Optimizing the Dealkalization Process of Red Mud: Controlling Calcium Compounds to Improve Solid–Liquid Separation Performance
Abstract
1. Introduction
2. Material and Methods
2.1. Materials and Reagents
2.2. Experimental Procedure
2.3. Analytical Methods and Characterization of Samples
3. Results and Discussion
3.1. Acid Leaching
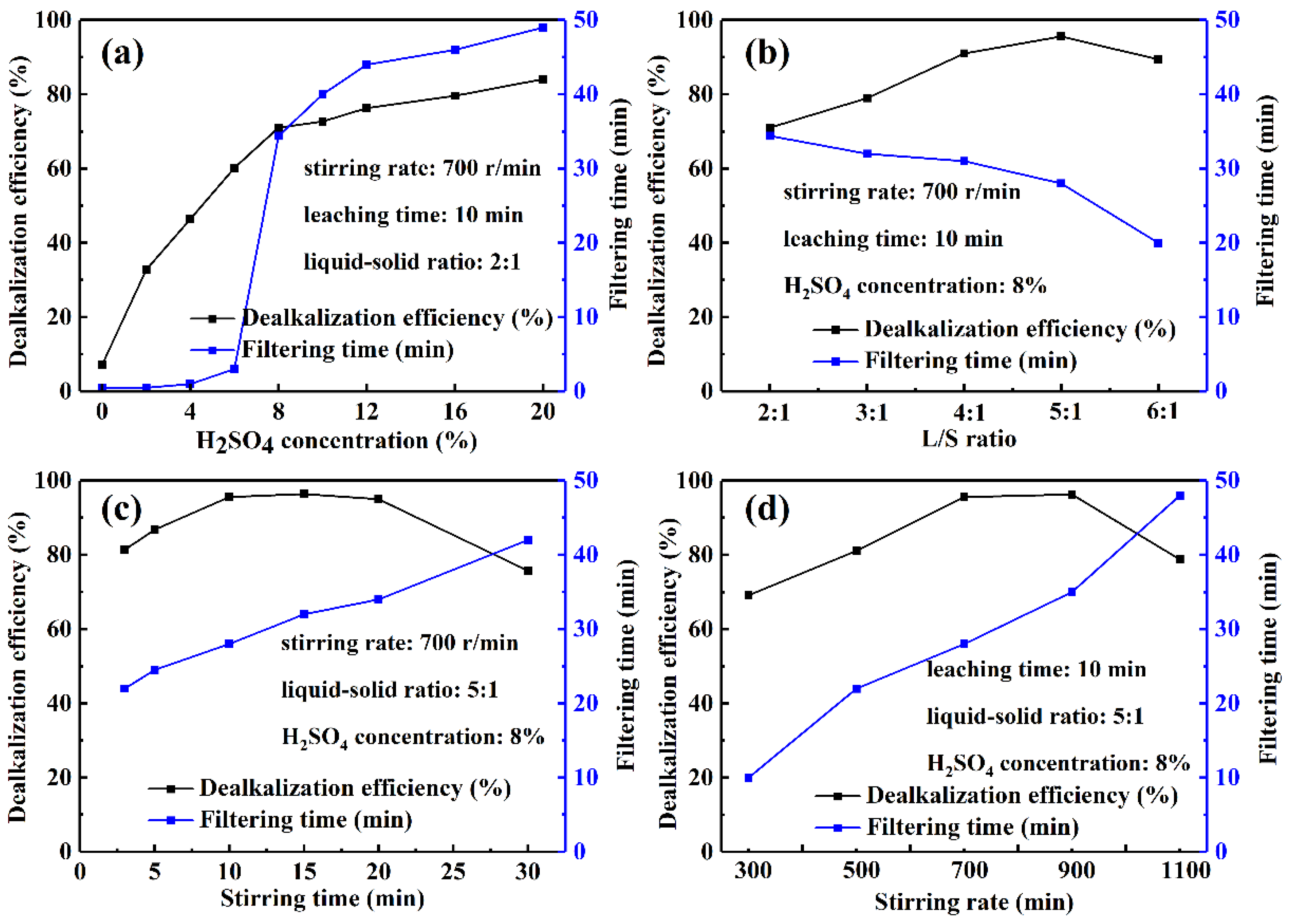
3.1.1. Factors Affecting the Leaching Using H2SO4
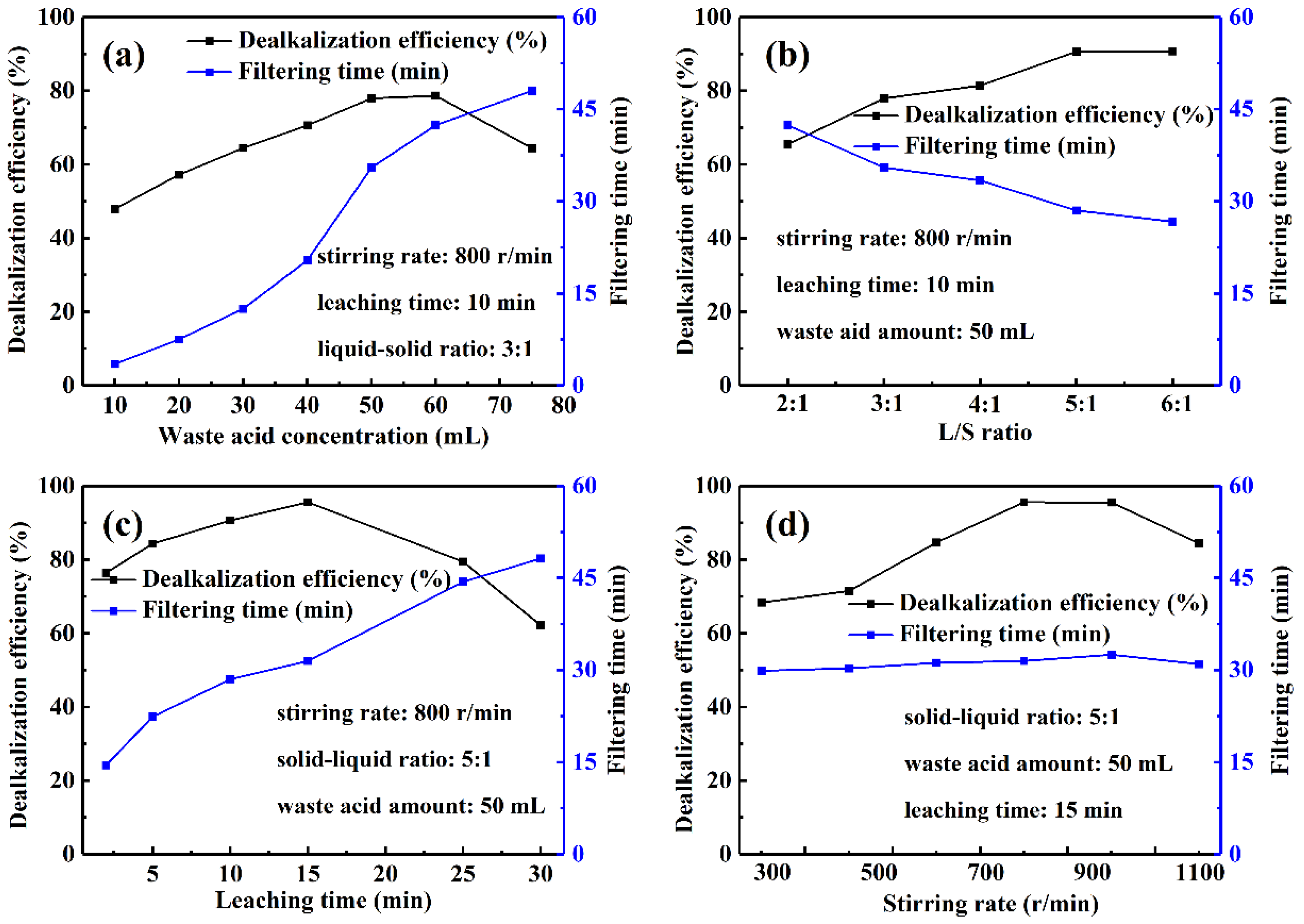
3.1.2. Factors Affecting Leaching Using Waste Acid
3.2. Acid Leaching with Calcium Compounds
3.3. Residue Analysis
3.4. Analysis of the Leachate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, G.-T.; Wang, Y.-L.; Qi, T.-G.; Zhou, Q.-S.; Liu, G.-H.; Peng, Z.-H.; Li, X.-B. Toward sustainable green alumina production: A critical review on process discharge reduction from gibbsitic bauxite and large-scale applications of red mud. J. Environ. Chem. Eng. 2023, 11, 109433. [Google Scholar] [CrossRef]
- Yulikasari, A.; Tangahu, B.V.; Nurhayati, E.; Arliyani, I.; Mashudi; Titah, H.S.; Lam, Y.M.; Wang, Y. A comprehensive review of integrated phytoremediation and nanoparticle methods for heavy metal in red mud. Ecotoxicol. Environ. Saf. 2024, 288, 117381. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.M.A.; Sun, T.; Dai, M.; Xu, W.; Ye, Y.; Ali, I.; Gao, F.; Peng, C. Technologies for recovery of iron from red mud: Processes, challenges and opportunities. Sustain. Mater. Technol. 2024, 41, e01053. [Google Scholar] [CrossRef]
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The composition, recycling and utilisation of bayer red mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Agrawal, S.; Dhawan, N. Evaluation of red mud as a polymetallic source—A review. Miner. Eng. 2021, 171, 107084. [Google Scholar] [CrossRef]
- Pan, X.; Wu, H.; Lv, Z.; Yu, H.; Tu, G. Recovery of valuable metals from red mud: A comprehensive review. Sci. Total Environ. 2023, 904, 166686. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, J.; Zhang, Y.; Peng, D.; Huang, T.; Sun, C. Ph-dependent leaching characteristics of major and toxic elements from red mud. Int. J. Environ. Res. Public Health 2019, 16, 2046. [Google Scholar] [CrossRef]
- Singh, M.; Upadhayay, S.N.; Prasad, P.M. Preparation of iron rich cements using red mud. Cem. Concr. Res. 1997, 27, 1037–1046. [Google Scholar] [CrossRef]
- Mayes, W.M.; Burke, I.T.; Gomes, H.I.; Anton, Á.D.; Molnár, M.; Feigl, V.; Ujaczki, É. Advances in understanding environmental risks of red mud after the ajka spill, hungary. J. Sustain. Metall. 2016, 2, 332–343. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, B. Development of unsintered construction materials from red mud wastes produced in the sintering alumina process. Constr. Build. Mater. 2008, 22, 2299–2307. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, N. Utilization of red mud in cement production: A review. Waste Manag. Res. 2011, 29, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Y.; Liu, Z. Red mud-based catalysts for the catalytic removal of typical air pollutants: A review. J. Environ. Sci. 2023, 127, 628–640. [Google Scholar] [CrossRef]
- Ruyters, S.; Mertens, J.; Vassilieva, E.; Dehandschutter, B.; Poffijn, A.; Smolders, E. The red mud accident in ajka (hungary): Plant toxicity and trace metal bioavailability in red mud contaminated soil. Environ. Sci. Technol. 2011, 45, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Winkler, D.; Bidlo, A.; Bolodar-Varga, B.; Erdo, A.; Horvath, A. Long-term ecological effects of the red mud disaster in hungary: Regeneration of red mud flooded areas in a contaminated industrial region. Sci. Total Environ. 2018, 644, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-Y.; Wu, C.-S. Stockpiling and comprehensive utilization of red mud research progress. Materials 2012, 5, 1232–1246. [Google Scholar] [CrossRef]
- Gelencser, A.; Kovats, N.; Turoczi, B.; Rostasi, A.; Hoffer, A.; Imre, K.; Nyiro-Kosa, I.; Csakberenyi-Malasics, D.; Toth, A.; Czitrovszky, A.; et al. The red mud accident in ajka (hungary): Characterization and potential health effects of fugitive dust. Environ. Sci. Technol. 2011, 45, 1608–1615. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Hualong, L.; Chenglin, Z. Research status and progress of red mud alkali removal technology. Multipurp. Util. Miner. Resour. 2014, 11–14+36. [Google Scholar]
- Wu, J.; Lei, T.; Wang, B.; Ma, S.; Lin, Y.; Lu, X.; Ye, Z. An eco-friendly acid leaching strategy for dealkalization of red mud by controlling phase transformation. Materials 2022, 15, 580. [Google Scholar] [CrossRef]
- Hao, Y.; Tian, Y.; Ma, R. Research status of bayer method of red mud dealkalization. Environ. Sci. Technol. 2023, 46, 194–199. [Google Scholar]
- Qiu, Z.; Peng, C.; Zhou, K.; Chen, W. Current situation and prospect of comprehensive utilization of titanium white iron resources by sulfuric acid process. Nonferrous Met. Sci. Eng. 1–11.
- Debadatta, D.; Pramanik, K. A study on chemical leaching of iron from red mud using sulphuric acid. Res. J. Chem. Environ. 2013, 17, 50–56. [Google Scholar]
- Zhang, X.-K.; Zhou, K.-G.; Chen, W.; Lei, Q.-Y.; Huang, Y.; Peng, C.-H. Recovery of iron and rare earth elements from red mud through an acid leaching-stepwise extraction approach. J. Cent. South Univ. 2019, 26, 458–466. [Google Scholar] [CrossRef]
- Lu, F.; Xiao, T.; Lin, J.; Li, A.; Long, Q.; Huang, F.; Xiao, L.; Li, X.; Wang, J.; Xiao, Q.; et al. Recovery of gallium from bayer red mud through acidic-leaching-ion-exchange process under normal atmospheric pressure. Hydrometallurgy 2018, 175, 124–132. [Google Scholar] [CrossRef]
- Kinnarinen, T.; Theliander, H.; Hakkinen, A.; Mattsson, T. Local properties of filter cakes formed from ph-adjusted bauxite residue slurries. Sep. Purif. Technol. 2018, 194, 1–9. [Google Scholar] [CrossRef]
- Patil, S.V.; Thorat, B.N. Mechanical dewatering of red mud. Sep. Purif. Technol. 2022, 294, 121157. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, F.; Cao, S.; Zhang, Y. The solid-liquid separation behaviors of the typical leach slurries in the alkaline processes for alumina. Hydrometallurgy 2017, 169, 229–238. [Google Scholar] [CrossRef]
- Botelho, A.B., Jr.; Romano Espinosa, D.C.; Soares Tenorio, J.A. Extraction of scandium from critical elements-bearing mining waste: Silica gel avoiding in leaching reaction of bauxite residue. J. Sustain. Metall. 2021, 7, 1627–1642. [Google Scholar] [CrossRef]
- Jiang, P.; Liao, C. Research on separation of valtahle metals from red mud by hydrochloric acid. China Min. Mag. 2011, 20, 85–87. [Google Scholar]
- Shao-Ming, L.I.; Li-Ying, G.E.; Xiang-Qin, Z. The simulation experiment about silica to the settling of red mud separation process. J. Guizhou Univ. Technol. (Nat. Sci. Ed.) 2007. [Google Scholar]
- Gu, H.; Wang, N.; Hargreaves, J.S.J. Sequential extraction of valuable trace elements from bayer process-derived waste red mud samples. J. Sustain. Metall. 2018, 4, 147–154. [Google Scholar] [CrossRef]
- Hongliang, C.; Ting, W.; Yang, K.; Shengbi, W. Technology conditions and mechanism discussion of sodium and iron extraction from red mud by acid. Inorg. Chem. Ind. 2016, 48, 44–48. [Google Scholar]
- Xie, W.; Zhang, N.; Li, J.; Zhou, F.; Ma, X.; Gu, G.; Zhang, W. Optimization of condition for extraction of aluminum and iron from red mud by hydrochloric acid leaching. Chin. J. Environ. Eng. 2017, 11, 5677–5682. [Google Scholar]
- Georgiou, D.; Aivazidis, A.; Hatiras, J.; Gimouhopoulos, K. Treatment of cotton textile wastewater using lime and ferrous sulfate. Water Res. 2003, 37, 2248–2250. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, G.M.; Hamzeh, A.; Semerjian, L. Post treatment of tannery wastewater using lime/bittern coagulation and activated carbon adsorption. Desalination 2011, 273, 359–365. [Google Scholar] [CrossRef]
- Ghosh, P.; Samanta, A.N.; Ray, S. Reduction of cod and removal of zn2+ from rayon industry wastewater by combined electro-fenton treatment and chemical precipitation. Desalination 2011, 266, 213–217. [Google Scholar] [CrossRef]
- Ayeche, R. Treatment by coagulation-flocculation of dairy wastewater with the residual lime of national algerian industrial gases company (nigc-annaba). Energy Procedia 2012, 18, 147–156. [Google Scholar] [CrossRef]
- Zeng, H.; Lyu, F.; Hu, G.; Tang, H.; Wang, L.; Sun, W.; Hu, Y.; Liu, R. Dealkalization of bauxite residue through acid neutralization and its revegetation potential. JOM 2020, 72, 319–325. [Google Scholar] [CrossRef]
- Zeng, H.; Tang, H.; Sun, W.; Wang, L. Strengthening solid–liquid separation of bauxite residue through the synergy of charge neutralization and flocculation. Sep. Purif. Technol. 2022, 285, 120296. [Google Scholar] [CrossRef]





| Compositions | Al2O3 | CaO | Fe2O3 | TiO2 | Na2O | SiO2 |
| Contents/% | 20.49 | 2.46 | 48.01 | 5.77 | 9.14 | 14.66 |
| Na | K | Si | Ti | Ni | Fe | Ca | |
| Feed, % | 12.63 | 0.09 | 6.55 | 3.56 | 0.08 | 26.50 | 2.98 |
| Residue, % | 0.96 | 0.07 | 1.41 | 5.22 | 0.60 | 27.90 | 3.27 |
| Mg | S | Mn | Cl | Zn | As | Cu | |
| Feed, % | 4.47 | 5.30 | 0.12 | 0.13 | 0.02 | / | 2.00 |
| Residue, % | 4.84 | 5.86 | 0.13 | 0.03 | 0.02 | / | 0.16 |
| Number | Photos | Na | Fe | Si | Al | Ca |
|---|---|---|---|---|---|---|
| Water leaching |  | 22.347 | 0.043 | 34.419 | 38.241 | / |
| H2SO4 leaching |  | 11,356 | 14,400 | 335.4 | 1532 | 446 |
| H2SO4-2% CaO |  | 12,085 | 0.145 | 0.937 | 0.329 | 562 |
| The Amounts of CaO/% | Test Days | ||||
|---|---|---|---|---|---|
| 0 | 1 | 4 | 6 | 40 | |
| 0 | 4.25 | 6.73 | 6.04 | 5.94 | 6.40 |
| 1 | 4.54 | 7.22 | 7.25 | 7.42 | 7.44 |
| 2 | 4.84 | 7.75 | 8.15 | 8.19 | 8.03 |
| 4 | 8.4 | 8.48 | 9.07 | 8.95 | 8.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Dai, M.; Guan, Q.; Zeng, H.; Sun, W.; Wang, L. Optimizing the Dealkalization Process of Red Mud: Controlling Calcium Compounds to Improve Solid–Liquid Separation Performance. Minerals 2025, 15, 150. https://doi.org/10.3390/min15020150
Zhou J, Dai M, Guan Q, Zeng H, Sun W, Wang L. Optimizing the Dealkalization Process of Red Mud: Controlling Calcium Compounds to Improve Solid–Liquid Separation Performance. Minerals. 2025; 15(2):150. https://doi.org/10.3390/min15020150
Chicago/Turabian StyleZhou, Jianfei, Mengmeng Dai, Qingjun Guan, Hua Zeng, Wei Sun, and Li Wang. 2025. "Optimizing the Dealkalization Process of Red Mud: Controlling Calcium Compounds to Improve Solid–Liquid Separation Performance" Minerals 15, no. 2: 150. https://doi.org/10.3390/min15020150
APA StyleZhou, J., Dai, M., Guan, Q., Zeng, H., Sun, W., & Wang, L. (2025). Optimizing the Dealkalization Process of Red Mud: Controlling Calcium Compounds to Improve Solid–Liquid Separation Performance. Minerals, 15(2), 150. https://doi.org/10.3390/min15020150









