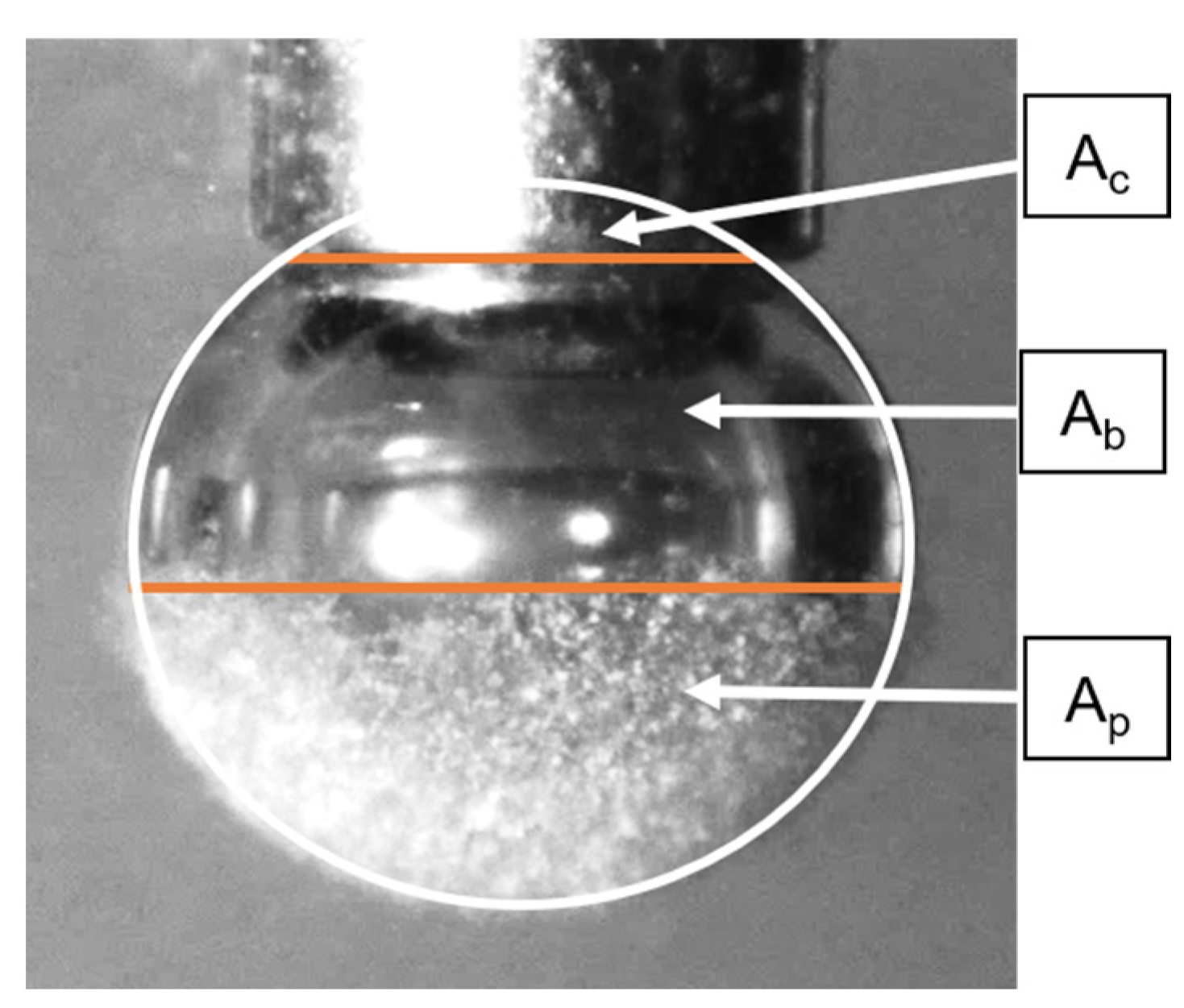
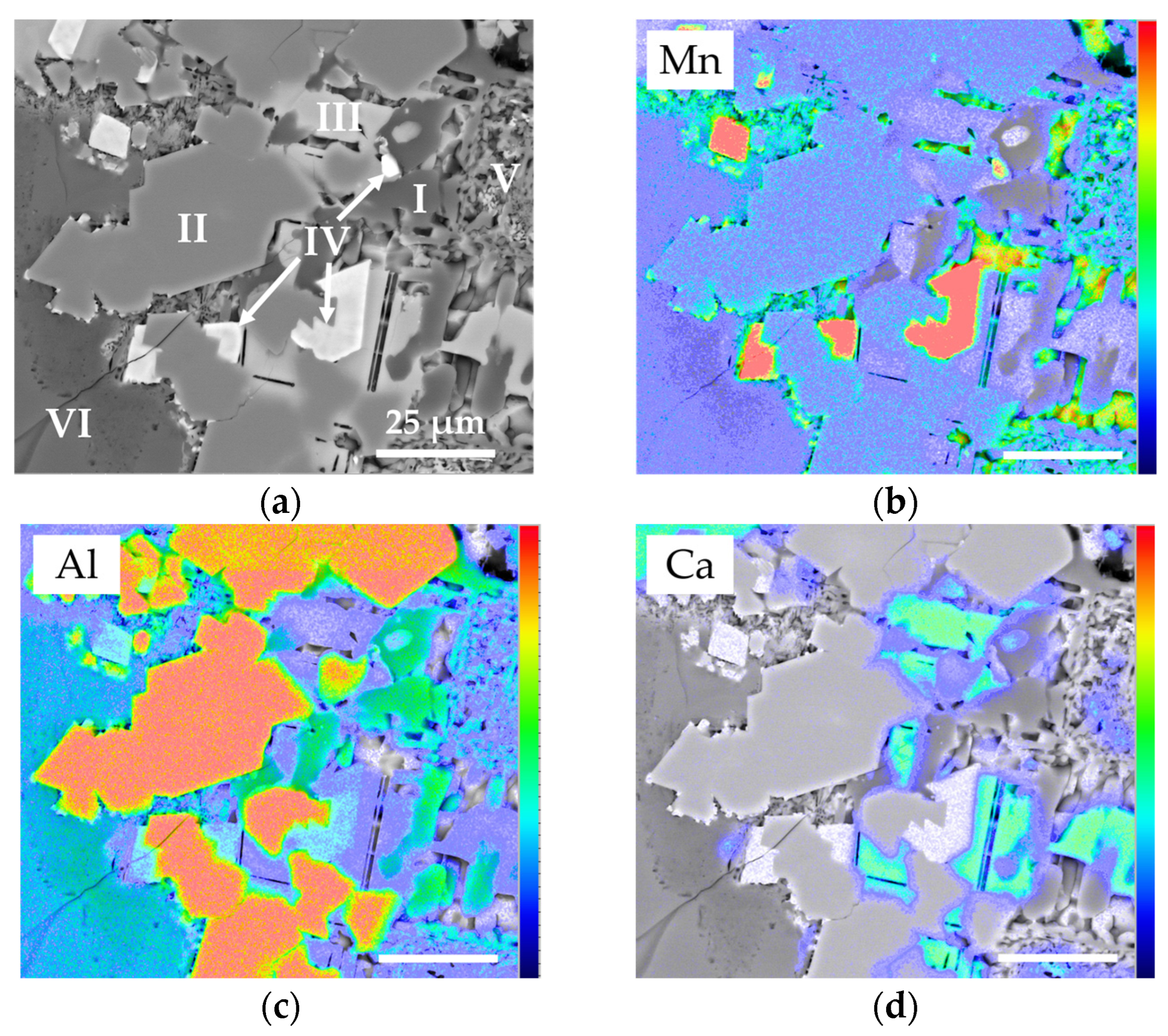
1. Introduction
The demand for lithium-ion batteries (LIBs) has doubled in the past five years due to their importance as energy storage systems for renewable energy and their utilization in the global shift to electric mobility [
1]. As a consequence, lithium was declared to be a critical element by the U.S. Department of Energy and the European Commission in 2020 [
2]. Thus, it is highly necessary to secure recycled lithium sources from LIBs and feed them into a more resource-efficient and sustainable circular economy to allow for the necessary shift to renewable energies and lower carbon emissions.
Flowsheets for LIB recycling employ a number of new and dynamic recycling strategies, which include mechanical, hydrometallurgical and pyrometallurgical treatments. Industrial-scale LIB recycling facilities, such as the Umicore battery recycling plant in Hoboken, Belgium, utilizes hydrometallurgical and pyrometallurgical processes to recover metals, such as nickel, copper and cobalt. However, major amounts of rather ignoble elements like lithium and manganese are commonly lost in the slag and are rarely recovered. Slags obtained from this recycling route generally contain higher concentrations of Li
2O (5–20)% (
w/
w) in comparison to natural phases, such as spodumene (8% (
w/
w) Li
2O [
3]. In addition to being sources of Li [
4] and Mn [
5], slags originating from other processes are known to contain valuable concentrations of copper [
6,
7,
8,
9] and rare earth elements [
10,
11]. Although slags can contain significant concentrations of valuable metals, the complexity of slag phases and microstructures hinders the beneficiation and extraction processes [
12]. In this contribution, a novel approach that involves the beneficiation of slag residues from recycled LIBs is presented. The solution is to engineer specific phases in synthetic slags in order to analyze the concentration of valuable elements in a limited number of well-defined phases within a model system. This approach enables the identification of key parameters for a slagging process that can effectively resolve microstructural challenges [
13]. The creation of engineered artificial mineral phases (EnAMs) is accomplished by adjusting the concentrations of certain slag additives and cooling rates during solidification [
7,
14]. The adjustment of these parameters can allow for the precise manipulation of crystal shapes and sizes, thereby increasing downstream beneficiation and mineral extraction efficiencies. When examining slag from Umicore’s pyrometallurgical recycling process, Elwert et al. [
4] found that Li crystallizes primarily within Li-aluminate (LiAlO
2) and that gehlenite (Ca
2Al
2SiO
7) is the dominant silicate gangue phase. Li-aluminate is a particularly important phase, not only because it represents the most abundant Li-bearing EnAM, but it also contains the highest molar ratio of Li and thus represents the primary target phase in Li-bearing slags [
15]. Although studies relating to the regulation of the crystal shape and size of Li-aluminate have been carried out [
16], the crystallization sequence of different EnAMs is not straightforward since it is dependent on the type and cathode chemistry of the recycled batteries. For example, varying the manganese (Mn) content can negatively influence Li-aluminate formation and instead promote the formation of spinel-type phases [
5]. This was observed in second-generation LIB slags, which contained additional manganese ions, and resulted in the formation of diverse EnAMs and corresponding crystallization textures [
4].
For slag beneficiation, froth flotation, which is regarded as one of the most important separation techniques in mineral processing, can be applied to Li-bearing EnAMs. Flotation reagents, such as collectors, depressants and activators, are crucial drivers for the separation efficiency in froth flotation. The hydrophobic particles will adhere to air bubbles and are recovered as concentrates, while hydrophilic particles will remain suspended in the aqueous phase and are collected as tailings. To date, there has been limited research on the flotation behavior of Li-aluminate and gehlenite, and information regarding their flotation response to various collector regimes is scarce [
17,
18,
19,
20,
21]. Although pure model materials have been used to investigate potential collectors [
21], the flotation behavior of Li-aluminate in real and synthetic Li-slags is poorly understood. Knowledge gained from the flotation of natural spodumene can serve as a valuable reference for the behavior of lithium-bearing minerals in flotation [
22,
23,
24,
25,
26,
27,
28,
29]. A prominent benchmark anionic collector, oleic acid, or its deprotonated form, sodium oleate, is widely used for spodumene flotation [
22,
23,
27,
29]. Sodium oleate was already successfully applied in the flotation of Li-aluminate in Li-slags [
13]. Recently, a novel cyclic betaine collector, punicine, was successfully trialed within microflotation and showed similar recovery rates to sodium oleate [
17,
19]. Punicine is a collector whose charge varies depending on the surrounding pH conditions: at alkaline pH levels of 9–14, it is negatively charged and can function as an anionic collector. In the pH range of 5–9, the molecule exhibits both positive and negative charges, while at pH levels of 2–5, it predominantly carries a positive charge and acts as a cationic collector [
30,
31]. Additionally, it is a light-sensitive molecule that becomes activated into a radical state under different wavelengths of light. Both of these properties are currently being studied to understand their interactions with mineral surfaces, particularly Li-aluminate, with the objective of making use of punicine as a pH-responsive and photo-switchable collector [
17,
19,
32]. In this study, we examine for the first time the application of the punicine collector in batch flotation tests for the selective recovery of Li-aluminate from a synthesized and engineered lithium-bearing slag. Additionally, the benchmark collector, oleic acid, is utilized for comparison. The flotation efficiency is discussed in the context of EnAM phase composition, phase association, phase liberation and particle size.
4. Discussion
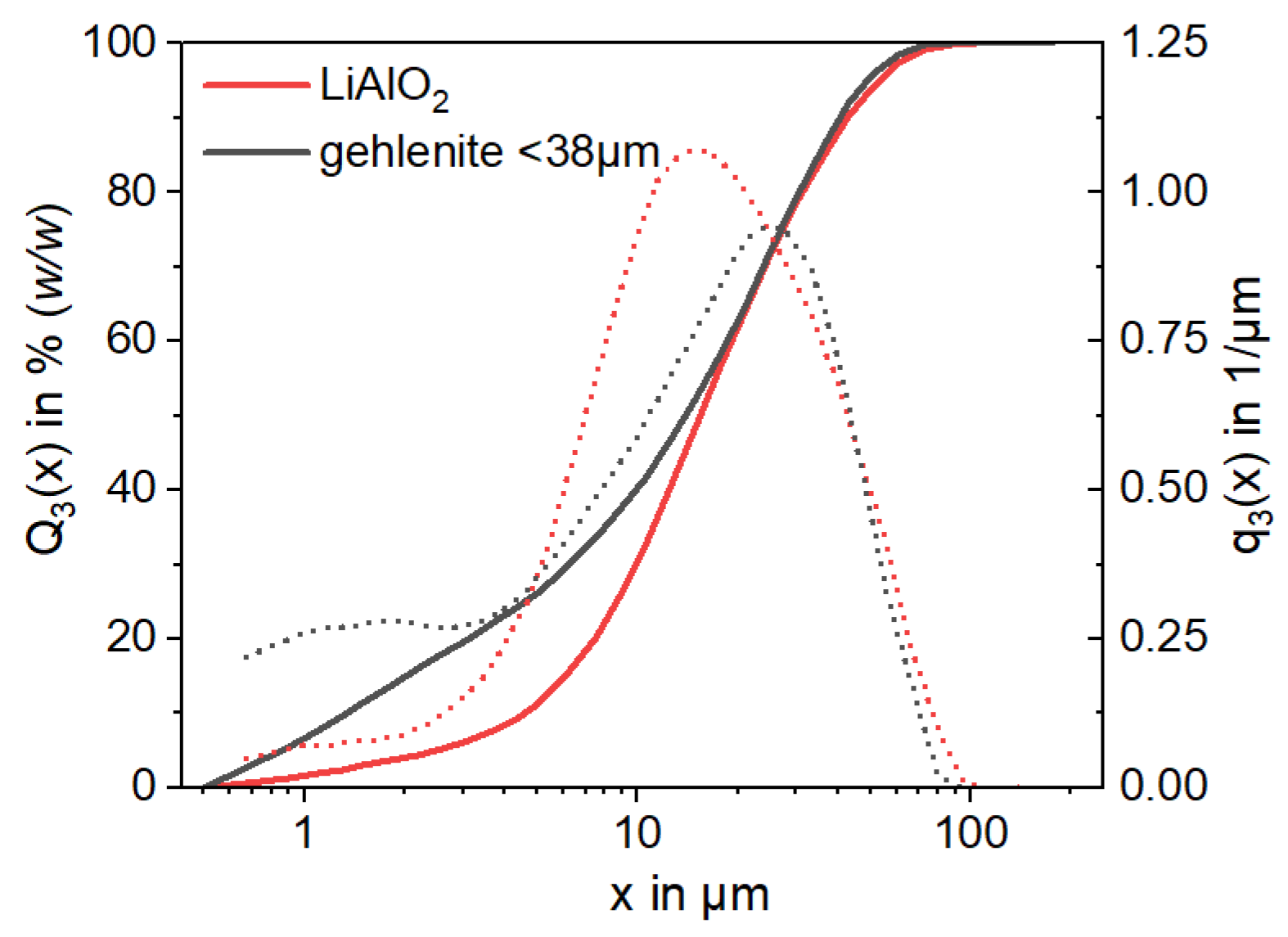
In agreement with the predicted EnAM phase formation in engineered Li-slags [
13], the main EnAM phases in the studied large scale synthetic slag (120 kg) are Li-aluminate, gehlenite, eucryptite and aluminum spinel (
Table 2). The Li-aluminate content characterized in the present sample (7.9% (
w/
w)) by XRD is relatively low compared to other studies on Li-bearing slags, which reported values from 11% (
w/
w) to 22% (
w/
w) [
13,
16,
47]. The Li-aluminate content in the slag was reduced by more than half compared to a slag with the same feed composition in a small batch size of 4.5 kg (13.2% (
w/
w) Li-aluminate) at the same cooling rate of 25 K/h [
16].
The composition of EnAM phases identified by MLA are comparable to the composition calculated by Wittkowski et al. and Schirmer et al. from lab-scale experiments [
5,
39,
40]. Li-aluminate, the target Li-bearing phase, contains minor amounts of silicon according to its published chemical formula Li
1 − x(Al
1 − xSi
x)O
2 [
40] and accounts for 6.0% (
w/
w) of the slag. Additional Li-host phases include spinel and eucryptite (Mn-poor and Mn-rich). The spinel phases are found as a solid solution according to (Li
(2x)Mn
2+(1 − x)(1 + x)(Al
(2 − z),Mn
3+(z))O
4 [
5]. These form 36.1% (
w/
w) of the slag sample. Eucryptite and Mn-rich eucryptite, following the formula Li
1 − x(Mn
3+y,Al
1 − (x + y))
1 − x(Si
1 + x)O
4 [
40], contain lithium depending on the incorporated amount of Mn. These minerals together compose 11.2% (
w/
w) of the slag. Furthermore, the silicate phase Li
2MnSiO
4, which was identified by XRD (13.5% (
w/
w)), could also represent a lithium-host phase in the slag. Li
2MnSiO
4 was also identified by XRD within synthesized slags of different feed compositions [
13,
16]. The identification of these Li-containing silicate phases confirms the predicted phase formation considering the composition of the feed used for the studied slag [
16].
Regarding the modal composition of the Li-slag sample studied, one of the main differences to other systems described in the literature is the abundance of mixed phases, which were which were observed in the MLA results MLA (14.1% (w/w)). These phases represent a challenge for characterization since the mixes contain finely disseminated phases, smaller than 1 µm, which are difficult to resolve using standard MLA measurement settings. Altogether, it is likely that one or more of the major phases represents a component of the mixed phases, and a proportion of the Li-content of the slag occurs within one or more of these mixed phases.
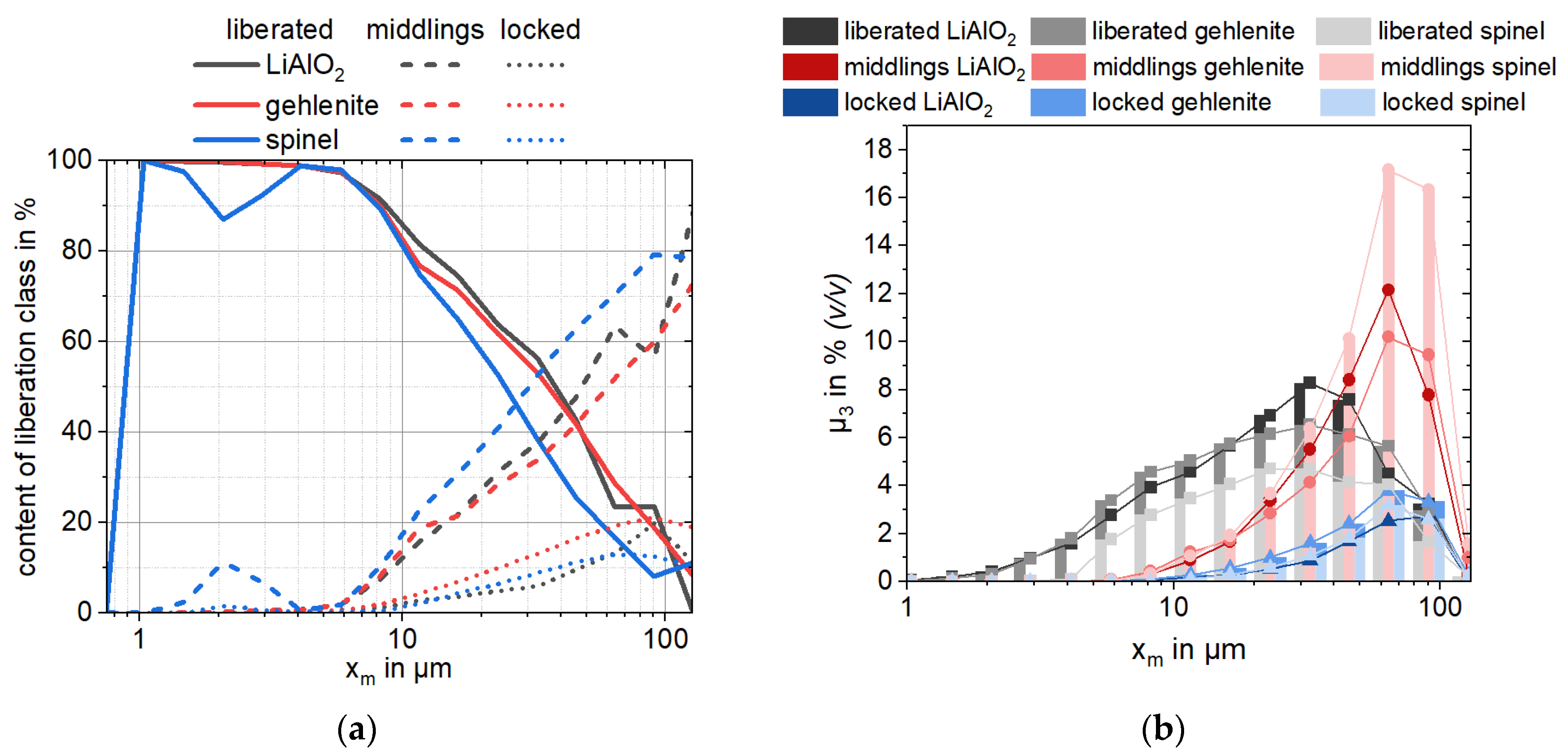
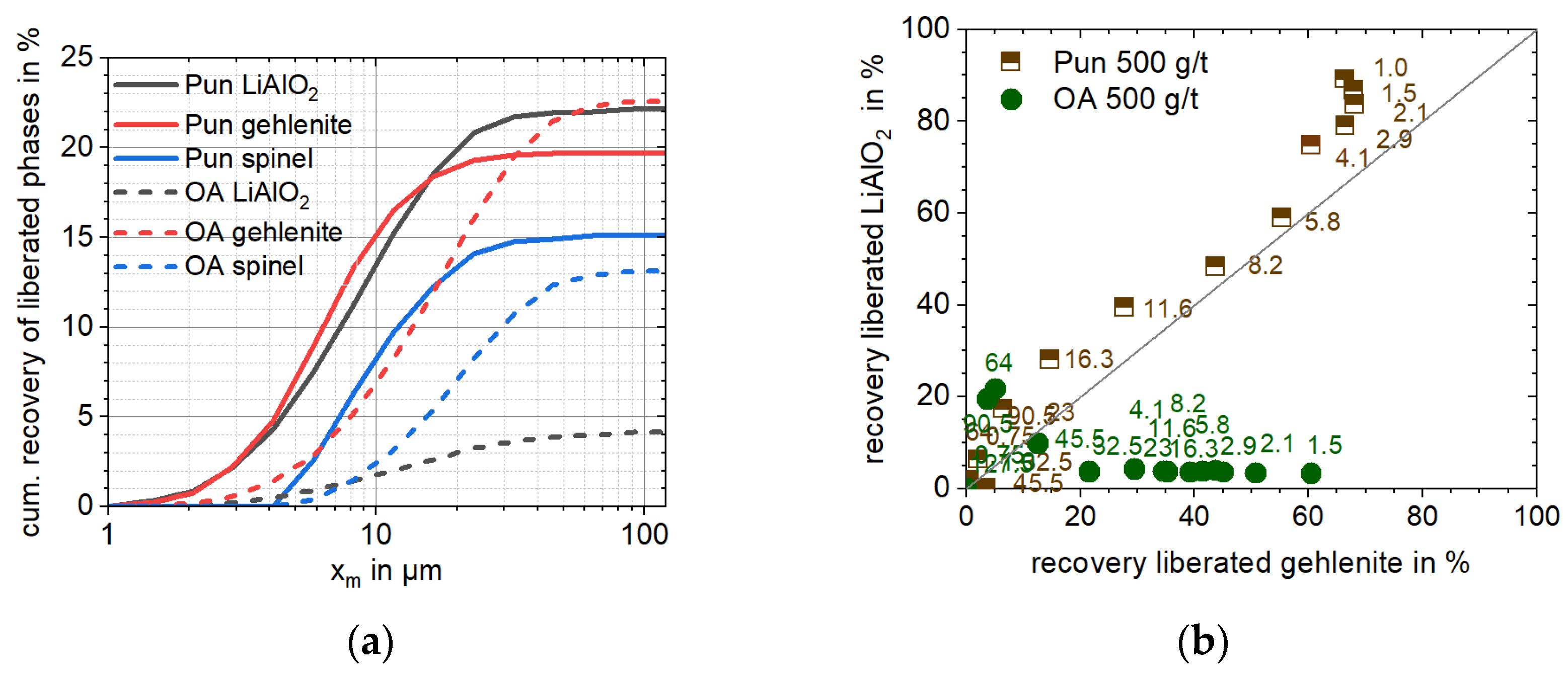
In context of slag beneficiation, it is noteworthy that the liberation characteristics of Li-aluminate and gehlenite are comparable over particle size, making it impossible to separate the target and gangue phases solely based on particle geometric properties (e.g., size or liberation). In contrast, spinel-type phases exhibit a different breakage behavior resulting in a high amount of middlings (60%,
Table 3) and coarse particle sizes. These distinctive characteristics could be explored when designing a comminution circuit for this type of material.
A full degree of liberation (>90% (
A/
A)) of all phases is achieved for particles finer than 9.6 µm. The target EnAM Li-aluminate is liberated to 50% (
Table 3) and represents only a minor association partner to other slag phases (<5%,
Table A2). Overall, Li-aluminate is slightly associated with gehlenite (16%,
Table A2), which can facilitate their selective separation via flotation under the proper reagent conditions. Eucryptite is the main phase associated with Li-aluminate after spinel (
Table A2). Since eucryptite and spinel also carry lithium, it is possible that this association does not drastically influence Li recovery but may limit its physical enrichment ability.
In context of EnAM flotation, the two different reagent regimes, oleic acid and punicine, render characteristic recovery trends to the phases of interest as a function of their particle size and liberation degree (
Figure 7b). The punicine regime demonstrates a notable tendency to recover fine particles, with an x
50,3 particle size of (8–13) µm, regardless of collector concentration (
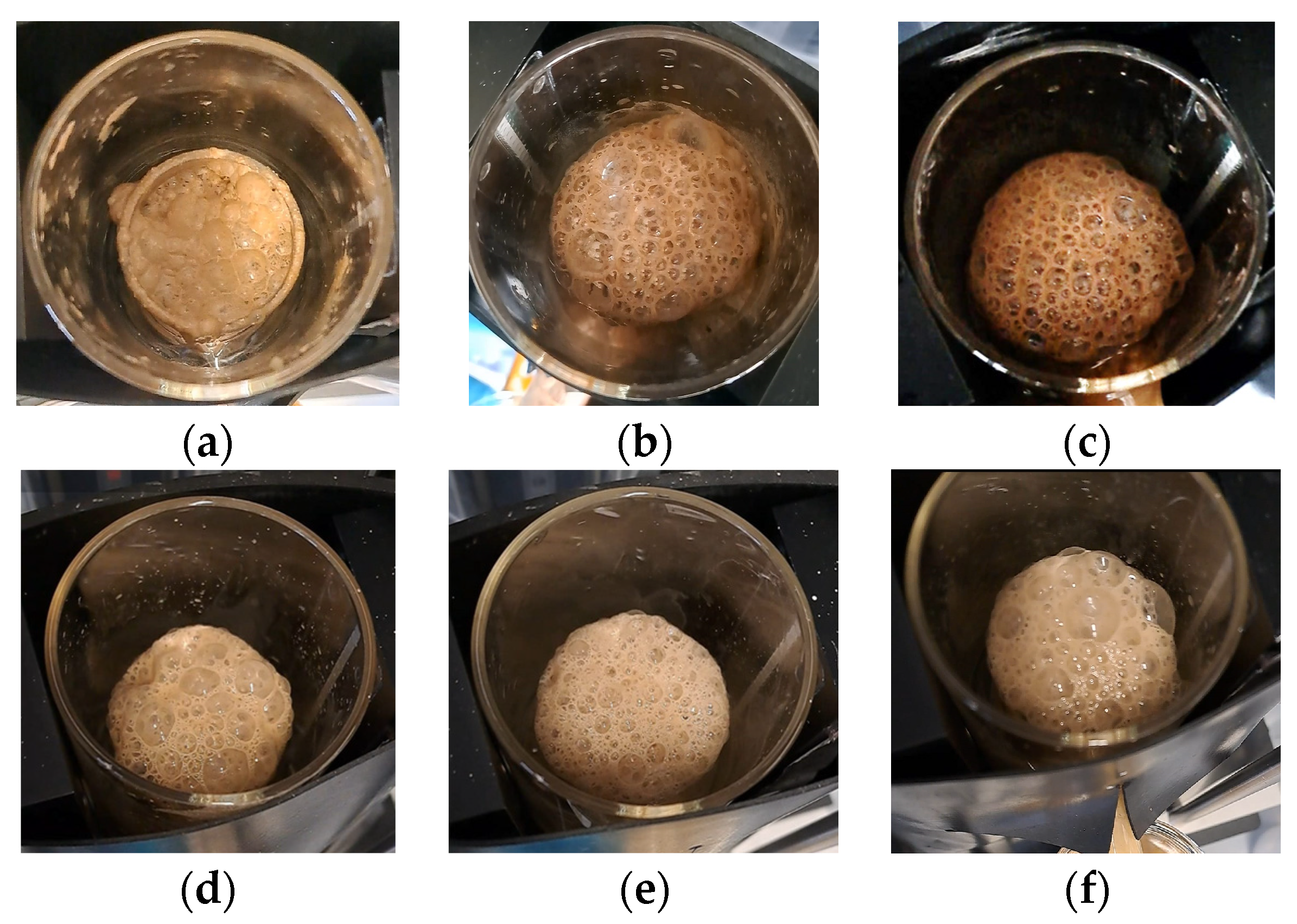
Figure 7b). The formation of smaller bubbles and a denser froth (
Figure A7d–f) increases the efficiency of fine particle capture [
45], distinguishing the punicine regime from oleic acid. This froth characteristic could support the typical phase-independent preferred recovery of liberated particles (with a degree of liberation higher than 80% (
A/
A)) by punicine. Conversely, the oleic acid regime renders a drier and particle-loaded froth (
Figure A7a–c), which results in a coarser concentrate (x
50,3 21 µm; 500 g/t oleic acid) relative to punicine (
Figure 7c).
Sodium oleate, a deionized form of oleic acid, has shown a higher affinity towards Li-bearing minerals in Li-slag flotation when using a mechanical small Denver-type flotation cell with collector concentrations between 200 g/t and 900 g/t and higher pulp density [
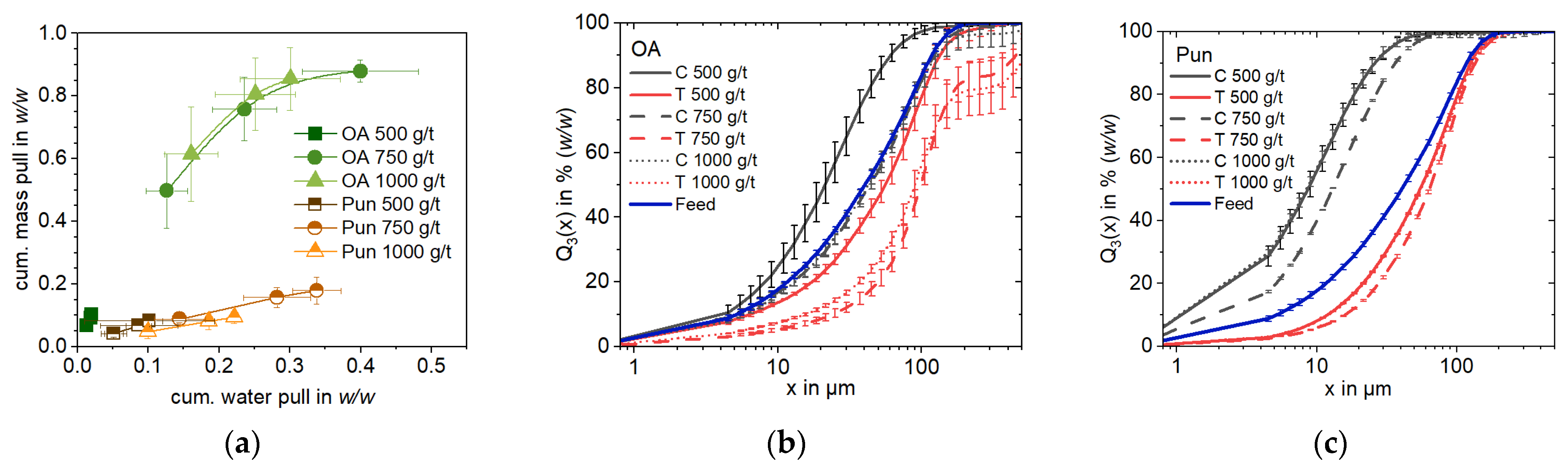
13]. Yet, the use of oleic acid in this study resulted in the selectiverecovery of Ca-bearing gehlenite particles in the lower dosage concentration (500 g/t), leading to the reverse flotation of Li-aluminate. At higher concentrations (750 g/t), the strong frothing characteristics of oleic acid at basic pH (10.5) dominates the process, leading to a high mass pull recovery, a similar concentrate composition to the feed and non-selectivity (
Figure 8). This indicates that increases in collector concentration do not yield additional benefits. Further investigations regarding the optimization of oleic acid dosage for this slag system should be limited to 750 g/t as a maximum dosage.
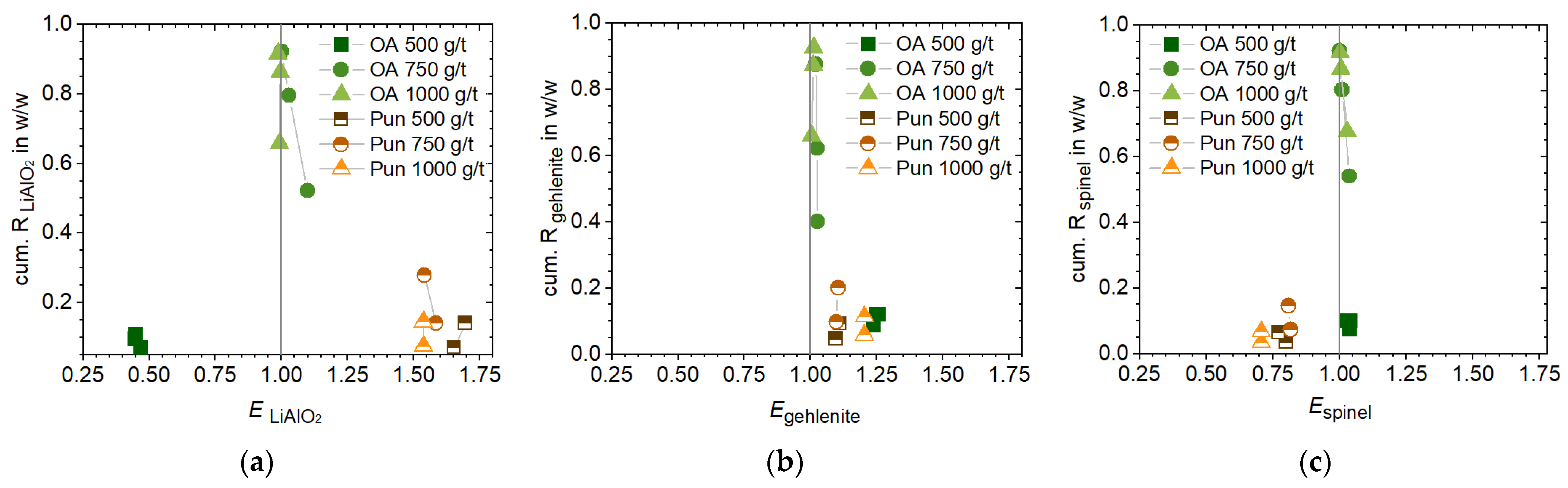
In contrast, the punicine regime enables the direct flotation of Li-aluminate from the slag. The highest upgrading of Li-aluminate (+4.2% (
w/
w)) was observed at 500 g/t. The optimum Li-aluminate selectivity and recovery obtained among the studied conditions was at 750 g/t of punicine (1.54 enrichment factor, 1.40 Fuerstenau coefficient, 28% (
w/
w) recovery) (
Figure 9a,b). However, higher punicine dosages lead to a higher gehlenite recovery. Overall, the mass recovery of Li-aluminate with the collector punicine is still quite low, but similar to oleic acid at 500 g/t. A change in reagent parameters in the future, e.g., by using depressants or activators, has the potential to increase selectivity or recovery. Additionally, the preceding desliming procedures can also improve flotation performance [
21], but may potentially compromise the overall recovery.
In conjunction with the previously discussed properties of punicine regarding the recovery of fine liberated particles (
Table 6), the system is found to be particularly selective towards liberated Li-aluminate particles smaller than 10 µm (
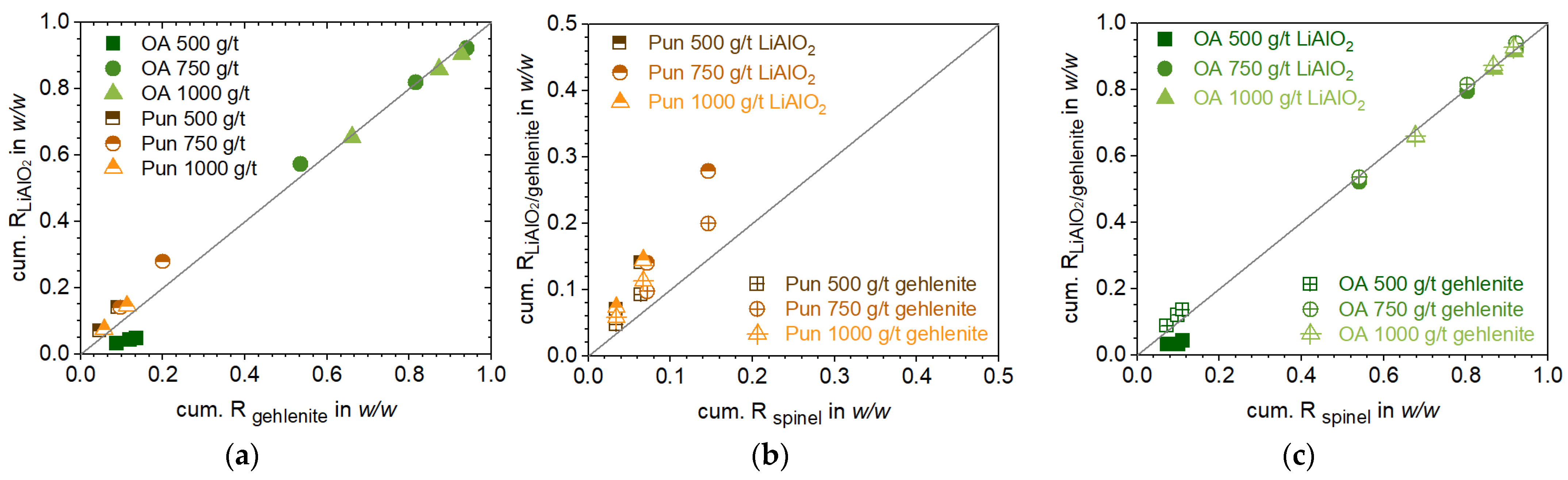
Figure 5b). Besides the real selectivity of the surfactant to Li-aluminate, i.e., true flotation, entrainment effects of fine, light, particles containing a higher number of liberated phases, such as Li-aluminate, can also be a driver of this behavior. To determine whether this effect has an impact on selectivity, the mass ratio of Li-aluminate to gehlenite was plotted across the particle size classes of both the feed and concentrate within a single liberation class. With punicine, the mass ratio increased across all particle sizes compared to the feed (
Figure A8b). Notably, the mass ratio within liberated particles in the size fractions of 10 µm < x < 64 µm rose, indicating that the selectivity towards Li-aluminate is not primarily driven by general entrainment effects in finer particle sizes. Eucryptite, as a Li-host phase, is enriched alongside Li-aluminate when using punicine, but not with oleic acid. In addition to microstructure-related factors, such as the strong association of Li-aluminate with eucryptite (
Table A2), these findings may also support punicine exhibiting selectivity towards Li-containing EnAMs, particularly since this trend was not observed with oleic acid.
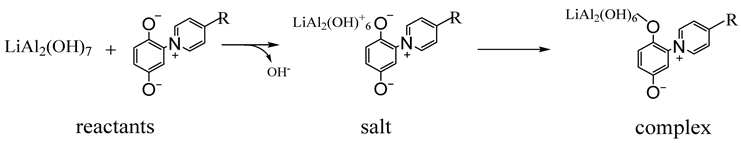
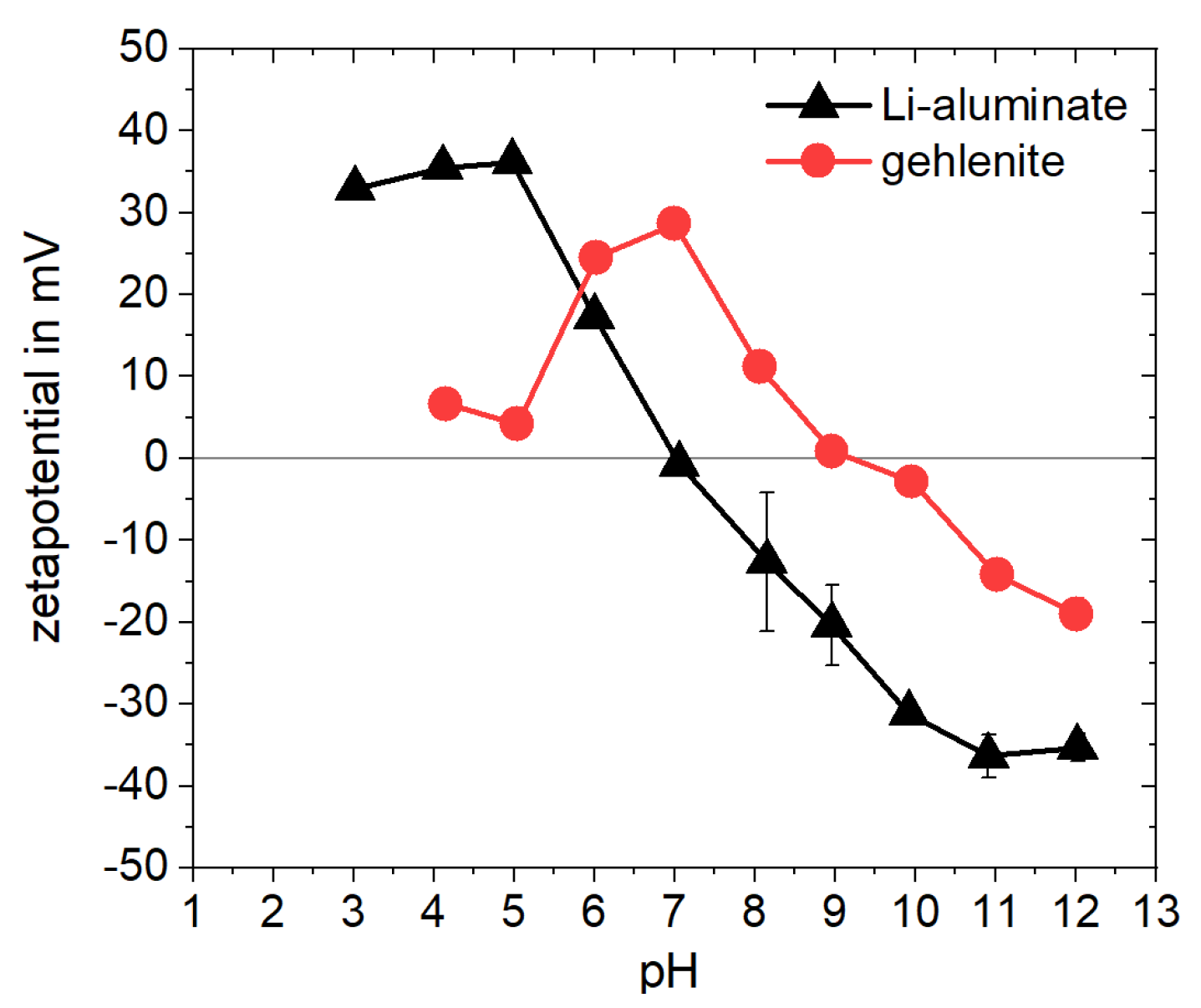
To determine the potential selectivity of collectors, it is important to consider their adsorption mechanism on mineral surfaces, as described by Schmidt and Zgheib et al. [
19] for punicine using the model materials Li-aluminate and gehlenite. For this mechanism, under humid conditions or in aqueous media, Li-aluminate forms hydrophilic species on the surface of the particles. In particular, Li-aluminate reacts to form lithium aluminum hydroxide hydrate (LiAl
2(OH)
7 × H
2O) with the release of lithium cations and hydroxide anions, which can explain the high pH of about 11 of suspensions of Li-aluminate in water according to Equation (2). Subsequent reactions with atmospheric CO
2 can result in the formation of carbonates [
48]. In addition, hydroxide leads to further conversions into LiAl
2(OH)
7 × H
2O. During this reaction, lithium cations are released under the formation of aluminum hydroxide anions according to Equation (3) [
49]. In the presence and addition of punicine, hydroxide groups of LiAl
2(OH)
7 can be substituted by the nucleophilic 2-olat group of the punicine according to Equation (4), which leads to the targeted surface hydrophobization [
19,
32]. The type of bond between the punicine and the hydrophilic particle surface can be described as a Coulomb interaction, for example, as a salt-like structure, or as a complex formation.
Therefore, the hydration, hydroxylation and accessibility of hydroxy groups on EnAM phases can play a crucial role in the hydrophobization of these phases during flotation with punicine. It is important to highlight that the hydroxylated aluminates required for complexation and hydrophobization can form not only on Li-bearing aluminate but also on aluminosilicates [
50], such as the gangue phase gehlenite. In addition to punicine, the adsorption of the benchmark collector oleic acid can also depend on the availability of hydroxyl groups. For instance, in the case of sillimanite, it was discussed that adsorption was facilitated by an ion exchange mechanism between oleate ions and surface hydroxyl sites [
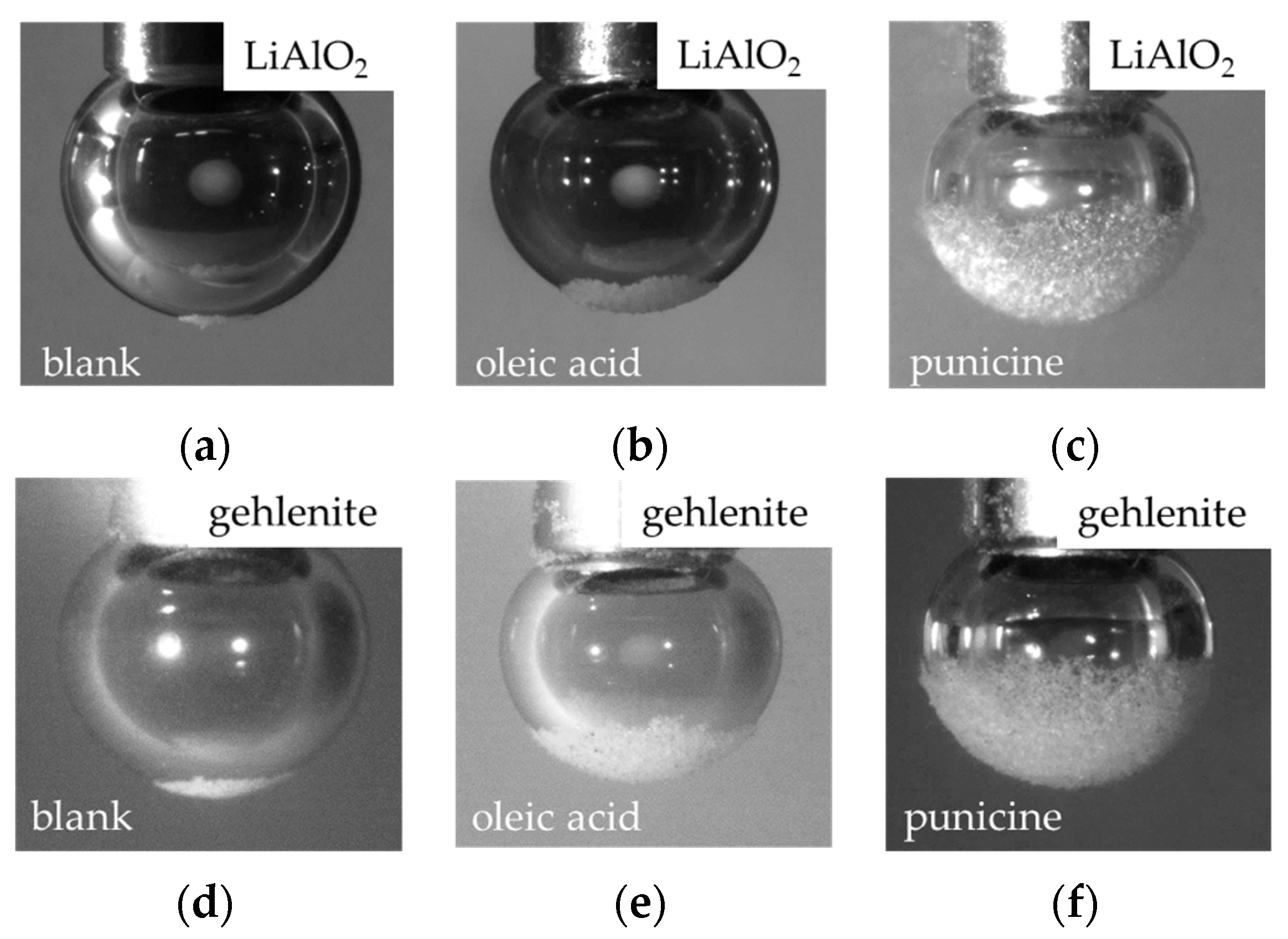
50]. The discussion on the comparable adsorption capabilities of collectors on aluminates and aluminosilicates is supported by the results of bubble–particle attachment tests. Experiments have demonstrated that both punicine and oleic acid interacted with the model minerals Li-aluminate and gehlenite, leading to an increase in bubble–particle attachment for both minerals (see
Section 3.2). Overall, the loading of bubbles with gehlenite particles was higher under all conditions, thus demonstrating a higher degree of dewettability. When comparing the two collectors, the mass pull obtained by punicine was twice as high as oleic acid. Similarly, a greater bubble loading towards gehlenite was observed with punicine. This affinity for gehlenite, which was not observed with punicine in complex slag systems during flotation, suggests that bubble–particle attachment tests using model materials can only provide an indication of a collector’s ability to effectively dewet target minerals. However, these tests cannot offer selectivity information for subsequent flotation experiments in more complex systems, such as slag. In summary, bubble–particle attachment tests confirm the successful hydrophobization of Li-aluminate with punicine, while the results of the flotation tests with complex synthetic slags show a noticeable selectivity of punicine for the EnAM Li-aluminate. Despite these promising findings, further fundamental studies are necessary and flotation experiments need to be reproduced, especially with Li-slag samples that exhibit contrasting compositions and liberation attributes. In particular, the adsorption behavior of punicine on EnAM phases of different surface characteristics requires further investigation.