Abstract
This study examines the influence of particle size on the flotation kinetics parameters of both raw and waste fine coal originating from the anthracite mine “Vrška Čuka”, Serbia. Flotation kinetics modeling was performed using MATLAB for nonlinear regression analysis, based on coal flotation test data. The correlation between total combustible recovery and flotation time was determined using the following models: Classical, Klimpel, Kelsall, Modified Kelsall, and Fully Mixed. The coefficients of determination range from 0.9724 (the Klimpel model) to 1 (the modified Kelsall model) for raw coal and from 0.8609 (the Klimpel model) to 0.9981 (the modified Kelsall model) for waste coal. Although both the Classical and Modified Kelsall models demonstrated a good correlation with the experimental data, the Modified Kelsall model provided a slightly better fit. The maximum values of the flotation rate constant (k) for both coals were obtained for the particle size-class (−0.1 + 0.053) mm for the Classical model and (−0.2 + 0.1) mm for the modified Kelsall model. The relation between flotation kinetics constant (k) and average particle size value (dsr) was estimated for the Classical model and the modified Kelsall model. It was observed that the flotation kinetics constant (k) for coal particle size could be predicted satisfactorily.
1. Introduction
Coal flotation is a very complex physicochemical process and difficult to control. Coal flotation plays an important role in coal processing and is a commonly used method in coal processing technologies for the recovery of fine coal below 0.5 mm [1] and in some cases even below 1 mm [2].
The coal flotation theory and parameters affecting the coal flotation performance are explained in detail by Polat [1] and Laskowski [3]. Generally, a wide range of parameters affects coal flotation [1]. The most critical parameter affecting the stability of bubble-particle aggregates is the particles’ size [4]. Particle size has an important role in coal flotation as well as flotation kinetics.
A number of kinetic models have been developed and tested for the coal flotation process. A comprehensive review on flotation kinetics was presented by Lynch et al. [5], Polat and Chander [6], and Drzymala [7]. A review of the available literature indicates that coal flotation typically follows first-order kinetics. In general, the best-fit kinetic model varies for different coal types and flotation conditions.
The relationship between particle size and flotation performance is complex, as demonstrated by numerous studies showing that variations in coal particle size distribution significantly affect flotation kinetics. Numerous studies have been carried out to investigate the influence of particle size on the coal flotation kinetics [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32].
Sokolović and Mišković [8] provided a comprehensive review of flotation kinetics models for coal presented in the literature, summarizing key findings from selected studies that examined the influence of particle size on flotation recovery, rate constants, and quality, while also emphasizing major developments and results achieved in this field over the past several decades.
Li et al. [17] studied the flotation kinetics and separation selectivity of three narrow coal size fractions. The values of R and k were determined using MATLAB R2010a software. They assert that during coal flotation, the organic component floats according to the first-order kinetics, while the inorganic component floats according to the second-order kinetics. The flotation rate constant of both combustible matter and ash-forming minerals increases with the particle size.
Ni et al. [19] used six kinetic models to coal flotation and found that the first-order kinetic model with rectangular distribution best described the process of the rougher and cleaner flotation of a bituminous coal.
Bu et al. [20] reported that the representative flotation kinetic models of 375, 188, 100, and 37 μm (average particle sizes) coal particles corresponded to the first order with rectangular distribution, non-integral order, non-integral order, and first order with rectangular distribution, respectively. Similarly, Bu et al. [22] concluded that the first-order model best fits conventional flotation of −74 µm coal fines, while more complex models are suited for carrier flotation.
Shahbazi and Chelgani [23] developed computational models correlating particle properties and hydrodynamic conditions with flotation performance, achieving high predictive accuracy (R2 = 0.85–0.99) for flotation rate and recovery.
Norori-McCormac et al. [24] demonstrated that particle size distribution substantially affects froth stability. Specifically, intermediate distributions (d80 ≈ 103.5 µm) generate more stable froths at lower air flow rates, while finer particles (d80 ≈ 89.6 µm) favor higher recovery at increased air rates.
The introduction of novel flotation technologies, such as oily bubble flotation [21,25] and nanobubble-assisted flotation [26], has further enhanced the recovery and kinetics of medium and fine coal particles. These technologies deliver higher flotation rate constants and improved combustible recovery compared to conventional air bubble flotation, emphasizing the role of particle size in flotation mechanism efficiency.
Studies by Sahoo et al. [27] and Bahrami et al. [28] confirmed that intermediate particle size fractions achieve the highest flotation rates and combustible recoveries in both bituminous and high-ash coals. In both cases, the first-order kinetic model, combined with a rectangular floatability distribution, provided the best fit to experimental data, validating the model’s applicability across coal types and particle size ranges.
Şahbaz and Demir [29] revealed that frother type and particle size jointly affect flotation kinetics in the Jameson cell. Their study found that aliphatic alcohol-based frothers improve the recovery of fine particles, whereas polypropylene glycol-based frothers are more effective for coarser fractions. According to Karaca et al. [30], the flotation rate constant increased significantly for medium and coarse particles in the Jameson cell compared to mechanical flotation cells.
Recent studies by Lu et al. [31] and Kazemi et al. [32] further emphasized the crucial role of particle size distribution and liberation characteristics. Lu et al. [31] demonstrated that medium-sized coal slime fractions exhibit superior hydrophobicity and flotation kinetics, while Kazemi et al. [32] showed that recovery efficiencies vary significantly between laboratory and industrial scales, influenced by particle size and liberation degree.
In summary, the flotation rate constant is strongly dependent on coal particle size. More recent studies have shown that the highest flotation rates are achieved within an intermediate particle size range, while significantly lower rates are observed for both fine and coarse particles. These trends are commonly attributed to the low collision probability between fine particles and bubbles, as well as the high likelihood of detachment of coarse particles from bubbles.
The relation between the flotation rate constant and cumulative recovery with particle size was found to be nonlinear [15]. Studies showed that the first-order kinetic model with a rectangular distribution of floatability gave the best fit to the flotation experimental data for coal with particle sizes between 37 and 375 µm [8]. Furthermore, the nonintegral-order equation fit the test data of fine coal with average particle sizes between 188 and 100 μm [15].
Understanding the relationship between particle size and flotation kinetics is crucial for optimizing the performance of the coal flotation process. Despite previous studies, inconsistencies remain in reports on the optimal particle sizes for maximum recovery and rate constants, partly due to variations in coal rank, surface chemistry, and experimental conditions. On the other hand, coal flotation kinetics are insufficiently studied on waste coals, i.e., coals that have been exposed to oxidation due to atmospheric influences.
Natural weathering processes can make coal surfaces more hydrophilic due to an increase in the content of hydrophilic functional groups (C-O, C=O, and COOH) and a decrease in the content of hydrophobic functional groups (C-C and C-H) on the coal surface [33]. These functional groups significantly reduce the wettability and hydrophobicity of coal [34] and, thus, negatively affect its flotation [35,36]. Weathering can affect the parameters used to model flotation kinetics, such as the flotation rate constant, thus reducing the flotation recovery of coal [37]. Sokolovic et al. [37] considered a classical first-order model and the modified Kelsall model for modeling raw and waste anthracite coal flotation. The application of a nonlinear model fitting approach showed that both models exhibited good correlation with the experimental data, with the Modified Kelsall model providing the best fit.
It is widely recognized that there is a strong relationship between particle size and the flotation kinetics. On the other hand, the flotation kinetics of waste coal have been little investigated. This paper investigates the effect of particle size on the flotation kinetics parameters of raw and waste coal from the anthracite mine “Vrška Čuka”, Serbia. The aim of this study is to systematically investigate the effect of particle size on the flotation kinetics of raw and waste coal, using well-defined particle size-classes and controlled flotation conditions. The results are expected to provide insights into the rate-limiting steps for different particle size ranges and guide process optimization for enhanced recovery and product quality. Understanding these interactions allows for more efficient control over flotation processes and better separation efficiency.
2. Materials and Methods
2.1. Materials
Coal samples of about 250 kg were collected from the anthracite coal mine “Vrška Čuka” in Serbia. The first representative samples were collected from raw coal, and the second from the coal waste ponds. Raw coal (RC) was sampled as feed from the “BSRI-1200” dense-medium separator, with a particle size range of 0–25 mm. Waste coal (WC) was sampled from the coal waste ponds, with a particle size range of 0–1 mm. The raw coal sample was screened at 1 mm for particle size analysis and flotation kinetics tests. The collected samples were subsampled using the coning and quartering method to obtain a representative sample for particle size-class analysis and laboratory flotation tests.
2.2. Methods
2.2.1. Particle Size-Class Analysis
The raw and waste coal samples were sieved to determine the particle size-class distribution. Wet sieving on sieves 0.5, 0.2, 0.1, and 0.053 mm was applied for determining the particle size distribution of both coal samples.
Laboratory sieve shaker Retsch AS200 was used with an amplitude of 2 mm for 25 min. After drying, the ash content was determined according to the ASTM D3174 [38] standard from 2014 at 815 °C.
The particle size distribution-wise ash content is shown in Table 1.

Table 1.
Particle size distribution and ash contents of raw coal (RC) and waste coal (WC) samples.
Based on the results presented in Table 1, it can be concluded that raw and waste coal show a similar mass distribution by size-class. By comparing the particle size distribution and ash contents by size-classes, it can be seen that a significant partition of both tested coal samples lies in the (−1 + 0.2) mm particle size range. Additionally, the presence of particles coarser than 0.5 mm was about 24% for both coals. The lowest ash content in both tested coal samples was observed in the (−0.5 + 0.2) mm particle size-class, amounting to 14.54% for raw coal and 29.60% for waste coal. It was noted that fine particle size-classes below 0.053 mm are 26.19% for raw coal and 22.77% for waste coal. Furthermore, higher ash content was observed in waste coal by particle size-classes. The average ash content in the waste coal sample was about 37%, compared to about 19% in the raw coal.
Higher ash content in waste coal may result from coal oxidation during weathering, a process whereby chemical changes occur in the coal’s structure upon prolonged exposure to atmospheric factors (such as oxygen, water, moisture, and other elements), particularly in coal storage ponds. This oxidation process induces the formation of oxygen-containing functional groups on the coal surface, leading to an increase in ash content and a subsequent reduction in flotation efficiency.
2.2.2. Proximate and Ultimate Analyses
The proximate and ultimate analyses of raw and waste coal were conducted at the Institute of Mining and Metallurgy (MMI) Bor, utilizing standard ASTM procedures to determine the coal’s composition. The CHNOS analysis (carbon, hydrogen, nitrogen, and sulfur) was performed using a LECO CHN628 elemental analyzer. The results of proximate and ultimate analyses of the raw and waste samples are given in Table 2.

Table 2.
Proximate and ultimate analyses of the raw and waste coal.
Table 2 shows that the highest carbon, hydrogen, and nitrogen content were observed in the raw coal. Also, the results of proximate analysis confirm the ash content values shown in Table 1. The carbon content was found to be higher (72.69%) in the raw coal, while the ash content was found to be higher (36.31%) in the waste coal. The results of proximate and ultimate analyses of the raw and waste coal show a reduction in C/H ratio (obtained from ultimate analysis) for about 1.30% in the waste coal, indicating the sensitivity of the coal surface to oxidation (weathering).
2.2.3. Flotation Tests
A laboratory flotation machine, Denver D-12, was used for flotation kinetics tests with the impeller speed of 1250 rpm. All coal flotation tests were conducted with feed slurries at 10% solids (by weight). The pH was adjusted to 7.5. The pH value of 7.5 was selected based on extensive experimental research [37] and corresponds to the isoelectric point of coal determined through zeta potential measurements, making it the optimal pH for flotation. Kerosene (1000 g/t), tannic acid (200 g/t), sodium silicate (200 g/t), and pine oil (45 g/t) were used as reagents in this study. The kerosene collector dosage of 1 kg/t was identified as the optimal value through flotation experiments [36] and reflects the industrial parameters used in the flotation process at the Vrška Čuka anthracite mine.
In each flotation kinetics test, the pulp was first agitated in the flotation cell for 3 min. Agitation was performed for 2 min, after which kerosene was added and the slurry was mixed for an additional 0.5 min. Subsequently, tannic acid, sodium silicate, and pine oil were introduced, followed by further stirring for 0.5 min. Air was introduced after the conditioning stage, and flotation concentrates were collected at 30, 90, 150, and 360 s. Concentrates and tailings obtained from froth flotation were processed by filtration, drying, and weighing, followed by ash content analysis for each sample. Upon completion of the flotation kinetics experiments, concentrates were subjected to dry sieving. Size-wise ash analysis for the five particle size-classes, 0.5, 0.2, 0.1, and 0.053 mm, was conducted for froth concentrates and the tailings. The sieve analysis was performed to determine the particle size distribution of the flotation products, which is critical for understanding the efficiency and selectivity of the flotation process. Based on the obtained results, the combustible recovery (R) of non-ash materials was calculated by:
where Y is the percentage yield of the concentrate, and Ac and Af are the percentage ash contents in concentrates and feed materials, respectively [12].
2.2.4. Flotation Kinetics
In this study, six different kinetic flotation models, Classical model (Zuniga, 1935) [44], Klimpel model (Klimpel, 1980) [45], Kelsall model (Kelsall, 1961) [46], Modified Kelsall model (Jowett, 1974) [47], and Fully mixed model (Imaizumi and Inoue, 1965) [48], were used to analyze batch flotation results and to optimize the flotation parameters using kinetics models.
- Classical model
A classical first-order kinetics model, introduced by Zuniga (1935) [44], given in Equation (2), is used to obtain the parameters of k and through fitting the flotation test data.
where R is the recovery [%] (dependent variable), is the ultimate (maximum) recovery of non-ash materials [%] (parameter 1), k is the flotation rate constant [min−1] (parameter 2, and t is the cumulative flotation time [min] (independent variable).
The classical first-order kinetics model was chosen to show the trends observed in the data due to its simplicity and the relatively good fit it provided.
- 2.
- Klimpel model
Klimpel model [45] used two parameters to describe flotation kinetics:
where is the ultimate (maximum) recovery of non-ash materials (%) (parameter 1), and k is the modified flotation rate constant (min−1) (parameter 2).
- 3.
- Kelsall model
Kelsall model [46] describes the flotation kinetics by considering the presence of two types of particles: fast-floating particles, with a rate constant kf, and slow-floating particles, with a rate constant ks. Additionally, the model introduces the parameter φ, which represents the fraction of slow-floating particles with the slow rate constant. Equation (4), which describes this model, can be expressed as follows:
In this model, the ultimate recovery () is not included. This model provides a slightly better description of experimental results compared to other models due to the additional parameter φ.
- 4.
- Modified Kelsall model
A modified Kelsall model [47] provides a slight improvement over the previous one, Equation (4), by introducing the value for ultimate recovery (), and is represented by Equation (5):
where kf is the fast flotation rate constant (min−1), ks is the slow flotation rate constant (min−1), and φ is the fraction of flotation components with the slow rate constant.
- 5.
- Fully mixed model
The fully mixed model [48], as the classical model, uses and k to describe flotation kinetics:
The MATLAB tool was used to fit the first-order kinetics model to the flotation test results to obtain and k values, i.e., k (first-order rate constant), kf (fast flotation rate constant), and ks (slow flotation rate constant) [49].
The coefficient of determination (r2) was used to evaluate the accuracy of the model fit, and if it was higher than 0.8, Equations (2)–(6) can be applied. Results are presented and discussed in the next section.
3. Results and Discussion
3.1. Particle Size Analysis of the Coal Flotation Products
Figure 1 and Figure 2 present a particle size-class analysis of the flotation products of raw and waste coal.
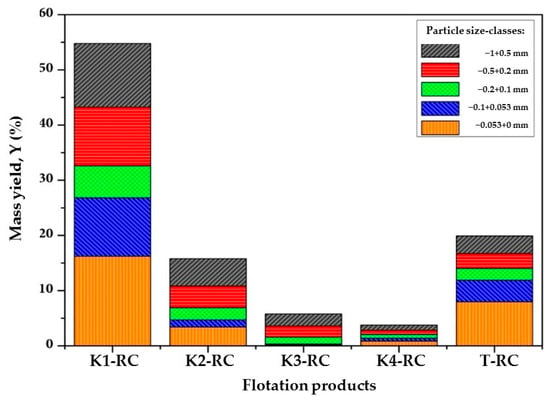
Figure 1.
Particle size-class analysis of the flotation products of raw coal.
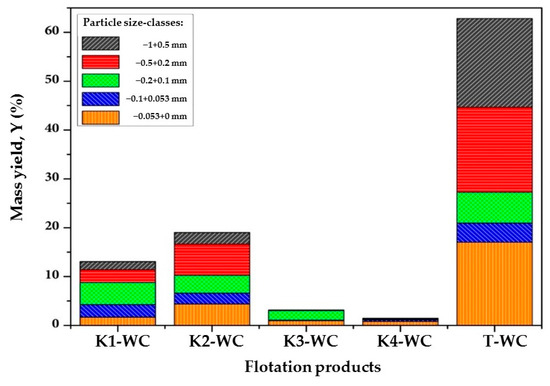
Figure 2.
Particle size-class analysis of the flotation products of waste coal.
From Figure 1, it can be seen that the dominant yield in the first minutes of raw coal flotation is composed of the coarsest and smallest coal particles. The significant presence of particle size-classes (−1 + 0.5) mm and (−0.5 + 0.2) mm in the coal flotation products obtained after 2.5 and 6 min of flotation, respectively, indicates that particles within these size-classes have the highest flotation rates. Compared to other particle size-classes, the cumulative shares of these particle size-classes in the final concentrates are 24.45% and 21.70%, respectively.
An analysis of the particle size-class distribution of the concentrate obtained from the flotation of waste coal (Figure 2) shows that coal particles from the size-classes (–0.5 + 0.2) mm and (−0.2 + 0.1) mm exhibit the highest flotation probability within the first few minutes. Notably, the (–0.5 + 0.2) mm size fraction also has the lowest ash content, which is advantageous for coal flotation. It is evident that the optimal size shifts towards intermediate and finer particle size-classes, specifically the (–0.2 + 0.1) mm and (–0.1 + 0.053) mm classes. Compared to raw coal, the mass yield of these size-classes in the final concentrates is higher, reaching approximately 28%. In contrast, the cumulative yield of the coarsest particles larger than 0.5 mm is 9.25% and 21.05% for the finest class (–0.053 + 0 mm).
3.2. Effect of Coal Particle Size on Flotation Recovery
The effect of coal particle size on the flotation recovery of raw and waste coal is presented in Table 3 and Table 4, respectively.

Table 3.
Flotation kinetics of different particle size-classes of raw coal (RC).

Table 4.
Flotation kinetics of different particle size-classes of waste coal (WC).
The cumulative recoveries for the individual particle size-classes were plotted as a function of flotation time. The curves of flotation kinetics of different particle size-classes of raw coal (RC) and waste coal (WC) are shown in Figure 3 and Figure 4.
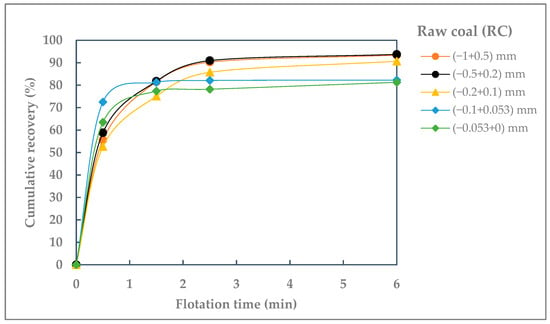
Figure 3.
Cumulative recovery of different particle size-classes of raw coal (RC) against flotation time.
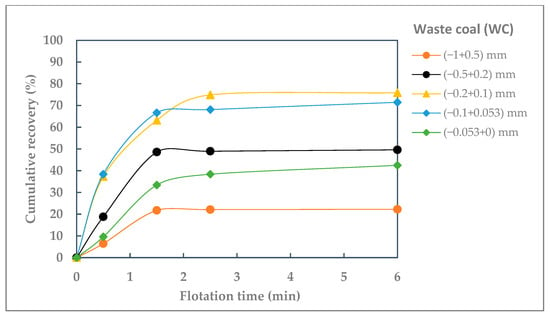
Figure 4.
Cumulative recovery of different particle size-classes of waste coal (WC) against flotation time.
The good flotation kinetics and achieved flotation recovery (over 80%) indicate the successful flotation of all coal particle sizes. The best flotation kinetics are observed for the particle size fraction (−0.5 + 0.2) mm.
However, the results show that fine, hydrophobic coal particles (below 0.1 mm) float at the beginning of the process, while the flotation rate of coarser particles (above 0.5 mm) increases after approximately 1.5 min, following the flotation of the finer particles.
For waste coal, different results were obtained. As shown in Figure 4, the difference in flotation recoveries is particularly pronounced for the coarsest and finest coal particles. Compared to raw coal, the flotation recovery of particles larger than 0.5 mm is significantly lower. The recovery of 22.23% for particles larger than 0.5 mm confirms this conclusion. The most efficient flotation of waste coal is observed in the size fractions (−0.2 + 0.1) mm and (−0.1 + 0.053) mm, which, from the point of flotation, represent the optimal particle size range. Compared to raw coal, the recovery of these fractions is lower, ranging from 71.49% to 75.86%. Furthermore, waste coal particles below 0.053 mm are more difficult to float compared to raw coal. This reduced floatability is primarily due to their higher hydrophilicity and ash content, both of which adversely affect flotation efficiency. The recovery for the finest particle size-class (−0.053 + 0) mm is 42.50%.
3.3. Effect of Coal Particle Size on Flotation Kinetics Parameters
Using the MATLAB tool for modeling first-order flotation kinetics, the values of the flotation rate constants and other flotation parameters were determined for five flotation kinetics models.
The obtained values of the flotation kinetics parameters for various particle size-classes of raw coal (RC) and waste coal (WC) samples are shown in Table 5 and Table 6.

Table 5.
Kinetics parameters generated by the first-order classical model for different particle size-classes of raw coal (RC) sample (r2—the coefficient of determination).

Table 6.
Kinetics parameters generated by the first-order classical model for different particle size-classes of waste coal (WC) sample (r2—the coefficient of determination).
A comparison of different kinetic models fitted to the experimental data for different particle size-classes of raw coal (RC) and waste coal (WC) is shown in Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13 and Figure 14.
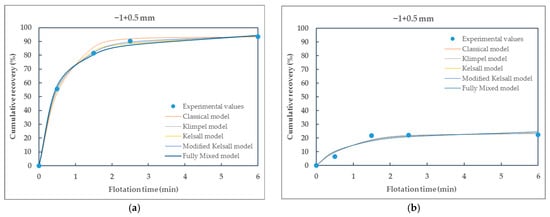
Figure 5.
Comparison of different kinetic models fitted to the experimental data of particle size-class −1 + 0.5 mm of (a) raw coal (RC) and (b) waste coal (WC) samples.
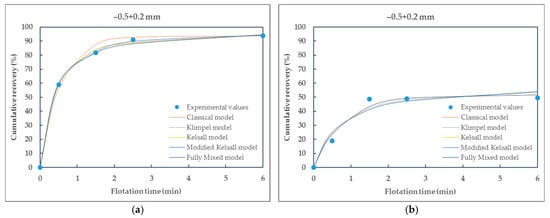
Figure 6.
Comparison of different kinetic models fitted to the experimental data of particle size-class −0.5 + 0.2 mm of (a) raw coal (RC) and (b) waste coal (WC) samples.
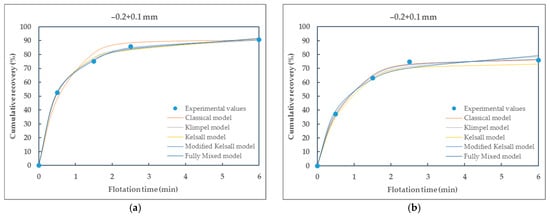
Figure 7.
Comparison of different kinetic models fitted to the experimental data of particle size-class −0.2 + 0.1 mm of (a) raw coal (RC) and (b) waste coal (WC) samples.
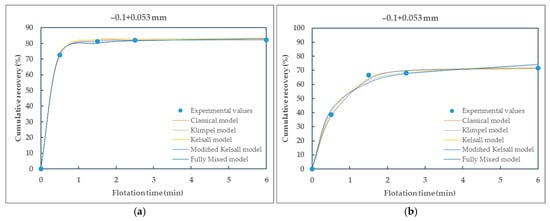
Figure 8.
Comparison of different kinetic models fitted to the experimental data of particle size-class −0.1 + 0.053 mm of (a) raw coal (RC) and (b) waste coal (WC) samples.
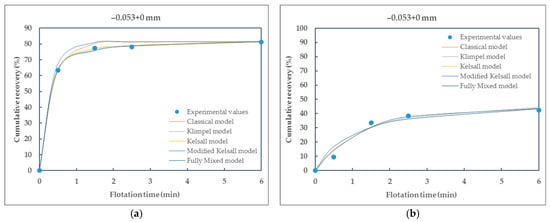
Figure 9.
Comparison of different kinetic models fitted to the experimental data of particle size-class −0.053 + 0 mm of (a) raw coal (RC) and (b) waste coal (WC) samples.
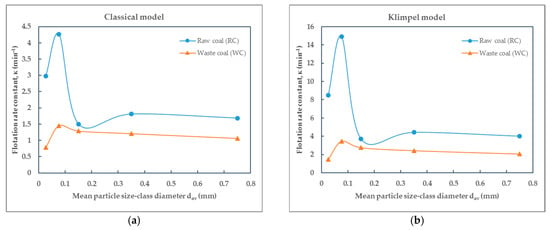
Figure 10.
Dependence of the flotation rate constant on particle size of raw (RC) and waste (WC) coal by (a) the Classical model and (b) the Klimpel model.
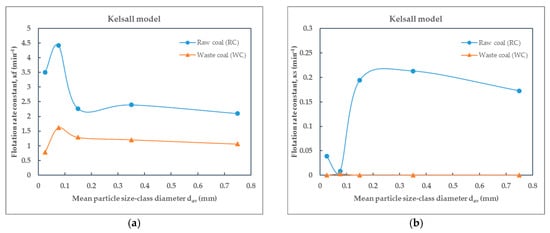
Figure 11.
Dependence of the flotation rate constant on particle size of raw (RC) and waste (WC) coal by the Kelsall model: (a) the fast and (b) the slow flotation rate constant.
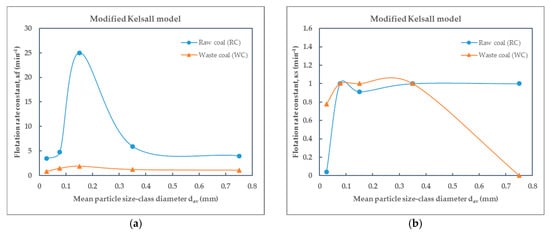
Figure 12.
Dependence of the flotation rate constant on particle size of raw (RC) and waste (WC) coal by the modified Kelsall model: (a) the fast and (b) the slow flotation rate constant.
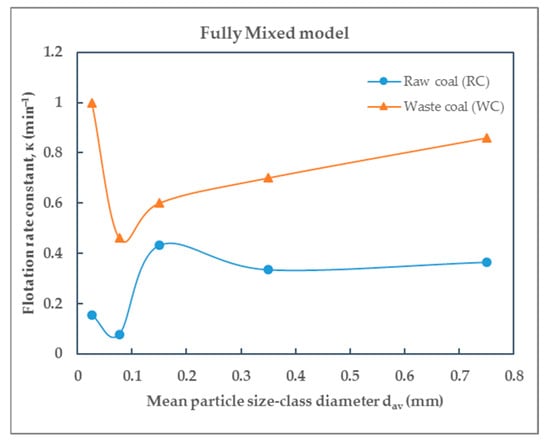
Figure 13.
Dependence of the flotation constant on particle size of raw (RC) and waste (WC) coal by the fully mixed model.
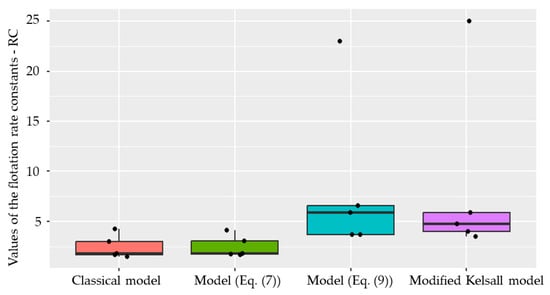
Figure 14.
Boxplot for the distribution of actual and predicted values of the flotation kinetics constants by the Classical model for raw and waste coal.
From the presented Table 5 and Table 6, it can be seen that all selected flotation kinetics models for describing first-order flotation kinetics show a good correlation with the experimental data. The obtained flotation kinetics curves are similar in shape, with the coefficients of determination being approximately equal to 1. Using the MATLAB tool, the coefficients of determination range from 0.9724 (the Klimpel model) to 1 (the modified Kelsall model) for raw coal and from 0.8609 (the Klimpel model) to 0.9981 (the modified Kelsall model) for waste coal. The poor fit of the Klimpel model (e.g., r2 = 0.8609 for waste coal) and deviations from the model assumptions most likely arise from the heterogeneity of the waste coal samples, which can lead to variability in flotation behavior and adversely affect the model’s accuracy.
Based on the coefficients of determination and the shape of the curves, it is observed that the classical and the modified Kelsall models best describe the first-order flotation kinetics. These models showed better applicability to the experimental data for raw and waste coal, with the coefficients of determination being approximately equal to 1.
Furthermore, the flotation kinetics of raw coal (RC) and waste coal (WC) across different particle size-classes demonstrate significant variation in cumulative recovery and model performance. Coarse particles (+0.5 mm) exhibit rapid flotation kinetics for RC, with most models providing a good fit; however, WC in the same particle size-class shows extremely poor recovery and model mismatch, indicating difficulty in floating coarse waste coal, likely due to poor hydrophobicity.
As particle size decreases, particularly in the (−0.2 + 0.1) mm range, RC samples achieve near-complete recovery with excellent agreement across all kinetic models, highlighting this size-class as the most favorable for flotation efficiency. Conversely, WC continues to exhibit lower recoveries, though performance improves slightly in this intermediate size range. For fine particles (−0.053 + 0 mm), both raw (RC) and waste (WC) coal show reduced flotation kinetics, likely due to increased oxidation and slime effects, with WC being particularly affected.
3.4. Effect of Coal Particle Size on Flotation Rate Constants
Based on the values of the flotation rate constants presented in Table 5 for raw coal and Table 6 for waste coal, obtained using different flotation kinetics models and for various particle size-classes, the effect of particle size—expressed by mean particle diameter—on the flotation rate constant of raw coal (RC) and waste coal (WC) is illustrated in the corresponding Figure 10, Figure 11, Figure 12 and Figure 13. The smooth lines in Figure 10, Figure 11, Figure 12 and Figure 13 represent trend lines, which connect the points based on the flotation rate constants data. This clarification ensures that the relationship between the variables is accurately represented, whether derived from experimental results or modeled trends.
The flotation kinetics of raw coal (RC) and waste coal (WC) across various particle size-classes reveal significant differences in flotation rate constants and model performance. By comparing the flotation rate constants of different particle size-classes, it can be concluded that all flotation rate constant values for raw coal are higher compared to those for waste coal.
The reduced flotation rate constants observed for waste coal are attributed to oxidation-induced hydrophilicity. Oxidation modifies the coal’s surface chemistry by increasing the concentration of oxygen-containing functional groups such as carboxyl, hydroxyl, and carbonyl. These groups enhance the coal’s surface hydrophilicity, diminishing its affinity for collector reagents and air bubbles. Consequently, both the flotation efficiency and the flotation rate constants are adversely affected.
Across all models, the Classical and the modified Kelsall models consistently provide better fitting with experimental data for both raw (RC) and waste (WC) coal. Analysis of flotation rate constants reveals that RC consistently peaks around 0.1 mm particle diameter across all models, confirming this as the optimal size for flotation. For WC, however, flotation rate constants remain lower, and there is no clear peak, indicating a fundamentally different and less efficient flotation response.
As can be seen from Figure 10, Figure 11a and Figure 12, the flotation rate constant (k), for raw coal, first increases sharply and then decreases, indicating an optimum particle size (about 0.1 mm), while for waste coal, the flotation rate constant values are lower and increase slightly with increasing particle size. The maximum flotation rate constant (k) values for raw and waste coal are 4.2624 min−1 and 1.4557 min−1, respectively, for the particle size-class of (−0.1 + 0.053) mm. The opposite findings were obtained for the Kelsall and fully mixed models. The lowest flotation rate constants were observed in this size-class.
The modified Kelsall model, which differentiates between fast and slow flotation components, offers the most comprehensive fit, capturing the heterogeneous flotation kinetics of both RC and WC. The fast rate constant shows a pronounced maximum for RC near 0.2 mm, followed by a decline with increasing or decreasing size. The slow rate constant shows modest variability in the WC. By applying the modified Kelsall model, the maximum values of the flotation rate constant k for raw and waste coal are 25 min−1 and 1.8889 min−1 and are obtained for the size-class (−0.2 + 0.1) mm. The fully mixed model also offers valuable insights, particularly for WC, where it more accurately reflects the slower, diffusion-limited nature of flotation.
Depending on the applied flotation kinetics model, it is possible to define the optimal particle size-class in the flotation process of raw and waste coal. For the classic model, the maximum values of the flotation rate constant of raw and waste coa006C are obtained for the size-class (−0.1 + 0.053) mm, while using the modified Kelsall model, it can be observed that the optimal particle size moves towards the size-class (−0.2 + 0.1) mm.
These findings showed that all flotation kinetics models showed good correlation; however, the Classical and the modified Kelsall models provided the best fit.
3.5. The Relationship Between Flotation Kinetics Constant and Coal Particle Size
The relationship between the flotation rate constant and particle size was investigated for both the classical and the modified Kelsall models. According to the results obtained for flotation kinetics parameters by the classical model, the relationship between flotation kinetics constant (k) and average particle size value (dsr) was estimated by Equations (7) and (8):
where a = 2.728965; b = −41.135466; c = 192.135603; d = −18.675380; e = 102.500493.
where a = −0.1258396; b = 0.0451993; c = −0.0014227; d = 1.1031061.
The relationship between the flotation rate constants and average particle size value for the Modified Kelsall model is expressed only for the fast flotation kinetic (kf) for both coals through Equations (9) and (10).
where a = 3.68734; b = 0.19295; c = 0.37274; d = 3.40367.
where a = 0.03926; b = 29.22031; c = −168.40056; d = 399.43937; e = -426.94274; f = −170.21096.
Analysis of variance of flotation kinetics constants is summarized in Table 7.

Table 7.
The values of the flotation kinetics constant of raw (RC) and waste (WC) coal calculated for different models.
Boxplots for the distribution of actual and predicted values of the flotation kinetics constants for raw and waste coal are shown in Figure 14 and Figure 15.
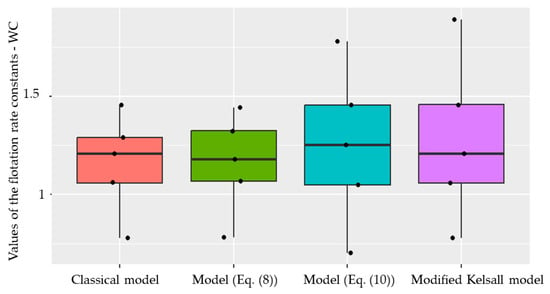
Figure 15.
Boxplot for the distribution of actual and predicted values of the flotation kinetics constants by the Modified Kelsall model for raw and waste coal.
For statistical data analysis, the Kruskal–Wallis (K-W) test and the Shapiro–Wilk (S-W) test were employed. The results of the statistical analysis are presented in Table 8.

Table 8.
The statistical test parameters for the raw and waste coal samples.
Based on the Shapiro–Wilk normality test (see the row in Table 8—“Shapiro–Wilk normality test = ‘+’ or ‘-’”), the four samples, obtained by four different methods, were checked for normality. For the sample that failed the normality test, we concluded that a parametric test like ANOVA could not be applied. Instead, we used the nonparametric alternative, the Kruskal–Wallis test. The results of the Kruskal–Wallis test are as follows: Kruskal–Wallis chi-squared = 10.109, df = 3, p-value = 0.01767. From this, we can conclude that there are significant differences between the models, as the distribution of the models is not the same.
Since all four samples (obtained by four methods) have normal distribution (as indicated by the Shapiro–Wilk normality test in Table 8—“Shapiro–Wilk normality test = ‘+’”), we applied the ANOVA test. The calculated F-value was 0.162, which is less than the critical value F_(3;16;0.05). Therefore, the ANOVA test indicates that the means of all four models are equal, suggesting no significant difference between them.
Comparison between actual and predicted values of the flotation kinetics constants for raw and waste coal, listed in Table 7, is given in Figure 16 and Figure 17.
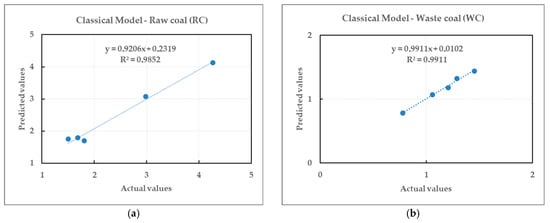
Figure 16.
Relationship between the actual and predicted values of the flotation rate constants for the classical model.
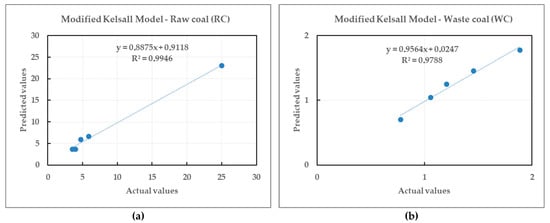
Figure 17.
Relationship between the actual and predicted values of the fast flotation rate constants for the modified Kelsall model.
The coefficient of determination (r2) and coefficient of correlation (ϱ) were used for statistical data analysis. The statistical parameters for raw (RC) and waste (WC) coal are given in Table 9 and Table 10.

Table 9.
Correlation between certain models for RC.

Table 10.
Correlation between certain models for WC.
The coefficient of determination (r2) values for the flotation kinetics constant for raw and waste coal by the classical model were 0.9852 and 0.9911, respectively. Based on the observed trend, the proposed mathematical functions given in Equations (7) and (8) are a good fit for both coals. The coefficient of correlation (ϱ) values for raw and waste coal were 0.9926 and 0.9955, respectively. The results indicate that both models are capable of predicting the flotation kinetics constant with minimal errors and high accuracy.
The r2 values for the fast flotation kinetics constant for raw and waste coal by the modified Kelsall model were 0.9946 and 0.9788, respectively. Based on the observed trend, both mathematical functions given in Equations (9) and (10) are a good fit for both coals. The analysis shows the coefficient of correlation (ϱ values obtained for raw and waste coal were 0.9973 and 0.9893, respectively). r2 and ϱ values showed that both mathematical functions give almost better estimates with smaller errors.
It was observed that the flotation kinetics constant (k) for individual particle sizes of the raw and waste coal could be predicted satisfactorily. Overall, these findings confirmed the importance of investigating the relationship between particle size and flotation rate constants across different kinetic models in order to optimize flotation performance, particularly in coal flotation.
4. Conclusions
The results of the study presented in this paper show that the classical and the modified Kelsall models can be successfully used as an effective tool for modeling the complex relation between the particle size and flotation rate constants in the flotation of raw and waste coal. The coefficient of determination (r2) values were obtained from 0.9904 to 0.9998 for raw coal and from 0.9555 to 0.9980 for waste coal, indicating a good relation.
The correlation between the flotation rate constant (k) and the average particle size value (dsr) depends on the type of coal. For the classical model, the maximum flotation rate constant (k) values for raw and waste coal are 4.2624 min−1 and 1.4557 min−1, respectively, for the particle size-class of (−0.1 + 0.053) mm. By the modified Kelsall model, the maximum values of the fast flotation rate constant k for raw and waste coal are 25 min−1 and 1.8889 min−1 and are obtained for the particle size-class (−0.2 + 0.1) mm.
This study shows that particle size has a key impact on the flotation performance of waste coal, with the (–0.2 + 0.1) mm size-class identified as optimal for both flotation rate and overall recovery. Recognizing this optimal size-class has significant industrial value, as it provides clear guidelines for more efficient coal classification in preparation plants, enabling improvements in flotation process efficiency, reductions in reagent consumption, and enhancements in concentrate quality. Additionally, applying this approach contributes to more sustainable and cost-effective utilization of oxidized coals.
Considering the established significance of particle size for flotation kinetics, the proposed mathematical models given in Equations (7) and (8) (the classical model) and Equations (9) and (10) (the modified Kelsall model) provide a precise mathematical relationship between the flotation kinetics constant (k) and average particle size value (dsr). Statistical analysis shows that these models can support the prediction and optimization of flotation kinetics with a high degree of accuracy in practical conditions.
Author Contributions
Conceptualization, J.S., I.Đ. and D.T.; methodology, J.S., I.Đ. and D.T.; software, I.Đ. and D.T.; validation, J.S. and Z.Š.; formal analysis, J.S., I.Đ. and D.T.; investigation, J.S. and I.I.; resources J.S. and I.I.; writing—original draft preparation, J.S., I.Đ., D.T. and Z.Š.; writing—review and editing, J.S., I.Đ., D.T. and Z.Š.; visualization, J.S. and Z.Š.; supervision, J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia, within the funding of the scientific research work at the University of Belgrade, Technical Faculty in Bor, according to the contract with registration number 451-03-137/2025-03/200131.
Data Availability Statement
All data are available upon request.
Acknowledgments
The research presented in this paper was performed with the financial support of the Ministry of Science, Technological Development and Innovation of the Republic of Serbia, within the funding of the scientific research work at the University of Belgrade, Technical Faculty in Bor, according to the contract with registration number 451-03-137/2025-03/200131.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Polat, M.; Polat, H.; Chander, S. Physical and chemical interactions in coal flotation. Int. J. Miner. Process. 2003, 72, 199–213. [Google Scholar] [CrossRef]
- Brozek, M.; Mlynarczykowska, A. An analysis of effect of particle size on batch flotation of coal. Physicochem. Probl. Miner. Process. 2013, 49, 341–356. [Google Scholar]
- LaskowskI, J.S. Coal Flotation and Fine Coal Utilization; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Polat, M.; Arnold, B.; Chander, S.; Hogg, R.; Zhou, R. Coal flotation kinetics-effect of particle size and specific gravity. Coal Sci. Technol. 1993, 24, 161–170. [Google Scholar]
- Lynch, A.J.; Johnson, N.W.; Manlapig, E.V.; Thorne, C.G. Mineral and Coal Flotation Circuits: Their Simulation and Control; Elsevier: Amsterdam, The Netherland, 1981. [Google Scholar]
- Polat, M.; Chander, S. First-order flotation kinetics models and methods for estimation of the true distribution of flotation rate constants. Int. J. Miner. Process. 2000, 58, 145–166. [Google Scholar] [CrossRef]
- Drzymala, J. Mineral Processing. Foundations of Theory and Practice of Minerallurgy, 2nd ed.; Ofic. Wyd. PWr: Wroclaw, Poland, 2018. [Google Scholar]
- Sokolović, J.; Miskovic, S. The effect of particle size on coal flotation kinetics: A review. Physicochem. Probl. Miner. Process. 2018, 54, 1172–1190. [Google Scholar]
- Panopoulos, G.; King, R.P.; Juckes, A. The effect of particle-size distribution on the flotation of two South African coals. J. S. Afr. Inst. Min. Metall. 1986, 86, 141–152. [Google Scholar]
- Vanangamudi, M.; Rao, T.C. Modelling of batch coal flotation operation. Int. J. Miner. Process. 1986, 16, 231–243. [Google Scholar] [CrossRef]
- Vanangamudi, M.; Kumar, S.S.; Rao, T.C. Effect of fines content on the froth flotation of coal. Powder Technol. 1989, 58, 99–105. [Google Scholar] [CrossRef]
- Rao, T.C.; Govindarajan, B.; Vanangamudi, M. A kinetic model for batch coal flotation. Miner. Eng. 1989, 2, 403–414. [Google Scholar] [CrossRef]
- Mohns, C.A. Effect of Particle Size on Coal Flotation Kinetics; Queen’s University: Kingston, ON, Canada, 1998. [Google Scholar]
- Chaves, A.P.; Ruiz, A.S. Considerations on the kinetics of froth flotation of ultrafine coal contained in tailings. Int. J. Coal Prep. Util. 2009, 29, 289–297. [Google Scholar] [CrossRef]
- Abkhoshk, E.; Kor, M.; Rezai, B. A study on the effect of particle size on coal flotation kinetics using fuzzy logic. Expert Syst. Appl. 2010, 37, 5201–5207. [Google Scholar] [CrossRef]
- Kor, M.; Abkhoshk, E.; Gharibie, K.; Shafaei, S.Z. An investigation of the particle size effect on coal flotation kinetics using multivariable regression. J. Min. Environ. Issues 2010, 1, 41–47. [Google Scholar]
- Li, Y.; Zhao, W.; Gui, X.; Zhang, X. Flotation kinetics and separation selectivity of coal size fractions. Physicochem. Probl. Miner. Process. 2013, 49, 387–395. [Google Scholar]
- Xia, W.; Xie, G.; Liang, C.; Yang, J. Flotation behavior of different size fractions of fresh and oxidized coals. Powder Technol. 2014, 267, 80–85. [Google Scholar] [CrossRef]
- Ni, C.; Xie, G.; Jin, M.; Peng, Y.; Xia, W. The difference in flotation kinetics of various size fractions of bituminous coal between rougher and cleaner flotation processes. Powder Technol. 2016, 292, 210–216. [Google Scholar] [CrossRef]
- Bu, X.; Xie, G.; Chen, Y.; Ni, C. The order of kinetic models in coal fines flotation. Int. J. Coal Prep. Util. 2017, 37, 113–123. [Google Scholar] [CrossRef]
- Xia, W.C.; Yang, J.G. Experiment design of oily bubble in oxidized coal flotation. Gospod. Surowcami Miner. 2013, 29, 129–135. [Google Scholar]
- Bu, X.; Wang, X.; Zhou, S.; Li, B.; Zhan, H.; Xie, G. Discrimination of six flotation kinetic models used In the conventional flotation and carrier flotation of −74 μm coal fines. Acs. Omega. 2020, 5, 13813–13821. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, B.; Chelgani, S.C. Modeling of fine coal flotation separation based on particle characteristics and hydrodynamic conditions. Int. J. Coal Sci. Technol. 2016, 3, 429–439. [Google Scholar] [CrossRef]
- Norori-McCormac, A.; Brito-Parada, P.R.; Hadler, K.; Cole, K.; Cilliers, J.J. The effect of particle size distribution on froth stability in flotation. Sep. Purif. Technol. 2017, 184, 240–247. [Google Scholar] [CrossRef]
- Liao, Y.; Cao, Y.; Liu, C.; Zhao, Y.; Zhu, G. Comparison of the effect of particle size on the flotation kinetics of a low-rank coal using air bubbles and oily bubbles. J. S. Afr. Inst. Min. Metall. 2017, 117, 561–566. [Google Scholar] [CrossRef]
- Han, H.; Liu, A.; Wang, C.; Yang, R.; Li, S.; Wang, H. Flotation kinetics performance of different coal size fractions with nanobubbles. Int. J. Miner. Metall. Mater. 2022, 29, 1502–1510. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Suresh, N.; Varma, A.K. Determining the best particle size-class for flotation of a high ash coal. Int. J. Coal Prep. Util. 2020, 40, 755–765. [Google Scholar] [CrossRef]
- Bahrami, A.; Ghorbani, Y.; Gulcan, E.; Kazemi, F.; Kakaei, H.; Farajzadeh, S. Effects of particle size distribution on flotation kinetics of bituminous coal. Iran. J. Chem. Eng. (IJChE) 2020, 17, 3–13. [Google Scholar]
- Şahbaz, O.; Demir, M.K. Effects of frothers and particle size on the flotation kinetics of the Jameson Cell. Physicochem. Probl. Miner. Process. 2020, 56, 829–838. [Google Scholar] [CrossRef]
- Karaca, S.; Ucar, A.; Sahbaz, O. Kinetic modelling of flotation column and Jameson cell in coal. Physicochem. Probl. Miner. Process. 2022, 58, 152848. [Google Scholar] [CrossRef]
- Lu, T.; Deng, Z.; Tang, Y.; Cheng, W. Effects of particle size on the flotation behavior of coal slime. Energy Explor. Exploit. 2023, 41, 1344–1355. [Google Scholar] [CrossRef]
- Kazemi, F.; Bahrami, A.; Ghorbani, Y.; Danesh, A.; Abdollahi, M.; Falah, H.; Salehi, M. The interaction and synergic effect of particle size on flotation efficiency: A comparison study of recovery by size, and by liberation between lab and industrial scale data. Rud.-Geološko-Naft. Zb. 2023, 38, 1–12. [Google Scholar] [CrossRef]
- Chang, Z.; Chen, X.; Peng, Y. Understanding and improving the flotation of coals with different degrees of surface oxidation. Powder Technol. 2017, 321, 190–196. [Google Scholar] [CrossRef]
- Xia, W.; Yang, J.; Liang, C. A short review of improvement in flotation of low rank/oxidized coals by pretreatments. Powder Technol. 2013, 237, 1–8. [Google Scholar] [CrossRef]
- Wen, B.; Xia, W.; Sokolovic, J.M. Recent advances in effective collectors for enhancing the flotation of low rank/oxidized coals. Powder Technol. 2017, 319, 1–11. [Google Scholar] [CrossRef]
- Sokolović, J.; Stanojlović, R.; Marković, Z. The effects of pretreatment on the flotation kinetics of waste coal. Int. J. Coal Prep. Util. 2012, 32, 130–142. [Google Scholar] [CrossRef]
- Sokolovic, J.; Stanojlovic, R.; Markovic, Z. Activation of oxidized surface of anthracite waste coal by attrition. Physicochem. Probl. Miner. Process. 2012, 48, 5–18. [Google Scholar]
- ASTM D3174-04(2010); Standard Test Method for Ash in the Analysis Sample of Coal and Coke from Coal. ASTM International: West Conshohocken, PA, USA, 2010.
- ISO 589:2008; Hard Coal—Determination of Total Moisture. International Organization for Standardization: Geneva, Switzerland, 2008.
- ASTM D3175-11; Standard Test Method for Volatile Matter in the Analysis Sample of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2011.
- ASTM D5373-08; Standard Test Methods for Instrumental Determination of Carbon, Hydrogen, and Nitrogen in Laboratory Samples of Coal. ASTM International: West Conshohocken, PA, USA, 2008.
- ASTM D4239-11; Standard Test Methods for Sulfur in the Analysis Sample of Coal and Coke Using High-Temperature Tube Furnace Combustion Methods. ASTM International: West Conshohocken, PA, USA, 2011.
- ASTM D5865-11; Standard Test Method for Gross Calorific Value of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2011.
- Zuniga, H.G. Flotation recovery is an exponential function of its rate. Boln. Soc. Nac. Min. Santiago Chile 1935, 47, 83–86. [Google Scholar]
- Klimpel, R.R. Selection of Chemical Reagents for Flotation. Mineral Processing Plant Design, 2nd ed.; SME-AIME: New York, NY, USA, 1980; pp. 907–934. [Google Scholar]
- Kelsall, D.F. Application of probability assessment of flotation systems. Trans. Am. Soc. Min. Metall. Eng. 1961, 70, 191–204. [Google Scholar]
- Jowett, A. Resolution of flotation recovery curves by a differential plot method. Trans. Am. Soc. Min. Metall. Eng. 1974, 85, C263–C266. [Google Scholar]
- Imaizumi, T.; Inoue, T. Kinetic consideration of froth flotation. In Proceedings of the 6th International Mineral Processing Congres, Cannes, France, 29 May–3 June 1965; pp. 563–579. [Google Scholar]
- Brezani, I.; Zelenak, F. MatLab Tool for Modeling First Order Flotation. 2010. Available online: https://www.mathworks.com/matlabcentral/fileexchange/28703-flotation-kinetics-modeling (accessed on 31 August 2011).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).