Terahertz Investigation of Cultural Heritage Synthetic Materials: A Case Study of Copper Silicate Pigments
Abstract
1. Introduction
2. Materials and Methods
2.1. Terahertz Continuous Wave Spectroscopy (THz-CW)
2.2. Energy Dispersive X-Ray Fluorescence (ED-XRF)
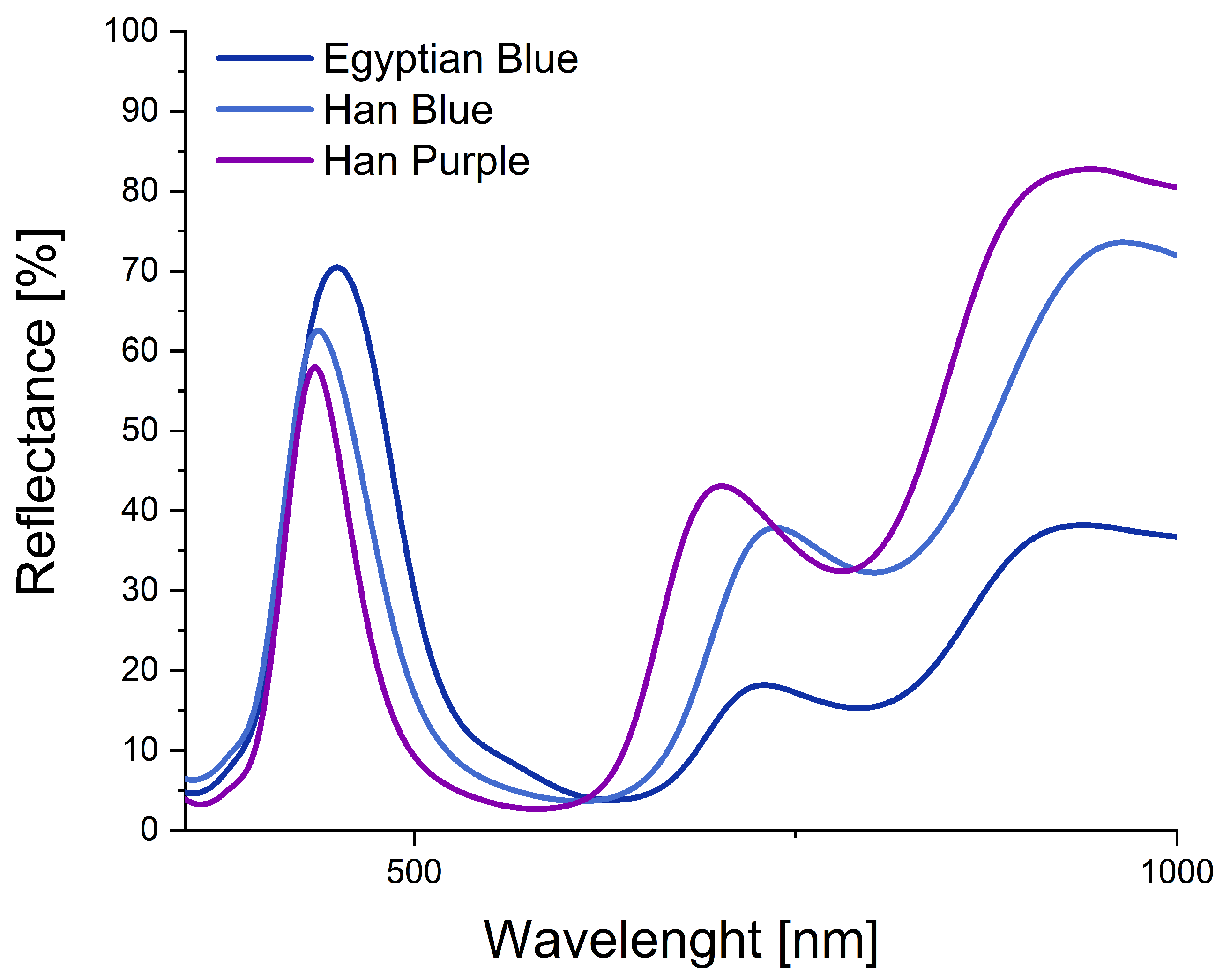
2.3. Fiber Optics Reflectance Spectroscopy (FORS)
2.4. Pigments Samples
3. Results
3.1. Egyptian Blue
3.2. Han Blue
3.3. Han Purple
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| THz-CW | Terahertz continuous wave |
| ED-XRF | Energy Dispersive X-Ray Fluorescence |
| FORS | Fiber Optics Reflectance Spectroscopy |
| EB | Egyptian blue |
| HB | Han Blue |
| HP | Han Purple |
| DFB | Distributed Feedback Lasers |
| PCA | Photoconductive antenna |
| PMs | Parabolic mirrors |
| Vis | Visible |
| SWIR | Short Wave Infrared |
| NIR | Near-infrared |
| FWHM | Full Width Half Maximum |
References
- Nikitkina, A.; Bikmulina, P.; Gafarova, E.; Kosheleva, N.; Efremov, Y.; Bezrukov, E.; Butnaru, D.; Dolganova, I.; Chernomyrdin, N.; Cherkasova, O.; et al. Terahertz radiation and the skin: A review. J. Biomed. Opt. 2021, 26, 043005. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Shi, C.; Wu, X.; Zhu, Y.; Zhuang, S. Terahertz Imaging and Spectroscopy in Cancer Diagnostics: A Technical Review. BME Front. 2021, 26, 2547609. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.; Qiu, Y.; Chen, X.; Zhao, Z.; Xia, L.; Shao, Y. Biomedical applications of terahertz technology. Appl. Spectrosc. Rev. 2020, 55, 418–438. [Google Scholar] [CrossRef]
- Yu, L.; Hao, L.; Meiqiong, M.T.; Jiaoqi, H.; Wei, L.; Jinying, D.; Xueping, C.; Weiling, F.; Yang, Z. The medical application of terahertz technology in non-invasive detection of cells and tissues: Opportunities and challenges. RSC Adv. 2019, 9, 9354–9363. [Google Scholar] [CrossRef] [PubMed]
- Bawuah, P.; Zeitler, J.A. Advances in terahertz time-domain spectroscopy of pharmaceutical solids: A review. TrAC Trends Anal. Chem. 2021, 139, 116272. [Google Scholar] [CrossRef]
- Afsah-Hejri, L.; Akbari, E.; Toudeshki, A.; Homayouni, T.; Alizadeh, A.; Ehsani, R. Terahertz spectroscopy and imaging: A review on agricultural applications. Comput. Electron. Agric. 2020, 177, 105628. [Google Scholar] [CrossRef]
- Zhong, S. Progress in terahertz nondestructive testing: A review. Front. Mech. Eng. 2019, 14, 273–281. [Google Scholar] [CrossRef]
- Tao, Y.H.; Fitzgerald, A.J.; Wallace, V.P. Non-Contact, Non-Destructive Testing in Various Industrial Sectors with Terahertz Technology. Sensors 2020, 20, 712. [Google Scholar] [CrossRef]
- D’Arco, A.; Rocco, D.; Magboo, P.F.J.; Moffa, C.; Ventura, G.D.; Marcelli, A.; Palumbo, L.; Mattiello, L.; Lupi, S.; Petrarca, M. Terahertz continuous wave spectroscopy: A portable advanced method for atmospheric gas sensing. Opt. Express 2022, 30, 19005–19016. [Google Scholar] [CrossRef]
- Fukunaga, K. THz Technology Applied to Cultural Heritage in Practice; Springer: Tokyo, Japan, 2016. [Google Scholar]
- Squires, A.D.; Kelly, M.; Lewis, R.A. Terahertz Analysis of Quinacridone Pigments. J. Infrared Millim. Terahertz Waves 2016, 38, 314–324. [Google Scholar] [CrossRef]
- Kleist, E.M.; Dandolo, C.L.K.; Guillet, J.; Mounaix, P.; Korter, T.M. Terahertz Spectroscopy and Quantum Mechanical Simulations of Crystalline Historical Pigments. J. Phys. Chem. A 2019, 123, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Lee, H.; Kim, J.; Jung, T.S.; Kim, J.H.; Kim, J.; Baek, N.Y.; Song, Y.N.; Lee, H.H.; Kim, J.H. Terahertz Spectroscopic Analysis of the Vermilion Pigment in Free Standing and Polyethylene-Mixed Form. ACS Omega 2021, 6, 13802–13806. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, K.; Picollo, M. Terahertz spectroscopy applied to the analysis of artists’ materials. Appl. Phys. A 2010, 100, 591–597. [Google Scholar] [CrossRef]
- Hong, T.; Choi, K.; Ha, T.; Park, B.C.; Sim, K.I.; Kim, J.H.; Kim, J.H. Terahertz Time-domain and Fourier-transform Infrared Spectroscopy of Traditional Korean Pigments. Korean Phys. Soc. 2014, 64, 727–731. [Google Scholar] [CrossRef]
- Ha, T.; Lee, H.; Sim, K.I.; Kim, J.; Jo, Y.C.; Kim, J.H. Optimal Methodologies for Terahertz Time-Domain Spectroscopic Analysis of Traditional Pigments in Powder Form. Korean Phys. Soc. 2017, 70, 866–887. [Google Scholar] [CrossRef]
- Yang, Y.; Zhai, D.; Zhang, Z.; Zhang, C. THz Spectra of Seven Red Mineral Pigments used in Ancient Chinese Artworks. J. Infrared Millim. Terahertz Waves 2017, 38, 1232–1240. [Google Scholar] [CrossRef]
- Squires, A.D.; Lewis, R.A. Terahertz Analysis of Phthalocyanine Pigments. Int. J. Infrared Millim. Waves 2019, 40, 738–751. [Google Scholar] [CrossRef]
- Bardon, T.; May, R.K.; Taday, P.F.; Strlič, M. Systematic study of terahertz time-domain spectra of historically informed black inks. Analyst 2013, 138, 4859–4869. [Google Scholar] [CrossRef]
- Taschin, A.; Bartolini, P.; Tasseva, J.; Striova, J.; Fontana, R.; Riminesi, C.; Torre, R. Drawing materials studied by THz spectroscopy. arXiv 2017, arXiv:1703.01770. [Google Scholar] [CrossRef]
- Kleist, E.M.; Korter, T.M. Quantitative Analysis of Minium and Vermilion Mixtures Using Low-Frequency Vibrational Spectroscopy. Anal. Chem. 2020, 92, 1211–1218. [Google Scholar] [CrossRef]
- Moffa, C.; Merola, C.; Magboo, F.P., Jr.; Chiadroni, E.; Giuliano, L.; Curcio, A.; Palumbo, L.; Felici, A.C.; Petrarca, M. Pigments, minerals, and copper-corrosion products: Terahertz continuous wave (THz-CW) spectroscopic characterization of antlerite and atacamite. J. Cult. Herit. 2024, 66, 483–490. [Google Scholar] [CrossRef]
- Moffa, C.; Curcio, A.; Merola, C.; Migliorati, M.; Palumbo, L.; Felici, A.C.; Petrarca, M. Discrimination of natural and synthetic forms of azurite: An innovative approach based on high-resolution terahertz continuous wave (THz-CW) spectroscopy for Cultural Heritage. Dye. Pigment. 2024, 229, 112287. [Google Scholar] [CrossRef]
- Moffa, C.; Urso, V.; Migliorati, M.; Palumbo, L.; Felici, A.; Zollo, G.; Petrarca, M. Terahertz time-domain investigation of atacamite: Spectral analysis and theoretical insights for cultural heritage applications. Appl. Phys. Lett. 2024, 125, 204102. [Google Scholar] [CrossRef]
- Manceau, J.M.; Nevin, A.; Fotakis, C.; Tzortzakis, S. Terahertz time domain spectroscopy for the analysis of cultural heritage related materials. Appl. Phys. B 2008, 90, 365–368. [Google Scholar] [CrossRef]
- Nicola, M.; Gobetto, R.; Masic, A. Egyptian blue, Chinese blue, and related two-dimensional silicates: From antiquity to future technologies. Part A: General properties and historical uses. Rend. Lincei. Sci. Fis. Nat. 2023, 34, 369–413. [Google Scholar] [CrossRef]
- Berke, H. The invention of blue and purple pigments in ancient times. Chem. Soc. Rev. 2007, 36, 15–30. [Google Scholar] [CrossRef]
- García-Fernandez, P.; Moreno, M.; Aramburu, J.A. Origin of the Anomalous Color of Egyptian and Han Blue Historical Pigments: Going beyond the Complex Approximation in Ligand Field Theory. J. Chem. Educ. 2016, 93, 111–117. [Google Scholar] [CrossRef]
- Zangh, Z.; Ma, Q.; Berke, H. Man-made blue and purple barium copper silicate pigments and the pabstite (BaSnSi3O9) mystery of ancient Chinese wall paintings from Luoyang. Herit. Sci. 2019, 7, 97. [Google Scholar]
- Kostomitsopoulou Marketou, A.; Giannici, F.; Handberg, S.; De Nolf, W.; Cotte, M.; Caruso, F. Synchrotron radiation-based micro-XANES and micro-XRF study of unsuccessfully produced Egyptian blue from the late hellenistic production site of Kos (Dodecanese, Greece). Anal. Chem. 2021, 93, 11557–11567. [Google Scholar] [CrossRef]
- Santos, H.C.; Caliri, C.; Pappalardo, L.; Rizzo, F.; Romano, F.P. MA-XRF and XRD analysis revealing a polychrome Centuripe vase. J. Archaeol. Sci. Rep. 2021, 35, 102760. [Google Scholar] [CrossRef]
- Dariz, P.; Schmid, T. Trace compounds in Early Medieval Egyptian blue carry information on provenance, manufacture, application, and ageing. Sci. Rep. 2021, 11, 11296. [Google Scholar] [CrossRef] [PubMed]
- Falcone, F.; Aquilino, M.; Stoppa, F. Exploring the composition of Egyptian faience. Minerals 2024, 14, 586. [Google Scholar] [CrossRef]
- Degenhart, G.; Heinemann, J.; Tropper, P.; Rodler-Rørbo, A.; Zerobin, B.; Auer, M.; Goldenberg, G. Mineralogical and Micro-Computer Tomographic (μCT) Texture Investigations of Egyptian Blue Spheres (Aguntum, East Tyrol; Retznei and Wagna, Flavia Solva, South Styria). Minerals 2025, 15, 302. [Google Scholar] [CrossRef]
- Xu, Y.; Basafa, H.; Wu, X.; Davari, M.S.; Wang, R. Faience and Egyptian blue frit from Khorasan: Scientific analysis on beads unearthed from Se Tepe site, northeast Iran. J. Archaeol. Sci. Rep. 2024, 55, 104496. [Google Scholar] [CrossRef]
- FitzHugh, E.W.; Zycherman, L.A. An early man-made blue pigment from China—Barium copper silicate. Stud. Conserv. 1983, 28, 15–23. [Google Scholar]
- Herm, C.; Thieme, C.; Emmerling, E.; Wu, Y.; Zhou, T.; Zhang, Z. Analysis of painting materials of the polychrome terracotta army of the first emperor Qin Shi Huang. In Proceedings of the International Symposium on The Ceramics Cultural Heritage of the 8th CIMTEC-World Ceramics Congress and Forum on New Materials, Florence, Italy, 28 June–2 July 1994; pp. 591–601. [Google Scholar]
- Cheng, X.; Xia, Y.; Ma, Y.; Lei, Y. Three fabricated pigments (Han purple, indigo and emerald green) in ancient Chinese artifacts studied by Raman microscopy, energy-dispersive X-ray spectrometry and polarized light microscopy. J. Raman Spectrosc. 2007, 38, 1274–1279. [Google Scholar] [CrossRef]
- Liu, Z.; Mehta, A.; Tamura, N.; Pickard, D.; Rong, B.; Zhou, T.; Pianetta, P. Influence of Taoism on the invention of the purple pigment used on the Qin terracotta warriors. J. Archaeol. Sci. 2007, 34, 1878–1883. [Google Scholar] [CrossRef]
- Xia, Y.; Ma, Q.; Zhang, Z.; Liu, Z.; Feng, J.; Shao, A.; Wang, W.; Fu, Q. Development of Chinese barium copper silicate pigments during the Qin Empire based on Raman and polarized light microscopy studies. J. Archaeol. Sci. 2014, 49, 500–509. [Google Scholar] [CrossRef]
- Ma, Q.; Portmann, A.; Wild, F.; Berke, H. Raman and SEM Studies of Man-Made Barium Copper Silicate Pigments in Ancient Chinese Artifacts. Stud. Conserv. 2006, 51, 81–98. [Google Scholar] [CrossRef]
- Masunaga, S.H.; Rebello, A.; Schye, A.T.; Prasai, N.; Neumeier, J.J.; Cohn, J.L. Heat capacity, thermal expansion and heat transport in the Han Blue (BaCuSi4O10): Observation of structural phase transitions. J. Phys. Chem. Solids 2015, 85, 69–74. [Google Scholar] [CrossRef]
- Pozza, G.; Ajò, D.; Chiari, G.; Zuane, F.D.; Favaro, M. Photoluminescence of the inorganic pigments Egyptian blue, Han blue and Han purple. J. Cult. Herit. 2000, 1, 393–398. [Google Scholar] [CrossRef]
- Jaime, M.; Correa, V.F.; Harrison, N.; Batista, C.D.; Kawashima, N.; Kazuma, Y.; Jorge, G.A.; Stern, R.; Heinmaa, I.; Zvyagin, S.A.; et al. Magnetic-Field-Induced Condensation of Triplons in Han Purple Pigment BaCuSi2O6. Phys. Rev. Lett. 2004, 93, 087203. [Google Scholar] [CrossRef]
- Rieck, B.; Pristacz, H.; Giester, G. Colinowensite, BaCuSi2O6, a new mineral from the Kalahari Manganese Field, South Africa and new data on wesselsite SrCuSi4O10. Mineral. Mag. 2015, 79, 1769–1778. [Google Scholar] [CrossRef]
- Puphal, P.; Allenspach, S.; Rüegg, C.; Pomjakushina, E. Floating Zone Growth of Sr Substituted Han Purple: Ba0.9Sr0.1CuSi2O6. Crystals 2019, 9, 273. [Google Scholar] [CrossRef]
- Allenspach, S.; Puphal, P.; Link, J.; Heinmaa, I.; Pomjakushina, E.; Krellner, C.; Lass, J.; Tucker, G.S.; Niedermayer, C.; Imajo, S.; et al. Revealing three-dimensional quantum criticality by Sr substitution in Han purple. Phys. Rev. Res. 2021, 3, 023177. [Google Scholar] [CrossRef]
- Kong, D.; Wu, X.; Wang, B.; Gao, Y.; Dai, J.; Wang, L.; Ruan, C.; Miao, J. High resolution continuous wave terahertz spectroscopy on solid-state samples with coherent detection. Opt. Express 2018, 26, 17964–17976. [Google Scholar] [CrossRef]
- Mirti, P.; Appolonia, L.; Casoli, A.; Ferrari, R.; Laurenti, E.; Canesi, A.A.; Chiari, G. Spectrochemical and structural studies on a Roman sample of Egyptian blue. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1995, 51, 437–446. [Google Scholar] [CrossRef]
- Radpour, R.; Delaney, J.K.; Kakoulli, I. Acquisition of high spectral resolution diffuse reflectance image cubes (350–2500 nm) from archaeological wall paintings and other immovable heritage using a field-deployable spatial scanning reflectance spectrometry hyperspectral system. Sensors 2022, 22, 1915. [Google Scholar] [CrossRef]
- La Rocca, R.; Pitman, R.; Shahbazi, S.; Lópes, T.; Dallerba, E.; Massi, M.; Smith, G.D.; Lewis, S.W. Preliminary investigations into the use of the ancient pigments Han blue and Han purple as luminescent dusting powders for the detection of latent fingermarks. Forensic Sci. Int. 2024, 362, 112172. [Google Scholar] [CrossRef]
- Aceto, M.; Agostino, A.; Fenoglio, G.; Idone, A.; Gulmini, M.; Picollo, M.; Ricciardi, P.; Delaney, J.K. Characterisation of colourants on illuminated manuscripts by portable fibre optic UV-visible-NIR reflectance spectrophotometry. Anal. Methods 2014, 6, 1488–1500. [Google Scholar] [CrossRef]
- Siozos, P.; Philippidis, A.; Anglos, D. Portable laser-induced breakdown spectroscopy/diffuse reflectance hybrid spectrometer for analysis of inorganic pigments. Spectrochim. Acta Part B At. Spectrosc. 2017, 137, 93–100. [Google Scholar] [CrossRef]
- Subramanian, M.A.; Li, J. YInMn blue-200 Years in the making: New intense inorganic pigments based on chromophores in trigonal bipyramidal coordination. Mater. Today Adv. 2022, 16, 100323. [Google Scholar] [CrossRef]
- García-Fernández, P.; Moreno, M.; Aramburu, J.A. Origin of the exotic blue color of copper-containing historical pigments. Inorg. Chem. 2015, 54, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Artesani, A.; Lamuraglia, R.; Menegazzo, F.; Bonetti, S.; Traviglia, A. Terahertz time-domain spectroscopy in reflection configuration for inorganic and mineral pigment identification. Appl. Spectrosc. 2023, 77, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, H.G.; Berke, H. Chemical and physical investigations of Egyptian and Chinese blue and purple. Monum. Sites 2001, 3, 154–171. [Google Scholar]
- Berke, H.; Corbiere, T.; Portmann, A.; Freisinger, E.; Wild, F.; Hutter, J.; Lu, P.; Yin, X.; Qinglin, M.; Zhiguo, Z. Man-made ancient Chinese Blue and Purple pigments. Wenbo 2009, 6, 251–265. [Google Scholar]






| Element | Counts | ||
|---|---|---|---|
| Si | K | 466.8 | 24.5 |
| Ca | K | 13,525.2 | 155.5 |
| Ti | K | 195.0 | 26.8 |
| Fe | K | 688.7 | 37.2 |
| Ni | K | 123.8 | 27.3 |
| Cu | K | 187,378.9 | 435.0 |
| Zn | K | 785.9 | 41.6 |
| Sr | K | 162.7 | 23.5 |
| Pb | L | 606.2 | 36.8 |
| Element | Counts | ||
|---|---|---|---|
| Si | K | 362.1 | 22.4 |
| Cu | K | 81,089.3 | 287.0 |
| Zn | K | 283.2 | 29.9 |
| Sr | K | 3972.71 | 94.1 |
| Ba | K | 8993.3 | 107.0 |
| Ba | L | 34,846.4 | 196.0 |
| Pb | L | 390.1 | 35.8 |
| Element | Counts | ||
|---|---|---|---|
| Si | K | 143.7 | 16.3 |
| Ca | K | 251.0 | 41.6 |
| Ti | K | 528.5 | 114.0 |
| Fe | K | 87.4 | 18.9 |
| Ni | K | 131.5 | 20.4 |
| Cu | K | 54,558.9 | 236.0 |
| Zn | K | 262.6 | 25.6 |
| Sr | K | 3060.9 | 61.2 |
| Ba | K | 7119.9 | 87.4 |
| Ba | L | 21,309.6 | 178.0 |
| Pb | L | 490.1 | 32.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moffa, C.; Felici, A.C.; Petrarca, M. Terahertz Investigation of Cultural Heritage Synthetic Materials: A Case Study of Copper Silicate Pigments. Minerals 2025, 15, 490. https://doi.org/10.3390/min15050490
Moffa C, Felici AC, Petrarca M. Terahertz Investigation of Cultural Heritage Synthetic Materials: A Case Study of Copper Silicate Pigments. Minerals. 2025; 15(5):490. https://doi.org/10.3390/min15050490
Chicago/Turabian StyleMoffa, Candida, Anna Candida Felici, and Massimo Petrarca. 2025. "Terahertz Investigation of Cultural Heritage Synthetic Materials: A Case Study of Copper Silicate Pigments" Minerals 15, no. 5: 490. https://doi.org/10.3390/min15050490
APA StyleMoffa, C., Felici, A. C., & Petrarca, M. (2025). Terahertz Investigation of Cultural Heritage Synthetic Materials: A Case Study of Copper Silicate Pigments. Minerals, 15(5), 490. https://doi.org/10.3390/min15050490







