Genetic Mineralogical Characteristics of Pyrite and Quartz from the Qiubudong Silver Deposit, Central North China Craton: Implications for Ore Genesis and Exploration
Abstract
1. Introduction
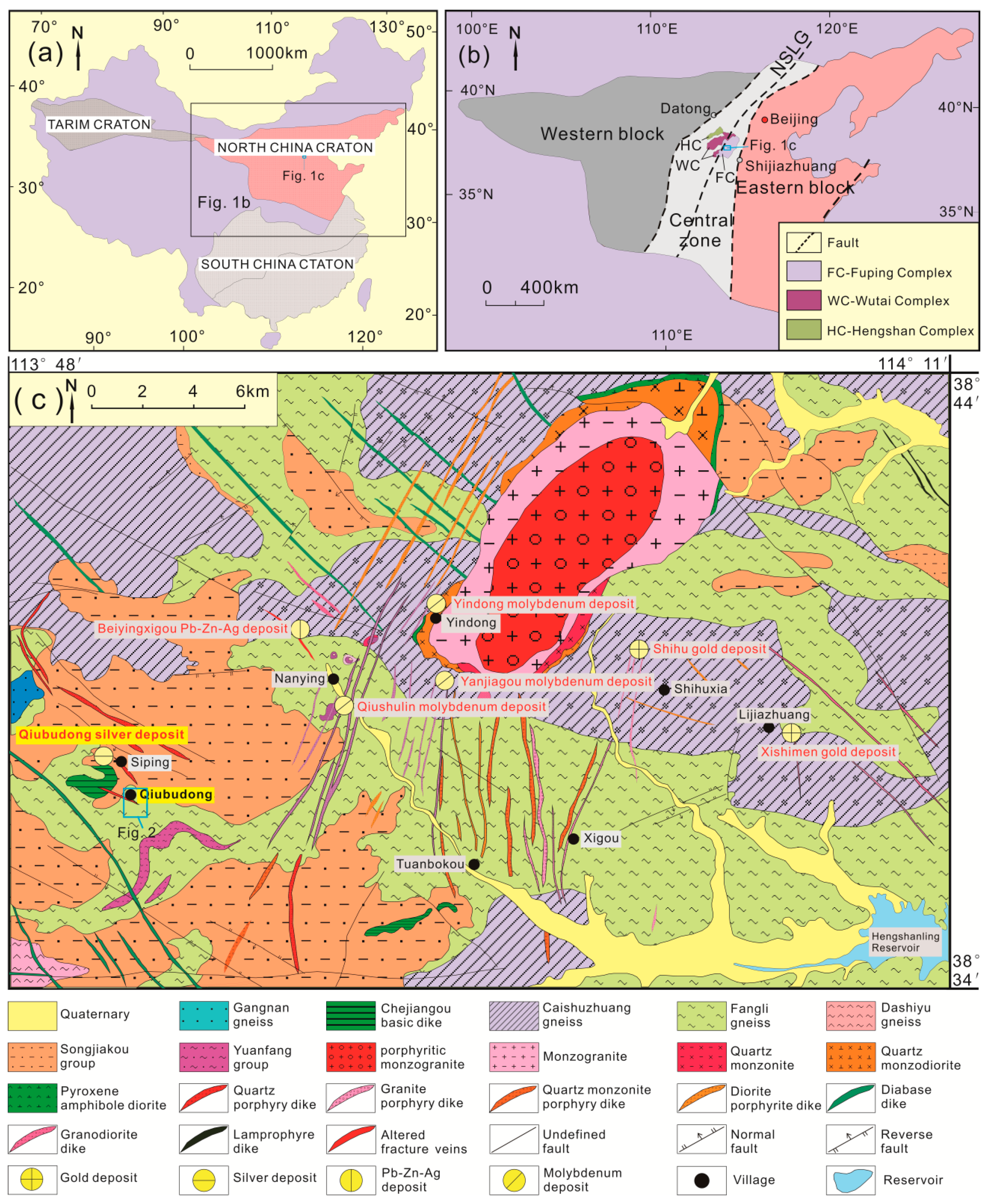
2. Geological Setting
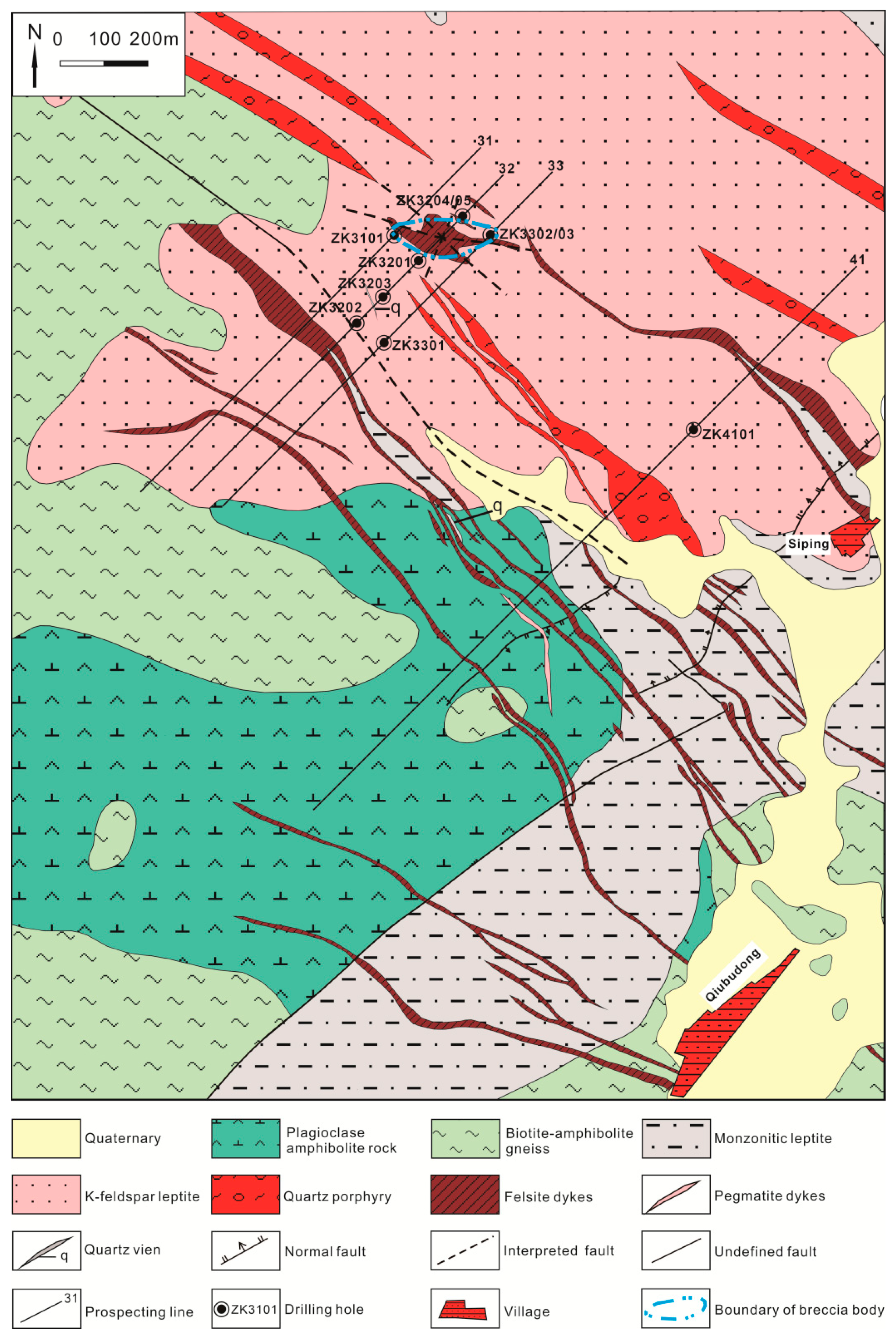
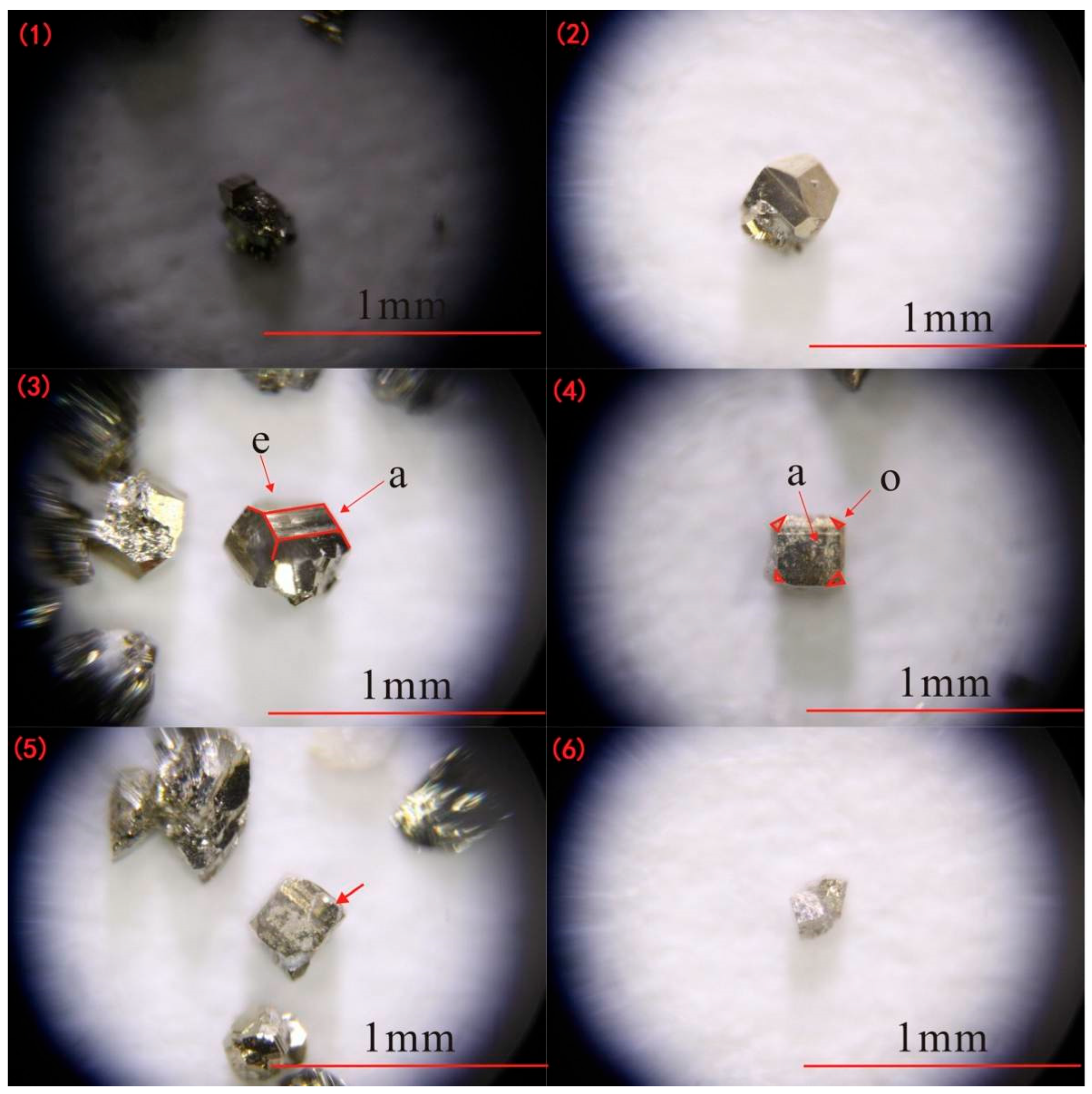
3. Occurrence Characteristics of Pyrite and Quartz
4. Samples and Analytical Methods
4.1. Major and Trace Element Analysis of Pyrite
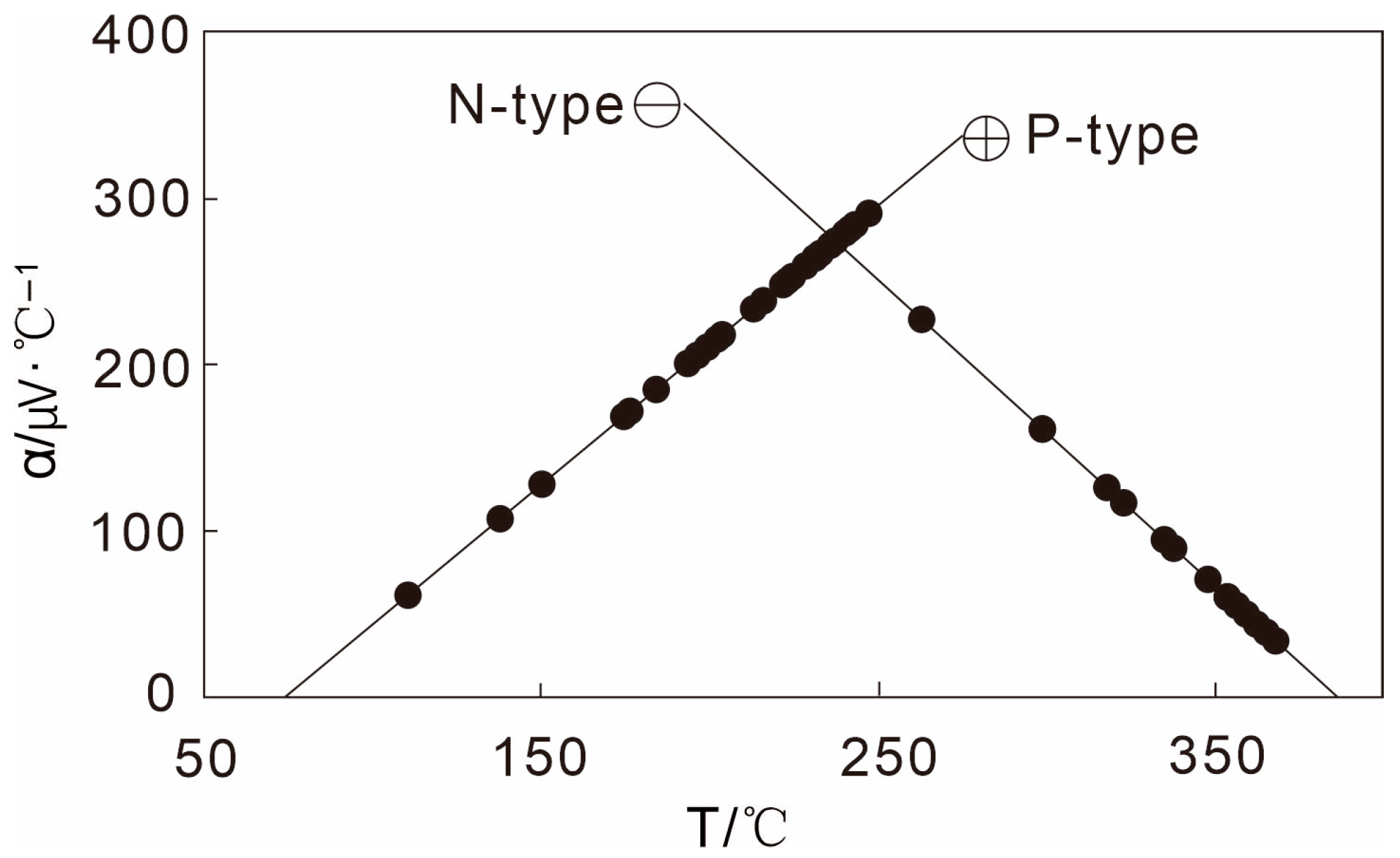
4.2. Thermoelectric Measurements of Pyrite
4.3. Thermoluminescence Measurements of Quartz
4.4. Microthermometry of Fluid Inclusions in Quartz
5. Results
6. Discussion
6.1. Characteristics of Ore-Forming Hydrothermal Fluids
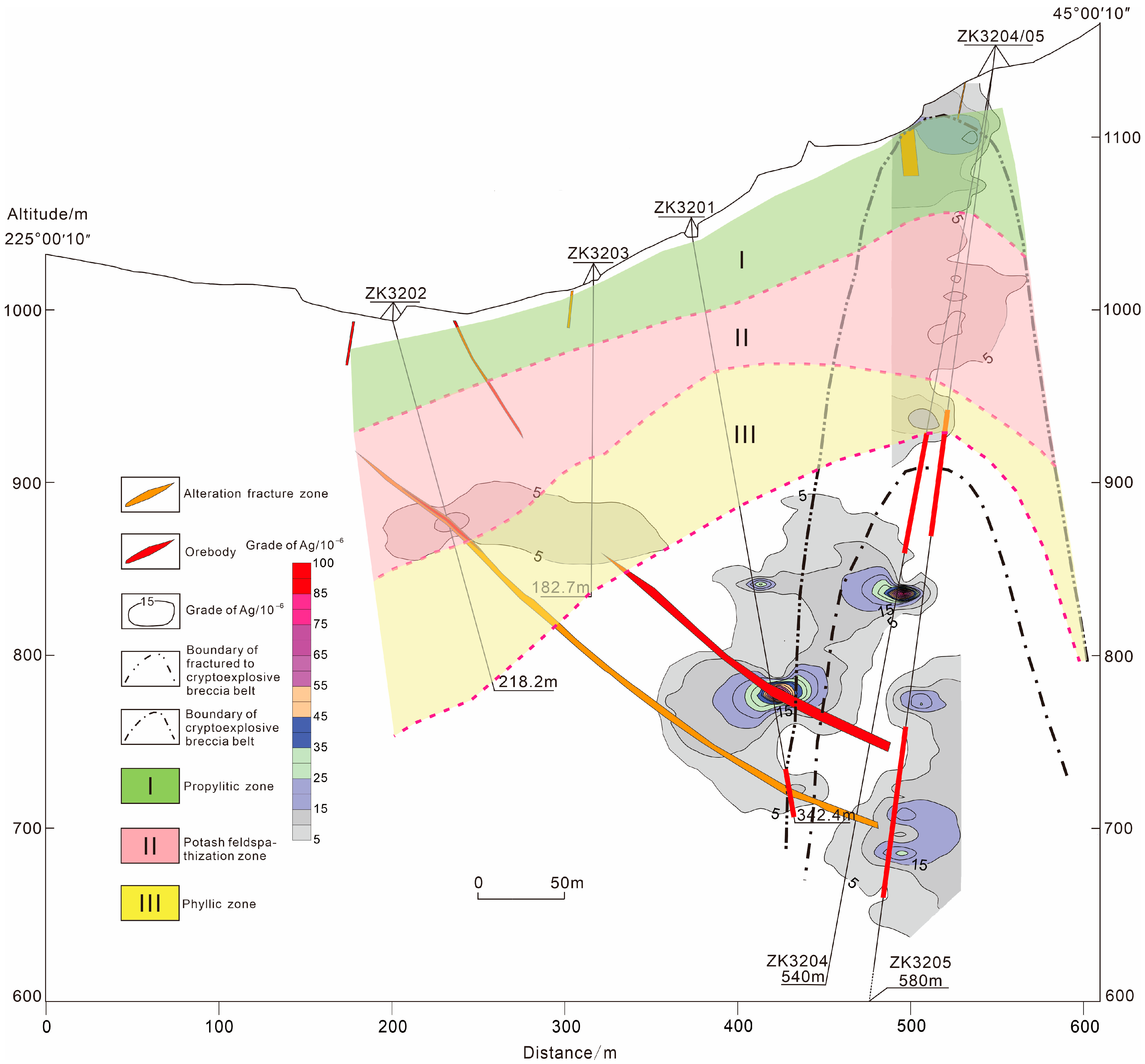
6.2. Ore Prospecting Indicators
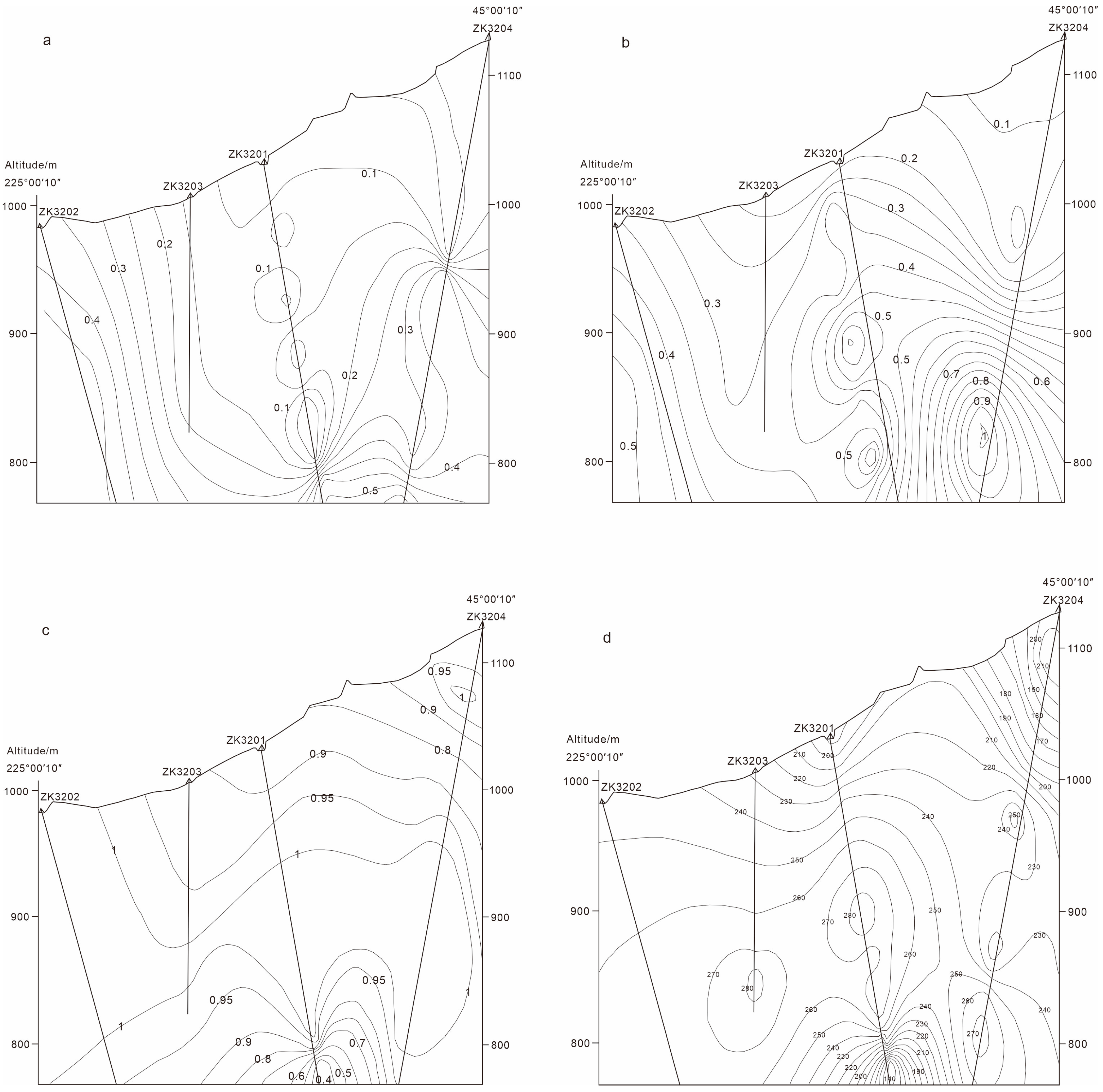
6.3. Prediction of Mineralization Potential
7. Conclusions
- Pyrite from the Qiubudong silver deposit exhibits systematic variations in crystal morphology (cubic, pentagonal dodecahedral, and intergrowths), chemical composition (Fe-enrichment, S-deficiency, Au-rich), and thermoelectric behavior (predominantly P-type with stage-specific transitions). These features collectively reflect dynamic hydrothermal evolution under a low-salinity, F-rich, structurally controlled environment at medium–low temperatures. The pentagonal dodecahedral habit, in particular, is strongly linked to main-stage mineralization and Au enrichment, offering a robust mineralogical indicator.
- Quartz thermoluminescence and fluid inclusion analyses reveal a progression from hotter, two-phase F-rich fluids in early stages to cooler, more confined low-density systems in late stages. The TL glow curves show characteristic bimodal patterns in ore-forming stages, while fluid inclusion data demonstrate consistent low salinities (10–12 wt.% NaCl eq.) and increasing fluid density over time. These thermal and compositional trends support a magmatic-hydrothermal system undergoing gradual cooling and partial fluid confinement.
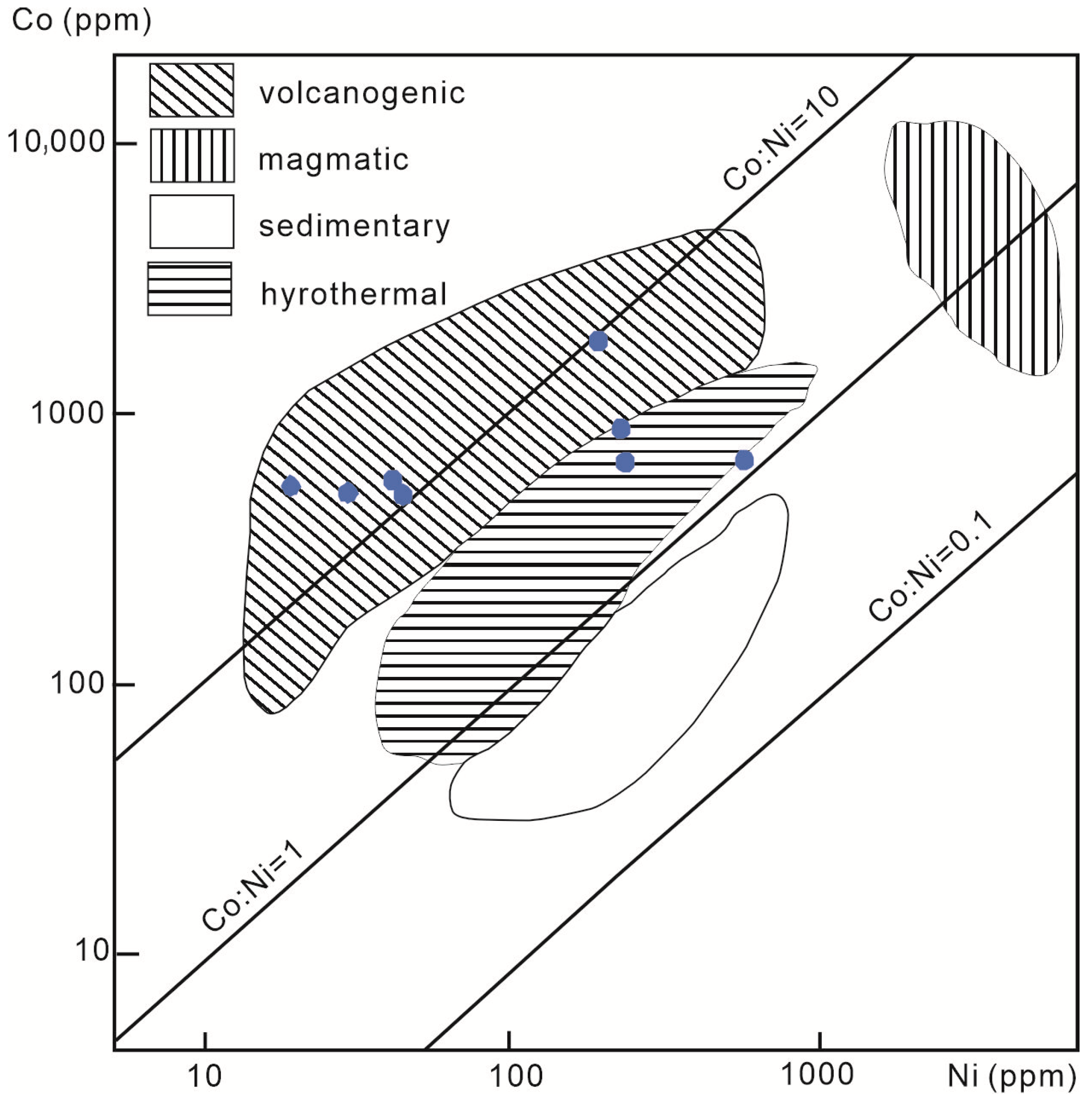
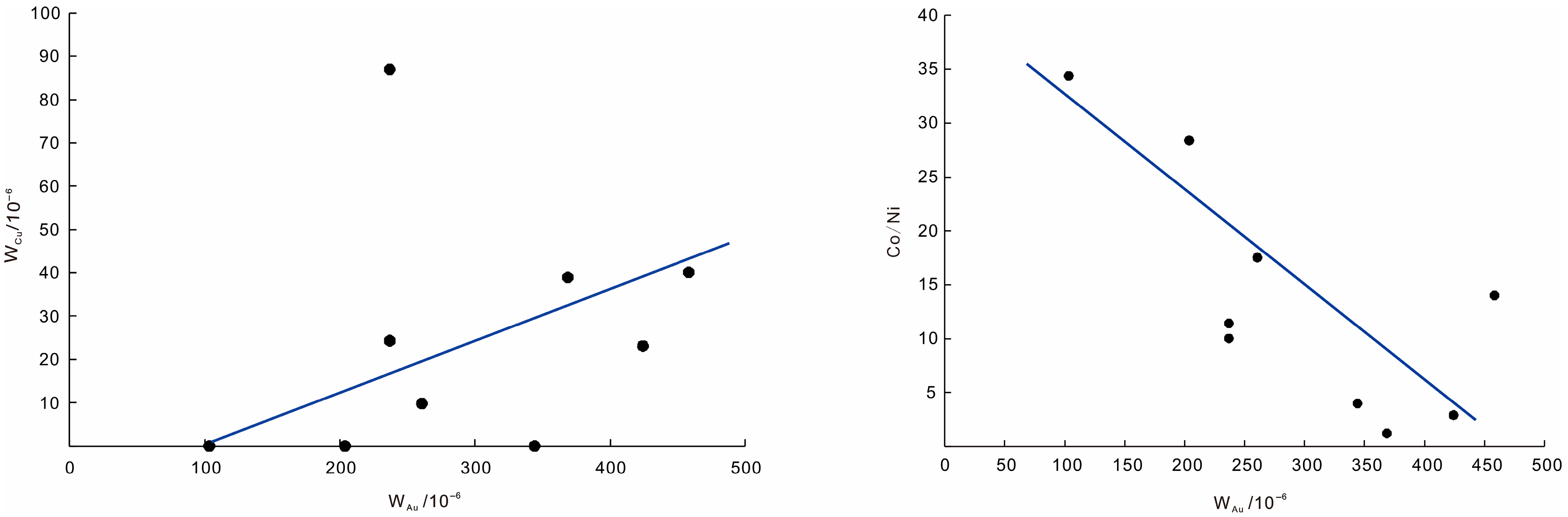
- Integrated geochemical and isotopic data, including Co/Ni and Se/Te ratios, sulfur–lead–hydrogen–oxygen isotopes, and trace element associations in pyrite, indicate that the ore-forming fluids were dominantly sourced from volcanic–magmatic systems, with limited meteoric water involvement. The geochemical proxies support a scenario of staged fluid evolution with increasing fluid–rock interaction at depth.
- Several mineralogical and geochemical indicators, such as the abundance of pentagonal dodecahedral pyrite, strong Au–Cu and inverse Au–Co/Ni correlations, complex quartz TL responses, and stage-specific thermoelectric polarity, can be applied as effective exploration tools. The integration of these attributes in 3D modeling has delineated a concealed ore potential zone below 800 m between drill holes ZK3201 and ZK3204. These results provide a practical framework for applying genetic mineralogy in deep prospecting of breccia-type Ag systems.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, G.Y.; Sun, D.S.; Yin, H.A. Genetic Mineralogy and Prospecting Mineralogy; Chongqing Publishing House: Chongqing, China, 1988. (In Chinese) [Google Scholar]
- Li, S.R. Crystallography and Mineralogy; Geological Publishing House: Beijing, China, 2008. (In Chinese) [Google Scholar]
- Gerasimov, B.; Beryozkin, V.; Kravchenko, A. Typomorphic Features of Placer Gold from the Billyakh Tectonic Melange Zone of the Anabar Shield and Its Potential Ore Sources (Northeastern Siberian Platform). Minerals 2020, 10, 281. [Google Scholar] [CrossRef]
- Gerasimov, B. The Use of Typomorphic Features of Placer Gold of the Anabar Region for Determining Its Sources. Minerals 2023, 13, 480. [Google Scholar] [CrossRef]
- Lalomov, A.; Grigorieva, A.; Kotov, A.; Ivanova, L. Typomorphic Features and Source of Native Gold from the Sykhoi Log Area Placer Deposits, Bodaibo Gold-Bearing District, Siberia, Russia. Minerals 2023, 13, 707. [Google Scholar] [CrossRef]
- Kondratieva, L.A.; Anisimova, G.S.; Kardashevskaia, V.N. Ore Mineralogy and Typomorphism of Native Gold of the Spokoininsky Cluster of the Aldan–Stanovoy Gold Province. Minerals 2023, 13, 543. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Pring, A. The mineralogy of pyrite and its significance in gold deposits. Minerals 2016, 6, 50. [Google Scholar]
- Yan, Y.T.; Li, S.R. Application of thermoelectricity in genetic mineralogy of pyrite. Acta Petrol. Mineral. 2011, 30, 403–414, (In Chinese with English Abstract). [Google Scholar]
- Reich, M.; Kesler, S.E.; Utsunomiya, S.; Palenik, C.S.; Chryssoulis, S.L.; Ewing, R.C. Solubility of gold in arsenian pyrite. Geochim. Cosmochim. Acta 2005, 69, 2781–2796. [Google Scholar] [CrossRef]
- Large, R.R.; Bull, S.W.; Maslennikov, V.V.; Thomas, H. A carbonaceous sediment-hosted gold deposit at Sukhoi Log, Russia: A large silica–pyrite–gold system of diagenetic origin. Miner. Depos. 2007, 42, 299–328. [Google Scholar]
- Large, R.R.; Maslennikov, V.; Robert, F.; Danyushevsky, L.; Chang, Z. Multistage sedimentary and metamorphic origin of pyrite and gold in the giant Sukhoi Log deposit, Russia. Econ. Geol. 2007, 102, 1233–1267. [Google Scholar] [CrossRef]
- Keith, M.; Smith, D.J.; Jenkin, G.R.; Holwell, D.A.; Dye, M.D. A review of Te and Se systematics in hydrothermal pyrite from precious metal deposits: Insights into ore-forming processes. Ore Geol. Rev. 2018, 96, 269–282. [Google Scholar] [CrossRef]
- Salje, E.K.H. Ferroelastic Twinning in Minerals: A Source of Trace Elements, Conductivity, and Unexpected Piezoelectricity. Minerals 2021, 11, 478. [Google Scholar] [CrossRef]
- Gorobets, B.S.; Rogovaya, M.L. Thermoluminescence of minerals and its application in ore geology. Geol. Ore Depos. 1992, 34, 203–212. [Google Scholar]
- Müller, W.F.; Grobety, B. Thermoluminescence and cathodoluminescence in quartz: Application to ore genesis. Mineral. Mag. 2002, 66, 859–874. [Google Scholar]
- Rusk, B. Cathodoluminescent Textures and Trace Elements in Hydrothermal Quartz; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Cao, W.T.; Hou, J.R. Genesis of the Qiubudong silver deposit in Pingshan County, Hebei Province. Geol. Explor. 2018, 54, 250–259, (In Chinese with English Abstract). [Google Scholar]
- Sun, W.Y.; Wang, C.Y.; Zhang, T. Structural framework and deep exploration potential of the silver-molybdenum metallogenic system in central Taihang Mountains. Acta Geol. Sin. 2021, 95, 4014–4032, (In Chinese with English Abstract). [Google Scholar]
- Zhang, T. Study on The Geological Characteristics and Origin of QiubudongSilver Deposit in Pingshan County, Hebei Province. Master’s Thesis, Jilin University, Changchun, China, 2018. (In Chinese with English Abstract). [Google Scholar]
- Sun, W.-Y.; Li, S.-R.; Santosh, M.; Wang, X.; Zhang, L.-J. Isotopic and geochemical constraints on the genesis of the Qiubudong silver deposit, central North China Craton: Implications for ore genesis and lithospheric dynamics. Ore Geol. Rev. 2014, 57, 229–242. [Google Scholar] [CrossRef]
- Kong, Z.Z. The Geoelectric Structure Characteristics and Mineral Prospecting Significance of the Sigou-Rock-Mass in Qiubodongsilver Deposit at Hebei. Master’s Thesis, Jilin University, Changchun, China, 2016. (In Chinese with English Abstract). [Google Scholar]
- Zhao, G.; Cawood, P.A.; Wilde, S.A.; Lu, L. Archean blocks and their boundaries in the North China Craton: Lithological, geochemical, structural and P–T metamorphic evidence. Precambrian Res. 2001, 107, 45–73. [Google Scholar] [CrossRef]
- Santosh, M.; Maruyama, S.; Zhao, D. The making and breaking of supercontinents: Some speculations based on superplume events. Gondwana Res. 2012, 21, 7–34. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Catchpole, H.J. Typomorphic features of ore minerals: A review of genetic significance and analytical approaches. Ore Geol. Rev. 2021, 135, 104244. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.M.; Scott, J.H.J.; Joy, D.C. Scanning Electron Microscopy and X-Ray Microanalysis, 4th ed.; Springer: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Jarvis, K.E. Inductively coupled plasma mass spectrometry: A new technique for the rapid or ultra-trace level determination of REEs in geological materials. Chem. Geol. 1992, 95, 47–60. [Google Scholar] [CrossRef]
- Eggins, S.M.; Woodhead, J.D.; Kinsley, L.P.J.; Mortimer, G.E.; Sylvester, P.; McCulloch, M.T.; Hergt, J.M.; Handler, M.R. A simple method for the precise determination of ≥40 trace elements in geological samples by ICPMS using enriched isotope internal standardisation. Chem. Geol. 1997, 134, 311–326. [Google Scholar] [CrossRef]
- Roedder, E. Fluid inclusions. Mineralogical Society of America. Rev. Mineral. 1984, 12, 644. [Google Scholar]
- Shepherd, T.J.; Rankin, A.H.; Alderton, D.H.M. A Practical Guide to Fluid Inclusion Studies; Blackie: Glasgow, UK, 1985. [Google Scholar]
- Zhou, X.W.; Shao, J.L.; Bian, Q.J. Study on typomorphic characteristics of pyriyte from Dongbeizhai gold deposit, Sichuan Province. Earth Sci.—J. China Univ. Geosci. 1994, 19, 52–59, (In Chinese with English Abstract). [Google Scholar]
- Bi, X.W.; Hu, R.Z.; Peng, J.T.; Wu, K.X. REE and HFSE geochemical characteristics of pyrites in Yao’an gold deposit: Tracing ore-forming fluid signatures. Bullutin Mineral. Petrol. Geochem. 2004, 23, 1–4, (In Chinese with English Abstract). [Google Scholar]
- Hall, D.L.; Sterner, S.M.; Bodnar, R.J. Freezing point depression of NaCl–KCl–H2O solutions. Geochim. Cosmochim. Acta 1988, 52, 495–504. [Google Scholar]
- Heinrich, C.A. The physical and chemical evolution of low-salinity magmatic fluids at the porphyry to epithermal transition: A thermodynamic study. Miner. Depos. 2005, 39, 864–889. [Google Scholar] [CrossRef]
- Goldstein, R.H.; Reynolds, T.J. Systematics of Fluid Inclusions in Diagenetic Minerals; SEPM Short Course; Geo Science World: McLean, VA, USA, 1994; Volume 31. [Google Scholar]
- Hegemann, E. The geochemistry of cobalt and nickel in pyrite. Econ. Geol. 1943, 38, 527–547. [Google Scholar]
- Davidson, D.T. Geochemical behavior of nickel and cobalt. Geochim. Cosmochim. Acta 1962, 26, 1–18. [Google Scholar]
- Bralia, A.; Sabatini, G.; Troja, F. Nickel and cobalt distribution in pyrite as a guide to ore genesis. Mineral. Mag. 1979, 43, 869–873. [Google Scholar]
- Wang, Y.; Xiong, L.; Zhong, Z.; Ren, S.; Zhang, G.; Wang, J.; Zhang, Y.; Song, C. Typomorphic Characteristics of Gold-Bearing Pyrite and Its Genetic Implications for the Fang’an Gold Deposit, the Bengbu Uplift, Eastern China. Minerals 2022, 12, 1196. [Google Scholar] [CrossRef]
- Gul, M.A.; Zhang, H.; Li, Y.; Yang, X.; Sun, C.; Zhao, X.; Ren, G.; Kanwal, A.; Hafeez, M.; Yang, Y.; et al. Machine learning-driven classification of Pb Zn ore deposits using pyrite trace elements and isotopic signatures: A case study of the Gunga deposit. J. Geochem. Explor. 2025, 272, 107693. [Google Scholar] [CrossRef]
- Shi, D.; Fan, S.; Li, G.; Zhu, Y.; Yan, Q.; Jia, M.; Faisal, M. Genesis of Yongping copper deposit in the Qin-Hang Metallogenic Belt, SE China: Insights from sulfide geochemistry and sulfur isotopic data. Ore Geol. Rev. 2024, 173, 15. [Google Scholar] [CrossRef]
- Bralia, A.; Sabatini, G.; Troja, F. A Revaluation of the Co/Ni Ratio in Pyrite as Geochemical Tool in Ore Genesis Problems. Miner. Depos. 1979, 14, 353–374. [Google Scholar] [CrossRef]
- Brill, B.A. Trace-element contents and partitioning of elements in ore minerals from the CSA Cu-Pb-Zn deposit, Australia. Can. Mineral. 1989, 27, 263–274. [Google Scholar] [CrossRef]
- Oreskes, N.; Einaudi, M.T. Origin of rare earth element-enriched hematite breccias at the Olympic Dam Cu-U-Au-Ag deposit, Roxby Downs, South Australia. Econ. Geol. 1990, 85, 1–28. [Google Scholar] [CrossRef]
- Keppler, H. Constraints from partitioning experiments on the composition of subduction-zone fluids. Nature 1996, 380, 237–240. [Google Scholar] [CrossRef]
- Андреев, Б.С. Пирит зoлoтoрудных местoрoждений; Изд.Наука: Мoсква, Russia, 1992; pp. 51–63. [Google Scholar]
- Wang, J.; Liu, Z.; Wang, K.; Zeng, X.; Liu, J.; Zhang, F. Typomorphic Characteristics of Pyrites from the Shuangwang Gold Deposit, Shaanxi, China: Index to Deep Ore Exploration. Minerals 2019, 9, 383. [Google Scholar] [CrossRef]



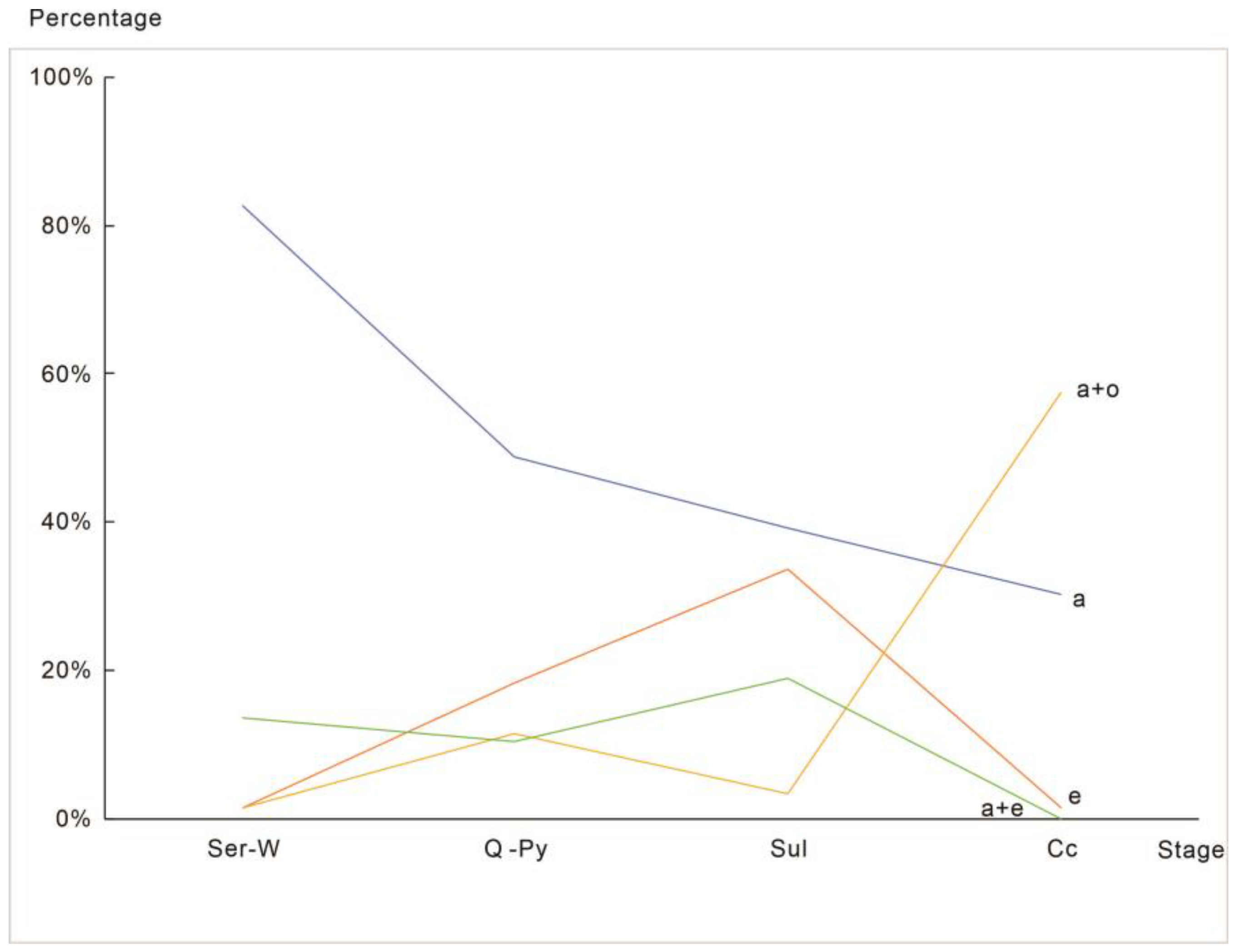


| Stage | Ser-W | Q-Py | Sul | Cc | Total | |
|---|---|---|---|---|---|---|
| Grains | 133 | 396 | 303 | 66 | 898 | |
| Single crystal | a | 110 (82.71%) | 193 (48.74%) | 119 (39.27%) | 20 (30.30%) | 442 (49.22%) |
| e | 2 (1.50%) | 72 (18.18%) | 102 (33.66%) | 1 (1.52%) | 177 (19.71%) | |
| o | — | 1 (0.25%) | — | — | 1 (0.11%) | |
| Combination | a + e | 18 (13.53%) | 41 (10.35%) | 57 (18.81%) | — | 116 (12.92%) |
| a + o | 2 (1.50%) | 45 (11.36%) | 10 (3.30%) | 38 (57.58%) | 95 (10.58%) | |
| o + e | — | 9 (2.27%) | 1 (0.33%) | 3 (4.55%) | 13 (1.45%) | |
| a + o + e | 1 (0.75%) | 10 (2.53%) | 14 (4.62%) | 4 (6.06%) | 29 (3.23%) | |
| Habitus | a | 98.50% | 72.98% | 66.01% | 93.94% | 75.95% |
| e | 15.79% | 33.33% | 57.43% | 12.12% | 37.31% | |
| o | 2.26% | 16.41% | 8.25% | 68.18% | 15.37% | |
| Single crystal | 84.21% | 67.17% | 72.94% | 31.82% | 69.04% | |
| Combination | 15.79% | 32.83% | 27.06% | 68.18% | 30.96% | |
| Stage | Q-Py | Sul | Cc | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample No. | ZK3201-10-3 | ZK3204-4-7 | ZK3302-20-3 | ZK3101-8-5 | ZK3204-5-4 | ZK3204-20-5 | ZK3101-8-2-2 | ZK3101-8-1-2 | ZK3201-2-3 |
| Co | 508.72 | 874.65 | 539.11 | 660.03 | 669.71 | 566.08 | 499.15 | 1861.56 | 548.96 |
| Ni | 29.03 | 219.3 | 18.98 | 227.53 | 546.71 | 40.38 | 43.68 | 185.69 | 15.96 |
| Au | 260.62 | 344.05 | 203.76 | 424.19 | 368.56 | 458.31 | 236.84 | 236.82 | 103.35 |
| Ag | 0 | 20.98 | 54.37 | 27 | 126.17 | 88.56 | 101.11 | 21.81 | 49.69 |
| Cu | 9.8 | 0 | 0 | 23.09 | 38.91 | 40.06 | 24.29 | 86.97 | 0 |
| Pb | 940.64 | 2372.56 | 322.17 | 1159.91 | 1660.71 | 1368.15 | 1429.96 | 2108.27 | 1261.65 |
| Zn | 132.79 | 1225.57 | 196.54 | 230.47 | 193.49 | 3.87 | 62.59 | 110 | 41.56 |
| Fe | 0.473 | 0.4685 | 0.4755 | 0.4674 | 0.4743 | 0.4716 | 0.4781 | 0.4821 | 0.4775 |
| S | 0.5141 | 0.523 | 0.5213 | 0.5294 | 0.5213 | 0.52 | 0.5184 | 0.5076 | 0.5198 |
| As | 10,528.66 | 2895.88 | 1389.79 | 289.43 | 444.23 | 5513.47 | 72.22 | 5165.47 | 468.31 |
| Sb | 61.28 | 83.73 | 84.49 | 67.86 | 88.12 | 103.83 | 87.45 | 501.85 | 99.11 |
| Se | 61.28 | 5.69 | 0 | 0 | 60.92 | 15.4 | 212.42 | 4.93 | 41.51 |
| Te | 0 | 133.59 | 123.4 | 23.03 | 29.24 | 42.35 | 173.33 | 7.34 | 69.57 |
| Bi | 302.52 | 287.2 | 258.64 | 15.08 | 204.05 | 115.99 | 568.13 | 53.86 | 0 |
| S/Fe | 1.893 | 1.944 | 1.909 | 1.973 | 1.914 | 1.92 | 1.889 | 1.834 | 1.896 |
| Chemical formula | Fe1.057 S2 | Fe1.029 S2 | Fe1.048 S2 | Fe1.014 S2 | Fe1.045 S2 | Fe1.041 S2 | Fe1.059 S2 | Fe1.091 S2 | Fe1.055 S2 |
| Co/Ni | 17.526 | 3.988 | 28.398 | 2.901 | 1.225 | 14.018 | 11.427 | 10.025 | 34.387 |
| Au/Ag | - | 16.402 | 3.748 | 15.711 | 2.921 | 5.175 | 2.342 | 10.86 | 2.08 |
| Se/Te | - | 0.043 | 0 | 0 | 2.083 | 0.364 | 1.226 | 0.671 | 0.597 |
| As/Sb | 171.818 | 34.587 | 16.449 | 4.265 | 5.041 | 53.101 | 0.826 | 10.293 | 4.725 |
| S/Se | 8390.228 | 91,923.243 | - | - | 8557.814 | 33,766.379 | 2440.608 | 103,011.513 | 12,522.46 |
| Fe + S | 0.9872 | 0.9915 | 0.9968 | 0.9969 | 0.9956 | 0.9916 | 0.9965 | 0.9897 | 0.9973 |
| Co + Ni | 537.74 | 1093.95 | 558.09 | 887.57 | 1216.41 | 606.46 | 542.84 | 2047.25 | 564.93 |
| Au + Ag | 260.62 | 365.03 | 258.13 | 451.18 | 494.73 | 546.86 | 337.95 | 258.63 | 153.05 |
| Cu + Pb + Zn | 1083.23 | 3598.13 | 518.71 | 1413.47 | 1893.11 | 1412.08 | 1516.84 | 2305.24 | 1303.21 |
| Se + Te | 61.28 | 139.28 | 123.4 | 23.03 | 90.16 | 57.75 | 385.74 | 12.27 | 111.08 |
| As + Sb + bi | 10,892.45 | 3266.81 | 1732.92 | 372.37 | 736.39 | 5733.29 | 727.81 | 5721.18 | 567.43 |
| Sample No. | 3204-20 | 3101-12 | 3201-10 | 3302-20 | 3101-6 | 3101-7 | 3201-20 | 3204-23 | 3101-8 |
|---|---|---|---|---|---|---|---|---|---|
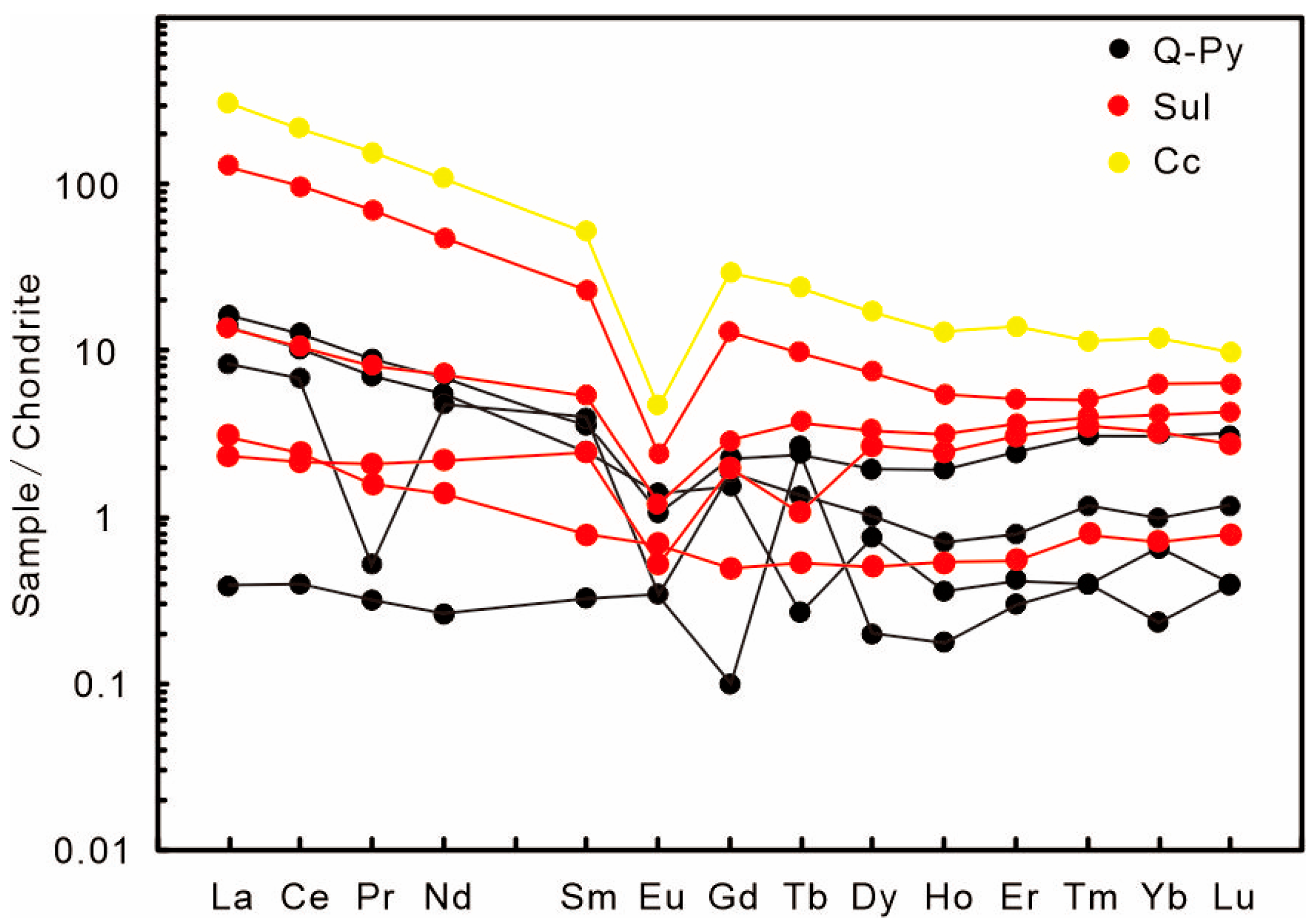
| Stage | Q-Py | Sul | Cc | ||||||
| La | 0.09 | 3.90 | 3.38 | 1.96 | 3.26 | 31.20 | 0.56 | 0.72 | 74.2 |
| Ce | 0.24 | 7.65 | 6.35 | 4.25 | 6.54 | 60.00 | 1.33 | 1.50 | 135.00 |
| Pr | 0.03 | 0.84 | 0.67 | 0.05 | 0.778 | 6.66 | 0.20 | 0.15 | 14.90 |
| Nd | 0.12 | 3.18 | 2.54 | 2.24 | 3.38 | 22.10 | 1.04 | 0.65 | 52.10 |
| Sm | 0.05 | 0.54 | 0.38 | 0.60 | 0.83 | 3.53 | 0.38 | 0.12 | 8.03 |
| Eu | 0.02 | 0.06 | 0.08 | 0.02 | 0.07 | 0.14 | 0.03 | 0.04 | 0.27 |
| Gd | 0.02 | 0.46 | 0.32 | 0.38 | 0.60 | 2.66 | 0.41 | 0.10 | 6.11 |
| Tb | 0.10 | 0.09 | 0.01 | 0.05 | 0.14 | 0.37 | 0.04 | 0.02 | 0.89 |
| Dy | 0.05 | 0.49 | 0.19 | 0.26 | 0.84 | 1.9 | 0.68 | 0.13 | 4.40 |
| Ho | 0.01 | 0.11 | 0.02 | 0.04 | 0.18 | 0.31 | 0.14 | 0.03 | 0.73 |
| Er | 0.05 | 0.41 | 0.07 | 0.13 | 0.60 | 0.83 | 0.51 | 0.09 | 2.37 |
| Tm | 0.01 | 0.08 | 0.01 | 0.03 | 0.10 | 0.13 | 0.09 | 0.02 | 0.29 |
| Yb | 0.04 | 0.52 | 0.11 | 0.17 | 0.70 | 1.09 | 0.56 | 0.12 | 2.02 |
| Lu | 0.01 | 0.08 | 0.01 | 0.03 | 0.11 | 0.16 | 0.07 | 0.02 | 0.25 |
| Y | 0.34 | 2.92 | 0.68 | 1.18 | 5.42 | 8.73 | 4.29 | 0.76 | 21.70 |
| ΣREE | 0.83 | 18.39 | 14.13 | 10.20 | 18.13 | 131.12 | 6.04 | 3.70 | 301.55 |
| LREE | 0.54 | 16.17 | 13.40 | 9.13 | 14.85 | 123.63 | 3.53 | 3.18 | 284.50 |
| HREE | 0.29 | 2.22 | 0.73 | 1.07 | 3.27 | 7.49 | 2.51 | 0.53 | 17.05 |
| LREE/HREE | 1.85 | 7.28 | 18.38 | 8.53 | 4.54 | 16.50 | 4.41 | 6.05 | 16.69 |
| LaN/YbN | 1.74 | 5.38 | 22.45 | 8.47 | 3.34 | 20.53 | 0.71 | 4.35 | 26.35 |
| δEu | 1.80 | 0.37 | 0.67 | 0.14 | 0.29 | 0.13 | 0.20 | 1.01 | 0.11 |
| δCe | 1.18 | 0.99 | 0.97 | 1.57 | 0.97 | 0.97 | 0.97 | 1.06 | 0.94 |
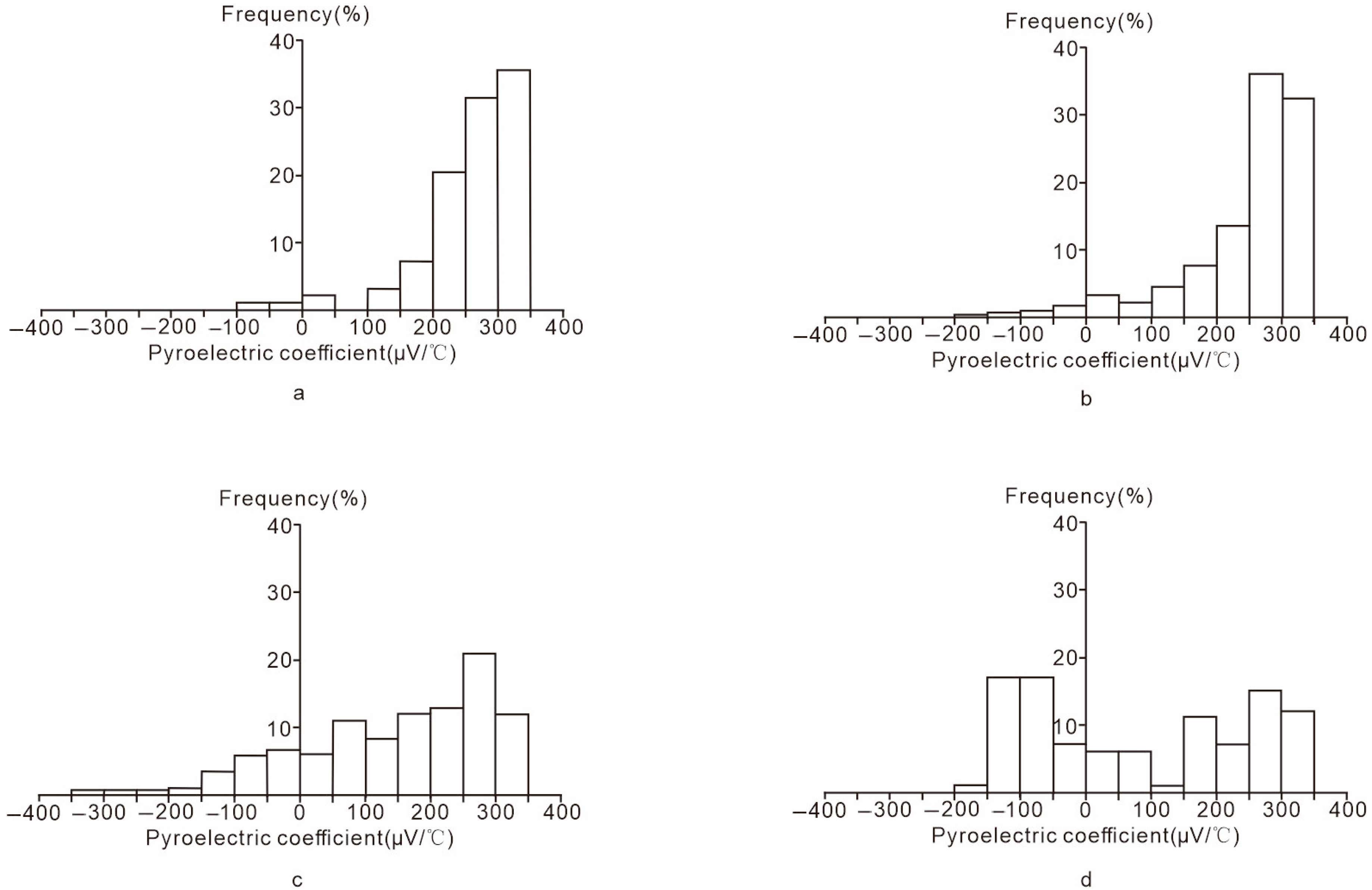
| Stage | Grains | N-type (μV/°C) | P-type (μV/°C) | Averages /Mean Value of α (μV/°C) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Minimum | Maximum | Mean | Occurrence rate | Minimum | Maximum | Mean | Occurrence rate | |||
| Ser | 100 | −69 | −6.7 | −37.85 | 2% | 5.1 | 333.3 | 258.5 | 98% | 251.7 |
| Q-Py | 520 | −168.1 | −8.3 | −59.8 | 2.69% | 1.7 | 335.6 | 257.1 | 97.31% | 248.6 |
| Sul | 490 | −328.9 | −6.7 | −87.4 | 18.16% | 6.7 | 335 | 200.6 | 81.84% | 148.3 |
| Cc | 100 | −165.3 | −11.6 | −90.2 | 42% | 6.8 | 328.4 | 209.1 | 58% | 83.4 |
| 1210 | −328.9 | −6.7 | −84.90 | 11.22% | 1.7 | 335.6 | 215.37 | 88.78% | 197.63 | |
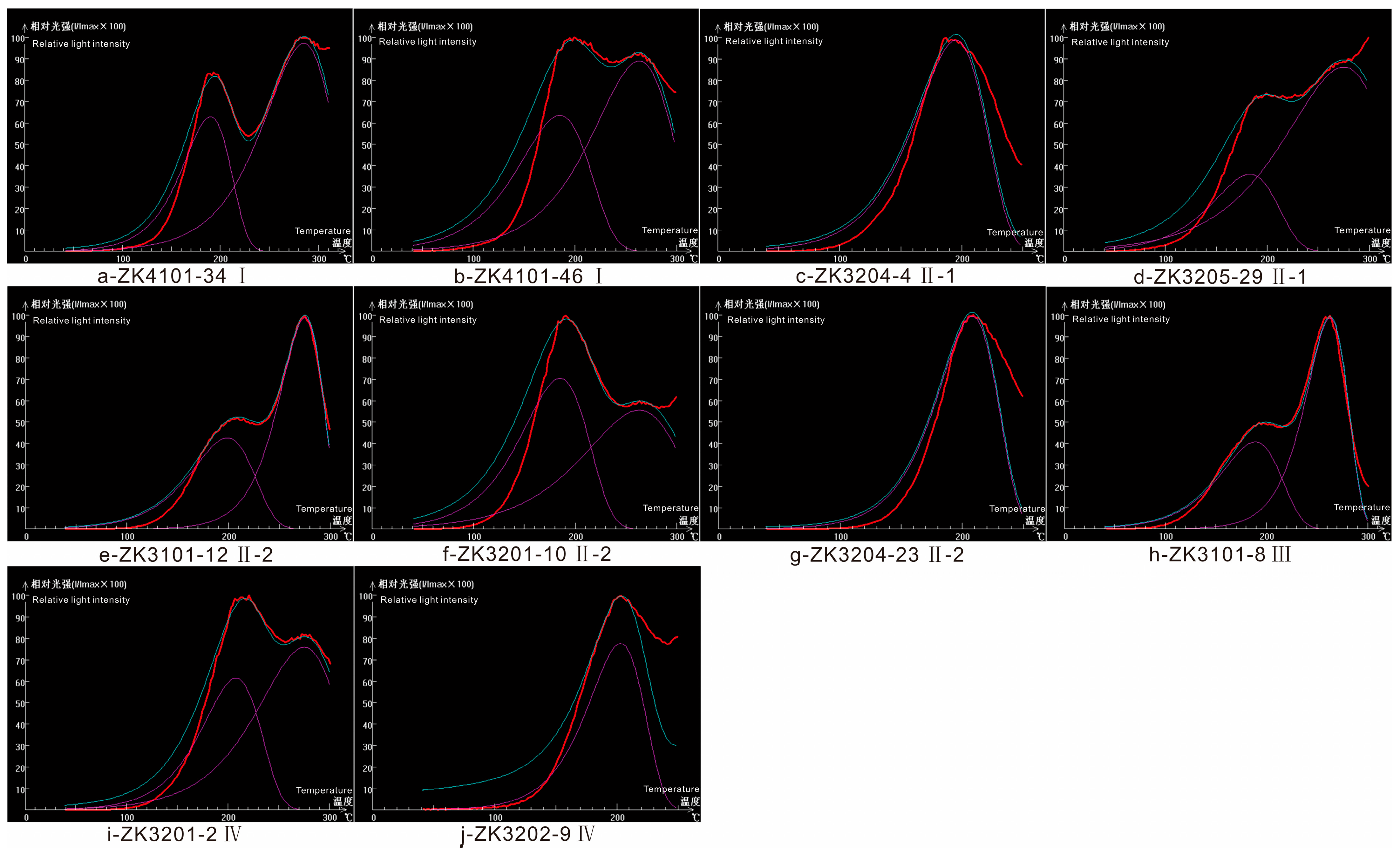
| Sample No. | Stage | Peak Type | Peak Temperature/°C | Half Peak Temperature/°C | Peak Strength/cps | Total Intensity/cps |
|---|---|---|---|---|---|---|
| ZK4101-34 | Ser-W | Bimodal | 190 | 60 | 700 | 46,667 |
| 285 | 85 | 1080 | 102,001 | |||
| ZK4101-46 | Ser-W | Bimodal | 185 | 85 | 550 | 51,945 |
| 263 | 95 | 770 | 81,279 | |||
| ZK3204-4 | Q-Py | Unimodal | 195 | 70 | 750 | 58,334 |
| ZK3205-29 | Q-Py | Bimodal | 183 | 78 | 360 | 31,200 |
| 275 | 120 | 860 | 114,668 | |||
| ZK3101-12 | Q-Py | Bimodal | 199 | 75 | 1250 | 104,168 |
| 275 | 52 | 2900 | 167,558 | |||
| ZK3201-10 | Q-Py | Bimodal | 185 | 80 | 520 | 46,223 |
| 263 | 115 | 410 | 52,389 | |||
| ZK3204-23 | Q-Py | Unimodal | 208 | 60 | 1150 | 76,667 |
| ZK3101-8 | Sul | Bimodal | 189 | 73 | 1650 | 133,835 |
| 263 | 50 | 4000 | 222,226 | |||
| ZK3201-2 | Cc | Bimodal | 208 | 70 | 425 | 33,056 |
| 275 | 95 | 525 | 55,417 | |||
| ZK3202-9 | Cc | Unimodal | 203 | 56 | 77 | 4791 |
| Stage | Homogenization Temperature/°C | Freezing Temperature/°C | Salinity (wt%NaCl) | Density (g/cm3) | |||
|---|---|---|---|---|---|---|---|
| Range | Mean | Range | Mean | Range | Mean | ||
| Q-Py | 170.2~359.8 | 254.5 | −13~−1.6 | 2.74~16.89 | 10.22 | 0.73~0.98 | 0.86 |
| Sul | 240.3~330.6 | 271.2 | −11.3~−4.9 | 7.73~15.27 | 11.8 | 0.80~0.94 | 0.87 |
| Cc | 146.7~258.4 | 204.7 | −9.6~−6.5 | 9.86~13.51 | 11.47 | 0.92~0.99 | 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Xue, J.; Tong, Z.; Zhang, X.; Wang, J.; Li, S.; Wang, M. Genetic Mineralogical Characteristics of Pyrite and Quartz from the Qiubudong Silver Deposit, Central North China Craton: Implications for Ore Genesis and Exploration. Minerals 2025, 15, 769. https://doi.org/10.3390/min15080769
Sun W, Xue J, Tong Z, Zhang X, Wang J, Li S, Wang M. Genetic Mineralogical Characteristics of Pyrite and Quartz from the Qiubudong Silver Deposit, Central North China Craton: Implications for Ore Genesis and Exploration. Minerals. 2025; 15(8):769. https://doi.org/10.3390/min15080769
Chicago/Turabian StyleSun, Wenyan, Jianling Xue, Zhiqiang Tong, Xueyi Zhang, Jun Wang, Shengrong Li, and Min Wang. 2025. "Genetic Mineralogical Characteristics of Pyrite and Quartz from the Qiubudong Silver Deposit, Central North China Craton: Implications for Ore Genesis and Exploration" Minerals 15, no. 8: 769. https://doi.org/10.3390/min15080769
APA StyleSun, W., Xue, J., Tong, Z., Zhang, X., Wang, J., Li, S., & Wang, M. (2025). Genetic Mineralogical Characteristics of Pyrite and Quartz from the Qiubudong Silver Deposit, Central North China Craton: Implications for Ore Genesis and Exploration. Minerals, 15(8), 769. https://doi.org/10.3390/min15080769





