Comparison and Mechanism Analysis of Three-Phase Contact Formation onto Hydrophilic/Hydrophobic Mineral Surfaces in the Presence of Cationic/Anionic Surfactants during Flotation Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Chemicals
2.2. Methods
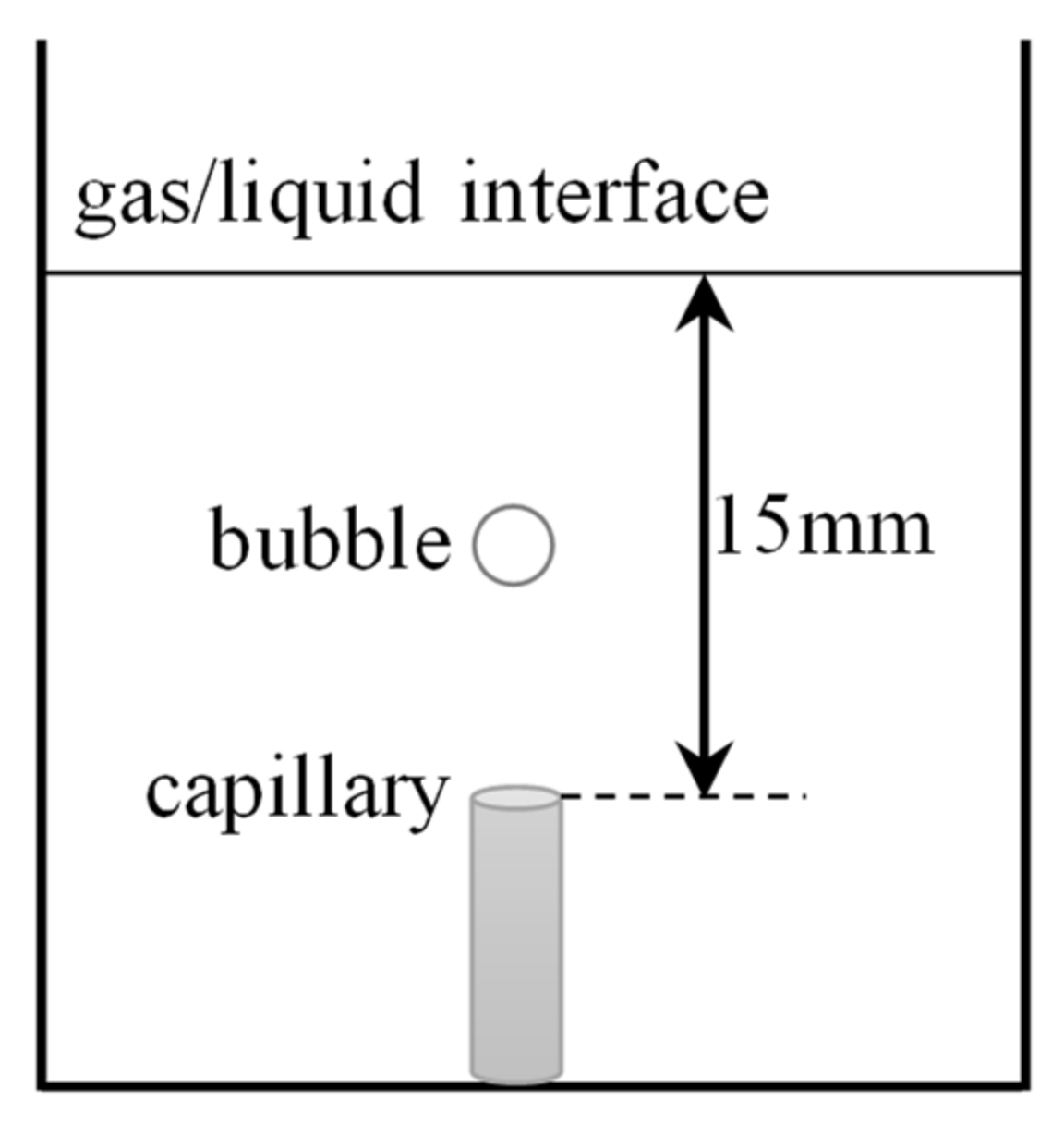
2.2.1. Experimental Apparatus
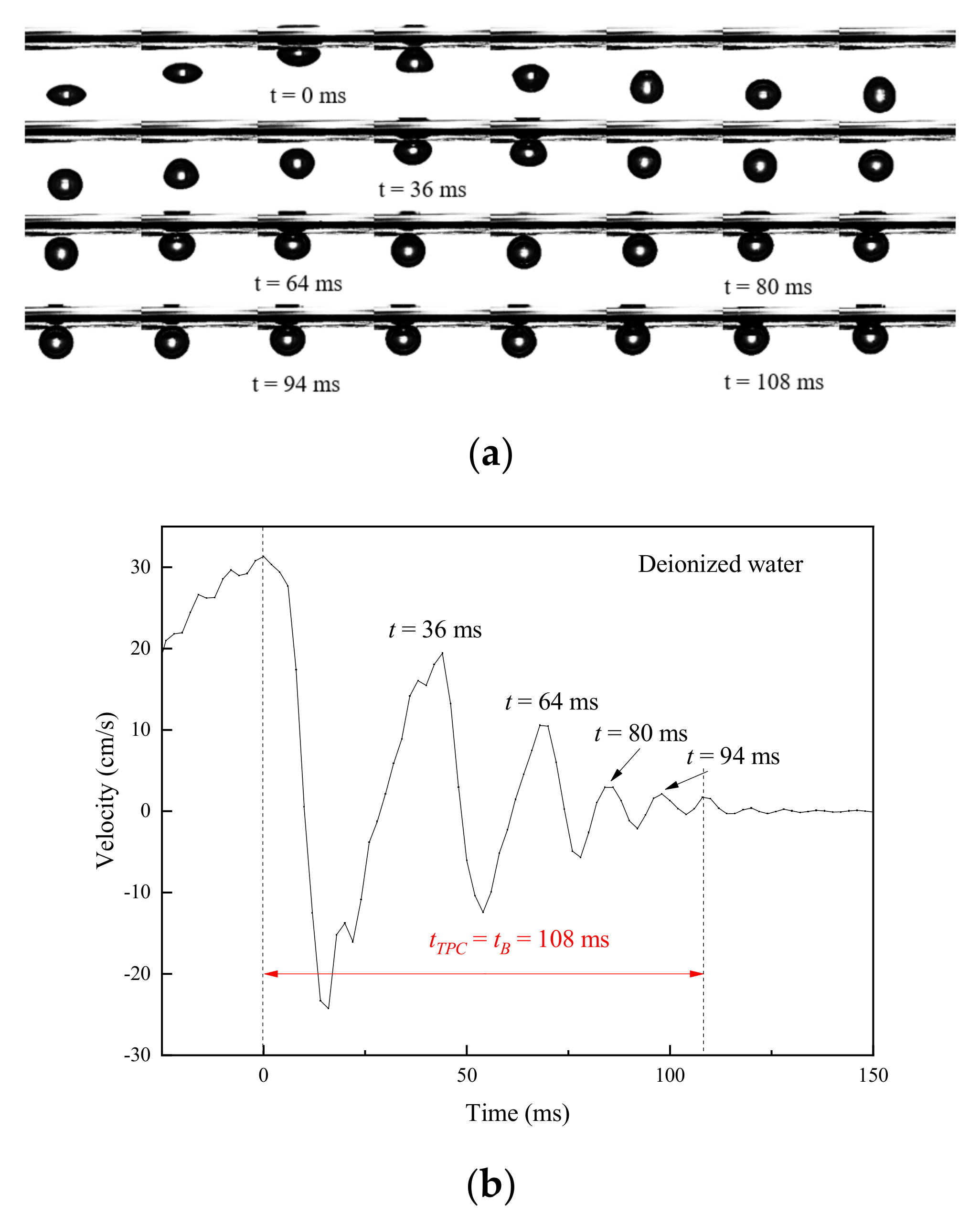
2.2.2. Measurements of Bubble Deformation
2.2.3. Stability Assessment of Bubble at the Gas/Liquid Interface
2.2.4. Three-Phase Contact Formation
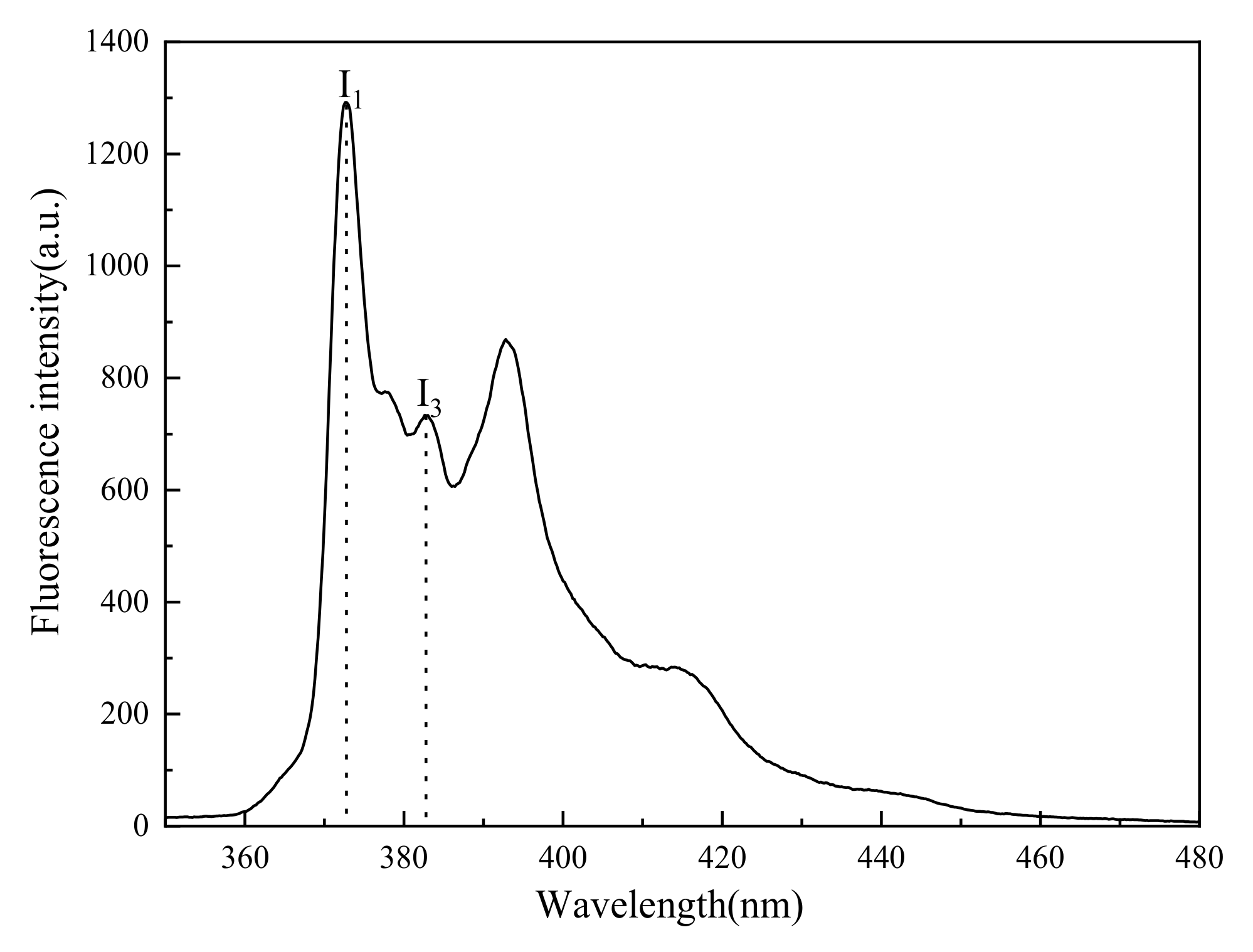
2.2.5. Fluorescence Spectrum Analysis
3. Results and Discussion
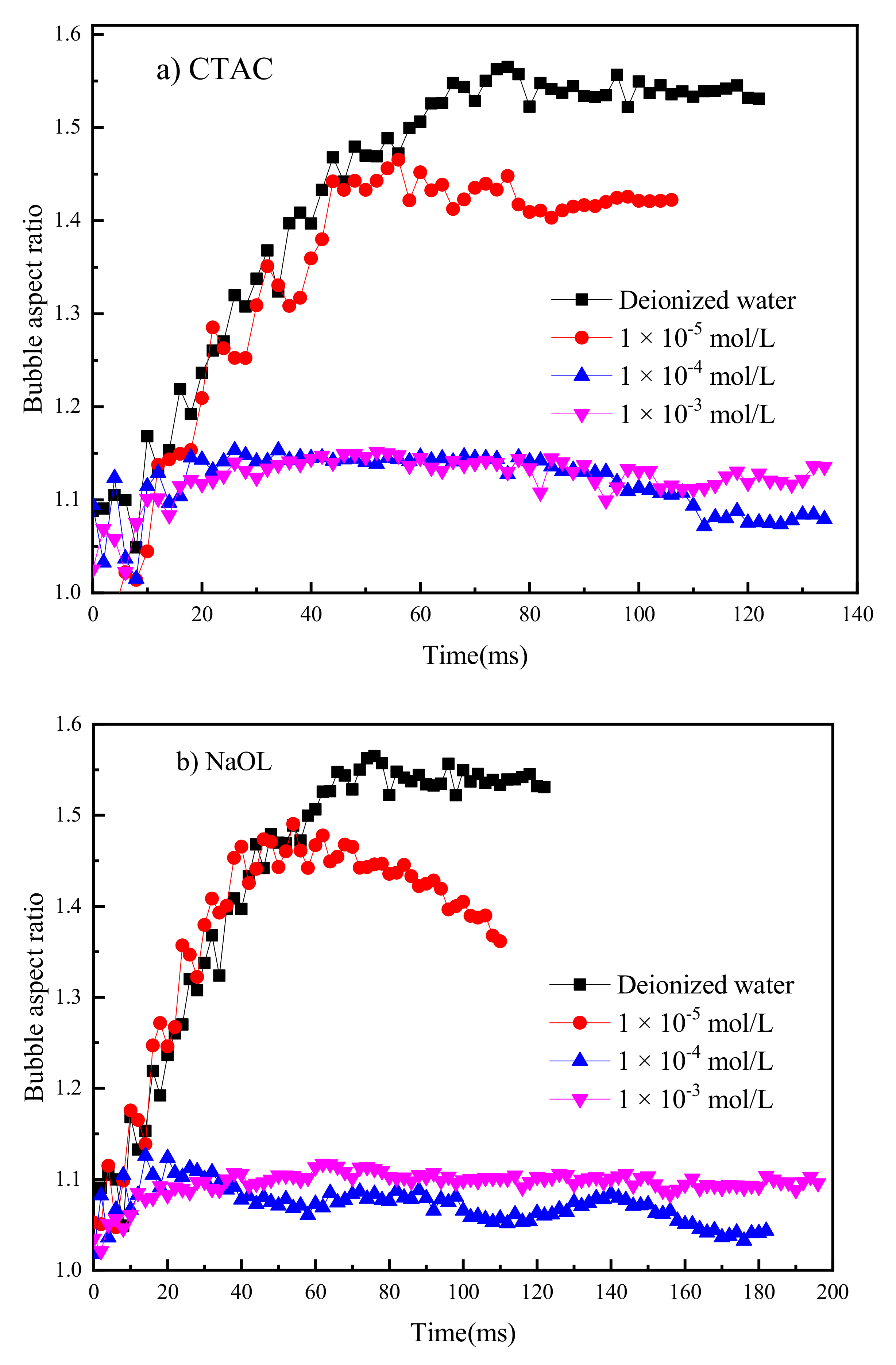
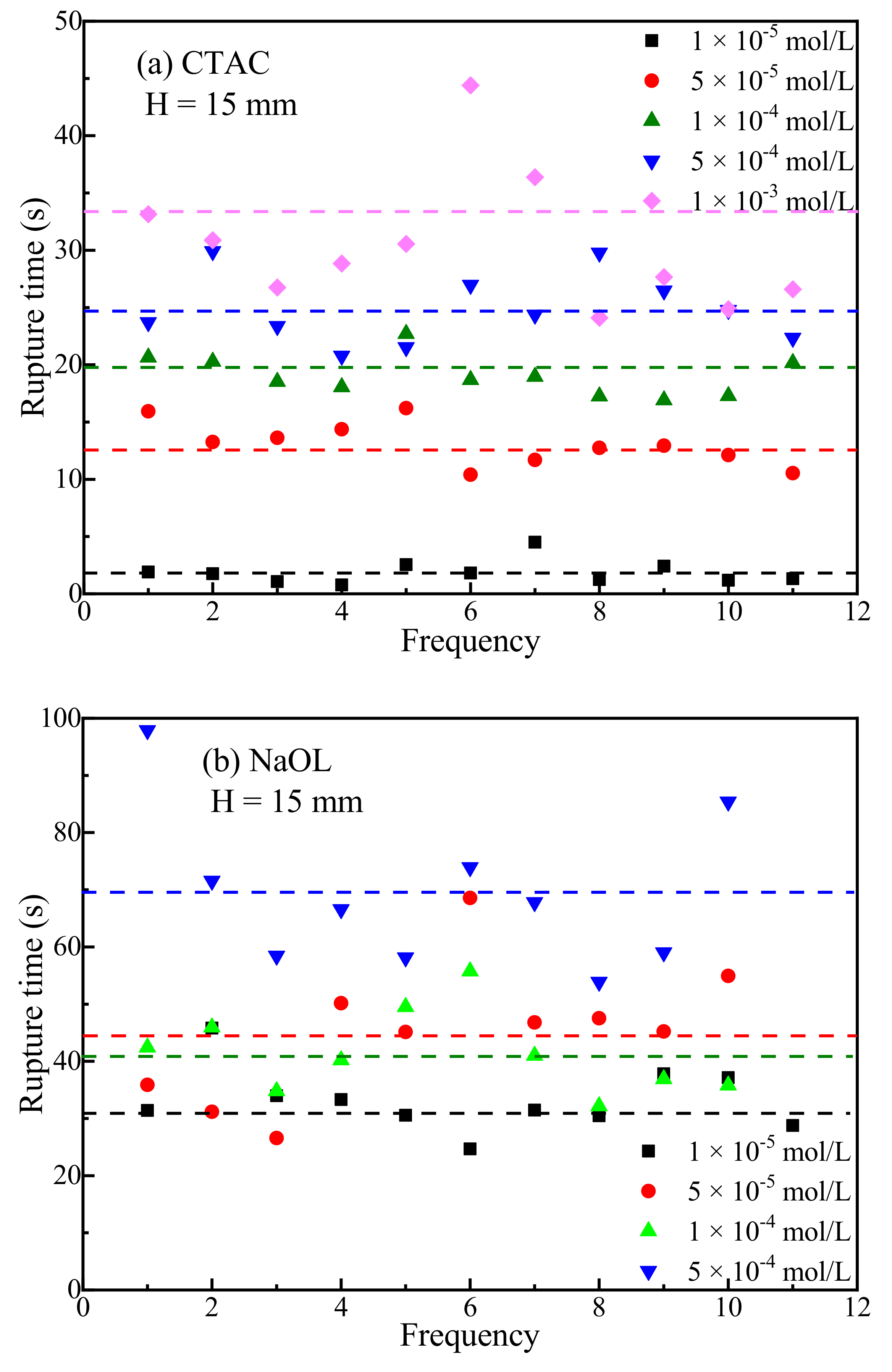
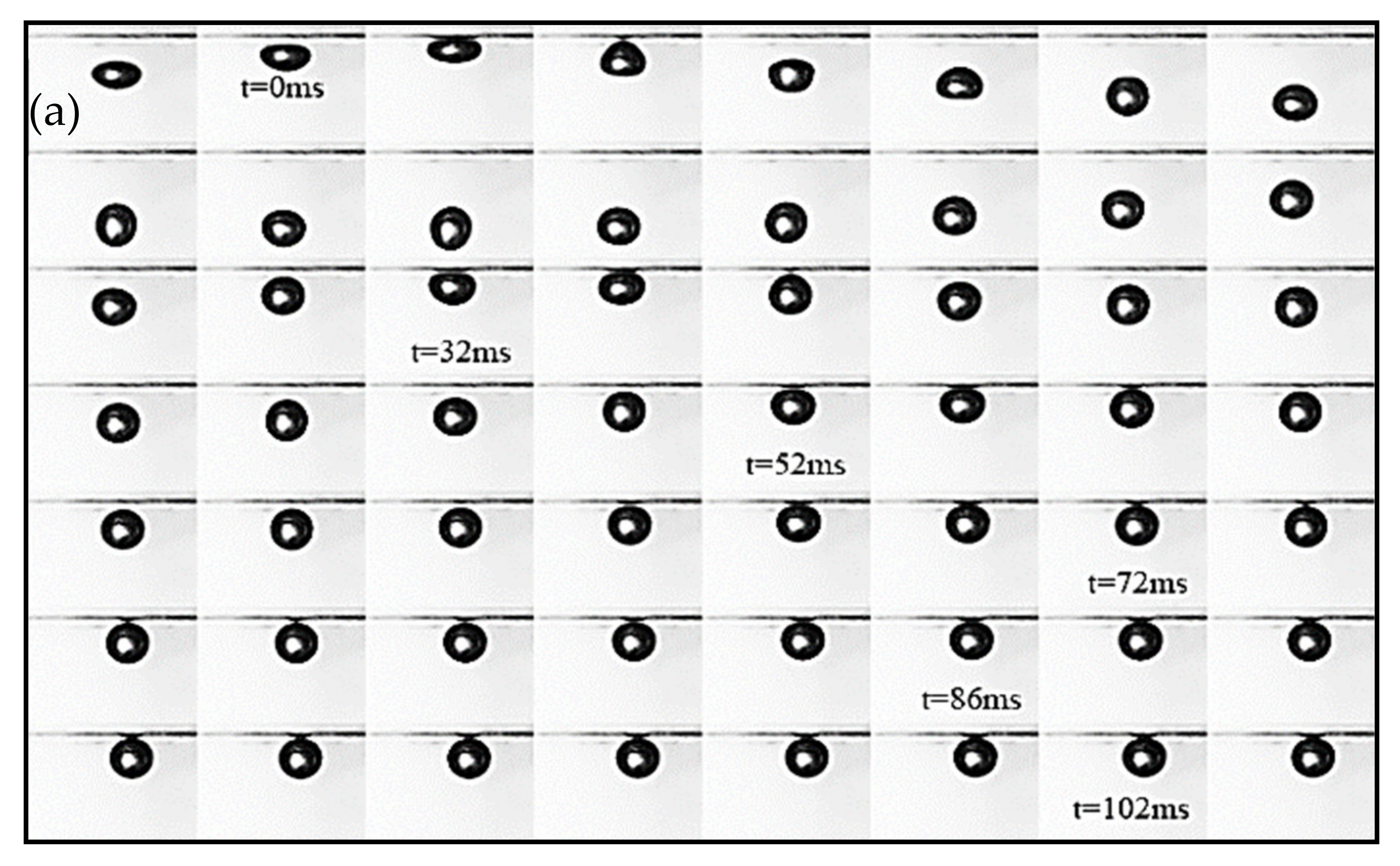
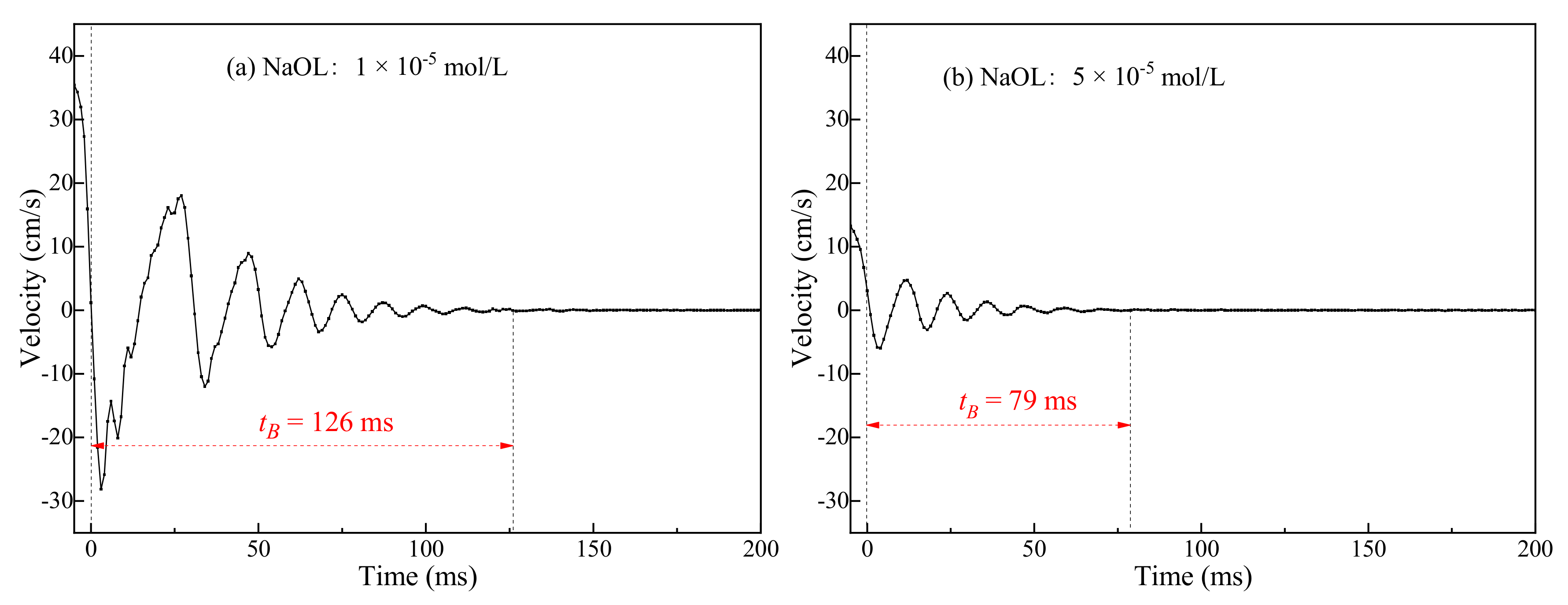
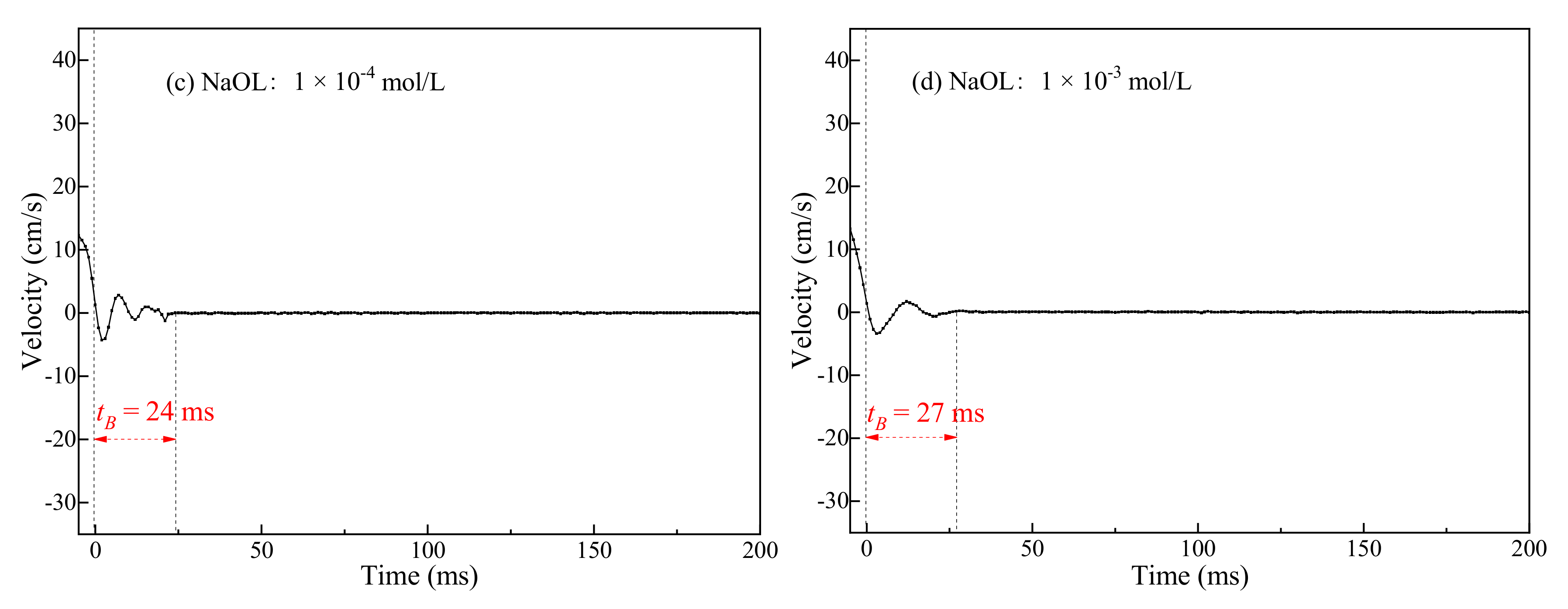
3.1. Bubble Deformation and Stability Analysis in Surfactant Solutions
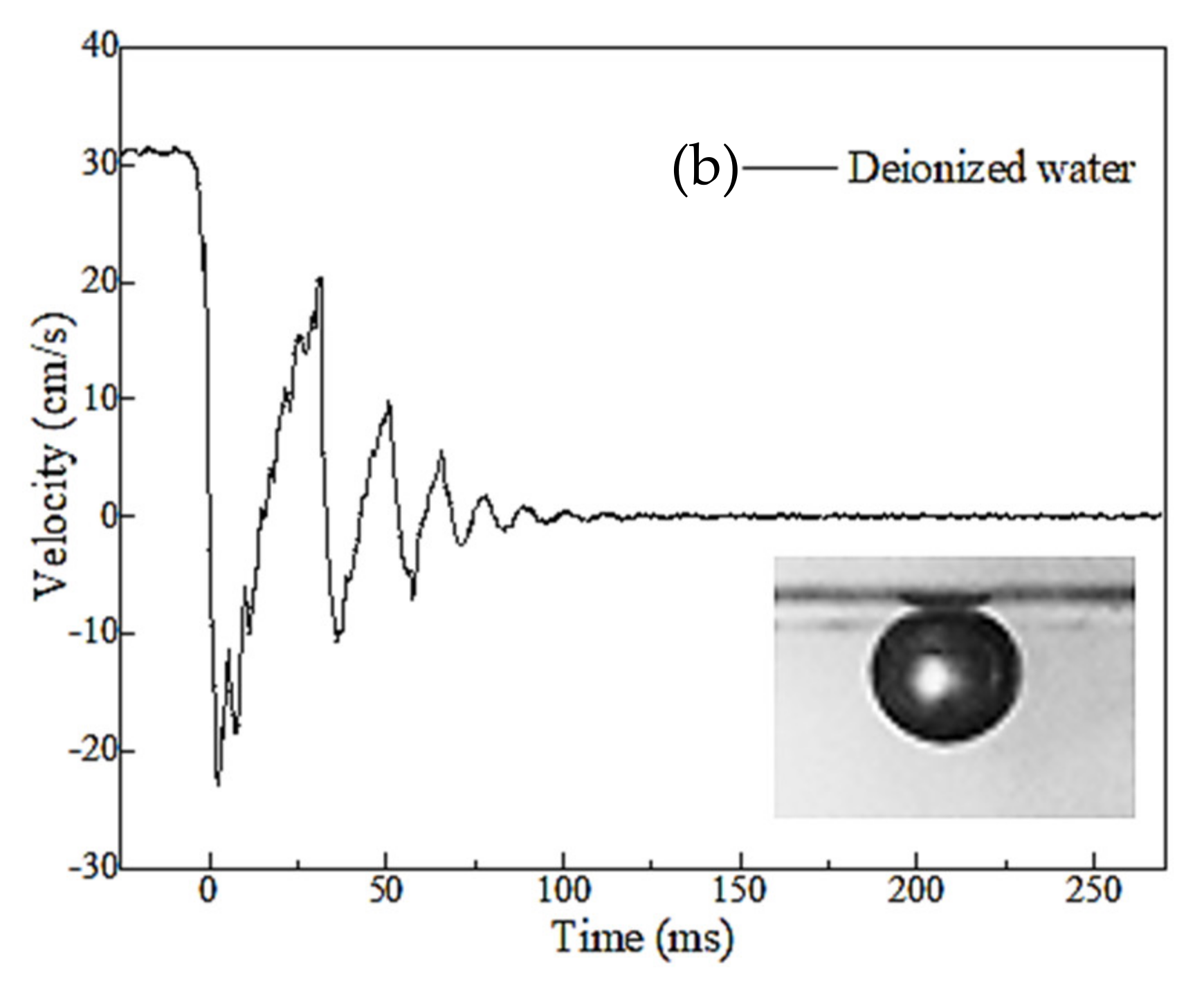
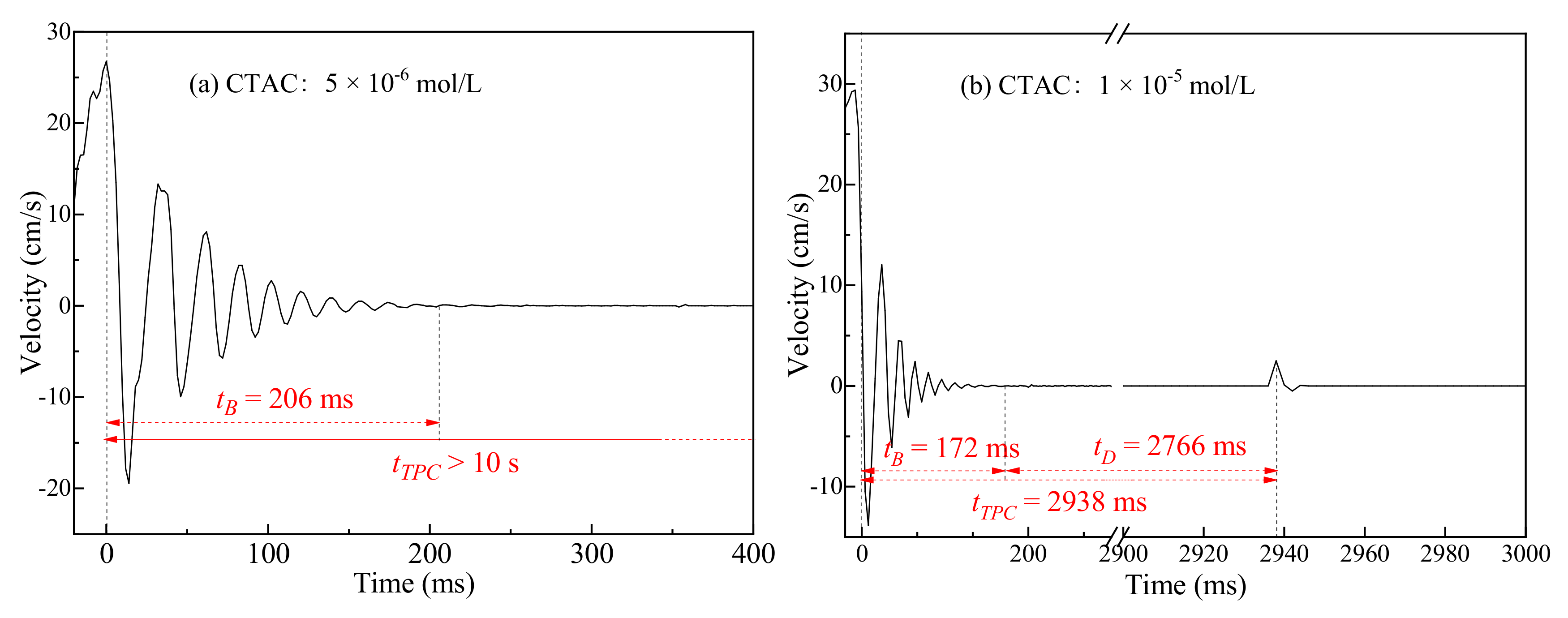
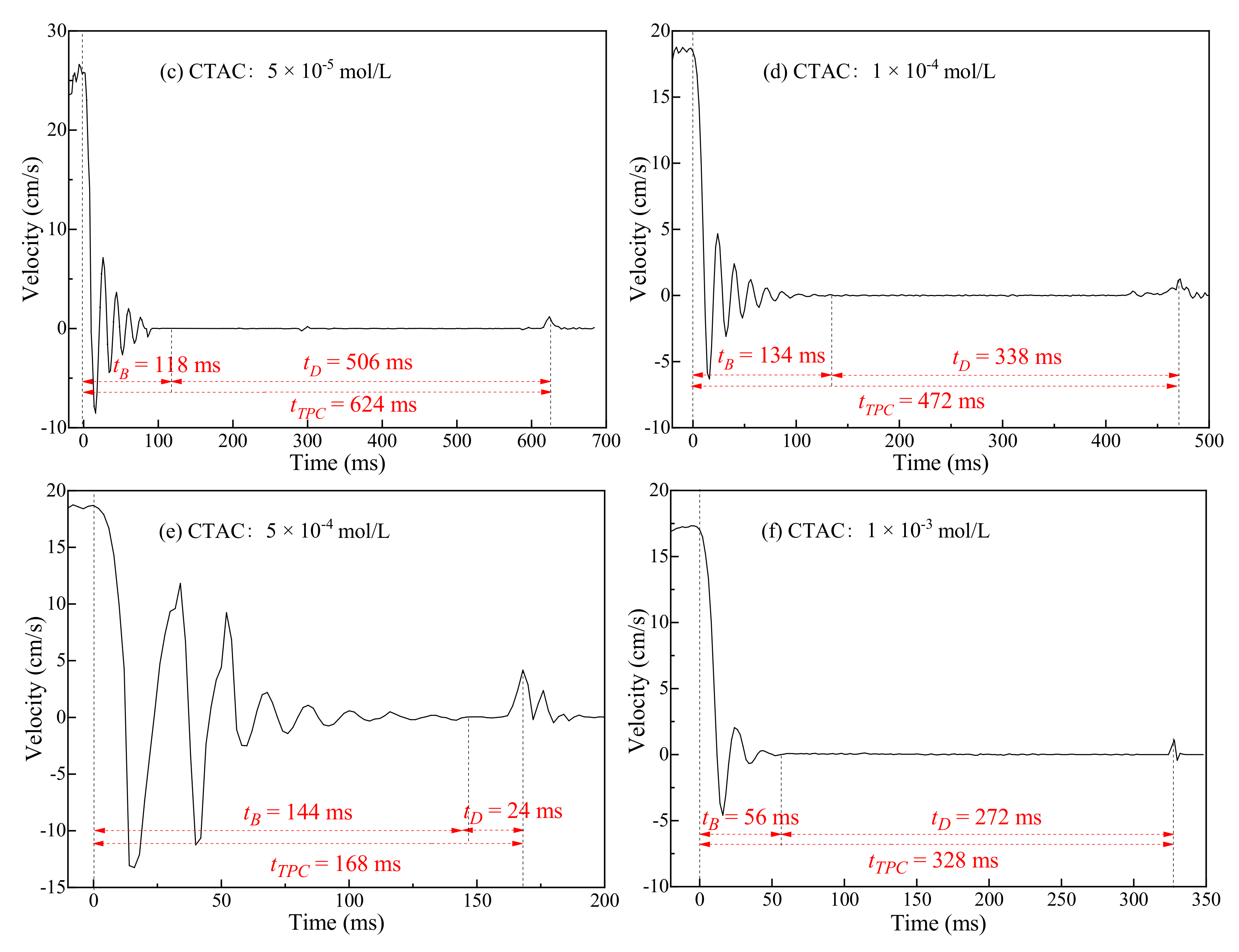
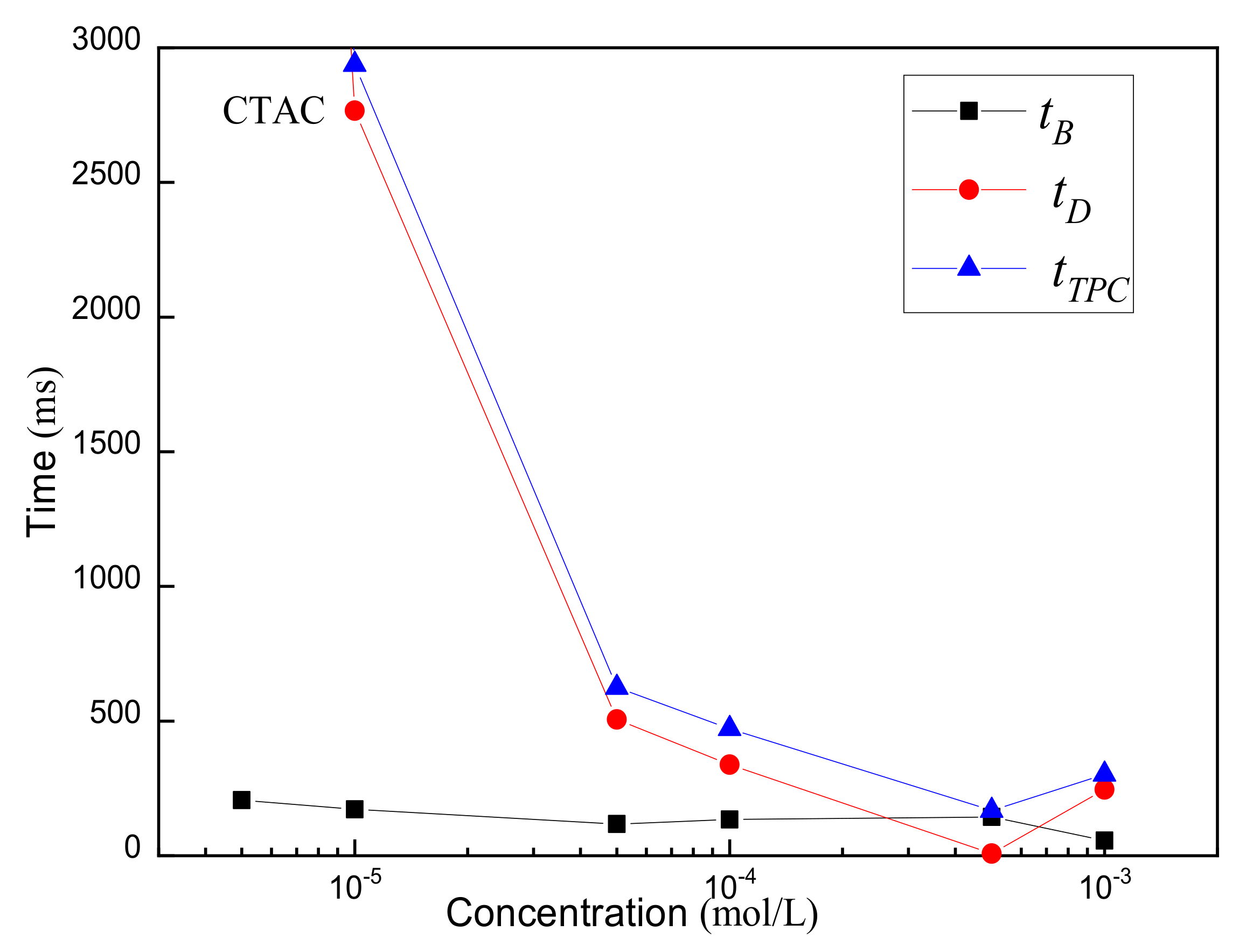
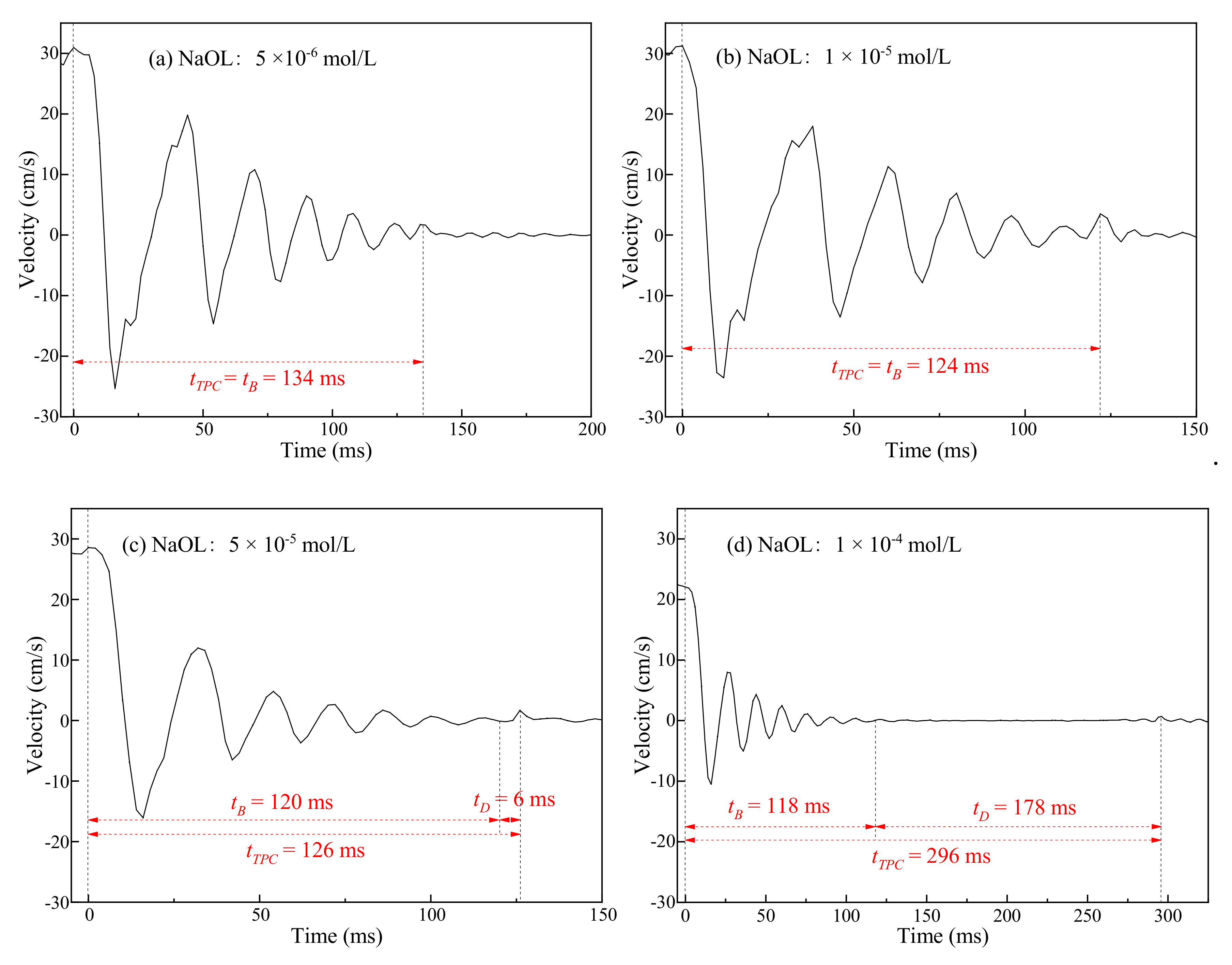
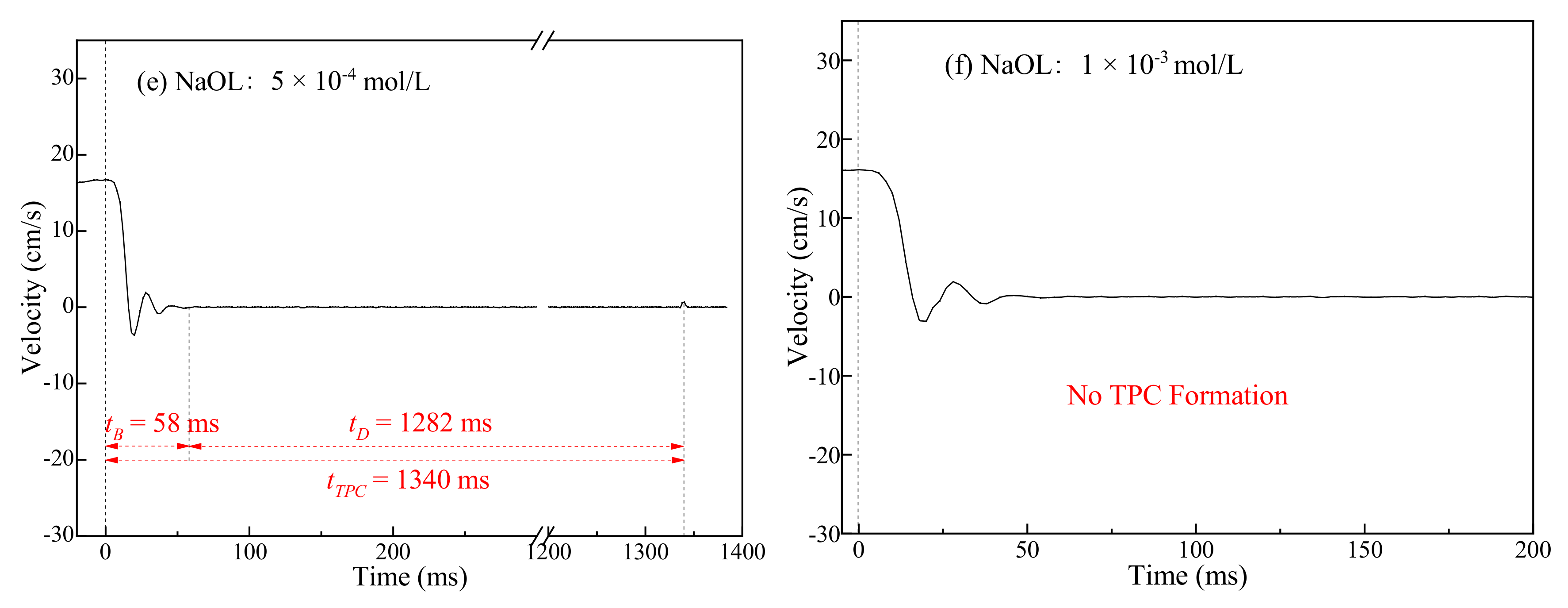
3.2. Three-Phase Contact Formation
3.2.1. TPC on Muscovite Surface
3.2.2. TPC on Talc Surface
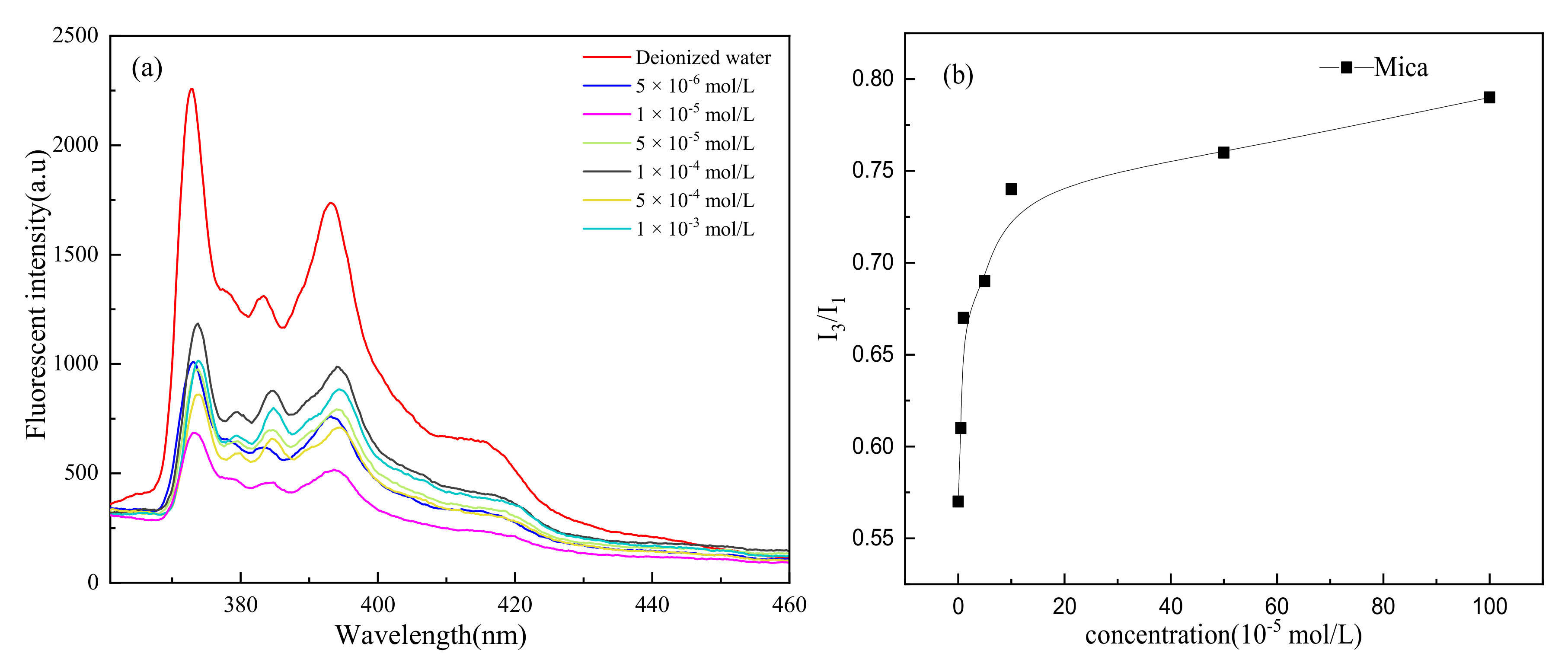
3.3. Discussion
4. Conclusions
- The increasing concentration of CTAC reduced the aspect ratio (AR) of the rising bubble, making it closer to the sphere.
- Bubbles may rebound and collide with the liquid level several times until they dissipate the kinetic energy and burst after a period of time. Due to the surface-tension gradient effect, the bubbles in NaOL were much more stable than those in CTAC, and the film thinning became slower.
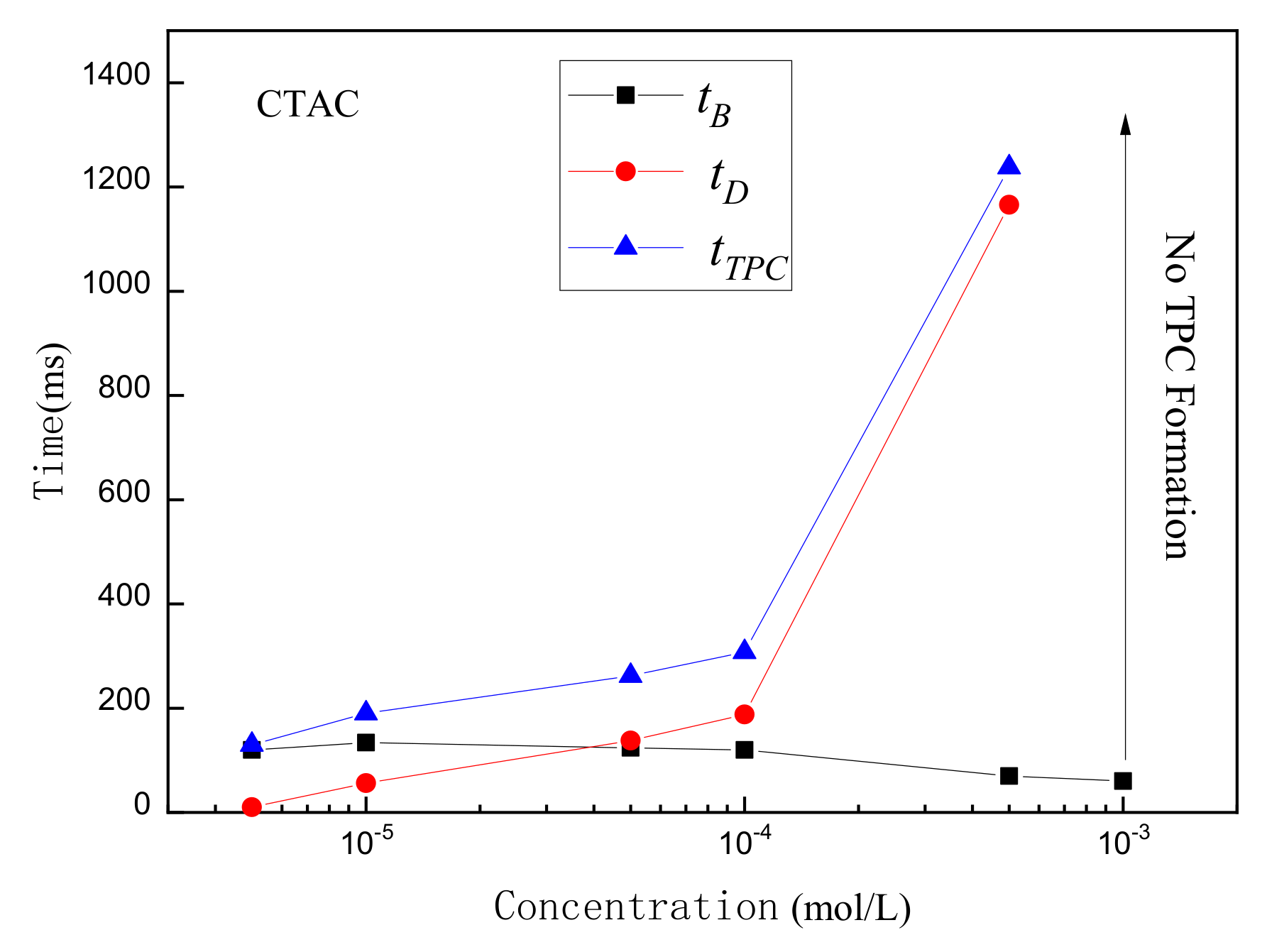
- The rupture of the liquid film between the bubbles and the mineral surface was a necessary condition for TPC formation. For hydrophilic muscovite, the TPC was formed in CTAC due to the hydrophobic attraction and electrostatic attraction, and the increasing concentration can shorten its formation time. However, it did not appear in water and NaOL due to electrostatic repulsion between the bubbles and the muscovite surface.
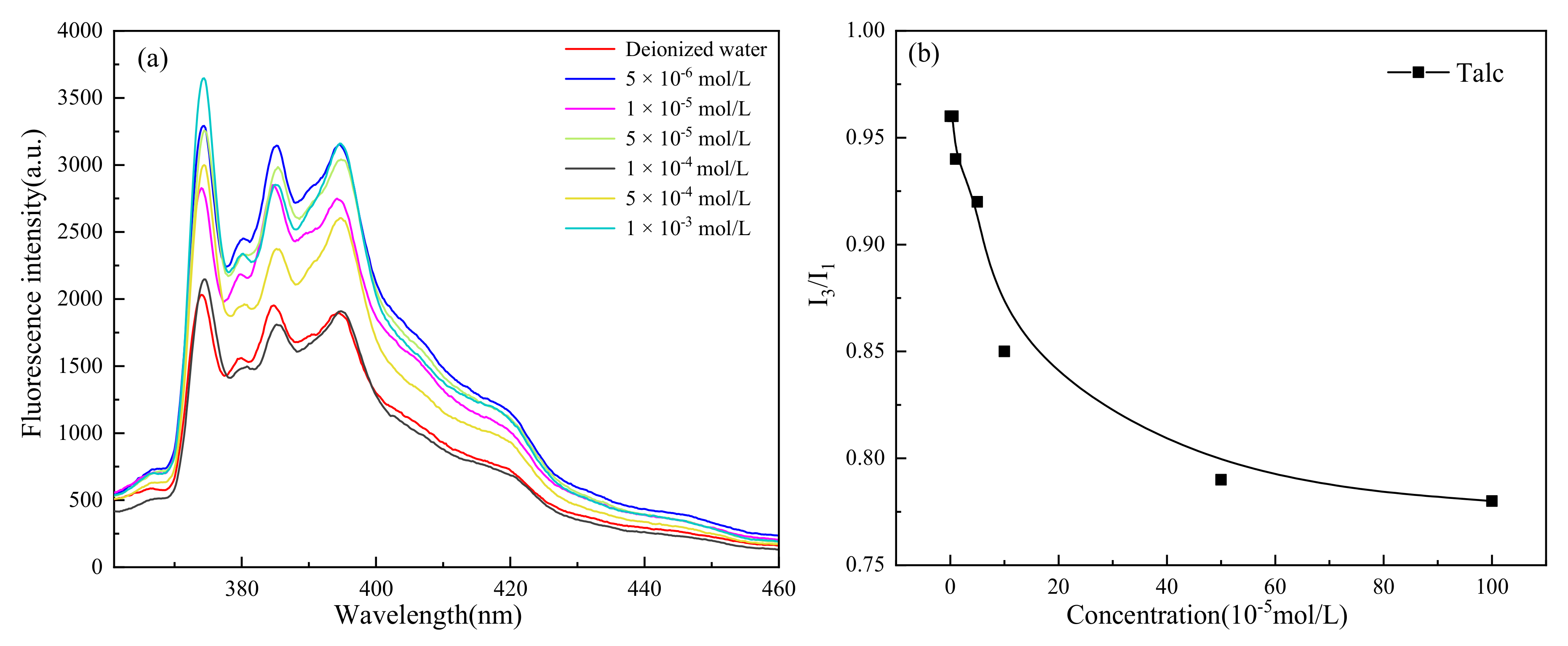
- Unlike muscovite, TPC was formed on the talc surface, either in deionized water or surfactants. Talc was a naturally hydrophobic mineral. When TPC was formed, the hydrophobic attraction played a major role and promoted the thinning and breaking of the hydration film between the bubbles and the talc. However, the multi-layer adsorption of high-concentration surfactants actually reduced its hydrophobicity with the result that the I3/I1 value decreased and the local micro-polarity enhanced. The hydrophobic attraction was weakened, and the TPC formation time was prolonged.
- The main reason for TPC formation was different for different minerals. Micro polarity changes on the mineral surface can be explained for different phenomena of TPC results via fluorescence spectrum analysis.
Author Contributions
Funding
Conflicts of Interest
References
- Albijanic, B.; Ozdemir, O.; Nguyen, A.; Bradshaw, D. A review of induction and attachment times of wetting thin films between air bubbles and particles and its relevance in the separation of particles by flotation. Adv. Colloid Interface Sci. 2010, 159, 1–21. [Google Scholar] [CrossRef]
- Zawala, J.; Drzymala, J.; Malysa, K. An investigation into the mechanism of the three-phase contact formation at fluorite surface by colliding bubble. Int. J. Miner. Process. 2008, 88, 72–79. [Google Scholar] [CrossRef]
- Wang, S.; Guo, J.; Tang, L.; He, H.; Tao, X. Effect of surface roughness of Chinese sub-bituminous coal on the kinetics of three-phase contact formation. Fuel 2018, 216, 531–537. [Google Scholar] [CrossRef]
- Kowalczuk, P.B.; Zawala, J.; Drzymala, J.; Malysa, K. Influence of hexylamine on kinetics of flotation and bubble attachment to the quartz surface. Sep. Sci. Technol. 2016, 51, 2681–2690. [Google Scholar] [CrossRef]
- Deryagin, B.V.; Gutop, Y.V. Theory of the breakdown (rupture) of free films. Kolloidn. Zh. 1962, 24, 370–374. [Google Scholar]
- Anfruns, J.F.; Kitchener, J.A. Rate of capture of small particles in flotation. Trans. I. MM. 1977, 86, c9–c15. [Google Scholar]
- Firouzi, M.; Nguyen, A.V.; Hashemabadi, S.H. The effect of microhydrodynamics on bubble–particle collision interaction. Miner. Eng. 2011, 24, 973–986. [Google Scholar] [CrossRef]
- Niecikowska, A.; Krasowska, M.; Ralston, J.; Malysa, K. Role of Surface Charge and Hydrophobicity in the Three-Phase Contact Formation and Wetting Film Stability under Dynamic Conditions. J. Phys. Chem. C 2012, 116, 3071–3078. [Google Scholar] [CrossRef]
- Malysa, K.; Krasowska, M.; Krzan, M. Influence of surface active substances on bubble motion and collision with various interfaces. Adv. Colloid Interface Sci. 2005, 114–115, 205–225. [Google Scholar] [CrossRef]
- Frumkin, A.; Vg, L. On surfactants an interfacial motion. Zh. Fiz. Khim. 1947, 21, 1183–1204. [Google Scholar]
- Dukhin, S.S.; Kretzschmar, G.; Miller, R. Dynamic of Adsorption at Liquid Interfaces; Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Malysa, K.; Zawała, J.; Krzan, M.; Krasowska, M. Bubbles rising in solutions; local and terminal velocities, shape variations and collisions with free surface. In Bubble and Drop Interfaces; CRC Press: Boca Raton, FL, USA, 2011; pp. 251–300. [Google Scholar]
- Dukhin, S.S.; Miller, R.; Loglio, G. Physico-chemical hydrodynamics of rising bubble. Stud. Interface Sci. 1998, 6, 367–432. [Google Scholar]
- Mierczynska-Vasilev, A.; Beattie, D.A. The effect of impurities and cleavage characteristics on talc hydrophobicity and polymer adsorption. Int. J. Miner. Process. 2013, 118, 34–42. [Google Scholar] [CrossRef]
- Khraisheh, M.; Holland, C.; Creany, C.; Harris, P.; Parolis, L. Effect of molecular weight and concentration on the adsorption of CMC onto talc at different ionic strengths. Int. J. Miner. Process. 2004, 75, 197–206. [Google Scholar] [CrossRef]
- Xu, L.; Wu, H.; Dong, F.; Wang, L.; Wang, Z.; Xiao, J. Flotation and adsorption of mixed cationic/anionic collectors on muscovite mica. Miner. Eng. 2013, 41, 41–45. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Hu, Y.-H.; Xu, L.-H. Adsorption mechanism of mixed anionic/cationic collectors in Muscovite – Quartz flotation system. Miner. Eng. 2014, 64, 44–50. [Google Scholar] [CrossRef]
- Xie, Z.; Jiang, H.; Sun, Z.C.; Yang, Q.H. Direct AFM measurements of morphology and interaction force at solid-liquid interfaces between DTAC/CTAC and mica. J. Cent. South Univ. 2016, 23, 2182–2190. [Google Scholar] [CrossRef]
- Pan, G.; Shi, Q.; Zhang, G.; Huang, G. Selective depression of talc in chalcopyrite flotation by xanthan gum: Flotation response and adsorption mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124902. [Google Scholar] [CrossRef]
- Peng, B.; Peng, J.; Zhang, W.; Ning, X.; Guo, Y.; Zhang, W. Use of locust bean gum in flotation separation of chalcopyrite and talc. Miner. Eng. 2018, 122, 79–83. [Google Scholar]
- Tang, X.; Chen, Y.; Liu, K.; Peng, Q.; Zeng, G.; Ao, M.; Li, Z. Reverse flotation separation of talc from molybdenite without addition of depressant: Effect of surface oxidation by thermal pre-treatment. Colloids Surfaces A: Physicochem. Eng. Asp. 2020, 594, 124671. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Song, S.; Li, H. A novel method to improve carboxymethyl cellulose performance in the flotation of talc. Miner. Eng. 2018, 131, 23–27. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, Y.; Yang, J.; Li, Y. Visualized study on the interaction between single bubbles and curved solid surface in flotation separation process. Water Ence Technol. 2014, 70, 627–633. [Google Scholar] [CrossRef]
- Krasowska, M.; Zawała, J.; Malysa, K. Air at hydrophobic surfaces and kinetics of three phase contact formation. Adv. Colloid Interface Sci. 2009, 147–148, 155–169. [Google Scholar] [CrossRef]
- Zawala, J.; Kosior, D.; Malysa, K. Formation and influence of the dynamic adsorption layer on kinetics of the rising bubble collisions with solution/gas and solution/solid interfaces. Adv. Colloid Interface Sci. 2015, 222, 765–778. [Google Scholar] [CrossRef]
- Elmahdy, A.M.; Mirnezami, M.; Finch, J. Zeta potential of air bubbles in presence of frothers. Int. J. Miner. Process. 2008, 89, 40–43. [Google Scholar] [CrossRef]
- Yang, C.; Dabros, T.; Li, D.; Czarnecki, J.; Masliyah, J.H. Measurement of the Zeta Potential of Gas Bubbles in Aqueous Solutions by Microelectrophoresis Method. J. Colloid Interface Sci. 2001, 243, 128–135. [Google Scholar] [CrossRef]
- Morga, M.; Adamczyk, Z. Monolayers of cationic polyelectrolytes on mica – Electrokinetic studies. J. Colloid Interface Sci. 2013, 407, 196–204. [Google Scholar] [CrossRef]
- Zembala, M.; Adamczyk, Z. Measurements of Streaming Potential for Mica Covered by Colloid Particles. Langmuir 1999, 16, 1593–1601. [Google Scholar] [CrossRef]
- Kowalczuk, P.B.; Zawala, J.; Kosior, D.; Drzymala, J.; Malysa, K. Three-Phase Contact Formation and Flotation of Highly Hydrophobic Polytetrafluoroethylene in the Presence of Increased Dose of Frothers. Ind. Eng. Chem. Res. 2016, 55, 839–843. [Google Scholar] [CrossRef]
- Jiang, H.; Gao, Y.; Yang, Q.; Khoso, S.A.; Liu, G.; Xu, L.; Hu, Y. Adsorption behaviors and mechanisms of dodecyltrimethyl ammonium chloride and cetyltrimethyl ammonium chloride on illite flotation. Powder Technol. 2018, 331, 218–225. [Google Scholar] [CrossRef]
- Sowmiya, M.; Tiwari, A.K.; Saha, S.K. Fluorescent probe studies of micro-polarity, premicellar and micellar aggregation of non-ionic Brij surfactants. J. Colloid Interface Sci. 2010, 344, 97–104. [Google Scholar] [CrossRef]
- Chandar, P.; Somasundaran, P.; Turro, N.J. Fluorescence probe studies on the structure of the adsorbed layer of dodecyl sulfate at the alumina—water interface. J. Colloid Interface Sci. 1987, 117, 31–46. [Google Scholar] [CrossRef]
- Misra, P.K.; Somasundaran, P. Fluorescence Probing of the Surfactant Assemblies in Solutions and at Solid-Liquid Interfaces. In Interfacial Processes and Molecular Aggregation of Surfactants; Narayanan, R., Ed.; Springer: Berlin, Germany, 2008; pp. 143–188. [Google Scholar]
- Jiang, H.; Xu, L.-H.; Hu, Y.-H.; Wang, D.-Z.; Li, C.-K.; Meng, W.; Wang, X.-J. Flotation and adsorption of quaternary ammonium cationic collectors on diaspore and kaolinite. Trans. Nonferrous Met. Soc. China 2011, 21, 2528–2534. [Google Scholar] [CrossRef]
- Jiang, H.; Gao, Y.; Khoso, S.A.; Ji, W.; Hu, Y. A new approach for characterization of hydrophobization mechanisms of surfactants on muscovite surface. Sep. Purif. Technol. 2019, 209, 936–945. [Google Scholar] [CrossRef]
- Jiang, H.; Gao, Y.; Khoso, S.; Ahmed, J.; Wanying, H. Interpretation of Hydrophobization Behavior of Dodecylamine on Muscovite and Talc Surface through Dynamic Wettability and AFM Analysis. Minerals 2018, 8, 391. [Google Scholar] [CrossRef] [Green Version]
- Albijanic, B.; Ozdemir, O.; Hampton, M.A.; Nguyen, P.T.; Nguyen, A.V.; Bradshawb, D. Fundamental Aspects of Bubble-particle Attachment Mechanism in Flotation Separation. Miner. Eng. 2014, 65, 187–195. [Google Scholar] [CrossRef]
- Yoon, R.H.; Yordan, J.L. Induction Time Measurement for the Quartz Amine Flotation System. J. Colloid Interface Sci. 1991, 141, 374–383. [Google Scholar] [CrossRef]
- Subasinghe, G.; Albijanic, B. Influence of the propagation of three phase contact line on flotation recovery. Miner. Eng. 2014, 57, 43–49. [Google Scholar] [CrossRef]
- Kowalczuk, P.B.; Zawala, J. A relationship between time of three-phase contact formation and flotation kinetics of naturally hydrophobic solids. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 371–377. [Google Scholar]



| Sample | Al2O3 | SiO2 | Fe2O3 | TiO2 | CaO | MgO | K2O | Na2O | H2O | LOI 1 |
|---|---|---|---|---|---|---|---|---|---|---|
| Muscovite | 37.46 | 44.55 | 2.30 | 0.74 | 0.19 | 0.97 | 6.53 | 2.24 | 2.33 | 2.69 |
| Talc | 0.84 | 60.61 | 0.71 | - | - | 29.07 | - | - | 6.65 | 2.12 |
| Concentration (mol/L) | 1 × 10−5 | 5 × 10−5 | 1 × 10−4 | 5 × 10−4 | 1 × 10−3 |
|---|---|---|---|---|---|
| CTAC | 1.866 | 13.075 | 19.045 | 24.910 | 30.376 |
| NaOL | 33.220 | 45.198 | 41.456 | 69.265 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Gao, Y.; Li, M.; Jiang, H.; Xie, J.; Xiang, G. Comparison and Mechanism Analysis of Three-Phase Contact Formation onto Hydrophilic/Hydrophobic Mineral Surfaces in the Presence of Cationic/Anionic Surfactants during Flotation Process. Minerals 2022, 12, 219. https://doi.org/10.3390/min12020219
Li X, Gao Y, Li M, Jiang H, Xie J, Xiang G. Comparison and Mechanism Analysis of Three-Phase Contact Formation onto Hydrophilic/Hydrophobic Mineral Surfaces in the Presence of Cationic/Anionic Surfactants during Flotation Process. Minerals. 2022; 12(2):219. https://doi.org/10.3390/min12020219
Chicago/Turabian StyleLi, Xianyuan, Ya Gao, Mei Li, Hao Jiang, Jiahui Xie, and Guoyuan Xiang. 2022. "Comparison and Mechanism Analysis of Three-Phase Contact Formation onto Hydrophilic/Hydrophobic Mineral Surfaces in the Presence of Cationic/Anionic Surfactants during Flotation Process" Minerals 12, no. 2: 219. https://doi.org/10.3390/min12020219
APA StyleLi, X., Gao, Y., Li, M., Jiang, H., Xie, J., & Xiang, G. (2022). Comparison and Mechanism Analysis of Three-Phase Contact Formation onto Hydrophilic/Hydrophobic Mineral Surfaces in the Presence of Cationic/Anionic Surfactants during Flotation Process. Minerals, 12(2), 219. https://doi.org/10.3390/min12020219





