A Genetic Model for the Biggenden Gold-Bearing Fe Skarn Deposit, Queensland, Australia: Geology, Mineralogy, Isotope Geochemistry, and Fluid Inclusion Studies
Abstract
1. Introduction
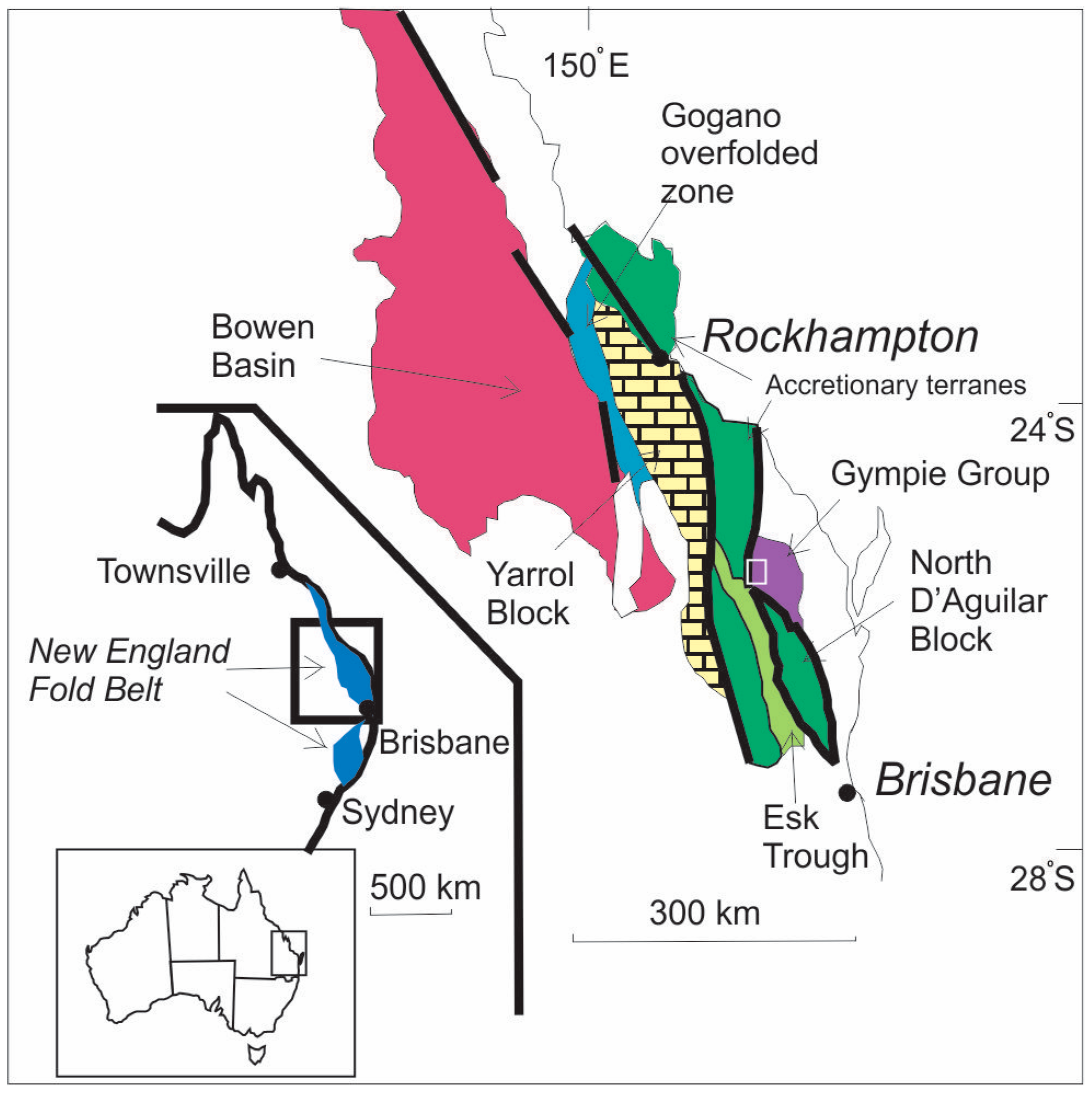
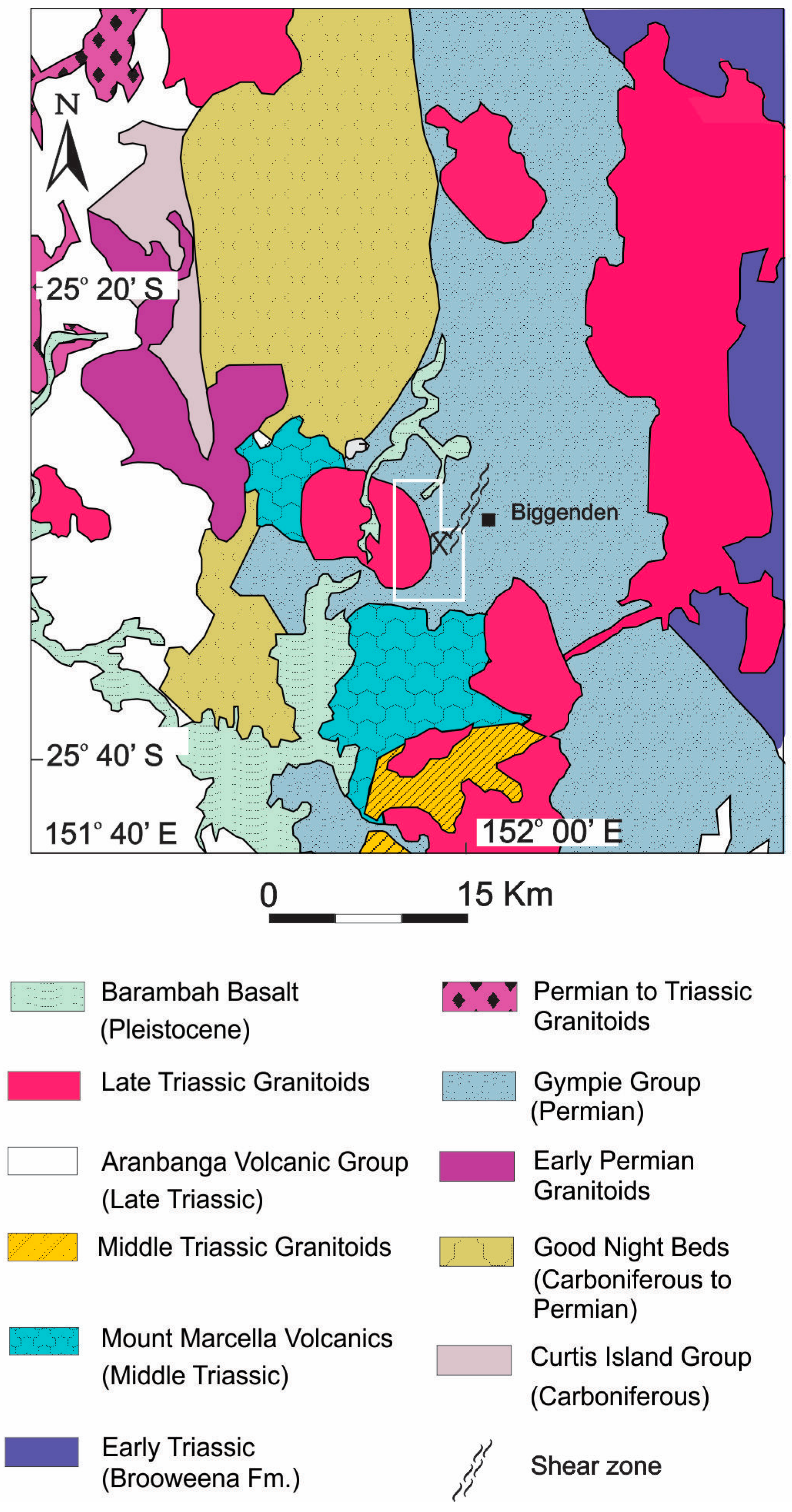
2. Regional Geological Setting
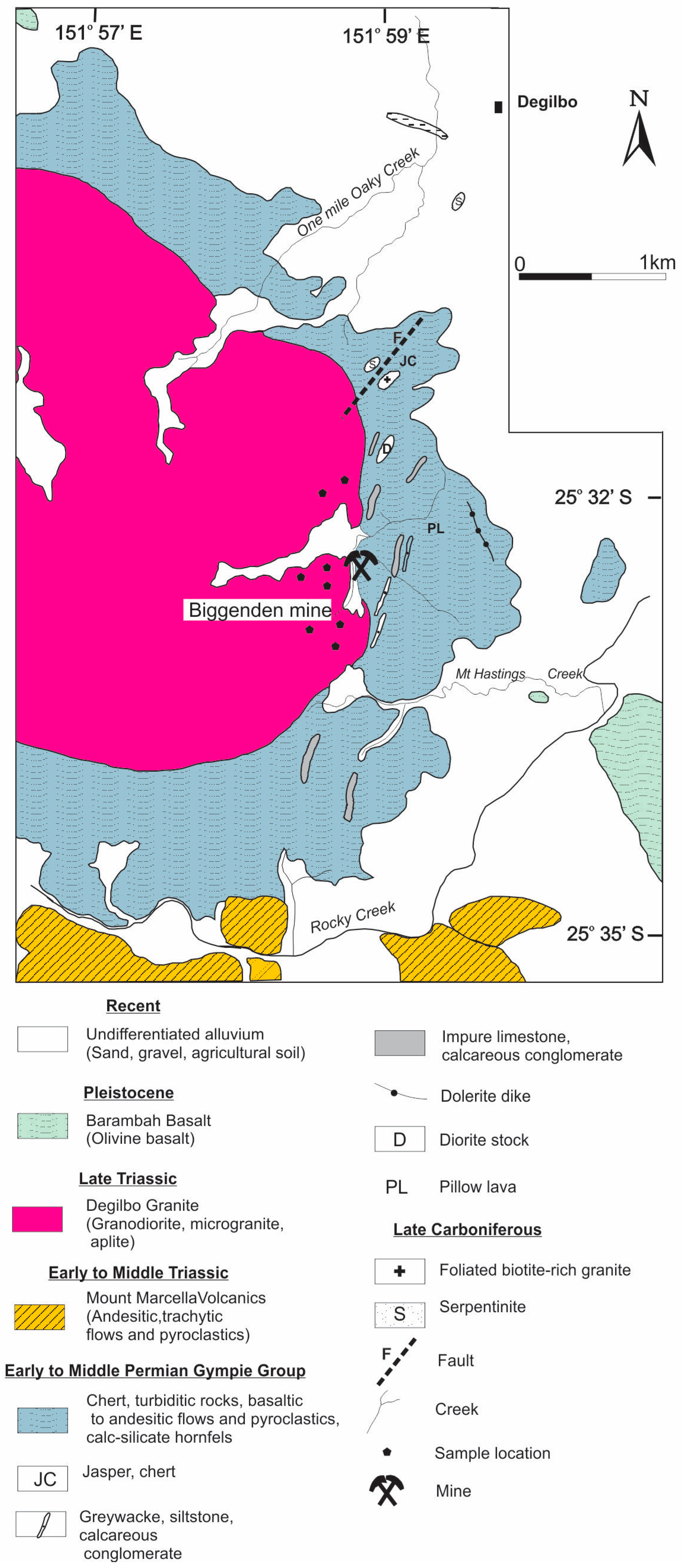
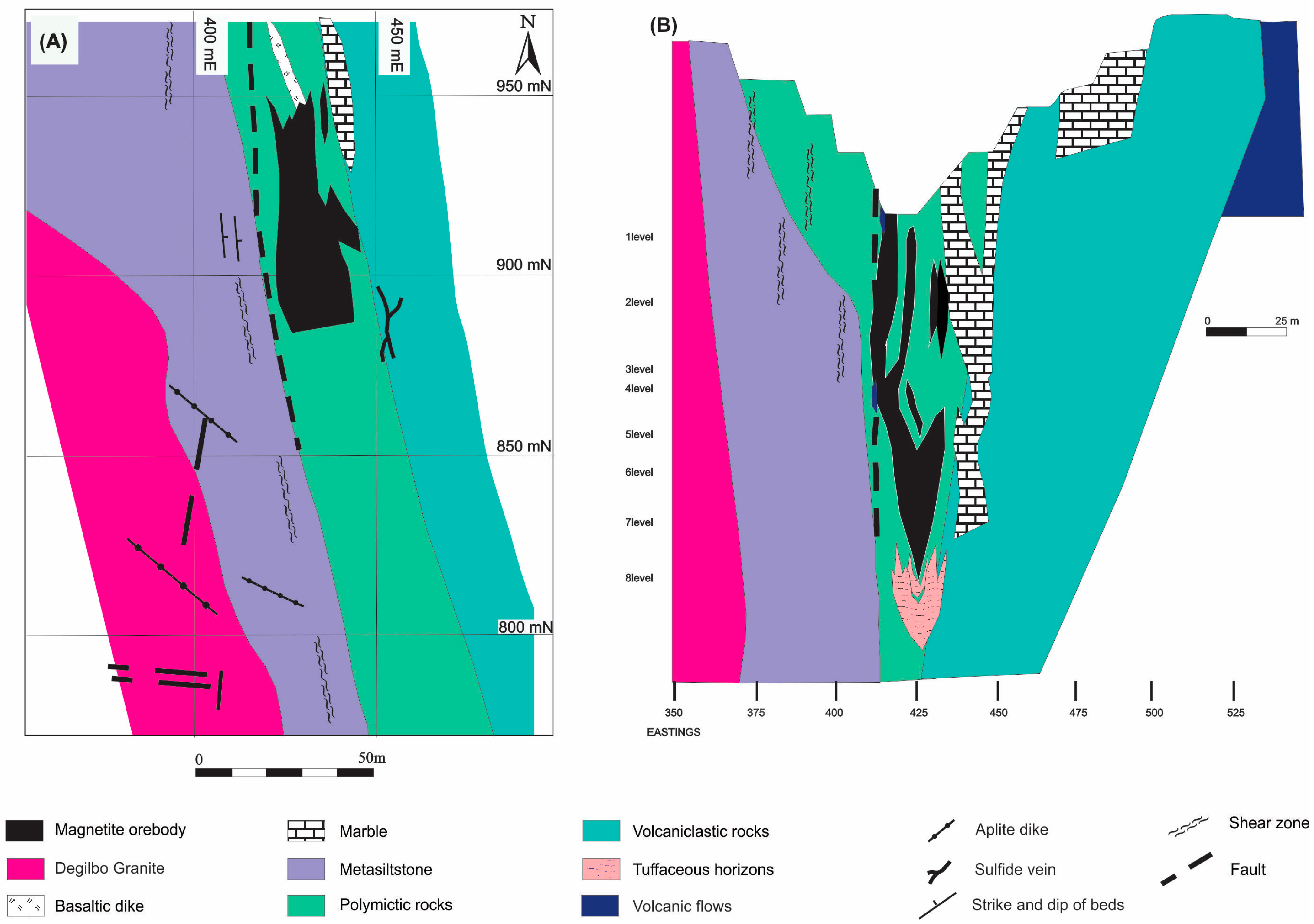
Mine Geology
- Lava Flows: These amygdaloidal basalts and andesites occur in the eastern part of the open pit and display coherent volcanic flow textures. They feature inter-granular, subophitic, or porphyritic textures, with albitized plagioclase laths and augite in a groundmass of altered plagioclase, ferromagnesian minerals, and accessory titanomagnetite and ilmenite. The amygdales, up to 1 cm, are filled mainly with epidote and chlorite.
- Volcaniclastic Rocks: These rocks are closely associated with the lava flows but differ in their dominant fragmental texture. The outcrops in the open pit transition from a gradational contact with the volcanic flows to a gradational contact with polymictic rocks.
- Polymictic Rocks: This unit, found between the granite contact and the volcaniclastic unit, includes a tuffaceous lithic wacke and breccia. These rocks are black to greenish-black, due to the presence of metamorphic hornblende, clinopyroxene, and biotite. The key difference from the volcaniclastic unit is the inclusion of non-volcanic clasts such as chert, siltstone, and limestone. Contact metamorphism has replaced some of these fragments with calc-silicate minerals.
- Limestone: The mine area features several large and small marble bodies, with a granoblastic polygonal texture. Over 98% of the rock is calcite, with disseminated garnet and other common calc-silicate minerals.
- Metasiltstone: This unit is an orange-brown, intensely jointed horizon in the open pit area. It has a granoblastic texture and locally contains up to 50% metamorphic biotite near the contact zone.
3. Materials and Methods
4. Results and Analysis
4.1. Mineralogy and Mineral Compositions
4.2. Geochemistry of the Degilbo Granite
4.3. Microthermometric Studies
4.3.1. Homogenization Temperatures
4.3.2. Salinities
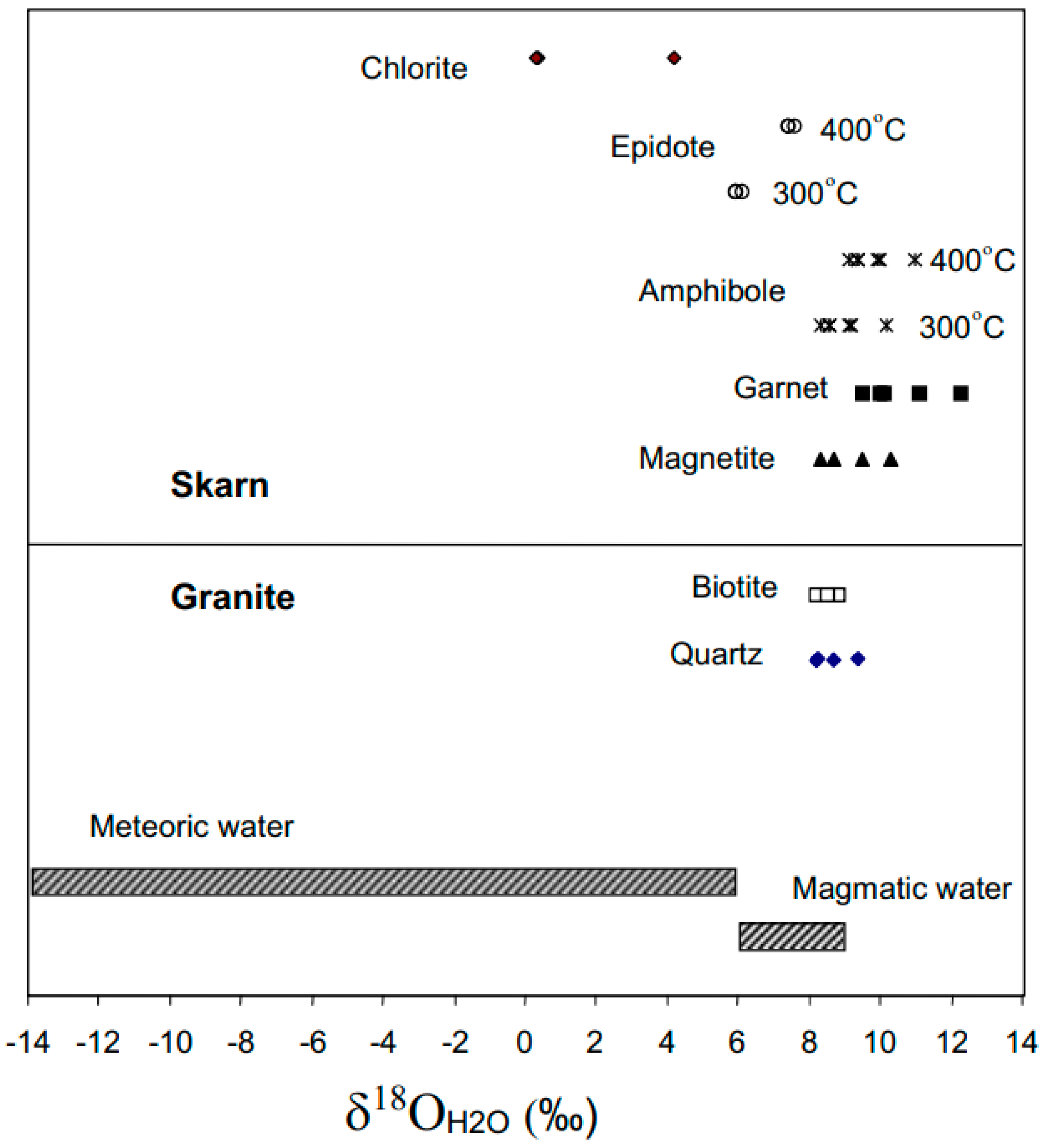
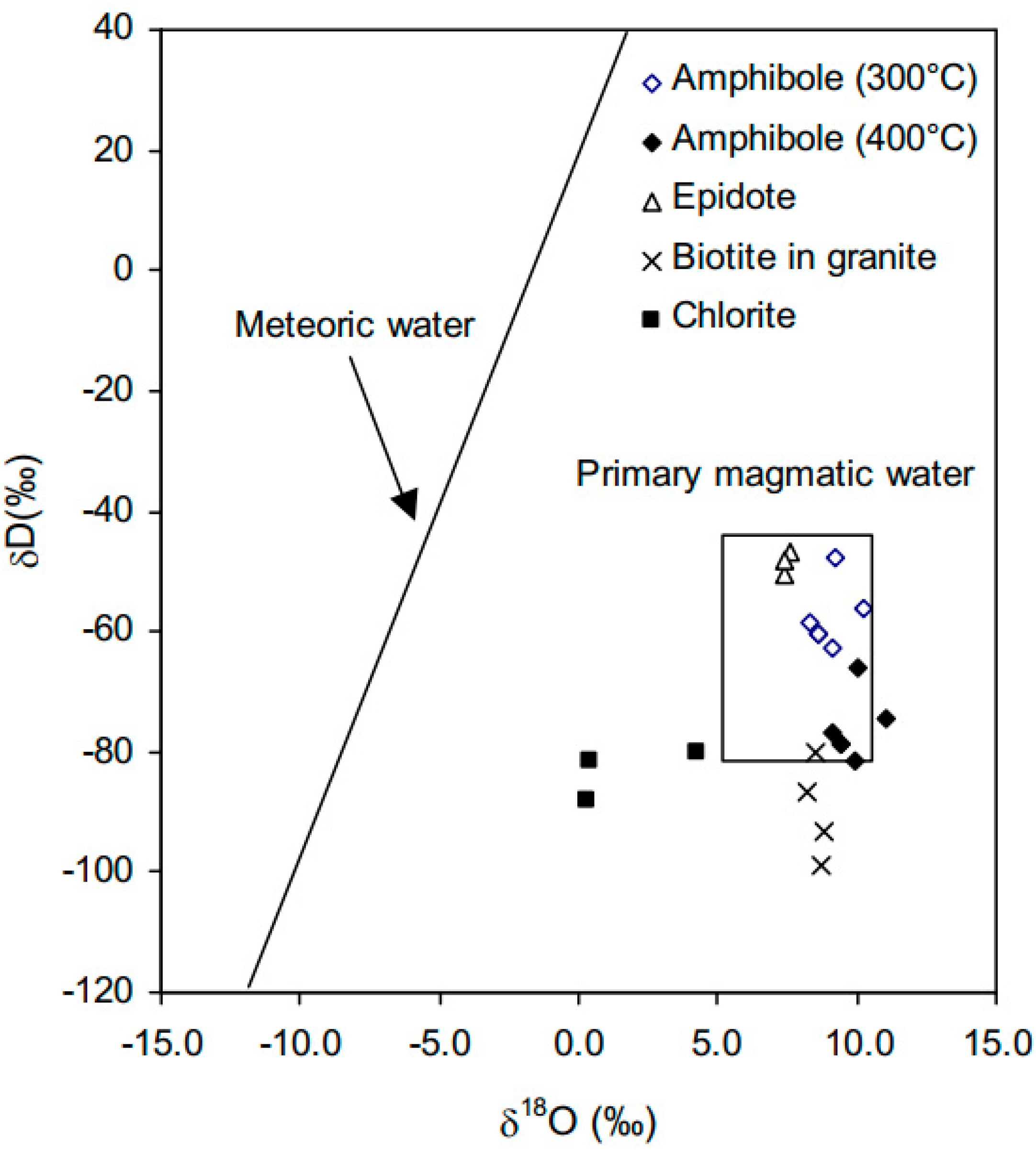
4.4. Isotope Geochemistry
5. Discussion
5.1. Isotope Interpretations
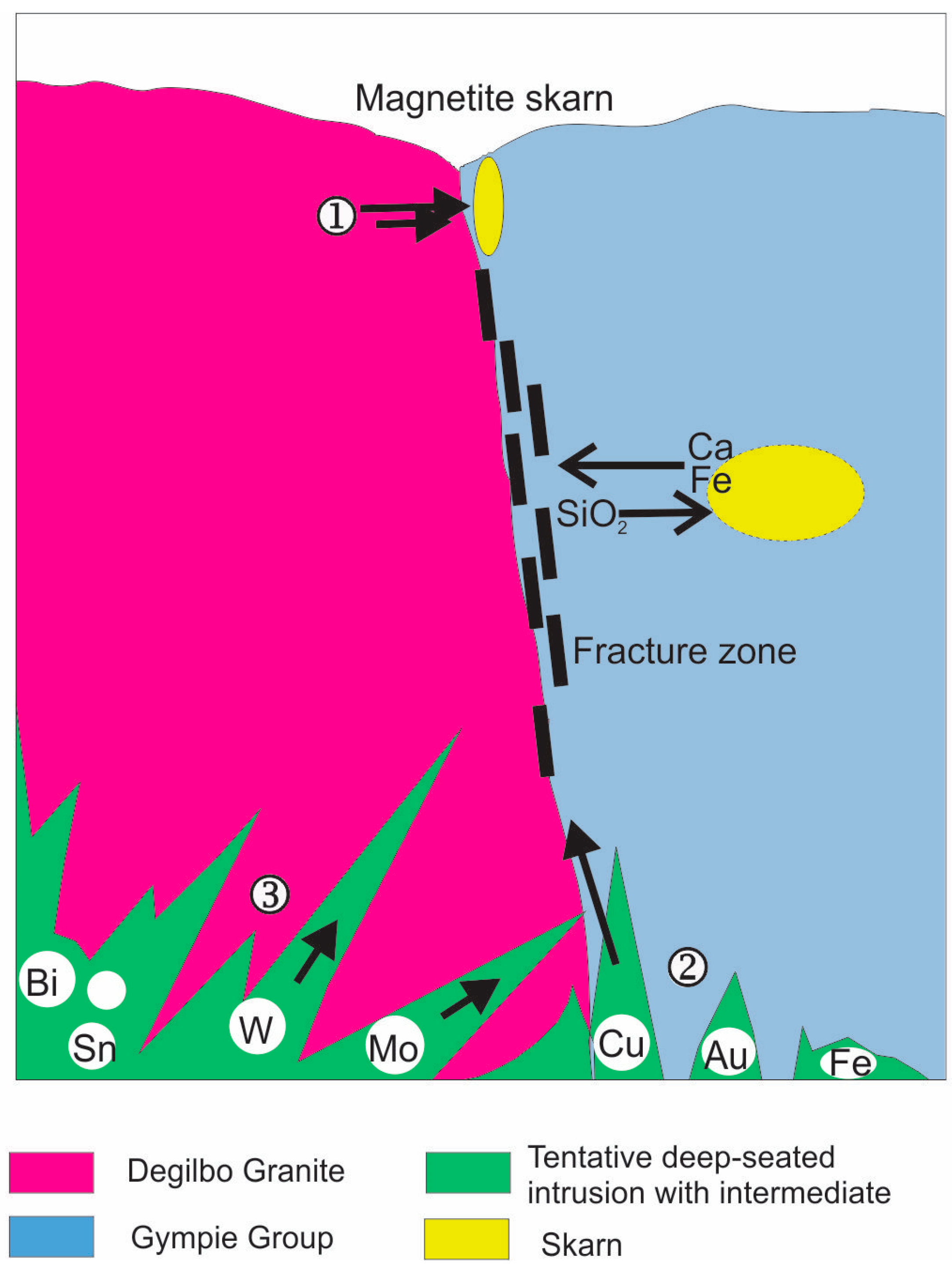
5.2. Classification of the Biggenden Skarn Deposit
5.3. Genetic Models
6. Conclusions
- The Biggenden Gold-Bearing Fe Skarn is a calcic magnetite skarn type that is hosted by the Early Permian volcanic and sedimentary rocks of the Gympie Group due to the intrusion of the Late Triassic Degilbo Granite.
- The skarn mineralogy varies from the prograde garnet, clinopyroxene, magnetite, and scapolite to retrograde epidote and ferropargasite, followed by chlorite, calcite, actinolite, quartz, sulfides, gold, nontronite, and quartz.
- The prograde mineralization occurred at a high temperature (500–600 °C) from high salinity (up to 45 equivalent wt % NaCl) hydrothermal fluids. Lower temperatures (280–360 °C) and lower salinity (5–15 equivalent wt % NaCl) solutions precipitated the retrograde sulfide minerals.
- The δ18O values of the fluids indicate that the prograde minerals were precipitated from magmatic water, whereas the retrograde minerals were mainly deposited from a meteoric fluid. Sulfur was likely derived from a magmatic source.
- Sr and Pb isotopes suggest that the volcanic and sedimentary rocks of the Gympie Group may have partially provided the metals to the hydrothermal solution.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Einaudi, M.T.; Meinert, L.D.; Newberry, R.J. Skarn deposits. Econ. Geol. 1981, 75, 317–391. [Google Scholar]
- Meinert, L.D. Skarns and skarn deposits. Geosci. Can. 1992, 19, 145–162. [Google Scholar]
- Meinert, L.D.; Dipple, G.M.; Nicolescu, S. World Skarn Deposits. In Economic Geology; Hedenquist, J.W., Thompson, J.F.H., Goldfarb, R.J., Richards, J.P., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2005; 100th Anniversary Volume, pp. 299–336. [Google Scholar]
- Ren, L.; Huang, J.; Wang, X.; Yang, S.; Yang, C.; Zhao, C.; Wang, L.; Mei, W.; Deng, M.; Zhou, Y. Magmatic control on orebody distribution of porphyry-skarn gold-copper deposit: A case study of Beiya deposit from Sanjiang metallogenic belt in the southwest China. Ore Geol. Rev. 2024, 175, 106350. [Google Scholar] [CrossRef]
- Sui, J.X.; Li, J.W.; Wen, G.; Jin, X.Y. The Dewulu reduced Au-Cu skarn deposit in the Xiahe-Hezuo district, West Qinling orogen, China: Implications for an intrusion-related gold system. Ore Geol. Rev. 2017, 80, 1230–1244. [Google Scholar] [CrossRef]
- Zhou, H.; Sun, X.; Wu, Z.; Huang, Q. Timing of skarn gold deposition in the giant Beiya polymetallic gold deposit, southwest China: Constraints from in situ monazite SIMS U-Th-Pb geochronology. Ore Geol. Rev. 2019, 106, 226–237. [Google Scholar] [CrossRef]
- Weekes, G. Biggenden magnetite mine, assessment of reserves March 1992 and preliminary proposal for final development, Commercial Minerals. 1992; Unpublished report No. 5/92. Brisbane, Queensland, Australia. [Google Scholar]
- Meinert, L.D. A review of skarns that contain gold. Mineral. Assoc. Can. Short Course Ser. 1988, 26, 359–414. [Google Scholar]
- Seccombe, P.K.; Downes, P.M.; Ashley, P.M.; Brathwaite, R.L.; Green, G.R.; Murray, C.G.; Rubenach, M.J. World Skarn Deposits: Skarns of the Southwest Pacific. In Economic Geology; Hedenquist, J.W., Thompson, J.F.H., Goldfarb, R.J., Richards, J.P., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2005; 100th Anniversary Volume, pp. 299–336. [Google Scholar]
- Clarke, D.E. The Geology of the Mount Biggenden Gold and Bismuth Mine and Environs. Unpublished Bachelor’s Thesis, University of Queensland, Brisbane, Australia, 1963; p. 137. [Google Scholar]
- Clarke, D.E. Geology of the Mount Biggenden Gold and Bismuth Mine and Environs; Report nr. 32; Geological Survey of Queensland: Brisbane, Australia, 1 January 1969; p. 16. [Google Scholar]
- Tellam, L. Geology of the Mt. Biggenden Magnetite Mine Environs, Southeast Queensland, Geochemical and Mineralogical Aspects. Unpublished Bachelor’s Thesis, University of Queensland, Brisbane, Australia, 1980; p. 71. [Google Scholar]
- Li, P.; Rosenbaum, G.; Yang, J.; Hoy, D. Australian-derived detrital zircons in the Permian-Triassic Gympie terrane (eastern Australia): Evidence for an autochthonous origin. Tectonics 2015, 34, 858–874. [Google Scholar] [CrossRef]
- Holcombe, R.J.; Stephens, C.J.; Fielding, C.R.; Gust, D.; Little, A.; Sliwa, R.; Kassan, J.; McPhie, J.; Ewart, A. Tectonic evolution of the northern New England fold belt: The Permian-Triassic Hunter-Bowen event. In Tectonics and Metallogenesis of the New England Orogen; Ashley, P.M., Flood, P.G., Eds.; Geological Society Special Publication: London, UK, 1997; Volume 19, pp. 52–65. [Google Scholar]
- Cranfield, L.C. 1: 250,000 Geological Series-Explanatory Notes, Maryborough; Queensland Sheet SG56-6; Department of Minerals and Energy: Brisbane, Australia, 1994; p. 120. [Google Scholar]
- Jell, P.A.; Cranfield, L.C. Gympie Province. In Geology of Queensland; Jell, P.A., Ed.; Geological Survey of Queensland: Brisbane, Australia, 2013; pp. 369–371. [Google Scholar]
- Sivell, W.J.; Waterhouse, J.B. Petrogenesis of Gympie Group volcanics; evidence for remnants of an Early Permian volcanic arc in eastern Australia. Lithos 1988, 21, 81–95. [Google Scholar] [CrossRef]
- Hoy, D.; Rosenbaum, G. Episodic behavior of Gondwanide deformation in eastern Australia: Insights from the Gympie Terrane. Tectonics 2017, 36, 1497–1520. [Google Scholar] [CrossRef]
- Edraki, M. Geochemistry, Mineralogy and Genesis of the Biggenden Au-Bi-Fe Skarn Deposit, Southeast Queensland, Australia. Unpublished Ph.D. Thesis, University of New England, Armidale, Australia, 2000; p. 318. [Google Scholar]
- Cranfield, L.C.; Murray, C.G. Geochemistry and tectonic setting of granitic rocks in the Maryborough 1:250 000 sheet area, Southeast Queensland—Caractéristiques géochimiques et tectoniques des roches granitiques sur la feuille au 1:250 000 de Maryborough, SE de Queensland. Queensl. Gov. Min. J. 1989, 90, 408–415. [Google Scholar]
- Webb, A.W.; McDougall, I. Isotopic dating evidence on the age of the Upper Permian and Middle Triassic. Earth and Planetary Sci. Lett. 1967, 2, 483–488. [Google Scholar] [CrossRef]
- Chappell, B.W.; White, A.J.R. I- and S-type granites in the Lachlan Fold Belt. Trans. R. Soc. Edinb. Earth Sci. 1992, 83, 1–26. [Google Scholar]
- Chappell, B.W.; White, A.J.R. Two contrasting granite types: 25 years later. Aust. J. Earth Sci. 2001, 48, 489–499. [Google Scholar] [CrossRef]
- Cobine, T.J. The Analytic Signal and Cross-Correlation Applied in Rapid, Detailed Analysis of Aeromagnetic and Radiometric Data. Unpublished Master’s Thesis, University of New England, Armidale, Australia, 1997; p. 75. [Google Scholar]
- Roedder, E. Fluid inclusions. Rev. Mineral. 1984, 12, 644. [Google Scholar]
- Clayton, R.N.; Mayeda, T.K. The use of bromine pentafluoride in the extraction of oxygen from oxides and silicates for isotopic analysis. Geochem. Cosmochim. Acta 1963, 27, 43–52. [Google Scholar] [CrossRef]
- Friedman, I. Deuterium content of natural waters. Geochim. Cosmochim. Acta 1953, 4, 89–103. [Google Scholar] [CrossRef]
- Robinson, B.W.; Kusakabe, M. Quantitative preparation of sulfur dioxide for 34S/32S analysis from sulfides by combustion with cuprous oxide. Anal. Chem. 1975, 47, 1179–1181. [Google Scholar] [CrossRef]
- McCrea, J.M. The isotope geochemistry of carbonates and a paleotemperature scale. J. Chem. Phys. 1950, 18, 849–857. [Google Scholar] [CrossRef]
- Sies, S.H.; Niklaus, T.R.; Sims, D.A.; Bruhn, F.; Suter, G.; Cripps, G. AUSTRALIS: A new tool for the study of isotopic systems and geochronology in mineral systems. Aust. J. Earth Sci. 2002, 49, 601–611. [Google Scholar]
- Theodore, T.G.; Orris, G.J.; Hammarstrom, J.M.; Bliss, J.D. Gold-Bearing Skarns, 1930th ed.; U.S. Government Printing Office: Washington, DC, USA, 1991; pp. 1–35. [Google Scholar]
- Nakano, T.; Yoshino, T.; Shimazaki, H.; Shimizu, M. Pyroxene composition as an indicator in the classification of skarn deposits. Econ. Geol. 1994, 89, 567–1580. [Google Scholar] [CrossRef]
- Matsueda, H. Pyrometasomatic iron-copper ore deposits of the Sampo Mine, Okayama Prefecture; II, The modes of occurrences, mineral paragenesis and chemical compositions of skarn and ore. Min. Geol. 1981, 6, 1–43. [Google Scholar]
- Einaudi, M.T.; Burt, D.M. Introduction—Terminology, classification, and composition of skarn deposits. Econ. Geol. 1982, 77, 745–754. [Google Scholar] [CrossRef]
- Meinert, L.D. Gold skarn deposits–geology and exploration criteria. Econ. Geol. 1989, 65, 537–552. [Google Scholar]
- Pan, Y. Scapolite in skarn deposits; petrogenetic and geochemical significance. Mineral. Assoc. Can. Short Course Handb. 1998, 26, 169–210. [Google Scholar]
- Pan, Y.; Fleet, M.E.; Ray, G.E. Scapolite in two Canadian gold deposits: Nickel Plate British Columbia and Hemlo. Ontario Can. Mineral. 1994, 32, 825–837. [Google Scholar]
- Meinert, L.D. Mineralogy and petrology of iron skarns in western British Columbia, Canada. Econ. Geol. 1984, 79, 869–882. [Google Scholar] [CrossRef]
- Cathelineau, M. The chlorite and illite geothermometers. Chem. Geol. 1988, 70, 182–183. [Google Scholar] [CrossRef]
- Le Bas, M.J.; Streckeisen, A.L. The IUGS systematics of igneous rocks. J. Geol. Soc. Lond. 1991, 148, 825–833. [Google Scholar] [CrossRef]
- Peccerillo, A.; Taylor, S.R. Geochemistry of Eocene calc-alkaline volcanic rocks from the Castamoun area, northern Turkey. Contrib. Mineral. Petrol. 1976, 58, 63–81. [Google Scholar] [CrossRef]
- Kwak, T.A.P. Fluid inclusions in skarns (carbonate replacement deposits). J. Metamorph. Geol. 1986, 4, 363–384. [Google Scholar] [CrossRef]
- Kwak, T.A.P.; Tan, T.H. The geochemistry of zoning in skarn minerals at the King Island (Dolphin) Mine. Econ. Geol. 1981, 76, 468–497. [Google Scholar] [CrossRef]
- Meinert, L.D. Skarn zonation and fluid evolution in the Groundhog Mine, Central mining district, New Mexico. Econ. Geol. 1987, 82, 523–545. [Google Scholar] [CrossRef]
- Potter, R.W.; Clynne, M.A.; Brown, D.L. Freezing point depression of aqueous sodium chloride solutions. Econ. Geol. 1978, 73, 284–285. [Google Scholar] [CrossRef]
- Bodnar, R. Revised equation and table for determining the freezing point depression of H2O-Nacl solutions. Geochim. Cosmochim. Acta 1993, 57, 683–684. [Google Scholar] [CrossRef]
- Lentz, D.R. Carbonatite genesis: A reexamination of the role of intrusion-related pneumatolytic skarn processes in limestone melting. Geology 1999, 27, 335–338. [Google Scholar] [CrossRef]
- Xu, X.; Xu, X.; Szmihelsky, M.; Yan, J.; Xie, Q.; Steele-MacInnis, M. Melt inclusion evidence for limestone assimilation, calc-silicate melts, and “magmatic skarn”. Geology 2023, 51, 491–495. [Google Scholar] [CrossRef]
- Ohmoto, H. Stable isotope geochemistry of ore deposits. In Stable Isotopes in High Temperature Geological Processes; Valley, J.W., Taylor, H.P., O’Neil, J.R., Eds.; Mineralogical Society of America: Chantilly, VA, USA, 1986; Volume 16, pp. 491–559. [Google Scholar]
- Valley, J.W. Stable isotope geochemistry of metamorphic rocks. Rev. Mineral. 1986, 16, 445–489. [Google Scholar]
- Taylor, H.P.; Sheppard, S.M.F. Igneous rocks, I. Processes of isotopic fractionation in isotope systematics. Rev. Mineral. 1986, 16, 227–271. [Google Scholar]
- Bottinga, Y.; Javoy, M. Comments on oxygen isotope geothermometry. Earth Planet. Sci. Lett. 1973, 20, 250–265. [Google Scholar] [CrossRef]
- Graham, C.M.; Sheppard, S.M.F.; Heaton, T.H.E. Experimental hydrogen isotope studies—I. Systematics of hydrogen isotope fractionation in the systems epidote-H2O, zoisite-H2O and AlO(OH)-H2O. Geochimica Cosmochimica Acta 1980, 44, 353–364. [Google Scholar] [CrossRef]
- Bottinga, Y.; Javoy, M. Oxygen isotope partitioning among the minerals in igneous and metamorphic rocks. Rev. Geophys. 1975, 13, 401–418. [Google Scholar] [CrossRef]
- Matsushisa, Y.; Goldsmith, N.; Tanaka, T. Oxygen isotope fractionation in the system quartz-albite-anorthite-water. Geochim. Cosmochim. Acta 1979, 43, 1131–1140. [Google Scholar] [CrossRef]
- Wenner, D.B.; Taylor, H.P., Jr. Temperatures of serpentinization of ultramafic rocks based on 18O/16O fractionation between coexisting serpentine and magnetite. Contrib. Mineral. Petrol. 1971, 32, 165–185. [Google Scholar] [CrossRef]
- Graham, C.M.; Harmon, R.S.; Sheppard, S.M.F. Experimental hydrogen isotope studies, hydrogen isotope exchange between amphibole and water. Am. Mineral. 1984, 69, 128–138. [Google Scholar]
- Suzuoki, T.; Epstein, S. Hydrogen isotope fractionation between OH-bearing minerals and water. Geochim. Cosmochim. Acta 1976, 40, 1229–1240. [Google Scholar] [CrossRef]
- Kyser, T.K. Equilibrium fractionation factors for stable isotopes. In Stable Isotope Geochemistry of Low Temperature Processes; Mineralogical Association of Canada: Montreal, QC, Canada, 1987; Volume 13, pp. 1–84. [Google Scholar]
- Ohmoto, H.; Goldhaber, M.B. Sulfur and carbon isotopes. In Geochemistry of Hydrothermal Ore Deposits, 3rd ed.; Barnes, H.L., Ed.; John Wiley and Sons: New York, NY, USA, 1997; pp. 517–612. [Google Scholar]
- Faure, G. Principles of Isotope Geology, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1986; p. 589. [Google Scholar]
- Carr, G.R.; Dean, J.A.; Suppel, D.W.; Heithersay, P.S. Precise lead isotope fingerprinting of hydrothermal activity associated with Ordovician to Carboniferous metallogenic events in the Lachlan Fold Belt of New South Wales. Econ. Geol. 1995, 90, 1467–1505. [Google Scholar] [CrossRef]
- Cartwright, I.; Weaver, T.R. Fluid-rock interaction between syenites and marbles at Stephen Cross Quarry, Québec, Canada: Petrological and stable isotope data. Contrib. Mineral. Petrol. 1993, 113, 533–544. [Google Scholar] [CrossRef]
- Baker, T.; Lang, J.R. Reconciling fluid inclusions, fluid processes and fluid source in skarns: An example from the Bismarck skarn deposit, Mexico. Miner. Depos. 2003, 38, 474–495. [Google Scholar] [CrossRef]
- Bowman, J.R. Stable-isotope systematics of skarns. Mineral. Assoc. Can. Short Course Ser. 1998, 26, 99–145. [Google Scholar]
- Bowman, J.R.; O’Neil, J.R.; Essene, E.J. Contact skarn formation at Elkhorn, Montana. II: Origin and evolution of C-O-H skarn fluids. Am. J. Sci. 1985, 285, 621–660. [Google Scholar]
- Vander-Auwera, J.; Andre, L. Trace elements (REE) and isotopes (O, C, Sr) to characterize the metasomatic fluid sources: Evidence from the skarn deposit (Fe, W, Cu) of Traversella (Ivrea, Italy). Contrib. Mineral. Petrol. 1991, 106, 325–339. [Google Scholar] [CrossRef]
- Taylor, B.E. Stable isotope geochemistry of ore-forming fluids. Mineral. Assoc. Can. Short Course Handb. 1987, 13, 377–445. [Google Scholar]
- Zaw, K.; Singoyi, B. Formation of Magnetite-Scheelite Skarn Mineralization at Kara, Northwestern Tasmania: Evidence from Mineral Chemistry and Stable Isotopes. Econ. Geol. 2000, 95, 1215–1230. [Google Scholar] [CrossRef]
- So, C.-S.; Rye, D.M.; Shelton, K.L. Carbon, hydrogen, oxygen, and sulfur isotope and fluid inclusion study of the Weolag tungsten-molybdenum deposit, Republic of Korea; fluid histories of metamorphic and ore-forming events. Econ. Geol. 1983, 78, 1551–1573. [Google Scholar] [CrossRef]
- Nabelek, P.I. Stable isotope monitors. In Contact Metamorphism; Kerrick, D.M., Ed.; Reviews in Mineralogy: Chantilly, VA, USA, 1991; Volume 26, pp. 395–435. [Google Scholar]
- Friedman, I.; O’Neil, J.R. Compilation of stable isotope fractionation factors of geochemical interest. In Data of Geochemistry; Fleischer, M., Ed.; United States Government Printing Office: Washington, DC, USA, 1977. [Google Scholar]
- Hall, D.L.; Cohen, L.H.; Schiffman, P. Hydrothermal alteration associated with Iron Hat skarn deposit, San Bernardino County, California. Econ. Geol. 1988, 83, 568–587. [Google Scholar] [CrossRef]
- Zürcher, L.; Ruiz, J.; Barton, M.D. Paragenesis, elemental distribution, and stable isotopes at the Peña Colorada iron skarn, Colima, Mexico. Econ. Geol. 2001, 96, 535–557. [Google Scholar] [CrossRef]
- Taylor, H.P. Comparison of hydrothermal systems in layered gabbros and granites and the origin of low 18O magmas. Geochem. Soc. Spec. Publ. 1987, 1, 337–357. [Google Scholar]
- Ashley, P.M.; Andrew, A.S. The Mt Ninderry acid sulphate alteration zone and its relation to epithermal mineralization in the North Arm Volcanics, southeast Queensland. Aust. J. Earth Sci. 1992, 39, 79–98. [Google Scholar] [CrossRef]
- Sun, S.S.; Eadington, P.J. Oxygen isotope evidence for the mixing of magmatic and meteoric waters during tin mineralization in the Mole Granite, New South Wales, Australia. Econ. Geol. 1987, 82, 45–52. [Google Scholar] [CrossRef]
- Taylor, H.P. Oxygen and hydrogen isotope studies of plutonic granite rocks. Earth Planet. Sci. Lett. 1974, 38, 177–210. [Google Scholar] [CrossRef]
- Taylor, B.E.; O’Neil, J.R. Stable isotope studies of metasomatic Ca-Fe-Al-Si skarns and associated metamorphic and igneous rocks, Osgood Mountains, Nevada. Contrib. Mineral. Petrol. 1977, 63, 1–49. [Google Scholar] [CrossRef]
- Baranov, E.N.; Grinenko, L.N.; Pavlov, G.P. Pyrite sulfur isotope compositions in Ural skarn-magnetite deposits. Geochem. Int. 1986, 23, 81–90. [Google Scholar]
- Cumming, G.L.; Richards, J.R. Ore lead isotope ratios in a continuously changing earth. Earth Planet. Sci. Lett. 1975, 28, 155–171. [Google Scholar] [CrossRef]
- Meinert, L.D. Compositional variation of igneous rocks associated with skarn deposits—Chemical evidence for a genetic connection between petrogenesis and mineralization. Mineral. Assoc. Can. Short Course 1995, 23, 401–418. [Google Scholar]
- Newberry, R.J. The formation of sub-calcic garnet in scheelite-bearing skarns. Can. Mineral. 1983, 21, 529–544. [Google Scholar]
- Frietsch, R.; Tuisku, P.; Martinsson, O.; Perdahl, J.-A. Early proterozoic Cu, Au, and Fe ore deposits associated with regional NaCl metasomatism in northern Fennoscandia. Ore Geol. Rev. 1998, 12, 1–34. [Google Scholar] [CrossRef]
- Steven, N.M.; Moore, J.M. Pan-African tungsten skarn mineralization at the Otjua Prospect, central Namibia. Econ. Geol. 1994, 89, 1431–1453. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, W.; Bi, C.; Li, D.; Jiang, C. Skarn Deposits of China; Chinese Academy Geological Sciences: Beijing, China, 1994. [Google Scholar]
- Kodera, P.; Rankin, A.H.; Lexa, J. Evolution of fluids responsible for iron skarn mineralization: An example from the Vyhne-Klokoc deposit, Western Carpathians, Slovakia. Mineral. Petrol. 1998, 64, 119–147. [Google Scholar] [CrossRef]
- Ashley, P.M.; Billington, W.G.; Graham, R.L.; Neale, R.C. Geology of the Coalstoun porphyry copper prospect, southeast Queensland, Australia. Econ. Geol. 1978, 73, 945–965. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, H.K.; Kim, S.J. Geochemistry and mineralization age of magnesian skarn-type iron deposits of the Janggun mine, Republic of Korea. Miner. Depos. 1998, 33, 379–390. [Google Scholar] [CrossRef]
- Meinert, L.D. Skarn, manto, and breccia pipe formation in sedimentary rocks of the Cananea mining district, Sonora, Mexico. Econ. Geol. 1982, 77, 919–949. [Google Scholar] [CrossRef]
- Mueller, A.G. The Savage Lode magnesian skarn in the Marvel Loch gold-silver mine, Southern Cross greenstone belt, Western Australia, Part 1; Structural setting, petrography, and geochemistry. Can. J. Earth Sci. 1991, 28, 659–685. [Google Scholar] [CrossRef]

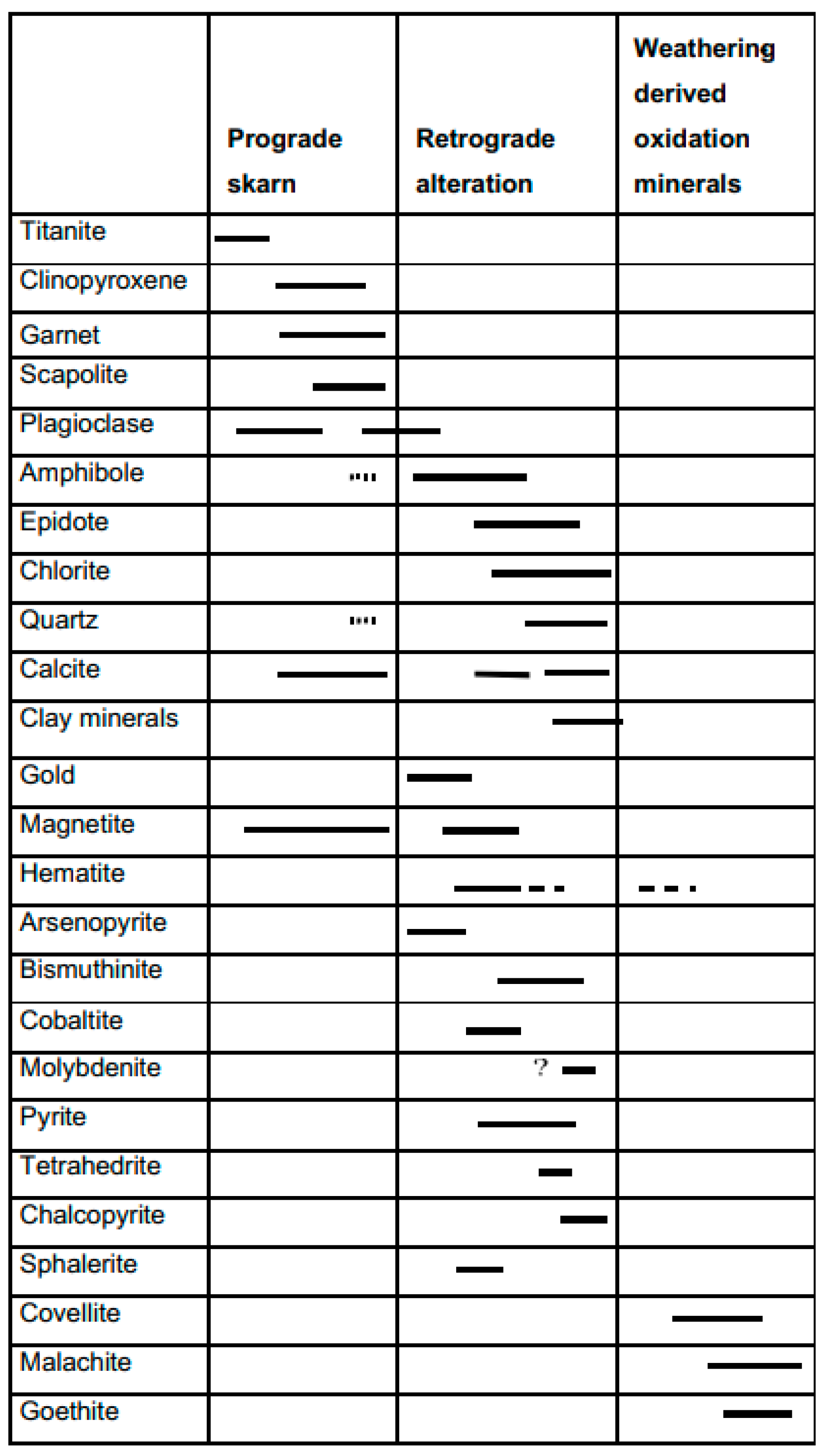





| Garnet | Clinopyroxene | |||||||
|---|---|---|---|---|---|---|---|---|
| Assemblage | Garnet-Pyroxene (2) | Magnetite Ore (2) | Garnet Skarn (2) | Calc-Silicate (2) | Garnet-Pyroxene (4) | Magnetite Ore (2) | Pyroxene-Plagioclase (4) | |
| SiO2 | 37.46–37.80 | 37.04–37.26 | 36.83–37.48 | 36.9–37.72 | SiO2 | 49.8–51.95 | 51.14–51.82 | 50.88–53.8 |
| TiO2 | 1.03–1.79 | 0.88–21.59 | 0.79–2.12 | 0.53–2.07 | TiO2 | ND-0.43 | ND-0.20 | ND |
| Al2O3 | 8.64–9.23 | 9.40–11.15 | 5.93–8.77 | 9.86–11.73 | Al2O3 | 1.22–1.85 | 1.50–2.87 | 0.20–0.92 |
| Fe2O3 * | 16.01–16.24 | 15.71–15.95 | 16.19–22.37 | 11.99–15.76 | Fe2O3 * | 0.85–1.92 | ND-1.88 | ND-1.30 |
| FeO | 0.32–0.72 | 0.89–2.04 | 0.28–0.65 | 1.01–6.79 | FeO * | 0.14–15.08 | 0.81–14.89 | 8.94–14.93 |
| MnO | 0.91–1.74 | 1.19–1.19 | 0.68–1.07 | 0.28–0.31 | MnO | ND-1.31 | 1.47–1.55 | 0.35–1.32 |
| MgO | ND | ND-0.96 | ND-0.31 | ND | MgO | 9.68–14.84 | 8.49–16.18 | 9.18–13.08 |
| CaO | 32.72–33.6 | 30.8–33.15 | 32.79–33.29 | 29.21–33.69 | CaO | 22.97–25.77 | 22.60–24.26 | 21.84–23.84 |
| Total | 98.91–99.30 | 99.21–100.00 | 99.57–100 | 98.49–99.36 | Total | 99.15–100.91 | 99.49–100.17 | 99.39–100.09 |
| Number of ions on the basis of 24 O | Cations on the basis of 6 O | |||||||
| Si | 6.02–6.05 | 5.94–5.96 | 5.99–6.03 | 5.94–6.02 | Si | 1.87–1.99 | 1.90–1.97 | 1.96–2.01 |
| Ti | 0.12–0.22 | 0.11–0.19 | 0.1–0.26 | 0.06–0.25 | Ti | ND-0.01 | ND-0.01 | ND |
| AlIV | 1.63–1.75 | 0.04–0.06 | 0.01–1.15 | 1.81–2.21 | AlIV | ND-0.07 | 0.03–0.10 | ND-0.04 |
| AlVI | ND | 1.71–2.06 | ND-1.64 | ND-0.06 | AlVI | ND-0.01 | 0.02–0.04 | ND-0.01 |
| Fe3+ | 2.15–2.17 | 1.85–2.14 | 2.17–2.76 | 1.60–2.12 | Fe3+ | 0.03–0.19 | ND-0.06 | ND-0.04 |
| Fe2+ | 0.04–0.10 | 0.12–0.31 | 0.04–0.09 | 0.13–0.92 | Fe2+ | 0.03–0.48 | 0.03–0.48 | 0.28–0.49 |
| Mn | 0.12–0.24 | 0.16–0.31 | 0.1–0.15 | 0.04 | Mn | ND-0.04 | 0.05 | 0.01–0.04 |
| Mg | ND | ND-0.23 | ND-0.08 | ND | Mg | 0.55–0.83 | 0.49–0.89 | 0.53–0.73 |
| Ca | 5.64–5.77 | 5.28–5.69 | 5.7–5.79 | 5.04–5.76 | Ca | 0.93–1.04 | 0.93–0.96 | 0.91–0.98 |
| Total | 16.01 | 16–16.01 | 16–16.03 | 16.00 | Total | 4.00 | 3.99–4.00 | 3.98–4.00 |
| Pyrope | 0.00 | 0.00–3.82 | 0.00–1.28 | 0.00 | Johannsenite | 0.0–4.5 | 4.5–5.0 | 1.1–3.9 |
| Almandine | 0.73–1.62 | 1.99–5.23 | 0.64–1.46 | 2.27–15.24 | Diopside | 51.4–77.8 | 47.9–87.3 | 50.1–71.5 |
| Spessartine | 2.08–3.97 | 2.70–2.71 | 1.59–2.44 | 0.64–0.70 | Hedenbergite | 20.5–47.4 | 8.2–47.1 | 27.4–45.9 |
| Grossularite | 36.75–37.19 | 36.79–39.14 | 24.69–34.93 | 29.35–50.34 | ||||
| Andradite | 57.23–60.44 | 49.11–58.51 | 61.17–71.8 | 46.76–54.71 | ||||
| Amphibole | Epidote (4) | Chlorite (5) | Scapolite | |||||
| Skarn (6) | Contact metamorphic (2) | |||||||
| SiO2 | 37.76–48.92 | 45.82–50.18 | SiO2 | 37.46–38.7 | SiO2 | 26.79–31.29 | SiO2 | 54.26–59.73 |
| TiO2 | ND-0.50 | ND | TiO2 | ND-0.27 | Al2O3 | 16.5–18.79 | Al2O3 | 25.09–26.34 |
| Al2O3 | 4.68–15.17 | 7.42–12.89 | Al2O3 | 22.54–24.7 | FeO * | 26.79–31.29 | CaO | 5.95–9.90 |
| FeO | 19.30–24.70 | 9.13–12.73 | Fe2O3 | 10.44–3.32 | MnO | 0.75–1.170 | Na2O | 7.56–8.92 |
| MnO | 0.71–1.03 | ND-0.57 | MnO | ND-0.33 | MgO | 6.75–15.01 | K2O | 0.21–1.04 |
| MgO | 4.84–9.44 | 13.74–15.97 | CaO | 23.1–24.61 | Total | 86.48–89.24 | Cl | ND-3.43 |
| CaO | 11.43–12.81 | 10.75–11.69 | Na2O | 0.41–0.64 | −O=Cl | ND-0.77 | ||
| Na2O | 0.33–2.74 | 1.57–3.62 | Total | 96.34–98.87 | Total | 99.24–100.82 | ||
| K2O | 0.28–1.87 | 0.22–0.26 | ||||||
| Cl | ND-0.72 | ND-0.22 | ||||||
| −O=Cl | ND-0.16 | ND-0.05 | ||||||
| Total | 97.16–99.26 | 98.33–98.40 | ||||||
| Number of ions on the basis of 23 O | Numbers of ions on the basis of 25 O | Number of ions on the basis of 25 O | ||||||
| Si | 5.84–7.37 | 6.53–7.25 | Si | 5.98–6.11 | Si | 5.73–5.95 | Si | 7.58–8.31 |
| AlIV | 0.63–2.16 | 0.75–1.47 | Ti | ND-0.03 | AlIV | 2.05–2.27 | Al | 4.12–4.34 |
| AlVI | 0.20–0.65 | 0.52–0.69 | Al | 4.22–4.64 | AlVI | 1.97–2.65 | Ca | 0.89–1.48 |
| Ti | ND-0.06 | ND | Fe3+ | 1.25–1.59 | Fe2+ | 4.75–6.34 | Na | 2.06–2.41 |
| Fe3+ * | 0.05–0.48 | 0.12–0.36 | Mn | ND-0.04 | Mn | 0.14–0.21 | K | 0.04–0.18 |
| Fe2+ | 1.97–3.09 | 0.56–1.41 | Ca | 3.93–4.18 | Mg | 2.44–4.74 | Cl | ND-0.82 |
| Mn | 0.09–0.13 | ND-0.07 | Na | 0.13–0.20 | Total | 15.65–16.45 | ||
| Mg | 1.12–2.12 | 2.96–3.39 | Total | 16.06–16.16 | Total cations | 19.24–19.71 | ||
| Ca | 1.91–2.07 | 1.64–1.81 | ||||||
| Na | 0.10–0.83 | 0.44–1.00 | Clinozoisite | 73.50–80.20 | ||||
| K | 0.05–0.37 | 0.04–0.05 | Pistacite | 19.80–26.50 | ||||
| Cl | 0.00–0.19 | ND-0.05 | Piemontite | 0–0.80 | ||||
| Total cations | 15.17–16.12 | 15.44–15.68 | ||||||
| Sample | R76342 | R76340 | R76343 | R76344 | R76345 | R76346 | R76410 | R76366 | R76458 | R76325 | R76440 | R76368 | R76337 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Granite | Microgranite Dike | Aplite Dike | |||||||||||
| Distance from Contact | 600 m | 500 m | 200 m | 150 m | 100 m | 80 m | 60 m | 50 m | 3 m | 1 m | 20 cm | ||
| SiO2 | 68.94 | 68.87 | 71.09 | 69.38 | 69.22 | 69.23 | 68.86 | 70.31 | 68.3 | 63.9 | 63.92 | 70.06 | 77.17 |
| TiO2 | 0.41 | 0.45 | 0.35 | 0.54 | 0.43 | 0.42 | 0.4 | 0.37 | 0.44 | 0.69 | 0.67 | 0.43 | 0.09 |
| Al2O3 | 15.44 | 15.37 | 14.73 | 14.49 | 15.2 | 15.42 | 15.19 | 14.59 | 15.65 | 16.82 | 18.16 | 15.23 | 12.49 |
| Fe2O3 | 1.49 | 1.19 | 1.12 | 1.34 | 1.30 | 1.34 | 1.30 | 1.13 | 1.16 | 1.42 | 1.25 | 0.08 | 0.35 |
| FeO | 1.43 | 1.77 | 1.41 | 2.12 | 1.72 | 1.66 | 1.57 | 1.55 | 1.72 | 2.85 | 2.20 | 1.78 | 0.25 |
| MnO | 0.06 | 0.07 | 0.05 | 0.07 | 0.06 | 0.05 | 0.05 | 0.07 | 0.06 | 0.11 | 0.09 | 0.04 | 0.03 |
| MgO | 0.79 | 0.95 | 0.66 | 1.03 | 0.85 | 0.79 | 0.89 | 0.79 | 0.86 | 1.31 | 1.25 | 0.77 | 0.11 |
| CaO | 2.14 | 2.03 | 1.74 | 1.93 | 2.06 | 2.00 | 1.94 | 1.69 | 2.33 | 3.15 | 3.12 | 2.24 | 0.63 |
| Na2O | 3.66 | 3.63 | 3.56 | 3.52 | 3.73 | 3.70 | 3.76 | 3.66 | 3.83 | 4.13 | 4.92 | 3.72 | 3.39 |
| K2O | 4.46 | 4.30 | 4.50 | 4.26 | 4.23 | 4.24 | 4.28 | 4.29 | 4.35 | 3.72 | 2.75 | 3.64 | 4.78 |
| P2O5 | 0.15 | 0.15 | 0.11 | 0.17 | 0.14 | 0.15 | 0.13 | 0.13 | 0.15 | 0.24 | 0.09 | 0.13 | 0.01 |
| S | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.14 | 0.02 |
| LOI | 0.73 | 0.94 | 0.58 | 0.74 | 0.67 | 0.71 | 0.77 | 0.77 | 0.74 | 1.00 | 1.05 | 0.85 | 0.56 |
| Total | 99.69 | 99.74 | 99.9 | 99.58 | 99.58 | 99.71 | 99.13 | 99.34 | 99.6 | 99.34 | 99.46 | 99.08 | 99.85 |
| Ba | 735 | 704 | 607 | 735 | 627 | 669 | 742 | 703 | 610 | 818 | 541 | 691 | 80 |
| Rb | 179 | 170 | 181 | 179 | 187 | 182 | 181 | 195 | 184 | 161 | 138 | 135 | 259 |
| Sr | 273 | 248 | 213 | 273 | 205 | 250 | 264 | 251 | 216 | 280 | 381 | 248 | 33 |
| Pb | 17 | 16 | 19 | 17 | 19 | 16 | 16 | 14 | 17 | 20 | 19 | 17 | 24 |
| Th | 17 | 20 | 33 | 17 | 22 | 18 | 15 | 18 | 23 | 16 | 21 | 58 | 62 |
| U | 3 | 4 | 4 | 3 | 4 | 4 | 2 | 3 | 5 | 4 | 4 | 6 | 17 |
| Zr | 240 | 249 | 218 | 240 | 304 | 246 | 233 | 239 | 215 | 244 | 541 | 211 | 87 |
| Nb | 10 | 10 | 13 | 10 | 13 | 10 | 10 | 9 | 11 | 8 | 16 | 12 | 9 |
| Y | 32 | 34 | 35 | 32 | 43 | 36 | 37 | 34 | 34 | 28 | 22 | 33 | 25 |
| La | 36 | 43 | 35 | 36 | 45 | 41 | 37 | 41 | 33 | 42 | 46 | 28 | 24 |
| Ce | 65 | 69 | 63 | 65 | 83 | 75 | 72 | 67 | 55 | 72 | 78 | 52 | 41 |
| Nd | 31 | 32 | 30 | 31 | 44 | 35 | 32 | 32 | 25 | 30 | 37 | 25 | 18 |
| Sc | 7 | 7 | 7 | 7 | 16 | 7 | 6 | 9 | 4 | 13 | 14 | 6 | 3 |
| V | 30 | 34 | 24 | 30 | 38 | 32 | 33 | 34 | 30 | 33 | 39 | 29 | 5 |
| Cr | 11 | 7 | 6 | 11 | 13 | 10 | 11 | 5 | 12 | 7 | 13 | 7 | 2 |
| Ni | 6 | 5 | 3 | 6 | 5 | 6 | 6 | 3 | 3 | 5 | 6 | 4 | 4 |
| Cu | 9 | 13 | 45 | 9 | 25 | 14 | 13 | 13 | 8 | 14 | 16 | 95 | 2 |
| Zn | 36 | 43 | 37 | 36 | 48 | 40 | 37 | 36 | 50 | 46 | 61 | 43 | 38 |
| Ga | 17 | 16 | 16 | 17 | 17 | 18 | 17 | 18 | 17 | 17 | 19 | 16 | 15 |
| Sn | <3 | <3 | 9 | <3 | 8 | 4 | <3 | 10 | <3 | <3 | <3 | <3 | <3 |
| As | 5 | 4 | 4 | 5 | 4 | 5 | 4 | 6 | 11 | 6 | 9 | 352 | 58 |
| Mo | 2 | 11 | 2 | 2 | 1 | <1 | 6 | 3 | 1 | 2 | 15 | 1 | 2 |
| Bi | 4 | 73 | 3 | 4 | 2 | 5 | <1 | <1 | 1 | 2 | 5 | 5 | 2 |
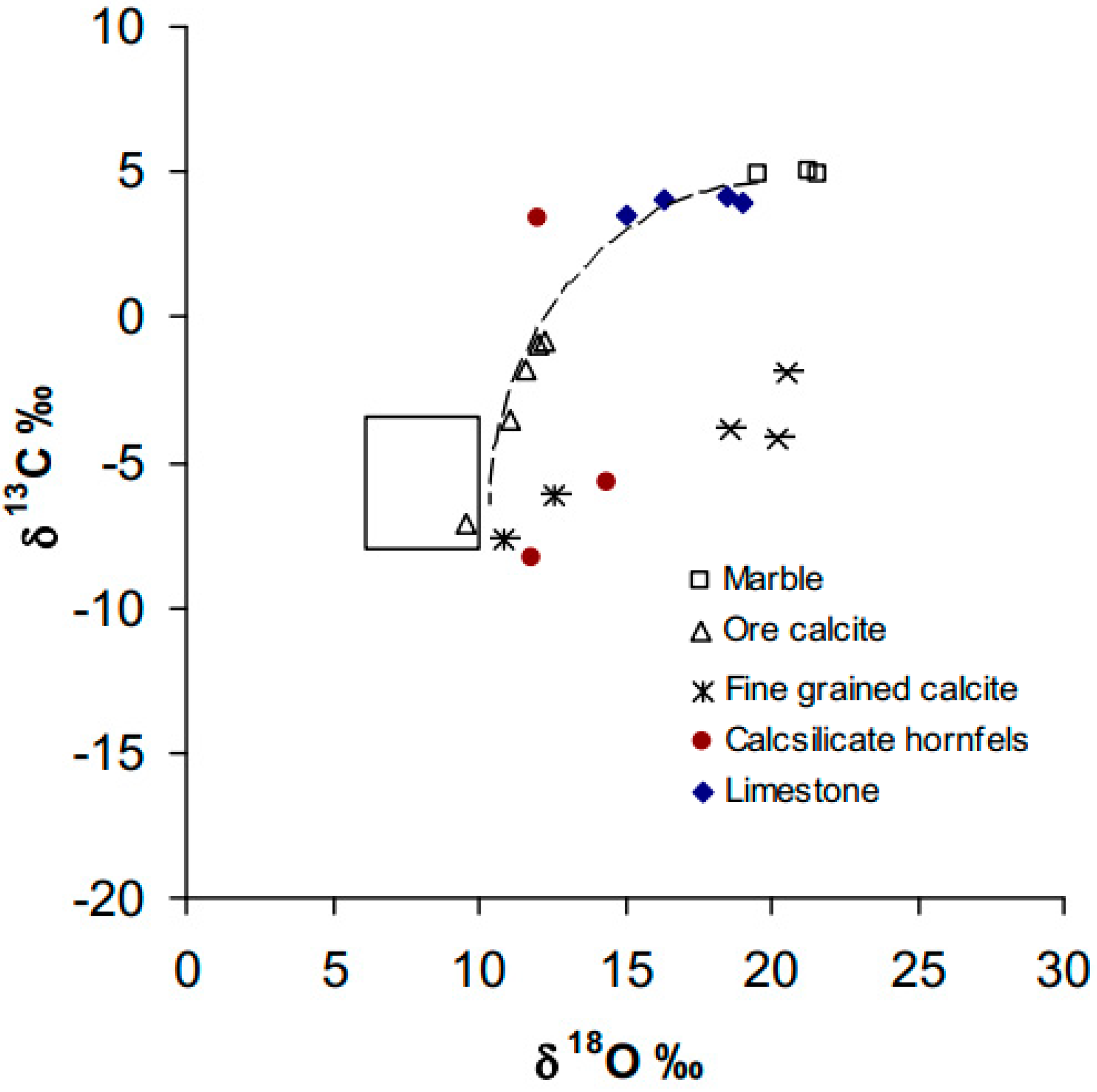
| Sample | Description | δ13C (% PDB) | δ18O (% SMOW) |
|---|---|---|---|
| R76483 | Limestone | 3.9 | 19.0 |
| R76477 | Limestone | 4.2 | 18.5 |
| R76477 | Limestone | 3.5 | 15.1 |
| R76467 | Limestone | 4.0 | 16.3 |
| R76329 | Marble | 4.9 | 21.6 |
| R76330 | Marble | 5.0 | 21.3 |
| R76318 | Marble | 4.9 | 19.6 |
| R76347 | Ore assemblage calcite | −3.5 | 11.1 |
| R76335 | Ore assemblage calcite | −0.8 | 12.3 |
| R76436 | Ore assemblage calcite | −7.1 | 9.6 |
| R76379 | Ore assemblage calcite | −0.9 | 12.0 |
| R76389 | Ore assemblage calcite | −1.8 | 11.6 |
| R76377 | Late-stage alteration calcite | −3.9 | 18.6 |
| R76363 | Late-stage alteration calcite | −4.2 | 20.2 |
| R76441 | Late-stage alteration calcite | −1.9 | 20.5 |
| R76364 | Fine-grained calcite | −7.7 | 10.9 |
| R76365 | Fine-grained calcite | −6.1 | 12.6 |
| R76468 | Calc-silicate hornfels | −8.3 | 11.8 |
| R76466 | Calc-silicate hornfels | −5.7 | 14.4 |
| R76488 | Calc-silicate hornfels | 3.4 | 12.0 |
| Sample | Mineral/Rock | δ18O (%) | δD (%) | T °C 1 | δ18O H2O (%) 2 | δDH2O (%) |
|---|---|---|---|---|---|---|
| R76296 | Amphibole | 8.6 | −133 | 300–400 | 9.1 to 9.9 | −63 to −81 |
| R76331 | Amphibole | 7.8 | −128 | 300–400 | 8.3 to 9.1 | −58 to −77 |
| R76388 | Amphibole | 9.7 | −126 | 300–400 | 10.2 to 11 | −56 to −75 |
| R76430 | Amphibole | 8.1 | −130 | 300–400 | 8.6 to 9.4 | −60 to −79 |
| R76435 | Amphibole | 8.7 | −117 | 300–400 | 9.2 to 10 | −48 to −66 |
| R76463 | Epidote | 8.0 | −100 | 300–400 | 5.9 to 7.4 | −50 |
| R76406 | Epidote | 8.2 | −97 | 300–400 | 6.1 to 7.6 | −47 |
| R76302 | Epidote | 8.0 | −98 | 300–400 | 5.9 to 7.4 | −48 |
| R76410 | Biotite | 6.2 | −133 | 650 | 8.7 | −99 |
| R76344 | Biotite | 6.3 | −128 | 650 | 8.8 | −93 |
| R76342 | Biotite | 5.7 | −121 | 650 | 8.2 | −87 |
| R76338 | Biotite | 6.0 | −114 | 650 | 8.5 | −80 |
| R76344 | Granite | 9.5 | - | - | - | - |
| R76307 | Chlorite | 4.1 | −120 | 310 | 4.2 | −80 |
| R76447 | Chlorite | 0.3 | −122 | 310 | 0.4 | −82 |
| R76441 | Chlorite | 0.2 | −128 | 310 | 0.3 | −88 |
| R76297 | Garnet | 9.2 | - | 550 | 11.1 | - |
| R76335 | Garnet | 8.1 | - | 550 | 10.0 | - |
| R76352 | Garnet | 8.2 | - | 550 | 10.1 | - |
| R76358 | Garnet | 7.6 | - | 550 | 9.5 | - |
| R76444 | Garnet | 10.4 | - | 550 | 12.3 | - |
| R76379 | Magnetite | 4.4 | - | 550 | 10.3 | - |
| R76427 | Magnetite | 3.6 | - | 550 | 9.5 | - |
| R76335 | Magnetite | 2.8 | - | 550 | 8.7 | - |
| R76353 | Magnetite | 2.4 | - | 550 | 8.3 | - |
| R76410 | Quartz | 10.6 | - | 650 | 8.7 | - |
| R76344 | Quartz | 10.0 | - | 650 | 8.1 | - |
| R76342 | Quartz | 10.3 | - | 650 | 8.4 | - |
| R76338 | Quartz | 10.4 | - | 650 | 8.5 | - |
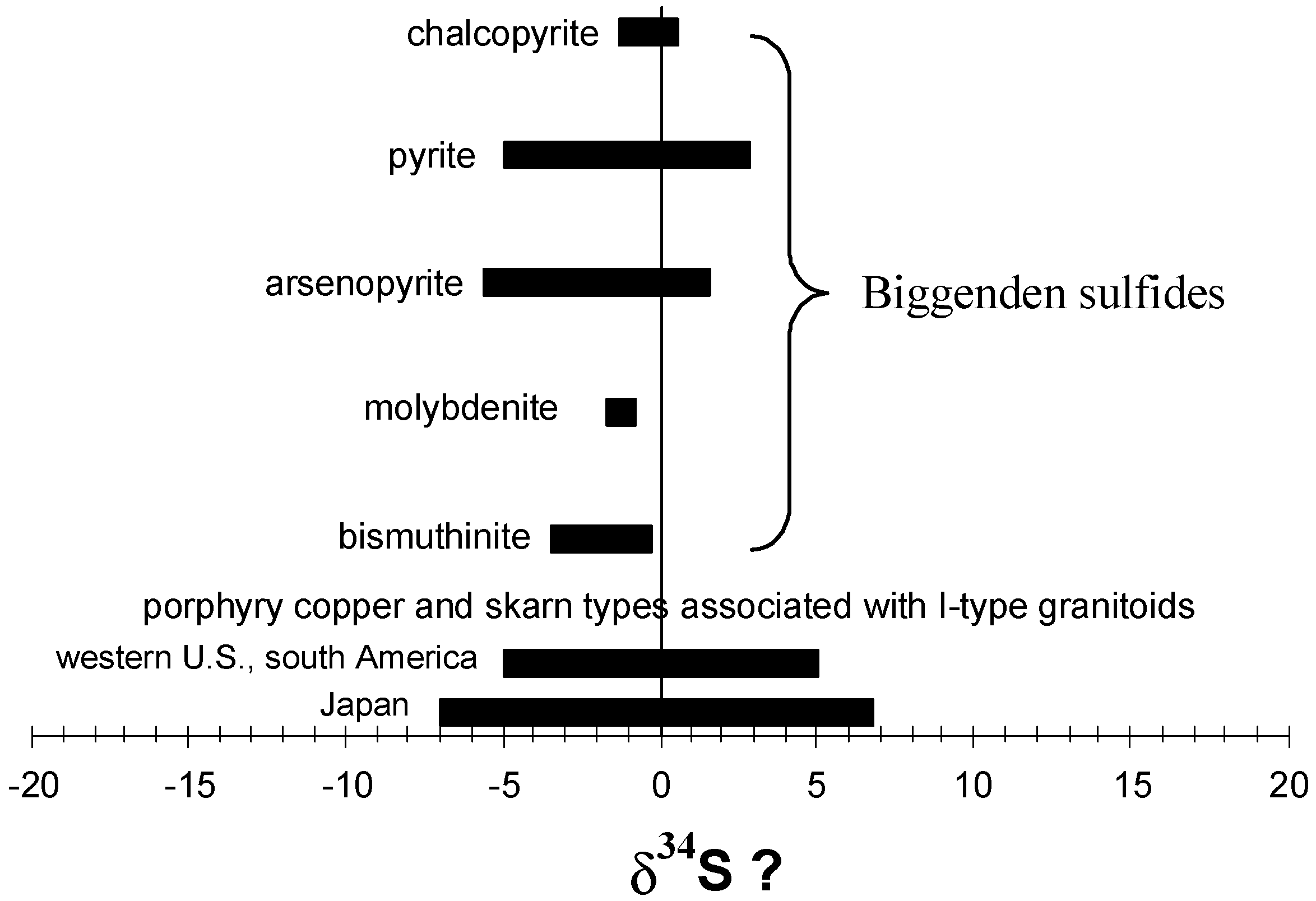
| Sample | Mineral | δ 34S (% CDT) |
|---|---|---|
| R76375 | Molybdenite | −1.6 |
| R76378 | Bismuthinite | −1.1 |
| R76380 | Bismuthinite | −1.1 |
| R76524 | Chalcopyrite | −1.1 |
| R76430 | Chalcopyrite | 0.5 |
| R76383 | Arsenopyrite | −5.6 |
| R76384 | Arsenopyrite | 1.4 |
| R76385 | Pyrite | −1.2 |
| R76447 | Pyrite | −5.0 |
| R76376 | Pyrite | −2.6 |
| R76486 | Pyrite | 2.3 |
| R76382 | Pyrite | −1.0 |
| R76381 | Pyrite | −1.8 |
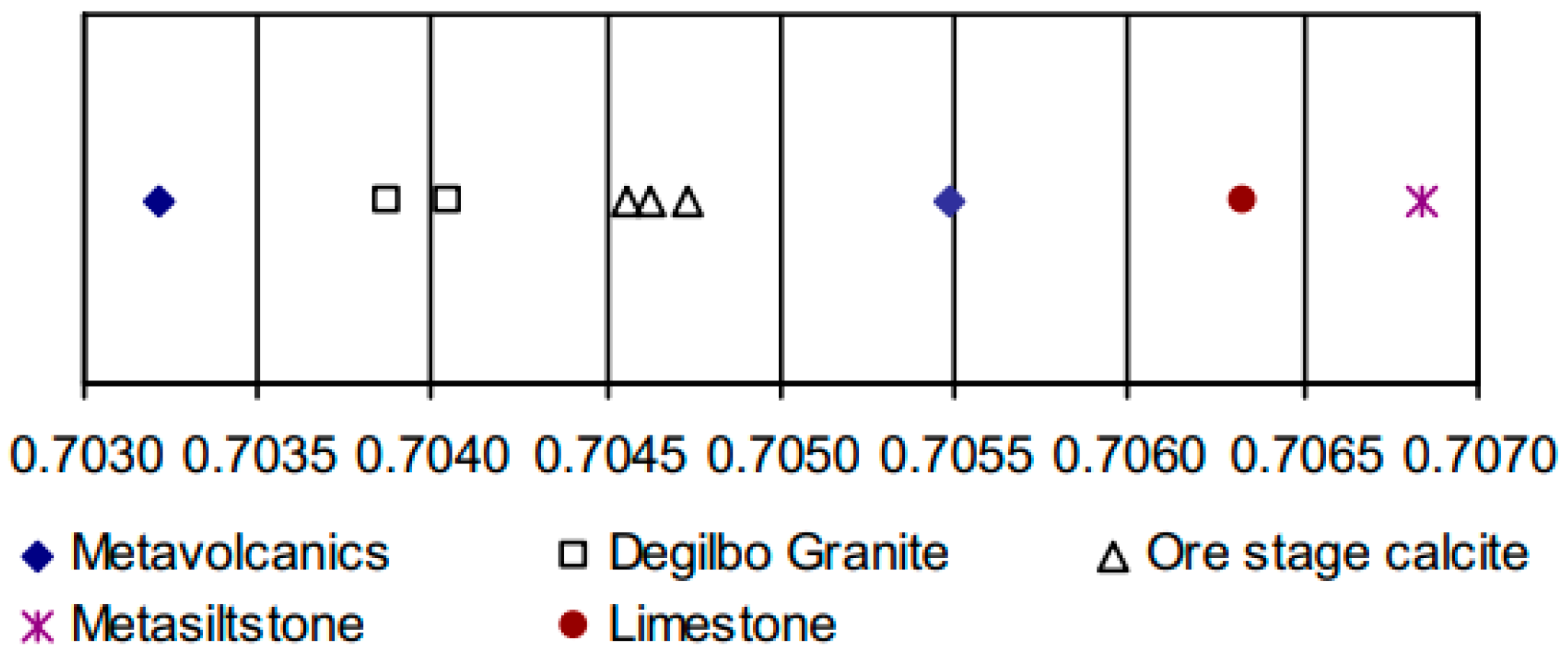
| Sample | Description | 87Rb/86Sr | 87Sr/86Sr | 87Sr/86Sr initial | Sr ppm | Rb ppm |
|---|---|---|---|---|---|---|
| R76472 | Metasiltstone | 1.9483 | 0.7128 | 0.70684 | 169 | 114 |
| R76402 | Metavolcanic | 0.2352 | 0.7062 | 0.70548 | 442 | 36 |
| R76501 | Metavolcanic | 0.0593 | 0.7034 | 0.70322 | 341 | 7 |
| R76410 | Granite | 2.2000 | 0.7106 | 0.70387 | 256 | 195 |
| R76344 | Granite | 2.6346 | 0.7121 | 0.70404 | 205 | 187 |
| R76483 | Limestone | 0.0543 | 0.7065 | 0.70633 | 319 | 6 |
| R76502 | Ore assemblage calcite | 0.0245 | 0.7047 | 0.70463 | 118 | <1 |
| R76335 | Ore assemblage calcite | 0.0162 | 0.7046 | 0.7046 | 178 | <1 |
| R76357 | Ore assemblage calcite | 0.0222 | 0.7048 | 0.70473 | 130 | <1 |
| Location | Sample | 206Pb/204Pb | 207Pb/204Pb | 208Pb/204Pb |
|---|---|---|---|---|
| Biggenden | R76448 | 18.505 | 15.602 | 38.369 |
| R76441 | 18.514 | 15.613 | 38.391 | |
| Ban Ban | BB1 | 18.472 | 15.589 | 38.315 |
| BB3 | 18.495 | 15.625 | 38.406 | |
| Mt Mudlo | MM22 | 18.466 | 15.602 | 38.350 |
| MM44A | 18.480 | 15.620 | 38.401 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edraki, M.; Somarin, A.K.; Ashley, P.M. A Genetic Model for the Biggenden Gold-Bearing Fe Skarn Deposit, Queensland, Australia: Geology, Mineralogy, Isotope Geochemistry, and Fluid Inclusion Studies. Minerals 2025, 15, 95. https://doi.org/10.3390/min15010095
Edraki M, Somarin AK, Ashley PM. A Genetic Model for the Biggenden Gold-Bearing Fe Skarn Deposit, Queensland, Australia: Geology, Mineralogy, Isotope Geochemistry, and Fluid Inclusion Studies. Minerals. 2025; 15(1):95. https://doi.org/10.3390/min15010095
Chicago/Turabian StyleEdraki, Mansour, Alireza K. Somarin, and Paul M. Ashley. 2025. "A Genetic Model for the Biggenden Gold-Bearing Fe Skarn Deposit, Queensland, Australia: Geology, Mineralogy, Isotope Geochemistry, and Fluid Inclusion Studies" Minerals 15, no. 1: 95. https://doi.org/10.3390/min15010095
APA StyleEdraki, M., Somarin, A. K., & Ashley, P. M. (2025). A Genetic Model for the Biggenden Gold-Bearing Fe Skarn Deposit, Queensland, Australia: Geology, Mineralogy, Isotope Geochemistry, and Fluid Inclusion Studies. Minerals, 15(1), 95. https://doi.org/10.3390/min15010095