Modified Montmorillonite as Drug Delivery Agent for Enhancing Antibiotic Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Organo-Montmorillonite Preparation
2.3. MNE/OMt Preparation
2.4. Chromatographic Measurements
2.5. Adsorption Isotherms
2.6. Kinetic of MNE Release
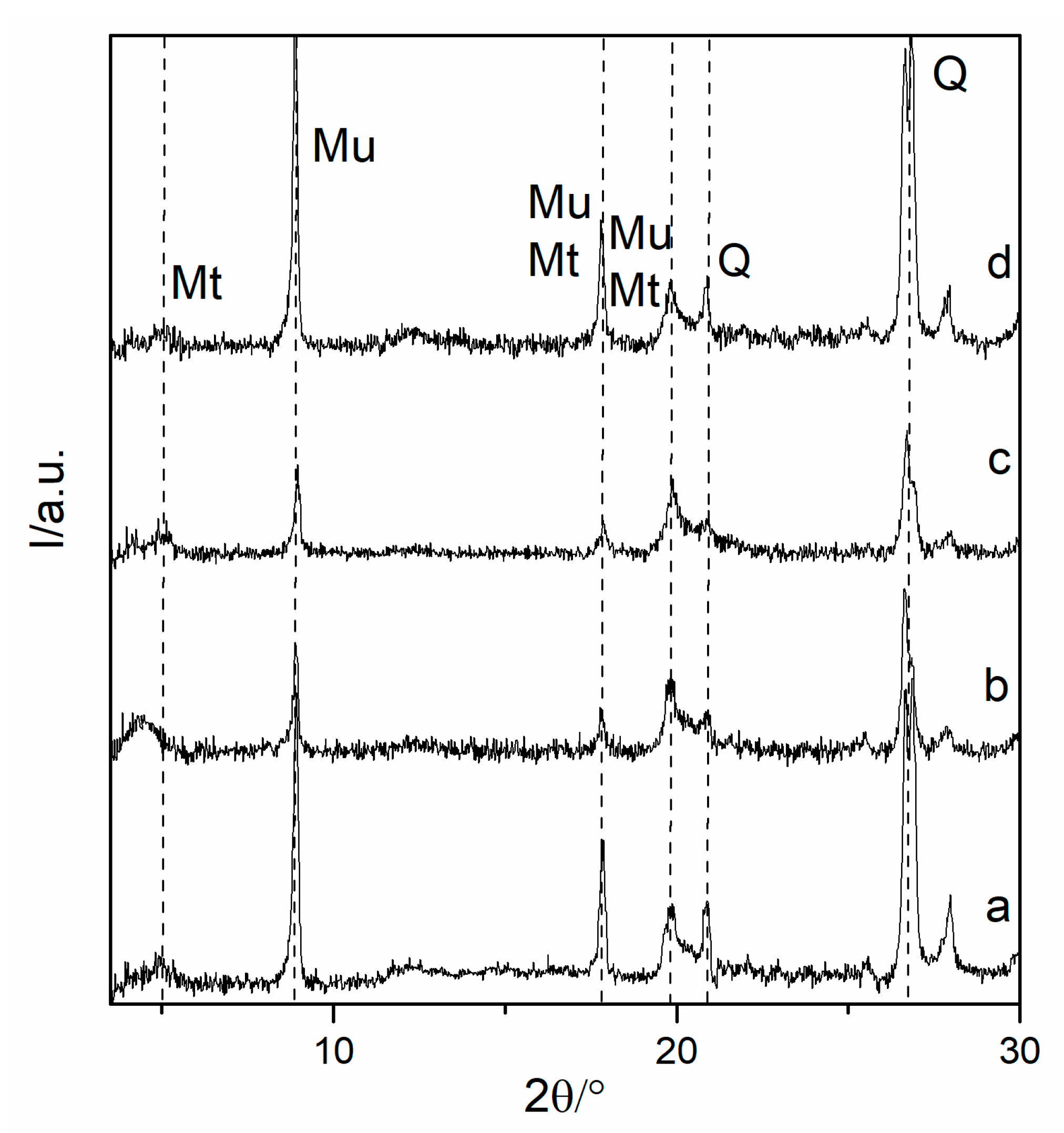
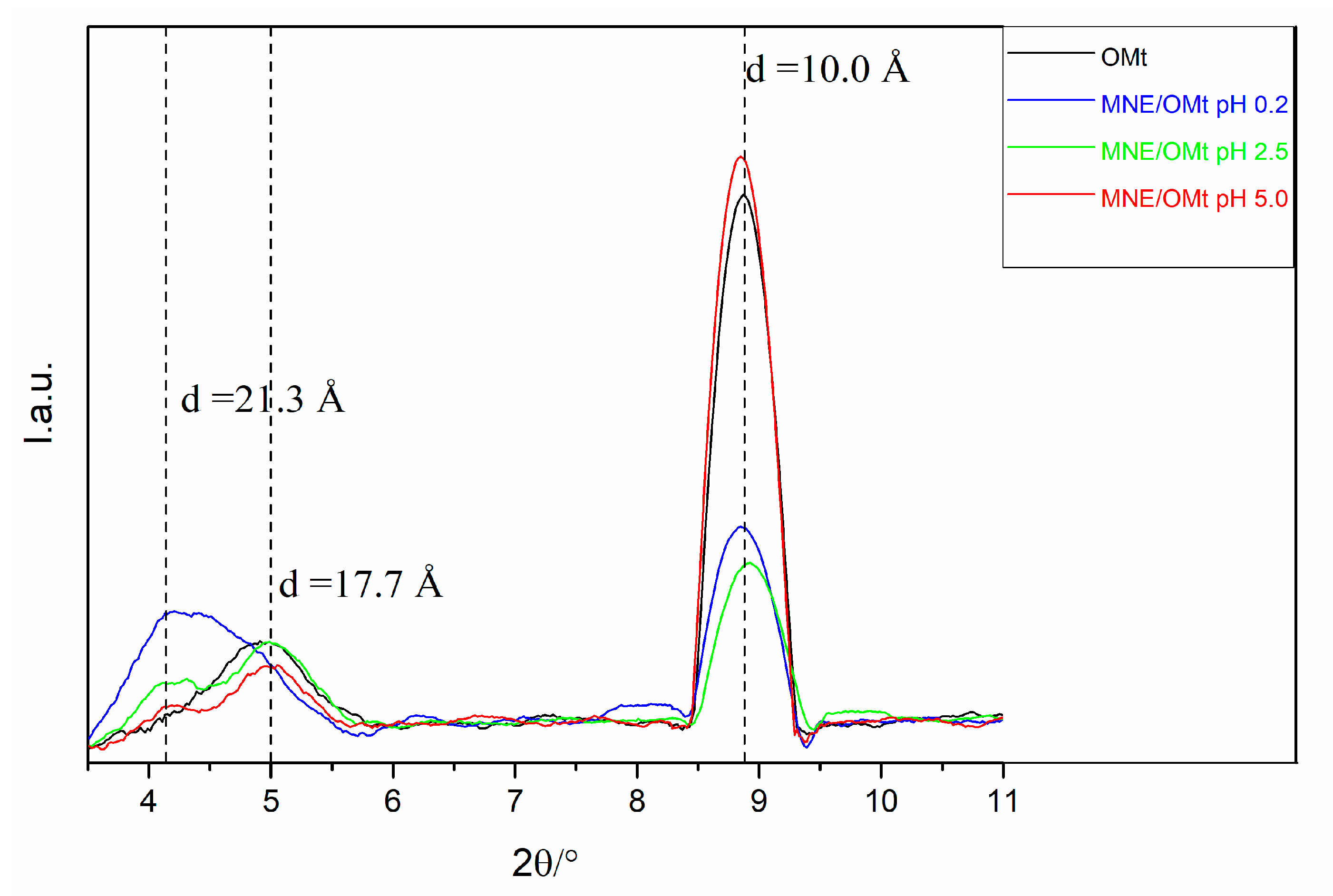
2.7. XRD Characterization
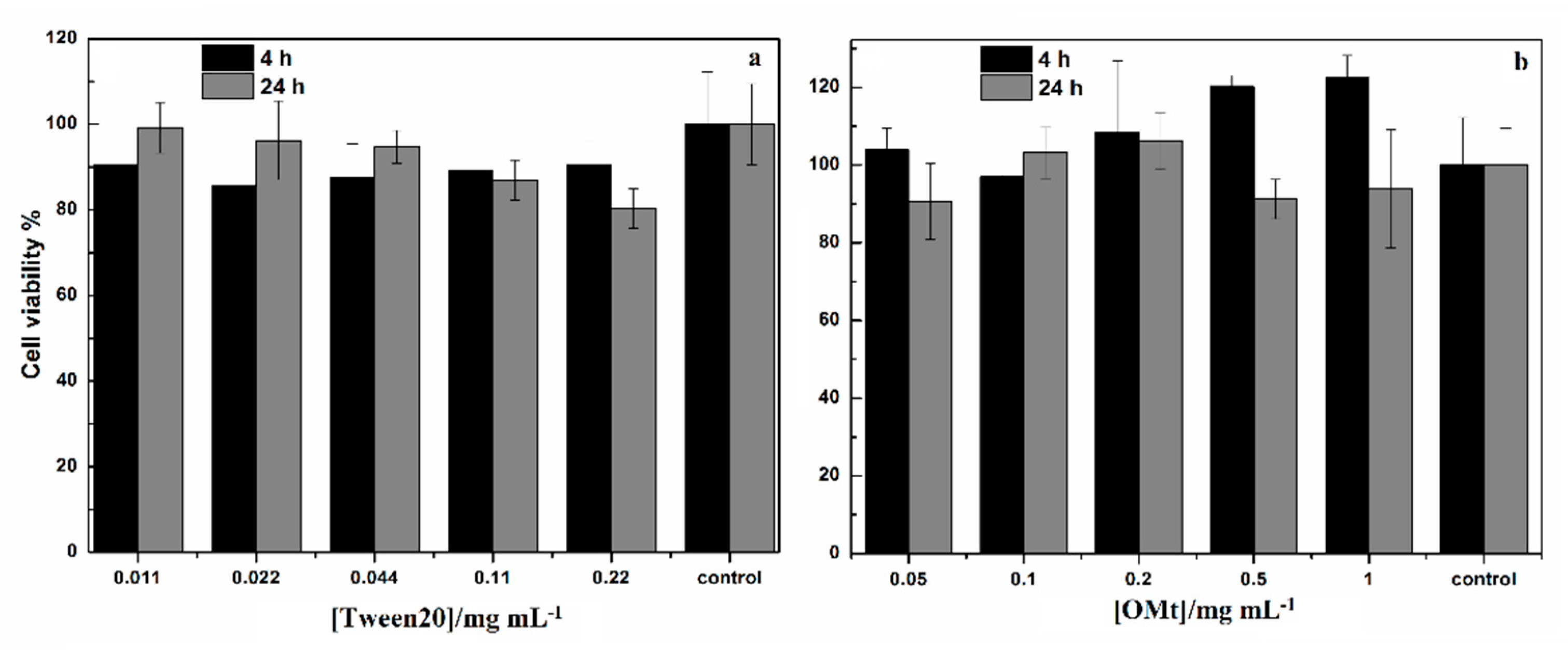
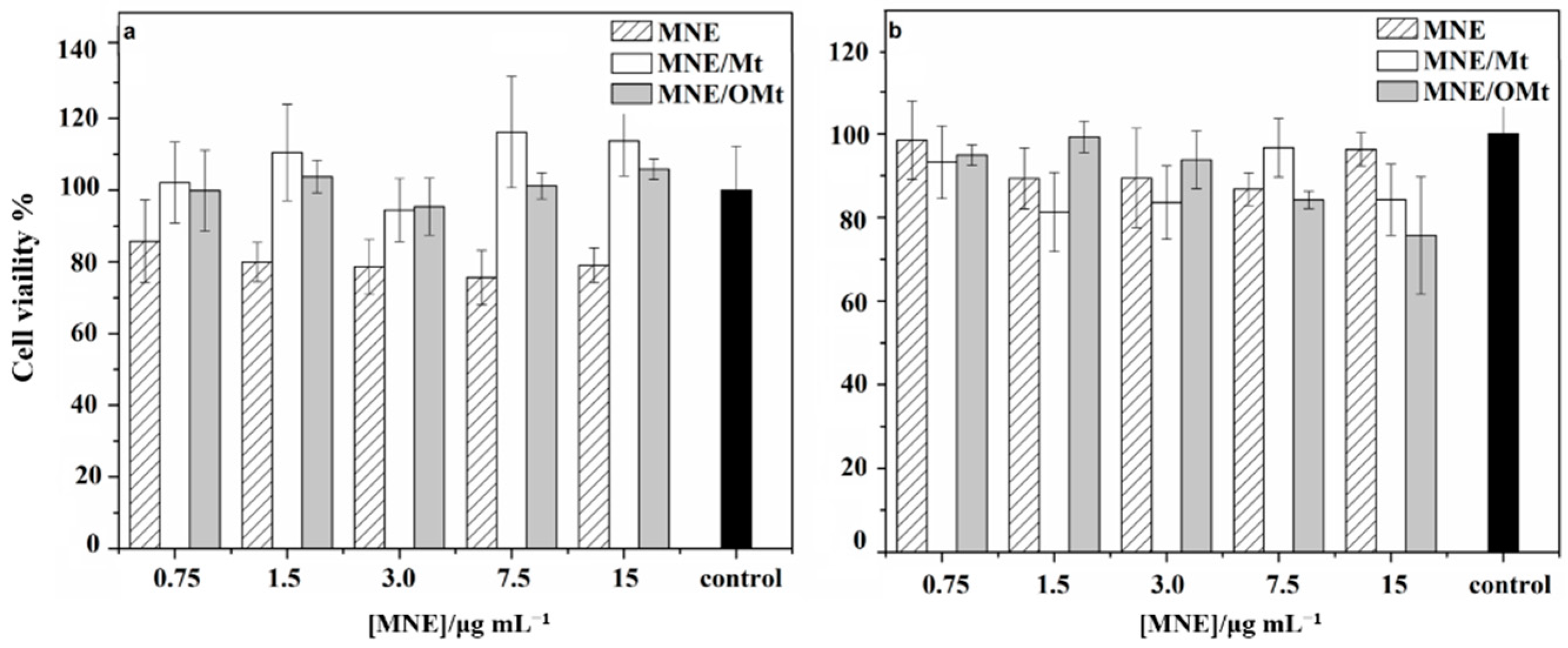
2.8. Cell Viability Assay on Human Colon Cancer (HCT116) Cells
3. Results
3.1. Adsorption Isotherms
3.2. Analysis of XRD Data
3.3. MNE Release Profiles from Organo-Clay Hybrids
3.4. Cell Viability Assay on Human Colon Cancer (HCT116) Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nikolaidis, A.K.; Koulaouzidou, E.A.; Achilias, D.S. Synthesis and Characterization of Novel Organomodified Nanoclays for Application in Dental Materials. Curr. Nanosci. 2019, 15, 512–524. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F.; Sciascia, L. Clay/Non-Ionic Surfactant Hybrid Nanocomposites. In Functionalized Nanomaterials I; Kumar, V., Guleria, P., Dasgupta, N., Ranjan, S., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 269–278. ISBN 9781351021623. [Google Scholar]
- Lobato-Aguilar, H.; Uribe-Calderón, J.A.; Herrera-Kao, W.; Duarte-Aranda, S.; Baas-López, J.M.; Escobar-Morales, B.; Cauich-Rodríguez, J.V.; Cervantes-Uc, J.M. Synthesis, Characterization and Chlorhexidine Release from Either Montmorillonite or Palygorskite Modified Organoclays for Antibacterial Applications. J. Drug Deliv. Sci. Technol. 2018, 46, 452–460. [Google Scholar] [CrossRef]
- De Oliveira, T.; Guégan, R.; Thiebault, T.; Milbeau, C.L.; Muller, F.; Teixeira, V.; Giovanela, M.; Boussafir, M. Adsorption of Diclofenac onto Organoclays: Effects of Surfactant and Environmental (PH and Temperature) Conditions. J. Hazard. Mater. 2017, 323, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hitzky, E.; Aranda, P.; Darder, M.; Rytwo, G. Hybrid Materials Based on Clays for Environmental and Biomedical Applications. J. Mater. Chem. 2010, 20, 9306. [Google Scholar] [CrossRef]
- Calabrese, I.; Gelardi, G.; Merli, M.; Liveri, M.L.T.; Sciascia, L. Clay-Biosurfactant Materials as Functional Drug Delivery Systems: Slowing down Effect in the In Vitro Release of Cinnamic Acid. Appl. Clay Sci. 2017, 135, 567–574. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Chen, X.; Bao, C.; Yi, J.; Lei, M.; Ke, C.; Zhang, W.; Lin, Q. Synthesis and Assessment of CTAB and NPE Modified Organo-Montmorillonite for the Fabrication of Organo-Montmorillonite/Alginate Based Hydrophobic Pharmaceutical Controlled-Release Formulation. Colloids Surf. B Biointerfaces 2020, 191, 110983. [Google Scholar] [CrossRef]
- Choy, J.; Choi, S.; Oh, J.; Park, T. Clay Minerals and Layered Double Hydroxides for Novel Biological Applications. Appl. Clay Sci. 2007, 36, 122–132. [Google Scholar] [CrossRef]
- De Sousa Rodrigues, L.A.; Figueiras, A.; Veiga, F.; de Freitas, R.M.; Nunes, L.C.C.; da Silva Filho, E.C.; da Silva Leite, C.M. The Systems Containing Clays and Clay Minerals from Modified Drug Release: A Review. Colloids Surf. B Biointerfaces 2013, 103, 642–651. [Google Scholar] [CrossRef]
- Hun Kim, M.; Choi, G.; Elzatahry, A.; Vinu, A.; Bin Choy, Y.; Choy, J.-H. Review of Clay-Drug Hybrid Materials for Biomedical Applications: Administration Routes. Clays Clay Miner. 2016, 64, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Carazo, E.; Borrego-Sánchez, A.; Sánchez-Espejo, R.; García-Villén, F.; Cerezo, P.; Aguzzi, C.; Viseras, C. Kinetic and Thermodynamic Assessment on Isoniazid/Montmorillonite Adsorption. Appl. Clay Sci. 2018, 165, 82–90. [Google Scholar] [CrossRef]
- Massaro, M.; Colletti, C.G.; Lazzara, G.; Riela, S. The Use of Some Clay Minerals as Natural Resources for Drug Carrier Applications. J. Funct. Biomater. 2018, 9, 58. [Google Scholar] [CrossRef] [Green Version]
- Cavalcanti, G.R.S.; Fonseca, M.G.; da Silva Filho, E.C.; Jaber, M. Thiabendazole/Bentonites Hybrids as Controlled Release Systems. Colloids Surf. B Biointerfaces 2019, 176, 249–255. [Google Scholar] [CrossRef]
- García-Villén, F.; Faccendini, A.; Aguzzi, C.; Cerezo, P.; Bonferoni, M.C.; Rossi, S.; Grisoli, P.; Ruggeri, M.; Ferrari, F.; Sandri, G.; et al. Montmorillonite-Norfloxacin Nanocomposite Intended for Healing of Infected Wounds. Int. J. Nanomed. 2019, 14, 5051–5060. [Google Scholar] [CrossRef] [Green Version]
- Peña-Parás, L.; Sánchez-Fernández, J.A.; Vidaltamayo, R. Nanoclays for Biomedical Applications. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 3453–3471. ISBN 9783319682556. [Google Scholar]
- De Melo Barbosa, R.; Alcântara, M.; Meirelles, L.; Zorato, N.; Raffin, F. Nanoclays in drug delivery systems. In Clay Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 185–202. ISBN 9780128167830. [Google Scholar]
- García-Villén, F.; Viseras, C. Clay-Based Pharmaceutical Formulations and Drug Delivery Systems. Pharmaceutics 2020, 12, 1142. [Google Scholar] [CrossRef]
- Khatoon, N.; Chu, M.Q.; Zhou, C.H. Nanoclay-Based Drug Delivery Systems and Their Therapeutic Potentials. J. Mater. Chem. B 2020, 8, 7335–7351. [Google Scholar] [CrossRef]
- Martín, S.A.; Pérez, I.; Rivera, A. Hosting of the Antibiotic Vancomycin by Bentonite: Characterization and Slow Release Study. Appl. Clay Sci. 2021, 202, 105965. [Google Scholar] [CrossRef]
- Aguzzi, C.; Cerezo, P.; Viseras, C.; Caramella, C. Use of Clays as Drug Delivery Systems: Possibilities and Limitations. Appl. Clay Sci. 2007, 36, 22–36. [Google Scholar] [CrossRef]
- Calabrese, I.; Cavallaro, G.; Lazzara, G.; Merli, M.; Sciascia, L.; Liveri, M.L.T. Preparation and Characterization of Bio-Organoclays Using Nonionic Surfactant. Adsorption 2016, 22, 105–116. [Google Scholar] [CrossRef]
- Gu, Z.; Gao, M.; Lu, L.; Liu, Y.; Yang, S. Montmorillonite Functionalized with Zwitterionic Surfactant as a Highly Efficient Adsorbent for Herbicides. Ind. Eng. Chem. Res. 2015, 54, 4947–4955. [Google Scholar] [CrossRef]
- Lazorenko, G.; Kasprzhitskii, A.; Yavna, V. Comparative Study of the Hydrophobicity of Organo-Montmorillonite Modified with Cationic, Amphoteric and Nonionic Surfactants. Minerals 2020, 10, 732. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, S.J. Delamination Behavior of Silicate Layers by Adsorption of Cationic Surfactants. J. Colloid Interface Sci. 2002, 248, 231–238. [Google Scholar] [CrossRef]
- Martos, R.; Guggenheim, S.; Sainz-Díaz, C. Interlayer Water Molecules in Organocation-Exchanged Vermiculite and Montmorillonite: A Case Study of Tetramethylammonium. Am. Mineral. 2013, 98, 1535. [Google Scholar] [CrossRef]
- Sciascia, L.; Casella, S.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Princivalle, F.; Parisi, F. Olive Mill Wastewaters Decontamination Based on Organo-Nano-Clay Composites. Thermophys. Asp. Funct. Ceram. Surf. 2019, 45, 2751–2759. [Google Scholar] [CrossRef]
- Dening, T.J.; Rao, S.; Thomas, N.; Prestidge, C.A. Montmorillonite-Lipid Hybrid Carriers for Ionizable and Neutral Poorly Water-Soluble Drugs: Formulation, Characterization and In Vitro Lipolysis Studies. Int. J. Pharm. 2017, 526, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Jayrajsinh, S.; Shankar, G.; Agrawal, Y.K.; Bakre, L. Montmorillonite Nanoclay as a Multifaceted Drug-Delivery Carrier: A Review. J. Drug Deliv. Sci. Technol. 2017, 39, 200–209. [Google Scholar] [CrossRef]
- Nielsen, R.B.; Kahnt, A.; Dillen, L.; Wuyts, K.; Snoeys, J.; Nielsen, U.G.; Holm, R.; Nielsen, C.U. Montmorillonite-Surfactant Hybrid Particles for Modulating Intestinal P-Glycoprotein-Mediated Transport. Int. J. Pharm. 2019, 571, 118696. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Sánchez, A.; Sánchez-Espejo, R.; García-Villén, F.; Viseras, C.; Sainz-Díaz, C.I. Praziquantel–Clays as Accelerated Release Systems to Enhance the Low Solubility of the Drug. Pharmaceutics 2020, 12, 914. [Google Scholar] [CrossRef]
- Lobato-Aguilar, H.A.; Lizama-Uc, G.; Uribe-Calderon, J.A.; Cauich-Rodriguez, J.; Rodriguez-Fuentes, N.; Cervantes-Uc, J.M. Antibacterial Properties and Release Kinetics of Chlorhexidine Diacetate from Montmorillonite and Palygorskite Clays. J. Biomater. Appl. 2020, 34, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Sabzevari, A.G.; Sabahi, H.; Nikbakht, M. Montmorillonite, a Natural Biocompatible Nanosheet with Intrinsic Antitumor Activity. Colloids Surf. B Biointerfaces 2020, 190, 110884. [Google Scholar] [CrossRef]
- Kumar, A.; Ramisetty, K.A.; Bordignon, S.; Hodnett, B.K.; Davern, P.; Hudson, S. Preparation, Stabilisation, Isolation and Tableting of Valsartan Nanoparticles Using a Semi-Continuous Carrier Particle Mediated Process. Int. J. Pharm. 2021, 597, 120199. [Google Scholar] [CrossRef]
- Sreekanth Reddy, O.; Subha, M.C.S.; Jithendra, T.; Madhavi, C.; Chowdoji Rao, K. Curcumin Encapsulated Dual Cross Linked Sodium Alginate/Montmorillonite Polymeric Composite Beads for Controlled Drug Delivery. J. Pharm. Anal. 2021, 11, 191–199. [Google Scholar] [CrossRef]
- Hazen, R.M.; Sverjensky, D.A.; Azzolini, D.; Bish, D.L.; Elmore, S.C.; Hinnov, L.; Milliken, R.E. Clay Mineral Evolution. Am. Mineral. 2013, 98, 2007–2029. [Google Scholar] [CrossRef]
- Calabrese, I.; Cavallaro, G.; Scialabba, C.; Licciardi, M.; Merli, M.; Sciascia, L.; Liveri, M.L.T. Montmorillonite Nanodevices for the Colon Metronidazole Delivery. Spec. Sect. Formul. Better Med. Child. 2013, 457, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Goodman, L.S.; Brunton, L.L.; Gilman, A.; Chabner, B.; Knollmann, B.C.; Goodman, L.S. Goodman & Gilman’s The Pharmacological Basis of Therapeutics; McGraw-Hill Medical: New York, NY, USA, 2011; ISBN 9780071593236. [Google Scholar]
- Samuelson, J. Why Metronidazole Is Active against Both Bacteria and Parasites. Antimicrob. Agents Chemother. 1999, 43, 1533–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amidon, S.; Brown, J.E.; Dave, V.S. Colon-Targeted Oral Drug Delivery Systems: Design Trends and Approaches. AAPS PharmSciTech 2015, 16, 731–741. [Google Scholar] [CrossRef]
- Awasthi, R.; Kumar, M. Development of Metronidazole-Loaded Colon-Targeted Microparticulate Drug Delivery System. Polym. Med. 2015, 45, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Holešová, S.; Hundáková, M.; Tarasiuk, Y.; Čech Barabaszová, K.; Pazdziora, E. Metronidazole/Clay Nanocomposites: Synthesis, Structure and Antibacterial Efficacy. Mater. Today Proc. 2021, 37, 21–27. [Google Scholar] [CrossRef]
- Latha, S.; Selvamani, P.; Kumar, C.S.; Sharavanan, P.; Suganya, G.; Beniwal, V.S.; Rao, P.R. Formulation Development and Evaluation of Metronidazole Magnetic Nanosuspension as a Magnetic-Targeted and Polymeric-Controlled Drug Delivery System. J. Magn. Magn. Mater. 2009, 321, 1580–1585. [Google Scholar] [CrossRef]
- Li, J.; Hao, X.; Wang, C.; Liu, H.; Liu, L.; He, X.; Sun, C.C. Improving the Solubility, Dissolution, and Bioavailability of Metronidazole via Cocrystallization with Ethyl Gallate. Pharmaceutics 2021, 13, 546. [Google Scholar] [CrossRef]
- Preisig, D.; Varum, F.; Bravo, R.; Hartig, C.; Spleiss, J.; Abbes, S.; Caobelli, F.; Wild, D.; Puchkov, M.; Huwyler, J.; et al. Colonic Delivery of Metronidazole-Loaded Capsules for Local Treatment of Bacterial Infections: A Clinical Pharmacoscintigraphy Study. Eur. J. Pharm. Biopharm. 2021, 165, 22–30. [Google Scholar] [CrossRef]
- Ali, A.; Kaldhone, P.; Shirode, A.; Kadam, V. Report on Pharmaceutical Approaches to Colon Targeted Drug Delivery Systems. J. Pharm. Res. 2010, 3, 470–473. [Google Scholar]
- Herculano, R.D.; Alencar de Queiroz, A.A.; Kinoshita, A.; Oliveira, O.N.; Graeff, C.F.O. On the Release of Metronidazole from Natural Rubber Latex Membranes. Mater. Sci. Eng. C 2011, 31, 272–275. [Google Scholar] [CrossRef]
- Krishnaiah, Y.S.R.; Bhaskar Reddy, P.R.; Satyanarayana, V.; Karthikeyan, R.S. Studies on the Development of Oral Colon Targeted Drug Delivery Systems for Metronidazole in the Treatment of Amoebiasis. Int. J. Pharm. 2002, 236, 43–55. [Google Scholar] [CrossRef]
- Al Jalali, V.; Zeitlinger, M. Systemic and Target-Site Pharmacokinetics of Antiparasitic Agents. Clin. Pharmacokinet. 2020, 59, 827–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnhill, G.; Starkey, E. What Do I Need to Know about Metronidazole? Arch. Dis. Child. Educ. Pract. Ed. 2018, 103, 307–309. [Google Scholar] [CrossRef]
- Goolsby, T.A.; Jakeman, B.; Gaynes, R.P. Clinical Relevance of Metronidazole and Peripheral Neuropathy: A Systematic Review of the Literature. Int. J. Antimicrob. Agents 2018, 51, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Hernández Ceruelos, A.; Romero-Quezada, L.C.; Ruvalcaba Ledezma, J.C.; López Contreras, L. Therapeutic Uses of Metronidazole and Its Side Effects: An Update. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 397–401. [Google Scholar] [CrossRef]
- Lamp, K.C.; Freeman, C.D.; Klutman, N.E.; Lacy, M.K. Pharmacokinetics and Pharmacodynamics of the Nitroimidazole Antimicrobials. Clin. Pharmacokinet. 1999, 36, 353–373. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.H.; Lam, N.P.; Piscitelli, S.C.; Wilkes, L.; Danziger, L.H. Clinical Pharmacokinetics of Metronidazole and Other Nitroimidazole Anti-Infectives. Clin. Pharmacokinet. 1992, 23, 328–364. [Google Scholar] [CrossRef]
- Calabrese, I.; Gelardi, G.; Merli, M.; Rytwo, G.; Sciascia, L.; Liveri, M.L.T. New Tailor-Made Bio-Organoclays for the Remediation of Olive Mill Waste Water. IOP Conf. Ser. Mater. Sci. Eng. 2013, 47, 012040. [Google Scholar] [CrossRef] [Green Version]
- Galamboš, M.; Paučová, V.; Kufčáková, J.; Rosskopfová, O.; Rajec, P.; Adamcová, R. Cesium Sorption on Bentonites and Mont morillonite K10. J. Radioanal. Nucl. Chem. 2010, 284, 55–64. [Google Scholar] [CrossRef]
- Golubeva, O.Y.; Pavlova, S.V.; Yakovlev, A.V. Adsorption and In Vitro Release of Vitamin B1 by Synthetic Nanoclays with Montmorillonite Structure. Appl. Clay Sci. 2015, 112–113, 10–16. [Google Scholar] [CrossRef]
- Leite, S.T.; do Nascimento, F.H.; Masini, J.C. Fe(III)-Polyhydroxy Cations Supported onto K10 Montmorillonite for Removal of Phosphate from Waters. Heliyon 2020, 6, e03868. [Google Scholar] [CrossRef]
- Madurai, S.L.; Joseph, S.W.; Mandal, A.B.; Tsibouklis, J.; Reddy, B.S.R. Intestine-Specific, Oral Delivery of Captopril/Montmorillonite: Formulation and Release Kinetics. Nanoscale Res. Lett. 2011, 6, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parisi, F. Adsorption and Separation of Crystal Violet, Cerium(III) and Lead(II) by Means of a Multi-Step Strategy Based on K10-Montmorillonite. Minerals 2020, 10, 466. [Google Scholar] [CrossRef]
- Parisi, F.; Lazzara, G.; Merli, M.; Milioto, S.; Princivalle, F.; Sciascia, L. Simultaneous Removal and Recovery of Metal Ions and Dyes from Wastewater through Montmorillonite Clay Mineral. Nanomaterials 2019, 9, 1699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popugaeva, D.; Manoli, K.; Kreyman, K.; Ray, A.K. Removal of Aluminum from Aqueous Solution by Adsorption on Montmorillonite K10, TiO2, and SiO2: Kinetics, Isotherms, and Effect of Ions. Adsorption 2019, 25, 1575–1583. [Google Scholar] [CrossRef]
- Sarma, G.; Sengupta, S.; Bhattacharyya, K. Montmorillonite K10: An Effective Adsorbent for Removal of a Toxic Reactive Mono-Azo Dye, Procion Red MX 5B, from Water. J. Surf. Sci. Technol. 2014, 30, 163–178. [Google Scholar]
- Alekseeva, O.; Noskov, A.; Grishina, E.; Ramenskaya, L.; Kudryakova, N.; Ivanov, V.; Agafonov, A. Structural and Thermal Properties of Montmorillonite/Ionic Liquid Composites. Materials 2019, 12, 2578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mucha, M.; Maršálek, R.; Bukáčková, M.; Zelenková, G. Interaction among Clays and Bovine Serum Albumin. RSC Adv. 2020, 10, 43927–43939. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S. Dispersions of Nanoclays of Different Shapes into Aqueous and Solid Biopolymeric Matrices. Extended Physicochemical Study. Langmuir 2011, 27, 1158–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, R.A.; Lattanzio, L.; McGee, H. Optimized Liquid-Chromatographic Determination of Metronidazole and Its Metabolites in Plasma. Clin. Chem. 1984, 30, 784. [Google Scholar] [CrossRef]
- Ognibene, M.C.; Rocco, F.; Craparo, E.F.; Picone, P.; Ceruti, M.; Giammona, G. Biocompatible Micelles Based on Squalene Portions Linked to PEGylated Polyaspartamide as Potential Colloidal Drug Carriers. Curr. Nanosci. 2011, 7, 747–756. [Google Scholar] [CrossRef]
- Tripodo, G.; Pitarresi, G.; Palumbo, F.; Craparo, E.; Giammona, G. UV-Photocrosslinking of Inulin Derivatives to Produce Hydrogels for Drug Delivery Application. Macromol. Biosci. 2005, 5, 1074–1084. [Google Scholar] [CrossRef]
- Bagchi, B.; Dey, S.; Bhandary, S.; Das, S.; Bhattacharya, A.; Basu, R.; Nandy, P. Antimicrobial Efficacy and Biocompatibility Study of Copper Nanoparticle Adsorbed Mullite Aggregates. Mater. Sci. Eng. C 2012, 32, 1897–1905. [Google Scholar] [CrossRef]
- Samstein, R.M.; Perica, K.; Balderrama, F.; Look, M.; Fahmy, T.M. The Use of Deoxycholic Acid to Enhance the Oral Bioavailability of Biodegradable Nanoparticles. Biomaterials 2008, 29, 703–708. [Google Scholar] [CrossRef]
- Merli, M.; Sciascia, L.; Turco Liveri, M.L. Regression Diagnostics Applied in Kinetic Data Processing: Outlier Recognition and Robust Weighting Procedures. Int. J. Chem. Kinet. 2010, 42, 587–607. [Google Scholar] [CrossRef]
- Ringot, D.; Lerzy, B.; Chaplain, K.; Bonhoure, J.-P.; Auclair, E.; Larondelle, Y. In Vitro Biosorption of Ochratoxin A on the Yeast Industry By-Products: Comparison of Isotherm Models. Bioresour. Technol. 2007, 98, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.; Heath, A.; Patureau, P.; Evernden, M.; Walker, P. Alkali Activation Behaviour of Un-Calcined Montmorillonite and Illite Clay Minerals. Appl. Clay Sci. 2018, 166, 250–261. [Google Scholar] [CrossRef]
- Sciascia, L.; Turco Liveri, M.L.; Merli, M. Kinetic and Equilibrium Studies for the Adsorption of Acid Nucleic Bases onto K10 Montmorillonite. Appl. Clay Sci. 2011, 53, 657–668. [Google Scholar] [CrossRef]
- Costa, P.; Lobo, S.; José, M. Modeling and Comparison of Dissolution Profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Novick, S.; Laster, B.; Quastel, M.R. Positive Cooperativity in the Cellular Uptake of a Boronated Porphyrin. Int. J. Biochem. Cell Biol. 2006, 38, 1374–1381. [Google Scholar] [CrossRef]
- Chen, T.; Chen, L.; Li, H.; Chen, Y.; Guo, H.; Shu, Y.; Chen, Z.; Cai, C.; Guo, L.; Zhang, X.; et al. Design and In Vitro Evaluation of a Novel Poly(Methacrylic Acid)/Metronidazole Antibacterial Nanogel as an Oral Dosage Form. Colloids Surf. B Biointerfaces 2014, 118, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Marcelo, G.; Ferreira, I.C.; Viveiros, R.; Casimiro, T. Development of itaconic acid-based molecular imprinted polymers using supercritical fluid technology for pH-triggered drug delivery. Int. J. Pharm. 2018, 542, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, B.; Ma, P.; Yang, W.; Liu, M.; Huang, Q.; Wei, S. Preparation and in vitro evaluation of macrocyclic metronidazole conjugates as an oral colon-specific delivery system. J. Incl. Phenom. Macrocycl. Chem. 2014, 78, 501–504. [Google Scholar] [CrossRef]



| Adsorption Isotherm Model | Figures of Merit | pH 0.2 | pH 2.5 | pH 5.0 |
|---|---|---|---|---|
| Langmuir | R2 | 0.98 | 0.98 | 0.94 |
| χ2 | 6.1 × 10−6 | 1.4 × 10−5 | 1.4 × 10−5 | |
| ESS | 2.4 × 10−5 | 6.2 × 10−5 | 5.7 × 10−5 | |
| ANOVA F value | 866 | 200 | 94 | |
| Freundlich | R2 | 0.94 | 0.98 | 0.94 |
| χ2 | 3.0 × 10−5 | 7.9 × 10−6 | 1.6 × 10−5 | |
| ESS | 1.2 × 10−4 | 3.2 × 10−5 | 6.3 × 10−5 | |
| ANOVA F value | 174 | 394 | 84 | |
| Dual mode Langmuir | R2 | 0.98 | 0.97 | 0.94 |
| χ2 | 6.8 × 10−6 | 2.1 × 10−5 | 1.8 × 10−5 | |
| ESS | 2.0 × 10−5 | 6.2 × 10−5 | 5.5 × 10−5 | |
| ANOVA F value | 522 | 100 | 49 | |
| Dual mode Freundlich | R2 | 0.98 | 0.99 | 0.94 |
| χ2 | 1.1 × 10−5 | 9.7 × 10−6 | 2.1 × 10−5 | |
| ESS | 3.1 × 10−5 | 2.9 × 10−5 | 6.3 × 10−5 | |
| ANOVA F value | 335 | 214 | 42 | |
| Hill | R2 | 0.99 | 0.99 | 0.99 |
| χ2 | 3.9 × 10−6 | 1.5 × 10−6 | 1.7 × 10−6 | |
| ESS | 1.2 × 10−5 | 5.8 × 10−6 | 5.2 × 10−6 | |
| ANOVA F value | 903 | 1217 | 519 |
| pH | 0.2 | 2.5 | 5.0 |
|---|---|---|---|
| qm/mmol∙g−1 | 0.061 ± 0.004 | 0.08 ± 0.01 | 0.033 ± 0.002 |
| KH/L∙mol−1 | 130 ± 20 | 33 ± 4 | 52 ± 3 |
| n | 1.3 ± 0.2 | 2.4 ± 0.3 | 4.1 ± 0.7 |
| R2 | 0.98985 | 0.99671 | 0.99138 |
| Kinetic Model | Figures of Merit | pH 0.2 | pH 2.5 | pH 5.0 |
|---|---|---|---|---|
| Zero order | R2 | 0.98 | 0.99 | 0.99 |
| ESS | 18 | 17 | 4 | |
| ANOVA F value | 186 | 291 | 2500 | |
| First order | R2 | 0.97 | 0.98 | 0.98 |
| χ2 | 5.3 | 1.1 | 0.8 | |
| ESS | 10.7 | 20.3 | 10 | |
| ANOVA F value | 54 | 254 | 1049 | |
| Second order | R2 | 0.94 | 0.95 | 0.99 |
| χ2 | 6.2 | 5.9 | 1.5 | |
| ESS | 18.6 | 17.8 | 5.5 | |
| ANOVA F value | 69 | 72 | 462 |
| Kinetic Model | Figures of Merit | pH 0.2 | pH 2.5 | pH 5.0 |
|---|---|---|---|---|
| First order | R2 | 0.99 | 0.99 | 0.99 |
| χ2 | 2.0 | 3.8 | 0.7 | |
| ESS | 12.3 | 22.98 | 3.6 | |
| ANOVA F value | 10,517 | 5410 | 23,700 | |
| Second order | R2 | 0.72 | 0.73 | 0.87 |
| χ2 | 296 | 256 | 88.9 | |
| ESS | 2078 | 1868 | 533 | |
| ANOVA F value | 105 | 112 | 289 | |
| Double exponential model | R2 | 0.99 | 0.99 | 0.99 |
| χ2 | 3.1 | 5.6 | 1.21 | |
| ESS | 12.1 | 22.6 | 365 | |
| ANOVA F value | 4213 | 2199 | 8536 |
| Simulated Gastric Fluids | Simulated Intestinal Fluids | |||||
|---|---|---|---|---|---|---|
| t120% | DE% | k0/min−1 | t480% | DE% | k1/min−1 | |
| Commercial formulation | 44 | 18 | 0.48 ± 0.03 | 100 | 72 | (1.1 ± 0.1) × 10−2 |
| MNE/OMt pH 0.2 | 20 | 9.5 | 0.20 ± 0.01 | 100 | 87 | (2.63 ± 0.07) × 10−2 |
| MNE/OMt pH 2.5 | 24 | 10 | 0.21 ± 0.01 | 100 | 89,5 | (2.6 ± 0.1) × 10−2 |
| MNE/OMt pH 5.0 | 26 | 14 | 0.23 ± 0.01 | 100 | 75 | (1.02 ± 0.05) × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciascia, L.; Calabrese, I.; Cavallaro, G.; Merli, M.; Scialabba, C.; Liveri, M.L.T. Modified Montmorillonite as Drug Delivery Agent for Enhancing Antibiotic Therapy. Minerals 2021, 11, 1315. https://doi.org/10.3390/min11121315
Sciascia L, Calabrese I, Cavallaro G, Merli M, Scialabba C, Liveri MLT. Modified Montmorillonite as Drug Delivery Agent for Enhancing Antibiotic Therapy. Minerals. 2021; 11(12):1315. https://doi.org/10.3390/min11121315
Chicago/Turabian StyleSciascia, Luciana, Ilaria Calabrese, Gennara Cavallaro, Marcello Merli, Cinzia Scialabba, and Maria Liria Turco Liveri. 2021. "Modified Montmorillonite as Drug Delivery Agent for Enhancing Antibiotic Therapy" Minerals 11, no. 12: 1315. https://doi.org/10.3390/min11121315
APA StyleSciascia, L., Calabrese, I., Cavallaro, G., Merli, M., Scialabba, C., & Liveri, M. L. T. (2021). Modified Montmorillonite as Drug Delivery Agent for Enhancing Antibiotic Therapy. Minerals, 11(12), 1315. https://doi.org/10.3390/min11121315







