Process Mineralogy Characteristics and Flotation Application of a Refractory Collophanite from Guizhou, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ore Samples
2.2. Reagents
2.3. Characterization Methods
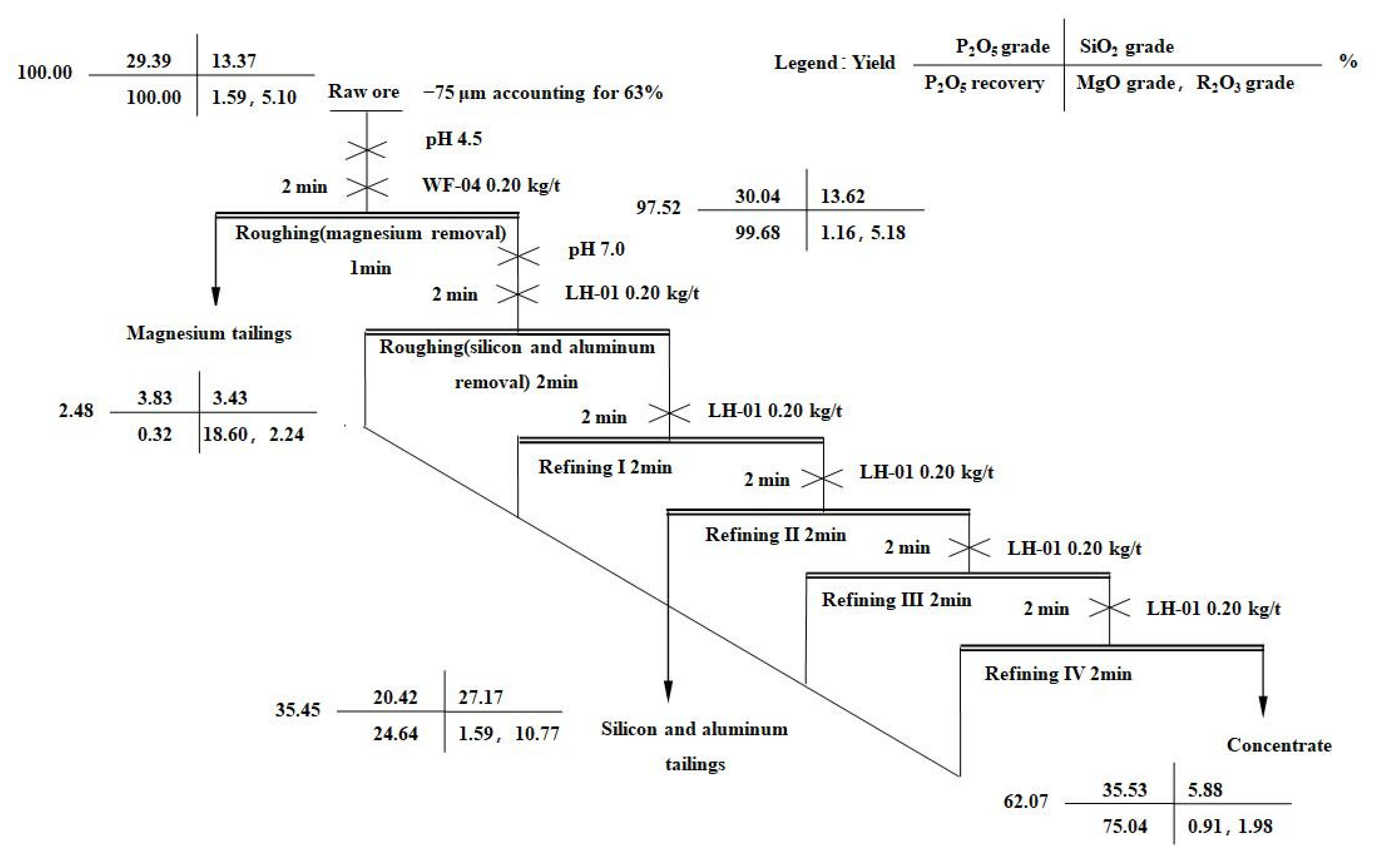
2.4. Flotation Tests
3. Results and Discussion
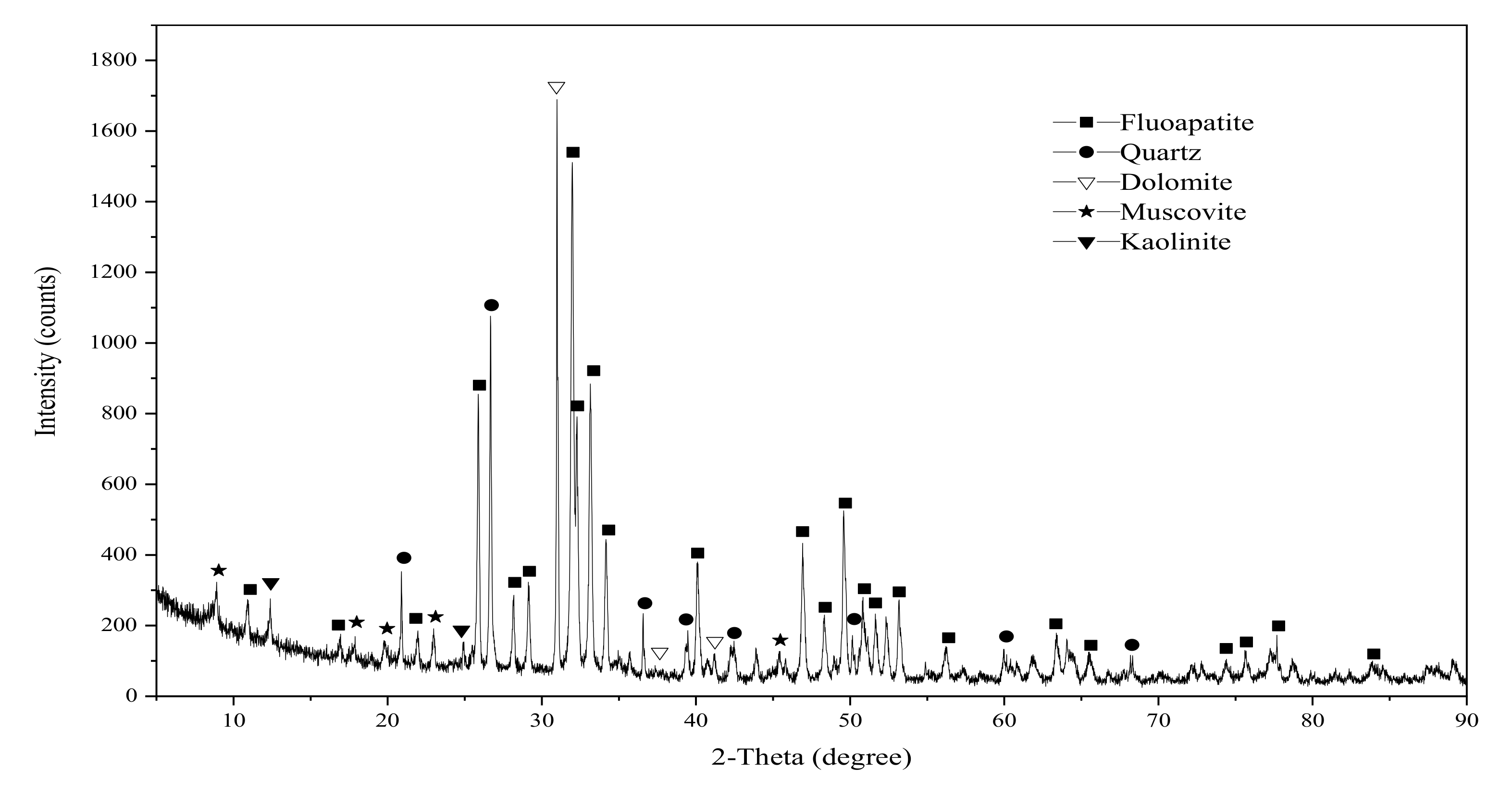
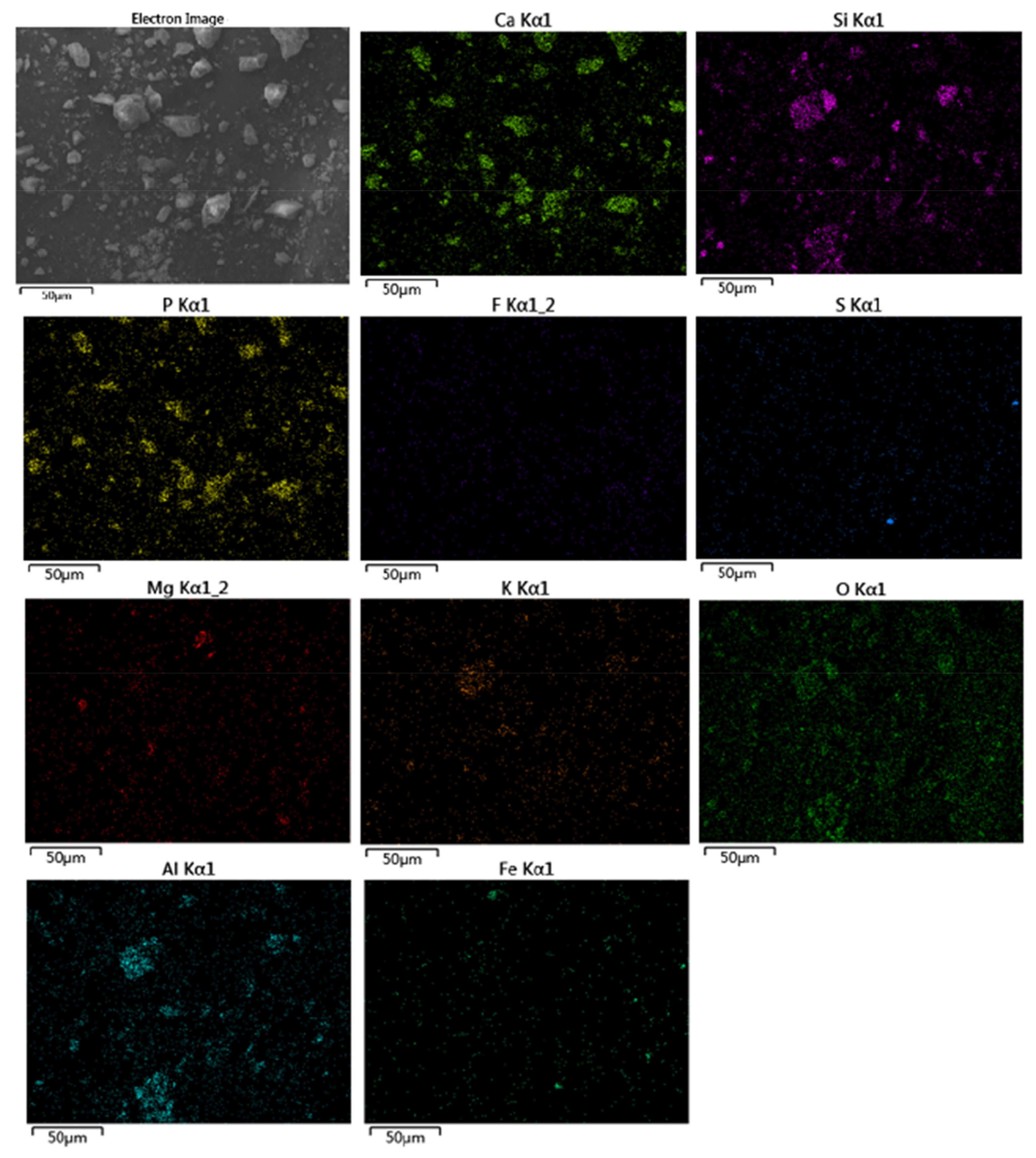
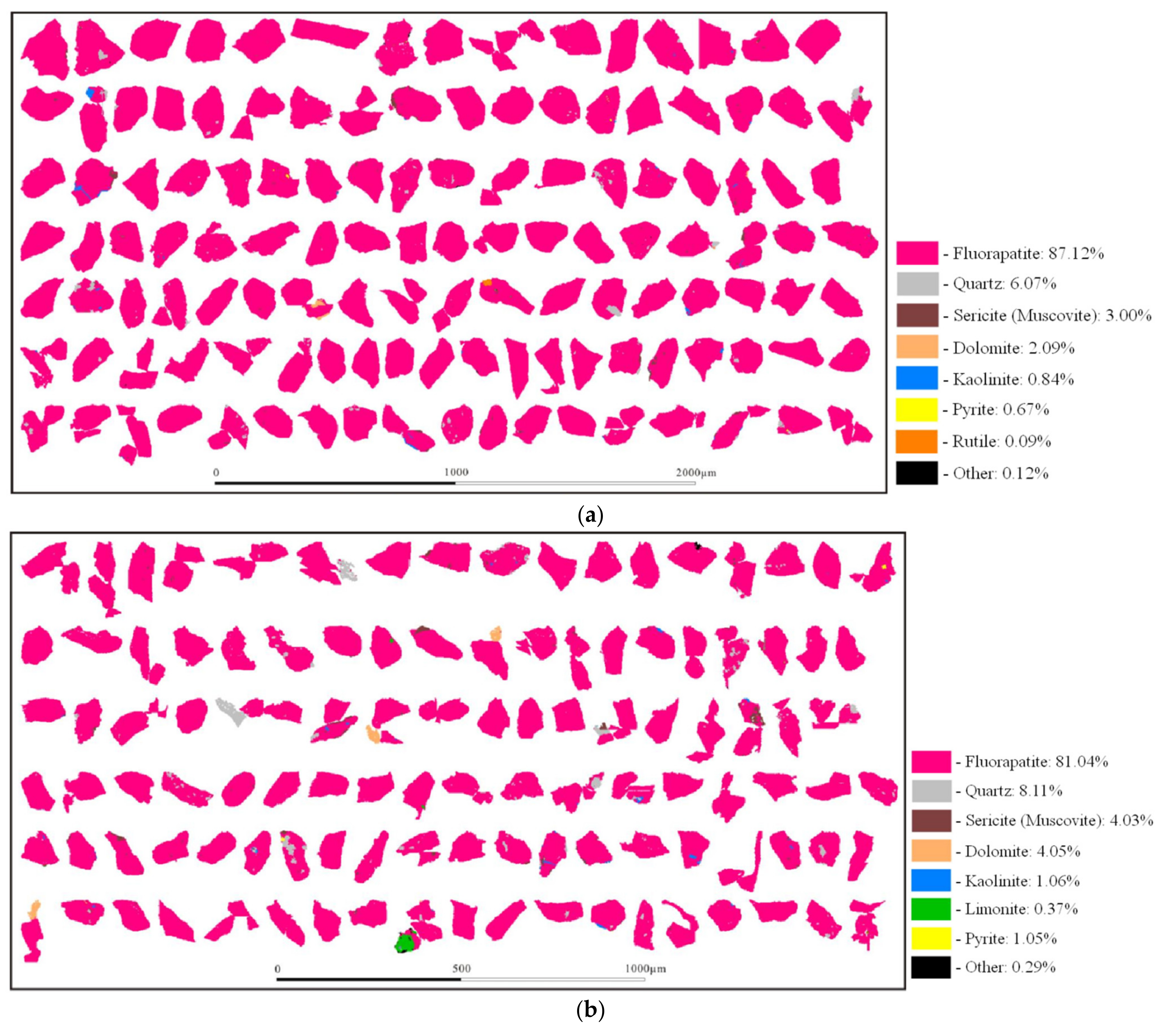
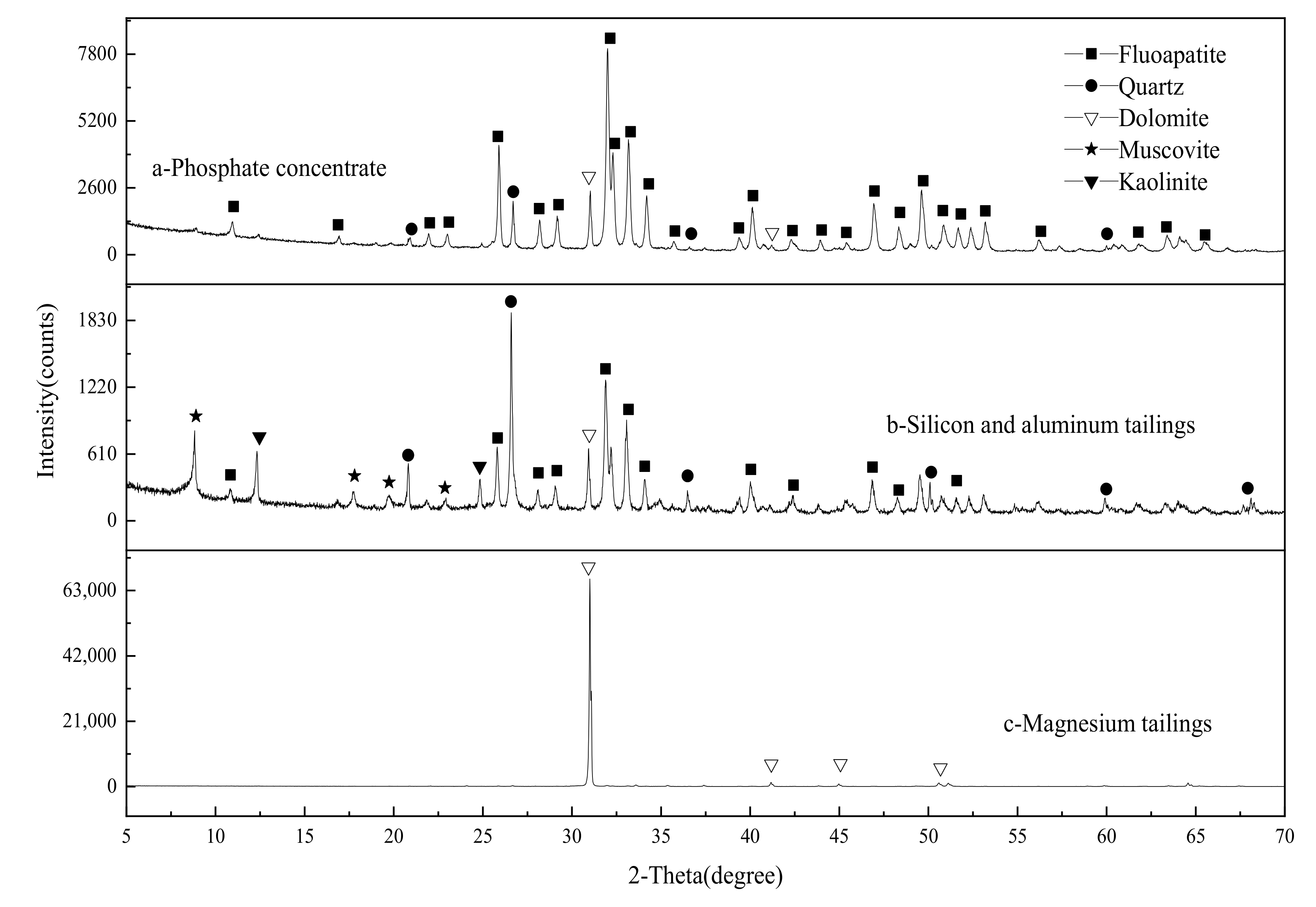
3.1. Mineral Composition and Element Occurrence
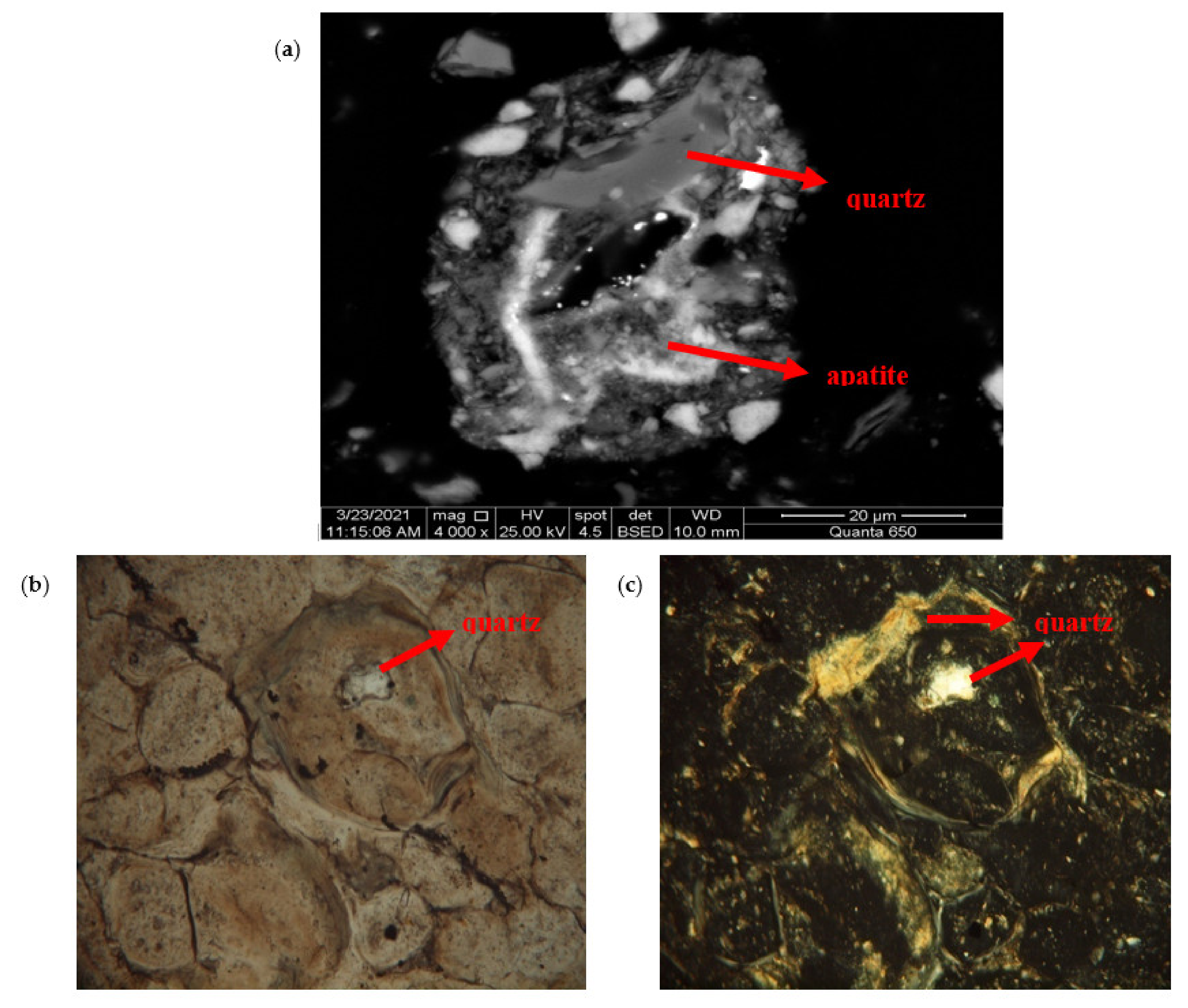
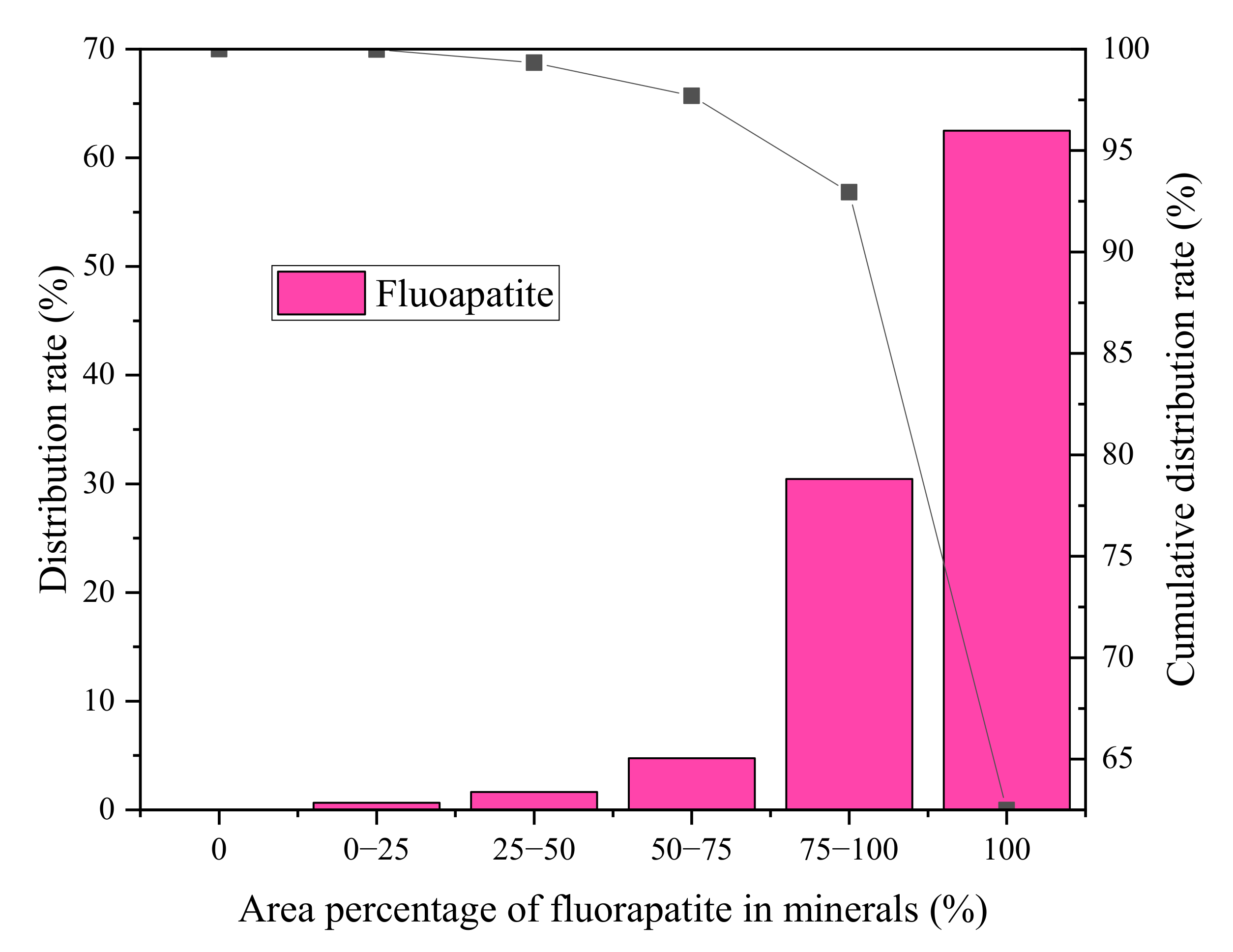
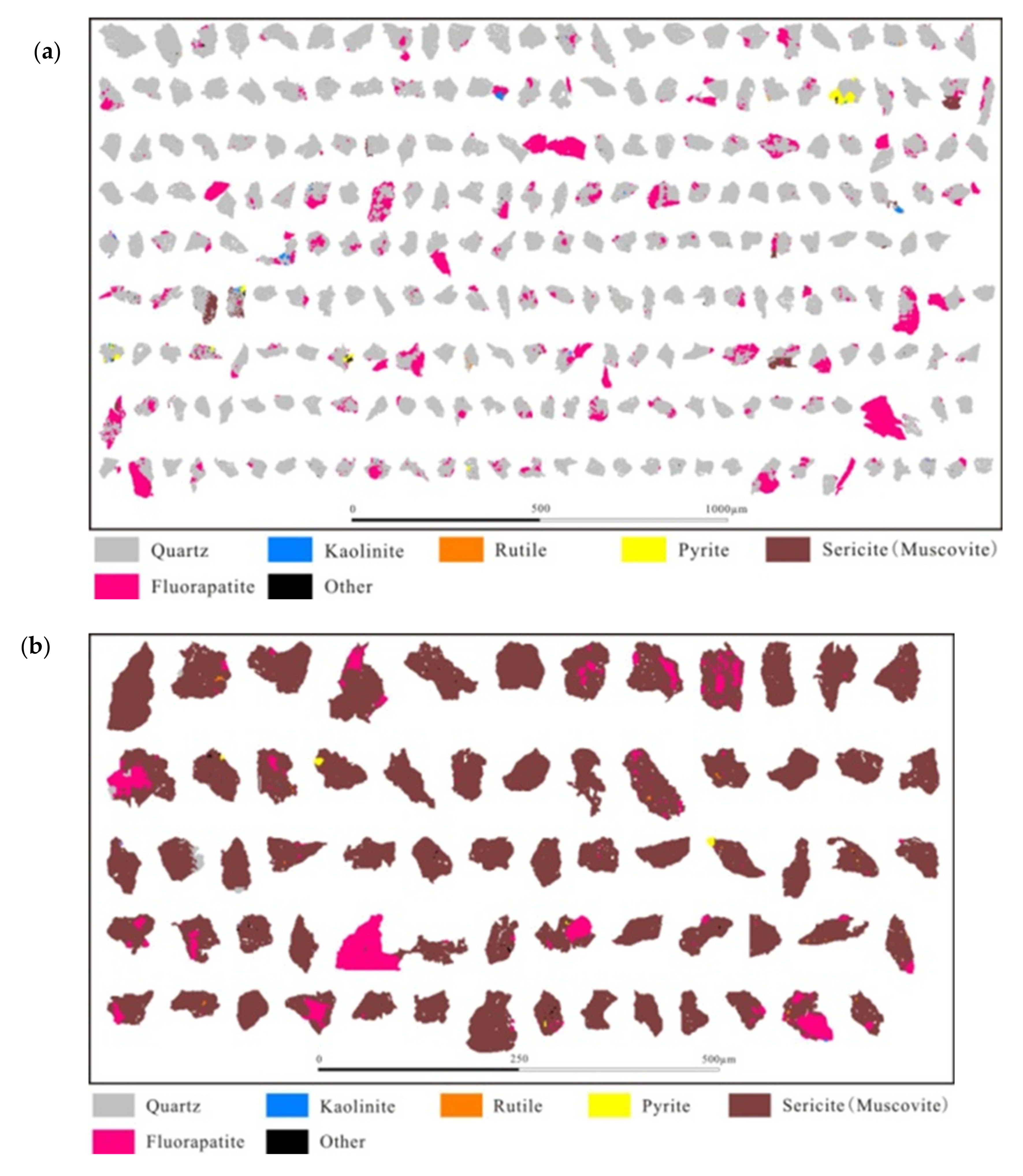
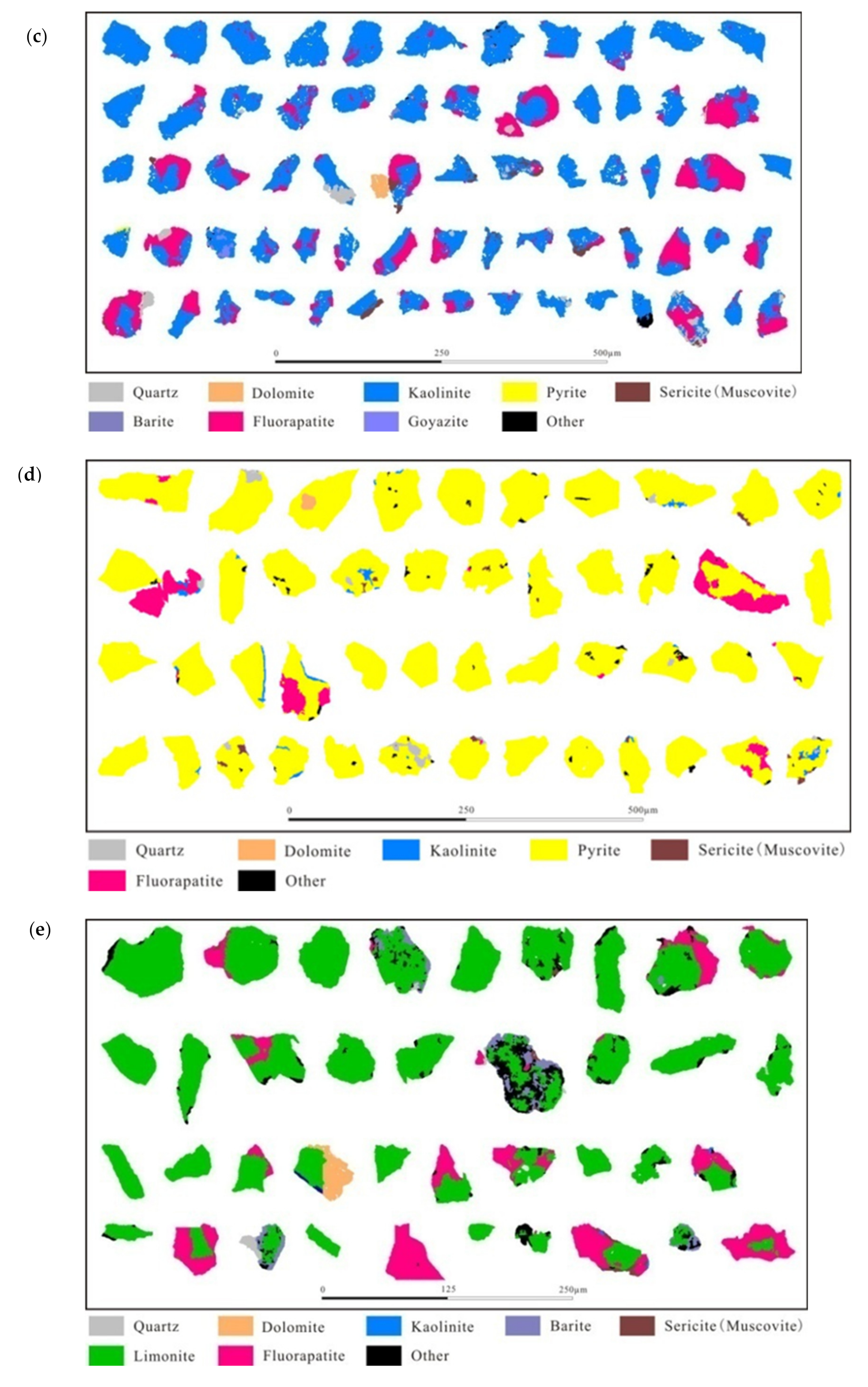
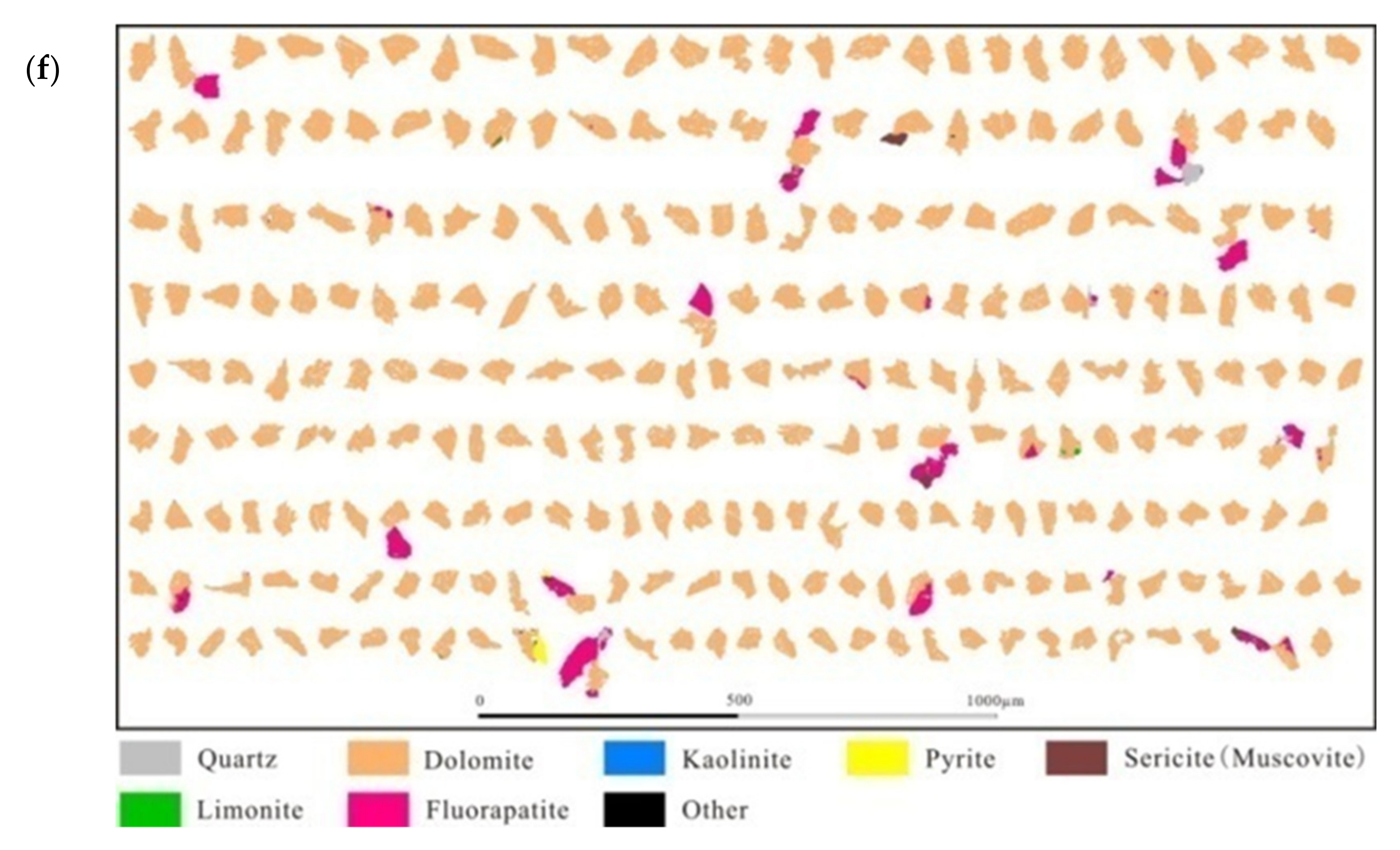
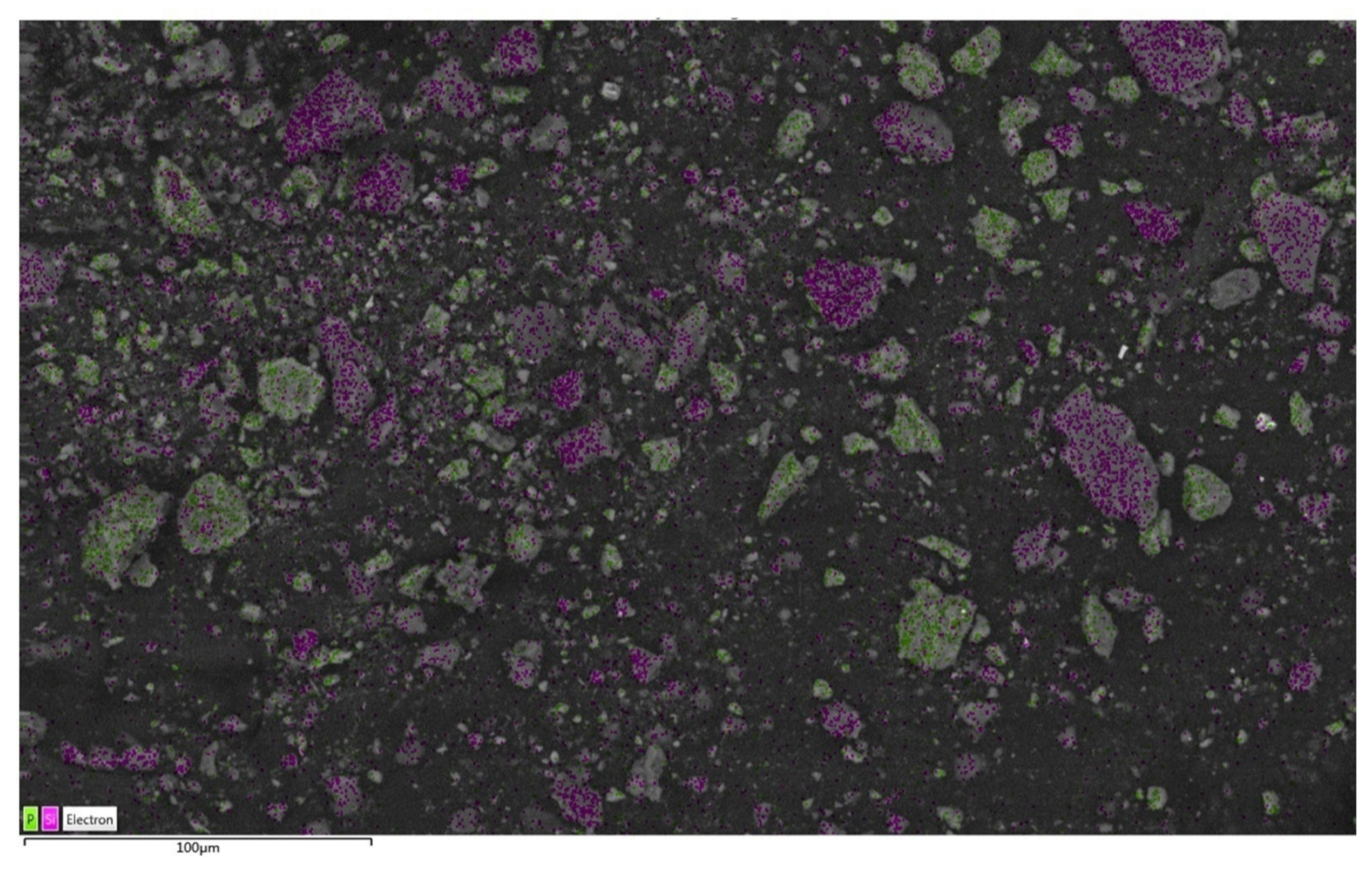
3.2. Disseminated Types and Liberation Characteristics of Fluorapatite
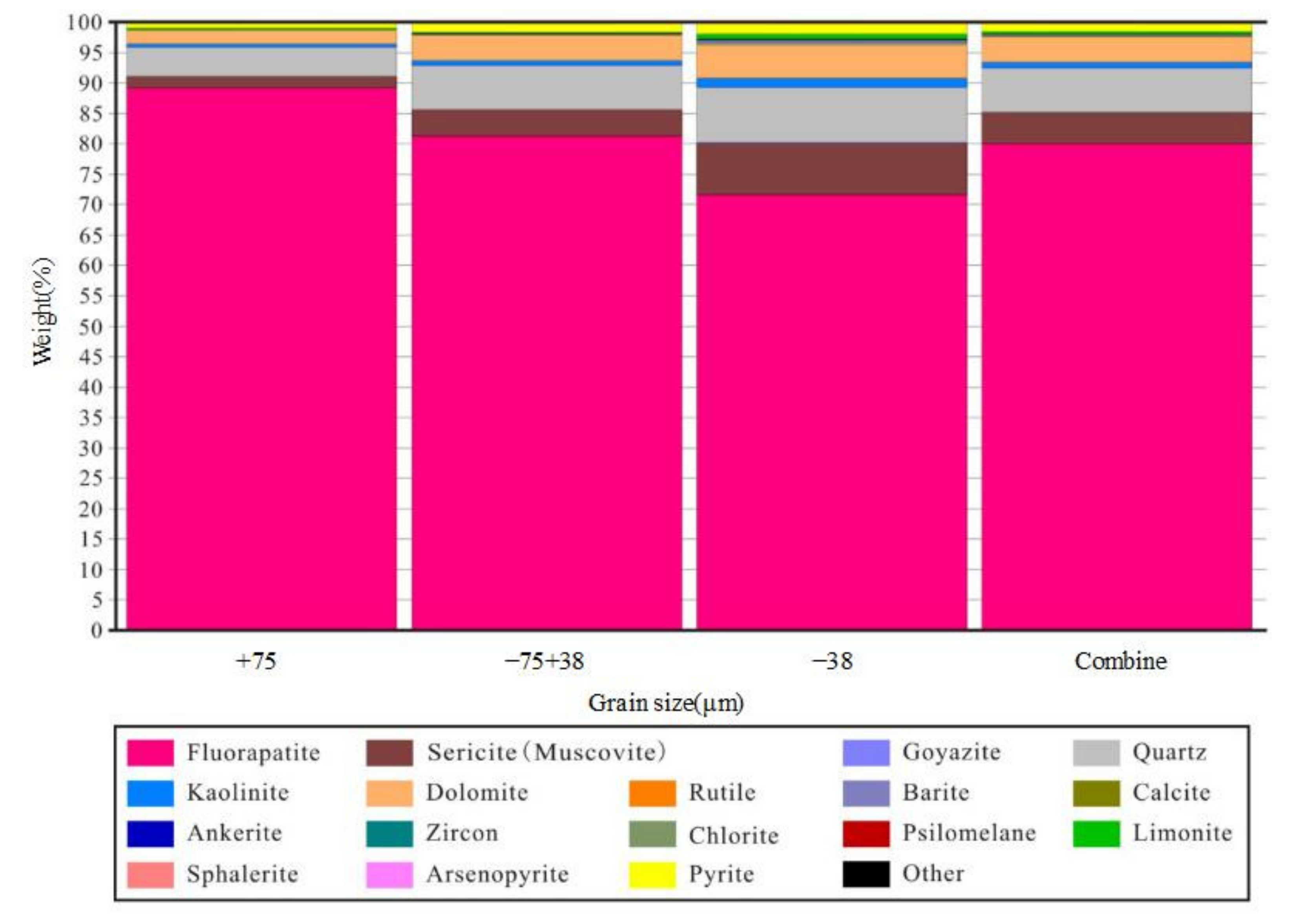
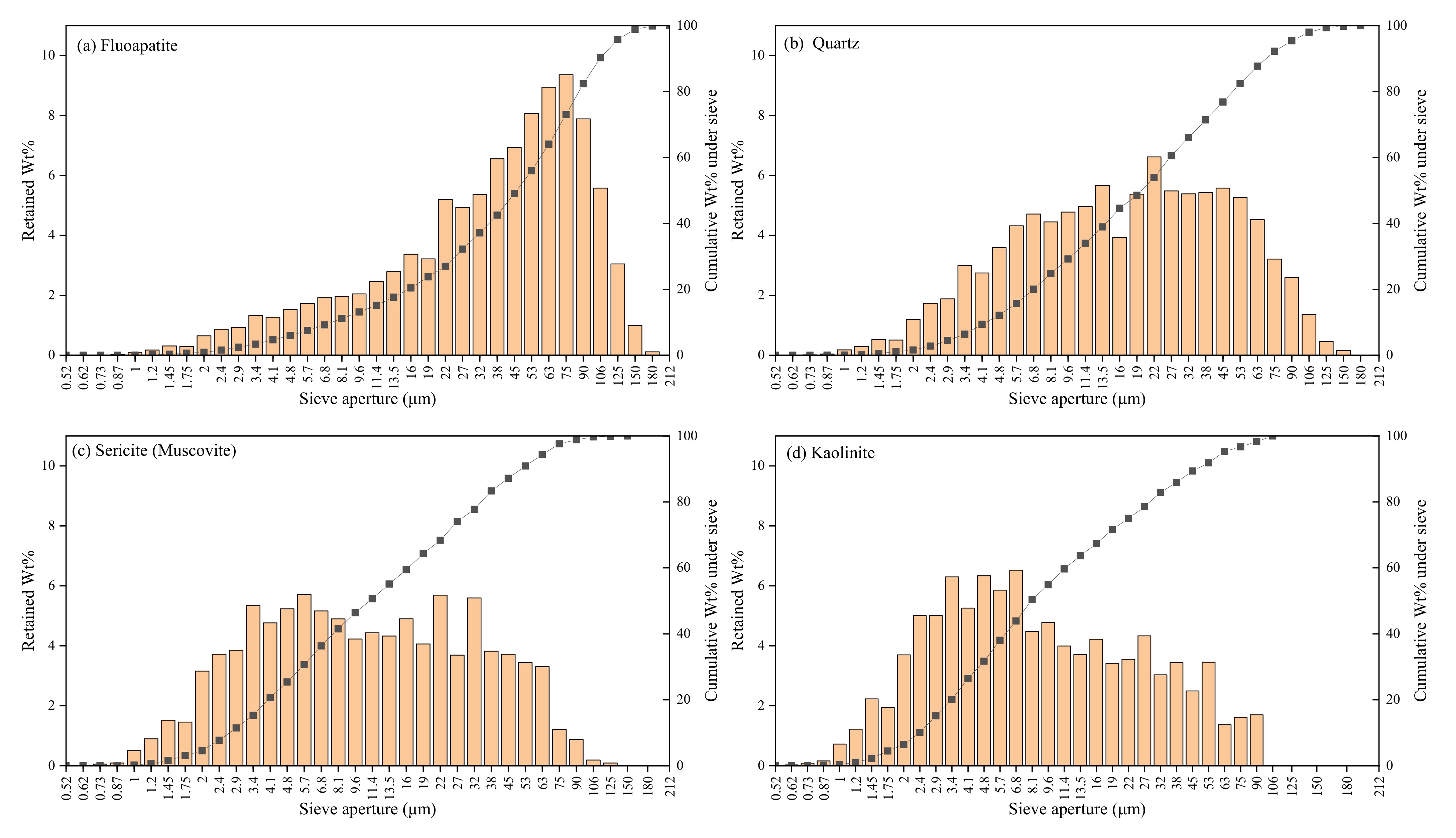
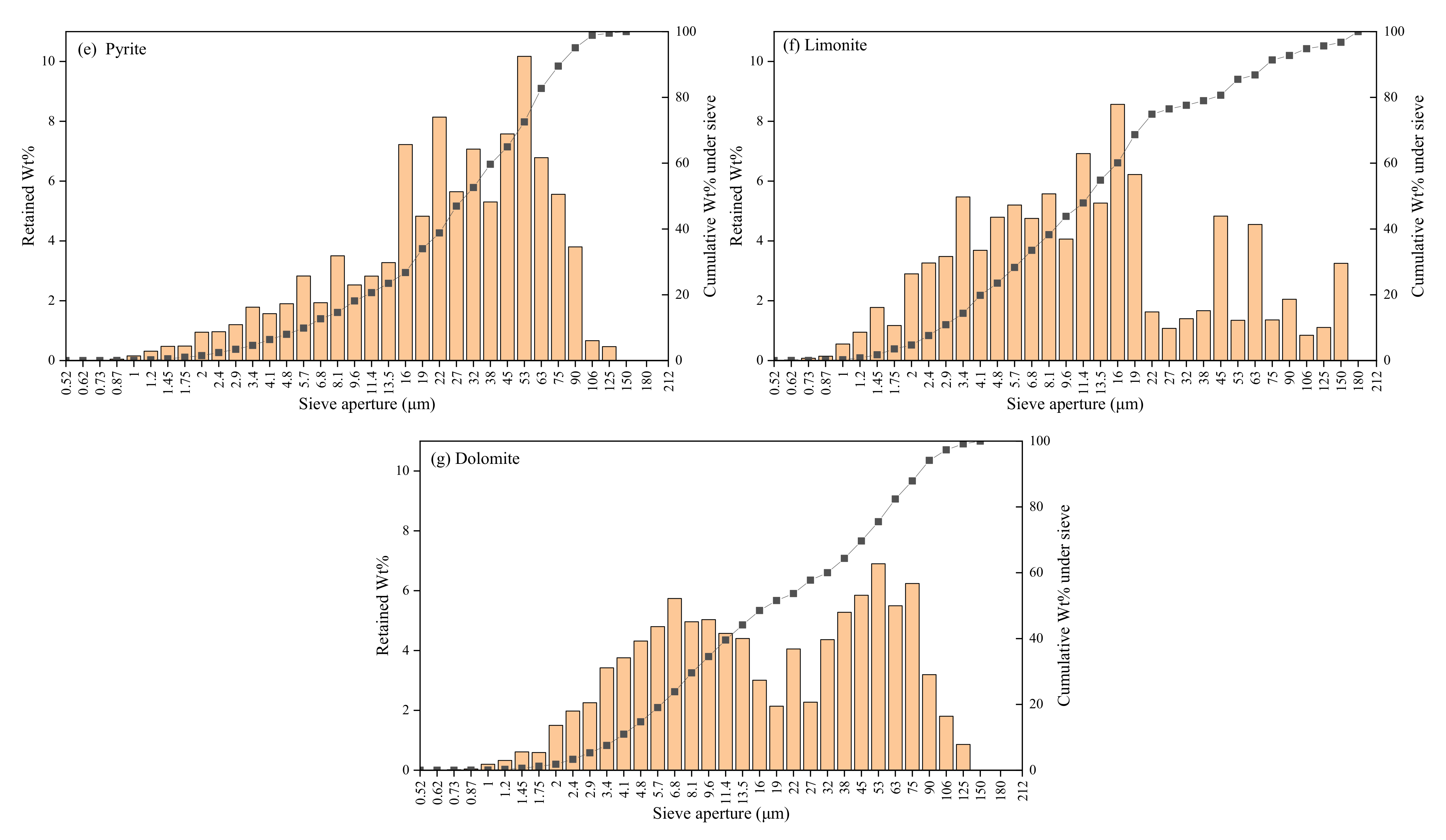
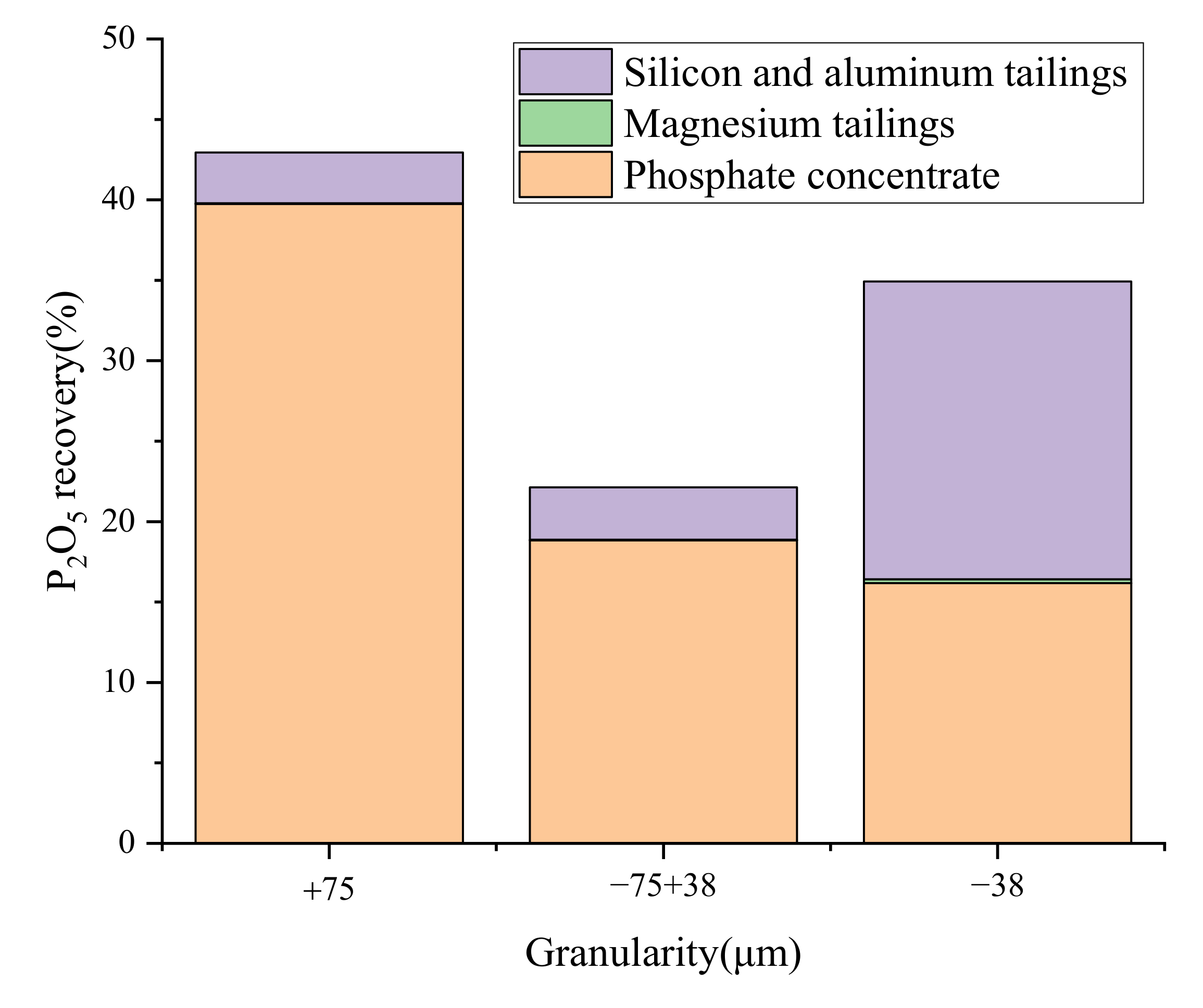
3.3. Characteristics of Disseminated Particle Size of Main Minerals
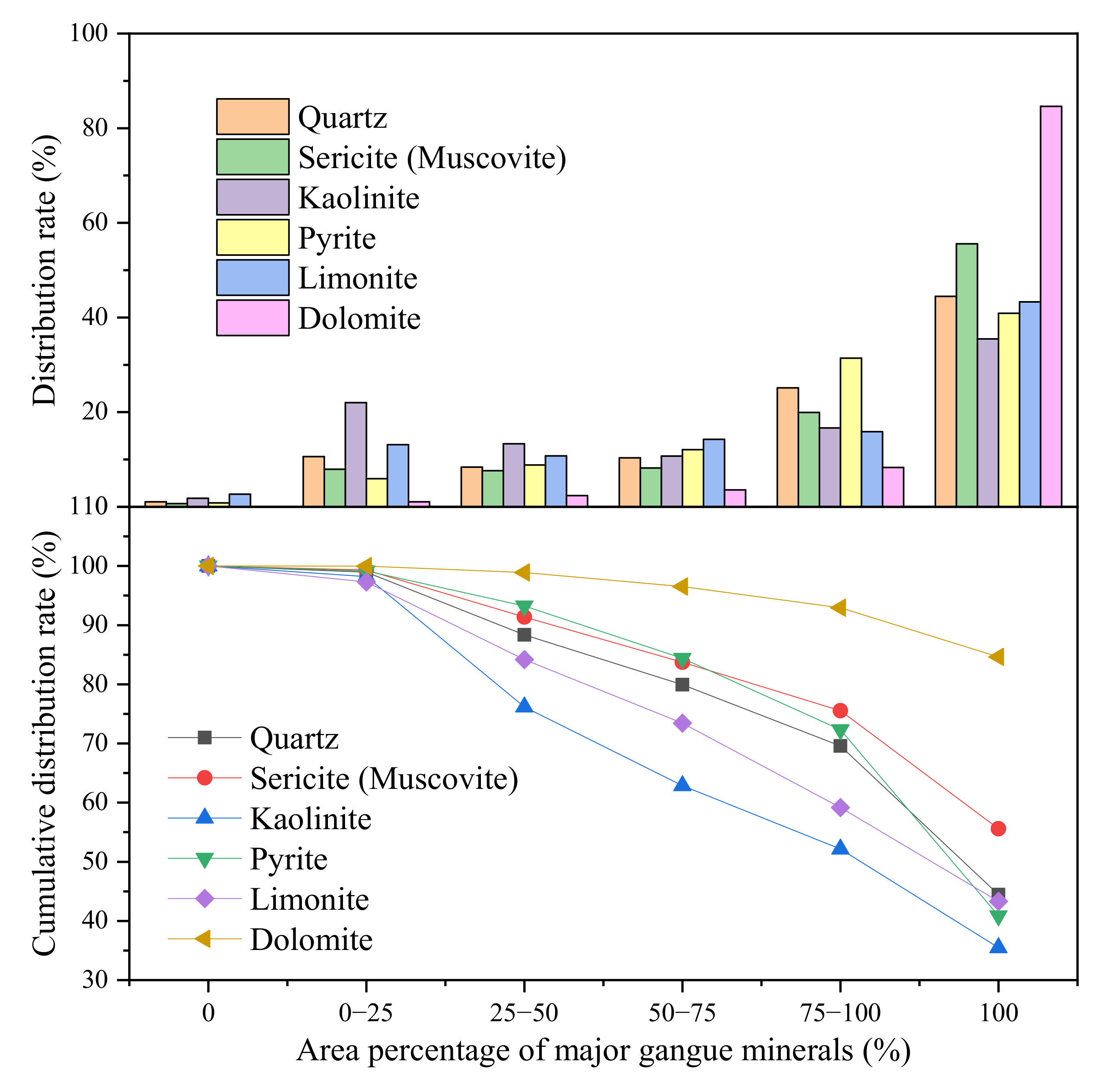
3.4. Liberation Degree and Intergrowth Characteristics of Main Gangue Minerals
3.5. Application in Flotation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cui, R.G.; Zhang, Y.F.; Guo, J.; Guo, Z.H.; Xiao, Y.P. Development strategy of phosphate rock in China under global allocation of resources. Eng. Sci. 2019, 21, 128–132. [Google Scholar] [CrossRef]
- Liu, X.; Li, C.X.; Luo, H.H.; Cheng, R.J.; Liu, F.Y. Selective reverse flotation of apatite from dolomite in collophanite ore using saponified gutter oil fatty acid as a collector. Int. J. Miner. Process. 2017, 165, 20–27. [Google Scholar] [CrossRef]
- Yang, H.Y.; Xiao, J.F.; Xia, Y.; Xie, Z.J.; Tan, Q.P.; Xu, J.B.; Guo, H.Y.; He, S.; Wu, S.W. Origin of the Ediacaran Weng’an and Kaiyang phosphorite deposits in the Nanhua basin, SW China. J. Asian Earth Sci. 2019, 182, 103931. [Google Scholar] [CrossRef]
- Wang, L.S.; Long, Z.Q.; Huang, X.W.; Yu, Y.; Cui, D.L.; Zhang, G.C. Recovery of rare earths from wet-process phosphoric acid. Hydrometallurgy 2010, 101, 41–47. [Google Scholar] [CrossRef]
- Abouzeid, A.Z.M. Physical and thermal treatment of phosphate ores—An overview. Int. J. Miner. Process. 2008, 85, 59–84. [Google Scholar] [CrossRef]
- Ruan, Y.Y.; He, D.S.; Chi, R.A. Review on Beneficiation Techniques and Reagents Used for Phosphate Ores. Minerals 2019, 9, 253. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Gao, H.; Luo, Y.J.; Gao, J. Status, trends and suggestions of phosphorus ore resources at home and abroad. China Min. Mag. 2015, 24, 6–10. [Google Scholar]
- Fang, F.Y.; Wang, J.M. The mineralogy characteristics of overflow product from hydrocyclone in the Yunnan phosphorite mine. Value Eng. 2019, 38, 162–166. [Google Scholar]
- Li, H.Q.; Zhang, W.; Zheng, H.F.; Ge, W.; Weng, X.Q.; Xiao, L.B.; Jiang, X.H. Process mineralogy study of phosphate ore in Dayukou area. Ind. Miner. Process. 2019, 48, 43–45. [Google Scholar]
- Yang, W.Q.; Fang, S.X.; Pang, J.T.; He, H.T.; Zhang, Z.Q. Determination of collophane monomer dissociation degree under different grinding fineness and its use in flotation. J. Wuhan Inst. Technol. 2014, 36, 31–34. [Google Scholar] [CrossRef]
- Han, M. Analysis of application of technological mineralogy in mineral processing. World Nonferrous Met. 2018, 13, 242–243. [Google Scholar] [CrossRef]
- Gu, Y. Automated scanning electron microscope based mineral liberation analysis an introduction to JKMRC/FEI mineral liberation analyser. J. Miner. Mater. Charact. Eng. 2003, 2, 33–41. [Google Scholar] [CrossRef]
- Zhou, L.G. Process Mineralogy, 3rd ed.; Metallurgical Industry Press: Beijing, China, 2007; pp. 1–7. [Google Scholar]
- Deng, J.; Zhang, K.C.; He, D.S.; Zhao, H.Q.; Hakkou, R.; Benzaazoua, M. Occurrence of sesquioxide in a mid-low grade collophane-sedimentary apatite ore from Guizhou, China. Minerals 2020, 10, 1038. [Google Scholar] [CrossRef]
- Leißner, T.; Hoang, D.H.; Rudolph, M.; Heinig, T.; Bachmann, K.; Gutzmer, J.; Schuberta, H.; Peuker, U.A. A mineral liberation study of grain boundary fracture based on measurements of the surface exposure after milling. Int. J. Miner. Process. 2016, 156, 3–13. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Q.; Zhao, W.Q.; Zhang, W.S.; Chen, K.X. Research progress of iron and aluminum removal from phosphate rock. Min. Metall. Eng. 2012, 32, 357–361. [Google Scholar] [CrossRef]
- Zhang, Q.; He, F.Y.; Mao, S.; Liu, J.; Chen, W.H.; Ye, J.J.; Liu, B.; Huang, X.F. Dissemination characteristics and grinding fineness of collophanite and dolomite. Ind. Miner. Process. 2010, 39, 8–10. [Google Scholar] [CrossRef]
- Wu, Z.X.; Tao, D.P. Mineralogical analysis of collophane in Yunnan using AMICS and exploration of difficult flotation mechanisms. Chin. J. Eng. 2021, 43, 503–511. [Google Scholar] [CrossRef]
- Hoang, D.H.; Kupka, N.; Peuker, U.A.; Rudolph, M. Flotation study of fine grained carbonaceous sedimentary apatite ore -Challenges in process mineralogy and impact of hydrodynamics. Miner. Eng. 2018, 121, 196–204. [Google Scholar] [CrossRef]
- Leistner, T.; Peuker, U.A.; Rudolph, M. How gangue particle size can affect the recovery of ultrafine and fine particles during froth flotation. Miner. Eng. 2017, 109, 1–9. [Google Scholar] [CrossRef]
- Yu, Y.X.; Ma, L.Q.; Zhang, Z.L.; Wang, L.Y.; Yao, L.Y. Mechanism of entrainment and slime coating on coal flotation. J. China Coal Soc. 2015, 40, 652–657. [Google Scholar] [CrossRef]
- Yao, J.; Xue, J.W.; Li, D.; Fu, Y.F.; Gong, E.P.; Yin, W.Z. Effects of fine-coarse particles interaction on flotation separation and interaction energy calculation. Part Sci. Technol. 2018, 36, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Yin, W.Z.; Li, D.; Luo, X.M.; Yao, J.; Sun, Q.Y. Effect and mechanism of siderite on reverse flotation of hematite. Int. J. Miner. Metall. Mater. 2016, 23, 373–379. [Google Scholar] [CrossRef]
- Hoang, D.H.; Hassanzadeh, A.; Peuker, U.A.; Rudolph, M. Impact of flotation hydrodynamics on the optimization of fine-grained carbonaceous sedimentary apatite ore beneficiation. Powder Technol. 2019, 345, 223–233. [Google Scholar] [CrossRef]
- Song, Z.X.; Han, J.K.; Wang, W.Z.; Zhang, R.H.; Li, X. Development and application status of flotation column technology. Met. Mine 2019, 6, 20–26. [Google Scholar] [CrossRef]
- Fan, M.M.; Tao, D.; Honaker, R.; Luo, Z.F. Nanobubble generation and its application in froth flotation (part II): Fundamental study and theoretical analysis. Min. Sci. Technol. 2010, 20, 159–177. [Google Scholar] [CrossRef]



| Element | P2O5 | CaO | MgO | SiO2 | Al2O3 | Fe2O3 | F | SO3 |
|---|---|---|---|---|---|---|---|---|
| Content (wt. %) | 29.40 | 42.05 | 1.47 | 13.37 | 3.73 | 1.18 | 2.90 | 2.16 |
| Element | K2O | Na2O | BaO | ZrO2 | SrO | MnO | LOI 1000 | |
| Content (wt. %) | 0.95 | 0.25 | 0.23 | 0.03 | 0.15 | 0.05 | 4.46 |
| Mineral | Fluorapatite | Quartz | Sericite (Muscovite) | Dolomite | Pyrite | Kaolinite | Limonite | Barite |
|---|---|---|---|---|---|---|---|---|
| Content (wt. %) | 79.76 | 7.17 | 5.18 | 4.02 | 1.53 | 1.04 | 0.48 | 0.29 |
| Mineral | Rutile | Goyazite | Calcite | Ankerite | Chlorite | Zircon | Other | |
| Content (wt. %) | 0.12 | 0.06 | 0.05 | 0.04 | 0.02 | 0.01 | 0.23 |
| Grain Size (Mesh) | Yield (wt. %) | Grade (wt. %) | Distribution Rate (wt. %) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Individual | Accumulation on Sieve | Accumulation under Sieve | P2O5 | MgO | SiO2 | Al2O3 | Fe2O3 | P2O5 | MgO | SiO2 | Al2O3 | Fe2O3 | |
| +200 | 36.93 | 36.93 | 100.00 | 33.90 | 0.90 | 8.45 | 1.93 | 0.96 | 42.91 | 22.23 | 23.30 | 19.14 | 22.93 |
| 200–400 | 21.88 | 58.81 | 63.07 | 30.94 | 1.40 | 11.29 | 2.51 | 1.35 | 23.21 | 20.42 | 18.43 | 14.76 | 19.06 |
| −400 | 41.19 | 100.00 | 41.19 | 23.98 | 2.09 | 18.95 | 5.96 | 2.18 | 33.88 | 57.35 | 58.27 | 66.10 | 58.01 |
| Total | 100.00 | 29.17 | 1.50 | 13.40 | 3.72 | 1.55 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Shi, B.; Tian, Y.; Chen, Y.; Li, S.; Cheng, Q.; Mei, G. Process Mineralogy Characteristics and Flotation Application of a Refractory Collophanite from Guizhou, China. Minerals 2021, 11, 1249. https://doi.org/10.3390/min11111249
Xu W, Shi B, Tian Y, Chen Y, Li S, Cheng Q, Mei G. Process Mineralogy Characteristics and Flotation Application of a Refractory Collophanite from Guizhou, China. Minerals. 2021; 11(11):1249. https://doi.org/10.3390/min11111249
Chicago/Turabian StyleXu, Wei, Bo Shi, Yan Tian, Yue Chen, Songqing Li, Qian Cheng, and Guangjun Mei. 2021. "Process Mineralogy Characteristics and Flotation Application of a Refractory Collophanite from Guizhou, China" Minerals 11, no. 11: 1249. https://doi.org/10.3390/min11111249
APA StyleXu, W., Shi, B., Tian, Y., Chen, Y., Li, S., Cheng, Q., & Mei, G. (2021). Process Mineralogy Characteristics and Flotation Application of a Refractory Collophanite from Guizhou, China. Minerals, 11(11), 1249. https://doi.org/10.3390/min11111249





