1. Introduction
Flotation is a widely used technique for mineral separation and wastewater treatment. The fundamental principle involves interactions between bubbles and solid particles, wherein mineral particles selectively attach to the bubble surface, forming particle–bubble aggregates, leading to the separation of target minerals from mixed ores [
1,
2]. The efficiency of the process depends on effective collisions and the adhesion between bubbles and particles, with the hydration film being a key factor in influencing these interactions [
3].
A hydration film is a thin layer of water molecules and dissolved substances that forms on the surface of particles, with a similar film developing on bubble surfaces. This film significantly affects the interfacial dynamics between bubbles and particles [
4]. Hydration films can negatively impact flotation by increasing the hydrophilicity of particle surfaces, thereby weakening the adhesion between bubbles and particles [
5,
6,
7]. In the presence of high concentrations of electrolytes or hydrating agents, the hydration film may thicken, hindering the stable attachment of bubbles to particle surfaces [
8,
9]. Some studies suggest that the chemical composition and surface properties of the hydration film may influence flotation selectivity and efficiency more than its thickness [
10]. The hydration film not only inhibits bubble attachment but also may alter flotation outcomes by modifying the wettability of the mineral surface [
11]. Furthermore, hydration films can alter the surface charge distribution of particles, thereby affecting electrostatic interactions between particles and bubbles, which reduces the ability of bubbles to capture particles [
12,
13]. These disruptions to bubble–particle interactions can lead to substantial changes in flotation efficiency. Additionally, the stability of the hydration film requires energy input to thin and rupture during the process [
14].
Although the hydration film plays a critical role in flotation, its underlying mechanism remains poorly understood. Some researchers propose that the hydration film acts as a physical barrier that impedes contact between bubbles and particles, whereas others emphasize the importance of the film’s chemical and charge properties in flotation performance [
15,
16]. Moreover, the formation and disruption of the hydration film exhibit varying characteristics under different flotation conditions, further complicating the understanding of its role [
17,
18].
Despite these challenges, overcoming the barrier posed by hydration films to bubble–particle collisions is crucial for efficient mineralization [
19]. However, given the complex nature of hydration films, finding effective solutions remains challenging. In this study, an innovative model of gas–liquid–solid collision is introduced. A novel pathway by which mineral particles can directly adhere to the internal gas–liquid interface of bubbles to achieve mineralization is explored. This method bypasses the need to overcome the interference of the hydration film in traditional flotation while also leveraging the hydration film to reduce the energy required for mineral particles to interact with the gas–liquid interface.
2. Proposal of Reverse Sequence Collision Flotation Model
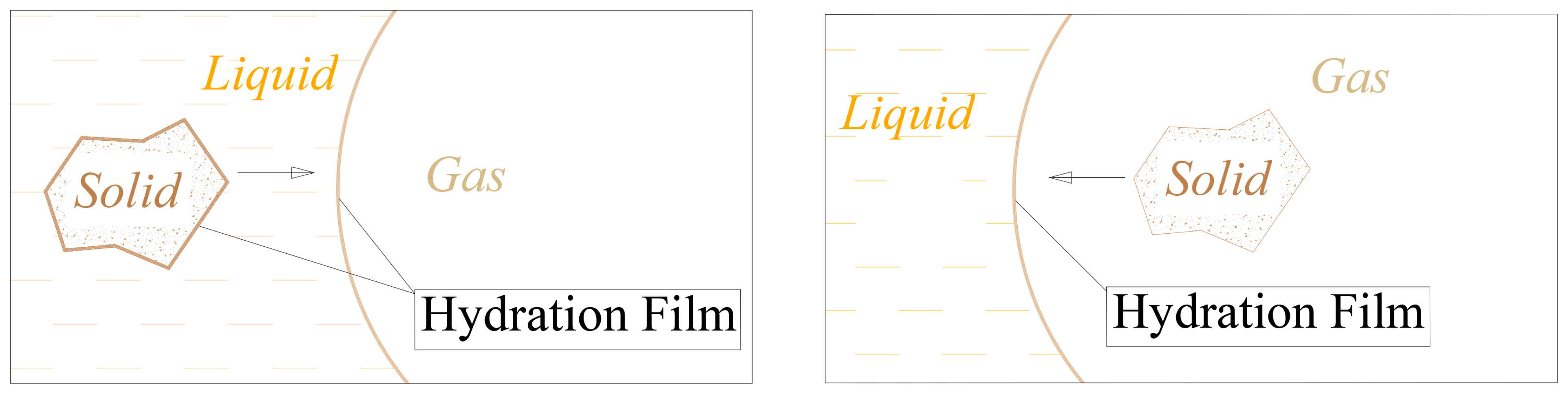
In conventional flotation processes, mineral particles and bubbles are introduced into the liquid phase, in which a hydration film forms on the surfaces of both the particles and the bubbles (
Figure 1). This hydration film acts as a barrier, impeding direct contact between the gas and solid phases. Overcoming this barrier requires sufficient energy and contact time. If the energy is insufficient, then mineral particles may rebound from the bubbles or slide along their edges. Conversely, excessive energy may cause particles to penetrate or escape from bubbles, preventing effective attachment. As a result, the effective particle size range for flotation is significantly restricted.
Studies indicate that conventional flotation is constrained by several factors, including mineral wettability, energy barriers, and contact time, which collectively increase the difficulty and energy required for bubble–particle collisions and adhesion. These factors are closely linked to the presence of the hydration film on the particle and bubble surfaces. Furthermore, the hydration film may influence the effectiveness of flotation reagents.
The aforementioned challenges are addressed in this study by introducing a novel concept: Reverse Sequence Collision Flotation (RSCF). This method alters the sequence of interactions between the gas, liquid, and solid phases, effectively overcoming many limitations of traditional flotation processes.
The key innovation of this model is that mineral particles collide with bubbles without being hindered by the hydration film. The reverse sequence collision process follows these stages: “(internal) approach → collision → adhesion → hydration film rupture → (nontarget mineral) detachment.” This sequence contrasts with the conventional approach: “(external) approach → collision → hydration film rupture → target mineral) adhesion”. In the RSCF model, target mineral particles interact directly with the gas–liquid interface inside the bubble, forming mineralized bubbles without the need for hydration film rupture.
In the proposed collision model, gas encapsulates solid particles as they enter the liquid phase. A hydration film initially forms at the gas–liquid interface, with solid particles subsequently migrating from the bubble’s interior to its periphery. At the bubble edge, the hydration film captures solid particles. During three-phase contact, mineral particles become progressively wetted, facilitating their interaction with the liquid phase. Depending on the mineral’s hydrophobicity or hydrophilicity, particles either remain adhered to the bubble edge or detach into the liquid phase.
3. Theoretical Analysis of RSCF Process
3.1. Existence and Effects of Hydration Film
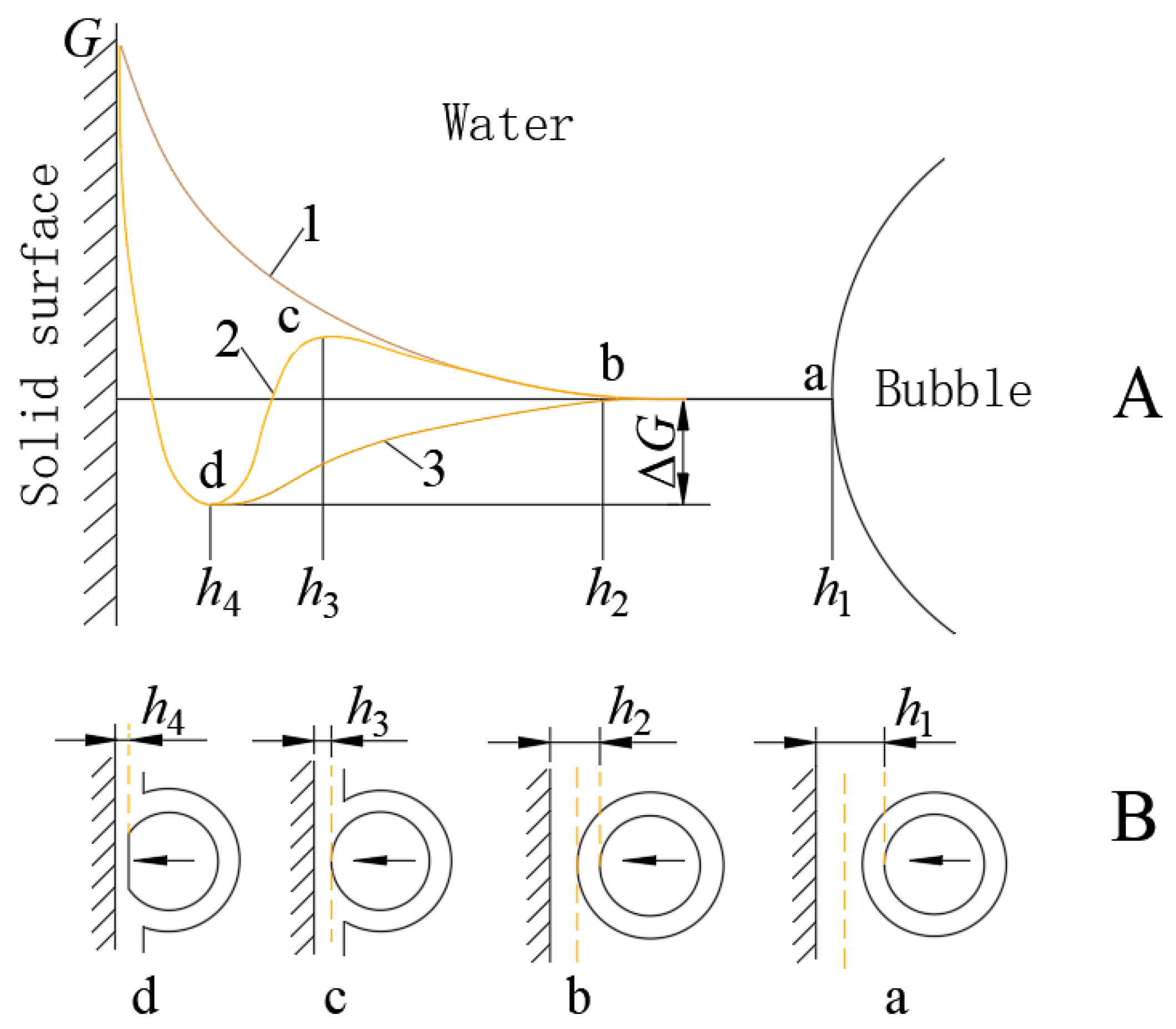
Hydration causes a unique hydration film to form on mineral surfaces, and a similar thin hydration film exists on the surface of bubbles. When a mineral particle collides with a bubble, the hydration layer between them must thin and break (partially or entirely), displacing the water to allow the mineral particle to adhere to the bubble and establish solid–gas contact. The hydration film, influenced by the surface bond energy of the mineral, is more viscous than regular water and exhibits elasticity similar to that of a solid. Although the hydration film appears to be liquid, its properties closely resemble those of a solid. Thinning and rupturing this stable hydration layer requires energy. The interaction between the particles and bubbles and the change in free energy within the hydration layer are illustrated in
Figure 2 [
20]. This finding demonstrates that the hydration film impedes contact between bubbles and particles, necessitating increased energy or prolonged contact. However, in the reverse sequence collision process, this limitation imposed by the hydration film is absent.
3.2. Work of Adhesion and Attachment of Bubbles to Mineral Particles
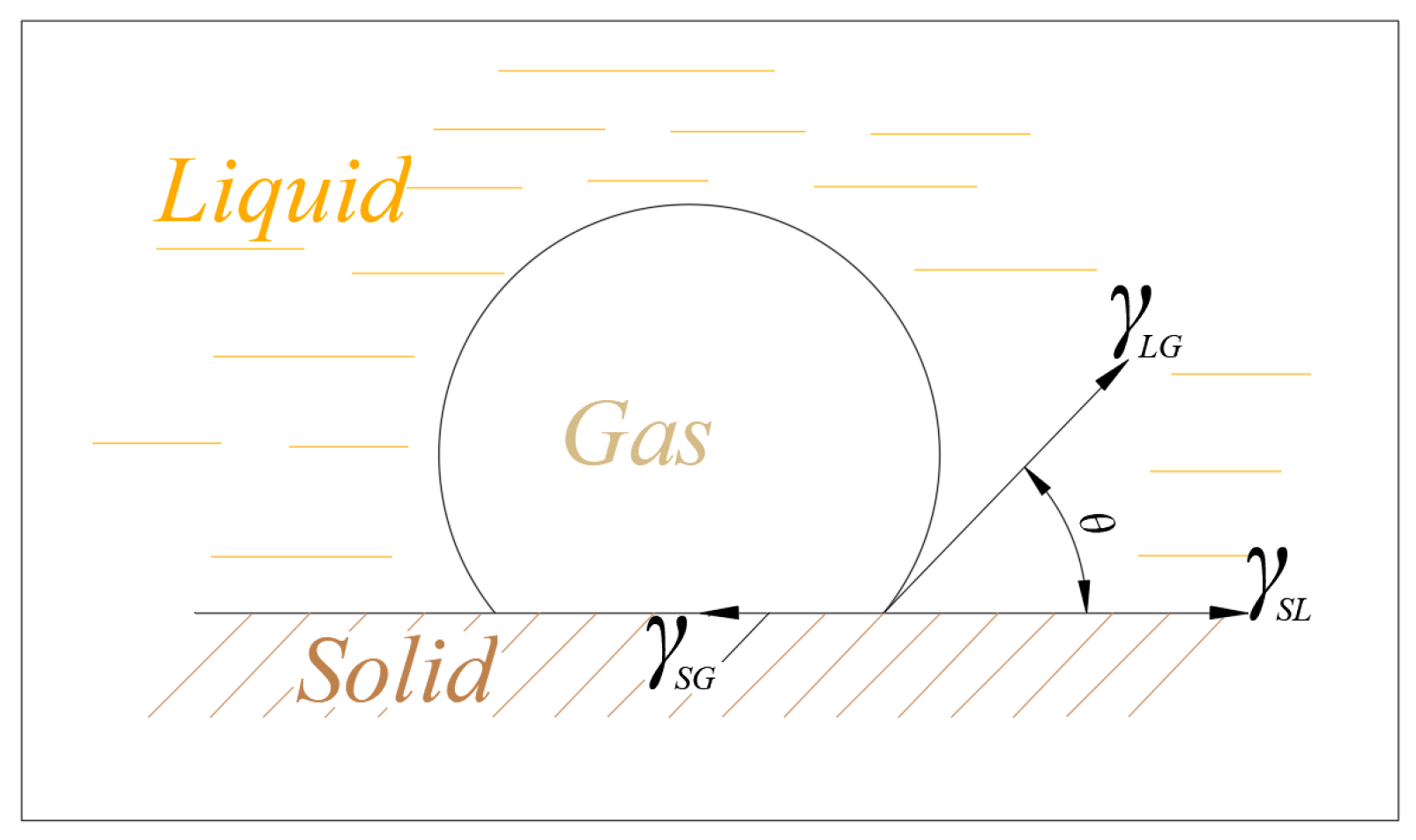
The adhesion between bubbles and particles is a thermodynamic process.
Figure 3 shows that at equilibrium, the interfacial tensions of the gas–liquid–solid three-phase system are described by Young’s equation, as follows [
21,
22]:
where
,
, and
represent the interfacial tensions (or surface free energies) of the solid–gas, solid–liquid, and liquid–gas interfaces, respectively. Equation (1) forms the basis for understanding wetting. Wetting work (
) refers to the maximum amount of work the system can perform during the adhesion of water to a solid surface, also known as the work of adhesion. The reduction in free energy (
) during the transition from liquid–gas and solid–gas interfaces to a solid–liquid interface is [
23]:
By substituting Equation (1) into Equation (2), the following expression for the work of wetting can be obtained:
During flotation, when mineral particles come into contact with bubbles, the work of adhesion (
) is used to measure the strength of this adhesion. This can also be quantified by the reduction in the system’s free energy [
24].
For the unit areas of the solid–gas, liquid–gas, and solid–liquid interfaces, the interfacial tensions are
,
and
, respectively:
Substituting Equation (1) into Equation (4) yields the following:
In conventional flotation, the adhesion between bubbles and mineral particles in water is driven by changes in the interfacial energy, which can be described by the work of adhesion. In reverse sequence flotation, however, this adhesion process is governed primarily by wetting and should therefore be evaluated by wetting. This distinction highlights the difference between reverse sequence flotation and conventional flotation processes.
According to thermodynamic principles (Equations (2) and (4), when > 0 the system’s free energy before adhesion exceeds the free energy after adhesion, indicating a spontaneous reduction in free energy. The second law of thermodynamics thus suggests a spontaneous tendency for mineral particles to adhere to bubbles. Moreover, as a mineral’s hydrophobicity increases (i.e., as the contact angle θ increases), the value of also increases. Therefore, the mineral adheres more readily to the bubble surface, with a stronger spontaneous tendency and firmer adhesion.
In conventional flotation, the work of adhesion is given by
, whereas in reverse sequence flotation, the work of adhesion (equivalent to the work of wetting) is
. For conventional flotation minerals (as shown in
Table 1), the contact angle is generally less than 90°. Thus,
> 0, and:
The work of adhesion in reverse sequence flotation is greater than in conventional flotation, indicating a stronger spontaneous tendency for adhesion and a more stable bond in the reverse sequence process.
3.3. Surface Free Energy Variation Analysis
The free energy variation (Δ
G) is determined by quantifying interfacial energy differences between initial and terminal configurations. For conventional flotation processes, this thermodynamic parameter characterizes the phase displacement mechanism where gaseous phases substitute liquid–solid interfaces. Starting with liquid–solid adhesion and terminating at gas–solid bonding states, the conventional free energy change Δ
Gcon can be mathematically formulated as follows:
In the Reverse Sequence Collision Flotation (RSCF) process, the free energy variation originates from interfacial restructuring where liquid phases replace gas–mineral bonding states. The system evolves from initial gas–solid adhesion to terminal liquid–solid stabilization states. The corresponding free energy change Δ
GRSCF is formulated as follows:
Through thermodynamic modeling, the substitution of Young’s equation (Equation (1)) into the derived expressions (Equations (7) and (8)) enables the calculation of free energy variations (Δ
G) during the gas–liquid–solid triple-phase boundary evolution:
The sign of the free energy change determines whether a process is spontaneous (Δ
G < 0 indicates spontaneity). Based on the data in
Table 1, conventional minerals exhibit contact angles
θ < 90°, from which it can be inferred that
, this indicates that in the conventional collision process, the displacement of liquid by gas to achieve solid contact does not occur spontaneously; and , whereas in the Reverse Sequence Collision Flotation (RSCF) process, the displacement of gas by liquid to achieve solid contact occurs spontaneously.
3.4. Effect of Contact Angle on Adhesion
The contact angle is influenced by the direction of movement of the gas–liquid–solid interface. Although contact angles are traditionally measured under static conditions, the interactions among the gas, liquid, and solid phases are dynamic in practice. This dynamic behavior often results in discrepancies between the measured contact angle and the actual wetting properties [
26].
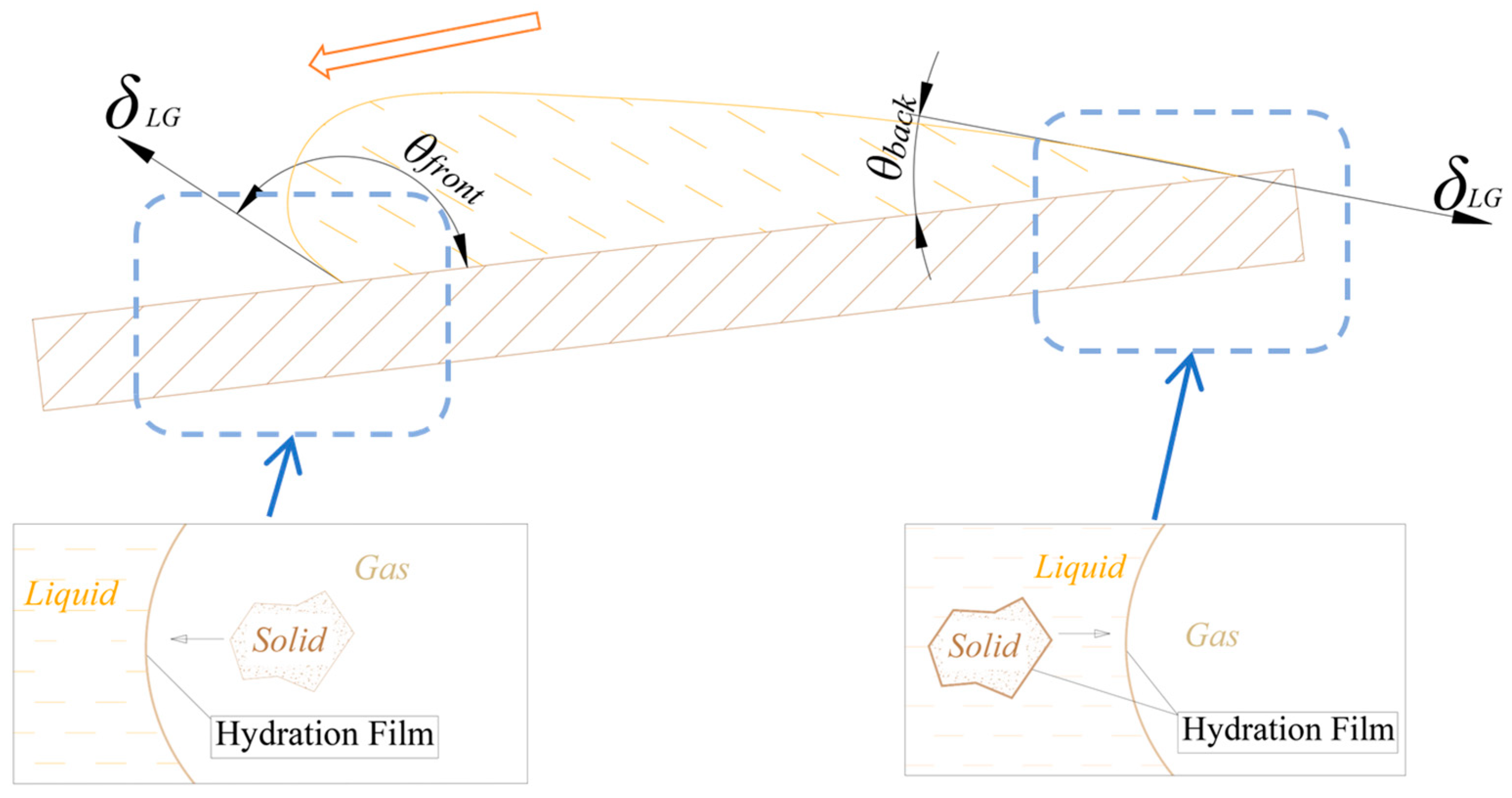
For a droplet on an inclined surface, as the droplet begins to move downhill (
Figure 4), and the solid surface in front of the droplet becomes dry, creating a “water replaces air” scenario. In contrast, at the rear of the droplet, the gas phase moves toward an already wetted surface, leading to a “gas replaces water” scenario. This situation results in a difference between the advancing contact angle (
) and the receding contact angle (
), with the advancing contact angle typically being larger:
This variation in the contact angle, caused by the relative motion of the liquid, is referred to as “dynamic wetting hysteresis.” In the RSCF model, the process mirrors the “water replaces air” mechanism, whereas conventional flotation follows the “gas replaces water” mechanism. As a result, reverse sequence flotation typically achieves a larger contact angle, enhancing the floatability of mineral particles.
4. Materials and Methods
4.1. Materials for Single Bubble Static Testing
The static reverse sequence collision process with a single bubble primarily involves qualitative analysis, where the reverse sequence collision process and the adhesion of particles to the bubble are visually assessed. In the testing process of this study, colored (blue and red) chalk powder was selected, which is mainly composed of calcium carbonate (limestone) and calcium sulfate (gypsum), as shown in
Figure 5. The choice of chalk powder, rather than real minerals, as the experimental subject was based on several of its characteristics which facilitate research, such as its low specific gravity, large volume, minimal impact on bubbles during the collision process, ease of observation, strong hydrophilic nature, and the ease with which the detachment process can be achieved and observed.
4.2. Materials for Continuous Dynamic Testing of Multiple Bubbles
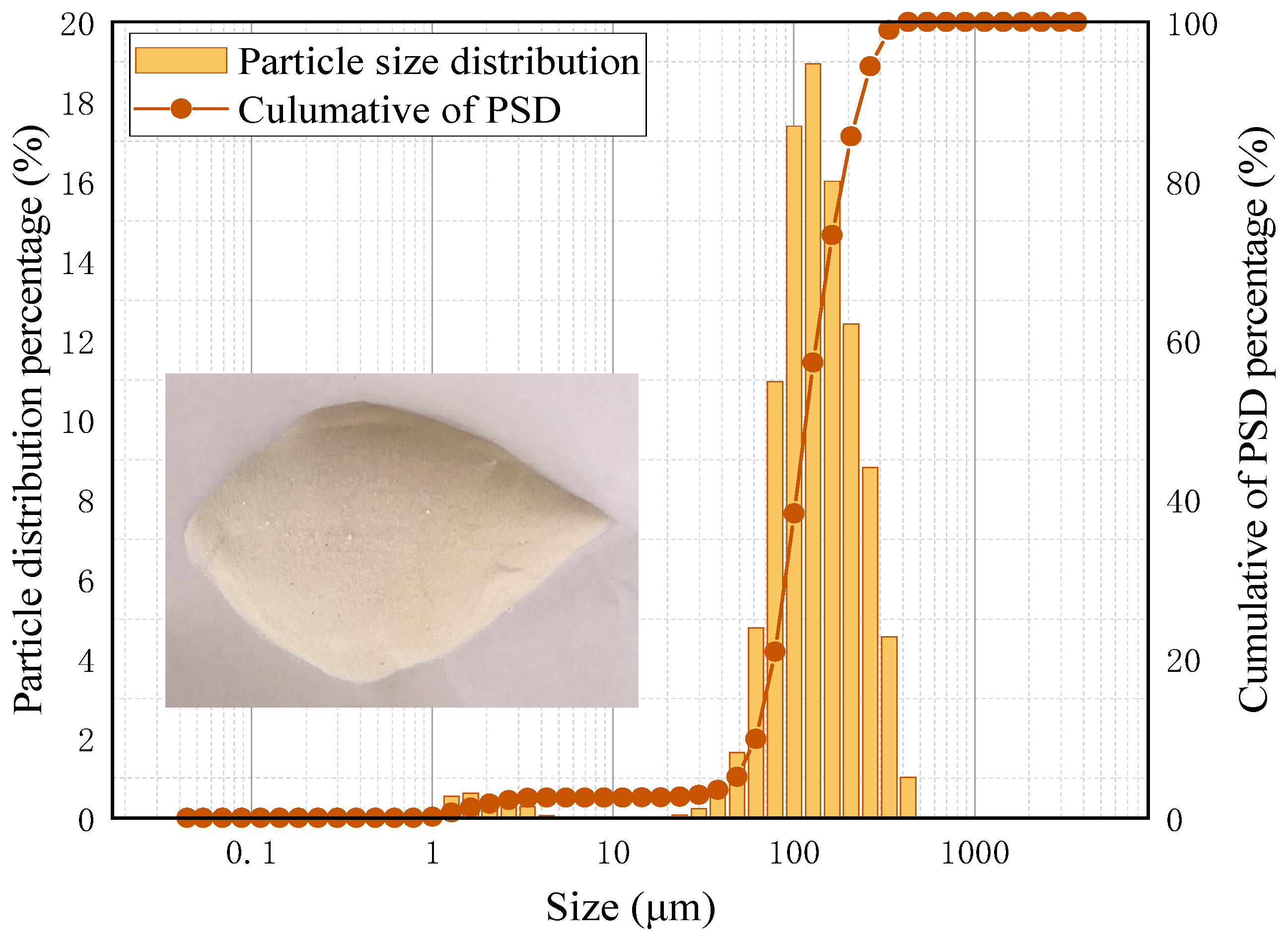
To investigate the effects of reverse sequence collision mineralization, continuous quantitative tests were conducted using pure quartz particles as the research material (purity: 98%, with a particle size distribution of −45 μm fraction: 22.24%, −75 μm fraction: 60.59%), as shown in
Figure 6. The yield and recovery of the separation process can be easily calculated by weighing the samples.
4.3. Single-Bubble Static RSCF Test System
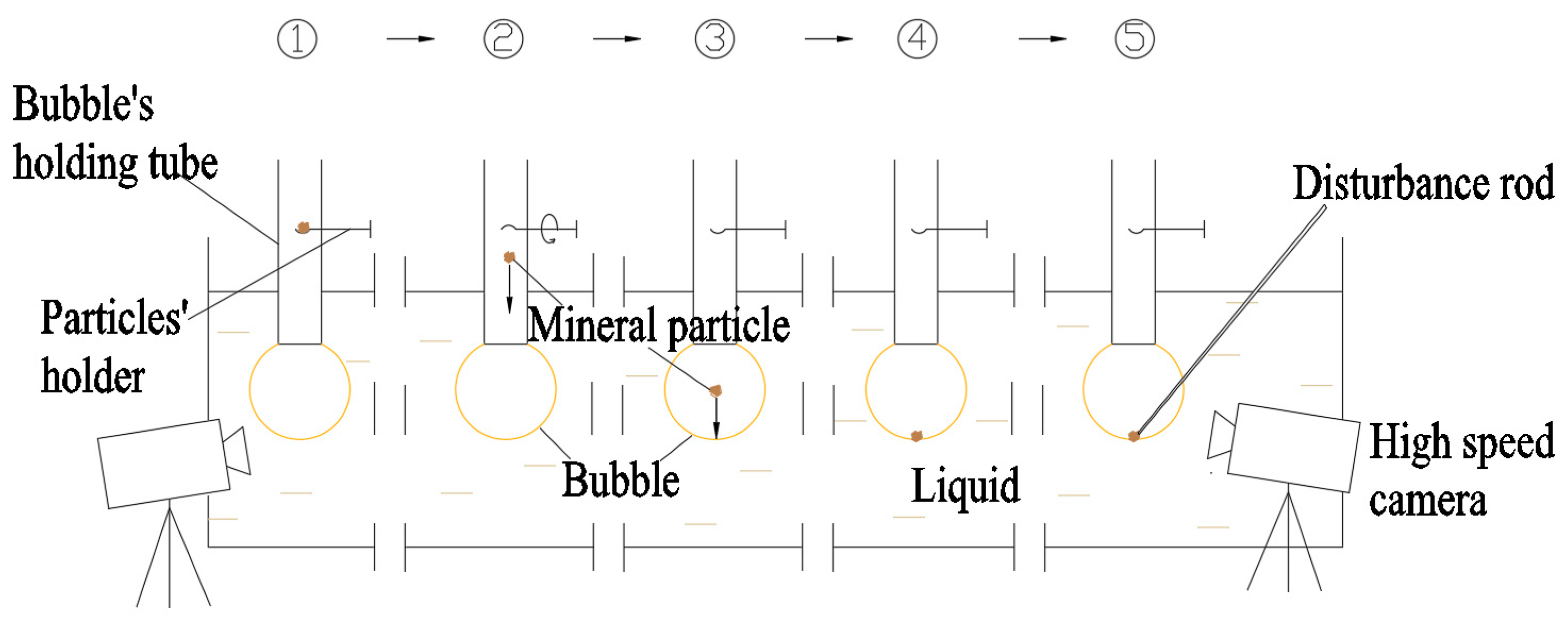
The reverse sequence collision process between mineral particles and bubbles can be examined by examining the direct entry of particles into the bubble interior. A qualitative experimental setup was designed for this purpose, and a single batch of mineral particles was introduced into a stationary bubble (
Figure 7).
A bubble-holding tube was submerged in water, with pressure applied to its opposite end to generate a stable bubble. A mineral particle holder was positioned mid-tube to preload the particles. When the bubble stabilized, the rotation of the holder released the particles, causing them to drop directly into the bubble, initiating reverse sequence collisions.
4.4. Multi-Bubble Dynamic RSCF Test System
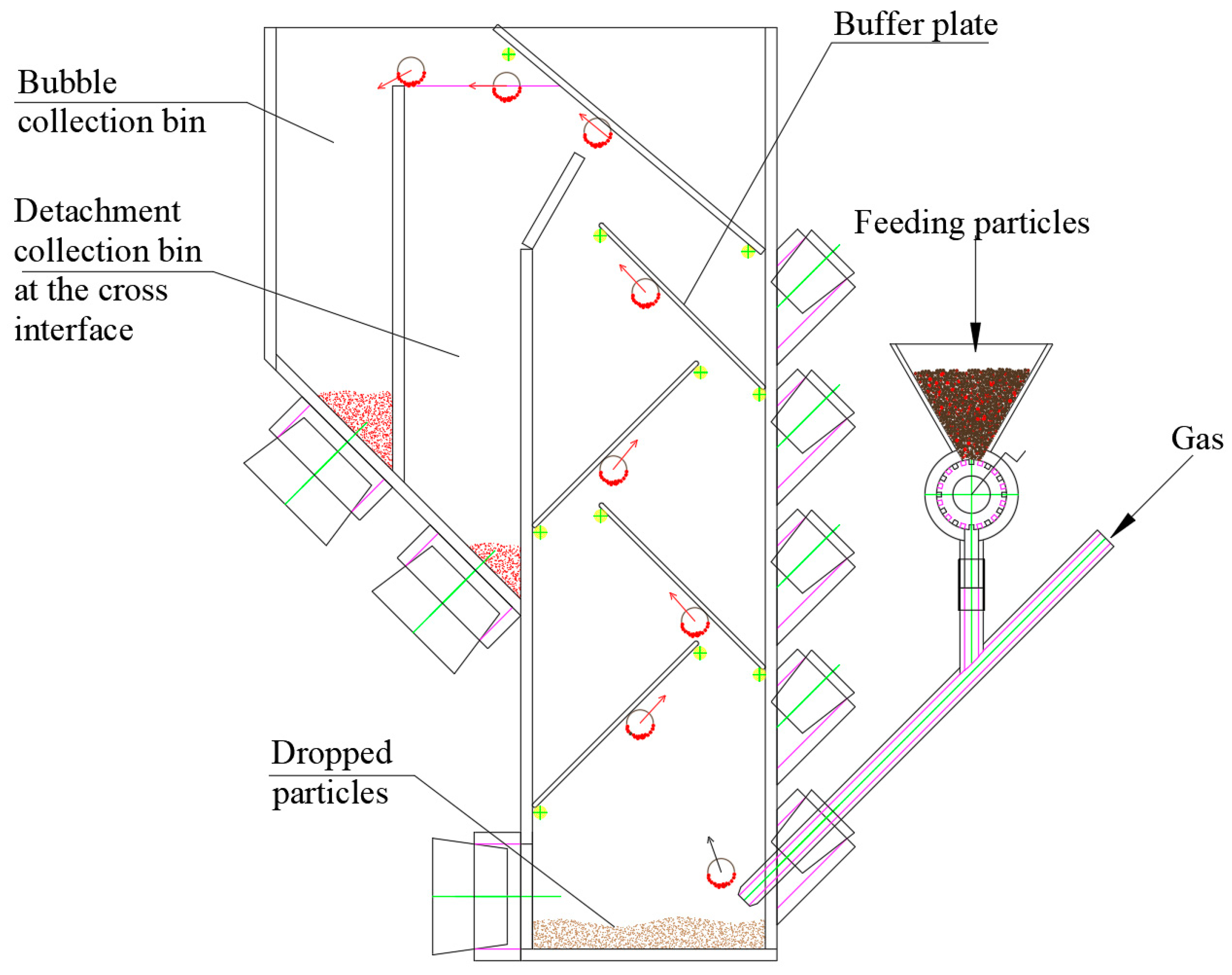
Although single-bubble static tests provide insights into the reverse sequence collision process, the limited amount of material and lack of collection mechanisms hinder the evaluation of separation efficiency. These issues are addressed by exploring the feasibility of continuous bubble generation and material feeding. A Y-shaped tube was designed, with one branch connected to the gas source and the other to the material storage tank. The gas and particles combine and enter the water, generating mineralized bubbles via reverse sequence collisions. Continuous gas flow and feeding allowed for repeated particle–bubble collisions, producing a substantial amount of mineralized bubbles. A collection device for mineralized bubbles was subsequently developed, enabling the analysis of the bubbles and their associated minerals to evaluate flotation performance (
Figure 8).
5. Results and Discussion
5.1. Qualitative Analysis of Single-Bubble Static Reverse Sequence Collision Process
Observing the collision process between a single bubble and mineral particles provides direct evidence of the effects of collision and adhesion. For both conventional collisions and reverse sequence collisions, this study conducted qualitative analysis on single bubbles separately.
5.1.1. Conventional Collision Test Between Bubbles and Particles
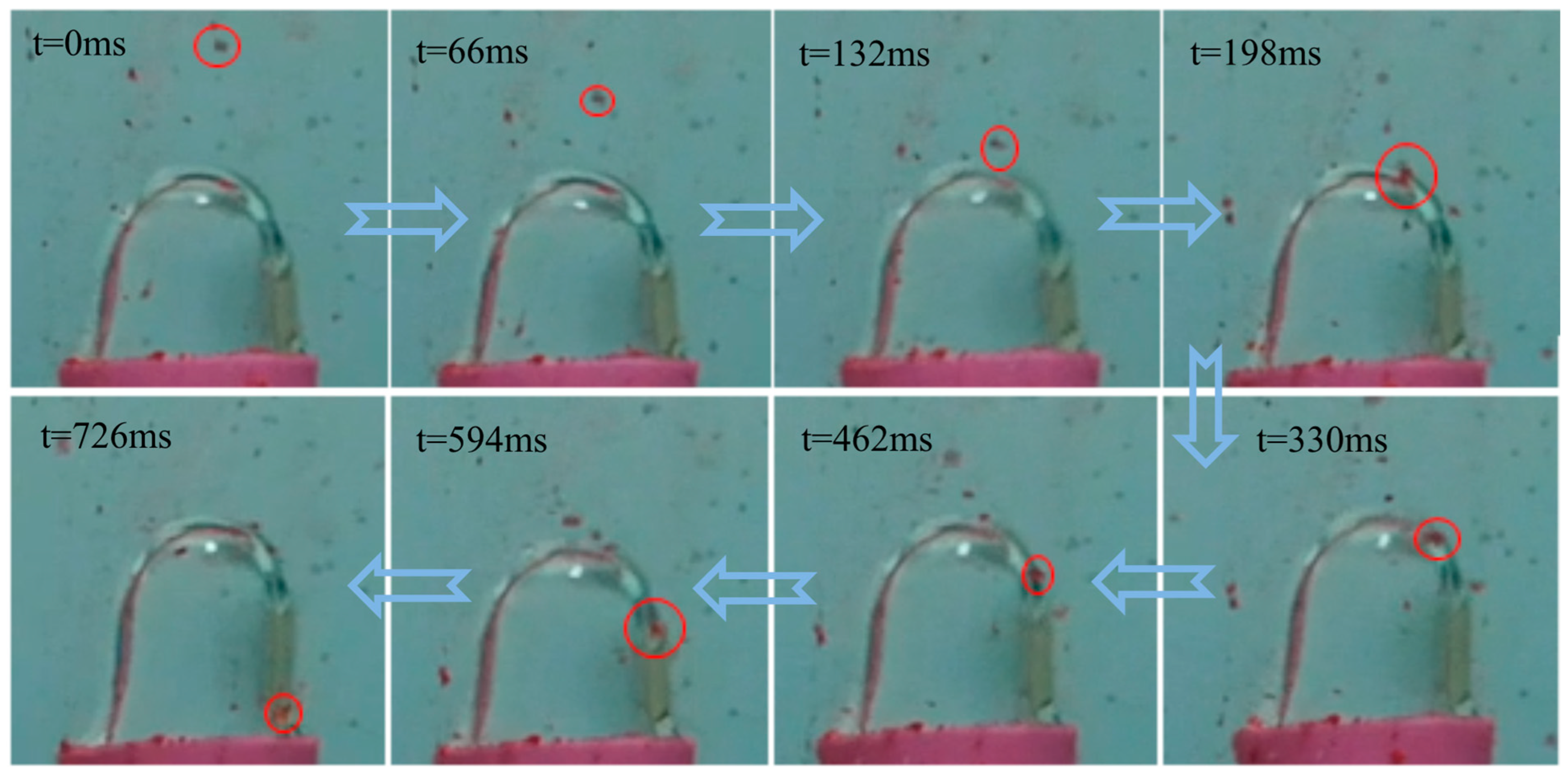
For these experiments, colored solid particles composed of calcium carbonate (limestone) and calcium sulfate (gypsum) were selected because of their strong hydrophilic properties. The particles were allowed to freely fall through the water and collide with the bubble surface.
Figure 9 shows the video observations. As the particles approach and collide with the bubble from the outside, most cannot penetrate the bubble wall or adhere to its surface. In some cases, the particles were directly repelled. The hydration film on both the bubble and particle surfaces significantly impedes collision and adhesion.
5.1.2. Reverse Sequence Collision Test Between Solid Particles and Bubbles
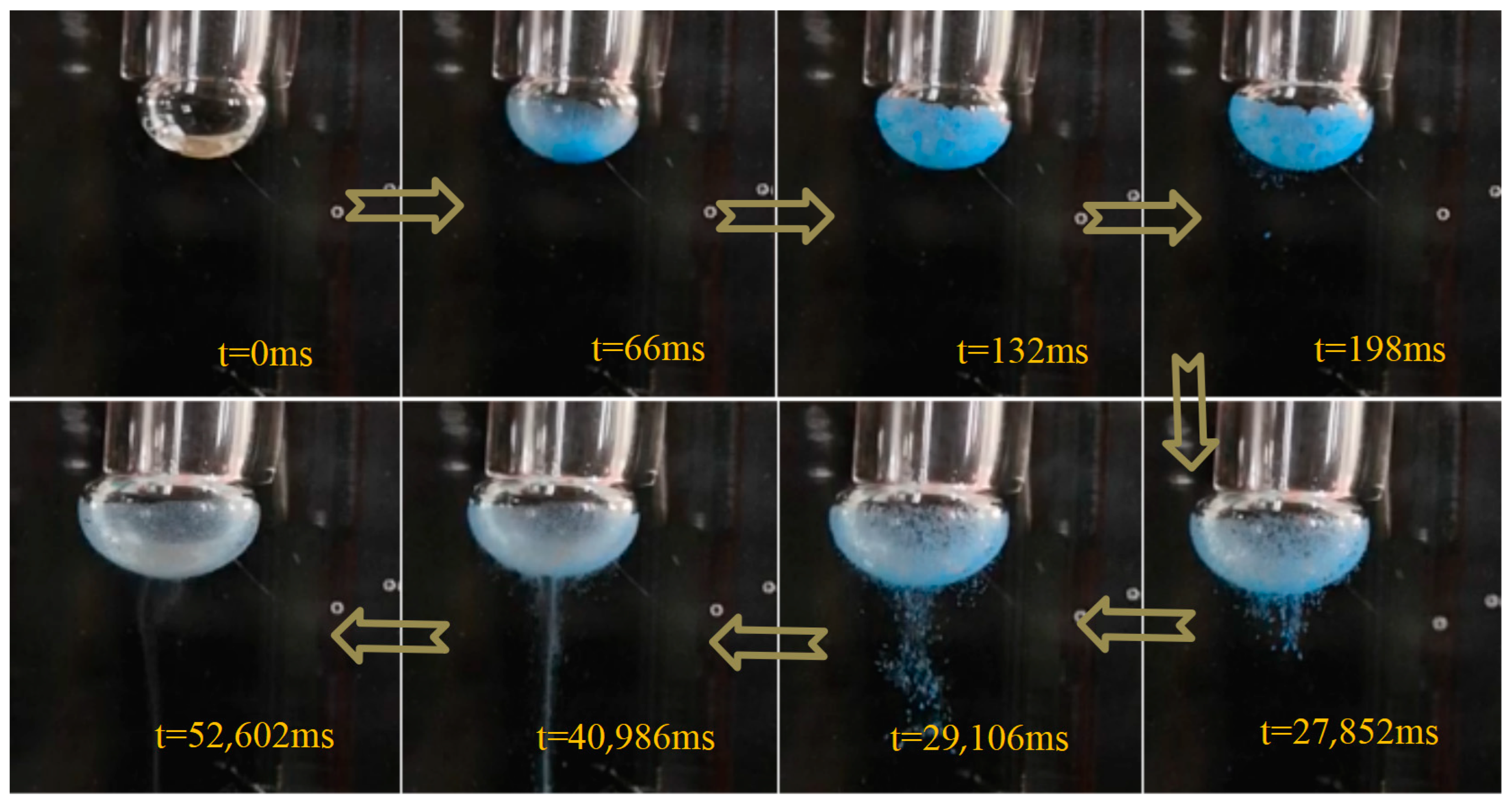
Using a single-bubble static reverse sequence collision apparatus, the interaction between solid particles and bubbles was tested (
Figure 10). The particles fell directly inside the bubble, and the hydration film on the outer bubble surface fully supported the particles, causing them to quickly spread across the bubble’s inner surface. Given that these materials are composed of calcium carbonate and calcium sulfate, which are highly hydrophilic, the dry particles gradually become wet after contact with the bubble’s liquid film, causing some particles to detach from the bubble surface and enter the water.
When mineral particles collide with the bubble’s edge from the inside, they disperse across the bubble surface rather than accumulate in one spot. This phenomenon promotes the selective adhesion of mineral particles to the bubble surface. The hydrophilic particles gradually detached during the wetting process, and more hydrophobic particles remained attached to the bubble. Mechanical agitation accelerated the detachment of weakly adhered particles. Thus, both the time spent in the water and the mechanical disturbances affected the detachment of the adhered particles from the bubble.
The reverse sequence collision process contrasts sharply with conventional collisions. Under conventional conditions, solid particles struggle to contact and adhere to the bubble surface. However, in reverse sequence collisions, the bubble surface captures all the particles, and as the wetting process or disturbances progress, the hydrophobic particles gradually detach.
5.2. Quantitative Analysis of Multi-Bubble Dynamic Reverse Sequence Collision Process
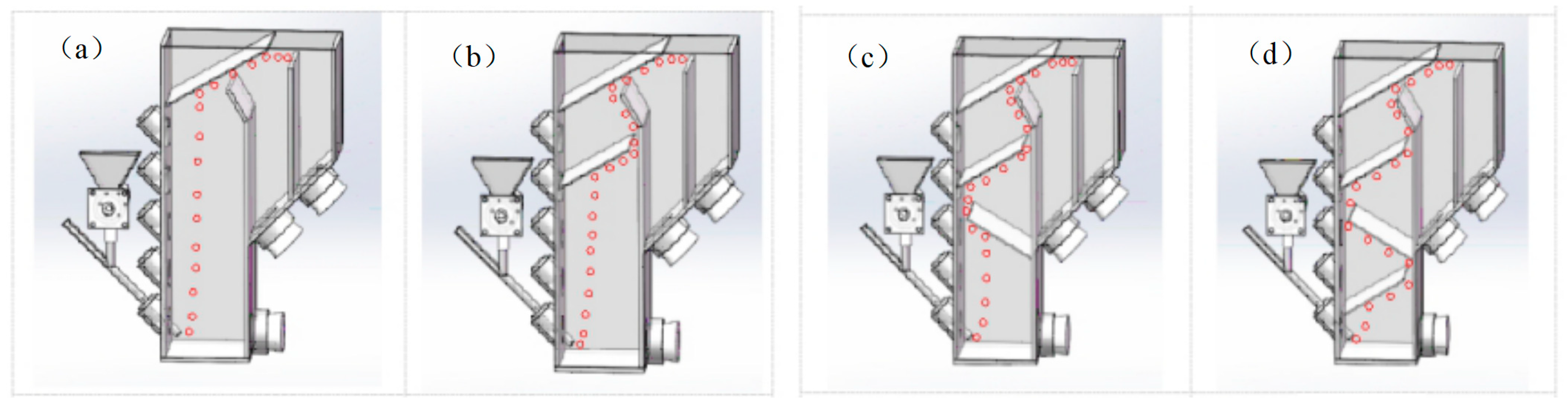
The RSCF system utilizes an outward-moving phase interface to ensure the full recovery of particles floating to the surface, thereby preventing detachment at the interface, which could compromise flotation process evaluation. How changes in the bubble path and its extension impact the regulation of the mineralized bubble load are investigated in this study (
Figure 11).
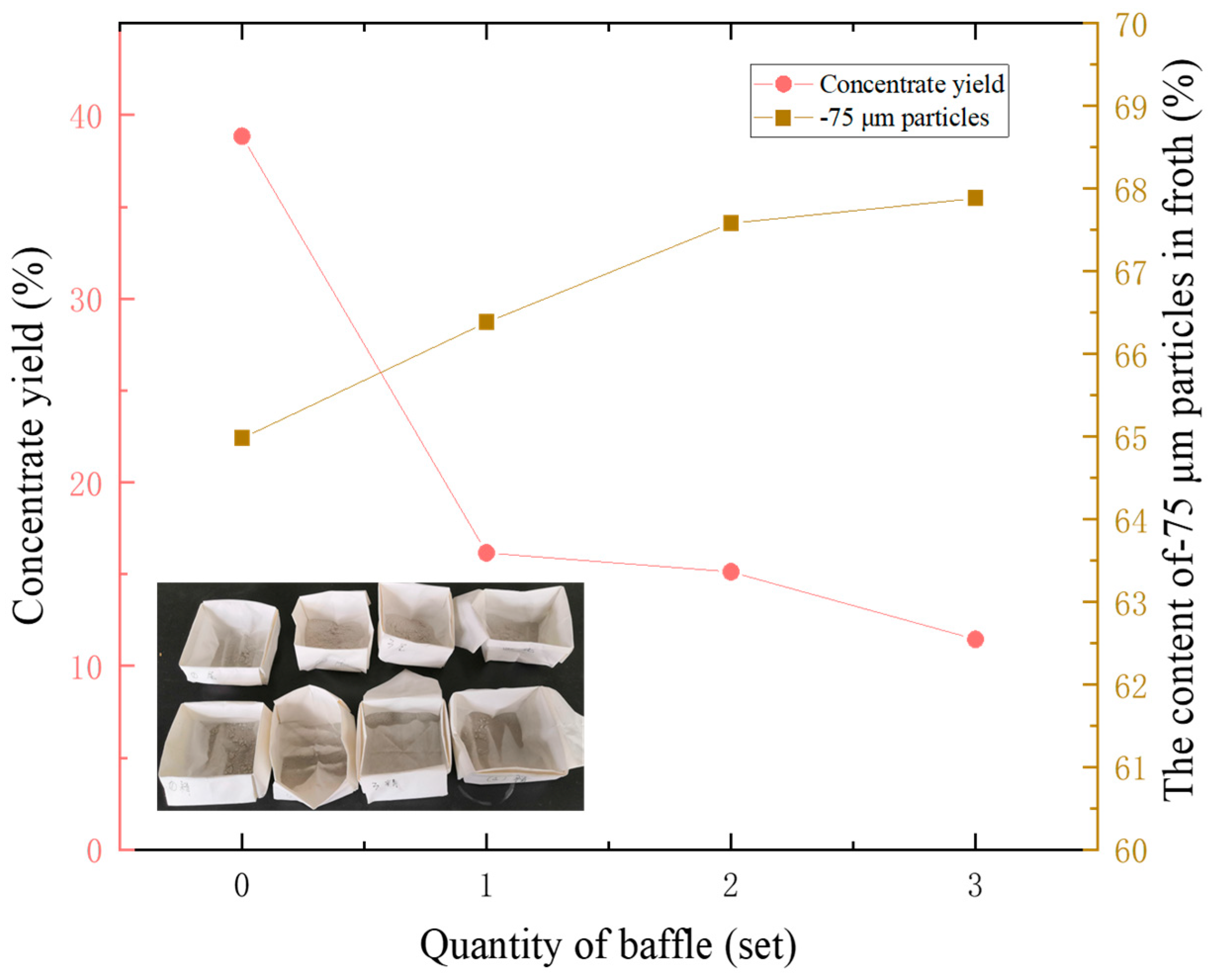
The experimental data indicate that, even without the addition of chemical reagents, the hydration film on the bubble surface alone can achieve a foam product yield exceeding 10%. As shown in
Table 2 and
Figure 12, increasing the number of baffles reduces the froth yield while increasing the proportion of particles smaller than 75 μm. The presence of baffles accelerates the detachment of particles from the bubble surface, particularly coarse-grained and hydrophilic minerals, which are more likely to fall off. The experiment confirms that the mechanical disturbance caused by the baffles affects the ascent of mineralized bubbles, allowing for the regulation of their load.
The primary objective of this study is to validate the feasibility of Reverse Sequence Collision Flotation (RSCF), through both single-bubble static tests and multi-bubble dynamic tests. For future industrial applications of RSCF technology, a key question arises: how to modify the hydrophobicity and hydrophilicity of mineral surfaces through reagent addition?
The authors propose that RSCF will fundamentally alter reagent dosing methods. Unlike conventional approaches where reagents bind to mineral surfaces via aqueous dissolution, RSCF may employ two novel strategies:
Gas-phase attachment: reagents are aerosolized or vaporized to directly adhere to dry mineral surfaces.
Pre-optimized slurry conditioning: tailoring the flotation solution environment for enhanced mineral separation efficiency.
6. Conclusions and Outlook
The innovative approach of the RSCF model was investigated in this study. Both a static single-bubble RSCF test system and a continuous dynamic multi-bubble RSCF test system were established. Different materials were used to validate this new approach, and both qualitative and quantitative assessments were conducted, leading to the following conclusions:
The RSCF process, in which mineral particles first interact with the bubble’s internal interface, was successfully demonstrated in both static and dynamic multi-bubble tests, confirming the technical feasibility of this method.
RSCF enhances the efficiency of the contact and adhesion between mineral particles and bubbles, with collision probabilities approaching 100%. The amount of particle adhesion is also significantly greater than that of conventional flotation processes.
In the RSCF process, mineral particles do not accumulate locally on the bubble surface but are evenly dispersed, facilitating the detachment of hydrophilic minerals and improving the overall mineral content of the bubbles.
The prolonged retention of mineralized bubbles in water promotes the detachment of hydrophilic minerals from the bubble surface and their re-entry into the water phase.
Mechanical disturbance accelerates the detachment of hydrophilic particles from mineralized bubbles, suggesting that the loading performance of bubbles post-RSCF can be effectively controlled.
The RSCF method alters the sequence of particle–bubble collisions, a fundamental process in flotation. A novel mineralization mechanism was proposed and preliminarily validated, allowing the enrichment process to be shifted from one dominated by the adhesion of target minerals to one centered on the detachment of nontarget minerals. By significantly reducing the energy required for particle–bubble collisions, this technique could expand the size range of minerals that can be processed via flotation, addressing the challenges of simultaneously floating both coarse and fine particles. As a new mineralization technique, RSCF has the potential to drive advancements in flotation processes, equipment, and reagent systems.
Author Contributions
Conceptualization, D.H. and S.S.; methodology, W.H.; validation, D.H. and S.C.; formal analysis, W.H.; investigation, C.S.; data curation, D.H.; writing—original draft preparation, D.H.; writing—review and editing, S.S.; supervision, W.H.; project administration, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the BGRIMM Technology Group (02-1923) and Key Fund of BGRIMM Technology Group (Y-JDFX-02-2022).
Data Availability Statement
Data are contained within the article.
Acknowledgments
This research is exploratory in nature and represents a bold attempt to develop a novel collision mineralization model. We are grateful to all the institutions that supported and encouraged this study.
Conflicts of Interest
Authors Dengfeng Han, Shuaixing Shi and Chuanyao Sun are affiliated with the BGRIMM company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Hu, X.G.; Huang, H.W.; Mao, J.F. Theory and Technology of Flotation; Central South University Press: Changsha, China, 1991. [Google Scholar]
- Wills, B.A.; Finch, J.A. Wills’ Mineral Processing Technology, 7th ed.; Butterworth-Heinemann: Oxford, UK, 2016. [Google Scholar]
- Basarova, P.; Zawala, J.; Zedníková, M. Interactions between a Small Bubble and a Greater Solid Particle during the Flotation Process. Miner. Process. Extract. Metall. Rev. 2019, 40, 410–426. [Google Scholar] [CrossRef]
- Xing, Y.; Gui, X.; Cao, Y. Hydration film measurement on mica and coal surfaces using atomic force microscopy and interfacial interactions. J. Cent. South Univ. 2018, 25, 1295–1305. [Google Scholar] [CrossRef]
- Lei, P.; Yoon, R. Measurement of hydrophobic forces in thin liquid films of water between bubbles and xanthate-treated gold surfaces. Miner. Eng. 2016, 98, 240–250. [Google Scholar]
- Basařová, P.; Váchová, T.; Moore, G.; Nannetti, G.; Pišlová, J. Bubble adhesion onto the hydrophobic surface in solutions of non-ionic surface-active agents. Colloids Surf. A Physicochem. Eng. Asp. 2016, 505, 64–71. [Google Scholar] [CrossRef]
- Li, S.; Jue, K.; Sun, C. Effect of Bubble Surface Properties on Bubble–Particle Collision Efficiency in Froth Flotation. Minerals 2020, 10, 367. [Google Scholar] [CrossRef]
- Møller, A.; Beheshti, A.; Skinner, W.; Franks, G.; Beattie, D.; Krasowska, M. Hydrophobic surfaces in mineral flotation: The effect of (physical and chemical) heterogeneity on wettability and wetting film stability. In Encyclopedia of Solid-Liquid Interfaces, 1st ed.; Wandelt, K., Bussetti, G., Eds.; Elsevier: Oxford, UK, 2024; pp. 494–504. [Google Scholar]
- Ralston, J.; Dukhin, S.; Mishchuk, N. Wetting film stability and flotation kinetics. Adv. Colloid Interface Sci. 2002, 95, 145–236. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Yin, Q.; He, Q.; Feng, X.; Yang, C.; Gui, X.; Xing, Y. Role of hydrophobic fine particles in coarse particle flotation: An analysis of bubble-particle attachment and detachment. Colloids Surf. A Physicochem. Eng. Asp. 2023, 662, 130980. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Li, D.; Chen, R.; Yan, X.; Zhang, H. Collisional interaction process between a bubble and particles with different hydrophobicity. Sep. Purif. Technol. 2022, 301, 121940. [Google Scholar] [CrossRef]
- October, L.; Manono, M.S.; Corin, K.C.; Schreithofer, N.; Wiese, J.G. The Influence of Specific Ions and Oxyhydroxo Species in Plant Water on the Bubble–Particle Attachment of Pyrrhotite. ACS Omega 2021, 6, 28496–28506. [Google Scholar] [CrossRef]
- Yin, W.; Tian, D.; Xie, Y.; Xue, M.; Yao, J. Research Progress of Induction Time in Flotation Process. Conserv. Util. Miner. Resour. 2023, 43, 1–9. [Google Scholar]
- Ajaev, V. Instability and rupture of thin liquid films on solid substrates. J. Colloid Interface Sci. 2013, 1, 81–92. [Google Scholar] [CrossRef]
- Manica, R.; Liu, B.; Li, M.; Chen, Z.; Liu, Q. Hydrodynamic collisions involving bubbles and mineral particles. Can. J. Chem. Eng. 2022, 100, 3270–3287. [Google Scholar] [CrossRef]
- Cui, W.; Chen, J. Insight into mineral flotation fundamentals through the DFT method. Int. J. Min. Sci. Technol. 2021, 31, 983–994. [Google Scholar] [CrossRef]
- Xie, L.; Wang, J.; Lu, Q.; Hu, W.; Yang, D.; Qiao, C.; Zeng, H. Surface interaction mechanisms in mineral flotation: Fundamentals, measurements, and perspectives. Adv. Colloid Interface Sci. 2021, 295, 102491. [Google Scholar] [CrossRef]
- Xing, Y.; Gui, X.; Pan, L.; Pinchasik, B.E.; Cao, Y.; Liu, J.; Butt, H.J. Recent experimental advances for understanding bubble-particle attachment in flotation. Adv. Colloid Interface Sci. 2017, 246, 105–132. [Google Scholar] [CrossRef]
- Yoon, R.H. The role of hydrodynamic and surface forces in bubble–particle interaction. Int. J. Miner. Process. 2000, 58, 129–143. [Google Scholar] [CrossRef]
- Sun, C. Mineral Processing Engineer’s Manual—Volume 1, Upper Volume—General Mineral Processing, 2nd ed.; Metallurgical Industry Press: Beijing, China, 2005. [Google Scholar]
- Young, M.L. Young’s equation revisited. J. Phys. Condens. Matter 2016, 28, 135001. [Google Scholar]
- Chau, T.T. A review of techniques for measurement of contact angles and their applicability on mineral surfaces. Miner. Eng. 2009, 22, 213–219. [Google Scholar] [CrossRef]
- Montero de Hijes, P.; Shi, K.; Noya, E.; Santiso, E.E.; Gubbins, K.E.; Sanz, E.; Vega, C. The Young-Laplace equation for a solid-liquid interface. J. Chem. Phys. 2020, 153, 191102. [Google Scholar] [CrossRef]
- Gomez-Flores, A.; Bradford, S.; Hwang, G.; Heyes, G.W.; Kim, H. Particle–bubble interaction energies for particles with physical and chemical heterogeneities. Miner. Eng. 2020, 155, 106472. [Google Scholar] [CrossRef]
- Hu, W. Flotation, 2nd ed.; Metallurgical Industry Press: Beijing, China, 1989. [Google Scholar]
- Drelich, J. Guidelines to measurements of reproducible contact angles using a sessile-drop technique. Surf. Innov. 2013, 1, 248–254. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).