Crystal Chemistry, High-Pressure Behavior, Water Content, and Thermal Stability of Natural Spodumene
Abstract
1. Introduction
2. Experimental Methods
3. Results and Discussion
4. Conclusions
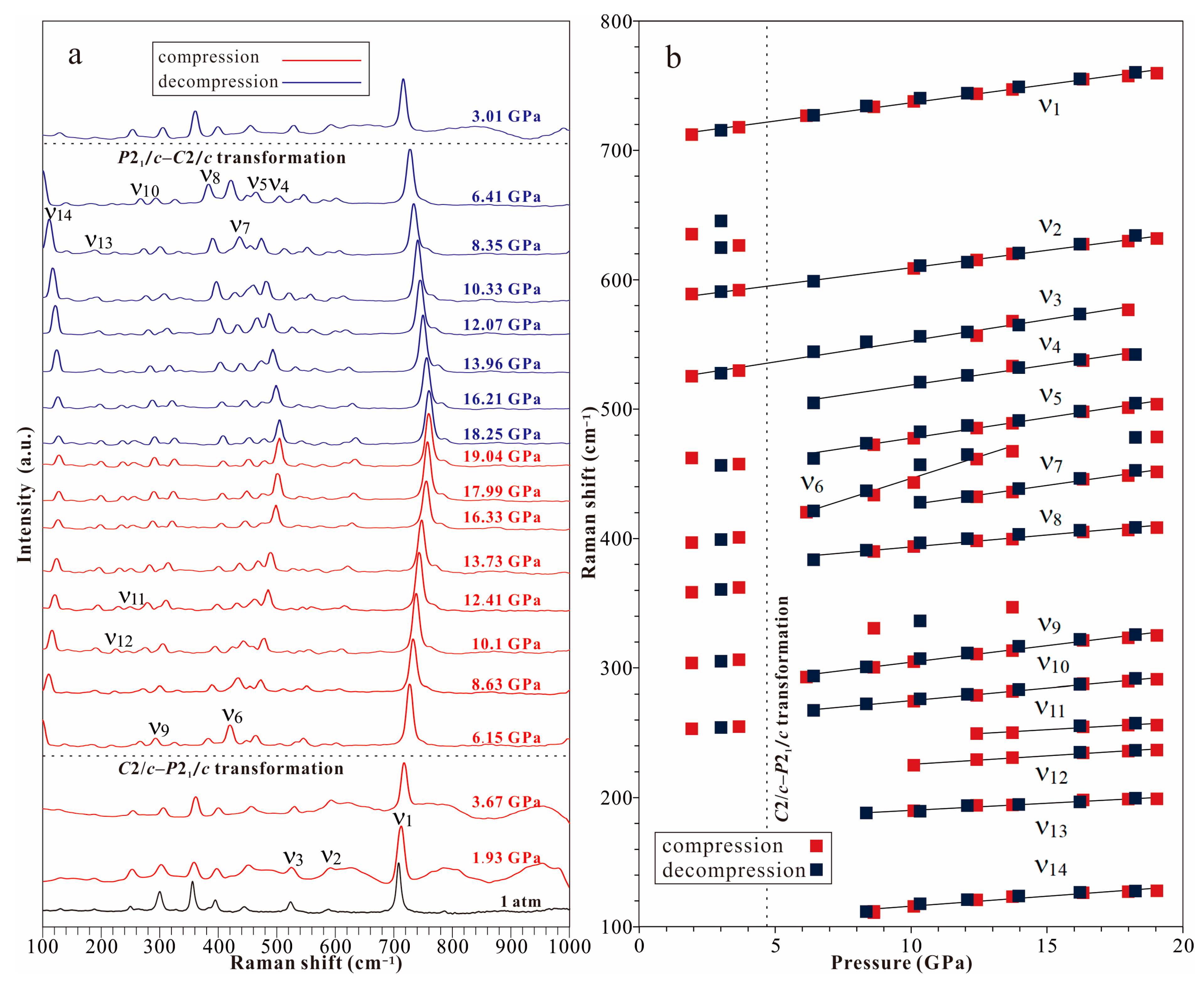
- In situ high-pressure Raman spectroscopy investigation demonstrates that spodumene, after the C2/c–P21/c transformation triggered at ~3.2 GPa, can remain stable under pressures up to 19 GPa at room temperature without undergoing decomposition, irreversible amorphization, or a second transformation.
- According to the results of FTIR analysis, the water content of the natural spodumene sample in this study is estimated to be 19–97 ppm wt. H2O. The relatively low wavenumbers of the OH bands (at 2580–3220 cm−1) indicate a strong hydrogen bond interaction. Hydrogen may primarily reside near the O-O edges with a length of 2.55–2.7 Å. O2 can be the potential site for hydrogen incorporation.
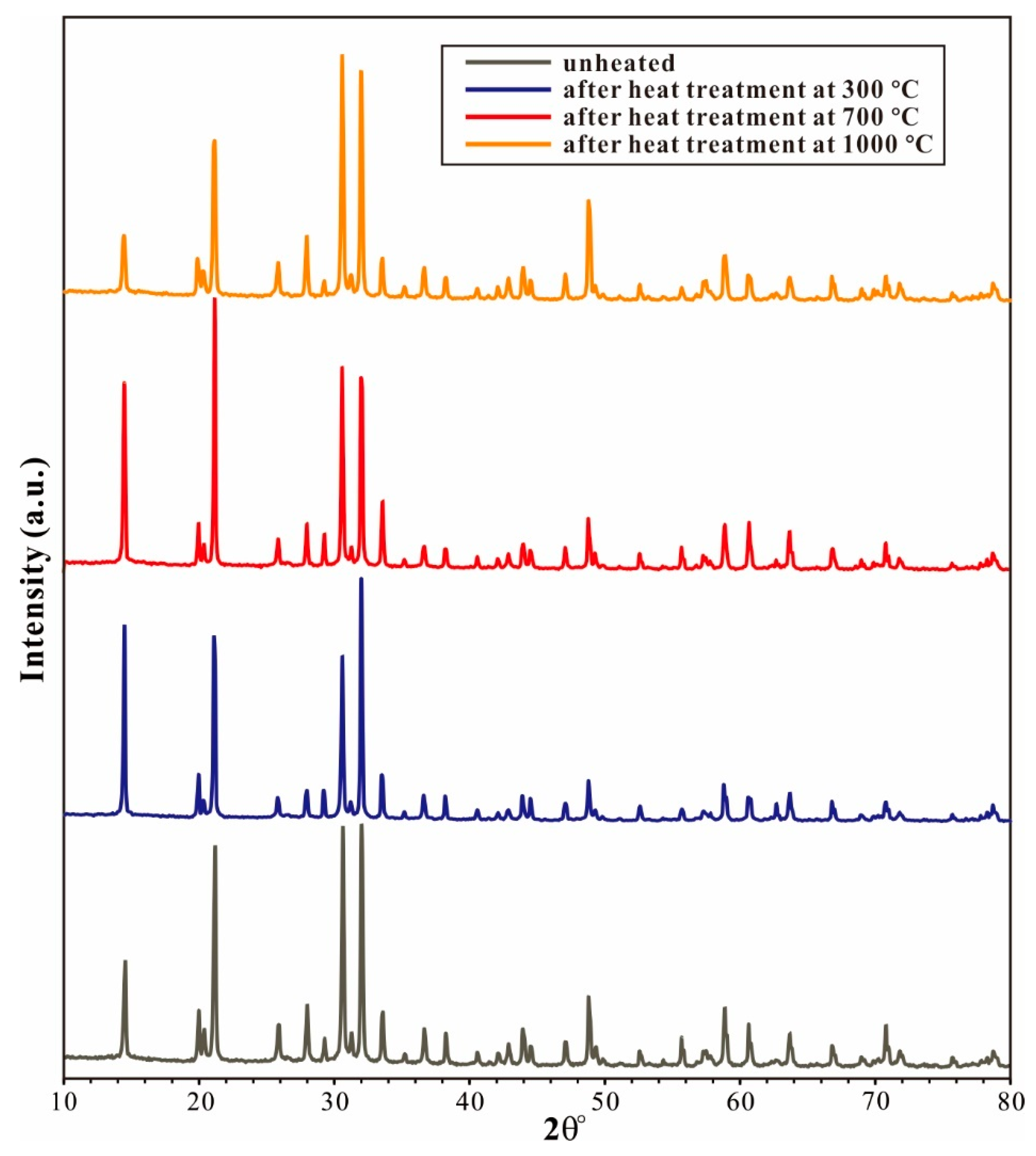
- Spodumene can retain a significant amount (up to ~100 ppm wt. H2O) of water under high-temperature conditions up to 1000 °C. This indicates that spodumene in subducted slabs is unlikely to undergo dehydration at temperatures below 1000 °C, and is therefore not expected to trigger partial melting. Thus, spodumene may act as an important carrier for Li, transporting it into the deep mantle without releasing Li into melts during subduction.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Asif, A.H.; Li, C.; Lim, H.; Sun, H. Australia’s spodumene: Advances in lithium extraction technologies, decarbonization, and circular Economy. Ind. Eng. Chem. Res. 2024, 63, 2073–2086. [Google Scholar] [CrossRef]
- Pommier, C.J.S.; Denton, M.B.; Downs, R.T. Raman spectroscopic study of spodumene (LiAlSi2O6) through the pressure-induced phase change from C2/c to P21/c. J. Raman. Spectrosc. 2003, 34, 769–775. [Google Scholar] [CrossRef]
- Tribaudino, M.; Nestola, F.; Prencipe, M. A single-crystal neutron-diffraction investigation of spodumene at 54 K. Can. Miner. 2003, 41, 521–527. [Google Scholar] [CrossRef]
- Arlt, T.; Angel, R.J. Displacive phase transitions in C-centred clinopyroxenes: Spodumene, LiScSi2O6 and ZnSiO3. Phys. Chem. Miner. 2000, 27, 719–731. [Google Scholar] [CrossRef]
- Ullrich, A.; Schranz, W.; Miletich, R. The nonlinear anomalous lattice elasticity associated with the high-pressure phase transition in spodumene: A high-precision static compression study. Phys. Chem. Miner. 2009, 36, 545–555. [Google Scholar] [CrossRef]
- Filip, J.; Novák, M.; Beran, A.; Zboil, R. Crystal chemistry and OH defect concentrations in spodumene from different granitic pegmatites. Phys. Chem. Miner. 2006, 32, 733–746. [Google Scholar] [CrossRef]
- Ingrin, J.; Skogby, H. Hydrogen in nominally anhydrous upper-mantle minerals: Concentrations levels and implications. Eur. J. Miner. 2000, 12, 543–570. [Google Scholar] [CrossRef]
- Johnson, E.A. Water in nominally anhydrous crustal minerals: Speciation, concentration, and geologic significance. Rev. Miner. Geochem. 2006, 62, 117–154. [Google Scholar] [CrossRef]
- Skogby, H.; Bell, D.R.; Rossman, G.R. Hydroxide in pyroxene: Variations in the natural environment. Am. Miner. 1990, 75, 764–774. [Google Scholar]
- Zhang, L.; Jin, Z.M. High-temperature metamorphism of the Yushugou ophiolitic slice: Late Devonian subduction of seamount and mid-oceanic ridge in the South Tianshan orogen. J. Asian Earth Sci. 2016, 132, 75–93. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL: Programs for Crystal Structure Analysis; University of Göttingen: Göttingen, Germany, 2018. [Google Scholar]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Prince, E. International Tables for Crystallography. Volume C: Mathematical Physical and Chemical Tables, 3rd ed.; Springer Science & Business Media: New York, NY, USA, 2004. [Google Scholar]
- Klotz, S.; Chervin, J.C.; Munsch, P.; Le Marchand, G. Hydrostatic limits of 11 pressure transmitting media. J. Appl. Phys. 2009, 42, 075413. [Google Scholar] [CrossRef]
- Chijioke, A.J.; Nellis, W.J.; Soldatov, A.; Silvera, I.F. The ruby pressure standard to 150 GPa. J. Appl. Phys. 2005, 98, 114905. [Google Scholar] [CrossRef]
- Yang, X.Z.; Xia, Q.K.; Deloule, E.; Dallai, L.; Fan, Q.C.; Feng, M. Water in minerals of the continental lithospheric mantle and overlying lower crust: A comparative study of peridotite and granulite xenoliths from the north China craton. Chem. Geol. 2008, 256, 33–45. [Google Scholar] [CrossRef]
- Li, W.; Jin, Z.M.; Li, H.M.; Tao, C.H. High water content in primitive mid-ocean ridge basalt from southwest Indian ridge (51.56°E): Implications for recycled hydrous component in the mantle. J. Earth Sci. 2017, 28, 411–421. [Google Scholar] [CrossRef]
- Paterson, M.S. The determination of hydroxyl by infrared absorption in quartz, silicate-glasses and similar materials. Bull. Minéral. 1982, 105, 20–29. [Google Scholar] [CrossRef]
- Bell, D.R.; Ihinger, P.D.; Rossman, G.R. Quantitative analysis of trace OH in garnet and pyroxenes. Am. Miner. 1995, 80, 465–474. [Google Scholar] [CrossRef]
- Gatta, G.D.; Ballaran, T.B.; Iezzi, G. High-pressure X-ray and Raman study of a ferrian magnesian spodumene. Phys. Chem. Miner. 2005, 32, 132–139. [Google Scholar] [CrossRef]
- Li, C.T.; Peacor, D.R. The crystal structure of LiAlSi2O6-II (“β spodumene″). Z. Kristallogr. 1968, 126, 1–6. [Google Scholar] [CrossRef]
- Clarke, P.T.; Spink, J.M. The crystal structure of β spodumene, LiAlSi2O6-II. Z. Kristallogr. 1969, 130, 420–426. [Google Scholar] [CrossRef]
- Johnson, E.A.; Rossman, G.R.; Dyar, M.D.; Valley, J.W. Correlation between OH concentration and oxygen isotope diffusion rate in diopsides from the Adirondack Mountains, New York. Am. Mineral. 2002, 87, 899–908. [Google Scholar] [CrossRef]
- Libowitzky, E. Correlation of O-H stretching frequencies and O-H···O hydrogen bond lengths in minerals. Monatsh. Chem. 1999, 130, 1047–1059. [Google Scholar] [CrossRef]
- Seaman, S.J.; Williams, M.L.; Jercinovic, M.J.; Koteas, G.C.; Brown, L.B. Water in nominally anhydrous minerals: Implications for partial melting and strain localization in the lower crust. Geology 2013, 41, 1051–1054. [Google Scholar] [CrossRef]
- Marschall, H.R.; Pogge von Strandmann, P.A.E.; Seitz, H.M.; Elliott, T.; Niu, Y.L. The lithium isotopic composition of orogenic eclogites and deep subducted slabs. Earth Planet. Sc. Lett. 2007, 262, 563–580. [Google Scholar] [CrossRef]
- Rauch, M.; Keppler, H. Water solubility in orthopyroxene. Contrib. Mineral. Petrol. 2002, 143, 525–536. [Google Scholar] [CrossRef]
- Zhang, B.H.; Matsuzaki, T.; Wu, X.P. Water solubility in orthopyroxene: Dependence on temperature and Al content. Chin. Sci. Bull. 2013, 58, 3895–3902. [Google Scholar] [CrossRef]


| Unit-Cell Parameters | |||
|---|---|---|---|
| a | b | c | β |
| 9.4727 (14) Å | 8.3967 (12) Å | 5.2239 (8) Å | 110.153 (3)° |
| Atom position parameters | |||
| x | y | z | |
| Si | 0.29410 (4) | 0.09342 (5) | 0.25604 (8) |
| Li | 0 | 0.2743 (6) | 0.25 |
| Al | 0 | 0.90671 (7) | 0.25 |
| O1 | 0.10956 (12) | 0.08230 (12) | 0.1404 (2) |
| O2 | 0.36487 (12) | 0.26709 (13) | 0.3005 (2) |
| O3 | 0.35660 (11) | 0.98669 (14) | 0.0584 (2) |
| Space Group: C2/c | |||
| Si | Li | Al | |||
|---|---|---|---|---|---|
| Si-O1 | 1.644 Å | Li-O1 (2) | 2.101 Å | Al-O1 (2) | 1.946 Å |
| Si-O2 | 1.588 Å | Li-O2 (2) | 2.281 Å | Al-O1 (2) | 1.997 Å |
| Si-O3 | 1.625 Å | Li-O3 (2) | 2.256 Å | Al-O2 (2) | 1.821 Å |
| Si-O3 | 1.629 Å | O1-O1 | 2.694 Å | O1-O1 (2) | 2.955 Å |
| O1-O2 | 2.750 Å | O1-O2 (2) | 2.700 Å | O1-O1 (2) | 2.504 Å |
| O1-O3 | 2.657 Å | O1-O2 (2) | 3.115 Å | O1-O1 | 2.694 Å |
| O1-O3 | 2.645 Å | O1-O3 (2) | 3.710 Å | O1-O2 (2) | 2.700 Å |
| O2-O3 | 2.661 Å | O2-O3 (2) | 2.537 Å | O1-O2 (2) | 2.666 Å |
| O2-O3 | 2.537 Å | O2-O3 (2) | 4.078 Å | O1-O2 (2) | 2.711 Å |
| O3-O3 | 2.621 Å | O3-O3 | 2.764 Å | O2-O2 | 2.786 Å |
| Tetrahedral Volume | 2.173 Å3 | Octahedral Volume | 10.792 Å3 | Octahedral Volume | 9.268 Å3 |
| Compression | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pressure (GPa) | 1 atm a | 1.9 | 3.7 | 6.2 | 8.6 | 10.1 | 12.4 | 13.7 | 16.3 | 18.0 | 19.0 |
| ν1 | 708.3 (0) | 712.1 (1) | 717.8 (1) | 726.6 (1) | 733.6 (1) | 737.8 (1) | 743.6 (0) | 747.0 (0) | 754.9 (0) | 757.3 (0) | 759.6 (0) |
| ν2 | 590.2 (1) | 588.9 (16) | 592.0 (13) | – | – | 608.7 (9) | 615.2 (6) | 619.9 (5) | 627.4 (5) | 629.7 (6) | 631.7 (5) |
| ν3 | 523.0 (0) | 525.4 (8) | 529.7 (9) | – | – | – | 556.7 (14) | 567.9 (9) | – | 576.7 (16) | – |
| ν4 | – | – | – | – | – | – | – | 533.3 (13) | 537.4 (10) | 542.2 (10) | – |
| ν5 | – | – | – | – | 472.3 (4) | 477.5 (2) | 485.2 (1) | 488.9 (1) | 497.9 (1) | 501.1 (1) | 503.8 (1) |
| ν6 | – | – | – | 420.3 (3) | 433.6 (3) | 443.2 (5) | 461.4 (3) | 467.4 (3) | – | – | – |
| ν7 | – | – | – | – | – | – | 432.2 (3) | 435.9 (3) | 445.8 (3) | 448.6 (4) | 451.4 (3) |
| ν8 | – | – | – | – | 389.9 (5) | 393.9 (4) | 398.2 (3) | 399.5 (5) | 405.0 (4) | 406.6 (4) | 408.4 (4) |
| ν9 | – | – | – | 292.9 (8) | 300.4 (6) | 304.8 (3) | 310.6 (3) | 313.3 (3) | 321.2 (3) | 323.2 (3) | 325.0 (3) |
| ν10 | – | – | – | – | – | 274.4 (6) | 279.0 (3) | 281.9 (3) | 287.8 (3) | 289.8 (3) | 291.1 (2) |
| ν11 | – | – | – | – | – | – | 249.5 (6) | 250.1 (5) | 254.6 (6) | 255.9 (6) | 256.0 (7) |
| ν12 | – | – | – | – | – | 225.0 (6) | 229.4 (5) | 230.8 (5) | 234.5 (4) | 235.8 (5) | 236.7 (5) |
| ν13 | – | – | – | – | – | 189.7 (7) | 194.0 (4) | 194.4 (5) | 198.2 (4) | 198.8 (3) | 199.0 (4) |
| ν14 | – | – | – | – | 110.8 (5) | 115.7 (1) | 120.8 (1) | 123.2 (1) | 126.3 (2) | 127.1 (2) | 127.7 (2) |
| Decompression | |||||||||||
| Pressure (GPa) | 3.0 | 6.4 | 8.4 | 10.3 | 12.1 | 14.0 | 16.2 | 18.3 | |||
| ν1 | – | – | 715.4 (0) | 726.9 (1) | 734.3 (1) | 740.2 (0) | 744.1 (0) | 749.0 (1) | 755.1 (1) | 760.1 (0) | – |
| ν2 | – | – | 590.7 (4) | 598.7 (32) | – | 610.8 (8) | 613.5 (10) | 620.6 (9) | 627.4 (6) | 634.1 (4) | – |
| ν3 | – | – | 527.7 (3) | 544.3 (11) | 552.0 (4) | 556.2 (4) | 559.6 (6) | 565.0 (11) | 573.5 (12) | – | – |
| ν4 | – | – | – | 504.7 (10) | – | 520.9 (3) | 526.1 (4) | 532.1 (6) | 538.3 (8) | 542.2 (6) | – |
| ν5 | – | – | – | 461.8 (9) | 473.6 (2) | 482.4 (1) | 487.3 (1) | 491.2 (2) | 498.3 (1) | 504.6 (1) | – |
| ν6 | – | – | – | 421.2 (3) | 436.8 (3) | 456.9 (3) | 464.9 (2) | – | – | – | – |
| ν7 | – | – | – | – | – | 427.9 (3) | 432.3 (2) | 438.5 (4) | 446.3 (5) | 452.6 (3) | – |
| ν8 | – | – | – | 383.6 (4) | 390.9 (2) | 396.7 (1) | 399.7 (2) | 403.2 (3) | 406.3 (4) | 408.6 (2) | – |
| ν9 | – | – | – | 293.7 (12) | 300.8 (4) | 307.2 (4) | 311.4 (5) | 316.6 (5) | 322.1 (5) | 325.7 (4) | – |
| ν10 | – | – | – | 267.4 (10) | 272.4 (5) | 276.2 (5) | 279.8 (5) | 283.3 (5) | 287.5 (3) | 291.9 (2) | – |
| ν11 | – | – | – | – | – | – | – | – | 255.4 (8) | 257.5 (7) | – |
| ν12 | – | – | – | – | – | – | – | – | 235.1 (6) | 236.6 (6) | – |
| ν13 | – | – | – | – | 188.1 (9) | 189.5 (8) | 193.7 (7) | 194.6 (11) | 196.7 (7) | 199.5 (5) | – |
| ν14 | – | – | – | – | 111.7 (1) | 117.7 (1) | 121.0 (1) | 123.8 (1) | 126.7 (2) | 127.6 (3) | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Yu, J.; Ouyang, Y.; Zhang, L.; Li, X.; Zhang, Z.; Li, Y. Crystal Chemistry, High-Pressure Behavior, Water Content, and Thermal Stability of Natural Spodumene. Minerals 2025, 15, 307. https://doi.org/10.3390/min15030307
Jiang Y, Yu J, Ouyang Y, Zhang L, Li X, Zhang Z, Li Y. Crystal Chemistry, High-Pressure Behavior, Water Content, and Thermal Stability of Natural Spodumene. Minerals. 2025; 15(3):307. https://doi.org/10.3390/min15030307
Chicago/Turabian StyleJiang, Yuhui, Jiayi Yu, Yuanze Ouyang, Li Zhang, Xiaoguang Li, Zhuoran Zhang, and Yunxuan Li. 2025. "Crystal Chemistry, High-Pressure Behavior, Water Content, and Thermal Stability of Natural Spodumene" Minerals 15, no. 3: 307. https://doi.org/10.3390/min15030307
APA StyleJiang, Y., Yu, J., Ouyang, Y., Zhang, L., Li, X., Zhang, Z., & Li, Y. (2025). Crystal Chemistry, High-Pressure Behavior, Water Content, and Thermal Stability of Natural Spodumene. Minerals, 15(3), 307. https://doi.org/10.3390/min15030307







