The Alkaline Fusion-Hydrothermal Synthesis of Blast Furnace Slag-Based Zeolite (BFSZ): Effect of Crystallization Time
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Blast Furnace Slag-Based Zeolite (BFSZ)
2.3. Characterization
3. Results and Discussion
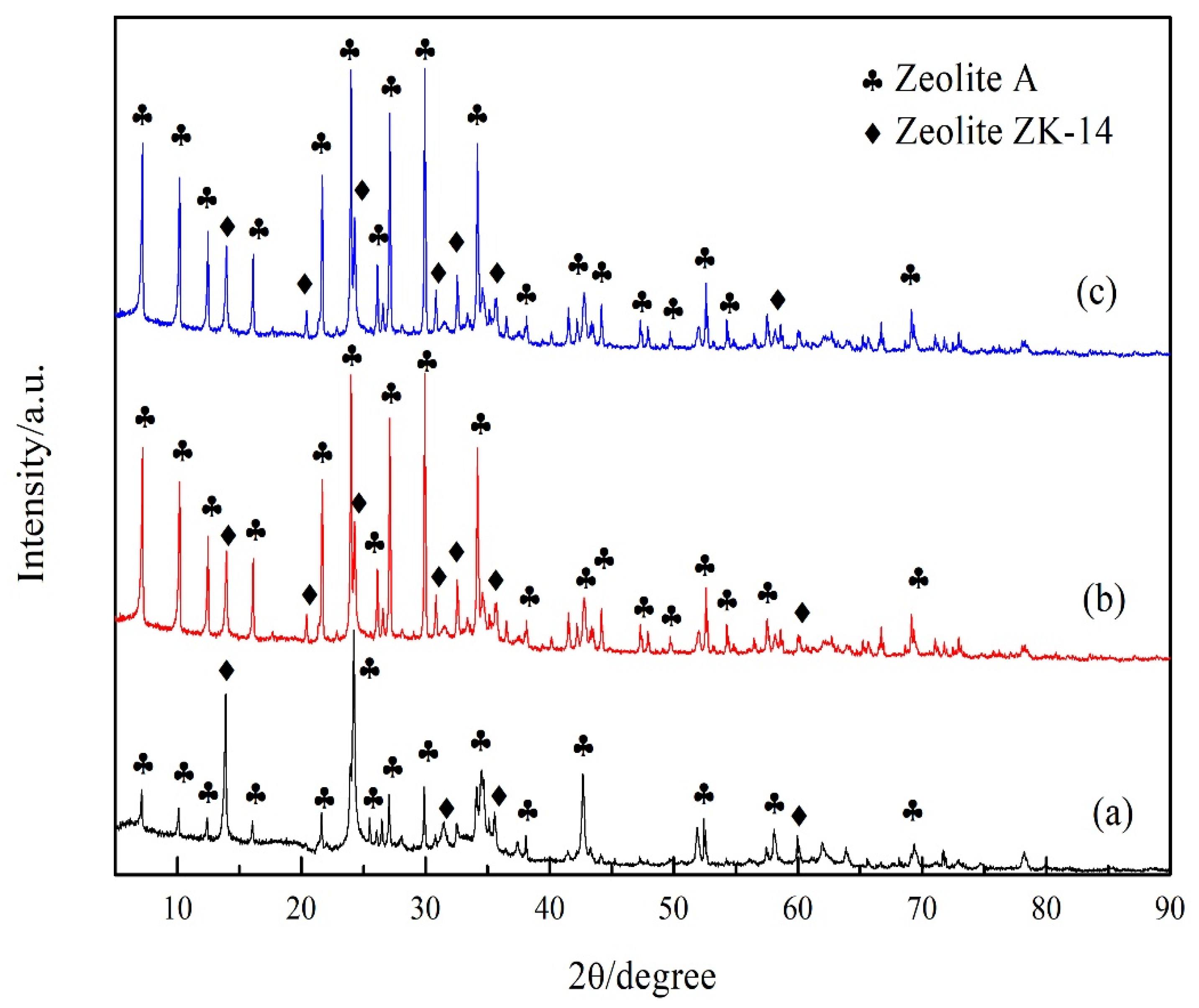
3.1. Crystalline Characteristics
3.2. Chemical Analysis
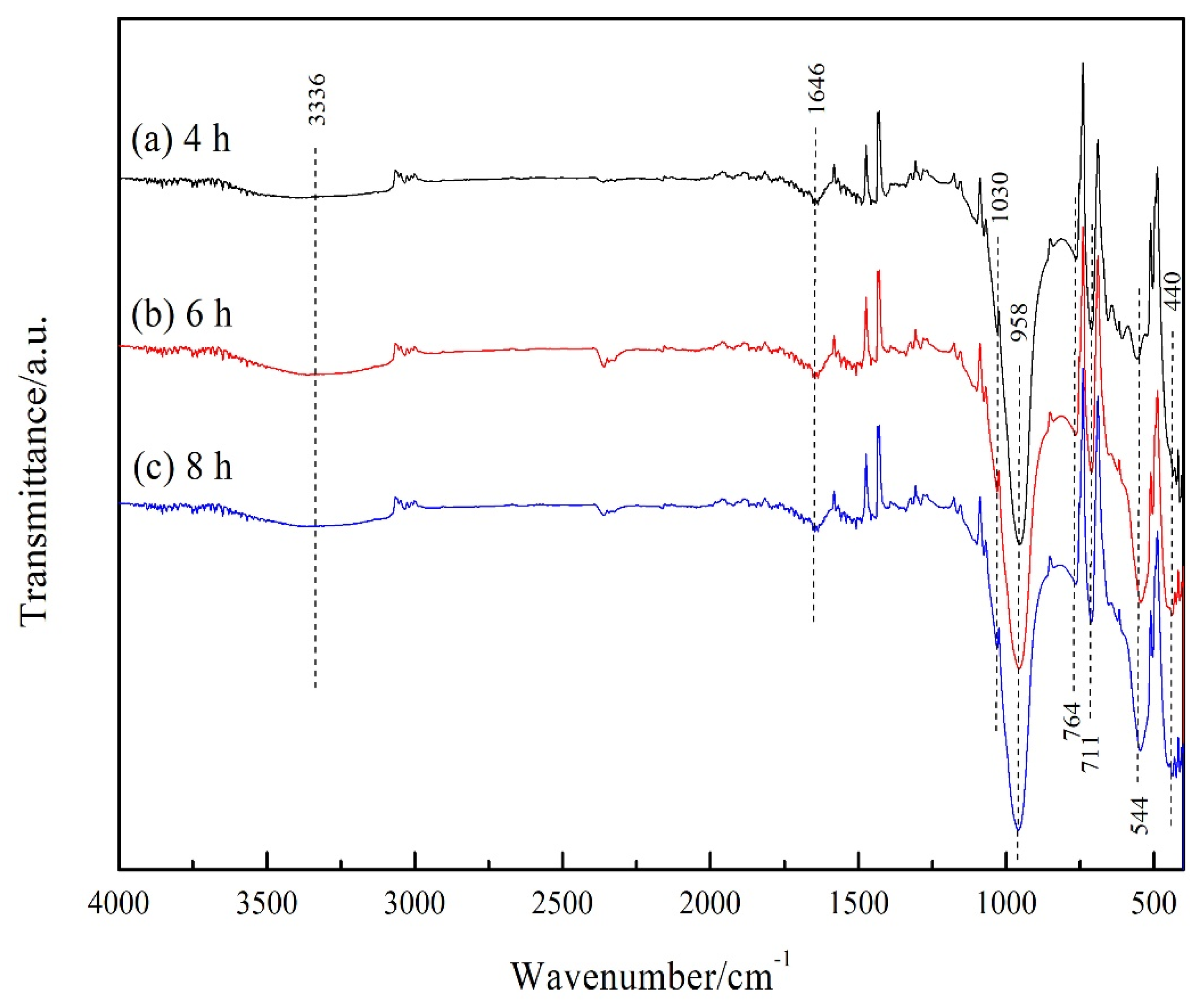
3.3. Fourier Transform Infrared (FT-IR) Analysis
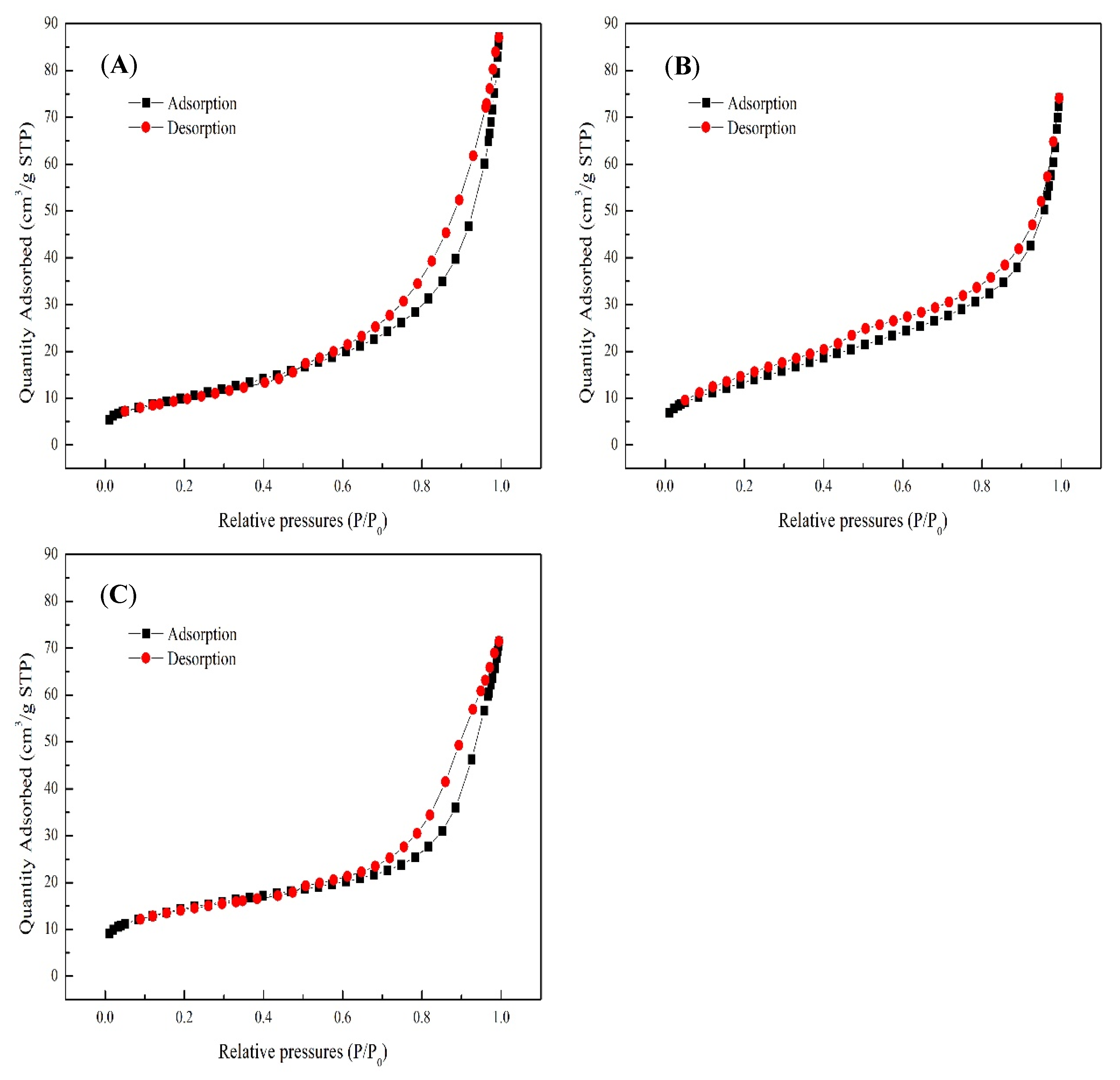
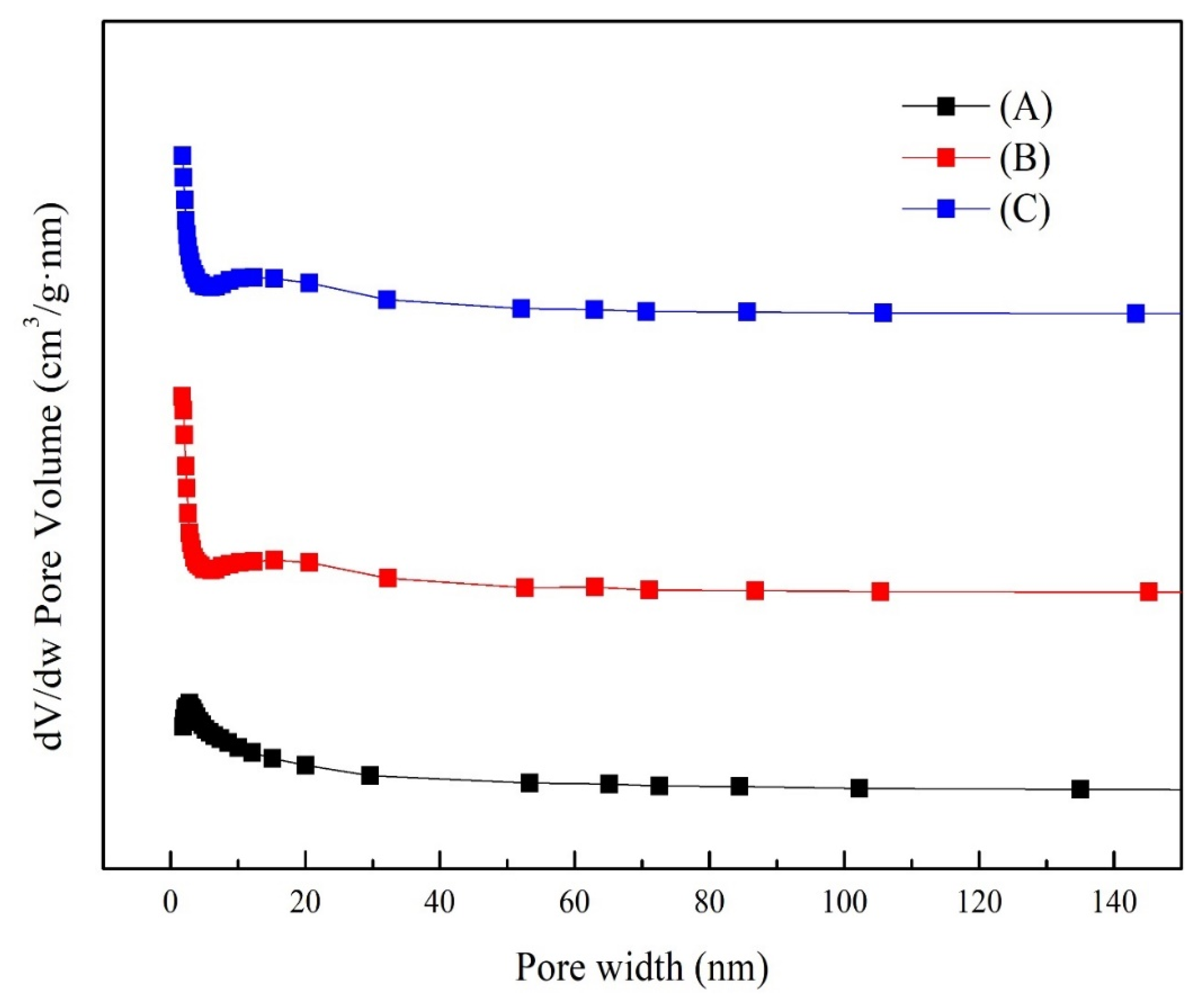
3.4. Microstructural Characteristics
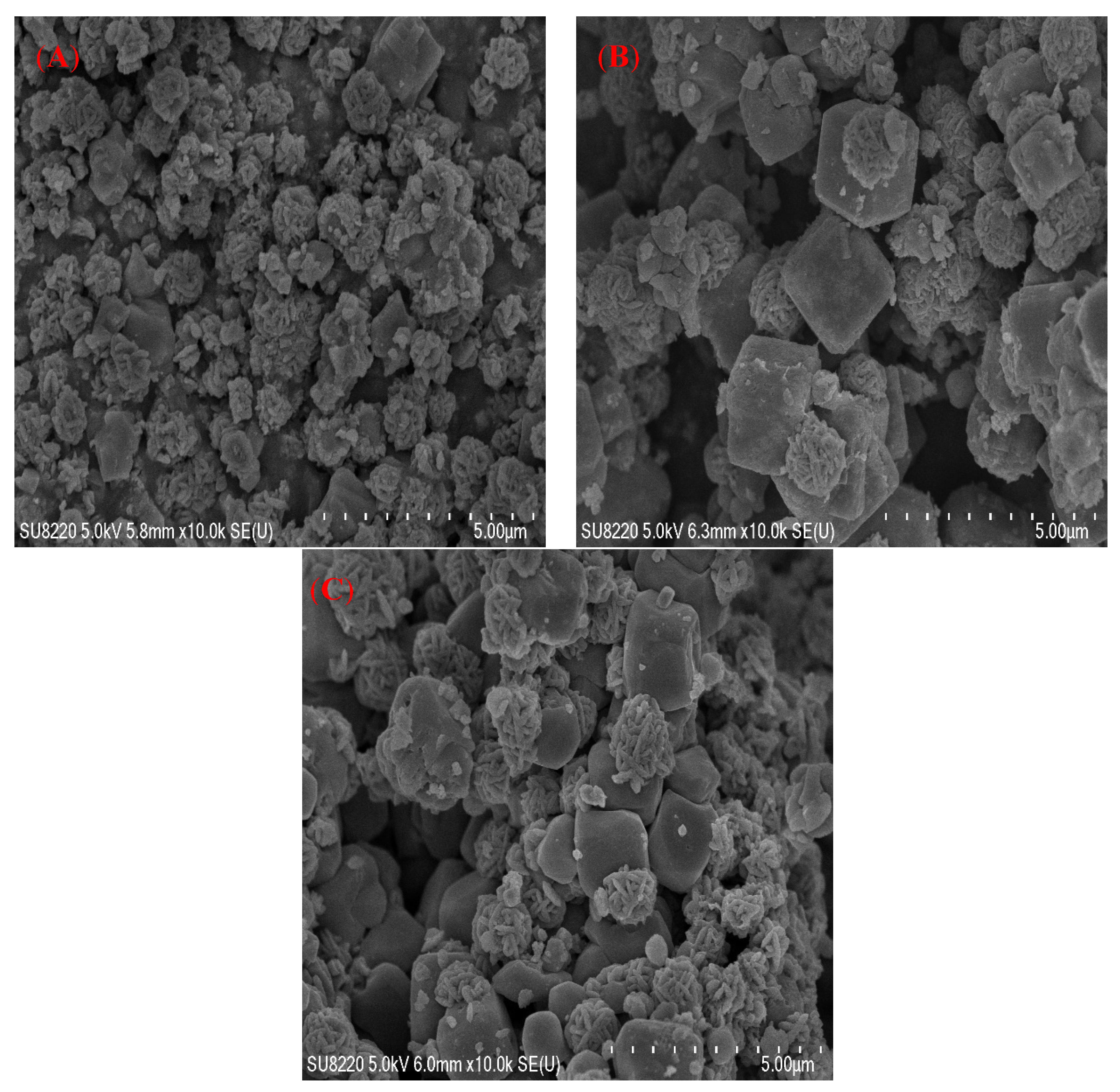
3.5. Morphology Analysis
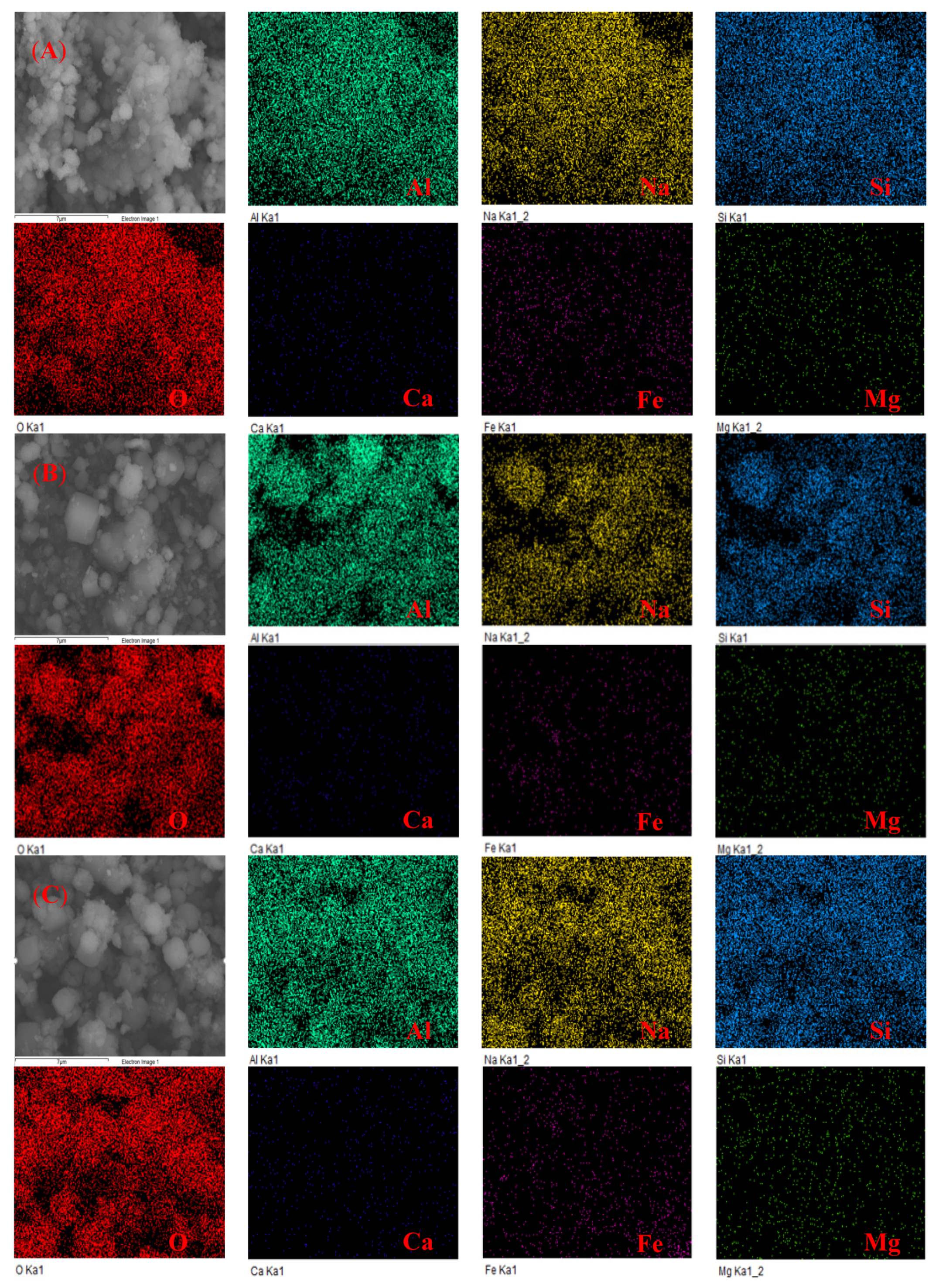
3.6. Elemental Mapping Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kuwahara, Y.; Ohmichi, T.; Kamegawa, T.; Mori, K.; Yamashita, H. A novel conversion process for waste slag: Synthesis of a hydrotalcite-like compound and zeolite from blast furnace slag and evaluation of adsorption capacities. J. Mater. Chem. 2010, 20, 5052–5062. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Hamdan, S.; Van Deventer, J.S.J. Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem. Concr. Comp. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Tian, P.; Dong, H.W.; Wu, X.M.; Huo, L.Q.; Xue, Y.K. Comprehensive utilization and control measures of iron and steel slag. Energ. Metall. Ind. 2020, 39, 9–13. (In Chinese) [Google Scholar]
- Czuma, N.; Katarzyna, Z.; Motak, M.; Gálvez, M.E.; Costa, P.D. Ni/zeolite X derived from fly ash as catalysts for CO2 methanation. Fuel 2020, 267, 117139. [Google Scholar] [CrossRef]
- Shawabkeh, R.; Al-Harahsheh, A.; Hami, M. Conversion of oil shale ash into zeolite for cadmium and lead removal from wastewater. Fuel 2004, 83, 981–985. [Google Scholar] [CrossRef]
- Kordatos, K.; Gavela, S.; Ntziouni, A.; Pistiolas, K.N. Synthesis of highly siliceous ZSM-5 zeolite using silica from rice husk ash. Micropor. Mesopor. Mat. 2008, 115, 189–196. [Google Scholar] [CrossRef]
- Belviso, C.; Perchiazzi, N.; Cavalcante, F. Zeolite from fly ash: An investigation on metastable behavior of the newly formed minerals in a medium-high-temperature range. Ind. Eng. Chem. Res. 2019, 58, 20472. [Google Scholar] [CrossRef]
- Belviso, C.; Abdolrahimi, M.; Peddis, D.; Gagliano, E.; Sgroi, M.; Lettino, A.; Roccaro, P.; Vagliasindi, F.G.A.; Falciglia, P.P.; Bella, G.D.; et al. Synthesis of zeolite from volcanic ash: Characterization and application for cesium removal. Micropor. Mesopor. Mat. 2021, 319, 111045. [Google Scholar] [CrossRef]
- Belviso, C.; Piancastelli, A.; Sturini, M.; Belviso, S. Synthesis of composite zeolite-layered double hydroxides using ultrasonic neutralized red mud. Micropor. Mesopor. Mat. 2020, 299, 110108. [Google Scholar] [CrossRef]
- Li, X.Y.; Jian, Y.; Liu, X.Q.; Shi, L.Y.; Zhang, D.Y.; Su, L.B. Direct synthesis of zeolites from a natural clay, attapulgite. Acs. Sustainable. Chem. Eng. 2017, 5, 6124–6130. [Google Scholar] [CrossRef]
- Anuwattana, R.; Balkus, K.J.; Asavapisit, S.; Khummongkol, P. Conventional and microwave hydrothermal synthesis of zeolite ZSM-5 from the cupola slag. Micropor. Mesopor. Mat. 2008, 111, 260–266. [Google Scholar] [CrossRef]
- Li, C.X.; Zhong, H.; Wang, S.; Xue, J.R.; Zhang, Z.Y. A novel conversion process for waste residue: Synthesis of zeolite from electrolytic manganese residue and its application to the removal of heavy metals. Colloid. Surface. A 2015, 470, 258–267. [Google Scholar] [CrossRef]
- Novembre, D.; Sabatino, B.D.; Gimeno, D.; Garcia-Vallès, M.; Martínez-Manent, S. Synthesis of Na–X zeolites from tripolaceous deposits (Crotone, Italy) and volcanic zeolitised rocks (Vico volcano, Italy). Micropor. Mesopor. Mat. 2004, 75, 1–11. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, M.G. Removal of Cu and Sr ions using adsorbent obtained by immobilizing zeolite synthesized from Jeju volcanic rocks in Polyacrylonitrile. J. Environ. Sci. Int. 2018, 27, 1215–1226. [Google Scholar] [CrossRef]
- Ayele, L.; Pérez-Pariente, J.; Chebude, Y.; Diaz, I. Synthesis of zeolite A using kaolin from Ethiopia and its application in detergents. New. J. Chem. 2016, 40, 3440–3446. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Ismail, A.A.; Kini, G.; Ibrahim, I.A.; Koopman, B. Synthesis of highly ordered cubic zeolite A and its ion-exchange behavior. Colloid. Surf. A 2009, 348, 87–92. [Google Scholar] [CrossRef]
- Liu, H.B.; Peng, S.C.; Shu, L.; Chen, T.H.; Bao, T.; Frost, R.L. Magnetic zeolite NaA: Synthesis, characterization based on metakaolin and its application for the removal of Cu2+, Pb2+. Chemosphere 2013, 91, 1539–1546. [Google Scholar] [CrossRef]
- Li, C.X.; Zhong, H.; Wang, S.; Xue, J.R.; Zhang, Z.Y. Removal of basic dye (methylene blue) from aqueous solution using zeolite synthesized from electrolytic manganese residue. J. Ind. Eng. Chem. 2015, 23, 344–352. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, J.H.; Kim, Y.T.; Riu, D.H.; Jung, S.J.; Lee, Y.J.; Chung, S.C.; Kim, Y.H. Synthesis of Si, Mg substituted hydroxyapatites and their sintering behaviors. Biomaterials 2003, 24, 1389–1398. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Ohmichi, T.; Mori, K.; Katayama, I.; Yamashita, H. Synthesis of zeolite from steel slag and its application as a support of nano-sized TiO2 photocatalyst. J. Mater. Sci. 2008, 43, 2407–2410. [Google Scholar] [CrossRef]
- Sugano, Y.; Sahara, R.; Murakami, T.; Narushima, T.; Iguchi, Y.; Ouchi, C. Hydrothermal synthesis of zeolite A using blast furnace slag. ISIJ Int. 2006, 45, 937–945. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Zhang, H.; Dan, X.; Lu, H.; Tian, B. Ammonium removal from aqueous solution by zeolites synthesized from low-calcium and high-calcium fly ashes. Desalination 2011, 277, 46–53. [Google Scholar] [CrossRef]
- Wang, W.Q.; Feng, Q.M.; Liu, K.; Zhang, G.F.; Liu, J. A novel magnetic 4A zeolite adsorbent synthesised from kaolinite type pyrite cinder (KTPC). Solid. State Sci. 2015, 39, 52–58. [Google Scholar] [CrossRef]
- Li, C.X.; Li, X.; Yu, Y.; Zhang, Q.W.; Li, L.; Zhong, H.; Wang, S. A novel conversion for blast furnace slag (BFS) to the synthesis of hydroxyapatite-zeolite material and its evaluation of adsorption properties. J. Ind. Eng. Chem. 2021, 105, 63–73. [Google Scholar] [CrossRef]
- Wan, X.K. Synthesis and characterization of composite molecular sieves with mesoporous and microporous structure from ZSM-5 zeolites by heat treatment. Micropor. Mesopor. Mat. 2003, 62, 157–163. [Google Scholar]
| Component | BFS/wt % a | ABFS/wt % a |
|---|---|---|
| CaO | 47.08 | 0.24 |
| SiO2 | 29.13 | 58.59 |
| Al2O3 | 20.59 | 41.56 |
| MgO | 1.11 | 0.14 |
| Fe2O3 | 1.58 | 0.58 |
| Na2O | 0.27 | 0.11 |
| Total | 99.76 b | 99.25 b |
| Crystallization Time (h) | Chemical Component a/wt % | Molar Ratio | Zeolite Content b /wt % | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | Na2O | Fe2O3 | MgO | Si/Al | Na/Al | ||
| 4 | 0.34 | 35.42 | 35.66 | 19.82 | 0.65 | 0.17 | 0.84 | 0.90 | 90.90 |
| 6 | 0.43 | 38.16 | 36.76 | 20.61 | 0.32 | 0.09 | 0.88 | 0.92 | 95.53 |
| 8 | 0.36 | 38.72 | 36.42 | 22.27 | 0.38 | 0.13 | 0.90 | 1.01 | 97.41 |
| Crystallization Time (h) | SSA (m²/g) a | Pore Volume (cm3/g) b | Average Pore Diameter (nm) b |
|---|---|---|---|
| 4 | 37.16 | 0.14 | 10.95 |
| 6 | 49.64 | 0.11 | 7.62 |
| 8 | 49.96 | 0.11 | 7.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Li, X.; Zhang, Q.; Li, L.; Wang, S. The Alkaline Fusion-Hydrothermal Synthesis of Blast Furnace Slag-Based Zeolite (BFSZ): Effect of Crystallization Time. Minerals 2021, 11, 1314. https://doi.org/10.3390/min11121314
Li C, Li X, Zhang Q, Li L, Wang S. The Alkaline Fusion-Hydrothermal Synthesis of Blast Furnace Slag-Based Zeolite (BFSZ): Effect of Crystallization Time. Minerals. 2021; 11(12):1314. https://doi.org/10.3390/min11121314
Chicago/Turabian StyleLi, Changxin, Xiang Li, Qingwu Zhang, Li Li, and Shuai Wang. 2021. "The Alkaline Fusion-Hydrothermal Synthesis of Blast Furnace Slag-Based Zeolite (BFSZ): Effect of Crystallization Time" Minerals 11, no. 12: 1314. https://doi.org/10.3390/min11121314
APA StyleLi, C., Li, X., Zhang, Q., Li, L., & Wang, S. (2021). The Alkaline Fusion-Hydrothermal Synthesis of Blast Furnace Slag-Based Zeolite (BFSZ): Effect of Crystallization Time. Minerals, 11(12), 1314. https://doi.org/10.3390/min11121314







