Trace Element Geochemistry and Stable Isotopic (δ13C and δ15N) Characterisation of Nevşehir Coals, Türkiye
Abstract
1. Introduction
2. Materials and Methods
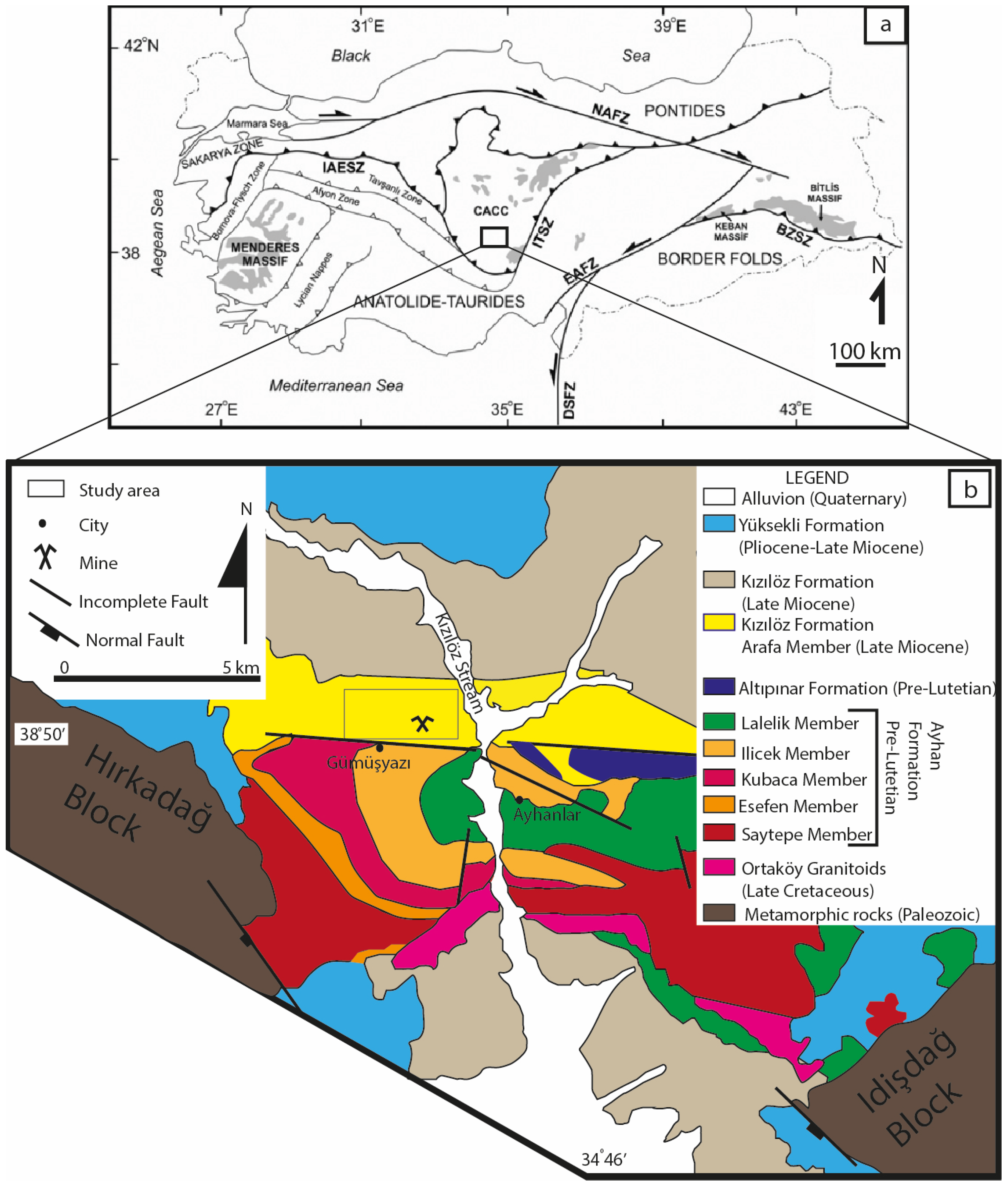
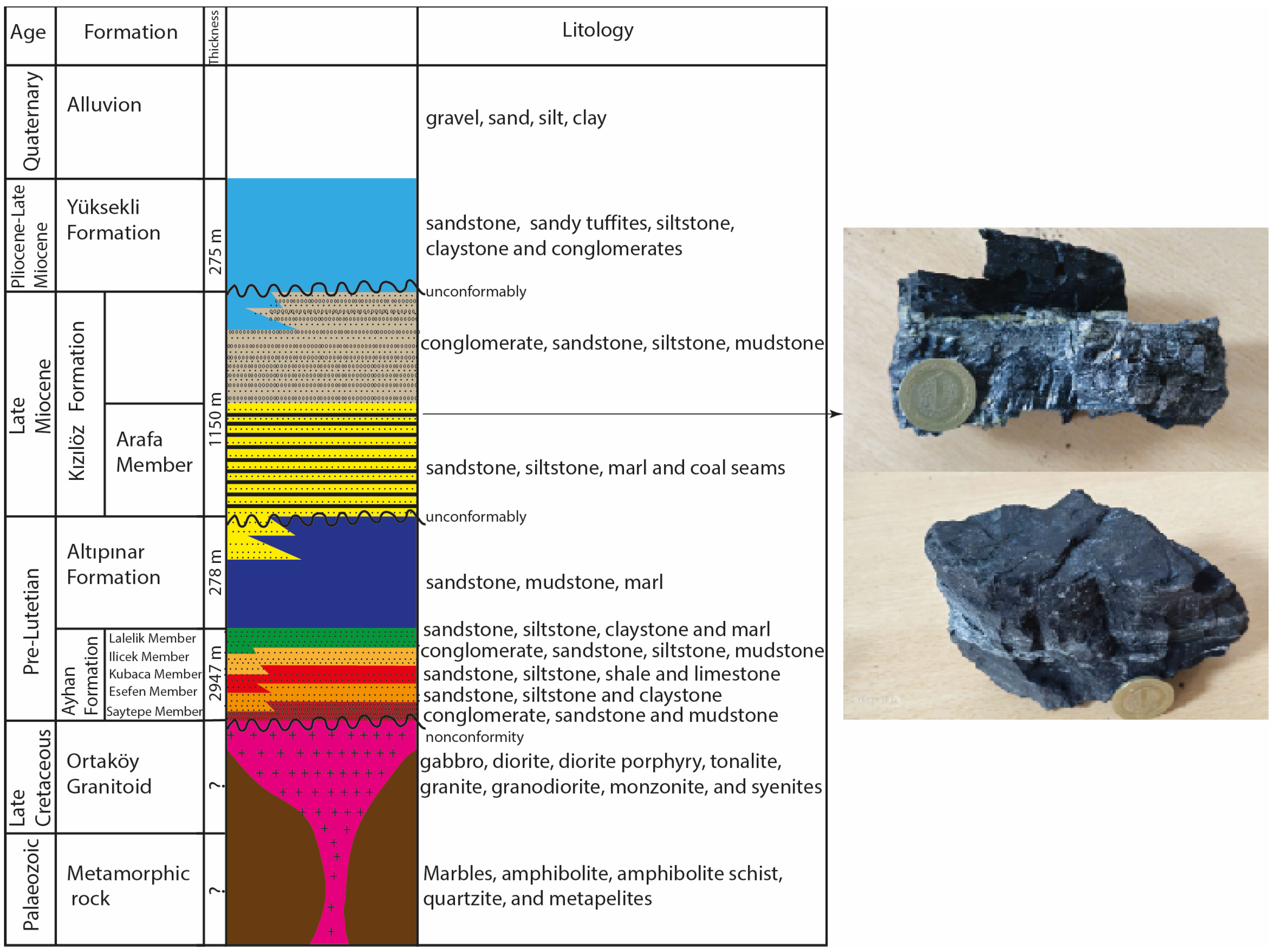
3. Geological Background
4. Results
4.1. Petrography
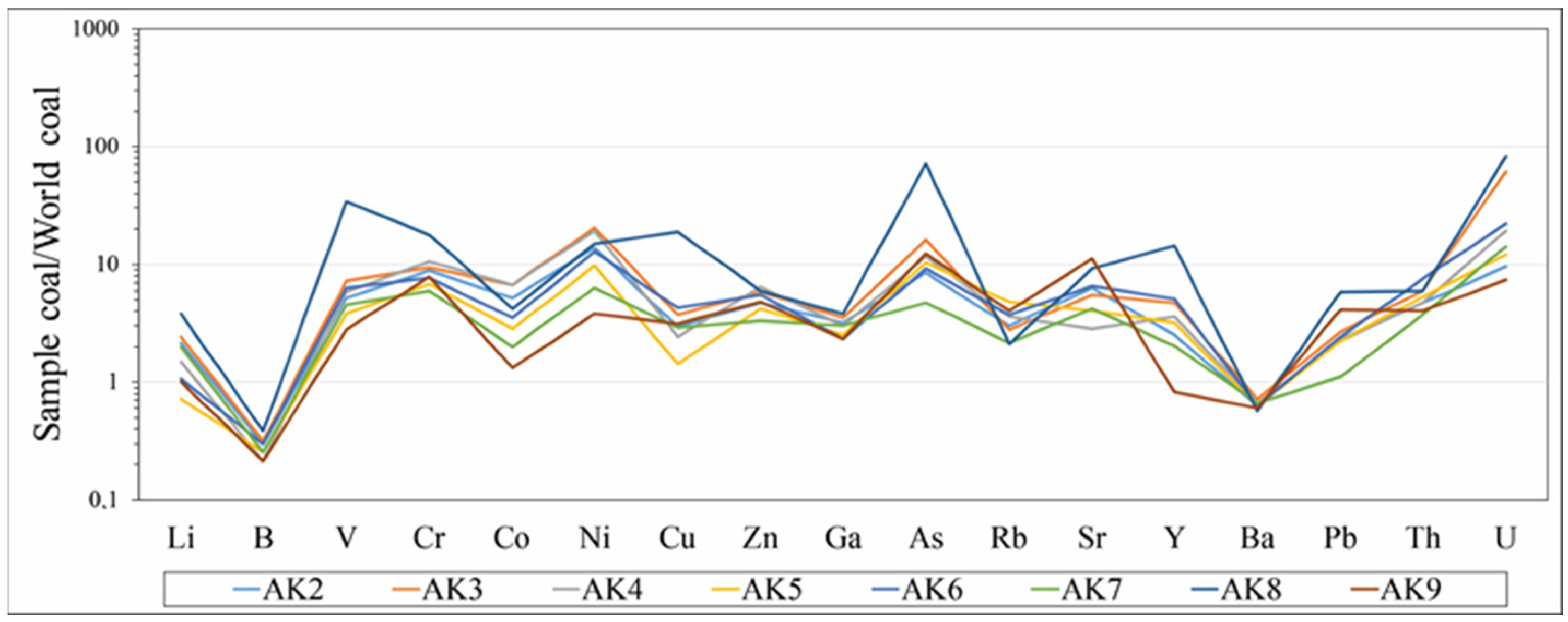
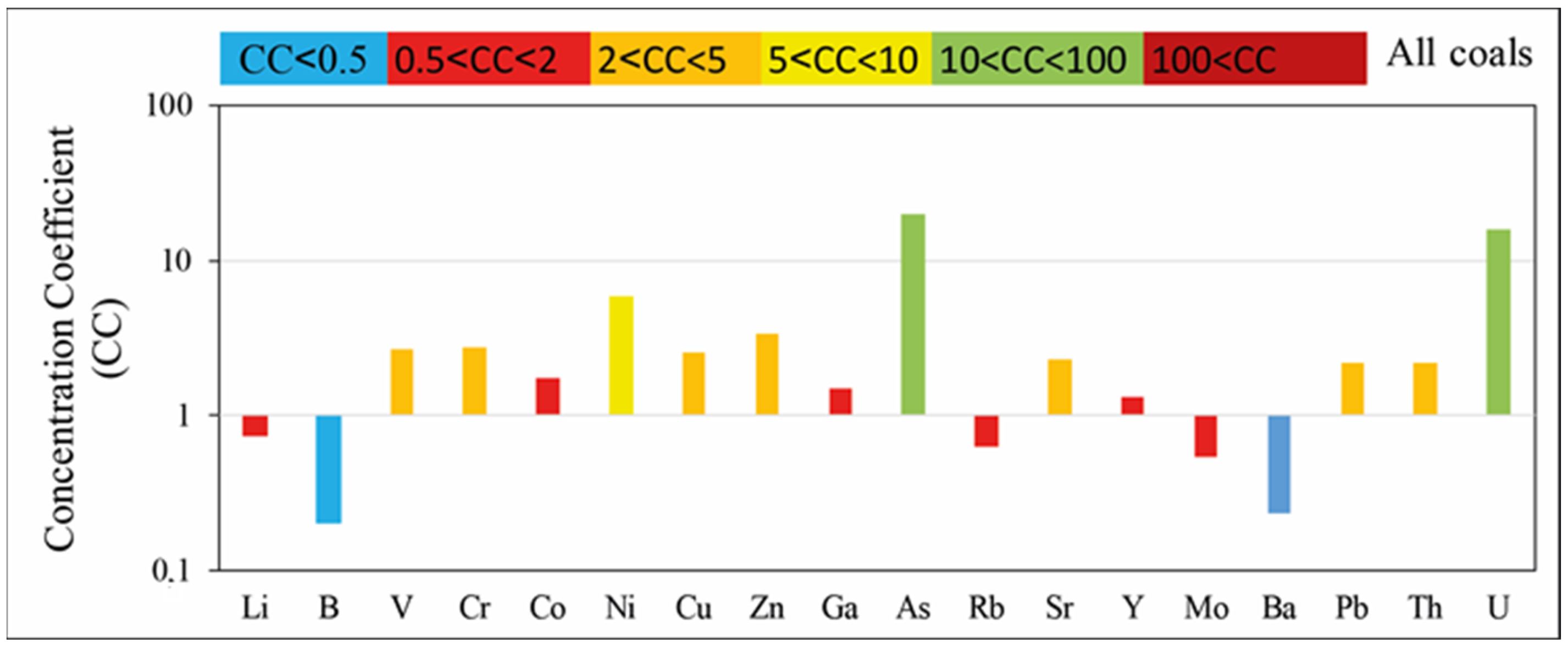
4.2. Geochemistry
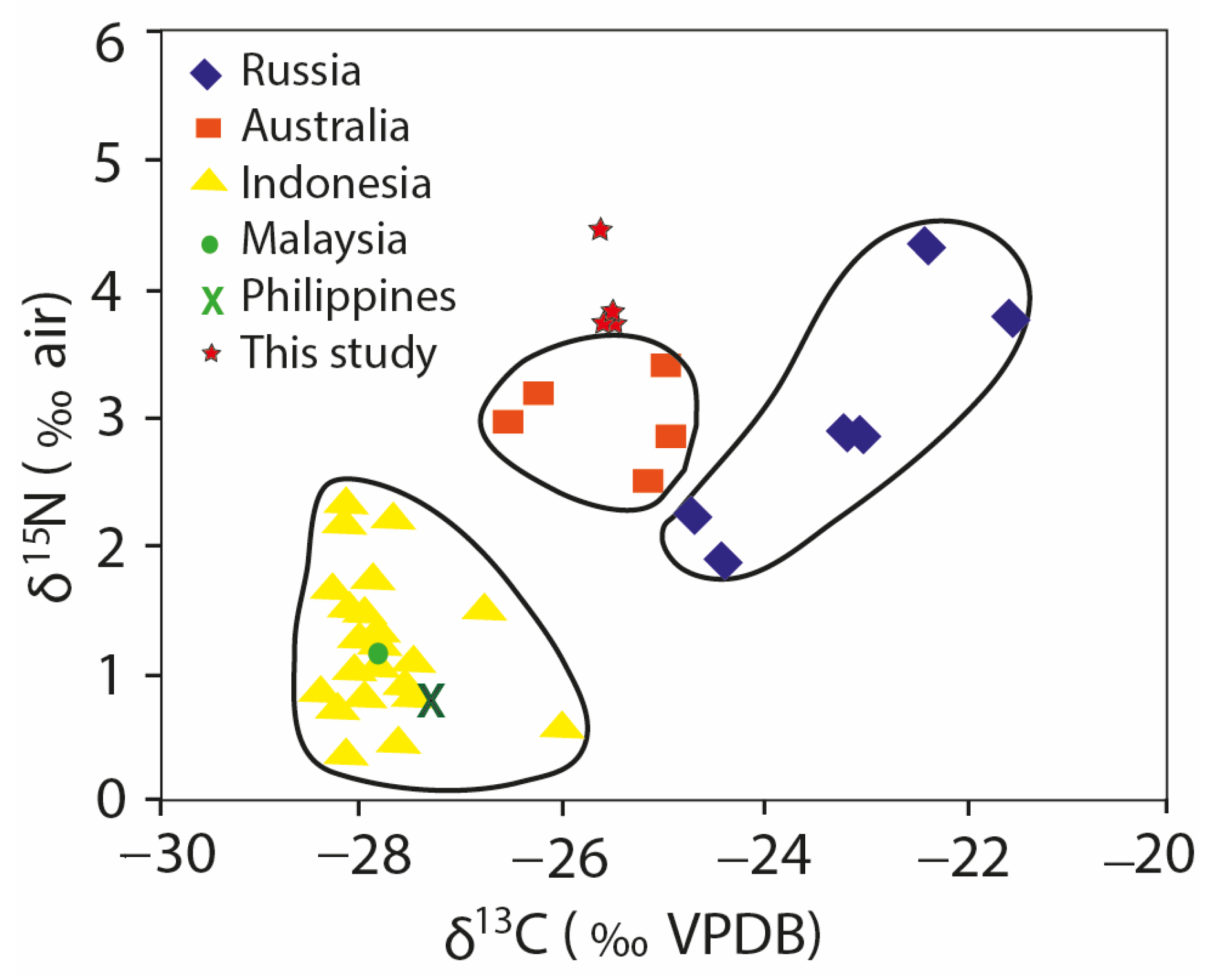
4.3. Nitrogen and Carbon Isotope Values
5. Discussion
5.1. Petrography of Coal
5.2. Major and Trace Element Geochemistry in Coal
5.3. Nitrogen and Carbon Isotope Characteristics of Nevşehir Coals
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, J.; Han, F.; Li, T.; Tian, H.; Li, Y. Mineralogical and geochemical compositions of the Lopingian coals in the Zhongliangshan Coalfield, Southwestern China. Minerals 2018, 8, 104. [Google Scholar] [CrossRef]
- Zou, J.; Cheng, L.; Guo, Y.; Wang, Z.; Tian, H.; Li, T. Mineralogical and geochemical characteristics of lithium and rare earth elements in high-sulfur coal from the Donggou mine, Chongqing, Southwestern China. Minerals 2020, 10, 627. [Google Scholar] [CrossRef]
- Liu, J.; Song, H.; Dai, S.; Nechaev, V.P.; Graham, I.T.; French, D.; Nechaeva, E.V. Mineralization of REE-Y-Nb-Ta-Zr-Hf in Wuchiapingian coals from the Liupanshui Coalfield, Guizhou, southwestern China: Geochemical evidence for terrigenous input. Ore Geol. Rev. 2019, 115, 103190. [Google Scholar] [CrossRef]
- Dai, S.; Zhou, P.; Zhang, M.; Wang, X.; Wang, J.; Song, X.; Jiang, Y.; Luo, Y.; Song, Z.; Yang, Z.; et al. A new type of Nb(Ta)-Zr(Hf)-REE-Ga polymetallic deposit in the late Permian coal-bearing strata, eastern Yunnan, southwestern China: Possible economic significance and genetic implications. Int. J. Coal Geol. 2010, 83, 55–63. [Google Scholar] [CrossRef]
- Wei, Y.; He, W.; Qin, G.; Fan, M.; Cao, D. Lithium enrichment in the No. 21 coal of the Hebi No. 6 Mine, Anhe Coalfield, Henan Province, China. Minerals 2020, 10, 521. [Google Scholar] [CrossRef]
- Zhou, M.; Zhao, L.; Wang, X.; Nechaev, V.P.; French, D.; Spiro, B.F.; Graham, I.T.; Hower, J.C.; Dai, S. Mineralogy and geochemistry of the Late Triassic coal from the Caotang mine, northeastern Sichuan Basin, China, with emphasis on the enrichment of the critical element lithium. Ore Geol. Rev. 2021, 139, 104582. [Google Scholar] [CrossRef]
- Seredin, V.; Finkelman, R.B. Metalliferous coals: A review of the main genetic and geochemical types. Int. J. Coal Geol. 2008, 76, 253–289. [Google Scholar] [CrossRef]
- Hu, R.; Qi, H.; Zhou, M.; Su, W.; Bi, X.; Peng, J.; Zhong, H. Geological and geochemical constraints on the origin of the giant Lincang coal seam-hosted germanium deposit, Yunnan, SW China: A review. Ore Geol. Rev. 2009, 36, 221–234. [Google Scholar] [CrossRef]
- Hower, J.C.; Granite, E.; Mayfield, D.; Lewis, A.; Finkelman, R. Notes on contributions to the science of rare earth element enrichment in coal and coal combustion byproducts. Minerals 2016, 6, 32. [Google Scholar] [CrossRef]
- Dai, S.; Finkelman, R.B. Coal as a promising source of critical elements: Progress and future prospects. Int. J. Coal Geol. 2018, 186, 155–164. [Google Scholar] [CrossRef]
- Spiker, E.G.; Hatcher, P.G. The effects of early diagenesis on the chemical and stable carbon isotopic composition of wood. Geochim. Cosmochim. Acta 1987, 51, 1385–1391. [Google Scholar] [CrossRef]
- Rimmer, S.M.; Rowe, H.D.; Taulbee, D.N.; Hower, J.C. Influence of maceral content on δ 13C and δ 15N in a Middle Pennsylvanian coal. Chem. Geol. 2006, 225, 77–90. [Google Scholar] [CrossRef]
- Skrzypek, G.; Jezierski, P.; Szynkiewicz, A. Preservation of primary stable isotope signatures of peat-forming plants during early decomposition—observation along an altitudinal transect. Chem. Geol. 2010, 273, 238–249. [Google Scholar] [CrossRef]
- Suto, N.; Kawashima, H. Global mapping of carbon isotope ratios in coal. J. Geochem. Explor. 2016, 167, 12–19. [Google Scholar] [CrossRef]
- Valentim, B.; Algarra, M.; Guedes, A.; Ruppert, L.F.; Hower, J.C. Notes on the origin of copromacrinite based on nitrogen functionalities and δ 13C and δ 15N determined on samples from the Peach Orchard coal bed, southern Magoffin County, Kentucky. Int. J. Coal Geol. 2016, 160, 63–72. [Google Scholar] [CrossRef]
- Lehmann, M.F.; Bernasconi, S.M.; Barbieri, A.; McKenzie, J.A. Preservation of organic matter and alteration of its carbon and nitrogen isotope composition during simulated and in situ early sedimentary diagenesis. Geochim. Cosmochim. Acta 2002, 66, 3573–3584. [Google Scholar] [CrossRef]
- Taylor, G.H.; Teichmüller, M.; Davis, A. Organic Petrology: A New Handbook Incorporating Some Revised Parts of Stach’s Textbook of Coal Petrology; Gebruder Borntraeger Verlagsbuchhandlung: Stuttgart, Germany, 1998; p. 704. [Google Scholar]
- Zheng, Q.; Liu, Q.; Huang, B.; Zhao, W. Isotopic composition and content of organic nitrogen in the coals of Qinshui Coalfield, North China. J. Geochem. Explor. 2015, 149, 120–126. [Google Scholar] [CrossRef]
- Gröcke, D.R. Carbon isotope analyses of fossil plants as a chemostratigraphic and palaeoenvironmental tool. Lethaia 1998, 31, 1–13. [Google Scholar] [CrossRef]
- Gröcke, D.; Hesselbo, S.P.; Jenkyns, H.C. Carbon isotope composition of Lower Cretaceous fossil wood: Ocean-atmosphere chemistry and relation to sea-level change. Geology 1999, 27, 155–158. [Google Scholar] [CrossRef]
- Gröcke, D.R. The carbon isotope composition of ancient CO2 based on higher-plant organic matter. Philos. Trans. R. Soc. A 2002, 360, 633–658. [Google Scholar] [CrossRef]
- Lücke, A.; Gerhard, H.; Schleser, G. Environmental history of the German Lower Rhine Embayment during the Middle Miocene as reflected by carbon isotopes in brown coal. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1999, 154, 339–352. [Google Scholar] [CrossRef]
- Nordt, L.; Tubbs, J.; Dworkin, S. Stable carbon isotope record of terrestrial organic materials for the last 450 Ma yr. Earth-Sci. Rev. 2016, 159, 103–117. [Google Scholar] [CrossRef]
- Schoell, M.; Shouten, S.; Damste, S. A molecular carbon record of organic isotope Miocene climate changes. Science 1994, 263, 1122–1125. [Google Scholar] [CrossRef] [PubMed]
- Van de Wetering, N.; Esterle, J.; Baublys, K. Decoupling δ13C response to palaeoflora cycles and climatic variation in coal: A case study from the Late Permian Bowen Basin, Queensland, Australia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 386, 165–179. [Google Scholar] [CrossRef]
- Widodo, S.; Bechtel, A.; Anggayana, K.; Püttmann, W. Reconstruction of floral changes during deposition of the Miocene Embalut coal from Kutai Basin, Mahakam Delta, East Kalimantan, Indonesia by use of organic matter. Org. Geochem. 2009, 40, 206–218. [Google Scholar] [CrossRef]
- Xiao, H.Y.; Liu, C.Q. The elemental and isotopic composition of sulfur and nitrogen in Chinese coals. Org. Geochem. 2011, 42, 84–93. [Google Scholar] [CrossRef]
- Freyer, H.D. Seasonal trends of NH4 and NO3 nitrogen isotope composition in rain collected at Jülich, Germany. Tellus 1978, 30, 83–92. [Google Scholar]
- Heaton, T.H.E. 15N/14N ratios of nitrate and ammonium in rain at Pretoria, South Africa. Atmos. Environ. 1987, 21, 843–852. [Google Scholar] [CrossRef]
- Chen, R.; Liu, G.; Shang, F.; Cao, Y. Nitrogen isotope compositions of the Upper Triassic Chang 7 Shale, Ordos Basin, North China: Implications for depositional redox conditions. Mar. Pet. Geol. 2019, 109, 279–290. [Google Scholar] [CrossRef]
- Li, M.; Chen, J.; Wang, T.; Zhong, N.; Shi, S. Nitrogen isotope and trace element composition characteristics of the Lower Cambrian Niutitang Formation shale in the upper-middle Yangtze region, South China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 501, 1–12. [Google Scholar] [CrossRef]
- Rigby, D.; Batts, B.D. The isotope composition of nitrogen in Australian coals and oil shales. Chem. Geol. 1986, 58, 273–282. [Google Scholar] [CrossRef]
- Algeo, T.; Rowe, H.; Hower, J.C.; Schwark, L.; Herrmann, A.; Heckel, P. Changes in ocean denitrification during Late Carboniferous glacial-interglacial cycles. Nat. Geosci. 2008, 1, 709–714. [Google Scholar] [CrossRef]
- Mineral Research and Exploration General Directorate (MTA). Dadağı (Nevşehir-Gülşehir) Kömür Zuhuru ile Ilgili bir Prospeksiyon Gezisine ait Rapor; Wedding, H., Ed.; Report No: 3927; MTA: Ankara, Türkiye, 1967. [Google Scholar]
- Nadkarni, R.A. Multitechnique multielemental analysis of coal and fly ash. Anal. Chem. 1980, 52, 929–935. [Google Scholar] [CrossRef]
- Şengör, A.M.C.; Yilmaz, Y. Thetyan evolution of Turkey: A plate tectonic approach. Tectonophysics 1981, 75, 181–241. [Google Scholar] [CrossRef]
- Okay, A.I. Geology of Turkey: A Synopsis. Anschnitt 2008, 21, 19–42. [Google Scholar]
- Köksal, S.; Göncüoğlu, C.M. Geology of the Idis Dağı–Avanos Area (Nevşehir–Central Anatolia). Bull. Miner. Res. Explor. 1997, 119, 41–58. [Google Scholar]
- Göncüoğlu, M.C.; Erler, A.; Toprak, G.M.V.; Olgun, E.; Kuşçu, İ. Orta Anadolu Masifinin batı Bölümünün Jeolojisi, Bölüm 1: Güney Kesim; Turkish Petroleum Corporation (TPAO) Report, No. 2909; TPAO: Ankara, Türkiye, 1991; p. 140. [Google Scholar]
- Atabey, E.; Tarhan, N.; Yusufoğlu, H.; Canpolat, M. Geology of Between Hacıbektaş, Gülşehir and Kalaba (Nevşehir)-Himmetdede (Kayseri); General Directory Mining Research and Exploration Report, Report No. 8523; MTA: Ankara, Türkiye, 1988. [Google Scholar]
- Demircioğlu, R. Gülşehir—Özkonak (Nevşehir) Çevresinde Kırşehir Masifi ve Örtü birimlerinin Jeolijisi ve Yapısal Özellikleri. Ph.D. Thesis, Selçuk Üniversitesi Fen Bilimleri Enstitüsü, Konya, Türkiye, 2014; p. 241. [Google Scholar]
- Whitney, D.L.; Bozkurt, E. Metamorphic history of the southern Menderes massif, western Turkey. Geol. Soc. Am. Bull. 2002, 114, 829–838. [Google Scholar] [CrossRef]
- Palmer, A.C.; Tuncalı, E.; Dennen, O.K.; Coburn, C.T.; Finkelman, R.B. Characterization of Turkish coals: A nationwide perspective. Int. J. Coal Geol. 2004, 60, 85–115. [Google Scholar] [CrossRef]
- Dai, S.; Ren, D.; Chou, C.-L.; Finkelman, R.B.; Seredin, V.V.; Zhou, Y. Geochemistry of trace elements in Chinese coals: A review of abundances, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- Ketris, M.P.; Yudovich, Y.E. Estimations of Clarkes for carbonaceous biolithes: World averages for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Swaine, D.J. Trace Elements in Coal; Butterworths: London, UK, 1990; p. 292. [Google Scholar]
- Sykorova, I.; Pıckel, W.; Chrıstanıs, K.; Wolf, M.; Taylor, G.H.; Flores, D. Classification of huminite-ICCP System. Int. J. Coal Geol. 1994, 62, 85–106. [Google Scholar] [CrossRef]
- Sağıroğlu, A.; Kara, H.; Kürüm, S. Mineralogy and geochemistry of the hematite muscovite schists of Malatya, Turkey: Could it be the first known banded iron formation (BIF) in the Taurids And Anatolia? Carpathian J. Earth Environ. Sci. 2013, 8, 49–58. [Google Scholar]
- Liu, J.; Dai, S.; Song, H.; Nevhaev, V.P.; French, D.; Spiro, B.F.; Graham, I.T.; Hower, J.C.; Shao, L.; Zhao, J. Geological factors controlling variations in the mineralogical and elemental compositions of Late Permian coals from the Zhijin-Nayong Coalfield, western Guizhou, China. Int. J. Coal Geol. 2021, 247, 103855. [Google Scholar] [CrossRef]
- Dai, S.; Ren, D.; Finkelman, R.B.; French, D.; Hower, J.C.; Graham, I.T.; Zhou, F. Modes of occurrence of elements in coal: A critical evaluation. Earth-Sci. Rev. 2021, 222, 103815. [Google Scholar] [CrossRef]
- Hayashi, K.I.; Fujisawa, H.; Holland, H.D.; Ohmoto, H. Geochemistry of ~1.9 Ga sedimentary rocks from northeastern Labrador, Canada. Geochim. Cosmochim. Acta 1997, 61, 4115–4137. [Google Scholar] [CrossRef]
- Dai, S.; Seredin, V.V.; Ward, C.R.; Hower, J.C.; Xing, Y.; Zhang, W.; Song, W.; Wang, P. Enrichment of U–Se–Mo–Re–V in coals preserved within marine carbonate successions: Geochemical and mineralogical data from the Late Permian Guiding Coalfield, Guizhou, China. Miner. Depos. 2015, 50, 159–186. [Google Scholar] [CrossRef]
- Orem, W.H.; Finkelman, R.B. Coal Formation and Geochemistry. In Sediments, Diagenesis, and Sedimentary Rocks: Treatise on Geochemistry, 7; Mackenzie, F.T., Ed.; Elsevier-Pergamon: Oxford, UK, 2003; pp. 191–222. [Google Scholar]
- Kalaitzidis, S.; Christanis, K.; Georgakopoulos, A.; Fernández-Turiel, J.L.; Papazisimou, S. Influence of geological conditions during peat accumulation on trace element affinities and their behavior during peat combustion. Energy Fuel 2002, 16, 1476–1482. [Google Scholar] [CrossRef]
- Altunsoy, M.; Sari, A.; Özçelik, O.; Engin, H.; Hökerek, S. Major and trace-element enrichments in the Karapınar coals (Konya, Turkey). Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 88–99. [Google Scholar] [CrossRef]
- Karayiğit, A.İ.; Bircan, C.; Oskay, R.G.; Türkmen, İ.; Querol, X. The geology, mineralogy, petrography, and geochemistry of the Miocene Dursunbey coal within fluvio-lacustrine deposits, Balıkesir (Western Turkey). Int. J. Coal Geol. 2020, 228, 103548. [Google Scholar] [CrossRef]
- Karayiğit, A.İ.; Spears, D.A.; Booth, C.A. Antimony and arsenic anomalies in the coal seams from the Gokler coalfield, Gediz, Turkey. Int. J. Coal Geol. 2000, 44, 1–17. [Google Scholar] [CrossRef]
- Yudovich, Y.E.; Ketris, M.P. Arsenic in coal: A review. Int. J. Coal Geol. 2005, 61, 141–196. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Ren, D.Y.; Zheng, C.G.; Zeng, R.S.; Chou, C.L.; Liu, J. Trace element abundances in major minerals of Late Permian coals from southwestern Guizhou Province, China. Int. J. Coal Geol. 2002, 53, 55–64. [Google Scholar] [CrossRef]
- Hower, J.C.; Campbell (Iain), J.L.; Teesdale, W.J.; Nejedly, Z.; Robertson, J.D. Scanning proton microprobe analysis of mercury and other trace elements in Fesulfides from a Kentucky coal. Int. J. Coal Geol. 2008, 75, 88–92. [Google Scholar] [CrossRef]
- Dai, S.; Wang, X.; Seredin, V.V.; Hower, J.C.; Ward, C.R.; O’Keefe, J.M.K.; Huang, W.; Li, T.; Li, X.; Liu, H.; et al. Petrology, mineralogy, and geochemistry of the Ge-rich coal from the Wulantuga Ge ore deposit, Inner Mongolia, China: New data and genetic implications. Int. J. Coal Geol. 2012, 90, 72–99. [Google Scholar] [CrossRef]
- Finkelman, R.B. Modes of Occurrence of Trace Elements in Coal; USGS Open-File Report, USGS Numbered Series; U.S. Geological Survey: Reston, VA, USA, 1981; pp. 81–99.
- Finkelman, R.B.; Palmer, C.A.; Wang, P. Quantification of the modes of occurrence of 42 elements in coal. Int. J. Coal Geol. 2018, 185, 138–160. [Google Scholar] [CrossRef]
- Arbuzov, S.I.; Volostnov, A.V.; Rikhvanov, L.P.; Mezhibor, A.M.; Ilenok, S.S. Geochemistry of radioactive elements (U, Th) in coal and peat of northern Asia (Siberia, Russian Far East, Kazakhstan, and Mongolia). Int. J. Coal Geol. 2011, 86, 318–328. [Google Scholar] [CrossRef]
- Alçiçek, Ö.N.; Kalender, L. Geochemical Characterization of the Uranium Mineralization in the Cenozoic Basin in the Central Anatolia, Turkey. Radiochemistry 2019, 61, 495–506. [Google Scholar] [CrossRef]
- Dai, S.; Li, T.; Jiang, Y.; Ward, C.R.; Hower, J.C.; Sun, J.; Liu, J.; Song, H.; Wei, J.; Li, Q.; et al. Mineralogical and geochemical compositions of the Pennsylvanian coal in the Hailiushu Mine, Daqingshan Coalfield, Inner Mongolia, China: Implications of sediment-source region and acid hydrothermal solutions. Int. J. Coal Geol. 2015, 137, 92–110. [Google Scholar] [CrossRef]
- Nicholls, G.D. The Geochemistry of Coal-Bearing Strata, in Coal and Coal-Bearing Strata; Murchison, D., Westoll, T.S., Eds.; Elsevier: New York, NY, USA, 1968; pp. 269–307. [Google Scholar]
- Bird, M.I.; Ascough, P.L. Isotopes in pyrogenic carbon: A review. Org. Geochem. 2012, 42, 1529–1539. [Google Scholar] [CrossRef]
- Moroeng, O.M.; Wagner, N.J.; Hall, G.; Roberts, R.J. Using δ15N and δ13C and nitrogen functionalities to support a fire origin for certain inertinite macerals in a No. 4 Seam Upper Witbank coal, South Africa. Org. Geochem. 2018, 126, 23–32. [Google Scholar] [CrossRef]
- Kohn, M.J. Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo)ecology and (paleo)climate. Proc. Natl. Acad. Sci. USA 2010, 107, 19691–19695. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Dai, S.; Hower, J.C.; Nechaev, V.P.; French, D.; Graham, I.T.; Wang, X.; Zhao, L.; Zuo, J. Nitrogen isotopic compositions in NH4+-mineral-bearing coal: Origin and isotope fractionation. Chem. Geol. 2021, 559, 119946. [Google Scholar] [CrossRef]
- Wang, Q.; Dai, S.; Nechaev, V.P.; French, D.; Graham, I.T.; Zhao, L.; Zhang, S.; Liang, Y.; Hower, J.C. Transformation of organic to inorganic nitrogen in NH4+-illite-bearing and Ga-Al-REE-rich bituminous coals: Evidence from nitrogen isotopes and functionalities. Chem. Geol. 2024, 660, 122169. [Google Scholar] [CrossRef]
- Coplen, T.B.; Shrestha, Y. Isotope-abundance variations and atomic weights of selected elements: 2016 (IUPAC Technical Report). Pure Appl. Chem. 2016, 88, 1203–1224. [Google Scholar] [CrossRef]
- Cheng, H.H.; Bremner, J.M.; Edwards, A.P. Variations of nitrogen-15 abundance in soils. Science 1964, 146, 1574–1575. [Google Scholar] [CrossRef]
- Guo, C.T. Coal Chemistry; Chemical Industrial Press of China: Beijing, China, 1992. (In Chinese) [Google Scholar]
- Wada, E.; Kadonaga, T.; Matsuo, S. 15N abundance in nitrogen of naturally occurring substances and global assessment of denitrification from isotopic viewpoint. Geochem. J. 1975, 9, 139–148. [Google Scholar] [CrossRef]
- Sweeney, R.E.; Liu, K.K.; Kaplan, I.R. Oceanic nitrogen isotopes and their uses in determining the source of sedimentary nitrogen. In Stable Isotopes in Earth Sciences; Robinson, B.W., Ed.; Bulletin No. 1978; DSIR Science Information Division: Auckland, New Zealand, 1978; Volume 220, pp. 9–26. [Google Scholar]
- Nissenbaum, A.; Kaplan, I.R. Chemical and isotopic evidence for the in situ origin of marine humic substances. Limnol. Oceanogr. 1972, 17, 570–582. [Google Scholar] [CrossRef]
- Miyake, Y.; Wada, E. The abundance of 15N/14N in marine environments. Rec. Oceanogr. Work. Jpn. 1967, 9, 37–53. [Google Scholar]
- Dai, S.; Li, T.; Seredin, V.V.; Ward, C.R.; Hower, J.C.; Zhou, Y.; Zhang, M.; Song, X.; Song, W.; Zhao, C. Origin of minerals and elements in the late Permian coals, tonsteins, and host rocks of the Xinde Mine, Xuanwei, eastern Yunnan, China. Int. J. Coal Geol. 2014, 121, 53–78. [Google Scholar] [CrossRef]
- Landergreen, S.; Manheim, F.T. On the dependence of the distribution of heavy metals on facies. Fortschr. Geol. Rheinl. Westfal. 1963, 10, 173–192. [Google Scholar]
- Goodarzi, F.; Swaine, D.J. The influence of geological factors on the concentration of boron in Australian and Canadian coals. Chem. Geol. 1994, 118, 301–318. [Google Scholar] [CrossRef]
- Whiticar, M.J. Stable Isotope Geochemistry of Coals, Humic Kerogens and Related Natural Gases. Int. J. Coal Geol. 1996, 32, 191–215. [Google Scholar] [CrossRef]
- Feng, L.; Li, H.; Yan, D. A Refinement of Nitrogen Isotope Analysis of Coal Using Elemental Analyzer/Isotope Ratio Mass Spectrometry and the Carbon and Nitrogen Isotope Compositions of Coals Imported in China. ACS Omega 2020, 5, 7636–7640. [Google Scholar] [CrossRef] [PubMed]

| Maceral Group (%) | Samples | ||||
|---|---|---|---|---|---|
| AK4 | AK8 | ||||
| Maceral (%) | Huminite | Humotelinite | Texinite | 1 | 2 |
| Ulminite | 10 | 16 | |||
| Humodetrinitis | Atrinite | 4 | 6 | ||
| Densinite | 2 | 3 | |||
| AK2 | AK3 | AK4 | AK5 | AK7 | AK6 | AK8 | AK9 | |
|---|---|---|---|---|---|---|---|---|
| SiO2 | 46 | 48 | 55.4 | 54.6 | nd | 46.6 | 42.2 | nd |
| TiO2 | 0.7 | 0.8 | 0.9 | 0.9 | nd | 0.8 | 0.7 | nd |
| Al2O3 | 19.6 | 21.4 | 21 | 22.8 | nd | 23.4 | 27.8 | nd |
| Fe2O3 | 9.9 | 16.2 | 13.4 | 11.2 | nd | 14 | 13.4 | nd |
| MnO | <0.1 | 0.1 | <0.1 | <0.1 | nd | <0.1 | <0.1 | nd |
| MgO | 1.9 | 2.3 | 2.7 | 2.6 | nd | 2.4 | 1.8 | nd |
| CaO | 9.1 | 5.5 | 2.5 | 3.4 | nd | 6.6 | 7.6 | nd |
| Na2O | 0.3 | 0.4 | 0.7 | 0.2 | nd | 0.3 | 0.2 | nd |
| K2O | 1 | 0.9 | 1.3 | 1.6 | nd | 1 | 0.4 | nd |
| P2O3 | 0.1 | 0.2 | 0.2 | 0.1 | nd | 0.1 | 0.1 | nd |
| SO3 | 11 | 3.8 | 1.6 | 2.3 | nd | 4.6 | 5 | nd |
| LOI | 0.4 | 0.4 | 0.3 | 0.3 | 0.2 | 0.8 | ||
| Al2O3/TiO2 | 28 | 26.75 | 23.33 | 25.33 | 29.25 | 39.71 | ||
| Li | 30 | 34 | 21 | <10 | 15 | 28 | 53 | 14 |
| B | 14 | 15 | <10 | 12 | 14 | 12 | 18 | <10 |
| V | 147 | 205 | 164 | 106 | 176 | 127 | 955 | 78 |
| Cr | 150 | 159 | 178 | 116 | 131 | 102 | 302 | 133 |
| Co | 31 | 40 | 40 | 17 | 21 | 12 | 25 | 8 |
| Ni | 237 | 348 | 330 | 167 | 217 | 107 | 253 | 65 |
| Cu | 47 | 60 | 39 | 23 | 68 | 46 | 302 | 50 |
| Zn | 133 | 164 | 182 | 118 | 153 | 93 | 167 | 135 |
| Ga | 19 | 21 | 18 | 15 | 14 | 18 | 23 | 14 |
| As | 71 | 135 | 96 | 85 | 76 | 39 | 589 | 103 |
| Se | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 |
| Rb | 54 | 50 | 64 | 87 | 67 | 39 | 38 | 72 |
| Sr | 630 | 547 | 282 | 401 | 654 | 420 | 921 | 1106 |
| Y | 21 | 40 | 30 | 27 | 43 | 17 | 122 | 7 |
| Mo | 8 | 15 | 11 | 8 | 8 | <5 | 15 | <5 |
| Cd | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 |
| Sb | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 |
| Ba | 95 | 108 | 99 | 95 | 94 | 100 | 86 | 91 |
| Pb | 21 | 24 | 20 | 20 | 22 | 10 | 53 | 37 |
| Th | 15 | 19 | 15 | 17 | 24 | 12 | 19 | 13 |
| U | 18 | 117 | 37 | 23 | 42 | 27 | 155 | 14 |
| Element | World Coals 1 | Türkiye Coals 2 | This Study | World 3 | Chinese 4 |
|---|---|---|---|---|---|
| As | 0.5–80 | 1.8–620 | 39–589 | 8.3 | 3.79 |
| B | 5–400 | 22–1200 | <10–18 | - | 53 |
| Ba | 20–1000 | 15–590 | 86–108 | 150 | 159 |
| Cd | 0.1–3 | - | <5 | 0.22 | 0.25 |
| Co | 0.5–30 | 0.87–51 | 8–40 | 5.1 | 7.08 |
| Cr | 0.5–60 | - | 10–302 | 15.4 | |
| Cu | 0.5–50 | 0.5–50 | 23–302 | 16 | 17.5 |
| Mo | 0.1–10 | 0.43–69 | <5–15 | 2.2 | 3.08 |
| Ni | 0.5–50 | 3.1–1600 | 65–348 | 13 | 13.7 |
| Pb | 2–80 | 0.95–58 | 10–53 | 7.8 | 15.1 |
| Th | 0.5–10 | - | 12–24 | 3.3 | 5.84 |
| U | 0.5–10 | 0.32–140 | 14–117 | 2.4 | 2.43 |
| V | 2–100 | 5.5–270 | 78–955 | 25 | 35.1 |
| Zn | 5–300 | 5.8–260 | 93–182 | 23 | 41.4 |
| Ga | - | - | 14–23 | 5.8 | 6.55 |
| Li | - | - | <10–53 | - | 31.8 |
| Sr | - | - | 282–1106 | 110 | 140 |
| C (%) | δ13C (‰) | N (%) | δ15N (‰) | |
|---|---|---|---|---|
| AK4 | 9.98 | −25.66 | 0.31 | 3.9 |
| AK5 | 19.07 | −25.78 | 0.5 | 3.6 |
| AK6 | 24.02 | −25.91 | 0.67 | 4.3 |
| AK8 | 17.95 | −25.86 | 0.48 | 3.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kara, H.; Kalender, L.; Yumutgan, M.Ç. Trace Element Geochemistry and Stable Isotopic (δ13C and δ15N) Characterisation of Nevşehir Coals, Türkiye. Minerals 2025, 15, 151. https://doi.org/10.3390/min15020151
Kara H, Kalender L, Yumutgan MÇ. Trace Element Geochemistry and Stable Isotopic (δ13C and δ15N) Characterisation of Nevşehir Coals, Türkiye. Minerals. 2025; 15(2):151. https://doi.org/10.3390/min15020151
Chicago/Turabian StyleKara, Hatice, Leyla Kalender, and Mehmet Çağay Yumutgan. 2025. "Trace Element Geochemistry and Stable Isotopic (δ13C and δ15N) Characterisation of Nevşehir Coals, Türkiye" Minerals 15, no. 2: 151. https://doi.org/10.3390/min15020151
APA StyleKara, H., Kalender, L., & Yumutgan, M. Ç. (2025). Trace Element Geochemistry and Stable Isotopic (δ13C and δ15N) Characterisation of Nevşehir Coals, Türkiye. Minerals, 15(2), 151. https://doi.org/10.3390/min15020151