africa: Evidence for two distinct styles of orogenic gold mineralization. Econ. Geol. 2013, 108, 199–227. [Google Scholar] [CrossRef]
- IAMGOLD. 2012. Available online: https://www.iamgold.com/French/exploitations/previsions/default.aspx (accessed on 27 July 2025).
- Loulo Gold Mine. 2012. Available online: https://www.barrick.com/English/home/default.aspx (accessed on 27 July 2025).
- Endeavour Mining. 2012. Available online: https://www.endeavourmining.com/ (accessed on 27 July 2025).
- Avion Gold. Inc. Technical Report and Updated Resource Estimate on the Kofi Project, Mali, Africa for Avion Gold Corp. Technical Report Kayes; PM Report; Avion Gold: Toronto, ON, Canada, 2012; p. 155. [Google Scholar]
- Dabo, M.; Aïfa, T. Structural styles and tectonic evolution of the Kolia-Boboti sedimentary basin, Kédougou-Kéniéba inlier, eastern Senegal. Comptes Rendus Geosci. 2010, 342, 796–805. [Google Scholar] [CrossRef]
- Taylor, P.N.; Moorbath, S.; Leube, A.; Hirdes, W. Early Proterozoic crustal evolution in the Birimian of Ghana: Constraints from geochronology and isotope geochemistry. Precambrian Res. 1992, 56, 97–111. [Google Scholar] [CrossRef]
- Hirdes, W.; Davis, D.W.; Lüdtke, G.; Konan, G. Two generations of Birimian (Paleoproterozoic) volcanic belts in northeastern Côte d’Ivoire (West Africa): Consequences for the Birimian controversy. Precambrian Res. 1996, 80, 173–191. [Google Scholar] [CrossRef]
- Abouchami, W.; Boher, M.; Michard, A.; Albarède, F. A major 2.1 Ga old event of mafic magmatic in West Africa: An early stage of crustal accretion. J. Geophys. Res. 1990, 95, 17605–17629. [Google Scholar] [CrossRef]
- Boher, M.; Abouchami, W.; Michard, A.; Albarède, F.; Arndt, N.T. Crustal growth in West Africa at 2.1 Ga. J. Geophys. Res. 1992, 97, 345–369. [Google Scholar] [CrossRef]
- Bassot, J.P. Etude géologique du Sénégal oriental et de ses confins guinéo-maliens. Mémoires BRGM 1966, 40, 332. (In French) [Google Scholar]
- Dammanget, A.; Diallo, L.; Guilloux, L.; Milési, J.P. Le Gisement de Loulo (Mali): Un Exemple de Concentration Aurifère Stratiforme Dans des Grès à Tourmaline du Birrimien de l’Afrique de l’Ouest; CIFEG Publication: Orleans, France, 1986; pp. 13–130. (In French) [Google Scholar]
- N’diaye, P.M.; Dia, A.; Vialette, Y.; Diallo, D.P.; Ngom, P.M.; Sylla, M.; Wade, S.; Dioh, E. Données topographiques, géochimiques et géochronologiques nouvelles sur les granitoïdes du Paléoprotérozoïque du Supergroupe de Dialé–Daléma (Sénégal Oriental): Implications pétro-génétiques et géodynamiques. J. Afr. Earth Sci. 1997, 25, 193–208. (In French) [Google Scholar] [CrossRef]
- Koné, A.Y.; Nasr, I.H.; Traoré, B.; Amiri, A.; Inoubli, M.H.; Sangaré, S.; Qaysi, S. Geophysical contributions to gold exploration in Western Mali according to airborne electromagnetic data interpretations. Minerals 2021, 11, 126. [Google Scholar] [CrossRef]
- Lefeuvre, N.; Truche, L.; Donzé, F.-V.; Vandenborre, J.; Gaucher, E.C.; Magnin, V. The contribution of mechanoradical reactions to crustal hydrogen generation. Earth Planet. Sci. Lett. 2025, 660, 119363. [Google Scholar] [CrossRef]
- Dejonghe, L.; Demaiffre, D.; Gorzawski, H. Géochimie isotopique (C, O, Sr) des dolomies frasniennes du Massif de Philippeville (Synclinorium de Dinant, Belgique). Ann. Soc. Géol. Belg. 1989, 112, 87–102. (In French). Available online: https://popups.uliege.be/0037-9395/index.php?id=990 (accessed on 27 July 2025).
- Fontboté, L.; Amstutz, G.C.; Cardozo, M.; Cedillo, E.; Frutos, J. (Eds.) Stratabound ore deposits in the Andes. In Special Publication of the Society for Geology Applied to Mineral Deposits; Springer: Berlin/Heidelberg, Germany, 1990; Volume 8, 815p, Available online: https://link.springer.com/book/10.1007/978-3-642-88282-1 (accessed on 27 July 2025).
- Cathelineau, M. Cation site occupancy in chlorites and illites as a function of temperature. Clay Miner. 1988, 23, 471–485. [Google Scholar] [CrossRef]
- Jowett, E.C. Fitting Iron and Magnesium Into the Hydrothermal Chlorite Geothermometer. In Proceedings of the Geological Association of Canada + MAC + SEG Joint Annual Meeting, Toronto, ON, USA, 1991; Volume Program with Abstracts 16. p. A62. [Google Scholar]
- De Caritat, P.; Hutcheon, I.; Walshe, J. Chlorite geochemistry: A review. Clays Clay Miner. 1993, 41, 219–239. [Google Scholar] [CrossRef]
- Klein, E.L.; Koppe, J.C. Chlorite geothermometry and physicochemical conditions of gold mineralization in the Paleoproterozoic Caxias Deposit, São Luȋs Craton, Northern Brazil. Geochim. Bras. 2000, 14, 219–232. [Google Scholar]
- Vidal, O.; Parra, T.; Trotet, F. A thermodynamic model for Fe-Mg aluminous chlorite using data from phase equilibrium experiments and natural pelitic assemblages in the 100° to 600 °C, 1 to 25 kb Range. Am. J. Sci. 2001, 301, 557–592. [Google Scholar] [CrossRef]
- Bourdelle, F.; Parra, T.; Christian Chopin, C.; Beyssac, O. A new chlorite geothermometer for diagenetic to low-grade metamorphic conditions. Contrib. Mineral. Petrol. 2013, 165, 723–735. [Google Scholar] [CrossRef]
- Henry, D.J.; Guidotti, C.V.; Thomson, J.A. The Ti-saturation surface for low-to-medium pressure metapelitic biotites: Implications for geothermometry and Ti-substitution mechanisms. Am. Mineral. 2005, 90, 316–328. [Google Scholar] [CrossRef]
- Massonne, H.J.; Schreyer, W. Phengite geobarometry based on the limiting assemblage with K-feldspar, phlogopite, and quartz. Contrib. Mineral. Petrol. 1987, 96, 212–224. [Google Scholar] [CrossRef]
- Kretschmar, U.; Scott, S.D. Phase relations involving arsenopyrite in the system Fe-As-S and their application. Can. Mineral. 1976, 14, 364–386. [Google Scholar]
- Nayak, B.; Sindern, S.; Wagner, T. Formation of late-stage hydrothermal mineralization in the Mesoarchean Boula-Nuasahi ultramafic complex, Odisha, India: Constraints from arsenopyrite geothermometry and trace element data. Ore Geol. Rev. 2021, 139, 104482. [Google Scholar] [CrossRef]
- Fridovsky, V.Y.; Polufuntikova, L.I.; Kudrin, M.V. Origin of Disseminated Gold-Sulfide Mineralization from Proximal Alteration in Orogenic Gold Deposits in the Central Sector of the Yana–Kolyma Metallogenic Belt, NE Russia. Minerals 2023, 13, 394. [Google Scholar] [CrossRef]
- Ai Zamruddin, N.N.S.; Zainal Abidin, N.S.; Endut, Z.; Makoundi, C.; Lok, L.K.; Ismail, M.S. Trace Element Analysis of Pyrite and Arsenopyrite Using the LA-ICPMS Technique in Pulai, Central Belt of Peninsular Malaysia. Minerals 2023, 13, 1026. [Google Scholar] [CrossRef]
- Wilkinson, B.H.; Kesler, S.E. Tectonic-diffusion estimate of orogenic gold resources. Econ. Geol. 2010, 105, 1321–1334. [Google Scholar] [CrossRef]
- Reeder, R.J. Carbonates: Mineralogy and chemistry: Short course notes. In Reviews in Mineralogy; Mineralogical Society of America: Chantilly, VA, USA, 1983; Volume 11, 400p, ISBN 0-939950-15-4. [Google Scholar]
- Murzin, V.; Chudnenko, K.; Palyanova, G.; Kissin, A.; Varlamov, D. Physicochemical model of formation of gold-bearing magnetite-chlorite-carbonate rocks at the Karabash Ultramafic Massif (Southern Urals, Russia). Minerals 2018, 8, 306. [Google Scholar] [CrossRef]
- Dick, J.M. CHNOSZ: Thermodynamic Calculations and diagrams for geochemistry. Front. Earth Sci. 2019, 7, 180. [Google Scholar] [CrossRef]
- Lawrence, D.M.; Lambert-Smith, J.S.; Treloar, P.J. A review of gold mineralization in Mali. In Mineral Deposits of North Africa; Bouabdellah, M., Slack, J., Eds.; Mineral Resource Reviews; Springer: Berlin/Heidelberg, Germany, 2016; pp. 327–351. [Google Scholar] [CrossRef]
- Groves, D.I.; Santosh, M.; Zhang, L. A scale-integrated exploration model for orogenic gold deposits based on a mineral system approach. Geosci. Front. 2020, 11, 719–738. [Google Scholar] [CrossRef]
- Camara, N.; Jébrak, M. Etude Minéralogique de l’or Dans le Birimien de Kofi. Master’s Thesis, University of Lorraine, Nancy, France, 2013; 53p. (In French). [Google Scholar]
- Lawrence, D.M.; Treloar, P.J.; Rankin, A.H.; Boyce, A.; Hardbidge, P. A fluid inclusion and stable isotope study at the Loulo Mining District, Mali, West Africa: Implications for multi-fluid sources in the generation of orogenic gold deposits. Econ. Geol. 2011, 108, 229–257. [Google Scholar] [CrossRef]
- Pokrovski, S.; Kokh, M.A.; Guillaume, D.; Borisova, A.Y.; Gisquet, P.; Hazemann, J.L.; Lahera, E.; Del Net, W.; Proux, O.; Testemale, D.; et al. Sulfur radical species form gold deposits on Earth. Proc. Natl. Acad. Sci. USA 2015, 112, 13484–13489. [Google Scholar] [CrossRef]
- Yu, H.D.; Fu, Y.Z.; Liu, W.Y.; Said, N.; Cao, H.W.; Liu, C.M.; Fang, W.X.; Liu, J.J.; Zou, H. The gold mineral, systems of the West Qinling orogen, central China: New insight from the Baguamiao gold deposit. Ore Geol. Rev. 2023, 162, 105705. [Google Scholar] [CrossRef]
- Gaillard, N.; Williams-Jones, A.E.; Clark, J.R.; Salvi, S.; Perrouty, S.; Linnen, R.L.; Olivo, G.R. The use of lithogeochemistry in delineating hydrothermal fluid pathways. Ore Geol. Rev. 2020, 120, 103351. [Google Scholar] [CrossRef]
- Atkins, P.; Jones, L.; Laverman, L. Chemical Principles, 7th ed.; Freeman: London, UK, 2016; pp. 399–461. ISBN 978-1-4641-8395-9. [Google Scholar]
- Bellehumeur, C. Application des Techniques Géostatistiques et D’analyse Multivariable à L’interprétation des Relevés Géochimiques Régionaux. Ph.D Thesis, Thèse Université du Québec à Montréal, Chicoutimi, QC, Canada, 1992; 441p. (In French). [Google Scholar]
- Saporta, G. Probabilités, Analyse des Données et Statistique Multivariable; Dunod: Malakoff, France, 2006; Volume 2, p. 656. (In French) [Google Scholar]
- Paradigm, Reservoir Characterization Petrophysics, Paradigm Training Program. 2022. Available online: https://fr.scribd.com/document/374908678/Reservoir-Characterization-Petrophysics (accessed on 27 July 2025).
- Matheron, G. Principles of geostatistics. Econ. Geol. 1963, 58, 1246–1266. [Google Scholar] [CrossRef]
- Journel, A.G.; Huijbregts, C.J. Mining Geostatistics. Academic Press: London, UK; New York, NY, USA, 1978; 600p. [Google Scholar]
- Mallet, J.L. Geomodeling. In Applied Geostatistics; Oxford University Press: New York, NY, USA, 2002; 624p. [Google Scholar]
- Mallet, J.L. Discrete modeling for natural objects. Math. Geol. 1997, 29, 199–219. [Google Scholar] [CrossRef]
- Royer, J.J. Cours de Géostatistique Minière; Cours ENSG; University of Lorraine: Nancy, France, 2013; t 1 & 2. (In French) [Google Scholar]
- 2022. Available online: https://miningdataonline.com/property/182/Tabakoto-Mine.aspx (accessed on 27 July 2025).
- Bessoles, B. Géologie de Afrique: Le craton Ouest-Africain. Mémoires BRGM 1977, 88, 402. (In French) [Google Scholar]
- Béziat, D.; Dubois, M.; Debat, P.; Nikiéma, S.; Salvi, S.; Tollon, F. Gold metallogeny in the Birimian craton of Burkina Faso (West Africa). J. Afr. Earth Sci. 2008, 50, 215–233. [Google Scholar] [CrossRef]
- Milési, J.P.; Ledru, P.; Dommanget, M.; Johan, V.; Diallo, M. Lower Proterozoic Succession in Senegal and Mali (West Africa): Position of Sediment Hosted Ag and Fe Deposits of Loulo Area and Significance in Terms of Crustal Evolution; Centre International pour la Formation et les Echanges en Géosciences (CIFEG): Orleans, France, 1989; p. 135. [Google Scholar]
- Ledru, P.; Pons, J.; Milesi, J.P.; Feybesse, J.L.; Johan, V.B. Transcurrent tectonics and polycyclic evolution in the Lower Proterozoic of Senegal-Mali. Precambrian Res. 1991, 50, 337–354. [Google Scholar] [CrossRef]
- Vidal, M.; Gumiaux, C.; Cagnard, F.; Pouclet, A.; Ouattara, G.; Pichon, M. Evolution of a Paleoproterozoic “weak type” orogeny in the west Africa Craton (Ivory Coast). Tectonophysics 2009, 477, 145–159. [Google Scholar] [CrossRef]
- Milési, J.P. Vers la Mesure de Potentialités Métallifères, Habilitation à Diriger des Recherches; Claude Bernard University Lyon I: Villeurbanne, France; BRGM: Orléans, France, 2001; 204p. (In French) [Google Scholar]
- Feybesse, J.L.; Milési, J.P. The Archaean/Proterozoic contact zone in West Africa: A mountain belt of décollement thrusting and folding on a continental margin related to 2.1 Ga convergence of Archaean cratons? Precambrian Res. 1994, 69, 199–227. [Google Scholar] [CrossRef]
- Feybesse, J.L.; Billa, M.; Guerrot, C.; Duguey, E.; Lescuyer, J.L.; Milési, J.P.; Bouchot, V. The Paleoproterozoic Ghanaian province: Geodynamic model and ore controls, including regional stress modeling. Precambrian Res. 2006, 149, 149–196. [Google Scholar] [CrossRef]
- Lompo, M. Paleoproterozoic structural evolution of the Man-Leo Shield (West-Africa). Key structures for vertical to transcurrent tectonics. J. Afr. Earth Sci. 2010, 58, 19–36. [Google Scholar] [CrossRef]
- Vanderhaeghe, O.; Ledru, P.; Tiéblemont, D.; Egal, E.; Cocherie, A.; Tegyey, M.; Milési, J.P. Contrasting mechanism of crustal growth: Geodynamic evolution of the Paleoproterozoic granite greenstone belts of French Guiana. Precambrian Res. 1998, 92, 165–193. [Google Scholar] [CrossRef]
- Théveniaut, H.; Delor, C. Le paléomagnétisme du Bouclier des Guyanes: État des connaissances et analyse critique des données. Géologie Fr. 2003, 2-3-4, 59–82. (In French). Available online: https://geolfrance.brgm.fr/sites/default/files/upload/documents/gf2-2-2003.pdf (accessed on 27 July 2025).
- Villeneuve, M.; Cornée, J.J. Structure, evolution and paleogeography of the West African craton and bordering belts during the Neoproterozoic. Precambrian Res. 1994, 69, 307–326. [Google Scholar] [CrossRef]
- Nomade, S.; Chen, Y.; Pouclet, A.; Féraud, G.; Théveniaut, H.; Daouda, B.Y.; Vidal, M.; Rigolet, C. The Guiana and the West African shield Paleoproterozoic grouping: New palaeomagnetic data for French Guiana and the Ivory Coast. Geophys. J. Int. 2003, 154, 677–694. [Google Scholar] [CrossRef]
- Oberthür, T.; Vetter, U.; Davis, D.W.; Amanor, J.A. Age constraints on gold mineralization and Paleoproterozoic crustal evolution in the Ashanti belt of southern Ghana. Precambrian Res. 1998, 89, 129–143. [Google Scholar] [CrossRef]
- Jébrak, M.; Marcoux, E. Géologie des Ressources Minérales; Ressources naturelles et Faune: Québec, QC, Canada, 2008; 667p. (In French) [Google Scholar]
- Gueye, M.; Ngom, P.M.; Diène, M.; Thiam, Y.; Siegesmund, S.; Wemmer, K.; Pawlig, S. Intrusive rocks and tectono-metamorphic evolution of the Mako Paleoproterozoic belt (Eastern Senegal, West Africa). J. Afr. Earth Sci. 2008, 50, 88–110. [Google Scholar] [CrossRef]
- Milési, J.P.; Ledru, P.; Feybesse, J.L.; Dommanget, A.; Marcoux, E. Early Proterozoic ore deposits and tectonics of Birimian orogenic belt, West Africa. Precambrian Res. 1992, 58, 305–344. [Google Scholar] [CrossRef]
- Leube, A.; Hirdes, W.; Mauer, R.; Kesse, G.O. The Early Proterozoic Birimian Supergroup of Ghana and some aspects of its associated gold mineralization. Precambrian Res. 1990, 46, 139–165. [Google Scholar] [CrossRef]
- Eisenlohr, B.N.; Hirdes, W. The structural development of the early Proterozoic Birimian and Tarkwaian rocks of southwest Ghana, West Africa. J. Afr. Earth Sci. 1992, 14, 313–325. [Google Scholar] [CrossRef]
- Loh, G.; Hirdes, W.; Anani, C.; Davis, D.W.; Vetter, U. Explanatory Notes for the Geological Map of Southwest Ghana 1:100,000, Sekondi (0402A) and Axim (0403B) Sheets; Geological Yearbook, Series B Heft 93; E. Schweizerbart: Stuttgart, Germany, 1999; 150p. [Google Scholar]
- Davis, D.W.; Hirdes, W.; Schaltegger, U.; Nunoo, E.A. U-Pb age constraints on deposition and provenance of Birimian and gold-bearing Tarkwaian sediments in Ghana, West Africa. Precambrian Res. 1994, 67, 89–107. [Google Scholar] [CrossRef]
- Perret, J.; Jessell, M.W.; Masurel, Q.; Hayman, P.C.; Thébaud, N.; Baratoux, L.; Kouamélan, A.; Eglinger, A.; André-Mayer, A.S.; Koffi, A.Y.; et al. Review of Paleoproterozoic tectonics in the southern West African Craton: Insights from multi-disciplinary data integration. 50th Anniversary. Precambrian Res. 2025, 422, 107707. [Google Scholar] [CrossRef]
- Hein, K.A.A.; Matsheka, I.R.; Bruguier, O.; Masurel, Q.; Bosch, D.; Caby, R.; Monié, P. The Yatela gold deposit: 2 billion years in the making. J. Afr. Earth Sci. 2015, 112, 548–569. [Google Scholar] [CrossRef]
- Masurel, Q.; Thébaud, N.; Miller, J.; Ulrich, S. The tectono-magmatic framework to gold mineralization in the Sadiola-Yatela gold camp and implications for the paleotectonic setting of the Kédougou-Kénieba inlier, West Africa. Precambrian Res. 2017, 292, 35–56. [Google Scholar] [CrossRef]
- Koné, J. Structure et Métamorphisme de la Ceinture Eburnéenne au Sénégal Oriental: Signification en Termes du Contexte Tectonique et de L’évolution Thermomécanique de la Croûte Eburnéenne. Ph.D Thesis, Université de Toulouse III Paul Sabatier, Toulouse, France, Université Cheikh Anta Diop de Dakar, Dakar, Senegal, 2020; 167p. (In French). [Google Scholar]
- Allibone, A.H.; Lawrence, D.; Scott, J.; Fanning, M.; Lambert-Smith, J.; Stenhouse, P.; Harbidge, R.; Vargas, C.; Turnbull, R.; Holliday, J. Chapter 7: Paleoproterozoic gold deposits of the Loulo District, western Mali. In Geology of the World’s Major Gold Deposits and Provinces; Society of Economic Geologists: Littleton, CO, USA, 2020; pp. 141–162. [Google Scholar] [CrossRef]
- Théveniaut, H.; Ndiaye, P.M.; Buscail, F.; Couëffé, R.; Delor, C.; Fullgraf, T.; Goujou, J.-C. Notice Explicative de la Carte Géologique du Sénégal Oriental à 1/500 000; Ministère des Mines, de l’Industrie, de l’Agro-Industrie et des PME, Direction des Mines et de la Géologie: Dakar, Senegal, 2010. (In French)
- Adadey, K.; Clarke, B.; Théveniaut, H.; Urien, P.; Delor, C.; Roig, J.Y.; Feybesse, J.L. Geological Map Explanation—Map Sheet 0503 B (1:100 000); No. MSSP/2005/GSD/5a; Geological Survey Department of Ghana (GSD): Accra, Ghana, 2009. [Google Scholar]
- Koné, J.; Vanderhaeghe, O.; Diatta, F.; Baratoux, L.; Thebaud, N.; Bruguier, O.; Ndiaye, P.M.; Duchene, S.; Pitra, P.; Ganne, J. Source and deposition age of the Dialé-Daléma metasedimentary series (Kédougou-Kéniéba Inlier, Senegal) constrained by U–Pb geochronology on detrital zircon grains. J. Afr. Earth Sci. 2020, 165, 103801. [Google Scholar] [CrossRef]
- Bassot, J.P. Le complexe volcano-plutonique calco-alcalin de la rivière Daléma (Est Sénégal): Discussion de sa signification géodynamique dans le cadre de l’orogénie éburnéenne (Protérozoïque inferieur). J. Afr. Earth Sci. 1987, 6, 505–519. (In French) [Google Scholar] [CrossRef]
- Diallo, M.; Baratoux, L.; Dufréchou, G.; Jessell, M.W.; Vanderhaeghe, O.; Ly, S.; Baratoux, D. Structure of the Paleoproterozoic Kédougou-Kéniéba Inlier (Senegal-Mali) deduced from gravity and aeromagnetic data. J. Afr. Earth Sci. 2020, 162, 103732. [Google Scholar] [CrossRef]
- Diatta, F.; Ndiaye, P.M.; Diene, M.; Amponsah, P.O.; Ganne, J. The structural evolution of the Diale-Dalema basin, Kédougou-Kénieba Inlier, eastern Senegal. J. Afr. Earth Sci. 2017, 129, 923–933. [Google Scholar] [CrossRef]
- Diallo, M.; Salvi, S.; Baratoux, L.; Béziat, D.; Vanderhaeghe, O.; Labou, I.; Baratoux, D.; Ly, S. Geology of the Tabakoto gold deposit, Kédougou-Kéniéba Inlier, West African Craton, Mali. Bull. Société Géologique Fr. 2024, 195, 24. [Google Scholar] [CrossRef]
- Debat, P.; Nikiéma, S.; Mercier, A.; Lompo, M.; Béziat, D.; Bourges, F.; Roddaz, M.; Salvi, S.; Tollon, F.; Wenmenga, U. A new metamorphic constraint for the Eburnean orogeny from Paleoproterozoic formations of the Man shield (Aribinda and Tampelga countries, Burkina Faso). Precambrian Res. 2003, 123, 47–65. [Google Scholar] [CrossRef]
- Hirdes, W.; Davis, D.W. U–Pb geochronology of Paleoproterozoic rocks in the southern part of the Kedougou-Kéniéba inlier, Sénégal, West Africa: Evidence for diachronous accretionary development of the Eburnean Province. Precambrian Res. 2002, 118, 83–99. [Google Scholar] [CrossRef]
- Bassot, J.P.; Dia, A.; Rocci, G. Lithostratigraphie des Complexes magmatiques de Sandikounda Laminia (Senegal oriental): Données Nouvelles sur le Protérozoïque Inférieur de la Fenêtre de Kédougou. In Proceedings of the 14th Colloquium of African Geology, Berlin, Germany, 18–22 August 1987; p. 46. (In French). [Google Scholar]
- Lambert-Smith, J.S.; Lawrence, D.M.; Müller, W.; Treloar, J.P. Paleotectonic setting of the south-eastern Kédougou-Kéniéba Inlier, West Africa: New insights from igneous trace element geochemistry and U-Pb zircon ages. Precambrian Res. 2015, 274, 110–135. [Google Scholar] [CrossRef]
- Le Mignot, E.; Reisberg, L.; André-Mayer, A.S.; Bourassa, Y.; Fontaine, A.; Miller, J. Re-Os Geochronological evidence for multiple Paleoproterozoic gold events at the scale of the West African Craton. Econ. Geol. 2017, 112, 145–168. [Google Scholar] [CrossRef]




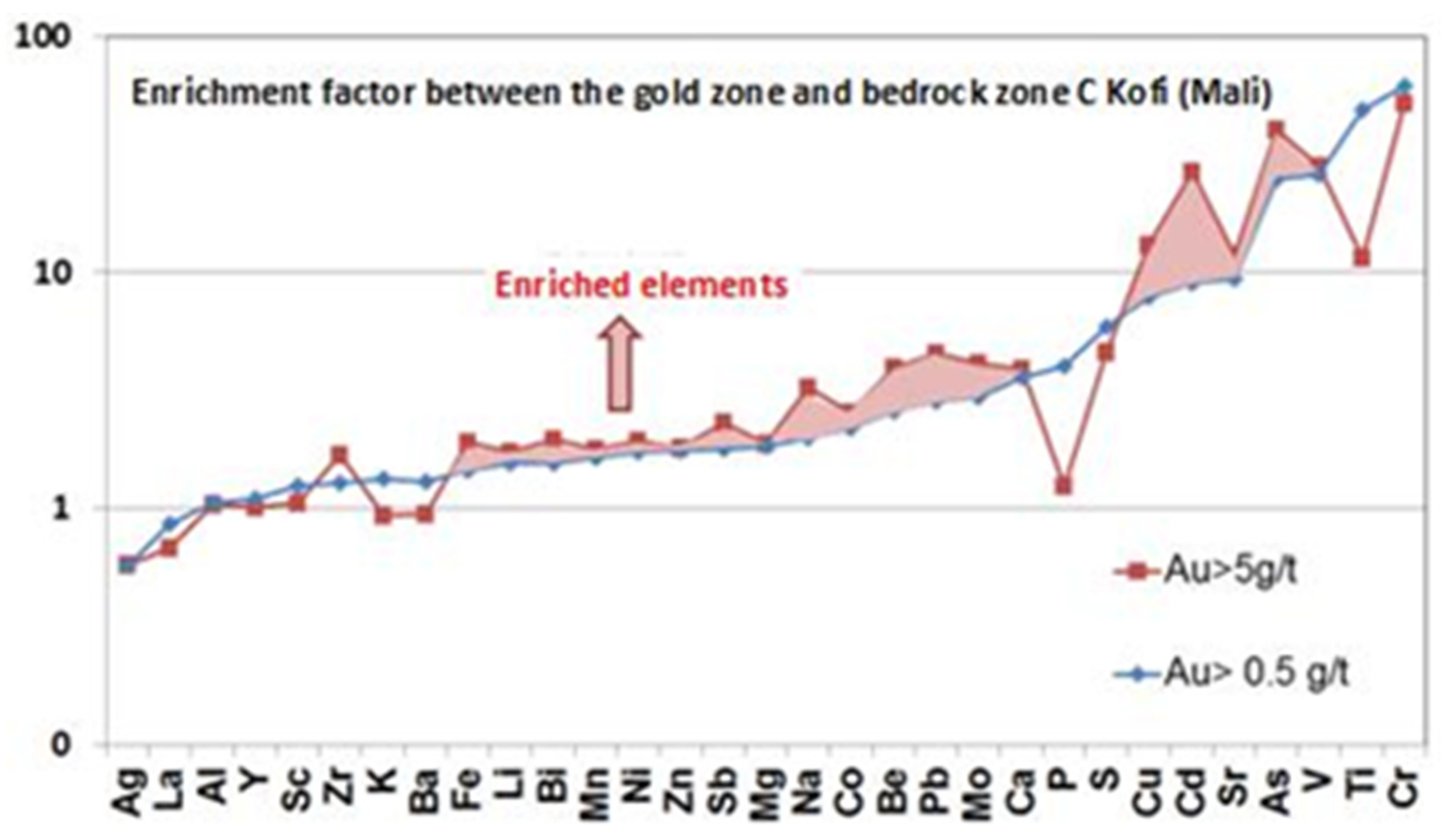
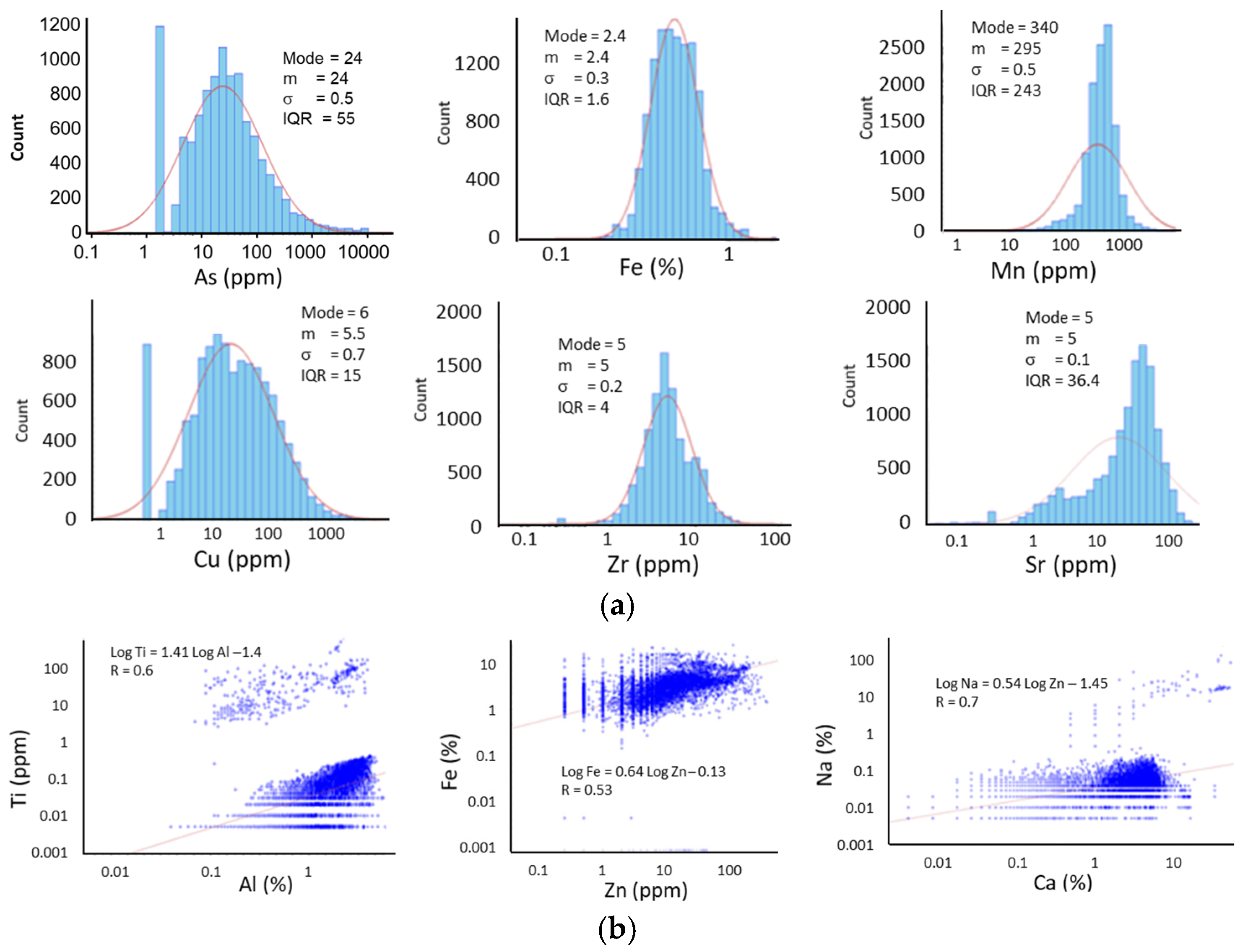
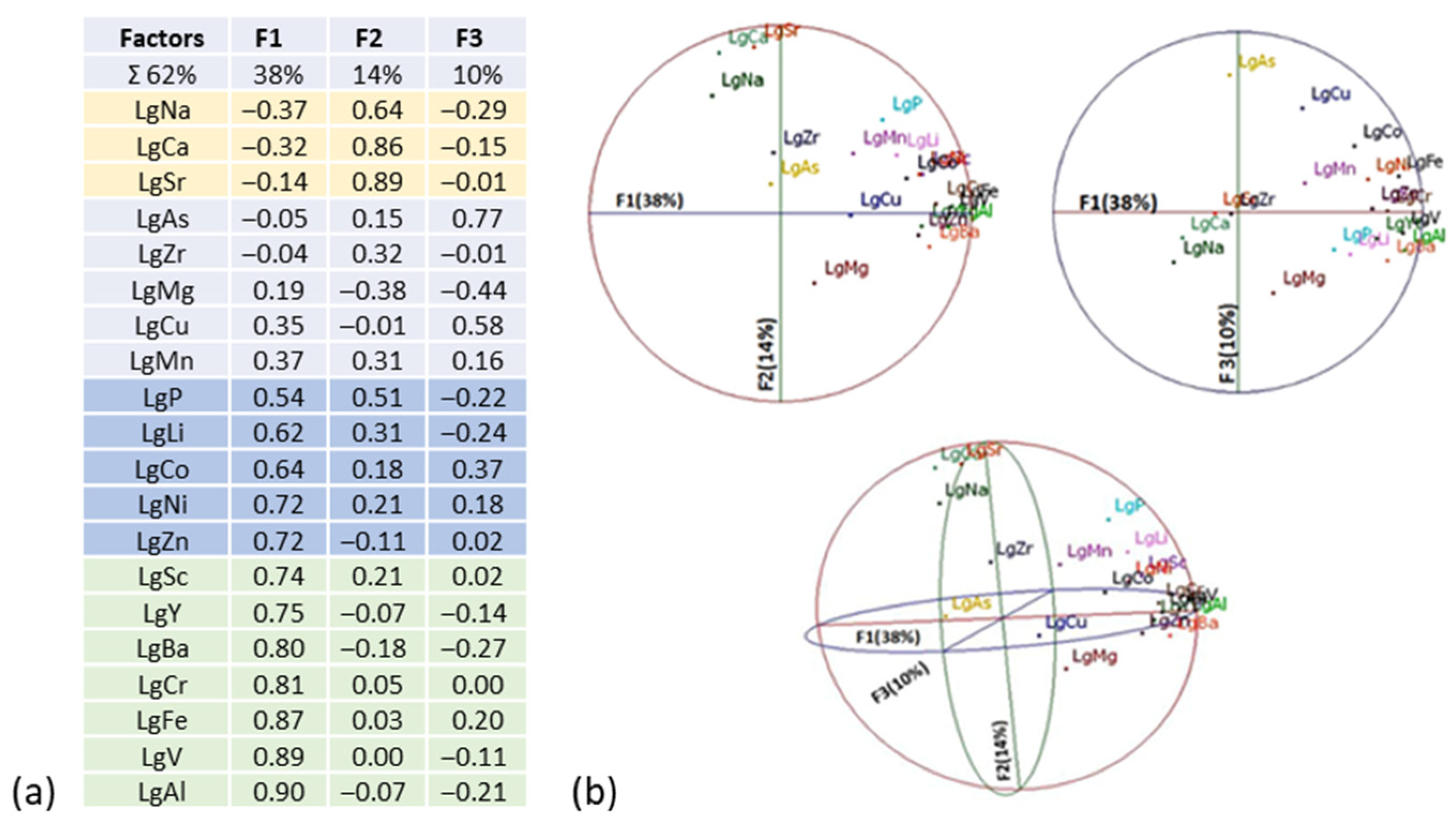
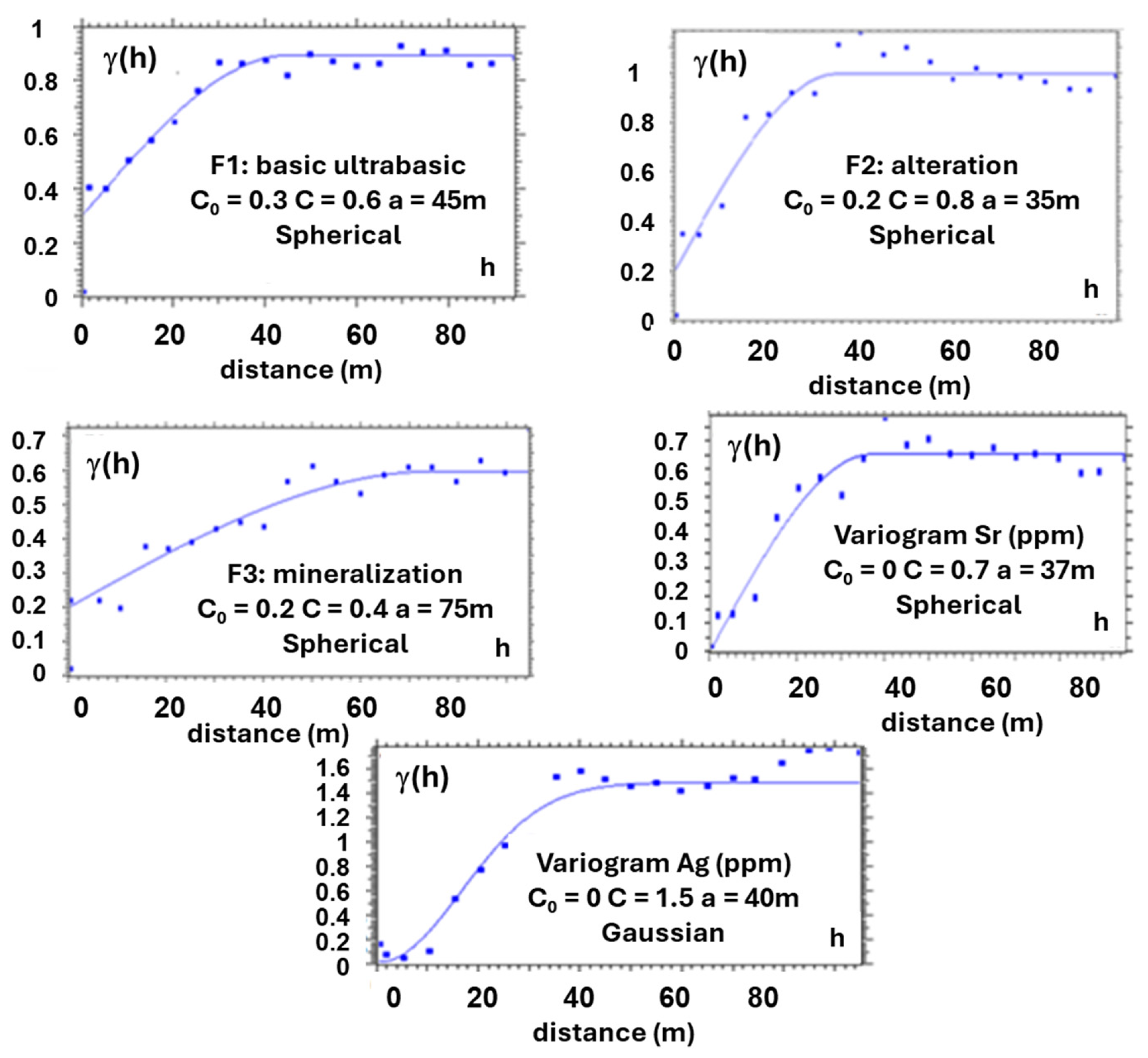

| Deposits | Tonnage (Mt) | Grade (g/t) | Source | Comments |
|---|---|---|---|---|
| Sadiola | 22.30 | 3.30 | [14] | AngloGold Ashanti Mali |
| Yatéla | 7.60 | 1.40 | [14] | Proven and probable resources |
| Loulo | 39.97 | 4.93 | [15] | Proven and probable resources |
| Tabakoto | 4.40 | 4.60 | [16] | Proven and probable resources |
| Equilibrium Curve | a0 | a1 | a2 | a3 | R2 |
|---|---|---|---|---|---|
| asp + py + As | −6430 | 525 | −14.0 | 0.1341 | 1 |
| ±e 1 | 2662 | 238 | 7.0 | 0.0695 | |
| asp + py | −17,280 | 1430 | −39.1 | 0.3661 | 0.999 |
| ±e | 2770 | 245 | 7.2 | 0.0700 |
| Method | Qore | Qmet | Qmet | Grade | Grade | Gain 1 | |
|---|---|---|---|---|---|---|---|
| (Mt) | (t) | (Moz) | (g/t) | (oz/t) | 2022 | 2025 | |
| Kriging | 1.29 | 8.20 | 0.289 | 6.36 | 0.22 | 110.2 | 501.8 |
| ±σes | 0.06 | 0.41 | 0.01 | 0.32 | 0.01 | 5.5 | 25.1 |
| SGS | 0.71 | 5.24 | 0.168 | 7.38 | 0.26 | 70.4 | 320.7 |
| Avon | 2.47 | 8.6 | 0.303 | 3.50 | 0.12 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Royer, J.-J.; Camara, N. Mineralogical, Petrological, 3D Modeling Study and Geostatistical Mineral Resources Estimation of the Zone C Gold Prospect, Kofi (Mali). Minerals 2025, 15, 843. https://doi.org/10.3390/min15080843
Royer J-J, Camara N. Mineralogical, Petrological, 3D Modeling Study and Geostatistical Mineral Resources Estimation of the Zone C Gold Prospect, Kofi (Mali). Minerals. 2025; 15(8):843. https://doi.org/10.3390/min15080843
Chicago/Turabian StyleRoyer, Jean-Jacques, and Niakalé Camara. 2025. "Mineralogical, Petrological, 3D Modeling Study and Geostatistical Mineral Resources Estimation of the Zone C Gold Prospect, Kofi (Mali)" Minerals 15, no. 8: 843. https://doi.org/10.3390/min15080843
APA StyleRoyer, J.-J., & Camara, N. (2025). Mineralogical, Petrological, 3D Modeling Study and Geostatistical Mineral Resources Estimation of the Zone C Gold Prospect, Kofi (Mali). Minerals, 15(8), 843. https://doi.org/10.3390/min15080843








