Fluid Inclusion Evidence of Deep-Sourced Volatiles and Hydrocarbons Hosted in the F–Ba-Rich MVT Deposit Along the Zaghouan Fault (NE Tunisia)
Abstract
1. Introduction
| Locality | Fluorite Generation | Mean Th ± 5 (°C) | Mean Salinity ± 1 (wt.% eq. NaCl) | Reference |
|---|---|---|---|---|
| Jebel Stah | 1 | 130 | 19.5 | [2,4] |
| 2 | 175 | 10 | ||
| Hammam–Zriba | 1 | 125 | 13 | [1] |
| 2 | 135 | 15 | ||
| 3 | 170 | 17 | ||
| Sidi Taya | - | 130 | 19.5 | [26] |
| Jebel Mecella | - | 138 | 18.75 | [27] |
| Jebel Oust | - | 202 | 32 | [4] |
| Oued M’tak | - | 120–140 | 14–17 | [27] |
2. Geological Setting
2.1. Regional Geology and Tectonic Events

2.2. Study Area: Geology and Ore Deposits
3. Sampling and Analytical Methods
3.1. Microthermometry
3.2. Raman Micro-Spectroscopy
4. Results
4.1. Petrography and Microthermometry of Fluid Inclusions
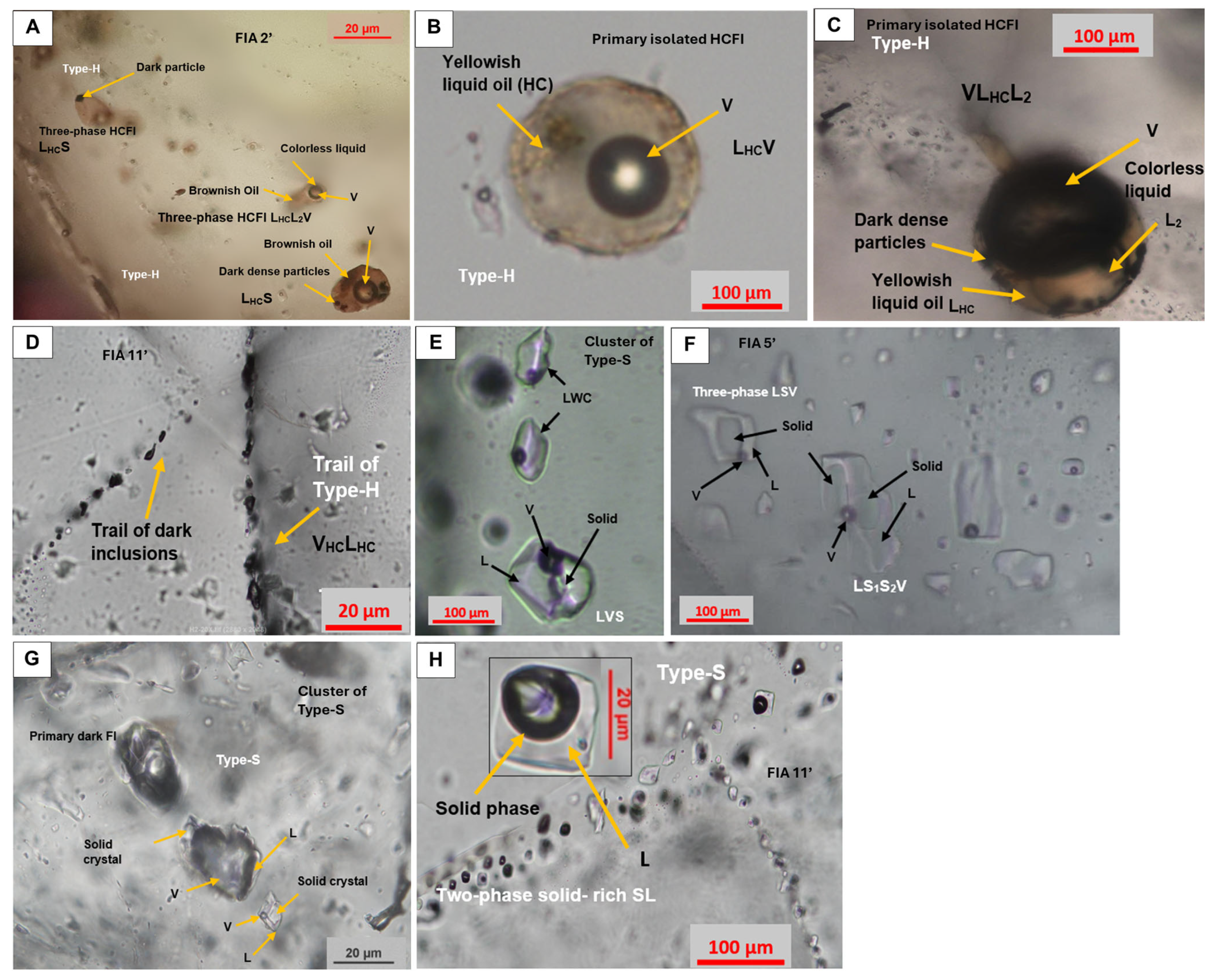
- Aqueous inclusions (Type-A): Primary two-phase LWC where liquid water is the dominant phase with a minor vapor CO2-rich phase (L: liquid; W: water; C: carbon-rich phase dominated by CO2) and two-phase LW inclusions with liquid water as the dominant phase, containing a water vapor phase. These inclusions are the most abundant (Figure 8A–D) in fluorite. They have distinctively large sizes (200–500 µm in diameter) and dominant negative crystal shapes. Secondary and pseudosecondary FIs are very abundant (Figure 8C–E). They are arranged along planar arrays in healed microcracks or scattered along cleavage planes. These inclusions are approximately 10 to 100 μm in diameter and are usually ellipsoidal and sometimes flat with irregular shapes. These FIs are typically LW and LWC (Figure 8D,E). Three-phase liquid-rich FIs LCW (Figure 8F) containing liquid water, liquid CO2, and vapor CO2. Monophasic liquid inclusions (Figure 8G) are also present.
- Hydrocarbon-bearing inclusions (Type-H): Primary and pseudosecondary three-phase liquid hydrocarbon-dominated FIs (LHCLV = two immiscible liquids + vapor) (Figure 8A and Figure 9A) usually have ellipsoidal or spherical shapes. Under the microscope, the hydrocarbon fluid looks yellowish and immiscible with another colorless liquid (either water or a supermature colorless hydrocarbon). Two-phase liquid–hydrocarbon-dominated fluid inclusions LHCV (LHC liquid hydrocarbon and VHC vapor hydrocarbon) are also present (Figure 9B). VLHCL2 inclusions are also present (Figure 8D and Figure 9). These FIs are three-phase vapor-dominated HCFIs containing two immiscible liquids (LHC and L2) in a vapor bubble. VHCLHC (Figure 9D) are also observed. These inclusions are vapor-dominated and composed of two phases. Three-phase LHCLS inclusions (Figure 9A) are composed of a dominant liquid hydrocarbon phase (LHC) and a second immiscible liquid phase (L), and contain several dark particles (S), possibly trapped OM. In this type of FI, the abbreviation ‘HC’ is used in a general sense to refer to all types of hydrocarbons and oils present in the fluid inclusions, including those that will be further identified and characterized, such as PAHs and evenkite, through Raman analysis in Section 4.2.
- Solid-bearing inclusions (Type-S): Primary three-phase inclusions: LWSHl (Figure 8A and Figure 9E) and poly-phase FIs: LS1S2V (Figure 9F,G). The secondary and pseudosecondary two-phase SL FIs are dominated by a dark solid phase and a colorless liquid phase (Figure 9H). Secondary and pseudosecondary monophasic solid inclusions (Figure 8D) have been also observed. Further characterization of the solid phases present in these inclusions is provided in Section 4.2 through Raman spectroscopic analysis.
4.2. Raman Micro-Spectroscopy (RMS)
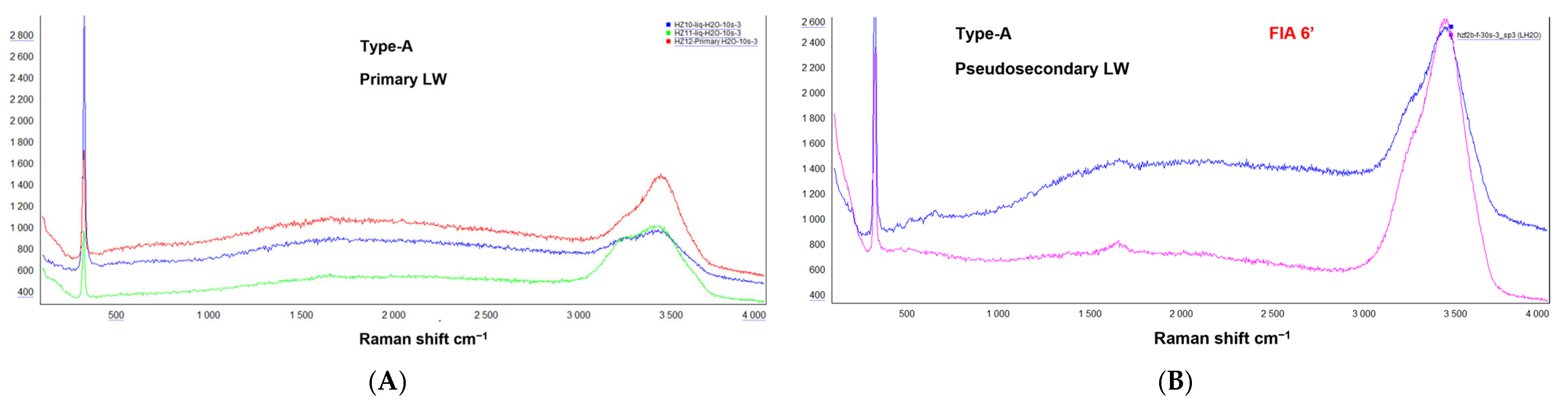
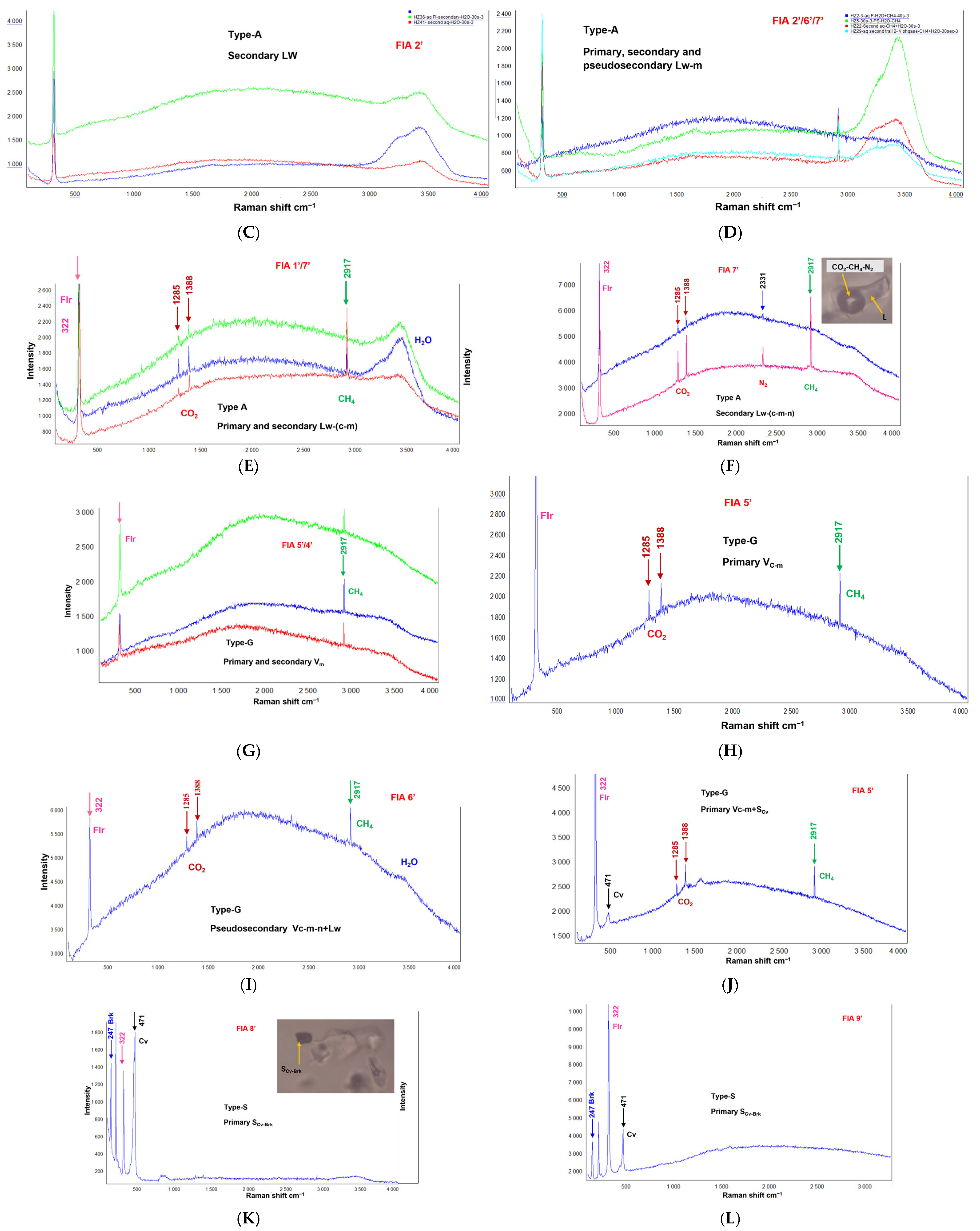
4.2.1. Aqueous-Rich Fluid Inclusions Type-A
4.2.2. Gas–Rich Fluid Inclusions Type-G
4.2.3. Solid-Rich Fluid Inclusions Type-S
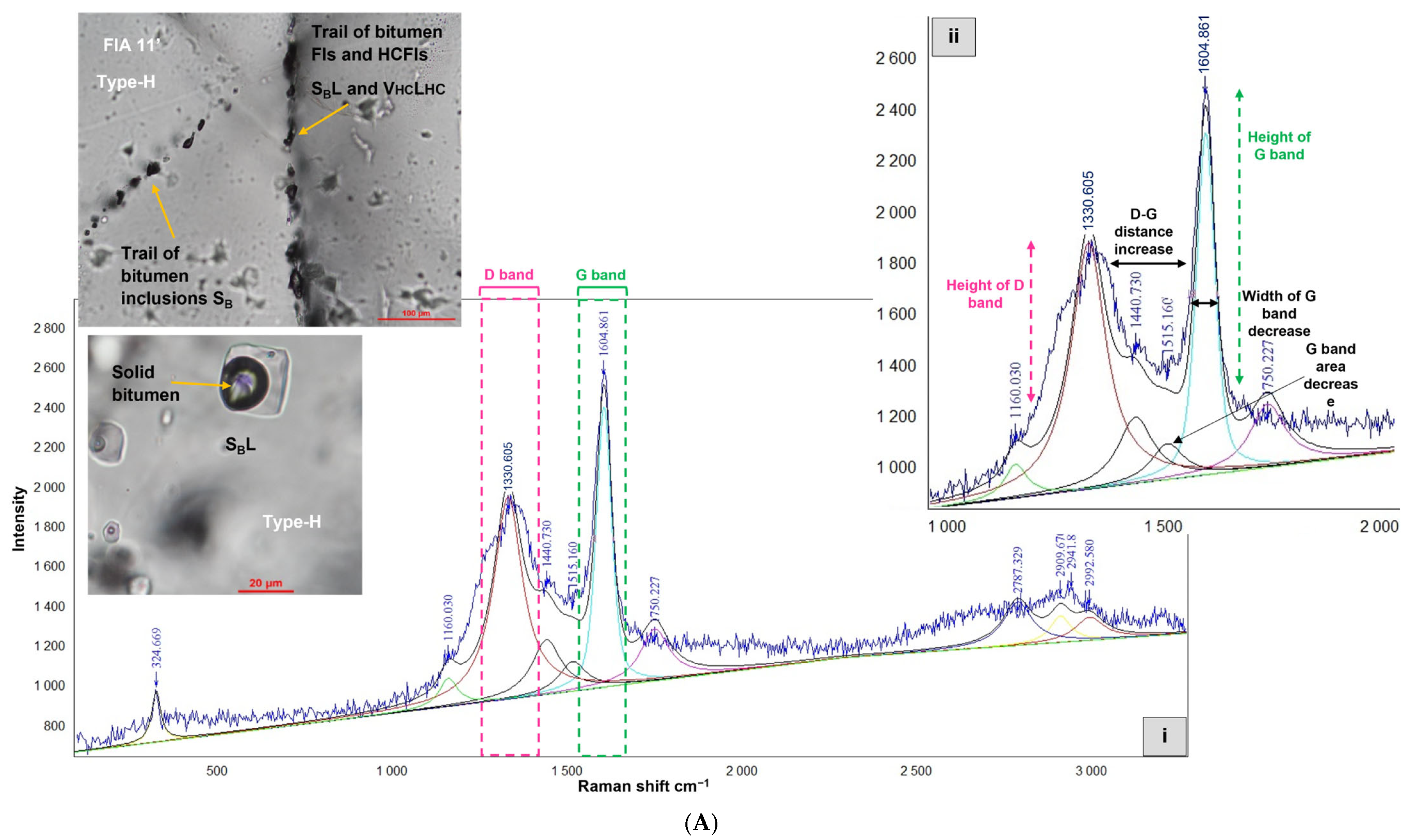
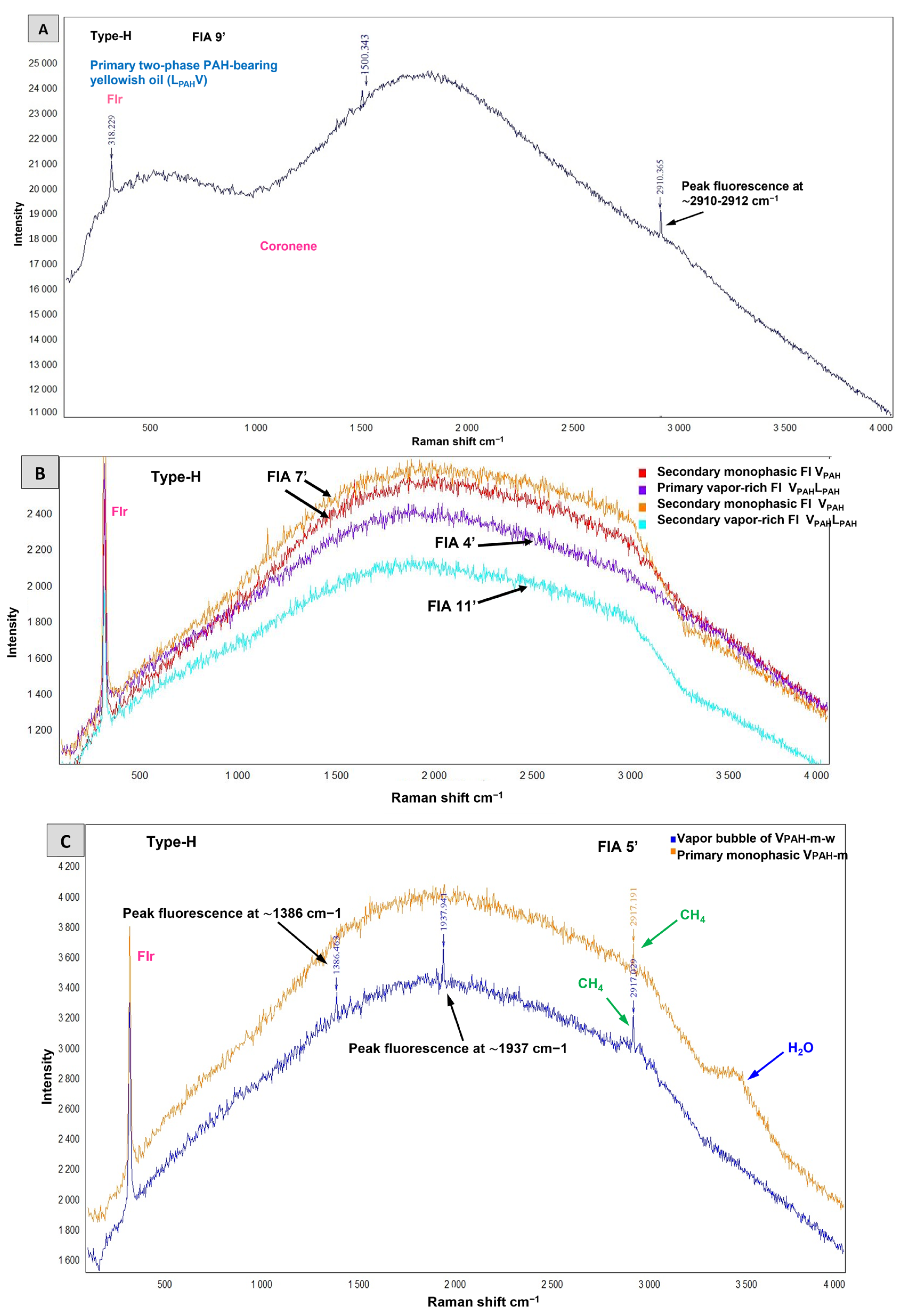
4.2.4. Hydrocarbon-Bearing Fluid Inclusions Type-H
5. Discussion
5.1. Immiscible Fluid Mixture of Brines, Oil, and Volatiles
5.2. Processes for the Formation of Multi-Volatile Systems in Vapor Phases
- CO2 and CH4 were likely produced from over-mature petroleum-bearing source rocks. These fluids are the most abundant gases found in the three upper-Cretaceous units, which are part of the regional petroleum system in central and northern Tunisia: the chalky Abiod Formation (Campanian–Maastrichtian), the shaly and calcareous Aleg Formation (Turonian–Santonian), and the shaly and calcareous Fahdene Formation (Albian–Cenomanian). The greatest concentrations of these gases were recorded in several wells within the Fahdene Formation, with CH4 (15%) and CO2 (89%) [94]. These fluids may be generated through hydrous thermal alteration of petroleum and by organic materials in the surrounding rocks. The sporadic presence of oil indicators in the regional setting suggests an organic origin for the dense volatile phases (CO2, CH4, and N2) [95]. In this sense, [51,96,97] documented petroleum wells were drilled in the Zaghouan area.
- 2.
- Trapped oil with brines in PAH-bearing fluid inclusions VPAH-m + Lw (Figure 14C) may undergo oxidation over time, leading to molecular fractionation [104]. Independent of temperature, CO2 can result from the chemical oxidation of hydrocarbons by water within inclusions or cracks [105]. Depending on the oil–water interaction, hydrolytic disproportionation occurs at 150 °C. This process enables the exchange of oxygen or hydrogen between hydrocarbons, water, and OM [106]. The CH4 trapped in FIs (Vm, Lw-(c-m-n), Lw-m, Lw-(c-m): Figure 12; VPAH-m: Figure 14C) could follow this mechanism. These conditions align with those proposed by [107], suggesting that CH4 is produced from the thermal decomposition of organic materials in the presence of chemical catalysts. This process follows an oxidation–hydration reaction sequence, as CH4 remains highly stable under hydrothermal conditions.
5.3. Sources of Hydrocarbon Fluids
5.4. Critical Insights into the Origin of Graphite
5.5. Insights into the Origins of Evenkite and Hatchettine
- Evenkite is often found in association with magmatic rocks or hydrothermal materials, e.g., [142,143,153]. Although most hydrocarbons are believed to be of biogenic origin, some studies suggest that hydrocarbon alkanes may also originate from mantle-derived rocks, e.g., [154,155]. The absence of evidence for magmatic activity during ore formation is one of the most notable characteristics of this extensive F-rich hydrothermal activity in the ZFP. However, the presence of deep-sourced CO2 and CH4 gases may point to a hidden undiscovered magmatic activity that occurred during fluorite precipitation.
- Long-chain n-alkanes can be produced from terrestrial and/or marine biogenic sources. Evenkite forms by the thermal cracking of OM, i.e., living organisms and marine flora in sedimentary rocks [142]. The carbon preference index (CPI) values obtained from the n-alkanes of the Fahdene and Bahloul Cretaceous source rocks [116] indicated natural rather than anthropogenic input [156,157]. Typically, photosynthetic-producing bacteria and aquatic algal phytoplankton are dominated by short-chain n-alkanes (SHC) < C23. Whereas terrestrial plants are dominated by long-chain n-alkanes (LHC) > C23 [157,158], suggesting a predominant terrestrial contribution to the studied n-alkanes and a more proximal environment. During the late Jurassic, the ZFP was dominated by a reef complex known as the Ressas Formation [159]. The Jurassic–Cretaceous boundary at Hammam–Zriba marks a transition from a relatively shallow carbonate platform to a more restricted environment [160]. In warm climates, vegetation produces longer chain n-alkanes than those found in cooler climates [161]. Since the abundance of n-alkanes in algae reflects the origin of OM [161,162], the referential biodegradation of high molecular weight (HMW) n-alkanes from terrestrial plants by bacterioplankton [163] could explain the occurrence of n-alkane evenkite in the paleofluids of MVT fluorite deposits.
5.6. Genetic Model of the Epigenetic Fluorite Deposit
6. Conclusions
- The fluid inclusions were analyzed using a combination of Raman spectroscopy, petrography, and microthermometry. These techniques revealed the heterogenous trapping of immiscible fluids, with four essential types of inclusions identified: liquid-, vapor-, and solid-dominated fluid inclusions, and hydrocarbon-bearing inclusions.
- Raman spectroscopy identified previously undetected organic compounds, including aliphatic hydrocarbons (evenkite C24H50 and/or hatchettine C38H78), polycyclic aromatic hydrocarbons (PAHs) (coronene C24H12), mixtures of aliphatic and aromatic compounds (bitumen: CnH2n+2 where n typically ranges between 20 and 40), and kerogen (the chemical formula of kerogen is not a specific and fixed formula due to its varied and complex composition), as well as water-containing low-molecular-weight (LMW) hydrocarbons (CH4) and PAH-containing water.
- Several hydrothermal stages of fluid circulation are recognized, involving the interaction of different fluid-end members: (i) The first major episode involves the H2O–NaCl–CaCl2 fluid system of hot (85 to 145 ± 5 °C) basinal brines (~13–22 wt.% eq. NaCl) derived from interactions with Triassic salts. (ii) Next is an episode of oil-bearing fluids associated with the expulsion of deep CO2–CH4–N2-rich fluids during Miocene compressional events. (iii) In later unmixing processes, paleofluids were linked to the accumulation of evenkite, bitumen, and graphite within the inclusions.
- The presence of the hydrocarbon mineral evenkite is a very rare case of hydrocarbon accumulation worldwide. Evenkite-bearing fluid inclusions are reported for the first time in Tunisian MVT deposits, which suggests the presence of deep-sourced long-chain n-alkanes. Some evidence suggests that evenkite may have formed from the same type of organic matter that produced oil at the Hammam–Zriba F–Ba(Zn–Pb) deposit.
- The presence of bitumen, kerogen, and graphite in coeval inclusions indicates that the oil and organic matter experienced varying stages of modification. The entrapped fluids preserved different generations of hydrocarbons, depending on their maturity. These hydrocarbons were most likely released at different stages of burial in the source rock. The Raman spectra allowed the identification of three oil families (I, II and III). Family I (aliphatic) is attributed to the lower-Eocene Bou Dabbous Formation and family II (aromatic) is attributed to the Albian Fahdene or Cenomanian–Turonian Bahloul source rocks, respectively. While family III is believed to be a mixture of both aliphatic and aromatic compounds produced by all three sources.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouhlel, S.; Fortuné, J.P.; Guilhaumou, N.; Touray, J.C. Les Minéralisations Stratiformes à F-Ba de Hammam Zriba, Jebel Guébli (Tunisie Nord Orientale): L’apport Des Études d’inclusions Fluides à La Modélisation Génétique. Miner. Depos. 1988, 23, 166–173. [Google Scholar] [CrossRef]
- Souissi, F.; Fortune, J.-P.; Sassi, R. La Minéralisation Fluorée de Type Mississippi Valley Du Jebel Stah (Tunisie Nord-Orientale). Bull. Soc. Géol. Fr. 1998, 169, 163–175. [Google Scholar]
- Souissi, F.; Souissi, R.; Dandurand, J.-L. The Genesis of the Mississippi Valley-Type Fluorite Ore at Jebel Stah (Zaghouan District, North-Eastern Tunisia) Constrained by Thermal and Chemical Properties of Fluids and REE and Sr Isotope Geochemistry; Geochemistry—Earth’s System Process; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Souissi, F.; Dandurand, J.L.; Fortuné, J.P. Thermal and Chemical Evolution of Fluids during Fluorite Deposition in the Zaghouan Province, North-Eastern Tunisia. Miner. Depos. 1997, 32, 257–270. [Google Scholar] [CrossRef]
- Souissi, F.; Souissi, R.; Dandurand, J.L. The Mississippi Valley-Type Fluorite Ore at Jebel Stah (Zaghouan District, North-Eastern Tunisia): Contribution of REE and Sr Isotope Geochemistries to the Genetic Model. Ore Geol. Rev. 2010, 37, 15–30. [Google Scholar] [CrossRef]
- Souissi, F.; Jemmali, N.; Souissi, R.; Dandurand, J.L. REE and Isotope (Sr, S, and Pb) Geochemistry to Constrain the Genesis and Timing of the F-(Ba-Pb-Zn) Ores of the Zaghouan District (NE Tunisia). Ore Geol. Rev. 2013, 55, 1–12. [Google Scholar] [CrossRef]
- Kenderes, S.M.; Appold, M.S. Fluorine Concentrations of Ore Fluids in the Illinois-Kentucky District: Evidence from SEM-EDS Analysis of Fluid Inclusion Decrepitates. Geochim. Cosmochim. Acta 2017, 210, 132–151. [Google Scholar] [CrossRef]
- Bau, M.; Romer, R.L.; Lüders, V.; Dulski, P. Tracing Element Sources of Hydrothermal Mineral Deposits: REE and Y Distribution and Sr-Nd-Pb Isotopes in Fluorite from MVT Deposits in the Pennine Orefield, England. Miner. Depos. 2003, 38, 992–1008. [Google Scholar] [CrossRef]
- González-Partida, E.; Carrillo-Chávez, A.; Grimmer, J.O.W.; Pironon, J.; Mutterer, J.; Levresse, G. Fluorite Deposits at Encantada-Buenavista, Mexico: Products of Mississippi Valley Type Processes. Ore Geol. Rev. 2003, 23, 107–124. [Google Scholar] [CrossRef]
- Kraemer, D.; Viehmann, S.; Banks, D.; Sumoondur, A.D.; Koeberl, C.; Bau, M. Regional Variations in Fluid Formation and Metal Sources in MVT Mineralization in the Pennine Orefield, UK: Implications from Rare Earth Element and Yttrium Distribution, Sr-Nd Isotopes and Fluid Inclusion Compositions of Hydrothermal Vein Fluorites. Ore Geol. Rev. 2019, 107, 960–972. [Google Scholar] [CrossRef]
- Bourdet, J.; Burruss, R.C.; Bodnar, R.J.; Eadington, P.J. Assessment of UV-Raman for Analysis of Petroleum Inclusions. In Proceedings of the European Current Research on Fluid Inclusions (ECROFI–XXI), Montanuniversität Leoben, Leoben, Austria, 9–11 August 2011; pp. 50–51. [Google Scholar]
- Liu, D.H.; Xiao, X.M.; Tian, H.; Min, Y.S.; Zhou, Q.; Cheng, P.; Shen, J.G. Sample Maturation Calculated Using Raman Spectroscopic Parameters for Solid Organics: Methodology and Geological Applications. Chin. Sci. Bull. 2013, 58, 1285–1298. [Google Scholar] [CrossRef]
- Gao, Y.; Zou, Y.R.; Liang, T.; Peng, P. Jump in the Structure of Type I Kerogen Revealed from Pyrolysis and 13C DP MAS NMR. Org. Geochem. 2017, 112, 105–118. [Google Scholar] [CrossRef]
- Richardson, C.K.; Rye, R.O.; Wasserman, M.D. The Chemical and Thermal Evolution of the Fluids in the Cave-in-Rock Fluorspar District, Illinois; Stable Isotope Systematics at the Deardorff Mine. Econ. Geol. 1988, 83, 765–783. [Google Scholar] [CrossRef]
- Wang, G.; Lei, Q.; Huang, Z.; Liu, G.; Fu, Y.; Li, N.; Liu, J. Genetic Relationship between Mississippi Valley-Type Pb–Zn Mineralization and Hydrocarbon Accumulation in the Wusihe Deposits, Southwestern Margin of the Sichuan Basin, China. Minerals 2022, 12, 1447. [Google Scholar] [CrossRef]
- Petrov, D.V. Raman Spectrum of Methane in Nitrogen, Carbon Dioxide, Hydrogen, Ethane, and Propane Environments. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 191, 573–578. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, N.; Borjigin, T.; Shen, B.; Xie, X.; Ma, Z.; Lu, C.; Yang, Y.; Yang, L.; Cheng, L.; et al. Integrated Assessment of Thermal Maturity of the Upper Ordovician–Lower Silurian Wufeng–Longmaxi Shale in Sichuan Basin, China. Mar. Pet. Geol. 2019, 100, 447–465. [Google Scholar] [CrossRef]
- Schito, A.; Romano, C.; Corrado, S.; Grigo, D.; Poe, B. Diagenetic Thermal Evolution of Organic Matter by Raman Spectroscopy. Org. Geochem. 2017, 106, 57–67. [Google Scholar] [CrossRef]
- Schito, A.; Muirhead, D.; Bowden, S.; Parnell, J. Hydrothermal Generation of Hydrocarbons in Basement Rocks, Southern Tuscany. Ital. J. Geosci. 2022, 141, 5–19. [Google Scholar] [CrossRef]
- Craddock, P.R.; Sauerer, B. Robust Determination of Kerogen Properties in Organic-Rich Mudrocks via Raman Spectroscopy. Org. Geochem. 2022, 169, 104381. [Google Scholar] [CrossRef]
- Touray, J.C.; Yajima, J. Hydrocarbures Liquides Inclus Dans Des Fluorites Tunisiennes. Miner. Depos. 1967, 2, 286–290. [Google Scholar] [CrossRef]
- Touray, J.C. Hydrocarbures Liquides et Gazeux En Inclusions Dans Les Minéraux. Bull. Cent. Rech. Pau. SNPA 1969, 3, 429–441. [Google Scholar]
- Touray, J.-C. Deep Origin CO_2 Trapped by Healed Cracks in Epigenetic Tunisian Fluorites. Proc. Int. Mineral. Ass. Int. Ass. Genesis Ore Deposits, Gen. Meeting. Tokyo Kyoto Publ. Soc. Min. Geol. 1971, 3, 351–354. [Google Scholar]
- Bejaoui, J.; Bouhlel, S.; Cardellach, E.; Canals, À.; Perona, J.; Piqué, À. Mineralization and Fluid Inclusion Studies of the Aptian Carbonate-Hosted PbZnBa Ore Deposits at Jebel Hamra, Central Tunisia. J. Geochem. Explor. 2013, 128, 136–146. [Google Scholar] [CrossRef]
- Souissi, F. Etude Gitologique et Conditions de Formation Des Gisements de Fluorine (Pb, Zn, Ba) Du Jebel Zaghouan (jJ. Stah et Sidi Taya) et Du Jebel Oust (Tunisie Nord-Orientale). Ph.D. Thesis, Université Paul Sabatier, Toulouse, France, 1987; 220p. [Google Scholar]
- Souissi, F.; Fortuné, J.P.; Sassi, R.; Bouhlel, S. Le Gisement de Fluorine et de Galène de Sidi Taya: Minéralogie et Étude Des Inclusions Fluides. Notes Du. Serv. Géologique Tunis. 1997, 63, 83–102. [Google Scholar]
- Bejaoui, J. Géologie, Minéralogie, Éléments En Traces, Inclusions Fluides, Isotopes et Modélisation Génétique Des Gisements à Pb-Zn et/Ou F, Ba de La Tunisie Centroseptentrionale (Fedj Hassène, Hamra, Ajered, Kohol, Mecella, Mokta et M’Tak). Ph.D. Thesis, Université de Tunis El Manar, Tunis, Tunisia, 2012; p. 260. [Google Scholar]
- Guilhaumou, N.; Touray, J.-C.; Bouhlel, S. Stretching of Hydrocarbon Fluid Inclusions in Fluorite at 200 and 400 Bars Confining Pressure. Application to Low Pressure Geobarometry. Bull. Mineral. 1988, 111, 421–426. [Google Scholar] [CrossRef]
- Guilhaumou, N.; Szydlowskii, N.; Pradier, B. Characterization of Hydrocarbon Fluid Inclusions by Infra-Red and Fluorescence Microspectrometry. Mineral. Mag. 1990, 54, 311–324. [Google Scholar] [CrossRef]
- Benchilla, L.; Guilhaumou, N.; Mougin, P.; Jaswal, T.; Roure, F. Reconstruction of Palaeo-Burial History and Pore Fluid Pressure in Foothill Areas: A Sensitivity Test in the Hammam Zriba (Tunisia) and Koh-i-Maran (Pakistan) Ore Deposits. Geofluids 2003, 3, 103–123. [Google Scholar] [CrossRef]
- Guilhaumou, N.; Dumas, P. Synchrotron FTIR Hydrocarbon Fluid Inclusion Microanalysis Applied to Diagenetic History and Fluid Flow Reconstruction in Reservoir Appraisal. Oil Gas Sci. Technol. 2005, 60, 763–779. [Google Scholar] [CrossRef]
- Han, Y.; Noah, M.; Lüders, V.; Horsfield, B.; Mangelsdorf, K. NSO-Compounds in Oil-Bearing Fluid Inclusions Revealed by FT-ICR-MS in APPI (+) and ESI (−) Modes: A New Method Development. Org. Geochem. 2020, 149, 104113. [Google Scholar] [CrossRef]
- Klett, T.R. Total Petroleum Systems of the Pelagian Province, Tunisia, Libya, Italy, and Malta; the Bou Dabbous, Tertiary and Jurassic-Cretaceous Composite; US Department of the Interior, US Geological Survey Bulletin: Reston, VA, USA, 2001; pp. 1–27. [CrossRef]
- Camprubí, A.; González-Partida, E.; Richard, A.; Boiron, M.-C.; González-Ruiz, L.E.; Aguilar-Ramírez, C.F.; Fuentes-Guzmán, E.; González-Ruiz, D.; Legouix, C. MVT-Like Fluorite Deposits and Oligocene Magmatic-Hydrothermal Fluorite–Be–U–Mo–P–V Overprints in Northern Coahuila, Mexico. Minerals 2019, 9, 58. [Google Scholar] [CrossRef]
- Piqué, A.; Tricart, P.; Guiraud, R.; Laville, E.; Bouaziz, S.; Amrhar, M.; Ait Ouali, R. The Mesozoic–Cenozoic Atlas Belt (North Africa): An Overview. Geodin. Acta 2002, 15, 185–208. [Google Scholar] [CrossRef]
- Melki, F.; Zouaghi, T.; Ben Chelbi, M.; Bédir, M.; Zargouni, F. Role of the NE-SW Hercynian Master Fault Systems and Associated Lineaments on the Structuring and Evolution of the Mesozoic and Cenozoic Basins of the Alpine Margin, Northern Tunisia. In Tectonics—Recent Advances; IntechOpen: London, UK, 2012; pp. 131–168. [Google Scholar]
- Zouaghi, T.; Bédir, M.; Melki, F.; Gabtni, H.; Gharsalli, R.; Bessioud, A.; Zargouni, F. Neogene Sediment Deformations and Tectonic Features of Northeastern Tunisia: Evidence for Paleoseismicity. Arab. J. Geosci. 2011, 4, 1301–1314. [Google Scholar] [CrossRef]
- Soumaya, A.; Ben Ayed, N.; Delvaux, D.; Ghanmi, M. Spatial Variation of Present-day Stress Field and Tectonic Regime in Tunisia and Surroundings from Formal Inversion of Focal Mechanisms: Geodynamic Implications for Central Mediterranean. Tectonics 2015, 34, 1154–1180. [Google Scholar] [CrossRef]
- Roure, F.; Casero, P.; Addoum, B. Alpine Inversion of the North African Margin and Delamination of Its Continental Lithosphere. Tectonics 2012, 31, 1–28. [Google Scholar] [CrossRef]
- Ben Ayed, N. Evolution Tectonique de l’avant-Pays de La Chaîne Alpine de Tunisie Du Début Du Mésozoïque à l’Actuel. Ph.D. Thesis, Université Paris Sud-Paris XI, Paris, France, 1986; 328p. [Google Scholar]
- Turki, M.M.; Delteil, J.; Truillet, R.; Yaich, C. Les Inversions Tectoniques de La Tunisie Centro-Septentrionale. Bull. Soc. Géol. Fr. 1988, 4, 399–406. [Google Scholar] [CrossRef]
- Coward, M.; Dietrich, D. Alpine Tectonics—An Overview. Geol. Soc. Lond. Spec. Publ. 1989, 45, 1–29. [Google Scholar] [CrossRef]
- Melki, F.; Zargouni, F. Tectonique Cassante Post-Jurassique de la Mine de Hammam Zriba. Bull. Soc. Geol. Fr. 1991, 162, 851–858. [Google Scholar]
- Morgan, M.A.; Grocott, J.; Moody, R.T.J. The Structural Evolution of the Zaghouan-Ressas Structural Belt, Northern Tunisia. Geol. Soc. Lond. Spec. Publ. 1998, 132, 405–422. [Google Scholar] [CrossRef]
- Frizon de Lamotte, D.; Mercier, E.; Outtani, F.; Addoum, B.; Ghandriche, H.; Ouali, J. Structural Inheritance and Kinematics of Folding and Thrusting along the Front of the Eastern Atlas Mountains (Algeria and Tunisia). In Stratigraphy and Evolution of the Peri-Tethyan Platforms, Peri-Tethys Mem. 3; Crasquin-Soleau, S., Barrier, E., Eds.; Muséum National d’Histoire Naturelle: Paris, France, 1998; Volume 177, pp. 237–252. [Google Scholar]
- Bouaziz, S.; Barrier, E.; Soussi, M.; Turki, M.M.; Zouari, H. Tectonic Evolution of the Northern African Margin in Tunisia from Paleostress Data and Sedimentary Record. Tectonophysics 2002, 357, 227–253. [Google Scholar] [CrossRef]
- Jolivet, L.; Faccenna, C.; Piromallo, C. From Mantle to Crust: Stretching the Mediterranean. Earth Planet. Sci. Lett. 2009, 285, 198–209. [Google Scholar] [CrossRef]
- Castany, G. Le Haut-Bassin Siculo-Tunisien:Étude de Morphologie et de Géologie Sous-Marines. Bull. Stn. Océanogr. Salammbô 1955, 2, 3–12. Available online: http://hdl.handle.net/1834/9018 (accessed on 9 January 2022).
- Mejri, F.; Burollet, P.F.; Ferjani, A.B. Petroleum Geology of Tunisia: A Renewed Synthesis. Enterp. Tunis. Act. Pet. 2006, 22, 233. [Google Scholar] [CrossRef]
- Chihi, L. Les Fossés Néogènes et Quaternaires de La Tunisie et de La Mer Pélagienne. Etude Structurale et Leur Signification Dans Le Cadre de La Géodynamique de La Méditerrané. Ph.D. Thesis, Faculty of Sciences of Tunis, University of Tunis, Tunis, Tunisia, 1995; 324p. [Google Scholar]
- Ouhibi, R.; Mzali, H.; Ghanmi, M.; Zargouni, F. Determining of a Complex Structure Consisting of Detachment, Fault-Bend and Fault Propagation Folds in the Collapse Structure of Zaghouan-Bouficha (Bouficha Region, North Eastern Tunisia). Model. Earth Syst. Environ. 2018, 4, 241–250. [Google Scholar] [CrossRef]
- Morgan, M.; Grocott, J.; Moody, R.T.J. The Structural Setting and Evolution of the Zaghouan-Ressas Structural Belt in the Zaghouan Area. Tunisian Atlas, Northern Tunisia; Memoir No. 5; Entreprise Tunisienne d’Activités Pétrolières (ETAP): Tunis, Tunisia, 1992; pp. 193–209. [Google Scholar]
- Mzali, H.; Harbi, A.; Alyahyaoui, S. Geometry Characterization and Depth Determination of Deep Water Reservoirs: The Case of the Jebel Sidi Abd Er Rahmane Region (Cap Bon, Northeastern Tunisia). Arab. J. Geosci. 2016, 9, 295. [Google Scholar] [CrossRef]
- Zouaghi, T.; Ferhi, I.; Bédir, M.; Youssef, M.B.; Gasmi, M.; Inoubli, M.H. Analysis of Cretaceous (Aptian) Strata in Central Tunisia, Using 2D Seismic Data and Well Logs. J. Afr. Earth Sci. 2011, 61, 38–61. [Google Scholar] [CrossRef]
- Decrée, S.; Marignac, C.; Abidi, R.; Jemmali, N.; Deloule, E.; Souissi, F. Tectonomagmatic Context of Sedex Pb–Zn and Polymetallic Ore Deposits of the Nappe Zone Northern Tunisia, and Comparisons with MVT Deposits in the Region. In Mineral Deposits of North Africa; Carranza, E.J.M., Sadeghi, M., Eds.; Springer: Cham, Switzerland, 2016; pp. 497–525. [Google Scholar] [CrossRef]
- Jemmali, N.; Carranza, E.J.M.; Zemmel, B. Isotope Geochemistry of Mississippi Valley Type Stratabound F-Ba-(Pb-Zn) Ores of Hammam Zriba (Province of Zaghouan, NE Tunisia). Chem. Erde 2017, 77, 477–486. [Google Scholar] [CrossRef]
- Gharbi, M.; Ben Yed, N.; Ben Said, E. Essai d’interprétation Tectonique Du Champ Filonien de Bou-Jabeur (Atlas Tunisien Central). In Proceedings of the 1st National Congress of Earth Sciences, Tunis, Tunisia, 15–22 September 1985; Office National des Mines: Tunis, Tunisia, 1985; pp. 393–403. [Google Scholar]
- Thibieroz, J. Reconstitution Chronologique du Rôle des Surfaces d’Émersion dans l’Histoire du Gisement de Fluorine de Ham-998 mam Zriba (Région de Zaghouan, Tunisie). Mém. H. Série Soc. Géol. Fr. 1976, 7, 33–37. [Google Scholar]
- Touhami, A. Contribution à l’étude Géologique et Métallogénique de La Province Fluorée Tunisienne; Rôle de l’altération Superficielle Dans La Formation Des Concentrations Fluorées. Ph.D. Thesis, University of Tunis, Tunis, Tunisia, 1979; p. 321. [Google Scholar]
- Bouhlel, S. Composition Chimique, Fréquence et Distribution Des Minéraux de La Série Barytine-Célestite Dans Les Gisements de Fluorine de Hammam Jédidi et Hammam Zriba-Jébel Guébli (Tunisie Nord-Orientale). Bull. Mineral. 1985, 108, 403–420. [Google Scholar] [CrossRef]
- Somrani, C.; Souissi, F.; Souissi, R.; De Giudici, G.; Ferreira da Silva, E.; Fancello, D.; Podda, F.; Santos, J.F.; Abu-Alam, T.; Ribeiro, S.; et al. The Geochemical Characteristics of Ore-Forming Fluids in the Jebel Stah Fluorite Deposit in Northeast Tunisia: Insights from LA-ICP-MS and Sr Isotope Analyses. Minerals 2025, 15, 331. [Google Scholar] [CrossRef]
- Thibieroz, J. Hammam Jedidi et Hammam Zriba: Etude Géologique et Minière de Deux Gisements Stratiformes Dans Le Cadre de La Province Fluorée Tunisienne; L’association Des Concentrations Fluorées Aux Surfaces D’émersion; Université Paris VI: Paris, France, 1974; 432p. [Google Scholar]
- Roedder, E. Volume 12: Fluid Inclusions. Rev. Miner. 1984, 12, 644. [Google Scholar]
- Goldstein, R.H.; Reynolds, T.J. Systematics of Fluid Inclusions in Diagenetic Minerals; SEPM Society for Sedimentary Geology: Tulsa, OK, USA, 1994; Volume 31, p. 199. [Google Scholar] [CrossRef]
- Goldstein, R.H. Petrographic Analysis of Fluid Inclusions. Fluid. Incl. Anal. Interpret. 2003, 32, 9–54. [Google Scholar]
- Van Den Kerkhof, A.M.; Hein, U.F. Fluid Inclusion Petrography. Lithos 2001, 55, 27–47. [Google Scholar] [CrossRef]
- Bakker, R.J. Raman Spectra of Fluid and Crystal Mixtures in the Systems H2O, H2O–NaCl and H2O–MgCl2 at Low Temperatures: Applications to Fluid-Inclusion Research. Can. Mineral. 2004, 42, 1283–1314. [Google Scholar] [CrossRef]
- Bakker, R.J. The Perfection of Raman Spectroscopic Gas Densimeters. J. Raman Spectrosc. 2021, 52, 1923–1948. [Google Scholar] [CrossRef]
- Cloutis, E.; Szymanski, P.; Applin, D.; Goltz, D. Identification and Discrimination of Polycyclic Aromatic Hydrocarbons Using Raman Spectroscopy. Icarus 2016, 274, 211–230. [Google Scholar] [CrossRef]
- Crawford, M.L. Phase Equilibria in Aqueous Fluid Inclusions. In Fluid Inclusions: Applications to Petrology; Hollister, L.S., Crawford, M.L., Eds.; Short Course Series; Mineralogical Association of Canada: Ottawa, Canada, 1981; Volume 6, pp. 75–100. [Google Scholar]
- Frezzotti, M.L.; Tecce, F.; Casagli, A. Raman Spectroscopy for Fluid Inclusion Analysis. J. Geochem. Explor. 2012, 112, 1–20. [Google Scholar] [CrossRef]
- Jehlička, J.; Urban, O.; Pokorný, J. Raman Spectroscopy of Carbon and Solid Bitumens in Sedimentary and Metamorphic Rocks. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 2341–2352. [Google Scholar] [CrossRef]
- Xiao, X.; Zhou, Q.; Cheng, P.; Sun, J.; Liu, D.; Tian, H. Thermal Maturation as Revealed by Micro-Raman Spectroscopy of Mineral-Organic Aggregation (MOA) in Marine Shales with High and over Maturities. Sci. China Earth Sci. 2020, 63, 1540–1552. [Google Scholar] [CrossRef]
- Jehlička, J.; Edwards, H.G.M.; Villar, S.E.J. Raman Spectroscopy of Natural Accumulated Paraffins from Rocks: Evenkite, Ozokerite and Hatchetine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 68, 1143–1148. [Google Scholar] [CrossRef]
- Jehlička, J.; Edwards, H.G.M.; Vítek, P. Assessment of Raman Spectroscopy as a Tool for the Non-Destructive Identification of Organic Minerals and Biomolecules for Mars Studies. Planet. Space Sci. 2009, 57, 606–613. [Google Scholar] [CrossRef]
- Bodnar, R.J.; Burnham, C.W.; Sterner, S.M. Synthetic Fluid Inclusions in Natural Quartz. III. Determination of Phase Equilibrium Properties in the System H2O-NaCl to 1000 °C and 1500 Bars. Geochim. Cosmochim. Acta 1985, 49, 1861–1873. [Google Scholar] [CrossRef]
- Diamond, L.W. Introduction to Gas-Bearing, Aqueous Fluid Inclusions. Fluid. Incl. Anal. Interpret. 2003, 32, 363–372. [Google Scholar]
- Pichavant, M.; Ramboz, C.; Weisbrod, A. Fluid Immiscibility in Natural Processes: Use and Misuse of Fluid Inclusion Data: I. Phase equilibria analysis—a theoretical and geometrical approach. Chem. Geol. 1982, 37, 1–27. [Google Scholar] [CrossRef]
- Xavier, R.P.; Foster, R.P. Fluid Evolution and Chemical Controls in the Fazenda Maria Preta (FMP) Gold Deposit, Rio Itapicuru Greenstone Belt, Bahia, Brazil. Chem. Geol. 1999, 154, 133–154. [Google Scholar] [CrossRef]
- Coulibaly, Y.; Boiron, M.C.; Cathelineau, M.; Kouamelan, A.N. Fluid Immiscibility and Gold Deposition in the Birimian Quartz Veins of the Angovia Deposit (Yaouré, Ivory Coast). J. Afr. Earth Sci. 2008, 50, 234–254. [Google Scholar] [CrossRef]
- Wilkinson, J.J. Fluid Inclusions in Hydrothermal Ore Deposits. Lithos 2001, 55, 229–272. [Google Scholar] [CrossRef]
- Ramboz, C.; Pichavant, M.; Weisbrod, A. Fluid Immiscibility in Natural Processes: Use and Misuse of Fluid Inclusion Data: II. Interpretation of fluid inclusion data in terms of immiscibility. Chem. Geol. 1982, 37, 29–48. [Google Scholar] [CrossRef]
- Goldstein, R.H. Fluid Inclusions in Sedimentary and Diagenetic Systems. Lithos 2001, 55, 159–193. [Google Scholar] [CrossRef]
- Chi, G.X.; Lu, H.Z. Validation and Representation of Fluid Inclusion Microthermometric Data Using the Fluid Inclusion Assemblage (FIA) Concept. Acta Petrol. Sin. 2008, 24, 1945–1953. [Google Scholar]
- Chi, G.; Diamond, L.W.; Lu, H.; Lai, J.; Chu, H. Common Problems and Pitfalls in Fluid Inclusion Study: A Review and Discussion. Minerals 2021, 11, 7. [Google Scholar] [CrossRef]
- Brown, P.E. Fluid Inclusion Modeling for Hydrothermal Systems. Rev. Econ. Geol. 1998, 10, 151–171. [Google Scholar]
- Nedkvitne, T.; Karlsen, D.A.; Bjørlykke, K.; Larter, S.R. Relationship between Reservoir Diagenetic Evolution and Petroleum Emplacement in the Ula Field, North Sea. Mar. Pet. Geol. 1993, 10, 255–270. [Google Scholar] [CrossRef]
- Wilkinson, J.J.; Lonergan, L.; Fairs, T.; Herrington, R.J. Fluid Inclusion Constraints on Conditions and Timing of Hydrocarbon Migration and Quartz Cementation in Brent Group Reservoir Sandstones, Columba Terrace, Northern North Sea. Geol. Soc. Spec. Publ. 1998, 144, 69–89. [Google Scholar] [CrossRef]
- Aplin, A.C.; Larter, S.R.; Bigge, M.A.; Macleod, G.; Swarbrick, R.E.; Grunberger, D. Confocal Microscopy of Fluid Inclusions Reveals Fluid-Pressure Histories of Sediments and an Unexpected Origin of Gas Condensate. Geology 2000, 28, 1047–1050. [Google Scholar] [CrossRef]
- Yardley, B.W.D. 100th Anniversary Special Paper: Metal Concentrations in Crustal Fluids and Their Relationship to Ore Formation. Econ. Geol. 2005, 100, 613–632. [Google Scholar] [CrossRef]
- González-Esvertit, E.; Fusswinkel, T.; Canals, À.; Casas, J.M.; Neilson, J.; Wagner, T.; Gomez-Rivas, E. Fluid Evolution and Halogen Fractionation in Orogenic Belts: A Comparative Fluid Inclusion Appraisal in the Eastern Pyrenees. Chem. Geol. 2025, 674, 122578. [Google Scholar] [CrossRef]
- Richardson, C.K.; Holland, H.D. Fluorite Deposition in Hydrothermal Systems. Geochim. Cosmochim. Acta 1979, 43, 1327–1335. [Google Scholar] [CrossRef]
- Dhia, H.B. The Geothermal Gradient Map of Central Tunisia: Comparison with Structural, Gravimetric and Petroleum Data. Tectonophysics 1987, 142, 99–109. [Google Scholar] [CrossRef]
- Kort, H.M.; El Asmi, A.M.; Ouazaa, N.L.; Gasquet, D.; Saidi, M. Hydrothermal History in the Eastern Margin of Tunisia: Inferred Magmatic Rocks Alterations, New Paragenesis and Associated Gas Occurrences. Arab. J. Geosci. 2015, 8, 8927–8942. [Google Scholar] [CrossRef]
- Perthuisot, V.; Guilhaumou, N.; Touray, J.C. Les Inclusions Fluides Hypersalines et Gazeuses Des Quartz et Dolomites Du Trias Evaporitique Nord-Tunisien; Essai d’interpretation Geodynamique. Bull. Soc. Géol. Fr. 1978, S7-XX, 145–155. [Google Scholar] [CrossRef]
- Mzali, H.; Zouari, H. Structural Cutting of a Region Showing Various Compound Tectonic Deformations (Example of Bouficha-Grombalia Region). Arab. J. Geosci. 2012, 5, 1399–1409. [Google Scholar] [CrossRef]
- Soltani, H.; Mabrouk El Asmi, A.; Asmi, E.; Haj, B.; Saidi, M.B.B. Evaluation of the Zaghouan Region Oil Potential: Inferred Thermal Maturity Through 1D Modeling; ResearchGate. 2018. Available online: https://www.researchgate.net/publication/329000536_Evaluation_of_the_Zaghouan_re-1080gion_oil_potential_Inferred_thermal_maturity_through_1D_modeling (accessed on 13 February 2025).
- Fourati, L.; Hamouda, F.; El Mahersi, C.; Saıdi, M. Ypresian Bou Dabbous Formation: An Active Petroleum System within the Thrust Belt Zone in Northern Tunisia-North Africa. In Proceedings of the 8th Tunisian Petroleum Exploration & Production Conference, Tunis, Tunisia, 2–4 March 2002; American Association of Petroleum Geologists: Denver, CO, USA, 2002; Available online: https://www.searchanddiscovery.com/abstracts/html/2001/annual/abstracts/0250.htm (accessed on 7 February 2023).
- Lewan, M.D. Experiments on the Role of Water in Petroleum Formation. Geochim. Cosmochim. Acta 1997, 61, 3691–3723. [Google Scholar] [CrossRef]
- Behar, F.; Beaumont, V.; Penteado, H.L.D.B. Rock-Eval 6 Technology: Performances and Developments. Oil Gas Sci. Technol. 2001, 56, 111–134. [Google Scholar] [CrossRef]
- Rddad, L.; Jemmali, N.; Jaballah, S. The Role of Organic Matter and Hydrocarbons in the Genesis of the Pb-Zn-Fe (Ba-Sr) Ore Deposits in the Diapirs Zone, Northern Tunisia. Minerals 2024, 14, 932. [Google Scholar] [CrossRef]
- Bilkiewicz, E.; Kowalski, T. Origin of Hydrocarbon and Non-Hydrocarbon (H2S, CO2 and N2) Components of Natural Gas Accumulated in the Zechstein Main Dolomite (Ca2) Strata in SW Part of the Polish Permian Basin: Stable Isotope and Hydrous Pyrolysis Studies. J. Pet. Sci. Eng. 2020, 192, 107296. [Google Scholar] [CrossRef]
- Souissi, F.; Sassi, R.; Dandurand, J.L.; Bouhlel, S.; Ben Hamda, S. Fluid Inclusion Microthermometry and Rare Earth Element Distribution in the Celestites of the Jebel Doghra Ore Deposit (Dome Zone, Northern Tunisia): Towards a New Genetic Model. Bull. La. Soc. Geol. Fr. 2007, 178, 459–471. [Google Scholar] [CrossRef]
- George, S.C.; Ruble, T.E.; Dutkiewicz, A.; Eadington, P.J. Assessing the Maturity of Oil Trapped in Fluid Inclusions Using Molecular Geochemistry Data and Visually-Determined Fluorescence Colours. Appl. Geochem. 2001, 16, 451–473. [Google Scholar] [CrossRef]
- Pokrovskii, V.A. The Oxidative Solubility of Petroleum in Formation Waters: Implications for Hydrothermal Transport, Diagenesis, and Development of Secondary Porosity in Reservoir Rocks. In Proceedings of the Geoscience for the Community 13th Australian Geological Convention, Canberra, Australia, 19–23 February 1996; Geological Society of Australia: Sydney, Australia, 1996; pp. 41–347. [Google Scholar]
- Helgeson, H.C.; Knox, A.M.; Owens, C.E.; Shock, E.L. Petroleum, Oil Field Waters, and Authigenic Mineral Assemblages Are They in Metastable Equilibrium in Hydrocarbon Reservoirs. Geochim. Cosmochim. Acta 1993, 57, 3295–3339. [Google Scholar] [CrossRef]
- Mango, F.D.; Hightower, J.W.; James, A.T. Role of Transition-Metal Catalysis in the Formation of Natural Gas. Nature 1994, 368, 536–538. [Google Scholar] [CrossRef]
- Burollet, P.F. Contribution à l’étude Stratigraphique de La Tunisie Centrale. Ann. Mines Géol. 1956, 18, 350. [Google Scholar]
- Mohamed, A.B.; Saidi, M.; Jrad, M.B.; Soussi, M.; Riahi, S.; Bouazizi, I.; Saadani, B. The Organic Geochemistry of Source Rocks, Bitumens, Asphalts and Oil Stains from Northern Tunisia. In Proceedings of the 77th EAGE Conference and Exhibition 2015, Madrid, Spain, 1–4 June 2015; European Association of Geoscientists & Engineers: Houten, The Netherlands, 2015; Volume 2015, pp. 1–5. Available online: https://www.earthdoc.org/content/papers/10.3997/2214-4609.201413144 (accessed on 2 May 2024).
- Bédir, M.; Soltani, A.; Mohamed, A.B.; Arbi, A.; Saidi, M. Cretaceous Petroleum System Modeling of Kairouan Basin in Eastern Tunisia. Arab. J. Geosci. 2020, 13, 683. [Google Scholar] [CrossRef]
- Rddad, L.; Belayouni, H. Characterization of Organic Matter and Metal Contents Preserved within the Albian-Vraconian and Cenomanian-Turonian Black Shales in North-Central Tunisia. In Proceedings of the Geo. Soc. Am. Northeastern Section 49th Annual Meeting, Lancaster, PA, USA, 23 March 2014. [Google Scholar]
- Razgallah, S.; Philip, J.; Thomel, G.; Zaghbib-Turki, D.; Chaabani, F.; Ben Haj Ali, N.; M’Rabet, A.; Ali, N.B.H.; M’rabet, A. La Limite Cénomanien-Turonien En Tunisie Centrale et Méridionale: Biostratigraphie et Paléoenvironnements. Cretac. Res. 1994, 15, 507–533. [Google Scholar] [CrossRef]
- Robaszynski, F.; Caron, M.; Dupuis, C.; Amédro, F.; González Donoso, J.-M.; Linares, D. A Tentative Integrated Stratigraphy in the Turonian of Central Tunisia: Formations, Zones and Sequential Stratigraphy in the Kalaat Senan Area. Bull. Des. Cent. Rech. Explor. Elf Aquitaine 1990, 4, 213–384. [Google Scholar]
- Zaïer, A.; Beji-Sassi, A.; Sassi, S.; Moody, R.T.J. Basin Evolution and Deposition during the Early Paleogene in Tunisia. Geol. Soc. Lond. Spec. Publ. 1998, 132, 375–393. [Google Scholar] [CrossRef]
- Affouri, H.; Montacer, M. Organic Matter Appraisal of the Lower Eocene (Ypresian) Bou Dabbous Formation in Tunisia. Arab. J. Geosci. 2014, 7, 5509–5542. [Google Scholar] [CrossRef]
- Hallek, T.; Akrout, D.; Ahmadi, R.; Montacer, M. Assessment of Sedimentary Environment from PAHs and Aliphatic Biomarkers: The Case Study of Fahdene Black Shales in Northern Tunisia. J. Afr. Earth Sci. 2020, 161, 103676. [Google Scholar] [CrossRef]
- Peters, K.E.; Moldowan, J.M. The Biomarker Guide: Interpreting Molecular Fossils in Petroleum and Ancient Sediments; Prentice Hall: Englewood Cliffs, NJ, USA, 1993; 363p. [Google Scholar]
- Peters, K.E.; Walters, C.C.; Moldowan, J.M. The Biomarker Guide; Cambridge University Press: Cambridge, UK, 2005; Volume 1. [Google Scholar]
- Stein, S. On the High Temperature Chemical Equilibria of Polycyclic Aromatic Hydrocarbons. J. Phys. Chem. 1978, 82, 566–571. [Google Scholar] [CrossRef]
- Stein, S.E.; Fahr, A. High-Temperature Stabilities of Hydrocarbons. J. Phys. Chem. 1985, 89, 3714–3725. [Google Scholar] [CrossRef]
- Quirico, E.; Rouzaud, J.N.; Bonal, L.; Montagnac, G. Maturation Grade of Coals as Revealed by Raman Spectroscopy: Progress and Problems. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 2368–2377. [Google Scholar] [CrossRef]
- Huang, E.P.; Huang, E.; Yu, S.C.; Chen, Y.H.; Lee, J.S.; Fang, J.N. In Situ Raman Spectroscopy on Kerogen at High Temperatures and High Pressures. Phys. Chem. Miner. 2010, 37, 593–600. [Google Scholar] [CrossRef]
- Chen, Y.; Chou, I.M. Quantitative Raman Spectroscopic Determination of the Composition, Pressure, and Density of CO2-CH4Gas Mixtures. J. Spectrosc. 2022, 2022, 7238044. [Google Scholar] [CrossRef]
- Kelemen, S.R.; Fang, H.L. Maturity Trends in Raman Spectra from Kerogen and Coal. Energy Fuels 2001, 15, 653–658. [Google Scholar] [CrossRef]
- Beyssac, O.; Goffé, B.; Chopin, C.; Rouzaud, J.N. Raman Spectra of Carbonaceous Material in Metasediments: A New Geothermometer. J. Metamorph. Geol. 2002, 20, 859–871. [Google Scholar] [CrossRef]
- Sauerer, B.; Craddock, P.R.; AlJohani, M.D.; Alsamadony, K.L.; Abdallah, W. Fast and Accurate Shale Maturity Determination by Raman Spectroscopy Measurement with Minimal Sample Preparation. Int. J. Coal Geol. 2017, 173, 150–157. [Google Scholar] [CrossRef]
- Wopenka, B.; Pasteris, J.D. Structural Characterization of Kerogens to Granulite-Facies Graphite: Applicability of Raman Microprobe Spectroscopy. Am. Mineral. 1993, 78, 533–557. [Google Scholar]
- Wang, J.; Liu, Y.; Yang, C.; Jiang, W.; Li, Y.; Xiong, Y.; Peng, P. Evolution of Mechanical Properties of Kerogen with Thermal Maturity. Mar. Pet. Geol. 2022, 145, 105906. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef]
- Guedes, A.; Valentim, B.; Prieto, A.C.; Rodrigues, S.; Noronha, F. Micro-Raman Spectroscopy of Collotelinite, Fusinite and Macrinite. Int. J. Coal Geol. 2010, 83, 415–422. [Google Scholar] [CrossRef]
- Khatibi, S.; Ostadhassan, M.; Tuschel, D.; Gentzis, T.; Carvajal-Ortiz, H. Evaluating Molecular Evolution of Kerogen by Raman Spectroscopy: Correlation with Optical Microscopy and Rock-Eval Pyrolysis. Energies 2018, 11, 1406. [Google Scholar] [CrossRef]
- Agrawal, V.; Sharma, S. Molecular Characterization of Kerogen and Its Implications for Determining Hydrocarbon Potential, Organic Matter Sources and Thermal Maturity in Marcellus Shale. Fuel 2018, 228, 429–437. [Google Scholar] [CrossRef]
- Witte, E.G.; Schenk, H.J.; Müller, P.J.; Schwochau, K. Structural Modifications of Kerogen during Natural Evolution as Derived from 13C CP/MAS NMR, IR Spectroscopy and Rock-Eval Pyrolysis of Toarcian Shales. Org. Geochem. 1988, 13, 1039–1044. [Google Scholar] [CrossRef]
- Takai, K.; Oga, M.; Sato, H.; Enoki, T.; Ohki, Y.; Taomoto, A.; Suenaga, K.; Iijima, S. Structure and Electronic Properties of a Nongraphitic Disordered Carbon System and Its Heat-Treatment Effects. Phys. Rev. B 2003, 67, 214202. [Google Scholar] [CrossRef]
- Cançado, L.G.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.A.; Mizusaki, H.; Speziali, N.L.; Jorio, A.; Pimenta, M.A. Measuring the Degree of Stacking Order in Graphite by Raman Spectroscopy. Carbon N. Y. 2008, 46, 272–275. [Google Scholar] [CrossRef]
- Graf, D.; Molitor, F.; Ensslin, K.; Stampfer, C.; Jungen, A.; Hierold, C.; Wirtz, L. Spatially Resolved Raman Spectroscopy of Single- and Few-Layer Graphene. Nano Lett. 2007, 7, 238–242. [Google Scholar] [CrossRef]
- Toboła, T.; Botor, D. Raman Spectroscopy of Organic Matter and Rare Minerals in the Kłodawa Salt Dome (Central Poland) Cap-Rock and Triassic Cover—Indicators of Hydrothermal Solution Migration. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 231, 118121. [Google Scholar] [CrossRef]
- Beyssac, O.; Rouzaud, J.-N.N.; Goffé, B.; Brunet, F.; Chopin, C. Graphitization in a High-Pressure, Low-Temperature Metamorphic Gradient: A Raman Microspectroscopy and HRTEM Study. Contrib. Mineral. Petrol. 2002, 143, 19–31. [Google Scholar] [CrossRef]
- Kouketsu, Y.; Mizukami, T.; Mori, H.; Endo, S.; Aoya, M.; Hara, H.; Nakamura, D.; Wallis, S. A New Approach to Develop the Raman Carbonaceous Material Geothermometer for Low-Grade Metamorphism Using Peak Width. Isl. Arc 2014, 23, 33–50. [Google Scholar] [CrossRef]
- Clough, A.; Sigle, J.L.; Jacobi, D.; Sheremata, J.; White, J.L. Characterization of Kerogen and Source Rock Maturation Using Solid-State NMR Spectroscopy. Energy Fuels 2015, 29, 6370–6382. [Google Scholar] [CrossRef]
- Solli, H.; Van Graas, G.; Leplat, P.; Krane, J. Analysis of Kerogens of Miocene Shales in a Homogenous Sedimentary Column. A Study of Maturation Using Flash Pyrolysis Techniques and Carbon-13 CP-MAS NMR. Org. Geochem. 1984, 6, 351–358. [Google Scholar] [CrossRef]
- Spangenberg, J.E. Geochemistry of the Organic Mineral Evenkite in Septarian Concretions in the Oxfordian Marls of the French Alps. Mineral. Mag. 1998, 62A, 1436–1437. [Google Scholar] [CrossRef]
- Skropyshev, A.V. A Paraffin from a Polymetallic Vein. Dokl. Acad. Nauk. SSSR 1953, 88, 717–719. [Google Scholar]
- Manuella, F.C. Serpentinization and Abiogenic Production of Hydrocarbons in the Ultramafic Basement of Hyblean Plateau: Mineralogical and Petrological Inferences from Xenoliths. Plinius 2010, 36, 1–6. [Google Scholar]
- Kotel’nikova, E.N.; Filatov, S.K. Crystal Chemistry of Paraffins: Research Methods, Results, and Behavior in Nature; Neva Publishing House: Saint Petersburg, Russia, 2002; 352p. [Google Scholar]
- Chazhengina, S.Y.; Kotelnikova, E.N.; Filippova, I.V.; Filatov, S.K. Phase Transitions of N-Alkanes as Rotator Crystals. J. Mol. Struct. 2003, 647, 243–257. [Google Scholar] [CrossRef]
- Platonova, N.V.; Kotel’nikova, E.N. Synthesis of the Organic Mineral Evenkite. Geol. Ore Depos. 2007, 49, 638–640. [Google Scholar] [CrossRef]
- Kotel’nikova, E.N.; Platonova, N.V.; Filatov, S.K. Identification of Biogenic Paraffins and Their Thermal Phase Transitions. Geol. Ore Depos. 2007, 49, 697–709. [Google Scholar] [CrossRef]
- Kruťa, T. O Nerostech z Ostravsko-Karvinského Revíru. Přirodověd. Sborn. Ostrav. Kraj. 1951, 12, 451–486. (In Czech) [Google Scholar]
- Houzar, S.; Hršelová, P.; Gilíková, H.; Buriánek, D.; Nehyba, S. Přehled Historie Výzkumů Permokarbonských Sedimentů Jižní 1204 Části Boskovické Brázdy (Část 2. Geologie a Petrografie). Acta Musei Morav. Sci. Geol. 2017, 102, 3–65. (In Czech) [Google Scholar]
- Welser, P.; Slobodník, M.; Malek, O.; Osovský, M.; Jirásek, J.; Matýsek, D. Epigenetic Minerals From the Czech Part of the Upper Silesian Basin. Acta Musei Morav. Sci. Geol. 2021, 106, 81–123. [Google Scholar]
- Houzar, S.; Hršelová, P. Přehled Historie Výzkumů Permokarbonských Sedimentů Jižní Části Boskovické Brázdy (Část 1. 1208 Historie Dolování a Mineralogie). Acta Musei Morav. Sci. Geol. 2016, 101, 3–32. (In Czech) [Google Scholar]
- Konnerup-Madsen, J.; Kreulen, R.; Rose-Hansen, J. Stable Isotope Characteristics of Hydrocarbon Gases in the Alkaline Ilímaussaq Complex, South Greenland. Bull. Mineral. 1988, 111, 567–576. [Google Scholar] [CrossRef]
- Kutcherov, V.G.; Krayushkin, V.A. Deep-Seated Abiogenic Origin of Petroleum: From Geological Assessment to Physical Theory. Rev. Geophys. 2010, 48, 1–30. [Google Scholar] [CrossRef]
- Kutcherov, V.G. Abiogenic Deep Origin of Hydrocarbons and Oil and Gas Deposits Formation; Kutcherov, V., Kolesnikov, A., Eds.; IntechOpen: Rijeka, Croatia, 2013; Chapter 1. [Google Scholar] [CrossRef]
- Colombo, J.C.; Pelletier, E.; Brochu, C.; Khalil, M.; Catoggio, J.A. Determination of Hydrocarbon Sources Using N-Alkane and Polyaromatic Hydrocarbon Distribution Indexes. Case Study: Rio de La Plata Estuary, Argentina. Environ. Sci. Technol. 1989, 23, 888–894. [Google Scholar] [CrossRef]
- Sakari, M.; Zakaria, M.P.; Lajis, N.H.; Mohamed, C.A.R.; Abdullah, M.H. Reconstruction of Aliphatic Hydrocarbons History and Sources from Sedimentary Record of the Johor Strait, Malaysia. Coast. Mar. Sci. 2012, 35, 142–152. [Google Scholar]
- Eglinton, G.; Gonzalez, A.G.; Hamilton, R.J.; Raphael, R.A. Hydrocarbon Constituents of the Wax Coatings of Plant Leaves: A Taxonomic Survey. Phytochemistry 1962, 1, 89–102. [Google Scholar] [CrossRef]
- Peybernès, B. The Jurassic of Tunisia: An Attempt at Reconstruction of the South Neotethyan Margin during and after the Rifting Phase. In The Geology of Libya; Salem, M.J., Busrewil, M.T., Ben Ashour, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 4, pp. 1681–1709. [Google Scholar]
- Abdesselam-mahdaoui, S.B.E.N.; Benzaggagh, M.; Bouhlel, S. Nouvelles Données Biostratigraphiques Sur Les Niveaux de Passage Jurassique- Crétacéet Les Calcaires Du Campanien Dans Le Secteur de Hammam Zriba-Jebel Guebli (Tunisie Septentrionale). Geo-Eco-Trop 2010, 34, 113–126. [Google Scholar]
- El Nemr, A.; Moneer, A.A.; Ragab, S.; El Sikaily, A. Distribution and Sources of N-Alkanes and Polycyclic Aromatic Hydrocarbons in Shellfish of the Egyptian Red Sea Coast. Egypt. J. Aquat. Res. 2016, 42, 121–131. [Google Scholar] [CrossRef]
- Choudhary, P.; Routh, J.; Chakrapani, G.J. Organic Geochemical Record of Increased Productivity in Lake Naukuchiyatal, Kumaun Himalayas, India. Environ. Earth Sci. 2010, 60, 837–843. [Google Scholar] [CrossRef]
- Zakaria, M.P.; Bong, C.-W.; Vaezzadeh, V. Chapter 16—Fingerprinting of Petroleum Hydrocarbons in Malaysia Using Environmental Forensic Techniques: A 20-Year Field Data Review; Stout, S.A., Wang, Z., Eds.; Butterworth-Heinemann: Oxford, UK, 2018; pp. 345–372. [Google Scholar] [CrossRef]
- Geddes, C.D.; Lakowicz, J.R.; Blamey, N.J.F.; Ryder, A.G. Hydrocarbon Fluid Inclusion Fluorescence: A Review. Rev. Fluoresc. 2007, 2009, 299–334. [Google Scholar]
- Moser, M.R.; Rankin, A.H.; Milledge, H.J. Hydrocarbon-Bearing Fluid Inclusions in Fluorite Associated with the Windy Knoll Bitumen Deposit, UK. Geochim. Cosmochim. Acta 1992, 56, 155–168. [Google Scholar] [CrossRef]
- Lenoir, L.; Blaise, T.; Chourio-Camacho, D.; Richard, A.; Tarantola, A.; Agrinier, P.; Rigaudier, T.; Monvoisin, G.; Bardoux, G.; Brigaud, B.; et al. The Origin of Fluorite-Barite Mineralization at the Interface between the Paris Basin and Its Variscan Basement: Insights from Fluid Inclusion Chemistry and Isotopic (O, H, Cl) Composition. Miner. Depos. 2024, 59, 397–417. [Google Scholar] [CrossRef]
- Machel, H.G. Bacterial and Thermochemical Sulfate Reduction in Diagenetic Settings—Old and New Insights. Sediment. Geol. 2001, 140, 143–175. [Google Scholar] [CrossRef]
- Sverjensky, D.A. Oil Field Brines as Ore-Forming Solutions. Econ. Geol. 1984, 79, 23–37. [Google Scholar] [CrossRef]
- Surdam, R.C.; Crossey, L.J. Organic-Inorganic Reactions during Progressive Burial: Key to Porosity and Permeability Enhancement and Preservation. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1985, 315, 135–156. [Google Scholar]
- Smith, J.T.; Ehrenberg, S.N. Correlation of Carbon Dioxide Abundance with Temperature in Clastic Hydrocarbon Reservoirs: Relationship to Inorganic Chemical Equilibrium. Mar. Pet. Geol. 1989, 6, 129–135. [Google Scholar] [CrossRef]
- Giordano, T.H. Metal Transport in Ore Fluids by Organic Ligand Complexation BT—Organic Acids in Geological Processes; Pittman, E.D., Lewan, M.D., Eds.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 319–354. [Google Scholar] [CrossRef]
- Di, J.; Ding, X. Complexation of REE in Hydrothermal Fluids and Its Significance on REE Mineralization. Minerals 2024, 14, 531. [Google Scholar] [CrossRef]
- González-Partida, E.; Carrillo-Chávez, A.; Grimmer, J.O.W.; Pironon, J. Petroleum-Rich Fluid Inclusions in Fluorite, Purisima Mine, Coahuila, Mexico. Int. Geol. Rev. 2002, 44, 755–764. [Google Scholar] [CrossRef]
- Bouabdellah, M.; Castorina, F.; Bodnar, R.J.; Banks, D.; Jébrak, M.; Prochaska, W.; Lowry, D.; Klügel, A.; Hoernle, K. Petroleum Migration, Fluid Mixing, and Halokinesis as the Main Ore-Forming Processes at the Peridiapiric Jbel Tirremi Fluorite-Barite Hydrothermal Deposit, Northeastern Morocco. Econ. Geol. 2014, 109, 1223–1256. [Google Scholar] [CrossRef]
- Belayouni, H. Oil Seeps and Associated Phenomena in Northern Tunisia. In Proceedings of the IIIème Journées de Géologie Tunisienne Appliquées à la Recherche des Hydrocarbures ETAP, Tunis, Tunisia, 8–9 May 1992; p. 18. [Google Scholar]
- Frizon de Lamotte, D.; Saint Bezar, B.; Bracène, R.; Mercier, E. The Two Main Steps of the Atlas Building and Geodynamics of the Western Mediterranean. Tectonics 2000, 19, 740–761. [Google Scholar] [CrossRef]
- Bouhlel, S. Gitologie, Mineralogie et Essai de Modélisation des Minéralisations à F-Ba-Sr-Pb-Zn-(S) Associées aux Carbonates (Jurassiques et Crétacées) et aux Diapirs Triassiques: Gisements de Stah-Kohol, Zriba-Guebli, Bou Jaber et Fedj-el-Adoum (Tunisie Septentrionale). Ph.D. Thesis, Faculty of Sciences of Tunis (FST), University of Tunis, Tunis, Tunisia, 1993; p. 303. Available online: https://www.researchgate.net/publication/277300260 (accessed on 27 September 2021).
- Paradis, S.; Hannigan, P.; Dewing, K. Mississippi Valley-Type Lead-Zinc Deposits. Miner. Depos. Canada A Synth. Major Depos. Dist. Metallog. Evol. Geol. Prov. Explor. Methods Geol. Assoc. Canada Miner. Depos. Div. Spec. Publ. 2007, 5, 185–203. [Google Scholar]
- De Graaf, S.; Lüders, V.; Banks, D.A.; Sośnicka, M.; Reijmer, J.J.G.; Kaden, H.; Vonhof, H.B. Fluid Evolution and Ore Deposition in the Harz Mountains Revisited: Isotope and Crush-Leach Analyses of Fluid Inclusions. Miner. Depos. 2020, 55, 47–62. [Google Scholar] [CrossRef]

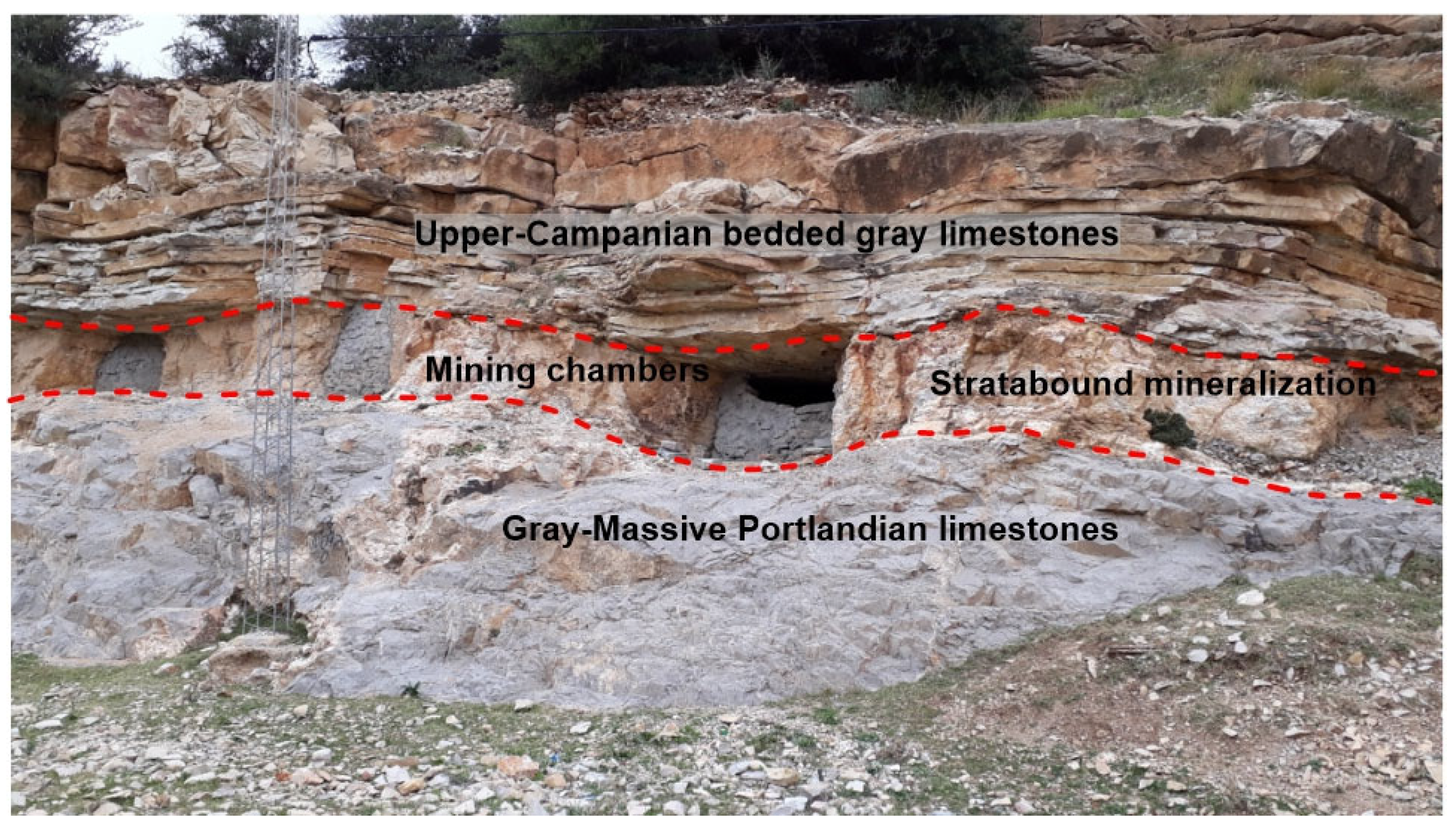
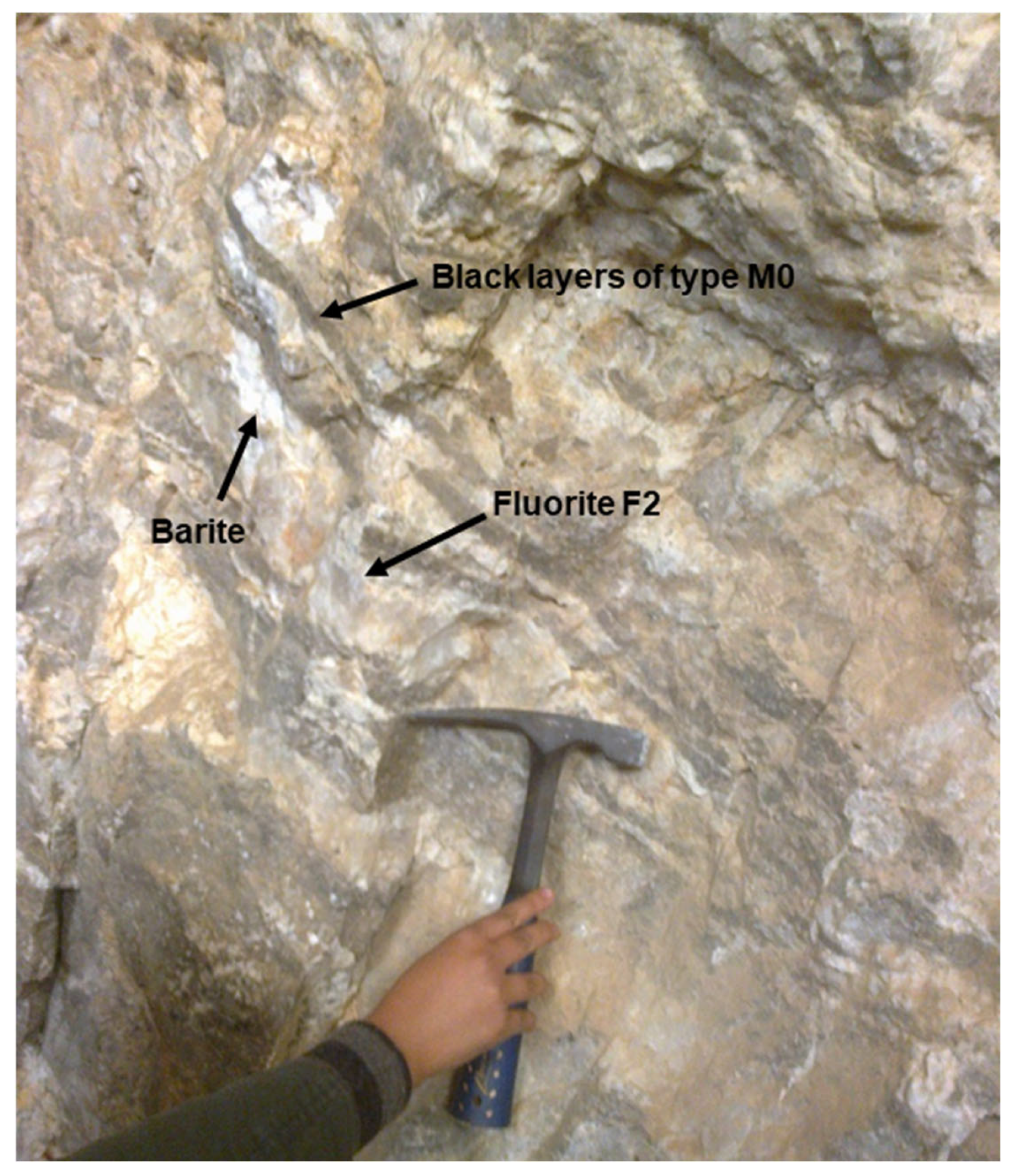




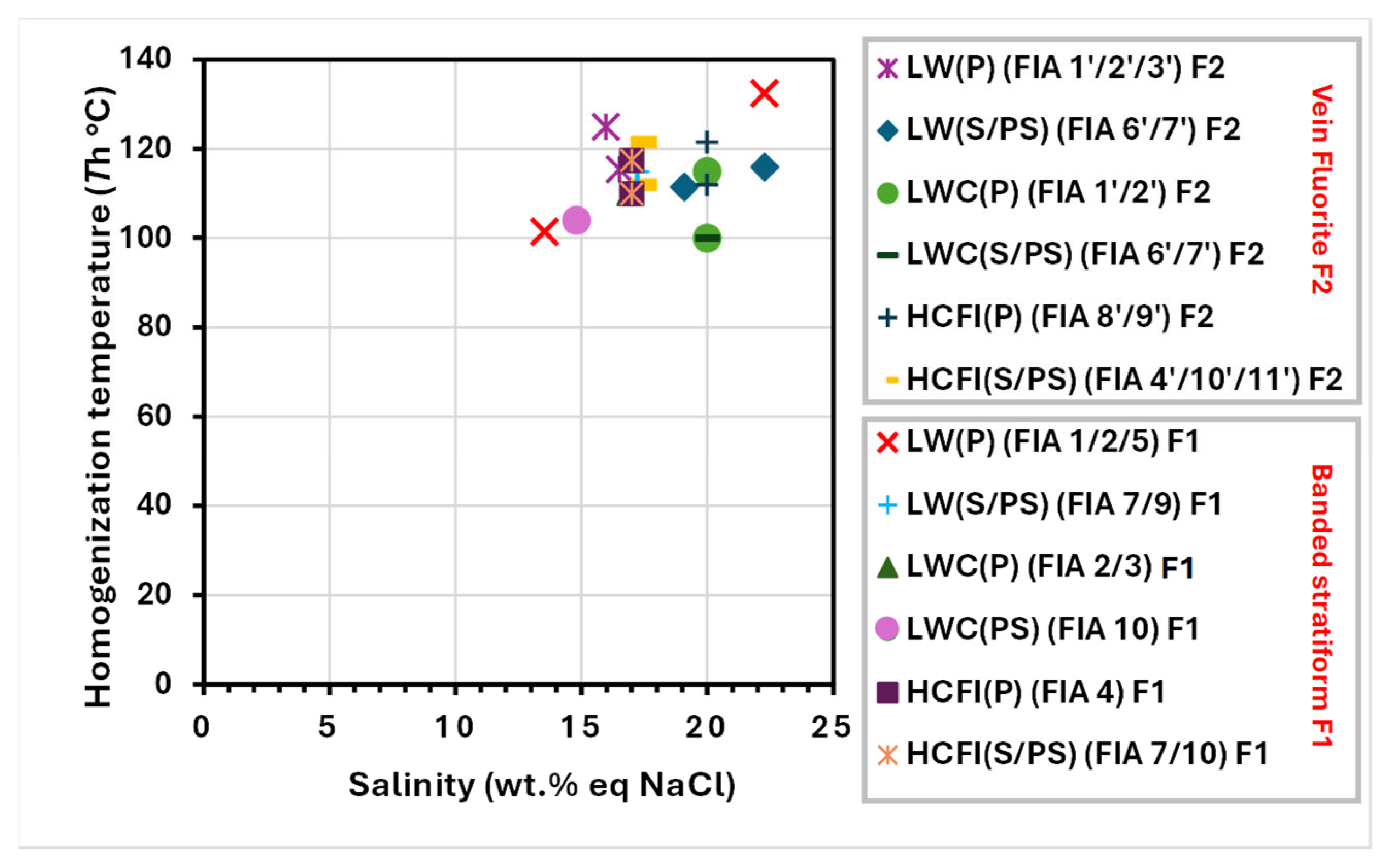


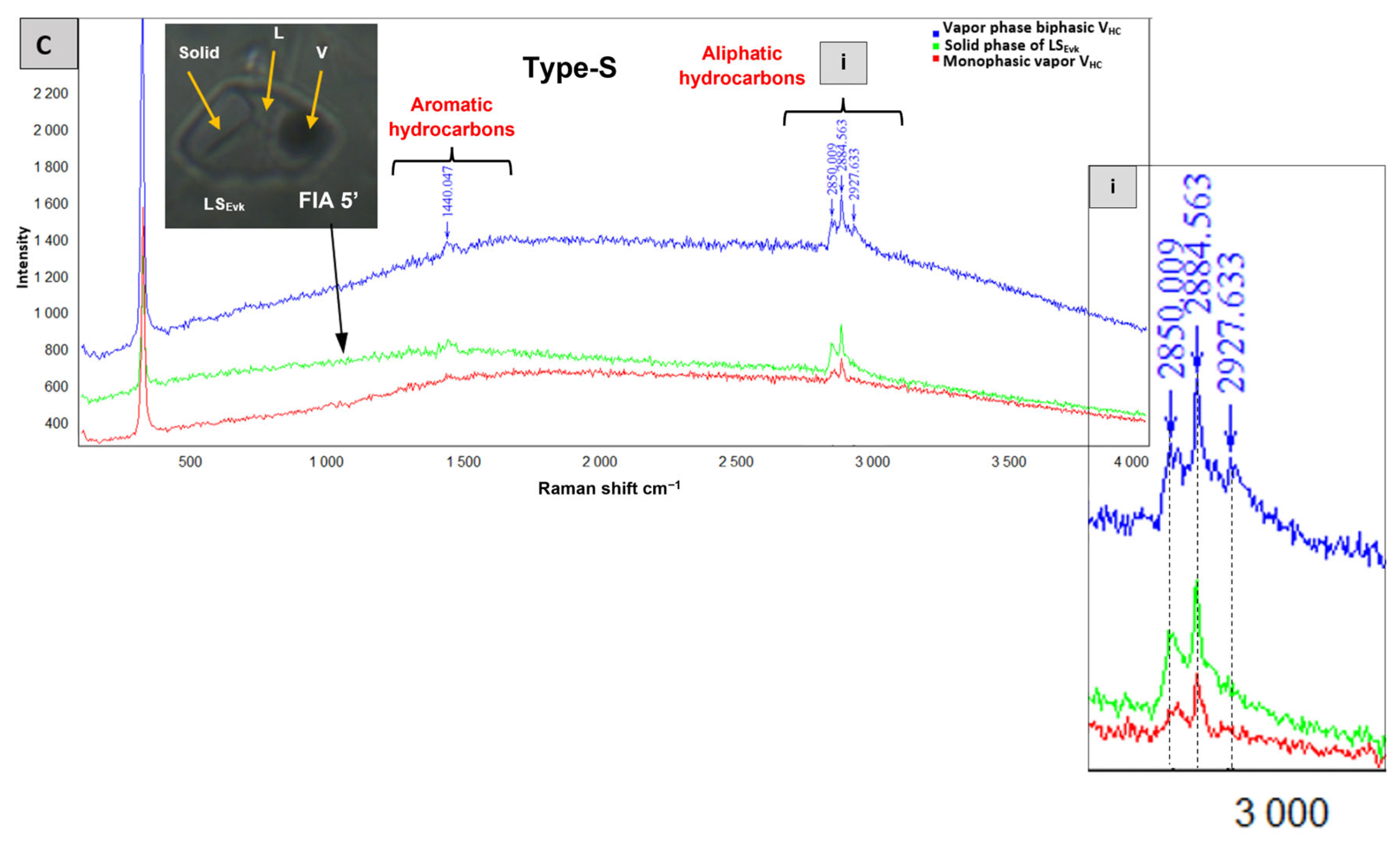
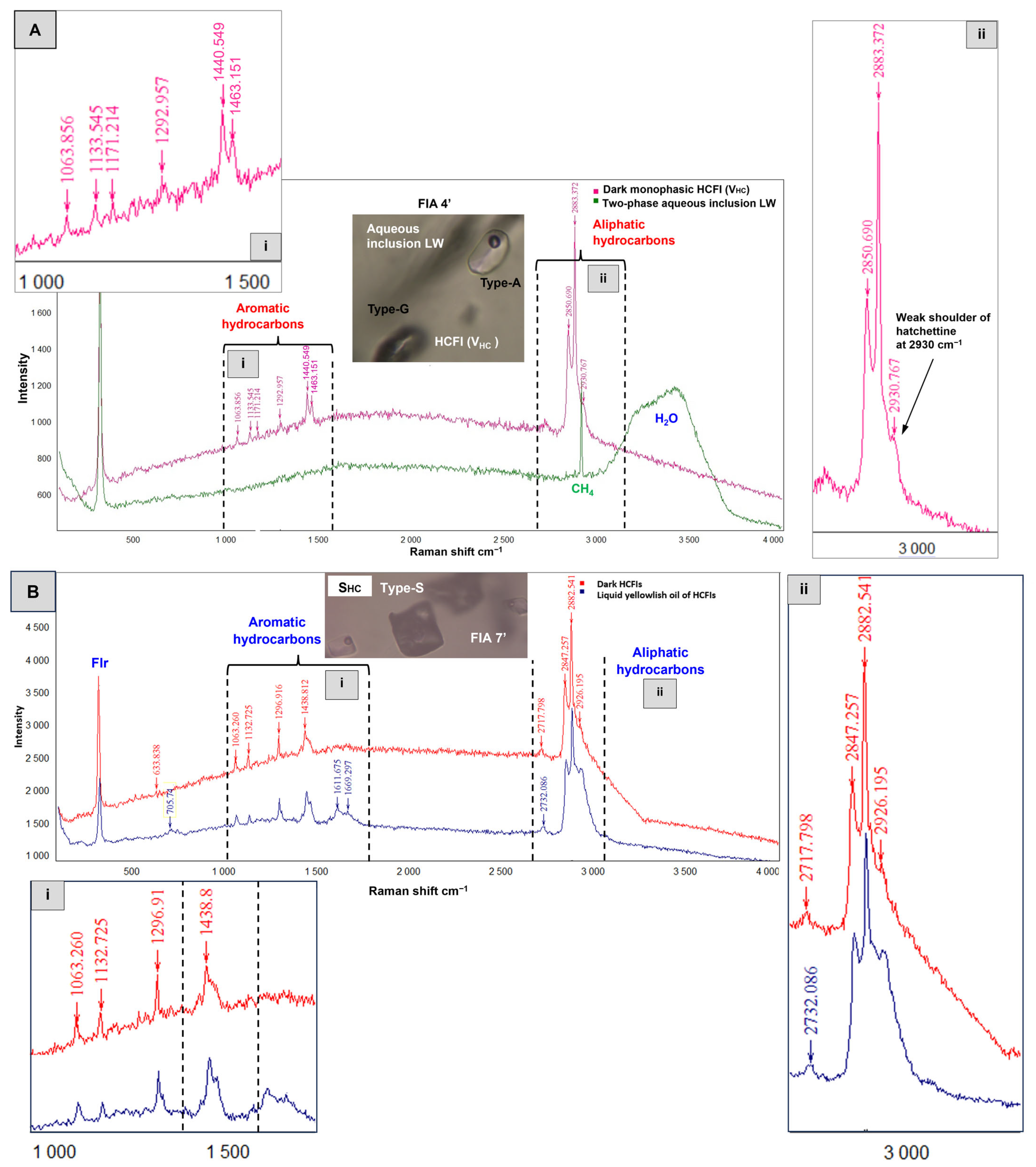


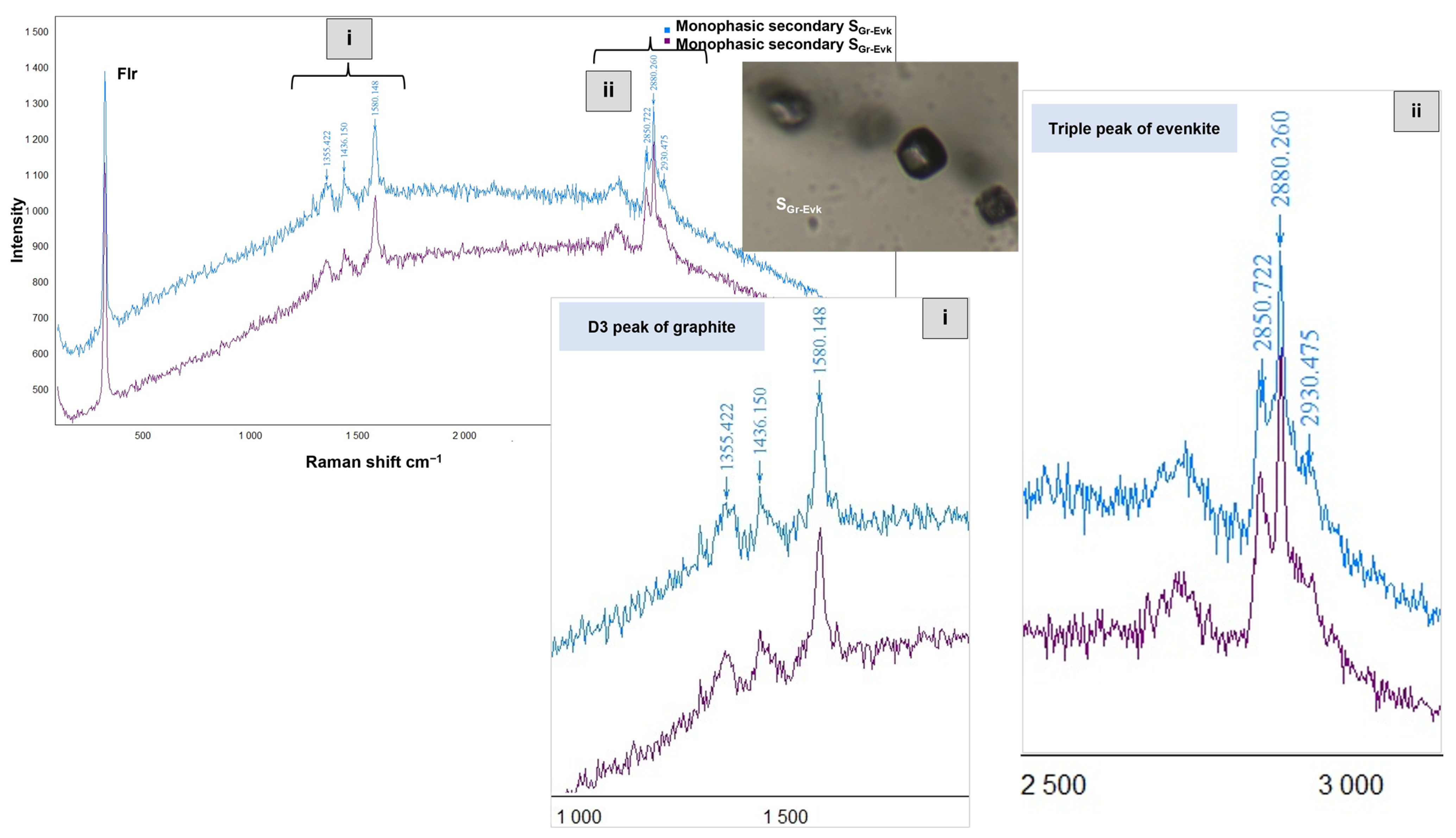
| Orebody (Sample Reference) | N° of Studied FIAs | N° of FIs Studied Within FIAs | FIs Type | Occurrence | Te (°C) | TmCO2 (°C) | Tmice (°C) | TmClathr (°C) | wt.% eq NaCl | Th Mode | ThCO2 (°C) | Thtotal (°C) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vein F2 (sample HZF2b) | FIA 1′ | 22 | LW | P | −50 to −52 | – | −12 | 15.95 | L | – | 110–140 | |
| FIA 3′ | 11 | LW | P | 50 to −52 | – | −12.1 | 16.5 | L | – | 112–119 | ||
| FIA 6′ | 20 | LW | PS | 50 to −52 | – | −15.6 | 19.1 | L | – | 90–133 | ||
| FIA 2′/7′/6′ | 15 | LW | P/S/PS | 50 to −52 | – | −19.9 | 22.3 | L | – | 95–137 | ||
| FIA 1′ | 15 | LWC | P | 50 to −52 | −70/−60.5 | −13.9 | −3.9 | 20 | L | – | 100–130 | |
| FIA 2′ | 13 | LWC | P | 50 to −52 | −70/−60.5 | −9.7 to−9.5 | −3.9 | 20 | L | – | 100 | |
| FIA 6′/7′ | 12 | LWC | S/PS | 50 to −52 | −70/−60.5 | – | −3.9 | 20 | L | – | – | |
| FIA 2′/4′ | 10 | VC | P/PS/S | – | −70/−60.5 | – | – | V | 25 to 29 | – | ||
| FIA 5′ | 11 | VC | P | – | −70/−60.5 | – | −1.2 | – | V | 24 to 28 | – | |
| FIA 10′ | 7 | LHCL2V | PS/S | 50 to −52 | – | −24.2 | −1.2 | 17 | L | – | 98–145 | |
| FIA 11′/4′ | 8 | LHCV | PS/S | 50 to −52 | – | −22.5 | −1.1 | 17 | L | – | 87–137 | |
| FIA 8′ | 17 | LHCL2V | P | 50 to −52 | −24.2 | −3.9 | 20 | L | – | 98–145 | ||
| FIA 9′ | 15 | LHCV | P | 50 to −52 | −22.5 | −3.9 | 20 | L | – | 87–137 | ||
| Banded stratiform F1 (sample HZMR18) | FIA 1/2 | 43 | LW | P | 50 to −52 | – | −9.7 to −9.5 | −2.4 | 13.6/13.4 | L | – | 90–113 |
| FIA 7/9 | 12 | LW | S/PS | 50 to −52 | – | −13.2 to−13.3 | −2.4 | 17.1/17.2 | L | – | 110–120 | |
| FIA 9 | 6 | LW | PS | 50 to −52 | – | −13.4 | −2.4 | 17.25 | L | – | 115 | |
| FIA 7 | 3 | LW | S | 50 to −52 | – | – | −2.4 | 3.3 | L | – | – | |
| FIA 10 | 21 | LWC | PS | 50 to −52 | −70/−60.5 | −10.8 | −2.4 | 14.8 | L | – | 95–113 | |
| FIA 3/2 | 43 | LWC | P | 50 to −52 | −70/−60.5 | −13 | −2.4 | 16.9 | L | – | 110 | |
| FIA 5 | 52 | LW | P | 50 to −52 | – | −19.9 | −2.4 | 22.3 | L | – | 115–150 | |
| FIA 8/9 | 11 | VC | S/PS | – | −70/−60.5 | – | −1.2 | V | 22.8 to 28.5 | – | ||
| FIA 1/2 | 9 | VC | P | – | −70/−60.5 | – | V | 22.8 to 28.5 | – | |||
| FIA 4 | 8 | LHCL2V | P | 50 to −52 | – | −24.2 | −1.2 | 17 | L | – | 90–145 | |
| FIA 4 | 7 | LHCV | P | 50 to −52 | – | −22.5 | −1.1 | 17 | L | – | 85–135 | |
| FIA 10/7 | 8 | LHCL2V | PS/S | 50 to −52 | – | −24.2 | −1.2 | 17 | L | – | 90–145 | |
| FIA 10/7 | 7 | LHCV | PS/S | 50 to −52 | – | −22.5 | −1.2 | 17 | L | – | 85–135 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somrani, C.; Souissi, F.; De Giudici, G.; Guedes, A.; Ferrero, S. Fluid Inclusion Evidence of Deep-Sourced Volatiles and Hydrocarbons Hosted in the F–Ba-Rich MVT Deposit Along the Zaghouan Fault (NE Tunisia). Minerals 2025, 15, 489. https://doi.org/10.3390/min15050489
Somrani C, Souissi F, De Giudici G, Guedes A, Ferrero S. Fluid Inclusion Evidence of Deep-Sourced Volatiles and Hydrocarbons Hosted in the F–Ba-Rich MVT Deposit Along the Zaghouan Fault (NE Tunisia). Minerals. 2025; 15(5):489. https://doi.org/10.3390/min15050489
Chicago/Turabian StyleSomrani, Chaima, Fouad Souissi, Giovanni De Giudici, Alexandra Guedes, and Silvio Ferrero. 2025. "Fluid Inclusion Evidence of Deep-Sourced Volatiles and Hydrocarbons Hosted in the F–Ba-Rich MVT Deposit Along the Zaghouan Fault (NE Tunisia)" Minerals 15, no. 5: 489. https://doi.org/10.3390/min15050489
APA StyleSomrani, C., Souissi, F., De Giudici, G., Guedes, A., & Ferrero, S. (2025). Fluid Inclusion Evidence of Deep-Sourced Volatiles and Hydrocarbons Hosted in the F–Ba-Rich MVT Deposit Along the Zaghouan Fault (NE Tunisia). Minerals, 15(5), 489. https://doi.org/10.3390/min15050489










