Abstract
Rare earth is an important strategic resource and a key mineral resource for global competition. As the depletion of primary rare-earth resources increases, a great number of rare-earth secondary resources, such as waste phosphor powder collected from fluorescent lamps, cathode-ray tubes, and other luminescent materials, continue to be generated and accumulated. How to achieve the low-carbon extraction and green and efficient utilization of these resources has become an urgent problem to be solved. In recent years, preliminary enrichment methods, such as flotation, magnetic separation, and adsorption, chemical methods, such as acid leaching and alkaline fusion, external-field-enhanced methods (including mechanical activation, microwave and oxidant, green solvent, etc.), and solvent extraction have been used for the separation and extraction of rare-earth elements (REEs), such as Y, Eu, Ce, Tb, La, and Ga, from waste phosphors. In this article, we systematically summarized the research progress of commonly used separation and extraction methods for REEs in waste phosphor powders, analyzed the advantages, disadvantages, and existing problems of different methods, and proposed potential directions for future research.
1. Introduction
Rare-earth elements (REEs) are a set of 17 elements, which include Y, Sc, and lanthanide family elements (such as La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu). Rare-earth resources are nonrenewable and scarce strategic resources, known as the vitamins or spices of industry and the mother of new materials [1]. They are widely used in energy, electricity, new materials, metallurgy, petrochemicals, and other fields [2,3,4]. Their sustainable development is directly related to a country’s economic and social development, industrial security, energy security, and national defense security [3,5,6,7]. For example, Figure 1 gives the plots of global REE reserves and rare-earth ore production for 2023 as announced by the United States Geological Survey (USGS) [8]. It can be seen from Figure 1 that China’s reserves account for 40% in 2023, followed by Vietnam, Brazil, Russia, and Australia. China’s rare-earth ore production will be 69% in 2023, followed by the United States, Myanmar, Australia, and Thailand. Over the last several years, as the rapid development of high-tech, economy, and society, the consumption of rare-earth mineral resources has accelerated, and the primary rare-earth resources are increasingly depleted. The resource utilization rate is still at a low level during the extraction process, but environmental pollution problems are becoming increasingly serious. The uneven application of REEs and the difficulty of high-purity and high-value rare-earth transformation are also significant. The protection and restrictions on rare earths are becoming increasingly strict in various countries in the world. Therefore, it is important to seek new ways to obtain more rare-earth resources.
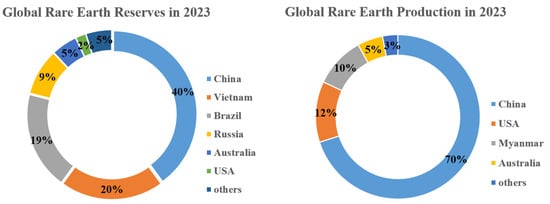
Figure 1.
Global rare-earth reserves and production in 2023.
During the production of rare-earth products and devices, the generation of a large amount of rare-earth waste is inevitable. At the same time, as the service life expires, a large number of retired or scrapped devices and equipment will be produced, such as waste/scrap rare-earth fluorescent powders, waste rare-earth magnetic materials, waste rare-earth hydrogen storage materials, waste rare-earth new alloys, etc. These solid wastes contain a large amount of renewable and recycled REEs. If these rare-earth secondary resources can be maximally recycled, it will significantly cut down the demand for nonrenewable primary rare-earth mineral resources, protect relatively scarce limited mineral resources, extend the service life of minerals, and reduce the impact of waste emissions on the natural environment [9,10].
With the execution of the Minamata Convention on Mercury, LCD smart TVs, display systems, and LED components are becoming increasingly used. At the same time, traditional cathode-ray tubes (CRTs) and fluorescent lamps made with rare-earth phosphors as solid luminescent materials are gradually being scrapped on a large scale. The traditional processing methods for these rare-earth fluorescent powder devices are mainly landfill and incineration, which lead to an enormous potential threat to the environment and human health [11,12,13,14]. However, these solid wastes contain a large amount of rare-earth fluorescent powder. The main REEs in these rare-earth fluorescent powders are Y, Eu, Ce, Tb, La, and Ga. According to statistics, the total content of REEs in waste rare-earth fluorescent powder can reach 23%, exceeding the lowest industrial grade of ion adsorption rare-earth ore by more than 250 times and the lowest industrial grade of primary rare-earth ores by more than 15 times [15,16,17,18]. These solid wastes contain many high-priced REEs, among which the content of recoverable REEs is more than 20%, mainly yttrium, europium, terbium, and cerium REEs. They are a rare-earth secondary resource with great potential for recycling [19]. Therefore, the recycling and recovery of REEs from waste phosphors were hot topics of global concern. In recent years, various methods for recovering REEs from secondary waste materials such as waste phosphors have been developed and have made definite progress [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115], and some review articles can be found in the references [20,21,22,23,24,25]. In this paper, we mainly focused on the progress, the flow diagram, and the advantages and disadvantages of each developed method used in recycling the REEs of Y, Eu, Ce, Tb, La, and Ga from waste phosphors in recent years. The prospects and direction for future development were also discussed.
2. Extraction and Separation of REEs from Waste Phosphors
Rare-earth fluorescent powders are mainly applied in fluorescent lamps as tricolor phosphors, accounting for over 90% of the phosphors. Rare-earth tricolor fluorescent powder is a mixture of red powders (33%~71%), green powder (29%~40%), and blue powder (0%~30%). Red powder is mainly composed of Eu and Y oxide system phosphors [20,21,22,23,24,25]. Blue and green powder contains not only rare-earth oxides but also a large amount of other metal oxides. Green powder and blue powder have various systems, such as the aluminate type and phosphate type, but the aluminate system is predominant [23,24,25,26]. The specific compositions and properties of fluorescent powders are shown in Table 1. The wavelengths and color coordinates of the fluorescent powders are the properties of the fluorescent powders, so we also included them in Table 1. The wavelength is the wavelength of the excitation light source. Color coordinates are one of the important characteristics of phosphors, which can help us accurately describe the color of the light emitted by phosphors. The color coordinates consist of three axes of the X, Y, and Z, which determine the color position of the fluorescent powder emitting light. Different phosphors have different color coordinates and can emit light of different colors to meet the needs of different application scenarios. The color coordinates of fluorescent powder not only affect its color performance but also have an impact on its brightness. The commonly used fluorescent powder systems are phosphate and aluminate, while borate and silicate are not yet fully developed. They can be used alone or in combination. The sintering temperature of phosphate is lower than that of the aluminate system, but its color development is not as good as that of the aluminate system, and its stability under high pressure is poor. Aluminum compounds are widely used due to their high UV irradiation intensity, strong resistance to UV aging, large luminous efficiency, and excellent stability at high temperatures. They have stable structures and complex compositions and are generally insoluble in acids or bases under normal conditions. So it also increases the difficulty of their recycling and utilization. Therefore, the focus and difficulty of recycling will be on the aluminate system and blue and green powders. The main REEs to be recovered from waste rare-earth fluorescent powders are Y, Eu, Ce, Tb, La, and Ga [30].

Table 1.
The classification of phosphors.
The recovery and recycling of REEs such as Y, Eu, Ce, Tb, La, and Ga from waste phosphor mainly involves three steps. First, physical methods are used to preliminarily enrich the REEs in the waste rare-earth fluorescent powder. Second, chemical methods are applied to deeply recover and recycle REEs from the waste rare-earth fluorescent powder [29]. Finally, the purification and product are prepared. The commonly used process for the extraction and separation of the REEs from waste phosphors is shown in Figure 2. There are four processes for treating the waste phosphors, such as dismantling, preliminary concentration, leaching, separation, and purification. The approaches include physical and chemical methods. Physical methods include flotation, adsorption, and magnetic separation [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. The preliminary enrichment of waste phosphor can be achieved through physical methods. Chemical methods include acid leaching, alkaline fusion, external field-enhanced methods, green solvent methods, solvent extraction methods, and so on [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115]. The application of chemical methods, such as leaching, extraction and stripping, precipitation, or electrolysis, can achieve high recovery efficiencies of REEs.
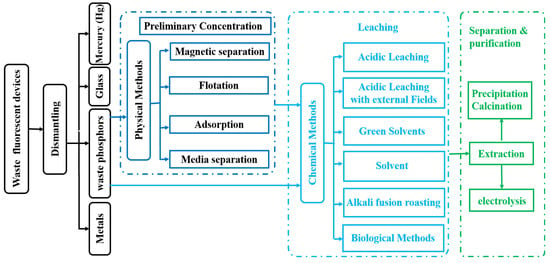
Figure 2.
Flow diagram of recycling waste fluorescent devices.
2.1. Physical Methods
The physical method mainly utilizes the physical property differences such as the particle size, density, magnetic susceptibility, surface hydrophilicity, and hydrophobicity of different types of fluorescent powders in waste phosphors for separation and enrichment. Commonly used methods include centrifugal separation, flotation, and magnetic separation [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45].
2.1.1. Magnetic Separation Method
Magnetic separation depends on the divergence in magnetic susceptibility and can effectively separate and recover different types of fluorescent powders with an HGMS (high-gradient magnetic separation) and magnetic Archimedes’s method. Tanaka [28] extracted REEs from waste phosphors with a high-gradient magnetic separator. Due to the higher magnetic susceptibility of green powder, after three repeated magnetic separations, it can be collected by rinsing with deionized water to obtain green powder with a purity greater than 99%. Masaru et al. [29] used HGMS technology to separate and recover green powder (LaPO4:Ce3+, Tb3+) from waste phosphors. The iron particles in the waste phosphors were removed by setting the magnetic-field strength at 0.02 T. After the suspension, the fluorescent powder precipitated into the solid layer for 3 h, while the fine Al particles remained in the suspension. Al particles were removed by solid–liquid separation. After repeating operations three times, the purity of LaPO4:Ce3+, Tb3+ reached 87%, and the luminescence intensity reached 97% of the original green powder. This indicates that the green powder recovered with flotation can be used for fluorescent lamp manufacturing.
HGMS has been employed in industrial production in Japan because of its advantages of high production efficiency and low cost in the separation and recycling of waste phosphors. However, this method can only separate blue and green powders with significantly different magnetic susceptibility, and the separation effect is poor for red powders with similar magnetic susceptibility. On this basis, the magnetic Archimedes’s method was proposed, which utilizes the difference in the suspension position of each target particle in the medium for separation. In the axisymmetric magnetic field generated by high-temperature superconductors, the suspension position of particles is determined by both magnetization and density [30,31]. Yamashita et al. [31] used the separation technology of a high-gradient magnetic separator to recover waste phosphors LaPO4:Ce3+, Tb3+ (LAP); impurities of iron oxide in waste phosphors can be effectively removed. After three repeated magnetic separations, it was found that the purity of the extracted waste phosphors could reach 87%. Wada et al. [32,33] successfully recovered a single fluorescent powder from a mixture of discarded fluorescent powders using the magnetic Archimedes method. Moreover, by combining the HGMS method, the separation of different fluorescent powders from waste fluorescent powders has been successfully achieved, providing a reference for its industrial application. However, due to its low processing efficiency and high cost, this method is unfit for large-scale industrial application, and further research is needed. Recently, Kuge et al. [34] designed a magnetic field-controlled chromatography method for separating rare-earth fluorescent powders from waste fluorescent lamps. By optimizing the inherent magnetization of the phosphor particles and process parameters, LaPO4:Ce3+, Tb3+ with a purity of up to 95.3% and a recovery rate of up to 93.6% was obtained.
2.1.2. Flotation Method
The flotation method is widely used in the field of mineral production and processing, depending on the differences in hydrophilicity and hydrophobicity of mineral surfaces, as well as the different binding abilities of mineral particles and bubbles, to achieve separation effects. Hirajima et al. [35] performed comparative research on the flotation effects of flotation agents such as dodecyl ammonium acetate (DDA), sodium oleate (NaOI), and sodium dodecyl sulfonate (SDS) on fluorescent powders under the condition of sodium silicate (Na2SiO3) as a dispersant. It showed that the pH of the solution has an important influence on the flotation effect. Under acidic conditions with a pH of 2.5, DDA had an 80% recovery effect on the red and green powders. Under alkaline conditions with a pH of 9.6, the recovery efficiency of SDS for rare-earth fluorescent powder can reach 90%. Due to the finer particle size of fluorescent powder compared to traditional mineral particles (diameter < 10 μm), a single flotation method is not effective in separating fluorescent powder.
Otsuki et al. [36] used dimethylformamide and heptane as incompatible phases to separate fluorescent powder using a two-step liquid-phase flotation method. DDA and sodium n-octane sulfonate were used as surfactants to sequentially separate blue and green powders, achieving the separation goal. A 90% efficiency of rare-earth fluorescent powder recovery can be achieved. Mei et al. [37] used a two-step dual-liquid flotation to separate tricolor fluorescent powders. The first step is to use the DDA as a cationic surfactant to extract and separate green fluorescent powders from the heptane/N, N-dimethylformamide (DMF) system. The second step is to use sodium 1-octanesulfonate as the anionic surfactant and a 99.5% ethanol treatment; the blue powder is then separated and collected, while the red powder remains in the DMF phase and is then subjected to two-step extraction through an artificial mixture. The experimental results showed that 90.9%, 95.2%, and 91.8% of red, blue, and green powders were separated, with grades of 95.3%, 90.0%, and 92.2%, respectively. At the same time, in the flotation research on fluorescent powder, two active agents were also used in combination [38], such as DDA + octadecylamine, sodium octane sulfonate + sodium octadecane sulfonate, etc. Wu et al. [39] used 1-dodecanyl-3-methylimidazolium chloride as a collector for flotation to recover the Y oxide (Y2O3) from fluorescent powder. With a pH 3 of, citric acid of 600 g·t−1, and 0.3 mg·L−1 dodecyl 3-methylimidazole chloride, the grade of Y2O3 increased from 30.51% to 56.58%, and the highest Y2O3 efficiency of recovery can reach 88.96%.
Common flotation agents for extracting waste phosphors included DAA, SDS, and sodium oleate. The flotation method can effectively separate various rare-earth phosphors and calcium halide phosphates from waste rare-earth phosphors. Compared to other methods, the process is simpler in terms of equipment and requires fewer chemical reagents, which is more conducive to environmental protection. However, due to the hydrophobicity and fine particle size of different types of rare-earth fluorescent powders and other oxides, flotation is difficult, resulting in low product purity and poor recovery efficiency.
2.1.3. Adsorption Method
The adsorption method is a method of using porous solids (adsorbents) to adsorb one or several solutes (adsorbents) in a solution in order to recover or remove these substances. Bai et al. [40] used diethylene glycol amino acid-modified chitosan sponge (CSs-DGAA) as an adsorbent to prepare a novel sponge-like adsorption device for REE recovery. This adsorbent can effectively recover Y and Eu from waste phosphors while avoiding environmental pollution caused by using organic solvents during liquid–liquid extraction. Arunraj et al. [41] applied a mixture of oxidized graphene Niger Aspergillus spores (GO-A, Niger spores) for adsorption and recovery of Eu. The maximum capacity of Eu (III) adsorption is 147.3 mg·g−1. The adsorbent used in this process can be extracted by EDTA, and so it has good reusability. Artiushenko et al. [42] used amino di (methylene phosphonic acid) silicon-based functional adsorbent (SiO2-AdPA) to extract REEs from waste phosphors. This adsorbent can effectively (over 90%) recover all REEs from strong acidic leachate with pH 2.0. It showed that 14 REEs were completely absorbed within 5 min at acidic conditions (pH greater than 2.0). The creation and usage of adsorbents can greatly improve the sustainability of rare-earth recovery processes and can be reused without causing environmental pollution. However, their adsorption and separation effects are far inferior to chemical methods, and most adsorbents cannot be utilized on a large scale, making practical application in engineering more difficult.
2.1.4. Media Separation Method
Besides flotation, magnetic separation, and adsorption, media separation (such as centrifugal and wind sorting) technology is commonly used to recover waste phosphors. Takahashi et al. [43] passed waste phosphors through a centrifugal wind sorting machine, and the study showed that the best separation effect of a tricolor fluorescent powder was achieved when the rotation speed and air flow rate were 5000 r·min−1 and 0.053 m3·s−1, respectively. As the enhancement of centrifugal speed increases, the efficiencies of rare-earth fluorescent powder recovery and halide phosphate fluorescent powder both increase, but the enrichment purity of rare-earth fluorescent powder decreases. This is mainly due to the influence of the shape of the fluorescent powder on the settling rate during wind separation, and its particle size is relatively fine, resulting in unclear separation efficiency. Hirajima et al. [44] applied centrifugal separation to extract and separate REEs from discarded fluorescent tubes. The results showed that when the concentration of NaOI was 5 × 10−5 mol·L−1, CH2I2 was used as the organic medium, the slurry density was 400 kg·m−3, and the centrifugal speed was 15,000 r·min−1, the waste phosphors were separated, and more than 97% of REEs were extracted. However, the method has high energy consumption, and CH2I2 is toxic, which can affect the environment, so it is used less frequently.
Currently, the strengths and weaknesses of the main physical methods for separating and recycling waste phosphors from fluorescent lamps are shown in Table 2. Although the physical separation method can achieve the recovery of waste rare-earth fluorescent powder and impurities, the recycled fluorescent powder products have different particle sizes, low purity, and low luminescence performance, and the regenerated fluorescent powder cannot meet production requirements. Therefore, physical methods mainly used for the enrichment of fluorescent powders. Improving the efficiency of rare-earth fluorescent powder recovery and decreasing the processing costs are the most prospective directions for the further development of fluorescent powder enrichment technology. To further recycle the waste rare-earth fluorescent powder, chemical methods were commonly used to recover the REEs.

Table 2.
The advantages and disadvantages of various physical methods.
2.2. Chemical Methods
2.2.1. Acid Leaching
Acid leaching is currently the most common method for recovering and recycling the REEs from waste phosphors, which involves leaching waste phosphors with different acids. Nowadays, sulfuric acid (H2SO4), hydrochloric acid (HCl), and nitric acid (HNO3) are commonly used as leaching agents [23]. It was shown that in rare-earth fluorescent powders, the red powder is more readily soluble in the inorganic acids (HCl, H2SO4, and HNO3), while blue and green powders have lower overall solubility. Moreover, there are significant differences in acid leaching methods between phosphate and aluminate fluorescent powders, with blue and green powders being more difficult to dissolve in aluminate systems [17].
Luidol et al. [46] used hot acid to dissolve green powders and blue powders. They found that the efficiencies of Tb, Ce, and Eu leaching were around 30% at 80 °C. Although they could dissolve some REEs in the fluorescent powder, the overall leaching efficiency was relatively low. In order to more efficiently recover REEs from waste phosphors, two-step acid leaching is proposed. The first step is to use low-concentration acid to dissolve Y and Eu oxides in the red powder. In the second step, high-concentration acid is used to leach and recover the REEs (La, Tb, Ce) in the poorly soluble blue and green powders. The chemical reaction [47] for leaching the REEs (Y, Eu, La, Tb, Ce) that occurs is illustrated in Equations (1) and (2). Equation (2) is mainly for the leaching of LaPO4:Ce2+, Tb3+ type green powder.
Ln2O3 + 6H+ = Ln3+ + 3H2O; Ln = Y, Eu
Ln(PO4) + 3H+ = Ln3+ + H3PO4; Ln = Ce, La, Tb
Yang et al. [48] used HCl and HNO3 to leach REEs from phosphate powders in a stepwise manner. The first step is acid leaching: Y is almost completely leached, and the leaching efficiency of Eu exceeds 95% in 5 mol·L−1 HSO4 at 100 °C for 6 h. Then, 5 mol·L−1 HNO3 is used to leach the residue at 100 °C, and all of the efficiencies of La, Tb, and Ce leaching exceed 90%. Liu et al. [49] employed a two-step acid dissolution method to recover and recycle the REEs from waste phosphate powders. The first step is to dissolve the red powder in acid. Then, the green powder and blue powder containing residues need to be roasted with sodium hydroxide and then leached with HCl solution. The total leaching efficiency of REEs using the two-step hydrochloric acid leaching method is 94.6%, which is 42.08 percentage points higher than the traditional method. A total of 99.05% Eu, 94.6% Y, 76.22% Tb, and 71.45% Ce was leached in the whole process.
Tunsu et al. [50] used acetic acid, ammonium chloride (NH4Cl), HCl, and HNO3, and pure water to recover REEs and mercury (Hg) from waste phosphors. It was found that pure water and 1 mol·L−1 NH4Cl are not good for REE leaching. The use of weak HNO3 and 0.5 mol·L−1 HCl solution is good for the leaching of the Eu and Y, with dissolution rates exceeding 95% and 97%, respectively, but not suitable for the REEs leaching of Tb, Gd, and Ce. HCl and HNO3 have the same leaching effect on REEs, while HCl has a higher recovery efficiency for mercury, with recovery efficiencies of 89.6% and 23.2% for HCl and HNO3, respectively. A 25% acetic acid solution can dissolve about 50% Eu, 75% Y, 10% other REEs, and slightly more than 2% Hg.
Innocenzi et al. [51] used HCl as the leaching solution for the separation of REEs from waste phosphors. It showed that Tb can basically completely dissolve, and recovery efficiency exceeded 70% with 2 mol·L−1 HCl, 5% slurry at 90 °C. Patil et al. [52] designed a method for simultaneously processing YOX and LAP with a hydrometallurgical approach to obtain high-purity rare earths. It was shown that effective recovery of Y, Eu, and Tb (target REEs) was achieved with 1, 25, and 55 stages in L/L extraction, respectively, with purities greater than 99%. In addition, the purity of Gd, La, Ce, and other REEs is also greater than 97%, 80%, and 60%, respectively. Lie et al. [53] studied the leaching of the REEs Eu and Y from waste cathode-ray tube phosphors (CRT) using response surface and central composite design methodology. It was found that temperature has the most impact on the leaching efficiencies of Eu and Y from waste CRT phosphors, followed by acid concentration and leaching time. All of the Eu and Y can be recovered with 1.15 mol·L−1 H2SO4, S/L ratio of 20 g·L−1 at 1 MPa for 27 minutes. The leaching efficiencies of Y and Eu can total 87.72% and 95.11% with pH 2 at 50 °C for 2 h precipitation, respectively. The insoluble REE (PO4) was formed in the present process.
The inorganic acids of H2SO4, HCl, and HNO3 were used to leach the REEs from the waste phosphors. From the published work, it can be seen that the acid leaching method can only achieve effective recovery of the REEs such as Eu and Y, and H2SO4 is the best among the three acids to leach the REEs such as Eu, Y, Ce, and Tb from waste phosphors. But the complex structure of aluminum salt of blue and green powders cannot be destroyed by the inorganic acid, resulting in the extremely low extraction rates of elements such as Ce and Tb with only 30%. The advantages of the acid leaching method for treating waste fluorescent powders are mainly high leaching efficiency of REEs, high purity of rare-earth products obtained, a mature process, low cost, and easy industrialization. However, a usually complicated stepwise leaching process should be used for the extraction and separation of the REEs, such as Ce, Tb, and Gd, from the blue and green powder. The high leaching efficiency of other impurity elements, such as aluminum, can be attained. The liquid-solid ratio and acid consumption are larger. The leaching efficiency of the Tb and Ce is still too low, and the further treatments of the leaching solution need to be performed and will produce a large amount of acidic wastewater, causing environmental pollution. For this reason, most researchers in the past have focused on the pretreatment of waste phosphors. Some scholars try to use alkali fusion roasting to treat fluorescent powders and to solve the problem of the low leaching efficiency of Tb, Ce, La, and Ga from blue and green powder by destroying their sharp crystal structures.
2.2.2. Alkaline Fusion
As mentioned in Section 2, the most commonly used blue and green fluorescent powder at present is the aluminate salts. From the discussion in the previous section, we know that rare earths in blue and green powders of the aluminate type are difficult to dissolve in traditional inorganic acids (such as HCl, H2SO4, and HNO3). To increase the leaching efficiency of REEs from the fluorescent powders and achieve rational resource utilization, alkaline fusion pretreatment is usually carried out before the process of acid leaching.
- (1)
- NaOH for Alkaline Fusion
Alkali fusion methods usually use alkali (including KOH, NaOH, Na2CO3, NaHCO3, etc.) to promote the dissociation of the aluminum salt structure in the fluorescent powder at a certain temperature, ultimately generating water-soluble compounds. Zhang et al. [54] used the “NaOH fusion-water leaching-acid leaching” method to separate REEs from the waste phosphors. After fusion roasting with a mass ratio of NaOH to waste powder of 2.5 at 1050 °C for 2 h and acid leaching at 65 °C for 2 h, the aluminum could be removed with water washing, and the total rare-earth leaching efficiency reached over 98%. Liao et al. [55] used the “alkaline fusion-acid leaching” approach to recover REEs from waste phosphors and revealed the reaction mechanism. It was shown that REEs and Al2O3 were changed into rare-earth oxides and NaAlO2 after alkaline fusion, respectively. The leaching efficiency of REEs reached 99.8% with 3 mol L−1 HCl in 1.5 h. Yu et al. [56] used NaOH to fusion roast the blue and green powders of waste fluorescent powder. It showed that the stable spinel structure of the green powder and blue powders was basically destroyed under the conditions of 70 wt.% NaOH and 280 °C for 4 h, and the obtained REEs and aluminum compounds can be dissolved in the leaching solution. The leaching efficiencies of Ce, Tb, Eu, and Al were 92.2%, 82.5%, 96.5%, and 99.4%, respectively.
Liu et al. [57] applied alkali fusion roasting to waste phosphors with NaOH at 600 °C for 2 h. The REE compounds in blue and green powders were decomposed to their oxides, and then HCl solution was used for leaching these oxides. The leaching efficiencies of Eu, Y, Tb, and Ce were 99%, 96%, 92%, and 81%, respectively. Shao et al. [58] applied the alkaline fusion with NaOH and the acid leaching approach to treat the waste phosphors; the separation efficiencies of Eu, Y, Tb, and Ce were 99.20%, 99.21%, 99.18%, and 99.11%, respectively, and the total leaching efficiency of REEs was 99.19%. A simple method for separating REEs from waste phosphors was developed by Xie et al. [59]. They used NaOH as the alkaline agent to fuse the waste phosphors to change the spinel structure of aluminum–magnesium compounds in the blue and the green powders. Then, the fusion slag is washed with water to eliminate aluminum, and the acid leaching is performed with FeCl2 as a reduction agent. The leaching efficiency of Ce, Eu, Y, and Tb reached 98.6%, 99.1%, 99.4%, and 98.8% in this process, respectively. Pramanik et al. [60] studied the process of recovering high-purity terbium oxide (Tb4O7) from fluorescent powders. The Y and Eu were selectively separated with 2 mol·L−1 HCl for 30 min, and then the leaching residue was roasted with NaOH. The impurities (such as Ba, Ca, Al, Mg, and P) were derived from the leaching residue containing Ce, La, and Tb by water washing. Finally, the residue was leached with 8 mol·L−1 HCl for 5 h to recover rare earths (La, Ce, and Tb). The Tb4O7 with a purity of 99.8% was obtained by oxalic acid precipitation and calcination.
As mentioned above, the chemical reactions between the NaOH and green and blue powders during alkaline fusion roasting are shown in Equations (3) and (4) [61,62]. The obtained principal products are Eu2O3, MgO, CeO2, Y2O3, and Tb2O3 [48] or Tb4O7 [62], BaO, and NaAlO2.
BMA + NaOH + CO2 → NaAlO2 + MgO + BaCO3 + Eu2O3 + H2O
CTMA + NaOH → NaAlO2 + MgO + CeO2 + Tb2O3 + H2O
- (2)
- Carbonates and Bicarbonates for Alkaline Fusion
Hua et al. [63] used sodium carbonate (Na2CO3) for alkaline fusion to decompose the stable spinel structure of the Ce0.67Tb0.33MgAl11O19. It was found that the Ce0.67Tb0.33MgAl11O19 was completely destroyed as Tb2O3, α-Al2O3, CeO2, and MgO at 1000 °C for 2 h roasting. Yurramendi et al. [64] applied the Na2CO3 to fusion roast phosphates [68]. The best effect was achieved with a sample and sodium carbonate mass ratio of 1:0.5 at 900 °C for 1 h. The fusion product was leaching with HCl and HSO4 solution. The ultimate leaching efficiency of La, Tb, and Ce was 81%, 85%, and 86%, respectively. When a waste rare-earth fluorescent powder is mixed with Na2CO3 for fusion roasting, Zeng et al. [69] found that the reaction between blue powder, green powder, and sodium carbonate during the calcination process can be expressed by Equations (5) and (6):
MgAl11O19:Ce3+, Tb3+ + Na2CO3 + O2 → CeO2 + Tb4O7 + NaAlO2 + MgO + CO2
BaMgAl10O19:Eu2+ + Na2CO3 → NaAlO2 + MgO + BaCO3 + Eu2O3 + H2O.
Using Na2CO3 and NaHCO3 as roasting agents [66] during the alkaline fusion process of waste phosphors, the Ce can be transformed to CeO2, Tb converted to Tb4O7, and Al to NaAlO2. With a NaOH and waste powder mass ratio of 2.5:1 at 900 °C for 2 h, the last leaching efficiency of REEs can attain 78.21%, and 99.05% of Al can be removed by water washing.
From the works published, through the Na2CO3 or NaHCO3 alkaline fusion process [62], the Ce, Eu, and Tb in waste phosphors are ultimately decomposed as CeO2, Eu2O3, and Tb4O7, respectively. The Al is in the form of K2Al2O4, while the Mg and Ba can be converted to MgO and BaCO3. Liu et al. [61] found that the temperature of the phase transition for alkaline fusion with Na2CO3, NaHCO3, and K2CO3 is at 900 °C, while it is only 500 °C for alkaline fusion with NaOH, as shown in Figure 3. So NaOH is better than other agents for alkaline fusion to decompose the magnesium aluminum spinel structure in blue and green powders at lower temperatures.
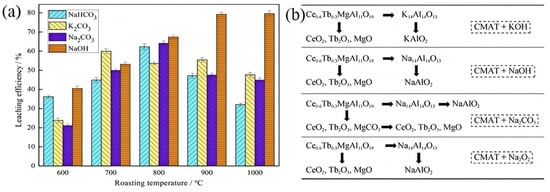
Figure 3.
(a) Effects of alkaline fusion of different chemicals on the leaching efficiency of REEs [Reproduced with permission from Ref. [59] published by Elsevier]. (b) Decomposition mechanism of BAM phosphors.
- (3)
- Other Agents for Alkaline Fusion
Wu et al. [70,71] used Na2O2 as an alkaline fusion agent to extract REEs from green powder ((Ce0.67Tb0.33)MgAl11O19). The REEs of Tb and Ce in the green powder were transformed into Na2CeO3 and Na2TbO with a mass ratio of Na2O2 to green powder of 1.5:1 at 650 °C for 50 min. A 99% leaching efficiency for REEs was obtained. The Ba(OH)2 was applied as a fusion agent to roast waste phosphors by Ippolito et al. [68]. The roasting product was leached by H2SO4, and the 80% Tb, 60% Ce, 63% Gd, 99% Y and Eu, and 65% La were achieved.
To avoid excessive oxidation, iron is applied as a reduction agent in the alkaline fusion roasting. Guo et al. [69] and Liang et al. [70] used the “reduced iron powder-alkaline fusion-acid leaching” approach to extract REEs from the waste phosphors. After alkaline fusion for 3 h at 700 °C with NaOH to iron powder mass ratio of 1.2:0.005, the residue was leached with HCl. It was shown that the leaching efficiency of Tb, Ce, Eu, and Y can be achieved as 99.37%, 98.12%, 99.93%, and 99.60% with leaching for 1 h in 3 mol·L−1 HCl, respectively. The total leaching efficiency of REEs achieved was about 99.35% with reduced iron powder involved.
In summary, the alkaline agents NaOH and KOH, sodium and potassium carbonates and bicarbonates, Na2O2, and iron powder are used for alkaline fusion. It can be found that the structure was destroyed for acid-insoluble blue and green powders in waste phosphors by using the alkaline fusion roasting approach. In addition, during fusion roasting, variable valence REEs such as Ce and Tb are inevitably oxidized by oxygen in the air to form high-valence rare-earth oxides of CeO2 and Tb4O7. The extraction efficiency of Tb and Ce in waste rare-earth fluorescent powder has significantly increased from about 30% with traditional acid leaching to about 80% through alkaline fusion roasting. Therefore, alkaline fusion roasting pretreatment is an effective way to extract and separate the REEs from waste blue or green fluorescent powder.
As shown in Figure 4, there are two processes of alkaline fusion, such as one-stage leaching and two-stage leaching. In one-stage leaching, the waste phosphors are roasted with the alkaline agents, and then go through water flashing, acid leaching, and filtration to obtain the mixture solution of REEs for further separation. In two-stage leaching, waste phosphors are first leached with inorganic acid to extract the Y and Eu (in the leaching solution). Then, the residues are dried and roasted with alkaline agents. The product of alkaline fusion is washed with water to remove the compounds of Ca and Al and so on. After that, the washing residue is leached with acid to extract REEs such as Tb, Ce, etc. From an economic viewpoint, it is recommended to use the one-stage leaching method, which is a shorter and energy-saving process that avoids the drying process.
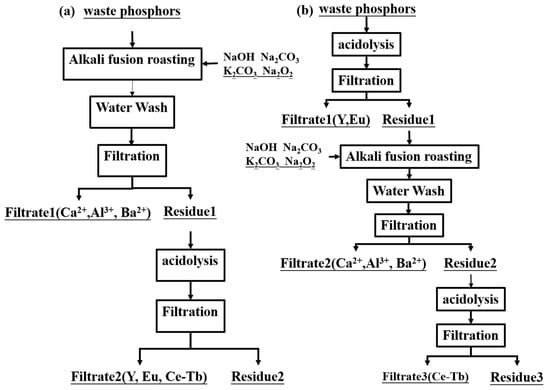
Figure 4.
Flow diagram of alkaline fusion roasting to recover REEs from waste phosphors. (a) alkaline fusion-water wash-acid leaching process (b) acid leaching-alkaline fusion-water wash process.
Owing to the generally higher price of potassium salt compared to sodium salt in the market, sodium salts are generally used in alkaline fusion research [71]. However, the alkaline fusion method usually requires a higher temperature, resulting in higher production and operation costs, although adding reduction agents such as iron powder during the alkaline fusion process can avoid the formation of high-valence rare-earth oxides and improve the extraction efficiency of REEs. However, the addition of reduction agents will introduce some new impurities into the system, thereby increasing the burden of the subsequent extraction and separation. Again, the alkaline fusion method consumes a large amount of alkaline agents, which increases the consumption of other chemical reagents in subsequent processes. Therefore, taking certain measures during the alkaline fusion process, without introducing new impurities, avoiding high-valence rare-earth oxide formation, improving the extraction efficiency of REEs, and exploring new technologies for green, efficient, and low-carbon treatment of waste phosphors, especially blue and green powders, are the directions for future efforts.
2.2.3. Acid Leaching with External Fields Enhanced Method
To avoid the problem that traditional acid leaching cannot treat the blue and green powders of waste phosphors, various methods such as adding hydrogen peroxide, mechanical activation, and microwave-assisted methods were used to destroy the complex structure of waste phosphors to improve the leaching efficiency of REEs in them [72].
- (1)
- Addition of H2O2 Oxidant
In hydrometallurgy, adding oxidants is a very effective method to improve the leaching efficiency. In order to improve the leaching efficiency of REEs in waste fluorescent powders, especially blue and green powders, some oxidants have been studied. Tian et al. [73] used an acid leaching process to leach the REEs from the fluorescent powder obtained from the waste cathode-ray tubes (CRTs). They added the H2O2 oxidant to the leaching system of H2SO4, which decreases the H2S release into the atmosphere. It showed that the leaching efficiency of Eu, Y, Al, and Zn can achieve 100%, 98.76%, 80%, and 45%, respectively. Miskufova et al. [74] found that approximately 90%–95% of Eu and Y was leached with 30 v/v% H2O2 and 0.4 M H2SO4 at 80 °C after 20 min leaching. Wu et al. [75] found that 61.2% of Eu and 99.9% Y can be leached from waste trichromatic phosphor powder with 1 mol/L HCl and 2 mL H2O2 (per 500 mL) for 4 h at 50 °C. Yin et al. applied H2SO4 and H2O2 to synergistically oxidize and extract the REEs [76]. The leaching efficiency of Eu and Y both attained 99% with 3 M H2SO4 and 4 vol% H2O2 addition at 55 °C for 1 h leaching. Choi [72] studied the effects of acidic medium and concentration, H2O2 addition, temperature, pulp density, and time on the selective leaching of red powder. It was found that 100% Y and over 95% Eu can be leached in the HCl system, while 92% Y and 96% Eu were obtained in the H2SO4 system. 96% Y and 91% Eu can be leached from waste phosphor powder in the HNO3 system. In these systems, the reaction kinetics are controlled by chemical reactions. From these studies, it can be seen that the addition of oxidants such as H2O2 significantly increases the leaching reaction rate and shortens the time.
- (2)
- Mechanical Activation
Mechanical activation mainly changes the surface, crystal structure, and particle size of substances through mechanical processes such as grinding, scraping, milling, polishing, and friction, and has wide applications in the field of comprehensive metal recovery from secondary resources [77,78,79]. Due to the stable and complex crystal structure of blue and green powders in rare-earth phosphors, they are substances that are difficult to dissolve in traditional acids or bases. Mechanical activation of rare-earth fluorescent powders has attracted much attention for its ability to effectively improve leaching efficiency and reaction efficiency and reduce reaction temperature, with good potential [80].
Waste phosphors with ball milling and without ball milling were leached with 1 mol·L−1 HCl solution for 1 h [80]. The leaching efficiency of Y and Eu for ball milling powder exceeded 70%, while those were less than 20% for powder without ball milling. By using the discrete element method to simulate and calculate the changes in collision energy during ball milling, it was found that the diameter of the grinding ball had a little effect on the Y leaching efficiency and was mainly affected by the energy of forward collision during ball milling [81].
The mechanical activation method often involves pretreatment of the waste phosphors and combining it with other treatment methods to improve the recovery efficiency of REEs. Li Y. et al. [82] used mechanically activated waste phosphors to recover REEs through a process of alkaline fusion with two-stage leaching. After roasting, the structure of aluminum magnesium spinel was destroyed, and then the roasting product was leached with sulfuric acid to separate the REEs. The leaching efficiency of Eu, Y, Tb, and Ce is significantly increased using their process. Tan et al. [83] employed mechanical activation to treat waste phosphors. Then, they used acid leaching to recover the REEs from the milled phosphors and found that mechanical activation can induce the spontaneous leaching of Tb, and the higher the rotational speed, the stronger the activation intensity, while the leaching efficiencies of Tb, Ce and La are all less than 1% with direct acid leaching. However, the leaching efficiencies of REEs Ce, Tb, and La increased to about 90% and the leaching efficiency of the Eu increased from 85% to 94.6%, and the leaching efficiencies of Y also increased from 80% to 93.1% with an activation time of 60 min and leaching 15 min. Song et al. [84] extracted REEs from tricolor phosphors using mechanical activation and acid leaching methods. Under the optimal conditions of 60 min ball milling, 2 mol·L−1 H2SO4, and leaching 60 min at 70 °C, the leaching efficiencies of Eu, Y, and Ce in waste phosphors were 91.1%, 96.3%, and 77.3%, respectively. The leaching efficiencies of Eu, Y, and Ce without ball milling were 42.3%, 46.7%, and 31.2%, respectively. They concluded that disordered crystallinity changes due to mechanical activation play a key role in improving the recovery efficiency of REEs in waste phosphors.
Van Loy et al. [85] showed that the leaching efficiencies of Tb were 16%, 81%, and 98% after a wet ball milling cycle method, ball milling leaching method, and mechanical activation with ball milling leaching method, respectively. It can be found that the method of simultaneous leaching and ball milling can significantly enhance the Tb recovery efficiency, and combining the leaching process with mechanical activation pretreatment shortens the time of the leaching process. He et al. [26] applied the alkaline and mechanical activation method to treat the waste phosphors. It showed that the recovery efficiency of REEs rapidly enhanced with the promotion of activation time and rotation speed. Under a 550 r·min−1 milling speed and a 3:1 mass ratio of alkaline to fluorescent powder for 60 min, the waste phosphors were pretreated to obtain activated slag. Then, the activated slag was leached with 2 mol·L−1 H2SO4 at 80 °C for 120 min. The total recovery efficiency of rare earths was 95.2%, and the best leaching efficiency of REEs Tb and Ce was about 89.8% and 85.0%, respectively.
Ippolito et al. [86] applied the H2SO4 leaching with the mechanical activation to prompt the extraction efficiency of REEs of Tb. The best conditions for the leaching were further identified, including 1 h of mechanical activation, 1 M H2SO4, 5% slurry density, and room temperature. Then, they used oxalic acid to precipitate and calcinate. The recovery efficiency of Tb using this method is 35%. Although this method has a lower recovery efficiency for REEs, if Ba(OH)2 is employed for thermal pretreatment, oxalic acid precipitation is leached with H2SO4 solution and calcined to obtain a rare-earth mixture, and the recovery efficiency of Tb is 66%. From these works, we can see that mechanical activation can minimize the acid consumption and effectively improve rare-earth leaching efficiency, but it has high energy consumption, and the mechanism is not fully understood.
Figure 5 shows a comparison between acid leaching, alkali activation, and mechanical activation. It can be seen from Figure 5 that alkali activation, such as alkaline fusion, has the best effect, followed by mechanical activation, and finally acid leaching.
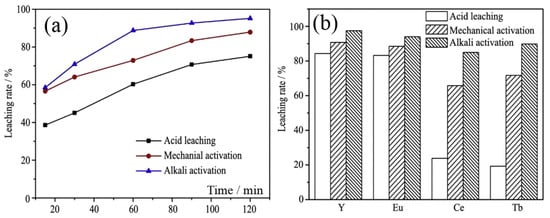
Figure 5.
Comparison of the effects of acid leaching, mechanical activation, and alkali activation on the leaching rate of rare earths (a) and leaching rates of REEs dissolved from waste phosphors (b). Reproduced with permission from Ref. [26] published by Elsevier.
- (3)
- Microwave-Assisted Extraction and Separation
The microwave-assisted method mainly utilizes the principle of microwave rapid heating, where components in the system are selectively heated owing to differences in their ability to absorb microwaves. Heating can change the structure of a substance and promote the rapid dissolution of volatile components in the solvent, thereby achieving the goal of rapid extraction.
Chen et al. [87] studied the waste of Lutetium Yttrium Orthosilicate (LYSO) scraps using a microwave-assisted acid leaching method. Over 99% of Lu ions can be recovered with 5 M HCl at 180 °C for 2 h of acid leaching under microwave assistance. Lie et al. [88] compared the microwave-assisted and conventional acid leaching of waste phosphors in waste cathode-ray tubes (CRTs). The leaching efficiencies of Eu and Y within 60 min were 100% and 78.07% with 2 mol·L−1 H2SO4 and a solid–liquid ratio of 10 g·L−1 and microwave power of 400 W, respectively. Compared to conventional leaching, microwave-assisted leaching shortens the reaction time by half. Shukla et al. [89] used a microwave-assisted acid roasting–acid leaching method to treat waste phosphors from waste fluorescent lamps. When the microwave roasted at 800 W for 3 min and the H2SO4 ratio was 1 mL·g−1, the dissolution rates of REEs of Tb, La, Eu, and Y were 93.6%, 39.6%, 100%, and 100%, respectively.
Lie et al. [90] recovered Eu and Y from waste CRT phosphors using the microwave-sealed leaching-oxalic acid precipitation calcination method. After leaching with a solid/liquid ratio of 10 g·L−1, 2 mol·L−1 H2SO4 for leaching for 30 min at 125 °C, the leaching efficiencies of Eu and Y were 100% and 86.67%, respectively. Compared to traditional heating methods, closed microwave leaching is a fast and efficient method for recovering Eu and Y from the waste CRT fluorescent powder. Bilen et al. [91] used the microwave-assisted leaching method to separate the Eu and Y from fluorescent lamp powders. It showed the highest efficiencies of Eu and Y recovery can be attained with 6 mol·L−1 HCl, a liquid–solid ratio of 100:1 for 90 min at 160 °C. The microwave extraction method has the advantages of rapid and green extraction and separation, but it requires high cost and is sensitive to solvent selection.
By enhancing the acid leaching method, the leaching effect of REEs from waste phosphors can be significantly prompted. Microwave-assisted, mechanical activation, and other methods can achieve higher leaching and recovery efficiencies than under traditional acid leaching in less time. However, the poor selectivity of the leaching process increases the difficulty of subsequent purification and separation. In addition, the treatment of waste liquid after leaching requires the use of a large amount of alkaline for neutralization, which increases the cost of treatment and equipment corrosion. To achieve efficient recovery of REEs from blue powders and green powders, it is necessary to destroy the stable lattice structure of the blue and green powders. Alkaline fusion is still the most frequently used and effective method.
2.3. Green Solvent Leaching Method
Green solvents, including the ionic liquids (ILs) and the deep eutectic solvents (DESs), have the characteristics of a broad liquid temperature range, high chemical and thermal stability, good solubility, and easy recovery and recycling. Green solvents are widely employed in various fields such as separation, electrochemistry, catalysis, etc. ILs are ion systems composed of small organic or inorganic anions and asymmetric big organic cations, which keep the liquid state at or near room temperature [92,93,94,95,96,97].
Deep eutectic solvents are formed by hydrogen bonding between donors and acceptors through the interactions of the hydrogen bond. Figure 6 shows the structural composition and characteristics of green solvents. Compared with traditional solvents, green solvents have the advantages of good solubility, design-ability, high stability, low vapor pressure, low volatility, and low environmental pollution. Moreover, various green solvents with different properties can be formed by modifying anions and cations or changing different donors or acceptors. Therefore, using green solvents to effectively recover REEs from waste phosphors can attain the goal of efficient and green separation and extraction.
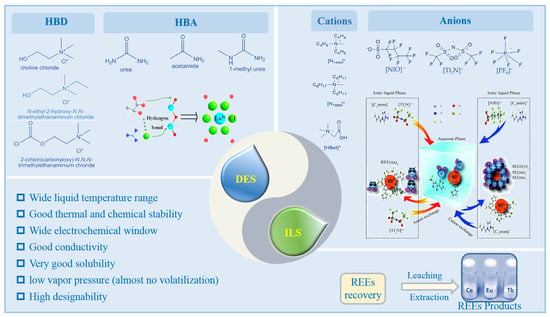
Figure 6.
Composition, property of green solvents for REE separation.The HBA:Hydrogen Bond Acceptors; HBD: Hydrogen Bond Donors; DES:Deep Eutectic Solvents; ILS: Ionic Liquids.
Dupont and Binnemans [98] used Brønsted acidic ionic liquid of [Hmim]HSO4 to selectively leach red powder in waste phosphors and then used oxalic acid to precipitate Y and Eu. Red powder can be obtained again after calcination. Schaeffer et al. [99] recovered 97.7 wt.% Eu and 91.6 wt.% Y by leaching waste phosphors with [Hmim]HSO4/H2O (1:1 wt.%) solution for 4 h. At the same time, the dissolved REEs are converted into oxide phase, forming recovered oxide (YOX) of (Y0.95Eu0.05)2O3 with only slight loss of leaching efficiency in four cycles, which can achieve the recycling of ILs. Pateli et al. [100] used the DESs of acetic acid/choline chloride to extract and separate the REEs from waste fluorescent powder. It was found that YOX phosphors have very high solubility and extremely low solubility for HALO phosphors with acetic acid/choline chloride (xChCl 0.33). They found that the activity of protons is more prominent in DES than in the metal ligand of chloride. Rodriguez et al. [101] provided a green approach to recover the REEs from waste fluorescent powder by methane sulfonic acid (MSA). YOX fluorescent powder can be selectively leached by adjusting the MSA concentration and temperature of leaching, HALO powder, and LAP fluorescent powder.
Jiang et al. [102] compared the methods of recovering REEs from red mud and waste phosphors by using the DESs of LevA/choline chloride. It was found that LevA is more suitable for the selective recovery of YOX. The leaching efficiency of REEs, except the Sc, was 100% after 60 h, while more than 98.7% of REEs from the fluorescent powder after leaching 96 h. Meanwhile, the selective leaching of YOX by hexylbetaine bis (trifluoromethanesulfonyl) imide ([Hbet]NTf2) was studied, and the leaching efficiency is also close to 100% within 48 h. Asadollahzadeh et al. [103] proposed a selective extraction technique for Y in waste fluorescent lamps by using an ionic liquid [C6mim]NTf2-supported membrane. A mixture of tributyl phosphates, imidazole ionic liquid, and D2EHPA was applied to make the carrier phase. The extraction of yttrium is 412.5 mg·L−1. It indicated that the supported IL membrane can be used as a low-cost and simple method for extracting Y from leachate. Hu [104] used the hydrophilic DESs of betaine/carrot acid to leach Eu and Y from waste phosphors. It was found that 93.12% of Eu and 99.38% of Y in the waste phosphors could be leached with a water content of 20% at 90 °C when leaching for 16 h. The behavior of the DESs was basically unchanged after five cycles.
Since the unique characteristics of the low volatility, good solubility, low cost, and easy availability of DESs, their effectiveness in leaching and extracting REEs is significant. The ILs or DESs used can be easily recycled and reused, with almost no impact on leaching efficiency and extraction efficiency. The research further confirms that deep eutectic solvents are an effective way to recovery REEs from secondary resources. However, the relevant work is only in the laboratory research stage, and the large-scale synthesis of green solvents has reduced costs and decreased viscosity to improve diffusion characteristics and promote leaching efficiency, which requires continuous attention.
2.4. Solvent Extraction
Solvent extraction is a separation technique for obtaining high-purity single REEs. The solvent extraction methods commonly used for REEs include supercritical fluid and solvent extraction. Commonly used extractants such as di-(2-ethylhexyl) phosphoric acid (D2EHPA), trialkyl-phosphine oxide mixture (Cyanex 923), N, N′, N′ tetraoctyl-3-oxoglutarylamide (TODGA), TBP (tributyl phosphonate), mixed phosphate oxide (Cyanex572), and 2-ethylhexyl phosphate mono-2-ethylhexyl ester (P507) can effectively extract and separate REEs from the leachate [105,106,107,108,109,110]. Innocenzi et al. [111] recovered Y from waste fluorescent lamps through solvent extraction. The separation of Ca and Y in the solution of acid-leaching fluorescent powder was studied by solvent extraction using extractant D2EHPA and Cyanex272 in kerosene. It showed that D2EHPA is more effective in separating Y than Cyanex272. The extracted Y can form the oxalate through oxalic acid precipitation, and the recovery efficiency of the leachate is about 90%. Yin et al. [112] used Cyanex 272 extraction to separate rare earths from H2SO4-H2O2 leaching solution. It showed that extraction efficiencies of Eu and Y were 87% and 99%, respectively, and can be obtained with 10/1 of A/O with Cyanex 272 for 20 min. Mishra et al. [112] used solvent extraction and the chemical reduction method to extract the Eu and Y from the acid leachate of waste phosphors. When the pH of the aqueous phase is 2.56 and 1 mol·L−1 D2EHPA is used, the separation factor of Eu3+ and Y3+ reaches as high as 60.6. Quantitative extraction of Y from the leachate was attained in two stages of counter current with a pH of 2.56 and an O/A of 1/1.5.
Due to the complex composition of metal ions in the leachate, in addition to rare-earth ions, there are also a large number of impurity ions (Al3+, Fe3+, Ca2+, etc.), which have an important effect on the efficiency of the extraction. Extractants such as D2EH-PA, P507, and TBP are easily affected by Al3+ and are prone to emulsification during the extraction process, leading to the appearance of a third phase and the inability to separate phases. Cyanex 923 and TODGA have lower saturation levels during the extraction process. PC-88A can effectively extract Y from the leachate after 15 stages of extraction, but the extraction and separation abilities for Tb and Eu are weak [108,109,110,111,112]. Although traditional extractants have beneficial effects on the extraction and separation of REEs in leachate, they also have drawbacks such as volatility, environmental pollution, and high toxicity.
Compared to traditional extractants, ionic liquids are the potential extractants for environmentally friendly and recyclable properties. Yang et al. [113] used bifunctional IL extractants (Bif ILEs) of [A336]P507 and [A336]P204 for the extraction and separation of REEs and metal ions. It showed that at lower pH, RE (III) in the loaded Bif ILEs can be effectively extracted, and the regenerated organic phase can be recycled. [A336]P507 and [A336]P204 are effective extractants for recovery and separation of REEs from waste phosphors. Wang et al. [114] successfully applied the bi-functional IL [N1888]CA12 for Y separation in highly concentrated rare-earth solutions. The approach based on the [N1888]CA12 IL has been tested to be 13 stages of extraction, 8 stages of washing, and 5 stages of stripping, which can obtain Y products with a purity of 99.1 mol%. Tunsu et al. [115] extracted REEs from the leaching solution of HNO3 with a mixture of 35% (v/v) of Cyanex 923/trialkyl-phosphine oxide. Oxides containing 99.96% REEs were obtained, which contained 94.61% Y and 5.09% Eu. The solvent extraction mechanism is described in Figure 7. It can be seen from Figure 7 that the structural characteristics of green solvents such as ILs enable their cations, anions, and themselves to bind well with rare-earth ions, thereby achieving selective and efficient extraction.
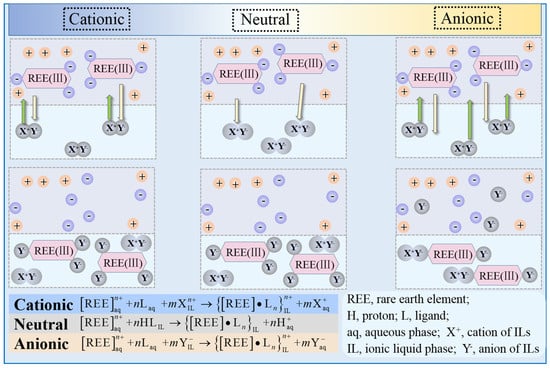
Figure 7.
The extraction mechanism of REEs in ILs.
As can be seen from published work, the use of extractants can efficiently extract REEs from the leaching solution. However, traditional organic extractants are very sensitive and volatile. ILs have better selectivity and saturation of rare-earth ions in leachate than traditional extractants and are environmentally friendly, making them highly potential extractants. However, the price of ionic liquid extractants is expensive, and there is a problem of dissolution in the liquid phase during the extraction process, leading to the loss of ionic liquids. The exploration of the extraction mechanism and the design and synthesis of new types of ILs with better performance are further development directions.
3. Conclusions and Perspective
REEs, as nonrenewable resources, are increasingly depleted due to increasing demand. But the fluorescent powders from waste fluorescent lamps are rich in rare earths. Directly discarding waste fluorescent lamps not only caused environmental pollution but also wasted the resources of REEs. The majority of rare-earth recycling processes involve the preliminary concentration of rare-earth compounds using physical methods, followed by the leaching of the target elements using chemical methods and extraction and purification. A series of methods has been developed to achieve the established goals for these three main processes, and some progress has been made. However, each of these methods has its own advantages and disadvantages, as shown in Table 2 and Table 3. Based on an analysis of the literature and practical experience, we recommend the following optimal process for the extraction and separation of REEs from waste fluorescent powder: The fluorescent powder first goes through external field-enhanced alkaline fusion, and then is leached by water to exclude the Ca, Al, and Ba elements. External field (microwave, ultrasound)-enhanced rapid acid leaching is used on the residue to obtain the REE-containing solution. The REE-containing solution is selectively extracted using green solvents and then using a reverse extraction process. The reverse extraction solution is precipitated and calcined to prepare oxides or electrolyzed to make metals and alloys. To better promote the comprehensive utilization of rare earths in waste phosphors, future research directions should mainly focus on the following aspects:

Table 3.
The advantages and disadvantages of various chemical methods.
- (1)
- Studying the reaction thermodynamics and kinetics of the process of extracting and separating REEs from waste phosphors. This should involve the thermodynamics of the reactions and the feasibility and pathways of multiphase reactions between REEs and impurity elements during alkaline fusion, machine activation, microwave-assisted, oxidant addition, and green solvent processing. It should also reveal the relevant reaction mechanisms under different conditions, especially the decomposition mechanisms of blue and green powders in pretreating (machine activation, alkaline fusion, and external field enhancement), leaching, and solvent extraction.
- (2)
- Much effort should made to account for the application of mechanical activation, alkaline fusion, ultrasound, microwave, oxidation, and other methods to disrupt the complex structure of the fluorescent powders and to promote the leaching efficiency of REEs and increase recovery efficiencies.
- (3)
- Developing new green solvents, such as ILs and DESs, for green and efficient extraction and separation. Reducing costs and viscosity while ensuring effectiveness is an important research direction for the future. DESs with a wide range of raw material sources, low prices, and relatively low viscosity are worth further development and have good application prospects. At the same time, the reagents balancing should be considered in the alkaline fusion-acid leaching method to avoid the excessive consumption of acid and alkaline and reduce the process costs.
- (4)
- Combining multiple extraction and separation methods to innovate and develop the new processes for green and efficient comprehensive utilization of waste fluorescent powder. Especially, improving the leaching efficiency of Ce, Tb, La, and Eu rare-earth elements in blue and green powders is the key point. More attention should also be paid to the behavior and direction of the REEs and the impurity elements of Al, Mg, and Sb in the system during processing to achieve efficient comprehensive recovery and high-value utilization as much as possible.
Author Contributions
Conceptualization, methodology, writing—original draft preparation, writing—review and editing, supervision, project administration, funding acquisition, G.T.; resources, data curation, writing—review and editing, Z.X. and X.L.; writing—review and editing, Z.H. and B.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 52364042, 51774158, 51264021).
Data Availability Statement
Data are contained within the article.
Acknowledgments
Guocai Tian would like to thank Chenguang Han for help in drawing some of the pictures in the paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Van Gosen, B.S.; Verplanck, P.L.; Long, K.R.; Gambogi, J.; Seal, R.R. The Rare-Earth Elements: Vital to Modern Technologies and Lifestyles; US Geological Survey: Reston, VA, USA, 2014; pp. 245–249. [Google Scholar]
- Dong, B.; Cao, B.; He, Y.; Liu, Z.; Li, Z.; Feng, Z. Temperature sensing and in vivo imaging by molybdenum sensitized visible upconversion luminescence of rare-earth oxides. Adv. Mater. 2012, 24, 1987–1993. [Google Scholar] [CrossRef] [PubMed]
- Alonso, E.; Sherman, A.M.; Wallington, T.J.; Everson, M.P.; Field, F.R.; Roth, R.; Kirchain, R.E. Evaluating rare-earth element availability: A case with revolutionary demand from clean technologies. Environ. Sci. Technol. 2012, 46, 3406–3414. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y. Rare-Earth Element Resources: Indian Context; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 26–31. [Google Scholar]
- Jha, A.R. Rare-Earth Materials: Properties and Applications; CRC Press: Boca Raton, FL, USA, 2014; pp. 326–329. [Google Scholar]
- Atwood David, A. The Rare-Earth Elements: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 6–9. [Google Scholar]
- Roesky Peter, W. Molecular Catalysis of Rare-Earth Elements; Springer: Berlin/Heidelberg, Germany, 2010; Volume 137, pp. 2–6. [Google Scholar]
- Rare Earths Statistics and Information. Available online: https://www.usgs.gov/centers/national-minerals-information-center/rare-earths-statistics-and-information (accessed on 30 December 2024).
- Tunsu, C.; Petranikova, M.; Gergorić, M.; Ekberg, C.; Retegan, T. Reclaiming rare-earth elements from end-of-life products: A review of the perspectives for urban mining using hydrometallurgical unit operations. Hydrometallurgy 2015, 156, 239–258. [Google Scholar] [CrossRef]
- Costis, S.; Mueller, K.K.; Coudert, L.; Neculita, C.M.; Reynier, N.; Blais, J.F. Recovery potential of rare-earth elements from mining and industrial residues: A review and cases studies. J. Geochem. Explor. 2021, 221, 106699. [Google Scholar] [CrossRef]
- Rebello, R.Z.; Lima, M.T.W.D.C.; Yamane, L.H.; Siman, R.R. Characterization of end-of-life LED lamps for the recovery of precious metals and rare-earth elements. Resour. Conserv. Recycl. 2020, 153, 104557. [Google Scholar] [CrossRef]
- Omodara, L.; Pitkäaho, S.; Turpeinen, E.M.; Saavalainen, P.; Oravisjärvi, K.; Keiski, R.L. Recycling and substitution of light rare-earth elements, cerium, lanthanum, neodymium, and praseodymium from end-of-life applications-A review. J. Clean. Prod. 2019, 236, 117573. [Google Scholar] [CrossRef]
- Rene, E.R.; Sethurajan, M.; Ponnusamy, V.K.; Kumar, G.; Dung, T.N.B.; Brindhadevi, K.; Pugazhendhi, A. Electronic waste generation, recycling and resource recovery: Technological perspectives and trends. J. Hazard. Mater. 2021, 416, 125664. [Google Scholar] [CrossRef]
- Pagano, G. Rare-Earth Elements in Human and Environmental Health: At the Crossroads Between Toxicity and Safety; CRC Press: Boca Raton, FL, USA, 2016; pp. 12–14. [Google Scholar]
- Tian, H.; Zhang, M.L.; Lai, L.; Zhao, Z. Treatment technology of waste phosphor by sulfation roasting-leaching. Chin. J. Process Eng. 2019, 19, 144–150. [Google Scholar]
- Dhawan, N.; Tanvar, H. A critical review of end-of-life fluorescent lamps recycling for recovery of rare-earth values. Sustain. Mater. Technol. 2022, 32, e00401. [Google Scholar] [CrossRef]
- Zhong, L.Q. Study on the cascade recover the rare earth from waste phosphors. Master’s Thesis, Jiangxi University of Science and Technology, Ganzhou, China, 2018. [Google Scholar]
- Innocenzi, V.; De Michelis, I.; Ferella, F.; Vegliò, F. Leaching of yttrium from cathode ray tube fluorescent powder: Kinetic study and empirical models. Int. J. Miner. Process. 2017, 168, 76–86. [Google Scholar] [CrossRef]
- Tang, Z.T. Study on Extraction of Rare-Earth Elements from Waste Phosphor by Sodium Carbonate Roasting and Molten Salts Chlorination. Master’s Thesis, Anhui University of Technology, Maanshan, China, 2017. [Google Scholar]
- Binnemans, K.; Jones, P.T. Perspectives for the recovery of rare earths from end-of-life fluorescent lamps. J. Rare Earths 2014, 32, 195–200. [Google Scholar] [CrossRef]
- Liao, C.F.; Zeng, Y.L.; Jiao, Y.F. The Latest Development of Rare-earth Recovery from Waste Rare-earth Phosphors. Rare Met. Cem. Carbides 2013, 41, 7–12. [Google Scholar]
- Zhao, Z.; Xu, G.L. Present Situation and Development Trend of Technology of Rare-earth Elements in Waste Phosphor. J. Chin. Soc. Rare Earths 2015, 33, 641–649. [Google Scholar]
- Xu, S.B.; Liu, C.; Sun, Z.X.; Li, Y.P.; Xie, Y.; Wang, X.G. Recovery of Rare-earth Elements in Waste Fluorescent Lamps: A Review. Chin. J. Rare Met. 2021, 45, 879–890. [Google Scholar]
- Zhang, W.J.; Du, Y.P.; Xie, X.; Feng, D.X.; Tong, X.; Cao, Y. A Review on Research of Rare-earth Element Recycling Technology in Waste Phosphor. Chin. J. Rare Met. 2023, 47, 1437–1452. [Google Scholar]
- Xu, H.; Hu, T.J.; Liu, Y.; Su, E.Y.; Xie, B.Y.; Wang, R.X.; Yan, K.; Xu, Z.F. Research progress on recovery of rare-earth elements from waste rare-earth phosphors. Nonferrous Met. (Extr. Metall.) 2024, 9, 25–36. [Google Scholar]
- He, L.; Ji, W.; Yin, Y.W.; Sun, W. Study on alkali mechanical activation for recovering rare-earth from waste fluorescent lamps. J. Rare Earths 2018, 36, 108–112. [Google Scholar] [CrossRef]
- Wang, W.B.; Guo, F.; Yan, S.R. The Technology Development and Research of Rare-earth Lamp Phosphor (I). China Light &Lighting. 2009, 6, 11–16. [Google Scholar]
- Tanaka, M.; Oki, T.; Koyama, K.; Narita, H.; Oishi, T. Recycling of rare earths from scrap. In Handbook on the Physics and Chemistry of Rare Earths; Bunzli, J.C.G., Pecharsky, V.K., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2013; Volume 43, pp. 159–211. [Google Scholar]
- Masaru, Y.; Tomoko, A.; Masaki, M.; Tatsuya, O. Recovery of LaPO4: Ce, Tb from waste phosphors using high-gradient magnetic separation. Waste Manag. 2018, 7, 164. [Google Scholar]
- Nishijima, S. Magnetic force control technology and its industrial applications. J. Mag. Soc. Jpn. 2013, 37, 333. [Google Scholar] [CrossRef]
- Yamashita, M.; Akai, T.; Imamura, T.; Murakami, M.; Oki, T. Recycling of waste phosphors using high gradient magnetic separation method. Trans. Mater. Res. Soc. 2014, 39, 35. [Google Scholar] [CrossRef][Green Version]
- Wada, K.; Mishoma, F.; Akiyama, Y.; Nishijima, S. Fundamental study of phosphor separation by controlling magnetic force. Physics C 2013, 494, 217. [Google Scholar] [CrossRef]
- Wada, K.; Mishoma, F.; Akiyama, Y.; Nishijima, S. The development of the separation apparatus of phosphor by controlling the magnetic force. Phys. Procedia 2014, 58, 252. [Google Scholar] [CrossRef]
- Kuger, L.; Franzreb, M. Design of a magnetic field-controlled chromatography process for efficient and selective fractionation of rare-earth phosphors from end-of-life fluorescent lamps. ACS Sustain. Chem. Eng. 2024, 12, 2988–2999. [Google Scholar] [CrossRef]
- Hirajima, T.; Bissombolo, A.; Sasaki, K.; Nakayam, K.; Hirai, H.; Tsunekawa, M. Floatability of rare-earth phosphors from waste fluorescent lamps. Int. J. Miner. Process. 2005, 77, 187. [Google Scholar] [CrossRef]
- Otuski, A.; Dodbiba, G.; Shibayama, A.; Fujita, T. Separation of rare-earth flourescent powders by tow-liquid flotation using organic solvents. Jpn. J. Appl. Phys. 2008, 47, 5093. [Google Scholar] [CrossRef]
- Mei, G.J.; Rao, P.; Mitsuaki, M.; Toyohisa, F. Separation of red (Y2O3:Eu3+), blue (Sr,Ca,Ba)10(PO4)6Cl2:Eu2+ and green (LaPO4:Tb3+,Ce3+) rare-earth phosphors by liquid/liquid extraction. J. Wuhan Univ. Technol.-Mater 2009, 24, 418–423. [Google Scholar] [CrossRef]
- Mei, G.J.; Rao, P.; Mitsuaki, M.; Toyohisa, F. Separation of red (Y2O3:Eu3+), blue (Sr,Ca,Ba)10(PO4)6Cl2:Eu2+ and green (CeMgAl10O17:Tb3+) rare-earth phosphors by liquid/liquid extraction. J. Wuhan Univ. Technol.-Mater. 2009, 24, 603–607. [Google Scholar] [CrossRef]
- Wu, M.; Yu, M.M.; Cheng, Q.; Yuan, Q.; Mei, G.; Liang, Q.; Wang, L. Flotation recovery of Y2O3 from waste phosphors using ionic liquids as collectors. Chem. Phys. Lett. 2023, 825, 140608. [Google Scholar] [CrossRef]
- Bai, R.X.; Yang, F.; Zhang, Y.; Yuan, Q.; Mei, G.; Liang, Q.; Wang, L. Preparation of elastic diglycolamic-acid modified chitosan sponges and their application to recycling of rare-earth from waste phosphor powder. Carbohydr. Polym. 2018, 190, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Arunraj, B.; Rajesh, V.; Rajesh, N. Potential application of graphene oxide and Aspergillus niger spores with high adsorption capacity for recovery of europium from red phosphor, compact fluorescent lamp and simulated radioactive waste. J. Rare earths 2023, 41, 157–166. [Google Scholar] [CrossRef]
- Artiushenko, O.; Rojano, W.S.; Nazarkovsky, M.; Azevedo, M.F.M.; Saint’Pierre, T.D.; Kai, J.; Zaitsev, V. Recovery of rare-earth elements from waste phosphors using phosphonic acid-functionalized silica adsorbent. Sep. Purif. Technol. 2024, 330, 125525. [Google Scholar] [CrossRef]
- Takahashi, T.; Takano, A.; Saito, T. Separation and recovery of rare-earth elements from phosphors in waste fluorescent lamp (part III)-separation and recovery of rare-earth elements by multistage countercurrent extraction. Rep. Hokkaido Ind. Res. Inst. 1991, 298, 37. [Google Scholar]
- Hirajima, T.; Sasaki, K.; Bissombolo, A.; Hira, H.; Hamada, M.; Tsunekawa, M. Feasibility of an efficient recovery of rare-earth-activated phosphors from waste fluorescent lamps through dense-medium centrifugation. Sep. Purif. Technol. 2005, 44, 197. [Google Scholar] [CrossRef]
- Patil, A.B.; Tarik, M.; Struis, R.P.W.J.; Ludwig, C. exploiting end-of-life lamps fluorescent powder e-waste as a secondary resource for critical rare-earth metals. Resour. Conserv. Recycl. 2020, 164, 105153. [Google Scholar] [CrossRef]
- Luidold, S.; Poscher, A.; Antrekowitsch, H. Concepts for the extraction of rare earths from spent phosphors. In Proceedings of the 51st Annual Conference of Metallurgists, Niagara Falls, NY, USA, 30 September–3 October 2012. [Google Scholar]
- Ida, D.M.; Ferella, F.; Ennio, F.V.; Veglio, F. Treatment of exhaust fluorescent lamps to recover yttrium: Experimental and process analyses. Waste Manag. 2011, 31, 2559. [Google Scholar]
- Yang, F.; Kubota, F.; Baba, Y.; Kamiya, N.; Goto, M. Selective extraction and recovery of rare-earth metals from phosphor powders in waste fluorescent lamps using an ionic liquid system. J. Hazard. Mater. 2013, 254, 79. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, S.G.; Pan, D.; Tian, J.; Yang, M.; Wu, M.; Volinsky, A.A. Rare-earth elements recycling from waste phosphor by dual hydrochloric acid dissolution. J. Hazard. Mater. 2014, 272, 96–101. [Google Scholar] [CrossRef]
- Tunsu, C.; Ekberg, C.; Retegan, T. Characterization and leaching of real fluorescent lamp waste for the recovery of rare-earth metals and mercury. Hydrometallurgy 2014, 144, 91–98. [Google Scholar] [CrossRef]
- Innocenzi, V.; Ippolito, N.M.; De Michelis, I.; Medici, F.; Vegliò, F. A hydrometallurgical process for the recovery of terbium from fluorescent lamps: Experimental design, optimization of acid leaching process and process analysis. J. Environ. Manag. 2016, 184, 552–559. [Google Scholar] [CrossRef]
- Lie, J.N.; Shuwanto, H.; Abdullah, H.; Soetaredjo, F.E.; Ismadji, S.; Wijaya, C.J.; Gunarto, C. Optimization of intensified leaching and selective recovery of Y and Eu from waste cathode ray tube phosphor. Miner. Eng. 2024, 209, 108620. [Google Scholar] [CrossRef]
- Zhang, S.G.; Liu, H.; Pan, D.A.; Tian, J.J. Complete recovery of Eu from BaMgAl10O17:Eu2+ by alkaline fusion and its mechanism. Rsc Adv. 2015, 5, 1113. [Google Scholar] [CrossRef]
- Zhang, Z.X. Process Research on Recovery of Rare-Earth of Waste Rare-Earth Fluorescent Powders by Alkali Fusion-Washing-Reduction Acid Leaching. Master’s Thesis, Jiangxi University of Science and Technology, Ganzhou, China, 2016. [Google Scholar]
- Liao, C.F.; Li, Z.Y.; Zeng, Y.L. Selective extraction and recovery of rare-earth metals from waste fluorescent powder using alkaline roasting-leaching process. J. Rare Earths 2017, 10, 1008. [Google Scholar] [CrossRef]
- Yu, M.M.; Mei, G.J.; Chen, X.D. Recovering rare earths and aluminum from waste BaMgAl10O17:Eu2+ and CeMgAl11O19:Tb3+ phosphors using NaOH sub-molten salt method. Miner. Eng. 2018, 117, 1. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Wang, B.; Wang, K.; Alex, A.V. Multiscale recycling rare-earth elements from real waste trichromatic phosphors containing glass. J. Clean. Prod. 2019, 238, 117998. [Google Scholar] [CrossRef]
- Shao, L.B. Study on Rare-Earth Extraction of Waste Rare-Earth Phosphor Based on Phase Regulation. Master’s Thesis, Jiangxi University of Science and Technology, Ganzhou, China, 2019. [Google Scholar]
- Xie, B.Y.; Liu, C.X.; Wei, B.H.; Wang, R.; Ren, R. Recovery of rare-earth elements from waste phosphors via alkali fusion roasting and controlled potential reduction leaching. Waste Manag. 2023, 163, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Kumari, A.; Sinha, M.K.; Munshi, B.; Sahu, S.K. Valorization of phosphor powder of waste fluorescent tubes with an emphasis on the recovery of terbium oxide (Tb4O7). Sep. Purif. Technol. 2023, 322, 124332. [Google Scholar] [CrossRef]
- Liu, H. Study on the Recycling and Alkali Melting Mechanism of Waste Rare-Earth Fluorescent Powder. Master’s Thesis, Beijing University of Science and Technology, Beijing, China, 2015. [Google Scholar]
- Liu, C.X. Research on Comprehensive Recovery of Rare-earth Elements from Waste Rare-earth Phosphor. Master’s Thesis, Jiangxi University of Science and Technology, Ganzhou, China, 2022. [Google Scholar]
- Hua, Z.S.; Geng, A.; Tang, Z.T.; Zhao, Z.; Liu, H.; Yao, Y.L.; Yang, Y.X. Decomposition behavior and reaction mechanism of Ce0.67Tb0.33MgAl11O19 during Na2CO3 assisted roasting: Toward efficient recycling of Ce and Tb from waste phosphor. J. Environ. Manag. 2019, 249, 109383. [Google Scholar] [CrossRef] [PubMed]
- Yurramendi, L.; Gijsemans, L.; Forte, F.; Aldana, J.L.; Río, C.d.; Binnemans, K. Enhancing rare-earth recovery from lamp phosphor waste. Hydrometallurgy 2019, 187, 38–44. [Google Scholar] [CrossRef]
- Zeng, Y.L. Study on the Process of Enhanced Leaching of Rare earths from Waste Rare-earth Fluorescent Powder. Master’s Thesis, Jiangxi University of Science and Technology, Ganzhou, China, 2014. [Google Scholar]
- Wu, Y.; Wang, B.; Zhang, Q.; Tian, J.J. Recovery of rare-earth elements from waste fluorescent phosphors: Na2O2 molten salt decomposition. J. Mater. Cycles Waste Manag. 2014, 16, 635. [Google Scholar] [CrossRef]
- Wu, Y.F.; Wang, B.L.; Zhang, Q.J.; Li, Q.; Yu, J.M. A novel process for high efficiency recovery of rare-earth metals from waste phosphors using a sodium peroxide system. RSC Adv. 2014, 4, 7927. [Google Scholar] [CrossRef]
- Ippolito, N.M.; Innocenzi, V.; De Michelis, I.; Medici, F.; Vegliò, F. Rare-earth elements recovery from fluorescent lamps: A new thermal pretreatment to improve the efficiency of the hydrometallurgical process. J. Clean. Prod. 2017, 153, 287–298. [Google Scholar] [CrossRef]
- Guo, D.D. Study on the Extraction of Rare-earth from Waste Rare-earth Phosphor Iron Powder by Alkali Fusion-Acid Leaching. Master’s Thesis, Jiangxi University of Science and Technology, Ganzhou, China, 2016; p. 1. [Google Scholar]
- Liang, Y.; Liu, Y.X.; Lin, R.D.; Guo, D.D.; Liao, C.F. Leaching of rare-earth elements from waste lamp phosphor mixtures by reduced alkali fusion followed by acid leaching. Hydrometallurgy 2016, 163, 99. [Google Scholar] [CrossRef]
- Yao, Z.; Li, J.; Zhao, X. Molten salt oxidation: A versatile and promising technology for the destruction of organic containing wastes. Chemosphere 2011, 84, 1167. [Google Scholar] [CrossRef]
- Choi, S.; Ilyas, S.; Kim, H. Intensive leaching of red phosphor rare-earth metals from waste fluorescent lamp: Parametric optimization and kinetic studies. JOM 2022, 74, 1054–1060. [Google Scholar] [CrossRef]
- Tian, X.M.; Yin, X.F.; Gong, Y.; Wu, Y.; Tan, Z.; Xu, P. Characterization, recovery potentiality, and evaluation on recycling major metals from waste cathode-ray tube phosphor powder by using sulphuric acid leaching. J. Clean. Prod. 2016, 135, 1210–1217. [Google Scholar] [CrossRef]
- Miskufova, A.; Kochmanova, A.; Havlik, T.; Horvathova, H.; Kuruc, P. Leaching of yttrium, europium and accompanying elements from phosphor coatings. Hydrometallurgy 2018, 176, 216–228. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Q.; Zuo, T. Selective recovery of Y and Eu from rare-earth tricolored phosphorescent powders waste via a combined acid-leaching and photo-reduction process. J. Clean. Prod. 2019, 226, 858–865. [Google Scholar] [CrossRef]
- Yin, X.; Tian, X.; Wu, Y.; Zhang, Q.; Wang, W.; Li, B.; Gong, Y.; Zuo, T. Recycling rare-earth elements from waste cathode ray tube phosphors: Experimental study and mechanism analysis. J. Clean. Prod. 2018, 205, 58–66. [Google Scholar] [CrossRef]
- Lee, C.; Jeong, M.; Fatih, K.M.; Lee, J.; Hong, H.; Hong, S. Recovery of indium from used lcd panel by a time efficient and environmentally sound method assisted Hebm. Waste Manag. 2013, 33, 730. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Rahman, I.M.M.; Egawa, Y.; Sawai, H.; Begum, Z.A.; Teruya, M.; Satoshi, M. Recovery of indium from end-of-life liquid-crystal display panels using aminopolycarboxylate chelants with the aid of mechanochemical treatment. Microchem. J. 2013, 106, 289. [Google Scholar] [CrossRef]
- Ou, Z.; Li, J. Synergism of mechanical activation and sulfurization to recover copper from waste printed circuit boards. RSC Adv. 2014, 4, 51970. [Google Scholar] [CrossRef]
- Zhang, Q.; Saito, F. Non-thermal extraction of rare-earth elements from fluorescent powder means of its mechanochemical treatment. Shigen-Sozai 1998, 114, 253. [Google Scholar] [CrossRef][Green Version]
- Mio, H.; Lee, J.; Nakagawa, T.; Kano, J.; Saito, F. Estimation of extraction rate of yttrium from fluorescent powder by ball milling. Mater. Trans. 2001, 42, 2460. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Xu, X.X.; Lei, Y.; Gao, Z.F. Research on the mechanical activation leaching of REOs from waste fluorescent powder. Chin. Rare Earths 2016, 37, 72–78. [Google Scholar]
- Tan, Q.Y.; Deng, C.; Li, J.H. Enhanced recovery of rare-earth elements from waste phosphors by mechanical activation. J. Clean. Prod. 2017, 142, 2187. [Google Scholar] [CrossRef]
- Song, G.H.; Yuan, W.Y.; Zhu, X.F.; Wang, X.Y.; Zhang, C.L.; Li, J.H.; Bai, J.F.; Wang, J.W. Improvement in rare-earth element recovery from waste trichromatic phosphors by mechanical activation. J. Clean. Prod. 2017, 151, 361. [Google Scholar] [CrossRef]
- Van Loy, S.; Binnemans, K.; Van Gerven, T. Mechanochemical-assisted leaching of lamp phosphors: A green engineering approach for rare-earth recovery. Engineering 2018, 4, 398–405. [Google Scholar] [CrossRef]
- Ippolito, N.M.; Ferella, F.; Innocenzi, V.; Trapasso, F.; Passeri, D.; Belardi, G.; Vegliò, F. Effect of mechanical activation on terbium dissolution from waste fluorescent powders. Miner. Eng. 2021, 167, 106906. [Google Scholar] [CrossRef]
- Chen, P.; Yang, F.; Liao, Q.; Zhao, Z.; Zhang, Y.; Zhao, P.; Guo, W.; Bai, R. Recycling and separation of rare-earth resources lutetium from LYSO scraps using the diglycol amic acid functional XAD-type resin. Waste Manag. 2017, 62, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Lie, J.; Ismadji, S.; Liu, J.C. Microwave-assisted leaching of rare-earth elements (Y and Eu) from waste cathode ray tube phosphor. J. Chem. Technol. Biotechnol. 2019, 94, 3859–3865. [Google Scholar] [CrossRef]
- Shukla, N.; Agrawal, S.; Dhawan, N. Microwave acid baking process for recovery of rare-earth concentrate from phosphor of end-of-life fluorescent lamps. J. Clean. Prod. 2021, 307, 127235. [Google Scholar] [CrossRef]
- Lie, J.N.; Liu, J.C. Recovery of Y and Eu from waste CRT phosphor using closed-vessel microwave leaching. Sep. Purif. Technol. 2021, 277, 119448. [Google Scholar] [CrossRef]
- Bilen, A.; Birol, B.; Saridede, M.N.; Kaplan, Ş.; Sönmez, M. Direct microwave leaching conditions of rare-earth elements in fluorescent wastes. J. Rare Earths 2024, 42, 1165–1174. [Google Scholar] [CrossRef]
- Tian, G.C.; Li, J.; Hua, Y. Application of ionic liquids in hydrometallurgy of nonferrous metals. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2010, 20, 513–520. [Google Scholar] [CrossRef]
- Li, R.X. Green Solvents: Synthesis and Application of Ionic Liquids; Chemical Industry Engineering Press: Beijing, China, 2004; pp. 27–39. [Google Scholar]
- Deng, Y.Q. Ionic Liquids: Properties, Preparation and Application; China SINOPEC Press: Beijing, China, 2006; pp. 20–45. [Google Scholar]
- Zhang, S.J.; Lv, X.M. Ionic Liquids from Fundamental Study to Industrial Application; Science Press: Beijing, China, 2006; pp. 35–95. [Google Scholar]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 2002, 99, 2071–2083. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Dupont, D.; Binnemans, K. Rare-earth recycling using a functionalized ionic liquid for the selective dissolution and revalorization of Y2O3:Eu3+ from lamp phosphor waste. Green Chem. 2015, 17, 856–868. [Google Scholar] [CrossRef]
- Schaeffer, N.; Feng, X.; Grimes, S.; Cheeseman, C. Recovery of an yttrium europium oxide phosphor from waste fluorescent tubes using a Brønsted acidic ionic liquid, 1-methylimidazolium hydrogen sulfate. J. Chem. Technol. Biotechnol. 2017, 92, 2731–2738. [Google Scholar] [CrossRef]
- Pateli, I.M.; Abbott, A.P.; Binnemans, K.; Rodriguez, N.R. Recovery of yttrium and europium from spent fluorescent lamps using pure levulinic acid and the deep eutectic solvent levulinic acid–choline chloride. RSC Adv. 2020, 10, 28879–28890. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.R.; Grymonprez, B.; Binnemans, K. Integrated process for recovery of rare-earth elements from lamp phosphor waste using methanesulfonic acid. Ind. Eng. Chem. Res. 2021, 60, 10319–10326. [Google Scholar] [CrossRef]
- Jiang, T.; Singh, S.; Dunn, K.A.; Liang, Y. Optimizing Leaching of Rare-earth Elements from Red Mud and Spent Fluorescent Lamp Phosphors Using Levulinic Acid. Sustainability 2022, 14, 9682. [Google Scholar] [CrossRef]
- Asadollahzadeh, M.; Torkaman, R.; Torab-Mostaedi, M. Recovery of yttrium ions from fluorescent lamp waste through supported ionic liquid membrane: Process optimisation via response surface methodology. Int. J. Environ. Anal. Chem. 2022, 102, 3161–3174. [Google Scholar] [CrossRef]
- Hu, M.; Zhao, Z.G.; Du, J.M.; Yang, F. Recovery of Rare-earth Elements from Wasted Lamp Phosphor Using Deep Eutectic Solvents. Chin. Rare Earths 2024, 45, 1–11. [Google Scholar] [CrossRef]
- Kinga, P.M.; Katarzyna, G.; Zbigniew, W. Potential management of waste phosphogypsum with particular focus on recovery of rare-earth metals. Pol. J. Chem. Technol. 2015, 17, 55. [Google Scholar]
- Daejin, K.; Lawrence, E.P.; Lætitia, H.D.; Eric, S.P.; Jim, H.; Ramesh, R.B. Selective extraction of rare-earth elements from permanent magnet scraps with membrane solvent extraction. Environ. Sci. Technol. 2015, 49, 9452. [Google Scholar]
- Rout, A.; Binnemans, K. Influence on the ionic liquid cation on the solvent extraction of trivalent rare-earth ions by mixtures of Cyanex 923 and ionic liquids. Dalton Trans. 2015, 44, 1379. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Nishihama, S.; Yoshizuka, K. Separation and recovery process for rare-earth metals from fluorescence material wastes using solvent extraction. Solvent Extr. Res. Dev. Jpn. 2007, 14, 105. [Google Scholar]
- Tunsu, C.; Lapp, J.B.; Ekberg, C.; Retegan, T. Selective separation of yttrium and europium using Cyanex 572 for applications in fluorescent lamp waste processing. Hydrometallurgy 2016, 166, 98. [Google Scholar] [CrossRef]
- Innocenzi, V.; Ippolito, N.M.; Pietrelli, L.; Centofanti, M.; Piga, L.; Vegliò, F. Application of solvent extraction operation to recover rare earths from fluorescent lamps. J. Clean. Prod. 2018, 172, 2840. [Google Scholar] [CrossRef]
- Innocenzi, V.; De Michelis, I.; Ferella, F.; Vegliò, F. Secondary yttrium from spent fluorescent lamps: Recovery by leaching and solvent extraction. Int. J. Miner. Process. 2017, 168, 87–94. [Google Scholar] [CrossRef]
- Mishra, B.B.; Devi, N.; Sarangi, K. Yttrium and europium recycling from phosphor powder of waste tube light by combined route of hydrometallurgy and chemical reduction. Miner. Eng. 2019, 136, 43–49. [Google Scholar] [CrossRef]
- Yang, H.; Wang, W.; Cui, H.; Zhang, D.; Liu, Y.; Chen, J. Recovery of rare-earth elements from simulated fluorescent powder using bifunctional ionic liquid extractants (Bif-ILEs). J. Chem. Technol. Biotechnol. 2012, 87, 198–205. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, C.; Li, F.; Dong, Y.; Zhao, Z.; Sun, X. The development of sustainable yttrium separation process from rare-earth enrichments using bifunctional ionic liquid. Sep. Purif. Technol. 2016, 162, 106–113. [Google Scholar] [CrossRef]
- Tunsu, C.; Petranikova, M.; Ekberg, C.; Retegan, T. A hydrometallurgical process for the recovery of rare-earth elements from fluorescent lamp waste fractions. Sep. Purif. Technol. 2016, 161, 172–186. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).