Heat-Induced Mn2+ and Fe2+ Oxidation in Heterophyllosilicates: Kupletskite and Kupletskite-(Cs)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Electron-Microprobe Analysis
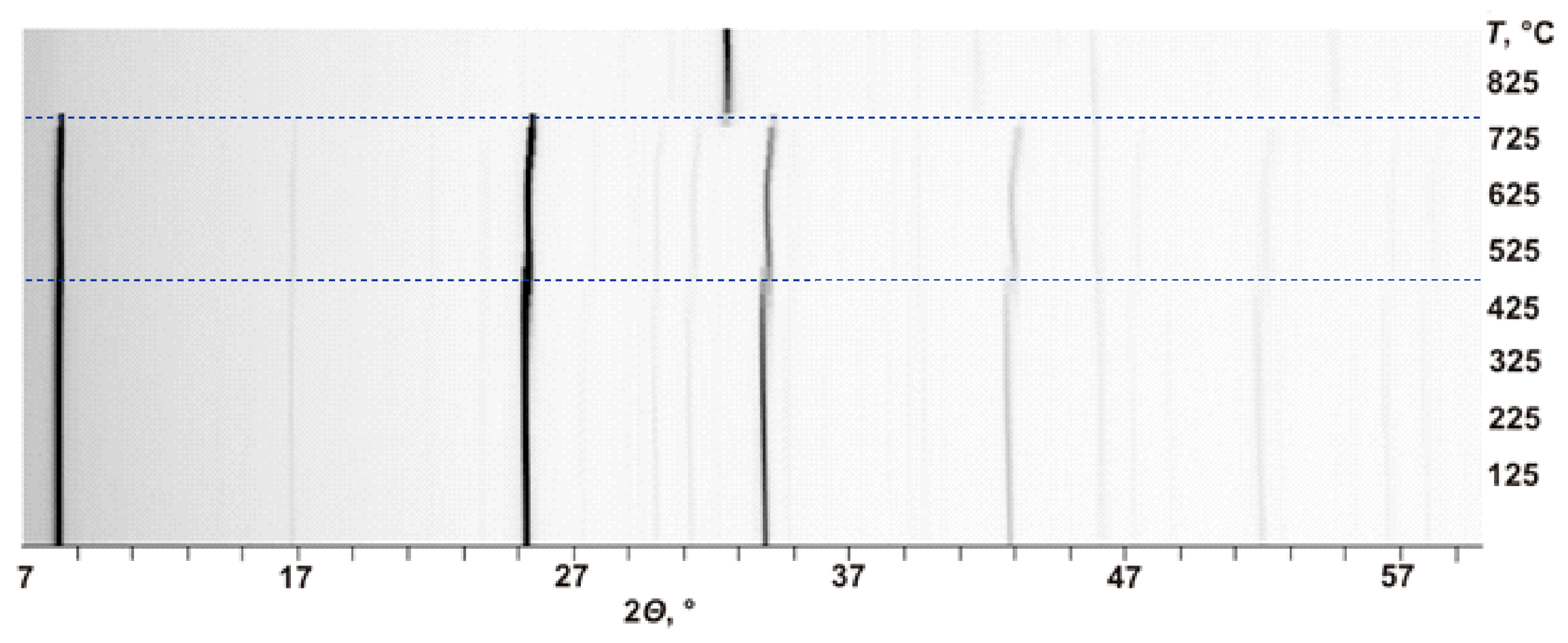
2.2.2. High-Temperature In Situ X-Ray Diffraction
2.2.3. Single-Crystal X-Ray Diffraction
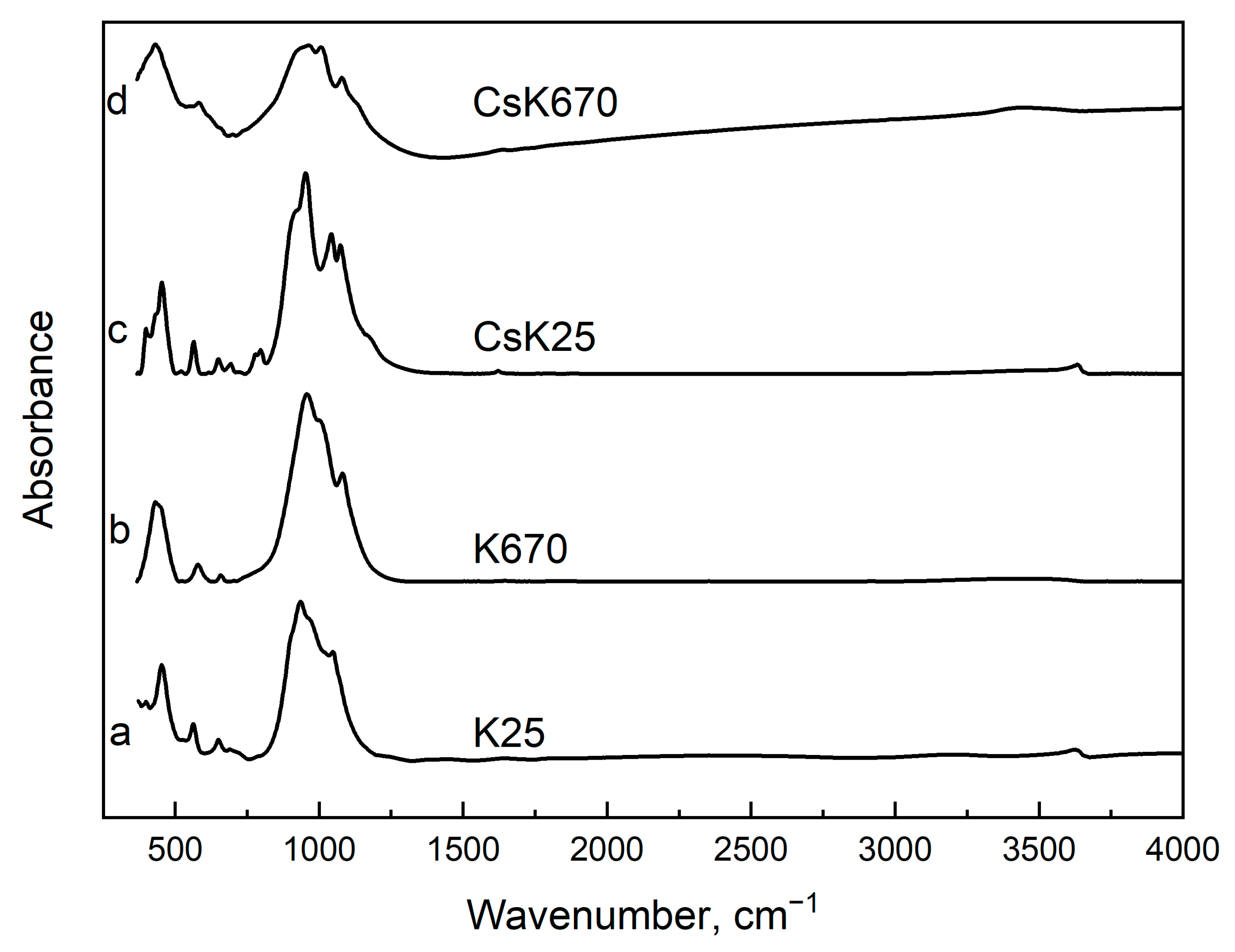
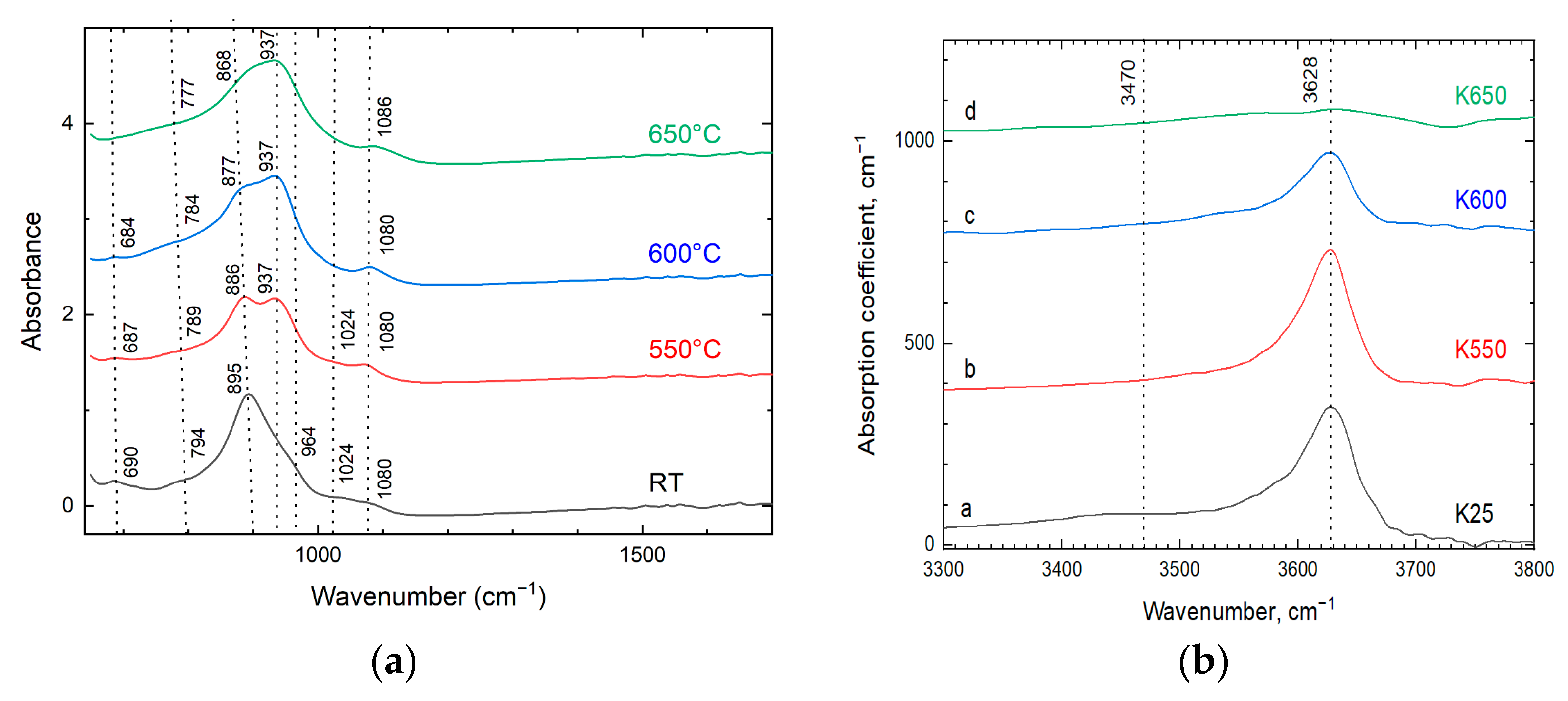
2.2.4. Infrared Spectroscopy
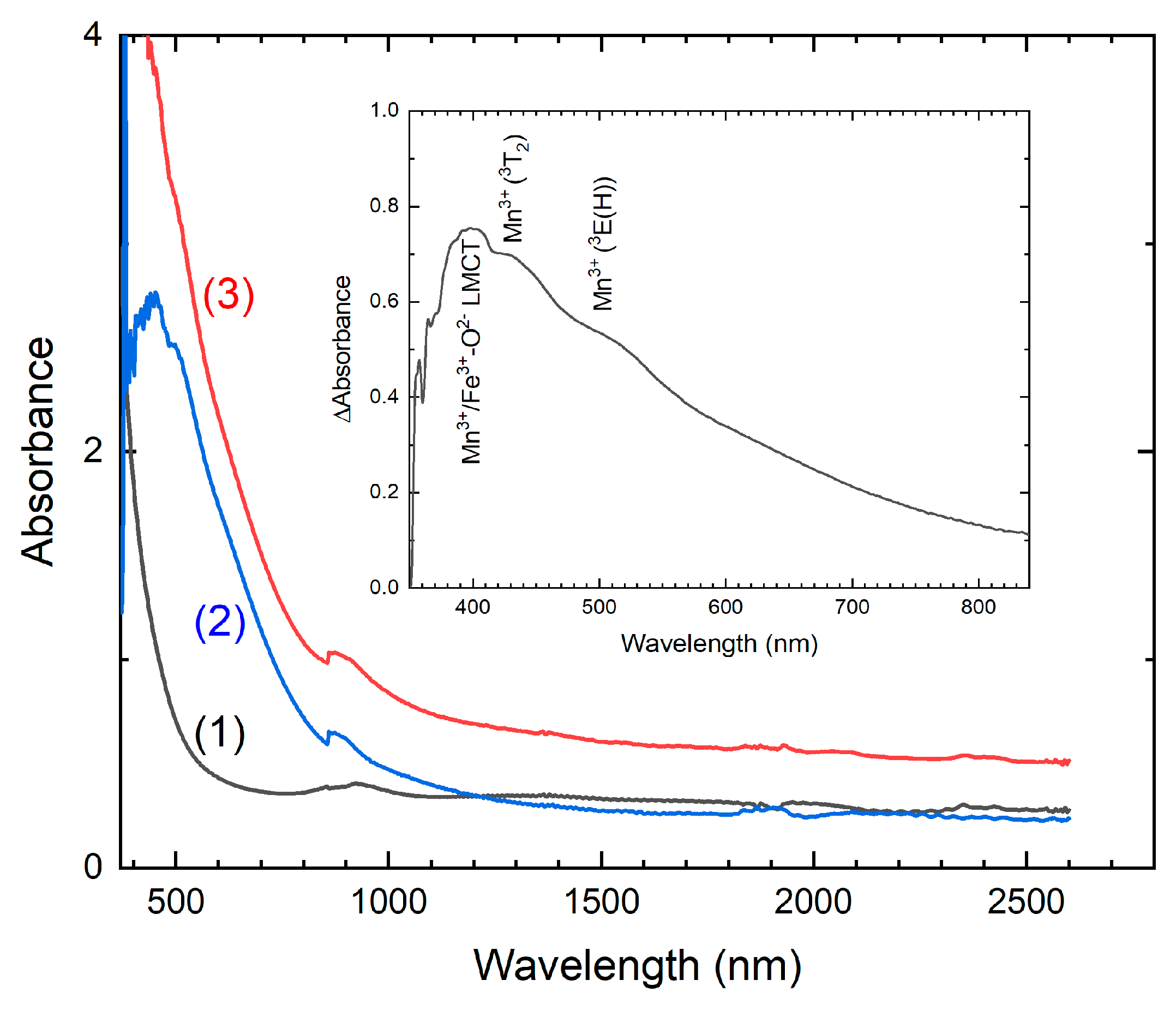
2.2.5. Optical Absorption Spectroscopy
3. Results
3.1. Chemical Composition
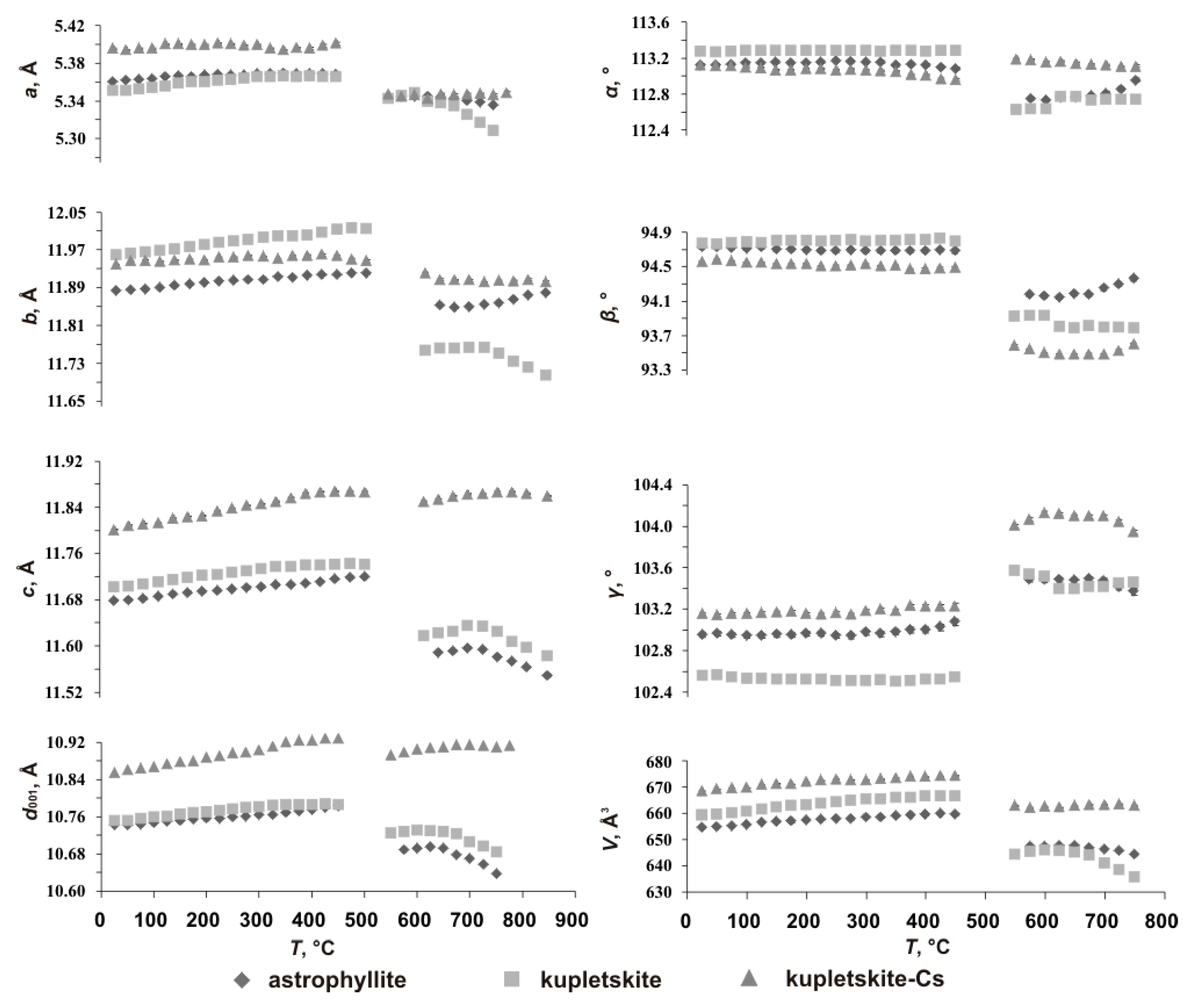
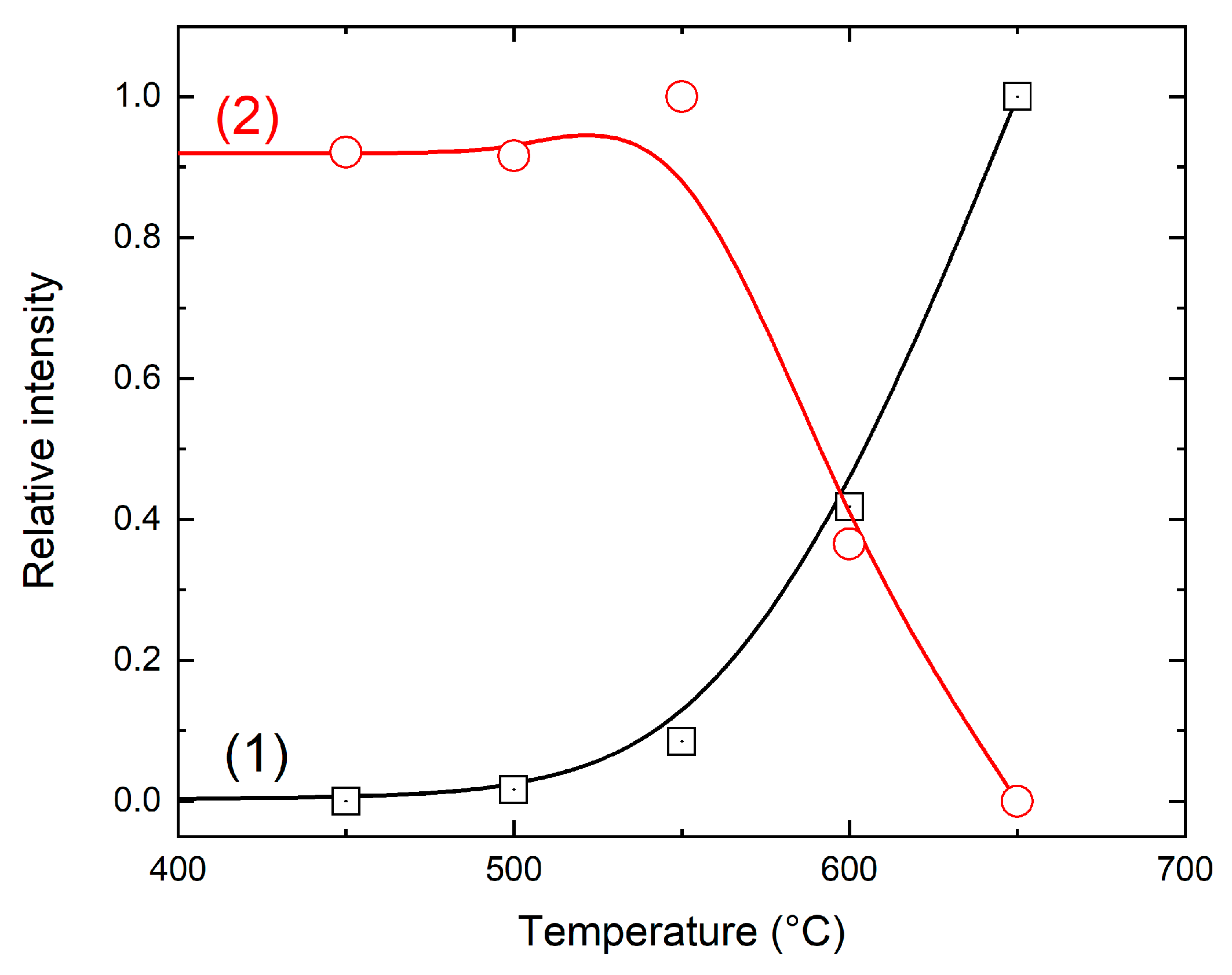
3.2. Thermal Evolution
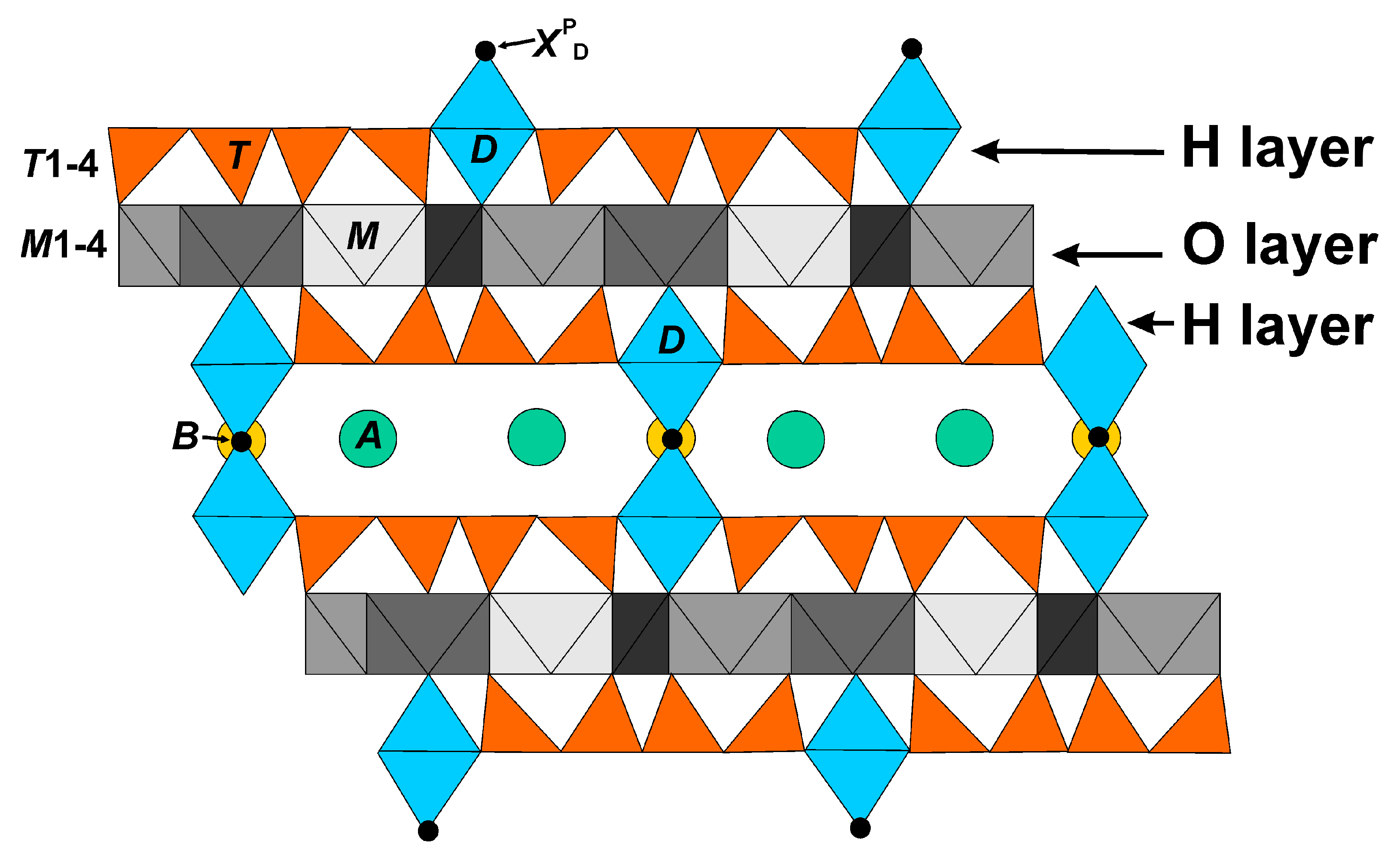
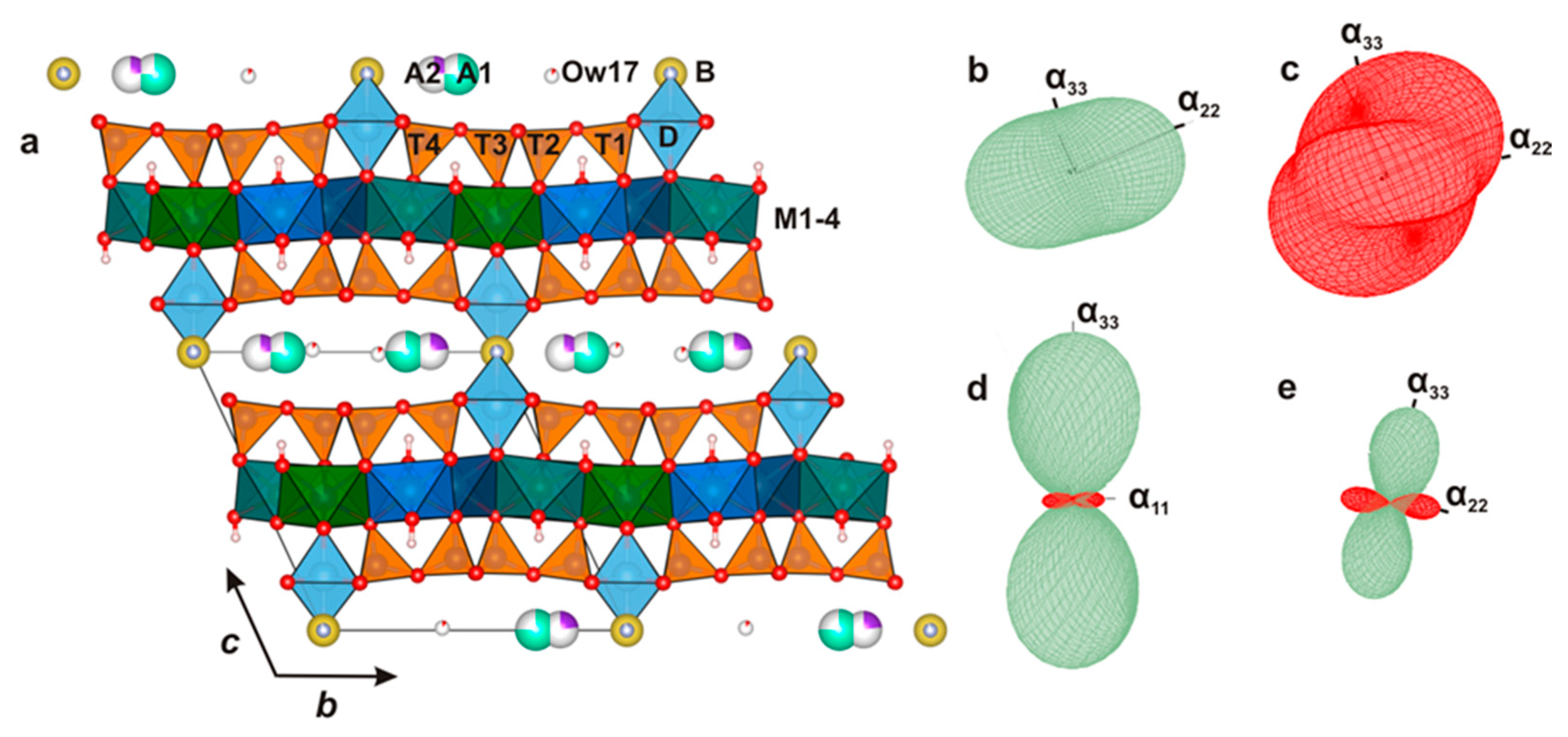
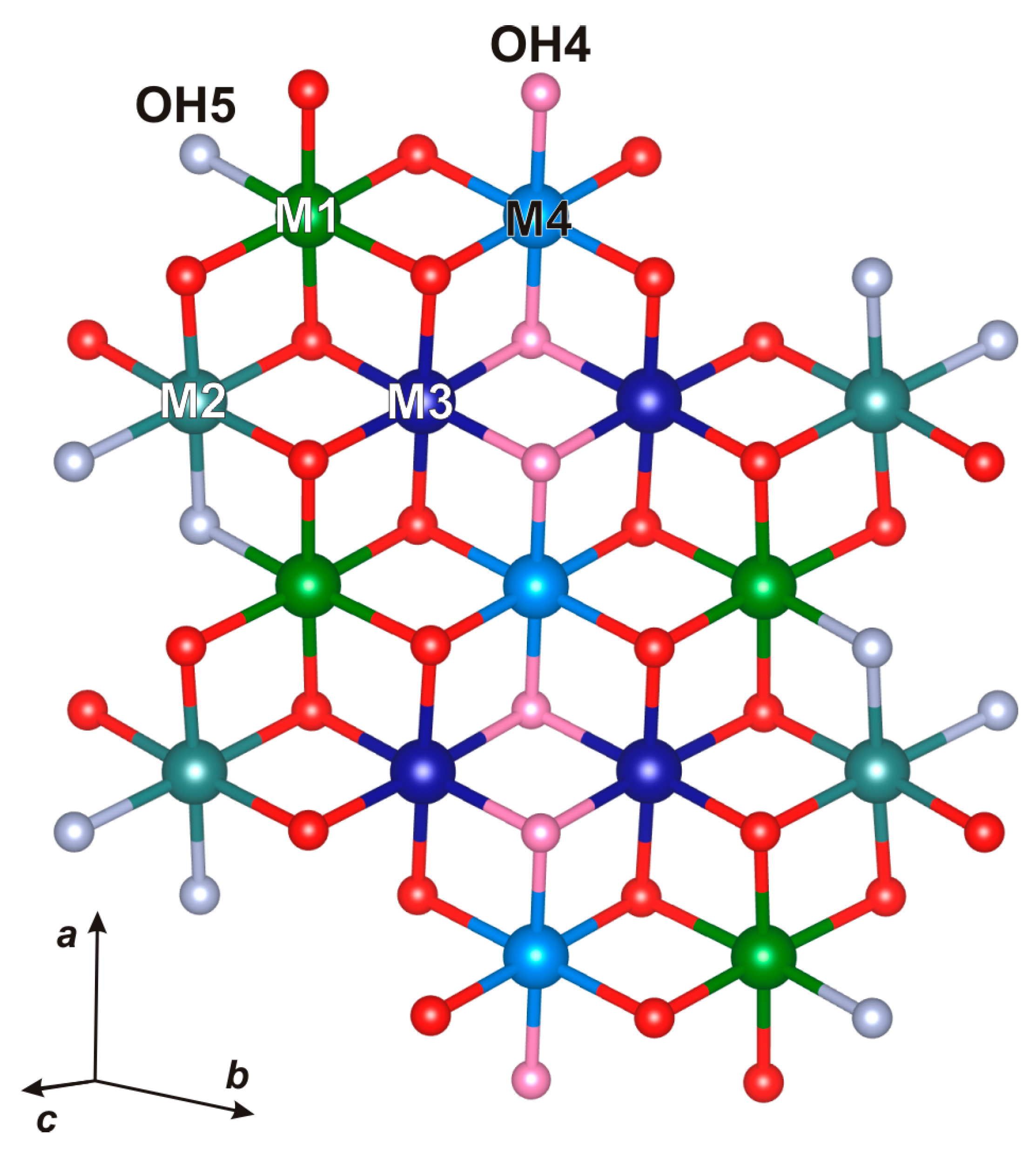
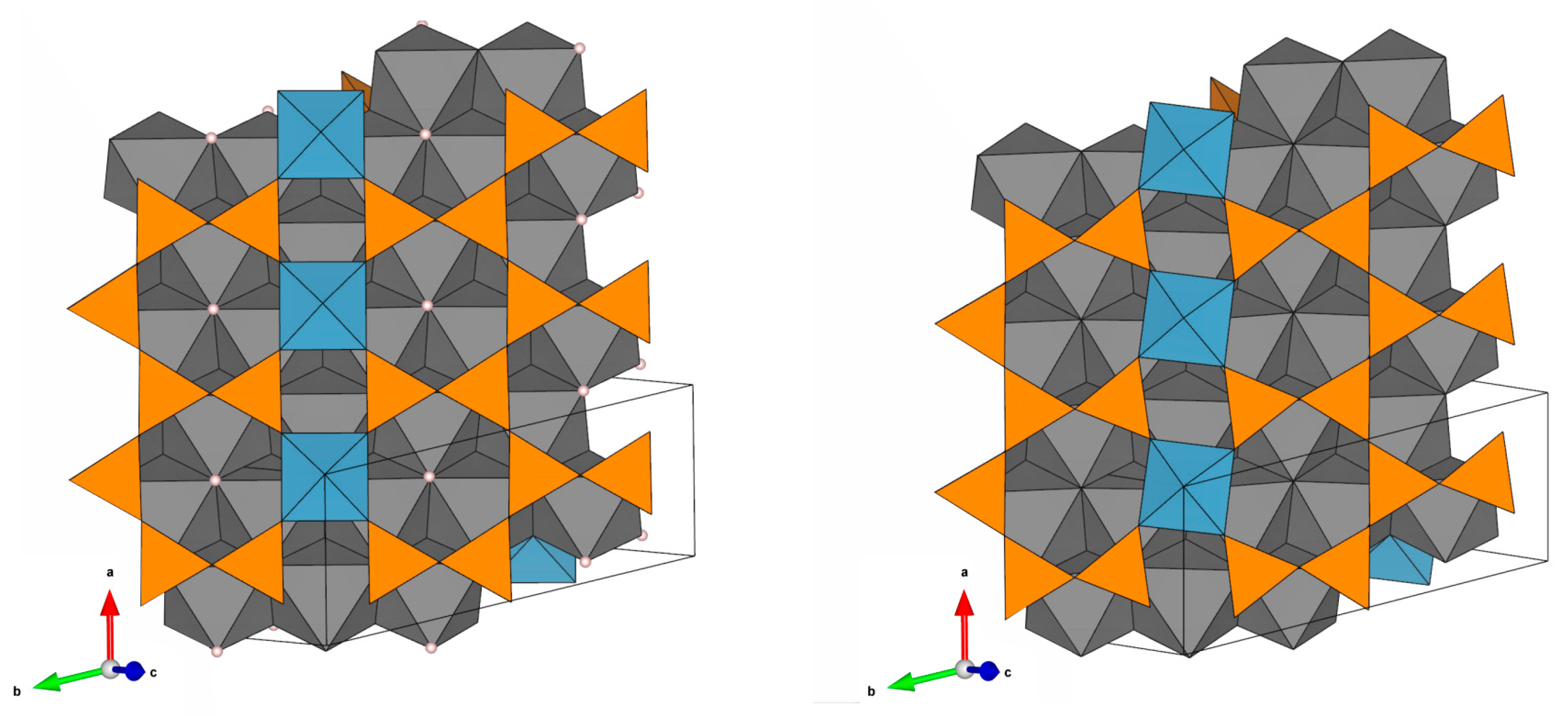
3.3. Crystal Structures
3.4. Dexydroxylation
3.5. The Mn Oxidation
4. Discussion
| Parameter | Sample | M(1) | M(2) | M(3) | M(4) |
|---|---|---|---|---|---|
| toct, Å | K | 2.49 → 2.38 | 2.47 → 2.29 | 2.47 → 2.32 | 2.42 → 2.31 |
| CsK | 2.49 → 2.36 | 2.48 → 2.28 | 2.47 → 2.30 | 2.42 → 2.29 | |
| Bond angle variance, ° | K | 59.3 → 93.7 | 46.5 → 94.6 | 41.9 → 88.0 | 39.1 → 37.3 |
| CsK | 59.7 → 94.7 | 47.1 → 101.0 | 45.1 → 90.7 | 43.7 → 44.18 | |
| Voctahedra, Å3 | K | 13.88 → 13.94 | 13.44 → 11.64 | 13.23 → 12.17 | 12.72 → 11.22 |
| CsK | 13.69 → 13.53 | 13.50 → 11.52 | 13.28 → 12.00 | 12.78 → 11.2 | |
| Distortion index | K | 0.007 → 0.017 | 0.026 → 0.072 | 0.017 → 0.029 | 0.014 → 0.041 |
| CsK | 0.006 → 0.018 | 0.027 → 0.079 | 0.019 → 0.031 | 0.015 → 0.042 | |
| Quadratic elongation | K | 1.0179 → 1.0299 | 1.0152 → 1.0382 | 1.0132 → 1.0280 | 1.0123 → 1.0156 |
| CsK | 1.0180 → 1.0304 | 1.0155 → 1.0437 | 1.0144 → 1.0292 | 1.0138 → 1.0179 | |
| T(1) | T(2) | T(3) | T(4) | ||
| Vtetrahedra, Å3 | K | 2.19 → 2.17 | 2.21 → 2.17 | 2.24 → 2.20 | 2.18 → 2.15 |
| CsK | 2.17 → 2.15 | 2.21 → 2.15 | 2.20 → 2.19 | 2.17 → 2.14 | |
| Distortion index | K | 0.008 → 0.011 | 0.006→ 0.004 | 0.008 → 0.005 | 0.007 → 0.010 |
| CsK | 0.009 → 0.012 | 0.011 → 0.003 | 0.009 → 0.006 | 0.009 → 0.009 | |
| Quadratic elongation | K | 1.0021 → 1.0018 | 1.0013 → 1.0015 | 1.0018 → 1.0030 | 1.0021 → 1.0015 |
| CsK | 1.0019 → 1.0018 | 1.0018→ 1.0022 | 1.0024 → 1.0040 | 1.0019 → 1.0015 | |
| D | A | B | |||
| Vpolyhedra, Å3 | K | 9.78 → 9.68 | 72.8 → 69.8 | 34.52 → 33.18 | |
| CsK | 9.89 → 9.88 | 74.8 → 72.5 | 35.65 → 34.33 | ||
| Distortion index | K | 0.026 → 0.011 | 0.091 → 0.82 | 0.013 → 0.060 | |
| CsK | 0.018 → 0.008 | 0.043 → 0.049 | 0.010 → 0.063 | ||
| Quadratic elongation | K | 1.0179 → 1.0102 | 1.1500 → 1.1510 | ||
| CsK | 1.0097 → 1.0049 | 1.1688 → 1.1770 |
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belov, N.V. Essays on Structural Mineralogy; Nauka: Moscow, Russia, 1976; pp. 1–344. (In Russian) [Google Scholar]
- Liebau, F. Structural Chemistry of Silicates: Structure, Bonding and Classification; Springer: Berlin, Germany, 1985. [Google Scholar]
- Ferraris, G. Modular structures–the paradigmatic case of the heterophyllosilicates. Z. Kristallogr. Cryst. 2008, 223, 76–84. [Google Scholar] [CrossRef]
- Sokolova, E.; Cámara, F.; Hawthorne, F.C.; Ciriotti, M.E. The astrophyllite supergroup: Nomenclature and classification. Mineral. Mag. 2017, 81, 143–153. [Google Scholar] [CrossRef]
- Piilonen, P.C.; Lalonde, A.E.; McDonald, A.M.; Gault, R.A.; Larsen, A.O. Insights into astrophyllite-group minerals. I. Nomenclature, composition and development of a standardized general formula. Can. Mineral. 2003, 41, 1–26. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Krivovichev, S.V.; Hawthorne, F.C.; Krzhizhanovskaya, M.G.; Zolotarev, A.A.; Abdu, Y.A.; Yakovenchuk, V.N.; Pakhomovsky, Y.A.; Goncharov, A.G. High-temperature behaviour of astrophyllite, K2NaFe72+Ti2(Si4O12)2O2(OH)4F: A combined X-ray diffraction and Mössbauer spectroscopic study. Phys. Chem. Miner. 2017, 44, 595–613. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Zolotarev, A.A.; Krivovichev, S.V.; Goncharov, A.G.; Gabdrakhmanova, F.A.; Vladykin, N.V.; Krzhizhanovskaya, M.G.; Shilovskikh, V.V.; Vlasenko, N.S.; Zolotarev, A.A. Temperature-induced iron oxidation in bafertisite Ba2Fe42+Ti2(Si2O7)2O2(OH)2F2: X-ray diffraction and Mössbauer spectroscopy study. Hyperfine Interact. 2017, 238, 1–12. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Zolotarev, A.A.; Hawthorne, F.C.; Krivovichev, S.V.; Yakovenchuk, V.N.; Goncharov, A.G. High-temperature Fe oxidation coupled with redistribution of framework cations in lobanovite, K2Na(Fe2+4Mg2Na)Ti2(Si4O12)2O2(OH)4—The first titanosilicate case. Acta Crystallogr. 2019, 75, 578–590. [Google Scholar] [CrossRef]
- Brown, I.D. The Chemical Bond in Inorganic Chemistry. The Bond Valence Model, 2nd ed.; Oxford University Press: Oxford, UK, 2016; pp. 1–344. [Google Scholar]
- Hawthorne, F.C. A bond-topological approach to theoretical mineralogy: Crystal structure, chemical composition and chemical reactions. Phys. Chem. Miner. 2012, 39, 841–874. [Google Scholar] [CrossRef]
- Hawthorne, F.C. Toward theoretical mineralogy: A bond-topological approach. Am. Mineral. 2015, 100, 696–713. [Google Scholar] [CrossRef]
- Russell, R.L.; Guggenheim, S. Crystal structures of near-endmember phlogopite at high temperatures and heat-treated Fe-rich phlogopite: The influence of the O, OH, F site. Can. Mineral. 1999, 37, 711–729. [Google Scholar]
- Chon, C.-M.; Lee, C.-K.; Song, Y.; Kim, S.A. Structural changes and oxidation of ferroan phlogopite with increasing temperature: In situ neutron powder diffraction and Fourier transform infrared spectroscopy. Phys. Chem. Miner. 2006, 33, 289–299. [Google Scholar] [CrossRef]
- Ventruti, G.; Zema, M.; Scordari, F.; Pedrazzi, G. Thermal behavior of a Ti-rich phlogopite from Mt. Vulture (Potenza, Italy): An in situ X-ray single-crystal diffraction study. Am. Mineral. 2008, 93, 632–643. [Google Scholar] [CrossRef]
- Zema, M.; Ventruti, G.; Lacalamita, M.; Scordari, F. Kinetics of Fe-oxidation/deprotonation process in Fe-rich phlogopite under isothermal conditions. Am. Mineral. 2010, 95, 1458–1466. [Google Scholar] [CrossRef]
- Murad, E.; Wagner, U. The thermal behaviuor of an Fe-rich illite. Clay Miner. 1996, 31, 45–52. [Google Scholar] [CrossRef]
- Güttler, B.; Niemann, W.; Redfern, S.A.T. EXAFS and XANES spectroscopy study of the oxidation and deprotonation of biotite. Mineral. Mag. 1989, 53, 591–602. [Google Scholar] [CrossRef]
- Veith, J.A.; Jackson, M.L. Iron oxidation and reduction effects on structural hydroxyl and layer charge in aqueous suspensions of micaceous vermiculites. Clays Clay Miner. 1974, 22, 345–353. [Google Scholar] [CrossRef]
- Korovushkin, V.V.; Kuzmin, V.; Belov, V.F. Mossbauer studies of structural features in tourmaline of various genesis. Phys. Chem. Miner. 1979, 4, 209–220. [Google Scholar] [CrossRef]
- Ferrow, E.A.; Annersten, H.; Gunawardane, R.P. Mössbauer effect study on the mixed valence state of iron in tourmaline. Mineral. Mag. 1988, 52, 221–228. [Google Scholar] [CrossRef]
- Bačik, P.; Ozdin, D.; Miglierini, M.; Kardošova, P.; Pentra, M.; Haloda, J. Crystallochemical effects of heat treatment on Fedominant tourmalines from Dolni Bory (Czech Republic) and Vlachovo(Slovakia). Phys. Chem. Miner. 2011, 38, 599–611. [Google Scholar] [CrossRef]
- Filip, J.; Bosi, F.; Novák, M.; Skogby, H.; Tuček, J.; Čuda, J.; Wildner, M. Iron redox reactions in the tourmaline structure: Hightemperature treatment of Fe3+-rich schorl. Geochim. Cosmochim. Acta 2012, 86, 239–256. [Google Scholar] [CrossRef]
- Oberti, R.; Della Ventura, G.; Dyar, M.D. Combining structure refinement and spectroscopies: Hints and warnings for more efficient tolls to decipher the mechanism of deprotonation in amphiboles. Period. Mineral. 2015, ECMS 2015, 131–132. [Google Scholar]
- Della Ventura, G. FTIR spectroscopy at HT: Applications and problems. Period. Mineral. 2015, ECMS 2015, 7–8. [Google Scholar]
- Susta, U.; Della Ventura, G.; Bellatreccia, F.; Hawthorne, F.C.; Oberti, R. HT-FTIR spectroscopy of riebeckite. Period. Mineral. 2015, ECMS 2015, 167–168. [Google Scholar]
- Della Ventura, G.; Redhammer, G.J.; Galdenzi, F.; Ventruti, G.; Susta, U.; Oberti, R.; Radica, F.; Marcelli, A. Oxidation or cation re-arrangement? Distinct behavior of riebeckite at high temperature. Am. Mineral. 2023, 108, 59–69. [Google Scholar] [CrossRef]
- Mihailova, B.; Della Ventura, G.; Waeselmann, N.; Bernardini, S.; Xu, W.; Marcelli, A. Polarons in rock-forming minerals: Physical implications. Condens. Matter 2022, 7, 68. [Google Scholar] [CrossRef]
- Della Ventura, G.; Galdenzi, F.; Marcelli, A.; Cibin, G.; Oberti, R.; Hawthorne, F.C.; Bernardini, S.; Mihailova, B. In situ simultaneous Fe K-edge XAS spectroscopy and resistivity measurements of riebeckite: Implications for anomalous electrical conductivity in subduction zones. Geochemistry 2023, 84, 126037. [Google Scholar] [CrossRef]
- Bernardini, S.; Della Ventura, G.; Schlüter, J.; Hawthorne, F.C.; Mihailova, B. The effect of A-site cations on charge-carrier mobility in Fe-rich amphiboles. Am. Mineral. 2024, 109, 1545–1553. [Google Scholar] [CrossRef]
- Bernardini, S.; Della Ventura, G.; Hawthorne, F.C.; Marcelli, A.; Salvini, F.; Mihailova, B. The effect of anisotropic electrical conductivity of amphiboles on geophysical anomalies observed in subduction zones. Sci. Rep. 2025; accepted. [Google Scholar] [CrossRef]
- Della Ventura, G.; Bernardini, S.; Redhammer, J.G.; Galdenzi, F.; Radica, F.; Marcelli, A.; Hawthorne, F.C.; Oberti, R.; Mihailova, B. The oxidation of iron in amphiboles at high temperatures: A review and implications for large-scale Earth processes. Rend. Lincei Sci. Fis. Nat. 2024, 35, 893–906. [Google Scholar] [CrossRef]
- Ferraris, G. Heterophyllosilicates, a potential source of nanolayers for materials science. In Minerals as Advanced Materials I.; Krivovichev, S.V., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 157–163. [Google Scholar]
- Ferraris, G.; Merlino, S. Micro- and Mesoporous Mineral Phases; Mineralogical Society of America: Washington, DC, USA, 2005; Volume 57, pp. 1–448. [Google Scholar]
- Lin, Z.; Paz, F.A.A.; Rocha, J. Layered titanosilicates. In Layered Mineral Structures and Their Application in Advanced Technologies; Brigatti, M.F., Mottana, A., Eds.; Mineralogical Society of Great Britain and Ireland: London, UK, 2011; Volume 11. [Google Scholar] [CrossRef]
- Aksenov, S.M.; Yamnova, N.A.; Chukanov, N.V.; Kabanova, N.A.; Kobeleva, E.A.; Deyneko, D.V.; Krivovichev, S.V. Theoretical analysis of cation-migration paths in microporous heterophyllosilicates with astrophyllite and veblenite type structures. J. Struct. Chem. 2022, 63, 293–301. [Google Scholar] [CrossRef]
- Yakovenchuk, V.; Ivanyuk, G.; Pakhomovsky, Y.; Men’shikov, Y. Khibiny; Laplandia Minerals: Apatity, Russia, 2005. [Google Scholar]
- Yefimov, A.F.; Dusmatov, V.D.; Ganzeyev, A.A.; Katayeva, Z.T. Cesium kupletskite, a new mineral. Dokl. Akad. Nauk. SSSR 1971, 197, 140–143. [Google Scholar]
- Bruker AXS. Topas V4.2: General Profile and Structure Analysis Software for Powder Diffraction Data; Bruker AXS: Karlsruhe, Germany, 2009. [Google Scholar]
- Belousov, R.; Filatov, S. Algorithm for calculating the thermal expansion tensor and constructing the thermal expansion diagram for crystals. Glass Phys. Chem. 2007, 33, 271–275. [Google Scholar] [CrossRef]
- Bubnova, R.S.; Firsova, V.A.; Filatov, S.K. Software for determining the thermal expansion tensor and the graphic representation of its characteristic surface (theta to tensor-TTT). Glass Phys. Chem. 2013, 39, 347–350. [Google Scholar] [CrossRef]
- Langreiter, T.; Kahlenberg, V. TEV—A program for the determination of the thermal expansion tensor from diffraction data. Crystals 2015, 5, 143–153. [Google Scholar] [CrossRef]
- Bruker AXS. APEX2, Version 2014.11-0; Bruker AXS: Madison, WI, USA, 2014. [Google Scholar]
- Sheldrick, G.M. Crystal structure solution with ShelXT. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analisis program. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Cámara, F.; Sokolova, E.; Abdu, Y.; Hawthorne, F.C. The crystal structures of niobophyllite, kupletskite-(Cs) and Sn-rich astrophyllite: Revisions to the crystal chemistry of the astrophyllite-group minerals. Can. Mineral. 2010, 48, 1–16. [Google Scholar] [CrossRef]
- Woodrow, P.J. The crystal structure of astrophyllite. Acta Crystallogr. 1967, 22, 673–678. [Google Scholar] [CrossRef]
- Piilonen, P.C.; McDonald, A.M.; Lalonde, A.E. Insights into astrophyllite-group minerals. II. Crystal chemistry. Can. Mineral. 2003, 41, 27–54. [Google Scholar] [CrossRef]
- Sokolova, E. Further developments in the structure topology of the astrophyllite-group minerals. Mineral. Mag. 2012, 76, 863–882. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Sokolova, E.; Day, M.C.; Hawthorne, F.C.; Kasatkin, A.V.; Downs, R.T.; Horváth, L.; Pfenninger-Horváth, E. Laverovite, K2NaMn7Zr2(Si4O12)2O2(OH)4F, a new astrophyllite-supergroup mineral from Mont Saint-Hilaire, Québec, Canada. Can. Mineral. 2019, 57, 201–213. [Google Scholar] [CrossRef]
- Hålenius, U.; Bosi, F. Color of Mn-bearing gahnite: A first example of electronic transitions in heterovalent exchange coupled IVMn2+–VIMn3+ pairs in minerals. Am. Mineral. 2014, 99, 261–266. [Google Scholar] [CrossRef]
- Czaja, M.; Lisiecki, R.; Chrobak, A.; Sitko, R.; Mazurak, Z. The absorption-and luminescence spectra of Mn3+ in beryl and vesuvianite. Phys. Chem. Miner. 2018, 45, 475–488. [Google Scholar] [CrossRef]
- Fridrichová, J.; Bačík, P.; Ertl, A.; Wildner, M.; Dekan, J.; Miglierini, M. Jahn-Teller distortion of Mn3+-occupied octahedra in red beryl from Utah indicated by optical spectroscopy. J. Mol. Struct. 2018, 1152, 79–86. [Google Scholar] [CrossRef]
- Gagné, O.C.; Hawthorne, F.C. Bond-length distributions for ions bonded to oxygen: Results for the transition metals and quantification of the factors underlying bond-length variation in inorganic solids. IUCrJ 2020, 7, 581–629. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Gagné, O.C. New ion radii for oxides and oxysalts, fluorides, chlorides and nitrides. Acta Crystallogr. 2024, B80, 326–339. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
| Sample | K25 | K650 | CsK25 | CsK650 |
|---|---|---|---|---|
| Crystal data | ||||
| Crystal system | Triclinic | Triclinic | Triclinic | Triclinic |
| Space group | P-1 | P-1 | P-1 | P-1 |
| Unit-cell dimensions a, b, c (Å), α, β, γ (°) | 5.3976(2) 11.9431(7) 11.7092(6) 113.066(5) 94.702(4) 103.086(4) | 5.3233(5) 11.8826(14) 11.5362(12) 112.756(10) 93.699(8) 104.462(9) | 5.3904(10) 11.946(2) 11.799(2) 113.135(5) 94.573(6) 103.115(17) | 5.312(2) 11.832(4) 11.739(5) 112.996(10) 93.587(10) 104.39(4) |
| Unit-cell volume (Å3) | 664.06(6) | 640.90(13) | 668.2(2) | 647.3(5) |
| Z | 1 | 1 | 1 | 1 |
| Calculated density (g/cm−3) | 3.274 | 3.354 | 3.635 | 3.726 |
| Absorption coefficient (μ/mm−1) | 4.992 | 4.481 | 6.204 | 6.244 |
| Data collection | ||||
| Diffractometer | Bruker APEX II | |||
| Temperature (K) | 293 K | |||
| Radiation | MoKα | |||
| 2θ range (°) | 3.852–72.8 | 3.892–72.88 | 3.864–59.99 | 3.832–59.986 |
| h, k, l ranges | −8 ≤ h ≤ 8, −19 ≤ k ≤ 19, −18 ≤ l ≤ 19 | −8 ≤ h ≤ 8, −19 ≤ k ≤ 19, −18 ≤ l ≤ 18 | −7 ≤ h ≤ 7, −16 ≤ k ≤ 16, −14 ≤ l ≤ 16 | −7 ≤ h ≤ 7, −16 ≤ k ≤ 16, −16 ≤ l ≤ 16 |
| F(000) | 634.0 | 625.0 | 693.0 | 686.0 |
| Total reflections collected | 12,050 | 11,358 | 8686 | 12,060 |
| Unique reflections (Rint) | 6044 (0.0325) | 5837 (0.0303) | 3591 (0.0518) | 3766 (0.0476) |
| Unique reflections F > 4σ(F) | 4768 | 4531 | 2667 | 2831 |
| Structure refinement | ||||
| Refinement method | Full-matrix least-squares on F2 | |||
| Data/restrains/parameters | 6044/2/253 | 5837/0/245 | 3591/2/256 | 3766/0/251 |
| R1 [F > 4σ(F)], wR2 [F > 4σ(F)] | 0.0433, 0.1119 | 0.0416, 0.1002 | 0.0323, 0.0715 | 0.0476, 0.1108 |
| R1 all, wR2 all | 0.0590, 0.1232 | 0.0611, 0.1110 | 0.0657, 0.0904 | 0.0697, 0.1229 |
| Goodness-of-fit on F2 | 1.051 | 1.037 | 1.061 | 1.044 |
| Largest diff. peak and hole (ēÅ−3) | 1.29/−1.81 | 1.57/−1.37 | 1.72/−1.45 | 1.29/−1.52 |
| Mineral | Kupletskite | Kupletskite-(Cs) | Constituent | Kupletskite | Kupletskite-(Cs) | ||
|---|---|---|---|---|---|---|---|
| n | 22 | 27 | Constituent | ||||
| Constituent, wt. % | average | range | average | range | apfu, calculated as Si + Al = 8 | ||
| Na2O | 2.77 | 2.64–2.93 | 2.11 | 1.76–2.83 | Na | 1.15 | 1.00 |
| K2O | 6.22 | 6.03–6.38 | 0.73 | 0.61–0.95 | K | 1.70 | 0.23 |
| CaO | 1.43 | 1.27–1.59 | 0.73 | 0.56–0.92 | Ca | 0.33 | 0.19 |
| Cs2O | - | - | 14.40 | 14.25–15.20 | Cs | - | 1.50 |
| PbO | - | - | 0.37 | 0–1.09 | Pb | - | 0.02 |
| MgO | 1.42 | 1.29–1.55 | 0.04 | 0–0.25 | Mg | 0.45 | 0.01 |
| MnO | 18.62 | 17.05–20.16 | 17.79 | 17.41–18.37 | Mn | 3.37 | 3.68 |
| FeO (1) | 11.82 | 14.23–17.20 | 8.74 | 10.54–11.72 | Fe2+ | 2.11 | 1.78 |
| Fe2O3 | 4.15 | 2.57 | Fe3+ | 0.67 | 0.47 | ||
| Li2O (2) | - | - | 0.74 | - | Li | - | 0.73 |
| Al2O3 | 0.81 | 0.64–1.19 | 0.12 | 0–0.48 | Al | 0.20 | 0.03 |
| TiO2 | 11.18 | 10.88–11.48 | 6.96 | 5.83–8.04 | Ti | 1.80 | 1.28 |
| Nb2O5 | 2.12 | 1.83–2.23 | 6.35 | 5.17–8.56 | Nb | 0.20 | 0.70 |
| SiO2 | 36.47 | 35.80–37.89 | 32.62 | 32.11–33.26 | Si | 7.80 | 7.97 |
| F | 1.19 | 0.68–1.63 | 0.84 | 0.65–1.19 | F | 0.80 | 0.65 |
| H2O (3) | 2.67 | - | 2.35 | - | OH | 3.80 | 3.83 |
| O (3) | 0.50 | - | 0.58 | - | O | 0.20 | 0.52 |
| 2F = O | −0.50 | - | −0.35 | - | |||
| Total | 100.87 | 99.60 | |||||
| T, °C | α11 | α22 | α33 | <α11a | <α22b | <α33c | αa | αb | αc | αα | αβ | αγ | αV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kupletskite | |||||||||||||
| 300 | 4.3 | 11.5 | 6.7 | 24.9 | 12.2 | 20 | 4.9(4) | 11.2(4) | 6.7(3) | −0.01(9) | 0.6(2) | 0.2(2) | 22.5(6) |
| Kupletskite-(Cs) | |||||||||||||
| 300 | −3.4 | 1.6 | 17.1 | 47 | 33.9 | 26.7 | −1(1) | 0.1(7) | 13.3(7) | −3.7(4) | −2.0(4) | 2.7(4) | 15(2) |
| High-temperature modification of kupletskite | |||||||||||||
| 650 | −39.2 | −19.9 | −11.8 | 27.3 | 35.4 | 22.3 | −34(2) | −23(1) | −15(2) | 5(2) | −9(2) | −6(1) | −71(3) |
| High-temperature modification of kupletskite-(Cs) | |||||||||||||
| 650 | 3.2 | −6.7 | 10.3 | 26.9 | 10.8 | 39.5 | 2(1) | −6(2) | 5.5(6) | −4.1(4) | 0.6(9) | −3(1) | 7(2) |
| Sample | Kupletskite | HT Kupletskite | Kupletskite-(Cs) | HT Kupletskite-(Cs) | Astrophyllite | HT Astrophyllite |
|---|---|---|---|---|---|---|
| V, Å3 | 664.1 | 640.9 | 668.2 | 647.3 | 655.5 | 633.0 |
| ΔV/V, % | −3.5 | −3.1 | −3.4 | |||
| Octahedra M(1)O6 | ||||||
| ē M(1) | 23.05 | 21.10 | 21.75 | 19.28 | 23.69 | 21.14 |
| Δē M(1) | −2.0 | −2.5 | −2.6 | |||
| <M(1)–O>, Å | 2.203 | 2.219 | 2.193 | 2.197 | 2.176 | 2.185 |
| Δ<M(1)–O>, Å | +0.016 | +0.004 | +0.009 | |||
| Octahedra M(2)O6 | ||||||
| ēM(2) | 25.00 | 23.83 | 25.00 | 24.61 | 25.06 | 23.01 |
| ΔēM(2) | −1.2 | −0.4 | −2.0 | |||
| <M(2)–O>, Å | 2.174 | 2.091 | 2.179 | 2.089 | 2.156 | 2.102 |
| Δ<M(2)–O>, Å | −0.083 | −0.090 | −0.054 | |||
| Octahedra M(3)O6 | ||||||
| ēM(3) | 24.61 | 24.48 | 23.83 | 24.61 | 24.18 | 24.28 |
| ΔēM(3) | −0.1 | +0.8 | +0.1 | |||
| <M(3)–O>, Å | 2.162 | 2.117 | 2.167 | 2.108 | 2.144 | 2.097 |
| Δ<M(3)–O>, Å | −0.045 | −0.059 | −0.047 | |||
| Octahedra M(4)O6 | ||||||
| ēM(4) | 23.31 | 24.09 | 25.00 | 24.22 | 23.51 | 24.15 |
| ΔēM(4) | +0.8 | −0.8 | +0.6 | |||
| <M(4)–O>, Å | 2.133 | 2.047 | 2.138 | 2.049 | 2.128 | 2.059 |
| Δ<M(4)–O>, Å | −0.086 | −0.089 | −0.069 | |||
| Octahedra Dφ6 | ||||||
| D–O(2), Å | 1.808 | 1.902 | 1.855 | 1.960 | 1.811 | 1.952 |
| D–XPD, Å | 2.088 | 2.002 | 2.051 | 1.993 | 2.100 | 1.982 |
| <D–φ>, Å | 1.958 | 1.946 | 1.959 | 1.964 | 1.958 | 1.945 |
| Tetrahedra TO4 | ||||||
| <T1–O>, Å | 1.623 | 1.620 | 1.618 | 1.614 | 1.615 | 1.607 |
| <T2–O>, Å | 1.629 | 1.618 | 1.627 | 1.613 | 1.625 | 1.620 |
| <T3–O>, Å | 1.637 | 1.627 | 1.627 | 1.625 | 1.632 | 1.624 |
| <T4–O>, Å | 1.620 | 1.614 | 1.618 | 1.611 | 1.614 | 1.606 |
| Extraframework sites A, B | ||||||
| <A1–φ>, Å | 3.283 | 3.225 | 3.336 | 3.318 | 3.298 | 3.243 |
| <A2–φ>, Å | 3.299 | 3.226 | 3.369 | 3.345 | - | - |
| <B–φ>, Å | 2.621 | 2.595 | 2.648 | 2.629 | 2.615 | 2.548 |
| Reference | This work | This work | [6] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhitova, E.S.; Zolotarev, A.A.; Sheveleva, R.M.; Shendrik, R.Y.; Hawthorne, F.C.; Nuzhdaev, A.A.; Vlasenko, N.S.; Kaneva, E.V.; Yakovenchuk, V.N. Heat-Induced Mn2+ and Fe2+ Oxidation in Heterophyllosilicates: Kupletskite and Kupletskite-(Cs). Minerals 2025, 15, 587. https://doi.org/10.3390/min15060587
Zhitova ES, Zolotarev AA, Sheveleva RM, Shendrik RY, Hawthorne FC, Nuzhdaev AA, Vlasenko NS, Kaneva EV, Yakovenchuk VN. Heat-Induced Mn2+ and Fe2+ Oxidation in Heterophyllosilicates: Kupletskite and Kupletskite-(Cs). Minerals. 2025; 15(6):587. https://doi.org/10.3390/min15060587
Chicago/Turabian StyleZhitova, Elena S., Andrey A. Zolotarev, Rezeda M. Sheveleva, Roman Yu. Shendrik, Frank C. Hawthorne, Anton A. Nuzhdaev, Natalia S. Vlasenko, Ekaterina V. Kaneva, and Victor N. Yakovenchuk. 2025. "Heat-Induced Mn2+ and Fe2+ Oxidation in Heterophyllosilicates: Kupletskite and Kupletskite-(Cs)" Minerals 15, no. 6: 587. https://doi.org/10.3390/min15060587
APA StyleZhitova, E. S., Zolotarev, A. A., Sheveleva, R. M., Shendrik, R. Y., Hawthorne, F. C., Nuzhdaev, A. A., Vlasenko, N. S., Kaneva, E. V., & Yakovenchuk, V. N. (2025). Heat-Induced Mn2+ and Fe2+ Oxidation in Heterophyllosilicates: Kupletskite and Kupletskite-(Cs). Minerals, 15(6), 587. https://doi.org/10.3390/min15060587








