Ore Genesis Based on Microtextural and Geochemical Evidence from the Hydrothermal As–Sb Mineralization of the Matra Deposit (Alpine Corsica, France)
Abstract
1. Introduction
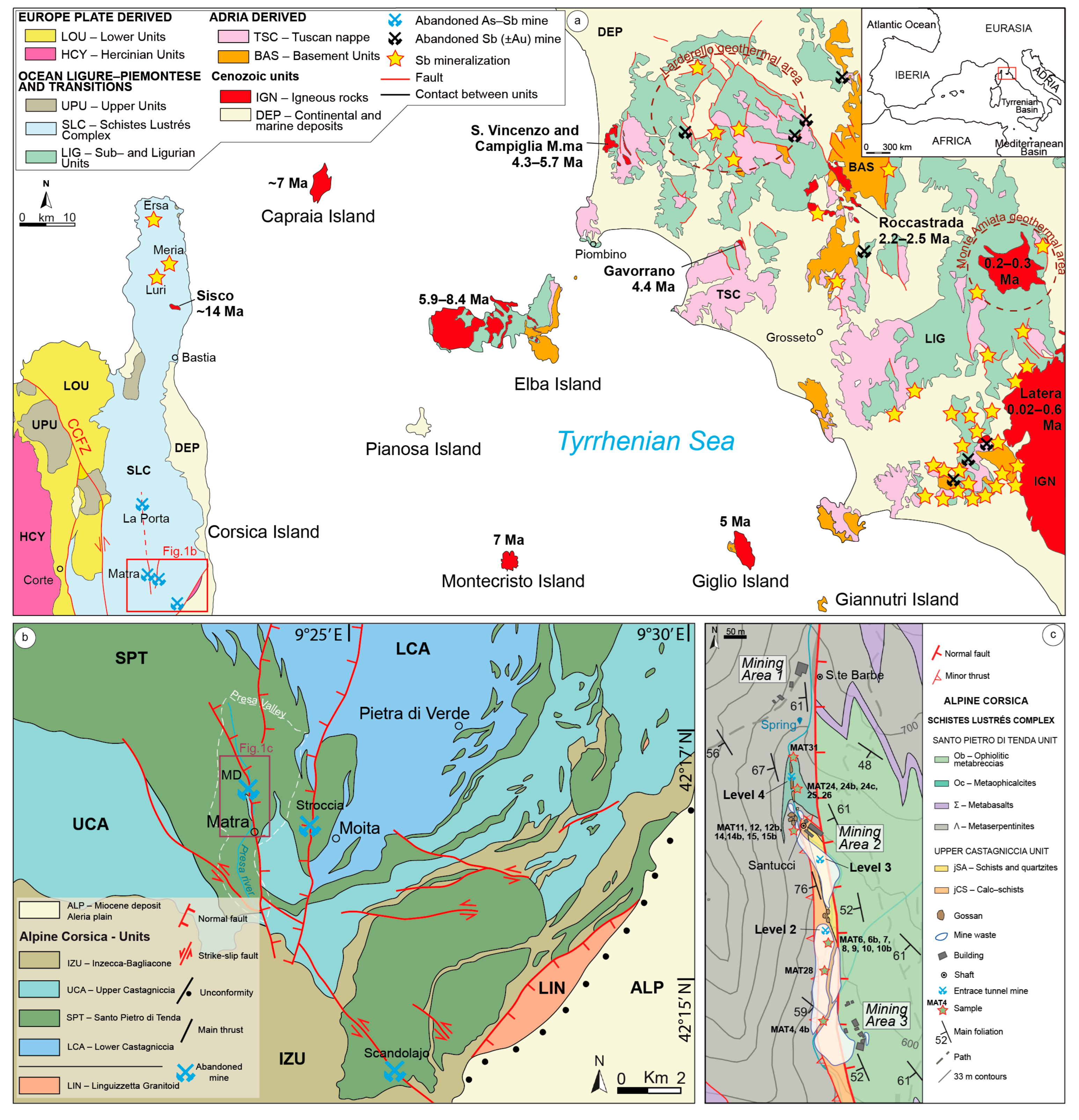
2. Geological Setting
3. Sampling and Analytical Methods
4. Mineralogy and Geochemistry of Ore Minerals
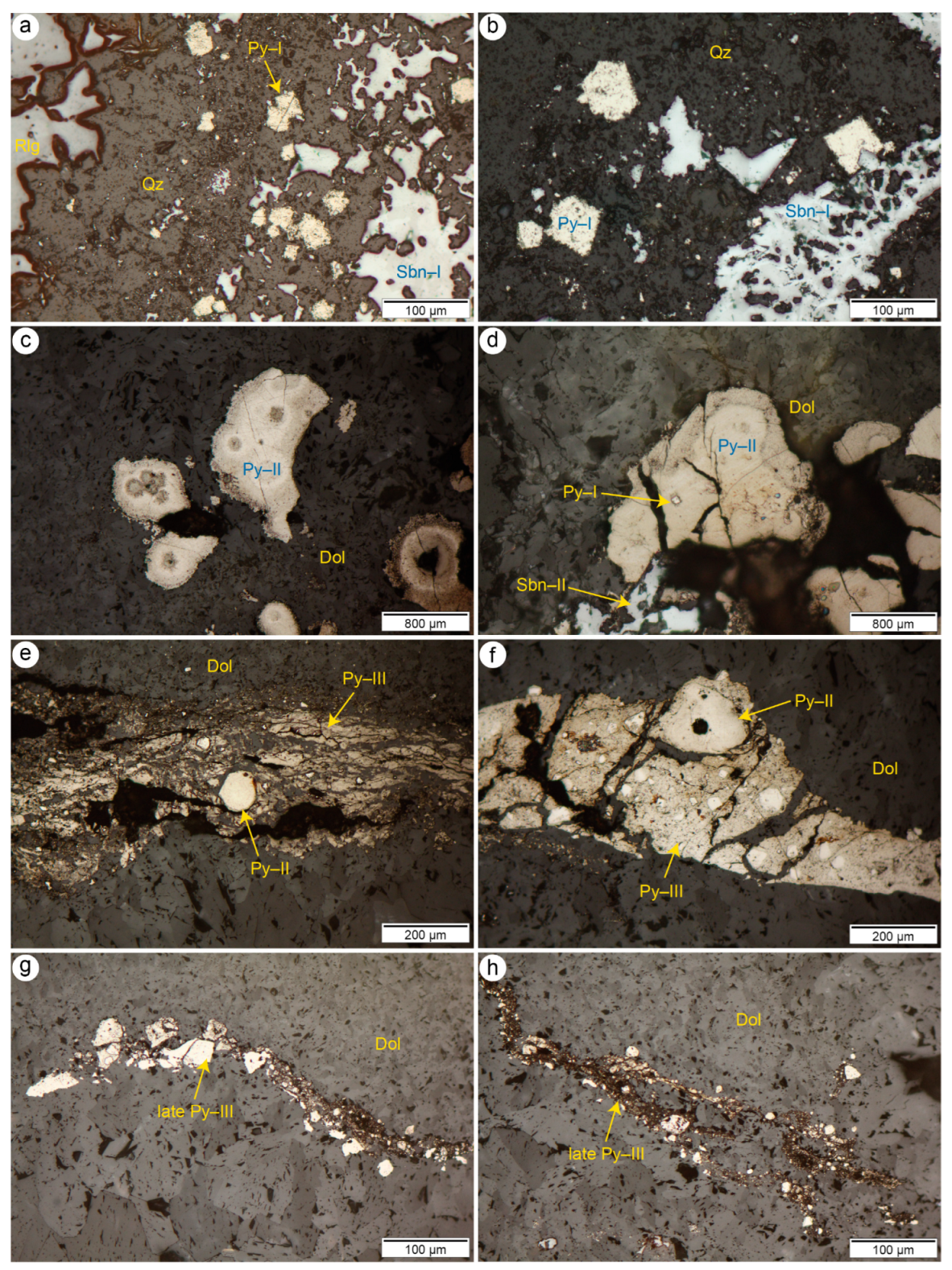
4.1. Pyrite
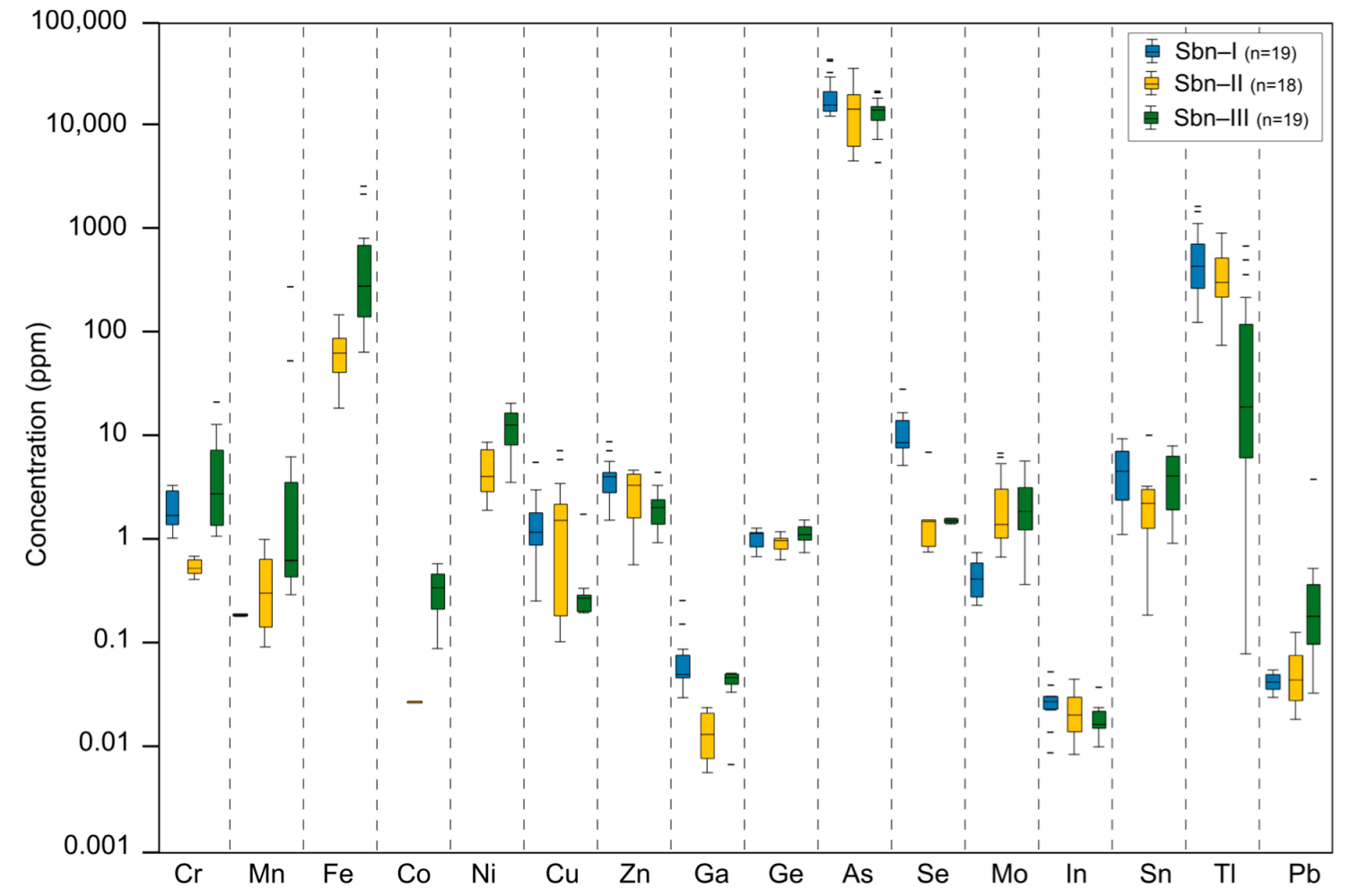
4.2. Stibnite
4.3. Arsenic Minerals
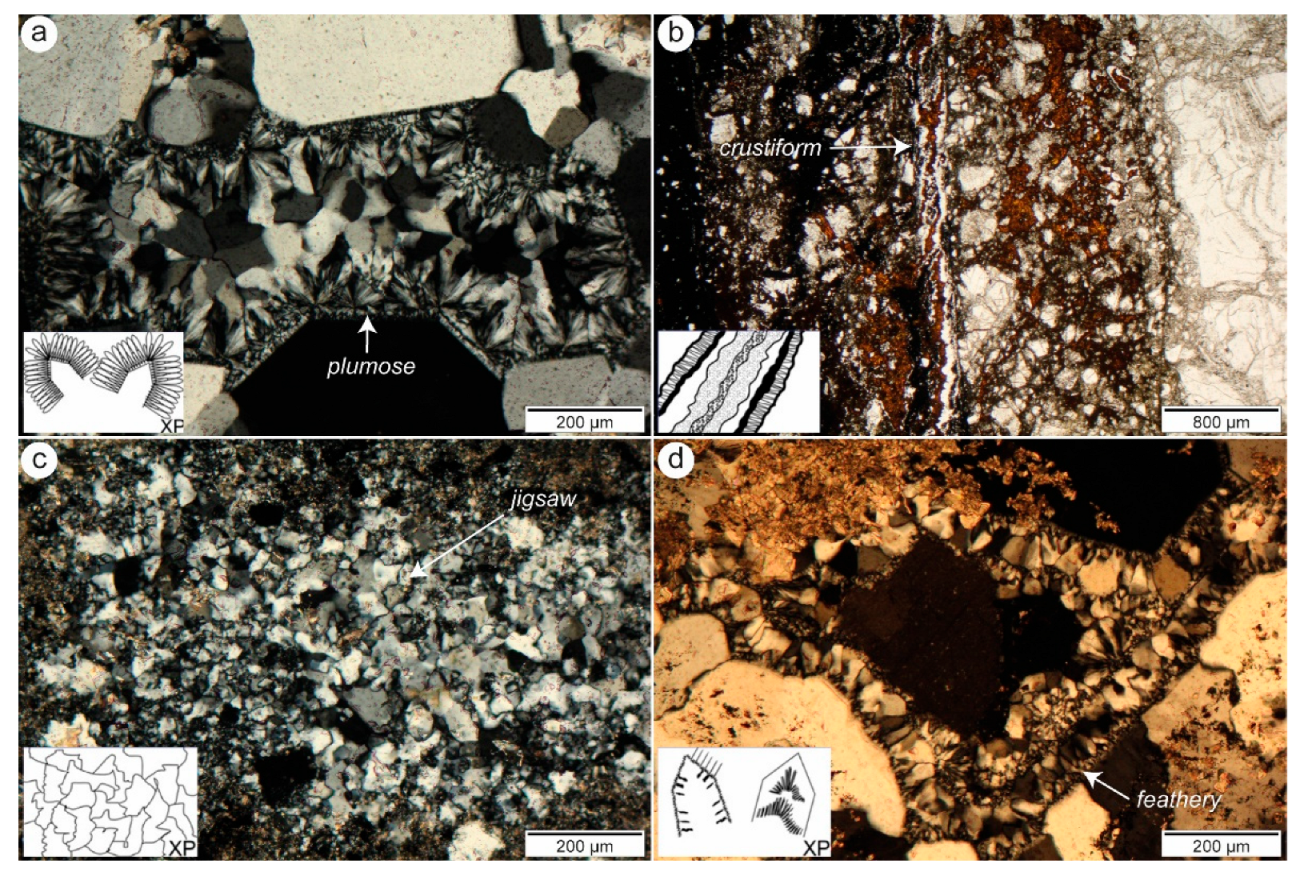
4.4. Gangue
4.5. Supergene Minerals
5. Discussion
5.1. Ore Textures and Geochemistry
5.2. Paragenetic Sequence
5.3. Geodynamic Evolution of the Matra Deposit
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mori, C.; Orsini, A.; Migon, C. Impact of arsenic and antimony contamination on benthic invertebrates in a minor Corsican river. Hydrobiologia 1999, 392, 73–80. [Google Scholar] [CrossRef]
- Orcel, J. The realgar deposit of Matra (Corsica). Bull. Société Française Minéralogie Cristallogr. 1921, 44, 98–104. (In French) [Google Scholar] [CrossRef]
- Orcel, J. Mineralogical and petrographic notes on Corsica. Bull. Société Des Sci. Hist. Naturelles Corse 1924, 44, 65–127. (In French) [Google Scholar]
- Feraud, J. Les Gisements De Sulfures D’arsenic Du Sud-Est De La France Mineralization Liées-Aux-Strates Et Gites Filoniens. Ph.D. Thesis, Universitè de Paris VI, Paris, France, 1974. (In French). [Google Scholar]
- Filimon, D.I.; Vannucci, G.; La Rosa, A.; Zarone, V.; Di Rosa, M. The geological setting of Matra ore deposit (France): Insights from lithostratigraphy and polyphase deformation history. J. Maps 2024, 20, 2394502. [Google Scholar] [CrossRef]
- Gueydan, F.; Brun, J.-P.; Phillippon, M.; Noury, M. Sequential extension as a record of Corsica Rotation during Apennines slab roll-back. Tectonophysics 2017, 710, 149–161. [Google Scholar] [CrossRef]
- Chamot-Rooke, N.; Gaulier, J.M.; Jestin, F. Constraints on Moho depth and crustal thickness in the Liguro-Provençal basin from a 3D gravity inversion: Geodynamic implications. Geol. Soc. Lond. Spec. Publ. 1999, 156, 37–61. [Google Scholar] [CrossRef]
- Lattanzi, P. Epithermal precious metal deposits of Italy–an overview. Miner. Depos. 1999, 34, 630–638. [Google Scholar] [CrossRef]
- Brogi, A.; Fulignati, P. Tectonic control on hydrothermal circulation and fluid evolution in the Pietratonda–Poggio Peloso (southern Tuscany, Italy) carbonate-hosted Sb-mineralization. Ore Geol. Rev. 2012, 44, 158–171. [Google Scholar] [CrossRef]
- Sillitoe, R.H.; Brogi, A. Geothermal systems in the northern Apennines, Italy: Modern analogues of Carlin-style gold deposits. Econ. Geol. 2021, 116, 1491–1501. [Google Scholar] [CrossRef]
- European Commission. Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs, Study on the Critical Raw Materials for the EU (2023)–Final Report; Publications Office of the European Union: Luxembourg, Luxembourg, 2023. [Google Scholar]
- Durand-Delga, M. Main features of Alpine Corsica and correlation with the Ligurian Alps. Mem. Della Soc. Geol. Ital. 1984, 28, 285–329. (In French) [Google Scholar]
- Rossi, P.; Oggiano, G.; Cocherie, A. A restored section of the “southern Variscan realm” across the Corsica–Sardinia microcontinent. Comptes Rendus. Géoscience 2009, 341, 224–238. [Google Scholar] [CrossRef]
- Malavieille, J.; Chemenda, A.; Larroque, C. Evolutionary model for the Alpine Corsica: Mechanism for ophiolite emplacement and exhumation of high-pressure rocks. Terra Nova 1998, 10, 317–322. [Google Scholar] [CrossRef]
- Marroni, M.; Pandolfi, L. The architecture of an incipient oceanic basin: A tentative reconstruction of the Jurassic Liguria-Piemonte basin along the Northern Apennines-Alpine Corsica transect. Int. J. Earth Sci. 2007, 96, 1059–1078. [Google Scholar] [CrossRef]
- Vitale Brovarone, A.; Beyssac, O.; Malavieille, J.; Molli, G.; Beltrando, M.; Compagnoni, R. Stacking and metamorphism of continuous segments of subducted lithosphere in a high-pressure wedge: The example of Alpine Corsica (France). Earth Sci. Rev. 2013, 116, 35–56. [Google Scholar] [CrossRef]
- Di Rosa, M.; Frassi, C.; Malasoma, A.; Marroni, M.; Meneghini, F.; Pandolfi, L. Syn-exhumation coupling of oceanic and continental units along the western edge of the Alpine Corsica: A review. Ofioliti 2020, 45, 71–102. [Google Scholar]
- Saccani, E.; Principi, G.; Garfagnoli, F.; Menna, F. Corsica ophiolites: Geochemistry and petrogenesis of basaltic and metabasaltic rocks. Ofioliti 2008, 33, 187–207. [Google Scholar]
- Saccani, E. A new method of discriminating different types of post-Archean ophiolitic basalts and their tectonic significance using Th–Nb and Ce–Dy–Yb systematics. Geosci. Front. 2015, 6, 481–501. [Google Scholar] [CrossRef]
- Ribes, C.; Manatschal, G.; Ghienne, J.F.; Karner, G.D.; Johnson, C.A.; Figueredo, P.H.; Incerpi, N.; Epin, M.A. The syn-rift stratigraphic record across a fossil hyper-extended rifted margin: The example of the northwestern Adriatic margin exposed in the Central Alps. Int. J. Earth Sci. 2019, 108, 2071–2095. [Google Scholar] [CrossRef]
- Cavazza, W.; Zattin, M.; Ventura, B.; Zuffa, G.G. Apatite fission track analysis of Neogene exhumation in northern Corsica (France). Terra Nova 2001, 13, 51–57. [Google Scholar] [CrossRef]
- Marroni, M.; Pandolfi, L. Deformation history of the ophiolite sequence from the Balagne Nappe, northern Corsica: Insights in the tectonic evolution of the Alpine Corsica. Geol. J. 2003, 38, 67–83. [Google Scholar] [CrossRef]
- Durand-Delga, M.; Peybernès, B.; Rossi, P. Arguments en faveur de la position, au Jurassique, des ophiolites de Balagne (Haute-Corse, France) au voisinage de la marge continentale européenne. Comptes Rendus De. Académié De. Sci. Paris. 1997, 325, 973–981. [Google Scholar] [CrossRef]
- Beccaluva, L.; Deriu, M.; Macciotta, G.; Savelli, C.; Venturelli, G. Geochronology and Magmatic Character of the Pliocene-Pleistocene Volcanism in Sardinia (Italy). Bull. Volcanol. 1977, 40, 153–168. [Google Scholar] [CrossRef]
- Venturelli, G.; Capedri, S.; Thorpe, R.S.; Potts, P.J. Rare-earth and other element distribution in some ophiolitic metabasalts of Corsica, Western Mediterranean. Chem. Geol. 1979, 24, 39–353. [Google Scholar] [CrossRef]
- Di Rosa, M.; Meneghini, F.; Marroni, M.; Frassi, C.; Pandolfi, L. The coupling of high-pressure oceanic and continental units in Alpine Corsica: Evidence for syn-exhumation tectonic erosion at the roof of the plate interface. Lithos 2020, 354, 105328. [Google Scholar] [CrossRef]
- Venturelli, G.; Thorpe, R.S.; Potts, P.J. Rare earth and trace element characteristics of ophiolitic metabasalts from the Alpine-Apennine belt. Earth Planet. Sci. Lett. 1981, 53, 109–123. [Google Scholar] [CrossRef]
- Guelfi, R.; Maremmani, A.; Di Rosa, M.; Meneghini, F.; Pandolfi, L.; Marroni, M. Adding pieces to the puzzle of the Western Tethys Oceanic Basin structure: The architecture of the Inzecca Unit in the Noceta-Vezzani area (Alpine Corsica, France). Episodes 2025, 48, 163–179. [Google Scholar] [CrossRef] [PubMed]
- De Cesari, F.; Di Rosa, M.; Sanità, E.; Pandolfi, L.; Marroni, M. Long-lived sedimentation in the Western Tethys oceanic basin: Revisiting the metasediments of Bagliacone-Riventosa Formation (Corsica, France). Int. Geol. Rev. 2024, 67, 906–932. [Google Scholar] [CrossRef]
- Ohnenstetter, M. La serie ophiolitifere de Rospigliani (Corse) est- elle un temoin des phenomenes tectoniques, sedimentaires et magmatiques lies au fonctionnement des zones transformantes? Comptes Rendus De. L’academie Des. Sci. De. Paris. 1979, 289, 1199–1202. (In French) [Google Scholar]
- Lahondére, D. Les schistes blues et les éclogites à lawsonite des unités continentals et océanique de la Corse alpine: Nouvelles donnée pétrologique et structurales (Corse). Ph.D. Thesis, Université Montpellier 2 Sciences et Techniques, Montpellier, France, 1996. [Google Scholar]
- Padoa, E. Les ophiolites du massif d’Inzecca (Corse alpine): Litostratigraphie, structure géologique et évolution géodynamique. Géologie De. Fr. 1999, 3, 37–48. [Google Scholar]
- Elter, P.; Pertusati, P.C. Considerations on the Alps–Apennines boundary and its relationship with the arc of the Western Alps. Mem. Della Soc. Geol. Ital. 1973, 12, 359–375. (In Italian) [Google Scholar]
- Lagabrielle, Y.; Polino, R. A structural diagram of the domain of lustrous ophiolitic shales to the northwest of the Mont Viso massif (Southwestern Alps) and its implications. Comptes Rendus De. L’académie Des. sciences. Série 2 Mécanique Phys. Chim. Sci. De. L’univers Sci. De. La. Terre 1988, 306, 921–928. (In French) [Google Scholar]
- Marroni, M.; Meneghini, F.; Pandolfi, L. A revised subduction inception model to explain the Late Cretaceous, double-vergent orogen in the precollisional western Tethys: Evidence from the Northern Apennines. Tectonics 2017, 36, 2227–2249. [Google Scholar] [CrossRef]
- Di Rosa, M.; Sanità, E.; Frassi, C.; Lardeaux, J.M.; Corsini, M.; Marroni, M.; Pandolfi, L. A journey of the continental crust to and from mantle depth: The P–T–t–d path of Venaco unit, Alpine Corsica (France). Geol. J. 2023, 59, 422–440. [Google Scholar] [CrossRef]
- Zarki-Jakni, B.; Van Der Beek, P.; Poupeau, G.; Sosson, M.; Labrin, E.; Rossi, P.; Ferrandini, J. Cenozoic denudation of Corsica in response to Ligurian and Tyrrhenian extension: Results from apatite fission track thermochronology. Tectonics 2004, 23, TC1003. [Google Scholar] [CrossRef]
- Lahondére, D.; Guerrot, C. Datation Sm-Nd du métamorphisme éclogitique en Corse Alpine: Un argument pour l’existence au Crétacé Supérieur d’une zone de subduction active localisée sous le bloc corso-sarde. Géologie De. Fr. 1997, 3, 3–11. (In French) [Google Scholar]
- Gueguen, E.; Doglioni, C.; Fernandez, M. On the post-25 Ma geodynamic evolution of the western Mediterranean. Tectonophysics 1998, 298, 259–269. [Google Scholar] [CrossRef]
- Fellin, M.G.; Picotti, V.; Zattin, M. Neogene to Quaternary rifting and inversion in Corsica: Retreat and collision in the western Mediterranean. Tectonics 2005, 24, TC1011. [Google Scholar] [CrossRef]
- Jolivet, L.; Daniel, J.M.; Fournier, M. Geometry and Kinematics of ductile extension in Alpine Corsica. Earth Planet. Sci. Lett. 1991, 104, 278–291. [Google Scholar] [CrossRef]
- Brunet, C.; Monié, P.; Jolivet, L.; Cadet, J.P. Migration of compression and extension in the Tyrrhenian Sea, insights from 40Ar/39Ar ages on micas along a transect from Corsica to Tuscany. Tectonophysics 2000, 321, 127–155. [Google Scholar] [CrossRef]
- Malinverno, A.; Ryan, W.B.F. Extension in the Tyrrhenian Sea and shortening in the Apennines as a result of arc migration driven by the sinking of the lithosphere. Tectonics 1986, 5, 227–245. [Google Scholar] [CrossRef]
- Kastens, K.A.; Mascle, J.; Auroux, C. Proc. ODP, Init. Repts. 107 College Station, TX; (Ocean Drilling Program): Brazos County, TX, USA, 1987. [Google Scholar] [CrossRef]
- Rosenbaum, G.; Lister, G.S.; Duboz, C. Relative motion of Africa, Iberia and Europe during Alpine orogeny. Tectonophysics 2002, 359, 117–129. [Google Scholar] [CrossRef]
- Serri, G.; Innocenti, F.; Manetti, P. Geochemical and petrological evidence of the subduction of delaminated Adriatic continental lithosphere in the genesis of the Neogene-Quaternary magmatism of central Italy. Tectonophysics 1993, 223, 117–147. [Google Scholar] [CrossRef]
- Peccerillo, A.; Poli, G.; Donati, C. The Plio-Quaternary magmatism of Southern Tuscany and Northern Latium: Compositional characteristics, genesis and geodynamic significance. Ofioliti 2001, 26, 229–238. [Google Scholar]
- Caron, J.M.; Delcey, R. Lithostratigraphy of the Corsican Schistes Lustrés: Diversity of post-ophiolitic sequences. Comptes Rendus De. Académié De. Sci. Paris. 1979, 288, 1525–1528. (In French) [Google Scholar]
- Caron, J.M.; Loye-Pilot, M.D.; Conchon, O.; Scius, H. Carte géologique de France, feuille Pietra-di-Verde (1115). BRGM, 1990; scale 1: 50000, 1 sheet. (In French) [Google Scholar]
- Warr, L.N. IMA–CNMNC approved mineral symbols. Mineral. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Lanari, P.; Vidal, O.; De Andrade, V.; Dubacq, B.; Lewin, E.; Grosch, E.G.; Schwartz, S. XMapTools: A MATLAB©-based program for electron microprobe X-ray image processing and geothermobarometry. Comput. Geosci. 2014, 62, 227–240. [Google Scholar] [CrossRef]
- Paton, C.; Hellstrom, J.; Paul, B.; Woodhead, J.; Hergt, J. Iolite: Freeware for the visualisation and processing of mass spectrometric data. J. Anal. At. Spectrom. 2011, 26, 2508–2518. [Google Scholar] [CrossRef]
- Belousov, I.; Danyushevsky, L.; Goemann, K.; Gilbert, S.; Olin, P.; Thompson, J.; Lounejecaa, E.; Garbe-Shonberg, D. STDGL3, a Reference Material for Analysis of Sulfide Minerals by Laser Ablation ICP-MS: An Assessment of Matrix Effects and the Impact of Laser Wavelengths and Pulse Widths. Geostand. Geoanalytical Res. 2023, 47, 493–508. [Google Scholar] [CrossRef]
- Kampman, N.; Bertier, P.; Busch, A.; Snippe, J.; Harrington, J.; Pipich, V.; Maskell, A.; Bickle, M. Validating reactive transport models of CO2-brine-rock reactions in caprocks using observations from a natural CO2 reservoir. Energy Procedia 2017, 114, 4902–4916. [Google Scholar] [CrossRef]
- Moncada, D.; Mutchler, S.; Nieto, A.; Reynolds, T.J.; Rimstidt, J.D.; Bodnar, R.J. Mineral textures and fluid inclusion petrography of the epithermal Ag–Au deposits at Guanajuato, Mexico: Application to exploration. J. Geochem. Explor. 2012, 114, 20–35. [Google Scholar] [CrossRef]
- Large, R.R.; Maslennikov, V.V.; Robert, F.; Danyushevsky, L.V.; Chang, Z. Multistage sedimentary and metamorphic origin of pyrite and gold in the giant Sukhoi Log Deposit, Lena Gold Province. Russia. Econ. Geol. 2007, 102, 1233–1267. [Google Scholar] [CrossRef]
- Reich, M.; Deditius, A.; Chryssoulis, S.; Li, J.; Ma, C.; Parada, M.A.; Barra, F.; Mittermayr, F. Pyrite as a record of hydrothermal fluid evolution in a porphyry copper system: A SIMS/EMPA trace element study. Geochem. Cosmochim. Acta 2013, 104, 42–62. [Google Scholar] [CrossRef]
- George, L.L.; Biagioni, C.; D’Orazio, M.; Cook, N.J. Textural and trace element evolution of pyrite during greenschist facies metamorphic recrystallization in the southern Apuan Alps (Tuscany, Italy): Influence on the formation of Tl-rich sulfosalt melt. Ore Geol. Rev. 2018, 102, 59–105. [Google Scholar] [CrossRef]
- Liu, K.; Huang, F.; Gao, S.; Zhang, Z.; Ren, Y.; An, B. Morphology of framboidal pyrite and its textural evolution: Evidence from the Logatchev area, Mid-Atlantic Ridge. Ore Geol. Rev. 2022, 141, 104630. [Google Scholar] [CrossRef]
- Fan, L.; Wang, G.; Holzheid, A.; Zoheir, B.; Shi, X.; Lei, Q. Systematic variation in trace element composition of pyrites from the 26°S hydrothermal field, Mid-Atlantic Ridge. Ore Geol. Rev. 2022, 148, 105006. [Google Scholar] [CrossRef]
- Palenik, C.S.; Utsunomiya, S.; Reich, M.; Kesler, S.E.; Wang, L.; Ewing, R.C. “Invisible” gold revealed: Direct imaging of gold nanoparticles in a Carlin-type deposit. Amer Min. 2004, 89, 1359–1366. [Google Scholar] [CrossRef]
- Deditius, A.P.; Reich, M. Constraints on the solid solubility of Hg, Tl, and Cd in arsenian pyrite. Amer Min. 2016, 101, 1451–1459. [Google Scholar] [CrossRef]
- Zhao, H.X.; Frimmel, H.E.; Jiang, S.Y.; Dai, B.Z. LA-ICP-MS trace element analysis of pyrite from the Xiaoqinling gold district, China: Implications for ore genesis. Ore Geol. Rev. 2011, 43, 142–153. [Google Scholar] [CrossRef]
- Picot, P.; Johan, Z. Atlas des Minéraux Métalliques; BRGM Elsevier: Amsterdam, The Netherlands, 1977. [Google Scholar]
- Loftus-Hills, G.; Solomon, M. Cobalt, nickel and selenium in sulphides as indicators of ore genesis. Miner. Depos. 1967, 2, 228–242. [Google Scholar] [CrossRef]
- Brill, B.A. Trace-element contents and partitioning of elements in ore minerals from the CSA Cu–Pb–Zn deposit, Australia, and implications for ore genesis. Canad Miner. 1989, 27, 263–274. [Google Scholar]
- Reich, M.; Simon, A.C.; Deditius, A.; Barra, F.; Chryssoulis, S.; Lagas, G.; Tardani, D.; Knipping, J.; Bilenker, L.; Sánchez-Alfaro, P.; et al. Trace element signature of pyrite from the Los Colorados iron oxide-apatite (IOA) deposit, Chile: A missing link between Andean IOA and iron oxide–copper–gold systems? Econ. Geol. 2016, 111, 743–761. [Google Scholar] [CrossRef]
- George, L.L.; Biagioni, C.; Lepore, G.O.; Lacalamita, M.; Agrosì, G.; Capitani, G.C.; Bonaccorsi, E.; d’Acapito, F. The speciation of thallium in (Tl, Sb, As)-rich pyrite. Ore Geol. Rev. 2019, 107, 364–380. [Google Scholar] [CrossRef]
- Mederski, S.; Pršek, J.; Majzlan, J.; Kiefer, S.; Dimitrova, D.; Milovský, R.; Bender Koch, C.; Kozień, D. Geochemistry and textural evolution of As–Tl–Sb–Hg-rich pyrite from a sediment-hosted As–Sb–Tl–Pb±Hg±Au mineralization in Janjevo, Kosovo. Ore Geol. Rev. 2022, 151, 105221. [Google Scholar] [CrossRef]
- D’Orazio, M.; Biagioni, C.; Dini, A.; Vezzoni, S. Thallium-rich pyrite ores from the Apuan Alps, Tuscany, Italy: Constraints for their origin and environmental concerns. Miner. Depos. 2017, 52, 687–707. [Google Scholar] [CrossRef]
- Reich, M.; Kesler, S.E.; Utsunomiya, S.; Palenik, C.S.; Chryssoulis, S.L.; Ewing, R.C. Solubility of gold in arsenian pyrite. Geochem. Cosmochim. Acta 2005, 69, 2781–2796. [Google Scholar] [CrossRef]
- Qian, G.; Brugger, J.; Testamale, D.; Skiner, W.; Pring, A. Formation of As(II)-pyrite during experimental replacement of magnetite under hydrothermal conditions. Geochim. Cosmochim. Acta 2013, 100, 1–10. [Google Scholar] [CrossRef]
- Deditius, A.P.; Utsunomiya, S.; Renock, D.; Ewing, R.C.; Ramana, C.V.; Becker, U.; Kesler, S.E. A proposed new type of arsenian pyrite: Composition, nanostructure and geological significance. Geochim. Cosmochim. Acta 2008, 72, 2919–2933. [Google Scholar] [CrossRef]
- Deditius, A.P.; Utsunomiya, S.; Ewing, R.C.; Kesler, S.E. Nanoscale “liquid” inclusions of As–Fe–S in arsenian pyrite. Amer Min. 2009, 94, 391–394. [Google Scholar] [CrossRef]
- Deditius, A.P.; Reich, M.; Kesler, S.E.; Utsunomiya, S.; Chryssoulis, S.L.; Walshe, J.; Ewing, R.C. The coupled geochemistry of Au and As in pyrite from hydrothermal ore deposits. Geochim. Cosmochim. Acta 2014, 140, 644–670. [Google Scholar] [CrossRef]
- Fu, S.; Hu, R.; Bi, X.; Sullivan, N.A.; Yan, J. Trace element composition of stibnite: Substitution mechanism and implications for the genesis of Sb deposits in southern China. Appl. Geochem. 2020, 118, 104637. [Google Scholar] [CrossRef]
- Fu, S.; Wang, T.; Yan, J.; Pan, L.; Wei, L.; Lan, Q.; Fu, S. Formation of the Banxi Sb deposit in Eastern Yangtze Block: Evidence from individual fluid inclusion analyses, trace element chemistry, and He-Ar-S isotopes. Ore Geol. Rev. 2022, 146, 104949. [Google Scholar] [CrossRef]
- Song, X.; Lai, J.; Xu, J.; Liu, X.; Li, B.; He, H.; Wang, Y.; Shi, J.; Wang, C.; Wen, C. Material Source and Genesis of the Daocaowan Sb Deposit in the Xikuangshan Ore Field: LA–ICP–MS Trace Elements and Sulfur Isotope Evidence from Stibnite. Minerals 2022, 12, 1407. [Google Scholar] [CrossRef]
- Silyanov, S.A.; Sazonov, A.M.; Naumov, E.A.; Lobastov, B.M.; Zvyagina, Y.A.; Artemyev, D.A.; Nekrasova, N.A.; Pirajno, F. Mineral Paragenesis, formation stages and trace elements in sulfides of the Olympiada gold deposit (Yenisei Ridge, Russia). Ore Geol. Rev. 2022, 143, 104750. [Google Scholar] [CrossRef]
- Stergiou, C.L.; Melfos, V.; Voudouris, P.; Papadopoulou, L.; Spry, P.G.; Peytcheva, I.; Dimitrova, D.; Stefanova, E. A fluid inclusion and critical/rare metal study of epithermal quartz-stibnite veins associated with the Gerakario Porphyry Deposit, Northern Greece. Appl. Sci. 2022, 12, 909. [Google Scholar] [CrossRef]
- Mederski, S.; Pršek, J.; Dimitrova, D. Distribution of In, Sn, Ga, Ge, and other critical metals in sulfide ores from epithermal listvenite-associated Badovc Pb–Zn–Sb–Ni deposit (Kosovo): Insights from mineralogy and geochemistry. Ore Geol. Rev. 2024, 164, 105824. [Google Scholar] [CrossRef]
- Wang, T.; Fu, S.; Sullivan, N.A.; Lan, J.; Wei, L. Deciphering the ore-forming process of Sb-W deposits through scheelite and stibnite trace element geochemistry. J. Geochem. Explor. 2024, 257, 107367. [Google Scholar] [CrossRef]
- Qui, Z.; Wu, J.; Voudouris, P.; Tombros, S.; Liu, J.; Zhai, D. LA-ICP-MS Trace Element Characteristics and Geological Significance of Stibnite in the Zhaxikang Pb–Zn–Ag–Sb Deposit, Southern Tibet, SW China. Minerals 2024, 14, 1294. [Google Scholar] [CrossRef]
- Ma, G.; Zhao, X.; Mao, Q.; Xue, C.; Wan, J.; Li, J. Gold and antimony metallogenic relationship of the Awanda Au (Sb) deposit, South Tianshan, NW China: Constraints from textures, in-situ trace elements and s-pb isotope systematics. Ore Geol. Rev. 2024, 176, 106384. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Wang, Q.; Zhang, H.; Zhu, F. Trace element composition and significance of quartz and stibnite in the Woxi Au-Sb-W deposit, Hunan. Ore Energy Resour. Geol. 2025, 18, 100082. [Google Scholar] [CrossRef]
- Mederski, S.; Pršek, J.; Kluza, K.; Dimitrova, D. LA-ICP-MS Trace Element Composition of Stibnite from the Kizhnica-Hajvalia Badovc Ore Field, Kosovo. In Proceedings of the Mineral Resources in a Changing World 17th Biennial SGA Meeting, Zurich, Switzerland; 2023; pp. 296–299. [Google Scholar]
- Rytuba, J.J. Mercury from mineral deposits and potential environmental impact. Environ. Geol. 2003, 43, 326–338. [Google Scholar] [CrossRef]
- Wilson, S.C.; Lockwood, P.V.; Ashley, P.M.; Tighe, M. The chemistry and behaviour of antimony in the soil environment with comparisons to arsenic: A critical review. Environ. Pollut. 2010, 158, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Filimon, D.I. Metallogenesis of As-Sb sulfides Ore Deposit of Matra (Alpine Corsica, France). Master’s Thesis, University of Pisa, Pisa, Italy, 2023. [Google Scholar]
- André-Mayer, A.S.; Leroy, J.; Bailly, L.; Chauvet, A.; Marcoux, E.; Grancea, L.; Llosa, F.; Rosas, J. Boiling and vertical mineralization zoning. A case study from the Apacheta low-sulfidation epithermal gold-silver deposit, south Peru. Miner. Depos. 2002, 37, 452–464. [Google Scholar] [CrossRef]
- Spycher, N.F.; Reed, M.H. Evolution of a broadlands-type epithermal ore fluid along alternative P–T paths; implications for the transport and deposition of base, precious, and volatile metals. Econ. Geol. 1989, 84, 328–359. [Google Scholar] [CrossRef]
- Vaughan, D.J.; Craig, J.R. Mineral Chemistry of Metal Sulfides. Cambridge University Press: Cambridge, UK, 1978. [Google Scholar]
- Jovanovski, G.; Makreski, P. Intriguing minerals: Photoinduced solid-state transition of realgar to pararealgar—Direct atomic scale observation and visualization. ChemTexts 2020, 6, 5. [Google Scholar] [CrossRef]
- Krupp, R.E. Solubility of stibnite in hydrogen sulfide solution, speciation, and equilibrium constants, from 25 to 350 °C. Geochim. Cosmochim. Acta 1988, 52, 3005–3015. [Google Scholar] [CrossRef]
- Wilson, N.; Webster-Brown, J.; Brown, K. Controls on stibnite precipitation at two New Zealand geothermal power stations. Geothermics 2007, 36, 330–347. [Google Scholar] [CrossRef]
- Yu, H.C.; Qiu, K.F.; Simon, A.C.; Wang, D.; Mathur, R.; Wan, R.Q.; Jiang, X.-Y.; Deng, J. Telescoped boiling and cooling mechanisms triggered hydrothermal stibnite precipitation: Insights from the world’s largest antimony deposit in Xikuangshan China. Amer Min. 2023, 108, 1213–1223. [Google Scholar] [CrossRef]
- Ferrill, D.A.; Morris, A.P.; Evans, M.A.; Burkhard, M.; Groshong, R.H., Jr.; Onasch, C.M. Calcite twin morphology: A low-temperature deformation geothermometer. J. Struct. Geol. 2004, 26, 1521–1529. [Google Scholar] [CrossRef]
- Hagemann, S.; Lüders, V. P-T-X conditions of hydrothermal fluids and precipitation mechanism of stibnite-gold mineralization at the Wiluna lode-gold deposits, Western Australia: Conventional and infrared microthermometric constraints. Miner. Depos. 2003, 38, 936–952. [Google Scholar] [CrossRef]
- Dickson, F.W.; Radke, A.S.; Weissberg, B.G.; Heropoulos, C. Solid solutions of antimony, arsenic, and gold in stibnite (Sb2S3) Orpiment (As2S3), and realgar (As2S2). Econ. Geol. 1975, 70, 591–594. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Normand, C. Controls of mineral parageneses in the system Fe–Sb–S–O. Econ. Geol. 1997, 92, 308–324. [Google Scholar] [CrossRef]
- Seward, T.M. The formation of lead (II) chloride complexes to 300 °C: A spectrophotometric study. Geochim. Cosmochim. Acta 1984, 48, 121–134. [Google Scholar] [CrossRef]
- Zhong, R.C.; Brugger, J.; Chen, Y.J.; Li, W.B. Contrasting regimes of Cu, Zn and Pb transport in ore-forming hydrothermal fluids. Chem. Geol. 2015, 395, 154–164. [Google Scholar] [CrossRef]
- Gliozzo, E.; Brogi, A.; Ruggieri, G.; Langone, A. Sb–Au-bearing chalcedonies in hot geothermal systems: Insights from the jasperoids of Poggio Peloso (southern Tuscany, Italy). Geol. Mag. 2023, 160, 712–731. [Google Scholar] [CrossRef]
- Di Rosa, M.; Filimon, D.I.; Groff, J.A.; Marroni, M. U–Pb dating of carbonate gangue with associated As–Sb mineralization in the Matra Fault (Alpine Corsica, France): Constraints for the rifting stage in the Tyrrhenian Sea. Journal of Geodynamics 2025, 165, 102110. [Google Scholar] [CrossRef]
- Moeller, S.; Grevemeyer, I.; Ranero, C.R.; Berndt, C.; Kleschen, D.; Sallares, V.; Zitellini, N.; de Franco, R. Early-stage rifting of the northern Tyrrhenian Sea Basin: Results from a combined wide-angle and multichannel seismic study. Geochem. Geophys. Geosystatistics 2013, 14, 3032–3052. [Google Scholar] [CrossRef]
- Dini, A.; Gianelli, G.; Puxeddu, M.; Ruggieri, G. Origin and evolution of Pliocene–Pleistocene granites from the Larderello geothermal field (Tuscan Magmatic Province, Italy). Lithos 2005, 81, 1–31. [Google Scholar] [CrossRef]






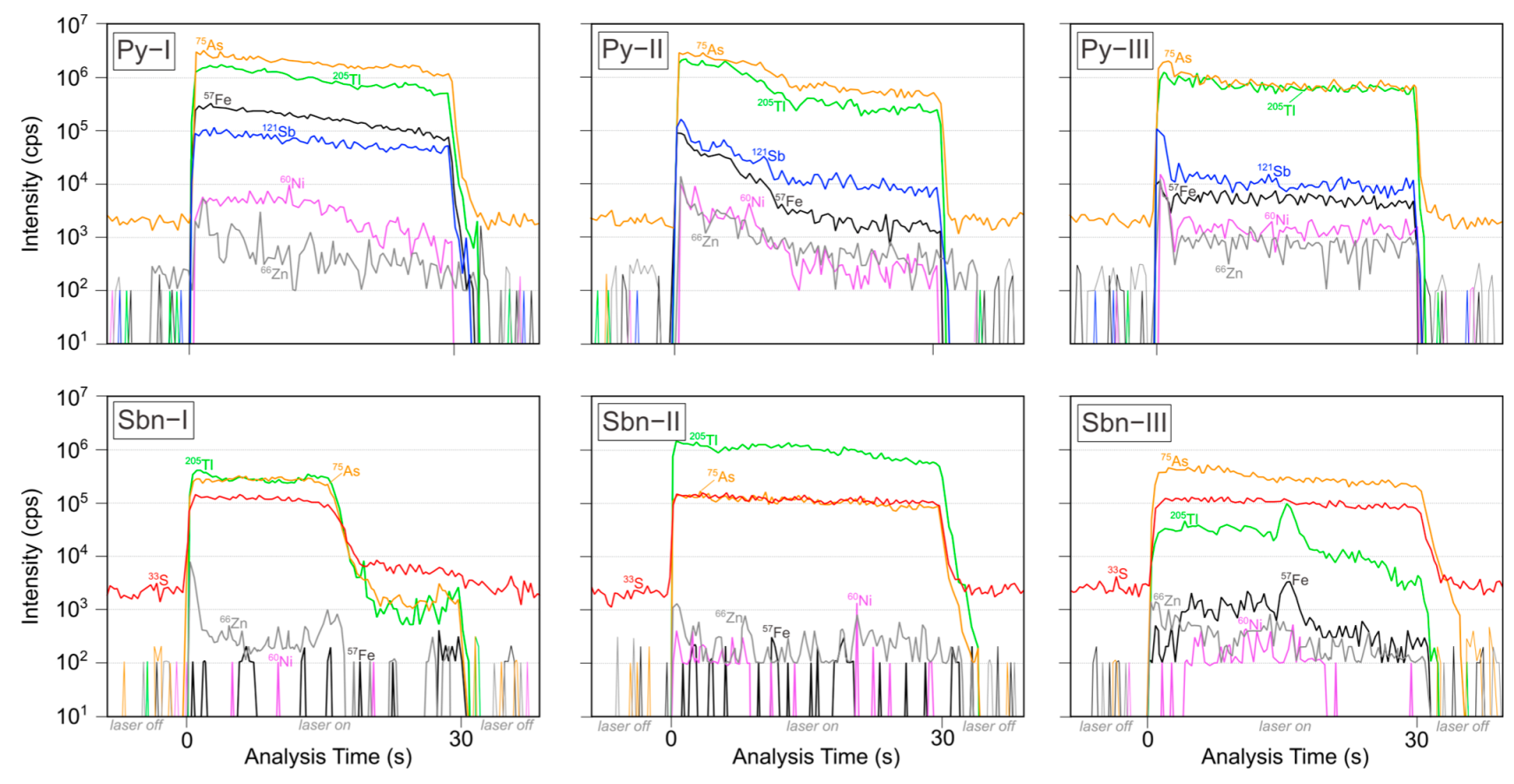
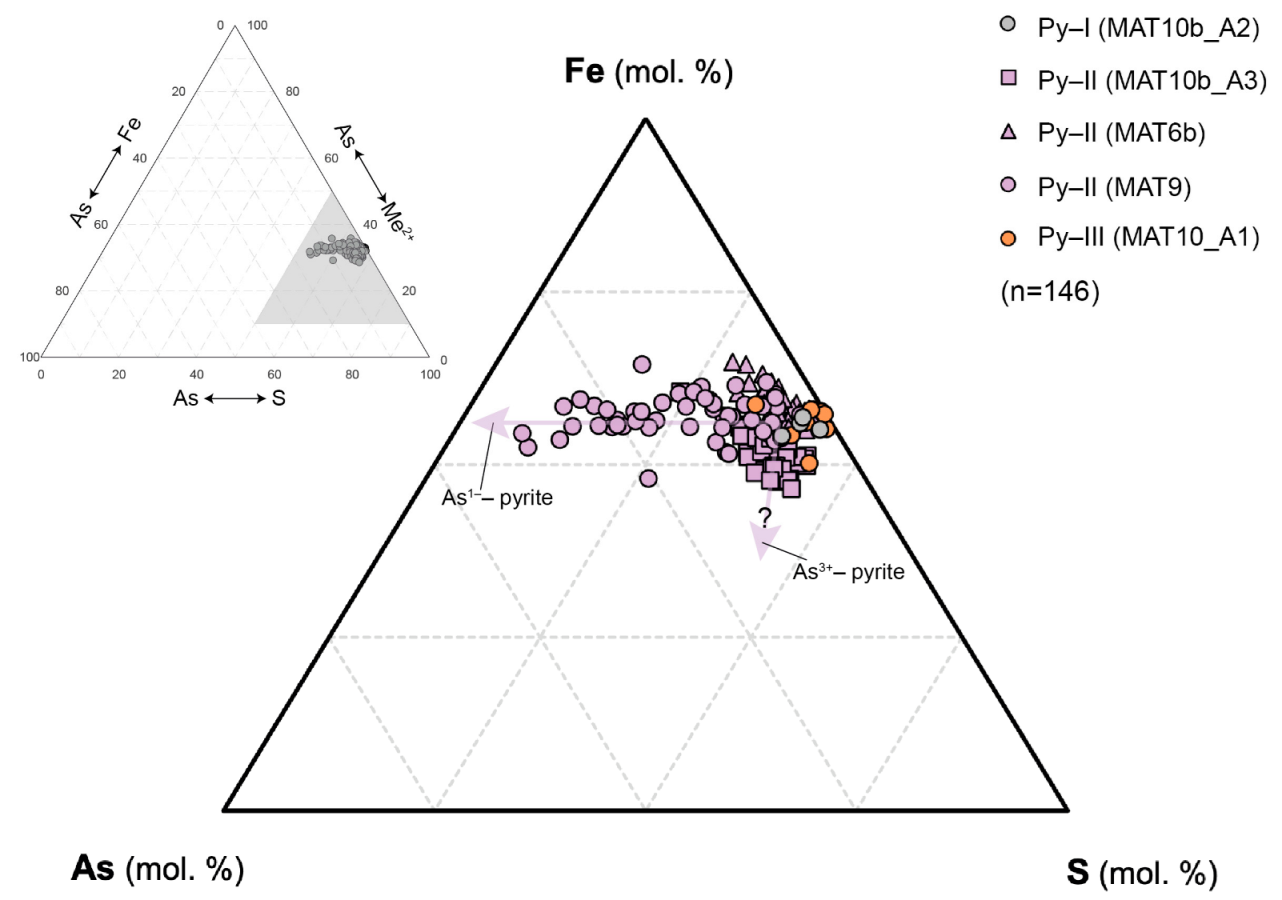
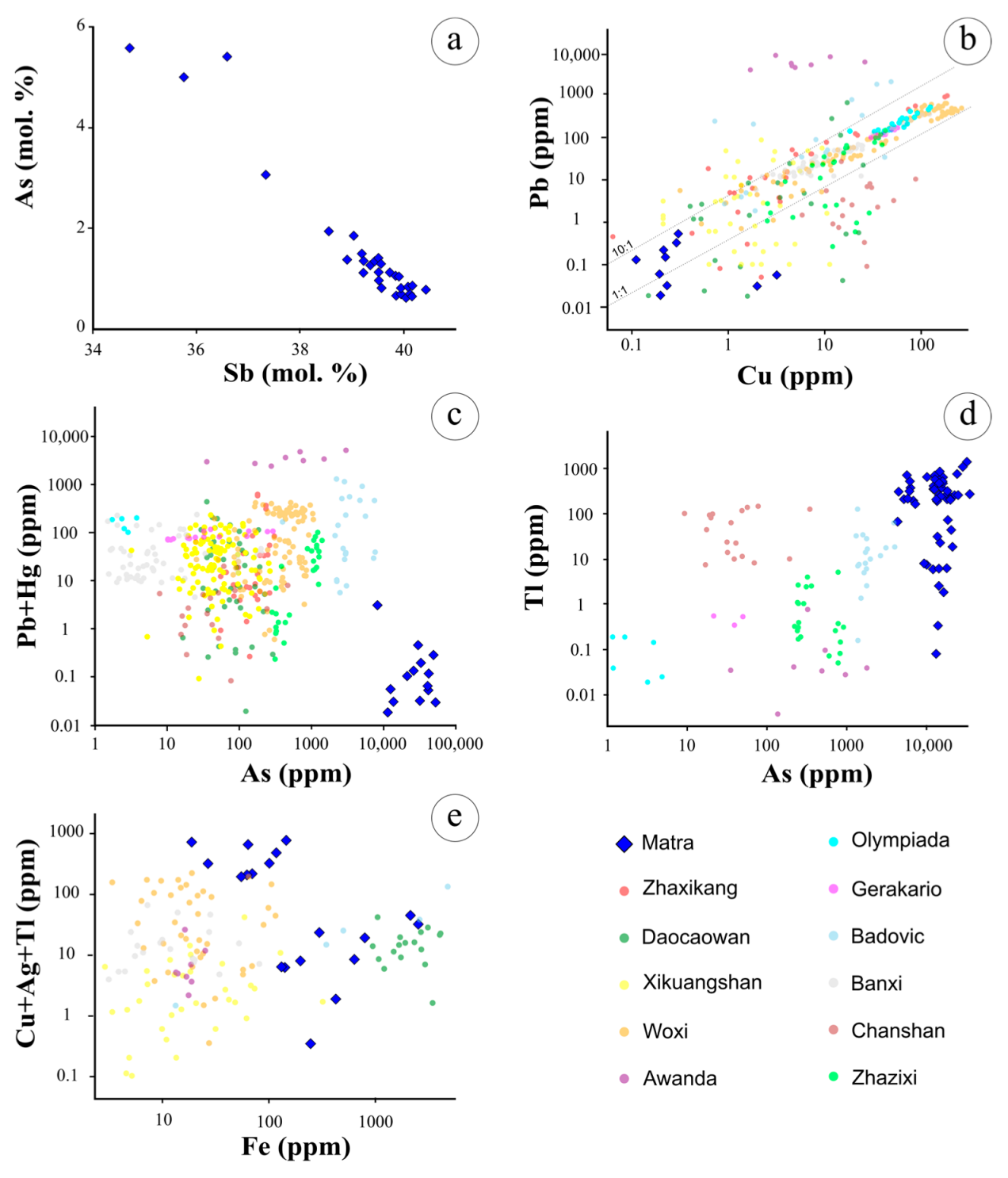

| Sample | Type | Description |
|---|---|---|
| MAT 4, 4b | Host rock | Foliated calc–schist of Upper Castagniccia Unit, characterized by different veins of realgar and stibnite. |
| MAT 6, 6b | Breccia | Breccia with quartz and dolomite clasts with the matrix containing traces of pyrite, realgar, and orpiment. |
| MAT 7 | Ore | Mineralized sample with dolomite gangue, showing veins of realgar and disseminated stibnite and pyrite. |
| MAT 8 | Ore | Framework of calcite veins with massive realgar. |
| MAT 9 | Ore | Dolomite gangue with interstitial stibnite, realgar, and crosscutting calcite veins. |
| MAT 10, 10b | Ore | Dolomite gangue with stibnite, realgar, and pyrite in veinlets or disseminations. |
| MAT 11 | Breccia | Breccia with quartz and dolomite clasts in a matrix containing traces of mineralization, including pyrite, realgar, and hörnesite. |
| MAT 12, 12b | Ore | Quartz gangue with disseminated stibnite and pyrite, along with nodules of realgar. |
| MAT 14, 14b | Gossan | Non–cohesive altered and mineralized rock, showing iron–oxide coloration with traces of green zones enriched with nickel oxides. |
| MAT 15, 15b | Host rock | Serpentinite sample of the Santo Pietro di Tenda Unit, with dolomite and realgar veins cutting the main foliation. |
| MAT 24, 24b, 24c | Ore breccia | Mineralized breccia composed of drusy dolomite gangue with clasts of pyrite, stibnite, and realgar, featuring slickensides and slickenfibers on quartz and stibnite. |
| MAT 25 | Gossan | Altered sample with iron–oxide coloration, containing drusy calcite veins. |
| MAT 26 | Gossan | Altered sample with iron–oxide coloration and quartz veins. |
| MAT 28 | Ore | Mineralized sample with dolomite and calcite, containing massive realgar and disseminated stibnite and pyrite. |
| MAT 31 | Gossan | Weathered, altered, with a reddish–brown iron–oxide coating. Contains residual quartz and calcite veinlets. |
| Minerals | N | Ti | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Mo | Ag | Cd | In | Sn | Sb | Te | W | Pt | Au | Tl | Pb | Bi | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Py–I | 20 | max | 3.83 | n.d. | 1.63 | n.d. | 3.17 | 219 | 1.46 | 386 | n.d. | n.d. | 49,417 | n.d. | 5.59 | n.d. | n.d. | 0.10 | 0.39 | 26,876 | n.d. | 0.12 | n.d. | n.d. | 1795 | 0.10 | n.d. |
| min | 2.00 | n.d. | 0.85 | n.d. | 0.18 | 11.5 | 0.34 | 1.74 | n.d. | n.d. | 11,601 | n.d. | 0.63 | n.d. | n.d. | 0.03 | 0.26 | 8999 | n.d. | 0.12 | n.d. | n.d. | 905 | 0.06 | n.d. | ||
| median | 3.00 | n.c. | 1.25 | n.c. | 0.53 | 35.5 | 0.51 | 2.91 | n.c. | n.c. | 19,793 | n.c. | 2.91 | n.c. | n.c. | 0.05 | 0.33 | 12,699 | n.c. | 0.12 | n.c. | n.c. | 1075 | 0.10 | n.c. | ||
| mean | 2.86 | n.c. | 1.23 | n.c. | 0.88 | 58.2 | 0.62 | 22.5 | n.c. | n.c. | 23,286 | n.c. | 2.92 | n.c. | n.c. | 0.05 | 0.33 | 13,261 | n.c. | 0.12 | n.c. | n.c. | 1165 | 0.09 | n.c. | ||
| stdev | 0.55 | n.c. | 0.21 | n.c. | 0.92 | 52.4 | 0.34 | 85.6 | n.c. | n.c. | 10,443 | n.c. | 1.32 | n.c. | n.c. | 0.02 | 0.09 | 4458 | n.c. | n.c. | n.c. | n.c. | 237 | 0.02 | n.c. | ||
| %adl | 100 | n.c. | 100 | n.c. | 85.0 | 100 | 45.0 | 100 | n.c. | n.c. | 100 | n.c. | 100 | n.c. | n.c. | 100 | 10.0 | 100 | n.c. | 5.00 | n.c. | n.c. | 100 | 15.0 | n.c. | ||
| Py–II | 20 | max | 12.7 | n.d. | 627 | n.d. | 55.0 | 2413 | 6.79 | 257 | n.d. | n.d. | 154,785 | n.d. | 1160 | n.d. | 0.60 | 1.16 | 11.9 | 76,967 | n.d. | 7.28 | n.d. | n.d. | 13,604 | 0.93 | n.d. |
| min | 1.93 | n.d. | 15.1 | n.d. | 1.24 | 0.58 | 0.34 | 4.73 | n.d. | n.d. | 26,996 | n.d. | 3.99 | n.d. | 0.60 | 0.14 | 0.41 | 2285 | n.d. | 0.11 | n.d. | n.d. | 780 | 0.07 | n.d. | ||
| median | 5.84 | n.c. | 154 | n.c. | 2.55 | 34.4 | 1.11 | 46.2 | n.c. | n.c. | 46,566 | n.c. | 33.8 | n.c. | 0.60 | 0.34 | 1.28 | 15,055 | n.c. | 1.32 | n.c. | n.c. | 3662 | 0.46 | n.c. | ||
| mean | 5.64 | n.c. | 224 | n.c. | 13.0 | 340 | 1.90 | 72.8 | n.c. | n.c. | 63,066 | n.c. | 115 | n.c. | 0.60 | 0.40 | 2.11 | 23,539 | n.c. | 2.90 | n.c. | n.c. | 4589 | 0.49 | n.c. | ||
| stdev | 2.50 | n.c. | 199 | n.c. | 20.0 | 710 | 2.07 | 70.7 | n.c. | n.c. | 39,703 | n.c. | 263 | n.c. | n.c. | 0.23 | 2.64 | 20,007 | n.c. | 3.44 | n.c. | n.c. | 3751 | 0.43 | n.c. | ||
| %adl | 100 | n.c. | 100 | n.c. | 40.0 | 75.0 | 50.0 | 100 | n.c. | n.c. | 100 | n.c. | 100 | n.c. | 5.00 | 95.0 | 90.0 | 100 | n.c. | 30.0 | n.c. | n.c. | 100 | 15.0 | n.c. | ||
| Py–III | 37 | max | 768 | n.d. | 780 | n.d. | 2869 | 11,405 | 442 | 907 | n.d. | n.d. | 320,187 | 3.70 | 2580 | 0.23 | 1.85 | 1.56 | 2.06 | 251,513 | 1.07 | 145 | n.d. | n.d. | 47,443 | 5.50 | n.d. |
| min | 1.98 | n.d. | 0.68 | n.d. | 0.47 | 10.3 | 0.43 | 1.87 | n.d. | n.d. | 4454 | 3.70 | 0.57 | 0.23 | 0.67 | 0.03 | 0.49 | 389 | 1.07 | 0.07 | n.d. | n.d. | 9.67 | 0.11 | n.d. | ||
| median | 7.82 | n.c. | 40.9 | n.c. | 68.0 | 2381 | 43.0 | 12.2 | n.c. | n.c. | 32,232 | 3.70 | 6.59 | 0.23 | 1.01 | 0.16 | 1.24 | 2319 | 1.07 | 1.69 | n.c. | n.c. | 928 | 0.59 | n.c. | ||
| mean | 40.0 | n.c. | 87.2 | n.c. | 153 | 3068 | 79.4 | 52.8 | n.c. | n.c. | 76,138 | 3.70 | 303 | 0.23 | 1.07 | 0.28 | 1.22 | 28,707 | 1.07 | 8.17 | n.c. | n.c. | 6620 | 1.32 | n.c. | ||
| stdev | 130 | n.c. | 144 | n.c. | 466 | 3217 | 114 | 151 | n.c. | n.c. | 90,896 | n.c. | 619 | n.c. | 0.42 | 0.30 | 0.52 | 61,702 | n.c. | 26.1 | n.c. | n.c. | 11,842 | 1.56 | n.c. | ||
| %adl | 91.9 | n.c. | 100 | n.c. | 100 | 100 | 86.5 | 100 | n.c. | n.c. | 100 | 0.00 | 81.1 | 2.70 | 18.9 | 97.3 | 16.2 | 100 | 2.70 | 81.1 | n.c. | n.c. | 100 | 73.0 | n.c. | ||
| Sbn–I | 19 | max | n.d. | 3.33 | 0.19 | n.d. | n.d. | n.d. | 3.04 | 8.87 | 0.26 | 1.30 | 44,078 | 28.6 | 0.76 | n.d. | n.d. | 0.05 | 9.30 | n.d. | n.d. | 0.04 | n.d. | n.d. | 1606 | 0.06 | n.d. |
| min | n.d. | 1.04 | 0.19 | n.d. | n.d. | n.d. | 0.26 | 1.55 | 0.03 | 0.69 | 12,247 | 5.30 | 0.24 | n.d. | n.d. | 0.01 | 1.15 | n.d. | n.d. | 0.04 | n.d. | n.d. | 126 | 0.03 | n.d. | ||
| median | n.c. | 1.75 | 0.19 | n.c. | n.c. | n.c. | 1.20 | 4.08 | 0.05 | 1.16 | 15,548 | 8.73 | 0.42 | n.c. | n.c. | 0.03 | 4.62 | n.c. | n.c. | 0.04 | n.c. | n.c. | 432 | 0.04 | n.c. | ||
| mean | n.c. | 2.13 | 0.19 | n.c. | n.c. | n.c. | 1.36 | 4.07 | 0.08 | 1.06 | 19,681 | 12.8 | 0.46 | n.c. | n.c. | 0.03 | 4.92 | n.c. | n.c. | 0.04 | n.c. | n.c. | 575 | 0.04 | n.c. | ||
| stdev | n.c. | 0.96 | n.c. | n.c. | n.c. | n.c. | 0.73 | 1.80 | 0.06 | 0.18 | 9755 | 10.6 | 0.24 | n.c. | n.c. | 0.01 | 3.65 | n.c. | n.c. | n.c. | n.c. | n.c. | 421 | 0.02 | n.c. | ||
| %adl | n.c. | 36.8 | 5.26 | n.c. | n.c. | n.c. | 100 | 100 | 84.2 | 68.4 | 100 | 21.1 | 21.1 | n.c. | n.c. | 52.6 | 31.6 | n.c. | n.c. | 5.26 | n.c. | n.c. | 100 | 10.5 | n.c. | ||
| Sbn–II | 18 | max | n.d. | 0.70 | 1.01 | 148 | 0.03 | 8.71 | 7.19 | 4.69 | 0.02 | 1.20 | 34,885 | 7.09 | 6.80 | 0.02 | 0.23 | 0.04 | 10.5 | n.d. | 0.29 | 0.03 | n.d. | n.d. | 898 | 0.13 | 0.005 |
| min | n.d. | 0.42 | 0.09 | 19.0 | 0.03 | 1.94 | 0.10 | 0.58 | 0.01 | 0.65 | 4508 | 0.78 | 0.69 | 0.02 | 0.14 | 0.01 | 0.19 | n.d. | 0.29 | 0.02 | n.d. | n.d. | 75.6 | 0.02 | 0.005 | ||
| median | n.c. | 0.56 | 0.31 | 63.0 | 0.03 | 4.06 | 1.56 | 3.35 | 0.01 | 0.98 | 14,180 | 1.53 | 1.40 | 0.02 | 0.14 | 0.02 | 2.25 | n.c. | 0.29 | 0.03 | n.c. | n.c. | 302 | 0.05 | 0.005 | ||
| mean | n.c. | 0.56 | 0.40 | 69.3 | 0.03 | 5.01 | 1.85 | 2.98 | 0.01 | 0.93 | 14,005 | 2.36 | 2.34 | 0.02 | 0.16 | 0.02 | 2.98 | n.c. | 0.29 | 0.03 | n.c. | n.c. | 378 | 0.06 | 0.005 | ||
| stdev | n.c. | 0.20 | 0.32 | n.c. | n.c. | 2.91 | 2.14 | 1.43 | 0.01 | 0.16 | 8774 | 2.67 | 1.98 | n.c. | 0.05 | 0.01 | 3.20 | n.c. | n.c. | 0.01 | n.c. | n.c. | 233 | 0.05 | n.c. | ||
| %adl | n.c. | 11.1 | 50.0 | 38.9 | 5.56 | 27.8 | 88.9 | 100 | 33.3 | 83.3 | 100 | 27.8 | 100 | 5.56 | 22.2 | 50.0 | 44.4 | n.c. | 5.56 | 11.1 | n.c. | n.c. | 100 | 22.2 | 5.56 | ||
| Sbn–III | 19 | max | n.d. | 21.3 | 273 | 2584 | 0.59 | 20.5 | 1.78 | 4.53 | 0.05 | 1.54 | 21,237 | 1.52 | 5.72 | n.d. | n.d. | 0.04 | 8.02 | n.d. | 0.25 | 4.32 | n.d. | n.d. | 681 | 3.82 | 0.01 |
| min | n.d. | 1.10 | 0.30 | 64.5 | 0.09 | 3.63 | 0.20 | 0.95 | 0.03 | 0.77 | 4383 | 1.52 | 0.38 | n.d. | n.d. | 0.01 | 0.57 | n.d. | 0.11 | 0.02 | n.d. | n.d. | 0.08 | 0.03 | 0.01 | ||
| median | n.c. | 2.78 | 0.63 | 276 | 0.35 | 12.8 | 0.28 | 2.06 | 0.05 | 1.12 | 13,775 | 1.52 | 1.90 | n.c. | n.c. | 0.02 | 4.13 | n.c. | 0.18 | 0.06 | n.c. | n.c. | 19.18 | 0.18 | 0.01 | ||
| mean | n.c. | 5.89 | 30.6 | 655 | 0.34 | 12.3 | 0.43 | 2.10 | 0.05 | 1.16 | 13,403 | 1.52 | 2.40 | n.c. | n.c. | 0.02 | 4.21 | n.c. | 0.18 | 0.82 | n.c. | n.c. | 113 | 0.66 | 0.01 | ||
| stdev | n.c. | 6.66 | 81.8 | 841 | 0.21 | 8.44 | 0.51 | 0.86 | 0.01 | 0.24 | 4122 | n.c. | 1.70 | n.c. | n.c. | 0.01 | 3.34 | n.c. | 0.10 | 1.72 | n.c. | n.c. | 194 | 1.29 | n.c. | ||
| %adl | n.c. | 52.6 | 57.9 | 63.2 | 21.05 | 15.8 | 0.00 | 94.7 | 26.3 | 80.0 | 100 | 5.26 | 73.7 | n.c. | n.c. | 31.6 | 21.1 | n.c. | 10.5 | 31.6 | n.c. | n.c. | 100 | 42.1 | 5.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filimon, D.I.; Groff, J.A.; Saccani, E.; Di Rosa, M. Ore Genesis Based on Microtextural and Geochemical Evidence from the Hydrothermal As–Sb Mineralization of the Matra Deposit (Alpine Corsica, France). Minerals 2025, 15, 814. https://doi.org/10.3390/min15080814
Filimon DI, Groff JA, Saccani E, Di Rosa M. Ore Genesis Based on Microtextural and Geochemical Evidence from the Hydrothermal As–Sb Mineralization of the Matra Deposit (Alpine Corsica, France). Minerals. 2025; 15(8):814. https://doi.org/10.3390/min15080814
Chicago/Turabian StyleFilimon, Danis Ionut, John A. Groff, Emilio Saccani, and Maria Di Rosa. 2025. "Ore Genesis Based on Microtextural and Geochemical Evidence from the Hydrothermal As–Sb Mineralization of the Matra Deposit (Alpine Corsica, France)" Minerals 15, no. 8: 814. https://doi.org/10.3390/min15080814
APA StyleFilimon, D. I., Groff, J. A., Saccani, E., & Di Rosa, M. (2025). Ore Genesis Based on Microtextural and Geochemical Evidence from the Hydrothermal As–Sb Mineralization of the Matra Deposit (Alpine Corsica, France). Minerals, 15(8), 814. https://doi.org/10.3390/min15080814








