Abstract
An improved method for producing high-purity quartz (Qtz) and potassium feldspar (Kfs) concentrates was developed using various chemical reagents. Froth flotation experiments on a Qtz–Kfs mixture showed that quartz could be selectively floated from Kfs in diluted hydrofluoric acid (HF) using a frother. Similarly, feldspar could be selectively floated from quartz. Recovery rates depended on reagent choice, pH levels, HF modifier dosage, and HF conditioning time. High recoveries and good grades were achieved for both quartz and feldspar at low pH levels. Among the five collectors tested, Brij C20 showed the highest recovery when used in conjunction with HF and a frother alone and a concentrate containing 82.8% quartz was obtained at a 96% recovery rate. Good recoveries were also achieved with collectors like Duomeen C and Flotigam V4343 for floating K-feldspar, with a Kfs concentrate of 99.9% purity at a 94% recovery. Lilaflot OT 55 and Duomeen TDO were less effective. The concentration of HF was 22 kg/t, which implies an important reduction with respect to the earlier research.
1. Introduction
Quartz and potassium feldspar usually occur associated in nature. They have large industrial applications, which depend on their purity. Quartz is mainly used in the manufacture of glass, as a filter material for water, and as a filler for the ceramic industry. Among other applications, high-purity quartz is used in photovoltaic cells [1]. Potassium feldspar (Kfs) is used to manufacture paints in the ceramic industry [2], especially for the removal of colored impurities [3] insulators and abrasives and for the production of electrodes [4]. Thus, due to the variety of industrial applications, high-purity quartz and Kfs are in high demand. In this regard, by using froth flotation technology, the purity of quartz and Kfs concentrates can be improved.
Various authors have investigated the separation of these minerals using froth flotation methods with anionic and cationic collectors [5,6,7,8], which is based on the conductivity of the surfaces of the minerals to float. Quartz and feldspar showcase a tetrahedral network, creating compensating three-dimensional structures, and potassium feldspar introduces a positive charge deficit compensated by K+ through aluminum substitution in the coordination tetrahedron on silica particle surfaces [9]. In this regard, the application of double-layer theory to quartz mineral surface speciation is a crucial concept in understanding the electrochemical interactions that influence the surface chemistry of quartz with surrounding ions. Quartz surfaces typically carry a negative surface charge due to the presence of silanol (SiOH) groups. The extent of this charge depends on factors like pH and the concentration of ions in the solution [10].
The speciation of the quartz surface also leads to understanding its reactivity, and that is the crucial part in the mineral processing field, especially in beneficiation stages, where it affects the adsorption of collector molecules and, ultimately, the separation of minerals. It is based on interaction optimization of anionic and cationic collectors with quartz surfaces. It helps in tailoring the surface chemistry to achieve selective mineral separation. In low-pH conditions, the quartz surface may have a more positive charge, leading to the protonation of silanol groups. In high-pH conditions, the surface becomes negatively charged as the silanol groups deprotonate [11].
On the other hand, the tetrahedra oxygens on the surface of the quartz particles are not fully compensated. In aqueous suspensions of feldspar and quartz particles, the free bonds are neutralized by OH− and H+ species. Partial or total particle surface hydroxylation can form silanol groups [Si(OH)n] [12], which is pure water dissociated through the reactions (1) and (2) [13]:
≡ SiOH + OH− ⇌ Si-O− + H2O
≡ SiOH + H+ ⇌ Si-OH2+ + H2O
For this reason, these particles may end up negatively charged in water suspension for basic pH and positively charged under acidic pH. These phenomena directly impact the behavior of silica suspensions. Considering that SiO2 is inherently an acidic oxide, an aqueous silica suspension pH is slightly acidic. However, whether surface planes are exposed on the fractured surface of particles, their atomic density, and the presence of a contaminated SiO2 source depend on the crystal structure.
Nowadays, the most efficient process for Kfs flotation from quartz applies amine as a collector in acidic conditions produced by HF acid [14,15]. Feldspar is floated from a feldspar–quartz binary mixture using HF acid, which acts like an activator and cationic collector (amine) at pH 2–3 [16]. It was determined that Kfs displays different surface and floatability properties due to some impurities on the mineral surface [5]. HF substantially produces a significant difference between the zeta potential of quartz and feldspar because this acid modifies the zeta potential of feldspar toward negative values [17]. The amine ion does not adsorb sufficiently on feldspar to promote froth flotation. These findings suggest that HF plays an essential role in the process, and different possible mechanisms have been proposed [18]. This occurs because HF removes positively charged cations from the feldspar surface while depositing fluoride ions [15]. As a result of this mechanism, feldspar tends to be effectively depressed in the presence of collectors. Quartz could be easily floated, without using collectors, with HF solution and frother Brij C58 [19]. Selective flotation can be performed on feldspar from other impurities in the system.
F− is both a pollutant and a corrosive agent in acidic conditions. For that reason, additional investment and production costs are adversely affected [8]. Utilizing cationic, anionic, and cationic–anionic collectors for feldspar flotation in an acidic environment without the need for HF aims to minimize or reduce the consumption of HF. However, it should be noted that even with efforts to use HF sparingly, achieving complete elimination or a significant reduction in its consumption remains a challenge [20].
HF alters the properties of the quartz surface, which carries a positive surface charge when exposed to the air, but when it is exposed to HF in an aqueous solution, HF dissociates into HF2−, H+, and F−. HF breaks down into negatively charged fluoride ions (F- and HF2−) and some positively charged ions (H+). The positively charged quartz surface attracts hydroxyl groups (OH−) in what is known as the Stern layer. The Stern layer is a region of charge separation near the mineral surface. As more ions are attracted to the surface, the potential near the quartz surface decreases, giving a place to the so-called Gouy layer [21], which essentially extends the charge distribution. Then, the interaction between the quartz surface and HF creates negatively charged silica tetrahedra [SiO4]4−, which carry two negative charges per unit. Under acidic conditions, some of the OH− groups on the surface of the silica tetrahedra can be replaced by F− ions [22]. This substitution can occur due to the dissociation of F− ions in the HF solution. The degree of dissociative ionization in the HF solution increases as the HF concentration increases, leading to a greater number of F− ions in the solution. The increased dissociation of HF results in silicon atoms on the quartz surface having coordination defects, which make the surface more receptive to further chemical reactions. Over time, continuous fluoride ions (F−) adsorbed onto the quartz surface leads to a fluorine-coated surface. This coating makes the quartz surface hydrophobic and allows minerals to attach to the quartz without the need for additional collector chemicals. This is the basis for the flotation of minerals from an ore mixture [13].
HF is challenging due to health and safety regulations. Hence, many studies discussed a preferred alternative acid (H2SO4 + NaF), by replacing HF, in the flotation of quartz with the combined reagent system [23,24,25]. However, HF still plays a significant role in beneficiating quartz, and Kfs with high-grade recovery is a fascinating topic. Conventionally, the recovery was attempted by more than 93% recovery of quartz at the consumption of HF acid 274 kg/t while frother dosage of Brij C20 was around 600 g/t at pH 1.5. However, the HF consumption was less in the collector system.
This research aims to achieve a new alternative process to produce high-purity quartz and Kfs concentrates using different chemical reagents. To this end, Brij C20, a cationic collector, Flotigam V4343. Duomeen C, Duomeen TDO and Lilaflot OT 55 as amine collectors were used in the experiments. A collectorless system means HF solution with frother (Brij C 20) and the collector system (Flotigam V4343, Duomeen C and Lilaflot OT 55) and HF mechanism also were tested for effective separation.
2. Materials and Methods
2.1. Materials
Quartz used in this work consisted of commercially available high-purity quartz from Jardi pond SL, Madrid, Spain of granular particle size ranging from 100 mm to 1000 µm. Kfs used was a commercial product from VICAR, S.A., Manises, Spain, which was in powdered form, 90% passing in 106 µm in size. Before froth flotation experiments, the quartz-rich material was milled in a ceramic laboratory-scale mill of 20 cm in diameter and 25 cm long. The 80% passing size in the feed was about 500 μm, and its top size was 1 mm. Ball-filling was kept constant at 40%, and the speed of the mill for all the experiments was maintained at about 77% of the critical speed. The ball top size used in the tests was 2.5 mm, and the lower one was 1 mm. The material was sieved after the milling, and a 38–106 µm size range of particles was used as flotation feed material.
The particle size distribution of the materials (Figure 1) was determined using the digital sieving equipment CISA BA-200. In contrast, the chemical composition was obtained from portable X-ray fluorescence equipment (XRF) using an Epsilon 1 model from Malvern Panalytical, Malvern, UK, (Table 1). The pH was measured by Lab Logistic Group Gmbh (Marchtrenk, Austria) pH meter 5, and a Malvern Panalytical Zetasizer Nano ZS ZEN3600 measured the zeta potential of the samples.

Figure 1.
Particle size distribution of initial raw material.

Table 1.
Chemical composition, in mass %, of quartz and Kfs raw materials.
HF 40% from Merck (Darmstadt, Germany) was used in concentrated form. Brij C20 from Acros Organics (Alcobendas, Madrid, Spain) was prepared as a 6 wt% solution with deionized water. Flotigam V4343 from Clariant (Muttenz, Switzerland) and Duomeen C, Duomeen TDO and Lilaflot OT 55 from Nuoryon solution (Itupeva-SP Brazil) were used as collectors. Pine oil used as a frother from I.C.L. Iberia, Spain. An overview of chemical reagents is shown in Table 2.

Table 2.
Chemical reagents used in the froth flotation experiments.
2.2. Methods
2.2.1. Froth Flotation
The batch flotation studies and kinetics used a W.E.M.C.O. laboratory flotation machine from FLSMIDTH Copenhagen, Denmark with a 4 L Plexiglas volume cell. All the flotation experiments were conducted by first conditioning individual 200 g batches of feed material with 0–100 mL of 40% HF and 3000 mL deionized water for 10 min. The conductivity of the deionized water used was 100 µS. Before flotation, the pH was measured with a Lab Logistic Group GmbH pH meter 5. The pH measurement was carried out under acidic conditions of 1.5 or less. The addition of HCL and NaOH adjusted the pH. The impeller speed was maintained at around 820 rpm. Deionized water was added to retain the level of pulp during flotation. A frother solution was added to the cell, followed by 3 min of conditioning. Following these two steps, the air was introduced to the cell at a superficial 6 L/min flow rate. Flotation was performed until foam was produced.
The addition of frother Brij C20 was carried out in three steps, with 2 mL (scale-down from a 200 g/t dosage) of frother in each step. The first flotation test (Float 1) was conducted after conditioning the sample with 23 mL (corresponding to 114 kg/t of fresh feed) HF for 5 min and two additional minutes with 2 mL Brij C20 solution. After 2 min of residence time, froth was achieved. The float material was collected as Float 1. Then, the air was stopped, and 2 mL of Brij C20 solution was added to the cell, followed by a 2 min conditioning time. The float material was collected as Float 2 up to a collection time of up to 3 min. The last dosage was two additional minutes with 2 mL Brij C20 solution. After this residence time, froth was achieved. The total froth collection was 9 min and was named Float 3. The remaining sink material in the cell was collected separately. All the products were dried at 80 °C for 7–8 h to be weighed in order to produce mass balance. To study the flotation kinetics, differences in froth collection time were tested by adding frother dosage in three stages.
The general evolution of the experimental procedures during the froth flotation test of quartz and feldspar is shown in Figure 2, which indicates the evolution of pH with the chemical reagents’ addition over time. Table 3 shows the total operational data required during the chemical reagent experiment.

Figure 2.
A general experimental procedure during the flotation test of quartz and feldspar.

Table 3.
Flotation operational procedures in a mixture of 50% quartz+ 50% feldspar of size fraction 38–106 µm.
2.2.2. Zeta Potential
The determination of the zeta potential was conducted at a temperature of 20 °C. A diluted silica solution was introduced into a 10.3N NaCl solution in order to set the silica ionic strength [26]. The introduction of NaCl at this specified concentration has two effects on charged particles: particles in a low-salt buffer show a higher zeta potential, and a higher-valency salt at the same molar concentration has a stronger impact. Consequently, reducing the ionic strength of a buffer enhances small zeta potential values and the pH of the suspension was adjusted using standard decimal solutions of HCl and NaOH. Calibration was measured by the zeta potential analyzer using standard reference materials or solutions. Calibration ensures the accuracy of the measurements. The zeta potential analyzer was set up according to the manufacturer’s instructions, ensuring the electrodes and cuvettes were clean and free of contaminants and the cuvette or sample cell was filled with the prepared colloidal suspension. The absence of air bubbles in the sample cell was checked, and the measurement parameters, including the applied electric field strength and the measurement duration, were defined. The analyzer applies an electric field to the sample, causing the charged particles to move. This movement was recorded and used to calculate the zeta potential. Controlling pH and ionic strength can influence zeta potential and, consequently, the stability of colloidal systems [27].
2.2.3. BET Surface Area
The BET (Brunauer–Emmett–Teller) method has been a widely utilized technique in materials science and surface chemistry to determine the specific surface area of porous materials [28]. With the utilization of the ASAP 2020 (Micromeritics, Shanghai, China), the BET method assisted us in quantifying the surface area properties of these particles, providing valuable insights into their behavior throughout the flotation process.
The sample was initially prepared to meet the required size specifications and ensure it was devoid of impurities. The equipment was connected and calibrated with the necessary gases, typically nitrogen. The instrument conducted gas adsorption and desorption measurements, recording the quantity of gas adsorbed by the sample as a function of pressure during the adsorption phase. These data were instrumental in constructing the adsorption isotherm. In the desorption phase, the instrument recorded the quantity of gas desorbed from the sample as the pressure was reduced, and these data were used to calculate the BET surface area. The ASAP 2020 Plus, V2.00 software was used for this calculation based on the BET equation, which relates the quantity of adsorbed gas to the sample’s surface area.
3. Results and Discussion
3.1. Zeta Potential
Results of the determination of the zeta potential show that this is near when pH is lower than 0.9, and specifically at about pH 0.85, for both quartz and Kfs (Figure 3). It reaches the isoelectric point where the negative [29,30,31,32,33] and positive charges created by the silanol groups are equal on the surface of the quartz particles. As the value increases from 1, the zeta potential becomes more negative, indicating increased negative charges on the particle surface. Because of the OH/SiO− acid/base dissociation equilibrium, more negative charges will result in higher surface energy and numbers of SiO− species [34].

Figure 3.
Zeta potential of quartz and feldspar.
As the zeta potential [35] is a crucial experimental tool for studying charged surfaces, the optimal pH for sulfides are around 8–10, where positive zeta potential occurs [17]. In colloidal systems, the zeta potential indicates stability, controlling particle deposition on surfaces, particularly in porous media. These potentials are key parameters for predicting colloid attachment kinetics within the Derjaguin–Landau–Verwey–Overbeek (DLVO) theory [35], offering insights into colloidal particle behavior, especially in quartz-containing liquid suspensions.
In mineral flotation, double-layer repulsion and hydrophobic attraction dynamics are pivotal, influenced by pH, collector selection, and mineral surface chemistry. The primary goal is to manage these interactions effectively for optimal mineral separation while minimizing undesired associations with nontarget minerals [36].
3.2. BET Surface Area
In this research, the particles of raw materials used ranged in size from 38 to 106 µm. This particular range was selected to capture the unique characteristics of quartz and feldspar particles, which are critical in flotation. The BET surface areas of the raw quartz and feldspar are indicated in Table 4.

Table 4.
BET surface area of quartz and feldspar sample.
3.3. Quartz and Feldspar Flotation
The grade and recovery of quartz and Kfs as a function of HF consumption and various chemical reagents are shown in Figure 4. In the quartz–Kfs separation, when no collector was used, quartz was obtained as the floating mineral and Kfs was a nonfloat material. The highest recovery of quartz concentrate was 89% with a grade of 99% with no collector at 274 kg/t of HF. The highest recovery of a feldspar concentrate was 89% with a grade of 99.9% using flotigam V4343 at 22 kg/t of HF. When utilizing Lilaflot OT 55 collectors, the quartz recovery rate was comparatively lower, but it exhibited a higher grade in the presence of reduced HF consumption. Nuoryon solutions Duomeen C was also used for quartz recovery, obtaining around 78% at 22 kg/t HF. When comparing all the results, the HF required dosage varied greatly without a frother system and with a collector system. The results indicate that less than 12 times the amount of HF consumption is required, making the operating cost of the process economical in practice.

Figure 4.
Recovery and grade characteristics of the flotation materials as a function of HF consumption using various chemical reagents. (a) Quartz grade, (b) Quartz recovery, (c) feldspar grade, (d) feldspar recovery.
Previous studies [24] explained that the HF-treated samples have higher selectivity in separation. Thus, a substantial amount of HF solution is required to float the quartz and feldspar. As shown in the present study, the new approach can produce a pure Kfs concentrate and could be especially valuable as a cleaning step to remove the last traces of quartz from the product. It shows that when other collectors were used, such as Duomeen TDO [37], Flotigam V4343 [38], Duomeen C, and Lilaflot OT 55, the Kfs was obtained as float material, but the quartz as nonfloat material. Using Clariant solutions (Flotigam V4343), Kfs recovery was obtained at around 99%. The grade was approximately 90% at 22 kg/t HF. Using Duomeen C Kfs, recovery was obtained at about 93%, while the grade was about 78% at 22 kg/t HF. Feldspar recovery without collectors was around 80%. However, using Lilaflot OT 55 and Duomeen TDO at 130 kg/t HF, where the grade was high, around 90%, encountered a substantial reduction in both recovery and grade. This concentrate of 89% quartz has to also contain about 11% feldspar. The disclosure of feldspar grade and recovery in the study indicates that a segment of the fraction indeed comprised feldspar, and this pattern holds true for the other conducted tests (Figure 4).
Quartz grade decreases as the mass recovery increases (Figure 5a). After the first flotation, a concentrate of more than 94% quartz was produced, while a concentrate of 80% feldspar was obtained after the third flotation. Similarly, the feldspar grade decreases as the mass recovery increases (Figure 5b). A concentrate of more than 99% feldspar was produced after the first flotation, while 94% quartz was produced after the third flotation. The total float collection time was 3 min. The quartz and feldspar recovery as a function of flotation time for the different chemical reagents is shown in Table 5.
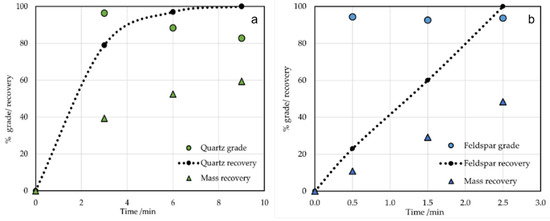
Figure 5.
(a) Quartz grade and recovery as a function of flotation time at 274 kg/t HF, and (b) feldspar grade and recovery as a function of flotation time at 22 kg/t HF.

Table 5.
Employing the least-squares methodology for fitting: linear regression models.
It is evidenced that using Brij C20 results in significantly faster flotation and a total recovery of 100%. The flotation is completed within 1.5 min. To compare the data against kinetic models, it is necessary to determine the total (i.e., the initial) concentration of floatable material in the froth flotation cell.
Based on the results shown in Table 6, it was assumed that 100% recovery of quartz material floats when using Brij C20 under the given conditions. The corresponding Flotigam V4343 and Lilaflot OT 55 attempted 100% recovery for feldspar while grade was 87.2 and 88.4%, respectively. The kinetic recovery data for all regents produced good fits when compared against first-order kinetics; however, none of the recovery curves would fit zero- or second-order kinetics.

Table 6.
Summary data of flotation separation results.
3.4. Effect of HF
Flotation experiments with different amounts of HF and Brij C20 dosages for recovering quartz show how high concentrations of HF are required to float quartz (Figure 6). Moreover, an increase in HF dosage markedly enhances quartz recovery, which is in concordance with the results of [19]. In addition, the increase in the amount of BrijC20 has a strong positive effect on the froth flotation of quartz. At 600 g/t Brij C20, a change from 114 to 224 kg/t HF dosage increases the recovery of quartz by approximately 21%. Following this pattern, continuing to increase the amount of HF from 224 to 274 kg/t represents an improvement in the recovery of quartz from 89.41% to 99.14%.

Figure 6.
Effect of HF and Brij C20 on the flotation of quartz.
The necessity for increased amounts of Brij C20 in the flotation of quartz can be attributed to various factors. The inherent hydrophilicity of quartz surfaces, characterized by negatively charged silanol groups, may require higher concentrations of the hydrophobic collector for effective flotation. Additionally, the presence of competing reactions or adsorption on other minerals within the ore matrix may limit the availability of Brij C20 for selective interaction with quartz surfaces. The liberation of quartz particles and their particle size distribution can also impact collector dosage, with finer particles potentially demanding higher concentrations for successful flotation. Furthermore, solution chemistry, particularly pH and ionic strength, plays a crucial role. Changes in pH influence the surface charge of minerals, while ionic strength affects the stability of collector micelles and their ability to interact with quartz surfaces. Without the specific pH reported in the study, these variables collectively contribute to the observed requirement for higher dosages in efficient quartz flotation, highlighting the intricate and nuanced nature of mineral processing conditions.
3.5. Effect of Acidic pH
Figure 7 shows the effect of pH on the recovery of quartz in materials floated at 274 kg/t HF. The decrease in pH positively affects its flotation. Using 300 g/t Brij C20, a decrease from 3 to 1.5 pH increases the recovery of quartz by approximately 30%, while at 600 g/t Brij C20, lower pH seems to increase the recovery of quartz by more than 58%, from 28% to 84%.

Figure 7.
Quartz recovery as a function of pH at 274 kg/t HF.
Mineral behavior in flotation processes is complex, influenced by factors like mineralogy and solution chemistry. At low pH levels, the selective flotation of quartz in the absence of a collector is a consequence of the distinctive surface properties inherent to this mineral. The extreme acidity promotes quartz dissolution, releasing individual particles into the solution. This, coupled with surface activation rendering quartz more hydrophobic, enhances particle adhesion during flotation. The changes in surface charge and chemistry under low-pH conditions create conditions conducive to the attachment of air bubbles, facilitating the flotation of quartz particles. For feldspars, when they are exposed to similar low-pH conditions, they do not exhibit a similar behavior. This divergence can be attributed to the unique surface chemistry of feldspar, which may not undergo a parallel hydrophobic transformation as observed in quartz.
4. Conclusions
This study demonstrates a high froth flotation separation selectivity between quartz and feldspar and that quartz can be readily floated in diluted HF solutions with several frothers. A comparative study was conducted between different chemical reagents with low consumption of modifier dosage of HF for high recovery. Flotation experiments were conducted with various cationic and amine collectors, such as Flotigam V4343, Duomen C, Lilaflot OT 55, and Duomeen TDO. The best results in concentration of quartz and feldspar were obtained using the Flotigam V4343 collector, with concentrates of 99.9% feldspar at a recovery of 94% and 94% quartz at a recovery of 87.24%. The concentration of HF was 22 kg/t, which implies an important reduction with respect to the earlier research.
By using Brij C20, frother concentrates of 80% feldspar at a recovery of 88.98% and 79% quartz at a recovery of 96% at 274 kg/t HF were obtained. By using Duomeen C, concentrates of 76.34% feldspar at a recovery of 95% and 74% quartz at a recovery of 80.16% were obtained at 16.41 kg/t HF. The parameters for flotation kinetics were determined for all the cases studied and are now ready for modeling these processes.
Author Contributions
Conceptualization, J.O., P.A. and C.H.S.; methodology, software, K.M., J.O., C.H.S. and P.A.; validation, J.O.; formal analysis, K.M.; investigation, J.O. and P.A.; data curation, J.O., C.H.S. and P.A.; writing—original draft preparation, K.M.; writing—review and editing, P.A., H.A. and C.H.S.; supervision, J.O.; project administration, J.O.; funding acquisition, J.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the ACCIÓ (Agència per a la Competitivitat de l’Empresa of Generalitat de Catalunya—Grant Agrement ACE014/20/000053) for their financial support. This research belongs to the SGR-01041 (RIIS) project of the Generalitat de Catalunya.
Data Availability Statement
Data are contained within the article.
Acknowledgments
Arenes Bellpuig (Lleida, Spain), Jardi pond SL (Madrid, Spain) Vicar S.A., (Manises, Spain) ICL Iberia, (Barcelona, Spain) Clariant Solutions (Muttenz, Switzerland) and Nuoryon (Itupeva-SP Brazil) companies are thanked for the materials and chemical supply and support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vatalis, K.I.; Charalambides, G.; Benetis, N.P. Market of high purity quartz innovative applications. Procedia Econ. Financ. 2015, 24, 734–742. [Google Scholar] [CrossRef]
- Baila, F.; Labbilta, T.; Darmane, Y. Feldspar Purification from Iron Impurities: A Review of Treatment Methods. Miner. Process. Extr. Metall. Rev. 2023, 1–9. [Google Scholar] [CrossRef]
- Kalyoncu, E.; Burat, F. Selective Separation of Coloring Impurities from Feldspar Ore by Innovative Single-stage Flotation. Miner. Process. Extr. Metall. Rev. 2022, 43, 910–915. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Sun, N.; Liu, R.; Wang, Z.; Wang, L.; Sun, W. Systematic review of feldspar beneficiation and its comprehensive application. Miner. Eng. 2018, 128, 141–152. [Google Scholar] [CrossRef]
- Rao, K.H.; Forssberg, K.S.E. Mixed collector systems in flotation. Int. J. Miner. Process. 1997, 51, 67–79. [Google Scholar] [CrossRef]
- Vidhyadhar, A.; Rao, K.H. Adsorption mechanism of mixed cationic/anionic collectors in feldspar-quartz flotation system. J. Colloid Interface Sci. 2007, 306, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Peretti, R.; Serci, A.; Zucca, A. Electrostatic K-feldspar/Na-feldspar and feldspar/quartz separation: Influence of feldspar composition. Miner. Process. Extr. Metall. Rev. 2012, 33, 220–231. [Google Scholar] [CrossRef]
- Heyes, G.W.; Allan, G.C.; Bruckard, W.J.; Sparrow, G.J. Review of flotation of feldspar. Miner. Process. Extr. Metall. 2007, 121, 72–78. [Google Scholar] [CrossRef]
- Worrall William, E. Ceramic Raw Materials: Institute of Ceramics Textbook Series; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Zhang, H.; Xu, Z.; Sun, W.; Zhu, Y.; Chen, D. Hydroxylation structure o quartz surface and its molecular hydrophobicity. Appl. Surf. Sci. 2023, 612, 155884. [Google Scholar] [CrossRef]
- Crundwell, K.F. On the mechanism of the dissolution of quartz and silica in aqueous solutions. Acs Omega 2017, 2, 1116–1127. [Google Scholar] [CrossRef]
- Bickmore, B.R.; Wheeler, J.C.; Bates, B.; Nagy, K.L.; Eggett, D.L. Reaction pathways for quartz dissolution determined by statistical and graphical analysis of macroscopic experimental data. Geochim. Et Cosmochim. Acta 2008, 72, 4521–4536. [Google Scholar] [CrossRef]
- Alves Junior, A.J.; Baldo, J.B. The Behavior of Zeta Potential of Silica Suspensions. New J. Glass Ceram. 2014, 4, 29–37. [Google Scholar] [CrossRef]
- Burat, F.; Kokkilic, O.; Kangal, O.; Gurakan, V.; Celik, M.S. Quartz-feldspar separation for the glass and ceramics industries. Miner. Metall. Process. 2007, 24, 75–80. [Google Scholar] [CrossRef]
- Wei, M.; Ban, B.; Li, J.; Sun, J.; Li, F.; Jiang, X.; Chen, J. Flotation behaviour, collector adsorption mechanism of quartz and feldspar-quartz systems using PEA as a novel green collector. Silicon 2020, 12, 327–338. [Google Scholar] [CrossRef]
- Vidhyadhar, A.; Rao, K.H.; Forsberg, K.S.E. Separation of feldspar from quartz: Mechanism of mixed cationic/anionic collector adsorption on minerals and flotation selectivity. Miner. Metall. Process. 2002, 19, 128–136. [Google Scholar] [CrossRef]
- Fuerstenau, D.W.; Pradip. Zeta potentials in the flotation of oxide and silicate minerals. Adv. Colloid Interface Sci. 2005, 114–115, 9–26. [Google Scholar] [CrossRef]
- Larsen, E.; Kleiv, R.A. Flotation of quartz from quartz-feldspar mixtures by the HF method. Miner. Eng. 2016, 98, 49–51. [Google Scholar] [CrossRef]
- Larsen, E.; Kleiv, R.A. Towards a new process for the flotation of quartz. Miner. Eng. 2015, 83, 13–18. [Google Scholar] [CrossRef]
- Li, Y.; Ren, J.; Xie, J.; Duan, T.; Gao, M.; Wu, X.; He, H. Application of mixed collectors on quartz-feldspar by fluorine-free flotation separation and their interaction mechanism: A review. Physicochem. Probl. Miner. Process. 2021, 57, 139–156. [Google Scholar] [CrossRef]
- Allagui, A.; Benaoum, H.; Olendski, O. On the Gouy-Chapman-Stern model of the electrical double-layer structure with a generalized Boltzmann factor. Phys. A Stat. Mech. Its Appl. 2021, 582, 126252. [Google Scholar] [CrossRef]
- Ibrahim, I.; Hussin, H.; Azizli, K.A.M.; Alimon, M. A study on the interaction of feldspar and quartz with mixed anionic/cationic collector. Malays. J. Fundam. Appl. Sci. 2014, 7, 101–107. [Google Scholar] [CrossRef]
- Wang, W.; Cong, J.; Deng, J.; Weng, X.; Lin, Y.; Huang, Y.; Peng, T. Developing effective separation of feldspar and quartz while recycling tailwater by HF pre-treatment. Minerals 2018, 8, 149. [Google Scholar] [CrossRef]
- Larsen, E.; Kowalczuk, B.P.; Kleiv, R.A. Non-HF collector less flotation of quartz. Miner. Eng. 2019, 133, 115–118. [Google Scholar] [CrossRef]
- Zhu, J.; Dai, S.; Li, P.; Yang, S. An experimental study of removing impurity from a quartz ore by microbial flotation-acid leaching. Physicochem. Probl. Miner. Proc. 2021, 57, 18–28. [Google Scholar] [CrossRef]
- Yukselen-Aksoy, Y.; Kaya, A. A study of factors affecting on the zeta potential of kaolinite and quartz powder. Environ. Earth Sci. 2011, 62, 697–705. [Google Scholar] [CrossRef]
- Kirby, J.B.; Hasselbrink, E. Zeta potential of microfluidic substrates: 1. Theory, experimental techniques, and effects on separations. Electrophoresis 2004, 25, 187–202. [Google Scholar] [CrossRef]
- Sinha, P.; Datar, A.; Jeong, C.; Deng, X.; Chung, Y.G.; Lin, L.C. Surface Area Determination of Porous Materials Using the Brunauer–Emmett–Teller (BET) Method: Limitations and Improvements. J. Phys. Chem. C 2019, 123, 20195–20209. [Google Scholar] [CrossRef]
- Gülgönül, I.; Karagüzel, C.; Cinar, M.; Çelik, M.S. Interaction of sodium ions with feldspar surfaces and its effect on the selective separation of Na- and K-feldspars. Miner. Process. Extr. Metall. Rev. 2012, 33, 233–245. [Google Scholar] [CrossRef]
- Jada, A.; Akbour, A.R.; Douch, J. Surface charge and adsorption from water onto quartz sand of humic acid. Chemosphere 2006, 64, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Jinkeun, K.F.; Lawler, D.F. Characteristics of Zeta Potential Distribution in Silica Particles. Bull. Korean Chem. Soc. 2005, 26, 1083–1089. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, L.; Xu, Z.; Liu, Q.; Chi, R. Reactive oily bubble technology for flotation of apatite, dolomite and quartz. Int. J. Miner. Process. 2015, 134, 74–81. [Google Scholar] [CrossRef]
- Salopek, B.; Krasić, D.; Filipović, S. Measurement and application of zeta-potential. Rud. Geološko-Naft. Zb. 1992, 4, 147–151. [Google Scholar]
- Dobias, B.; Jakbos, U.; Oberndorfer, H.; Petzenhauser, R.; Weirer, K. New Aspects in the Theory of Mineral Flotation. Miner. Process. Extr. Metall. Rev. Int. J. 1992, 10, 71–86. [Google Scholar] [CrossRef]
- Elimelech, M.; Nagai, M.; Ko, C.H.; Ryan, J.N. Relative insignificance of mineral grain zeta potential to colloid transport in geochemically heterogeneous porous media. Environ. Sci. Technol. 2000, 34, 2143–2148. [Google Scholar] [CrossRef]
- Xie, L.; Wang, J.; Lu, Q.; Hu, W.; Yang, D.; Qiao, C.; Peng, Q.; Wang, T.; Sun, W.; Zhang, H.; et al. Surface interaction mechanisms in mineral flotation: Fundamentals, measurements, and perspectives. Adv. Colloid Interface Sci. 2021, 295, 102491. [Google Scholar] [CrossRef] [PubMed]
- Kangal, O.M.; Bulut, G.; Yeşilyurt, Z.; Güven, O.; Burat, F. An Alternative Source for Ceramics and Glass Augen-Gneiss. Minerals. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Sekulic, Z.; Canic, N.; Bartulovic, Z.; Dakovic, A. Application of different collectors in the flotation concentration of feldspar, mica and quartz sand. Miner. Eng. 2004, 17, 77–80. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).