The Role of Calcite Dissolution and Halite Thermal Expansion as Secondary Salt Weathering Mechanisms of Calcite-Bearing Rocks in Marine Environments
Abstract
:1. Introduction
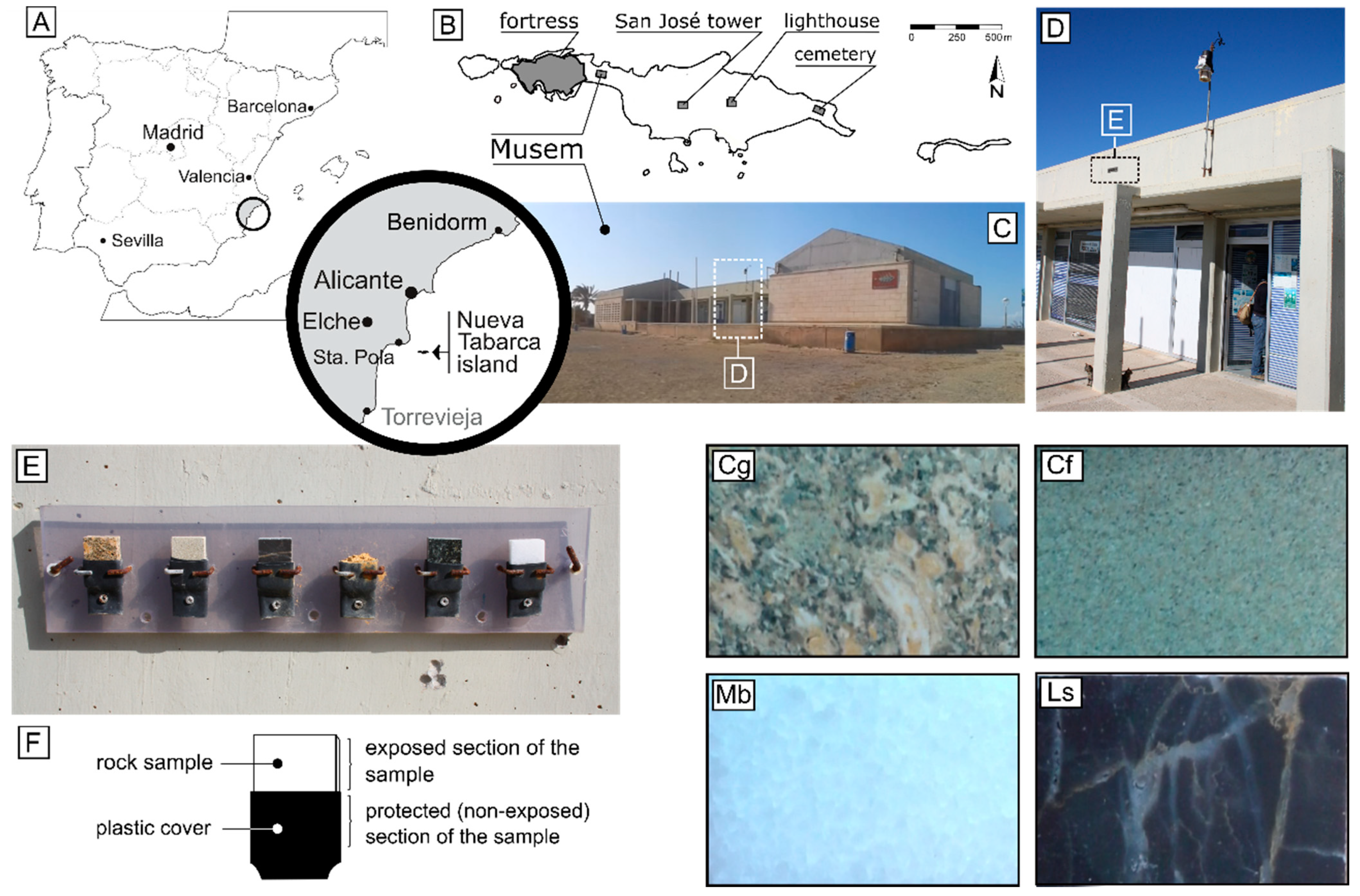
2. Materials
3. Methodology
3.1. In Situ Rock Weathering Monitoring
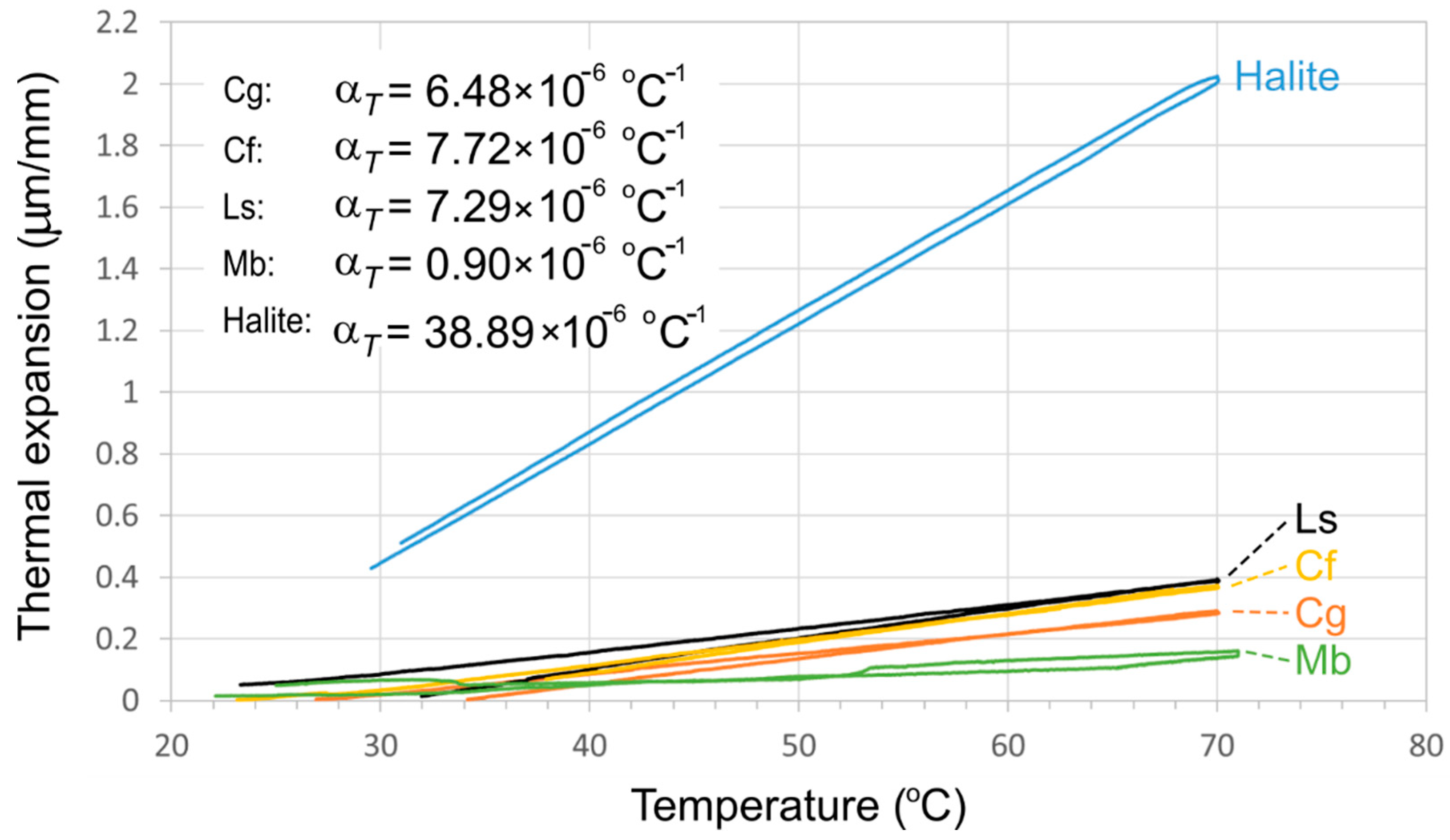
3.2. Laboratory Simulation: Differential Thermal Expansion
3.3. Laboratory Simulation: Chemical Dissolution
4. Results and Discussion
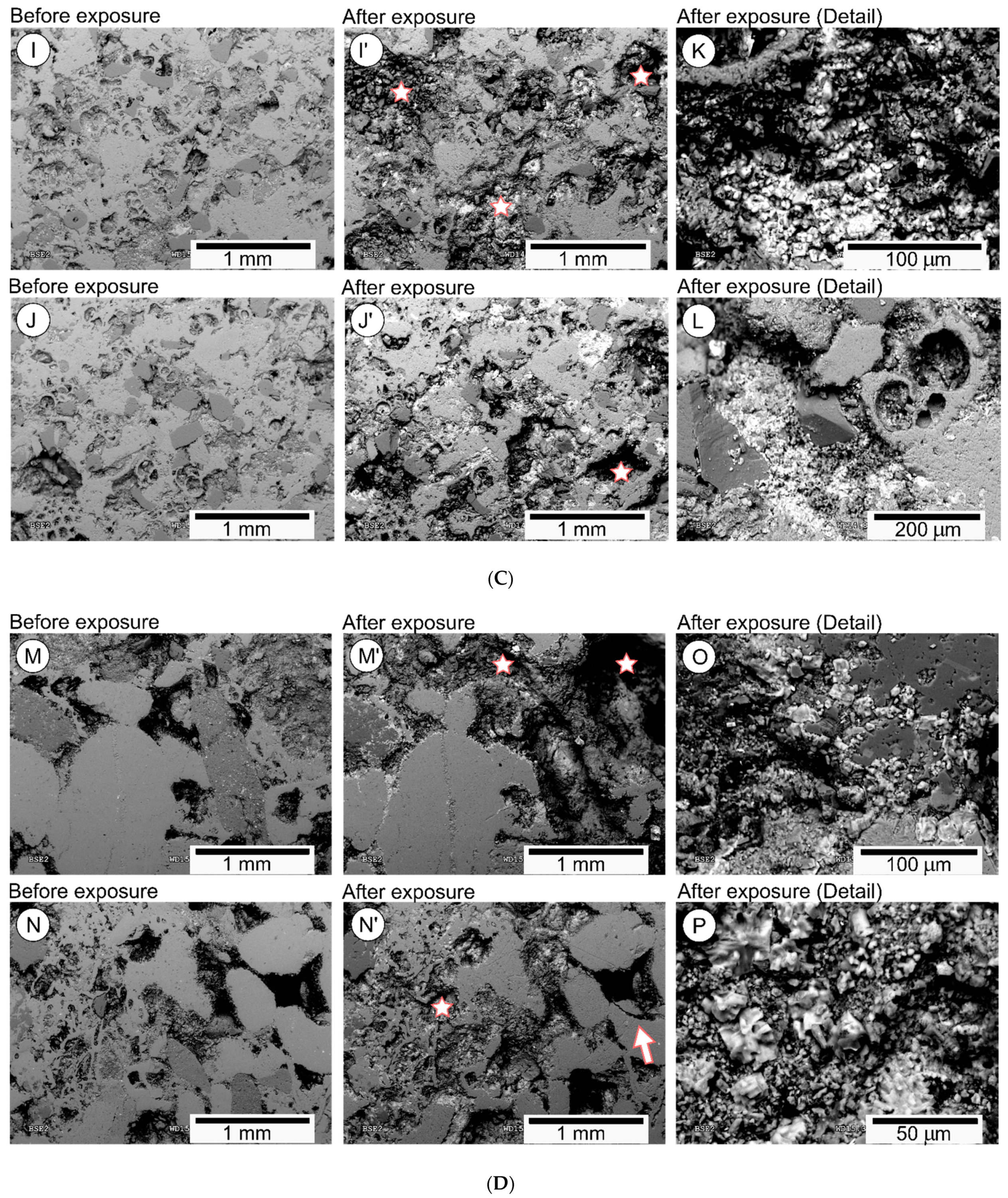
4.1. Rock Textural Changes after Direct Exposure to Marine Environment
4.2. Rock Weathering during Thermal Cycles
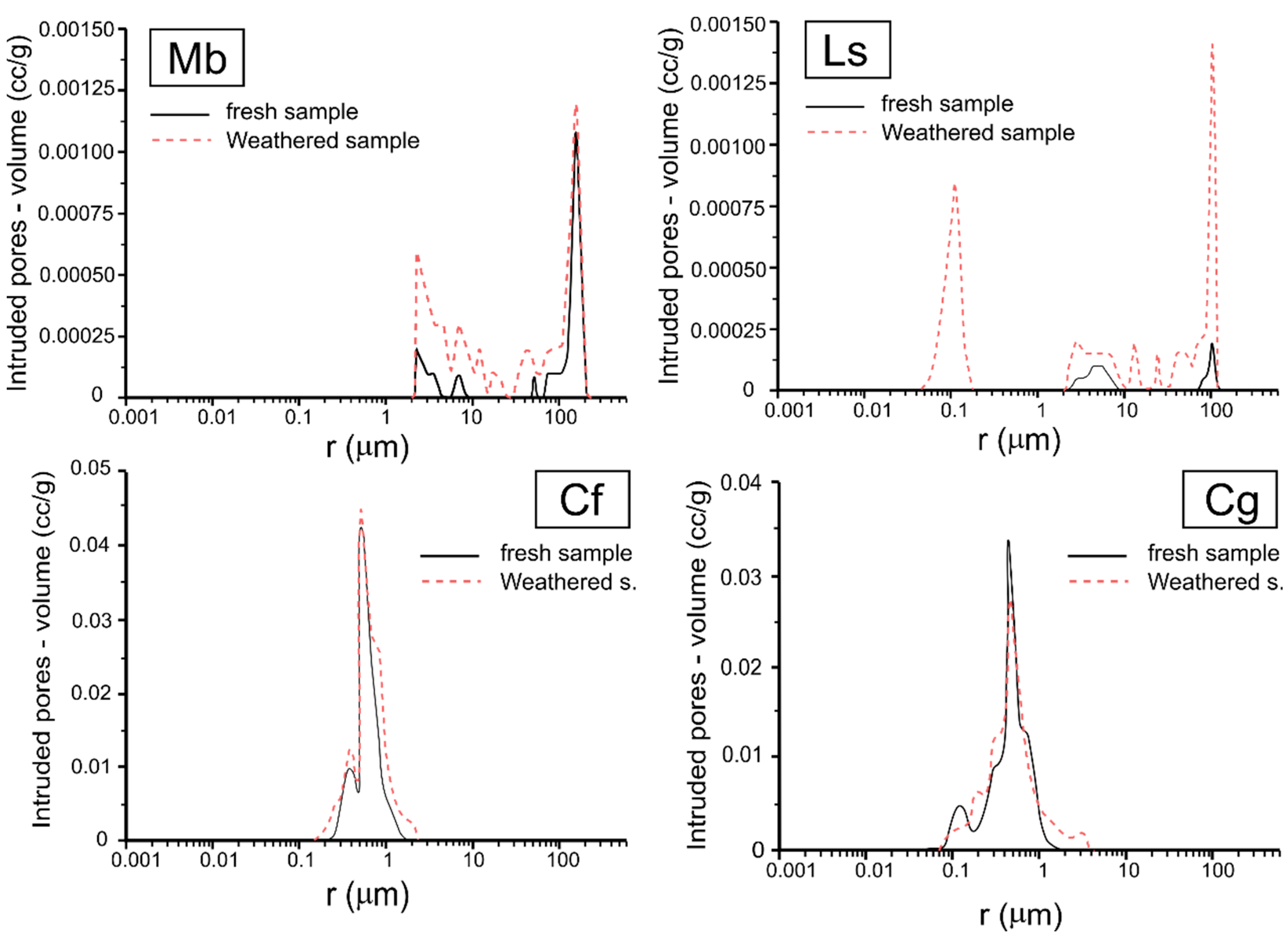
4.3. Rock Weathering during Brine Immersion Test
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cardell, C.; Delalieux, F.; Roumpopoulos, K.; Moropoulou, A.; Auger, F.; Van Grieken, R. Salt-induced decay in calcareous stone monuments and buildings in a marine environment in SW France. Constr. Build. Mater. 2003, 17, 165–179. [Google Scholar] [CrossRef]
- Andriani, G.F.; Walsh, N. The effects of wetting and drying, and marine salt crystallization on calcarenite rocks used as building material in historic monuments. Geol. Soc. Lond. Spec. Publ. 2007, 271, 179–188. [Google Scholar] [CrossRef]
- Rothert, E.; Eggers, T.; Cassar, J.; Ruedrich, J.; Fitzner, B.; Siegesmund, S. Stone properties and weathering induced by salt crystallization of Maltese Globigerina Limestone. In Building Stone Decay: From Diagnosis to Conservation; Prikryl, R., Smith, B.J., Eds.; Geological Society: London, UK, 2015; pp. 189–198. [Google Scholar]
- Stefanis, N.A.; Theoulakis, P.; Pilinis, C. Dry deposition effect of marine aerosol to the building stone of the medieval city of Rhodes, Greece. Build. Environ. 2009, 44, 260–270. [Google Scholar] [CrossRef]
- Martínez-Martínez, J.; Benavente, D.; Jiménez-Gutiérrez, S.; García-del-Cura, M.A.; Ordóñez, S. Stone weathering under Mediterranean semiarid climate in the fortress of Nueva Tabarca island (Spain). Build. Environ. 2017, 121, 262–276. [Google Scholar] [CrossRef] [Green Version]
- Colston, B.J.; Watt, D.S.; Munro, H.L. Environmentally-induced stone decay: The cumulative effects of crystallization–hydration cycles on a Lincolnshire oopelsparite limestone. J. Cult. Herit. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Grossi, C.M.; Brimblecombe, P.; Menéndez, B.; Benavente, D.; Harris, I.; Déqué, M. Climatology of salt transitions and implications for stone weathering. Sci. Total. Environ. 2011, 409, 2577–2585. [Google Scholar] [CrossRef]
- Goudie, A.S.; Viles, H.A. Salt Weathering Hazards; John Wiley: Chichester, UK, 1997; p. 241. [Google Scholar]
- Rodriguez-Navarro, C.; Doehne, E.; Sebastián, E. Origins of honeycomb weathering: The role of salts and wind. GSA Bull. 1999, 111, 1250–1255. [Google Scholar] [CrossRef]
- Doehne, E. Salt weathering: A selective review. In Natural Stone, Weathering Phenomena, Conservation Strategies and Case Studies; Siegesmund, S., Weisss, T., Vollbrecht, A., Eds.; Geological Society Special Publication: London, UK, 2002; p. 256. [Google Scholar]
- Scherer, G.W.; Flatt, R.; Wheeler, G. Materials science research for the conservation of sculpture and monuments. MRS Bull. 2001, 26, 44–50. [Google Scholar] [CrossRef]
- Benavente, D.; Sanchez-Moral, S.; Fernández-Cortes, A.; Canaveras, J.C.; Elez, J.; Saiz-Jimenez, C. Salt damage and microclimate in the Postumius Tomb, Roman Necropolis of Carmona, Spain. Environ. Earth Sci. 2011, 63, 1529–1543. [Google Scholar] [CrossRef] [Green Version]
- Benavente, D.; Brimblecombe, P.; Grossi, C.M. Termodynamic calculations for the salt crystallisation damage in porous built heritage using PHREEQC. Environ. Earth Sci. 2015, 74, 2297–2313. [Google Scholar] [CrossRef]
- Liang, Y.; Baer, D.R. Anisotropic dissolution at the CaCO3 {104}–water interface. Surf. Sci. 1997, 373, 275–287. [Google Scholar] [CrossRef]
- Benavente, D.; Martínez-Verdú, F.; Bernabéu, A.; Viqueira, V.; Fort, R.; García del Cura, M.A.; Illueca, C.; Ordóñez, S. Influence of Surface roughness on color changes in builing stones. Color Res. Appl. 2002, 28, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Martínez, J.; Benavente, D.; Pérez-Huerta, A.; Cueto, N.; García-del-Cura, M.A. Changes on the Surface properties of foliated marbles at different cutting orientations. Constr. Build. Mater. 2019, 222, 493–499. [Google Scholar] [CrossRef]
- Liu, A.; Yuan, D.; Dreybrodt, W. Comparative study of dissolution rate-determining mechanisms of limestone and dolomite. Environ. Geol. 2005, 49, 274–279. [Google Scholar] [CrossRef]
- Luttge, A.; Winkler, U.; Lasaga, A.C. An interferometric study of the dolomite dissolution: A new conceptual model for mineral dissolution. Geochim. Cosmochim. Acta 2003, 67, 1099–1116. [Google Scholar] [CrossRef]
- Viles, H.A. Can Stone decay be chaotic? In Stone Decay in the Architectural Environment; Turkington, A.V., Ed.; Geological Society of America, Special Publication: Boulder, CO, USA, 2005; p. 390. [Google Scholar]
- Smith, B.J.; Gomez-Heras, M.; McCabe, S. Understanding the decay of stone-built cultural heritage. Prog. Phys. Geog. 2008, 32, 361–439. [Google Scholar] [CrossRef]
- Smith, B.J.; Gomez-Heras, M.; Viles, H.A. Underlying Issues on the Selection Use and Conservation of Building Limestone; Geological Society, Special Publication: London, UK, 2010; p. 331. [Google Scholar]
- Martínez-Martínez, J.; Corbí, H.; Martín-Rojas, I.; Baeza-Carratalá, J.F.; Giannetti, A. Stratigraphy, petrophysical characterization and 3D geological modelling of the historical quarry of Nueva Tabarca island (western Mediterranean): Implications on heritage conservation. Eng. Geol. 2017, 231, 88–99. [Google Scholar] [CrossRef] [Green Version]
- Benavente, D.; Cueto, N.; Martínez-Martínez, J.; García-del-Cura, M.A.; Cañaveras, J.C. The influence of petrophysical properties on the salt weathering of porous building rocks. Environ. Earth Sci. 2007, 52, 215–224. [Google Scholar] [CrossRef]
- Millero, F.J.; Milne, P.J.; Thurmond, V.L. The solubility of calcite, strontianite and witherite in NaCl solutions at 25 °C. Geochim. Cosmochim. Acta 1984, 48, 1141–1143. [Google Scholar] [CrossRef]
- Sanchez-Moral, S.; Soler, V.; Canaveras, J.C.; Sanz-Rubio, E.; Van Grieken, R.; Gysels, K. Inorganic deterioration affecting the Altamira Cave, N Spain: Quantitative approach to wall-corrosion (solutional etching) processes induced by visitors. Sci. Total. Environ. 1999, 244, 67–84. [Google Scholar] [CrossRef]
- Arvidson, R.S.; Ertan, I.E.; Amonette, J.E.; Luttge, A. Variation in calcite dissolution rates: A fundamental problem? Geochim. Cosmochim. Acta 2003, 67, 1623–1634. [Google Scholar] [CrossRef]
- Angeli, M.; Benavente, D.; Bigas, J.P.; Menéndez, B.; Hébert, R.; David, C. Modification of the porous network by salt crystallization in experimentally weathered sedimentary stones. Mater. Struct. 2008, 41, 1091–1108. [Google Scholar] [CrossRef] [Green Version]



| Month | Temperature (°C) | RH (%) | Rainfall (mm) | ||||
|---|---|---|---|---|---|---|---|
| Mean | Min | Max | Mean | Min | Max | ||
| January | 12.5 | 4.3 | 20.2 | 78.0 | 36.0 | 96.8 | 45.8 |
| February | 12.6 | 5.5 | 21.1 | 74.9 | 32.1 | 94.8 | 67.5 |
| March | 13.0 | 6.0 | 21.1 | 78.9 | 34.7 | 97.3 | 74.6 |
| April | 16.9 | 13.2 | 21.2 | 74.0 | 37.5 | 94.4 | 0.0 |
| May | 19.3 | 13.7 | 23.2 | 81.9 | 41.2 | 95.6 | 3.2 |
| Jun | 23.2 | 18.3 | 29.8 | 80.1 | 36.2 | 94.1 | 0.0 |
| July | 26.1 | 22.8 | 31.6 | 81.9 | 48.3 | 93.8 | 0.2 |
| August | 26.8 | 23.4 | 30.2 | 78.3 | 50.0 | 90.1 | 4.2 |
| September | 25.0 | 22.2 | 31.1 | 71.5 | 38.4 | 86.5 | 0.0 |
| October | 21.0 | 16.8 | 24.9 | 72.9 | 38.3 | 92.7 | 4.6 |
| November | 17.9 | 10.3 | 26.6 | 70.5 | 32.5 | 92.1 | 18.2 |
| December | 12.3 | 3.6 | 19.3 | 75.0 | 34.6 | 94.2 | 11.0 |
| Sample | Initial State | After Salt Saturation | After Cabinet Test | |||
|---|---|---|---|---|---|---|
| With Salts | After Salt Removing | |||||
| Ls | Weight (g) | A | 34.1 | 34.66 | 34.66 | 34.11 |
| B | 34.46 | 34.98 | 34.98 | 34.48 | ||
| C | 24.86 | 25.29 | 25.28 | 24.85 | ||
| Mean Weight increment (%) | 1.69 | 1.61 | 0.02 | |||
| Cf | Weight (g) | A | 38.91 | 41.88 | 41.01 | 38.13 |
| B | 44.85 | 47.68 | 46.55 | 43.97 | ||
| C | 42.69 | 45.55 | 44.6 | 41.89 | ||
| Mean Weight increment (%) | 6.88 | 4.55 | −1.95 | |||
| Cg | Weight (g) | A | 54.03 | 58.98 | 57.84 | 53.15 |
| B | 49.66 | 54.5 | 53.58 | 49.02 | ||
| C | 50.07 | 54.89 | 53.78 | 49.2 | ||
| Mean Weight increment (%) | 9.51 | 7.45 | −1.55 | |||
| Mb | Weight (g) | A | 48.96 | 48.98 | 48.98 | 48.97 |
| B | 47.51 | 47.5 | 47.5 | 47.5 | ||
| C | 52.81 | 52.84 | 52.84 | 52.82 | ||
| Mean Weight increment (%) | 0.02 | 002 | 0.01 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Martínez, J.; Arizzi, A.; Benavente, D. The Role of Calcite Dissolution and Halite Thermal Expansion as Secondary Salt Weathering Mechanisms of Calcite-Bearing Rocks in Marine Environments. Minerals 2021, 11, 911. https://doi.org/10.3390/min11080911
Martínez-Martínez J, Arizzi A, Benavente D. The Role of Calcite Dissolution and Halite Thermal Expansion as Secondary Salt Weathering Mechanisms of Calcite-Bearing Rocks in Marine Environments. Minerals. 2021; 11(8):911. https://doi.org/10.3390/min11080911
Chicago/Turabian StyleMartínez-Martínez, Javier, Anna Arizzi, and David Benavente. 2021. "The Role of Calcite Dissolution and Halite Thermal Expansion as Secondary Salt Weathering Mechanisms of Calcite-Bearing Rocks in Marine Environments" Minerals 11, no. 8: 911. https://doi.org/10.3390/min11080911
APA StyleMartínez-Martínez, J., Arizzi, A., & Benavente, D. (2021). The Role of Calcite Dissolution and Halite Thermal Expansion as Secondary Salt Weathering Mechanisms of Calcite-Bearing Rocks in Marine Environments. Minerals, 11(8), 911. https://doi.org/10.3390/min11080911








