Recycling Quarry Dust as a Supplementary Cementitious Material for Cemented Paste Backfill
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Analytical Methods
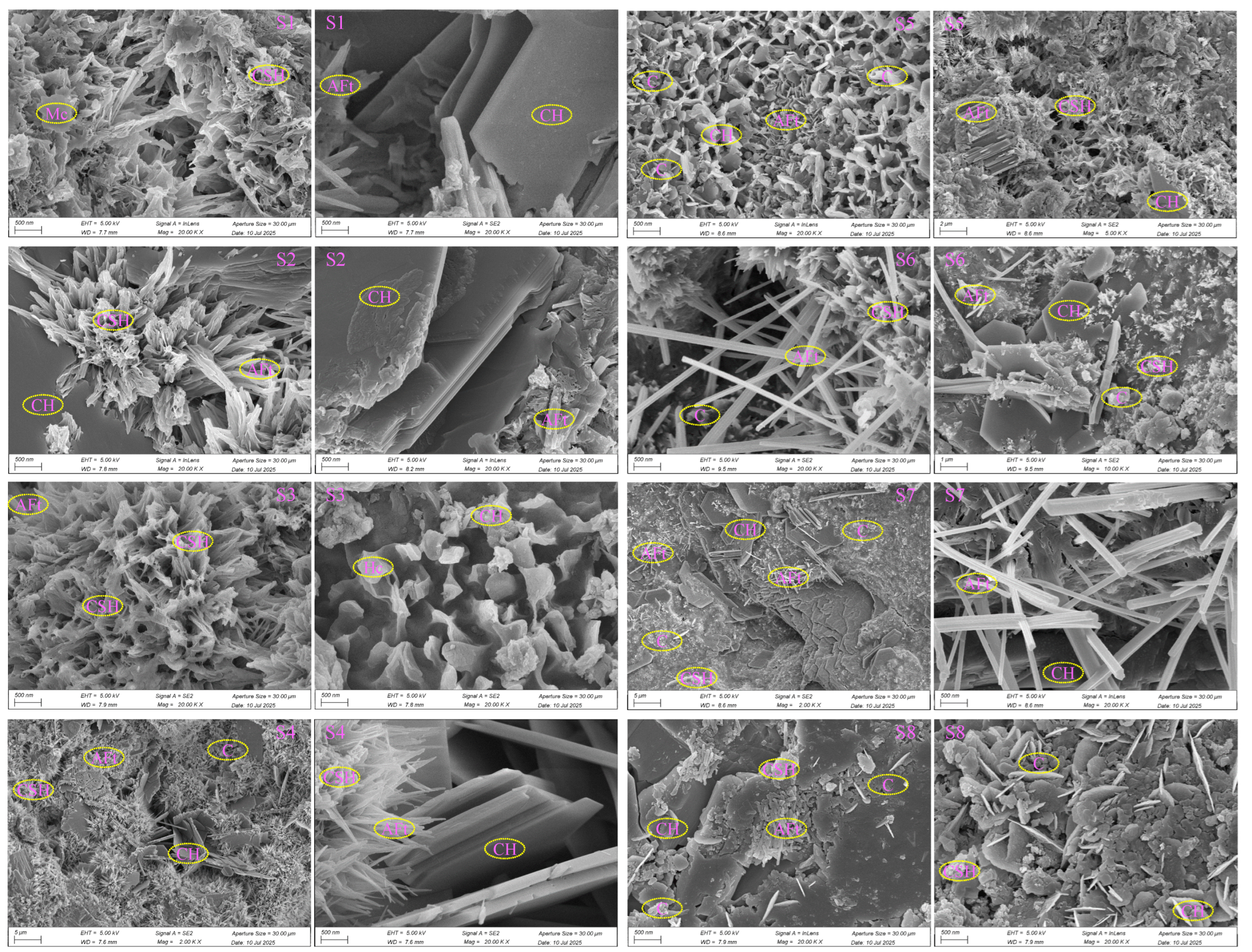
3. Results and Discussion
3.1. MQD Mineral Phase Transformation
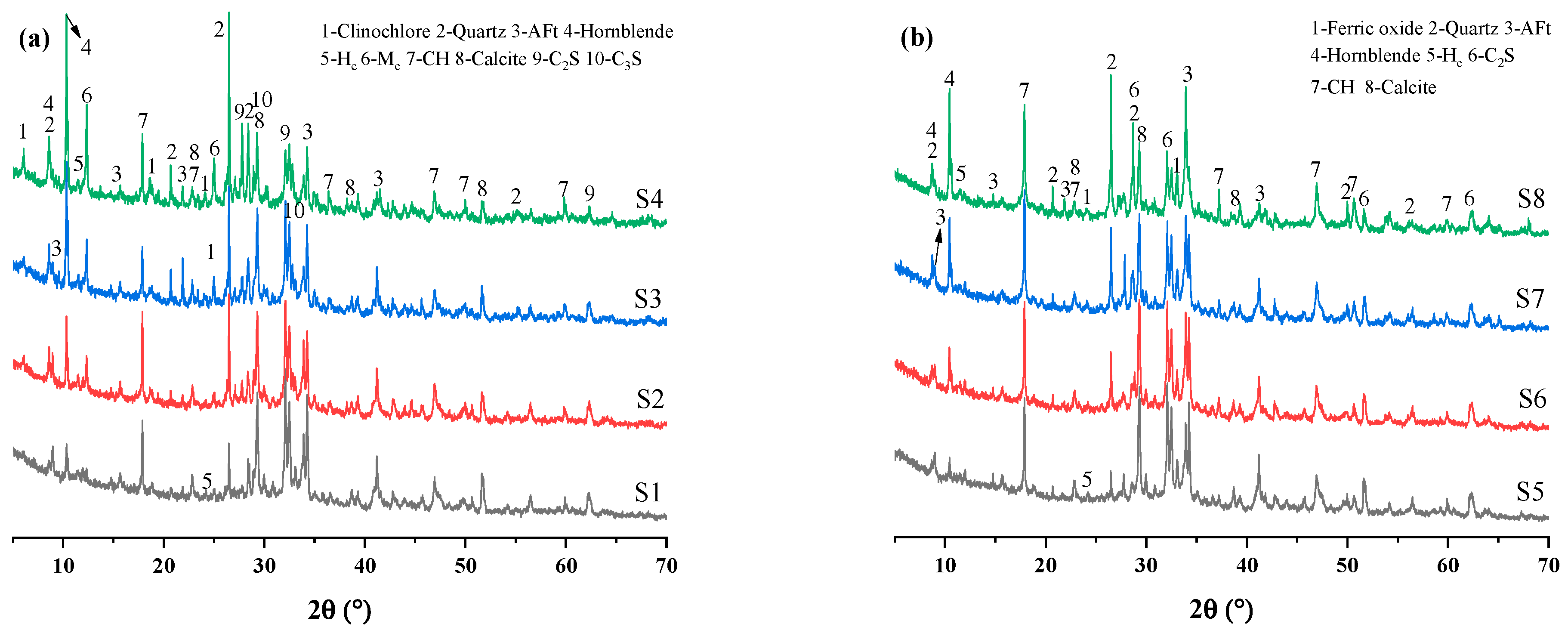
3.1.1. XRD Analysis of QD/MQD
3.1.2. FTIR Analysis
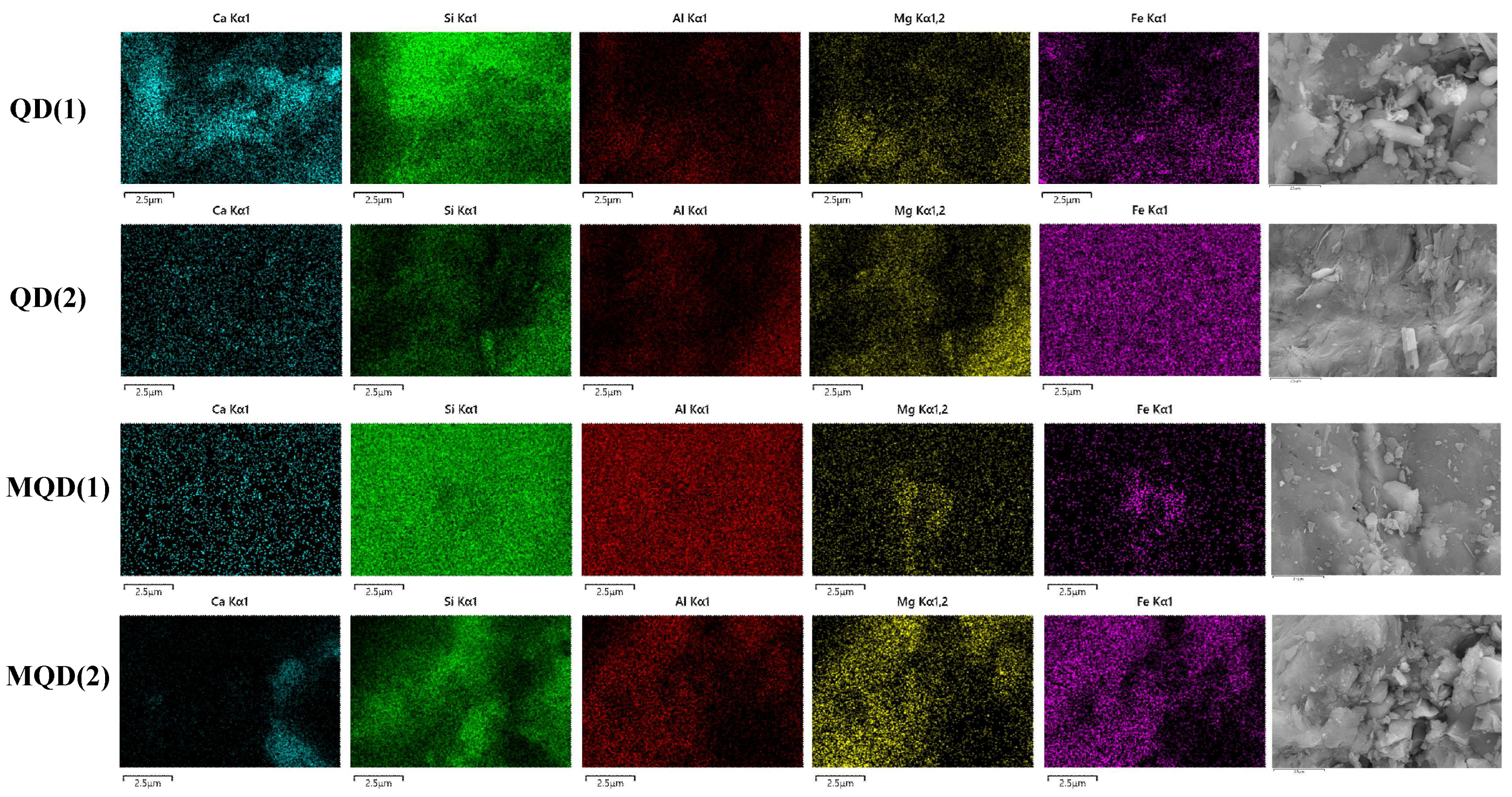
3.1.3. SEM-EDS Analysis
3.2. Hydration Evolution of QD/MQD-Containing CPB
3.2.1. XRD Analysis of QD/MQD-Containing CPB
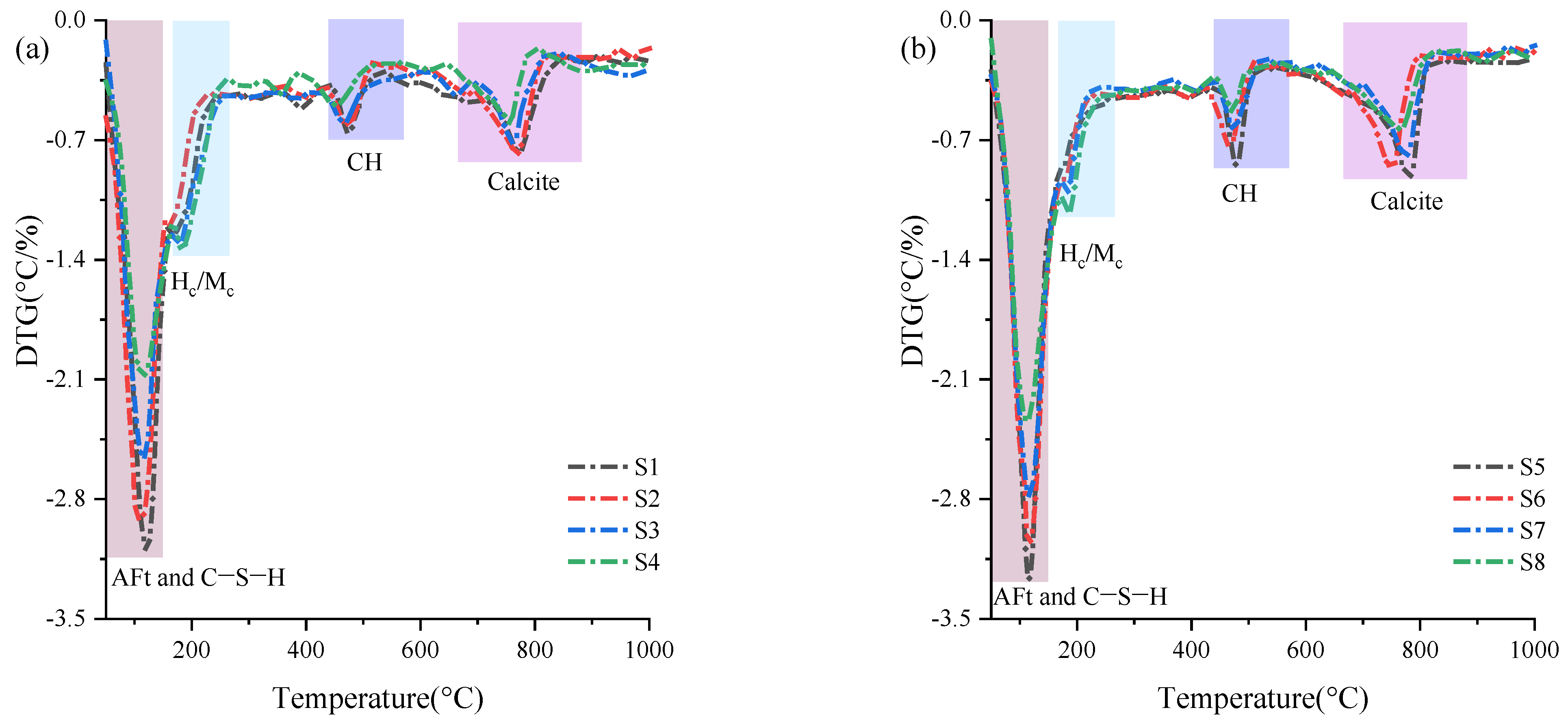
3.2.2. TG Analysis
3.3. Workability of QD/MQD-Containing CPB
3.3.1. Fluidity
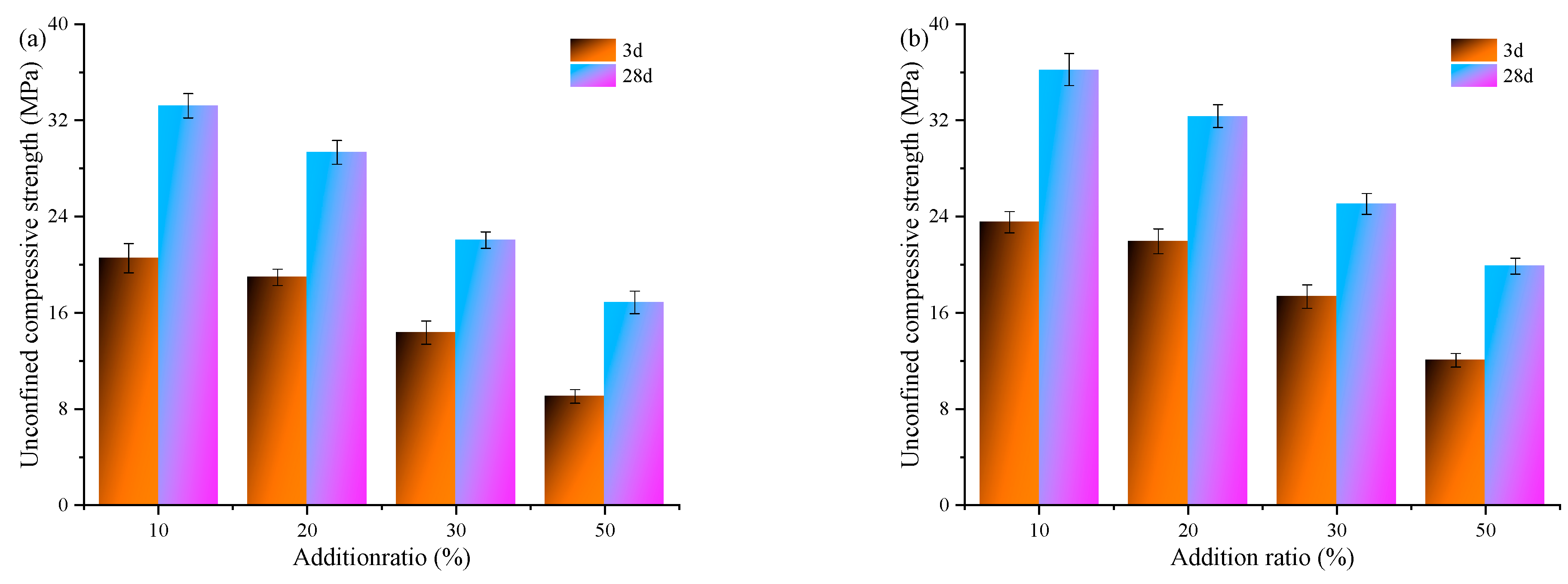
3.3.2. Compressive Strength
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mustasem, E.-F.; Salah, S.; Chahine, W. Environmental management of quarries as waste disposal facilities. Environ. Manag. 2001, 27, 515–531. [Google Scholar] [CrossRef]
- Nur Fatin Amira Mohamed, Y.; Aidah, J.; Ummu Raihanah, H.; Muhd Norhasri Muhd, S.; Haris, N.A. Morphological Characteristics and Mechanical Properties of Quarry Dust Waste as Sand Replacement in Mortar. Int. J. Integr. Eng. 2024, 16, 213–221. [Google Scholar] [CrossRef]
- Thete Swapnil, T.; Arpitha, D.; Suman, S.; Rajasekaran, C. Suitability of Quarry Dust as a Partial Replacement of Fine Aggregate in Self Compacting Concrete. Appl. Mech. Mater. 2018, 877, 248–253. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, S.; Zhao, Y. Recycling arsenic-containing bio-leaching residue after thermal treatment in cemented paste backfill: Structure modification, binder properties and environmental assessment. Int. J. Miner. Metall. Mater. 2024, 31, 2136–2147. [Google Scholar] [CrossRef]
- Guo, Z.; Qiu, J.; Jiang, H.; Zhang, S.; Ding, H. Improving the performance of superfine-tailings cemented paste backfill with a new blended binder. Powder Technol. 2021, 394, 149–160. [Google Scholar] [CrossRef]
- Qiu, J.; Guo, Z.; Yang, L.; Jiang, H.; Zhao, Y. Effects of packing density and water film thickness on the fluidity behaviour of cemented paste backfill. Powder Technol. 2020, 359, 27–35. [Google Scholar] [CrossRef]
- Aamar, D.; Togay, O.; Mohammad Ali, M.; Muhammad Usama, S.; Muhammed, B.; Jung Heum, Y.; Komael, J. Sustainability benefits and commercialization challenges and strategies of geopolymer concrete: A review. J. Build. Eng. 2022, 58, 105005. [Google Scholar] [CrossRef]
- Magdalena, D.; Bagcal, O.; Ahmet, B.; Dimitrios, G.G.; Köksal, F.; Błażej, P.; Hüsamettin, Ü. Utilization of rock dust as cement replacement in cement composites: An alternative approach to sustainable mortar and concrete productions. J. Build. Eng. 2023, 69, 106180. [Google Scholar] [CrossRef]
- Burak, F. Utilisation of high volumes of limestone quarry wastes in concrete industry (self-compacting concrete case). Resour. Conserv. Recycl. 2007, 51, 770–791. [Google Scholar] [CrossRef]
- Job, T.; Harilal, B. Properties of cold bonded quarry dust coarse aggregates and its use in concrete. Cem. Concr. Compos. 2015, 62, 67–75. [Google Scholar] [CrossRef]
- Diogo, S.; Filipe, G.; Jorge de, B. Mechanical Properties of Structural Concrete Containing Fine Aggregates from Waste Generated by the Marble Quarrying Industry. J. Mater. Civ. Eng. 2014, 26, 04014008. [Google Scholar] [CrossRef]
- Naganathan, S.; Abdul Razak, H.; Abdul Hamid Siti, N. Effect of quarry dust addition on the performance of controlled low-strength material made from industrial waste incineration bottom ash. Int. J. Miner. Metall. Mater. 2012, 19, 536–541. [Google Scholar] [CrossRef]
- Martins, P. Structural and Geo-Environmental Applications of Waste Quarry Dust. Ph.D. Thesis, Cardiff University, Cardiff, UK, 2014. [Google Scholar]
- Kenneth Miebaka, O.; Tamunoemi Alu, L.; Kaniyeh Anthony, I. Suitability of Saw Dust Ash and Quarry Dust as Mineral fillers in Asphalt Concrete. Int. J. Eng. Manag. Res. 2022, 12, 24–29. [Google Scholar] [CrossRef]
- Yong-Heng, L.; Shenxu, B.; Shuo, L.; Yimin, Z.; Shefeng, L.; Yang, P. Enhancing reactivity of granite waste powder toward geopolymer preparation by mechanical activation. Constr. Build. Mater. 2024, 414, 134981. [Google Scholar] [CrossRef]
- Rana Muhammad, W.; Mohammed, K.A.; Eyad, A.; Faheem, B.; Faisal, S. Experimental investigation of quarry rock dust incorporated fly ash and slag based fiber reinforced geopolymer concrete circular columns. Dent. Sci. Rep. 2024, 14, 20953. [Google Scholar] [CrossRef]
- Zhang, S.; Ren, F.; Zhao, Y.; Qiu, J.; Guo, Z. The effect of stone waste on the properties of cemented paste backfill using alkali-activated slag as binder. Constr. Build. Mater. 2021, 283, 122686. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Bai, H.; Wang, K. Resource utilization of stone waste and loess to prepare grouting materials. J. Environ. Manag. 2024, 354, 120452. [Google Scholar] [CrossRef]
- Yingliang, Z.; Jingping, Q.; Hui, L.; Zhengyu, M. Utilization of quarry dust for low carbon cementitious materials and the immobilization capacity for mine tailings. Constr. Build. Mater. 2020, 262, 120091. [Google Scholar] [CrossRef]
- Jiansong, Z.; Yuzhu, Z.; Yue, L.; Chen, T.; Du, P.; Qianqian, R. Characterization of Physical and Chemical Properties of Multi-Source Metallurgical Dust and Analysis of Resource Utilization Pathways. Superalloys 2024, 14, 1378. [Google Scholar] [CrossRef]
- Daniel, F.-G.; Íñigo, R.-B.; Javier, M.; González-Gasca, C.; Verdeja, L.F. Iron Ore Sintering: Process. Miner. Process. Extr. Metall. Rev. 2017, 38, 215–227. [Google Scholar] [CrossRef]
- C39/C39M-18; Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens. ASTM International: West Conshohocken, PA, USA, 2018.
- Omer, D.H.; Mohammad, Y.O.; Yara, I.O. Metamorphic Evolution of the Garnet Amphibolite Schist from Mawat Ophiolite, Kurdistan Region, Northeast Iraq: Geochemistry, Mineral Chemistry and Thermodynamic Approach. Iraqi Geol. J. 2023, 56, 127–148. [Google Scholar] [CrossRef]
- Vázquez, F.; Torres, L.M.; Garza, L.L.; Martínez, A.; López, W. A Mexican kaolin deposit: XANES characterization, mineralogical phase analysis and applications. Mater. Constr. 2009, 59, 113–121. [Google Scholar] [CrossRef]
- Abel da Silveira, R.; Evaristo, L.; Soria Pereira, A.; Buchner, S. Pressure-induced transformations in a glass: In situ X-Ray diffraction and Raman spectroscopy. Ceram. Int. 2024, 50, 15642–15648. [Google Scholar] [CrossRef]
- Chen, Y.; Kanan, M. Thermal Ca2+/Mg2+ Exchange Reactions to Transform Abundant Silicates Into Alkaline Materials for Carbon Dioxide Removal. ChemRxiv 2024. [Google Scholar] [CrossRef]
- Khodorevskaya, L.I.; Varlamov, D.A.; Safonov, O.G. Experimental Investigation of the Interaction of Amphibole with Highly Salted H2O-NaCl-KCl Fluid at 750°C, 700 MPa: Application to Alkaline Metasomatism of Amphibole Rocks. Петрoлoгия 2023, 31, 388–407. [Google Scholar] [CrossRef]
- Li, Y. Bulk metallic glasses Eutectic coupled zone and amorphous formation. JOM 2005, 57, 60–63. [Google Scholar] [CrossRef]
- Scaltsoyiannes, A.; Lemonidou, A. CaCO3 decomposition for calcium-looping applications: Kinetic modeling in a fixed-bed reactor. Chem. Eng. Sci. X 2020, 8, 100071. [Google Scholar] [CrossRef]
- Ventura, G.D.; Robert, J.L.; Hawthorne, F.C.; Prost, R. Short-range disorder of Si and Ti in the tetrahedral double chain unit of synthetic Ti-bearing potassium-richterite. Am. Mineral. 1996, 81, 56–60. [Google Scholar] [CrossRef]
- Li, Y.; Huang, F.; Gao, W.; Zhu, Q.; Shen, C.; Li, M.; Sun, X.; Wang, X. Raman spectroscopy and XPS study of the thermal decomposition of Mg-hornblende into augite. J. Raman Spectrosc. 2022, 53, 820–831. [Google Scholar] [CrossRef]
- Du, M.; Xiao, Y.; Liu, X.; Ma, Y.; Han, Y.; Li, Z.; Wei, T.; Zou, Y.; Li, Y.; Gao, D.; et al. Effects of calcination treatments on phases and properties of (La0.2Nd0.2Sm0.2Eu0.2Gd0.2)2Zr2O7/YSZ mixture, and of pressure on photoluminescence emission of calcined mixture. Ceram. Int. 2024, 50, 14980–14986. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Frost, R.L. Thermal decomposition of Ferrian chamosite an infrared emission spectroscopic study. Contrib. Mineral. Petrol. 2000, 138, 59–67. [Google Scholar] [CrossRef]
- Liu, S.-y.; Xue, W.-h.; Wang, L.-j.; Chou, K.-c. Synthesis of mullite by high temperature reaction to realize nontoxic and efficient recycling of vanadium slag chlorination residue. Trans. Nonferrous Met. Soc. China 2023, 33, 2523–2534. [Google Scholar] [CrossRef]
- Martinez, J.; Wardini, J.L.; Zheng, X.; Moghimi, L.; Rakowsky, J.; Means, J.; Guo, H.; Kuzmenko, I.; Ilavsky, J.; Zhang, F.; et al. Precision Calcination Mechanism of CaCO3 to High-Porosity Nanoscale CaO CO2 Sorbent Revealed by Direct In Situ Observations. Adv. Mater. Interfaces 2024, 11, 2300811. [Google Scholar] [CrossRef]
- Golub, N.P.; Golub, E.O.; Kozma, A.A.; Kuznietsova, A.O.; Hurch, A.V. Infrared Spectroscopic Analysis of Complex Oxide Catalyst 50%Cu3(PO4)2•50%Ni3(PO4)2. Sci. Bull. Uzhhorod Univ. Ser. Chem. 2023, 48, 95–99. [Google Scholar] [CrossRef]
- Isaienko, O.; Borguet, E. Hydrophobicity of Hydroxylated Amorphous Fused Silica Surfaces. Langmuir 2013, 29, 7885–7895. [Google Scholar] [CrossRef]
- Iezzi, G. Low-T neutron powder-diffraction and synchrotron-radiation IR study of synthetic amphibole Na(NaMg)Mg5Si8O22(OH)2. Am. Mineral. 2005, 90, 695–700. [Google Scholar] [CrossRef]
- Lin, P.-J.; Yeh, C.-H.; Jiang, J.-C. Theoretical insight into hydroxyl production via H2O2 decomposition over the Fe3O4(311) surface. RSC Adv. 2021, 11, 36257–36264. [Google Scholar] [CrossRef]
- Shan, T.; Sun, L.; Liu, C.; Fang, J.; Li, Z.; Wen, Y.; Wang, B.; Guo, S.; Zhang, J. Microstructure and mechanical properties of SiC joint using anorthite based glass-ceramic and first principles calculation of joint interface. Ceram. Int. 2023, 49, 40149–40157. [Google Scholar] [CrossRef]
- Balassa, C.; Kristály, F.; Németh, N. Mineral Composition of Rare Element Enriched Rock Bodies from the Bükk Mts., NE Hungary. Geosci. Eng. 2022, 10, 22–36. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Krzhizhanovskaya, M.G.; Pekov, I.V.; Varlamov, D.A.; Van, K.V.; Ermolaeva, V.N.; Vozchikova, S.A. Extremely Al-Depleted Chlorites from Dolomite Carbonatites of the Kovdor Ultramafic-Alkaline Complex, Kola Peninsula, Russia. Clays Clay Miner. 2024, 68, 81–88. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Affronte, M.; Elmi, C.; Malferrari, D.; Laurora, A. Trioctahedral Fe-rich micas: Relationships between magnetic behavior and crystal chemistry. Am. Mineral. 2015, 100, 2231–2241. [Google Scholar] [CrossRef]
- Duncanson, S.; Brengman, L.; Johnson, J.; Eyster, A.; Fournelle, J.; Moy, A. Reconstructing diagenetic mineral reactions from silicified horizons of the Paleoproterozoic Biwabik Iron Formation, Minnesota. Am. Mineral. 2023, 109, 339–358. [Google Scholar] [CrossRef]
- Swansbury, L.A.; Mountjoy, G. Homogeneity of modifier ion distributions and the mixed alkaline earth effect in MgO–CaO–SiO2 silicate glasses using molecular dynamics. Phys. Chem. Glas. Eur. J. Glass Sci. Technol. Part B 2017, 58, 165–170. [Google Scholar] [CrossRef]
- Fourmont, J.; Blanc, W.; Guichaoua, D.; Chaussedent, S. Phase-separated Ca and Mg-based nanoparticles in SiO2 glass investigated by molecular dynamics simulations. Sci. Rep. 2022, 12, 11959. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wei, X.; Guo, X.M. Competitive Formation Behavior of Calcium Silicate and Calcium Ferrite in Sintering of Iron Ore Fines. Steel Res. Int. 2024, 95, 2400086. [Google Scholar] [CrossRef]
- De Keyser, W.L. La Synthèse Thermique des Silicates de Calcium. Bull. Des Sociétés Chim. Belg. 2010, 62, 235–252. [Google Scholar] [CrossRef]
- Giammaria, G.; Lefferts, L. Catalytic effect of water on calcium carbonate decomposition. J. CO2 Util. 2019, 33, 341–356. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. The AFm phase in Portland cement. Cem. Concr. Res. 2007, 37, 118–130. [Google Scholar] [CrossRef]
- de Souza Oliveira, A.; Dweck, J.; de Moraes Rego Fairbairn, E.; da Fonseca Martins Gomes, O.; Toledo Filho, R.D. Crystalline admixture effects on crystal formation phenomena during cement pastes’ hydration. J. Therm. Anal. Calorim. 2019, 139, 3361–3375. [Google Scholar] [CrossRef]
- Salzmann, M.F.; Kahlenberg, V.; Krüger, B.; Krüger, H.; Grabowski, J. Ca2.68Fe10.32Si1.00O20—A strongly disordered SFCA-related phase in the system CaO-Fe2O3-SiO2. Mineral. Petrol. 2024, 118, 485–499. [Google Scholar] [CrossRef]
- Marinos, D.; Kotsanis, D.; Alexandri, A.; Balomenos, E.; Panias, D. Carbonation of Sodium Aluminate/Sodium Carbonate Solutions for Precipitation of Alumina Hydrates—Avoiding Dawsonite Formation. Crystals 2021, 11, 836. [Google Scholar] [CrossRef]
- Mehdizadeh, H.; Jia, X.; Mo, K.H.; Ling, T.C. Effect of water-to-cement ratio induced hydration on the accelerated carbonation of cement pastes. Environ. Pollut. 2021, 280, 116914. [Google Scholar] [CrossRef] [PubMed]
- De Weerdt, K.; Haha, M.B.; Le Saout, G.; Kjellsen, K.O.; Justnes, H.; Lothenbach, B. Hydration mechanisms of ternary Portland cements containing limestone powder and fly ash. Cem. Concr. Res. 2011, 41, 279–291. [Google Scholar] [CrossRef]
- Okada, Y.; Ishida, H.; Sasaki, K.; Young, J.F.; Mitsuda, T. Characterization of C-S-H from Highly Reactive β-Dicalcium Silicate Prepared from Hillebrandite. J. Am. Ceram. Soc. 2005, 77, 1313–1318. [Google Scholar] [CrossRef]
- Hao, H.; Wang, Y.; Jin, Z.; Wang, X. Interfacial reaction of alumina with Ag-Cu-Ti. J. Mater. Sci. 1995, 30, 1233–1239. [Google Scholar] [CrossRef]
- Ambarita, M.; Pusparizkita, Y.M.; Schmahl, W.W.; Lamura, M.D.P.; Jamari, J.; Bayuseno, A.P. The effect of carbon dioxide absorption using buton asphalt residue through carbonation on calcium carbonate precipitated efficiency. IOP Conf. Ser. Earth Environ. Sci. 2023, 1268, 012037. [Google Scholar] [CrossRef]
- Wong, H.H.; Kwan, A.K. Packing density of cementitious materials: Part 1—Measurement using a wet packing method. Mater. Struct. 2008, 41, 689–701. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Corti, D.S.; Franses, E.I. Use of Close-Packed Vesicular Dispersions to Stabilize Colloidal Particle Dispersions against Sedimentation. Langmuir 2015, 31, 8802–8808. [Google Scholar] [CrossRef]
- Ghasemi, Y.; Emborg, M.; Cwirzen, A. Effect of water film thickness on the flow in conventional mortars and concrete. Mater. Struct. 2019, 52, 62. [Google Scholar] [CrossRef]
- Ye, H.; Gao, X.; Wang, R.; Wang, H. Relationship among particle characteristic, water film thickness and flowability of fresh paste containing different mineral admixtures. Constr. Build. Mater. 2017, 153, 193–201. [Google Scholar] [CrossRef]
- Wang, C.; Ren, Z.; Huo, Z.; Zheng, Y.; Tian, X.; Zhang, K.; Zhao, G. Properties and hydration characteristics of mine cemented paste backfill material containing secondary smelting water-granulated nickel slag. Alex. Eng. J. 2021, 60, 4961–4971. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, S.; Sun, G.; Wang, X.; Fu, S. Preparation of Paste Backfill Material from Mix-Calcined Sludge Ash. Waste Biomass Valoriz. 2020, 12, 1633–1646. [Google Scholar] [CrossRef]
- Zhu, Z.; Gu, X.; Wang, S.; Xu, X.; Wang, H.; Liu, J.; Zhan, K. Investigation of synergistic effects of lithium slag and granulated blast furnace slag from the perspectives of physics and hydration. J. Build. Eng. 2024, 89, 109337. [Google Scholar] [CrossRef]
- Long, G.; Gao, Y.; Xie, Y. Designing more sustainable and greener self-compacting concrete. Constr. Build. Mater. 2015, 84, 301–306. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Ren, C.; Wang, W.; Yao, Y.; Wu, S.; Qamar; Yao, X. Complementary use of industrial solid wastes to produce green materials and their role in CO2 reduction. J. Clean. Prod. 2020, 252, 119840. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, J.; Xing, J.; Sun, X. Chemical activation of binary slag cement with low carbon footprint. J. Clean. Prod. 2020, 267, 121455. [Google Scholar] [CrossRef]





| Oxides | SiO2 | Fe2O3 | Al2O3 | MgO | CaO | Na2O | K2O | TiO2 | P2O5 | SO3 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| QD | 48.46 | 19.75 | 12.20 | 7.05 | 6.55 | 2.47 | 1.39 | 0.91 | 0.61 | 0.27 | 4.53 |
| OPC | 20.85 | 3.68 | 5.28 | 3.08 | 61.54 | 0.21 | 0.68 | - | - | 1.53 | - |
| Sample | OPC | QD | MQD | Solid Concentration |
|---|---|---|---|---|
| S1 | 90 | 10 | - | 75 |
| S2 | 80 | 20 | - | 75 |
| S3 | 70 | 30 | - | 75 |
| S4 | 50 | 50 | - | 75 |
| S5 | 90 | - | 10 | 75 |
| S6 | 80 | - | 20 | 75 |
| S7 | 70 | - | 30 | 75 |
| S8 | 50 | - | 50 | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, K.; Shi, Z.; Zhang, S. Recycling Quarry Dust as a Supplementary Cementitious Material for Cemented Paste Backfill. Minerals 2025, 15, 817. https://doi.org/10.3390/min15080817
Zhang Y, Wang K, Shi Z, Zhang S. Recycling Quarry Dust as a Supplementary Cementitious Material for Cemented Paste Backfill. Minerals. 2025; 15(8):817. https://doi.org/10.3390/min15080817
Chicago/Turabian StyleZhang, Yingying, Kaifeng Wang, Zhengkun Shi, and Shiyu Zhang. 2025. "Recycling Quarry Dust as a Supplementary Cementitious Material for Cemented Paste Backfill" Minerals 15, no. 8: 817. https://doi.org/10.3390/min15080817
APA StyleZhang, Y., Wang, K., Shi, Z., & Zhang, S. (2025). Recycling Quarry Dust as a Supplementary Cementitious Material for Cemented Paste Backfill. Minerals, 15(8), 817. https://doi.org/10.3390/min15080817






