Uranium Mineral Particles Produced by Weathering in Sierra Peña Blanca, Chihuahua, Mexico: A Synchrotron-Based Study
Abstract
1. Introduction
2. Materials and Methods
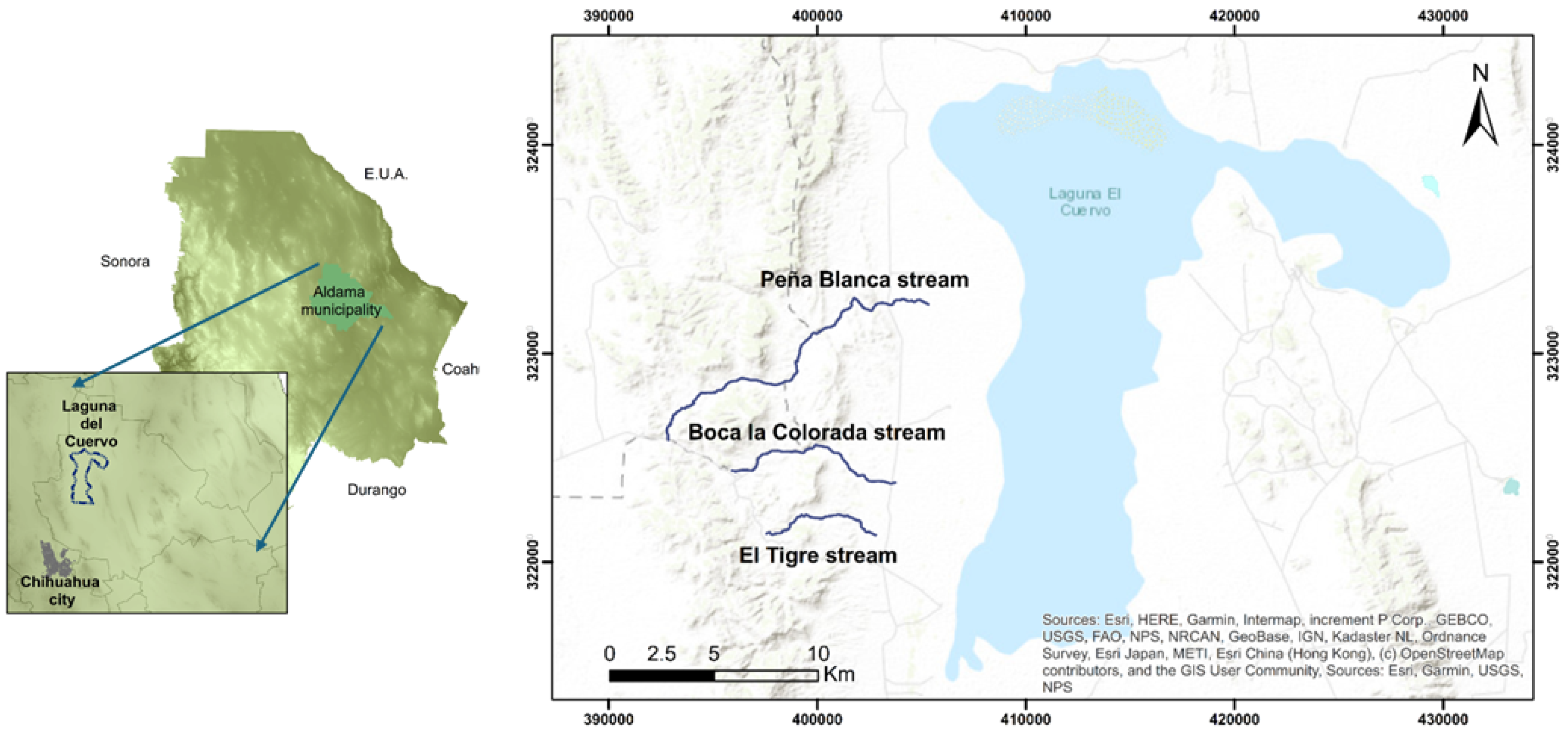
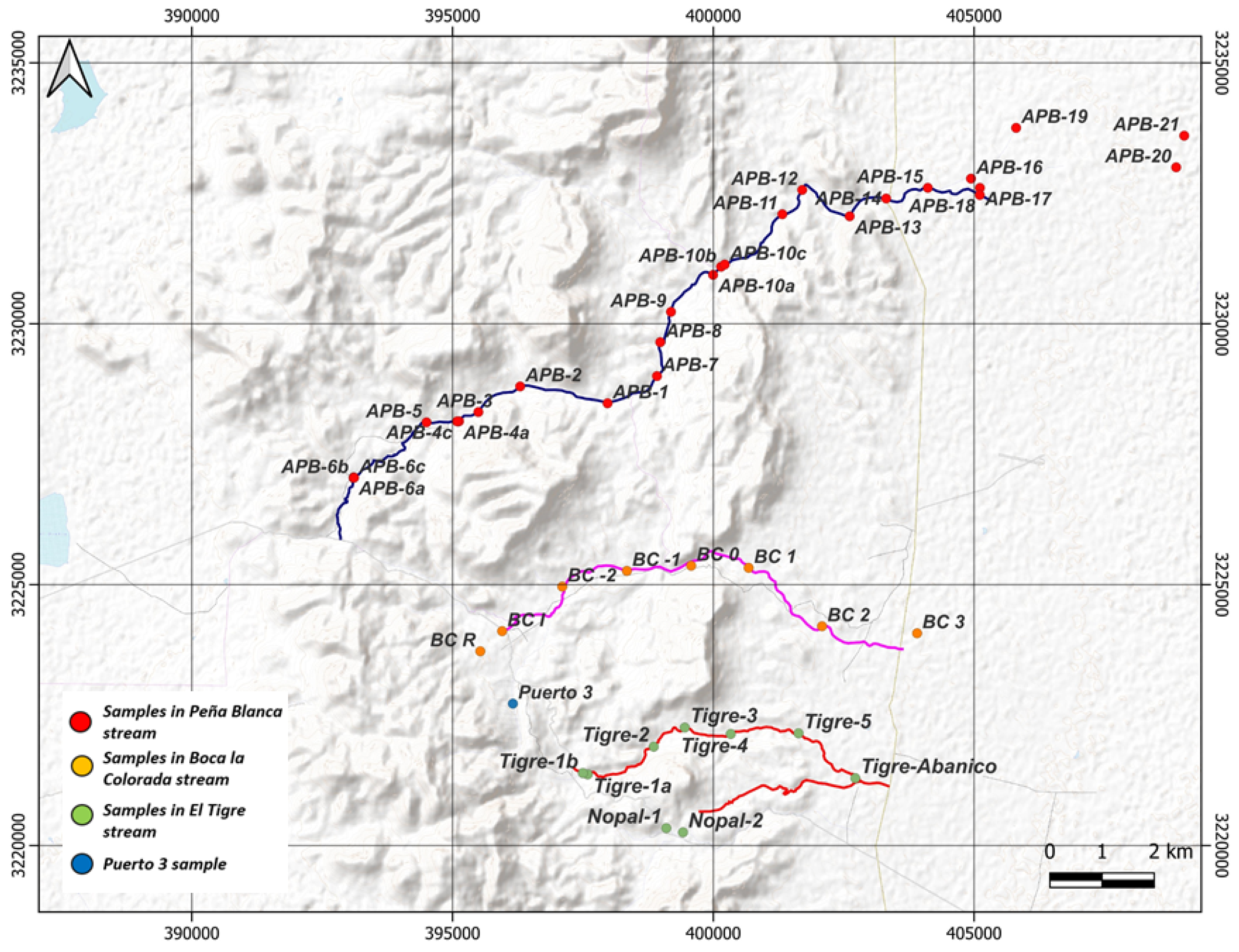
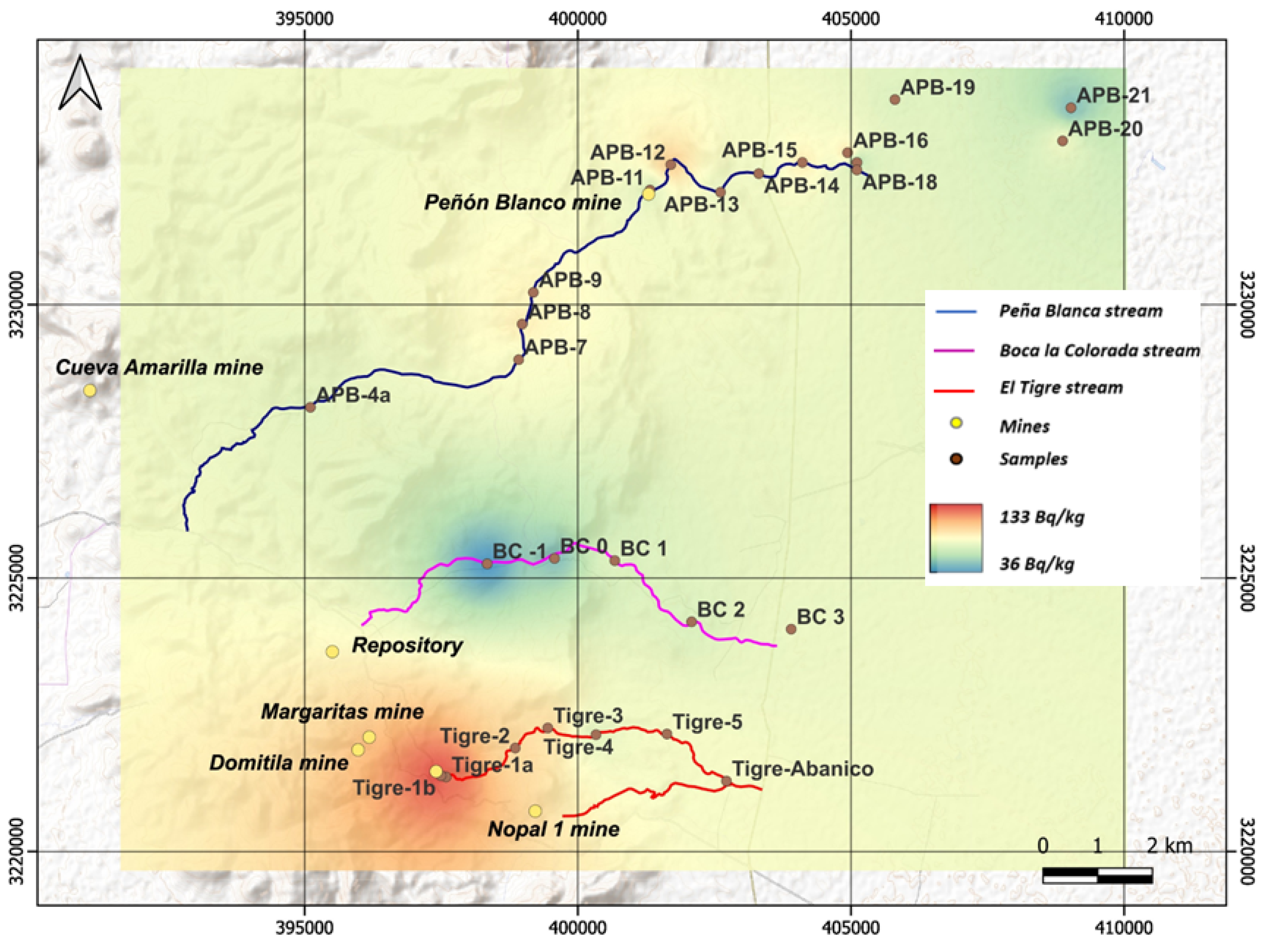
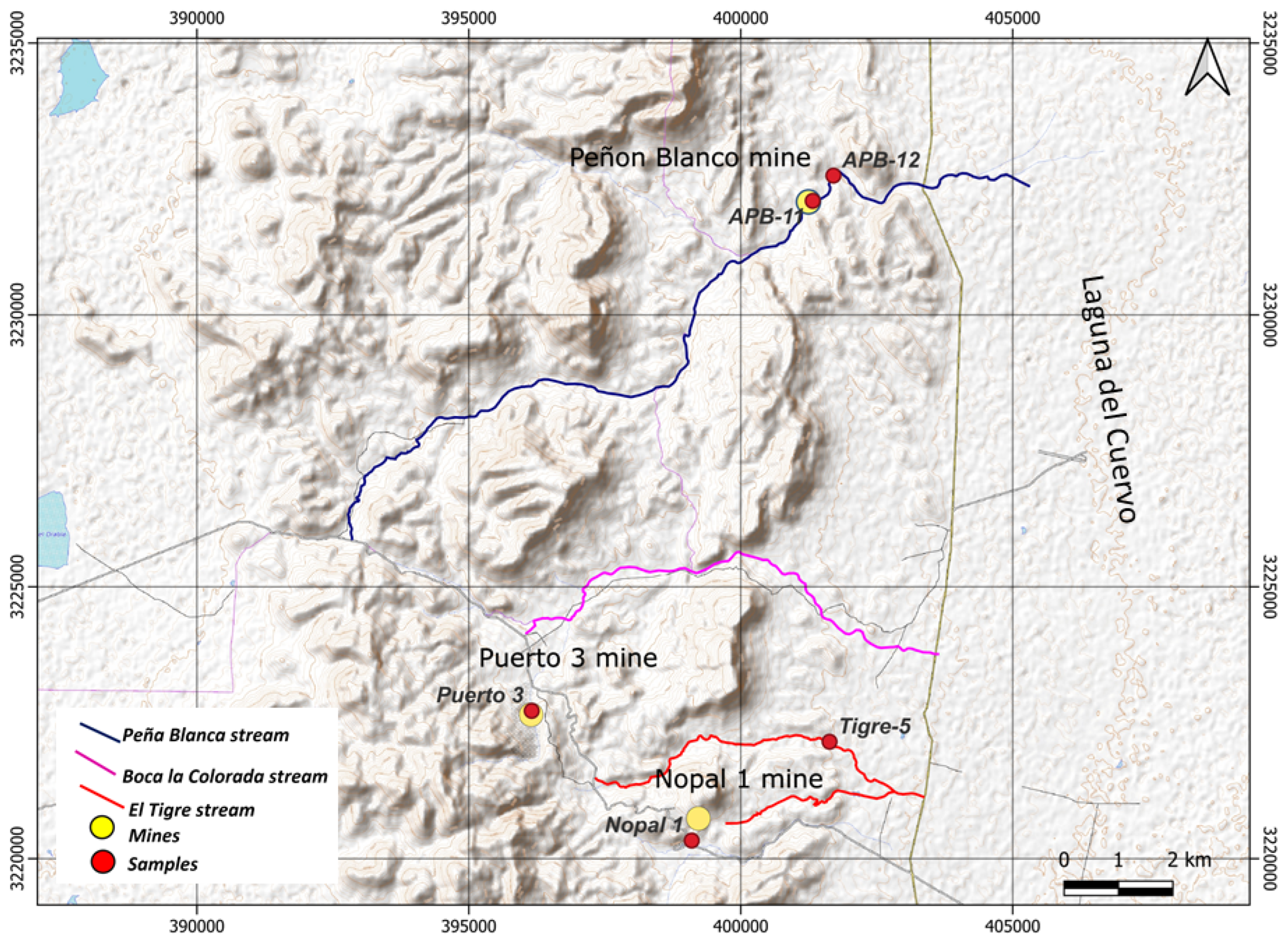
2.1. Sampling and Conditioning of Sediments
2.2. Characterization of Sediment Samples
2.2.1. X-Ray Diffraction (XRD)
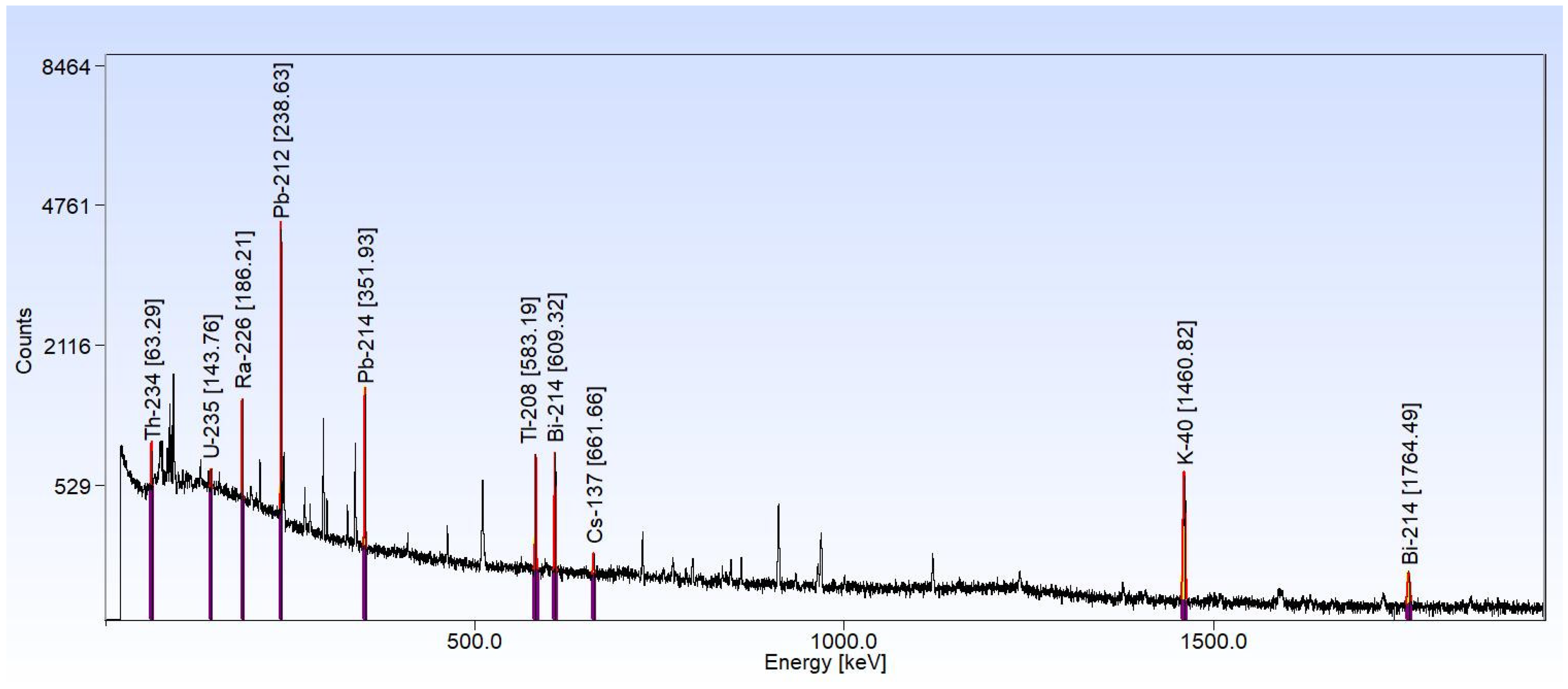
2.2.2. High-Resolution Gamma Spectrometry on Granulometric Fractions of Sediments
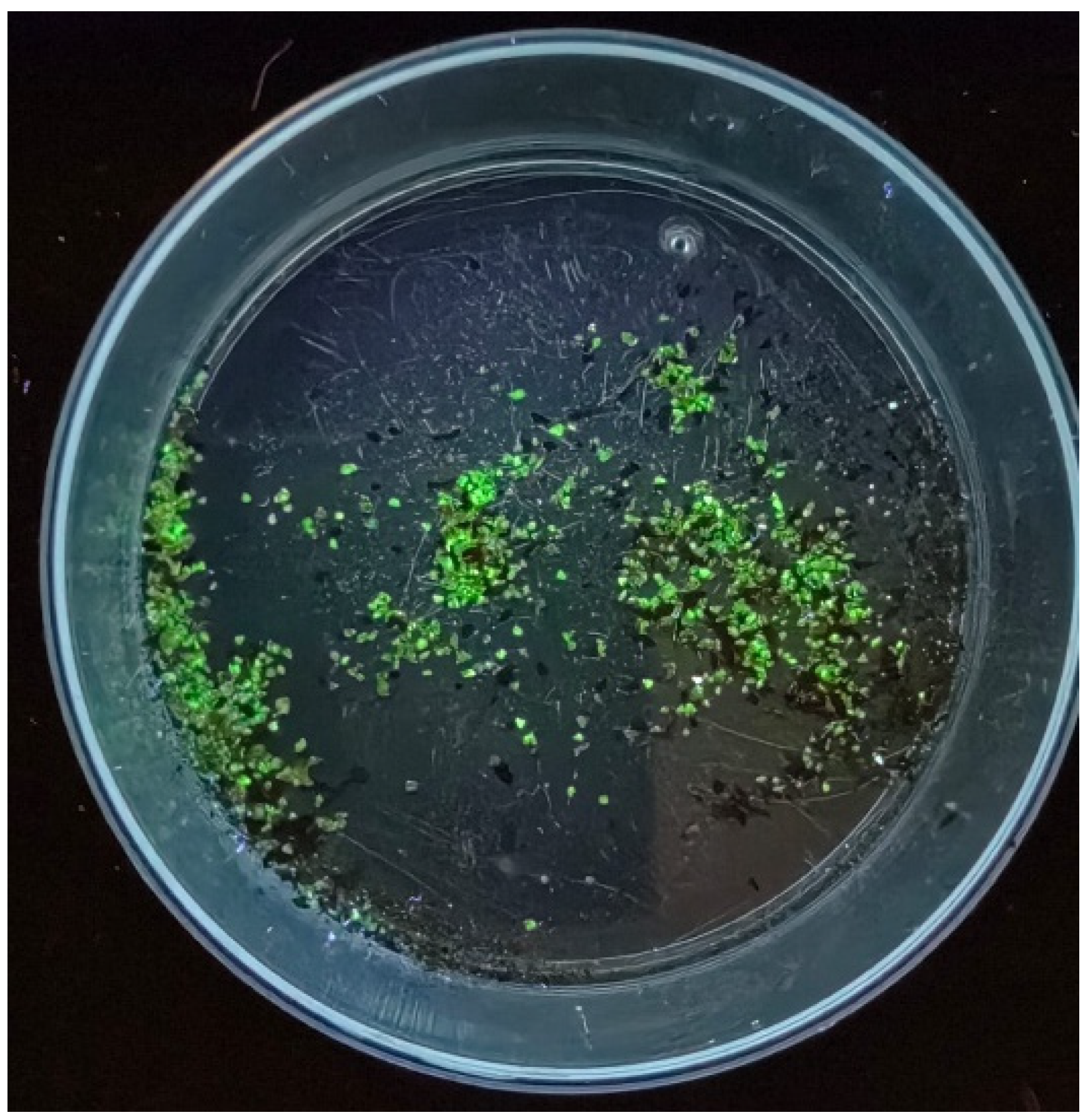
2.3. Selection of U Mineral Particles
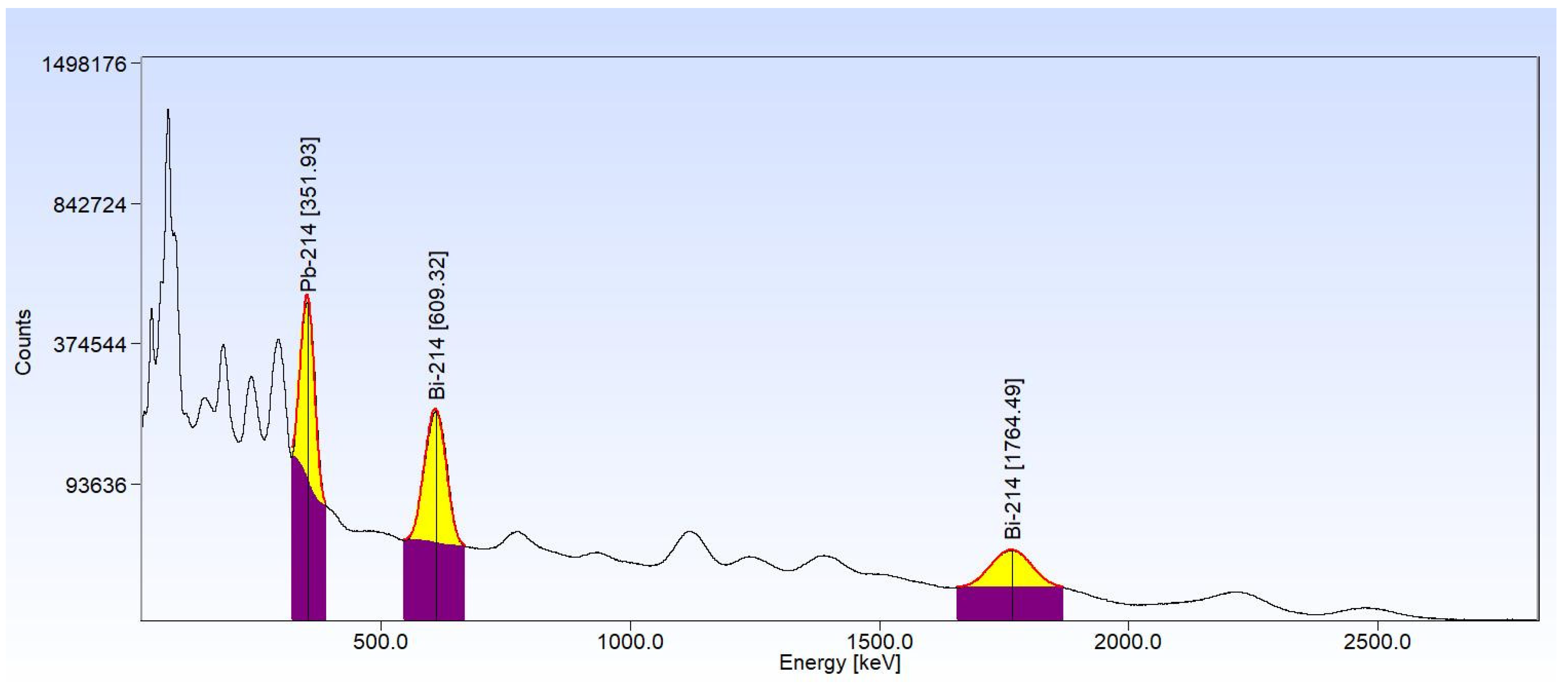
2.4. Quantification of U Particle Density in Fine Sand by Gamma Spectrometry
- A fine sand sample was selected from a remote position outside the mine drainage pattern, which displayed the lowest U activity concentration in the study area obtained separately by γ-ray spectrometry. This sample is a sediment “matrix blank” with the inherent U content of igneous rocks, of activity concentration (1.79 ± 0.02) Bq/g.
- From the fraction with grain size 0.279 mm < d < 1.19 mm of sample APB-11, 3336 particles were identified and extracted according to the procedure mentioned in Section 2.3. The extracted particles, whose total mass was 0.3764 ± 0.0001 g, were added to an aliquot of the matrix blank. The mixture was homogenized, placed in a vial, and measured in the same geometry. The added activity due to the particles was 0.26 ± 0.02 Bq. The estimated mass per particle was 0.112 ± 0.001 mg, and the assessed 238U activity per particle was Acteach particle = (0.77 ± 0.06) × 10−4 Bq/part.
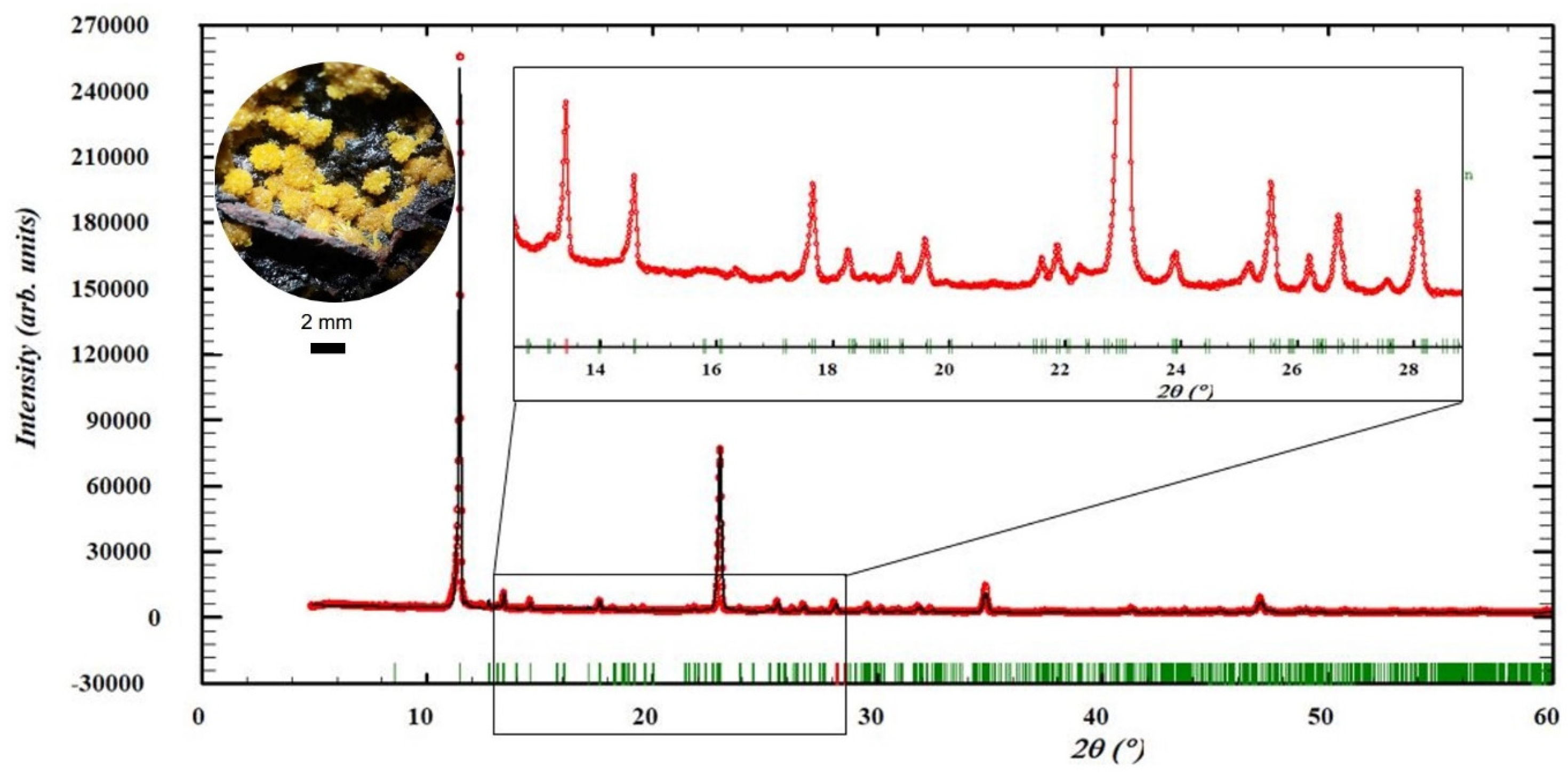
- An activity concentration reference sample was prepared with the same matrix blank described in paragraph 1 of this section. In this case, a mass of 0.9554 ± 0.0001 g of pure parauranophane crystals extracted from Peña Blanca was added to the blank, and its purity was determined by XRD. The diffraction pattern of parauranophane (URP) reference material analyzed by the Rietveld method is presented in Appendix A.5, Figure A3. With the resulting concentration of pure parauranophane, the activity of added 238U was ActURP = 6565 ± 65 Bq.
2.5. Morphology and Composition of Individual Particles
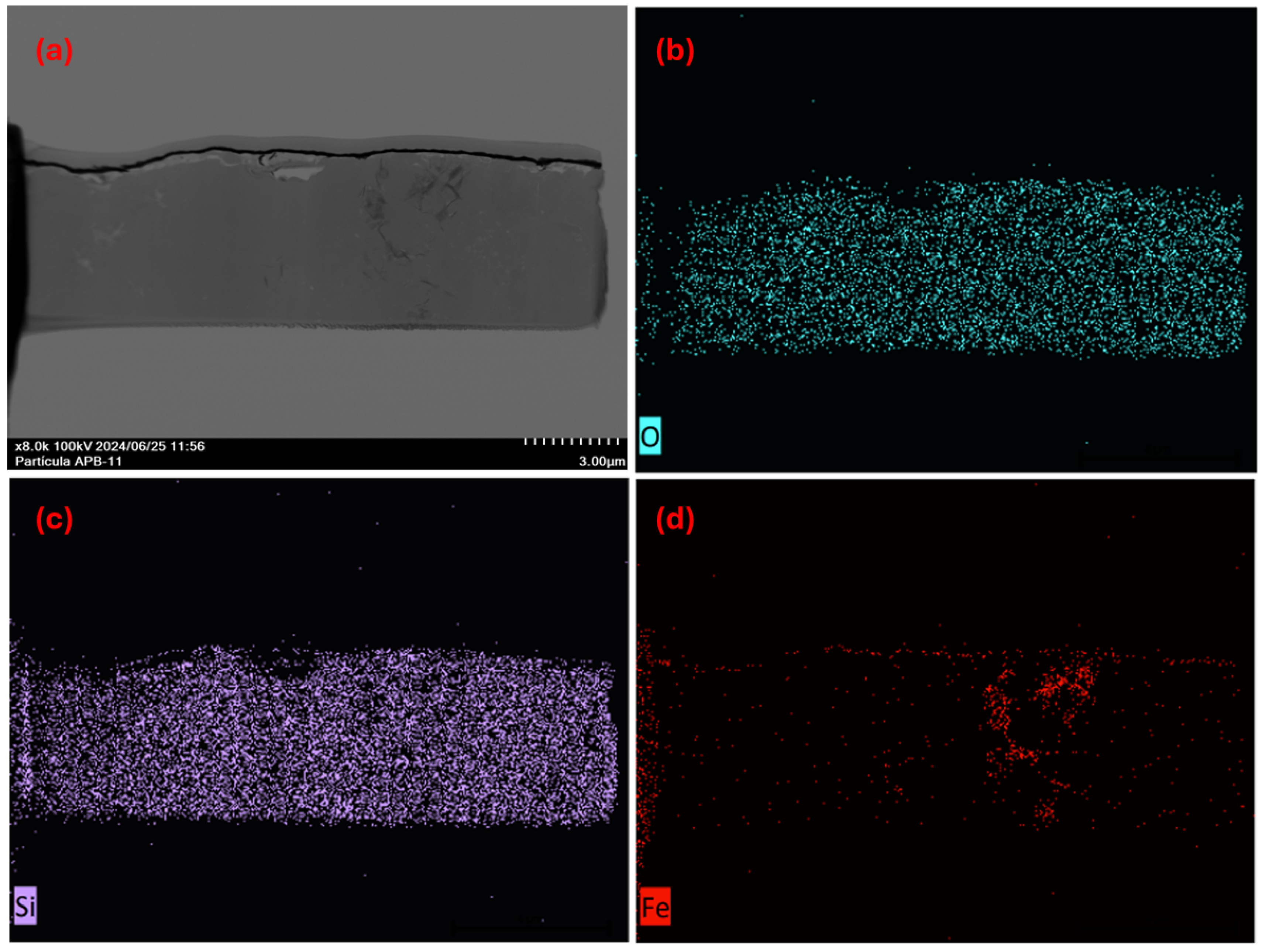
2.5.1. Focused Ion Beam and Scanning-Transmission Electron Microscopes
2.5.2. X-Ray Absorption Spectroscopy (XAS)
2.5.3. X-Ray Fluorescence Micro Computed Tomography (XRF µ-CT)
Sample Preparation
Data Collection at I18 Beamline, Diamond Light Source
3. Results
3.1. X-Ray Diffraction (XRD)
3.2. High-Resolution Gamma-Ray Spectrometry
3.3. Quantification of U Particle Density
3.4. Morphology and Composition of Individual Particles
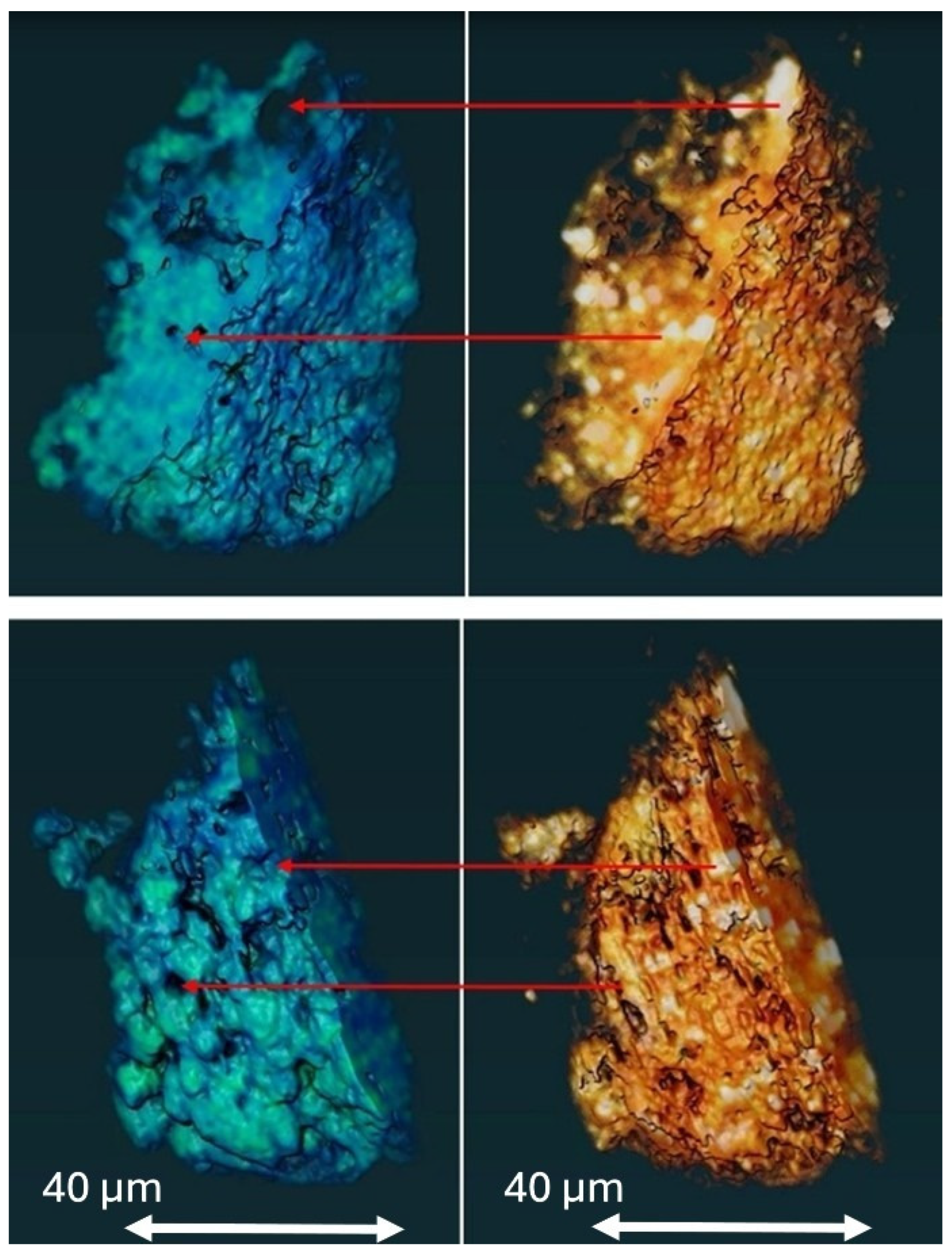
3.4.1. Microscopic Characterization of Fragmented Mineral Particles by FIB-STEM
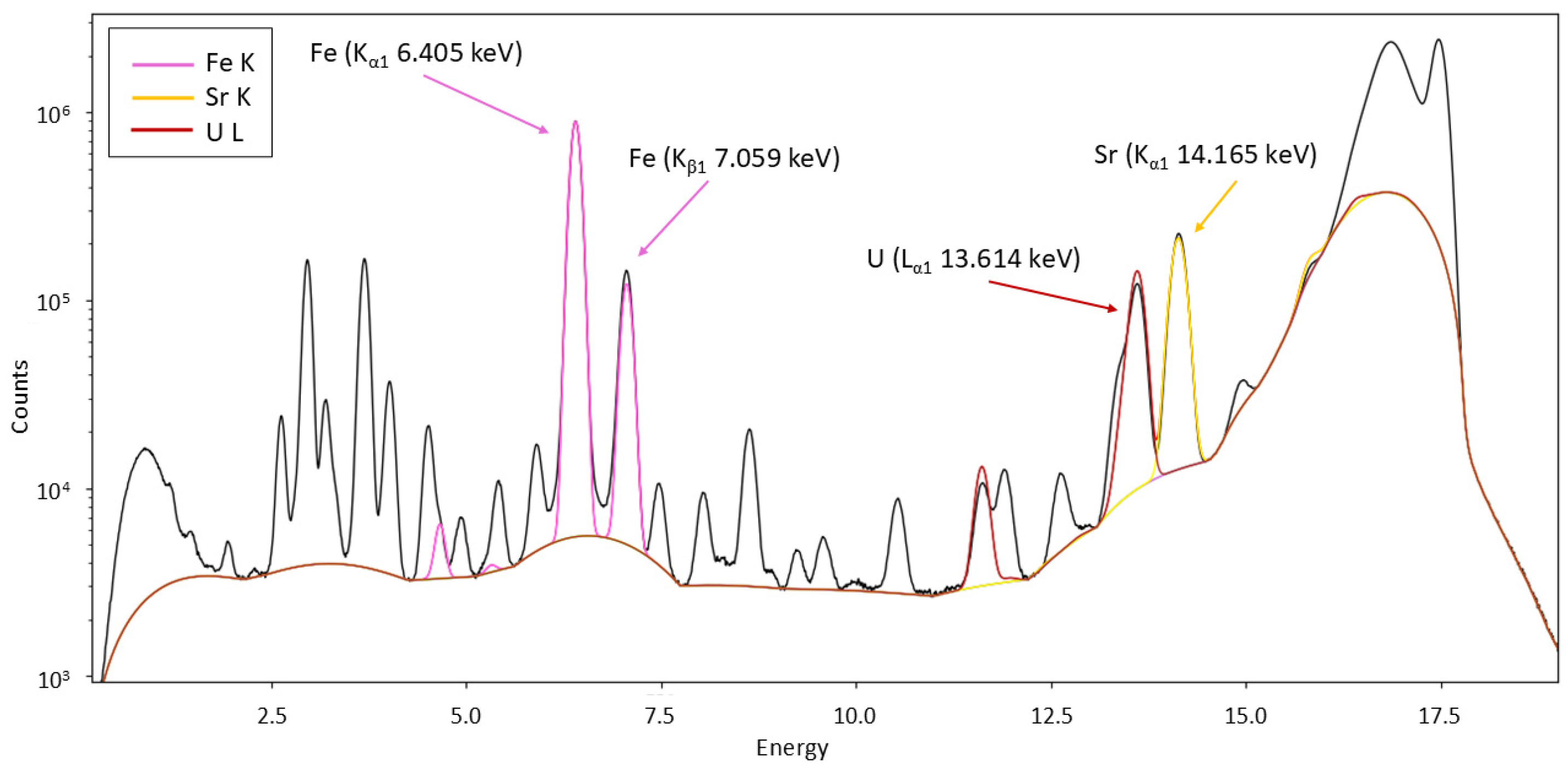
3.4.2. X-Ray Absorption Spectroscopy (XAS)
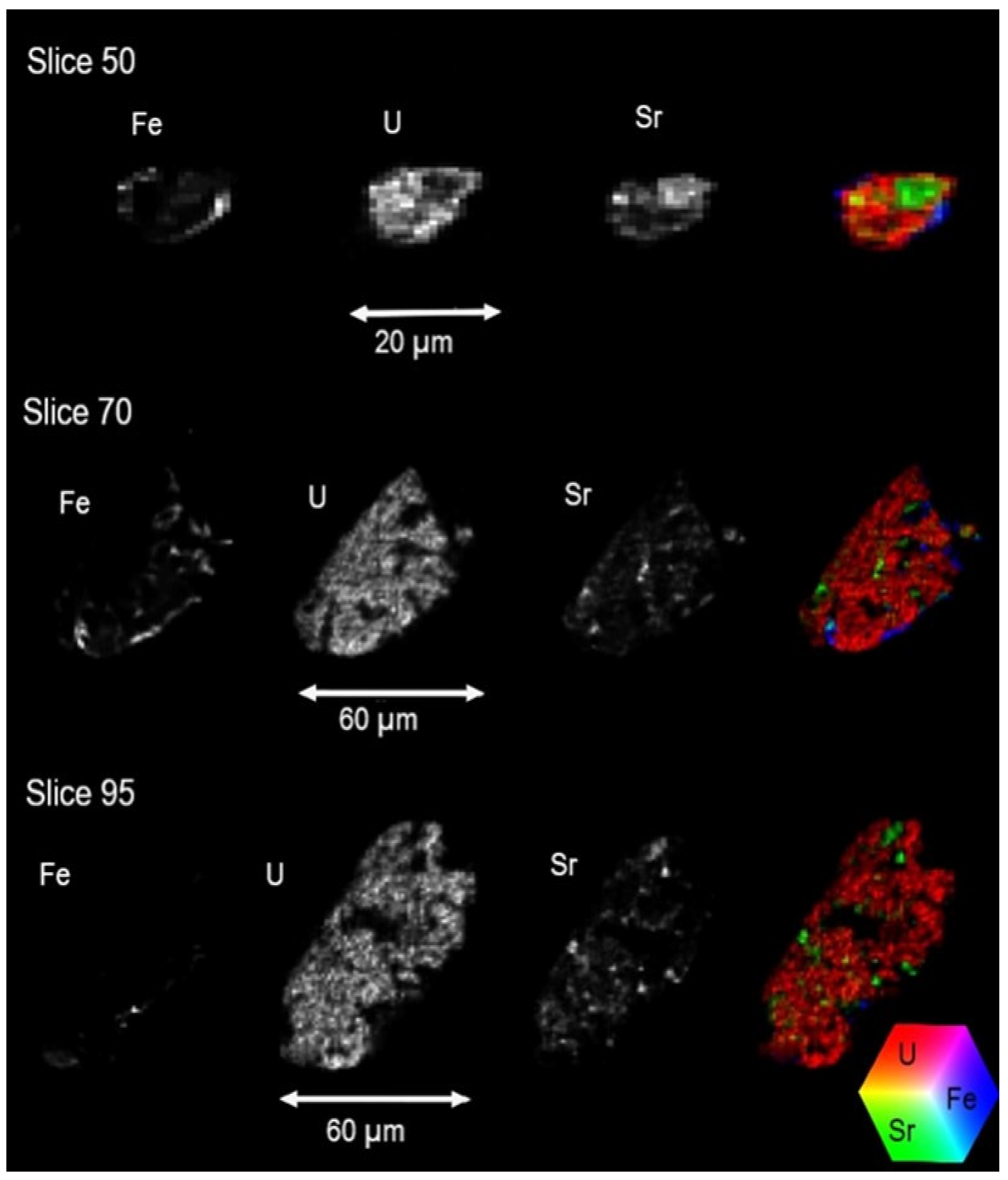
3.4.3. X-Ray Fluorescence MicroTomography (XRF-µCT)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| SPB | Sierra Peña Blanca |
| XRD | X-ray diffraction |
| ICP-OES | Inductively coupled plasma atomic emission spectroscopy |
| FIB | Focused ion beam microscopy |
| STEM | Scanning transmission electron microscopy |
| XAS | X-ray absorption spectrometry |
| XANES | X-ray Absorption Near Edge Structure |
| EXAFS | Extended X-ray Absorption Fine Structure |
| XRF-µCT | X-ray fluorescence microtomography |
| CIMAV | Centro de Investigación en Materiales Avanzados |
| SEM | Scanning electron microscopy |
| EDS | Energy dispersive X-ray spectroscopy |
| RM | Rietveld method |
| Ab | Albite |
| An | Anorthite |
| Cal | Calcite |
| Hly | Halloysite |
| Kln | Kaolinite |
| Mag | Magnetite |
| Mnt | Montmorillonite |
| Ms | Muscovite |
| Or | Orthoclase |
| Qz | Quartz |
| Sa | Sanidine |
| DL | Detection Limit |
Appendix A. Details of the Materials’ Characterization
Appendix A.1. Sediment Sampling in Streams

Appendix A.2. Calculation of Activities of the Isotopes 214Pb and 214Bi by the Relative Method in the HPGe and XtRa Spectrometers
Appendix A.3. Fluorescence of U Minerals Coated with Silica in SPB
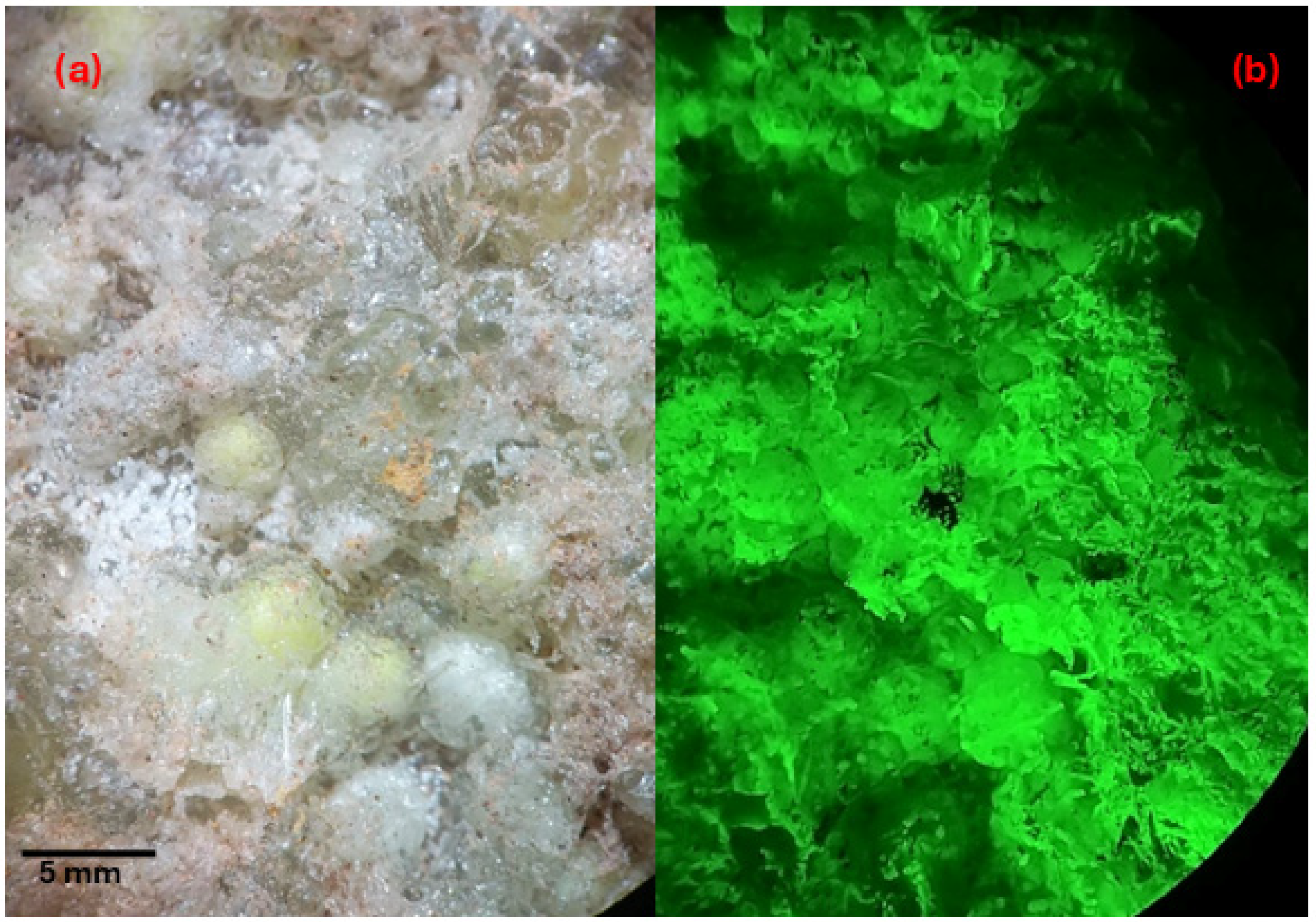
Appendix A.4. Calculation of Particle Concentration in Sediment Samples in the NaI(Tl) Detector
Appendix A.5. XRD Pattern of Parauranophane
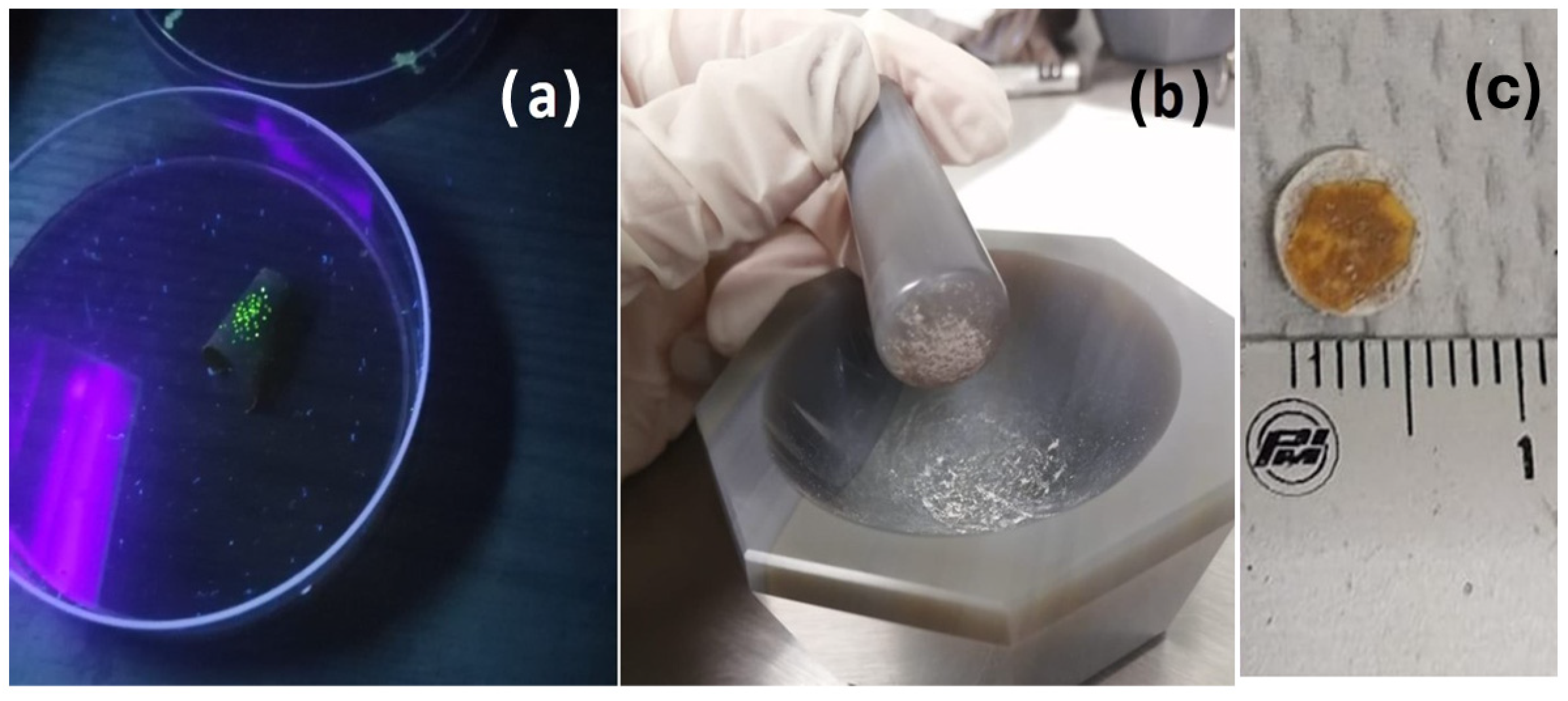
Appendix A.6. Sample Preparation for FIB-STEM

Appendix A.7. Sample Preparation for XAS and XRF-μCT

Appendix B. Additional XRD Results
| Location | Total Number of Samples | Type | Mineral Phase (%) | |||||||
| Qz | Cal | Mnt | Mag | Ab | Sa | Kln | An | |||
| APB This work | 19 | FSC | X | X | X | X | X | X | X | X |
| AET Rodríguez-Guerra [27] | 9 | FSD | ||||||||
| CSC | ||||||||||
| FSC | X | X | X | X | X | X | X | X | ||
| ABLC Hernández-Hernández [28] | 7 | FSD | X | X | X | X | X | |||
| CSC | X | X | X | X | X | |||||
| FSC | X | X | X | X | X | X | ||||
| ABLC Pérez-Reyes [29] | 4 | Mud | X | X | X | X | X | X | ||
Appendix C. Table of Results for the Activity Concentration
| Location | Sample | Aconc of 214Pb (Bq/kg) |
| APB This work | APB-4a | 76 |
| APB-7 | 83 | |
| APB-8 | 90 | |
| APB-9 | 88 | |
| APB-11 | 75 | |
| APB-12 | 100 | |
| APB-13 | 79 | |
| APB-14 | 80 | |
| APB-15 | 90 | |
| APB-16 | 94 | |
| APB-17 | 60 | |
| APB-18 | 79 | |
| APB-19 | 74 | |
| APB-20 | 79 | |
| APB-21 | 50 | |
| AET Rodríguez-Guerra [27] | Tigre-1a | 120 |
| Tigre-1b | 133 | |
| Tigre-2 | 98 | |
| Tigre-3 | 96 | |
| Tigre-4 | 76 | |
| Tigre-5 | 82 | |
| Tigre-Abanico | 83 | |
| ABLC Hernández-Hernández [28] | BC −1 | 37 |
| BC 0 | 50 | |
| BC 1 | 74 | |
| BC 2 | 73 | |
| BC 3 | 79 | |
| BC 4 | 33 |
Appendix D. Silica in SPB

References
- Angiboust, S.; Fayek, M.; Power, I.M.; Camacho, A.; Calas, G.; Southam, G. Structural and biological control of the Cenozoic epithermal uranium concentrations from the Sierra Peña Blanca, Mexico. Miner. Depos. 2012, 47, 859–874. [Google Scholar] [CrossRef]
- Alvarez, M. Provincias fisiograficas de la Republica Mexicana. Bol. Soc. Geol. Mex. 1961, 24, 3–20. [Google Scholar]
- INEGI Síntesis de Información Geográfica del Estado de Chihuahua. 2003. Available online: https://www.inegi.org.mx/contenidos/productos/prod_serv/contenidos/espanol/bvinegi/productos/historicos/2104/702825224332/702825224332_5.pdf (accessed on 19 March 2025).
- CONAGUA Actualización de la Disponibilidad Media Anual de Agua en el Acuífero Laguna de Hormigas (0824), Estado de Chihuahua. 2020. Available online: https://sigagis.conagua.gob.mx/gas1/Edos_Acuiferos_18/chihuahua/DR_0824.pdf (accessed on 19 March 2025).
- Goodell, P.C. Chihuahua City Uranium Province, Chihuahua, Mexico. In Proceedings of the Technical Committee Meeting on Uranium Deposits in Volcanic Rocks, El Paso, TX, USA, 2–5 April 1984; pp. 97–124. [Google Scholar]
- Reyes-Cortés, M. Deposito de molibdeno asociado con uranio en Peña Blanca, Mexico. In Proceedings of the Technical Committee Meeting on Uranium Deposits in Volcanic Rocks, El Paso, TX, USA, 2–5 April 1984; IAEA: Vienna, Austria, 1985; pp. 161–174, ISBN 92-0-041085-5. [Google Scholar]
- Cárdenas-Flores, D. Volcanic stratigraphy and U-Mo mineralization of the Sierra de Peña Blanca district, Chihuahua, México. In Proceedings of the Technical Committee Meeting on Uranium Deposits in Volcanic Rocks, El Paso, TX, USA, 2–5 April 1984; IAEA: Vienna, Austria, 1985; pp. 125–136, ISBN 92-0-041085-5. [Google Scholar]
- Dobson, P.F.; Fayek, M.; Goodell, P.C.; Ghezzehei, T.A.; Melchor, F.; Murrel, M.T.; Oliver, R.; Reyes-Cortés, I.A.; De La Garza, R.; Simmons, A. Stratigraphy of the PB-1 Well, Nopal I Uranium Deposit, Sierra Peña Blanca, Chihuahua, Mexico. Int. Geol. Rev. 2008, 50, 959–974. [Google Scholar]
- Faudoa, F.G. Modelo de la Evolución de las Especies Minerales Superficiales de Uranio de la Sierra Peña Blanca, Chihuahua, México. Master´s Thesis, Centro de Investigación en Materiales Avanzados, Chihuahua, México, 2023. [Google Scholar]
- George-Aniel, B.; Leroy, J.L.; Poty, B. Volcanogenic uranium mineralizations in the Sierra Pena Blanca District, Chihuahua, Mexico; three genetic models. Econ. Geol. 1991, 86, 233–248. [Google Scholar] [CrossRef]
- Schmidt, R.H. A climatic delineation of the ‘real’ Chihuahuan Desert. J. Arid Environ. 1979, 2, 243–250. [Google Scholar] [CrossRef]
- Hernandez Herrera, C.; Canche Tello, J.G.; Cabral Lares, R.M.; Rodriguez Guerra, Y.; Perez Reyes, V.; Faudoa Gomez, F.G.; Esparza Ponce, H.E.; Reyes Cortez, I.A.; Hernandez Cruz, D.; Loredo Portales, R.; et al. Interpretation of X-ray Absorption Spectra by synchrotron radiation of the uranium mineral species transported by the main stream “El Tigre”, in Peña Blanca, Chihuahua, Mexico. Supl. Rev. Mex. Física 2024, 5, 1–7. [Google Scholar] [CrossRef]
- Nichols, G. Sedimentology and Stratigraphy, 2nd ed.; Wiley-Blackwell: Chichester, UK, 2010; ISBN 978-1-4051-3592-4. [Google Scholar]
- Guzmán-Martínez, F.; Arranz-González, J.-C.; Tapia-Téllez, A.; Prazeres, C.; García-Martínez, M.-J.; Jiménez-Oyola, S. Assessment of potential contamination and acid drainage generation in uranium mining zones of Peña Blanca, Chihuahua, Mexico. Environ. Monit. Assess. 2023, 195, 386. [Google Scholar] [CrossRef] [PubMed]
- Schindler, M.; Fayek, M.; Hawthorne, F.C. Uranium-rich opal from the Nopal I uranium deposit, Peña Blanca, Mexico: Evidence for the uptake and retardation of radionuclides. Geochim. Cosmochim. Acta 2010, 74, 187–202. [Google Scholar] [CrossRef]
- Schindler, M.; Fayek, M.; Courchesne, B.; Kyser, K.; Hawthorne, F.C. Uranium-bearing opals: Products of U-mobilization, diffusion, and transformation processes. Am. Mineral. 2017, 102, 1154–1164. [Google Scholar] [CrossRef]
- Dawood, Y. Factors Controlling Uranium and Thorium Isotopic Composition of the Streambed Sediments of the River Nile, Egypt. J. King Abdulaziz Univ.-Earth Sci. 2010, 21, 77–103. [Google Scholar] [CrossRef]
- Chabaux, F.; Bourdon, B.; Riotte, J. Chapter 3 U-Series Geochemistry in Weathering Profiles, River Waters and Lakes. In Radioactivity in the Environment; Elsevier: Amsterdam, The Netherlands, 2008; Volume 13, pp. 49–104. ISBN 978-0-08-045012-4. [Google Scholar]
- Bosia, C.; Chabaux, F.; Pelt, E.; France-Lanord, C.; Morin, G.; Lavé, J.; Stille, P. U–Th–Ra variations in Himalayan river sediments (Gandak river, India): Weathering fractionation and/or grain-size sorting? Geochim. Cosmochim. Acta 2016, 193, 176–196. [Google Scholar] [CrossRef]
- Adams, J.A.S. The uranium geochemistry of latten Volcanic National Park, California. Geochim. Cosmochim. Acta 1955, 8, 74–85. [Google Scholar] [CrossRef]
- Hurley, P.M. Direct radiometric measurement by gamma-ray scintillation spectrometer: Part i: Uranium and thorium series in equilibrium. Bull. Geol. Soc. Am. 1956, 67, 395–404. [Google Scholar] [CrossRef]
- Hurley, P.M. Direct radiometric measurement by gamma-ray scintillation spectrometer: Part ii: Uranium, thorium, and potassium in common rocks. Bull. Geol. Soc. Am. 1956, 67, 405–412. [Google Scholar] [CrossRef]
- Adams, J.A.S.; Richardson, J.E.; Templeton, C.C. Determinations of thorium and uranium in sedimentary rocks by two independent methods. Geochim. Cosmochim. 1958, 13, 270–279. [Google Scholar] [CrossRef]
- Mero, J.L. Uses of the gamma-ray spectrometer in mineral exploration. Geophysics 1960, 25, 1054–1076. [Google Scholar] [CrossRef]
- United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and Effects of Ionizing Radiation, United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) 2008 Report; United Nations Scientific Committee on the Effects of Atomic Radiation: Vienna, Austria, 2010; Volume 1, p. 471. [Google Scholar] [CrossRef]
- Colmenero, S.L.; Montero, C.M.E.; Villalba, L. Natural Radioactivity in Soils of the Main Cities of the State of Chihuahua. In Proceedings of the Technical Committee Meeting on 14. Annual Congress of the SNM; 21. Annual Meeting of the SMSR, Energia Nuclear y Seguridad Radiologica: Nuevos Retos y Perspectivas, Guadalajara, Mexico, 10–13 September 2003; p. 8. [Google Scholar]
- Rodríguez-Guerra, Y. Transporte de Isótopos Radiactivos de la Serie del Uranio Desde Peña Blanca Hasta Laguna del Cuervo a Través del Arroyo El Tigre, Chihuahua. Master´s Thesis, Centro de investigación en Materiales Avanzados, Chihuahua, México, 2023. [Google Scholar]
- Hernández-Hernández, D. Transporte y Equilibrio Radioactivo del Uranio Desde el Yacimiento de Peña Blanca Hasta Laguna del Cuervo, Chihuahua. Master´s Thesis, Centro de investigación en Materiales Avanzados, Chihuahua, México, 2019. [Google Scholar]
- Pérez-Reyes, V.; Cabral-Lares, R.M.; Méndez-García, C.G.; Caraveo-Castro, C.D.R.; Reyes-Cortés, I.A.; Carrillo-Flores, J.; Montero-Cabrera, M.E. Transport and concentration of uranium isotopes in the Laguna del Cuervo, Chihuahua, México. Supl. Rev. Mex. Física 2022, 3, 010606. [Google Scholar] [CrossRef]
- Pérez-Reyes, V.; Cabral-Lares, R.M.; Canche-Tello, J.G.; Rentería-Villalobos, M.; González-Sánchez, G.; Carmona-Lara, B.P.; Hernández-Herrera, C.; Faudoa-Gómez, F.; Rodríguez-Guerra, Y.; Vázquez-Olvera, G.; et al. Uranium Mineral Transport in the Peña Blanca Desert: Dissolution or Fragmentation? Simulation in Sediment Column Systems. Appl. Sci. 2025, 15, 609. [Google Scholar] [CrossRef]
- Salbu, B.; Lind, O.C. Analytical techniques for charactering radioactive particles deposited in the environment. J. Environ. Radioact. 2020, 211, 106078. [Google Scholar] [CrossRef]
- Brown, G.E.; Calas, G.; Waychunas, G.A.; Petiau, J. X-ray absorption spectroscopy: Applications in mineralogy and geochemistry Reviews. In Mineralogy and Geochemistry; Hawthorne, F.C., Ed.; BookCrafters, Inc.: Chelsea, MI, USA, 1988; Volume 18, pp. 431–512. [Google Scholar]
- Manceau, A.; Marcus, M.A.; Tamura, N. Quantitative Speciation of Heavy Metals in Soils and Sediments by Synchrotron X-ray Techniques. Rev. Mineral. Geochem. 2002, 49, 341–428. [Google Scholar] [CrossRef]
- Nascimento-Dias, B.L.; Araujo, O.M.O.; Machado, A.S.; Oliveira, D.F.; Anjos, M.J.; Lopes, R.T.; Assis, J.T. Analysis of two meteorite fragments (lunar and martian) using X-Ray microfluorescence and X-Ray computed microtomography techniques. Appl. Radiat. Isot. 2019, 152, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Dent, A.J.; Ramsay, J.D.F.; Swanton, S.W. An EXAFS study of uranyl ion in solution and sorbed onto silica and montmorillonite clay colloids. J. Colloid Interface Sci. 1992, 150, 45–60. [Google Scholar] [CrossRef]
- Farges, F.; Ponader, C.W.; Calas, G.; Brown, G.E., Jr. Structural environments of incompatible elements in silicate glass/melt systems: II. U(IV), U(V), and U(VI). Geochim. Cosmochim. Acta 1992, 56, 4205–4220. [Google Scholar]
- Bertsch, P.M.; Hunter, D.B.; Sutton, S.R.; Bajt, S.; Rivers, M.L. In situ Chemical Speciation of Uranium in Soils and Sediments by Micro X-ray Absorption Spectroscopy. Environ. Sci. Technol. 1994, 28, 980–984. [Google Scholar] [CrossRef]
- ISO-18400-102; Soil Quality—Sampling—Part 102: Selection and Application of Sampling Techniques. International Organization for Standardization: Geneva, Switzerland, 2017; p. 71.
- Wentworth, C.K. A Scale of Grade and Class Terms for Clastic Sediments. J. Geol. 1922, 30, 377–392. [Google Scholar] [CrossRef]
- Folk, R.L. The Distinction between Grain Size and Mineral Composition in Sedimentary-Rock Nomenclature. J. Geol. 1954, 62, 344–359. [Google Scholar] [CrossRef]
- Switzer, A.D. 14.19 Measuring and Analyzing Particle Size in a Geomorphic Context. In Treatise on Geomorphology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 224–242. ISBN 978-0-08-088522-3. [Google Scholar]
- Rodríguez-Carbajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar]
- Gilmore, G. Practical Gamma-Ray Spectroscopy, 2nd ed.; John Wiley & Sons.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Cook, M.; Etschmann, B.; Ram, R.; Ignatyev, K.; Gervinskas, G.; Conradson, S.D.; Cumberland, S.; Wong, V.N.L.; Brugger, J. The nature of Pu-bearing particles from the Maralinga nuclear testing site, Australia. Sci. Rep. 2021, 11, 10698. [Google Scholar] [CrossRef]
- Newville, M. Fundamentals of XAFS. Rev. Mineral. Geochem. 2014, 78, 33–74. [Google Scholar] [CrossRef]
- Townsend, L.T.; Smith, K.F.; Winstanley, E.H.; Morris, K.; Stagg, O.; Mosselmans, J.F.W.; Livens, F.R.; Abrahamsen-Mills, L.; Blackham, R.; Shaw, S. Neptunium and Uranium Interactions with Environmentally and Industrially Relevant Iron Minerals. Minerals 2022, 12, 165. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Solé, V.A.; Papillon, E.; Cotte, M.; Walter, P.; Susini, J. A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim. Acta Part B At. Spectrosc. 2007, 62, 63–68. [Google Scholar] [CrossRef]
- Gürsoy, D.; De Carlo, F.; Xiao, X.; Jacobsen, C. TomoPy: A framework for the analysis of synchrotron tomographic data. J. Synchrotron Radiat. 2014, 21, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Basham, M.; Filik, J.; Wharmby, M.T.; Chang, P.C.Y.; El Kassaby, B.; Gerring, M.; Aishima, J.; Levik, K.; Pulford, B.C.A.; Sikharulidze, I.; et al. Data Analysis WorkbeNch (DAWN). J. Synchrotron Radiat. 2015, 22, 853–858. [Google Scholar] [CrossRef]
- Thermo Scientific Avizo Software, Version 9; Thermo Fisher: Waltham, MA, USA, 2018.
- Bès, R.; Rivenet, M.; Solari, P.-L.; Kvashnina, K.O.; Scheinost, A.C.; Martin, P.M. Use of HERFD–XANES at the U L3- and M4-Edges to Determine the Uranium Valence State on [Ni(H2 O)4]3[U(OH,H2O)(UO2)8O12(OH)3]. Inorg. Chem. 2016, 55, 4260–4270. [Google Scholar] [CrossRef]
- Catalano, J.G.; Brown, G.E., Jr. Analysis of uranyl-bearing phases by EXAFS spectroscopy: Interferences, multiple scattering, accuracy of structural parameters, and spectral differences. Am. Mineral. 2004, 89, 1004–1021. [Google Scholar] [CrossRef]
- Ginderow, D. Structure de l’uranophane alpha, Ca(UO2)2(SiO3OH)2·5H2O. Acta Crystallogr. C 1988, 44, 421–424. [Google Scholar] [CrossRef]
- Kelly, S.D. Uranium Chemistry in Soils and Sediments. In Developments in Soil Science; Elsevier: Amsterdam, The Netherlands, 2010; Volume 34, pp. 411–466. ISBN 978-0-444-53261-9. [Google Scholar]
- Lind, O.C.; De Nolf, W.; Janssens, K.; Salbu, B. Micro-analytical characterisation of radioactive heterogeneities in samples from Central Asian TENORM sites. J. Environ. Radioact. 2013, 123, 63–70. [Google Scholar] [CrossRef]
- Haynes, W.M. (Ed.) CRC Handbook of Chemistry and Physics, 93rd ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-0-429-11122-8. [Google Scholar]
- Mariner, P.E.; Holzmer, F.J.; Jackson, R.E.; Meinardus, H.W.; Wolf, F.G. Effects of High pH on Arsenic Mobility in a Shallow Sandy Aquifer and on Aquifer Permeability along the Adjacent Shoreline, Commencement Bay Superfund Site, Tacoma, Washington. Environ. Sci. Technol. 1996, 30, 1645–1651. [Google Scholar] [CrossRef]
- Gómez, V.R.; Núñez López, D.; Gutiérrez, M. Changes in the vegetation cover and quality of aquifers in the drylands of Mexico: Trends in an urbanized complex of three socio-ecological systems within the chihuahuan desert. In Stewardship of Future Drylands and Climate Change in the Global South: Challenges and Opportunities for the Agenda 2030; Springer: Berlin/Heidelberg, Germany, 2019; pp. 57–77. [Google Scholar]
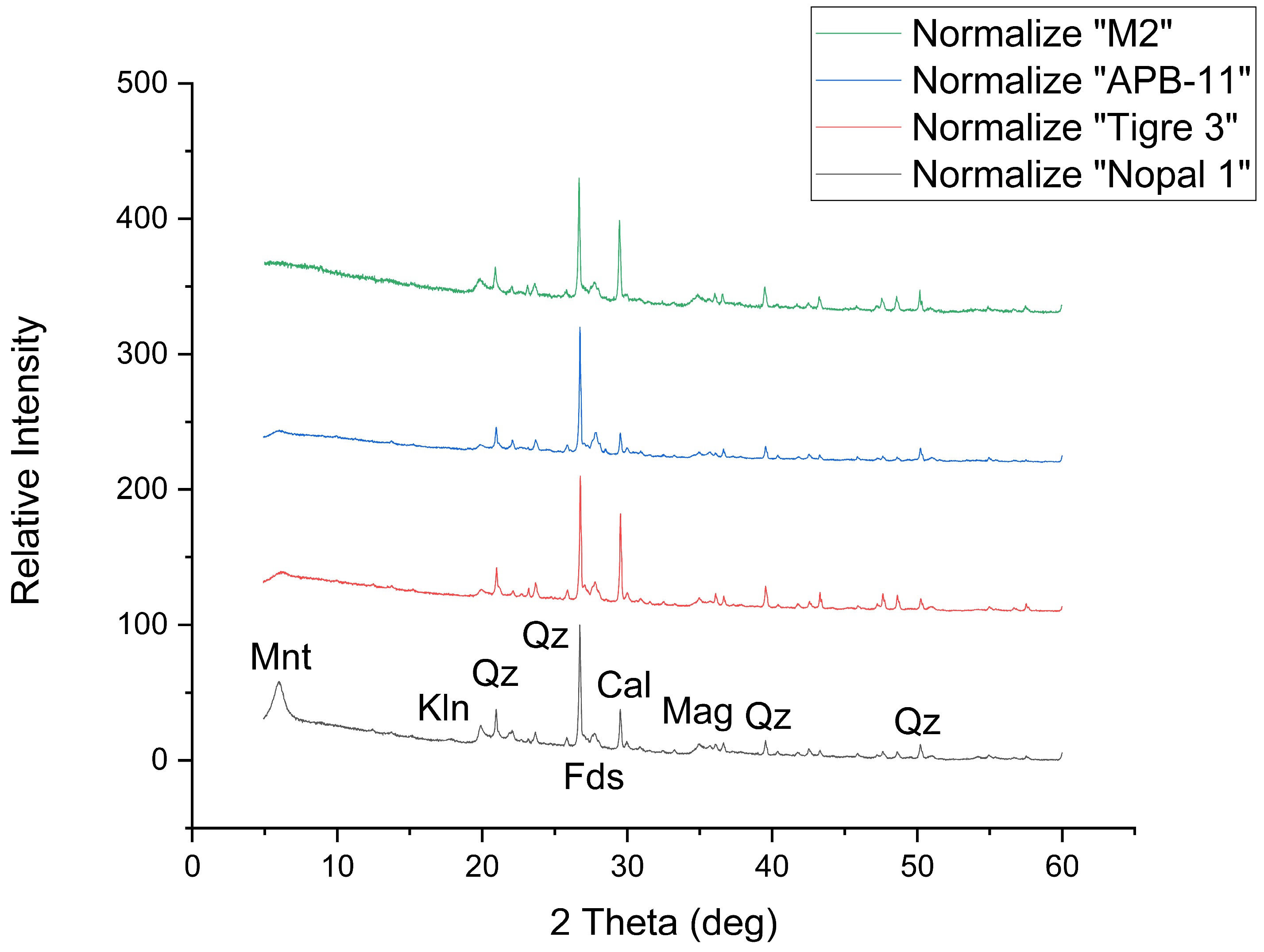


| Author | Sample | Stream | Mineral Phase (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qz | Cal | Mnt | Mag | Ab | Sa | Kln | An | |||
| This work | APB-11 | APB | 26.7 (0.2) | 8.4 (0.2) | 4.1 (0.2) | 2.6 (0.2) | 32.6 (0.7) | 15.8 (0.7) | 3.9 (0.9) | 5.3 (0.9) |
| Rodríguez-Guerra [27] | Tigre 3 | AET | 19.8 (0.4) | 16.0 (0.9) | 1.0 (0.2) | 1.0 (0.5) | 10.4 (0.1) | 11.2 (0.4) | 1.8 (0.5) | - |
| Rodríguez-Guerra [27] | Nopal 1 | AET | 26.7 (0.3) | 9.0 (0.7) | 11.0 (0.9) | 2.3 (0.7) | 12.3 (0.1) | 9.6 (0.1) | 4.3 (0.9) | - |
| Pérez-Reyes [29] | M2 | ABLC | 23.5 (0.7) | 22.8 (0.4) | - | - | - | 22.7 (0.9) | 14.7 (0.2) | 16 (1) |
| Author | Stream | Granulometry | Activity Concentration (Bq/kg) | |
|---|---|---|---|---|
| Highest AConc | Lowest AConc | |||
| This work | APB | FSC | 100 ± 2 | 50 ± 1 |
| CSC | 77 ± 2 | 51 ± 1 | ||
| FSD | 51 * | 51 * | ||
| Rodríguez-Guerra [27] | AET | FSC | 133 ± 2 | 76 ± 2 |
| FSD | 217 ± 1 | 71 ± 1 | ||
| Hernández-Hernández [28] | ABLC | FSC | 79 ± 1 | 36 ± 1 |
| CSC | 71 ± 1 | 31 ± 1 | ||
| Sample | Particle Density (g−1) |
|---|---|
| Puerto 3 | 2500 ± 250 |
| Nopal 1-d | 828 ± 82 |
| APB-11 | 386 ± 39 |
| APB-12 | 187 ± 18 |
| Tigre-5 | 124 ± 12 |
| Name | N (*) | S02 (*) | σ2 (Å2) | E0 (eV) (*) | ΔR (Å) | Reff (Å) | Reff + ΔR (Å) | R uncertainty (Å) |
|---|---|---|---|---|---|---|---|---|
| U_Oax | 2 | 1.05 | 0.0053(6) | 9 | 0.009 | 1.8045 | 1.814 | 0.004 |
| U_Oeq1 | 1 | 1.05 | 0.004(1) | 9 | −0.04 | 2.2411 | 2.201 | 0.01 |
| U_Oeq2 | 2 | 1.05 | 0.004(1) | 9 | −0.04 | 2.2952 | 2.255 | 0.01 |
| U_Oeq3 | 2 | 1.05 | 0.009(5) | 9 | −0.056 | 2.4498 | 2.394 | 0.019 |
| U_Si | 1 | 1.05 | 0.012(3) | 9 | 0.03 | 3.1444 | 3.174 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Herrera, C.; Canché-Tello, J.G.; Rodríguez-Guerra, Y.; Faudoa-Gómez, F.G.; Eichert, D.M.; Ignatyev, K.; Cabral-Lares, R.M.; Pérez-Reyes, V.; Esparza-Ponce, H.E.; Montero-Cabrera, M.-E. Uranium Mineral Particles Produced by Weathering in Sierra Peña Blanca, Chihuahua, Mexico: A Synchrotron-Based Study. Minerals 2025, 15, 333. https://doi.org/10.3390/min15040333
Hernández-Herrera C, Canché-Tello JG, Rodríguez-Guerra Y, Faudoa-Gómez FG, Eichert DM, Ignatyev K, Cabral-Lares RM, Pérez-Reyes V, Esparza-Ponce HE, Montero-Cabrera M-E. Uranium Mineral Particles Produced by Weathering in Sierra Peña Blanca, Chihuahua, Mexico: A Synchrotron-Based Study. Minerals. 2025; 15(4):333. https://doi.org/10.3390/min15040333
Chicago/Turabian StyleHernández-Herrera, Cristina, Jesús G. Canché-Tello, Yair Rodríguez-Guerra, Fabián G. Faudoa-Gómez, Diane M. Eichert, Konstantin Ignatyev, Rocío M. Cabral-Lares, Victoria Pérez-Reyes, Hilda E. Esparza-Ponce, and María-Elena Montero-Cabrera. 2025. "Uranium Mineral Particles Produced by Weathering in Sierra Peña Blanca, Chihuahua, Mexico: A Synchrotron-Based Study" Minerals 15, no. 4: 333. https://doi.org/10.3390/min15040333
APA StyleHernández-Herrera, C., Canché-Tello, J. G., Rodríguez-Guerra, Y., Faudoa-Gómez, F. G., Eichert, D. M., Ignatyev, K., Cabral-Lares, R. M., Pérez-Reyes, V., Esparza-Ponce, H. E., & Montero-Cabrera, M.-E. (2025). Uranium Mineral Particles Produced by Weathering in Sierra Peña Blanca, Chihuahua, Mexico: A Synchrotron-Based Study. Minerals, 15(4), 333. https://doi.org/10.3390/min15040333









