Occurrence State and Genesis of Large Particle Marcasite in a Thick Coal Seam of the Zhundong Coalfield in Xinjiang
Abstract
1. Introduction
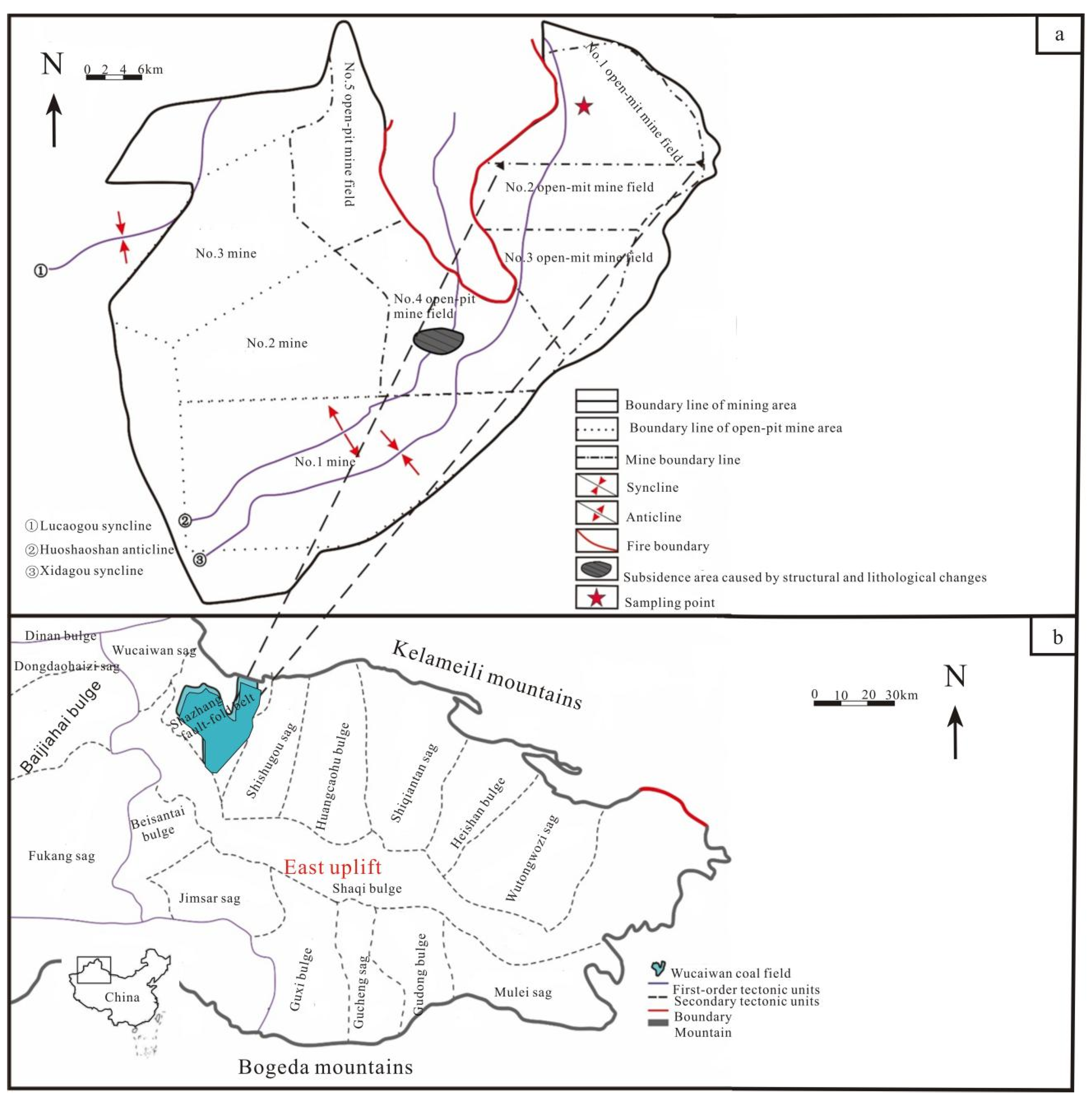
2. Geological Background
3. Samples and Research Methods
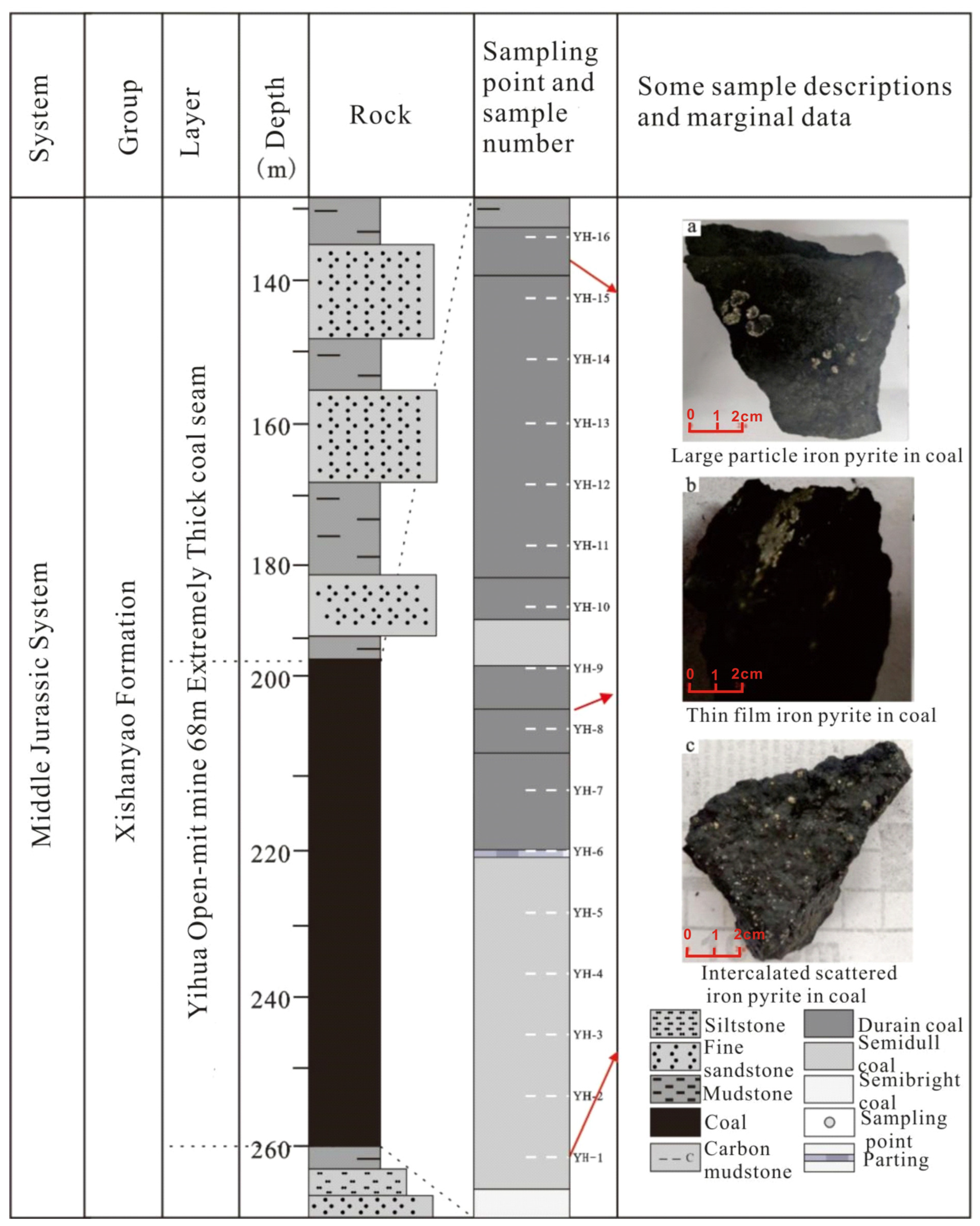
3.1. Sample Collection
3.2. Research Method
3.2.1. Determination of Major Elements
3.2.2. Determination of Microelements
3.2.3. Preparation of Pulverized Coal Light Sheet
3.2.4. Observation of Light Microscopy
3.2.5. Scanning Electron Microscopy-Raman Spectroscopy System
3.2.6. LA-MC-ICP-MS Isotope Analysis of Sulphide Minerals
4. Results
4.1. Coal Property
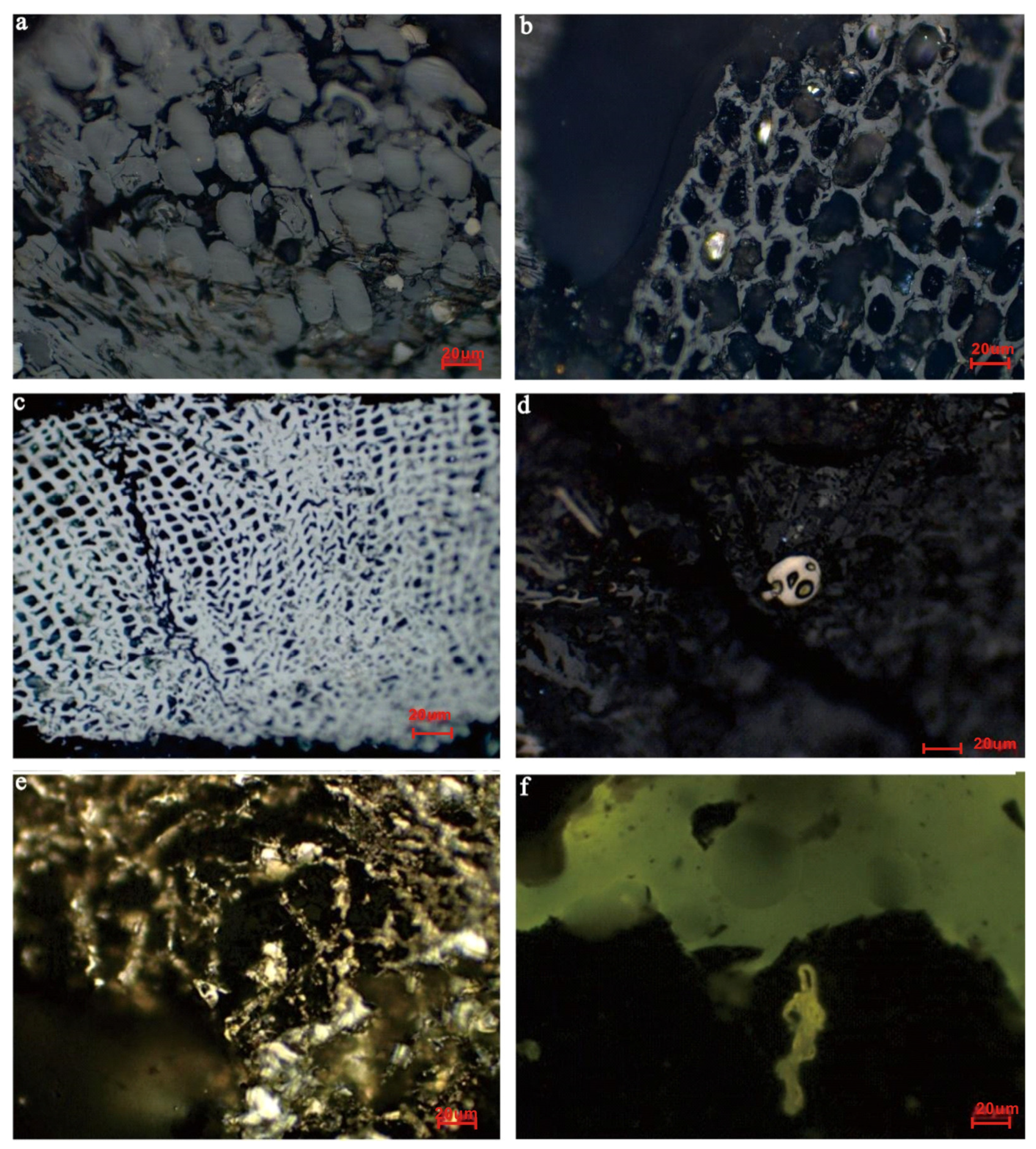
4.2. Mineralogy
4.3. Geochemistry
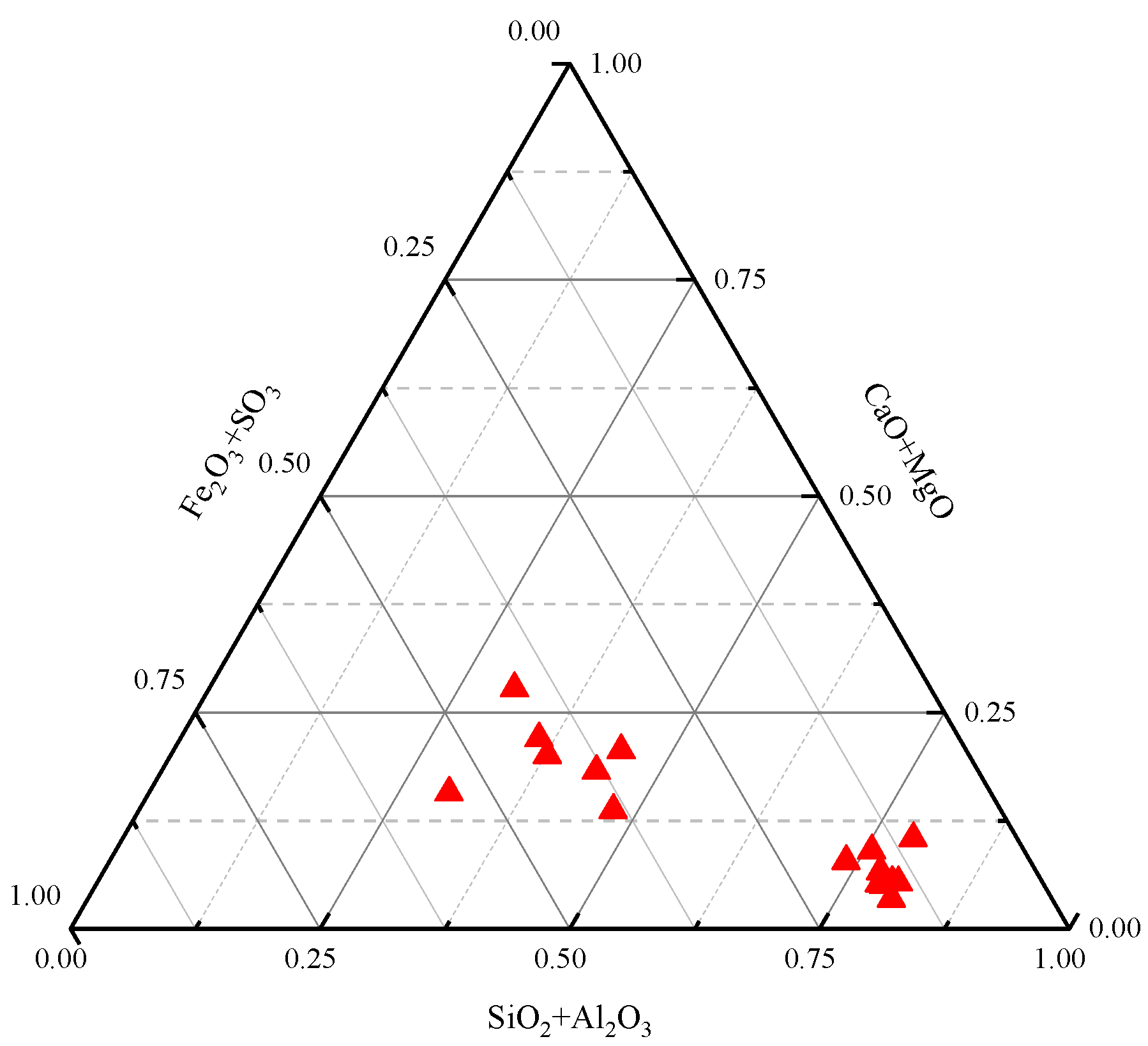
4.3.1. Content Characteristics of Major Elements
4.3.2. Content Characteristics of Trace Elements
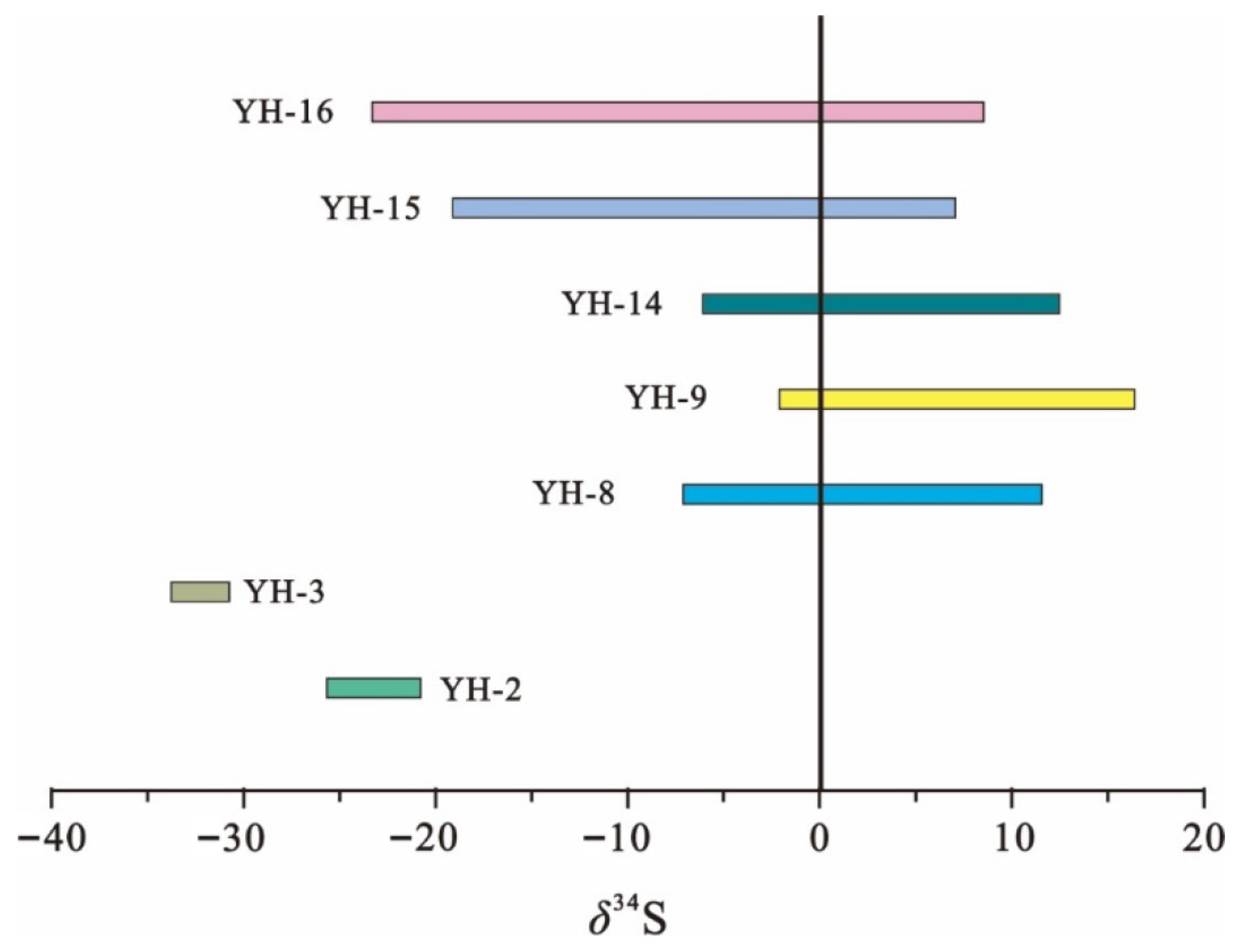
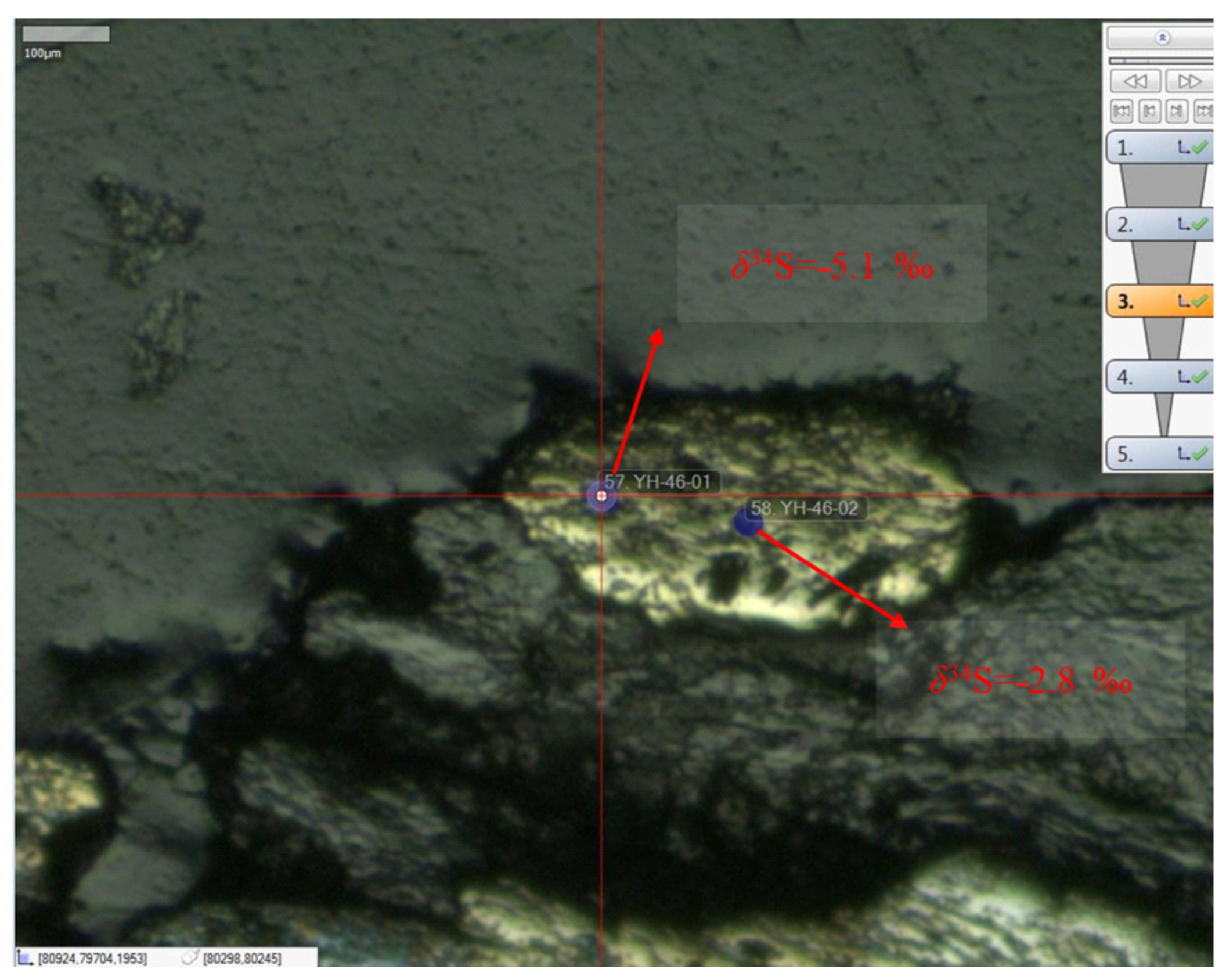
4.3.3. The Sulfur Isotope Characteristics
5. Discussion
5.1. Occurrence State of Marcasite in Thick Coal Seam
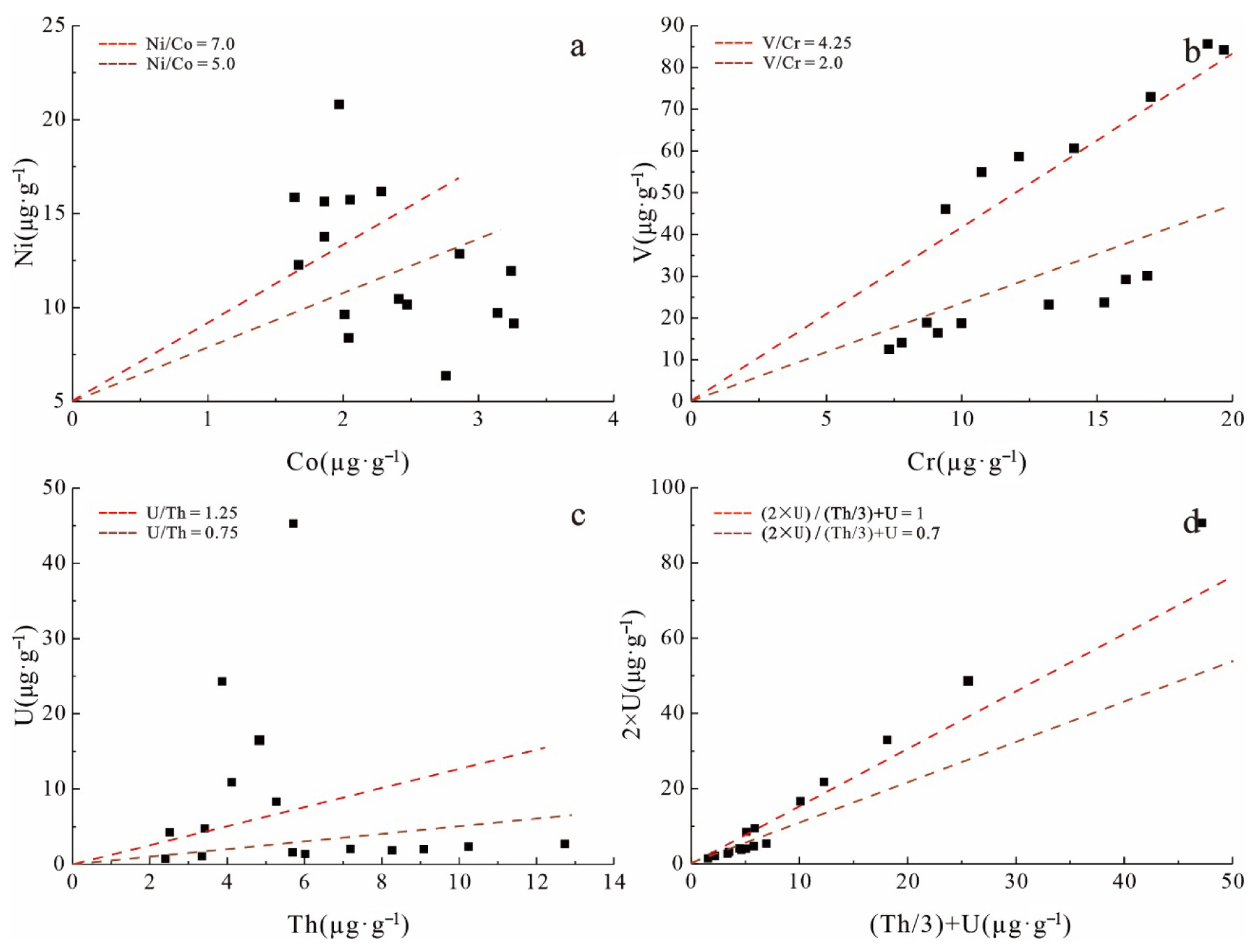
5.2. Environmental Indication of the Sulfur Isotope of Marcasite in the Extremely Thick Coal Seam
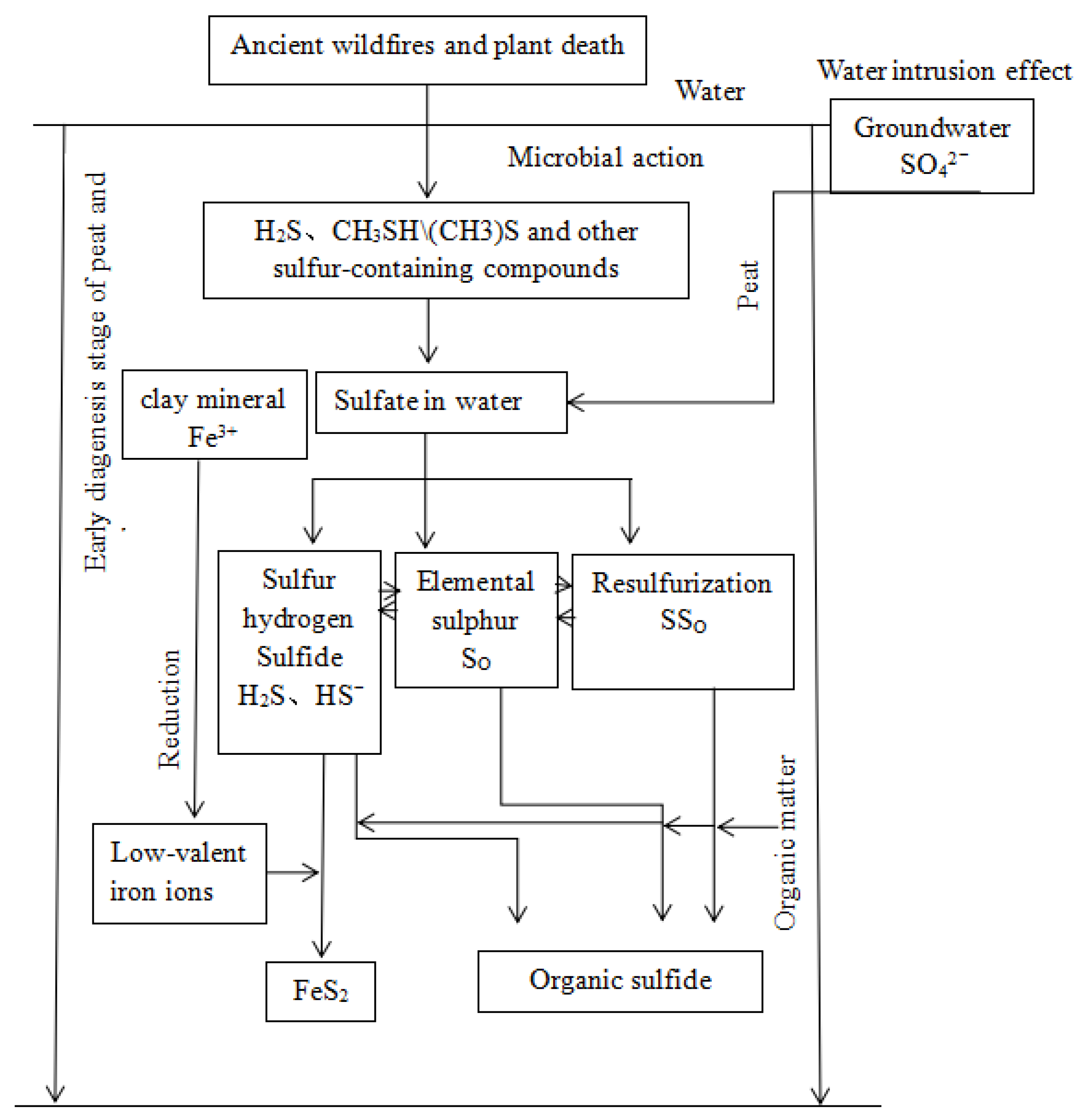
5.3. Genesis of Marcasite in Thick Coal Seam
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kolker, A. Minor element distribution in iron disulfides in coal: A geochemical review. Int. J. Coal Geol. 2012, 94, 32–43. [Google Scholar] [CrossRef]
- Chou, C.L. Sulfur in coals: A review of geochemistry and origins. Int. J. Coal Geol. 2012, 100, 1–13. [Google Scholar] [CrossRef]
- Ward, C.R. Analysis and significance of mineral matter in coal seams. Int. J. Coal Geol. 2002, 50, 135–168. [Google Scholar] [CrossRef]
- Zhang, T.; Wen, H.J.; Cheng, W.; Li, X.H.; Xie, J. A review on the advances in the occurrence modes and bebeficiation of associated elements in medium-high sulfur coals. Int. J. China Univ. Min. Technol. 2022, 51, 491–502. [Google Scholar]
- Diehl, S.F.; Goldhaber, M.B.; Koenig, A.E.; Lowers, H.A.; Ruppert, L.F. Distribution of arsenic, selenium, and other trace elements in high pyrite Appalachian coals: Evidence for multiple episodes of pyrite formation. Int. J. Coal Geol. 2012, 94, 238–249. [Google Scholar] [CrossRef]
- Pan, X.L.; Cheng, W.; Hou, H. Study on the mineralogical characteristics of pyrite in coal measures. Nonferrous Met. (Miner. Process Part) 2024, 11, 21–32. [Google Scholar]
- Qi, X. Study on the Crystal Characteristics of Pyrite and the Occurrence of Organic Sulfur in High-Sulfur Coal in the Eastern Sichuan Mining Area. Ph.D. Thesis, China University of Mining and Technology, Beijing, China, 2021. [Google Scholar]
- Liu, B.; Huang, W.H.; Ao, W.H.; Yan, D.Y.; Xu, Q.L. The geochemical characteristics of sulfur in Late Paleozoic coal in Qinshui Basin and its effect on the enrichment of harmful trace elements. Int. J. Geol. Front. 2016, 23, 59–67. [Google Scholar]
- Tao, X.X.; Tang, L.F.; Xie, M.H.; Xu, N.; Guo, J.F.; Chen, L. Dielectric response characteristics of sulfur-containing model compounds in coal and their effects on microwave desulfurization. Int. J. J. China Coal Soc. 2017, 42, 760–767. [Google Scholar]
- He, H.P.; Xian, H.; Zhu, J.X.; Tan, W.; Liang, X.L.; Chen, M. From mineral powder crystal surface reactivity to mineral crystal surface reactivity-Taking the crystal surface difference of pyrite oxidation behavior as an example. Int. J. Petrol. J. 2019, 35, 129–136. [Google Scholar]
- Zhu, L.Z.; Richardson, B.; Tanumihardja, J.; Yu, Q.M. Controlling morphology and phase of pyrite FeS2 hierarchical particles via the combination of structure-direction and 128 chelating agents. Crystengcomm 2012, 14, 4188–4195. [Google Scholar] [CrossRef]
- Fleet, M.E. Structural aspects of The Marcasite-Pyrite Transformation. Int. J. Am. Miner. 1969, 10, 225–231. [Google Scholar]
- Macfarlane, R.M. Resonant Raman Scattering From FeS2(Pyrite). Int. J. Solid State Commun. 1974, 14, 851–855. [Google Scholar] [CrossRef]
- Tossell, J.A.; Vaughan, D.J.; Burdett, J.K. Pyrite, Marcasite, and Arsenopyrite Type Minerals: Crystal Chemical and Structural Principles. Int. J. Phys. Chem. Miner. 1981, 7, 177–184. [Google Scholar] [CrossRef]
- Kathlee. Pyrite/Marcasite Size, Form, and Microlihotype Association in Western Kentuky Prepared Coals. Int. J. Fuel Process Technol. 1985, 10, 269–283. [Google Scholar]
- Zhuang, J. Pyrite minerals in coal seam and their genesis. Int. J. Mineral. 1985, 3, 245–250. [Google Scholar]
- White, R.N.; Smith, J.V.; Spears, D.A.; Rivers, M.L.; Sutton, S.R. Analysis of iron sulphides from UK coal by synchrotron radiation X-ray fluorescence. Int. J. Fuel 1989, 68, 1480–1486. [Google Scholar] [CrossRef]
- Blowes, D.W.; Al, T.; Lortie, L.; Gould, W.D.; Jambor, J.L. Microbiological, chemical, and mineralogical characteri-zation of the Kidd-Creek mine-tailings impoundment, Timmins area, Ontario. Int. J. Geomicrobiol. 1995, 13, 13–31. [Google Scholar] [CrossRef]
- Liu, D.M.; Yang, Q.; Zhou, C.G.; Tang, D.Z.; Kang, X.D. Occurrence and geological genesis of pyritesin Late Paleozoic coals in North China. Int. J. Geochem. 1999, 28, 340–350. [Google Scholar]
- Wang, H.D. The occurrence characteristics of sulfur in 8 ~ # coal in Gujiao mining area, Shanxi Province. Int. J. Coal 2005, 14, 17–19. [Google Scholar]
- Zhang, J.S.; Zhu, Y.M.; Zhou, X.N.; Wang, M.; Wang, H. Occurrence characteristics of strawberry pyrite in Permian coal seam of Zhijin, Guizhou. Int. J. Energy Technol. Manag. 2010, 3, 6–8. [Google Scholar]
- Zhao, W.Y. The surface morphology of pyrite in Xishan No.8 coal seam of Taiyuan. Int. J. Coal 2011, 20, 42–43. [Google Scholar]
- Wang, W.; Sang, S.; Bian, Z.; Duan, P.; Qin, Y. Fine-grained pyrite in some Chinese coals. Energy Explor. Exploit. 2016, 34, 54–65. [Google Scholar] [CrossRef]
- Chou, C.L. Abundances of sulfur, chlorine, and trace elements in Illinois Basin coals, USA. In Proceedings of the 14th Annual Pittsburgh Coal Conference, Taiyuan, China, 23–27 September 1997. [Google Scholar]
- Swaine, D. Contents of trace elements in coals. Trace Elem. Coal 1990, 24, 77–183. [Google Scholar]
- Dai, S.F.; Ren, D.; Tang, Y. Eological evolution model of sulfur in high sulfur coal-Taking Wuda mining area in Inner Mongolia as an example. Int. J. Geol. Rev. 2001, 4, 383–387. [Google Scholar]
- Querol, X.; Chinchon, S.; Lopez-Soler, A. Fe-sulphide minerals precipitation sequence in Albian coals from the Maestrazgo Basin, southeastern Iberian Range, northeastern Spain. Int. J. Coal Geol. 1989, 11, 171–189. [Google Scholar] [CrossRef]
- Grady, W.C. Petrography of West Virginia coals. In Carboniferous Coal Short Course and Guidebook; Donaldson, A., Presley, M.W., Renton, J.J., Eds.; West Virginia Geological & Economic Survey: Morgantown, WV, USA, 1979; Volume 37, pp. 240–277. [Google Scholar]
- King, H.M.; Renton, J.J. The mode of occurrence and distribution of sulfur in West Virginia coals. In Carboniferous Coal Short Course and Guidebook; Donaldson, A., Presley, M.W., Renton, J.J., Eds.; West Virginia Geological & Economic Survey: Morgantown, WV, USA, 1979; Volume 37, pp. 278–301. [Google Scholar]
- Boctor, N.Z.; Kullerud, G.; Sweany, J.L. Sulfide minerals in Seelyville Coal III, Chinook mine, Indiana. Miner. Deposita 1976, 11, 249–266. [Google Scholar] [CrossRef]
- Parratt, R.L.; Kullerud, G. Sulfide minerals in Coal Bed V, Minnehaha mine, Sullivan County, Indiana. Miner. Deposita 1979, 14, 195–206. [Google Scholar] [CrossRef]
- Lett, R.E.; Fletcher, W.K. Syngenetic sulphide minerals in a copper-rich bog. Miner. Deposita 1980, 15, 61–67. [Google Scholar] [CrossRef]
- Liu, D.M.; Liu, Z.H.; Li, Y.Y. Study Progress of Harmful Substances in Coal and Their Effects on Environment. Int. J. Adv. Earth Sci. 2002, 17, 840–847. [Google Scholar]
- Zhou, Q. Study on the occurrence state of sulfur and nitrogen in Chinese coal. Int. J. Clean Coal Technol. 2008, 1, 73–77. [Google Scholar]
- Wang, H. Sedimentological Characteristics and Paleoenvironmental Significance of Late Permian Coal in Eastern Yunnan-Western Guizhou. Ph.D. Thesis, China University of Mining and Technology, Beijing, China, 2011. [Google Scholar]
- Nielsen, H. Isotopes in nature. In Handbook of Geochemistry; Wedepohl, K.H., Ed.; Springer: Berlin, Germany, 1978; pp. 16B1–16B40. [Google Scholar]
- Goldhaber, M.B.; Kaplan, I.R. The sulfur cycle. In The Sea: Ideas and Observations on Progress in the Study of the Seas; Interscience/Wiley: New York, NY, USA, 1974; Volume 26, pp. 569–655. [Google Scholar]
- Chambers, L.A.; Trudinger, P.A. Microbiological fractionation of stable sulfur isotopes: A review and critique. Geomicrobiol. J. 1979, 23, 249–293. [Google Scholar] [CrossRef]
- Chukhrov, F.V.; Ermilova, L.P. Zur Schwefel-Isotope nzusammensetzung in Konkretionen. Ber. Dtsch. Ges. Geol. Wiss. 1970, 15, 255. [Google Scholar]
- Smith, J.W.; Batts, B.D. The distribution and isotopic composition of sulfur in coal. Geochim. Cosmochim. Acta 1974, 38, 121–133. [Google Scholar] [CrossRef]
- Hunt, J.W.; Smith, J.W. 34S/32S ratios of low-sulfur Permian Australian coals in relatio to depositional environments. Chem. Geol. 1985, 58, 137–144. [Google Scholar] [CrossRef]
- Gluskoter, H.J. Inorganic sulfur in coal. Int. J. Energy Sources 1977, 3, 125–131. [Google Scholar] [CrossRef]
- Love, L.G.; Murray, J.W. Biogenic pyrite in recent sediments of Christchurch Harbour, England. Am. J. Sci. 1963, 261, 433–448. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Wang, W.F. Structural evolution and pool forming in the Junggar basin. Int. J. J. Univ. Pet. 2004, 28, 4–8. [Google Scholar]
- Gan, H.J.; Wang, H.; Chen, J.; Zhuang, X.; Cao, H.; Jiang, S. Geochemical characteristics of Jurassic coal and its paleoenvironmental implication in the eastern Junggar Basin, China. J. Geochem. Explor. 2018, 188, 73–86. [Google Scholar] [CrossRef]
- Dai, S.F.; Hou, X.Q.; Ren, D.Y.; Tang, Y.G. Surface analysis of pyrite in the No. 9 coal seam, Wuda Coalfield, Inner Mongolia, China, using high-resolution time-of-flight secondary ion mass spectrometry. Int. J. Coal Geol 2003, 55, 139–150. [Google Scholar] [CrossRef]
- Dai, S.F.; Hower, J.C.; Finkelman, R.B.; Graham, I.T.; French, D.; Ward, C.R.; Eskenazy, G.; Wei, Q.; Zhao, L. Organic associations of non-mineral elements in coal: A review. Int. J. Coal Geol. 2020, 218, 103347. [Google Scholar] [CrossRef]
- Dai, S.F.; Bechtel, A.; Eble, C.F.; Flore, R.M.; French, D.; Graham, I.T.; Hood, M.M.; Hower, J.C.; Korasidis, V.A.; Moore, T.A.; et al. Recognition of peat depositional environments in coal: A review. Int. J. Coal Geol. 2020, 219, 103383. [Google Scholar] [CrossRef]
- Dai, S.F.; Finkelman, R.B.; French, D.; Hower, J.C.; Graham, I.T.; Zhao, F.H. Modes of occurrence of elements in coal: A critical evaluation. Int. J. Earth Sci. Rev. 2021, 222, 103815. [Google Scholar] [CrossRef]
- Liu, J.J.; Ward, C.R.; Graham, I.T.; French, D.; Dai, S.F.; Song, X.L. Modes of occurrence of non-mineral inorganic elements in lignites from the Mile Basin, Yunnan Province, China. Fuel 2018, 222, 146–155. [Google Scholar] [CrossRef]
- Jia, R.K.; Liu, J.J.; Han, Q.C.; Zhao, S.M.; Shang, N.D.; Tang, P.Q.; Zhang, Y.Q. Mineral matter transition in lignite during ashing process: A case study of Early Cretaceous lignite from the Hailar Basin, Inner Mongolia, China. Fuel 2022, 328, 125252. [Google Scholar] [CrossRef]
- Guay, R. Acid mine drainage: Microbiological teamwork. In Proceedings of the11th Annual Meeting of BIOMINET, Ottawa, ON, Canada, 16 January 1995; pp. 63–68. [Google Scholar]
- Luo, Z.J.; Chen, H.; Jiang, Y.X.; Lian, L.X. Study on the development characteristics of Xishanyao Formation coal seam in Zhundong area. Int. J. Coal Technol. 2015, 34, 121–122. [Google Scholar]
- GB/T 482-2008; Discussion on National Standard of Sampling of Coal Seams. Coal Quality Technology: Beijing, China, 2008.
- GB/T 14506.30-2010; Chemical Analysis Method of Silicate Rock. Standardization Administration of China: Beijing, China, 2010.
- GB/T 16773-2008; Methods for Preparing Coal Samples for Coal Petrographic Analysis. Standardization Administration of China: Beijing, China, 2008.
- Jia, R.K.; Liu, J.J.; Hower, J.C.; Jiang, Y.F.; Zhao, S.M.; Han, Q.C.; Shang, N.D.; Feng, J.W.; Teng, K.Y. Sedimentary conditions and palaeoenvironment during the Early Cretaceous: Evidence from macerals and organic carbon isotopes of the coal from Hailar Basin, Northeast China. Cretac. Res. 2025, 171, 106118. [Google Scholar] [CrossRef]
- ICCP. The new vitrinite classification (ICCP System 1994). Fuel 1998, 77, 349–358. [Google Scholar] [CrossRef]
- Tian, J.J.; Yang, S.G. Sequence stratigraphic framework and coal accumulation law of Lower-Middle Jurassic in the southern margin of Junggar Basin. Int. J. Coal J. 2011, 36, 58–64. [Google Scholar]
- Wang, X.T. Permian-Jurassic Stratigraphic Framework and Sedimentary Evolution Around Bogda Mountain. Ph.D. Dissertation, China University of Petroleum (East China), Qingdao, China, 2017. [Google Scholar]
- Dai, S.F.; Ren, D.Y.; Chou, C.L. Geochemistry of trace elements in Chinese coals: A review of abundances, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- Dai, S.F.; Zhou, Y.P.; Ren, D.Y. Geochemistry and mineralogy of the late Permian coals from the Songzao Coalfield, Chongqing, southwestern China. Sci. China Ser. D Earth Sci. 2007, 50, 678–688. [Google Scholar] [CrossRef]
- Yang, B.; Tian, J.J.; Feng, S.; Yang, J.H.; Li, Y.Z. Ancient wildfire events recorded in the Middle Jurassic coal in the eastern Junggar Basin. Int. J. Coal Sci. Technol. 2022, 50, 261–270. [Google Scholar]
- Hatch, J.R.; Leventhal, J.S. Relationship between inferred redox potential of the depositional environment and geochemistry of the Upper Pennsylvanian (Missourian) stark shale member of the Dennis limestone, Wabaunsee County, Kansas, U.S.A. Chem. Geol. 1992, 99, 65–82. [Google Scholar] [CrossRef]
- Jones, B.; Manning, D.A.C. Comparison of geochemical indices used for the interpretation of paleoredox conditions in ancient mudstones. Chem. Geol. 1994, 111, 111–129. [Google Scholar] [CrossRef]
- Zhao, Z.H. Geochemical Study of Rare Earth Elements in Coal-Bearing Rock Series; Coal Industry Press: Beijing, China, 2002. [Google Scholar]
- Fan, M.M.; Bu, J.; Zhao, X.Y.; Kang, B.; Li, W.H.; Zhang, W.G. Trace element geochemical characteristics and environmental significance of Yanchang Formation in southeastern Ordos Basin. Int. J. Northwest Univ. (Nat. Sci. Ed.) 2019, 49, 633–642. [Google Scholar]
- Kimura, H.; Watanabe, Y. Ocean anoxia at the Precambrian-Cambrian boundary. Int. J. Geol. 2001, 29, 995–998. [Google Scholar]
- Chang, H.J.; Chu, X.L.; Feng, L.J.; Huang, J.; Zhang, Q.R. Redox sensitive trace elements indication significance for paleo-marine sedimentary environment. Int. J. Geol. Rev. 2009, 55, 91–99. [Google Scholar]
- Liu, A.; Li, X.B.; Wang, C.S.; Wei, K.; Wang, B.Z. Analysis of geochemical characteristics and sedimentary environment of Cambrian source rocks in western Hunan and Hubei. Int. J. Sediment. J. 2013, 31, 1122–1132. [Google Scholar]
- Xu, C.K.; Liu, C.Y.; Guo, P.; Li, M.W.; Huang, L. Geochemical characteristics and geological significance of inter-salt mudstone in the Paleogene Qianjiang Formation of Qianjiang Sag. Int. J. Sedimentol. J. 2018, 36, 617–629. [Google Scholar]
- Wignall, P.B.; Twttchett, R.J. Oceanic anoxia and the end Permian mass extinction. Science 1996, 272, 1155–1158. [Google Scholar] [CrossRef]
- Rimmer, S.M. Geochemical paleoredox indicators in Devonian-Mississippian black shales. Central Appalachian Basin (USA). Chem. Geol. 2004, 206, 373–391. [Google Scholar] [CrossRef]
- Scheffler, K.; Buehmann, D.; Schwark, L. Analysis of late Palaeozoic glacial to postglacial sedimentary successions in South Africa by geochemical proxies: Response to climate evolution and sedimentary environment. Palaeogeogr. Palaeoclim. Palaeoecol. 2006, 240, 184–203. [Google Scholar] [CrossRef]
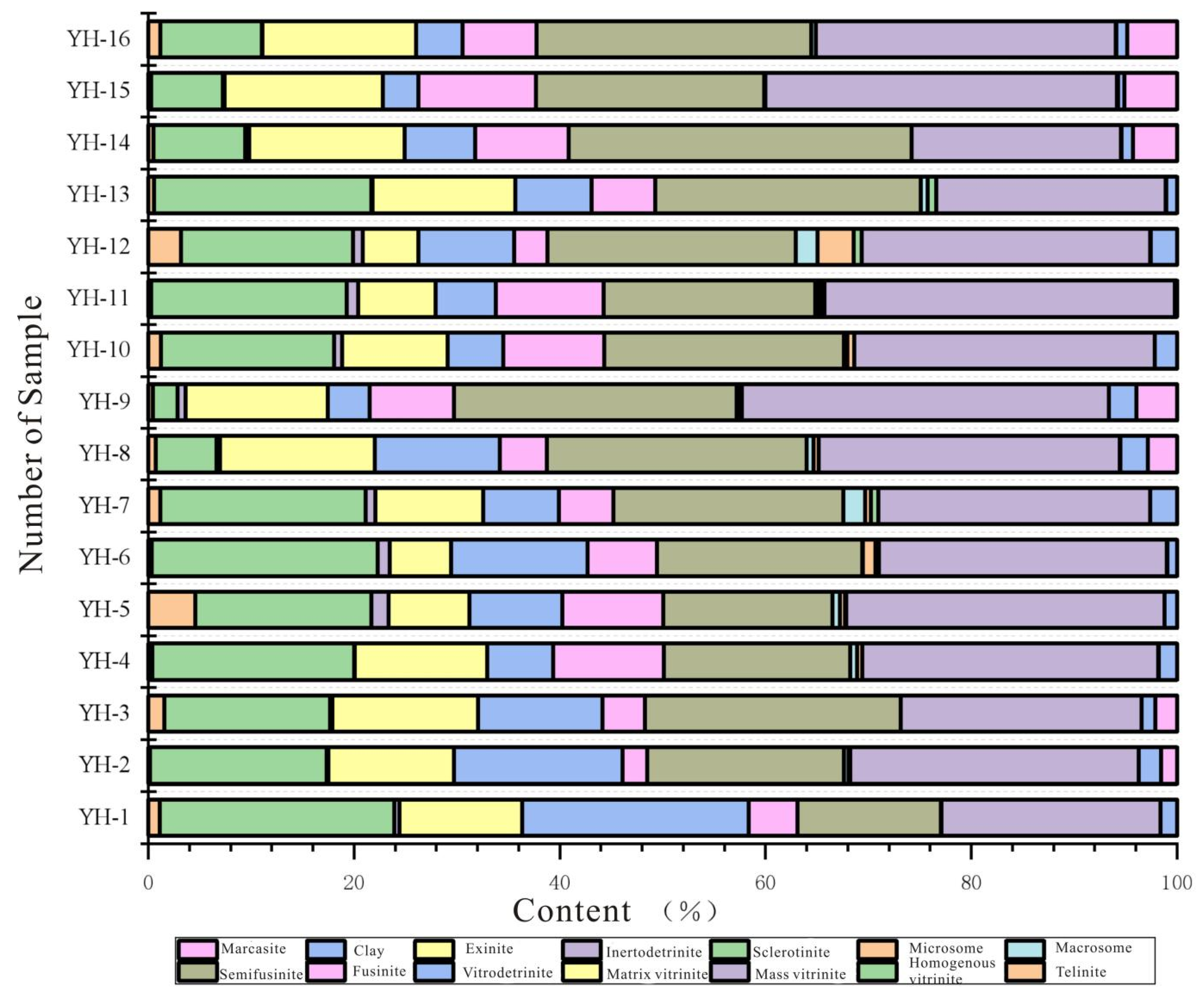

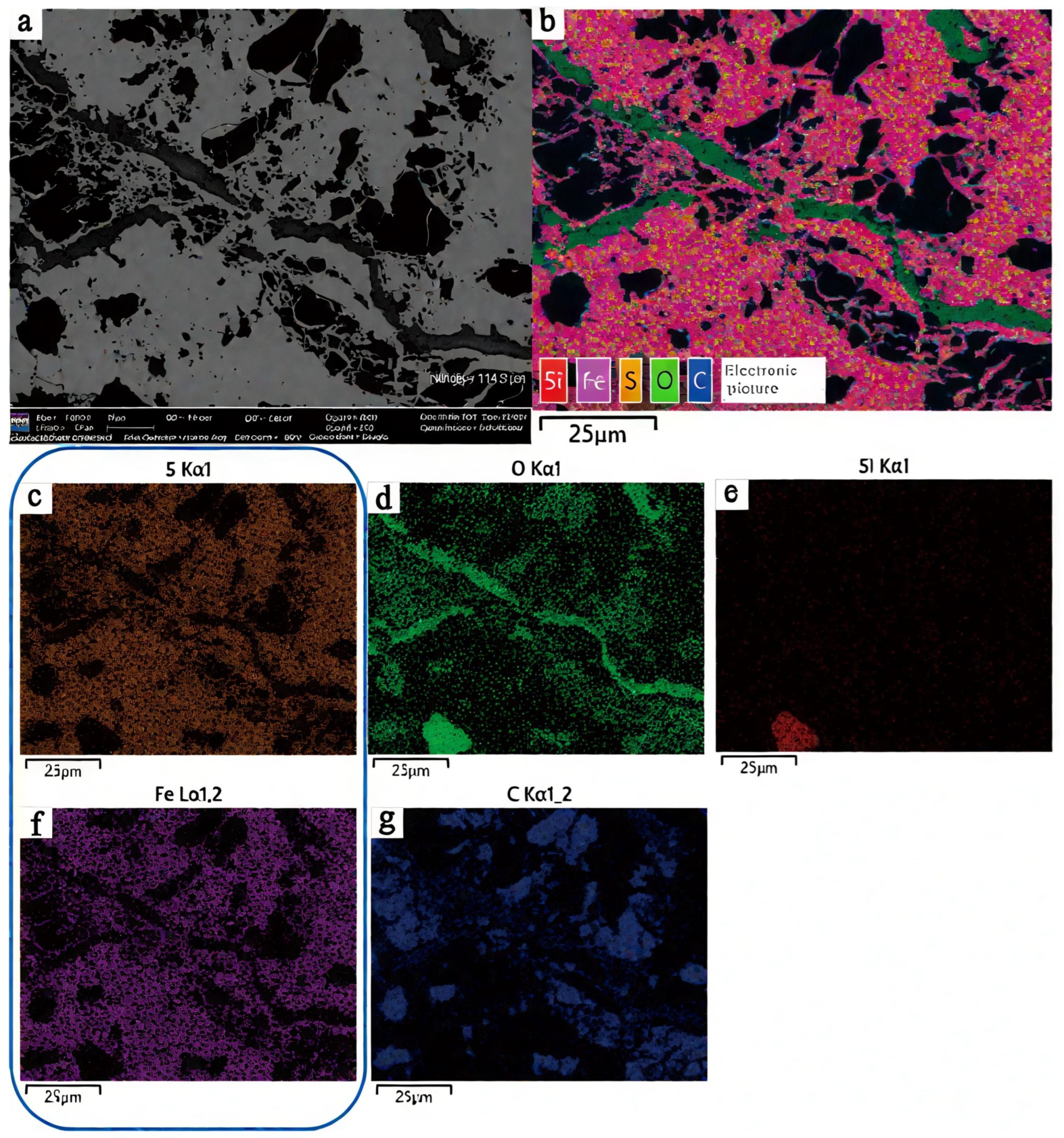
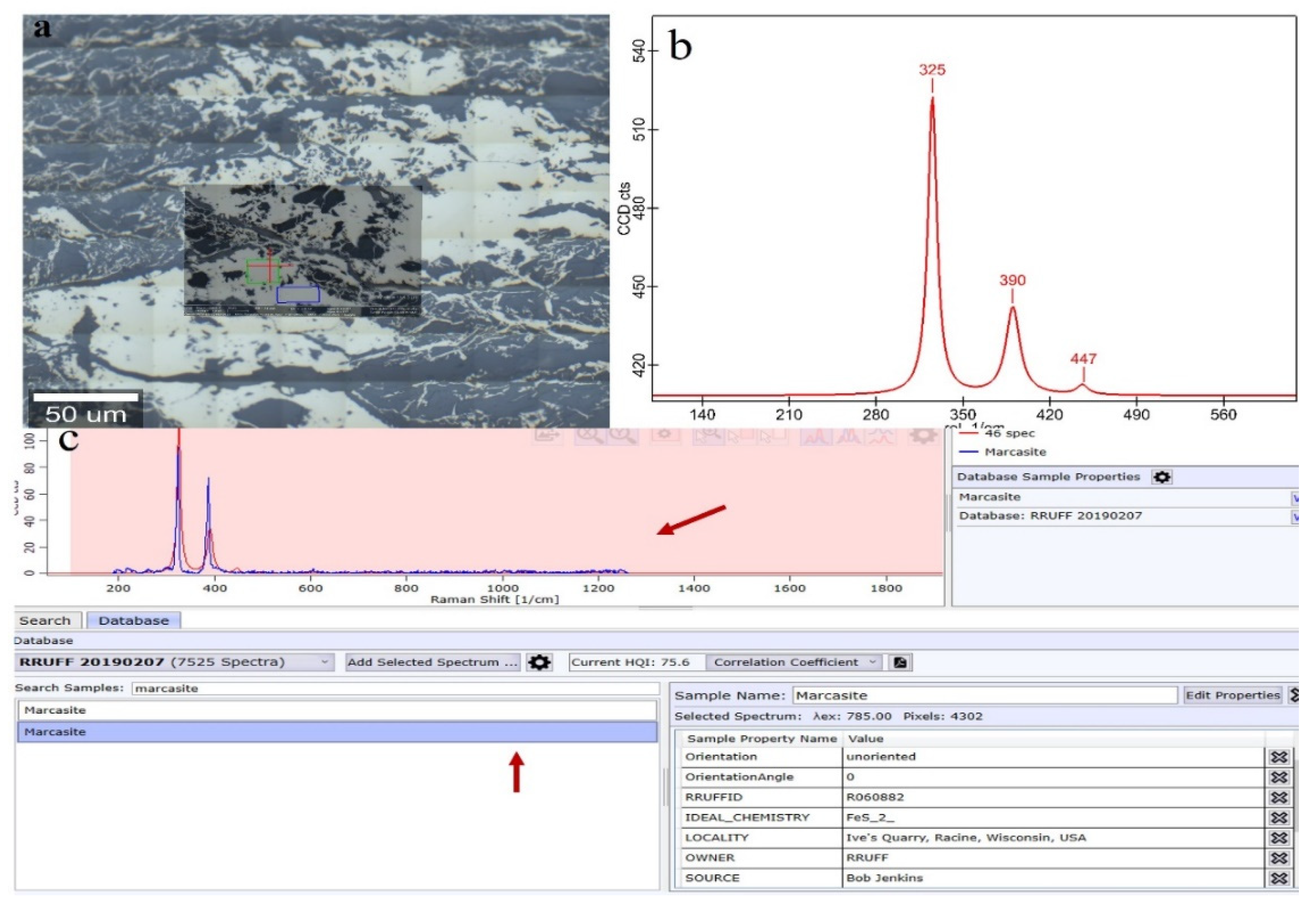
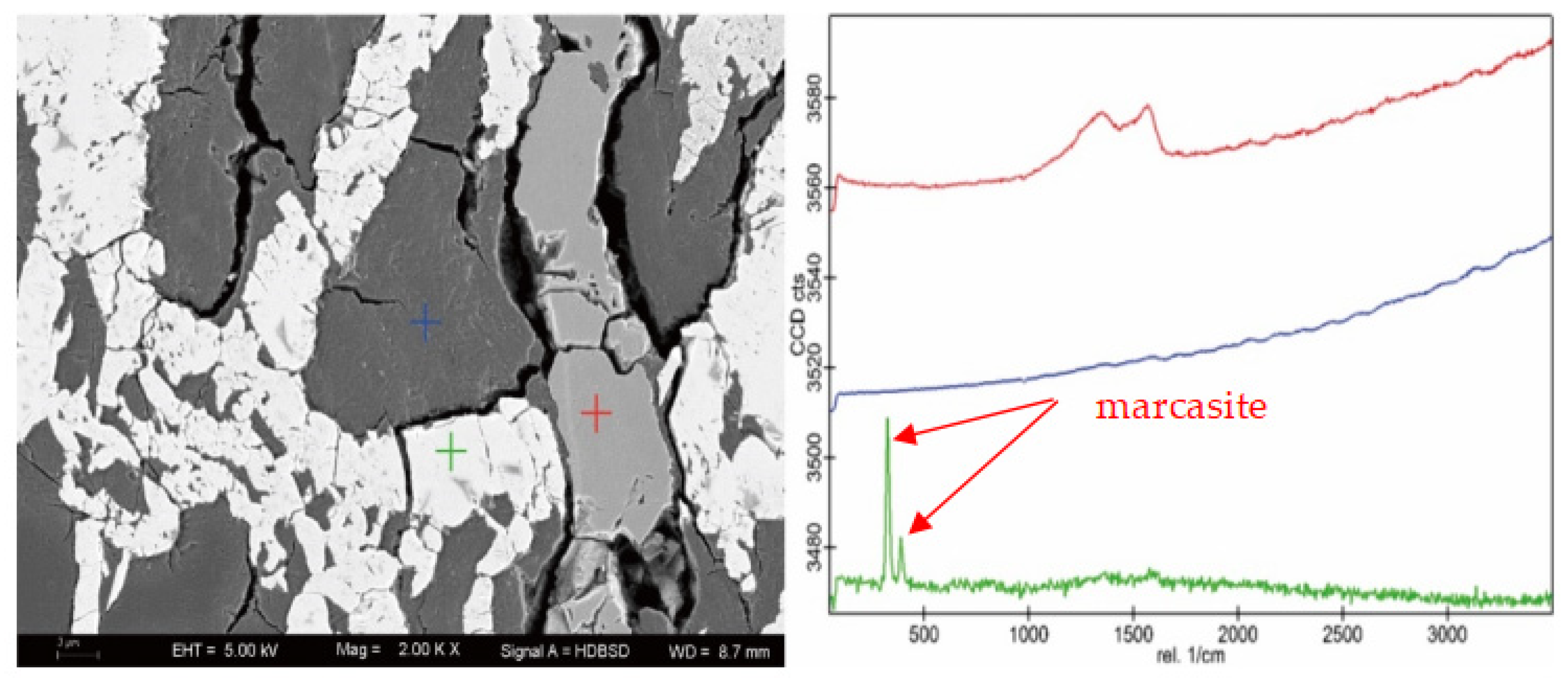
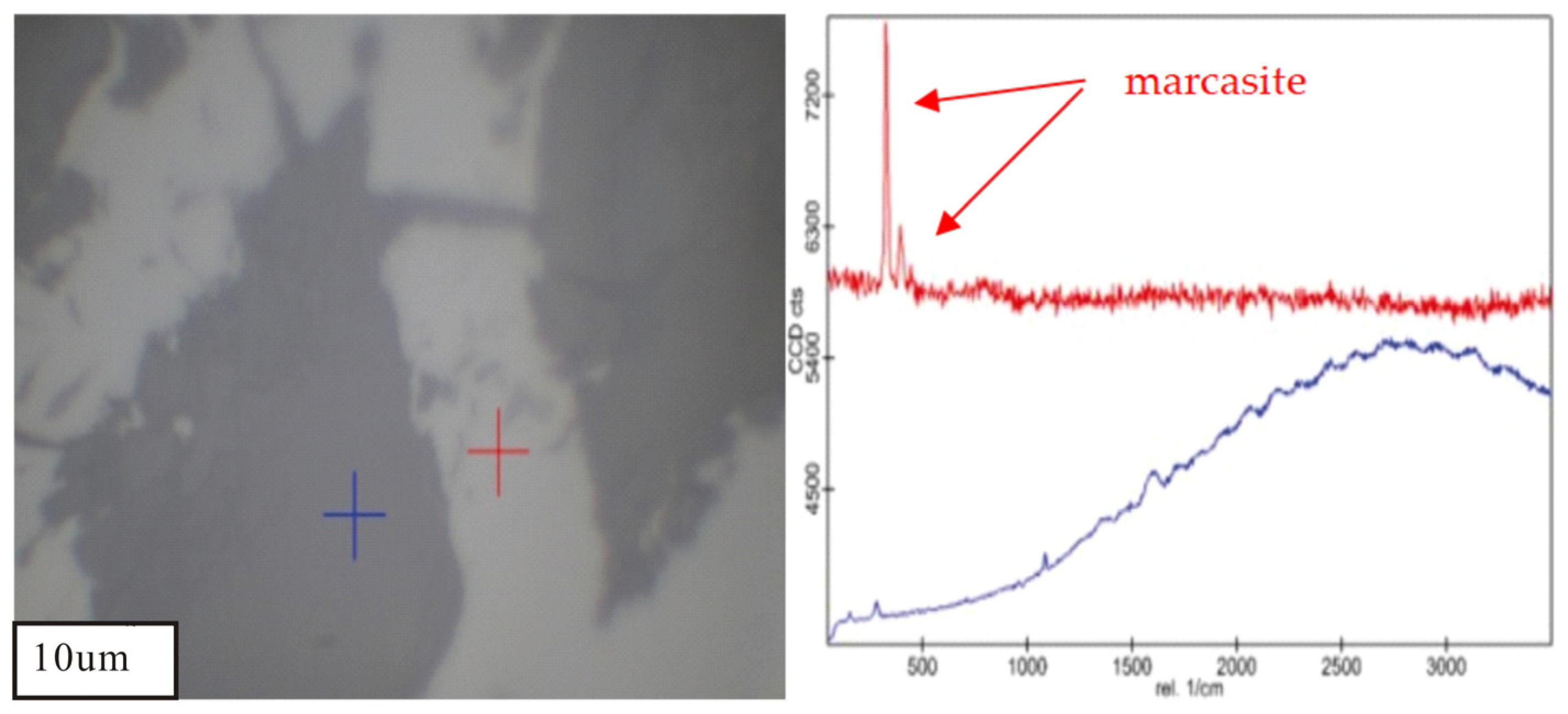
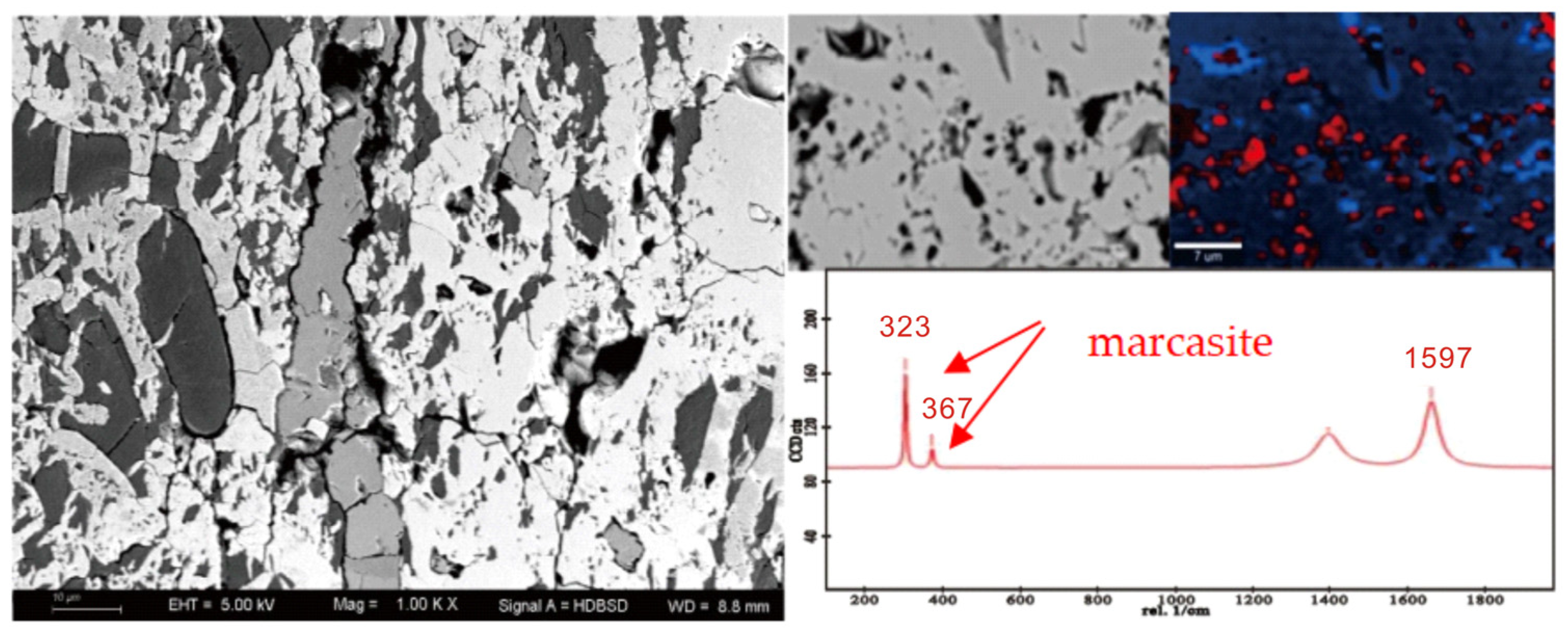
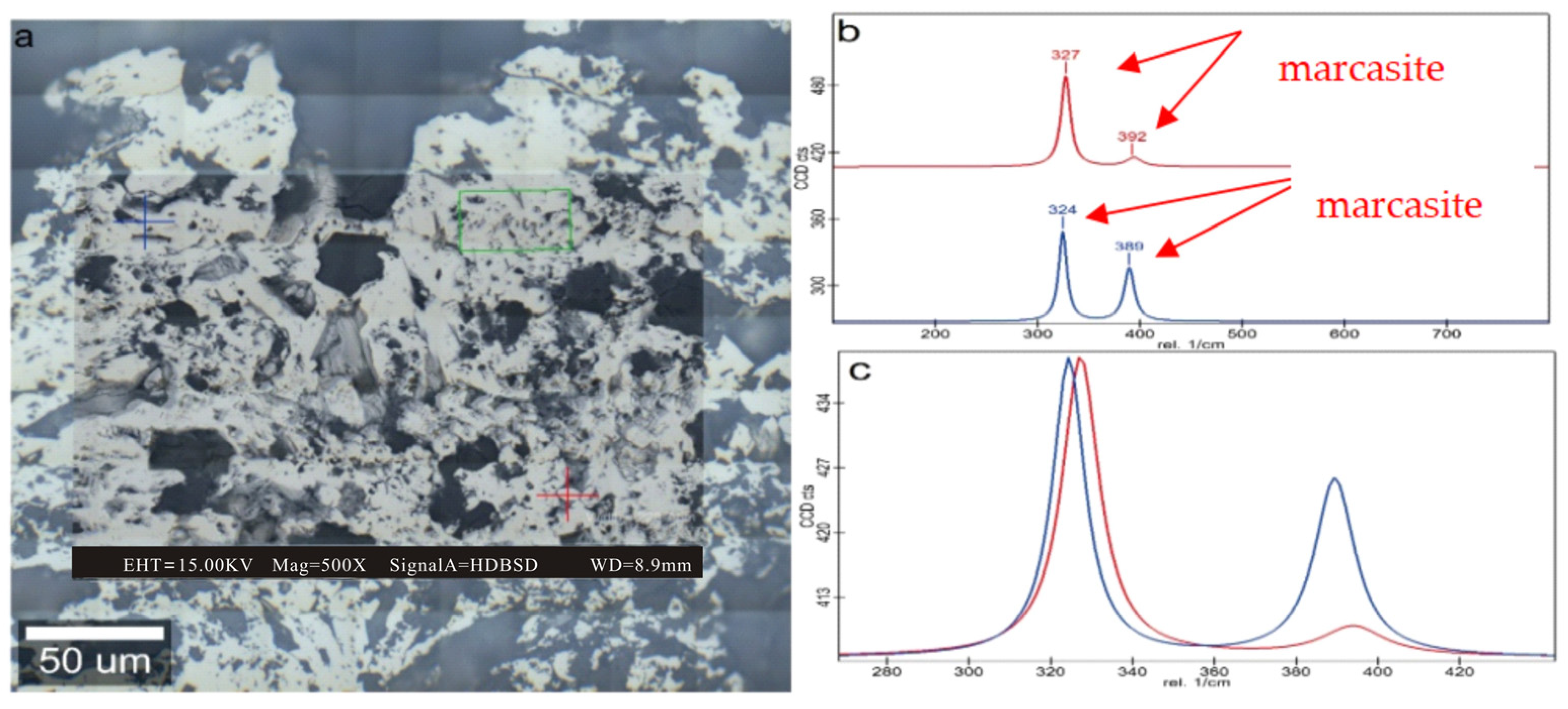
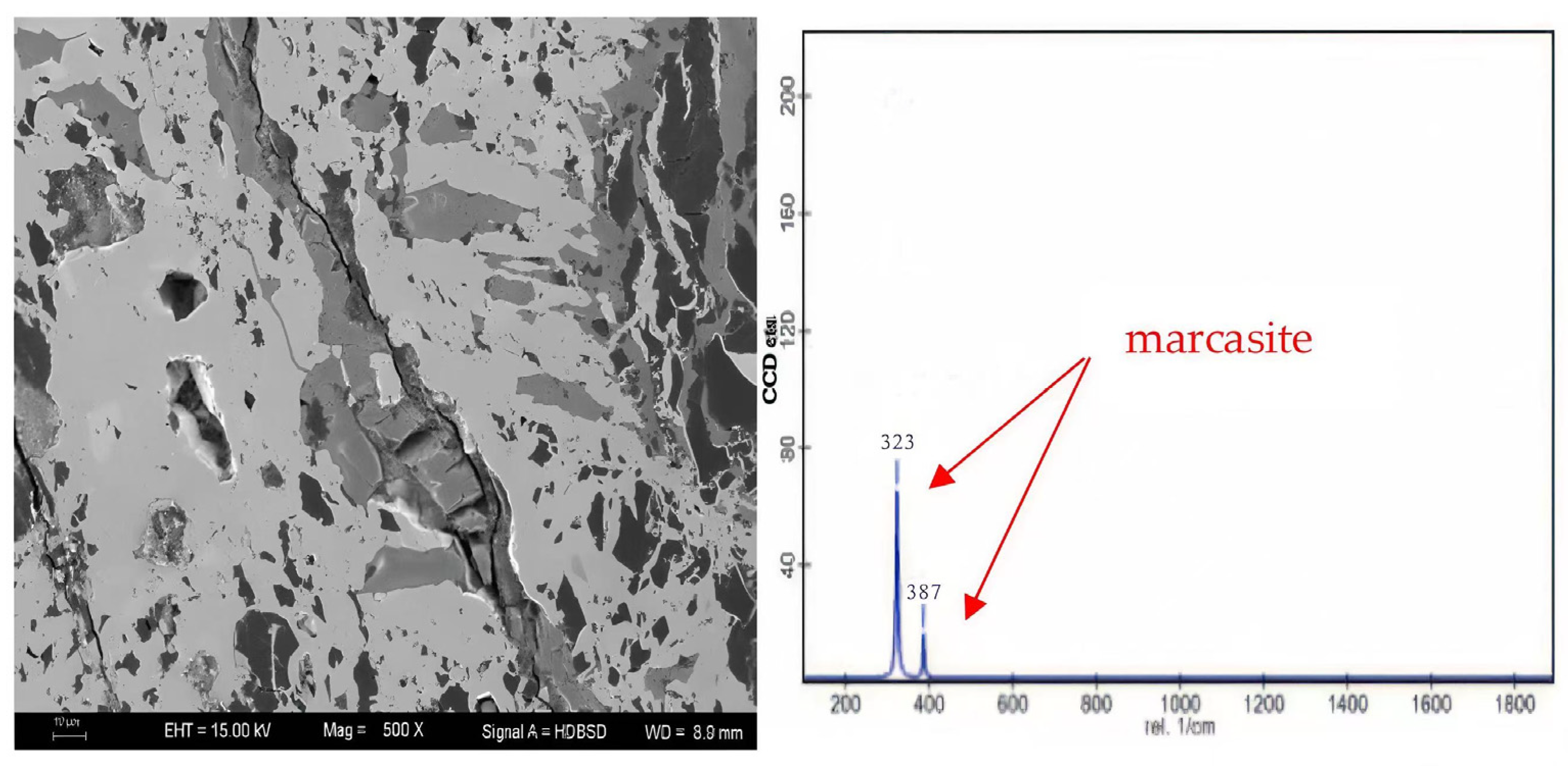
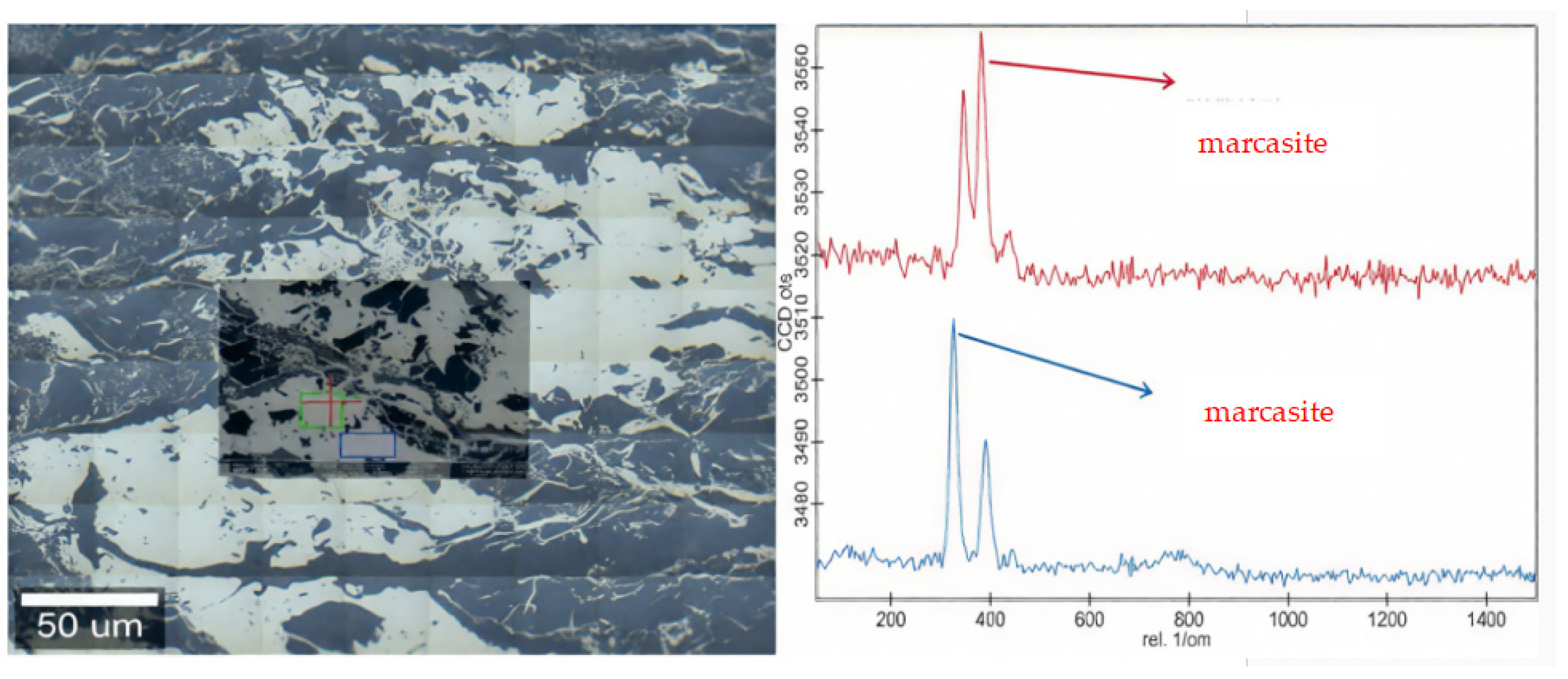
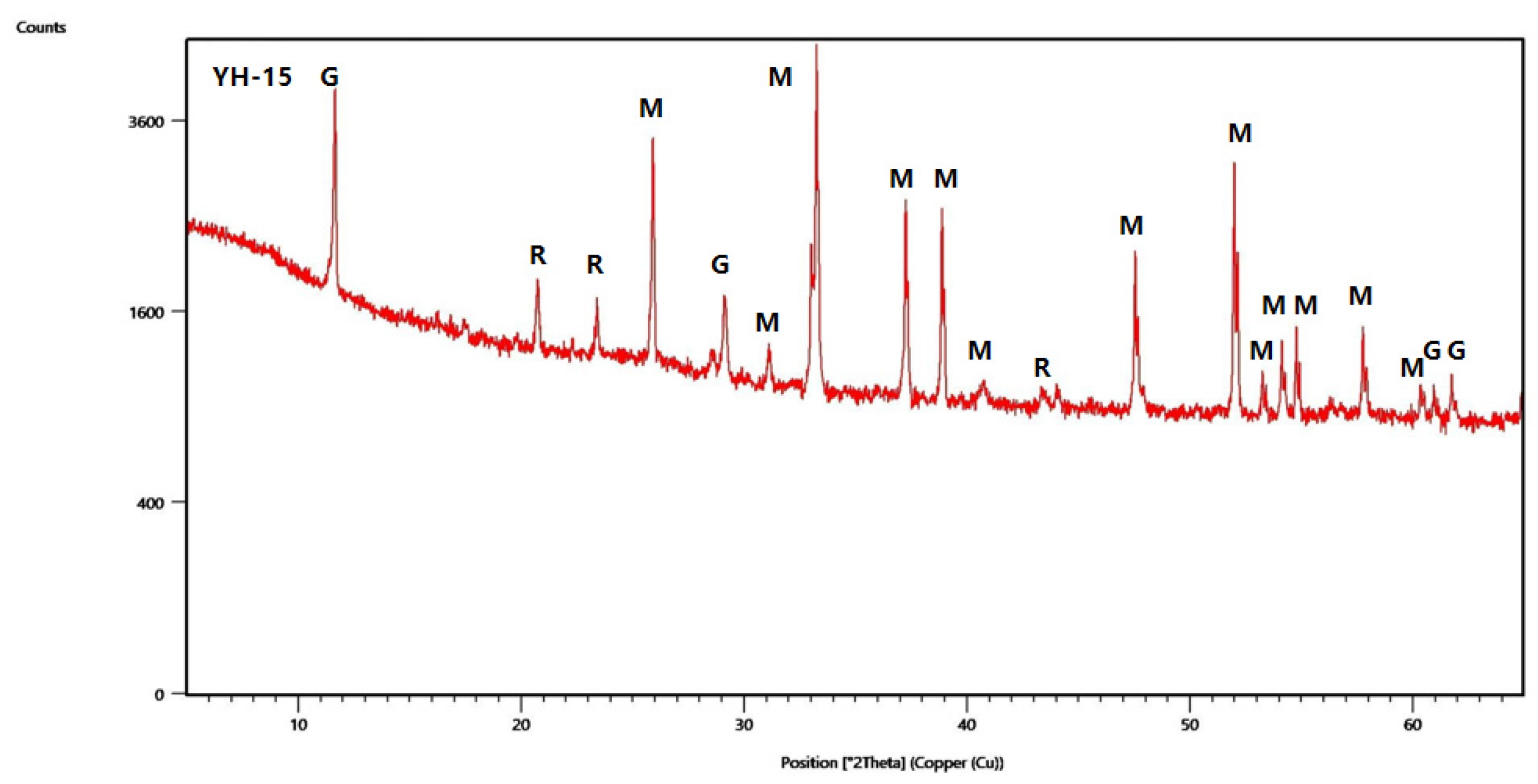
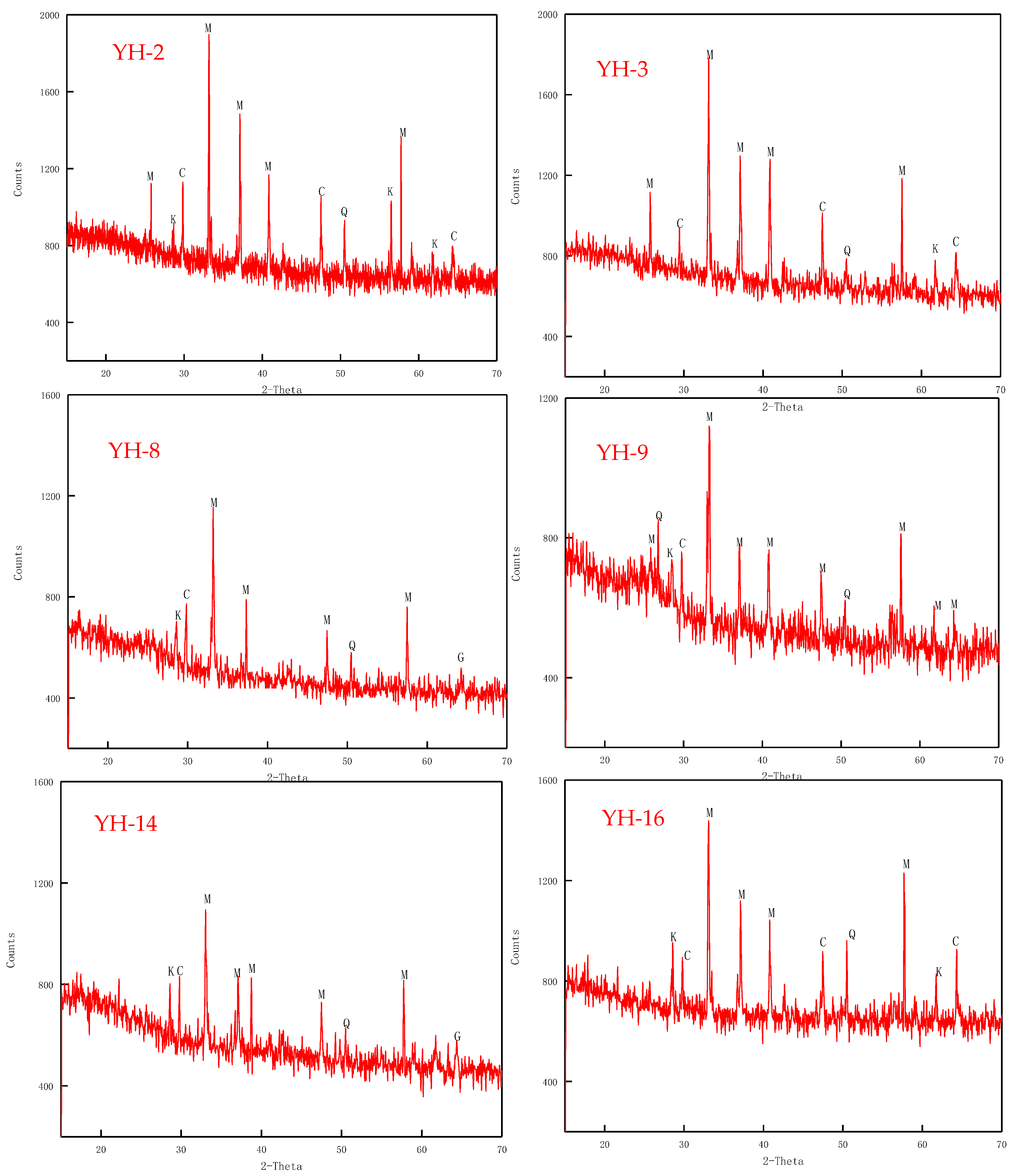
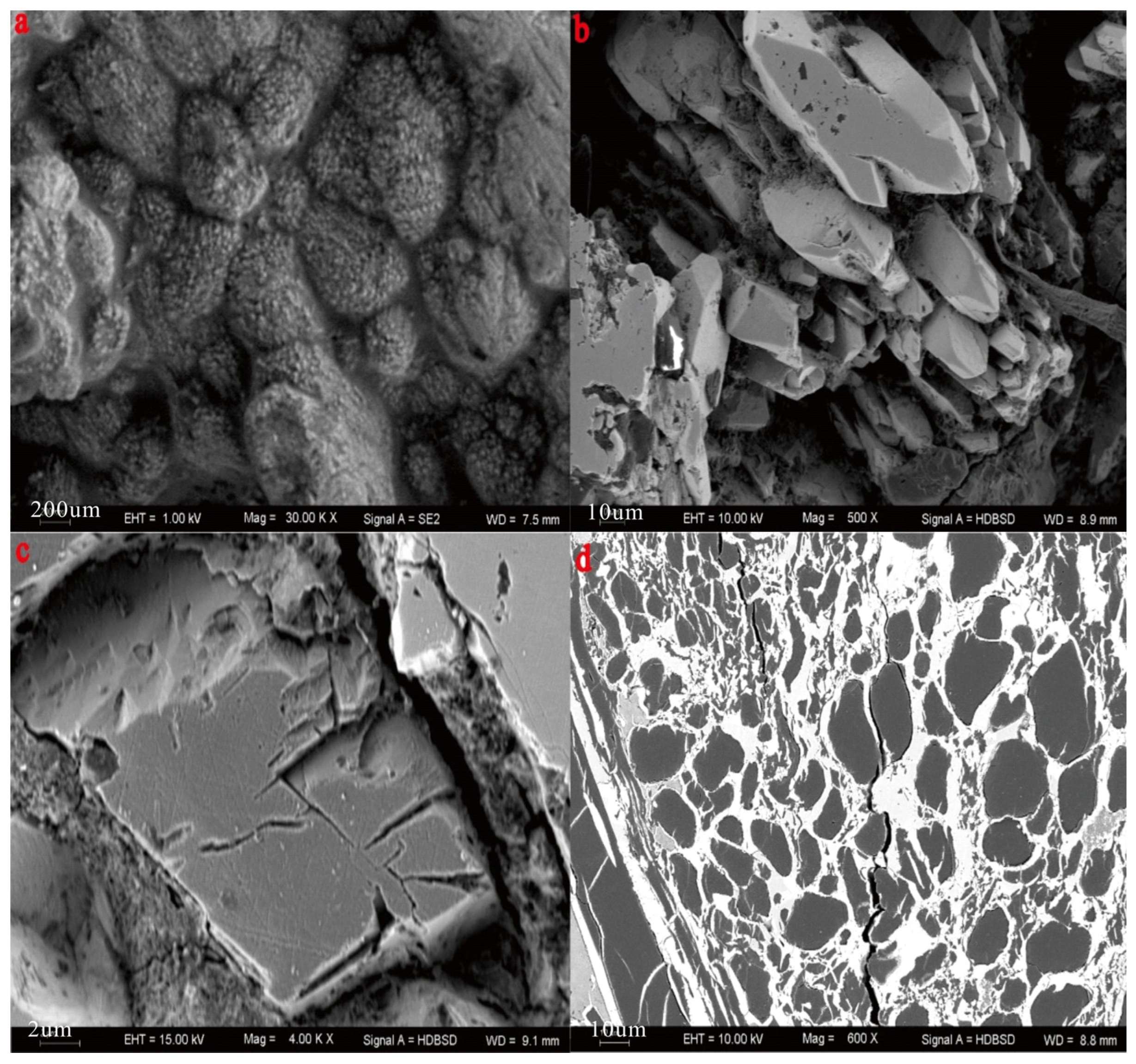
| Sample | Vitrinite (%) | Inertinite (%) | Liptinite (%) | Clay (%) | Marcasite (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TE | TEL | CO | DE | VI | SUM | FU | SE | MA | MI | FUN | IN | SUM | ||||
| YH-1 | 0.19 | 10.13 | 0.18 | 16.21 | 15.37 | 42.08 | 9.38 | 21.14 | 0.39 | 0.10 | 0.07 | 25.03 | 56.11 | 0.02 | 1.15 | - |
| YH-2 | 1.12 | 13.79 | 0.49 | 3.93 | 8.02 | 27.35 | 11.69 | 23.94 | 0.03 | 0.07 | - | 31.23 | 66.96 | - | 2.64 | 2.58 |
| YH-3 | 0.28 | 13.94 | 1.12 | 7.53 | 5.85 | 28.72 | 10.47 | 20.52 | 0.37 | 0.32 | 0.16 | 34.03 | 65.87 | 0.02 | 2.20 | 2.21 |
| YH-4 | 0.37 | 19.57 | 0.04 | 12.86 | 6.36 | 39.20 | 10.72 | 18.06 | 0.70 | 0.46 | 0.00 | 28.68 | 58.62 | 0.07 | 1.77 | - |
| YH-5 | 4.55 | 17.09 | 1.71 | 7.81 | 9.09 | 40.25 | 9.76 | 16.48 | 0.73 | 0.46 | 0.16 | 30.91 | 58.50 | 0.01 | 1.20 | - |
| YH-6 | 0.33 | 21.79 | 1.19 | 5.91 | 13.22 | 42.44 | 6.69 | 19.80 | 0.08 | 1.17 | 0.37 | 27.78 | 55.89 | 0.06 | 0.94 | - |
| YH-7 | 1.14 | 19.76 | 0.95 | 10.41 | 7.26 | 39.52 | 5.25 | 22.15 | 2.08 | 0.55 | 0.76 | 26.14 | 56.93 | 0.04 | 1.57 | - |
| YH-8 | 1.22 | 8.72 | 0.81 | 10.23 | 5.34 | 26.32 | 9.78 | 26.17 | 0.37 | 0.64 | 0.00 | 32.10 | 69.06 | - | 1.83 | 2.76 |
| YH-9 | 1.14 | 6.76 | 0.95 | 5.41 | 12.26 | 26.52 | 7.25 | 24.15 | 2.08 | 3.55 | 0.76 | 30.04 | 67.83 | 0.04 | 2.07 | 2.55 |
| YH-10 | 0.55 | 21.09 | 0.09 | 13.91 | 7.36 | 43.00 | 6.18 | 25.82 | 0.63 | 0.03 | 0.84 | 22.27 | 55.77 | 0.01 | 1.08 | - |
| YH-11 | 0.52 | 26.12 | 0.21 | 13.13 | 16.07 | 56.05 | 4.11 | 19.82 | - | - | - | 18.37 | 42.30 | 0.01 | 1.34 | - |
| YH-12 | 3.70 | 24.87 | 0.35 | 10.43 | 13.07 | 52.42 | 7.56 | 23.06 | 0.70 | 0.46 | - | 14.08 | 45.86 | 0.04 | 0.78 | - |
| YH-13 | 4.73 | 21.98 | 0.73 | 11.16 | 10.04 | 48.64 | 5.13 | 20.34 | 0.31 | - | 0.17 | 23.49 | 49.44 | 0.01 | 1.65 | - |
| YH-14 | 0.49 | 4.89 | 0.39 | 6.13 | 11.85 | 23.75 | 12.10 | 26.34 | - | - | - | 32.35 | 70.79 | 0.02 | 1.14 | 4.28 |
| YH-15 | 0.26 | 1.99 | 0.16 | 3.07 | 6.45 | 11.93 | 10.39 | 32.18 | 0.11 | - | - | 37.16 | 79.84 | 0.05 | 2.64 | 5.13 |
| YH-16 | 1.14 | 1.90 | 0.03 | 11.84 | 8.82 | 23.73 | 16.19 | 24.70 | 0.45 | - | - | 29.16 | 70.50 | 0.01 | 2.07 | 3.56 |
| Sample | SiO2 | Al2O3 | Fe2O3 | CaO | Na2O | MgO | TiO2 | K2O | P2O5 | MnO |
|---|---|---|---|---|---|---|---|---|---|---|
| YH-1 | 0.56 | 0.28 | 0.21 | 2.05 | 0.46 | 1.12 | 0.006 | 0.005 | 0.012 | 0.003 |
| YH-2 | 0.45 | 0.36 | 0.92 | 2.52 | 0.43 | 1.23 | 0.004 | 0.006 | 0.016 | 0.005 |
| YH-3 | 0.36 | 0.25 | 1.68 | 2.35 | 0.42 | 1.04 | 0.008 | 0.008 | 0.102 | 0.002 |
| YH-4 | 0.85 | 0.65 | 0.21 | 2.36 | 0.41 | 1.15 | 0.006 | 0.007 | 0.026 | 0.003 |
| YH-5 | 0.42 | 0.32 | 0.19 | 2.12 | 0.38 | 1.06 | 0.007 | 0.006 | 0.066 | 0.016 |
| YH-6 | 0.31 | 0.22 | 0.12 | 2.45 | 0.21 | 1.02 | 0.005 | 0.005 | 0.0196 | 0.065 |
| YH-7 | 0.52 | 0.28 | 0.42 | 4.32 | 0.35 | 1.21 | 0.008 | 0.058 | 0.008 | 0.009 |
| YH-8 | 0.36 | 6.62 | 1.65 | 2.40 | 0.46 | 0.42 | 0.005 | 0.156 | 0.026 | 0.008 |
| YH-9 | 0.54 | 0.32 | 0.72 | 2.38 | 0.47 | 1.18 | 0.009 | 0.007 | 0.016 | 0.007 |
| YH-10 | 0.48 | 0.25 | 0.21 | 2.32 | 0.15 | 1.16 | 0.005 | 0.008 | 0.006 | 0.006 |
| YH-11 | 0.21 | 0.18 | 0.34 | 15.06 | 1.08 | 0.62 | 0.006 | 0.002 | 0.009 | 0.008 |
| YH-12 | 0.96 | 0.42 | 0.20 | 2.45 | 0.36 | 1.16 | 0.011 | 0.008 | 0.038 | 0.009 |
| YH-13 | 0.35 | 0.12 | 0.38 | 1.82 | 0.54 | 1.18 | 0.012 | 0.007 | 0.018 | 0.106 |
| YH-14 | 0.32 | 0.25 | 0.05 | 2.56 | 0.26 | 0.96 | 0.015 | 0.006 | 0.008 | 0.007 |
| YH-15 | 0.33 | 0.32 | 4.12 | 12.6 | 0.25 | 0.82 | 0.018 | 0.003 | 0.005 | 0.13 |
| YH-16 | 0.62 | 0.41 | 0.62 | 2.62 | 0.35 | 1.06 | 0.013 | 0.006 | 0.002 | 0.006 |
| Ave | 0.48 | 0.70 | 0.75 | 3.89 | 0.41 | 1.02 | 0.01 | 0.02 | 0.02 | 0.02 |
| China | 8.47 | 5.98 | 4.85 | 1.23 | 0.16 | 0.22 | 0.33 | 0.19 | 0.19 | 0.19 |
| Sample | Li | Sr | Ni | Ga | V | Cr | Co | Ba | U | Th |
|---|---|---|---|---|---|---|---|---|---|---|
| YH-1 | 33.92 | 106.82 | 9.63 | 17.76 | 29.19 | 16.06 | 2.01 | 191.16 | 1.07 | 3.34 |
| YH-2 | 40.19 | 95.46 | 13.77 | 20.91 | 46.08 | 9.40 | 1.86 | 204.05 | 4.26 | 2.51 |
| YH-3 | 49.46 | 108.34 | 15.87 | 18.85 | 54.94 | 10.73 | 1.64 | 257.19 | 4.75 | 3.42 |
| YH-4 | 55.16 | 126.46 | 10.16 | 17.96 | 12.46 | 7.31 | 2.47 | 216.74 | 0.75 | 2.40 |
| YH-5 | 57.46 | 111.27 | 9.72 | 19.09 | 23.73 | 15.26 | 3.14 | 210.43 | 1.61 | 5.69 |
| YH-6 | 65.46 | 127.06 | 6.37 | 17.79 | 16.47 | 9.11 | 2.76 | 247.88 | 2.36 | 10.24 |
| YH-7 | 80.47 | 136.17 | 8.38 | 21.09 | 23.24 | 13.21 | 2.04 | 259.05 | 1.36 | 6.02 |
| YH-8 | 85.43 | 115.44 | 12.28 | 23.32 | 60.65 | 14.14 | 1.67 | 248.42 | 10.90 | 4.12 |
| YH-9 | 73.09 | 127.81 | 15.64 | 18.06 | 85.64 | 19.08 | 1.86 | 291.83 | 8.34 | 5.27 |
| YH-10 | 83.46 | 140.49 | 10.46 | 19.67 | 30.1 | 16.85 | 2.41 | 245.76 | 2.71 | 12.73 |
| YH-11 | 70.76 | 144.84 | 12.86 | 17.82 | 18.92 | 8.71 | 2.86 | 251.46 | 1.88 | 8.26 |
| YH-12 | 61.26 | 116.76 | 9.16 | 21.86 | 14.10 | 7.78 | 3.26 | 234.37 | 2.03 | 9.08 |
| YH-13 | 60.16 | 122.39 | 11.95 | 17.09 | 18.78 | 9.98 | 3.24 | 217.92 | 2.05 | 7.18 |
| YH-14 | 48.72 | 104.82 | 16.17 | 22.94 | 58.68 | 12.11 | 2.28 | 208.47 | 16.5 | 4.83 |
| YH-15 | 36.43 | 99.75 | 20.82 | 24.76 | 72.96 | 16.98 | 1.97 | 227.72 | 24.3 | 3.87 |
| YH-16 | 40.79 | 82.49 | 15.74 | 21.43 | 84.21 | 19.68 | 2.05 | 211.79 | 45.3 | 5.71 |
| Ave | 58.89 | 116.65 | 12.44 | 20.06 | 40.63 | 12.90 | 2.35 | 232.77 | 8.14 | 5.92 |
| China Coal | 43.91 | 140.20 | 13.72 | 6.64 | 34.97 | 15.35 | 7.07 | 158.70 | 2.41 | 5.84 |
| Sample Description | Test Items | Unit | δ34S | Mean Value |
|---|---|---|---|---|
| YH-2 | Sulfur isotopic | ‰ | −25.4~−21.6 | −23.5 |
| YH-3 | −34.6~−31.6 | −33.1 | ||
| YH-8 | −7.2~+12.8 | 2.8 | ||
| YH-9 | −3.5~+16.7 | 6.6 | ||
| YH-14 | −6.3~+13.1 | 3.4 | ||
| YH-15 | −19.5~+6.6 | 12.9 | ||
| YH-16 | −21.6~+8.2 | 13.4 |
| Microelement | Reducing Environment | Weak Oxidation–Weak Reduction Environment | Oxidizing Milieu | Sample | |
|---|---|---|---|---|---|
| Min–Max | Mean Value | ||||
| δU | >1.0 | 0.7~0.0 | <0.7 | 0.80~1.92 | 1.26 |
| V/Cr | >4.25 | 2.0~4.25 | <2.0 | 1.79~5.12 | 3.73 |
| Ni/Co | >7.0 | 5.0~7.0 | <5.0 | 2.31~10.57 | 5.75 |
| U/Th | >1.25 | 0.75~1.25 | <0.75 | 0.21~7.93 | 1.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Lü, N.; Feng, S.; Wang, W.; Tian, J.; Li, X.; Xadethan, H. Occurrence State and Genesis of Large Particle Marcasite in a Thick Coal Seam of the Zhundong Coalfield in Xinjiang. Minerals 2025, 15, 816. https://doi.org/10.3390/min15080816
Wu X, Lü N, Feng S, Wang W, Tian J, Li X, Xadethan H. Occurrence State and Genesis of Large Particle Marcasite in a Thick Coal Seam of the Zhundong Coalfield in Xinjiang. Minerals. 2025; 15(8):816. https://doi.org/10.3390/min15080816
Chicago/Turabian StyleWu, Xue, Ning Lü, Shuo Feng, Wenfeng Wang, Jijun Tian, Xin Li, and Hayerhan Xadethan. 2025. "Occurrence State and Genesis of Large Particle Marcasite in a Thick Coal Seam of the Zhundong Coalfield in Xinjiang" Minerals 15, no. 8: 816. https://doi.org/10.3390/min15080816
APA StyleWu, X., Lü, N., Feng, S., Wang, W., Tian, J., Li, X., & Xadethan, H. (2025). Occurrence State and Genesis of Large Particle Marcasite in a Thick Coal Seam of the Zhundong Coalfield in Xinjiang. Minerals, 15(8), 816. https://doi.org/10.3390/min15080816







