Diagenesis and Mineralization of the Neoarchean Bushy Park Lead-Zinc Deposit, Northern Cape Province, South Africa
Abstract
1. Introduction
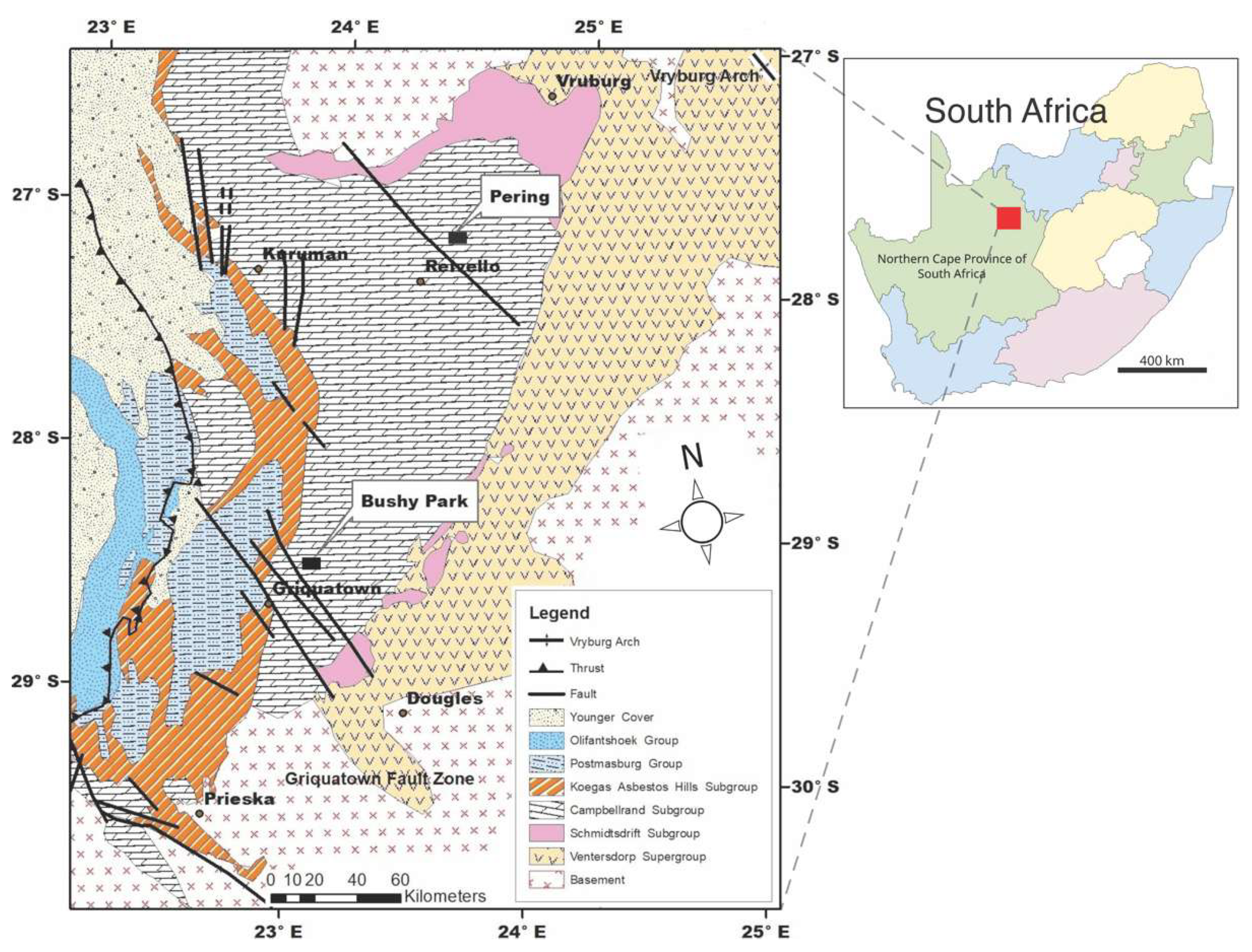
2. Geological Setting
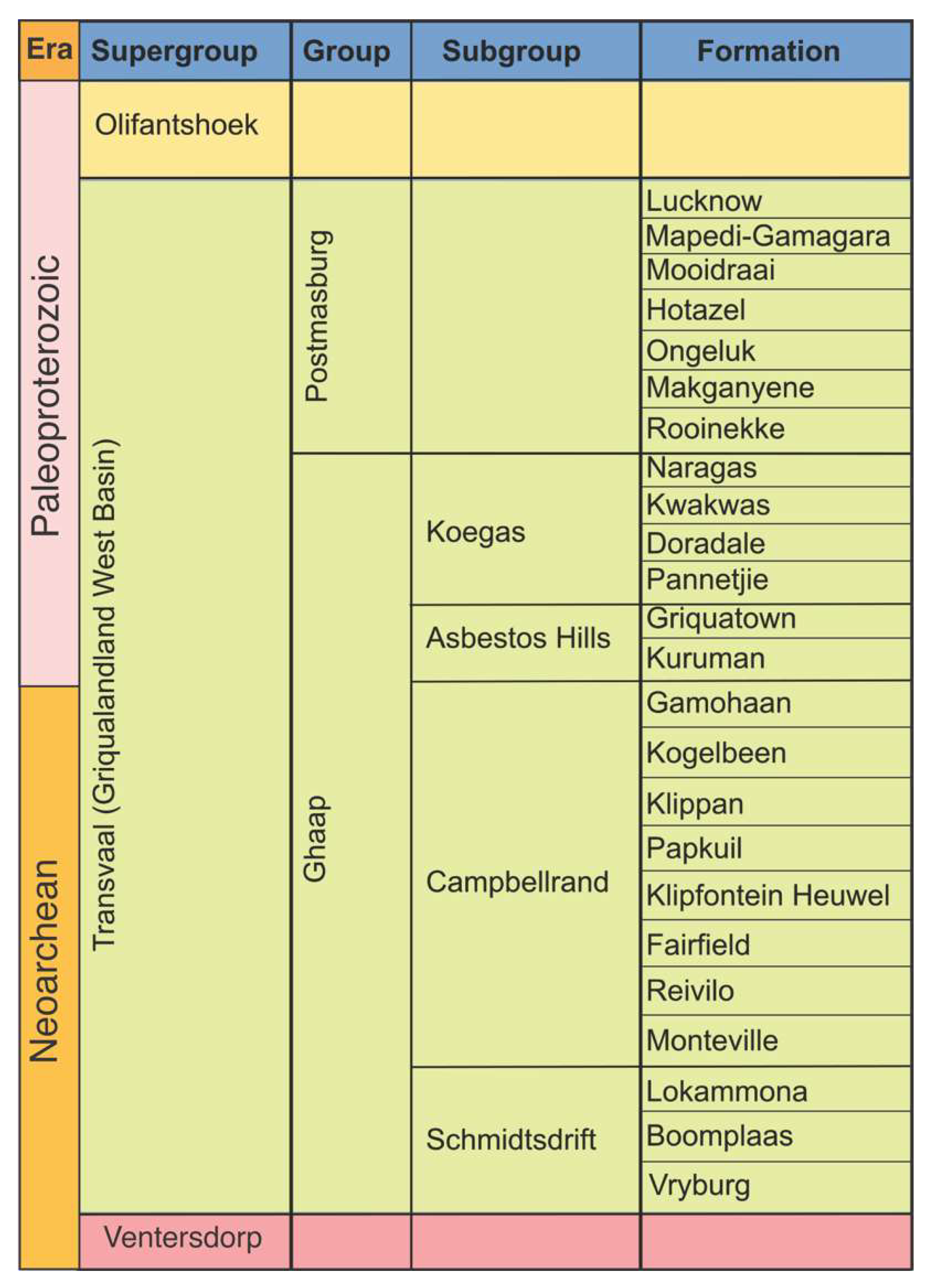
2.1. Tectonic Setting and Stratigraphy
2.2. Previous Work on Mineral Deposits in the Griqualand West Basin
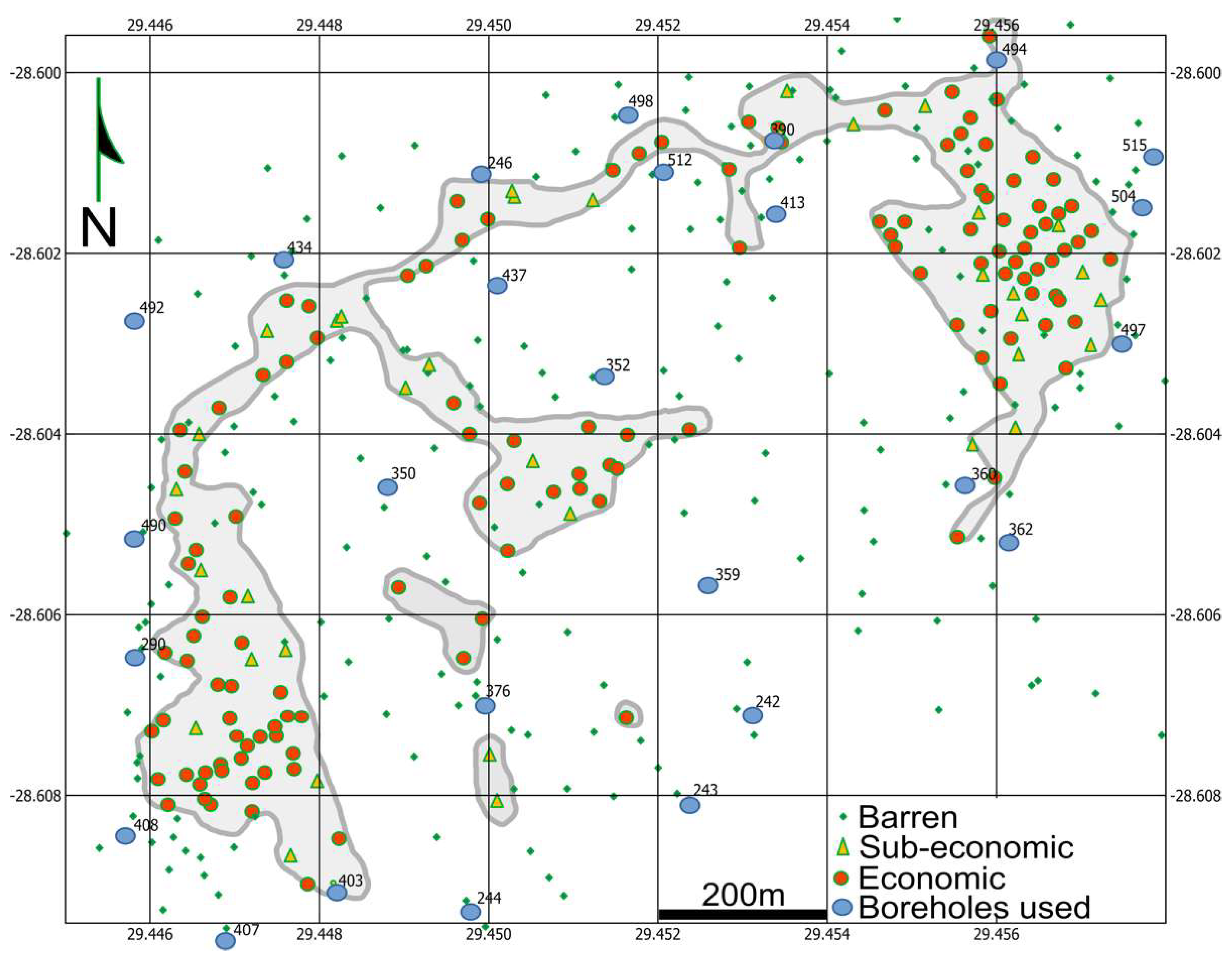
3. Materials and Methods
4. Results
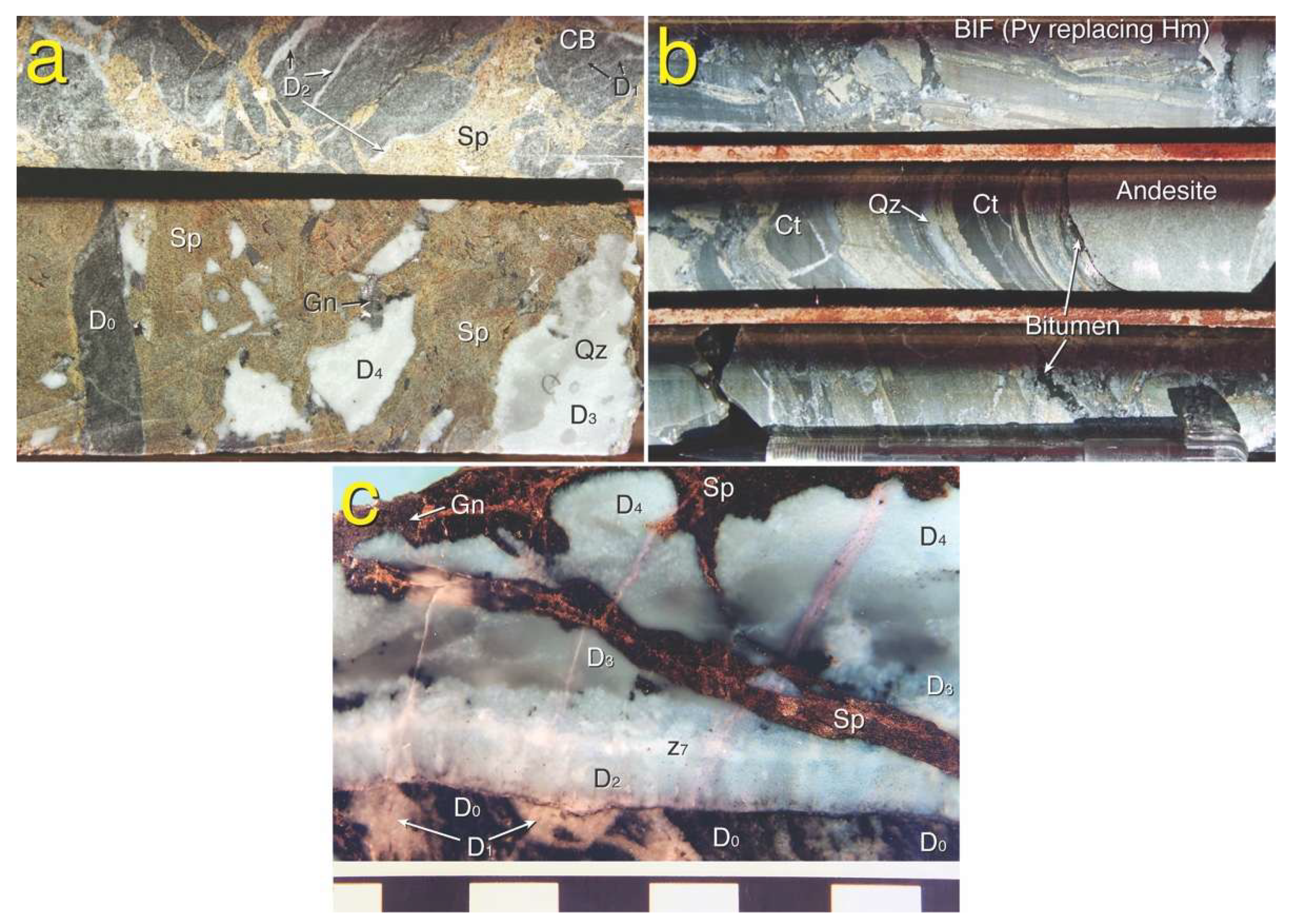
4.1. Breccias
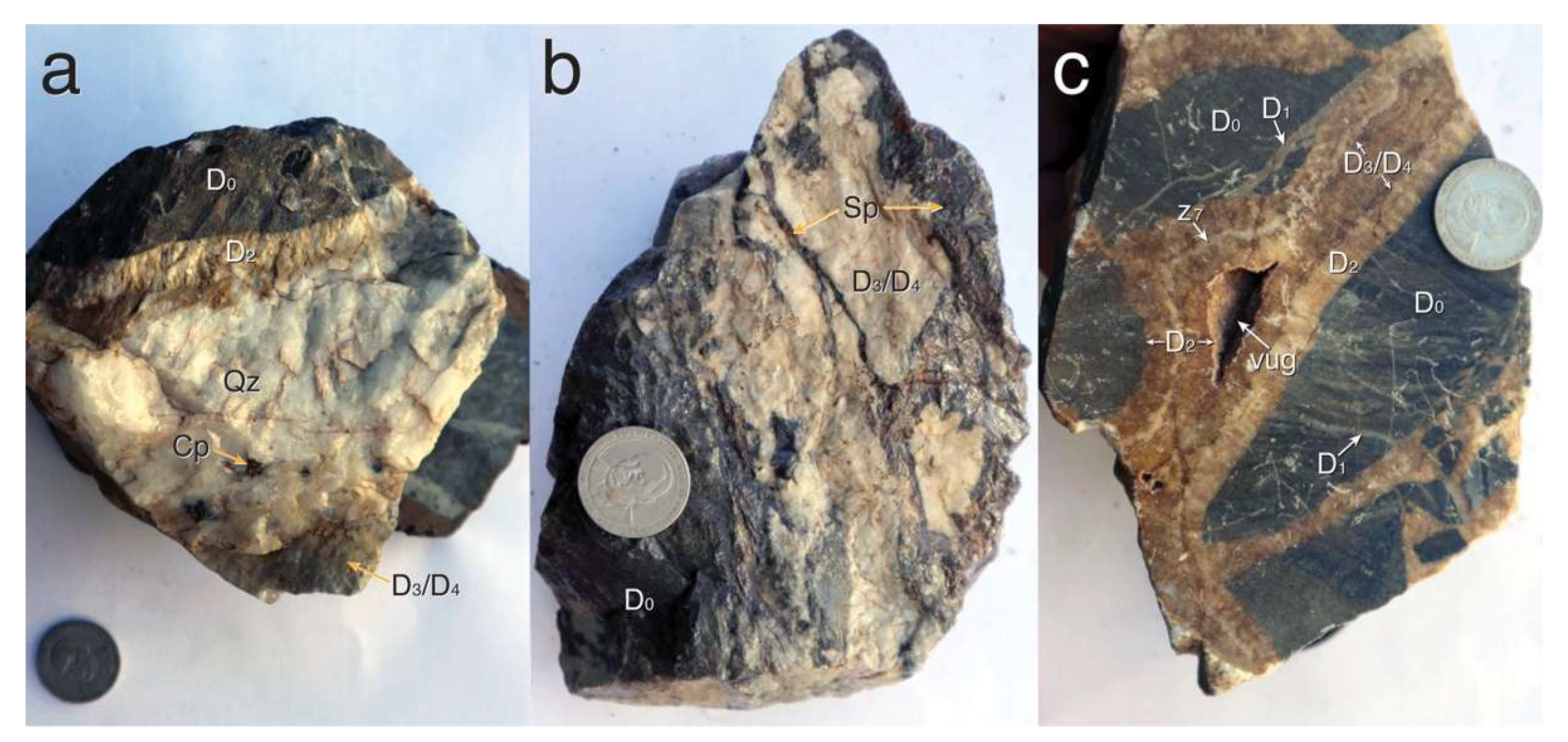
4.2. Hand Specimen Petrology
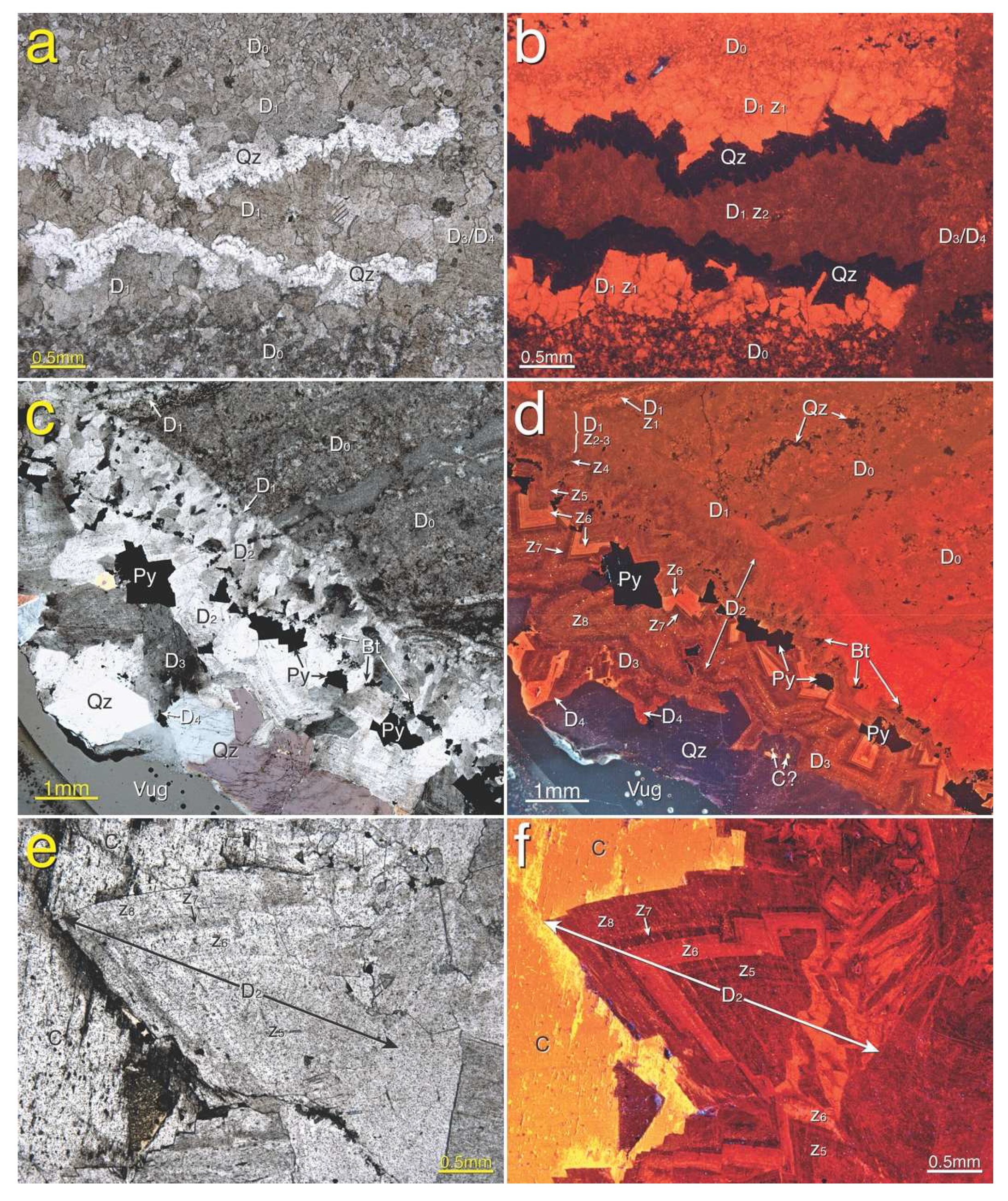
4.3. Petrography
4.3.1. Host Rock
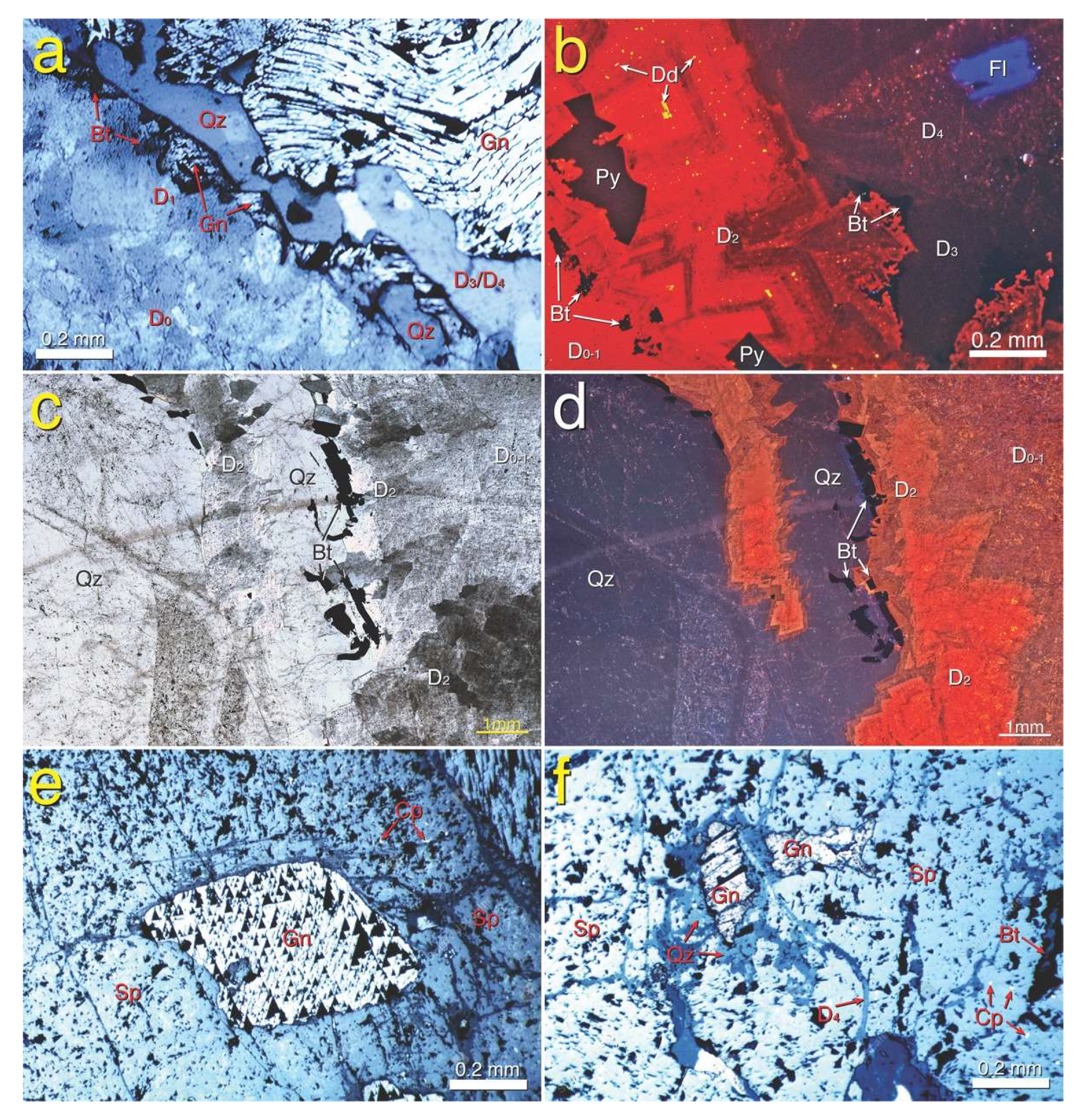
4.3.2. Gangue Minerals
4.3.3. Sulfides and Ore Minerals
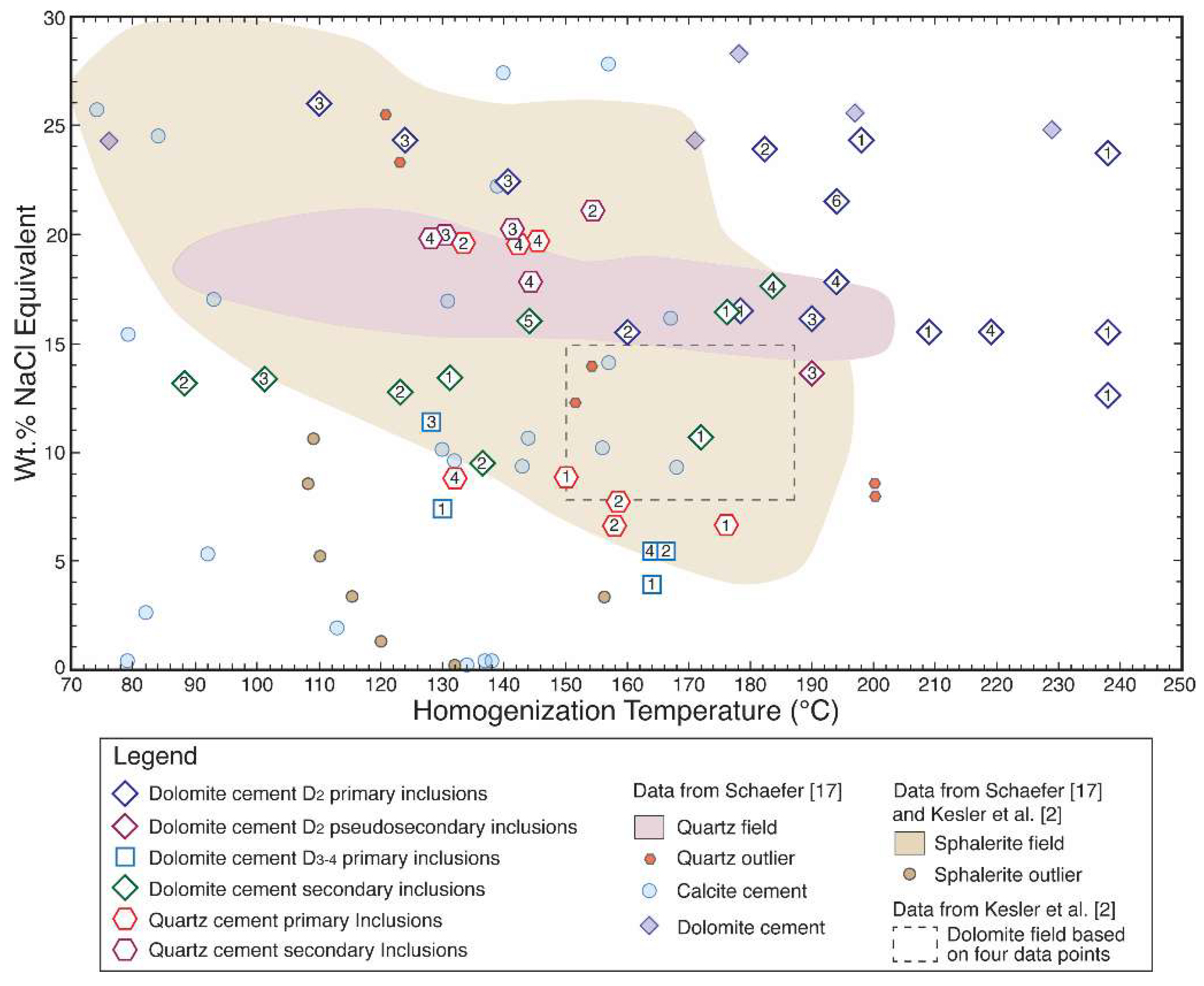
4.4. Fluid Inclusion Microthermometry
5. Discussion
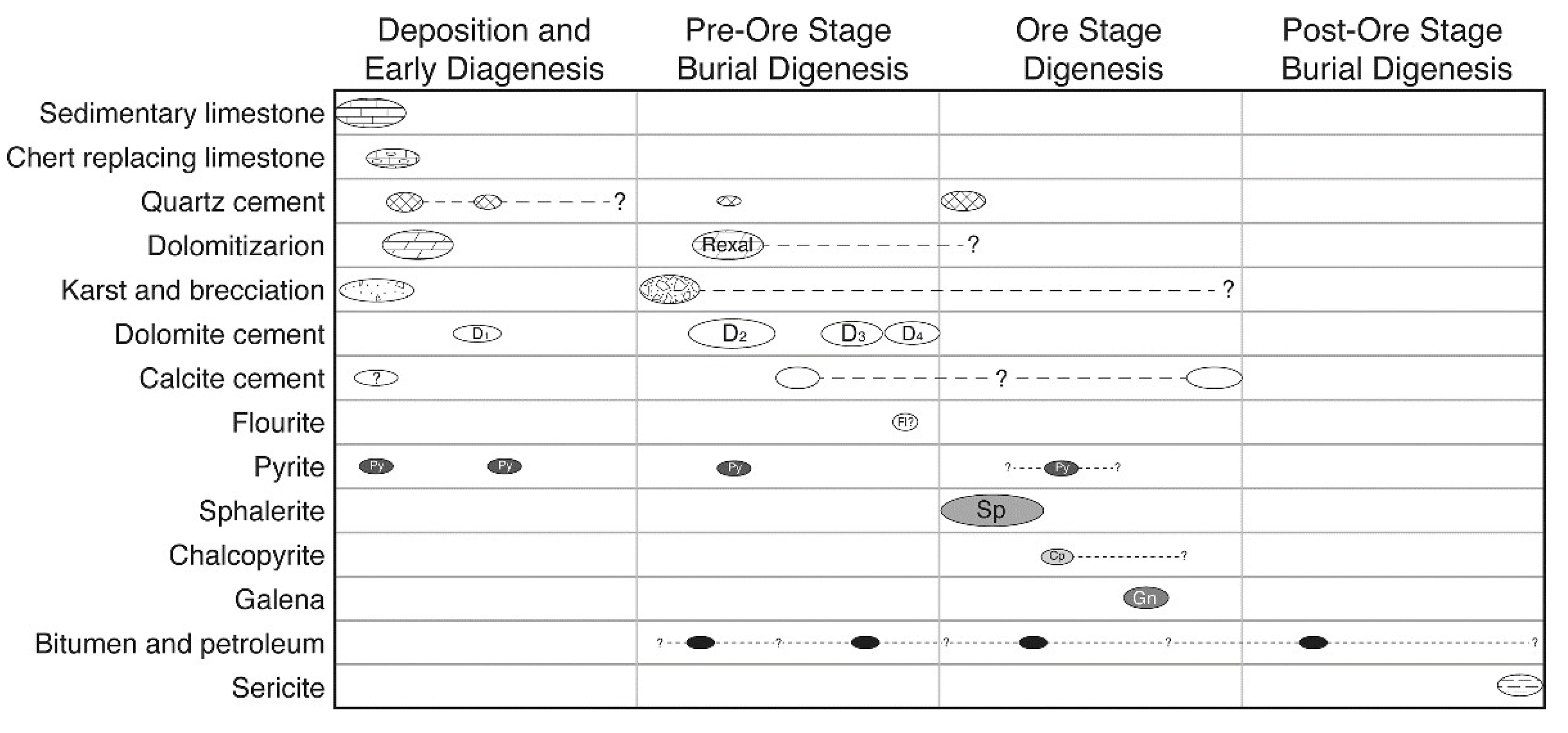
5.1. Paragenesis
5.2. Deposition and Early Diagenesis
5.3. Structurally Controlled Collapse Brecciation
5.4. Mineralizing Fluids
5.5. Timing of Mineralization at Bushy Park
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baugaard, W.D.; Gregg, J.M.; Ahler, B.A. Sequence stratigraphy and metre-scale cyclicity of Neoarchean dolomite strata at Bushy Park, Griqualand West Basin, Transvaal, South Africa. J. Afr. Earth Sci. 2024, 220, 1–16. [Google Scholar] [CrossRef]
- Kesler, S.E.; Reich, M.; Jean, M. Geochemistry of fluid inclusion brines from Earth’s oldest Mississippi Valley-type (MVT) deposits, Transvaal Supergroup, South Africa. Chem. Geol. 2007, 237, 274–288. [Google Scholar] [CrossRef]
- Anderson, G.M.; Macqueen, R.W. Mississippi Valley-Type Lead-Zinc Deposits, Ore Deposit Models. In Ore Deposit Models; Roberts, R.G., Sheahan, P.A., Eds.; Geoscience Canada Reprint Series 3; Geologican Association of Canada: Ottawa, ON, Canada, 1988; pp. 79–90. [Google Scholar]
- Sangster, D.F. (Ed.) Carbonate-Hosted Lead-Zinc Deposits, 75th Anniversary Volume; Special Publication 4; Society of Economic Geologists: Littleton, CO, USA, 1996; p. 664. [Google Scholar]
- Leach, D.L.; Bradley, D.C.; Huston, D.; Pisarevsky, S.A.; Taylor, R.D.; Gardoll, S.J. Sediment-Hosted Lead-Zinc Deposits in Earth History. Econ. Geol. 2010, 105, 593–625. [Google Scholar] [CrossRef]
- Gregg, J.M.; Shelton, K.L. Mississippi Valley-type mineralization and ore deposits in the Cambrian-Ordovician Great American Carbonate Bank. In The Great American Carbonate Bank: The Geology and Economic Resources of the Cambrian-Ordovician Sauk Megasequence of Laurentia; Derby, J.R., Fritz, R.D., Longacre, S.A., Morgan, W.A., Sternach, C.A., Eds.; Memoir 98; AAPG: Tulsa, OK, USA, 2012; pp. 163–186. [Google Scholar]
- Leach, D.L.; Sangster, D.F.; Kelley, K.D.; Large, R.R.; Garven, G.; Allen, C.; Gutzmer, J.; Walters, S. Sediment-hosted lead-zinc deposits: A global perspective. In Economic Geology One Hundredth Anniversary Volume 1905–2005; Hedenquist, J.W., Thompson, J.F.H., Goldfarb, R.J., Richards, J.P., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2005; pp. 561–607. [Google Scholar]
- Paradis, S.; Hannigan, P.; Dewing, K. Mississippi Valley-type lead-zinc deposits (MVT). In Mineral Deposits of Canada a Synthesis of Major Deposit-Types, District Metallogeny, the Evolution of Geological Provinces, and Exploration Methods; Goodfellow, W.D., Ed.; Special Publication 5; Geological Survey of Canada: Ottawa, ON, Canada, 2007; pp. 185–204. [Google Scholar]
- Leach, D.L.; Bradley, D.; Lewchuk, M.T.; Symons, D.T.A.; Marsily, G.d.; Brannon, J. Mississippi Valley-type lead-zinc deposits through geological time: Implications from recent age-dating research. Miner. Depos. 2001, 36, 711–740. [Google Scholar] [CrossRef]
- Sverjensky, D.A. Genesis of Mississippi Valley-type lead-zinc deposits. Annu. Rev. Earth Planet. Sci. 1986, 14, 177–199. [Google Scholar] [CrossRef]
- Mohammadi, S.; Hollenbach, A.M.; Goldstein, R.H.; Möller, A.; Burberry, C.M. Controls on timing of hydrothermal fluid flow in south-central Kansas, north-central Oklahoma, and the Tri-State Mineral District. Midcont. Geosci. 2022, 3, 1–26. [Google Scholar] [CrossRef]
- Gregg, J.M.; Shelton, K.L. Minor and trace element distributions in the Bonneterre Dolomite (Cambrian), southeast Missouri: Evidence for possible multiple basin fluid sources and pathways during lead-zinc mineralization. Geol. Soc. Am. Bull. 1989, 101, 221–230. [Google Scholar] [CrossRef]
- Bradley, D.C.; Leach, D.L. Tectonic controls of Mississippi Valley-type lead-zinc mineralization in orogenic forelands. Miner. Depos. 2003, 38, 652–667. [Google Scholar] [CrossRef]
- Beukes, N.J. Stratigraphy and lithofacies of the Campbellrand Subgroup of the Proterophytic Ghaap Group, Northern Cape (in Africaans). Trans. Geol. Soc. S. Afr. 1980, 83, 141–170. [Google Scholar]
- Schaefer, M.O.; Gutzmer, J.; Beukes, N.J.; Greyling, L.N. Mineral chemistry of sphalerite and galena from Pb-Zn mineralization hosted by the Transvaal Supergroup in Griqualand West, South Africa. S. Afr. J. Geol. 2004, 107, 341–354. [Google Scholar] [CrossRef]
- Schaefer, M.; Gutzmer, J.; Beukes, N.J. Genesis of carbonate-hosted Pb-Zn deposits in the late Archean Transvaal Supergroup, Northern Cape Province, South Africa. In Mineral Deposits at the Beginning of the 21st Century; Piestrzynski, A., Ed.; Swets and Zeitlinger: Krakow, Poland, 2001; pp. 177–180. [Google Scholar]
- Schaefer, M. Paleoproterozoic Mississippi Valley-Type Pb-Zn Deposits of the Ghaap Group, Transvaal Supergroup in Griqualand West, South Africa. Ph.D. Thesis, Rand Afrikaans University, Johannesburg, South Africa, 2002. [Google Scholar]
- Gutzmer, J. The Paleoproterozoic carbonate-hosted Pering Zn–Pb deposit, South Africa: I. Styles of brecciation and mineralization. Miner. Depos. 2006, 40, 664–685. [Google Scholar] [CrossRef]
- Baugaard, W.D. Stratigraphy and diagenesis of the Paleoproterozoic Bushy Park zinc–lead deposit, South Africa. M.S. Thesis, University of Missouri-Rolla, Rolla, MO, USA, 2002. [Google Scholar]
- Armstrong, R.A.; Compston, W.; Retief, E.A.; Williams, I.S.; Welke, H.J. Zircon ion microprobe studies bearing on the age and evolution of the Witwatersrand triad. Precambrian Res. 1991, 53, 243–266. [Google Scholar] [CrossRef]
- Clendenin, C.W.; Charlesworth, E.G.; Maske, S. An early proterozoic three-stage rift system, Kaapvaal Craton, South Africa. Tectonophysics 1988, 145, 73–76. [Google Scholar] [CrossRef]
- Altermann, W.; Siegfried, H.P. Sedimentology, facies development and a type-section of an Archean shelf-carbonate platform transition, Kaapvaal craton, as deduced from a deep borehole at Kathu, South Africa. J. Afr. Earth Sci. 1997, 24, 391–410. [Google Scholar] [CrossRef]
- Sumner, D.Y.; Beukes, N.J. Sequence stratigraphic development of the Neoarchean Transvaal carbonate platform Kaapvaal Craton, South Africa. S. Afr. J. Geol. 2006, 109, 11–22. [Google Scholar] [CrossRef]
- Wheatley, C.J.V.; Friggens, P.J.; Dooge, F. The Bushy Park carbonate-hosted zinc-lead deposit, Griqualand West. In Mineral Deposits of Southern Africa; Anhaeusser, C.R., Maske, S., Eds.; Geological Society of South Africa: Johannesburg, South Africa, 1986; pp. 891–900. [Google Scholar]
- Huizenga, J.M.; Gutzmer, J.; Banks, D.; Greyling, L.N. The Paleoproterozoic carbonate-hosted Pering Zn–Pb deposit, South Africa. II. Fluid inclusion, fluid chemistry and stable isotope constraints. Miner. Depos. 2006, 40, 686–706. [Google Scholar] [CrossRef]
- Kruger, F.J.; Duane, M.J.; Whitelaw, H. The c.2 Ga Kheis tectonism in southern Africa and associated MVT mineralisation. In Proceedings of the 5th Biennial SGA Meeting and the Tenth Quadrennial IAGOD Symposium, London, UK, 22–25 August 1999. [Google Scholar]
- Duane, M.J.; Turner, A.M.; Roberts, P.J.; Kruger, F.J.; Verhagen, F.J. Precambrian MVT Pb-Zn(F) and hydrocarbon deposits; evidence for early crustal-scale fluid transport in Southern Africa. In Precambrian Sedimentary Basins in Southern Africa; Eriksson, P.G., Ed.; Blackwell Scientific publications: Oxford, UK, 1991; pp. 9–10. [Google Scholar]
- Duane, M.J.; Kruger, F.J.; Turner, A.M.; Whitelaw, H.T.; Coetzee, H.; Verhagen, B.T. The timing and isotopic character of regional hydrothermal alteration and associated epigenetic mineralization in the western sector of the Kaapvaal Craton (South Africa). J. Afr. Earth Sci. 2004, 38, 461–476. [Google Scholar] [CrossRef]
- Gleason, J.D.; Gutzmar, J.; Kesler, S.E.; Zwingmann, H. 2.05-Ga isotopic ages for transvaal Mississippi valley–type deposits: Evidence for large-scale hydrothermal circulation around the bushveld igneous complex, South Africa. J. Geol. 2011, 119, 69–80. [Google Scholar]
- Kah, L.C.; Lyons, T.W.; Frank, T.D. Evidence for low marine sulphate and protracted oxygenation of the Proterozoic biosphere. Nature 2004, 431, 834–838. [Google Scholar] [CrossRef]
- Kesler, S.E.; Reich, M.H. Precambrian Mississippi Valley-type deposits: Relation to changes in composition of the hydrosphere and atmosphere. In Evolution of Early Earth’s Atmosphere, Hydrosphere and Biosphere—Constraints from Ore Deposits; Kesler, S.E., Ohmoto, H., Eds.; Memoir 198, Geological Society of America: Boulder, CO, USA, 2006; pp. 185–204. [Google Scholar]
- Holland, H.D. Why the atmosphere became oxygenated: A proposal. Geochim. Cosmochim. Acta 2009, 73, 5241–5255. [Google Scholar] [CrossRef]
- Sibley, D.F.; Gregg, J.M. Classification of dolomite rock textures. J. Sediment. Petrol. 1987, 57, 967–975. [Google Scholar]
- Folk, R.L. Practical Petrographic Classification of Limestones. AAPG Bull. 1959, 43, 1–38. [Google Scholar]
- Shelton, K.L.; Orville, P.M. Formation of synthetic fluid inclusions in natural quartz. Am. Mineral. 1980, 65, 1233–1236. [Google Scholar]
- Roedder, E. Fluid Inclusions; Reviews in Mineralogy Volume 12; Mineralogical Society of America: Washington, DC, USA, 1984; p. 644. [Google Scholar]
- Goldstein, R.H.; Reynolds, T.J. Systematics of Fluid Inclusions in Diagenetic Minerals; Short Course 31; SEPM: Tulsa, OK, USA, 1994; p. 199. [Google Scholar]
- Bodnar, R.J. Revised equation and table for determining the freezing point depression of H2O-NaCl solutions. Geochim. Cosmochim. Acta 1993, 57, 683–684. [Google Scholar] [CrossRef]
- Eldridge, C.S.; Bourcier, W.L.; Ohmoto, H.; Barnes, H.L. Hydrothermal Inoculation and Incubation of the Chalcopyrite Disease in Sphalerite. Econ. Geol. 1988, 83, 978–989. [Google Scholar] [CrossRef]
- Voss, R.L.; Hagni, R.D.; Gregg, J.M. Sequential deposition of zoned dolomite and its relationship to sulfide mineral paragenetic sequence in the Viburnum Trend, southeast Missouri. Carbonates Evaporites 1989, 4, 195–209. [Google Scholar] [CrossRef]
- Gerdemann, P.E.; Myers, H.E. Relationships of carbonate facies patterns to ore distribution and to ore genesis in the southeast Missouri lead district. Econ. Geol. 1972, 67, 426–433. [Google Scholar] [CrossRef]
- Hagni, R.D.; Grawe, O.R. Mineral paragenesis in the Tri-state district Missouri, Kansas, Oklahoma. Econ. Geol. 1964, 59, 449–457. [Google Scholar] [CrossRef]
- Hagni, R.D. Tri-State ore deposits: The character of their host rocks and their genesis. In Handbook of Strata-Bound and Strataform Ore Deposits; Wolf, K.H., Ed.; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1976; pp. 457–494. [Google Scholar]
- Mohammadi, S.; Gregg, J.M.; Shelton, K.L.; Appold, M.S.; Puckette, J.O. Influence of late diagenetic fluids on Mississippian carbonate rocks on the Cherokee–Ozark Platform, NE Oklahoma, NW Arkansas, SW Missouri, and SE Kansas. In Mississippian Reservoirs of the Mid-Continent, Memoir 122; Grammer, G.M., Gregg, J.M., Puckette, J.O., Jaiswal, P., Pranter, M., Mazzullo, S.J., Goldstein, R.H., Eds.; AAPG: Tulsa, OK, USA, 2019; pp. 325–352. [Google Scholar]
- Fowler, G.M. Oil and oil structures in Oklahoma-Kansas zinc-lead mining field. AAPG Bull. 1933, 17, 1436–1445. [Google Scholar]
- Hitzman, M.W.; Redmond, P.B.; Beaty, D.W. The carbonate-hosted Lisheen Zn-Pb-Ag deposit, County Tipperary, Ireland. Econ. Geol. 2002, 97, 1627–1655. [Google Scholar] [CrossRef]
- Wilkinson, J.J. On diagenesis, dolomitisation and mineralisation in the Irish Zn-Pb orefield. Miner. Depos. 2003, 38, 968–983. [Google Scholar] [CrossRef]
- Nagy, Z.R.; Gregg, J.M.; Becker, S.; Shelton, K.L.; Johnson, A.W.; Somerville, I.D.; Wright, W.R. Early dolomitisation and fluid migration through the Lower Carboniferous carbonate platform in the southeast Irish Midlands: Implications for reservoir attributes. In The Geometry and Petrogenesis of Dolomite Hydrocarbon Reservoirs; Special Publication 235; Braithwaite, C.J.R., Rizzi, G., Darke, G., Eds.; The Geological Society of London: London, UK, 2004; pp. 367–392. [Google Scholar]
- Gregg, J.M.; Shelton, K.L.; Johnson, A.W.; Somerville, I.D.; Wright, W.R. Dolomitization of the Waulsortian Limestone (Lower Carboniferous) Irish Midlands. Sedimentology 2001, 48, 745–766. [Google Scholar] [CrossRef]
- Ebers, M.L.; Kopp, O.C. Cathodoluminescent microstratigraphy in gangue dolomite, the Mascot-Jefferson City District, Tennessee. Econ. Geol. 1979, 74, 908–918. [Google Scholar] [CrossRef]
- Machel, H.G. Application of cathodoluminescence to carbonate diagenesis. In Cathodoluminescence in Geosciences; Pagel, M., Barbin, V., Philippe Blanc, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 271–301. [Google Scholar]
- Gregg, J.M. Regional epigenetic dolomitization in the Bonneterre Dolomite (Cambrian), southeastern Missouri. Geology 1985, 13, 503–506. [Google Scholar] [CrossRef]
- Wright, W.R.; Somerville, I.D.; Gregg, J.M.; Johnson, A.W.; Shelton, K.L. Dolomitization and neomorphism of Irish Lower Carboniferous (Early Mississippian) Limestones: Evidence from petrographic and isotopic data. In Permo-Carboniferous Carbonate Platforms and Reefs; Ahr, W.M., Harris, P.M., Morgan, W.A., Somerville, I.D., Eds.; Special Publication/Memoir 78/83; SEPM/AAPG: Tulsa, OK, USA, 2003; pp. 395–408. [Google Scholar]
- Lonnee, J.; Machel, H.G. Pervasive dolomitization with subsequent hydrothermal alteration in the Clarke Lake gas field, Middle Devonian Slave Point Formation, British Columbia, Canada. AAPG Bull. 2006, 90, 1739–1761. [Google Scholar] [CrossRef]
- Bathurst, R.G.C. Carbonate Sediments and Their Diagenesis; Elsevier Scientific Publishing Co.: New York, NY, USA, 1975; p. 658. [Google Scholar]
- Sumner, D.Y.; Grotzinger, J.P. Implications for Neoarchaean ocean chemistry from primary carbonate mineralogy of the Campbellrand-Malmani Platform, South Africa. Sedimentology 2004, 51, 1273–1299. [Google Scholar] [CrossRef]
- Overstreet, R.B.; Oboh-Ikuneobe, F.E.; Gregg, J.M. Sequence stratigraphy and depositional facies of Lower Ordovician cyclic carbonate rocks, southern Missouri, U.S.A. J. Sediment. Res. 2003, 73, 420–432. [Google Scholar] [CrossRef]
- Wang, Y.; Thompson, T.; Grammer, G.M. Fracture characterization and prediction in unconventional reservoirs of the “Mississippian limestone,” north-central Oklahoma, United States. In Mississippian Reservoirs of the Midcontinent; Grammer, G.M., Gregg, J.M., Puckette, J.O., Jaiswal, P., Mazzullo, S.J., Pranter, M.J., Goldstein, R.H., Eds.; Memoir 122; AAPG: Tulsa, OK, USA, 2019; pp. 271–300. [Google Scholar]
- Gregg, J.M.; Howard, S.A.; Mazzullo, S.J. Early diagenetic recrystallization of Holocene (<3000 years old) peritidal dolomites, Ambergris Cay, Belize. Sedimentology 1992, 39, 143–160. [Google Scholar]
- Kaczmarek, S.E.; Gregg, J.M.; Bish, D.L.; Machel, H.G.; Fouke, B.W. Dolomite, very high-magnesium calcite, and microbes–implications for the microbial model of dolomitization. In Characterization and Modeling of Carbonates–Mountjoy Symposium 1; MacNeil, A.J., Lonnee, J., Wood, R., Eds.; Special Publication 109; SEPM: Tulsa, OK, USA, 2017; pp. 7–20. [Google Scholar]
- Montañez, I.P.; Read, J.F. Eustatic control on early dolomitization of cyclic peritidal carbonates–Evidence from the Early Ordovician Upper-Knox Group, Appalachians. Geol. Soc. Am. Bull. 1992, 104, 872–886. [Google Scholar] [CrossRef]
- He, Z.; Gregg, J.M.; Shelton, K.L.; Palmer, J.R. Sedimentary facies control of fluid flow and mineralization in Cambro-Ordovician strata, southern Missouri. In Basinwide Fluid Flow and Associated Diagenetic Patterns: Integrated Petrologic, Geochemical and Hydrologic Considerations; Montañez, I.P., Gregg, J.M., Shelton, K.L., Eds.; Special Publication 57; SEPM: Tulsa, OK, USA, 1997; pp. 81–99. [Google Scholar]
- Melim, L.M.; Scholle, P.A. Dolomitization of the Capitan Formation forereef facies (Permian, west Texas and New Mexico): Seepage reflux revisited. Sedimentology 2002, 49, 1207–1227. [Google Scholar] [CrossRef]
- Illing, L.V.; Wells, A.J.; Taylor, J.C.M. Penecontemporary dolomite in the Persian Gulf. In Dolomitization and Limestone Diagenesis; Pray, L.C., Murray, R.C., Eds.; Special Publication 13; SEPM: Tulsa, OK, USA, 1965; pp. 89–111. [Google Scholar]
- McKenzie, J.A.; Hsü, K.J.; Schneider, J.F. Movement of subsurface waters under the sabkha, Abu Dhabi, UAE. In Concepts and Models of Dolomitization; Zenger, D.H., Dunham, J.B., Ethington, R.L., Eds.; Special Publication 28; SEPM: Tulsa, OK, USA, 1980; pp. 11–30. [Google Scholar]
- Furman, F.C.; Woody, R.E.; Rasberry, M.A.; Keller, D.J.; Gregg, J.M. Carbonate and evaporite mineralogy of Holocene (<1900 RCYBP) sediments at Salt Pond, San Salvador Island, Bahamas: Preliminary study. In Proceedings of the Sixth Symposium on the Geology of the Bahamas, San Salvador Island, Bahamas, June 1993; pp. 67–74. [Google Scholar]
- Shields, G.; Veizer, J. Precambrian marine carbonate isotope database: Version 1.1. Geochem. Geophys. Geosyst. 2002, 3, 1031. [Google Scholar] [CrossRef]
- Dupeyron, J.; Decraene, M.-N.; Marin-Carbonne, J.; Busigny, V. Formation pathways of Precambrian sedimentary pyrite: Insights from in situ Fe isotopes. Earth Planet. Sci. Lett. 2023, 609, 118070. [Google Scholar] [CrossRef]
- Roedder, E. Metastable superheated ice in liquid-water inclusions under high negative pressure. Science 1967, 155, 1413–1417. [Google Scholar] [CrossRef]
- Wilkinson, J.J. Fluid inclusions in hydrothermal ore deposits. Lithos 2001, 55, 229–272. [Google Scholar] [CrossRef]
- Shelton, K.L.; Bauer, R.M.; Gregg, J.M. Fluid inclusion studies of regionally extensive epigenetic dolomites, Bonneterre Dolomite (Cambrian), southeast Missouri: Evidence of multiple fluids during dolomitization and lead-zinc mineralization. Geol. Soc. Am. Bull. 1992, 104, 675–683. [Google Scholar] [CrossRef]
- Johnson, A.W.; Shelton, K.L.; Gregg, J.M.; Somerville, I.D.; Wright, W.R.; Nagy, Z.R. Regional studies of dolomites and their included fluids: Recognizing multiple chemically distinct fluids during the complex diagenetic history of the Irish Zn-Pb ore field. Mineral. Petrol. 2009, 96, 1–18. [Google Scholar] [CrossRef]
- Shelton, K.L.; Beasley, J.M.; Gregg, J.M.; Appold, M.S.; Crowley, S.F.; Hendry, J.P. Evolution of a carboniferous carbonate-hosted sphalerite breccia deposit, Isle of Man. Miner. Depos. 2011, 46, 859–880. [Google Scholar] [CrossRef]
- Shelton, K.L.; Hendry, J.P.; Gregg, J.M.; Truesdale, J.P.; Somerville, I.D. Fluid circulation and fault-fracture–related diagenesis in Mississippian syn-rift carbonate rocks on the northeast margin of the metaliferous Dublin Basin, Ireland. J. Sediment. Res. 2019, 89, 508–536. [Google Scholar] [CrossRef]
- Kesler, S.E.; Martini, A.M.; Appold, M.S.; Walter, L.M.; Huston, T.J.; Furman, F.C. Na–Cl–Br systematics of fluid inclusions from Mississippi Valley-type deposits, Appalachian Basin: Constraints on solute origin and migration paths. Geochim. Et Cosmochim. Acta 1996, 60, 225–233. [Google Scholar] [CrossRef]
- Shelton, K.L.; Gregg, J.M.; Johnson, A.W. Replacement dolomites and ore sulfides as recorders of multiple fluids and fluid sources in the Southeast Missouri Mississippi Valley-Type district: Halogen-87Sr/86Sr-δ18O-δ34S systematics in the Bonneterre Dolomite. Econ. Geol. 2009, 104, 733–748. [Google Scholar] [CrossRef]
- Meyer, F.M.; Robb, L.J. The geochemistry of black shales from the Chuniespoort Group, Transvaal Sequence, Eastern Transvaal, South Africa. Econ. Geol. 1996, 91, 111–121. [Google Scholar] [CrossRef]
- Roberts, P.J.; Gize, A.P.; Duane, M.J. Precambrian hydrocarbon residues associated with Mississippi Valley-type mineralization in the Transvaal Sequence, South Africa. S. Afr. J. Geol. 1993, 96, 57–60. [Google Scholar]
- Burger, A.J.; Coertze, F.J. Age determinations—April 1972 to march 1974. Ann. Geol. Surv. S. Afr. 1975, 10, 135–141. [Google Scholar]
- Walraven, F.; Armstrong, R.A.; Kruger, F.J. A chronostratigraphic framework for the north-central Kaapvaal Craton, the Bushveld Complex and Vredefort Structure. Tectonophysics 1990, 171, 23–48. [Google Scholar] [CrossRef]
- Van Niekerk, H.S.; Beukes, N.J. Revised definition/outline of the Kheis Terrane along the western margin of the Kaapvaal Craton and lithostratigraphy of the newly proposed Keis Supergroup. S. Afr. J. Geol. 2019, 122, 187–220. [Google Scholar] [CrossRef]




| Sample | Mineral | Assemblage | Th | Tfm | Tmice | NaCl eq | Description |
|---|---|---|---|---|---|---|---|
| 376-FI-1 | Dolomite | 1 | 166.0 | - | −3.3 | 5.4 | Primary |
| chip 1 | Stage 3/4 | 1 | 166.0 | - | −3.3 | 5.4 | Primary |
| 2 | 164.0 | - | −3.3 | 5.4 | Primary | ||
| 2 | 164.0 | - | −3.3 | 5.4 | Primary | ||
| 2 | 164.0 | - | −3.3 | 5.4 | Primary | ||
| 2 | 164.0 | - | −3.3 | 5.4 | Primary | ||
| 3 | 166.0 | −18.3 | −2.3 | 3.9 | Primary | ||
| 350-FI-2 | Dolomite | 1 | 140.0 | - | −20.0 | 22.4 | Primary |
| chip 1 | Stage 2 | 1 | 140.0 | - | −20.0 | 22.4 | Primary |
| 1 | 142.0 | - | −20.0 | 22.4 | Primary | ||
| 2 | 110.0 | - | −27.0 | 26.8 | Primary | ||
| 2 | 110.0 | - | −25.0 | 25.6 | Primary | ||
| 2 | 110.0 | - | −25.0 | 25.6 | Primary | ||
| 3 | 194.0 | - | −14.0 | 17.8 | Primary | ||
| 3 | 194.0 | - | −14.0 | 17.8 | Primary | ||
| 3 | 194.0 | - | −14.0 | 17.8 | Primary | ||
| 3 | 194.0 | - | −14.0 | 17.8 | Primary | ||
| 4 | 194.0 | - | −18.7 | 21.5 | Primary | ||
| 4 | 194.0 | - | −18.7 | 21.5 | Primary | ||
| 4 | 194.0 | - | −18.7 | 21.5 | Primary | ||
| 4 | 194.0 | - | −18.7 | 21.5 | Primary | ||
| 4 | 194.0 | - | −18.7 | 21.5 | Primary | ||
| 4 | 194.0 | - | −18.7 | 21.5 | Primary | ||
| 5 | 124.0 | - | −23.0 | 24.3 | Primary | ||
| 5 | 124.0 | - | −23.0 | 24.3 | Primary | ||
| 5 | 124.0 | - | −23.0 | 24.3 | Primary | ||
| 6 | 198.0 | −36.0 | −23.0 | 24.3 | Primary | ||
| 7 | 124.0 | - | - | - | Secondary | ||
| 7 | 124.0 | - | - | - | Secondary | ||
| 350-FI-2 | Dolomite | 1 | 238.0 | - | −8.8 | 12.6 | Primary |
| chip 2 | Stage 2 | 2 | 238.0 | - | −11.5 | 15.5 | Primary |
| 3 | 209.0 | - | −11.5 | 15.5 | Primary | ||
| 4 | 160.0 | - | −11.5 | 15.5 | Primary | ||
| 4 | 160.0 | - | −11.5 | 15.5 | Primary | ||
| 5 | 190.0 | - | −12.2 | 16.1 | Primary | ||
| 5 | 190.0 | - | −12.2 | 16.1 | Primary | ||
| 5 | 190.0 | - | −12.2 | 16.1 | Primary | ||
| 6 | 219.0 | - | −11.5 | 15.5 | Primary | ||
| 6 | 219.0 | - | −11.5 | 15.5 | Primary | ||
| 6 | 219.0 | - | −11.5 | 15.5 | Primary | ||
| 6 | 219.0 | - | −11.5 | 15.5 | Primary | ||
| 7 | 238.5 | - | −22.0 | 23.7 | Primary | ||
| 515-FI-5 | Dolomite | 1 | 183.6 | - | −24.5 | 25.3 | Primary |
| chip 1 | Stage 2 | 1 | 181.0 | - | −20.2 | 22.5 | Primary |
| 2 | 204.0 | - | - | - | Pseudosecondary | ||
| 2 | 204.0 | - | - | - | Pseudosecondary | ||
| 3 | 190.0 | - | −9.7 | 13.6 | Pseudosecondary | ||
| 3 | 190.0 | - | −9.7 | 13.6 | Pseudosecondary | ||
| 3 | 190.0 | - | −9.7 | 13.6 | Pseudosecondary | ||
| 4 | 183.6 | - | −13.8 | 17.6 | Secondary | ||
| 4 | 183.6 | - | −13.8 | 17.6 | Secondary | ||
| 4 | 183.6 | - | −13.8 | 17.6 | Secondary | ||
| 4 | 183.6 | - | −13.8 | 17.6 | Secondary | ||
| chip 2 | 1 | 98.0 | - | −9.5 | 13.4 | Secondary | |
| 1 | 78.0 | −13.0 | −9.0 | 12.8 | Secondary | ||
| 2 | 123.0 | −13.4 | −9.0 | 12.8 | Secondary | ||
| 2 | 123.0 | −13.4 | −9.0 | 12.8 | Secondary | ||
| 3 | 143.0 | - | - | - | Secondary | ||
| 4 | 172.0 | −28.4 | −7.2 | 10.7 | Secondary | ||
| 5 | 136.0 | - | −6.2 | 9.5 | Secondary | ||
| 5 | 136.0 | - | −6.2 | 9.5 | Secondary | ||
| 6 | 142.0 | −13.8 | −12.0 | 16.0 | Secondary | ||
| 6 | 142.0 | −13.8 | −12.0 | 16.0 | Secondary | ||
| 6 | 142.0 | −13.8 | −12.0 | 16.0 | Secondary | ||
| 6 | 142.0 | −13.8 | −12.0 | 16.0 | Secondary | ||
| 6 | 142.0 | −13.8 | −12.0 | 16.0 | Secondary | ||
| 7 | - | - | −4.4 | 7.0 | Secondary | ||
| 7 | - | - | −4.4 | 7.0 | Secondary | ||
| 7 | - | - | −4.4 | 7.0 | Secondary | ||
| 7 | - | - | −4.4 | 7.0 | Secondary | ||
| 8 | 176.0 | −13.0 | −12.4 | 16.3 | Secondary | ||
| 9 | 131.0 | - | −9.5 | 13.4 | Secondary | ||
| 10 | 105.0 | - | −9.5 | 13.4 | Secondary | ||
| 10 | 105.0 | - | −9.5 | 13.4 | Secondary | ||
| 10 | 94.0 | - | −9.5 | 13.4 | Secondary | ||
| 11 | 178.0 | - | −12.5 | 16.4 | Primary | ||
| 12 | - | - | −9.4 | 13.3 | Primary | ||
| 13 | 102.0 | - | - | - | Secondary | ||
| 515-FI-1 | Dolomite | 1 | 75.0 | −17.1 | - | - | Secondary-leaked |
| chip 1 | Stage 3/4 | 2 | 130.0 | −23.3 | −4.7 | 7.4 | Primary |
| 3 | 134.0 | −20.0 | −7.1 | 10.6 | Primary | ||
| 3 | 130.0 | - | - | - | Primary-leaked | ||
| 3 | 122.0 | −27.0 | −8.3 | 12.0 | Primary | ||
| 376-FI-1 | Quartz | 1 | 158.0 | - | −4.2 | 6.7 | Primary |
| chip 2 | 1 | 158.0 | - | −4.2 | 6.7 | Primary | |
| 2 | 176.0 | - | −4.2 | 6.7 | Primary | ||
| 3 | 150.0 | - | −5.8 | 8.9 | Primary | ||
| 4 | 132.0 | - | −5.8 | 8.9 | Primary | ||
| 4 | 132.0 | - | −5.8 | 8.9 | Primary | ||
| 4 | 132.0 | - | −5.8 | 8.9 | Primary | ||
| 4 | 132.0 | - | −5.8 | 8.9 | Primary | ||
| 5 | 167.0 | - | −4.2 | 6.7 | Primary | ||
| 5 | 150.0 | - | −5.8 | 8.9 | Primary | ||
| 515-FI-1 | Quartz | 1 | 128.0 | −22.0 | −16.2 | 19.6 | Secondary |
| chip 1 | 1 | 128.0 | −22.0 | −16.2 | 19.6 | Secondary | |
| 1 | 128.0 | - | −17.0 | 20.2 | Secondary | ||
| 1 | 128.0 | - | −17.0 | 20.2 | Secondary | ||
| 2 | 154.0 | - | −16.2 | 19.6 | Secondary | ||
| 2 | 154.0 | - | −20.0 | 22.4 | Secondary | ||
| 3 | 141.0 | - | −17.0 | 20.2 | Secondary | ||
| 3 | 141.0 | - | −17.0 | 20.2 | Secondary | ||
| 3 | 141.0 | - | −17.0 | 20.2 | Secondary | ||
| 4 | 144.0 | −23.0 | −13.3 | 17.2 | Secondary | ||
| 4 | 144.0 | −23.0 | −13.3 | 17.2 | Secondary | ||
| 4 | 144.0 | −23.0 | −13.3 | 17.2 | Secondary | ||
| 4 | 144.0 | - | −16.4 | 19.8 | Secondary | ||
| 5 | 123.0 | - | −16.7 | 20.0 | Secondary | ||
| 5 | 123.0 | - | −16.7 | 20.0 | Secondary | ||
| 5 | 144.0 | - | −16.7 | 20.0 | Secondary | ||
| 6 | 142.0 | −29.0 | −18.5 | 21.3 | Primary | ||
| 6 | 142.0 | - | −15.5 | 19.0 | Primary | ||
| 6 | 142.0 | - | −15.5 | 19.0 | Primary | ||
| 6 | 142.0 | - | −15.5 | 19.0 | Primary | ||
| 7 | 133.0 | −21.5 | −15.1 | 18.7 | Primary | ||
| 7 | - | - | −15.1 | 18.7 | Primary | ||
| 8 | 145.8 | - | −19.3 | 21.9 | Primary | ||
| 8 | 145.8 | - | −15.5 | 19.0 | Primary | ||
| 8 | 145.8 | - | −15.5 | 19.0 | Primary | ||
| 8 | 145.8 | - | −15.5 | 19.0 | Primary | ||
| 9 | - | −22.5 | −16.4 | 19.8 | Primary |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baugaard, W.; Mohammadi, S.; Gregg, J.M. Diagenesis and Mineralization of the Neoarchean Bushy Park Lead-Zinc Deposit, Northern Cape Province, South Africa. Minerals 2025, 15, 468. https://doi.org/10.3390/min15050468
Baugaard W, Mohammadi S, Gregg JM. Diagenesis and Mineralization of the Neoarchean Bushy Park Lead-Zinc Deposit, Northern Cape Province, South Africa. Minerals. 2025; 15(5):468. https://doi.org/10.3390/min15050468
Chicago/Turabian StyleBaugaard, William, Sahar Mohammadi, and Jay M. Gregg. 2025. "Diagenesis and Mineralization of the Neoarchean Bushy Park Lead-Zinc Deposit, Northern Cape Province, South Africa" Minerals 15, no. 5: 468. https://doi.org/10.3390/min15050468
APA StyleBaugaard, W., Mohammadi, S., & Gregg, J. M. (2025). Diagenesis and Mineralization of the Neoarchean Bushy Park Lead-Zinc Deposit, Northern Cape Province, South Africa. Minerals, 15(5), 468. https://doi.org/10.3390/min15050468









