Magnetite Texture and Geochemistry in the Takab Ore Deposit (NW Iran): Implications for a Complex Hydrothermal Evolution
Abstract
1. Introduction
2. Materials and Methods
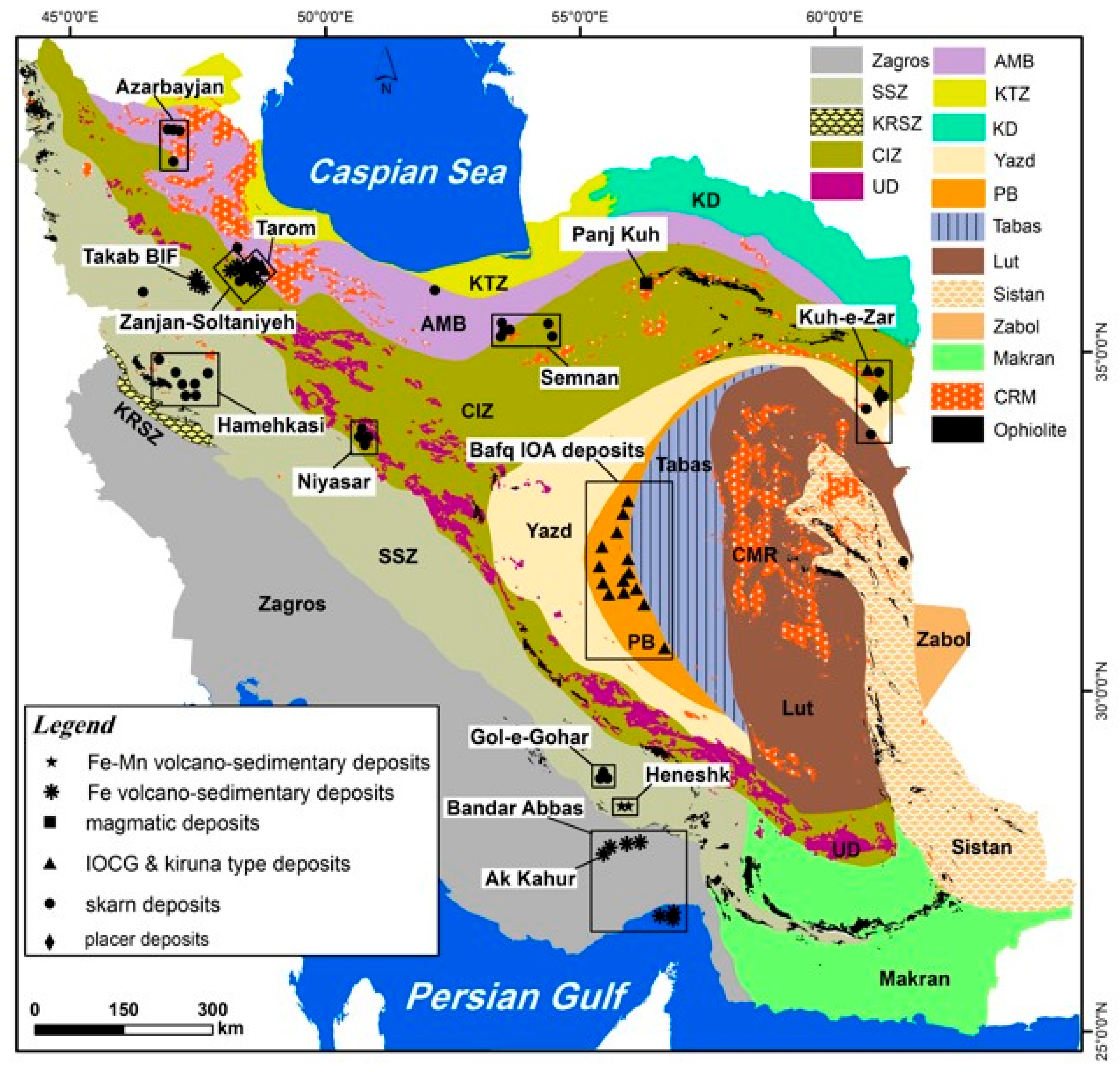
2.1. Geological Settings
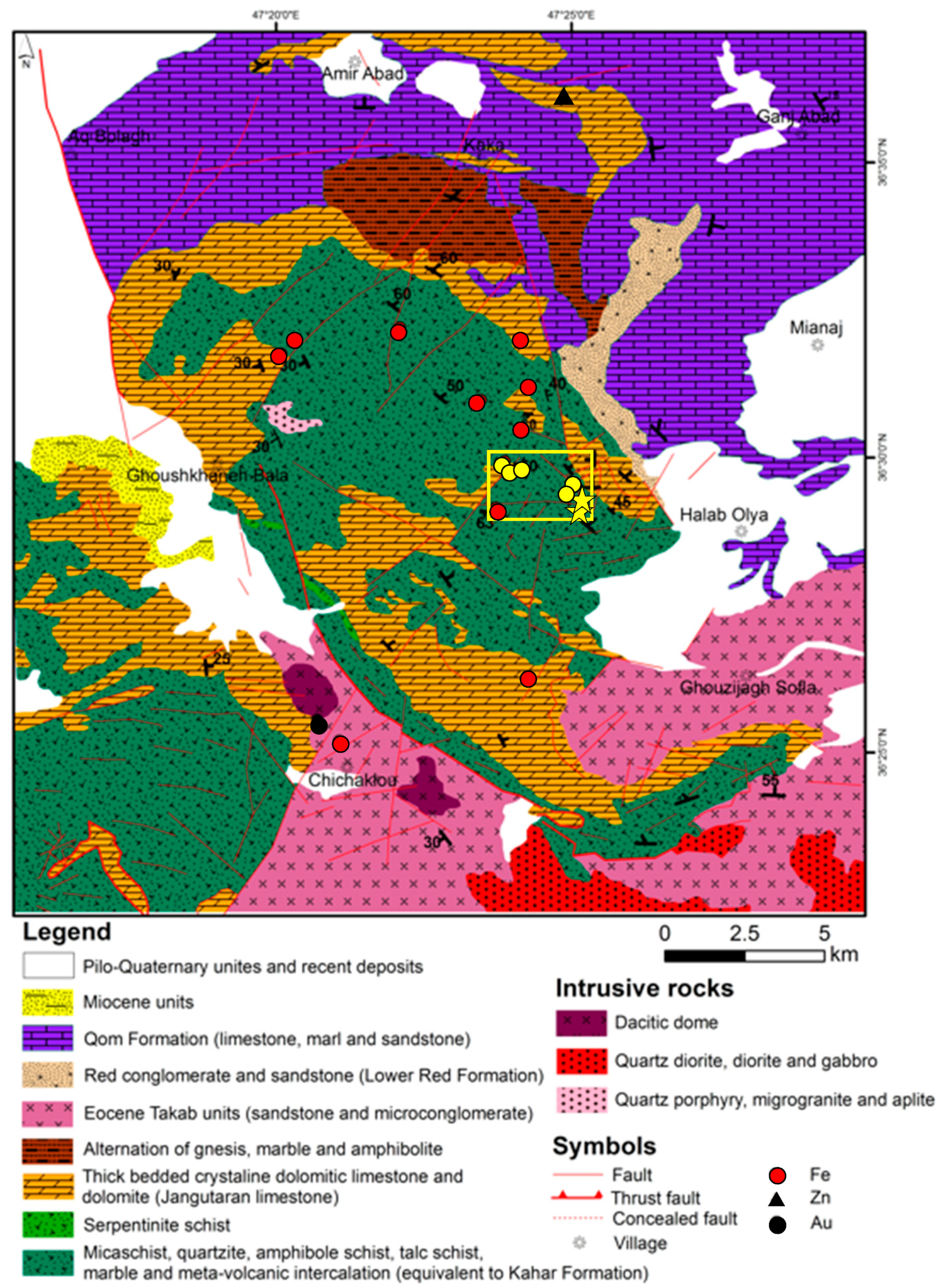
2.2. Ore Deposit and Sample Material
2.3. Analytical Methods
2.3.1. Electron Microprobe Analysis
2.3.2. Scanning Electron Microscopy and Electron Backscattered Diffraction Analysis
3. Results
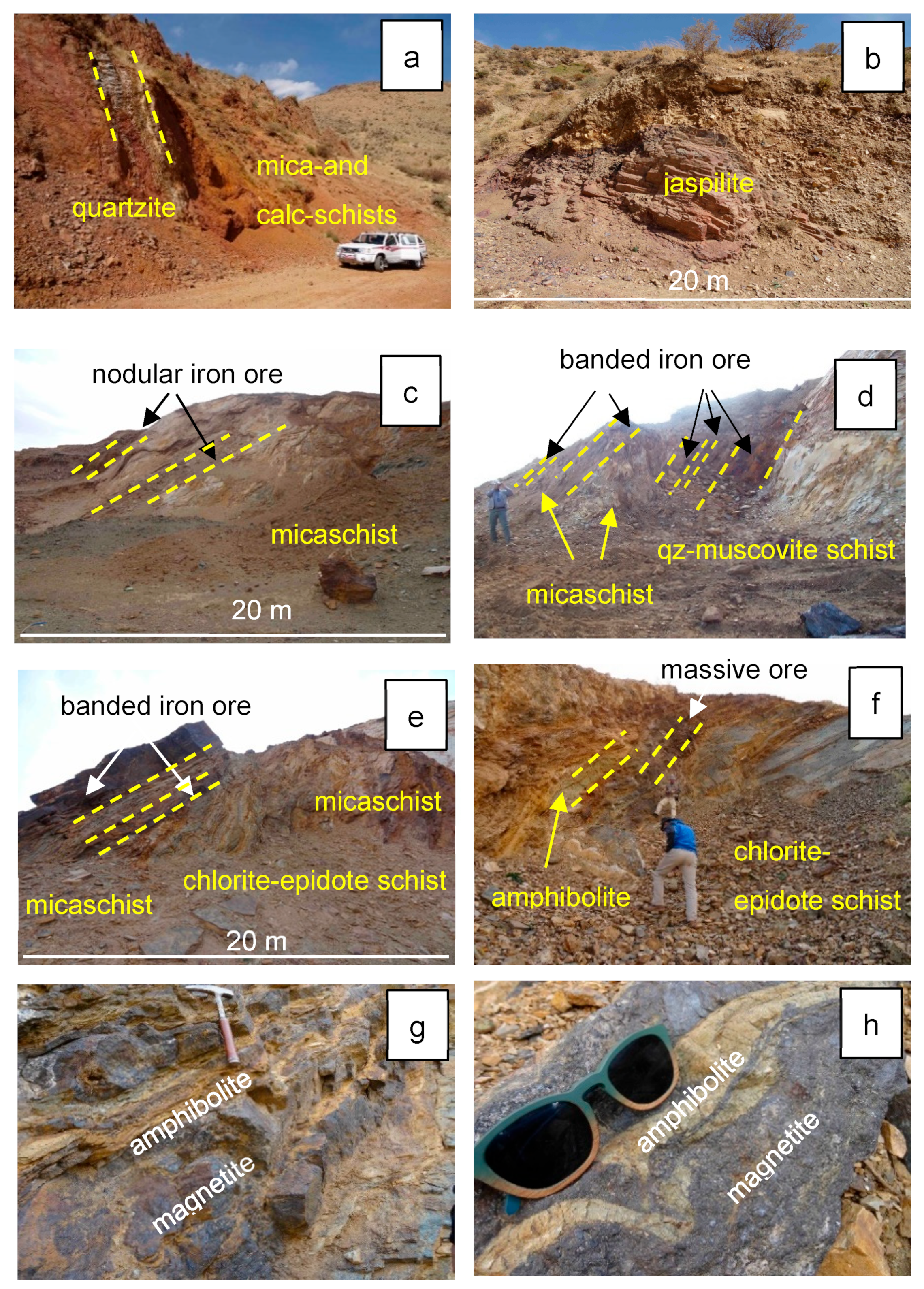
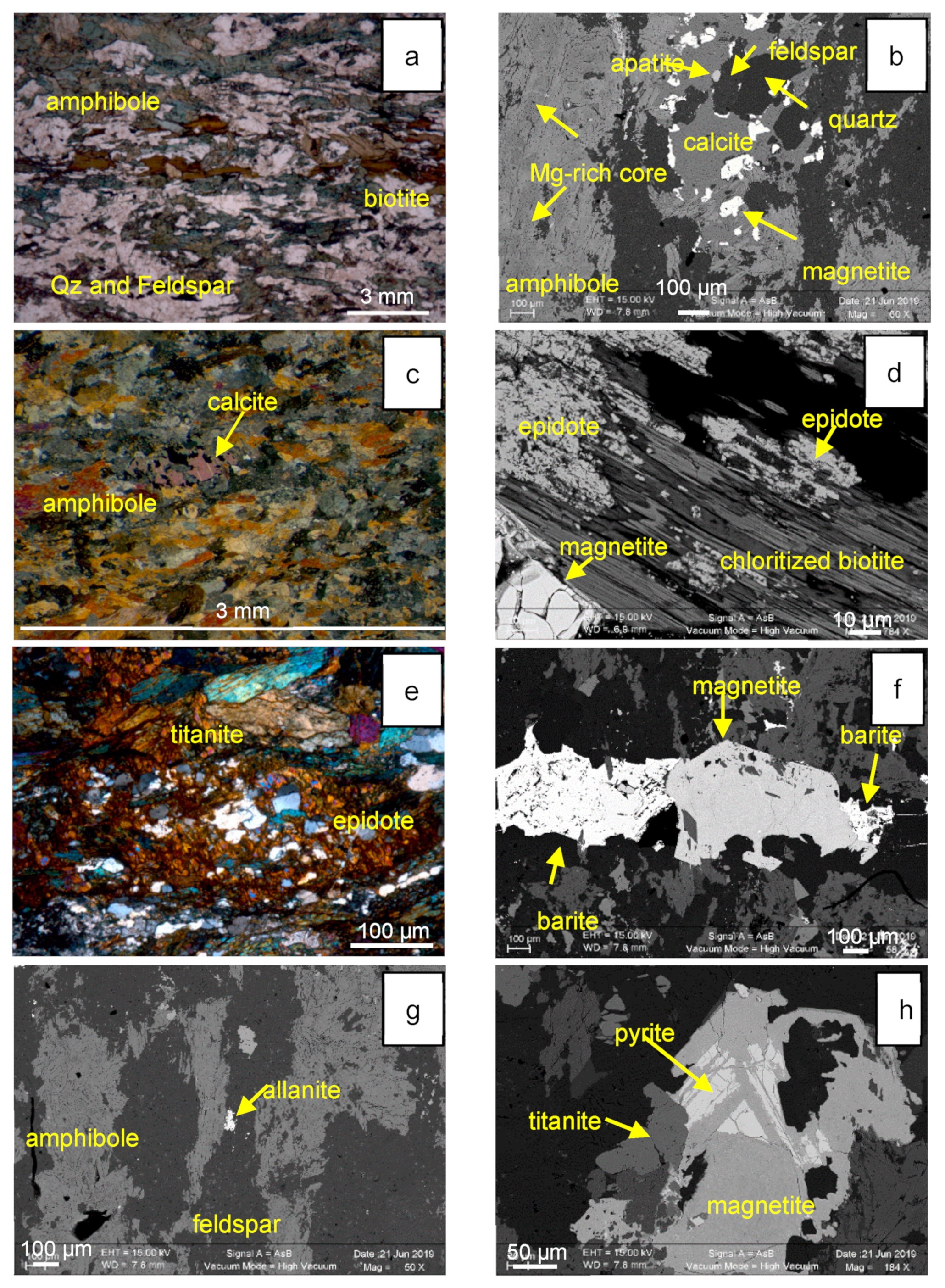
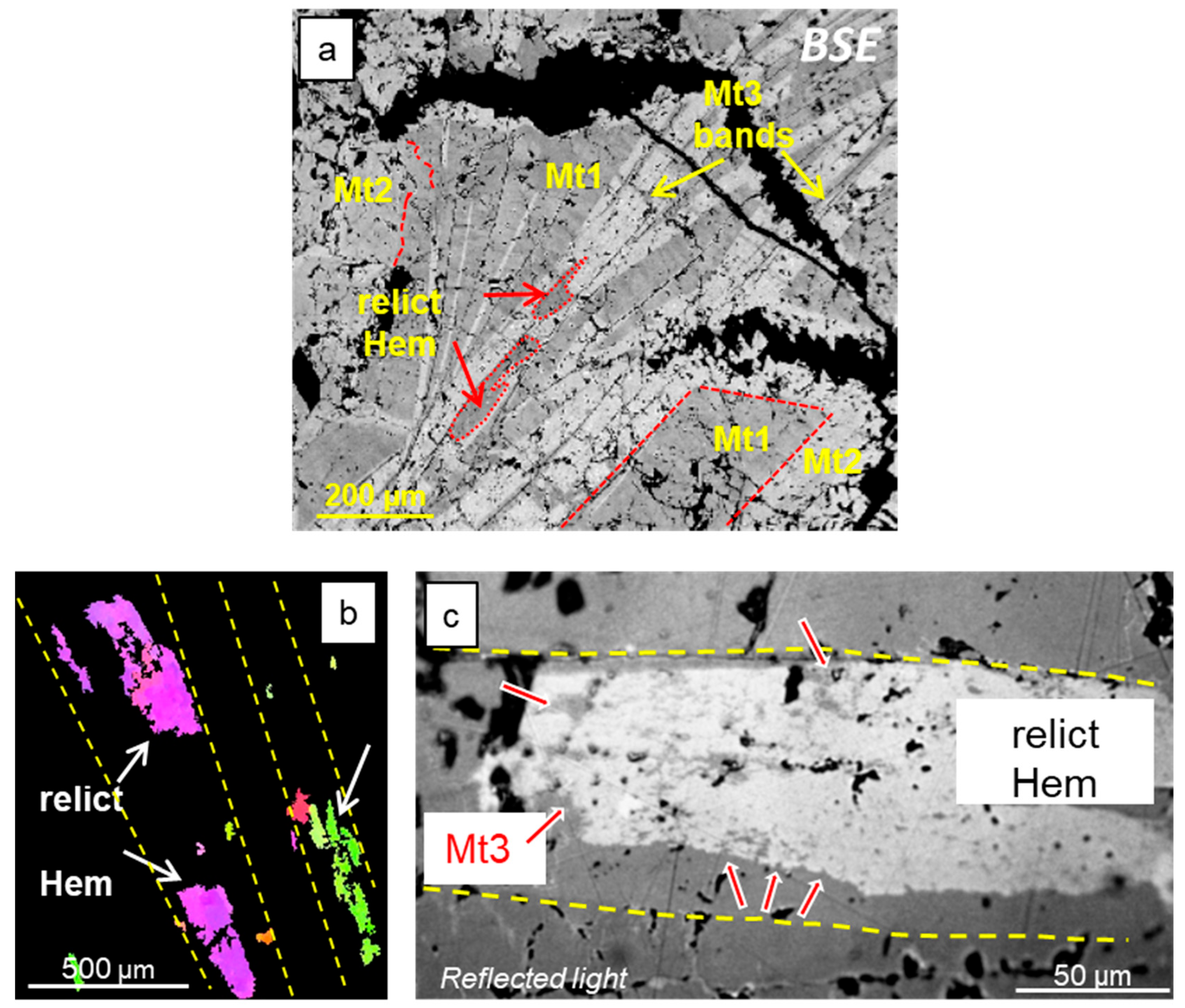
3.1. Morphology and Textures of Magnetite
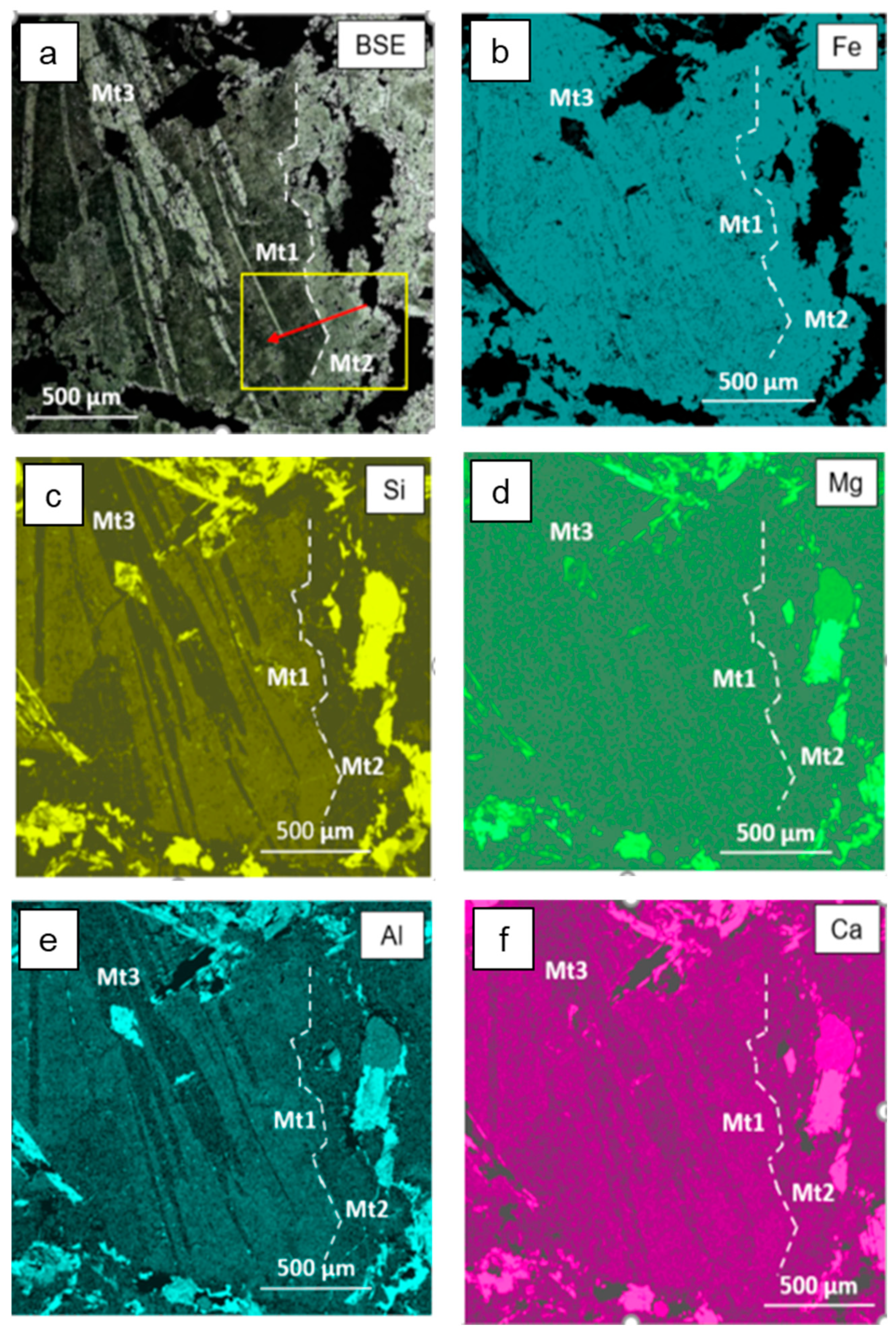
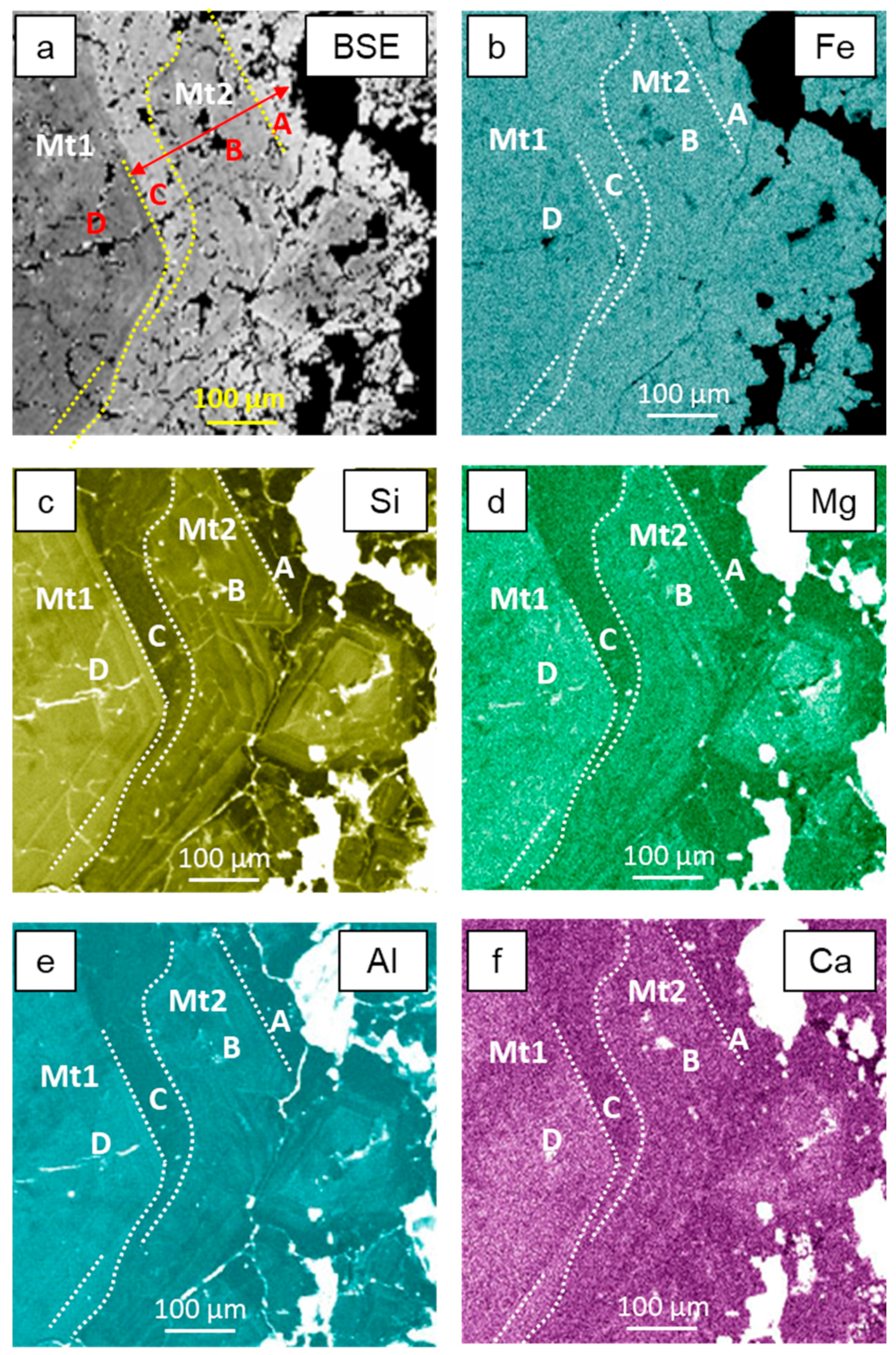
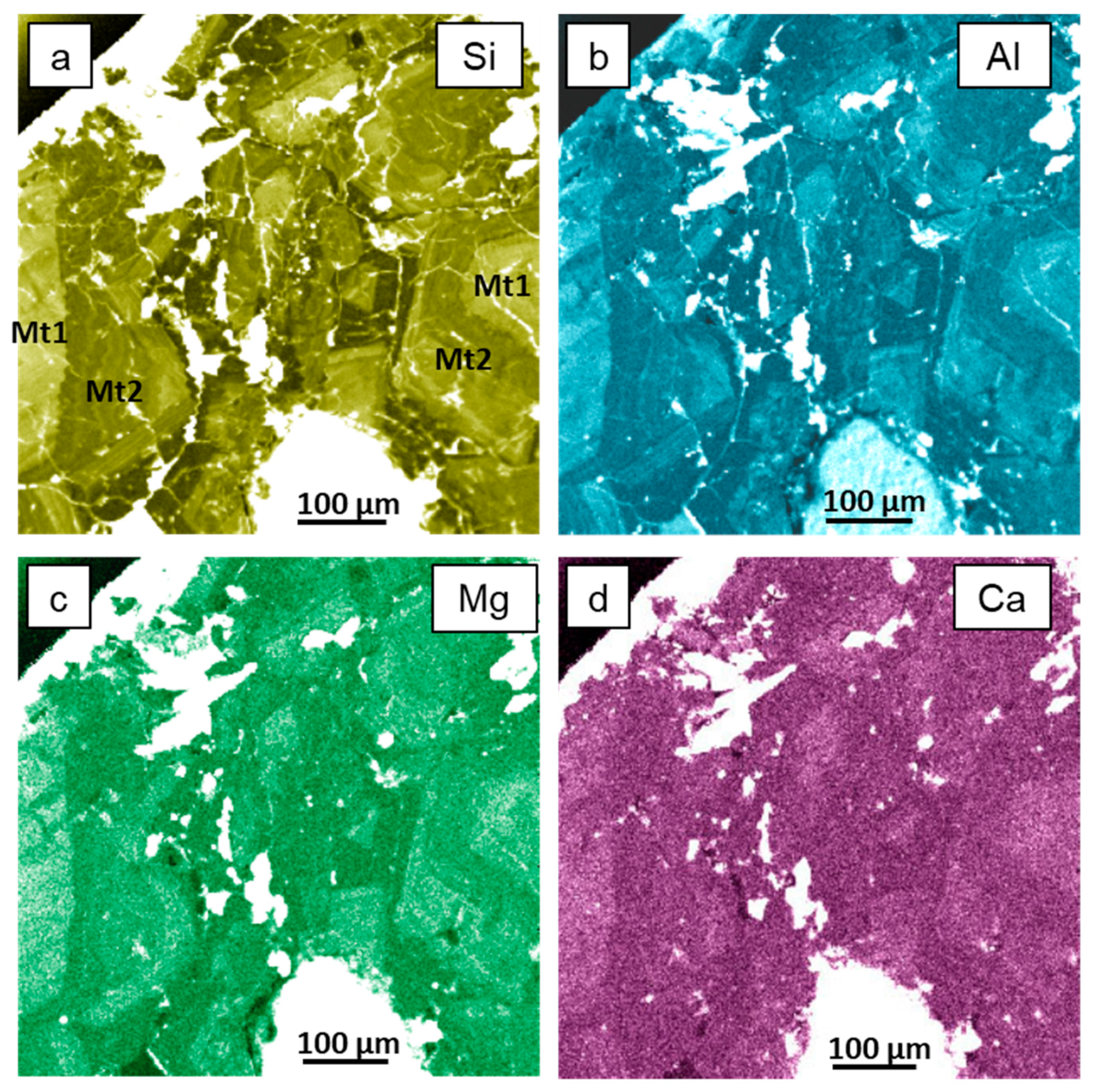
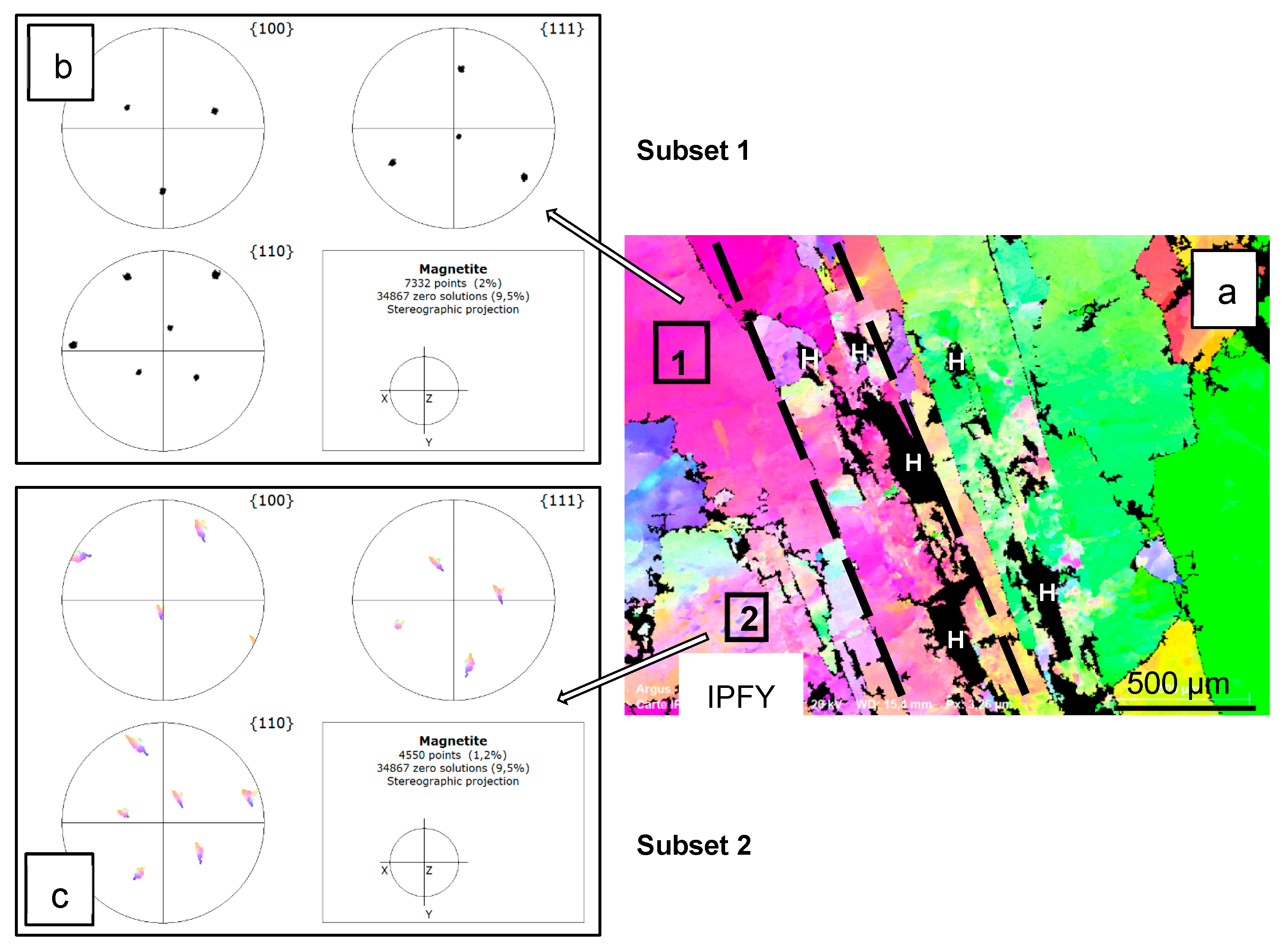
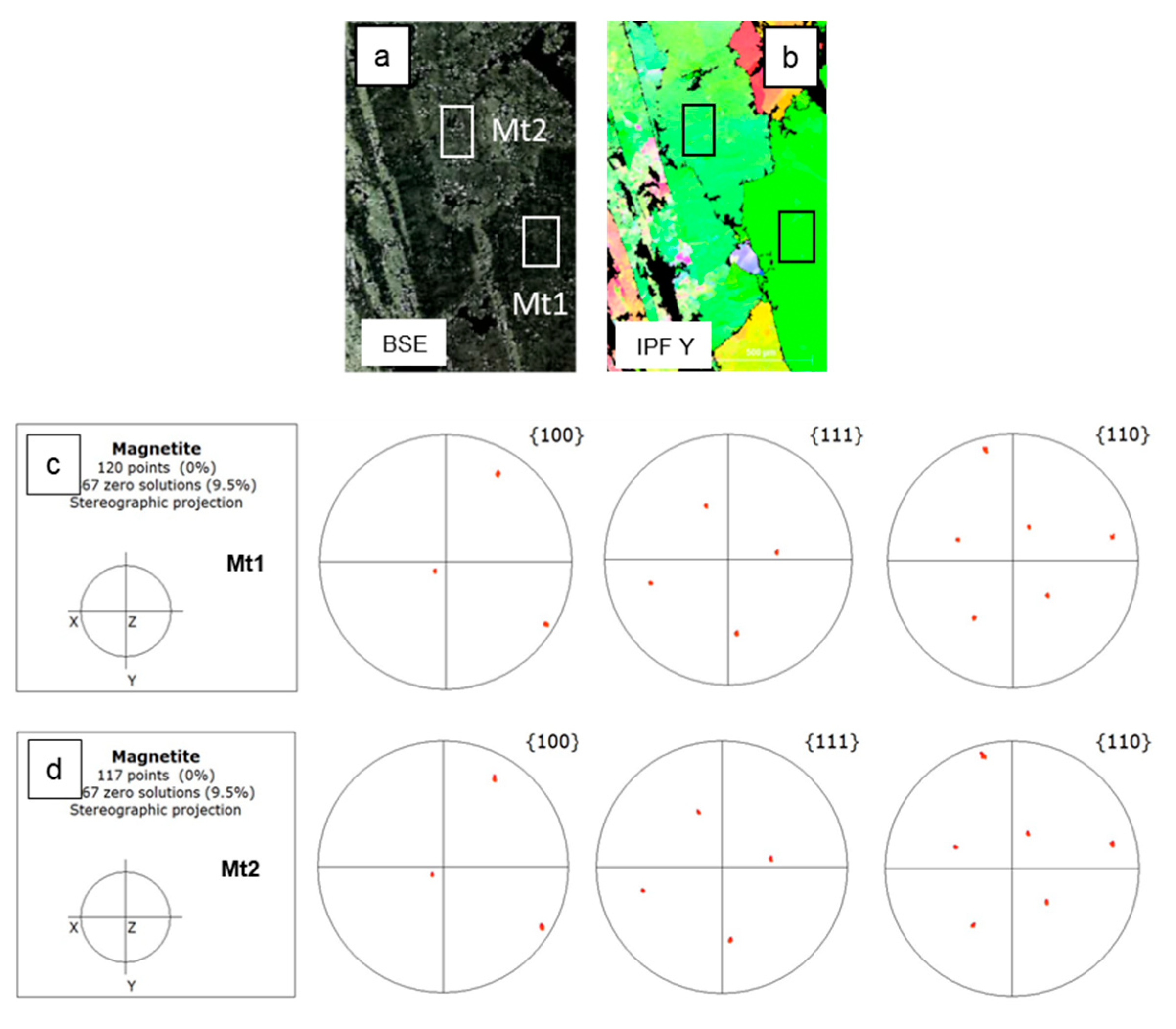
3.2. Electron Backscatter Diffraction Mapping
3.3. Chemical Composition of Magnetite
4. Discussion
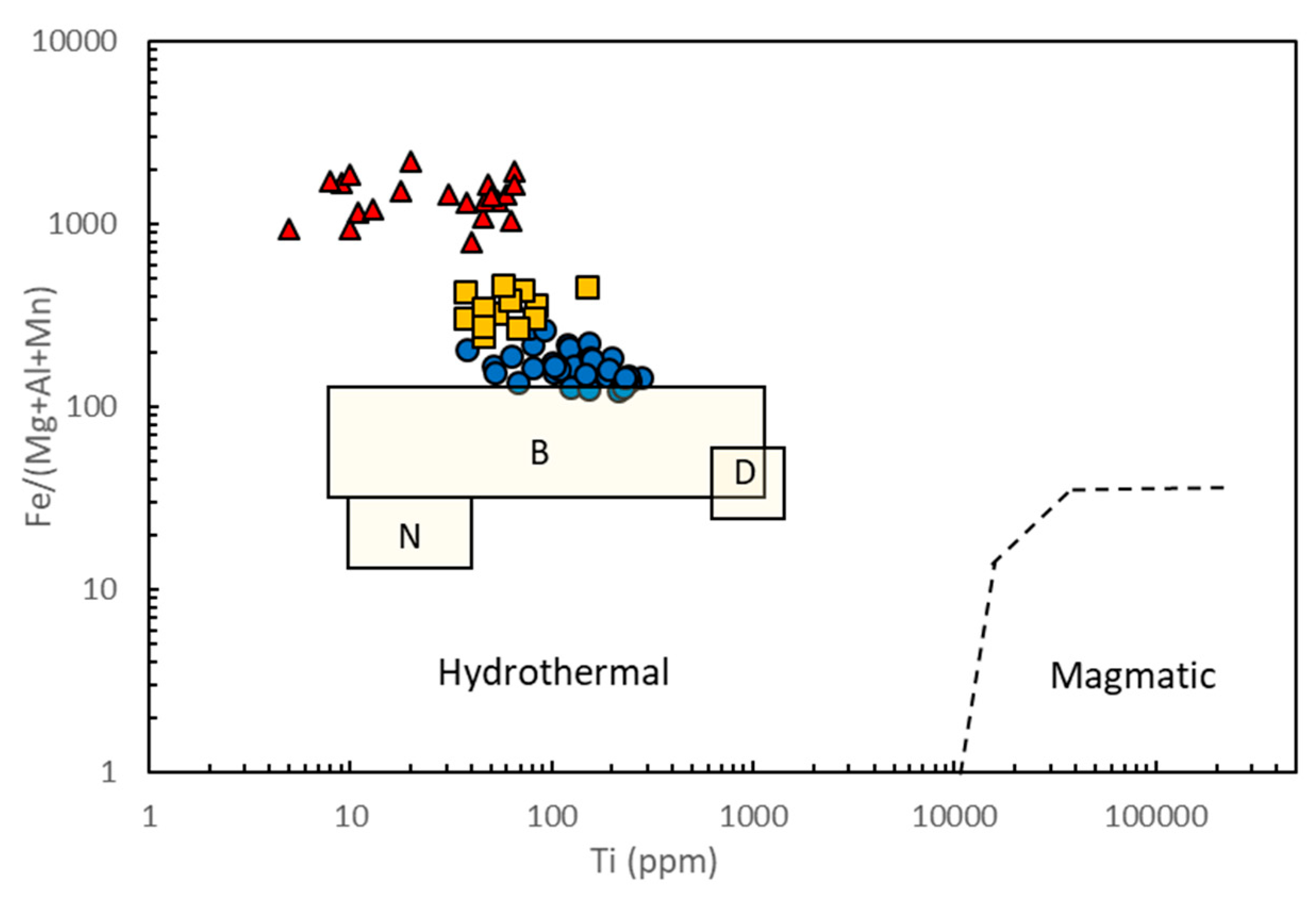
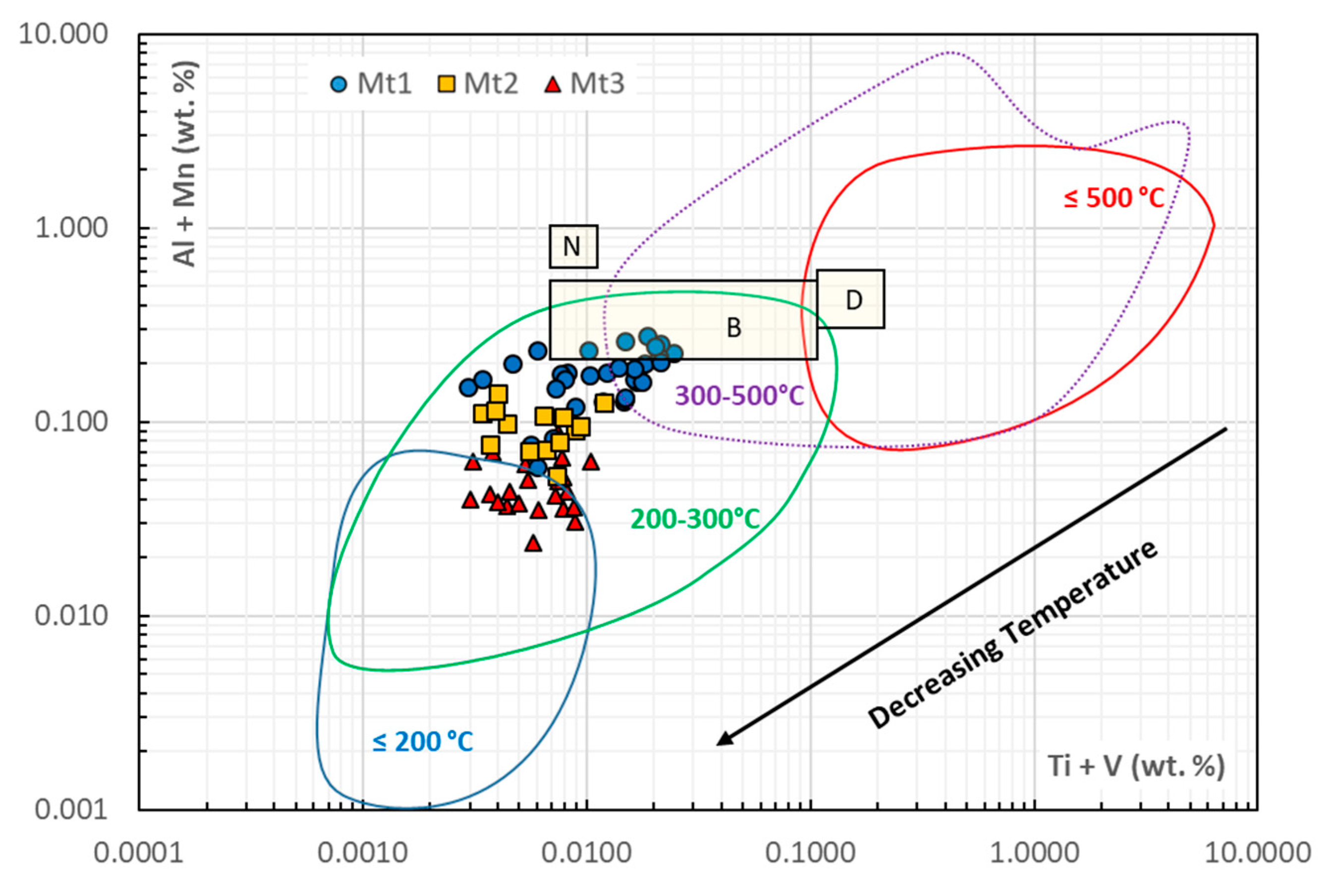
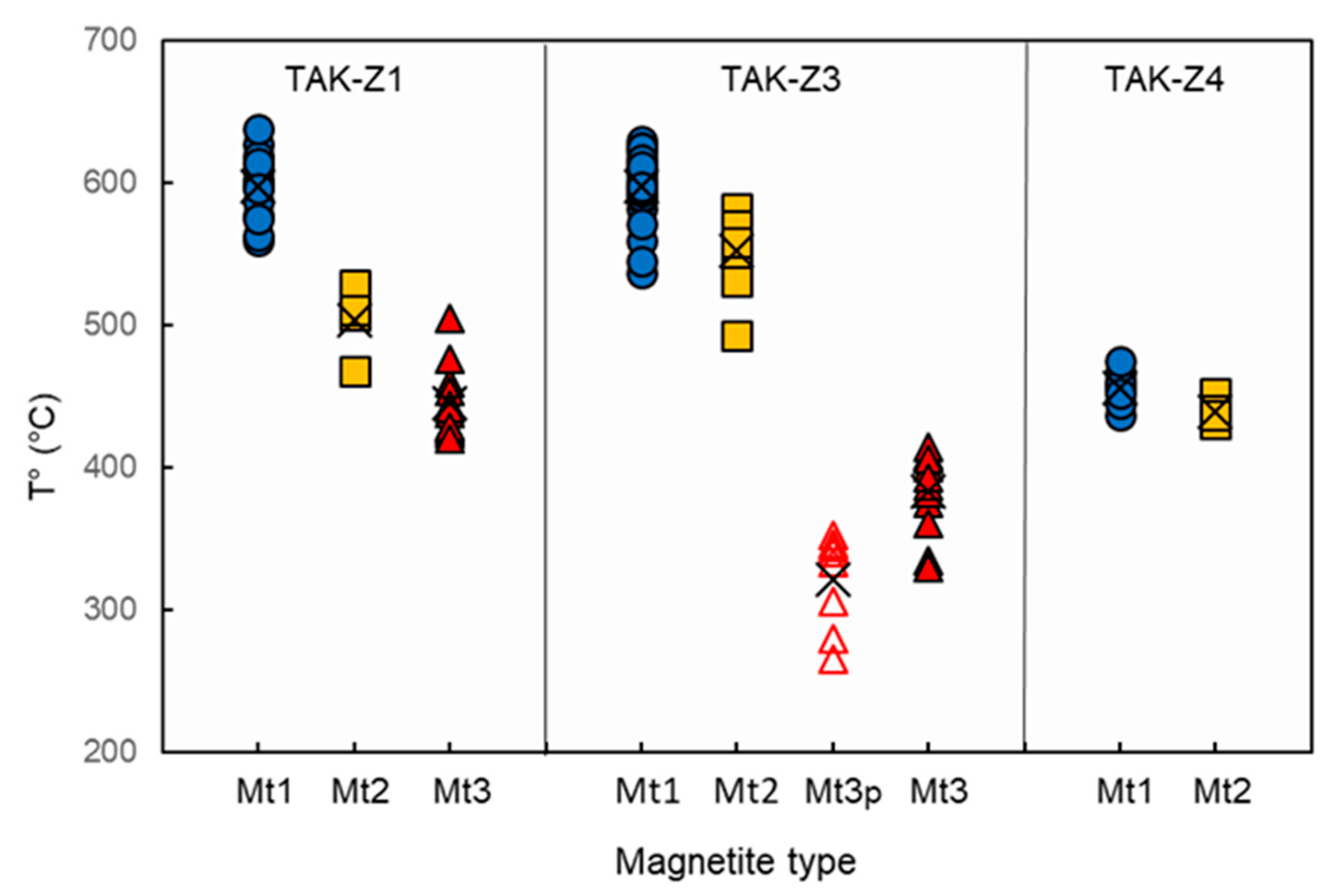
4.1. Magnetite Formation Conditions
4.2. Occurrence of Si and Elemental Substitution Mechanisms in Magnetite Mt1
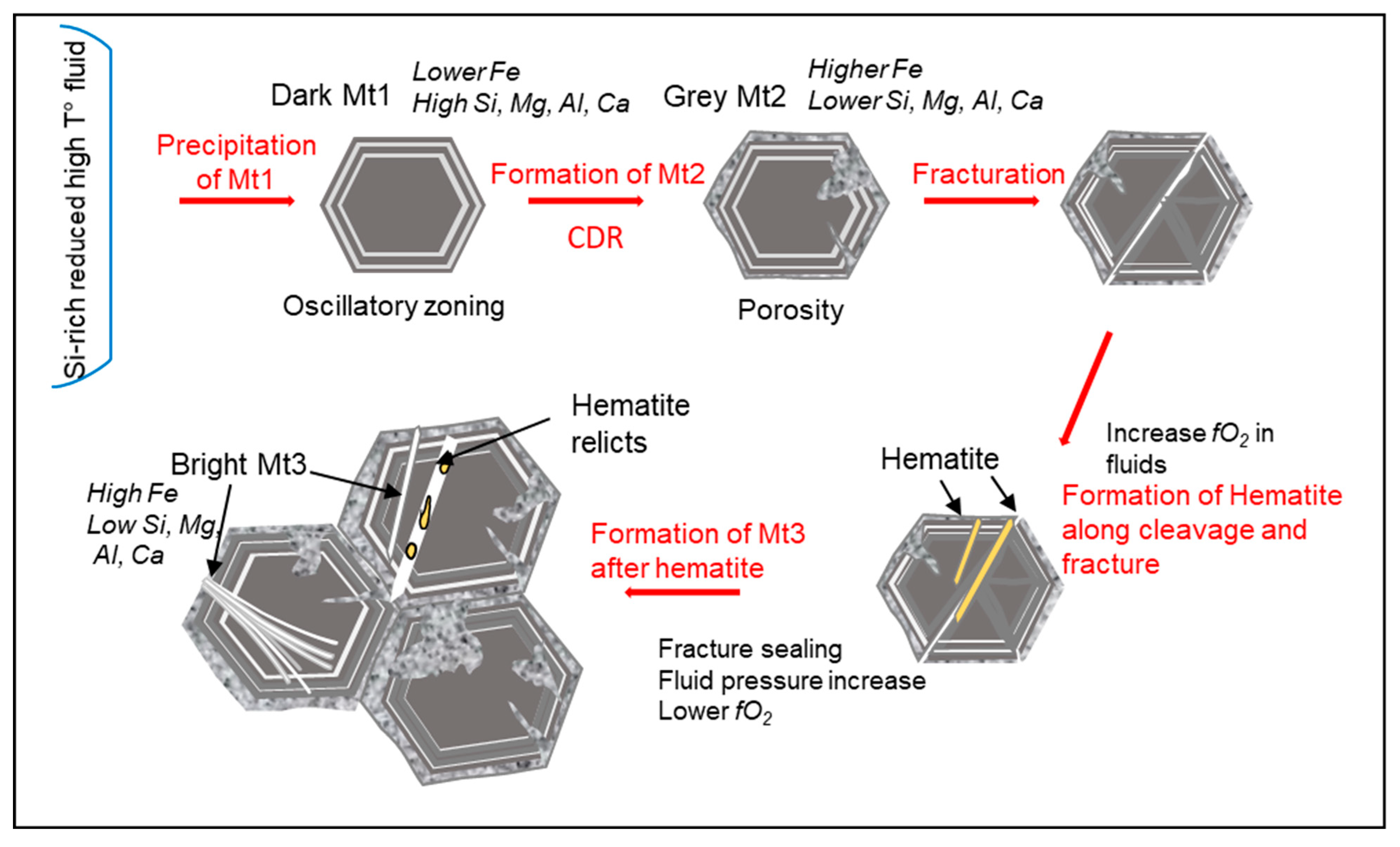
4.3. Dissolution–Reprecipitation Replacement of Mt1 by Mt2
4.4. Origin of Oscillatory Zoning in Magnetite Mt1
4.5. Origin of Magnetite Mt3 (Mushketovite)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dupuis, C.; Beaudouin, G. Discriminant diagrams for iron oxide trace element finger printing of mineral deposit types. Miner. Deposita 2011, 46, 319–335. [Google Scholar] [CrossRef]
- Dare, S.A.S.; Barnes, S.-J.; Beaudoin, G.; Méric, J.; Boutroy, E.; Potvin-Doucet, C. Trace elements in magnetite as petrogenetic indicators. Miner. Deposita 2014, 49, 785–796. [Google Scholar] [CrossRef]
- Nadoll, P.; Angerer, T.; Mauk, J.L.; French, D.; Walshe, J. The chemistry of hydrothermal magnetite: A review. Ore Geol. Rev. 2014, 16, 1–32. [Google Scholar] [CrossRef]
- Hu, X.; Chen, H.; Beaudouin, G.; Zhang, Y. Textural and compositional evolution of iron oxides at Mina Justa (Peru): Implications for mushketovite and formation of IOGC deposits. Am. Mineral. 2020, 105, 397–408. [Google Scholar] [CrossRef]
- Huang, X.W.; Beaudoin, G. Textures and chemical compositions of magnetite from iron oxide copper-gold (IOCG) and Kiruna-type iron oxide-apatite deposits and their implications for ore genesis and magnetite classification schemes. Econ. Geol. 2019, 114, 953–979. [Google Scholar] [CrossRef]
- Zhang, Y.; Hollings, P.; Shao, Y.; Li, D.; Chen, H.; Li, H. Magnetite texture and trace-element geochemistry fingerprint of pulsed mineralization in the Xinqiao Cu-Fe-Au deposit, Eastern China. Am. Mineral. 2020, 105, 1712–1723. [Google Scholar] [CrossRef]
- Liang, P.; Wu, C.; Hu, X.; Xie, Y. Textures and geochemistry of magnetite: Indications for genesis of the Late Paleozoic Laoshankou Fe-Cu-Au deposit, NW China. Ore Geol. Rev. 2020, 124, 103632. [Google Scholar] [CrossRef]
- Honarmand, M.; Nabatian, N.; Wagner, C.; Monsef, I.; Delpech, G.; Bayon, G.; Orberger, B. Late Ediacaran iron formation, NW Iran: Origin, depositional age and tectonic/climate significance. Precambrian Res. 2024, 406, 107382. [Google Scholar] [CrossRef]
- Karimpour, M. Applied Economic Geology; Javid Publication: Mashhad, Iran, 1989; 404p. [Google Scholar]
- Nabatian, G.; Rastad, E.; Neubauer, F.; Honarmand, M.; Ghaderi, M. Iron and Fe-Mn mineralisation in Iran: Implications for Tethyan metallogeny. Aust. J. Earth Sci. 2015, 62, 211–241. [Google Scholar] [CrossRef]
- Tuck, C.C. Iron ore. In Mineral Commodity Summaries; USGS Reports; USGS Reports; National Minerals Information Center: Reston, VA, USA, 2023. [Google Scholar]
- Foster, H.; Jafarzadeh, A. The Bafq mining district in Central Iran—A highly mineralized Infracambrian volcanic field. Econ. Geol. 1994, 89, 1697–1721. [Google Scholar] [CrossRef]
- Mazaheri, S.A.; Andrew, A.S.; Chenhall, B.E. Petrological Studies of Sangan Iron Ore Deposit; Center for Isotope Studies Research Report: Sydney, NSW, Australia, 1994; pp. 48–52. [Google Scholar]
- Daliran, F. Kiruna-Type Iron Oxide-Apatite Ores and Apatitites of the Bafq District, Iran, with an Emphasis on the REE Geochemistry of Their Apatites. In Hydrothermal Iron Oxide Copper Gold and Related Deposits. A Global Perspective; Porter, T.M., Ed.; PGC Publishing: Adelaide, SA, Australia, 2002; Volume 2, pp. 303–320. [Google Scholar]
- Daliran, F.; Stosch, H.G.; Williams, P.; Jamli, H.; Dorri, M.B. Lower Cambrian Iron Oxide-Apatite-REE (U) Deposits of the Bafq District, East-Central Iran. In Exploring for Iron Oxide Copper-Gold Deposits: Canada and Global Analogues; Corriveau, L., Mumin, H., Eds.; Geological Association of Canada Short Course Notes: St John’s, NL, Canada, 2010; Volume 20, pp. 143–155. [Google Scholar]
- Alavi, M. Sedimentary and structural characteristics of the Paleo-Tethys remnants in northeastern Iran. GSA Bull. 1991, 103, 983–992. [Google Scholar] [CrossRef]
- Berberian, M.; King, G.C.P. Towards a paleogeography and tectonic evolution of Iran. Can. J. Earth Sci. 1981, 18, 210–265. [Google Scholar] [CrossRef]
- Jafari, A.; Karimpour, M.H.; Mazaheri, S.A.; Shafaroudi, A.M.; Ren, M. Geochemistry of metamorphic rocks and mineralization in the Gol-Gohar iron ore deposit (No. 1), Sirjan, SE Iran: Implications for Paleotectonic setting and ore genesis. J. Geochem. Explor. 2019, 205, 106330. [Google Scholar] [CrossRef]
- Nabatian, G.; Ghaderi, M.; Daliran, F.; Rashidnejad-Omran, N. Sorkhe-Dizaj iron oxide-apatite ore deposit in the Cenozoic Alborz-Azarbaijan magmatic belt, NW Iran. Resour. Geol. 2013, 63, 42–56. [Google Scholar] [CrossRef]
- Nabatian, G.; Li, X.-H.; Honarmand, M.; Melgarejo, J.C. Geology, mineralogy and evolution of iron skarn deposits in the Zanjan district, NW Iran: Constraints from U-Pb dating, Hf and O isotope analyses of zircons and stable isotope geochemistry. Ore Geol. Rev. 2017, 84, 42–66. [Google Scholar] [CrossRef]
- Sarjoughian, F.; Habibi, H.; Lentz, D.R.; Azizi, H.; Esna-Ashari, A. Magnetite compositions from the Baba Ail iron deposit in the Sanandaj-Sirjan zone, western Iran: Implications for ore genesis. Ore Geol. Rev. 2020, 126, 103728. [Google Scholar] [CrossRef]
- Maanijou, M.; Khodaei, L. Mineralogy and electron microprobe studies of magnetite in the Sarab-3 iron Ore deposit, southwest of the Shahrak mining region (east Takab). J. Econ. Geol. 2018, 10, 267–293. (In Persian) [Google Scholar]
- Maanijou, M.; Salemi, R. Mineralogy, chemistry of magnetite and genesis of Korkora-1 iron deposit, east of Takab, NW Iran. J. Econ. Geol. 2014, 6, 355–374. (In Persian) [Google Scholar]
- Ghaderi Piraghoum, Z.; Kouhestani, H.; Tofighi, F. Geology, geochemistry and genesis of the Kosaj Fe occurrence, Takab‒Takht-e Soleiman‒Angouran metallogenic zone, SW Zanjan. Adv. Appl. Geol. 2020, 10, 294–313, (In Persian with English abstract). [Google Scholar]
- Pourmohammad, F.; Kouhestani, H.; Azimzadeh, A.M.; Nabatian, G.; Mokhtari, M.A.A. Mianaj iron occurrence, southwest of Zanjan: Metamorphosed and deformed volcano-sedimentary type of mineralization in Sanandaj-Sirjan zone. Sci. Q. J. Geosci. 2019, 28, 161–174, (In Persian with English abstract). [Google Scholar]
- Biparva, M. Geochemistry, Mineralogy and Genesis of Fe Deposits, East of Takab (Halab, Mianaj and Quzijan Villages). Master’s Thesis, Department of Geology, Shahid Beheshti University, Teheran, Iran, 2013. Unpublished (In Persian with English abstract). [Google Scholar]
- Fereiduni, Z. Geology, Mineralogy and Geochemistry of Halab Iron Mineralization, SW Zanjan. Master’s Thesis, Department of Geology, University of Zanjan, Zanjan, Iran, 2017. Unpublished (In Persian with English abstract). [Google Scholar]
- Wagner, C.; Villeneuve, J.; Boudouma, O.; Rividi, N.; Orberger, B.; Nabatian, G.; Honarmand, M.; Monsef, I. In Situ Trace Element and Fe-O Isotope Studies on Magnetite of the Iron-Oxide Ores from the Takab Region, North Western Iran: Implications for Ore Genesis. Minerals 2023, 13, 774. [Google Scholar] [CrossRef]
- Hassanzadeh, J.; Stockli, D.F.; Horton, B.K.; Axen, G.J.; Stockli, L.D.; Grove, M.; Schmitt, A.K.; Walker, J.D. U-Pb zircon geochronology of late Neoproterozoic-early Cambrian granitoids in Iran: Implications for paleogeography, magmatism, and exhumation history of Iranian basement. Tectonophysics 2008, 451, 71–96. [Google Scholar] [CrossRef]
- Hajialioghli, R.; Moazzen, M.; Jahangiri, A.; Oberhansli, R.; Mocek, B.; Altenberger, U. Petrogenesis and tectonic evolution of metaluminous sub-alkaline granitoids from the Takab complex, NW Iran. Geol. Mag. 2011, 148, 250–268. [Google Scholar] [CrossRef]
- Moazzen, M.; Hajialioggli, R. Zircon SHRIMP Dating of Mafic Migmatites from NW Iran: Reporting the Oldest Rocks from the Iranian Crust. In Proceedings of the 5th Annual Meeting AOGS, Busan, Korea, 16–20 June 2008. [Google Scholar]
- Babakhani, A.R.; Ghalamghash, J. Explanatory Text of Takht-e-Soleiman. In Geological Quadrangle Map 1:100000, No.5463; Geological Survey of Iran: Tehran, Iran, 2005. (In Persian) [Google Scholar]
- Till, J.L.; Moskowitz, B.M. Deformation microstructures and magnetite texture development in synthetic shear zones. Tectonophysics 2014, 629, 211–223. [Google Scholar] [CrossRef]
- Hu, X.; Xiao, B.; Jiang, H.; Huang, J. Magnetite texture and trace element evolution in the Shaquanzi Fe-Cu deposit, Eastern Tianshan, NW China. Ore Geol. Rev. 2023, 154, 105306. [Google Scholar] [CrossRef]
- Nielsen, R.L.; Forsythe, L.M.; Gallahan, W.E.; Fisk, M.R. Major element and trace element magnetite-melt equilibria. Chem. Geol. 1994, 117, 167–191. [Google Scholar] [CrossRef]
- Toplis, M.J.; Caroll, M.R. An experimental study of oxygen fugacity on Fe-Ti oxide stability, phase relations, and mineral-melt equilibria in ferro-basaltic systems. J. Petrol. 1995, 36, 1137–1170. [Google Scholar] [CrossRef]
- Deditius, A.P.; Reich, M.; Simon, A.C.; Suvorova, A.; Knipping, J.; Roberts, M.P.; Rubanov, S.; Dodd, A.; Saunders, M. Nanogeochemistry of hydrothermal magnetite. Contrib. Mineral. Petrol. 2018, 173, 46. [Google Scholar] [CrossRef]
- Xu, H.; Shen, Z.; Konishi, H. Si-magnetite nano-precipitates in silician magnetite from banded iron formation: Z-contrast imaging and ab-initio study. Amer. Mineral. 2014, 99, 2196–2202. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Verdugo-Ihl, M.R.; Slattery, A.; Cook, N.J.; Ehrig, K.; Courtney-Davies, L.; Wade, B.P. Silician Magnetite: Si–Fe-Nanoprecipitates and Other Mineral Inclusions in Magnetite from the Olympic Dam Deposit, South Australia. Minerals 2019, 9, 311. [Google Scholar] [CrossRef]
- Canil, D.; Lacourse, T. Geothermometry using minor and trace elements in igneous and hydrothermal magnetite. Chem. Geol. 2020, 541, 119576. [Google Scholar] [CrossRef]
- Huberty, J.M.; Konishi, H.; Heck, P.R.; Fournelle, J.H.; Valley, J.W.; Xu, H. Silician magnetite from the Dales Gorge Member of the Brockman Iron Hamersley Group, Western Australia. Am. Mineral. 2012, 97, 26–37. [Google Scholar] [CrossRef]
- Whittaker, E.J.W.; Muntus, R. Ionic radii for use in geochemistry. Geochim. Cosmochim. Acta 1970, 34, 945–956. [Google Scholar] [CrossRef]
- Hu, X.; Chen, H.; Zhang, W. Texture and composition of magnetite in the Duotoushan deposit, NW China: Implications for ore genesis of Fe-Cu deposits. Min. Mag. 2020, 84, 398–411. [Google Scholar] [CrossRef]
- Shimazaki, H. On the occurrence of silician magnetite. Resour. Geol. 1998, 48, 23–29. [Google Scholar] [CrossRef]
- Ohkawa, M.; Miyahara, M.; Ohta, E.; Hoshino, K. Silicon-substituted magnetite and accompanying iron oxides and hydroxides from the Kumano mine, Yamaguchi Prefecture, Japan: Reexamination of the so-called maghemite (g-Fe2O3). J. Mineral. Petrol. Sci. 2007, 102, 182–193. [Google Scholar] [CrossRef]
- Sun, S.; Li, Y.-L. Microstructures, crystallography and geochemistry of magnetite in 2500 to 2200 million-year old banded iron formation from South Africa, Western Australian and North China. Prec. Res. 2017, 298, 292–305. [Google Scholar] [CrossRef]
- Putnis, A. Mineral replacement reactions. In Thermodynamics and Kinetics of Water-Rock Interaction; Oelkers, E., Schott, J., Eds.; Reviews in Mineralogy & Geochemistry, Mineralogical Society of America: Chantilly, VA, USA, 2009; Volume 70, pp. 87–124. [Google Scholar]
- Putnis, A. Transient porosity resulting from fluid-mineral interaction and its consequences. In Pore-Scale Geochemical Processes; Steefel, C.I., Emmanuel, S., Anovitz, L.M., Eds.; Reviews in Mineralogy & Geochemistry, Mineralogical Society of America: Chantilly, VA, USA, 2009; Volume 80, pp. 1–23. [Google Scholar]
- Altree-Williams, A.; Pring, A.; Ngothai, Y.; Brugger, J. Textural and compositional complexities resulting from coupled dissolution-reprecipitation reactions in geomaterials. Earth Sci. Rev. 2015, 150, 628–651. [Google Scholar] [CrossRef]
- Hu, H.; Li, J.W.; Lentz, D.; Ren, Z.; Zhao, X.F.; Deng, X.D.; Hall, D. Dissolution-re precipitation process of magnetite from the Chengchao iron deposit: Insights into ore genesis and implication for in-situ chemical analysis of magnetite. Ore Geol. Rev. 2014, 57, 393–405. [Google Scholar] [CrossRef]
- Hu, H.; Lentz, D.; Li, J.-W.; McCarron, T.; Zhao, X.-F.; Hall, D. Reequilibration processes in magnetite from iron skarn deposits. Econ. Geol. 2015, 110, 1–8. [Google Scholar] [CrossRef]
- Yin, S.; Ma, C.Q.; Robinson, P.T. Textures and high field strength elements in hydrothermal magnetite from a skarn system: Implications for coupled dissolution-reprecipitation reactions. Am. Miner. 2017, 102, 1045–1056. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, H.; Xiao, B.; Zhao, L.; Hu, X.; Li, J.; Gong, L. Textural and chemical evolution of magnetite from the Paleozoic Shuanglong Fe-Cu deposit: Implications for tracing ore-forming fluids. Am. Miner. 2023, 108, 178–191. [Google Scholar] [CrossRef]
- Ohmoto, H. Nonredox transformations of magnetite-hematite in hydrothermal systems. Econ. Geol. 2003, 98, 157–161. [Google Scholar] [CrossRef]
- Mücke, A.; Cabral, A.R. Redox and non-redox reactions of magnetite and hematite in rocks. Geochemistry 2005, 65, 271–278. [Google Scholar] [CrossRef]



| Host | Iron Ore | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Actinolite | Tr | Mhb | Actinolite | ||||||||||
| core | core | acicular crystals | core | ||||||||||
| SiO2 | 54.44 | 51.83 | 50.8 | 53.9 | 55.97 | 55.97 | 57.32 | 49.36 | 47.34 | 48.45 | 52.55 | 51.75 | 55.93 |
| TiO2 | 0.12 | 0.11 | 0.08 | 0.1 | 0 | 0 | 0.05 | 0.37 | 0.41 | 0.43 | 0.05 | 0.06 | 0.03 |
| Al2O3 | 1.51 | 2.97 | 3.61 | 1.27 | 2.85 | 2.85 | 3.02 | 7.42 | 7.63 | 8.05 | 2.1 | 2.83 | 3.81 |
| Cr2O3 | 0.04 | 0.11 | 0.05 | 0.06 | 0.03 | 0.03 | 0.01 | 0.13 | 0.13 | 0.14 | 0.03 | 0 | 0.02 |
| FeO | 15.96 | 18.01 | 18.34 | 15.6 | 5.9 | 5.9 | 1.97 | 13.93 | 16.36 | 13.9 | 16.81 | 17.02 | 3.82 |
| MnO | 0.19 | 0.12 | 0.23 | 0.16 | 0.1 | 0.1 | 0.1 | 0.5 | 0.38 | 0.57 | 0.12 | 0.12 | 0 |
| MgO | 13.95 | 12.53 | 12.31 | 14.04 | 20.21 | 20.21 | 22.53 | 13.79 | 12.4 | 13.49 | 13.46 | 12.97 | 20.83 |
| CaO | 12.02 | 11.65 | 11.87 | 12.33 | 12 | 12 | 12.63 | 11.28 | 11.57 | 11.33 | 12.11 | 12.36 | 12.46 |
| Na2O | 0.45 | 0.67 | 0.84 | 0.38 | 1.18 | 1.18 | 1.13 | 1.5 | 1.28 | 1.48 | 0.49 | 0.64 | 1.38 |
| K2O | 0.08 | 0.17 | 0.16 | 0.1 | 0.26 | 0.26 | 0.08 | 0.16 | 0.34 | 0.15 | 0.15 | 0.13 | 0.17 |
| Total | 98.76 | 98.16 | 98.3 | 97.95 | 98.5 | 98.5 | 98.85 | 98.44 | 97.84 | 98 | 97.87 | 97.88 | 98.45 |
| mg# | 61.1 | 55.6 | 54.7 | 61.8 | 86.1 | 86.1 | 95.4 | 64.1 | 57.7 | 63.6 | 59 | 57.8 | 90.7 |
| Sample | TAK-Z1 | TAK-Z1 | TAK-Z1 | TAK-Z4 | TAK-Z4 | |
|---|---|---|---|---|---|---|
| Mt type | Mt1 | Mt2 | Mt3 | Mt1 | Mt2 | |
| n analyses | 12 | 4 | 10 | 8 | 6 | |
| Fe (wt. %) | Average | 68.0 | 69.8 | 70.8 | 67.6 | 69.6 |
| Range | 66.1–69.4 | 69.3–70.4 | 69.9–71.3 | 67.2–68.7 | 69.0–70.1 | |
| O (wt. %) | Average | 29.4 | 28.6 | 28.5 | 29.1 | 28.7 |
| Range | 29.0–29.7 | 28.2–28.8 | 28.0–29.1 | 28.0–29.6 | 27.8–29.1 | |
| Si | Average | 11,017 | 4336 | 214 | 9942 | 3550 |
| sd | 1107 | 409 | 195 | 1444 | 397 | |
| DL = 11 | Range | 8899–12779 | 3846–4840 | 11–426 | 8274–11,941 | 3149–4045 |
| Ti | Average | 130 | 86 | 26 | 293 | 104 |
| sd | 61 | 35 | 20 | 120 | 18 | |
| DL = 4 | Range | 51–276 | 37–126 | BDL-63 (2) | 149–474 | 73–106 |
| Al | Average | 2489 | 1031 | 275 | 2321 | 985 |
| sd | 479 | 76 | 94 | 364 | 173 | |
| DL = 12 | Range | 1428–3103 | 947–1120 | 187–434 | 1617–2773 | 818–1301 |
| Mn | Average | 262 | 227 | 261 | 279 | 223 |
| sd | 26 | 59 | 107 | 54 | 27 | |
| DL= 5 | Range | 198–289 | 161–305 | 166–519 | 198–368 | 193–273 |
| Mg | Average | 1263 | 410 | 34 | 1225 | 382 |
| sd | 320 | 134 | 26 | 378 | 131 | |
| DL = 19 | Range | 807–1882 | 234–560 | BDL-71 (4) | 825–1794 | 205–553 |
| Ca | Average | 2677 | 1289 | 313 | 2799 | 1192 |
| sd | 585 | 109 | 95 | 720 | 294 | |
| DL = 3 | Range | 1606–3529 | 1127–1349 | 194–484 | 1760–3821 | 1018–1155 |
| V | Average | 22 | 19 | 34 | nd | nd |
| sd | 10 | 5 | 6 | |||
| DL = 9 | Range | 13–43 | 12–24 | 22–42 | ||
| Sample | TAK-Z3 | TAK-Z3 | TAK-Z3 | TAK-Z3 | ||
| Mt type | Mt1 | Mt2 | Mt3 porous | Mt3 | ||
| n analyses | 23 | 10 | 8 | 13 | ||
| Fe (wt. %) | Average | 68.2 | 69.7 | 71 | 71.1 | |
| Range | 67.5–69.2 | 69.5–70.2 | 70.1–71.5 | 70.4–71.8 | ||
| O (wt. %) | Average | 29.4 | 29.0 | 28.7 | 28.8 | |
| Range | 28.9–29.9 | 28.6–29.2 | 28.3–29.7 | 28.4–29.5 | ||
| Si | Average | 11806 | 5430 | 587 | 432 | |
| sd | 1231 | 936 | 445 | 274 | ||
| DL = 11 | Range | 9769–13,785 | 3949–7170 | 12–870 | 55–884 | |
| Ti | Average | 143 | 56 | 9 | 38 | |
| sd | 61 | 13 | 5 | 22 | ||
| DL = 4 | Range | 38–246 | 37–82 | BDL-17 (2) | 8–65 | |
| Al | Average | 2781 | 1190 | 278 | 239 | |
| sd | 500 | 252 | 95 | 104 | ||
| DL = 12 | Range | 1745–3480 | 759–1621 | 175–468 | 105–519 | |
| Mn | Average | 222 | 240 | 198 | 181 | |
| sd | 23 | 32 | 45 | 45 | ||
| DL= 5 | Range | 181–262 | 196–276 | 148–292 | 101–261 | |
| Mg | Average | 1272 | 775 | 42 | 62 | |
| sd | 308 | 220 | 24 | 28 | ||
| DL = 19 | Range | 614–1738 | 342–1064 | BDL-70 (2) | 18–89 | |
| Ca | Average | 2866 | 1661 | 473 | 416 | |
| sd | 521 | 353 | 213 | 179 | ||
| DL = 3 | Range | 1966–3575 | 1150–2226 | 249–824 | 228–797 | |
| V | Average | 24 | 21 | nd | 27 | |
| sd | 10 | 8 | 7 | |||
| DL = 9 | Range | 11–39 | 11–30 | 22–42 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, C.; Boudouma, O.; Rividi, N.; Orberger, B.; Nabatian, G.; Honarmand, M.; Monsef, I. Magnetite Texture and Geochemistry in the Takab Ore Deposit (NW Iran): Implications for a Complex Hydrothermal Evolution. Minerals 2025, 15, 137. https://doi.org/10.3390/min15020137
Wagner C, Boudouma O, Rividi N, Orberger B, Nabatian G, Honarmand M, Monsef I. Magnetite Texture and Geochemistry in the Takab Ore Deposit (NW Iran): Implications for a Complex Hydrothermal Evolution. Minerals. 2025; 15(2):137. https://doi.org/10.3390/min15020137
Chicago/Turabian StyleWagner, Christiane, Omar Boudouma, Nicolas Rividi, Beate Orberger, Ghasem Nabatian, Maryam Honarmand, and Iman Monsef. 2025. "Magnetite Texture and Geochemistry in the Takab Ore Deposit (NW Iran): Implications for a Complex Hydrothermal Evolution" Minerals 15, no. 2: 137. https://doi.org/10.3390/min15020137
APA StyleWagner, C., Boudouma, O., Rividi, N., Orberger, B., Nabatian, G., Honarmand, M., & Monsef, I. (2025). Magnetite Texture and Geochemistry in the Takab Ore Deposit (NW Iran): Implications for a Complex Hydrothermal Evolution. Minerals, 15(2), 137. https://doi.org/10.3390/min15020137






