Serpentinite Applications: Effects of Surface-Ions-Modified Natural Silicate Minerals on Cultivation of Magnesium–Manganese-Enriched Garlics
Abstract
1. Research Background and Objectives
1.1. Growth Characteristics of Garlic
1.2. Varieties and Flavors of Garlic
- China: China is the world’s largest producer of garlic. It grows several types of garlic, including red, white, and green garlic. Red garlic is one of the most prominent varieties of garlic and is known for its strong, spicy flavor and firm texture. White garlic is another common variety with a milder taste and softer texture. Green garlic comprises tender stalks that are harvested at an early stage; it is highly nutritious and offers a fresh taste.
- France: French garlic is best represented by Rose de Lautrec, a variety distinguished by its strong fragrance. Rose de Lautrec is mainly grown in certain regions in southern France, where the climate and soil are particularly well suited for garlic growth.
- Spain: The most famous variety in Spain is Provence Wight, a white garlic known for its intense spiciness and aroma.
1.3. Chemical Composition and Health Benefits of Garlic [4,5,6]
1.4. Preservation and Consumption of Garlic [7]
1.5. Importance of Magnesium and Manganese to the Human Body [8,9]
1.6. Characteristics of Natural Serpentinite Materials [10,11,12,13,14,15,16,17,18,19]
1.7. Cultivation and Characteristics of Magnesium–Manganese-Enriched Garlic
2. Experimental Procedures and Methods
2.1. Preparation of Serpentinite Powder and Immersion Plating and Sintering
2.2. Ion Release Test
2.3. Garlic Cultivation Test and Composition Analysis
3. Results and Discussion
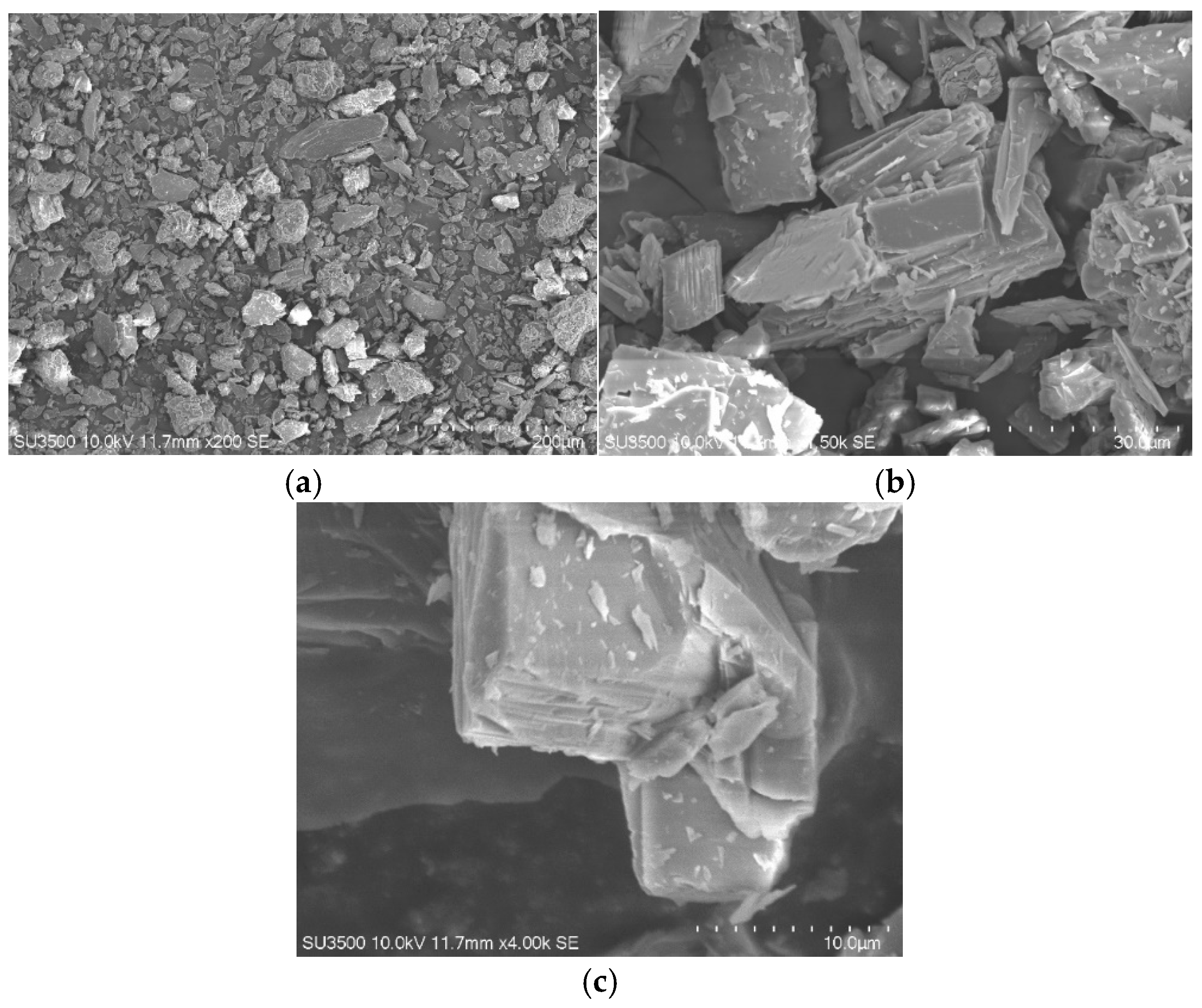
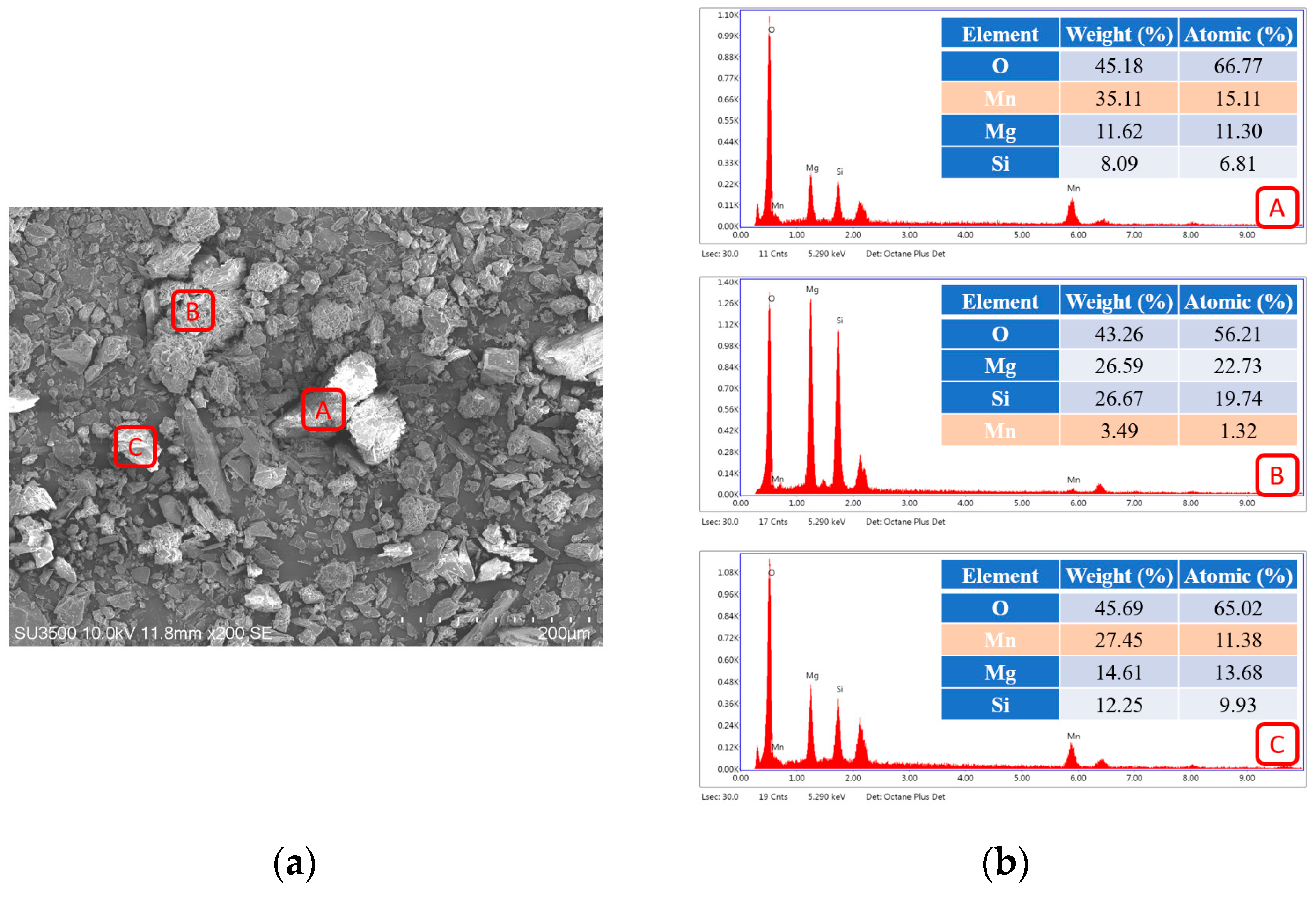
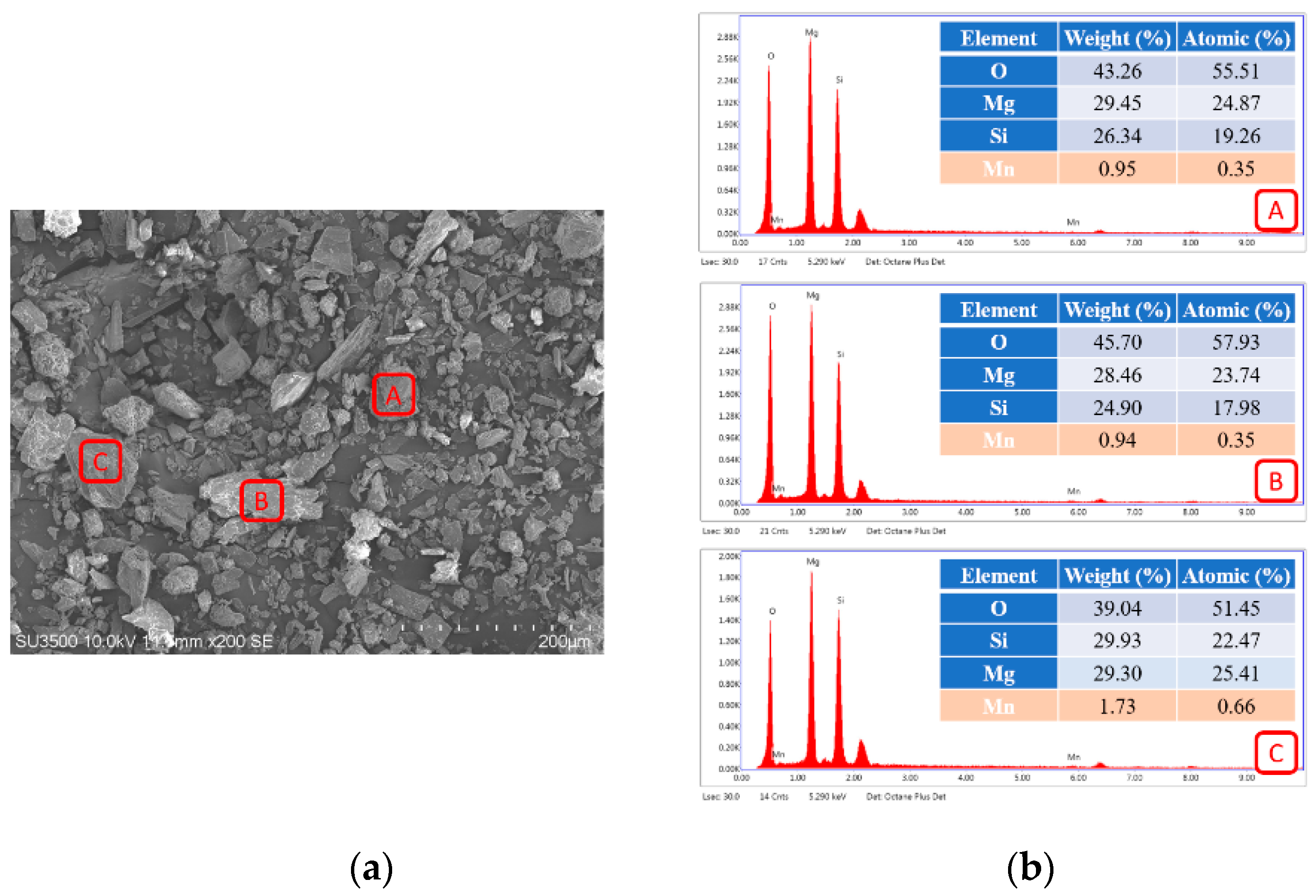
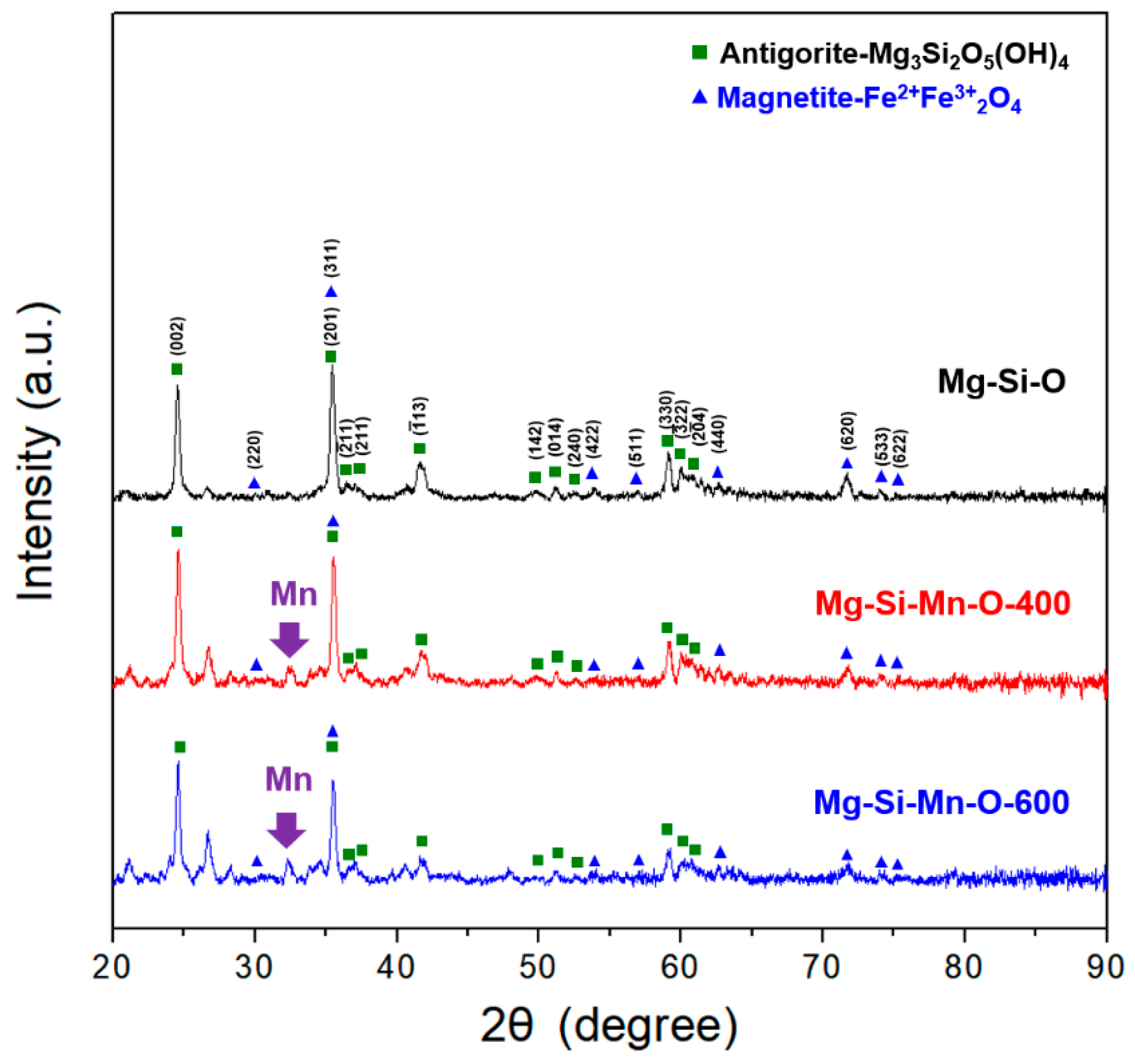
3.1. Characteristics of Serpentinite Powder and Surface Manganese Modification
3.2. Cultivation and Growth Characteristics of Magnesium–Manganese Garlic
4. Conclusions and Future Prospects
Funding
Data Availability Statement
Conflicts of Interest
References
- Labu, Z.K.; Rahaman, M. Proven Health Benefits of Garlic—A Review; Department of Pharmacy, World University of Bangladesh (WUB): Dhaka, Bangladesh, 2019. [Google Scholar]
- Petrovska, B.B.; Cekovska, S. Extracts from the history and medical properties of garlic. Pharmacogn. Rev. 2010, 4, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yao, H.-P.; Huang, F.-F.; Wu, W.; Gao, Y.; Chen, Z.-B.; Liang, Z.-Y.; Liang, T.-B. Allicin, a major component of garlic, inhibits apoptosis in vital organs in rats with trauma/hemorrhagic shock. Crit. Care Med. 2008, 36, 3226–3232. [Google Scholar] [CrossRef] [PubMed]
- Supakul, L.; Pintana, H.; Apaijai, N.; Chattipakorn, S.; Shinlapawittayatorn, K.; Chattipakorn, N. Protective effects of garlic extract on cardiac function, heart rate variability, and cardiac mitochondria in obese insulin-resistant rats. Eur. J. Nutr. 2014, 53, 919–928. [Google Scholar] [CrossRef]
- Padiya, R.; Khatua, T.N.; Bagul, P.K.; Kuncha, M.; Banerjee, S.K. Garlic improves insulin sensitivity and associated metabolic syndromes in fructose fed rats. Nutr. Metab. 2011, 8, 53. [Google Scholar] [CrossRef]
- Madkor, H.R.; Mansour, S.W.; Ramadan, G. Modulatory effects of garlic, ginger, turmeric and their mixture on hyperglycaemia, dyslipidaemia and oxidative stress in streptozotocin–nicotinamide diabetic rats. Br. J. Nutr. 2010, 105, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Fumia, A.; Cascio, P.L.; Miller, A. Immunostimulant and Antidepressant Effect of Natural Compounds in the Management of COVID-19 Symptoms. J. Am. Nutr. Assoc. 2022, 41, 840–854. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C. Combating COVID-19 and Building Immune Resilience: A Potential Role for Magnesium Nutrition? J. Am. Coll. Nutr. 2020, 39, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Kiss, S.A.; Forster, T.; Dongó, A. Absorption and Effect of the Magnesium Content of a Mineral Water in the Human Body. J. Am. Coll. Nutr. 2004, 23, 758S–762S. [Google Scholar] [CrossRef] [PubMed]
- Micke, O.; Vormann, J.; Kisters, K. Magnesium and COVID-19–Some Further Comments–A Commentary on Wallace TC. Combating COVID-19 and Building Immune Resilience: A Potential Role for Magnesium Nutrition? J. Am. Coll. Nutr. 2021, 40, 732–734. [Google Scholar] [CrossRef] [PubMed]
- Morandi, N.; Felice, G. Serpentine minerals from veins in serpentinite rocks. Mineral. Mag. 2018, 43, 135–140. [Google Scholar] [CrossRef]
- Harrison, B.M.; Priest, F.G. Composition of peats used in the preparation of malt for Scotch whisky production-Influence of geographical source and extraction depth. J. Agric. Food Chem. 2009, 57, 2385–2391. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, S.; Honda, T.; Fujii, K.; Funakawa, S.; Iwai, K.; Kosaki, T. Does soil chemical characteristics affect the brewer’s rice quality? A case study in Hyogo prefecture. Int. J. Tour. Sci. 2016, 9, 125–129. [Google Scholar]
- Emami, S.M.; Ramezani, A.; Nemat, S. Sintering behavior of waste serpentine from abdasht chromite mines Abdasht chromite mines and kaolin blends. Ceram. Int. 2017, 43, 15189–15193. [Google Scholar] [CrossRef]
- Chittenden, E.T.; Stanton, D.J.; Watson, J.; Dodson, K.J. Serpentine and dunite as magnesium fertilizer. N. Z. J. Agric. Res. 2012, 10, 160–171. [Google Scholar] [CrossRef]
- Masoud, M.A.; Rashad, A.M.; Sakr, K.; Shahien, M.G.; Zayed, A.M. Possibility of using different types of Egyptian serpentine as fine and coarse aggregates for concrete production. Mater. Struct. 2020, 53, 87. [Google Scholar] [CrossRef]
- El Maghrabi, A.H.; El-Rabiee, M.M.; Metwally, B.S.; Masoud, M.A.; Abdelaziz, M.H.; Petrounias, P.; Koukouzas, N.; Zayed, A.M. From Hazardous Chrysotile and Polyamide Wastes into Sustainable Serpentine/Polyamide Nanocomposite Membrane: Fabrication, Characterization, and Environmental Application. Sustainability 2023, 15, 7060. [Google Scholar] [CrossRef]
- Masoud, M.A.; El-Khayatt, A.M.; Shahien, M.G.; Bakhit, B.R.; Suliman, I.I.; Zayed, A.M. Radiation Attenuation Assessment of Serpentinite Rocks from a Geological Perspective. Toxics 2022, 10, 697. [Google Scholar] [CrossRef] [PubMed]
- O’Hanley, D.S. Serpentinites: Records of Tectonic and Petrological History; Oxford Monographs on Geology and Geophysics; Oxford University Press: Oxford, UK, 1996; Volume 34, p. xiii + 277. [Google Scholar]



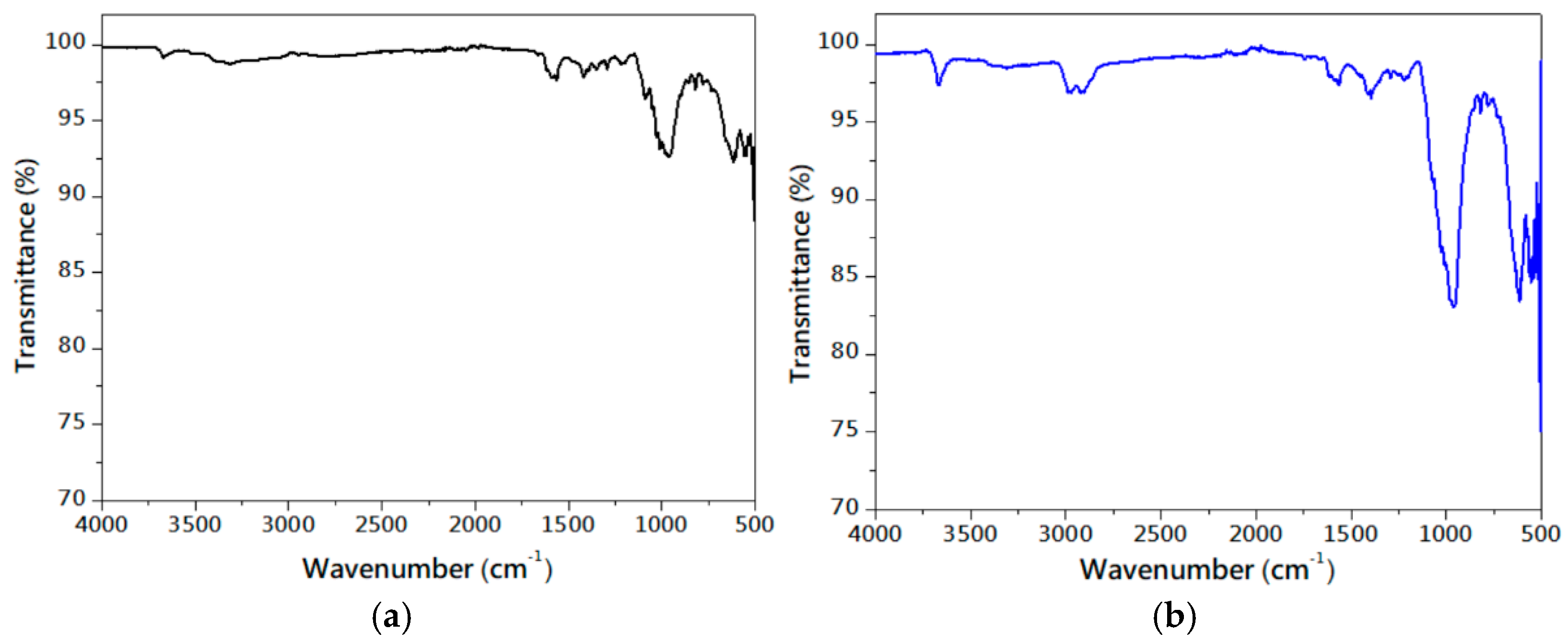
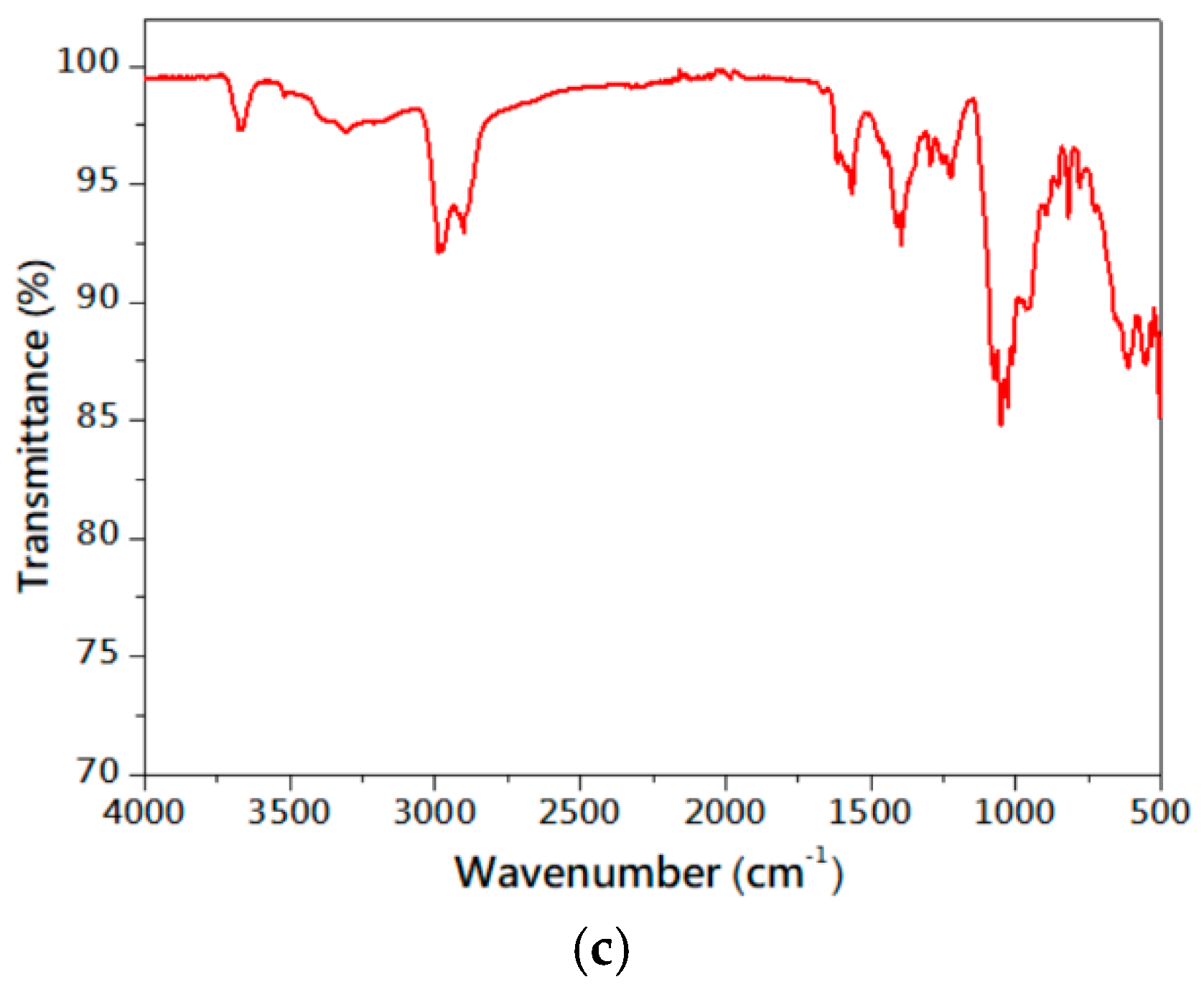
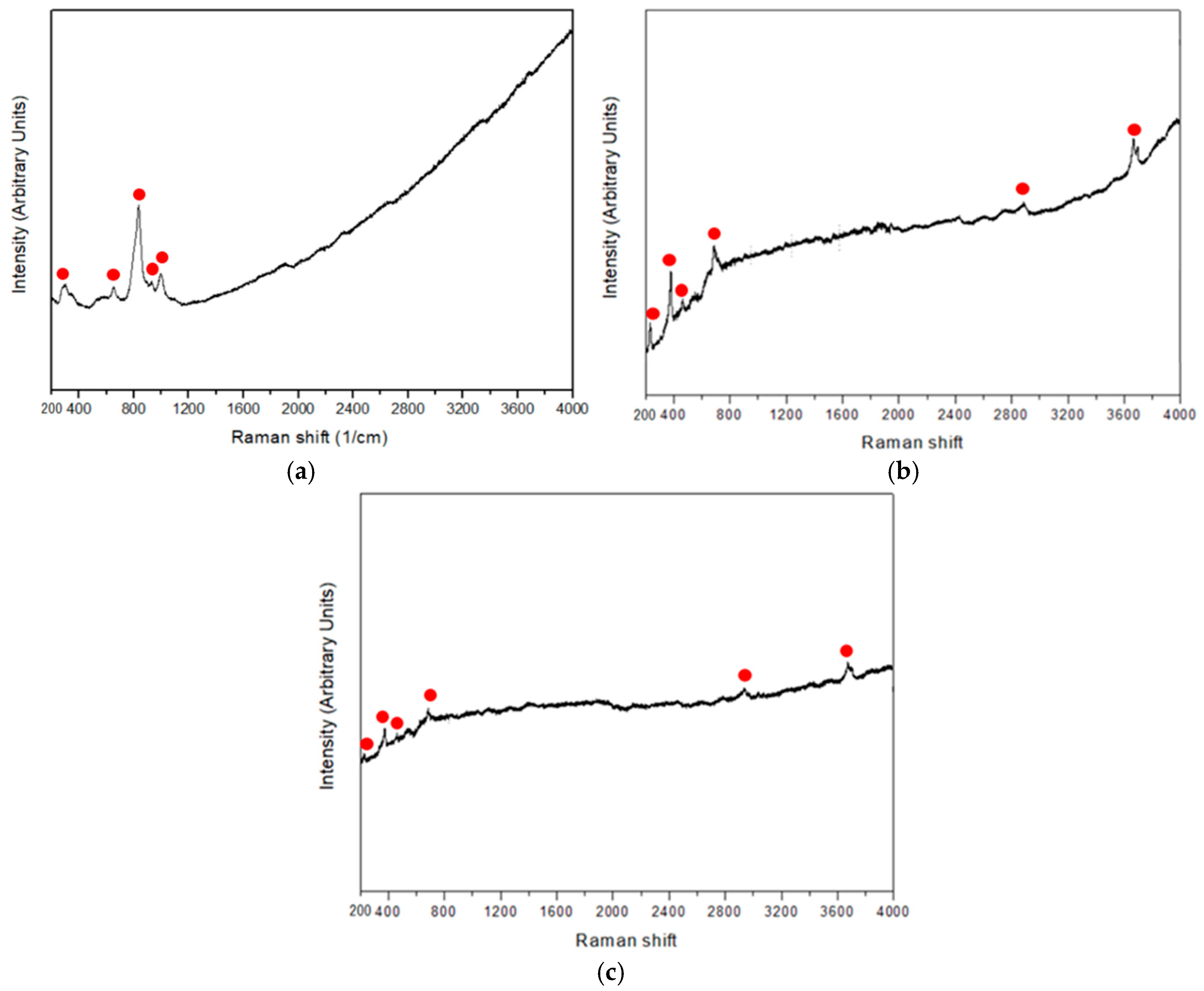

| mg/L | Mg-Si-O | Mg-Si-Mn-O-400 | Mg-Si-Mn-O-600 | Mg-Si-Mn-O-800 |
|---|---|---|---|---|
| Mg ion | 80.33 | 110.92 | 133.81 | 90.18 |
| Mn ion | N/A | 38.76 | 16.59 | 1.90 |
| Mg-Si-O | Mg-Si-Mn-O-400 | Mg-Si-Mn-O-600 | Mg-Si-Mn-O-800 | |
|---|---|---|---|---|
| m2/g | 42.82 | 61.50 | 60.92 | 56.47 |
| Na | P | K | Ca | Mg | Fe | Zn | Mn | pH | |
|---|---|---|---|---|---|---|---|---|---|
| mg/g | 10.2 | 35.4 | 158.2 | 111.7 | 0.5 | 1.6 | 0.8 | 0.0 | 7.2 |
| Mg Ion (mg/L) | Mn Ion (mg/L) | Allicin Content (mg/g) | Sulfur Compounds (mg/g) | Moisture Content (%) | |
|---|---|---|---|---|---|
| Magnesium–manganese water–wet garlic | 38.22 | 17.42 | 12.85 | 8.53 | 42.15 |
| Distilled water–wet garlic | 1.81 | 0 | 10.14 | 7.34 | 40.57 |
| Magnesium–manganese water–dried garlic | 42.92 | 11.72 | 11.40 | 8.04 | 26.00 |
| Distilled water–dried garlic | 0.46 | 0 | 5.70 | 6.01 | 24.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, F.S. Serpentinite Applications: Effects of Surface-Ions-Modified Natural Silicate Minerals on Cultivation of Magnesium–Manganese-Enriched Garlics. Minerals 2025, 15, 62. https://doi.org/10.3390/min15010062
Hung FS. Serpentinite Applications: Effects of Surface-Ions-Modified Natural Silicate Minerals on Cultivation of Magnesium–Manganese-Enriched Garlics. Minerals. 2025; 15(1):62. https://doi.org/10.3390/min15010062
Chicago/Turabian StyleHung, Fei Shuo. 2025. "Serpentinite Applications: Effects of Surface-Ions-Modified Natural Silicate Minerals on Cultivation of Magnesium–Manganese-Enriched Garlics" Minerals 15, no. 1: 62. https://doi.org/10.3390/min15010062
APA StyleHung, F. S. (2025). Serpentinite Applications: Effects of Surface-Ions-Modified Natural Silicate Minerals on Cultivation of Magnesium–Manganese-Enriched Garlics. Minerals, 15(1), 62. https://doi.org/10.3390/min15010062









