Abstract
Clay minerals have a great influence on the resulting properties of ceramic bodies. Fly ash as a waste material from burning black coal in power plants is a potentially valuable source of oxides (Al2O3, SiO2 or Fe2O3) for this production. Considering the process of ceramic firing, it is important to understand the thermal behavior of individual ingredients. The thermal behavior of natural smectite minerals (montmorillonite, beidellite, hectorite and nontronite) and their mixtures with added fly ash at different ratios (10%, 30% and 50%) was investigated. The phase analysis was obtained using X-ray diffraction and FT-IR spectroscopy. Under heating to 1200 °C, the structural changes of smectites were divided into four steps including dehydration, dehydroxylation, decomposition and crystallization of new phases. The addition of fly ash caused a shift in the reaction temperatures for all the mentioned phases. These changes were most noticeable for mixtures with hectorite; on the contrary, they were least noticeable for beidellite mixtures. Total mixture mass loss continually decreased with increasing fly ash amount. The obtained experimental thermal data can be applicable not only in the production of ceramic bodies or energy waste processing but also in construction and ensuring the safety of municipal waste landfills.
1. Introduction
Clay minerals are the most widespread minerals forming the earth’s crust rock system. Smectites are a diverse group of clay minerals including, in addition to the well-known montmorillonite, beidellite, nontronite, hectorite, saponite and other less industrially useful minerals. Undoubtedly, the main reasons that evoke interest in these clay minerals are their easy availability, environmentally friendly nature, low cost and extraordinary properties such as considerable sorption capacity, internal swelling in contact with water, high plasticity and cohesion [1,2]. These unique physico-chemical properties are the result of variability of internal chemical composition, variations in types of exchangeable ions, high cation exchange capacity and large surface area [3,4]. Smectites belong to the group of 2:1 expansible phyllosilicates, the basic structural unit of which consists of an octahedral sheet sandwiched between two tetrahedral sheets [5,6]. The tetrahedral sheet is composed of silicon–oxygen tetrahedra linked to neighboring tetrahedra by sharing three corners, resulting in a hexagonal network. The remaining fourth corner of each tetrahedron forms a part connecting to an adjacent octahedral sheet. The octahedral sheet is usually composed of aluminium in six-fold coordination with oxygen from the tetrahedral sheet and with hydroxyl [7,8]. Located in the interlayer are the hydrated exchangeable cations Ca2+, Na+, Mg2+, and K+, respectively. Smectites have permanent layer charge between 0.2 and 0.6 per half unit cell, which arises due to isomorphic substitutions in the octahedral and tetrahedral sheets [9,10]. All smectite minerals are identical in their basic structure but differ in chemical composition and the number of vacancies in tetrahedral and/or octahedral sheets. This group is represented by montmorillonite, beidellite, nontronite (dioctahedral), saponite, hectorite (trioctahedral) and laponite (synthetic form) [11,12,13,14].
Clay minerals are an essential component of fired bricks, giving adequate plasticity, porosity and strength to the final products [2,15]. Ceramics based on clays with a predominant amount of clay raw materials are in demand mainly for their capacity for sintering and shaping [16,17]. Recently, clay minerals have been partially replaced by solid wastes, thereby reducing production costs and the amount of industrial waste from a global perspective. Moreover, adding solid wastes (fly ash, sewage sludge, construction waste, etc.) can save clay mineral resources and also stabilize heavy metals [18,19]. Fly ash is a product of burning powdered anthracite or black or brown coal. It is captured in thermal power plant flue electrostatic gas separators. Due to its similar mineralogical composition to traditional ceramic raw materials, it can be widely used as a ceramic additive [20,21]. Generally, fly ash contains aluminum oxides, quartz and various salts. This inorganic solid waste containing silicates and aluminates, fusible at high temperatures, can be the source of the formation of complex aluminosilicates in bricks [22,23,24,25]. The brick production process with waste materials is similar to traditional brick-making procedures. The powdered waste material is blended with other raw materials, such as clay and sand. The mixture is molded into the desired shape, then dried and fired at a high temperature into hard bricks. Firing conditions such as final temperature and heating rates should be adjusted based on the input materials used [26,27]. Although smectite minerals are not used in large volumes due to their higher plasticity and swellability, in smaller amounts they give strength and contribute to the desired vitrification or color properties [17,28]. The determining the relationship of ceramic properties to structure and sintering temperature is facilitated by methods of thermal analysis. Information on the dehydration of brick clays connected to the weight loss during drying and firing provide insight into the behavior of raw materials when heated [29]. Thanks to the combination of thermal methods, it was then possible to design a suitable mode of firing brick clays in a tunnel kiln [30].
The relationship between water absorption and resistance to the inflection as a function of the firing temperature was examined in order to enhance the quality of the final products and optimize the production process. During sintering, a number of phase transformations occur, controlled by different mechanisms according to temperature changes, which play an important role in densification [31].
A full understanding of interaction of fly ash, smectite and their thermal behavior for brick production (including thermal mass loss and thermal phase transition) is essential for accurate quality control and mitigation of waste streams [32,33].
This paper is focused on the thermal behavior of mixtures of smectites and fly ash additive in the production of clay ceramics. The original smectites as well as their fly ash mixtures were characterized by X-ray fluorescence analysis, X-ray powder diffraction and infrared spectroscopy to obtain information about their composition and phase analysis. Simultaneous thermogravimetry and differential thermal analysis was used to describe the clay material changes during heating process. Dehydration, dehydroxylation and possibly recrystallization temperatures of the used smectite material were determined in order to assess the complex thermal behavior. It has to be emphasized that very careful study and experience are required to interpret thermal curves of smectites because thermal inflections cannot be easily interpreted in some cases. Therefore, the aim of this work was to obtain precise thermal data in order assess the thermal behavior of smectite/fly ash mixtures and to explore the possibilities of usage of such mixtures for the preparation of ceramic bodies.
2. Materials and Methods
2.1. Materials
The fly ash (PT) used in this study originated from the burning of black coal collected on an electrostatic precipitator in a power station (Czech Republic). Clay minerals were obtained from The Clay Minerals Society: montmorillonite (MMT), nontronite (NAU), beidellite (BEI) and hectorite (HEC). Localities and origin of tested smectite samples are described in Table 1. Full description and information of geological occurrences and clay deposits is summarized by Moll [34]. Fly ash mixtures were prepared by mixing weighed quantities (PT solid-weight percentage 10, 30 and 50%). Each mixture was homogenized in a planetary ball mill (Fritch-Pulverisette 6, Idar-Oberstein, Germany) for 30 min at a rotation speed of 200 r·min−1. Obtained samples were named MMT-PT10, BEI-PT10, HEC-PT10, NAU-PT10, MMT-PT30, BEI-PT30, HEC-PT30, NAU-PT30, MMT-PT50, BEI-PT50, HEC-PT50 and NAU-PT50. The powder PT/clay mixtures were sintered in a programmable laboratory electric furnace LH 30/15 Nabertherm (air atmosphere) with a delay of 2 h at a final temperature 1200 °C into the crystalline and poorly crystalline phases in ceramic samples. Obtained samples were named MMT-PT10-H, BEI-PT10-H, HEC-PT10-H, NAU-PT10-H, MMT-PT30-H, BEI-PT30-H, HEC-PT30-H, NAU-PT30-H, MMT-PT50-H, BEI-PT50-H, HEC-PT50-H and NAU-PT50-H.

Table 1.
Locality and origin of tested smectite samples.
2.2. Methods
The chemical composition of untreated clay minerals and fly ash samples was determined by X-ray fluorescence using an X-ray fluorescence spectrometer ARL PERFORM’X 4200 W (Thermo Scientific, Waltham, MA, USA). The fusion bead method was preferred for the preparation of pressed pellets in order to eliminate heterogeneity due to grain size and a mineralogical effect and to reduce inter-element effects by dilution of the sample. Fused beads (40 mm) were prepared by fusion of the samples with lithium tetraborate on a VULCAN 4M fusion machine in Pt/Au crucibles.
X-ray powder diffraction (XRPD) analysis of mineral phases was performed using diffractometer Ultima IV (RIGAKU, Tokyo, Japan) with CuKα radiation, scintillation detector and NiKβ filter, operated at 40 kV and 40 mA, Bragg–Brentano arrangement. Samples in a standard holder were measured in ambient atmosphere, with a scanning rate of 2.0° min−1, a measuring range of 2°–70° 2θ and a step of 0.02° 2θ.
The infrared spectra of clay minerals, recorded in the mid-range 4000–400 cm−1 on a Nicolet 6700 FT-IR spectrometer (Thermo Scientific, USA), equipped with a DTGS/KBr detector, were measured using the KBr pressed-disc technique. Approximately 1 mg of the powdered sample homogenized in 200 mg of dried KBr was compressed into a transparent pellet of 13 mm diameter using a hydraulic press at a pressure of 10 tons for one minute. The spectral resolution was 4 cm−1 and 64 scans were co-added for each sample.
Thermogravimetry and differential thermal curves (TG/DTA) were obtained using a thermal analyzer Setsys 24 Evolution (Setaram, Caluire-et-Cuire, France), equipped with the thermocouple Pt-Pt90/Rh10. Samples with a mass of about 30 mg in an alumina crucible were heated to the final temperature of 1200 °C at a heating rate 10 °C.min−1 under an inert atmosphere (Ar).
3. Results and Discussion
3.1. Chemical and Mineralogical Composition of Smectites and PT/Smectite Mixtures
3.1.1. X-ray Fluorescence
The content of major elements in raw materials, expressed in oxides form, obtained from X-ray fluorescence analysis is presented in Table 2. BEI and MMT show an amount of SiO2 of about 60%, BEI also has the highest amount of Al2O3, about 21%, in contrast to HEC with only less than 1%. The largest amounts of Mg and Ca were found in BEI. Calcium can exist in exchange sites. Because the cation exchange capacity of hectorite is low, very large amounts of Ca indicate the presence of other minerals in this sample [35]. According to Guggenheim et al. [36], the presence of dolomite and calcite in this sample was determined. As the Mg content is greater than the Ca content, considerable amounts of Mg are likely to be present in the silicate clay structure. According to their composition, HEC, BEI and MMT have typical white-beige-gray color; only NAU is greenish-brown in color due to higher amounts of Fe2O3 (Figure 1).

Table 2.
Content of major elements of the smectite samples and fly ash (PT).
Figure 1.
Photograph of the origin smectite and fly ash samples.
3.1.2. X-ray Powder Diffraction
The PT samples come from the high-temperature process of burning coal; therefore, the majority of the organic matter has been burned off or converted during this burning process. The XRD pattern of the PT samples proved the presence of quartz, mullite, anorthite and hematite, and a non-crystalline amorphous halo was observed. The XRD pattern of the MMT sample showed the presence of montmorillonite, muscovite and quartz. The BEI sample was composed of beidellite, quartz and kaolinite. The HEC sample contained, in addition to hectorite, kaolinite and quartz, a calcite impurity. The presence of calcite and/or dolomite is due to hectorite’s geological forming in magnesium-, calcium- or carbonate-rich environments [37]. In the XRD pattern of the NAU sample, nontronite, kaolinite and quartz were identified. Similar results were reported in the works of other authors [38,39]. In case of heat-treated clay mineral samples, the XRD pattern showed the presence of quartz, mullite and cristobalite for the BEI-H sample. The presence of quartz, cristobalite and mullite was also proven for the MMT-H sample. The HEC-H sample contained enstatite, quartz and mullite, and the sample of NAU-H contained cristobalite, mullite and quartz. The phase analysis of heat-treated mixtures of smectites with PT corresponded to the combination of phase analyses of individual heat-treated smectite and PT samples. The XRD patterns of the original smectites, heat-treated smectites and heat-treated mixtures are shown in Figure 2.
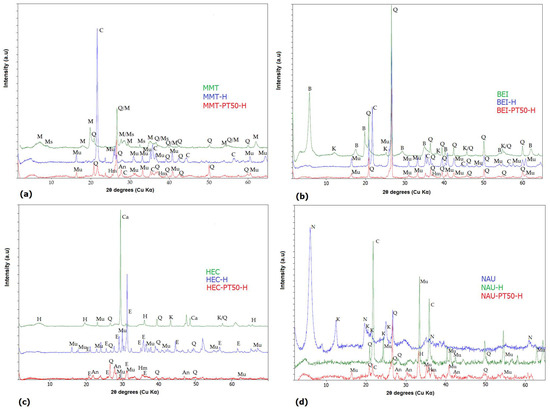
Figure 2.
XRD patterns: (a) MMT, MMT-H and MMT-PT50-H; (b) BEI, BEI-H and BEI-PT50-H; (c) HEC, HEC-H and HEC-PT50-H; (d) NAU, NAU-H and NAU-PT50-H. M—montmorillonite, B—beidellite, H—hectorite, N—nontronite, K—kaolinite, Q—quartz, Mu—mullite, E—enstatite, An—anorthite, Hm—hematite, C—cristobalite, Ms—muscovite, Ca—calcite.
3.1.3. FT-IR Spectroscopy
IR spectroscopy was used to detect mineral impurities and characterize structural changes caused by heating on selected clay minerals. For illustration, selected FT-IR spectra are shown in Figure 3. The admixture of carbonate in the hectorite sample HEC confirms the presence of a moderately intense absorption band at 1419 cm−1 together with bands located at 875 cm−1 and 712 cm−1 and weak absorption at 2875 cm−1, 2515 cm−1 and 1719 cm−1 [40,41]. Traces of carbonates (1433 cm−1) are also evident in the spectrum of the NAU sample [42]. The presence of kaolinite was confirmed in the BEI, NAU and HEC samples. For the BEI sample, there are absorption bands at 3700 cm−1, 3670 cm−1, 3648 cm−1 and 3622 cm−1, which correspond to the stretching vibrations of the structural OH groups, and bands appear in the region of lattice vibration Si-O and Al-O at 1114 cm−1, 1007 cm−1, 913 cm−1, 694 cm−1, 534 cm−1 and 472 cm−1, similar to the NAU spectrum [43]. In the HEC sample, kaolinite was detected only by a band at 3686 cm−1.
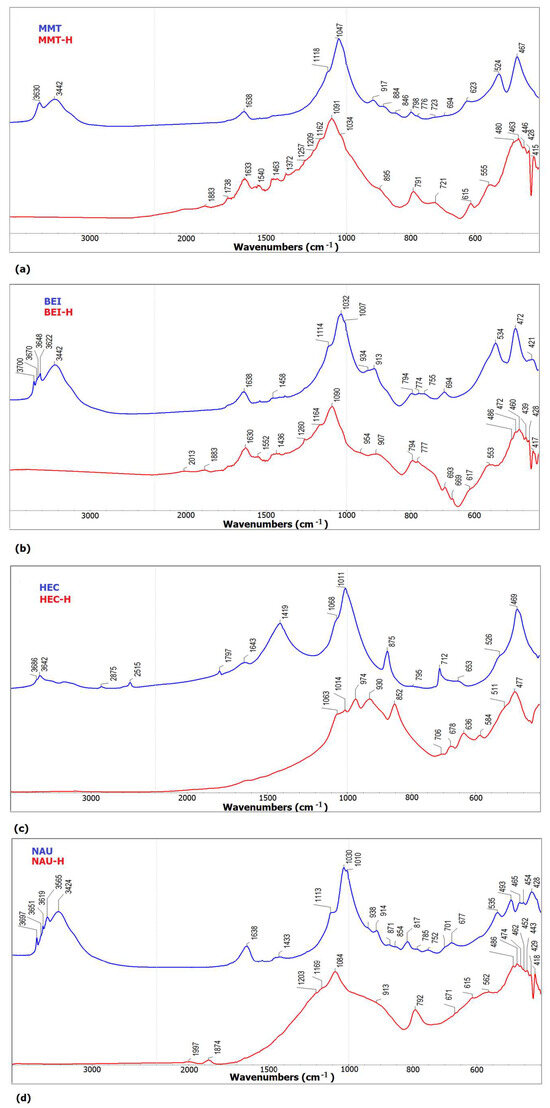
Figure 3.
FT-IR spectra: (a) MMT and MMT-H; (b) BEI and BEI-H; (c) HEC and HEC-H; (d) NAU and NAU-H.
The IR spectra of samples exposed to a temperature of 1200 °C show the destruction of the phyllosilicate structure associated with the formation of new high-temperature phases [44]. Absorption bands at 1063 cm−1, 1014 cm−1, 974 cm−1, 930 cm−1, 852 cm−1 and 511 cm−1 observed in HEC-H are identical to the enstatite mineral phase [37], while the bands at 1209 cm−1, 1091 cm−1, 792 cm−1, 615 cm−1 and 480 cm−1 occurring in MMT-H and similarly at 1091 cm−1, 794 cm−1, 617 cm−1 and 485 cm−1 in BEI-H correspond to cristobalite [45]. In the case of the NAU-H sample, the transformation to cristobalite also took place, but it is not so unambiguous. Other spectral bands found in the spectra MMT-H and BEI-H at about 1164, 907, 721, 554 cm−1 and 460 cm−1 were assigned to the high-temperature mullite phase based on literature sources [46,47,48].
3.2. Thermal Analysis and Transformation of Smectites and PT/Smectite Mixtures
Thermal treatment of clay matter leads to structural changes, divided into steps of dehydration, dehydroxylation, decomposition and crystallization/transformation of new mineral phases. The principal thermal reactions of clay minerals and the approximate temperature ranges are generally considered in the following categories: low-temperature interval below 400 °C (loss of adsorbed water and molecular water between layers), intermediate-temperature interval of 400–750 °C (dehydroxylation and the formation of quasi-stable dehydroxylated phases) and high-temperature interval above 750 °C (recrystallization and formation of new phases) [49,50]. The temperature, shape and intensity of the endotherm peaks on DTA curves, especially for montmorillonite, are closely connected to the presence of cations such as Mg2+, Ca2+, Sr2+, Ba2+, Be2+, Na+ and Li+ [51]. In the interval of OH groups removal, the position of the endo effect depends mainly on the presence of Fe3+, which isomorphically replaces Al3+. As the iron content increases, the temperature of endo effects in this region decreases, especially in nontronite samples. Subsequent exo reactions lead to the formation of variable products such as cristobalite, mullite, cordierite, spinel, enstatite, etc. [11,52].
The results from DTA curves of MMT samples showed that the addition of PT caused a decrease in both dehydration temperatures. Recrystallization temperatures of dehydrated substances to cristobalite and mullite also decreased with the amount of PT. In contrast, this addition did not affect the temperatures of the dehydroxylation process at all. For BEI samples, the first peak temperature of dehydration increased while the second peak decreased with the amount of PT. The temperatures of removal of OH groups decreased with PT amount, but transformation temperatures to cristobalite and mullite were not influenced. In the case of HEC samples, the addition of PT influenced all decomposition steps, with the dehydration, dehydroxylation and recrystallization to enstatite and mullite decreasing with increasing PT amount. Only the temperature of decomposition of carbonate increased with the amount of PT. The dehydroxylation step was accompanied by the decomposition step of calcite with the simultaneous release of CO2. For NAU sample, two endotherm peaks related to dehydroxylation were found; these peaks pointed out that the dehydroxylation of the NAU sample occurs as a continuous process. The same facts were also observed by the authors of [53]. The lower values of dehydroxylation temperatures compared to other measured smectites were connected to the higher amount of iron. In general, with a higher Fe content, dehydroxylation takes place at lower temperature intervals [51,54]. The temperatures of dehydration and dehydroxylation processes decreased with increasing PT amount. A very small and weak peak of transformation to cristobalite was found for the NAU and NAU-PT10 samples, but for NAU-PT30 and NAU-PT50, this transformation was not detectable on DTA curves (Figure 4), although it was proven according to X-ray and FT-IR analyses. The presence of kaolinite detected by X-ray and FT-IR analyses in the BEI, NAU and HEC samples was not confirmed by the thermal analysis due to the small amount of kaolinite in the sample. Also, kaolinite dehydroxylates are in the same interval as smectite minerals and therefore are not detectable on DTA curve in mixtures with a small quantity [55]. The temperatures of endotherm and exotherm peaks obtained from DTA curves are summarized in Table 3.
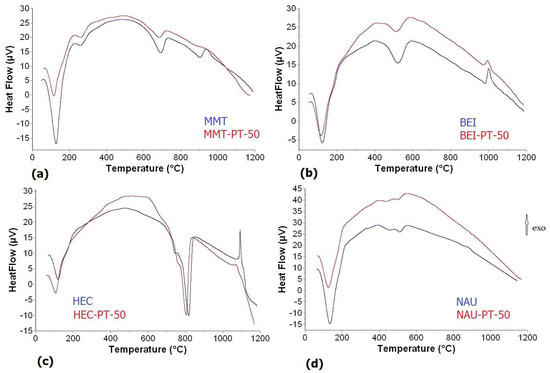
Figure 4.
DTA curves: (a) MMT and MMT-PT50; (b) BEI and BEI-PT50; (c) HEC and HEC-PT50; (d) NAU and NAU-PT50.

Table 3.
Temperatures of endo and exo peaks maxima for measured samples.
Figure 5 presents the TG curves of the measured samples. The mass loss percentages obtained from TG curves which characterize the thermal decomposition steps of the measured samples are, for illustrative purposes, presented in Table 4. For the MMT sample, a greater mass loss was recorded for the lower temperature interval (25–500 °C) due to a large amount of adsorbed water in the smectite interlayer. The residue of mass loss in the higher temperature interval (500–850 °C) corresponded to the removal of OH residues. Similarly, for the BEI sample, which is analogous to the montmorillonite structure, a significant weight loss due to the release of interlayer water was observed. The HEC sample showed the highest total mass loss from all measured smectites, which was mainly due to its structure, its composition and its content of calcite admixture. The amount of interlayer water is minimal, with only 2% mass loss. In the higher temperature interval, not only the removal of OH groups but also the decomposition of carbonates occurred, which corresponds well to the studies of other authors [29,36,56]. For the NAU sample, there was a higher mass loss in the initial temperature interval thanks to the two-stage dehydroxylation, after which the complete collapse of the original structure occurred. This collapse was accompanied by only a negligible mass loss.
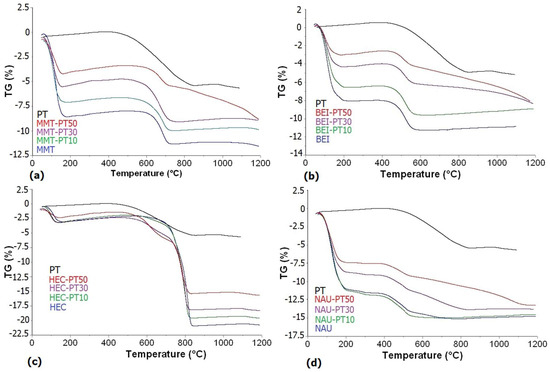
Figure 5.
TG curves: (a) PT, MMT and its PT mixtures; (b) PT, BEI and its PT mixtures; (c) PT, HEC and its PT mixtures; (d) PT, NAU and its PT mixtures.

Table 4.
Mass loss percentages obtained from TG curves in different intervals.
For the PT sample, in the lower temperature interval, mass loss was not detected, while in the higher interval, a mass loss of about 5% was observed. The crucial PT reactions including two endotherm maxima, both around 1300 °C, which are assigned to melting and mullite crystallization [57]. Since temperatures above 1200 °C are rarely used in the brick industry, these reactions are thus irrelevant from this point of view [32,58]. For PT/smectite mixtures, the total mass loss decreased with increasing PT amount, which is a consequence of the addition of PT material with a relatively low weight loss in different ratios. This finding is consistent with the results of other authors [59,60].
4. Conclusions
The fly ash, original smectite samples and smectites heat-treated to 1200 °C were subjected to composition and phase analysis by XRD and FTIR analytical methods. The high-temperature major phase developed during the heating of montmorillonite, beidellite and nontronite was observed as cristobalite; only hectorite has transformed to an enstatite phase. The addition of fly ash influenced the endotherm and exotherm reactions obtained from DTA curves. With increasing PT amount, the temperature of dehydration steps decreased for all tested smectite samples, with the exception of HEC, where only the first step of dehydration decreased, and BEI, where the first step even increased. The dehydroxylation temperatures decreased with PT amount for BEI, HEC and NAU; the addition of PT did not affect the removal of OH groups only for the MMT sample. The temperatures of transformation and recrystallization decreased with PT amount for the MMT and HEC samples; for BEI, no effect was observed, and for NAU, the temperatures was detectable only for NAU and NAU-PT10. The MMT and HEC samples appeared to be more suitable for the production of ceramic bodies due to their composition and being less vulnerable to changes during heating, especially those connected to recrystallization temperatures. In the HEC sample, a more pronounced effect of ash on the formation of new phases was found, which may be related to a higher volume of carbonates. Also, the higher iron content in the NAU sample together with fly ash had an effect on the deterioration of nontronite recrystallization. These facts can lead to a limitation of the applicability of these smectites for the production of traditional ceramic bodies.
The obtained thermal data, together with XRD and FT-IR analyses, can provide valuable monitoring data offering a better understanding of the preparation of ceramic materials and the prediction of the effect of smectite minerals on the key properties of ceramic bodies, in addition to the advantage of ecological utilization of waste material such as fly ash.
Author Contributions
Conceptualization, E.P.; methodology, E.P. and L.V.; analysis, E.P. and L.V.; data curation, L.V.; writing—original draft preparation, E.P.; writing—review and editing, E.P. and L.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Project of the Czech Academy of Sciences—Strategy AV21 and also supported by the Mobility programme of the Czech Academy of Sciences and the Polish Academy of Sciences, Mobility Plus Project, no. PAN-24-22.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We give special thanks to George Laynr for checking the English in this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Murali, K.; Sambath, K.; Hashir, S.A. Review on Clay and its Engineering Significance. Int. J. Sci. Res. Public 2018, 8, 8–11. [Google Scholar]
- Singh, N.B. Clays and clay minerals in construction industry. Minerals 2022, 12, 301. [Google Scholar] [CrossRef]
- Laird, D.A. Layer charge influences on the hydration of expandable 2:1 phyllosilicates. Clay Clay Miner. 1999, 47, 630–636. [Google Scholar] [CrossRef]
- Luo, W.; Zeng, Z.; Bian, L. Effect of Lattice Substitution on Adsorption of Hexavalent Chromium by Montmorillonite, Nontronite, and Beidellite. Minerals 2021, 11, 1407. [Google Scholar] [CrossRef]
- Dellisanti, F.; Minguzzi, V.; Valdre, G. Thermal and structural properties of Ca-rich Montmorillonite mechanically deformed by compaction and shear. Appl. Clay Sci. 2006, 31, 282–289. [Google Scholar] [CrossRef]
- Viani, B.E.; Low, P.F.; Roth, C.B. Direct measurement of the relation between interlayer force and interlayer distance in the swelling of montmorillonite. J. Colloid Interface Sci. 1983, 96, 229–244. [Google Scholar] [CrossRef]
- Wu, J.; Low, P.F.; Roth, C.B. Effects of octahedral-iron reduction and swelling pressure on interlayer distances in Na-montmorillonite. Clay Clay Miner. 1989, 37, 211–218. [Google Scholar]
- Guggenheim, S.; Martin, R.T. Definition of Clay and Clay Mineral: Joint Report of the Aipea Nomenclature and CMS Nomenclature Committees. Clay Clay Miner. 1995, 43, 255–256. [Google Scholar] [CrossRef]
- Velde, B. Introduction to Clay Minerals: Chemistry—Origins, Uses and Environmental Significance, 1st ed.; Chapman&Hall: London, UK, 1992; p. 92. [Google Scholar]
- Tao, L.; Xiao-Feng, T.; Yu, Z.; Tao, G. Swelling of K+, Na+ and Ca2+-montmorillonites and hydration of interlayer cations: A molecular dynamics simulation. Chin. Phys. B 2010, 19, 109101. [Google Scholar] [CrossRef]
- Grim, R.E. Clay Mineralogy. International Series in the Earth and Planetary Sciences, 1st ed.; McGraw-Hill Book Company: New York, NY, USA, 1986; pp. 77–92. [Google Scholar]
- Odom, I.E. Smectite clay minerals: Properties and uses. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1984, 311, 391–409. [Google Scholar]
- Ombaka, O. Characterization and classification of clay minerals for potential applications in Rugi Ward, Kenya. Afr. J. Environ. Sci. Technol. 2016, 10, 415–431. [Google Scholar]
- Murray, H.H. Overview, clay mineral applications. Appl. Clay Sci. 1991, 5, 379–395. [Google Scholar] [CrossRef]
- El Hammouti, A.; Charai, M.; Channouf, S.; Horma, O.; Nasri, H.; Mezrhab, A.; Karkri, M.; Tankari, M.A. Laboratory-testing and industrial scale performance of different clays from eastern Morocco for brick manufacturing. Constr. Build. Mater. 2023, 370, 130624. [Google Scholar] [CrossRef]
- Zhang, L.Y. Production of bricks from waste materials—A review. Constr. Build. Mater. 2013, 47, 643–655. [Google Scholar] [CrossRef]
- Wang, S.; Gainey, L.; Mackinnon, I.D.R.; Allen, C.; Gu, Y.; Xi, Y. Thermal behaviors of clay minerals as key components and additives for fired brick properties: A review. J. Build. Eng. 2023, 66, 105802. [Google Scholar] [CrossRef]
- Kijo-Kleczkowska, A.; Szumera, M.; Gnatowski, A.; Sadkowski, D. Comparative thermal analysis of coal fuels, biomass, fly ash and polyamide. Energy 2022, 259, 124840. [Google Scholar] [CrossRef]
- Zacco, A.; Borgese, L.; Giaconcelli, A.; Struis, R.P.W.J.; Depero, L.E.; Bontempi, E. Review of fly ash inertisation treatments and recycling. Environ. Chem. Lett. 2014, 12, 153–175. [Google Scholar] [CrossRef]
- Wang, H.; Yhu, M.; Sun, Z.; Ji, R.; Liu, L.; Wang, X. Synthesis of a ceramic tile base based on high-alumina fly ash. Constr. Build. Mater. 2017, 155, 930–938. [Google Scholar] [CrossRef]
- Hossain, S.S.; Roy, P. Sustainable ceramics derived from solid wastes: A review. J. Asian Ceram. Soc. 2020, 8, 984–1009. [Google Scholar] [CrossRef]
- Sun, Z.; Zhou, H.; Jiang, H.H.; Zhang, W.Y.; Mao, L.Q. Recycling municipal solid waste incineration fly ash in fired bricks: An evaluation of physical-mechanical and environmental properties. Construct. Build. Mater. 2021, 294, 1234765. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, F.; Zhou, H.; Li, Q.; Shang, S. Study on the performance and reaction mechanism of alkali-activated clay brick with steel slag and fly ash. Constr. Build. Mater. 2024, 411, 134406. [Google Scholar] [CrossRef]
- Muñoz Velasco, P.; Morales Ortíz, M.P.; Mendívil Giró, M.A.; Muńoz Velasco, L. Fired clay bricks manufactured by adding wastes as sustainable construction material—A review. Constr. Build. Mater. 2014, 63, 97–107. [Google Scholar] [CrossRef]
- Jimenez-Garcia, E.; Arellano-Vazquez, D.; Titotto, S.; Vilchis-Nestor, A.; Mayorga, M.; Romero-Salazar, L.; Arteaga-Arcos, J. A low environmental impact admixture for the elaboration of unfired clay building bricks. Constr. Build. Mater. 2023, 407, 133470. [Google Scholar] [CrossRef]
- Ibrahim, J.E.; Tihtih, M.; Tihtih, M.; Şahin, E.; Basyooni, M.A.; Kocserha, I. Sustainable zeolitic tuff incorporating tea waste fired ceramic bricks: Development and investigation. Case Stud. Construct. Mater. 2023, 19, e02238. [Google Scholar] [CrossRef]
- Hmeid, H.A.; Akokad, M.; Baghour, M. Preliminary characterization and potential use of different clay materials from North-Eastern Morocco in the ceramic industry. Mater. Today—Proc. 2022, 58, 1277–1284. [Google Scholar] [CrossRef]
- Lahcen, D.; Hicham, E.E.; Latifa, S.; Abderrahmane, A.; Jamal, B.; Mohamed, W.; Meriam, E.; Nathalie, F. Characteristics and ceramic properties of clayey materials from Amezmiz region (Western High Atlas, Morocco). Appl. Clay Sci. 2014, 102, 139–147. [Google Scholar] [CrossRef]
- Wang, S.; Gainey, L.; Baxter, D.; Wang, X.; Mackinnon, I.D.; Xi, Y. Thermal behaviours of clay mixtures during brick firing: A combined study of in-situ XRD, TGA and thermal dilatometry. Constr. Build. Mater. 2021, 299, 124319. [Google Scholar] [CrossRef]
- Vasić, M.V.; Pezo, L.; Zdravković, J.; Bačkalić, Z.; Radojević, Z. The study of thermal behavior of montmorillonite and hydromica brick clays in predicting tunnel kiln firing curve. Constr. Build. Mater. 2017, 150, 872–879. [Google Scholar] [CrossRef]
- Baccour, H.; Medhioub, M.; Jamoussi, F.; Mhiri, T. Influence of firing temperature on the ceramic properties of Triassic clays from Tunisia. J. Mater. Process. Technol. 2009, 209, 2812–2817. [Google Scholar] [CrossRef]
- Wang, S.; Gainey, L.; Wang, X.; Mackinnon, I.D.; Xi, Y. Influence of palygorskite on in-situ thermal behaviours of clay mixtures and properties of fired bricks. Appl. Clay Sci. 2022, 216, 106384. [Google Scholar] [CrossRef]
- Christogerou, A.; Lampropoulou, P.; Papoulis, D.; Angelopoulos, G.N. Feasibility Study on the Potential Replacement of Primary Raw Materials in Traditional Ceramics by Clayey Overburden Sterile from the Prosilio Region (Western Macedonia, Greece). Minerals 2021, 11, 961. [Google Scholar] [CrossRef]
- Moll, W.F. Baseline studies of the clay minerals society source clays: Geological origin. Clay Clay Miner. 2001, 49, 374–380. [Google Scholar] [CrossRef]
- Mermut, A.; Cano, A.F. Studies of the clay minerals society source clays: Chemical analysis of major elements. Clay Clay Miner. 2001, 49, 381–386. [Google Scholar] [CrossRef]
- Guggenheim, S.; Koster van Groos, A.F. Baseline studies of The Clay Minerals Society Source Clays: Thermal analysis. Clay Clay Miner. 2001, 49, 430–440. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Frost, R.L.; Kickey, L. Infrared emission spectroscopic study of the dehydroxylation of some hectorites. Thermochim. Acta 2000, 345, 145–156. [Google Scholar] [CrossRef]
- Chipera, S.J.; Bish, D.L. Studies of the clay minerals society source clays: Powder X-ray diffraction analysis. Clay Clay Miner. 2001, 49, 398–409. [Google Scholar] [CrossRef]
- Mukasa-Tebandeke, I.Z.; Mukasa-Tebandeke, I.Z.; Ssebuwufu, P.J.M.; Nyanzi, S.A.; Schumann, A.; Nyakairu, G.W.A.; Ntale, M.; Lugolobi, F. The Elemental, Mineralogical, IR, DTA and XRD Analyses Characterized Clays and Clay Minerals of Central and Eastern Uganda. Adv. Mater. Phys. Chem. 2015, 5, 67–86. [Google Scholar] [CrossRef]
- Madejová, J.; Bujdák, J.; Janek, M.; Komadel, P. Comparative FT-IR study of structural modifications during acid treatment of dioctahedral smectites and hectorite. Spectrochim. Acta A 1998, 54, 1397–1406. [Google Scholar] [CrossRef]
- Vaculíková, L.; Plevová, E.; Ritz, M. Characterization of Montmorillonites by Infrared and Raman Spectroscopy for Preparation of Polymer-Clay Nanocomposites. J. Nanosci. Nanotechnol. 2019, 5, 2775–2781. [Google Scholar] [CrossRef]
- Apeiranthitis, N.; Greenwell, H.C.; Carteret, C. Far- and mid-infrared examination of nontronite-1 clay mineral Redox and cation saturation effects. Appl. Clay Sci. 2022, 228, 106628. [Google Scholar] [CrossRef]
- Madejová, J.; Komadel, P. Baseline study of the clay minerals society source clays: Infrared methods. Clay Clay Miner. 2001, 49, 410–432. [Google Scholar] [CrossRef]
- Che, C.; Greenwell, H.C.; Carteret, C. Spectroscopic study of the dehydration and/or dihydroxylation of phyllosilicate and zeolite minerals. J. Geophys. Res. 2011, 116, E05007. [Google Scholar] [CrossRef]
- Yahagi, Y.; Yagi, T. Infrared absorption spectra of the high/pressure phases of cristobalite and their coordination numbers of silicon atoms. Solid State Commun. 1994, 89, 945–948. [Google Scholar] [CrossRef]
- Gören, R.; Ersoy, B.; Özgür, C.; Alp, T. Colloidal stability–slip casting behavior relationship in slurry of mullite synthesized by the USP method. Ceram. Int. 2012, 38, 679–685. [Google Scholar] [CrossRef]
- Xi, H.J.; Gao, L.; Guo, J.K. The structural change of diphasic mullite gel studied by XRD and IR spectrum analysis. J. Eur. Ceram. Soc. 2002, 22, 1307–1311. [Google Scholar]
- Ritz, M. Infrared and raman spectroscopy of mullite ceramics synthesized from fly ash and kaolin. Minerals 2023, 13, 864. [Google Scholar] [CrossRef]
- Giggis, B.S.; Felix, N.S.; Barawy, K.A. Dehydroxylation kinetics of some pure smectites. Thermochim. Acta 1987, 112, 265–274. [Google Scholar] [CrossRef]
- Vaculíková, L.; Plevová, E. Identification of clay minerals and micas in sedimentary rocks. Acta Geodyn. Geomater. 2005, 2, 167–175. [Google Scholar]
- Blažek, A. Book of Thermal Analysis, 1st ed.; SNTL: Prague, Czech Republic, 1974; pp. 208–209. [Google Scholar]
- Šajnor, V.S.; Jesenak, K. Differential thermal analysis of montmorillonite. J. Therm. Anal. Calorim. 1996, 46, 489–493. [Google Scholar]
- Ding, Z.; Frost, L.R. Controlled rate thermal analysis of nontronite. Thermochim. Acta 2002, 389, 185–193. [Google Scholar] [CrossRef]
- Gavin, P.; Chevrier, V.; Rochette, P.; Keck, W.M. Thermally transformed nontronite as a component of red dust layer on Mars. In Proceedings of the 38th Lunar and Planetary Science Conference, (Lunar and Planetary Science XXXVIII), League City, TX, USA, 12–16 March 2007; Volume 38, p. 2295. [Google Scholar]
- Plevová, E.; Vaculíková, L.; Valovičová, V. Thermal analysis and FTIR spectroscopy of synthetic clay mineral mixtures. J. Therm. Anal. Calorim. 2020, 142, 507–518. [Google Scholar] [CrossRef]
- Achik, M.; Benmoussa, H.; Oulmekki, A.; Ijjaali, M.; El Moudden, N.; Touache, A.; Álvaro, G.G.; Rivera, F.G.; Infantes-Molina, A.; Eliche-Quesada, D.; et al. Evaluation of technological properties of fired clay bricks containing pyrrhotite ash. Constr. Build. Mater. 2021, 269, 121312. [Google Scholar] [CrossRef]
- Valášková, M.; Blahůšková, V.; Vlček, J. Effects of Kaolin Additives in Fly Ash on Sintering and Properties of Mullite Ceramics. Minerals 2021, 11, 887. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, K.K.; Ramachandrarao, P. Effects of fly ash additions on the mechanical and other properties of porcelainised stoneware tiles. J. Mater. Sci. 2001, 36, 5917–5922. [Google Scholar] [CrossRef]
- Koukouzas, N.; Ketikidis, C.; Itskos, G.; Spiliotis, X.; Karayannis, V.; Papapolymerou, G. Synthesis of CFB-Coal Fly Ash Clay Bricks and Their Characterisation. Waste Biomass Valoriz. 2011, 2, 87–94. [Google Scholar] [CrossRef]
- Pranav, S.; Singhal, A.; Routroy, S.; Bhunia, D.; Rotta Loria, F.A.; Lahoti, M. Fired clay bricks synergistically valorizing hazardous nickel chrome-plating sludge and fly ash: Performance assessment. Constr. Build. Mater. 2024, 423, 135817. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).