Abstract
The Xiaobaihegou fluorite deposit is located in the southwest of the Altyn-Tagh Orogen, NW China. However, the provenance, thermodynamic properties, and enrichment mechanisms of the ore-forming fluids in this deposit remain unclear. Fluorite mineralization primarily occurs in the vicinity of the contact zone between the granite and the wall rocks. The zircon U-Pb age of the alkali-feldspar granite in the Xiaobaihegou fluorite deposit is 482.3 ± 4.1 Ma. The ore-hosting lithologies are mainly calcareous rock series of the Altyn Group. The ore bodies are controlled by NE-trending faults and consist primarily of veined, brecciated, massive, and banded ores. The ore mineral assemblage is primarily composed of calcite and fluorite. The rare earth element (REE) patterns of fluorite and calcite in the Xiaobaihegou deposit exhibit right-dipping LREE enrichment with distinct negative Eu anomalies, which closely resemble those of the alkali-feldspar granite. This similarity suggests that the REE distribution patterns of fluorite and calcite were likely inherited from the pluton. The ore-forming process can be divided into an early stage and a late stage. The massive ores formed in the early stage contain mainly gas-rich two-phase fluid inclusions and CO2-bearing three-phase inclusions, with homogenization temperatures ranging from 235 °C to 426 °C and salinities from 28.59% to 42.40% NaCl equivalent. In the late stage, brecciated and stockwork ores were formed. They host liquid-rich two-phase and gas-rich two-phase fluid inclusions, with homogenization temperatures ranging from 129 °C to 350 °C and salinities from 0.88% to 21.61% NaCl equivalent. The results of hydrogen and oxygen isotope studies indicate that the ore-forming fluids were derived from a mixture of magmatic–hydrothermal and meteoric water. Fluorite precipitation in the early stage was mainly due to the mixing of magmatic–hydrothermal solution and meteoric water, as well as a water–rock reaction. In the late stage, fluid mixing further occurred, resulting in a decrease in temperature and the formation of brecciated and stockwork ores. The 87Sr/86Sr and 143Nd/144Nd ratios of fluorite from the deposit range from 0.71033 to 0.71272 and 0.511946 to 0.512073, respectively, indicating that the ore-forming material originates from the crust. Based on the ore-forming characteristics, it is proposed that Ca may be primarily leached from the strata formation, while F may predominantly originate from magmatic–hydrothermal solutions. The formation of fluorite deposits is closely related to the transition of the Central Altyn-Tagh Block and Qaidam Block from a compressional orogenic environment to an extensional tectonic environment.
1. Introduction
Fluorite (CaF2), serving as the primary natural source of fluorine, is predominantly utilized in the production of anhydrous hydrogen fluoride (AHF). Aqueous hydrogen fluoride derived from AHF constitutes the principal feedstock for synthesizing most fluorine-containing chemicals, notably refrigerants and fluoropolymers. Additional industrial applications of fluorite include its use as a flux in steelmaking, a raw material in cement production and glass manufacturing, a component in enamel formulations, an agent in iron and steel foundry operations, and a constituent in welding rod coatings [1,2,3,4,5,6]. The accelerated advancement of high-technology industries has generated escalating demand for fluorite resources, necessitating intensified exploration efforts to identify new fluorite deposits.
According to data published by the U.S. Geological Survey, by the end of 2024, the global fluorite reserves were estimated to be 320 × 106 t, with nearly half of the reserves distributed in Mexico, China, and South Africa [6]. In China, the identified fluorite resources were mainly distributed in Inner Mongolia, Jiangxi, Zhejiang, Hunan, Fujian, and Henan provinces [2]. These fluorite deposits are predominantly situated in the central and eastern regions of China. In recent years, substantial advancements have been made in the exploration of fluorite mineral resources within the Altyn-Tagh Orogenic Belt of northwestern China, including the Kaerqiaer, Kumutashi, and Xiaobaihegou deposits [5,7,8,9,10].
This study presents the discovery and geological characteristics of the Xiaobaihegou fluorite deposit in the western segment of the Altyn-Tagh Orogen. Geochemical analyses, fluid inclusion studies, and C-H-O-Sr-Nd isotopic systematics were employed to characterize the deposit genesis, decipher the ore-forming processes in detail, and provide insights for exploring analogous large-scale fluorite deposits in China.
2. Geological Background
The Altyn-Tagh Orogen lies at the junction of Xinjiang, Qinghai, and Gansu provinces, bordering the Tarim, Kunlun, Qaidam, North Qaidam, and North Qilian regions (Figure 1a,b). The Altyn-Tagh Orogen preserves a complex tectonic history encompassing Neoproterozoic extensional and collisional events, Early Paleozoic oceanic plate subduction and continental collision, as well as Mesozoic to Cenozoic faulting activities [11,12,13,14,15,16,17].
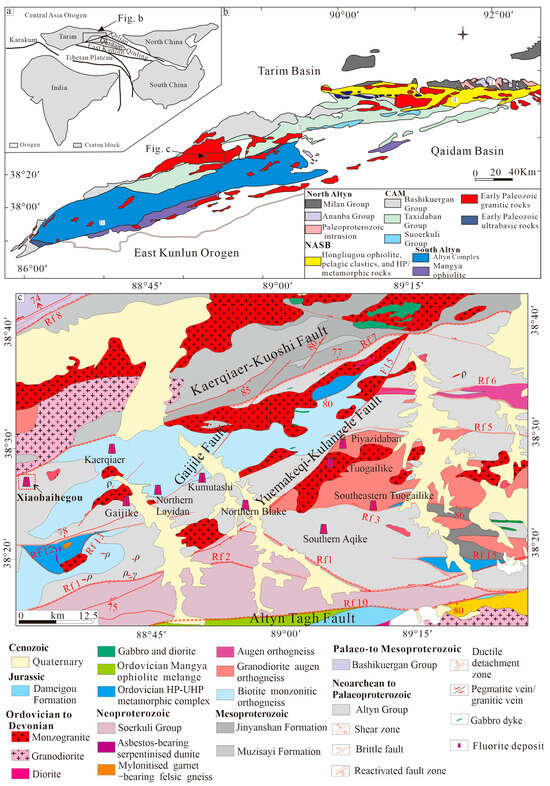
Figure 1.
Geological and tectonic map of the Altyn-Tagh Orogen (a,b), and geological map of the giant Kaerqiaer fluorite ore belt (c) (modified after [5,13,16]).
Through comprehensive regional geological, geochemical, and geochronological investigations, the Altyn-Tagh Orogen has been categorized into the North Altyn Terrane, Hongliugou-Lapeiquan Ophiolitic Melange, Middle Altyn Terrane, Jianggasiyi-Bashwak High-Pressure Accretionary Complex, and Apa-Mangya Ophiolitic Melange [16,18,19] (Figure 1b). The Xiaobaihegou fluorite deposit is situated in the northwestern part of the Altyn-Tagh Orogen, approximately 75 km southeast of Ruoqiang Town, and falls within the Middle Altyn Terrane subdivision (Figure 1c).
The strata in the Middle Altyn Terrane primarily consist of the Altyn Group, Bashikuergan Group, Taxidaban Group, and Suoerkuli Group. The Altyn Group is composed of biotite–plagioclase gneiss, garnet–sillimanite–biotite gneiss, monzonitic quartz schist, and plagioclase amphibolite. The Bashikuergan Group primarily consists of gray biotite quartz schist, two-mica quartz schist interbedded with grayish-white marble, quartzite, plagioclase amphibolite, and phyllite. The Taxidaban Group is characterized by grayish-white medium-thick bedded quartzite, two-mica quartz schist, phyllite, albite quartz schist, siltstone, and slate. The Suoerkuli Group is predominantly composed of carbonate rocks, mainly including marble, dolomite, and limestone. The Xiaobaihegou area is characterized by several significant regional tectonic activities that are complex and long-lived. These activities involve various orogenic events dating back to the Archaean, accompanied by records of widespread Neoproterozoic and Paleozoic intermediate to felsic magmatism. In the northern part of the Xiaobaihegou fluorite deposit, the belt is bounded by the Kaerqiaer-Kuoshi Fault, with the Gaijile, Yuemakeqi-Kulangele, and Altyn-Tagh Faults occurring sequentially to the south. The Kaerqiaer-Kuoshi Fault, a structure genetically linked to fluorite mineralization, trends northeast for approximately 70 km. It cuts through Paleozoic intermediate-to-felsic plutons and displays a sinuous surface morphology, indicating its multi-phase activity history (Figure 1c). Extensional secondary faults are pervasively developed between these regional faults. The Xiaobaihegou fluorite deposit is spatially associated with granites. The Altyn-Tagh Terrane has undergone multiple tectonic events, accompanied by the emplacement of felsic-to-intermediate plutons during the Neoproterozoic, Palaeozoic, and Mesozoic eras [5,10].
3. Geology of the Xiaobaihegou Fluorite Deposit
In the Xiaobaihegou fluorite deposit, most fluorite veins are hosted in biotite–plagioclase gneiss, with alkali-feldspar granite dykes frequently associated in the vicinity of these fluorite veins. The alkali-feldspar granite dykes and fluorite veins intrude into NE- and ESE-trending faults that cut through Neoarchaean to Palaeoproterozoic gneisses within the Altyn Complex (Figure 2). These units are overlain by Quaternary eluvium and diluvium exposed in gullies. In the mining area, the exposed intrusive rocks are predominantly alkali-feldspar granite, which shows a close spatial association with fluorite veins.
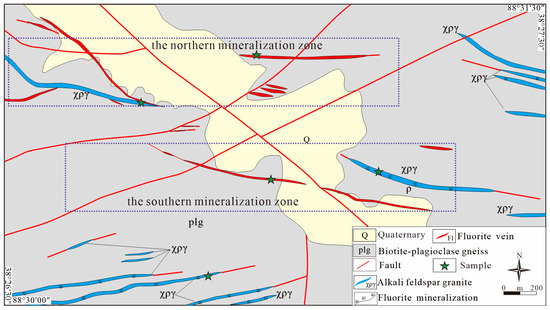
Figure 2.
Simplified geological map of the Xiaobaihegou fluorite deposit.
The mining area contains three major faults: two striking in the NE direction and one striking in the NW direction. Fluorite veins are closely associated with these three faults. The southern zone extends approximately 2.5 km in length and 0.4 km in width, striking northeast. The northern mineralization zone is about 0.4 km wide and 1.7 km long, trending nearly east (Figure 2). The fluorite ore body strikes nearly east–west, with a northerly dip of 30–40°. The ore bodies generally occur in lenticular, vein-like, and lenticular shapes, exhibiting good continuity with minor variations in attitude. They contain a few internal interlayers, exhibit bulging and constriction phenomena, and locally display branching and recombination. The overall morphology of the ore bodies is moderately complex.
The deposit is characterized by the occurrence of high-grade ore, with CaF2 grades exceeding 30% and locally reaching over 90%. The fluorite ore mainly occurs as veinlets filling the contact zone between alkali-feldspar granite veins (Figure 3a–e) and biotite–plagioclase gneiss. The ore types are dominated by massive and layered ores, with fluorite as the main mineral, accompanied by locally occurring calcite and minor quartz. Fluorite is white, green, purple, purple black, and so on. The ore exhibits a coarse-grained structure, characterized by euhedral-to-subhedral granular textures (Figure 3f–h). The main industrial types of ore are CaF2 and CaF2-CaCO3.

Figure 3.
Field, ore hand specimen, and micrographic photos of the Xiaobaihegou fluorite deposit: (a,b,d,e) fluorite veins; (c) the drill cores of Xiaobaihegou fluorite deposit; (f) purple fluorite ore; (g) fluorite and calcite closely associated; (h) photomicrograph of fluorite vein; (i) biotite–plagioclase gneiss; (j) photomicrograph of biotite–plagioclase gneiss; (k) alkali-feldspar granite; (l) photomicrograph of alkali-feldspar granite; Cal—calcite; Fl—fluorite; Pl—plagioclase; Bt—biotite; Ep—epidote; Qz—quartz; Kfs—K-feldspar.
Ore-forming processes can be divided into two stages based on the types of ore. Early stage: Ores are predominantly massive, mainly distributed in the central part of the ore veins (Figure 3f). They exhibit good crystallization with a coarse-grained texture. Late stage: Ores are dominated by brecciated and stockwork types, occurring in the marginal parts of the ore veins (Figure 3g). A gradual transition from massive to brecciated textures is observed from the center toward both sides (Figure 3e). The ores exhibit moderate crystallization, predominantly with euhedral–subhedral, anhedral granular, and cataclastic textures.
The country rocks of the fluorite ore bodies are biotite–plagioclase gneiss and alkali-feldspar granite (Figure 3i–l). The gneiss has a fine-grained lamellar granular structure, mainly composed of quartz, plagioclase, biotite, and microplagioclase, with the remainder comprising sphene and other minor minerals. The quartz particle size ranges from 0.06 to 2.80 mm; it is heteromorphic granular, with the long axis slightly oriented. The plagioclase particle size ranges from 0.10 to 1.60 mm; it is heteromorphic granular, with cluster twin crystals, sericitization on the surface, and a slightly oriented distribution along the long axis. The biotite particle size ranges from 0.04 to 1.00 mm, and it is scaly and sheet-like with a directional distribution. Microplagioclase has a particle size of 0.10–0.20 mm, with lattice twinned, and its long axis is slightly oriented among the plagioclase grains (Figure 3i,j).
The granite exhibits a medium-fine-grained subhedral granular texture and massive structure, predominantly composed of alkali feldspar, plagioclase, quartz, and biotite, with accessory minerals including apatite. The alkaline feldspar has a grain size of 0.9–5.0 mm, occurring as subhedral platy crystals with carlsbad twinning, grid twinning, or perthitic texture, and is predominantly microcline. The plagioclase has a grain size of 0.5–2.0 mm, occurs as subhedral platy crystals, and exhibits a reticulate edge texture. The quartz has a grain size of 0.5–4.5 mm, occurring as anhedral granular and elongated prismatic crystals. The biotite has a grain size of 0.4–3.5 mm, occurs as lamellar crystals and has undergone sericitization and chloritization (Figure 3k,l).
The alteration in biotite–plagioclase gneiss shows multiple-stage alteration superposition. Pre-ore alterations are primarily manifested as K-feldspathization and biotitization, while ore-stage alterations include epidotization, chloritization, carbonatization, fluoritization, followed by sericitization, pyritization, etc. K-feldspathization is the most widely distributed alteration type in the mining area, with K-metasomatism occurring during the emplacement of alkali-feldspar granite magma. It has indistinct boundaries and a diffuse distribution, with a bandwidth of 1–2 m, characterized by percolation–diffusion-dominated metasomatism, where plagioclase and other minerals in metamorphic rocks form K-feldspar through K-metasomatism. Silicification is mainly manifested as silicified quartz filling and cementing country rock breccia in structural fracture zones. During the fluorite mineralization stage, the country rock alterations in alkali-feldspar granite are primarily epidotization, chloritization, carbonatization, fluoritization, and kaolinization. In country rock marble, alteration phenomena caused by fluorite–calcite veins filling along fractures are commonly observed.
4. Sampling and Analytical Methods
All samples were obtained from field outcrops, drilling cores, and underground mine workings throughout the mining area. Over 100 doubly polished sections were prepared to facilitate microscopic observation and research on fluid inclusion. Fluorite and calcite separation was conducted at Xi’an Kuangpu Geological Exploration Technology Co., Ltd. (Xi’an, China). The separated fluorite and calcite grains were carefully selected for analyses of Sr-Nd isotopes, C-H-O isotopes, and trace elements.
4.1. Zircon U-Pb Geochronology
This study conducted zircon U-Pb dating on alkali-feldspar granites from the Xiaobaihegou fluorite deposit. Zircon grains were isolated via conventional electromagnetic and heavy fluid separation techniques at Xi’an Kuangpu Geological Exploration Technology Co., Ltd. Subsequent handpicking was performed under a binocular microscope. The selected zircon grains were embedded in epoxy resin, polished to expose their surfaces, and then photographed under transmitted and reflected light, followed by cathode luminescence (CL) imaging. CL images, integrated with transmission and reflected light photographs, were utilized to mark inclusion-free and fracture-free spots for laser ablation.
Zircon U-Pb isotopic dating was conducted via LA-ICP-MS at the Key Laboratory of Magmatism Mineralisation and Prospecting, Ministry of Natural Resources. A Geolas excimer solid sampling system served as the laser ablation device, coupled to a Thermo Fisher X Series II (Thermo Scientific, Bremen, Germany) quadrupole inductively coupled plasma mass spectrometer (ICP-MS). The laser parameters were set as a 32 μm beam diameter, 8 Hz frequency, and helium as the carrier gas. The American National Standard NIST610 was employed for equipment optimization and external standardization of trace element measurements [20]. Zircon standard 91,500 (206Pb/238U = 1065 Ma) was used for external calibration, with standard zircon GJ-1 serving as the monitoring sample [21]. The ICPMSDataCal 8.3 software was utilized for data processing [22], while age calculations and concordia diagrams were generated using Isoplot 3.0 [23]. Uncertainties for individual analyses are reported at the 1σ level, and errors on weighted mean ages are provided at 2σ (95% confidence level) [24].
4.2. Analysis of Major and Trace Elements
The major element analysis of the granite was conducted at the Xi’an Mineral Resources Survey, China Geological Survey, Xi’an, Shaanxi Province, China. Fresh samples were crushed and sieved through a 200-mesh sieve. After calcination, a fluxing agent was added to the samples, which were then melted to prepare glass disks. Major elements were analyzed using a pressed powder pellet method with an X-ray fluorescence spectrometer, with a relative error of less than 5%.
Trace element geochemical analyses of collected fluorite and calcite samples were completed at Nanjing Jupu Geological Services Ltd., Nanjing, China. Initially, the samples were manually crushed into smaller particles and then purified under a binocular microscope. Purified grains were further ground in an agate mortar until they reached a powder fineness of less than 200 mesh. Trace and rare earth elements were analyzed using inductively coupled plasma mass spectrometry (ICP-MS), with analytical errors of less than 10%.
4.3. Fluid Inclusion Analysis
Fluid inclusion petrography and microthermometric analyses were performed using a Linkam THMS 600 (Linkam, Godalming, Surrey, UK) cooling–heating stage mounted on an Olympus BX53 microscope (Olympus Corporation, Tokyo, Japan) at the Key Laboratory of Western Mineral Resources and Geological Engineering, Ministry of Education, Chang’an University, Xi’an, Shaanxi Province, China. The study samples are primarily composed of fluorite and calcite from the Xiaobaihegou fluorite deposit, encompassing representative samples from various ore-forming stages and exhibiting distinct spatial characteristics. Firstly, the mineral samples were ground into about 0.2 mm thick inclusions, and the large primary inclusions at each stage were observed under a microscope. The micro-temperature measurement experiment and the determination of gas-phase composition by laser Raman spectroscopy were carried out. Before the experiment, temperature correction was performed by pure H2O inclusion (0 °C), pure CO2 inclusion (−56.6 °C), and critical H2O inclusion (374 °C). The measured temperature range is −195 ~ +600 °C, and the analysis accuracy is ±0.2 °C, <30 °C; ±1 °C, <300 °C; ±2 °C, <600 °C. In the freezing–heating process, the temperature rise and fall rates were set to not exceed 20 °C/min, and the rate was reduced to less than 1 °C/min near the phase transition point. Laser Raman probing for single fluid inclusion composition analysis was conducted at the Metallogenesis and Dynamics Laboratory of Chang’an University. The analysis was conducted using a LabRAM HR Evolution, a next-generation high-resolution Raman spectrometer manufactured by HORIBA (Kyoto, Japan). The experiment was conducted under controlled conditions of 23 °C and 65% relative humidity.
4.4. Isotope Analysis
Calcite single mineral with a purity of more than 99% was selected for carbon and oxygen isotopic measurement, and it was fully ground to below 200-mesh powder. The carbon and oxygen isotope analysis was completed at the State Key Laboratory of Continental Dynamics, Northwest University. The isotopic compositions of calcite were determined by a Delta V Advantage stable isotope mass spectrometer from Thermo Fisher. The brief analysis steps are as follows: Weigh an appropriate amount of dried to constant weight calcite sample powder (150–250 μg) into the reaction bottle, seal it with a lid with a silicone spacer, inject high-purity helium gas into the reaction bottle with a draining needle (the blowing time of each reaction bottle is about 30 s), and then empty the air in the reaction bottle successively. Following emptying, 2 to 3 drops of saturated phosphoric acid were added manually to the reaction vessel. The reaction was conducted at 70 °C for approximately 1 h. After a period of equilibration, CO2 generated from the reaction of saturated phosphoric acid with carbonates was separated from impurity gases using a chromatographic column. The purified CO2 was then directed for analysis. Using standard samples for quality control, the test accuracy of δ13C and δ18O was better than ±0.1‰ and ±0.2‰, respectively, as determined by repeated testing of standard substances.
H-O isotope analysis of fluorite inclusions was conducted at the State Key Laboratory of Continental Dynamics, Northwestern University, using a Thermo 253plus gas stable isotope mass spectrometer (Thermo Scientific, Bremen, Germany). Oxygen isotope analysis employed the BrF5 method: oxygen was generated, collected in a vacuum using a sample tube packed with 5A molecular sieves, and its δ18O value was measured on the 253plus mass spectrometer. The external precision for standard samples was better than ±0.2‰, referenced to V-SMOW, with a single-sample internal precision of 0.05‰. For hydrogen isotope analysis, the explosive method was used to extract water and produce hydrogen. Samples were baked in a high-temperature (1420 °C) cracking furnace (Flash EA, Thermo) filled with glass carbon particles. Upon cracking and releasing the mineral inclusion water, H2 and CO formed via instantaneous reaction with glass carbon, transported by high-purity helium (5N) through a chromatographic column to the mass spectrometer (253plus, Thermo) for H2 isotope ratio (δD) determination. The testing precision for international standard materials (polyethylene, IAEA-CH-7, δDVSMOW = −100.3‰) was better than 1‰.
Sr and Nd isotope chemical pretreatment and mass spectrometry analyses were conducted at Nanjing Poly Spectrum Detection Technology Co., Ltd. The contents of Sr and Nd, along with Sr isotope ratios, were calculated via the isotope dilution method. The 87Sr/86Sr and Nd isotope ratios were determined on a Nu Plasma II MC-ICP-MS. During the measurements, a 86Sr/88Sr ratio of 0.1194 was used for internal calibration of instrumental mass fractionation, with the Sr isotope standard NIST SRM 987 serving as the external standard to correct for instrumental drift [25]. For Nd isotopes, 146Nd/144Nd = 0.7219 was applied for internal calibration, and the international standard material JNdi-1 was used as the external standard to correct instrumental drift.
5. Results
5.1. Zircon U-Pb Ages
Zircon grains within the alkali-feldspar granite samples exhibit a columnar habit, featuring a long-axis diameter ranging from 50 to 100 μm and a length-to-width ratio varying between 1:1.2 and 1:3. Their cathodoluminescence (CL) imaging reveals typical oscillatory zoning patterns (Figure 4a). The results of the zircon U-Pb isotopic analyses are documented in Table 1, indicating that the thorium (Th) and uranium (U) contents range from 1010 to 4182 ppm and 2189 to 8068 ppm, respectively. The Th/U ratios (0.32–0.65) all exceed 0.1, which is characteristic of a magmatic origin. Among the analytical data, 16 measurement points plot on or near the concordia curve (Figure 4b), with spot ages ranging from 481 to 484 million years (Ma). The weighted mean age is 482.3 ± 4.1 Ma (n = 16, MSWD = 0.016), which is interpreted to represent the emplacement time of the granite (Figure 4b,c).
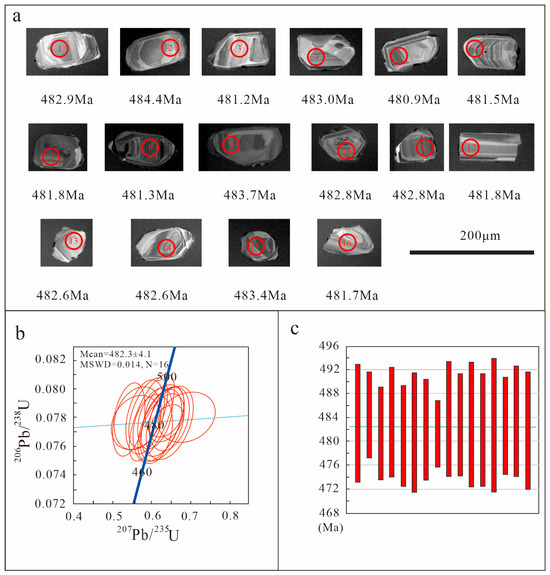
Figure 4.
Alkali-feldspar granite zircon CL images (a), U-Pb concordia (b), and weighted 206Pb/238U average age (c) diagram of the alkali-feldspar granite from the Xiaobaihegou fluorite deposit.

Table 1.
LA-ICP-MS zircon U-Pb isotopic data of the alkali-feldspar granite in the Xiaobaihegou fluorite deposit.
5.2. Major and Trace Elements
Table 2 presents the geochemical results of rare earth elements (REE) for fluorite and calcite samples from the Xiaobaihegou deposit. The corresponding chondrite-normalized REE patterns are illustrated in Figure 5.
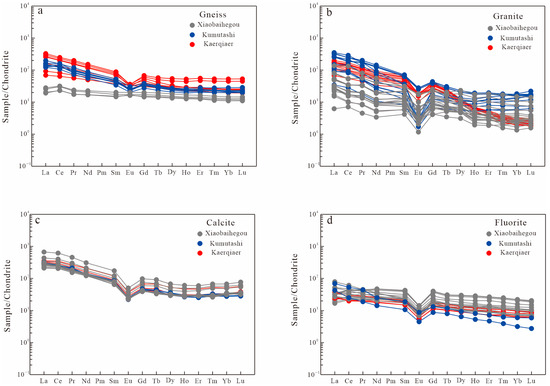
Figure 5.
Chondrite-normalized REE patterns of fluorite deposits from the Xiaobaihegou area (the data in Figure 5a is derived from refs. [5,7,8,9,10]). (a)—REE patterns of gneiss; (b) REE patterns of granite; (c) REE patterns of calcite; (d) REE patterns of fluorite.
The total rare earth element (ΣREE) contents of early-stage fluorite range from 91.5 to 97.3 ppm, whereas those of late-stage fluorite range from 45.1 to 79.5 ppm. Early-stage fluorite from the Xiaobaiheigou deposit exhibits LREE/HREE ratios, (La/Yb)N values, and δEu values of 2.42–7.49, 1.44–9.66, and 0.34–0.46, respectively. In contrast, late-stage fluorite from the deposit has LREE/HREE ratios, (La/Yb)N values, and δEu values of 2.80–6.09, 1.77–7.14, and 0.36–0.43, respectively. The ΣREE concentrations in calcite range from 1465 to 1579 ppm. The calcite’s LREE/HREE ratios, (La/Yb)N, and δEu in Xiaobaihegou deposit range from 12.5~13.5, 5.59~8.65, and 0.38~0.43, respectively.
The REE geochemical characteristics of fluorite in the Xiaobaihegou deposit reveal that the REE distribution patterns of fluorite and calcite are analogous to those of alkali-feldspar granite [9,10]. Both exhibit LREE-enriched patterns with distinct negative Eu anomalies (Figure 5), indicating that the REE signatures of fluorite and calcite in the fluorite ore belt likely inherit from the source rock.
Table 3 presents the results of major elements for granite samples from the Xiaobaihegou deposit. The Xiaobaihegou granites are characterized by high SiO2 (73.08%–75.30%), high K2O (3.91%–7.76%), and high alkali contents (K2O + Na2O = 8.33%–10.18%), coupled with low CaO (0.43%–1.2%) and low MgO (0.10%–0.69%). The ΣREE concentrations in granite range from 12.7 to 98.3 ppm. The granite’s LREE/HREE ratios, (La/Yb)N, and δEu in Xiaobaihegou deposit range from 2.08~5.81, 0.63~4.83, and 0.18~0.68, respectively. They have an A/CNK ratio of 0.6–0.75 and are further distinguished by depletion in Sr, Eu, Ba, Ti, and P and enrichment in high-field-strength elements (HFSEs), thus exhibiting characteristics typical of A-type granites [26].

Table 2.
Trace (ppm) element compositions of the fluorites and calcites at the Xiaobaihegou fluorite deposit.
Table 2.
Trace (ppm) element compositions of the fluorites and calcites at the Xiaobaihegou fluorite deposit.
| Sample | XBH-1 | XBH-2 | XBH-3 | XBH-4 | XBH-5 | XBH-6 | XBH-7 | XBH-8 | XBH-9 | XBH-10 | XBH-11 | XBH-12 | XBH-13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early Fluorite | Early Fluorite | Early Fluorite | Early Fluorite | Early Fluorite | Late Fluorite | Late Fluorite | Late Fluorite | Late Fluorite | Late Fluorite | Late Fluorite | Late Fluorite | Late Fluorite | |
| Cs | 0.01 | 0.02 | 0.08 | 0.03 | 2.29 | 0.09 | 0.01 | 0.01 | 0.01 | 1.06 | 0.25 | 0.31 | 0.35 |
| Pb | 3.35 | 4.78 | 5.69 | 5.78 | 26.9 | 5.78 | 5.67 | 2.25 | 4.96 | 17.5 | 3.11 | 1.99 | 9.18 |
| Rb | 0.23 | 0.14 | 0.19 | 0.23 | 49.8 | 2.78 | 0.46 | 0.57 | 0.13 | 23.2 | 5.46 | 6.22 | 3.70 |
| Ba | 1.12 | 1.35 | 2.18 | 1.08 | 92.1 | 2.86 | 31.4 | 0.93 | 1.18 | 40.7 | 12.3 | 11.6 | 5.97 |
| Th | 0.24 | 0.19 | 0.10 | 0.09 | 2.20 | 0.65 | 0.28 | 0.22 | 0.07 | 1.29 | 0.59 | 0.51 | 2.70 |
| U | 0.25 | 0.26 | 0.19 | 0.33 | 1.51 | 0.71 | 0.64 | 0.69 | 0.53 | 2.13 | 1.10 | 0.61 | 1.85 |
| Ta | 0.15 | 0.14 | 0.08 | 0.09 | 0.04 | 0.20 | 0.12 | 0.09 | 0.18 | 0.04 | 0.01 | 0.01 | 0.01 |
| Nb | 0.19 | 0.18 | 0.16 | 0.18 | 3.48 | 0.55 | 0.59 | 0.25 | 0.28 | 4.82 | 1.29 | 0.47 | 3.79 |
| Zr | 4.04 | 2.97 | 1.79 | 1.57 | 8.75 | 10.9 | 10.8 | 4.83 | 1.27 | 4.20 | 0.73 | 0.84 | 0.79 |
| Hf | 0.36 | 0.32 | 0.27 | 0.26 | 0.26 | 0.38 | 0.38 | 0.25 | 0.15 | 0.12 | 0.02 | 0.03 | 0.02 |
| Ti | 0.00 | 0.00 | 0.00 | 22.3 | 95.0 | 0.00 | 918 | 23.4 | 0.00 | 53.4 | 16.1 | 15.4 | 8.08 |
| Sr | 358 | 354 | 323 | 371 | 348 | 405 | 402 | 414 | 394 | 384 | 419 | 388 | 425 |
| La | 7.46 | 8.26 | 8.40 | 7.97 | 18.7 | 4.47 | 5.12 | 4.00 | 5.16 | 12.0 | 12.4 | 7.83 | 8.78 |
| Ce | 26.0 | 27.1 | 26.3 | 26.4 | 37.8 | 14.1 | 16.2 | 13.3 | 16.9 | 25.6 | 29.1 | 22.5 | 20.4 |
| Pr | 4.13 | 4.24 | 3.91 | 4.22 | 4.33 | 2.18 | 2.47 | 2.10 | 2.57 | 3.14 | 3.78 | 3.48 | 2.66 |
| Nd | 21.9 | 22.2 | 20.4 | 22.1 | 16.7 | 10.9 | 12.3 | 10.5 | 12.9 | 12.9 | 16.3 | 17.5 | 11.4 |
| Sm | 6.33 | 6.57 | 5.99 | 6.43 | 3.30 | 3.07 | 3.35 | 2.99 | 3.69 | 2.78 | 3.70 | 4.80 | 2.68 |
| Eu | 0.82 | 0.83 | 0.79 | 0.83 | 0.51 | 0.40 | 0.43 | 0.39 | 0.47 | 0.40 | 0.53 | 0.68 | 0.39 |
| Gd | 8.10 | 8.30 | 7.61 | 8.21 | 3.40 | 3.67 | 3.98 | 3.55 | 4.39 | 2.83 | 4.01 | 5.88 | 2.92 |
| Tb | 1.14 | 1.15 | 1.06 | 1.16 | 0.48 | 0.50 | 0.56 | 0.49 | 0.61 | 0.42 | 0.60 | 0.86 | 0.43 |
| Dy | 7.46 | 7.65 | 6.91 | 7.53 | 2.85 | 3.22 | 3.49 | 3.20 | 3.94 | 2.49 | 3.59 | 5.47 | 2.58 |
| Ho | 1.60 | 1.63 | 1.48 | 1.61 | 0.59 | 0.69 | 0.72 | 0.66 | 0.84 | 0.52 | 0.75 | 1.15 | 0.54 |
| Er | 4.33 | 4.44 | 4.05 | 4.35 | 1.71 | 1.86 | 1.98 | 1.82 | 2.27 | 1.50 | 2.21 | 3.40 | 1.57 |
| Tm | 0.64 | 0.63 | 0.58 | 0.62 | 0.23 | 0.27 | 0.29 | 0.27 | 0.33 | 0.20 | 0.30 | 0.46 | 0.21 |
| Yb | 3.71 | 3.77 | 3.49 | 3.75 | 1.39 | 1.61 | 1.71 | 1.62 | 1.96 | 1.20 | 1.85 | 2.70 | 1.26 |
| Lu | 0.50 | 0.52 | 0.47 | 0.52 | 0.19 | 0.22 | 0.24 | 0.22 | 0.27 | 0.17 | 0.26 | 0.38 | 0.18 |
| Y | 133 | 133 | 124 | 133 | 41.6 | 54.5 | 55.1 | 52.8 | 65.1 | 38.1 | 57.8 | 98.8 | 42.4 |
| ΣREE | 94.1 | 97.3 | 91.5 | 95.7 | 92.2 | 47.2 | 52.8 | 45.1 | 56.3 | 66.2 | 79.5 | 77.1 | 56.0 |
| LREEs | 66.6 | 69.2 | 65.8 | 68.0 | 81.3 | 35.1 | 39.9 | 33.3 | 41.7 | 56.9 | 65.9 | 56.8 | 46.4 |
| HREEs | 27.5 | 28.1 | 25.7 | 27.8 | 10.9 | 12.0 | 13.0 | 11.8 | 14.6 | 9.33 | 13.6 | 20.3 | 9.68 |
| LREE/HREE | 2.42 | 2.46 | 2.56 | 2.45 | 7.49 | 2.92 | 3.08 | 2.81 | 2.85 | 6.09 | 4.86 | 2.80 | 4.79 |
| (La/Yb)N | 1.44 | 1.57 | 1.73 | 1.52 | 9.66 | 1.99 | 2.15 | 1.77 | 1.89 | 7.14 | 4.81 | 2.08 | 4.98 |
| δEu | 0.35 | 0.34 | 0.36 | 0.35 | 0.46 | 0.36 | 0.36 | 0.36 | 0.36 | 0.43 | 0.42 | 0.39 | 0.42 |
| δCe | 1.15 | 1.12 | 1.13 | 1.12 | 1.03 | 1.11 | 1.12 | 1.13 | 1.14 | 1.02 | 1.04 | 1.06 | 1.04 |
| Sample | XBH-20 | XBH-21 | XBH-22 | XBH-23 | XBH-24 | XBH-25 | XBH-26 | XBH-27 | XBH-28 | XBH-29 | XBH-30 | ||
| Calcite | Calcite | Calcite | Calcite | Calcite | Calcite | Calcite | Calcite | Calcite | Calcite | Calcite | |||
| Cs | 0.06 | 0.05 | 0.05 | 0.06 | 0.05 | 0.05 | 0.06 | 0.06 | 0.09 | 0.04 | 0.61 | ||
| Pb | 64.8 | 56.8 | 54.6 | 64.6 | 53.2 | 52.8 | 53.1 | 57.8 | 62.5 | 60.2 | 56.5 | ||
| Rb | 0.10 | 0.49 | 0.25 | 0.13 | 0.65 | 0.35 | 0.62 | 0.82 | 1.02 | 0.10 | 3.83 | ||
| Ba | 48.3 | 41.4 | 53.9 | 57.0 | 62.7 | 71.4 | 51.2 | 44.1 | 30.7 | 40.9 | 26.1 | ||
| Th | 0.10 | 0.10 | 0.08 | 0.08 | 0.17 | 0.08 | 0.09 | 0.03 | 0.03 | 0.05 | 0.04 | ||
| U | 0.05 | 0.04 | 0.04 | 0.04 | 0.05 | 0.03 | 0.04 | 0.03 | 0.03 | 0.03 | 0.07 | ||
| Ta | 0.02 | 0.01 | 0.05 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | ||
| Nb | 0.02 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | ||
| Zr | 0.11 | 0.00 | 0.22 | 0.23 | 0.03 | 0.10 | 0.20 | 0.20 | 0.26 | 0.06 | 0.12 | ||
| Hf | 0.06 | 0.09 | 0.07 | 0.04 | 0.05 | 0.07 | 0.04 | 0.09 | 0.06 | 0.03 | 0.07 | ||
| Ti | 0.54 | 0.54 | 0.60 | 0.66 | 0.47 | 0.70 | 0.71 | 0.53 | 0.41 | 0.48 | 0.54 | ||
| Sr | 1198 | 1191 | 1173 | 1211 | 1193 | 1240 | 1188 | 1204 | 1173 | 1233 | 1220 | ||
| La | 51.8 | 63.4 | 62.9 | 60.8 | 67.8 | 56.4 | 49.9 | 65.1 | 63.0 | 68.9 | 74.1 | ||
| Ce | 131 | 146 | 142 | 150 | 150 | 143 | 125 | 148 | 143 | 153 | 168 | ||
| Pr | 15.6 | 15.6 | 15.8 | 17.0 | 16.7 | 15.9 | 14.5 | 16.7 | 16.0 | 17.0 | 17.6 | ||
| Nd | 59.2 | 60.5 | 60.6 | 62.8 | 63.3 | 60.1 | 56.5 | 62.8 | 61.7 | 64.8 | 67.0 | ||
| Sm | 10.1 | 11.5 | 11.1 | 12.4 | 11.9 | 11.5 | 9.95 | 12.1 | 11.4 | 11.2 | 11.6 | ||
| Eu | 1.36 | 1.30 | 1.35 | 1.38 | 1.46 | 1.35 | 1.34 | 1.41 | 1.44 | 1.41 | 1.32 | ||
| Gd | 8.61 | 8.53 | 8.03 | 8.21 | 8.48 | 8.91 | 8.50 | 8.63 | 8.42 | 8.47 | 8.35 | ||
| Tb | 1.27 | 1.25 | 1.36 | 1.37 | 1.38 | 1.36 | 1.32 | 1.33 | 1.29 | 1.40 | 1.44 | ||
| Dy | 7.79 | 7.59 | 7.65 | 8.30 | 7.53 | 8.21 | 7.47 | 7.79 | 8.03 | 7.49 | 7.91 | ||
| Ho | 1.62 | 1.55 | 1.62 | 1.70 | 1.72 | 1.75 | 1.68 | 1.73 | 1.69 | 1.75 | 1.67 | ||
| Er | 4.82 | 4.69 | 5.15 | 4.84 | 5.07 | 4.74 | 4.91 | 4.95 | 4.88 | 4.89 | 4.89 | ||
| Tm | 0.74 | 0.73 | 0.73 | 0.71 | 0.73 | 0.67 | 0.75 | 0.77 | 0.70 | 0.75 | 0.77 | ||
| Yb | 5.42 | 5.65 | 5.73 | 6.03 | 5.86 | 5.30 | 6.06 | 5.85 | 5.50 | 5.81 | 5.82 | ||
| Lu | 0.85 | 0.86 | 0.81 | 0.85 | 0.88 | 0.88 | 0.93 | 0.82 | 0.84 | 0.88 | 0.86 | ||
| Y | 41.7 | 41.4 | 41.5 | 42.3 | 42.6 | 42.6 | 43.0 | 42.9 | 42.3 | 42.3 | 42.6 | ||
| ΣREE | 1487 | 1509 | 1486 | 1536 | 1523 | 1549 | 1465 | 1531 | 1489 | 1569 | 1579 | ||
| LREEs | 1381 | 1401 | 1378 | 1423 | 1411 | 1440 | 1364 | 1418 | 1379 | 1456 | 1462 | ||
| HREEs | 106 | 108 | 108 | 113 | 113 | 109 | 101 | 113 | 110 | 114 | 117 | ||
| LREE/HREE | 13.1 | 13.0 | 12.8 | 12.6 | 12.6 | 13.2 | 13.5 | 12.6 | 12.5 | 12.8 | 12.5 | ||
| (La/Yb)N | 6.49 | 7.63 | 7.46 | 6.85 | 7.86 | 7.23 | 5.59 | 7.56 | 7.78 | 8.05 | 8.65 | ||
| δEu | 0.43 | 0.38 | 0.42 | 0.39 | 0.42 | 0.39 | 0.43 | 0.40 | 0.43 | 0.42 | 0.39 | ||
| δCe | 1.10 | 1.09 | 1.06 | 1.11 | 1.05 | 1.14 | 1.11 | 1.06 | 1.06 | 1.05 | 1.09 | ||
Notes: LaN/YbN values are La/Yb ratios normalized to chondrite values after [27], Eu/Eu* = 2 × w(Eu)N/[w(Sm)N + w(Gd)N], Ce/Ce* = 2 × w(Ce)N/[w(La)N + w(Pr)N].

Table 3.
Major element (%), trace element (ppm), and REE (ppm) analysis results of granites at the Xiaobaihegou fluorite deposit.
Table 3.
Major element (%), trace element (ppm), and REE (ppm) analysis results of granites at the Xiaobaihegou fluorite deposit.
| Sample | XB1 | XB2 | XB3 | XB4 | XB5 | XB6 | XB7 |
|---|---|---|---|---|---|---|---|
| Major element (wt.%) | |||||||
| SiO2 | 73.1 | 74.7 | 74.3 | 75.3 | 75.0 | 73.6 | 73.1 |
| Al2O3 | 15.0 | 14.7 | 14.6 | 14.0 | 14.3 | 12.6 | 15.0 |
| Fe2O3t | 1.68 | 0.76 | 0.99 | 0.79 | 0.88 | 1.73 | 0.85 |
| CaO | 0.43 | 0.54 | 0.62 | 0.63 | 0.59 | 1.20 | 0.54 |
| MgO | 0.10 | 0.14 | 0.14 | 0.14 | 0.13 | 0.69 | 0.13 |
| K2O | 3.91 | 5.07 | 4.51 | 4.20 | 4.12 | 7.76 | 6.57 |
| Na2O | 5.54 | 3.79 | 4.33 | 4.13 | 4.50 | 1.28 | 3.61 |
| P2O5 | 0.08 | 0.10 | 0.12 | 0.09 | 0.09 | 0.21 | 0.10 |
| TiO2 | 0.02 | 0.02 | 0.03 | 0.02 | 0.03 | 0.26 | 0.02 |
| LOI | 0.62 | 0.56 | 0.43 | 0.50 | 0.60 | 1.08 | 0.40 |
| Total | 99.9 | 99.8 | 99.6 | 99.3 | 99.6 | 99.3 | 99.9 |
| A/CNK | 0.60 | 0.67 | 0.64 | 0.64 | 0.63 | 0.75 | 0.68 |
| A/NK | 1.13 | 1.25 | 1.22 | 1.23 | 1.21 | 1.20 | 1.15 |
| ALK | 9.45 | 8.86 | 8.84 | 8.33 | 8.62 | 9.04 | 10.2 |
| Mg# | 10.6 | 26.7 | 21.9 | 26.0 | 22.6 | 44.1 | 23.3 |
| Trace element (ppm) | |||||||
| Bi | 1.65 | 0.22 | 0.45 | 0.23 | 0.39 | 0.10 | 2.63 |
| Li | 9.07 | 10.8 | 15.3 | 16.0 | 12.2 | 10.9 | 9.00 |
| Be | 5.93 | 9.01 | 8.51 | 6.82 | 8.17 | 1.09 | 7.25 |
| Sc | 1.81 | 1.32 | 0.81 | 0.76 | 0.83 | 4.20 | 0.96 |
| Co | 1.60 | 2.61 | 2.02 | 1.92 | 2.01 | 5.45 | 1.96 |
| Cu | 3.48 | 6.08 | 3.48 | 2.77 | 3.89 | 4.58 | 2.33 |
| Zn | 8.04 | 34.8 | 42.5 | 29.7 | 30.8 | 31.7 | 43.2 |
| Ga | 16.7 | 20.6 | 18.0 | 19.3 | 20.5 | 13.5 | 23.4 |
| Rb | 279 | 475 | 427 | 372 | 396 | 297 | 498 |
| Sr | 57.7 | 21.1 | 16.6 | 20.8 | 24.9 | 80.6 | 19.0 |
| Zr | 23.8 | 17.1 | 17.8 | 16.1 | 18.8 | 105 | 10.8 |
| Nb | 12.1 | 17.5 | 16.0 | 11.9 | 15.3 | 5.11 | 21.1 |
| Cs | 9.56 | 22.9 | 14.1 | 11.5 | 12.2 | 6.97 | 18.6 |
| Ba | 77.9 | 76.8 | 61.5 | 91.4 | 84.4 | 670 | 75.7 |
| Hf | 1.22 | 1.29 | 1.09 | 0.97 | 1.16 | 3.48 | 0.62 |
| Ta | 2.94 | 1.73 | 1.59 | 1.11 | 1.44 | 0.35 | 2.01 |
| W | 20.0 | 1.17 | 1.13 | 0.83 | 1.12 | 0.75 | 1.26 |
| Tl | 1.55 | 2.01 | 1.74 | 1.53 | 1.64 | 1.42 | 2.18 |
| Pb | 36.5 | 37.9 | 35.1 | 31.1 | 31.0 | 27.1 | 32.2 |
| Th | 2.86 | 3.54 | 3.52 | 3.77 | 3.71 | 7.72 | 5.61 |
| U | 10.6 | 5.91 | 5.25 | 7.36 | 4.67 | 1.39 | 7.70 |
| La | 1.48 | 5.18 | 6.48 | 6.82 | 6.71 | 19.3 | 6.01 |
| Ce | 4.36 | 10.9 | 10.2 | 10.5 | 10.5 | 33.2 | 10.4 |
| Pr | 0.43 | 1.26 | 1.44 | 1.49 | 1.46 | 5.37 | 1.49 |
| Nd | 1.59 | 3.90 | 4.35 | 4.52 | 4.32 | 18.9 | 4.26 |
| Sm | 0.64 | 1.28 | 1.60 | 1.62 | 1.38 | 4.77 | 1.88 |
| Eu | 0.07 | 0.13 | 0.16 | 0.16 | 0.16 | 1.06 | 0.12 |
| Gd | 0.84 | 1.37 | 1.70 | 1.72 | 1.51 | 4.77 | 2.14 |
| Tb | 0.19 | 0.21 | 0.25 | 0.26 | 0.22 | 0.66 | 0.37 |
| Dy | 1.21 | 1.18 | 1.38 | 1.50 | 1.27 | 4.63 | 2.29 |
| Ho | 0.23 | 0.15 | 0.17 | 0.20 | 0.16 | 0.77 | 0.30 |
| Er | 0.67 | 0.45 | 0.47 | 0.52 | 0.48 | 2.43 | 0.78 |
| Tm | 0.11 | 0.06 | 0.06 | 0.07 | 0.06 | 0.30 | 0.10 |
| Yb | 0.76 | 0.42 | 0.45 | 0.50 | 0.45 | 1.86 | 0.67 |
| Lu | 0.10 | 0.06 | 0.07 | 0.07 | 0.07 | 0.26 | 0.09 |
| Y | 6.10 | 5.94 | 6.81 | 7.73 | 6.49 | 25.4 | 11.3 |
| ∑REE | 12.7 | 26.6 | 28.8 | 30.0 | 28.8 | 98.3 | 30.9 |
| LREEs | 8.57 | 22.7 | 24.2 | 25.1 | 24.5 | 82.6 | 24.2 |
| HREEs | 4.11 | 3.90 | 4.55 | 4.84 | 4.22 | 15.7 | 6.74 |
| LREE/HREE | 2.08 | 5.81 | 5.33 | 5.19 | 5.81 | 5.27 | 3.58 |
| (La/Yb)N | 0.63 | 4.00 | 4.67 | 4.42 | 4.83 | 3.36 | 2.91 |
| δEu | 0.28 | 0.30 | 0.30 | 0.29 | 0.34 | 0.68 | 0.18 |
| δCe | 1.31 | 1.03 | 0.80 | 0.79 | 0.81 | 0.78 | 0.84 |
Notes: Mg# = Mg/(Mg + Fe2+) × 100.
5.3. Fluid Inclusions
The ore samples from the mining area are abundant in fluid inclusions (FIs), making them ideal for petrographic and microthermometric studies of FIs. Based on phase relationships at 25 °C, optical observations of more than 300 inclusions identified three major types of FIs in Xiaobaihegou. Liquid-rich (L-type) inclusions are dominant (up to 90%) in host minerals across all mineralization stages. These two-phase L-type inclusions, with a vapor phase comprising 5–20 vol% of the total inclusion volume, are significantly more abundant than other inclusion types observed in the samples. They vary in size from 5 to 60 μm and commonly exhibit elongated, triangular, elliptical, negative crystal, or irregular shapes (Figure 6a,b).
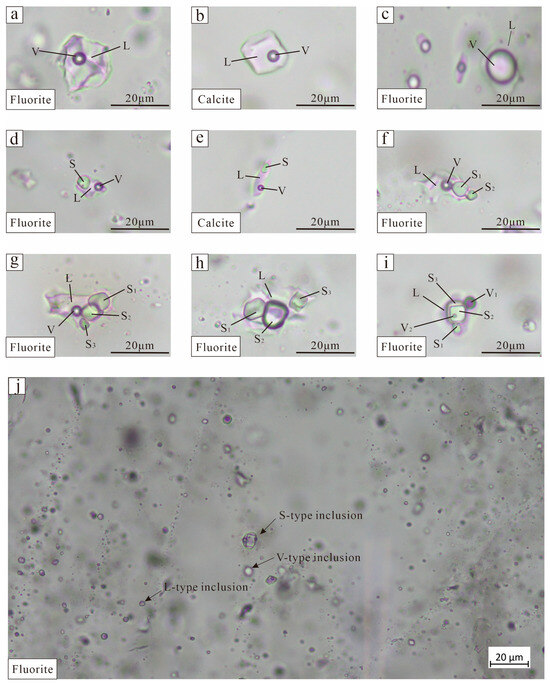
Figure 6.
Photomicrographs showing major fluid inclusion types in calcite and fluorite from the Xiaobaihegou district (V—vapor; L—liquid; S—solid). (a,b) Liquid-rich inclusions; (c) Vapor-rich inclusions; (d–i) Solid-bearing inclusions; (j) Photomicrograph of fluorite.
Vapor-rich two-phase (V-type) inclusions mostly occur as isolated entities in early-stage fluorite and calcite crystals, exhibiting rounded or regular morphologies (Figure 6c). They are less than 40 μm in size, with a vapor volume percentage ranging from 55% to 90%. Solid-bearing inclusions (S-type) are three-phase FIs that contain daughter minerals (Figure 6d–i).
Multiphase fluid inclusions are mostly found in purple–black and purple fluorite minerals, and a small amount can be seen in calcite and quartz. They are mostly elliptical or long strips, ranging in size from 2 to 120 μm, and can be roughly divided into two categories: those measuring 2 to 5 μm in size, grouped together, and those measuring 20 to 120 μm in dispersion.
Laser Raman microspectroscopy was employed to characterize the chemical composition of fluid inclusions. Multiphase daughter-mineral-bearing inclusions and CO2-rich inclusions were specifically targeted for analysis. Raman spectral signatures reveal the following diagnostic peaks: The characteristic Raman peak of fluorite is at 315.8 cm−1 (Figure 7a); for CH4, the characteristic peak is at 2914 cm−1 (Figure 7a); for H2O, the characteristic peak is at 3441 cm−1 (Figure 7b); for CO2, the characteristic peaks are at 1279 and 1382 cm−1 (Figure 7c); the daughter minerals contain gypsum, with a characteristic Raman peak at 1018 cm−1 (Figure 7d). Due to the lack of distinctive Raman scattering signals for halide salts, the presence of NaCl and KCl was inferred primarily from petrographic observations. NaCl daughter minerals are colorless, cubic, and homogeneous, with a particle size of about 5–10 μm. KCl daughter mineral is colorless or light green, homogeneous, cubic crystal, but because of corrosion, the edge of the daughter mineral is often ground into a rounded shape, with a particle size of about 5–50 μm; gypsum daughter mineral is colorless, plate, with a particle size of about 2–10 μm. In general, the appearance of the above daughter minerals indicates the existence of a high-salinity fluid.
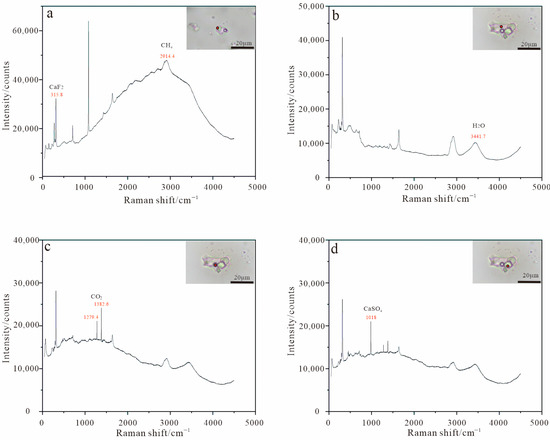
Figure 7.
Laser Raman spectra of fluid inclusions in calcite and fluorite of the Xiaobaihegou fluorite deposit. (a,c) vapor phases, (b) liquid phases, (d) solid phase.
Based on petrological characteristics, these inclusions formed synchronously with the host mineral, distributed regularly along crystal growth textures, and they displayed homogeneous compositional and physicochemical properties. A total of over 100 primary FIs were identified via microscopic observation and freezing–heating experiments [28] (Figure 8). Microthermometric data are presented in Table 4. Notably, the homogenization temperatures and salinities of FIs in different fluorite deposits within the Kaerqiaer district exhibit consistent characteristics. Liquid-rich (L-type) FIs were homogenized to the liquid phase, whereas vapor-rich (V-type) FIs were homogenized to the vapor phase.
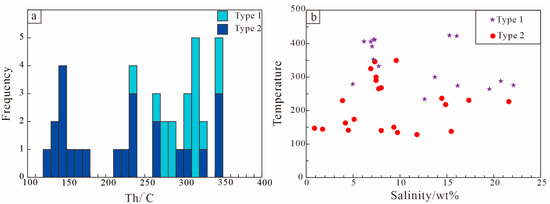
Figure 8.
Histograms of homogenization temperatures (Th) (a) and temperature–salinity diagrams (b) of fluid inclusions in calcite and fluorite of the Xiaobaihegou fluorite deposit (Type 1: early ore-forming stage; Type 2: late ore-forming stage).

Table 4.
Microthermometric data for primary fluid inclusions of the fluorite deposits in the Kaerqiaer district.
As mentioned above, the ore-forming process is subdivided into two stages: an early stage and a late stage. Early-stage massive ores are characterized by gas-rich two-phase inclusions and three-phase inclusions containing daughter crystals, with homogenization temperatures ranging from 235 to 426 °C, salinities of 28.59–42.40 wt.% NaCl eq., and densities of 0.93–1.03 g/cm3. In the late stage, brecciated and stockwork ores host predominantly liquid-rich two-phase and gas-rich two-phase fluid inclusions. These inclusions exhibit homogenization temperatures ranging from 129 to 350 °C, salinities of 0.88 to 21.61 wt% NaCl eq., and densities of 0.61–0.93 g/cm3. For individual deposits in the Kaerqiaer district, both homogenization temperatures and salinities show a gradual decrease from the early to late stages.
5.4. C-H-O Isotopes Geochemistry
The carbon and oxygen isotopic compositions of calcite samples obtained from the Xiaobaihegou are compiled in Table 5. The δ13CV-PDB and δ18OV-SMOW values of the calcite in Xiaobaihegou vary from −9.53‰ to −3.75‰ (mean −5.31‰) and from 9.76 to 17.45 (mean 12.90), respectively.

Table 5.
Carbon and oxygen isotopic compositions of calcites from the Xiaobaihegou fluorite deposit.
The obtained hydrogen and oxygen isotopic compositions of fluorite from the Xiaobaihegou are presented in Table 6. The δ18OV-SMOW and δDV-SMOW values of the fluorite in Xiaobaihegou vary from −9.4‰ to 0.5‰ (mean −3.3‰) and from −91.1‰ to −47.9‰ (mean −76.5‰), respectively.

Table 6.
Oxygen and hydrogen isotopic compositions of fluorites from the Xiaobaihegou fluorite deposit.
5.5. Sr-Nd Isotopic Compositions
The Rb-Sr isotopic data of fluorite from the Xiaobaihegou deposit are presented in Table 7. The 87Sr/86Sr ratios of fluorite from this deposit range from 0.71033 to 0.71272, with a mean value of 0.71112. The 143Nd/144Nd ratios vary from 0.511946 to 0.512073, with a mean of 0.512004.

Table 7.
Sr-Nd isotopic composition of fluorites from Xiaobaihegou deposit.
6. Discussion
6.1. Timing of Fluorite Mineralization and Geodynamic Setting
LA-ICP-MS zircon U-Pb dating of the alkali-feldspar granite in the Xiaobaihegou fluorite mining area in this study shows that the rock-forming age is 482.3 ± 4.1 Ma, indicating that the rock was formed in the early Paleozoic Ordovician. Generally, the metallogenic age of magmatic–hydrothermal fluorite ore is later than the formation age of the ore-forming rock body. Flesh-red alkali-feldspar granite vein bodies are found in all the fluorite mining areas in the Kaerqiaer area. Fluorite mineralization mainly occurs near the contact zone between the inner and outer rock bodies, and fluorite–calcite veins are interspersed in alkali-feldspar granite vein bodies (Figure 3b–d). The alkali-feldspar granite exhibits intense hydrothermal alteration, characterized by carbonatization, fluoritization, silicification, and other associated alteration types. These results indicate a close genetic relationship between the alkali-feldspar granite and fluorite mineralization in this area. Meanwhile, studies on the Kumutashi fluorite deposit indicate that the alkali-feldspar granite in the mining area is a fluorine-rich rock with an average w(F) > 0.1%. Its magmatic emplacement age is constrained to 450 ± 2.7 Ma [10], which is consistent with the LA-ICP-MS U-Pb age of apatite from fluorite ore bodies (448 ± 27 Ma) [5]. However, no magmatic rocks or wall-rock alterations associated with ~450 Ma magmatism (analogous to that in the Kumutashi area) have been identified in the study area, nor have late-stage dikes, hydrothermal veins with zircon ages of 450 Ma, or isotopic signatures of mantle/crustal melting during this stage been detected in our sampling and analysis. There is a 30 Ma age discrepancy between the study area and the Kumutashi region. Although the formation age of fluorite deposits is typically slightly postdated that of their host granites, a 30 Ma interval exceeds the expected temporal offset. We hypothesize that the mineralization timing of the Xiaobaihegou fluorite deposit is distinct from that of the Kumutashi deposit. Specifically, the Xiaobaihegou deposit likely formed at ~480 Ma, whereas the Kumutashi deposit formed at ~450 Ma. However, the granites from the Xiaobaihegou and Kumutashi fluorite deposits exhibit similar geochemical characteristics. They are characterized by high SiO2, high K2O, and high alkali contents, coupled with low CaO and low MgO; they are enriched in large ion lithophile elements (LILEs), relatively depleted in high field strength elements (HFSEs), with a high total rare earth element content (ΣREE), enriched in light rare earth elements (LREEs), relatively depleted in heavy rare earth elements (HREEs), and generally exhibit a strong negative Eu anomaly, thus exhibiting characteristics typical of A-type granites. Therefore, their similar granite properties may be one of the important reasons why both can form fluorite deposits.
Regionally, large-scale early Paleozoic magmatic rocks developed along the southwest margin of the Altyn-Tagh Orogen are the products of magmatic activity during the oceanic to continental transition between the Middle Altyn Block and the Qaidam Block [13,18,32,33]. Studies of regional ultra-high-pressure metamorphic rocks indicate that the peak metamorphic age is concentrated between 504 and 486 Ma, and the retrograde metamorphic age is ~450 Ma [13,18]. Studies of granites near Kaerqiaer show that monzonitic granites in the Paxialayidang area yield a zircon U-Pb age of 460 ± 4 Ma. These rocks formed in a tectonic environment transitioning from a compressive to an extensional system [32]. The zircon U-Pb age of the granitic rocks in the Qingshuiquan area is 451 ± 4 Ma, indicating that they were formed in an extensional tectonic regime [33]. In contrast, the mafic and ultramafic intrusions, dated at 468–454 Ma, suggest that the collisional orogeny had shifted to the extensional stage by this time [34]. All the above studies indicate that during the Middle and Late Ordovician, the Middle Altyn Block and Qaidam Block transitioned from a compressional orogenic regime to an extensional tectonic setting. The Kaerqiaer super-large fluorite belt thus represents the product of magmatic activity during this tectonic transition. In addition, large-scale pegmatite dyke swarms formed during the Early and Middle Ordovician collisional orogenic stage occur in the region. For example, the zircon U-Pb age of the ore-forming biotite monzonite in the Tugeman Li-Be rare metal deposit is 475–482 Ma. The U-Pb age of niobium–tantalite in ore-bearing pegmatite vein is 472 ± 8 Ma, and the U-Pb age of kainite is 468 ± 8.7 Ma [17,34,35]. In summary, the Early Paleozoic Caledonian orogeny represents a critical period for the mineralization of regional fluorite and Li-Be rare metal deposits.
6.2. Source of Ore-Forming Fluids
FIs within fluorite (the ore mineral) provide more direct and reliable constraints on ore-forming fluid characteristics while minimizing uncertainties [36,37]. Thus, this FIs study focused primarily on fluorite-hosted inclusions, with minor analysis of calcite-hosted inclusions. In the Kaerqiaer district, FIs are predominantly L-type, with minor V-type and S-type inclusions.
The homogenization temperatures and salinities of FIs in the early ore-forming stage of the Xiaobaiheigou deposit range from 235 to 426 °C and 28.59 to 42.40 wt.% NaCl eq., respectively, indicating a medium–high-temperature and medium–high-salinity fluid system. In the late stage, these parameters decrease to 129–350 °C and 0.88–21.61 wt.% NaCl eq., reflecting a medium–low-temperature and medium–low-salinity fluid regime [4].
For the Kaerqiaer deposit, liquid-rich FIs exhibit homogenization temperatures of 135–237 °C and salinities of 2.07–7.59 wt.% NaCl eq., indicating low-temperature and low-salinity hydrothermal fluids. Minor CO2-bearing three-phase fluid inclusions in fluorite exhibit homogenization temperatures of 240–359 °C and salinities of 2.58–3.39 wt.% NaCl eq., suggesting the presence of a moderate-temperature and low-salinity fluid end-member [7,8].
In the Kumutashi fluorite deposit, early-stage FIs have homogenization temperatures of 225.1–410.8 °C and salinities of 5.20–11.00 wt.% NaCl eq., indicating a medium–high-temperature and medium–low-salinity fluid. Late-stage FIs show lower homogenization temperatures (117.2–291.2 °C) and salinities (0.53–12.73 wt.% NaCl eq.), consistent with medium–low-temperature and low-salinity fluids [29].
Regionally, both homogenization temperature and salinity decrease from the early to late ore-forming stages. The early-stage fluids were NaCl-H2O-CO2 systems with medium-to-high temperatures and salinities, while the late-stage fluids evolved into NaCl-H2O-CO2 systems with medium-to-low temperatures and salinities.
In the Xiaobaihegou deposit, calcite occurs in coexistence with fluorite, both forming cross-cutting bands or veins. Hydrothermal-derived calcite is typically enriched in LREEs [38]. The calcite analyzed in this study exhibits LREE enrichment (Figure 5). Oxygen, carbon, and hydrogen isotope compositions are used to constrain fluid sources [39,40]. Carbon–oxygen isotopes in calcite indicate that the ore-forming fluids were predominantly of magmatic origin (Figure 9). For hydrogen–oxygen isotopes, δ18OV-SMOW and δDV-SMOW values of samples from the Kaerqiaer district (encompassing Xiaobaiheigou, Kumutashi, and Kaerqiaer deposits) plot between magmatic fluids and atmospheric precipitations (Figure 10), suggesting that the ore-forming fluid represents a mixture of magmatic water and atmospheric precipitation.
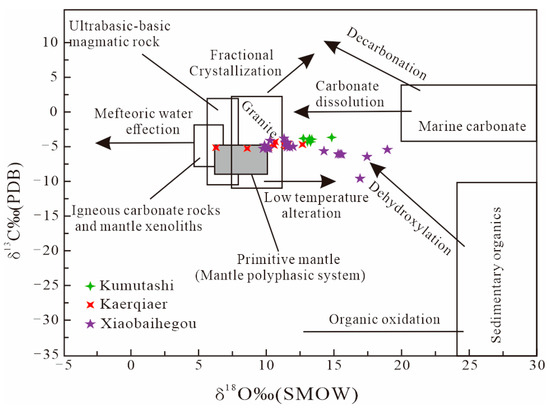
Figure 9.
δ18O versus δ13C diagram of calcites from the Kaerqiaer district. (The fields are after [40], and the data of the Kumutashi deposit and Kaerqaier deposit are derived from [5,7,8,9,10,29]).
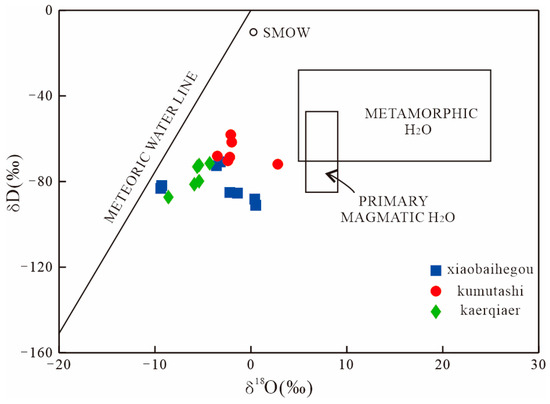
Figure 10.
Hydrogen and oxygen isotope composition of ore-forming fluids from Kaerqiaer district. (The fields are after [39], and the data of the Kumutashi deposit and Kaerqaier deposit are derived from [5,7,8,9,10,29]).
Regional fluorite mineralization is closely associated with alkali-feldspar granite, with ore bodies primarily developed in fault tectonic belts of the rock mass. Early-stage ore-forming fluids were characterized by medium-to-high temperatures, indicating they were predominantly magmatic water. The late-stage ore-forming fluids exhibited medium-to-low temperature characteristics due to the incorporation of substantial atmospheric precipitation, thus forming a mixed-fluid system of magmatic water and atmospheric precipitation.
In the Xiaobaihegou fluorite deposit, fluorite formed during the early mineralization stage has higher total rare earth element (REE) contents, whereas that formed during the late mineralization stage has relatively lower total REE contents (Table 2). The REE distribution patterns of fluorite from the early and late stages are similar, with both stages of fluorite precipitated from fluids evolved from the same parent magmatic–hydrothermal system. Consistent REE distribution patterns indicate a common fluid source, which is likely derived from the exsolution of the same alkali-feldspar granite magma. However, differences in ore textures and fluid inclusions between the early and late stages reflect the progressive evolution of the fluid over time, such as decreasing temperature and salinity. The Tb/La vs. Tb/Ca bivariate diagram, established based on studies of over 150 fluorite deposits worldwide, is used to determine the genetic origins of fluorite and identify water–rock reactions between ore-forming fluids and host rocks [41]. This diagram classifies fluorite deposits into three genetic types: (1) pegmatite (gas–liquid) origin; (2) hydrothermal origin; and (3) sedimentary genesis. Here, the Tb/Ca atomic ratio reflects the chemical environment during fluorite crystallization, carrying genetic information, while the Tb/La ratio indicates the degree of REE fractionation in ore-forming fluids, implying assimilative mixing of the liquids with surrounding rocks during mineralization.
Fluorite samples from the Xiaobaihegou deposit plot within the hydrothermal origin field (Figure 11), demonstrating that the fluorite deposits in this area formed through magmatic–hydrothermal processes. The significant variation in Tb/Ca ratios suggests that water–rock interactions occurred between ore-forming fluids and wall rocks. The fluorite samples plotted in Figure 11 include both early- and late-stage varieties from the Xiaobaihegou district. As expected, their data points are distributed along both the primary crystallization and remobilization trends. This is well illustrated in Figure 11: those of early fluorites lie along the primary crystallization trend, while late-stage fluorite data points align with the remobilization trend. Additionally, these fluorites are compared with samples from Iran’s Bozijan deposit and the United States’ Sierra deposit; both are hydrothermal fluorite deposits with analogous geochemical characteristics [42,43].
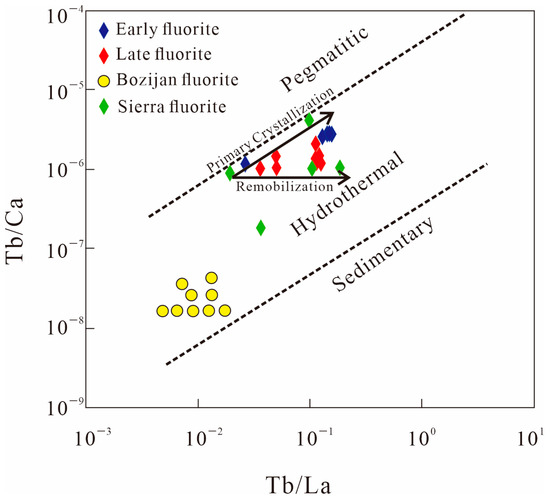
Figure 11.
Tb/Ca vs. Tb/La diagram for fluorite from the Xiaobaihegou deposit. Trends are taken from [41], fluorite data for Bozijan and Sierra are from [42,43].
Sr-Nd isotopic analyses of the Xiaobaihegou fluorite deposit reveal 87Sr/86Sr ratios ranging from 0.71033 to 0.71272 and 143Nd/144Nd ratios from 0.511946 to 0.512073, indicating crustal origin characteristics [44,45]. This is consistent with findings from the Kumutashi and Kaerqiaer fluorite deposits (Figure 12). Data in Table 7 reveal the Sr and Nd isotopic compositions of the ore veins. The measured radiogenic isotope characteristics closely reflect the source and are unlikely to have been significantly modified by wall-rock assimilation or hydrothermal overprinting. Initial isotopic values of the ore vein material show little variation and form a limited data cluster on conventional diagrams (Figure 12). These similarities indicate that the Xiaobaihegou, Kumutashi, and Kaerqiaer fluorite deposits were formed in the same tectonic setting and share a generally consistent source of ore-forming material.
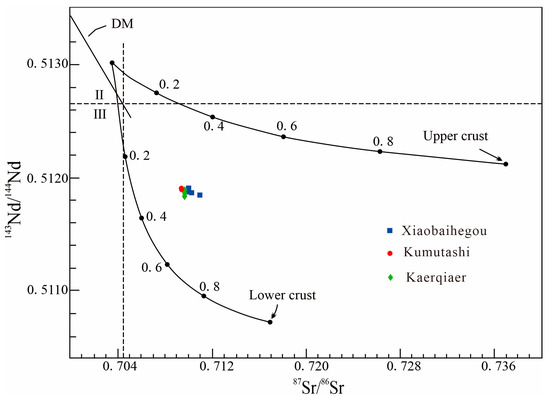
Figure 12.
87Sr/86Sr–143Nd/144Nd diagram of fluorite from the Xiaobaihegou area. (The fields are after [9], and the data of the Kumutashi and Kaerqaier deposit are derived from [9]).
6.3. Fluid Evolution and Precipitation Mechanisms
The principal mechanisms governing fluorite precipitation from ore-bearing hydrothermal fluids can be categorized as follows: (1) water–rock interaction between hydrothermal fluids and host lithologies; (2) thermobaric changes within the hydrothermal system; and (3) mixing of chemically distinct hydrothermal fluid populations [46]. Among these, water–rock interaction is proposed to be the dominant precipitation mechanism [47], as evidenced by typical fluorite deposits, including, but not limited to, the Xiaobeigou deposit in Inner Mongolia, the Yixian deposit in Liaoning Province, and the Wuyi deposit in Zhejiang Province. These deposits are characterized by medium–low-temperature, low-salinity, and low-density fluid regimes, where water–rock interaction plays a pivotal role in fluorite deposition [48].
Chinese fluorite deposits predominantly occur in association with medium- to low-temperature (homogenization temperatures generally <300 °C) and low-salinity hydrothermal systems [2,47,48,49,50,51,52,53,54,55,56,57,58,59]. For instance, the fluorite metallogenic belt in southeastern Sichuan and the Shuanghe barite–fluorite deposit in northeastern Guizhou exhibit narrow ranges of fluid inclusion homogenization temperatures, collectively indicative of low-temperature fluid characteristics. Upwelling ore-forming fluids, after enriching ore-forming elements, migrate to favorable stratigraphic horizons, where subsequent cooling induces fluorite precipitation [2,47,48,49,50,51].
Fluid mixing typically involves two end-member fluids: high-temperature/high-salinity and low-temperature/low-salinity, as documented in the Sumochagan Aobao fluorite deposit [2]. The presence of these dual-fluid end-members in the Xiaobaihegou fluorite deposit suggests a plausible role of fluid mixing in promoting fluorite precipitation.
Fluid inclusion studies reveal that, in the Xiaobaihegou fluorite deposit, primary fluid inclusions from the early mineralization stage have homogenization temperatures (Th) ranging from 235 to 426 °C, whereas those from the late stage exhibit lower Th values (129–350 °C), indicating a progressive cooling trend. The early ore-forming fluids are characterized by medium-to-high salinities (28.59–42.40 wt.% NaCl equivalent), which decrease to 0.88–21.61 wt.% NaCl equivalent in the late stage. Fluorite (CaF2) precipitates when the ion activity product (aCa2+ × aF−) exceeds its solubility product (Ksp) in the fluid, and in this study area, this process is primarily triggered by the following changes in fluid conditions: (1) Cooling of the ore-forming fluids. Fluorite solubility decreases significantly with decreasing temperature. During the early stage, fluids migrate at 235–426 °C, where Ca2+ and F− remain in solution due to high solubility; as these fluids ascend to shallower crustal levels (lower pressure) and cool to 129–350 °C via heat loss to wall rocks, their capacity to retain Ca2+ and F− diminishes. This cooling-driven reduction in solubility causes aCa2+ × aF− to exceed Ksp, thereby triggering fluorite precipitation. (2) Fluid mixing with meteoric water. Late-stage fluids exhibit lower salinities and Th values compared to the early stage, accompanied by shifts in H-O isotopes, which indicate mixing with meteoric water (with regional δ18O values of −9.4 to 0.5‰ and δD values of −91.1 to −47.9‰). The input of meteoric water dilutes the fluid, reduces salinity, and lowers temperature, further decreasing fluorite solubility; additionally, meteoric water introduces minor Ca2+ from altered wall rocks (e.g., carbonated country rocks), which increases aCa2+ and pushes aCa2+ × aF− above Ksp.
6.4. Genetic Model of the Xiaobaihegou Fluorite Deposit
Based on the above observations, the genetic model of fluorite mineralization in the Xiaobaihegou deposit is synthesized as follows. The fluorite ore-forming process represents a complex geochemical evolution. According to the metallogenic background, ore-forming characteristics, and specific conditions of the Altyn fluorite belt, the ore-forming model is established in Figure 13, summarizing the process as follows.
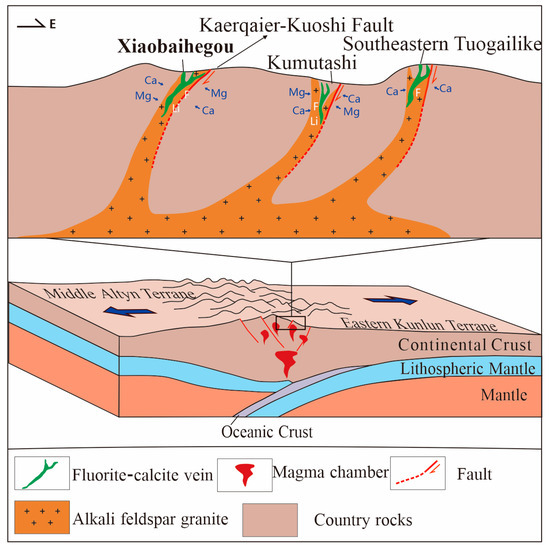
Figure 13.
Mineralization model and tectonic background of the Kaerqiaer fluorite belt (modified after [5]).
In the Xiaobaihegou area, magma migrated upward and mixed with crustal materials under strong extensional tectonics, forming A-type granitic magma that ascended and emplaced along regional deep faults to the shallow crust [5,12,13,14,15]. During upward emplacement, metallogenic elements and volatile components were highly enriched in magmatic–hydrothermal solutions. As the F-rich magmatic–hydrothermal fluids migrated along tectonic fractures, they continuously reacted with Ca-rich strata (e.g., biotite plagioclase gneiss and marble of the Altyn Group), activating and extracting Ca, Mg, Na, and other elements to form F-bearing ore-forming hydrothermal fluids [5,7,8,9,10]. The escape of F-rich fluids from magma involves several key processes, including the enrichment of F in residual magma during fractional crystallization, fluid exsolution triggered by decreasing pressure and temperature, and the role of F in reducing magma viscosity to facilitate fluid migration.
Since different types of inclusions can be found coexisting within the same fluorite grain (Figure 6j), and as further illustrated in Figure 8b, all data points are not distributed along a single cooling/dilution trend line; these observations collectively strongly indicate that phase separation (immiscibility) occurred within a specific temperature interval, thereby trapping inclusions of different phases. The ore-forming fluids underwent fluid unmixing, where F played a dual role: acting as a catalyst for immiscibility and as a complexing agent with metallogenic elements to facilitate their migration, and as the physico-chemical conditions of the hydrothermal system (e.g., acid–base properties, redox state, pH) changed, the ore-bearing fluids migrated to suitable ore-hosting environments, interacting with host rocks to induce carbonatization, which, in turn, triggered the decomposition of F-containing complexes, releasing F− and Ca2+ ions that combined to form CaF2, precipitating and filling favorable fractures [57,58,59].
Fluid inclusion studies indicate that the early-stage ore-forming fluids belong to a medium–high-temperature and -salinity fluid system, whereas the late-stage ore-forming fluids are characterized as medium–low-temperature and -salinity fluids. For the early-stage, stable isotope compositions and fluid inclusion component analysis indicate the fluids were weakly acidic and existed in a relatively reducing environment. These conditions favored the transport of fluorine as F− and metal–fluoride complexes (e.g., CaF+). For the late-stage, influx of meteoric water and fluid mixing caused a shift to near-neutral to weakly alkaline conditions. The redox state became more oxidizing, as indicated by the appearance of calcite in late veins. This pH increase reduced the solubility of fluorite by decreasing F− activity, while the oxidizing shift destabilized metal–fluoride complexes: both are critical triggers for late-stage fluorite precipitation. These physicochemical parameters are closely related to the migration and precipitation of fluorine in the fluids [52,53,54,55,56,57,58,59].
7. Conclusions
- (1)
- The Xiaobaihegou fluorite deposit is controlled by NE-trending secondary faults, which provide space for the orebodies. Fluorite mineralization is closely related to alkali-feldspar granite, and fluorite veins occur in fractures within or near the fracture zones of alkali-feldspar granite dykes. Most ore bodies extend >10 km in a discontinuous N-S trend. Mineralogy is simple, dominated by fluorite and calcite.
- (2)
- The formation age of the alkali-feldspar granite related to mineralization in Xiaobaihegou fluorite mine is 482.3 ± 4.1 Ma (MSWD = 0.016), which was formed in the Ordovician era.
- (3)
- The REE distribution pattern of the Xiaobaihegou deposit is of a right-leaning, LREE-enriched type with a significant negative Eu anomaly. This pattern is highly similar to that of the ore-forming alkali-feldspar granite, suggesting that the REE characteristics of fluorite and calcite may have been inherited from the granite.
- (4)
- The ore-forming process can be divided into an early and a late stage. Fluid inclusion studies indicate that the early-stage ore-forming fluids belong to a medium–high-temperature and -salinity fluid system, whereas the late-stage ore-forming fluids are characterized as medium–low-temperature and -salinity fluids. The ore-forming fluid primarily originates from magmatic–hydrothermal fluids and atmospheric precipitation.
- (5)
- The Sr-Nd isotopic composition of fluorite in the Xiaobaiheogou area shows that the ore-forming material originates from the crust. It is suggested that Ca may mainly comes from the leaching and extraction of the strata formation by the magmatic–hydrothermal solution, while F may mainly comes from the magmatic–hydrothermal solution.
Author Contributions
K.C., Y.W., L.Z., Y.J., Y.Z., N.D. and J.W.: Conceptualization, Methodology, Investigation, Writing—Original Draft, Supervision, Writing—Review and Editing. W.S., Y.G. and M.L.: Conceptualization, Methodology, Modelling, Supervision, Writing—Review and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research is financially supported by grants from Science and Technology Innovation Foundation of Comprehensive Survey & Command Center for Natural Resources (program No. KC20230011), Natural Science Basic Research Program of Shaanxi (program Nos. 2025JC-YBQN-415), and China Geology Survey projects (grant Nos. DD20243309).
Data Availability Statement
The datasets analyzed in this current study are available from the corresponding author.
Acknowledgments
We thank Ma, Q., Dang, P.B., Liu, P.W., and Li, X.B. for their assistance in fieldwork, sampling, and analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Magotra, R.; Namga, S.; Singh, P.; Arora, N.; Srivastava, P.K. A New Classification Scheme of Fluorite Deposits. Int. J. Geosci. 2017, 8, 599–610. [Google Scholar] [CrossRef]
- Han, B.B.; Shang, P.Q.; Gao, Y.Z.; Jiao, S.; Yao, C.M.; Zou, H.; Li, M.; Wang, L.; Zheng, H.Y. Fluorite deposits in China: Geological features, metallogenic regularity, and research progress. China Geol. 2020, 3, 473–489. [Google Scholar]
- Redina, A.A.; Nikolenko, A.M.; Doroshkevich, A.G.; Prokopyev, I.R.; Wohlgemuth-Ueberwasser, C.; Vladykin, N.V. Conditions for the crystallization of fluorite in the Mushgai-Khudag complex (Southern Mongolia): Evidence from trace element geochemistry and fluid inclusions. Geochem. Interdiscip. J. Chem. Probl. Geosci. Geoecol. 2020, 80, 125666. [Google Scholar] [CrossRef]
- Zou, H.; Li, M.; Santosh, M.; Zheng, D.; Cao, H.W.; Jiang, X.W.; Chen, H.F.; Li, Z.Q. Fault-controlled carbonate-hosted barite-fluorite mineral systems: The Shuanghe deposit, Yangtze Block, South China. Gondwana Res. Int. Geosci. J. 2022, 101, 26–43. [Google Scholar] [CrossRef]
- Gao, Y.B.; Zhang, L.; Bagas, L.; Hattori, K.; Liu, M.; Wu, H.H.; Wang, Y.W.; Chen, Z.Y.; Zhao, X.M.; Zhang, Y.; et al. Mineralogy and 40Ar-39Ar dating of the recently discovered tainiolite occurrences in the Kaerqiaer fluorite Belt, western Altyn Tagh Terrane. Ore Geol. Rev. 2024, 167, 105990. [Google Scholar] [CrossRef]
- Mineral Commodity Summaries 2025. Available online: https://pubs.usgs.gov/publication/mcs2025 (accessed on 3 March 2025).
- Wu, Y.P.; Zhang, L.C.; Yuan, B.; Zhou, Y.B.; Zhong, L.; Chen, S.Z.; Yang, G.J.; Yan, Y.W.; Zhang, X. Geological Character-istics and Genesis of the Super-large Kalqiar Fluorite Deposit in Altyn Tagh Area of Xinjiang, China. J. Earth Sci. Environ. 2021, 43, 962–977. [Google Scholar]
- Wu, Y.P.; Zhang, L.C.; Zhou, Y.B.; Zhu, M.T.; Chen, S.Z.; Zhong, L.; Yang, G.Q.; Yan, Y.W.; Liu, J.F. Study on fluid charac teristics and metallogenic mechanism of the super-large Kalqiar fluorite deposit in Altyn Tagh area. Chin. J. Geol. 2022, 57, 495–509. [Google Scholar]
- Zhao, X.M.; Gao, Y.B.; Yan, Z.Q.; Zhang, J.W.; Wang, B.; Jin, M.S. Genesis of Kalqiaer Super-large Fluorite Zone in Altyn Tagh Area: Chronology, Rare Earth Elements and Sr-Nd Isotopes Constraints. Northwestern Geol. 2023, 56, 31–47. [Google Scholar]
- Gao, Y.B.; Zhao, X.M.; Wang, B.; Zhang, J.W.; Jin, M.S.; Yang, S.F.; Yan, Z.Q.; Teng, J.X.; Zhao, H.B.; Chao, Y.Y. Geological characteristics, associated granites and the prospecting potential of the super-large Kaerqiaer-Kumutashi fluorite minerali-zation belt in the West Altyn-Tagh Orogen, NW China. Geol. China 2023, 50, 704–729. [Google Scholar]
- Yue, Y.; Liou, J.G. Two-stage evolution model for the Altyn Tagh fault, China. Geology 1999, 27, 227–230. [Google Scholar] [CrossRef]
- Liu, L.; Wang, C.; Chen, D.; Zhang, A.; Liou, J. Petrology and geochronology of HP-UHP rocks from the South Altyn Tagh, northwestern China. J. Asian Earth Sci. 2009, 35, 232–244. [Google Scholar] [CrossRef]
- Liu, L.; Wang, C.; Cao, Y.T.; Chen, D.L.; Kang, L.; Yang, W.Q.; Zhu, X.H. Geochronology of multi-stage metamorphic events: Constraints on episodic zircon growth from the UHP eclogite in the South Altyn, NW China. Lithos 2012, 136, 10–26. [Google Scholar] [CrossRef]
- Wang, C.; Liu, L.; Yang, W.Q.; Zhu, X.H.; Cao, Y.T.; Kang, L.; Chen, S.F.; Li, R.S.; He, S.P. Provenance and ages of the Altyn Complex in Altyn Tagh: Implications for the early Neoproterozoic evolution of northwestern China. Precambrian Res. 2013, 230, 193–208. [Google Scholar] [CrossRef]
- Wang, C.; Liu, L.; Xiao, P.X.; Cao, Y.T.; Yu, H.Y.; Meert, J.G.; Liang, W.T. Geochemical and geochronologic constraints for Paleozoic magmatism related to the orogenic collapse in the Qimantagh–South Altyn region, northwestern China. Lithos 2014, 202, 1–20. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, L.; Wang, C.; Zhang, C.; Kang, L.; Yang, W.; Zhu, X. Multi-Stage Metamorphism of the UHP Pelitic Gneiss from the Southern Altyn Tagh HP/UHP Belt, Western China: Petrological and Geochronological Evidence. J. Earth Sci. 2019, 30, 603–620. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, X.; Bagas, L.; Wang, Y.; Jin, M.; Zhang, J.; Lu, L.; Gao, Y.; Yan, Z.; Teng, J. Newly discovered Ordovician Li-Be deposits at Tugeman in the Altyn-Tagh Orogen, NW China. Ore Geol. Rev. 2021, 139, 104515. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.; Xu, Z.; Yang, J.; Cui, J. Petrology and geochronology of eclogites from the western segment of the Altyn Tagh, northwestern China. Lithos 2001, 56, 187–206. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, J.; del Real, P.G.; Zhao, X.; Hou, K.; Gong, J.; Li, Y. The Grenvillian orogeny in the Altun–Qilian–North Qaidam mountain belts of northern Tibet Plateau: Constraints from geochemical and zircon U–Pb age and Hf isotopic study of magmatic rocks. J. Asian Earth Sci. 2013, 73, 372–395. [Google Scholar] [CrossRef]
- NIST610; Certificate of Analysis: Standard Reference Material 610-Trace Elements in Glass (Revision 2023-01). National Institute of Standards and Technology (NIST): Gaithersburg, MD, USA, 1993.
- Wiedenbeck, M.; Alle, P.; Corfu, F.; Griffin, W.L.; Meier, M.; Oberli, F.V.; Quadt, A.V.; Roddick, J.; Spiegel, W. Three natural zircon standards for U-Th-Pb, Lu-Hf, trace element and REE analyses. Geostand. Newsl. 1995, 19, 1–23. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, S.; Hu, Z.; Gao, C.; Zong, K.; Wang, D. Continental and oceanic crust recycling-induced melt–peridotite interactions in the Trans-North China Orogen: U–Pb dating, Hf isotopes and trace elements in zircons from mantle xenoliths. J. Petrol. 2010, 51, 537–571. [Google Scholar] [CrossRef]
- Ludwig, K.R. User’s Manual for ISOPLOT 3.00: A Geochronological Toolkit for Microsoft Excel, Special Publication No. 4; Berkeley Geochronology Center: Berkeley, CA, USA, 2003; p. 71. [Google Scholar]
- Ludwig, K.R. Mathematical–statistical treatment of data and errors for 230Th/U geochronology. Rev. Mineral. Geochem. 2003, 52, 631–656. [Google Scholar] [CrossRef]
- NIST SRM 987; Certificate of Analysis: Standard Reference Material 987-Strontium Carbonate Isotopic Standard. National Institute of Standards and Technology (NIST): Gaithersburg, MD, USA, 2023.
- Zhai, M.G. Granites: Leading study issue for continental evolution. Acta Petrol. Sin. 2017, 33, 1369–1380. [Google Scholar]
- McDonough, W.F.; Sun, S.S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Roedder, E. Volume 12: Fluid inclusions. Rev. Miner. 1984, 12, 644. [Google Scholar]
- Zhang, Y.; Gao, Y.B.; Liu, M.; Wang, Y.W.; Chen, K.; Zhang, L.; Jing, Y.K.; Liu, J.Y. The Characteristics of Ore-forming Fluids and Metallogenic Mecha nism of the Kumutashi Fluorite Deposit in West Altyn-Tagh, China. Northwestern Geol. 2024, 57, 21–36. [Google Scholar]
- Goldstein, S.; O’nions, R.; Hamilton, P. A Sm-Nd isotopic study of atmospheric dusts and particulates from major river systems. Earth Planet. Sci. Lett. 1984, 70, 221–236. [Google Scholar] [CrossRef]
- Peucat, J.; Jegouzo, P.; Vidal, P.; Bernard-Griffiths, J. Continental crust formation seen through the Sr and Nd isotope systematics of S-type granites in the Hercynian belt of western France. Earth Planet. Sci. Lett. 1988, 88, 60–68. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Zeng, Z.C.; Chen, N.; Li, Q.; Wang, T.Y.; Zhao, J.L. The discovery of Middle-Late Ordovician syenogranite on the southern margin of Altun orogenic belt and its geological significance. Geol. Bull. China 2018, 37, 545–558. [Google Scholar]
- Wang, L.; Yang, P.; Duan, X.; Long, X.; Sun, J. Isotopic age and genesis of plagiogranite from Qingshuiquan area in the middle of South Altyn Tagh. Acta Petrol. Sin. 2016, 32, 759–774. [Google Scholar]
- Li, H.; Hong, T.; Yang, Z.Q.; Liu, S.K.; Wang, X.H.; Ma, Y.C.; Niu, L.; Xu, X.W. Multi-stage magmatism-mineralization and tectonic setting of the North Tugeman granitic pegmatite lithium-beryllium deposit in the middle of Altyn Tagh. Acta Petrol. Sin. 2022, 38, 3085–3103. [Google Scholar]
- Xu, X.; Hong, T.; Zhang, P.; Liu, S.; Yang, Z.; Ma, Y.; Kang, K.; Li, H. Metallogeny and resource potential of lithium-beryllium granites and pegmatites in the Altyn Tagh. Acta Petrol. Sin. 2024, 40, 2679–2702. [Google Scholar] [CrossRef]
- Liu, J.; Li, W.; Zhu, X.; Li, C.; Zhou, Q.; Yang, F. Origin and evolution of ore-forming fluids of the Larong W-(Mo) deposit, eastern Tibet: Constraints from fluid inclusions, HO isotopes, and scheelite geochemistry. Ore Geol. Rev. 2020, 124, 103620. [Google Scholar] [CrossRef]
- Schönenberger, J.; Köhler, J.; Markl, G. REE systematics of fluorides, calcite and siderite in peralkaline plutonic rocks from the Gardar Province, South Greenland. Chem. Geol. 2008, 247, 16–35. [Google Scholar] [CrossRef]
- Zhong, S.; Mucci, A. Partitioning of rare earth elements (REEs) between calcite and seawater solutions at 25 C and 1 atm, and high dissolved REE concentrations. Geochim. Cosmochim. Acta 1995, 59, 443–453. [Google Scholar] [CrossRef]
- Chacko, T.; Cole, D.R.; Horita, J. Equilibrium oxygen, hydrogen and carbon isotope fractionation factors applicable to geologic systems. Rev. Mineral. Geochem. 2001, 43, 1–81. [Google Scholar] [CrossRef]
- Wang, X.; Li, B.; Tan, S.; Tang, G.; Xiang, Z.; Liu, Y. Characteristics of antimony mineralization in the Yangla polymetallic deposit, northwestern Yunnan, SW China: Insights from calcite Sm-Nd dating and CO-Sr isotopes. Ore Geol. Rev. 2024, 173, 106266. [Google Scholar] [CrossRef]
- Möller, P.; Parekh, P.; Schneider, H.-J. The application of Tb/Ca-Tb/La abundance ratios to problems of fluorspar genesis. Miner. Depos. 1976, 11, 111–116. [Google Scholar] [CrossRef]
- Ehya, F. Variation of mineralizing fluids and fractionation of REE during the emplacement of the vein-type fluorite deposit at Bozijan, Markazi Province, Iran. J. Geochem. Explor. 2012, 112, 93–106. [Google Scholar] [CrossRef]
- Hill, G.T.; Campbell, A.R.; Kyle, P.R. Geochemistry of southwestern New Mexico fluorite occurrences implications for precious metals exploration in fluorite-bearing systems. J. Geochem. Explor. 2000, 68, 1–20. [Google Scholar] [CrossRef]
- Ito, E.; White, W.M.; Göpel, C. The O, Sr, Nd and Pb isotope geochemistry of MORB. Chem. Geol. 1987, 62, 157–176. [Google Scholar] [CrossRef]
- Nelson, D.R.; McCulloch, M.T.; Sun, S.S. The origins of ultrapotassic rocks as inferred from Sr, Nd and Pb isotopes. Geochim. Cosmochim. Acta 1986, 50, 231–245. [Google Scholar] [CrossRef]
- Richardson, C.K.; Holland, H. Fluorite deposition in hydrothermal systems. Geochim. Cosmochim. Acta 1979, 43, 1327–1335. [Google Scholar] [CrossRef]
- Zou, H.; Xiao, B.; Gong, D.X.; Huang, C.C.; Li, M.; Yu, L.M.; Tian, E.Y.; Liu, C.M.; Chen, H.F.; Hu, C.H. Origin and tectonic setting of Pingqiao fluorite-lithium deposit in the Guizhou, southwest Yangtze Block, China. Ore Geol. Rev. 2022, 143, 104755. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Fang, Y.; Tang, L. Integrated Exploration Model for Concealed Ore Deposit: A Case Study from Shuitou Fluorite Deposit, Inner Mongolia, North China. J. Earth Sci. 2021, 32, 370–389. [Google Scholar] [CrossRef]
- Zou, H.; Fang, Y.; Zhang, S.T.; Zhang, Q. The source of Fengjia and Langxi barite–fluorite deposits in southeastern Sichuan, China: Evidence from rare earth elements and S, Sr, and Sm–Nd isotopic data. Geol. J. 2017, 52, 470–488. [Google Scholar] [CrossRef]
- Zou, H.; Tang, L.; Cao, H.W.; Santosh, M. Mesozoic to Cenozoic mineralization in China: Preface. Ore Geol. Rev. 2022, 148, 105052. [Google Scholar] [CrossRef]
- Pei, Q.; Zhang, S.; Santosh, M.; Cao, H.; Zhang, W.; Hu, X.; Wang, L. Geochronology, geochemistry, fluid inclusion and C, O and Hf isotope compositions of the Shuitou fluorite deposit, Inner Mongolia, China. Ore Geol. Rev. 2017, 83, 174–190. [Google Scholar] [CrossRef]
- Fang, Y.; Zou, H.; Bagas, L.; Said, N.; Li, Y.; Liu, H. Fluorite deposits in the Zhejiang Province, southeast China: The possible role of extension during the late stages in the subduction of the Paleo-Pacific oceanic plate, as indicated by the Gudongkeng fluorite deposit. Ore Geol. Rev. 2020, 117, 103276. [Google Scholar] [CrossRef]
- Xu, C.; Taylor, R.N.; Li, W.; Kynicky, J.; Chakhmouradian, A.R.; Song, W. Comparison of fluorite geochemistry from REE deposits in the Panxi region and Bayan Obo, China. J. Asian Earth Sci. 2012, 57, 76–89. [Google Scholar] [CrossRef]
- Duan, Z.P.; Jiang, S.Y.; Su, H.M.; Zhu, X.Y.; Jiang, B.B. Textural features and in situ trace element analysis of fluorite from the Wujianfang fluorite deposit, Inner Mongolia (NE China): Insights into fluid metasomatism and ore-forming process. Ore Geol. Rev. 2022, 147, 104982. [Google Scholar] [CrossRef]
- Yan, H.; Ding, X.; Ling, M.; Li, C.; Harlov, D.E.; Sun, W. Three late-Mesozoic fluorite deposit belts in southeast China and links to subduction of the (paleo-) Pacific plate. Ore Geol. Rev. 2021, 129, 103865. [Google Scholar] [CrossRef]
- Ye, X.; Bai, F. Spectral characteristics, rare earth elements, and ore-forming fluid constrains on the origin of fluorite deposit in Nanlishu, Jilin Province, China. Minerals 2022, 12, 1195. [Google Scholar] [CrossRef]
- Subías, I.; Fanlo, I.; Billström, K. Ore-forming timing of polymetallic-fluorite low temperature veins from Central Pyrenees: A Pb, Nd and Sr isotope perspective. Ore Geol. Rev. 2015, 70, 241–251. [Google Scholar] [CrossRef]
- Fawzy, K.M. The genesis of fluorite veins in Gabal El Atawi granite, central Eastern Desert, Egypt. J. Afr. Earth Sci. 2018, 146, 150–157. [Google Scholar] [CrossRef]
- Dill, H.G.; Weber, B.; Eigler, G.; Kaufhold, S. The fluorite deposits NE of Regensburg, SE Germany—A mineralogical and chemical comparison of unconformity-related fluorite vein-type deposits. Geochemistry 2012, 72, 261–278. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).