Abstract
Calcium orthophosphates and clays and their composites are one of the most important groups in the field of new, modern, and technologically advanced materials that are accessible, inexpensive, and environmentally friendly. This review provides a summary of recent research on calcium orthophosphate–clay composites, their preparation, characterisation, and use in various applications. An introduction to the subject is followed by a detailed description of the chemical and physical properties of calcium orthophosphates, clays, and clay minerals. This is followed by a general summary of preparation methods for calcium orthophosphate–clay composites. Particular attention is paid to the description of individual applications, i.e., environmental applications, biomaterials science (tissue engineering, pharmacology), and other emerging applications. Finally, future perspectives are summarised and discussed.
1. Introduction
The preparation of new, modern, and technologically advanced materials, which are accessible, effective, environmentally friendly, minimise process time, and are cheap, is the main subject of interest of numerous scientific and industrial centres. Calcium orthophosphates (CaPs) and clays, their modifications, and composites are some of the most important groups in this field [1]. Just as there are biomimetic materials, which refer to man-made processes, substances, devices, and systems that imitate natural and biological phenomena, the idea of calcium orthophosphate–clay composites (CaPCCs) is inspired by the geological environment in which these two materials commonly coexist [2]. Natural CaPs occur in most geological environments, usually as accessory minerals (<5%), mostly as deposits of apatites belonging to the igneous rocks.
There are many applications for clay-based products, ranging from traditional ceramics to modern functional nanocomposites. Recently, scientists have focused on the properties of clay mineral nanoparticles with research in adsorption, catalysis, and biology. Clay minerals have excellent properties such as high adsorption/desorption and ion exchange capacity, low or no toxicity, and good biocompatibility. They have been shown to be effective inorganic hosts for proteins, drugs, bioactive molecules, and magnetic nanoparticles [3] and have been widely used in pharmaceutical and biological applications [4].
CaPs are chemical compounds of particular interest in many interdisciplinary fields of science, including geology, chemistry, biology, and medicine. They are also particularly important because they are the major inorganic constituents of vertebrate hard tissues and have remarkable bioactivity and biocompatibility due to their close chemical similarity to biological calcified tissues. Materials scientists are exploiting this property to construct artificial bone grafts that either consist entirely of or are surface-coated with biologically relevant CaPs. The ease of atomic ion exchange or substitution and the high ionic promiscuity of CaPs open up this mineral to a wide range of applications. Another important feature of apatite is the adsorption phenomena, which provide a way of binding drugs and other active ingredients. CaPs are similar to clay minerals in that they also have excellent stability, reinforcement, and carrier properties.
The combination of the physico-chemical properties of CaPs and clay minerals results in a synergistic effect [5], which has great potential for application in composite science and research [6]. Current research on CaPCCs focuses not only on the discovery of new applications but also on the development and improvement of new methods for their preparation. From classical methods such as mixing and sintering, other newly developed methods such as 3D printing, electrophoretic deposition, and the use of a biomimetic approach are gradually being applied to the production of CaPCCs.
The aim of this paper is to provide a review of the existing literature on the subject, with particular reference to the use and application of CaPCCs in various areas of human society. This review focuses mainly on studies published in the last 20 years. The review is organised into a number of sections, the first of which is the introduction. The next two sections are devoted to the physico-chemical properties of CaPs and clays/clay minerals; the fourth section summarises the current state of knowledge on methods for the preparation of CaPCCs. The fifth section provides a detailed description, derived from the relevant literature, of the application of these composites in the field of biomedical materials engineering, the environment, and other emerging applications. Finally, the summary presents concluding remarks on this area of research and discusses possible future developments. The text contains numerous references to enable those interested to obtain links to more detailed information. The chapters are supplemented with illustrative figures to aid understanding of the subject. The text contains many abbreviations; these are summarised and explained in the Abbreviation part.
2. Calcium Orthophosphates
Calcium orthophosphates are abundant in nature and in living organisms. In the ternary system CaO–P2O5–H2O, there are eleven known non-ion-substituted calcium orthophosphates with Ca/P molar ratios between 0.5 and 2.0, with different basicity/acidity, solubility, density, and crystallographic data. A detailed classification of individual CaPs is beyond the scope of this review and can be found in the publication by Dorozhkin [7]. Most natural CaPs occur as small polycrystalline structures, and they usually have the crystal structure of apatite (hexagonal system, space group P63/m) [7]. Most of all, natural CaPs in geological environments are not pure compounds; they always contain admixtures of other elements. Cations of calcium may be partially replaced by Sr, Ba, Mg, Mn, K, Na, or Fe; anions of orthophosphate may be partially replaced by AsO43−, CO32−, and VO43− anions of hydroxide in the anion channel by chloride, bromide, carbonate, and oxide. Substitution in the apatitic structure in minerals from the apatite group and synthetic apatites was investigated by Pan and Fleet [8], and they found that substitution included 53 elements, about half of the 109 elements in the periodic table. Substitution within the apatite lattice, which occurs in living organisms in the form of teeth, bones, horns, and antlers (bioapatites—a type of hydroxyapatite) [9], can be varied, but not with the same randomness and flexibility. Synthetic CaPs can also be prepared [10].
The term “apatite” refers to a group of several minerals that are of multidisciplinary research interest and diverse applications in mineralogy, geology, biomineralisation, medicine, and biomaterials. The typical representative of the CaPs with apatitic structure is hydroxylapatite (Ca5(PO4)3OH), which is often written as Ca10(PO4)6(OH)2 to show that there are two formula units in the crystallographic unit cell. There are four different crystallographic positions in the apatitic unit cell [11]. Four calcium cations (marked Ca(1)) are parallel to the c-axis and surrounded by nine oxygen atoms. Six other calcium cations (marked Ca(2)) per unit cell form two equilateral triangles at ¼ and ¾ of the c-axis, called anion channels, within which are two monovalent anions (most commonly OH−, F−, and Cl−) and potentially bivalent CO32−. The structure further contains six PO43− anions, with each P atom coordinated to four oxygen atoms (Figure 1A). This phosphate group can be replaced by various anionic complexes. The degree of substitution in the Ca(1) and Ca(2) positions is determined by the type of cation (element, size, and charge) and by its concentration. More on this subject is discussed in the literature [9].
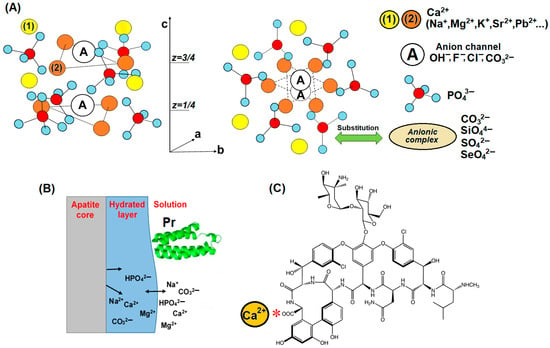
Figure 1.
(A) Sketch of the three-dimensional structure atomic structure of apatite showing substitution possibilities in geological environment and in living organisms; viewed perpendicular to the c-axis and down the c-axis; (B) the non-apatitic environment -hydrated layer and surface reactions containing loosely bound ions that can be exchanged by foreign ions and molecules, such as charged groups of proteins (Pr) [12]. (C) The chemical structure of vancomycin, “*” indicates the binding site of calcium to the carboxyl group of vancomycin.
The striking feature of nanocrystalline apatites (both biological and synthetic) is the presence of “non-apatitic” environments [12]. These environments (Figure 1B) correspond to hydrated domains of the nanocrystals, which are distinct from apatite domains containing relatively mobile ions. These hydrated surface layers allow a wide range of interactions (surface ion exchange), such as crystal–crystal; crystal–substrate; crystal–organic molecules, e.g., growth factors, proteins (Figure 1B), drugs (Figure 1C); and crystal–cell interactions [7,12]. Interacting molecules can play a key role in the recruitment and expression of cells that influence the biointegration of implants. The chemisorption of drugs, growth factors, etc., onto the surface of CaP [13] can be used in pharmacology as a delivery system.
In general, the crystal structure of apatite can incorporate half of the periodic table, and the cations and anions in the crystal structure can be altered by ion exchange (substitution) [7,9]. Ion exchange within the apatitic lattice, surface ion exchange, and adsorption processes in the hydrated layer leave this mineral open to a wide range of compositions and cause it to exhibit diverse performance.
3. Clays and Clay Minerals
Clays are naturally occurring, inexpensive materials composed mainly of fine-grained minerals. Clays are generally plastic at appropriate water content and harden after drying or intense heating [14]. They usually contain phyllosilicates but may also contain other inorganic and organic materials. Clay minerals are the youngest minerals in the Earth’s crust. They occur in all types of sediments and sedimentary rocks, as well as in hydrothermal deposits. Clay minerals are layered silicates (phyllosilicates).
A basic structural unit has been defined as a layer plus interlayer space [15]. The atomic structure of clay minerals consists of two basic units: a tetrahedral (T) and an octahedral (O) sheet (Figure 2). The tetrahedral sheet is composed of a tetrahedron with the silicon and aluminium atom at the centre [SiO4]4−, [AlO4]5−; the central atom is equidistant from four oxygens or possibly hydroxyls. These tetrahedrons are arranged in a hexagonal network repeated in two horizontal directions. The octahedral sheet consists of closely packed oxygens and hydroxyls in which aluminium, iron, and magnesium atoms are arranged in octahedral coordination [MA6]−. The octahedrons are connected to each other by edges and tips, and the octahedral network is formed by two closely arranged planes of O2− and OH− anions. The most common central cations in octahedrons (M) are aluminium, iron, and magnesium. The tetrahedral and octahedral sheets are joined by sharing the apical oxygens or hydroxyls to form two structural modifications called the 1:1 clay mineral layer (i.e., TO) or the 2:1 clay mineral layer (i.e., TOT), as seen in Figure 2. The interlayer space between the layers is filled with interlayer cations to balance the negative charge of the layers and with water molecules. Phyllosilicates are classified on the basis of the structural and chemical properties of their structural units. The structural property is the type of silicate layer, while the chemical properties are the size of the charge on the layers and the nature of the material in the interlayer space [16].
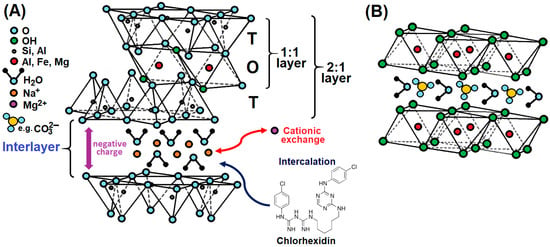
Figure 2.
Diagrammatic sketch of the structure of (A) cationic and (B) anionic clay minerals with possible interlayer reaction.
The atomic structure of clay minerals allows ion exchange of one ion for another on the surface or in the interlayer space. Clay minerals can sorb cations and, to a lesser extent, anions from solution without affecting the crystal structure because the exchangeable ions are attracted to a negatively charged clay surface. The negative charges on the clay surface appear to result from cation substitutions within the clay lattice structure (cationic clays). The substitution of Al3+ for Si4+ in the tetrahedral sheet and of lower valence ions (usually Mg2+) for Al3+ in the octahedral sheet results in an overall negative charge on the surface layer (Figure 2A) [17]. The cation exchange capacity (CEC) is one of the most important properties of cationic clays. This is the quantitative number of cations that a clay mineral can anchor to a negatively charged surface.
The simplest layered compounds contain alkaline earth metal cations or their hydroxides. A common example is the brucite structure Mg(OH)2, where the metal ions are held in an octahedrally coordinated hydroxide layer, while the anions and water of hydration occur in an anionic interlayer (anionic clays) [18]. The net positive charge of the metal ion layer is compensated by the net-negative charge of the anion–water interlayer (Figure 2B).
Natural phyllosilicates are ionic and hydrophilic in nature, leading to water swelling. Chemical modification can lead to an increase in the d-spacing and induce organophilic properties in the clay, which is a fundamental reaction for making clay minerals hydrophobic, as required in the preparation of polymer–clay composites [19]. Organic compounds can interact with clay minerals through various types of reactions, such as adsorption on the external and internal surfaces, exchangeable ion exchange on the external and internal surfaces, and grafting reactions with silanol and aluminol groups, leading to covalent bonds and intercalation. Intercalation refers to the penetration of organic molecules into the interlayer space of clay minerals. Polar organic molecules are able to displace the water molecules in the interlayer space, while neutral organic ligands can form complexes with the interlayer cations, but the interlayer cations can also be exchanged by different types of organic cations. The physico-chemical principles of these reactions have been described in detail by Lagaly et al. [20]. The interaction of clay minerals with various types of proteins, drugs (Figure 2A), etc., has been intensively studied.
The low or no toxicity of clay minerals is generally declared in the literature. With regard to the use of clay minerals in composites for biomedical applications, the release of cations (e.g., Na+, K+, Ca2+, Mg2+, Al3+, and Fe2+) and anions (e.g., SiO44−, SO42−, and Cl−) in an aqueous medium with a neutral pH is an important aspect. This issue has been studied in detail by Gaboreau et al. [21] for the most commonly used clay minerals, such as kaolinite, smectite, illite, vermiculite, and chlorite, where they were equilibrated in aqueous media in long-term batch experiments of up to seven years. Equilibria established under acidic and alkaline conditions may be important for understanding the processes involved in the transport of trace elements from sites of chemical weathering on land to marine depositional environments [22].
A detailed classification of phyllosilicates is beyond the scope of this review and can be found in the publication by Martin et al. [23].
4. Preparation of Calcium Orthophosphate–Clay Composites
Many different methods and modifications of these methods have been developed over the last few decades with the aim of obtaining CaPCCs. The preparation methods can be divided into two main groups.
- The first group includes methods based on physical principles, such as mixing, followed by the application of temperature and pressure. One technique that uses all of these parameters is sintering. Sintering is the process of compacting and shaping a solid mass of material by pressure or heat without melting it to the point of liquefaction. The particles in the sintered material diffuse across the particle boundaries, fusing the particles together to form a solid piece. CaPs and clay minerals are sintered at relatively high temperatures of 900–1400 °C [24,25] and pressures in the tens to hundreds of MPa. In some cases, a mixture of clay minerals is used and a ball mill is used for homogenisation [26]. Although the high temperatures chosen do not lead to melting, transformations can occur in both clay minerals and CaPs. The results of Chouia et al. [27] show that reaction sintering of kaolin and natural phosphate mixtures is a feasible route to hydroxyapatite (HA)/anorthite materials that can be used in the electronics industry, industrial heat exchangers, and biomedical applications. Jamil et al. [28] have proved the dehydroxylation of Na-montmorillonite (Na-MMT) and the transformation of calcium-deficient hydroxyapatite (CDH)—(Ca9(HPO4)(PO4)5(OH))—into β-tricalcium phosphate (β-TCP) during calcination at 900 °C. They also demonstrated the insertion of clay ions into the β-TCP structure. This substitution led to changes in lattice parameters, index distortion, and crystallite size depending on the concentration of the clay mineral. The compositional changes led to an increase in the temperature of the allotropic transformation from β-TCP to α-TCP shifts.The simple mixing of CaPs and clay can be modified by the addition of other components that increase the interfacial interactions. Li et al. [29] designed HA modified with the amino acid arginine (Arg) and studied its interactions with laponite nanoparticles (LAPs). Their results showed that Ca2+ cations allow attractive electrostatic interaction with the negatively charged surfaces of LAP clay, but this interaction may be limited by the repulsive forces of PO43− anions. Arg can strongly enhance the interfacial adhesion between HA and LAP through the interaction of guanidinium cationic groups (Figure 3A) with the negatively charged surfaces of LAPs and the PO43− anions in HA. The carboxyl groups of Arg interact with the Ca2+ cations in HA (Figure 3B).
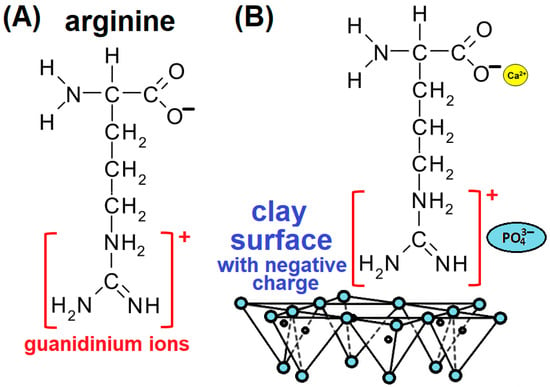 Figure 3. Schematic diagram of the structure of (A) arginine with marked guanidinium ions and (B) possible interactions of arginine with the clay surface proposed by Li et al. [29].In recent years, 3D printing has become widely used and popular as a powerful technique for producing prototypes with accuracy and complexity of internal and external structures. Currently, 3D printing has been very successful in the production of bone scaffolds for orthopaedic applications [30] and is being used to produce innovative medical devices. Faksawat et al. [31] have used the 3D-printing technique to produce a specific bone shape by mixing raw clay and HA followed by sintering. Different clay/HA ratios and sintering temperatures affect the physical and mechanical properties. An interesting modification of 3D printing is fused filament fabrication, also known as fused deposition modelling. This 3D-printing process uses a continuous filament of thermoplastic material (polymer), which is usually heated to a molten state and then extruded through the nozzle of the 3D printer. Printers extrude a thermoplastic filament in a series of layers over a build plate to create a three-dimensional object. This technique has been used to build polymer-based scaffolds, such as PLA [32] and PCL [33,34], which contain clay and HA as fillers.
Figure 3. Schematic diagram of the structure of (A) arginine with marked guanidinium ions and (B) possible interactions of arginine with the clay surface proposed by Li et al. [29].In recent years, 3D printing has become widely used and popular as a powerful technique for producing prototypes with accuracy and complexity of internal and external structures. Currently, 3D printing has been very successful in the production of bone scaffolds for orthopaedic applications [30] and is being used to produce innovative medical devices. Faksawat et al. [31] have used the 3D-printing technique to produce a specific bone shape by mixing raw clay and HA followed by sintering. Different clay/HA ratios and sintering temperatures affect the physical and mechanical properties. An interesting modification of 3D printing is fused filament fabrication, also known as fused deposition modelling. This 3D-printing process uses a continuous filament of thermoplastic material (polymer), which is usually heated to a molten state and then extruded through the nozzle of the 3D printer. Printers extrude a thermoplastic filament in a series of layers over a build plate to create a three-dimensional object. This technique has been used to build polymer-based scaffolds, such as PLA [32] and PCL [33,34], which contain clay and HA as fillers. - The second group relates to processes based on chemical principles. Charged powder particles dispersed in a liquid medium are attracted and deposited onto an oppositely charged conductive substrate by the application of an electric field. Electrophoretic deposition (EPD) is an interesting electrochemical method for processing bulk and coating materials that is attracting increasing interest due to its simplicity, ability to produce smooth coatings, and ability to deposit on complex shaped parts. Despite the simplicity of this method, a large number of parameters can influence the deposition properties [35,36]. Molaei et al. [37] used EPD to develop a novel chitosan (CHS)-bioactive glass-HA-halloysite nanotube (HNT) composite layer deposited on titanium substrate to enhance corrosion resistance and bioactivity. In their previous work [38], the authors demonstrated that the presence of HNTs in the CHS coating prepared by EPD provided better apatite-inducing ability compared to pure CHS. They proved the deposition of carbonated HA around the HNT particles. CaPs can be formed by combining calcium cations (Ca2+) with phosphate anions (PO43−) from simulated body fluid (SBF).The electrostatic interaction, without the use of an electric field, between Ca2+ cations and clay anionic groups enables in situ precipitation of apatite nuclei, and the electrostatic interaction between Ca2+ and PO43− ions induces the growth of apatite structure. This process is referred to as a “biomimetic approach”, which refers to man-made processes, substances, devices, and systems inspired by natural phenomena, such as biomineralisation [39]. Wan and Chen [40] were the first to publish the use of clay (surface-modified sepiolite—SEP) for in situ growth of HA. A negative surface charge may allow the growth of HA nanocrystals with controlled sizes. Surface-modified (acid activation) SEP particles with higher porosity, caused by acid extraction of magnesium (Mg2+) ions from the octahedral sheets and abundant silanol groups on the surface, make them more promising to induce HA biomineralisation. The authors have proposed the nucleation mechanism of HA on SEP. Si-OH groups at the edge of surface channels and some pore defects in the original SEP may interact with Ca2+ cations through electrostatic interactions and act as nucleation sites for HA formation (Figure 4A). The abundant Si-OH groups on the acid-activated SEP are able to attract more Ca2+ cations into the mesopores. In addition, Ca2+ cations could enter the octahedral positions originally occupied by Mg2+ cations, leading to local supersaturation of Ca2+ cations and facilitating the nucleation and growth of HA nanocrystals. The clay mineral therefore influences the precipitation process. As a result, clay enables the formation of smaller HA particles that bind to the clay particles and influence the resulting texture of the HA particles, as demonstrated by Broda et al. [41]. Jamil et al. [42] studied the influence of Na-MMT concentration on the formation of the crystalline structure of CaPs and the incorporation of clay ions into the apatitic structure. The addition of Na-MMT affects the size of the apatitic nanoparticles; a low concentration (2 wt%) improved their dispersion, while a higher concentration (10 wt%) promoted their agglomeration. CaP precipitation also affects the original structure of the clay mineral when exfoliation or delamination occurs (Figure 4C). Exfoliation (Figure 4B) is the decomposition of large aggregates into smaller particles, whereas delamination is the process of separation of individual layers from the particles [43]. Pazourková et al. [44] demonstrated by X-ray diffraction (XRD) and scanning electron microscopy (SEM) that CDH was precipitated on the surface of Na- and Mg-MMT, but they could not demonstrate the intercalation of CDH into the MMT interlayer using infrared analysis. Infrared bands were superimposed, and spectra fitting showed no significant changes. Jamil et al. [42] also applied the annealing process up to 1100 °C followed by biomimetic preparation, which induced a change from the original CaP phase (HA) to β-TCP. Rumi et al. [45] have used annealing temperatures from 600 to 1000 °C to form the backbone of the phosphate binder, in a system containing perlite and kaolinitic clay, using calcium carbonate in reaction with phosphoric acid. The biomimetic approach can also be used when clays need to be modified with organic surfactants. Liu et al. [46] have synthesised cetyltrimethylammonium bromide (CTAB)-modified HA-bentonite (BNT) composites using a one-step hydrothermal method. Although the intercalation of CTAB into the BNT interlayer space changes the charges from negative to positive and the properties from hydrophilic to hydrophobic, HA was successfully precipitated by Liu et al. [46]. The fact that clays promote the precipitation of CaPs was confirmed by Paluszkiewicz et al. [47]. Using 2D infrared correlation analysis of the CHS-MMT nanocomposite, they showed that the formation of the apatite structure in pure CHS takes longer than in the case of the CHS-MMT nanocomposite. It is possible to use proteins, peptides, and amino acids to precipitate CaPs within the clay gallery. Kalpana Katti et al. [48] intercalated Na-MMT with 5-aminovaleric acid inspired by biomimetic bone formation.
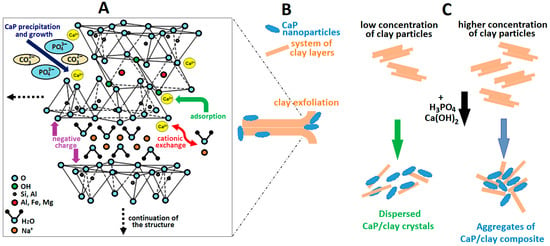 Figure 4. Schematic illustration of (A) CaP nanoparticle precipitation on the clay surface and interlayer, (B) exfoliation of the clay layer system, and (C) formation of dispersed and aggregated CaP/clay particles, as proposed by Jamil et al. [42].
Figure 4. Schematic illustration of (A) CaP nanoparticle precipitation on the clay surface and interlayer, (B) exfoliation of the clay layer system, and (C) formation of dispersed and aggregated CaP/clay particles, as proposed by Jamil et al. [42].
5. Applications of Calcium Orthophosphate–Clay Composites
Clays and clay minerals have low or no toxicity; CaPs contribute bioactivity and biocompatibility due to a high chemical and structural similarity to calcified tissues. These properties, together with excellent adsorption/desorption capacity, high ionic exchange capacity, and their mutual synergy, predetermine CaPCCs for applications in biomaterials engineering as hard tissue substitutes and in pharmacology for the formulation of delivery systems and antibacterial and antifungal agents. Finally, these composites are widely used in environmental applications as sorbents for inorganic and organic pollutants. For environmental applications, these composites are very often used in the CaP-clay composition alone, but for biomaterial or pharmaceutical applications, this material pair is very often combined with other components, most often with organic degradable polymers, as described in the following chapters.
5.1. Environmental Applications
A serious threat to the environment is the pollution of ground and surface water, soil, and the atmosphere. One of the most common pollutants is inorganic heavy metal ions due to their highly toxic nature and uncontrolled release into the environment. Organic pollutants such as dyes, which are widely used in textile, tanning, and food industries, are also an unavoidable problem. However, most of these tend to produce toxins and carcinogens, and most do not biodegrade. Today, contaminants such as plasticisers, pesticides, surfactants, hormones, and antibiotics are becoming increasingly important research topics due to their serious adverse effects on ecosystems and human health. Contamination of natural waters with antibiotics can create antibiotic-resistant genes in living organisms. Several technologies have been developed to remove harmful contaminants, including ion exchange, reverse osmosis, chemistry, electrodialysis, solvent extraction, and adsorption. In recent years, adsorption has been intensively studied as it is recognised to be effective, efficient, and cost-effective. Many different inorganic and organic adsorbents, such as minerals, industrial by-products, agricultural wastes, biomass, polymeric materials, and composite materials, have been studied in terms of their adsorption kinetics and effectivity. Due to their physico-chemical properties, as described in Chapters 2 and 3, CaP-clay composites meet these requirements. As widely available natural materials, they can offer economic and environmental benefits. The most commonly used clays for these applications include BNTs and kaolins. Biomimetic precipitation of CaPs in situ for the preparation of CaPCCs is becoming increasingly relevant [46,49,50,51,52,53,54,55,56,57,58,59]. Another alternative is the use of CaPs prepared from biowastes [60], such as animal bones [61,62,63] or eggshells [64]. CaPCCs are used for the adsorption of inorganic [46,49,50,51,52,53,55,59,61,62] and organic [56,57,58,63,64] pollutants, both in cationic [46,50,51,52,53,55,62] and anionic [49,59,61] form.
Inorganic anion removal refers to the removal of fluoride anions (F−) from drinking water. Fluoride anions are present in all natural waters at some concentration, but in many regions of the world, the higher concentrations are due to anthropogenic activities such as industrial and agricultural activities or a combination of both. High levels of fluoride in drinking water cause dental fluorosis, a disorder characterised by hypomineralisation of the enamel, resulting in physical damage to the teeth. Yakub and Soboyejo [59] presented a novel adsorbent based on sintered clay/HA and demonstrated that this porous adsorbent can effectively remove not only fluoride anions but also microbial pathogens (E. coli). They also found that the amount of fluoride adsorbed increased as the concentration of HA in the composite increased. The principles of adsorption by this composite are based on the ion-exchange substitution of F− ions by OH− ions and the formation of calcium fluoride. Similarly, clays can substitute their OH− groups for F− anions. One of the disadvantages of these composites, which must be considered, is that the pH of the water increases as the concentration of OH− increases. The effect of pH on the fluoride adsorption capacity, the point of zero charge (pHPZC), and the adsorption isotherm of sintered kaolin/HA in both powder and moulded form has been studied in detail by Laonapakul et al. [61]. The pHPZC is the pH at which the net surface charge of a material is zero under given conditions (temperature, applied pressure, and aqueous solution composition). Materials with low pHPZC are suitable for the adsorption of cations, whereas substrates with high pHPZC are more effective for the adsorption of anions.
Arsenic (As) contamination of groundwater is a widespread problem in many parts of the world as a result of both natural effects (weathering and geochemical reactions, biological activities, volcanic emissions) and anthropogenic activities (mining, discharge of industrial wastes, and application of arsenic herbicides and pesticides). The most abundant forms of arsenic in natural waters are arsenate As5+ and arsenite As3+. The As5+ species occurs in the form of oxyanions (H2AsO4−) or (HAsO42−), and the As3+ species occurs in the form of H3AsO3. Under oxidising and aerated conditions, the As5+ form is predominant, whereas under reducing and waterlogged conditions, the As3+ form, which is more toxic compared to As5+, predominates. Hokkanen et al. [49] prepared a HA/BNT system incorporated in cellulose (CL) polymer for As3+ removal from water and investigated the adsorption efficiency of this composite as a function of solution pH, time, As3+ concentration, and temperature. Using CL as a template for the incorporation of HA/BNT particles, the same authors were inspired by their previous work [50], where they used this type of composite for the adsorption of heavy metals. Pollution by heavy metal cations is a serious threat to the environment because they have cumulative properties and cause various diseases. Heavy metals include lead (Pb), nickel (Ni), cadmium (Cd), zinc (Zn), and copper (Cu). Their main sources are galvanic bath waste and waste water from electrical, chemical, printing, textile, leather, fur, oil refining, paper, and rubber industries.
The cationic efficacy of CaPCCs for heavy metals based on BNT [50,51,53], MMT [52], and vermiculite (VER) [52] has been investigated. Choudhury et al. [53] synthesised composites with the addition of glutaraldehyde, which helped bind the active groups of the HA and BNT particles together. An innovative approach was taken by Beni and Esmaeili [62], who used bioapatite isolated from bovine bones together with clay and other bio-wastes, such as Kenaf fibre and hair, to create a ceramic substrate that serves as a carrier for the growth of a biofilm containing micro-organisms. This whole reactor can be used to treat waste water where heavy metal contamination is metabolised by various micro-organisms where it is destroyed, transformed, or accumulated. A very serious problem is the sorption of uranyl cations (UO22+) from wastewater because of the extensive use of uranium as an energy source. Broda et al. [55] demonstrated that HA/kaolin composite has a higher adsorption capacity than pure clay. The presence of HA increases the adsorption efficiency in terms of (UO22+) on the HA/kaolin composite due to the reaction with the phosphate group and the formation of H2(UO2)2(PO4)2·10 H2O precipitates. Glacial meltwater, rivers, salt lake brines, and seawater often contain abundant (bi)carbonate and carbonate anions and, due to their alkalinity, these environments allow the formation of UO2(CO3)22− and UO2(CO3)34− anions rather than UO22+ cations. The negative charge in BNT, one of the inexpensive and readily available buffer materials for radioactive waste, limits its application. Therefore, Liu et al. [46] overcame this problem by modifying BNT with CTAB, which changed the charge within the BNT interlayers from negative to positive.
An interesting approach is the preparation of antibacterial silver (Ag)-doped HA/diatomite-kaolin composite using one-step coprecipitation (Ag-HA) followed by mixing and sintering to produce a ceramic for water filtration. The incorporation of Ag+ into CaP ceramics has antimicrobial activity comparable to that of antibiotic treatment [65]. Deng et al. [54] found that Ag-HA/diatomaceous earth/kaolin composites contain numerous channels in the particles after sintering. The bactericidal rates of block and powder Ag-HA/diatomite clay composite against E. coli and S. aureus were 60.76% and 100% within 1 h, respectively, both reaching 100% after 3 h. This promising filter for water treatment has persistent antibacterial properties within 24 h.
Much more complex interactions occur between the CaP-clay system and complex organic molecules such as drugs [56,57] and dyes. Thermodynamic analysis by Laab et al. [56] showed that the adsorption of the antibiotic Ciprofloxacin on HA/MMT was spontaneous and endothermic with a physisorption character. The binding mechanism at the liquid/composite interface involved the synergistic interplay of electrostatic forces, hydrophobic interactions, n-π electron donor–acceptor interactions, and H-bonding, which was strongly influenced by the pH of the solution (Figure 5A) [57]. Dyes used in the textile, tanning, and food industries tend to generate toxins and carcinogens. Wastes containing organic dyes reduce the concentration of dissolved oxygen in natural waters, thereby preventing light penetration and inhibiting photosynthesis in plants. They can also form coordination compounds with some toxic metal ions. Methylene blue (MB) is a commonly used cationic dye for dyeing textiles such as cotton, wool, and silk. Cationic dyes are reported to be more toxic than anionic dyes.
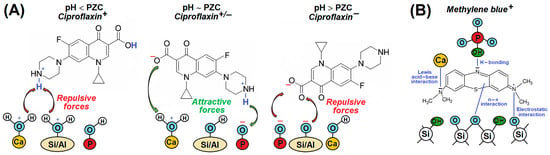
Figure 5.
Schematic mechanisms of (A) behaviour of Ciproflaxin drug at different pH and (B) adsorption of Methylene blue dye on CaP-clay surface, based on literature [57,58,64,66].
As dyes are not biodegradable, it is necessary to introduce measures for their analysis and control. Vezentsev et al. [58] have investigated the adsorption model of MB on BNT/HA composite and proved that the combination of BNT and HA is 1.3 and 17.8 times higher than that of BNT and HA, respectively. The schematic representation of the adsorption mechanism of MB by the HA/clay composite is shown in Figure 5B. This mechanism was proposed by Annan et al. [64] after considering the mechanism of MB adsorption by HA [58] and clay [66] separately. The HA/clay composite embodies the negative charge in the alkaline medium, which promotes the adsorption of MB cations. Although the positively charged surface is unable to adsorb MB efficiently, a reasonable amount of MB was absorbed, as demonstrated by Annan et al. [64]. They found that BNTs had the highest degree of adsorption compared to HNT and kaolinite (KAO) due to the 2:1 layer type compared to HNT and KAO, which are 1:1 layer type clays.
An example of the use of readily available and inexpensive material for the manufacture of an adsorption filter for the treatment of waste water from rubber processing was published by Chankachang et al. [63]. They used Lampang kaolinitic clay collected from Lampang (a province in northern Thailand) and pig bones for CaP production, while the CaPCC was prepared by sintering. Rubber wastewater has a high concentration of ammonia, nitrate, phosphorus, and suspended solids. The Lampang clay/HA composite completely removed total Kjehldahl nitrogen and total phosphorus, while the suspended solids removal was almost 98%, and the biochemical oxygen demand was reduced by 76%.
5.2. Applications in Biomaterial Engineering
The need for bioactive and non-toxic materials is nowadays in high demand in biomaterials engineering. One of the major challenges in the use of CaPs in engineering applications is their brittleness. The combination of CaP with clays reduces brittleness and confers special properties such as mechanical properties [24,30,40], high surface area, crystallinity, high porosity, and biocompatibility [67]. Furthermore, for biomaterial or pharmaceutical applications, the CaP-clay pair is very often modified with other components, e.g., proteins and saccharides [29,48], and they can also be mixed with degradable organic polymers as fillers [68] to produce CaP-clay-polymer composites with the required mechanical and degradable properties.
5.2.1. Tissue Engineering
CaPCCs for tissue engineering applications can be used in two ways. In a smaller number of cases, they are used as CaP-clay-bicomponent composites [24,25,30,36,69,70]. The mechanical properties of CaP-clay bicomponent composites predestine them for load-bearing applications, such as implants for orthopaedic [69] or dental [71] applications. Bone tissue engineering is a rapidly developing field of science that regenerates new tissue using scaffolds enriched with bioactive molecules and cells. Scaffolds should provide the support for cells to proliferate and maintain their differentiated functions. An ideal bone scaffold should mimic the natural extracellular environment of bone tissue, which consists of both organic and inorganic phases. One attempt to mimic this composition and structure is the production of composites that also contain organic components, mainly based on polymers. Recently, however, natural polymers have replaced synthetic polymers due to their improved biocompatibility and the non-toxic nature of their degradation products. Among natural polymers, CHS-based materials have been widely studied and used, both alone [72,73,74,75,76,77] and in combination with other polymers [78,79,80,81]. CHS is a natural biodegradable and biocompatible polysaccharide derived from chitin by partial or complete deacetylation; it consists of glucosamine and N-acetylglucosamine linked in a β(1–4) linkage. Chitosan was first used in combination with CaP/clay filler by Katti et al. [75]. They demonstrated significant interactions and proposed their mechanism between CHS, HA, and MMT. Other natural carbohydrate-based polymers used include starch [82] and carboxymethylcellulose [83].
Synthetic polyesters such as polycaprolactone (PCL) [48,68,84,85,86] and polylactide (PLA) [87,88] are still widely used for these applications. PCL is a semi-crystalline and hydrophobic biocompatible polymer that has attracted attention for its biocompatibility and superior mechanical properties. The weaknesses of PCL include its slow in vivo degradation rate and lack of bioactivity. The degradation properties of PCL can be improved by combining it with HA and clay nanoparticles or by combining it with other rapidly degrading polymers. Saturated poly(α-hydroxy esters) such as PLA are currently one of the most promising polymers due to their combination of controlled degradation (thanks to the possibility of combining different crystallinities) and ease of melt processing into complex shapes. Another synthetic polymer used in bone implant surgery is high-density polyethylene (HDPE). Balakrishnan et al. [89] investigated the introduction of organically modified MMT into HDPE/HA composites and demonstrated that the addition of MMT increased the strength and stiffness of HDPE/HA composites with a deterioration in the impact strength and elongation at break values.
Modification of clays with organic surfactants, e.g., maleic anhydride [89] or CTAB [46], leads to changes in interlayer charges and hydrophilic properties, allowing better miscibility of clay particles with polymers. Sandomierski et al. [71] modified MMT particles with silane to improve their adhesion to methacrylic resin for dental applications. Similarly, the functional groups present in a modifier can potentially serve as sites for the growth and precipitation of CaP, as shown in Figure 3 and described in detail in Section 4. The scientific group around Katti and Ambre has published many papers on the use of unnatural amino acids such as 5-aminovaleric acid [48,78,81,85,86] and 2-aminopimelic and 2-aminocaprylic acids [80] for organomodified clays as components of nanocomposite biomaterials. An increasing number of hydrogel/polymer hybrid composites have been fabricated to improve mechanical properties and osteogenic performance. Same et al. [90] developed 3D porous amphiphilic scaffolds based on a PCL/polyethylene/gelatine multipolymer system for the incorporation of HNT/HA/Fe2O3 to improve hydrogel surface properties in cell adhesion, migration, and osteogenesis.
The most commonly used clays for these applications include MMT [24,68,71,72,73,74,75,78,79,80,81,85,86,87,88,89,91], HNT [36,70,76,77,84,90], and, rarely, kaolins [25] and LAP [29]. Wang et al. [69] investigated and compared the effect of the addition of HNT, LAP, and SEP on the self-setting time of magnesium phosphate bone cements (MPCs). All these clays provide adequate setting time but influence the morphologies of the deposited CaP in SBF. Composite with SEP embody the highest compressive strength (33.45 ± 2.87 MPa, which increased by 116% compared to pure MPCs. Economical approaches include the use of natural rocks based on anorthite and β-TCP from dolomitic phosphate and kaolin rocks [25] or the use of bioapatite isolated from fish bones [87,88]. Chozhanathmisra et al. [36] prepared cerium (Ce) substituted HA for electrodeposition of HNT/Ce-HA composite layer on Ti alloy substrate as a bioactive coating with improved antibacterial activity and anti-corrosion stability in SBF. Antibacterial properties have also been demonstrated on organically modified clays [74]. Postoperative complications due to infection are among the most common problems following dental and orthopaedic surgery. The prevention of postoperative infections is, therefore, a critical need. For this purpose, HNTs have been loaded with antibiotics (gentamicin sulphate and neomycin sulphate) [70], dexamethasone (steroidal anti-inflammatory drug) [77], and curcumin (antioxidant, anti-inflammatory, and anti-tumorigenic properties) [84]. The CaP-clay/drug composite systems serve as biocompatible substitutes and, at the same time, release the drugs systematically and act directly at the targeted site, such as in local delivery treatments [70].
5.2.2. Pharmacology
In recent years, the study of controlled release of drugs and other bioactive agents has attracted many researchers from around the world. Controlled drug release includes sustained release over days/weeks/months/years and specific targeting of drugs (e.g., to a tissue cell or tumour cells). Controlled drug release systems [92] are needed to reduce the amount of drug required to achieve the same therapeutic effect. Many of the controlled release systems exhibit an initial large release of the drug immediately upon placement in the medium, followed by a stable release profile. This phenomenon is typically referred to as “burst release” [93]. Such unpredictable and uncontrolled release is often undesirable due to the potential for overdose and subsequent patient health complications. Chen et al. [94] have developed a tunable, biocompatible, biodegradable, and bioresorbable CHS/Cloisite Na+/β-TCP/PCL scaffold composite for the sustained release of doxorubicin hydrochloride (DH). DH is a widely used anthracycline antibiotic with broad-spectrum antitumour activity for the treatment of various types of malignancies. They demonstrated that scaffolds containing nanoclay released up to 45% of the drug for up to 2 months, while the scaffold without nanoclay released 95% of the drug within 4 days.
The clay particles are the main reservoir for the drug, while the CaP particles act as a filler, improving biocompatibility and influencing the mechanical properties of the final product. Drug and polymer molecules can intercalate and exfoliate the clay particles to form a stable aqueous suspension and improve the aqueous solubility of the drug. The choice of polymer for effective clay dispersion is a very important issue. Clay–drug interaction mechanisms, including hydrophobic interactions, hydrogen bonding, and ligand exchange, as well as the charge of the drug, the clay exchange capacity, and the pH of the medium, determine the drug release kinetics. For anionic drugs and polymers, instead of a cation exchanger such as Na-MMT, an anion exchanger such as layered double hydroxides (described in Section 3) can be used.
Pazourková et al. [95] compared two clay minerals, MMT and VER, in terms of their ability to intercalate chlorhexidine diacetate (CD), an antiseptic and disinfectant commonly used to reduce the risk of infection against a wide range of bacteria, fungi, and some viruses. Such formulations are becoming increasingly popular. They found that MMT had the ability to incorporate a higher amount of CD into the structure compared to VER. Clay/CD systems were decorated with biomimetically precipitated CDH particles. Furthermore, they confirmed [96] that after the fusion of hybrid nanofillers (MMT/CD/CDH) with polyethylene (PE), an intense exfoliation of the MMT layers occurred, and a higher dispersibility of the MMT particles in PE was observed compared to the VER/CD/CDA composite. Similarly, the MMT/CD/CDH/PE composite showed higher antibacterial efficacy against Staphylococcus aureus.
The effect of different pH on the release of doxorubicin and curcumin from bioapatite isolated from fish bone/MMT/sodium alginate (SA) composite was investigated by Fiaz Ahamed et al. [97]. They found that drug release was strongly pH-dependent and demonstrated that composites significantly improved release compared to pure bioapatite. The authors attributed this to water molecules in the MMT interlayer, which provide the various interactions, such as electrostatic interactions and hydrogen bonding, of the SA with the MMT and bioapatite. There is currently not much work on the use of CaP-clay composites for pharmacological applications, but this is definitely one of the developing areas.
Drug delivery may also include systems for cosmetic applications. Paientko et al. [98] prepared nanostructured composites based on KAO/quartz/HA/acai. Acai berries contain many trace elements such as Ca, P, K, Mg, Zn, B vitamins, beta-carotene, and anthocyanins. It has a moisturising and nourishing effect on the skin and can relieve the symptoms of various skin diseases and allergic skin manifestations; it has an anti-inflammatory effect; it protects against UV rays. The composites with HA have been considered better systems for cosmetic and medicinal preparations than composites with KAO clay alone because the morphological, electrochemical, and structural characteristics allow us to expect high efficiency in the delivery characteristics.
5.3. Other Applications
To date, CaPCCs have been used mainly for environmental and biomaterial engineering applications. However, the physico-chemical properties of CaPCCs described in Section 2 and Section 3 open up other new and promising applications. The first application relates to chemical fertilisers used in agriculture. Technical-grade CaPs are already a very popular mineral fertiliser in their own right. Phosphorus is a primary (main) plant nutrient, while calcium is a secondary nutrient, and together with C, H and O (available from air and water), they are termed macronutrients [99]. However, the efficiency of nutrient use by plants, particularly with regard to N, P, and K, is estimated to be very low. Up to 70% of N is lost through leaching, volatilisation as ammonia and nitrous oxide, and long-term incorporation into soil organic matter. Excessive use of phosphate fertilisers can lead to disruption of the natural nitrogen and phosphorus nutrient cycles due to massive inputs of phosphorus and nitrogen and the unintended release of heavy metals and radionuclides [100]. Scientists have predicted that nanocomposites could solve this problem, as the high surface area to volume ratio of nanoparticles allows plants to take up fertiliser on demand and in sustained doses. Therefore, Madusanka et al. [101] realised the synthesis of an improved nanohybrid composite based on MMT/HA encapsulating the nutrient (urea) and supporting the cation balance in the soil, and they proved that this composite system is structurally and functionally valuable for slow and sustained release of urea into the soil.
Another application is in geopolymer science. A geopolymer is the term for all inorganic polymeric materials used as environmentally friendly geopolymer concrete. Geopolymers exhibit high resistance to acids and salt solutions, low shrinkage, low thermal conductivity, and fire resistance, making these materials strong candidates to replace Portland cements. They have the ability to form insulating foams to immobilise hazardous wastes and also find application as adhesives, strongly bonding to ceramics, metals, and polymers. They are prepared from aluminosilicate materials (clays) via their geopolymerisation in an alkaline environment at normal temperature and pressure. Bhuiya et al. [102] prepared potassium-based geopolymer composites based on different metakaolin and different calcium phosphates (bone ash, HA, and brushite). They compared the physico-chemical characterisation (chemical analysis, XRD, thermogravimetry, and microstructure) and mechanical properties (compressive and flexural strength) of both types of composites: geopolymerised at room temperature and those after heat treatment (1150 °C). The authors suggest that one of the proposed composite types could also be further investigated for use as a hard tissue substitute.
6. Conclusions and Future Perspectives
Calcium orthophosphates, clays, and their derivatives have been of general importance for several decades in many interdisciplinary fields of science, including geology, chemistry, ecology, biology, and medicine. They exhibit excellent physicochemical properties such as adsorption/desorption capacity and high ionic exchange capacity combined with low or no toxicity, bioactivity, and biocompatibility, and have great application potential in many areas of the pharmaceutical, cosmetic, and food industries and the engineering of biocomposites.
A conservative approach using a combination of just these two components is most commonly used for applications involving the purification of groundwater, surface water, wastewater, soils, and the atmosphere from inorganic and organic contaminants. Purification methods have shifted from simple adsorption of heavy metal cations and organic dyes to sorption of complex organic substances such as pharmaceuticals and hormones, which have devastating effects on various parts of the ecosystem. The combination of CaP and clay has also proved beneficial in dental and orthopaedic applications. The disadvantage of the high brittleness of CaP can be compensated by the addition of clay mineral and, thanks to the synergistic effect of both components, it is possible to produce biocomposites with optimal mechanical properties, high surface area, high porosity, and biocompatibility, which are crucial for biomaterial engineering. Modification of this pair of materials with organic components further enhances the biocompatibility and degradation profile. Optimal degradation of the composite material, exchange capacity, and adsorption/desorption characteristics are important factors in the preparation of controlled release systems for pharmaceutical applications.
In addition to these standard applications, other interesting and promising applications have emerged in recent years. ACC nanoparticles can encapsulate the fertiliser, and due to the high surface area-to-volume ratio, these nanoparticles can act as systems for the controlled release of nutrients into the soil in a slow and sustained manner. Such a paradigm shift in fertiliser practices can be more efficient, leading to cost savings and less environmental damage. The optimal mechanical properties, high resistance to acids and salt solutions, low shrinkage, low thermal conductivity, and fire resistance of CaPCCs allow them to be used in geopolymer science. Geopolymers, such as environmentally friendly inorganic polymers, have a carbon footprint that is 80% lower than traditional Portland cement, an industry that contributes 8% of the world’s carbon dioxide emissions.
Because clays or clay minerals and CaPs are natural materials, they are readily available and inexpensive. The preparation of their composites is also not economically demanding. They can be prepared using both conservative methods, such as mixing, sintering and precipitation, and newer methods, such as electrophoresis and 3D printing. The isolation of CaPs from bio-waste such as animal bones or eggshells is becoming increasingly interesting and used. This effective use of bio-waste is necessary because it is accumulating, and it would be very beneficial to reuse it.
Since there is a wide range of clay minerals with different chemical and structural properties, such as layer charge and ion exchange capacity, and a wide range of CaPs with different Ca/P molar ratios, solubility, stability in media, etc., the final properties of CaPCCs can be tailored according to the required applications. The synergistic effect of the combination of CaP and clay minerals, as well as the further modification of CaPCCs by various organic components, would provide a new idea for the development of better nanofunctional materials and the production of new composites with a multifunctional property, which have great promise in many fields of human society.
Funding
This study was supported by the Long-term Conceptual Development Research Organization under project No. RVO: 67985891.
Data Availability Statement
The author declares that all analytical data supporting the findings of this study are available within the paper.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviation
| CaPCCs | Calcium orthophosphate–clay composites. |
| CaPs | Calcium orthophosphates. |
| α,β-TCP | α,β-tricalcium phosphate. |
| HA | Hydroxyapatite. |
| CDH | Calcium-deficient hydroxyapatite. |
| MPCs | Magnesium phosphate bone cements. |
| LAP | Laponite (clay mineral). |
| MMT | Montmorillonite (clay mineral). |
| HNT | Halloysite (clay mineral) nanotube. |
| SEP | Sepiolite (clay mineral). |
| BNT | Bentonite (clay). |
| VER | Vermiculite (clay mineral). |
| KAO | Kaolinite (clay mineral). |
| CTAB | Cetyltrimethylammonium bromide. |
| CHS | Chitosan (polysaccharide). |
| CL | Cellulose (polysaccharide). |
| PCL | Polycaprolactone (polymer). |
| PLA | Polylactide (polymer). |
| HDPE | High-density polyethylene (polymer). |
| MB | Methylene blue (dye). |
| SA | Sodium alginate (sodium salt form of alginic acid). |
| DH | Doxorubicin hydrochloride (drug). |
| CD | Chlorhexidine diacetate (drug). |
| SBF | Simulated body fluid. |
| EPD | Electrophoretic deposition. |
References
- Skwarek, E.B. Clay, Hydroxyapatite and Their Composites—Brief Review. In Nanooptics and Photonics, Nanochemistry and Nanobiotechnology, and Their Applications; Part of the Springer Proceedings in Physics Book Series (SPPHY, volume 247); Springer: Cham, Switzerland, 2020; pp. 255–272. [Google Scholar]
- Farsang, S.; Pekker, P.; Lampronti, G.I.; Molnár, Z.; Milovský, R.; Pósfai, M.; Ozdín, D.; Raub, T.D.; Redfern, S.A.T. Inclusions in calcite phantom crystals suggest role of clay minerals in dolomite formation. Am. Miner. 2022, 107, 1369–1377. [Google Scholar] [CrossRef]
- Nomicisio, C.; Ruggeri, M.; Bianchi, E.; Vigani, B.; Valentino, C.; Aguzzi, C.; Viseras, C.; Rossi, S.; Sandri, G. Natural and Synthetic Clay Minerals in the Pharmaceutical and Biomedical Fields. Pharmaceutics 2023, 15, 1368. [Google Scholar] [CrossRef]
- Choy, J.H.; Choi, S.J.; Oh, J.M.; Park, T. Clay minerals and layered double hydroxides for novel biological applications. Appl. Clay Sci. 2007, 36, 122–132. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, W.; Ding, J.; Wang, W.; Wang, A. Hydroxyapatite Nanomaterials: Synthesis, Properties, and Functional Applications. In Nanomaterials from Clay Minerals, A New Approach to Green Functional Materials, Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 10; pp. 485–536. [Google Scholar]
- Ahmed, A.S.A.; Hashem, F.S.M. Biodegradable Inorganic Nanocomposites for Industrial Applications. In Handbook of Biodegradable Materials; Ali, G.A.M., Makhlouf, A.S.H., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphates in Nature, Biology and Medicine. Materials 2009, 2, 399–498. [Google Scholar] [CrossRef]
- Pan, Y.; Fleet, M.E. Compositions of the apatite-group minerals: Substitution mechanisms and controlling factors. In Phosphates: Geochemical, Geobiological and Materials Importance; Series: Reviews in Mineralogy and Geochemistry; Hughes, J.M., Kohn, M., Rakovan, J., Eds.; Mineralogical Society of America: Washington, DC, USA, 2002; Volume 48, pp. 13–50. [Google Scholar] [CrossRef]
- Šupová, M. Substituted hydroxyapatites for biomedical applications: A review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.T.; Dinpanah-Khoshdargi, E.; Jamshid, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.M.; Rakovan, J. The Crystal Structure of Apatite, Ca5(PO4)3(F, OH, Cl). In Phosphates: Geochemical, Geobiological and Material Importance, Reviews in Mineralogy and Geochemistry; Kohn, M.J., Rakovan, J., Hughes, J.M., Eds.; Mineralogical Society of America: Washington, DC, USA, 2002; Volume 48, pp. 1–12. [Google Scholar]
- Rey, C.; Combes, C.; Drouet, C.; Sfihi, H.; Barroug, A. Physico-chemical properties of nanocrystalline apatites: Implications for biominerals and biomaterials. Mater. Sci. Eng. C 2007, 27, 198–205. [Google Scholar] [CrossRef]
- Stigter, M.; Bezemer, J.; de Groot, K.; Layrolle, P. Incorporation of different antibiotics into carbonated hydroxyapatite coatings on titanium implants, release and antibiotic efficacy. J. Control. Release 2004, 99, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Guggenheim, S.; Martin, R.T. Definition of Clay and Clay Mineral: Joint Report of the Aipea Nomenclature and CMS Nomenclature Committees. Clays Clay Miner. 1995, 43, 255–256. [Google Scholar] [CrossRef]
- Brindley, G.W.; Brown, G. Crystal Structures of Clay Minerals and Their X-Ray Identification. In Mineralogical Society Monograph No. 5; GeoScienceWorld: London, UK, 1980; Chapter 6. [Google Scholar] [CrossRef]
- Murray, H.H. Structure and Composition of the Clay Minerals and their Physical and Chemical Properties. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2006; Volume 2, pp. 7–31. [Google Scholar] [CrossRef]
- Whitworth, T.M. Clay minerals: Ion exchange. In Geochemistry. Encyclopedia of Earth Science; Springer: Dordrecht, The Netherlands, 1998. [Google Scholar] [CrossRef]
- Reichle, W.T. Synthesis of anionic clay minerals (mixed metal hydroxides, hydrotalcite). Solid State Ion. 1986, 22, 135–142. [Google Scholar] [CrossRef]
- Chiu, C.W.; Huang, T.K.; Wang, Y.C.; Alamani, B.G.; Lin, J.J. Intercalation strategies in clay/polymer hybrids. Prog. Polym. Sci. 2014, 39, 443–485. [Google Scholar] [CrossRef]
- Lagaly, G.; Ogawa, M.; Dékány, I. Chapter 10.3—Clay Mineral-Organic Interactions. Dev. Clay Sci. 2013, 5, 215–225. [Google Scholar] [CrossRef]
- Gaboreau, S.; Gailhanou, H.; Blanc, P.; Vieillard, P.; Madé, B. Clay mineral solubility from aqueous equilibrium: Assessment of the measured thermodynamic properties. Appl. Geochem. 2020, 113, 104465. [Google Scholar] [CrossRef]
- Hao, W.; Mand, K.; Swaren, L.; Myers, K.D.; Lalonde, S.; Wilmeth, D.T. Trace elemental partitioning on clays derived from hydrothermal muds of the El Tatio Geyser Field, Chile. J. Geophys. Res. Solid Earth 2021, 126, e2020JB021422. [Google Scholar] [CrossRef]
- Martin, R.T.; Bailey, S.W.; Eberl, D.D. Report of the Clay Minerals Society Nomenclature Committee: Revised Classification of Clay Materials. Clays Clay Miner. 1991, 39, 333–335. [Google Scholar] [CrossRef]
- Jamil, M.; Elouahli, A.; Abida, F.; Khallok, H.; Gourri, E.; Kheribech, A.; Hatim, Z. Development of Triphasic Hydroxyapatite/(α and β)-Tricalcium Phosphate Based Composites by Sintering Powder of Calcium-Apatite in the Presence of Montmorillonite. J. Inorg. Organomet. Polym. 2020, 30, 2489–2498. [Google Scholar] [CrossRef]
- El-Mehalawy, N.; Sayed, M.; Esmat, A.; El-Anwar, A.; Soliman, A.A.F.; Naga, S.M. Preparation and characterization of bioceramic composites based on anorthite and β-TCP from dolomitic phosphate and kaolin rocks. Mater. Chem. Phys. 2024, 312, 128625. [Google Scholar] [CrossRef]
- Ebadzadeh, T.; Behnamghader, A.; Nemati, R. Preparation of porous hydroxyapatite ceramics containing mullite by reaction sintering of clay, alumina and hydroxyapatite. Ceram. Int. 2011, 37, 2887–2889. [Google Scholar] [CrossRef]
- Chouia, F.; Belhouchet, H.; Sahnoune, F.; Bouzrara, F. Reaction sintering of kaolin-natural phosphate mixtures. Ceram. Int. 2015, 41, 8064–8069. [Google Scholar] [CrossRef]
- Jamil, M.; Elouahli, A.; Khallok, H.; El-ouatlia, B.; Hatim, Z. Characterization of β-tricalcium phosphate-clay mineral composite obtained by sintering powder of apatitic calcium phosphate and montmorillonite. Surf. Interfaces 2019, 17, 100380. [Google Scholar] [CrossRef]
- Li, S.; Chen, C.; Zhang, Z.; Wang, D.; Lv, S. Illustration and application of enhancing effect of arginine on interactions between nano-clays: Self-healing hydrogels. Soft Matter 2019, 15, 303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Han, K.; Dong, L.; Li, X. Preparation and Characterization of β-Tricalcium Phosphate/Nano Clay Composite Scaffolds via Digital Light Processing Printing. J. Inorg. Mater. 2022, 37, 1116–1122. Available online: https://www.jim.org.cn/EN/10.15541/jim20210745 (accessed on 25 October 2023). [CrossRef]
- Faksawat, K.; Limsuwan, P.; Naemchanthara, K. 3D printing technique of specific bone shape based on raw clay using hydroxyapatite as an additive materiál. Appl. Clay Sci. 2021, 214, 106269. [Google Scholar] [CrossRef]
- Nguyen, N.M.; Kakarla, A.B.; Nukala, S.G.; Kong, C.; Baji, A.; Kong, I. Evaluation of Physicochemical Properties of a Hydroxyapatite Polymer Nanocomposite for Use in Fused Filament Fabrication. Polymers 2023, 15, 3980. [Google Scholar] [CrossRef] [PubMed]
- Haq, R.H.A.; Wahab, M.S.; Jaimi, N.I. Fabrication Process of Polymer Nano-Composite Filament for Fused Deposition Modeling. Appl. Mech. Mater. 2014, 465–466, 8–12. Available online: https://www.scientific.net/AMM.465-466.8 (accessed on 5 December 2023). [CrossRef]
- Haq, R.H.A.; Wahab, M.S.; Wahit, M.U. Impact Test and Bioactivity Properties of Polycaprolactone (PCL) by Addition of Nano-Montmorillonite (MMT) and Hydroxyapatite (HA). Appl. Mech. Mater. 2014, 446–447, 1129–1133. Available online: https://www.scientific.net/AMM.446-447.1129 (accessed on 10 December 2023). [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Chozhanathmisra, M.; Murugan, N.; Karthikeyan, P.; Sathishkumar, S.; Anbarasu, G.; Rajavel, R. Development of antibacterial activity and corrosion resistence properties of electrodeposition of mineralized hydroxyapatite coated on titanium alloy for biomedical applications. Mater. Today Proc. 2017, 4, 12393–12400. [Google Scholar] [CrossRef]
- Molaei, A.; Yari, M.; Reza Afshar, M. Investigation of halloysite nanotube content on electrophoretic deposition (EPD) of chitosan-bioglass-hydroxyapatite-halloysite nanotube nanocomposites films in surface engineering. Appl. Clay Sci. 2017, 135, 75–81. [Google Scholar] [CrossRef]
- Molaei, A.; Amadeh, A.; Yari, M.; Reza Afshar, M. Structure, apatite inducing ability, and corrosion behavior of chitosan/halloysite nanotube coatings prepared by electrophoretic deposition on titanium substrate. Mater. Sci. Eng. C 2016, 59, 740–747. [Google Scholar] [CrossRef]
- Šupová, M. The Significance and Utilisation of Biomimetic and Bioinspired Strategies in the Field of Biomedical Material Engineering: The Case of Calcium Phosphate—Protein Template Constructs. Materials 2020, 13, 327. [Google Scholar] [CrossRef]
- Wan, C.; Chen, B. Synthesis and characterization of biomimetic hydroxyapatite/sepiolite nanocomposites. Nanoscale 2011, 3, 693–700. Available online: https://pubs.rsc.org/en/content/articlehtml/2011/nr/c0nr00650e (accessed on 23 October 2023). [CrossRef] [PubMed]
- Broda, E.; Skwarek, E.; Payentko, V.V.; Gunko, V.M. Synthesis and selected physicochemical properties of hydroxyapatite and white clay composite. Physicochem. Probl. Miner. Process. 2019, 55, 1475–1483. [Google Scholar] [CrossRef]
- Jamil, M.; Elouahli, A.; Abida, F.; Assaoui, J.; Gourri, E.; Hatim, Z. Apatitic calcium phosphate/montmorillonite nano-biocomposite: In-situ synthesis, characterization and dissolution properties. Heliyon 2022, 8, e10042. [Google Scholar] [CrossRef]
- Gardolinski, J.E.F.C.; Lagaly, G. Grafted organic derivates of kaolinite: II. Intercalation of primary n-alkylamines and delamination. Clay Miner. 2005, 40, 547–556. [Google Scholar] [CrossRef]
- Pazourková, L.; Peikertová, P.; Hundáková, M.; Martynková, G.S. Preparation of calcium deficient hydroxyapatite on the montmorillonite substrate: Structure and morphology. Mater. Today Proc. 2021, 37, 35–41. [Google Scholar] [CrossRef]
- Rumi, M.K.; Urazaeva, E.M.; Irmatova, S.K.; Nurmatov, S.R.; Zufarov, M.A.; Mansurova, E.P.; Ziyovaddinov, Z.K. Influence of heat treatment on the structure and properties of ceramic heat-insulating composites based on phosphate bound expanded perlite-expanded clay. Open Ceram. 2023, 14, 100344. [Google Scholar] [CrossRef]
- Liu, J.; Shi, S.; Li, C.; Hong, X.; Gu, Z.; Li, F.; Zhai, J.; Zhang, Q.; Liao, J.; Liu, N.; et al. U(VI) adsorption by one-step hydrothermally synthesized cetyltrimethylammonium bromide modified hydroxyapatite-bentonite composites from phosphate-carbonate coexisted solution. Appl. Clay Sci. 2021, 203, 106027. [Google Scholar] [CrossRef]
- Paluszkiewicz, A.; Wesełucha-Birczyńska, A.; Stodolak-Zych, E.; Hasika, M. 2D IR correlation analysis of chitosan-MMT nanocomposite system. Vib. Spectrosc. 2012, 60, 185–188. [Google Scholar] [CrossRef]
- Katti, K.S.; Ambre, A.H.; Payne, S.; Katti, D.R. Vesicular delivery of crystalline calcium minerals to ECM in biomineralized nanoclay composites. Mater. Res. Express 2015, 2, 045401. Available online: https://iopscience.iop.org/article/10.1088/2053-1591/2/4/045401 (accessed on 25 October 2023). [CrossRef]
- Hokkanen, S.; Doshi, B.; Srivastava, V.; Puro, L.; Koivula, R. Arsenic (III) removal from water by hydroxyapatite-bentonite clay-nanocrystalline cellulose. Environ. Prog. Sustain. Energy 2019, 38, 13147. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Srivastava, V.; Suorsa, V.; Sillanpää, M. Removal of Cd2+, Ni2+ and PO43− from aqueous solution by hydroxyapatite-bentonite clay-nanocellulose composite. Int. J. Biol. Macromol. 2018, 118, 903–912. [Google Scholar] [CrossRef]
- Vezentsev, A.I.; Thuy, D.M.; Peristaya, L.F.; Peristyj, V.A.; Alateya, A.T.; Minh, P.T. Investigation of sorption of Cu2+, Zn2+ and Cd2+ ions by a composite adsorbent obtained from bentonite-like clay and hydroxyapatite. J. Eng. Sci. Technol. 2019, 14, 520–530. [Google Scholar]
- Pazourková, L.; Kupková, J.; Hundáková, M.; Seidlerová, J.; Martynková, G.S. Sorption of Cd2+ on Clay Mineral/Hydroxyapatite Nanocomposites. J. Nanosci. Nanotechnol. 2016, 16, 7788–7791. [Google Scholar] [CrossRef]
- Choudhury, P.R.; Mondal, P.; Majumdar, S. Synthesis of bentonite clay based hydroxyapatite nanocomposites cross-linked by glutaraldehyde and optimization by response surface methodology for lead removal from aqueous solution. RSC Adv. 2015, 5, 100838. [Google Scholar] [CrossRef]
- Deng, C.; Qi, X.; Li, Q.; Song, Q.; Deng, Y. Preparation and antibacterial properties of Ag doped hydroxyapatite/diatomite-kaolin composite ceramics. Acta Mater. Compos. Sin. 2016, 11, 2591–2599. Available online: https://fhclxb.buaa.edu.cn/cn/article/doi/10.13801/j.cnki.fhclxb.20160125.003 (accessed on 22 November 2023).
- Broda, E.; Gładysz-Płaska, A.; Skwarek, E.; Payentko, V.V. Structural properties and adsorption of uranyl ions on the nanocomposite hydroxyapatite/white clay. Appl. Nanosci. 2022, 12, 1101–1111. [Google Scholar] [CrossRef]
- Laab, M.; Brahmi, Y.; El Ibrahimi, B.; Hsini, A.; Toufik, E.; Abdellaoui, Y.; Abou Oualid, H.; El Ouardi, M.; Albourine, A. A novel mesoporous Hydroxyapatite@Montmorillonite hybrid composite for high-performance removal of emerging Ciprofloxacin antibiotic from water: Integrated experimental and Monte Carlo computational assessment. J. Mol. Liq. 2021, 338, 116705. [Google Scholar] [CrossRef]
- Ersan, M.; Guler, U.A.; Acıkel, U.; Sarioglu, M. Synthesis of hydroxyapatite/clay and hydroxyapatite/pumice composites for tetracycline removal from aqueous solutions. Process Saf. Environ. Prot. 2015, 96, 22–32. [Google Scholar] [CrossRef]
- Vezentsev, A.I.; Thuy, D.M.; Goldovskaya-Peristaya, L.F.; Glukhareva, N.A. Adsorption of Methylene Blue on the Composite Sorbent Based on Bentonite-Like Clay and Hydroxyapatite. Indones. J. Chem. 2018, 18, 733–741. [Google Scholar] [CrossRef]
- Yakub, I.; Soboyejo, W. Adsorption of Fluoride from Water Using Sintered Clay-Hydroxyapatite Composites. J. Environ. Eng. ASCE 2013, 139, 995–1003. [Google Scholar] [CrossRef]
- Šupová, M. Isolation and Preparation of Nanoscale Bioapatites from Natural Sources: A Review. J. Nanosci. Nanotechnol. 2014, 14, 546–563. [Google Scholar] [CrossRef]
- Laonapakul, T.; Suthi, T.; Otsuka, Y.; Mutoh, Y.; Chaikool, P.; Chindaprasirt, P. Fluoride adsorption enhancement of Calcined-Kaolin/Hydroxyapatite composite. Arab. J. Chem. 2022, 15, 104220. [Google Scholar] [CrossRef]
- Beni, A.A.; Esmaeili, A. Design and optimization of a new reactor based on biofilm-ceramic for industrial wastewater treatment. Environ. Pollut. 2019, 255, 113298. [Google Scholar] [CrossRef] [PubMed]
- Chankachang, P.; Chantara, S.; Punyanitya, S.; Saelee, C.; Thiansem, S. Treatment of wastewater from rubber processing using hydroxyapatite and lampang clay nanocomposite filters. Key Eng. Mater. 2016, 675–676, 81–84. Available online: https://www.scientific.net/KEM.675-676.81 (accessed on 25 October 2023). [CrossRef]
- Annan, E.; Arkorful, G.K.; Konadu, D.S.; Asimeng, B.; Dodoo-Arhin, D.; Egblewogbe, M. Synthesis and Characterization of Hydroxyapatite- (HAP-) Clay Composites and Adsorption Studies on Methylene Blue for Water Treatment. J. Chem. 2021, 2021, 3833737. [Google Scholar] [CrossRef]
- Ewald, A.; Hösel, D.; Patel, S.; Grover, L.M.; Barralet, J.E.; Gbureck, U. Silver-doped calcium phosphate cements with antimicrobial aktivity. Acta Biomater. 2011, 7, 4064–4070. [Google Scholar] [CrossRef] [PubMed]
- Jawad, A.H.; Abdulhameed, A.S. Mesoporous Iraqi red kaolin clay as an efficient adsorbent for methylene blue dye: Adsorption kinetic, isotherm and mechanism study. Surf. Interfaces 2020, 18, 100422. [Google Scholar] [CrossRef]
- Ofudje, E.A.; Akande, J.A.; Sodiya, E.F.; Ajayi, G.O.; Ademoyegun, A.J.; Al-Sehemi, A.G.; Kavil, Y.N.; Bakheet, A.M. Bioactivity properties of hydroxyapatite/clay nanocomposites. Sci. Rep. 2023, 13, 19896. [Google Scholar] [CrossRef]
- Haq, R.H.A.; Wahab, M.S.; Wahit, M.U. Improvement of Mechanical Properties of Polycaprolactone (PCL) by Addition of Nano-Montmorillonite (MMT) and Hydroxyapatite (HA). Appl. Mech. Mater. 2013, 315, 815–819. Available online: https://www.scientific.net/AMM.315.815 (accessed on 3 December 2023).
- Wang, X.; Zhu, Y.; Mu, B.; Wang, A. Incorporation of clay minerals into magnesium phosphate bone cement for enhancing mechanical strength and bioaktivity. Biomed. Mater. 2023, 18, 025002. [Google Scholar] [CrossRef] [PubMed]
- Tappa, K.K.; Jammalamadaka, U.M.; Mills, D.K. Design and Evaluation of a Nanoenhanced Anti-Infective Calcium Phosphate Bone Cements. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; Available online: https://ieeexplore.ieee.org/document/6944481 (accessed on 24 October 2023).
- Sandomierski, M.; Buchwald, Z.; Voelkel, A. Calcium montmorillonite and montmorillonite with hydroxyapatite layer as fillers in dental composites with remineralizing potential. Appl. Clay Sci. 2020, 198, 105822. [Google Scholar] [CrossRef]
- Paluszkiewicz, C.; Stodolak-Zych, E.; Kwiatek, W.; Jelen, P. Bioactivity of a Chitosan based Nanocomposite. J. Biomim. Biomater. Biomed. Eng. 2011, 10, 95–106. Available online: https://www.scientific.net/JBBTE.10.95 (accessed on 1 December 2023). [CrossRef]
- Amin, A.; Kandil, H.; Awad, H.M.; Ismail, M.N. Preparation and characterization of chitosan–hydroxyapatite–glycopolymer/Cloisite 30 B, nanocomposite for biomedical applications. Polym. Bull. 2015, 72, 1497–1513. Available online: https://link.springer.com/article/10.1007/s00289-015-1351-2 (accessed on 25 October 2023). [CrossRef]
- Bhowmick, A.; Banerjee, S.L.; Pramanik, N.; Jana, P.; Mitra, T.; Gnanamani, A.; Das, M.; Kundu, P.P. Organically modified clay supported chitosan/hydroxyapatite-zinc oxide nanocomposites with enhanced mechanical and biological properties for the application in bone tissue engineering. Int. J. Biol. Macromol. 2018, 106, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Katti, K.S.; Katti, D.R.; Dash, R. Synthesis and characterization of a novel chitosan/montmorillonite/hydroxyapatite nanocomposite for bone tissue engineering. Biomed. Mater. 2008, 3, 034122. Available online: https://iopscience.iop.org/article/10.1088/1748-6041/3/3/034122/pdf (accessed on 25 October 2023). [CrossRef]
- Zheng, J.; Wu, F.; Li, H.; Liu, M. Preparation of bioactive hydroxyapatite@halloysite and its effect on MC3T3-E1 osteogenic differentiation of chitosan film. Mater. Sci. Eng. C 2019, 105, 110072. [Google Scholar] [CrossRef]
- Jammalamadaka, U.; Tappa, K.; Mills, D. Osteoinductive calcium phosphate clay nanoparticle bone cements (CPCs) with enhanced mechanical properties. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; Available online: https://ieeexplore.ieee.org/document/6944480 (accessed on 24 October 2023).
- Ambre, A.; Katti, K.S.; Katti, D.R. In situ mineralized hydroxyapatite on amino acid modified nanoclays as novel bone biomaterials. Mater. Sci. Eng. C 2011, 31, 1017–1029. Available online: https://www.sciencedirect.com/science/article/pii/S0928493111000695 (accessed on 25 October 2023). [CrossRef]
- Oladn, A.; Azhar, F.F. The synergetic effect of bioactive ceramic and nanoclay on the properties of chitosan–gelatin/nanohydroxyapatite–montmorillonite scaffold for bone tissue engineering. Ceram. Int. 2014, 40, 10061–10072. [Google Scholar] [CrossRef]
- Katti, K.S.; Ambre, A.H.; Peterka, N.; Katti, D.R. Use of unnatural amino acids for design of novel organomodified clays as components of nanocomposite biomaterials. Philos. Trans. R. Soc. A 2010, 368, 1963–1980. [Google Scholar] [CrossRef]
- Ambre, A.H.; Katti, K.S.; Katti, D.R. Nanoclay Based Composite Scaffolds for Bone Tissue Engineering Applications. J. Nanotechnol. Eng. Med. 2010, 1, 031013. [Google Scholar] [CrossRef]
- Prabakaran, S.; Rajan, M.; Geng, Z.; Liu, Y. Fabrication of substituted hydroxyapatite-starch-clay bio-composite coated titanium implant for new bone formation. Carbohydr. Polym. 2021, 271, 118432. [Google Scholar] [CrossRef] [PubMed]
- Logeshwaran, A.; Elsen, R.; Nayak, S. Mechanical and biological characteristics of 3D fabricated clay mineral and bioceramic composite scaffold for bone tissue applications. J. Mech. Behav. Biomed. Mater. 2023, 138, 105633. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.; Fombuena, V.; Vallés-Lluch, A.; Ellingham, T. Improvement of mechanical and biological properties of Polycaprolactone loaded with Hydroxyapatite and Halloysite nanotubes. Mater. Sci. Eng. C 2017, 75, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Ambre, A.H.; Katti, D.R.; Katti, K.S. Biomineralized hydroxyapatite nanoclay composite scaffolds with polycaprolactone for stem cell-based bone tissue engineering. J. Biomed. Mater. Res. A 2015, 103, 2077–2101. Available online: https://onlinelibrary.wiley.com/doi/10.1002/jbm.a.35342 (accessed on 25 October 2023). [CrossRef] [PubMed]
- Kundu, K.; Afshar, A.; Katti, D.R.; Edirisinghe, M.; Katti, K.S. Composite nanoclay-hydroxyapatite-polymer fiber scaffolds for bone tissue engineering manufactured using pressurized gyration. Compos. Sci. Technol. 2021, 202, 108598. [Google Scholar] [CrossRef]
- Ng, H.M.; Bee, S.T.; Sin, L.T.; Ratnam, C.T.; Rahmat, A.R. Interaction Effect of Scomberomorus Guttatus-Derived Hydroxyapatite and Montmorillonite on the Characteristics of Polylactic Acid Blends for Biomedical Application. J. Polym. Res. 2020, 27, 215. [Google Scholar] [CrossRef]
- Ng, H.M.; Bee, S.T.; Sin, L.T.; Ratnam, C.T.; Rahmat, A.R. Optimization study on properties of poly (lactic acid) (PLA) composites filled with Scomberomorus guttatus-derived hydroxyapatite and montmorillonite (MMT) under electron beam irradiation. Polym. Bull. 2022, 79, 8823–8864. [Google Scholar] [CrossRef]
- Balakrishnan, H.; Husin, M.R.; Wahit, M.U.; Kadir, M.R.A. Preparation and Characterization of Organically Modified Montmorillonite-Filled High Density Polyethylene/Hydroxyapatite Nanocomposites for Biomedical Applications. Polym. Plast. Technol. Eng. 2014, 53, 790–800. [Google Scholar] [CrossRef]
- Same, S.; Kadkhoda, J.; Navidi, G.; Abedi, F.; Aghazadeh, M.; Milani, M.; Akbarzadeh, A.; Davaran, S. The fabrication of halloysite nanotube-based multicomponent hydrogel scaffolds for bone healing. J. Appl. Biomater. Funct. Mater. 2022, 20, 1–15. [Google Scholar] [CrossRef]
- Wei, M.; Tang, Y.; Chen, L.; Zhang, B.; Zhang, S.; Zhao, K.; Wu, Z. Enhanced mechanical properties and anti-washout of calcium phosphate cement/montmorillonite composite bone-cement for bone-repair applications. Ceram. Int. 2022, 48, 35185–35197. [Google Scholar] [CrossRef]
- Bhattacharjee, S. Understanding the burst release phenomenon: Toward designing effective nanoparticulate drug-delivery systems. Ther. Deliv. 2021, 12, 21–36. Available online: https://www.future-science.com/doi/10.4155/tde-2020-0099 (accessed on 24 November 2023). [CrossRef]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Le1, D.Q.S.; Hein, S.; Li, P.; Nygaard, J.V.; Kassem, M.; Kjems, J.; Besenbacher, F.; Bünger, C. Fabrication and characterization of a rapid prototyped tissue engineering scaffold with embedded multicomponent matrix for controlled drug release. Int. J. Nanomed. 2012, 7, 4285–4297. [Google Scholar] [CrossRef] [PubMed]
- Pazourková, L.; Reli, M.; Hundáková, M.; Pazdziora, E.; Predoi, D.; Martynková, G.S.; Lafdi, K. Study of the Structure and Antimicrobial Activity of Ca-Deficient Ceramics on Chlorhexidine Nanoclay Substrate. Materials 2019, 12, 2996. [Google Scholar] [CrossRef]
- Klecandová, L.; Nakonieczny, D.S.; Reli, M.; Simha Martynková, G. Antibacterial and Biocompatible Polyethylene Composites with Hybrid Clay Nanofillers. Materials 2023, 16, 5179. [Google Scholar] [CrossRef]
- Ahamed, A.F.; Manimohan, M.; Kalaivasan, N. Fabrication of Biologically Active Fish Bone Derived Hydroxyapatite and Montmorillonite Blended Sodium Alginate Composite for In-Vitro Drug Delivery Studies. J. Inorg. Organomet. Polym. 2022, 32, 3902–3922. [Google Scholar] [CrossRef]
- Paientko, V.; Oranska, O.I.; Gun’ko, V.M.; Skwarek, E. Selected Textural and Electrochemical Properties of Nanocomposite Fillers Based on the Mixture of Rose Clay/Hydroxyapatite/Nanosilica for Cosmetic Applications. Molecules 2023, 28, 4820. [Google Scholar] [CrossRef]
- Ptáček, P. Utilization of Apatite Ores. In Apatites and their Synthetic Analogues—Synthesis, Structure, Properties and Applications; InTech: London, Uk, 2016. [Google Scholar] [CrossRef]
- Savci, S. Investigation of effect of chemical fertilizers on environment. APCBEE Procedia 2012, 1, 287–292. [Google Scholar] [CrossRef]
- Madusanka, N.; Sandaruwan, C.; Kottegoda, N.; Sirisena, D.; Munaweera, I.; De Alwis, A.; Karunaratne, V.; Amaratunga, G.A.J. Urea–hydroxyapatite-montmorillonite nanohybrid composites as slow release nitrogen compositions. Appl. Clay Sci. 2017, 150, 303–308. [Google Scholar] [CrossRef]
- Bhuiya, A.W.; Hu, M.; Sankar, K.; Keane, P.F.; Ribero, D.; Kriven, W.M. Bone ash reinforced geopolymer composites. J. Am. Ceram. Soc. 2021, 104, 2767–2779. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).