Enhanced Flame Retardancy of Silica Fume-Based Geopolymer Composite Coatings Through In Situ-Formed Boron Phosphate from Doped Zinc Phytate and Boric Acid
Abstract
1. Introduction
2. Experiment and Methods
2.1. Raw Materials
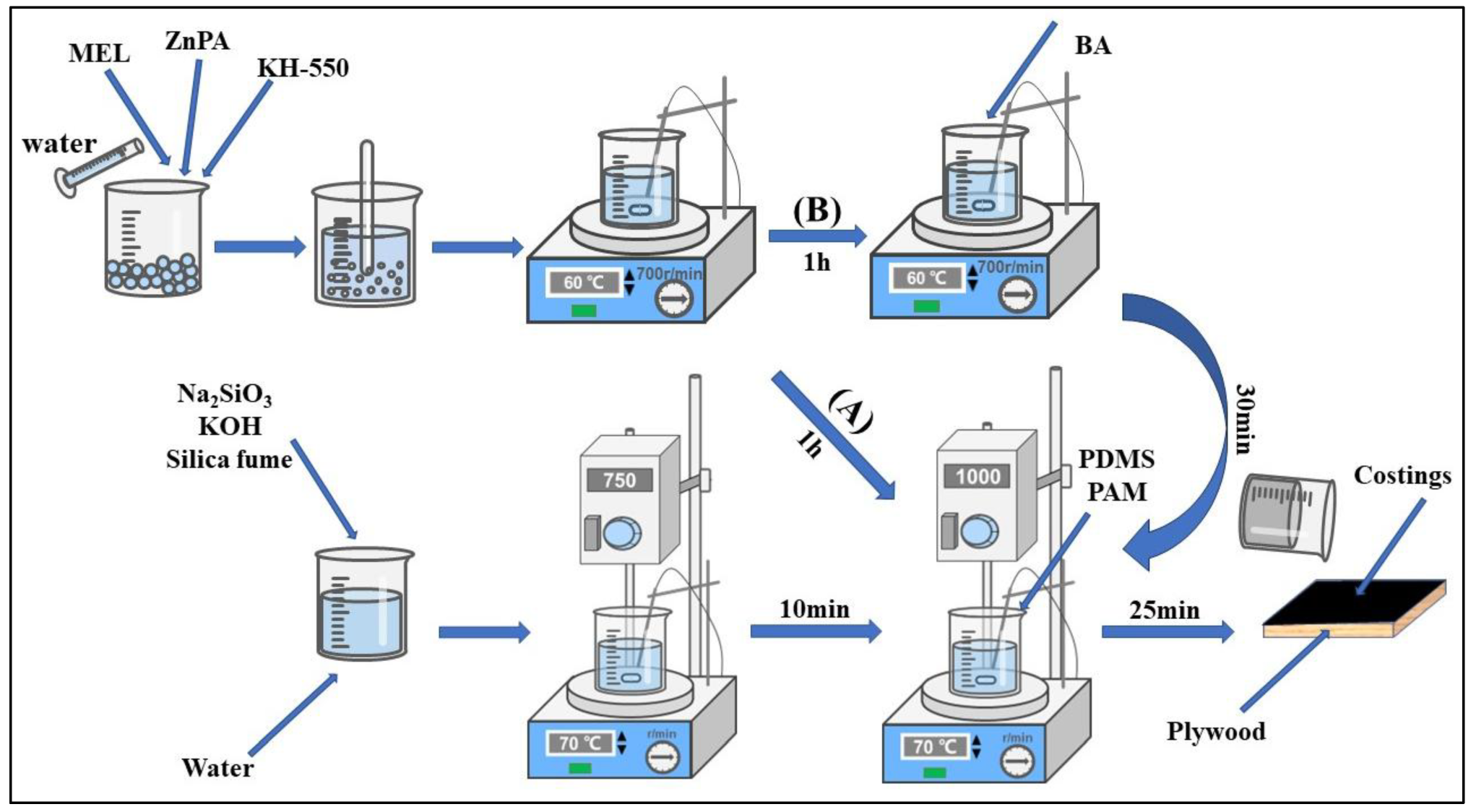
2.2. Preparation of Geopolymer Composite Coating
2.3. Characterizations
2.3.1. Flame Retardancy Testing
2.3.2. Microstructure Testing
3. Results
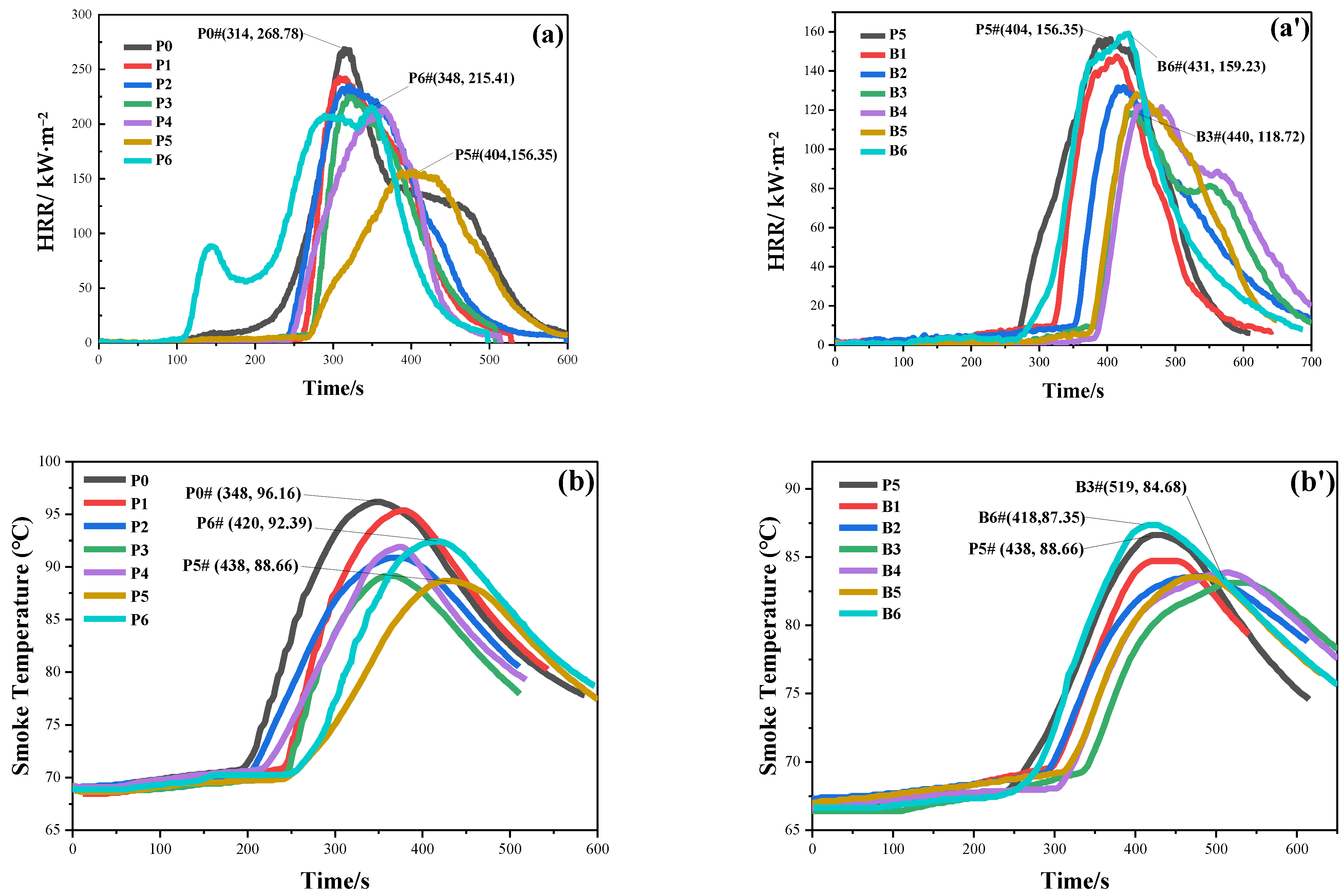
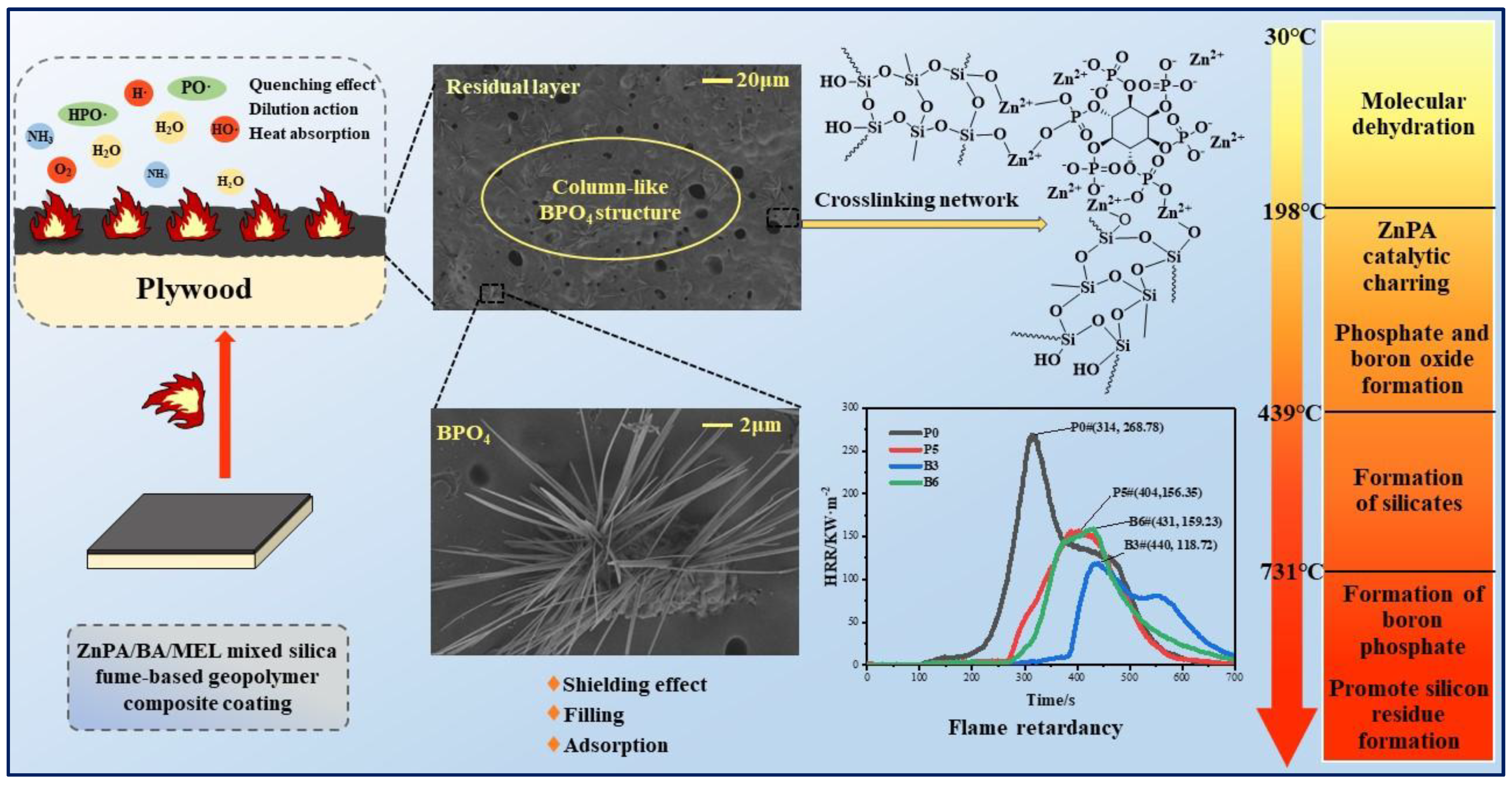
3.1. Flame Retardancy of Samples
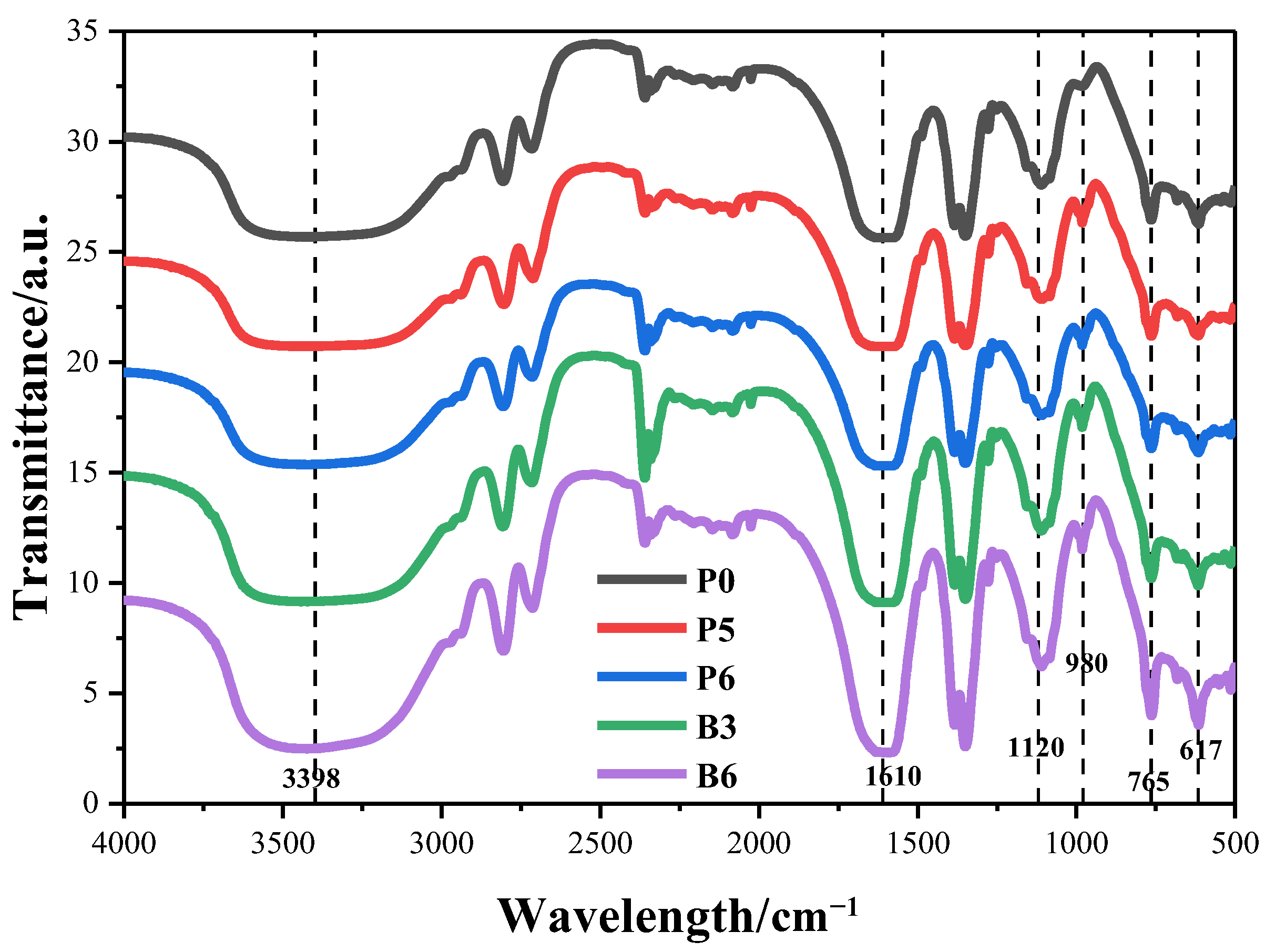
3.2. FTIR Spectra of Samples
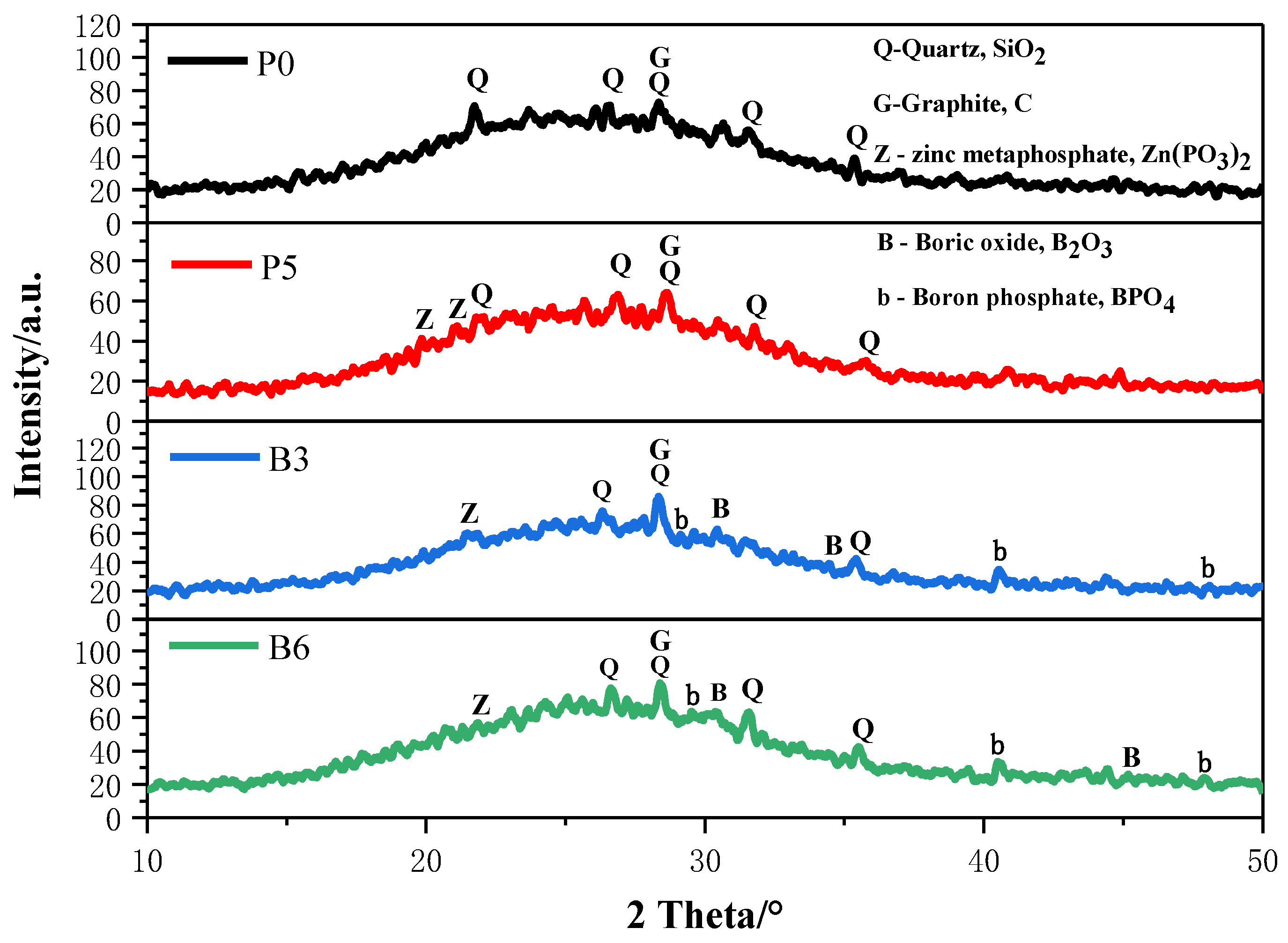
3.3. XRD Analysis
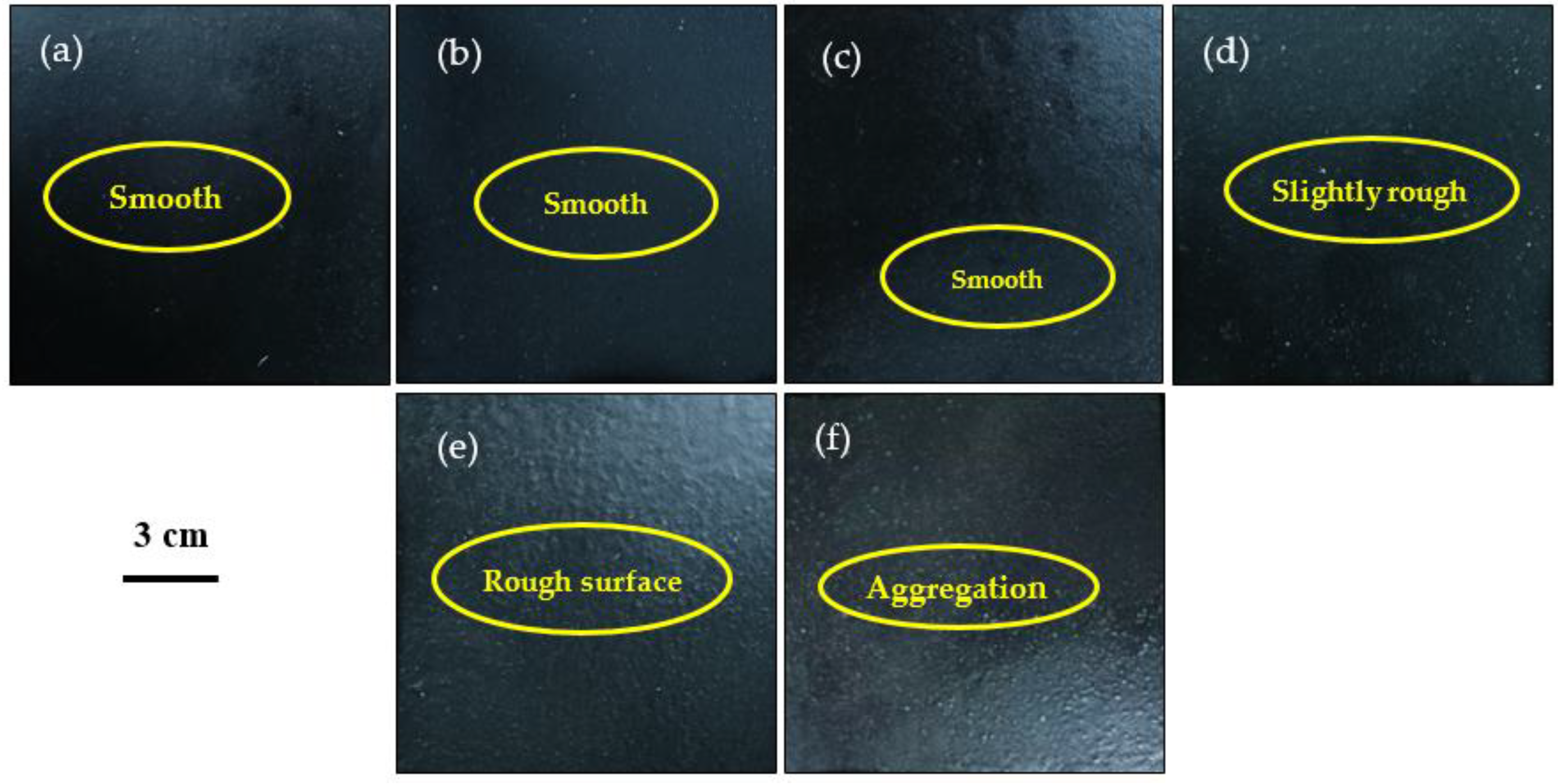
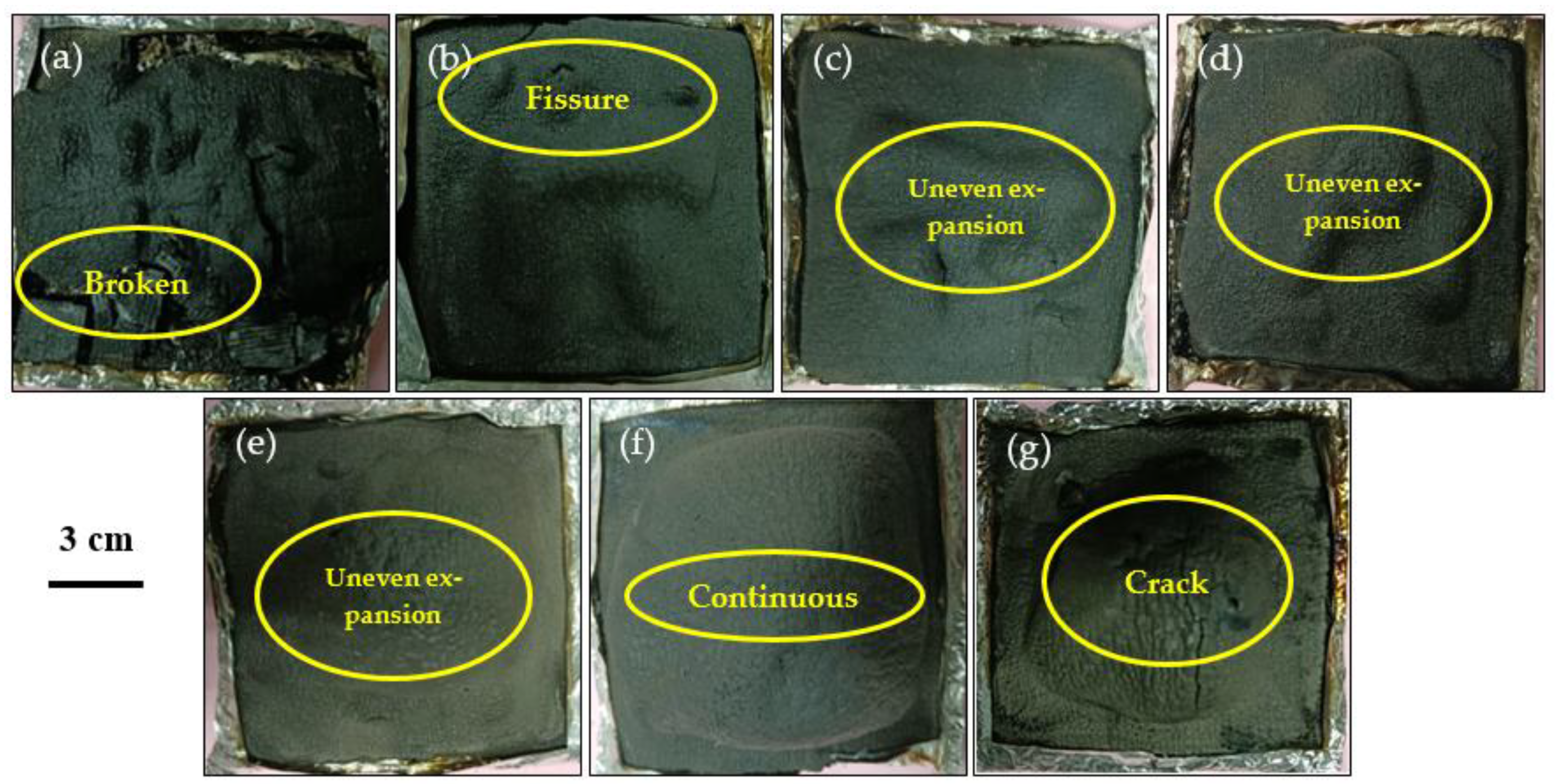
3.4. Appearance
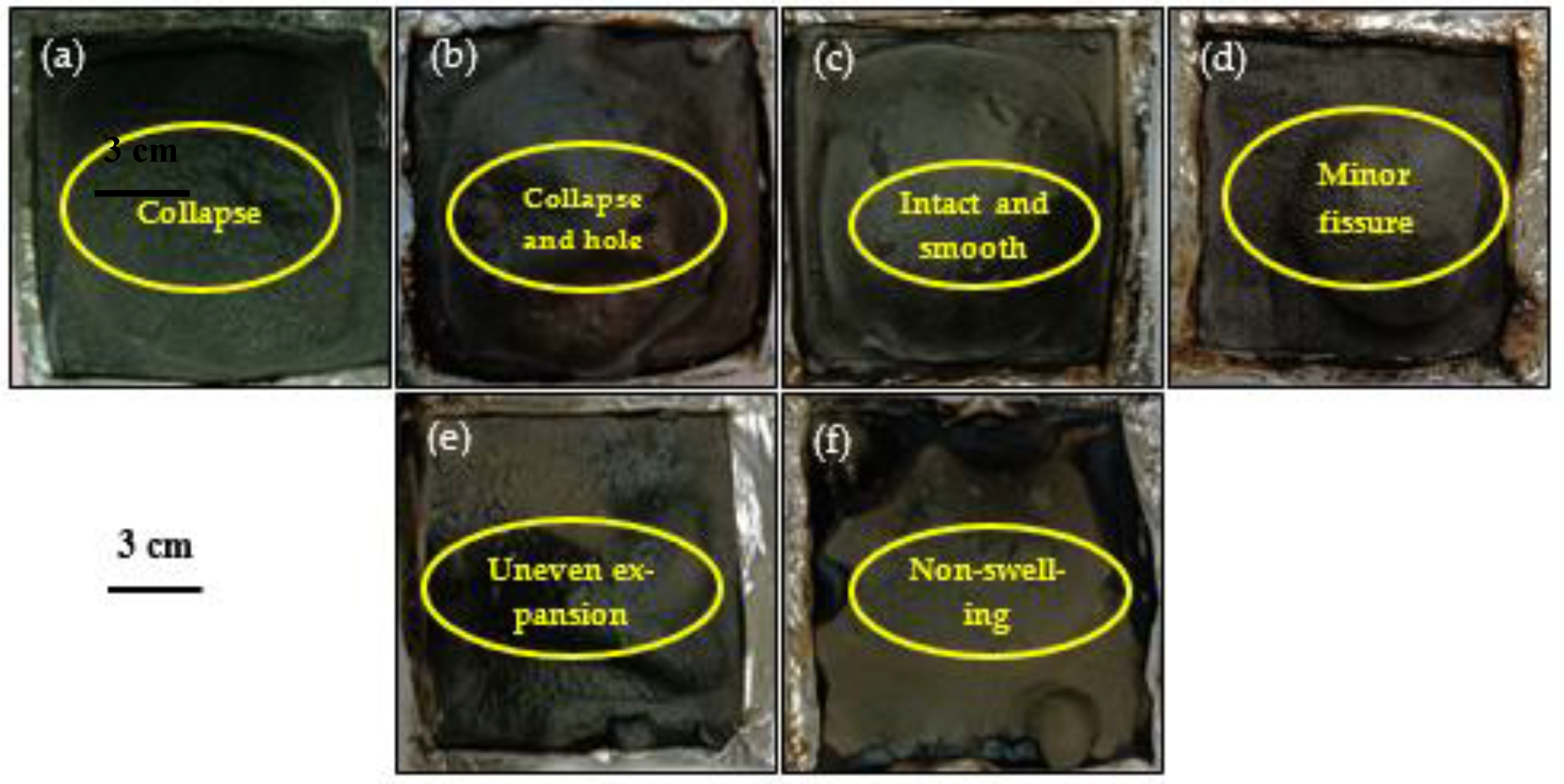
3.5. Residual Appearance of Samples
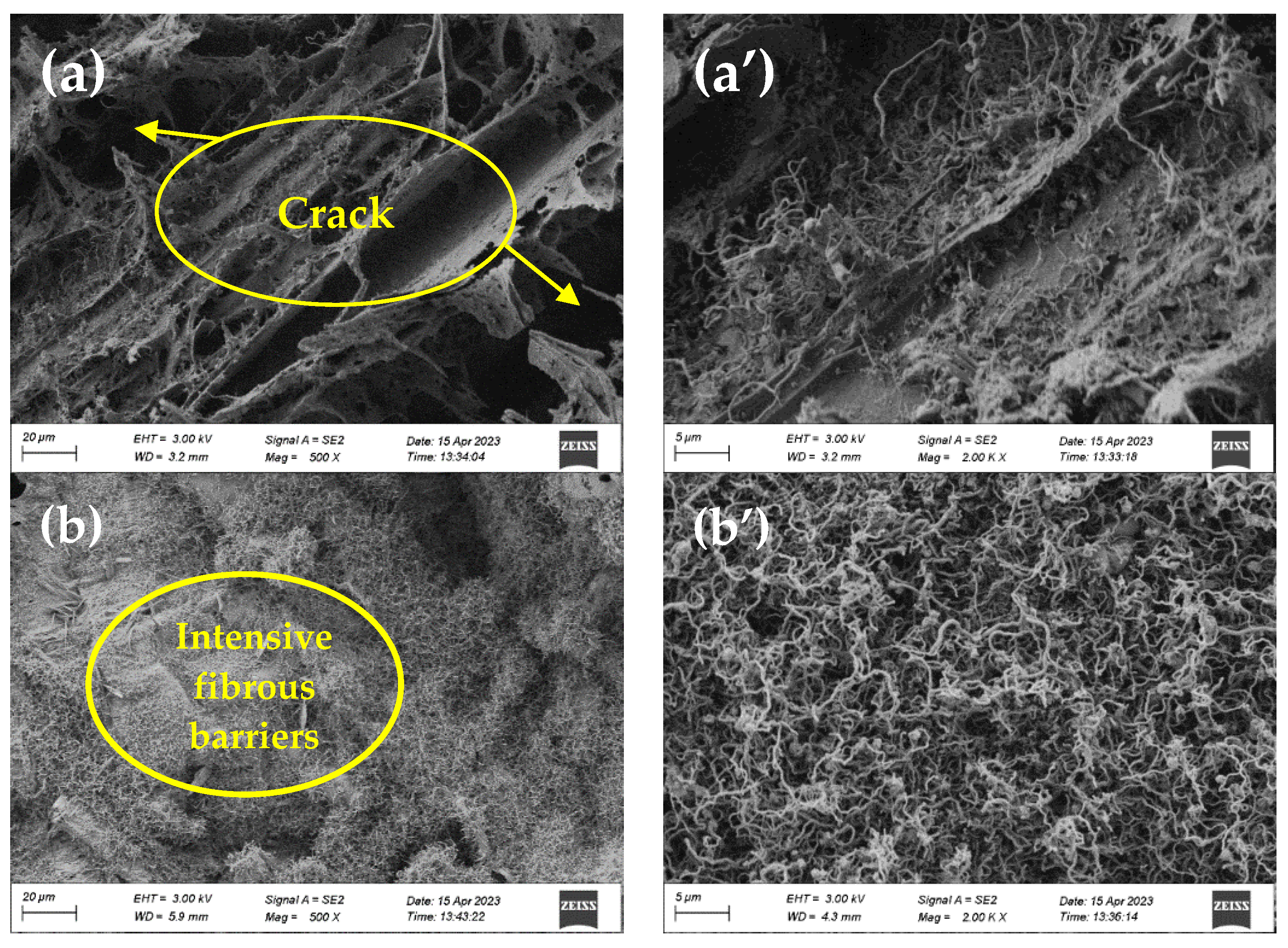
3.6. SEM of Residues
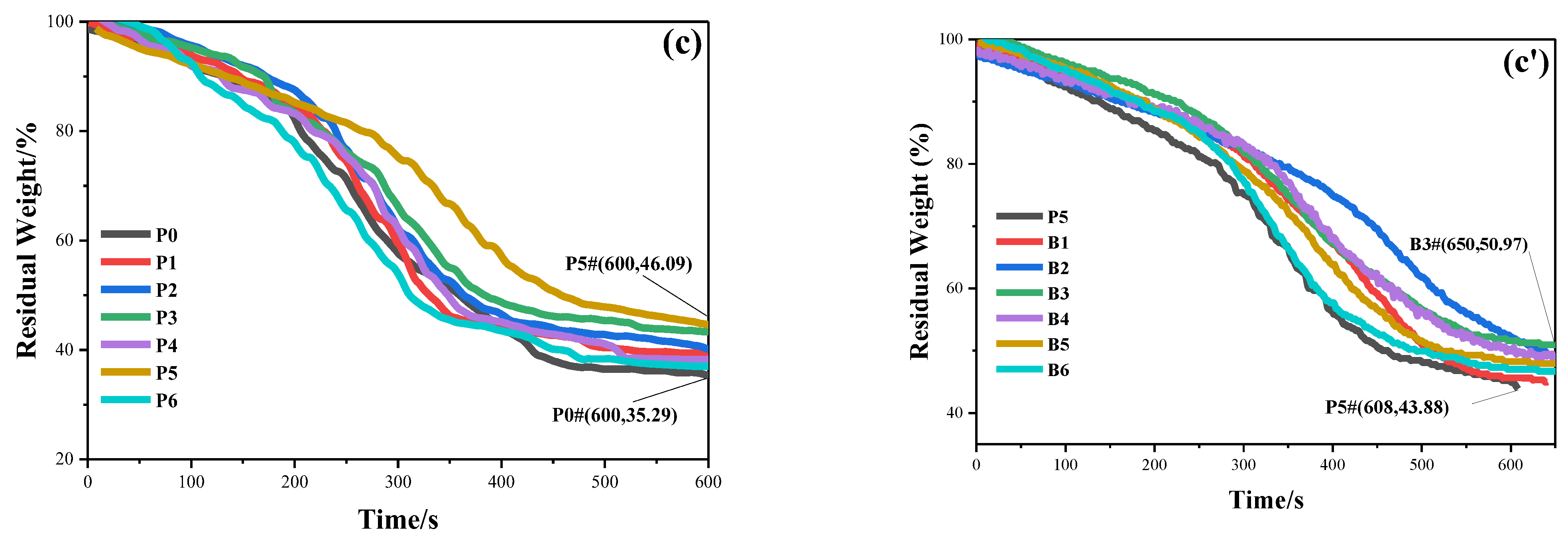
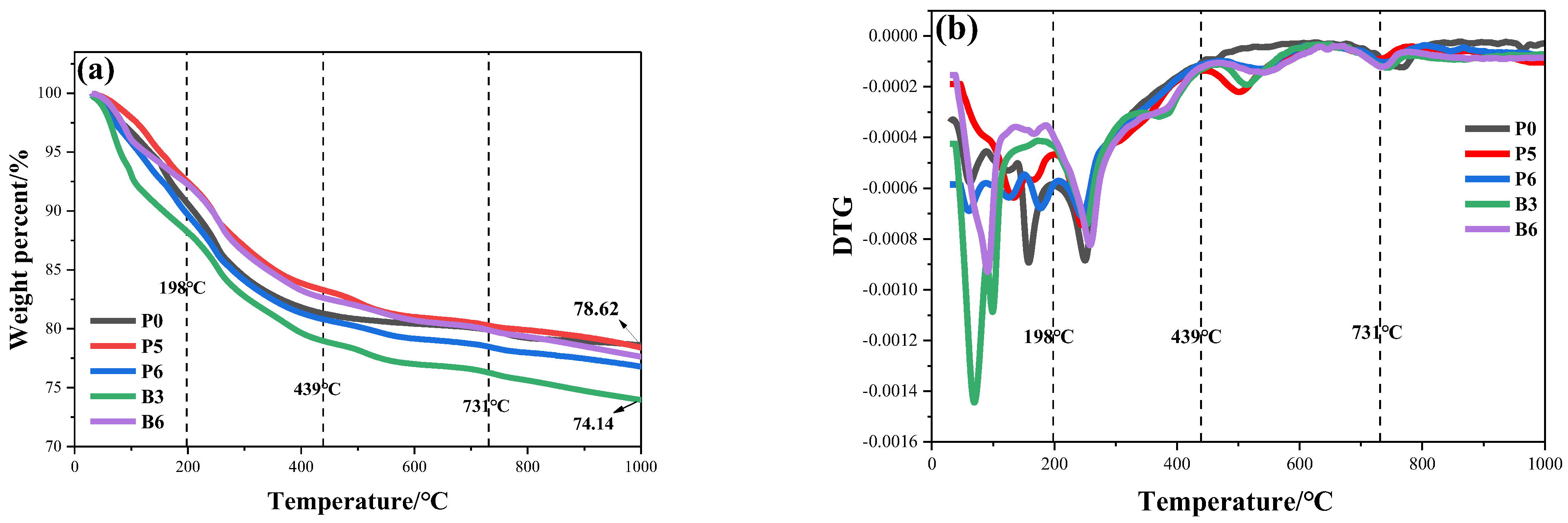
3.7. Thermal Performance Analysis
3.8. Pyrolysis Kinetics
4. Discussion
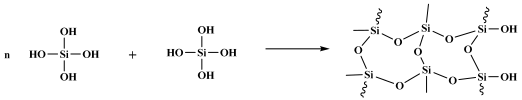
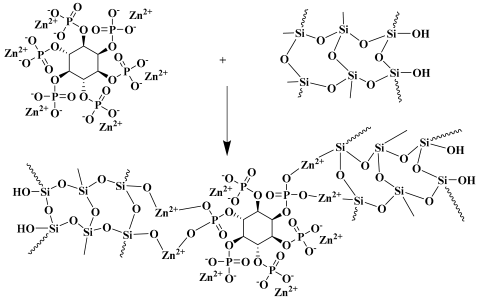
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, X.; Gan, W.; Shang, Y.; Tang, J.; Wang, Y.; Cao, Z.; Xie, Y.; Liu, J.; Bai, L.; Li, J.; et al. Low-value wood for sustainable high-performance structural materials. Nat. Sustain. 2022, 5, 628–635. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, T.; Wang, Q.; Guo, C. Synthesis of biobased flame-retardant carboxylic acid curing agent and application in wood surface coating. ACS Sustain. Chem. Eng. 2019, 7, 14727–14738. [Google Scholar] [CrossRef]
- Al-Kaseasbeh, Q.; Al-Qaralleh, M. Valorization of hydrophobic wood waste in concrete mixtures: Investigating the micro and macro relations. Results Eng. 2023, 17, 100877. [Google Scholar] [CrossRef]
- Sykam, K.; Hussain, S.S.; Sivanandan, S.; Narayan, R.; Basak, P. Non-halogenated UV-curable flame retardants for wood coating applications: Review. Prog. Org. Coat. 2023, 179, 107549. [Google Scholar] [CrossRef]
- He, Z.; Shen, A.; Guo, Y.; Lyu, Z.; Li, D.; Qin, X.; Zhao, M.; Wang, Z. Cement-based materials modified with superabsorbent polymers: A review. Constr. Build. Mater. 2019, 225, 569–590. [Google Scholar] [CrossRef]
- Ding, Z.; Xu, M.-R.; Dai, J.-G.; Dong, B.-Q.; Zhang, M.-J.; Hong, S.-X.; Xing, F. Strengthening concrete using phosphate cement-based fiber-reinforced inorganic composites for improved fire resistance. Constr. Build. Mater. 2019, 212, 755–764. [Google Scholar] [CrossRef]
- Bajpai, R.; Choudhary, K.; Srivastava, A.; Sangwan, K.S.; Singh, M. Environmental impact assessment of fly ash and silica fume based geopolymer concrete. J. Clean. Prod. 2020, 254, 120147. [Google Scholar] [CrossRef]
- Shahidi, S.S.; Mohammadi, S. Synergistic effect of nano hybrid multi-layered graphene oxide/talc and silica fume on the fire and water-resistance of intumescent coatings. Prog. Org. Coat. 2023, 183, 107736. [Google Scholar] [CrossRef]
- Zhang, D.R.; Zhu, H.J.; Wu, Q.S.; Yang, T.; Yin, Z.F.; Tian, L. Investigation of the hydrophobicity and microstructure of fly ash-slag geopolymer modified by polydimethylsiloxane. Constr. Build. Mater. 2023, 369, 130540. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Y.; Zhang, T.; Yang, Q. Engineering and microstructural properties of carbon-fiber-reinforced fly-ash-based geopolymer composites. J. Build. Eng. 2023, 79, 107883. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, H.; Tao, B.; Yin, R.; Liu, L.; Li, B. Safe and economical preparation of amino acid-derived bio-based triazine char-forming agent for efficient intumescent flame retardant polypropylene. Constr. Build. Mater. 2025, 484, 141876. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, Y.; Xu, K.; Yan, C.; Qin, A.; Du, C.; Xu, M.; Wang, C.; Li, B.; Liu, L. Fire-resistant and thermal-insulating alginate aerogel with intelligent bionic armor for exceptional mechanical and fire early-warning performance. Chem. Eng. J. 2024, 498, 155181. [Google Scholar] [CrossRef]
- Huo, S.; Wang, C.; Shi, Q.; Yu, L.; Liu, Z.; Fang, Z.; Wang, H. A novel hyperbranched phosphorus-boron polymer for transparent, flame-retardant, smoke-suppressive, robust yet tough epoxy resins. Compos. Part B Eng. 2021, 227, 109395. [Google Scholar] [CrossRef]
- Peng, H.; Mao, Y.; Wang, D.; Fu, S. B-N-P-linked covalent organic frameworks for efficient flame retarding and toxic smoke suppression of polyacrylonitrile composite fiber. Chem. Eng. J. 2022, 430, 133120. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.; Peng, Y.; Wang, W.; Cao, J. Thermal behavior and flame retardancy of poplar wood impregnated with furfuryl alcohol catalyzed by boron/phosphorus compound system. Ind. Crops Prod. 2022, 176, 114361. [Google Scholar] [CrossRef]
- Huo, S.; Sai, T.; Ran, S.; Guo, Z.; Fang, Z.; Song, P.; Wang, H. A hyperbranched P/N/B-containing oligomer as multifunctional flame retardant for epoxy resins. Compos. Part B Eng. 2022, 234, 109701. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.; Ding, M.; Huang, Y.; Li, L.; Wang, M. In-situ incorporation of metal phytates for green and highly efficient flame-retardant wood with excellent smoke-suppression property. Ind. Crops Prod. 2022, 187, 115287. [Google Scholar] [CrossRef]
- Jiang, G.; Xiao, Y.; Qian, Z.; Yang, Y.; Jia, P.; Song, L.; Hu, Y.; Ma, C.; Gui, Z. A novel phosphorus-, nitrogen- and sulfur-containing macromolecule flame retardant for constructing high-performance epoxy resin composites. Chem. Eng. J. 2023, 451, 137823. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Li, F.; Zhao, J. In-situ polymerized zinc phytate chelated Si-C-P geopolymer hybrid coating constructed by incorporating chitosan oligosaccharide and DOPO for flame-retardant plywood. Constr. Build. Mater. 2023, 397, 132416. [Google Scholar] [CrossRef]
- Wang, K.; Wang, S.; Meng, D.; Chen, D.; Mu, C.; Li, H.; Sun, J.; Gu, X.; Zhang, S. A facile preparation of environmentally-benign and flame-retardant coating on wood by comprising polysilicate and boric acid. Cellulose 2021, 28, 11551–11566. [Google Scholar] [CrossRef]
- Savas, L.A.; Dogan, M. Flame retardant effect of zinc borate in polyamide 6 containing aluminum hypophosphite. Polym. Degrad. Stab. 2019, 165, 101–109. [Google Scholar] [CrossRef]
- Wang, Y.; Kou, X.; Deng, J.; Zhao, J.P.; Shi, H. Ammonium polyphosphate/expandable graphite/TiO2 blended silica fume-based geopolymer coating for synergistically flame-retarding plywood. Constr. Build. Mater. 2022, 317, 125941. [Google Scholar] [CrossRef]
- ISO 5660-1:2015; Reaction-to-Fire Tests—Heat Release, Smoke Production and Mass Loss Rate. Part 1: Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement). ISO: Geneva, Switzerland, 2015.
- Yuan, X.; Luo, K.; Zhang, K.; He, J.; Zhao, Y.; Yu, D. Combinatorial Vibration-Mode Assignment for the FTIR Spectrum of Crystalline Melamine: A Strategic Approach toward Theoretical IR Vibrational Calculations of Triazine-Based Compounds. J. Phys. Chem. A 2016, 120, 7427–7433. [Google Scholar] [CrossRef]
- Ong, H.R.; Prasad, R.; Khan, M.M.R.; Chowdhury, M.N.K. Effect of Palm Kernel Meal as Melamine Urea Formaldehyde Adhesive Extender for Plywood Application: Using a Fourier Transform Infrared Spectroscopy (FTIR) Study. Appl. Mech. Mater. 2012, 121–126, 493–498. [Google Scholar] [CrossRef]
- Lei, J.; Song, H.; Wei, Y.; Zhao, S.; Qi, H. A novel strategy to enhance hydrothermal stability of Pd-doped organosilica membrane for hydrogen separation. Microporous Mesoporous Mater. 2017, 253, 55–63. [Google Scholar] [CrossRef]
- Carli, L.; Schnitzler, E.; Ionashiro, M.; Szpoganicz, B.; Rosso, N. Equilibrium, thermoanalytical and spectroscopic studies to characterize phytic acid complexes with Mn (II) and Co (II). J. Braz. Chem. Soc. 2009, 20, 1515–1522. [Google Scholar] [CrossRef]
- Elbeyli, İ.Y. Production of crystalline boric acid and sodium citrate from borax decahydrate. Hydrometallurgy 2015, 158, 19–26. [Google Scholar] [CrossRef]
- Yu, K.; Wang, Y.; Li, F.; Zhao, J. In-situ grown SiC whiskers enhance flame retardancy of alkali-activated gold tailings geopolymer composite coatings by incorporating expanded graphite. Constr. Build. Mater. 2023, 392, 131936. [Google Scholar] [CrossRef]
- Yang, F.; Yao, Y.; Xu, Y.; Wang, C.; Wang, M.; Ren, J.; Zhang, C.; Wu, F.; Lu, J. Evolution of the porous structure for phosphoric acid etching carbon as cathodes in Li-O2 batteries: Pyrolysis temperature-induced characteristics changes. Carbon Energy 2023, 6, e372. [Google Scholar] [CrossRef]
- Larsson, E.; Donzel-Gargand, O.; Heinrichs, J.; Jacobson, S. Tribofilm formation of a boric acid fuel additive-Material characterization; challenges and insights. Tribol. Int. 2022, 171, 107541. [Google Scholar] [CrossRef]
- Hu, J.; Xia, H.; Hou, X.; Yang, T.; Si, K.; Wang, Y.; Wang, L.; Shi, Z. Enhanced thermal management performance of nanofibrillated cellulose composite with highly thermally conductive boron phosphide. J. Mater. Chem. A 2021, 9, 27049–27060. [Google Scholar] [CrossRef]
- Ullah, S.; Ahmad, F.; Shariff, A.M.; Bustam, M.A.; Gonfa, G.; Gillani, Q.F. Effects of ammonium polyphosphate and boric acid on the thermal degradation of an intumescent fire retardant coating. Prog. Org. Coat. 2017, 109, 70–82. [Google Scholar] [CrossRef]
- Tong, X.-M.; Zhang, T.; Yang, M.-Z.; Zhang, Q. Preparation and characterization of novel melamine modified poly (urea–formaldehyde) self-repairing microcapsules. Colloids Surf. A Physicochem. Eng. Asp. 2010, 371, 91–97. [Google Scholar] [CrossRef]
- Yan, M.; Pan, Y.; Cheng, X.; Zhang, Z.; Deng, Y.; Lun, Z.; Gong, L.; Gao, M.; Zhang, H. “Robust–soft” anisotropic nanofibrillated cellulose aerogels with superior mechanical, flame-retardant, and thermal insulating properties. ACS Appl. Mater. Interfaces 2021, 13, 27458–27470. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Hong, W.B.; Yan, H.; Wu, S.Y.; Chen, X.M. Preparation and microwave dielectric properties of BPO4 ceramics with ultra-low dielectric constant. J. Mater. Sci. Mater. Electron. 2021, 32, 6660–6667. [Google Scholar] [CrossRef]
- Schmidt, M.; Ewald, B.; Prots, Y.; Cardoso-Gil, R.; Armbrüster, M.; Loa, I.; Zhang, L.; Huang, Y.-X.; Schwarz, U.; Kniep, R. Growth and characterization of BPO4 single crystals. Z. Anorg. Und Allg. Chem. 2004, 630, 655–662. [Google Scholar] [CrossRef]
- Chen, Y.; Duan, H.; Ji, S.; Ma, H. Novel phosphorus/nitrogen/boron-containing carboxylic acid as co-curing agent for fire safety of epoxy resin with enhanced mechanical properties. J. Hazard. Mater. 2021, 402, 123769. [Google Scholar] [CrossRef]
- Kong, D.; Liu, J.; Zhang, Z.; Wang, S.; Lu, Z. Preparation of synergistic silicon, phosphorus and nitrogen flame retardant based on cyclosiloxane and its application to cotton fabric. Cellulose 2021, 28, 8115–8128. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.-Y.; Yang, X.-M.; Wang, Y.-L.; Hao, J.-W. Application of TG/FTIR TG/MS and cone calorimetry to understand flame retardancy and catalytic charring mechanism of boron phosphate in flame-retardant PUR–PIR foams. J. Therm. Anal. Calorim. 2017, 130, 1817–1827. [Google Scholar] [CrossRef]
- Bifulco, A.; Parida, D.; Salmeia, K.A.; Nazir, R.; Lehner, S.; Stämpfli, R.; Markus, H.; Malucelli, G.; Branda, F.; Gaan, S. Fire and mechanical properties of DGEBA-based epoxy resin cured with a cycloaliphatic hardener: Combined action of silica, melamine and DOPO-derivative. Mater. Des. 2020, 193, 108862. [Google Scholar] [CrossRef]
- Guo, H.Z.; Lukovic, M.; Mendoza, M.; Schleputz, C.M.; Griffa, M.; Xu, B.W.; Gaan, S.; Herrmann, H.; Burgert, I. Bioinspired Struvite Mineralization for Fire-Resistant Wood. Acs Appl. Mater. Interfaces 2019, 11, 5427–5434. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wang, J.; Yang, Y.; Zhang, Y.; Zhao, C.; Yu, Y.; Wang, S. Comparison of the thermal degradation behaviors and kinetics of palm oil waste under nitrogen and air atmosphere in TGA-FTIR with a complementary use of model-free and model-fitting approaches. J. Anal. Appl. Pyrolysis 2018, 134, 12–24. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, S.; Hu, Y.; Zhang, S.; Li, Z.; Li, L.; Feng, J. Excellent antioxidizing, thermally insulating and flame resistance silica-polybenzoxazine aerogels for aircraft ablative materials. J. Appl. Polym. Sci. 2022, 139, e52499. [Google Scholar] [CrossRef]
- Rath, S.S.; Singh, S.; Rao, D.S.; Nayak, B.B.; Mishra, B.K. Adsorption of heavy metals on a complex Al-Si-O bearing mineral system: Insights from theory and experiments. Sep. Purif. Technol. 2017, 186, 28–38. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, W.; Meng, W.; Xie, W.; Cui, Y.; Xu, J.; Qu, H. Core-shell graphitic carbon nitride/zinc phytate as a novel efficient flame retardant for fire safety and smoke suppression in epoxy resin. Polymers 2020, 12, 212. [Google Scholar] [CrossRef]
- Piao, J.; Lai, Y.; Ren, J.; Wang, Y.; Feng, T.; Wang, Y.; Liu, W.; Dong, H.; Chen, W.; Jiao, C.; et al. Zn-doped carbon microspheres as synergist in intumescent flame-retardant thermoplastic polyurethane composites: Mechanism of char residues layer regulation. Compos. Commun. 2022, 32, 101173. [Google Scholar] [CrossRef]
- Han, G.; Zhao, X.; Feng, Y.; Ma, J.; Zhou, K.; Shi, Y.; Liu, C.; Xie, X. Highly flame-retardant epoxy-based thermal conductive composites with functionalized boron nitride nanosheets exfoliated by one-step ball milling. Chem. Eng. J. 2021, 407, 127099. [Google Scholar] [CrossRef]
- Song, F.; Zhao, Q.; Zhu, T.; Bo, C.; Zhang, M.; Hu, L.; Zhu, X.; Jia, P.; Zhou, Y. Biobased coating derived from fish scale protein and phytic acid for flame-retardant cotton fabrics. Mater. Des. 2022, 221, 110925. [Google Scholar] [CrossRef]
- Yu, R.; Wen, X.; Zhu, Y.; Lou, S.; Li, Y.; Wang, S.; Liu, J.; Tang, T. Boron-doped copper phenylphosphate as temperature-response nanosheets to fabricate high fire-safety polycarbonate nanocomposites. Compos. Part A 2023, 175, 107812. [Google Scholar] [CrossRef]
- Decsov, K.; Takács, V.; Marosi, G.; Bocz, K. Microfibrous cyclodextrin boosts flame retardancy of poly (lactic acid). Polym. Degrad. Stab. 2012, 191, 109655. [Google Scholar] [CrossRef]



| Samples | Na2SiO3·9H2O/g | KOH/g | Silica Fume/g | KH-550/g | MEL/g | PAM/g | H2O/g | PDMS/g | ZnPA/g | BA/g |
|---|---|---|---|---|---|---|---|---|---|---|
| P0 | 14.21 | 5.61 | 30 | 0.5 | 1 | 0.2 | 45 | 0.25 | 0.48 | 0.0 |
| P1 | 14.21 | 5.61 | 30 | 0.5 | 1 | 0.2 | 45 | 0.25 | 0.95 | 0.0 |
| P2 | 14.21 | 5.61 | 30 | 0.5 | 1 | 0.2 | 45 | 0.25 | 1.43 | 0.0 |
| P3 | 14.21 | 5.61 | 30 | 0.5 | 1 | 0.2 | 45 | 0.25 | 1.9 | 0.0 |
| P4 | 14.21 | 5.61 | 30 | 0.5 | 1 | 0.2 | 45 | 0.25 | 2.38 | 0.0 |
| P5 | 14.21 | 5.61 | 30 | 0.5 | 1 | 0.2 | 45 | 0.25 | 2.85 | 0.0 |
| P6 | 14.21 | 5.61 | 30 | 0.5 | 1 | 0.2 | 45 | 0.25 | 2.38 | 0.0 |
| B1 | 14.21 | 5.61 | 30 | 0.5 | 1 | 0.2 | 45 | 0.25 | 2.38 | 0.5 |
| B2 | 14.21 | 5.61 | 30 | 0.5 | 1 | 0.2 | 45 | 0.25 | 2.38 | 1.0 |
| B3 | 14.21 | 5.61 | 30 | 0.5 | 1 | 0.2 | 45 | 0.25 | 2.38 | 1.5 |
| B4 | 14.21 | 5.61 | 30 | 0.5 | 1 | 0.2 | 45 | 0.25 | 2.38 | 2.0 |
| B5 | 14.21 | 5.61 | 30 | 0.5 | 1 | 0.2 | 45 | 0.25 | 2.38 | 2.5 |
| B6 | 14.21 | 5.61 | 30 | 0.5 | 1 | 0.2 | 45 | 0.25 | 2.38 | 3.0 |
| Samples | TTI/s | TP/s | p-HRR/kW·m−2 | FPI/s·m2·kW−1 | FGI/kW·m−2·s−1 | WL/g | THR/MJ·m−2 | AEHC/kW·kg−1 | FRI |
|---|---|---|---|---|---|---|---|---|---|
| P0 | 158 | 314 | 268.78 | 0.59 | 0.86 | 30.93 | 36.67 | 1.19 | 1.00 |
| P1 | 219 | 308 | 242.31 | 0.90 | 0.79 | 29.16 | 31.27 | 1.07 | 1.80 |
| P2 | 201 | 319 | 234.90 | 0.86 | 0.74 | 29.93 | 35.93 | 1.20 | 1.49 |
| P3 | 232 | 321 | 223.93 | 1.04 | 0.70 | 26.04 | 27.92 | 1.07 | 2.31 |
| P4 | 205 | 364 | 213.97 | 0.96 | 0.59 | 24.56 | 27.79 | 1.13 | 2.15 |
| P5 | 228 | 404 | 156.35 | 1.46 | 0.39 | 27.12 | 27.79 | 1.02 | 3.27 |
| P6 | 78 | 348 | 215.41 | 0.36 | 0.62 | 31.37 | 40.44 | 1.29 | 0.56 |
| B1 | 266 | 414 | 147.49 | 1.80 | 0.36 | 26.84 | 22.42 | 0.84 | 5.02 |
| B2 | 294 | 423 | 131.89 | 2.23 | 0.31 | 29.46 | 23.44 | 0.80 | 5.93 |
| B3 | 336 | 440 | 118.72 | 2.83 | 0.27 | 24.30 | 20.83 | 0.86 | 8.48 |
| B4 | 327 | 453 | 123.31 | 2.65 | 0.27 | 23.90 | 22.62 | 0.95 | 7.31 |
| B5 | 318 | 443 | 128.29 | 2.48 | 0.29 | 25.01 | 20.68 | 0.83 | 7.48 |
| B6 | 260 | 431 | 159.23 | 1.63 | 0.37 | 26.51 | 27.63 | 1.04 | 3.69 |
| Samples | a Td5%/℃ | b Td10%/℃ | c Td20%/℃ | d Ti | e Tf | f Tmax/℃ | Char Residue/wt.% |
|---|---|---|---|---|---|---|---|
| P0 | 133.3 | 210.0 | 718.0 | 30 | 998.3 | 250.7 | 78.61 |
| P5 | 151.3 | 243.7 | 774.3 | 30 | 999.3 | 245.0 | 78.40 |
| P6 | 113.3 | 194.0 | 515.0 | 30 | 998.3 | 244.3 | 76.81 |
| B3 | 80.7 | 160.7 | 393.0 | 30 | 997.0 | 252.3 | 74.14 |
| B6 | 125.0 | 243.0 | 721.3 | 30 | 999.0 | 258.7 | 77.64 |
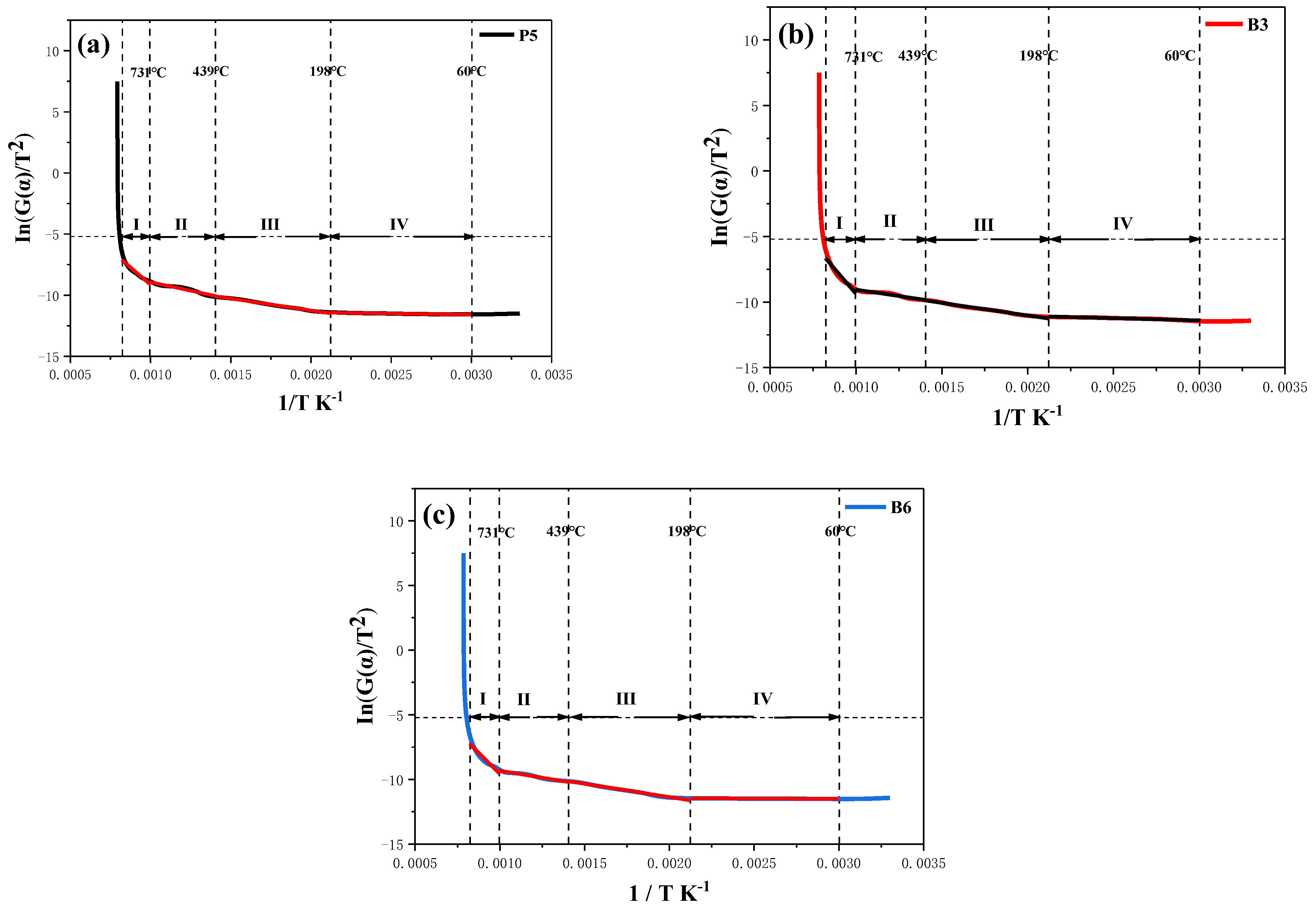
| Sample | Temperature | Intercept | Slope | Adj.R2 | Eα/kJ·mol−1 |
|---|---|---|---|---|---|
| P5 | 60–198 °C | −11.02 | −190.19 | 0.95 | 1.58 |
| 198–439 °C | −7.35 | −1948.92 | 0.99 | 16.20 | |
| 439–731 °C | −6.13 | −2800.53 | 0.95 | 23.28 | |
| 731–940 °C | 2.20 | −11,339.87 | 0.94 | 94.28 | |
| B3 | 60–198 °C | −10.40 | −333.17 | 0.95 | 2.77 |
| 198–439 °C | −7.19 | −1916.77 | 0.99 | 15.94 | |
| 439–731 °C | −7.13 | −1929.49 | 0.93 | 16.04 | |
| 731–940 °C | 5.91 | −15,284.84 | 0.93 | 127.08 | |
| B6 | 60–198 °C | 11.32 | −61.67 | 0.94 | 0.51 |
| 198–439 °C | −7.24 | −2062.03 | 0.99 | 17.14 | |
| 439–731 °C | −7.15 | −2172.26 | 0.98 | 18.06 | |
| 731–940 °C | 3.42 | −12,986.68 | 0.91 | 107.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Qu, Y.; Wang, C.; Dou, J. Enhanced Flame Retardancy of Silica Fume-Based Geopolymer Composite Coatings Through In Situ-Formed Boron Phosphate from Doped Zinc Phytate and Boric Acid. Minerals 2025, 15, 735. https://doi.org/10.3390/min15070735
Wang Y, Qu Y, Wang C, Dou J. Enhanced Flame Retardancy of Silica Fume-Based Geopolymer Composite Coatings Through In Situ-Formed Boron Phosphate from Doped Zinc Phytate and Boric Acid. Minerals. 2025; 15(7):735. https://doi.org/10.3390/min15070735
Chicago/Turabian StyleWang, Yachao, Yufei Qu, Chuanzhen Wang, and Juan Dou. 2025. "Enhanced Flame Retardancy of Silica Fume-Based Geopolymer Composite Coatings Through In Situ-Formed Boron Phosphate from Doped Zinc Phytate and Boric Acid" Minerals 15, no. 7: 735. https://doi.org/10.3390/min15070735
APA StyleWang, Y., Qu, Y., Wang, C., & Dou, J. (2025). Enhanced Flame Retardancy of Silica Fume-Based Geopolymer Composite Coatings Through In Situ-Formed Boron Phosphate from Doped Zinc Phytate and Boric Acid. Minerals, 15(7), 735. https://doi.org/10.3390/min15070735









