The Synthesis of Biodegradable Poly(L-Lactic Acid)-Polyethylene Glycols Copolymer/Montmorillonite Nanocomposites and Analysis of the Crystallization Properties
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of the Organophilic Montmorillonite
2.3. Synthesis of Copolymers and Preparation of Composite Materials
2.3.1. Synthesis of Copolymers
2.3.2. Preparation of PLLA-PEG Copolymer Nanocomposites
2.4. Characterizations
2.4.1. X-ray Diffraction (XRD)
2.4.2. Gel Permeation Chromatography (GPC)
2.4.3. Fourier Transform Infrared Spectrometer (FT-IR)
2.4.4. 1H-NMR Spectrometer
2.4.5. Transmission Electron Microscope (TEM)
2.4.6. Non-Isothermal and Isothermal Crystallization Experiments
3. Results and Discussion
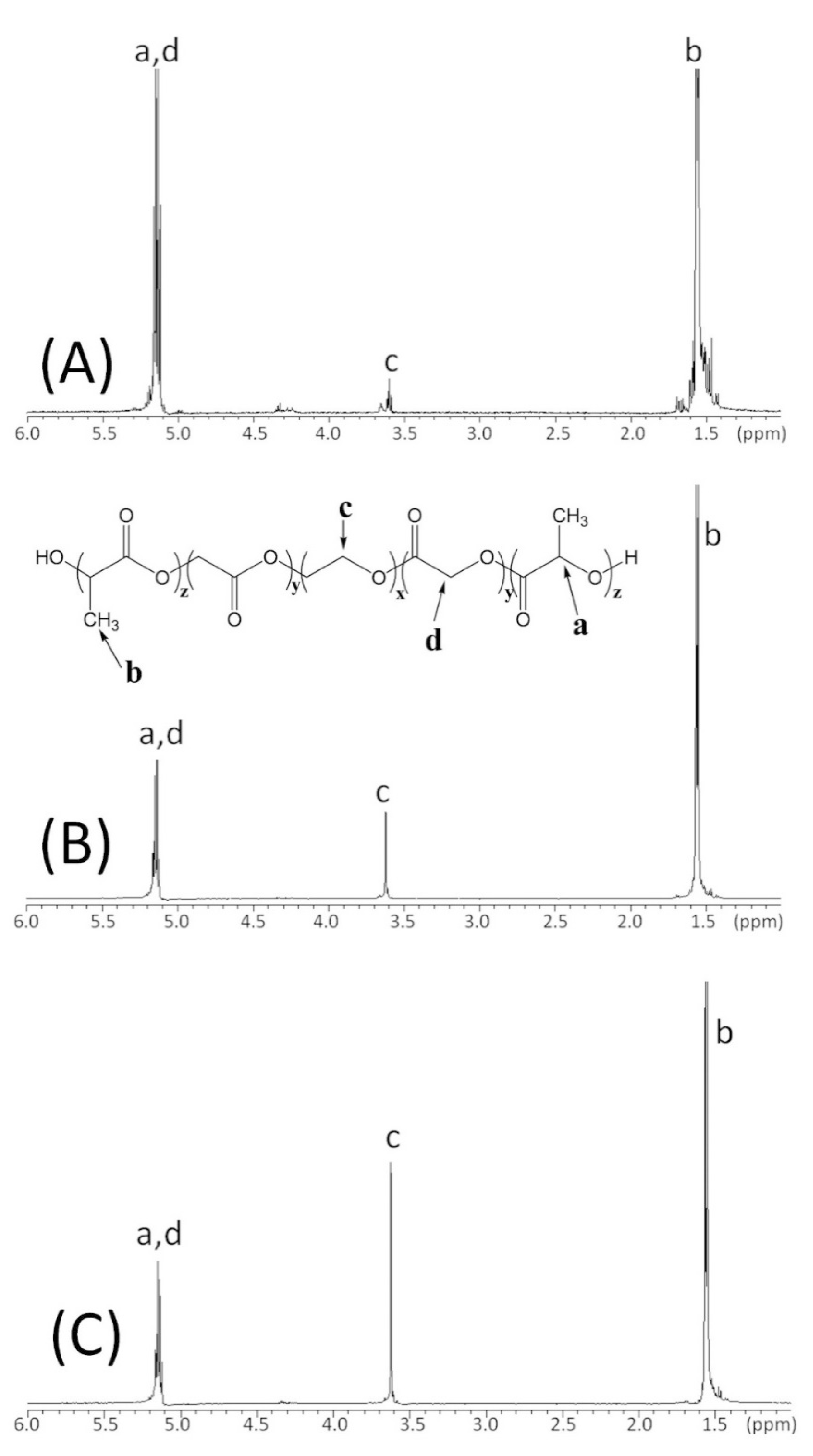
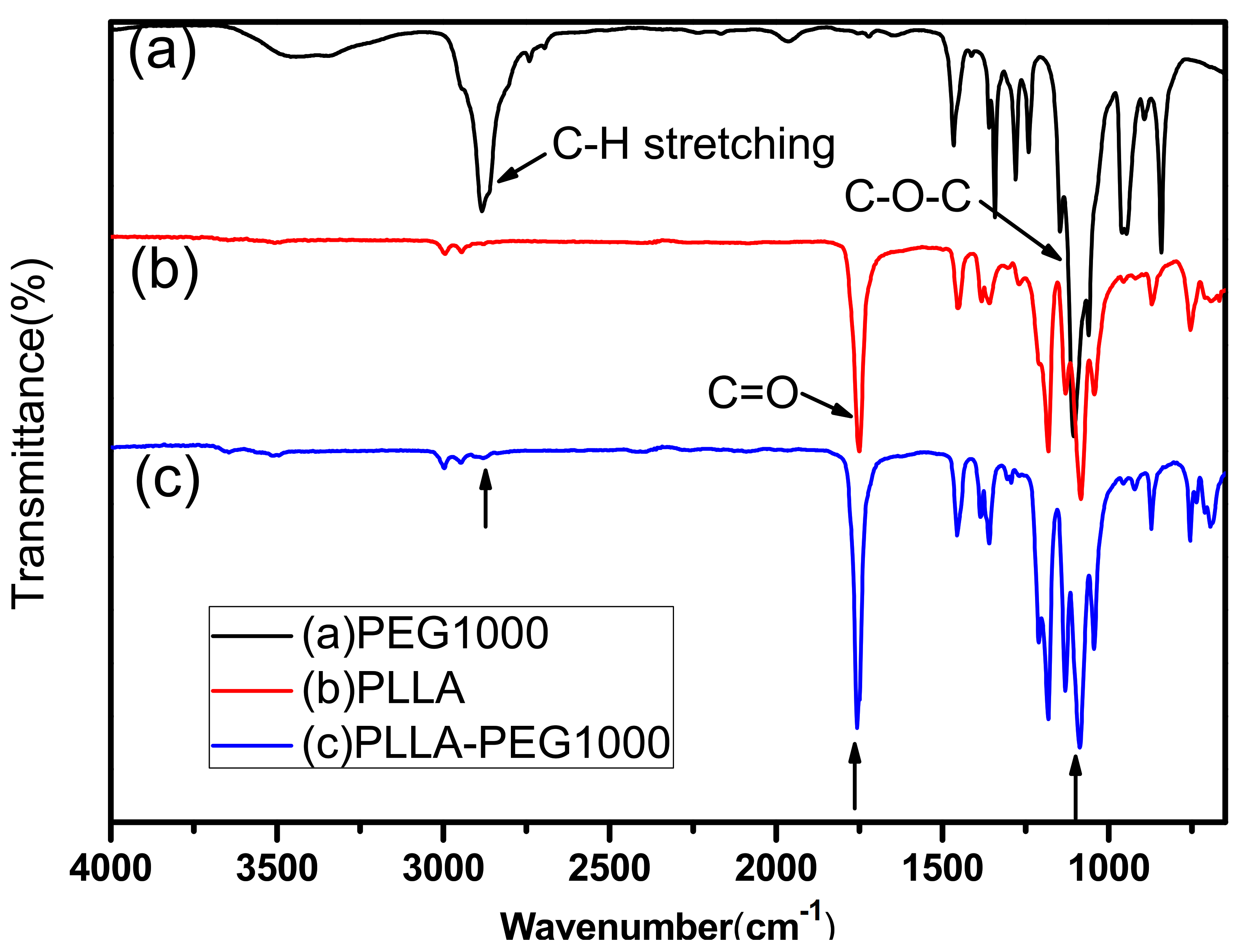
3.1. PLLA-PEG Copolymer Synthesis and Characterization
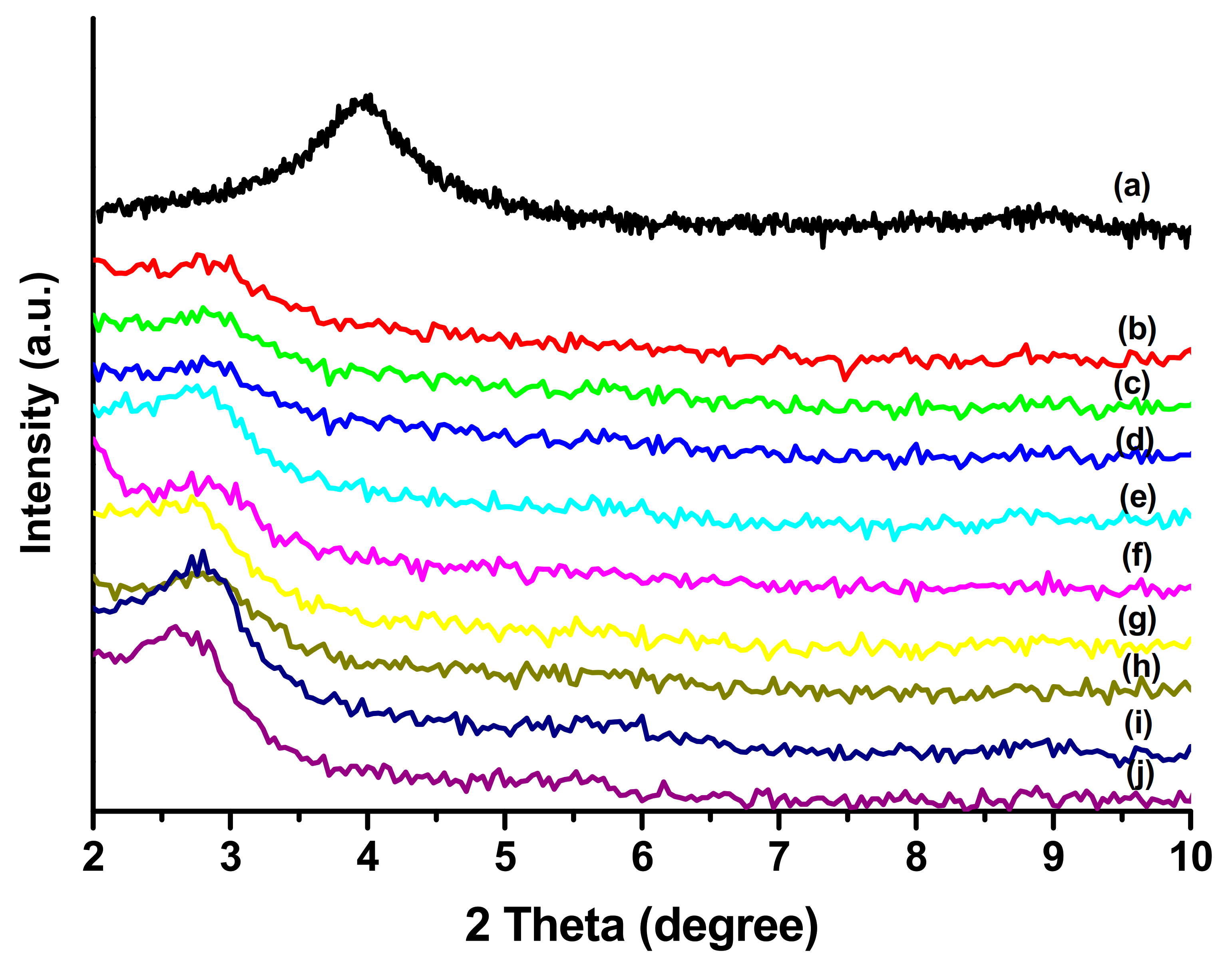
3.2. X-ray Diffraction of the PLLA-PEG/o-MMT Nanocomposites
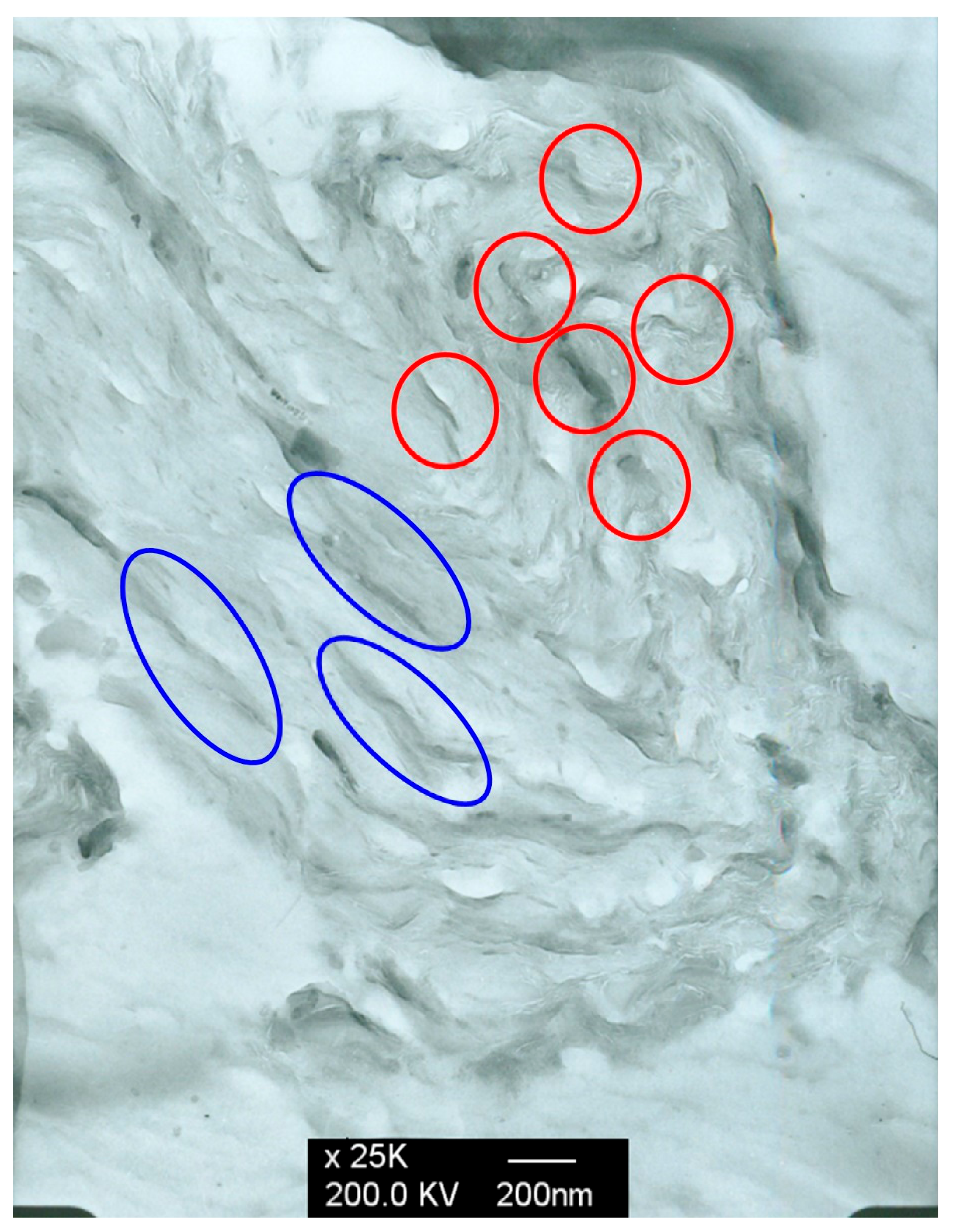
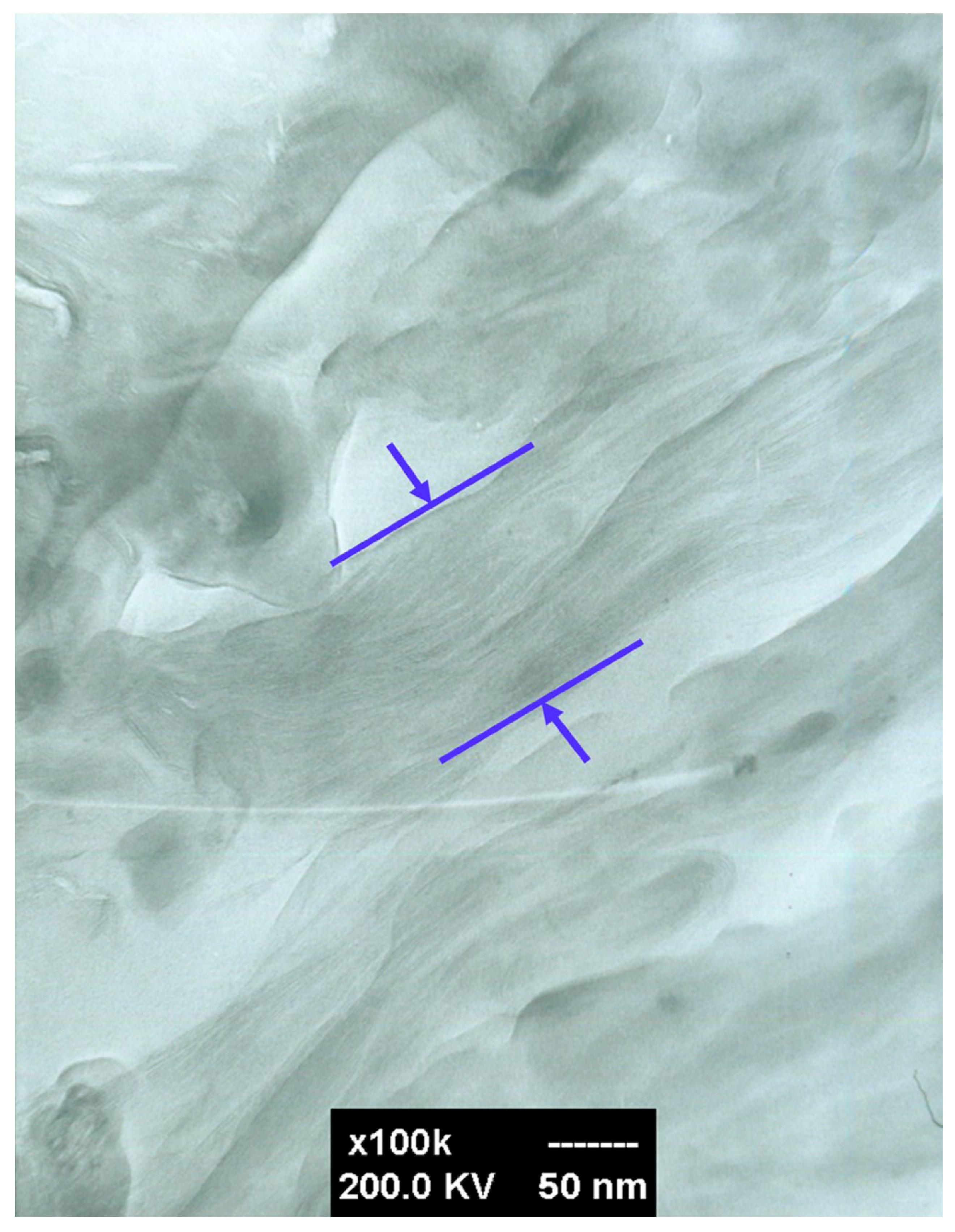
3.3. The Morphology of the PLLA-PEG/o-MMT Nanocomposites
3.4. Non-Isothermal Crystallization of the PLLA-PEG/o-MMT Nanocomposites
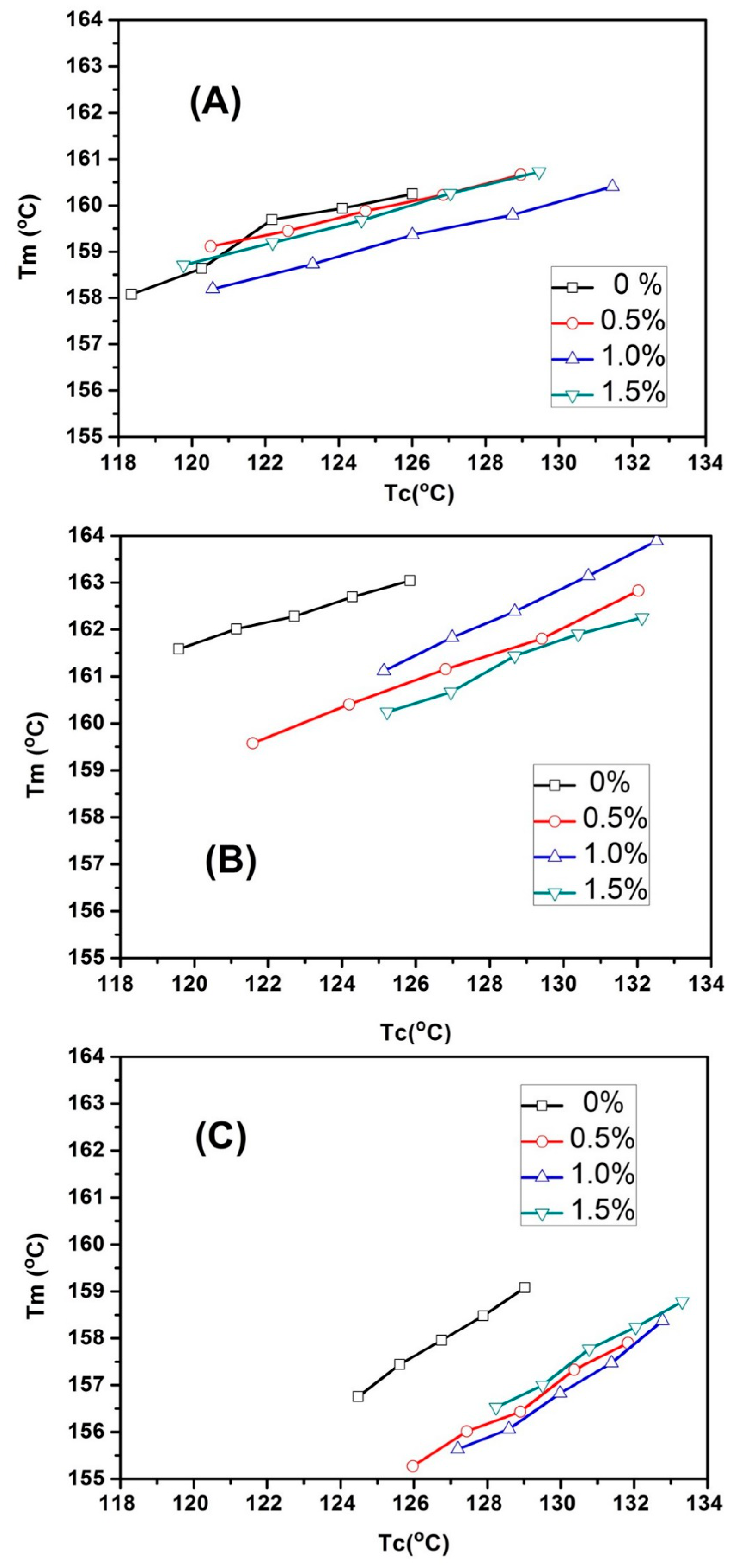
3.5. Isothermal Crystallization of the PLLA-PEG/o-MMT Nanocomposites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ye, H.Q. 2010 Framework of Sustainable Energy Policy; Bureau of Energy; Ministry of Economic Affairs: Taipei, Taiwan, 2010; Chapter 2. [Google Scholar]
- Derraik, J.G.B. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef]
- Scott, L.; Martin, W. Environmental performance of bio-based and biodegradable plastics: The road ahead. Chem. Soc. Rev. 2017, 46, 6855–6871. [Google Scholar]
- Havstad, M.R. Biodegradable plastics. In Plastic Waste and Recycling; Letcher, T.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 97–129. [Google Scholar]
- Scaffaro, R.; Maio, A.; Sutera, F.; Gulino, E.F.; Morreale, M. Degradation and Recycling of Films Based on Biodegradable Polymers: A Short Review. Polymers 2019, 11, 651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahman, Y. Poly(Lactic Acid): Green and Sustainable Plastics. Ferment. Technol. 2012, 2. [Google Scholar] [CrossRef] [Green Version]
- Siakeng, R.; Jawaid, M.; Ariffin, H.; Sapuan, S.M.; Asim, M.; Saba, N. Natural fiber reinforced polylactic acid composites: A review. Polym. Compos. 2019, 40, 446–463. [Google Scholar] [CrossRef]
- Wu, W.Y. Environmentally friendly new fiber materials. Sci. Dev. 2003, 362, 12–17. [Google Scholar]
- Seyednejad, H.; Ghassemi, A.H.; van Nostrum, C.F.; Vermonden, T.; Hennink, W.E. Functional aliphatic polyesters for biomedical and pharmaceutical applications. J. Control. Release 2011, 152, 168–176. [Google Scholar] [CrossRef]
- Kricheldorf, H.R. Syntheses and application of polylactides. Chemosphere 2001, 43, 49–54. [Google Scholar] [CrossRef]
- Ray, S.S.; Bosmina, M. Biodegradable polymers and their layered silicate nanocomposites: In greening the 21st century materials world. Prog. Mater. Sci. 2005, 50, 962. [Google Scholar]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental Impact of Food Packaging Materials: A Review of Contemporary Development from Conventional Plastics to Polylactic Acid Based Materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applica-tions-A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Zhang, B.; Li, M.; Li, J.; Zhang, C.; Han, Y.; Wang, L.; Wang, K.; Zhou, C.; Liu, L.; et al. 3D printing of PLA/n-HA composite scaffolds with customized mechanical properties and biological functions for bone tissue engineering. Compos. Part B Eng. 2021, 224, 109192. [Google Scholar] [CrossRef]
- Pluta, M. Morphology and properties of polylactide modified by thermal treatment, filling with layered silicates and plasticization. Polymer 2004, 45, 8239–8251. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, J.; Feng, H.; Zhang, M.; Lei, L.; Wu, X. Improve of tensile and thermal properties of poly(lactic acid) composties with admicellar-treated rice straw fiber. Chem. Eng. J. 2011, 173, 659–666. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J. Research progress in toughening modification of poly(lactic acid). J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1051–1083. [Google Scholar] [CrossRef]
- Darie-Niţă, R.N.; Vasile, C.; Irimia, A.; Lipşa, R.; Râpă, M. Evaluation of some eco-friendly plasticizers for PLA films processing. J. Appl. Polym. Sci. 2016, 133, 11. [Google Scholar] [CrossRef]
- Bai, T.; Zhu, B.; Liu, H.; Wang, Y.; Song, G.; Liu, C.; Shen, C. Biodegradable poly (lactic acid) nanocomposites rein-forced and toughened by carbon nanotubes/clay hybrids. Int. J. Biol. Macromol. 2020, 151, 628–634. [Google Scholar] [CrossRef]
- Nofar, M.; Salehiyan, R.; Ciftci, U.; Jalali, A.; Durmus, A. Ductility improvements of PLA-based binary and ternary blends with controlled morphology using PBAT, PBSA, and nanoclay. Compos. Part B Eng. 2020, 182, 107661. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, A.S.; Asif, A.; Yar, M.; Haycock, J.W.; Rehman, I.U. Recent concepts in biodegradable polymers for tissue en-gineering paradigms: A critical review. Int. Mater. Rev. 2019, 64, 91–126. [Google Scholar] [CrossRef] [Green Version]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Lasprilla, A.J.; Martinez, G.A.; Lunelli, B.H.; Jardini, A.L.; Maciel Filho, R. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Kutikov, A.B.; Song, J. Biodegradable PEG-Based Amphiphilic Block Copolymers for Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2015, 1, 463–480. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.; Xiao, L.Q.; Tan, L.; Sun, W.; Wu, T.; Chen, L.W.; Mei, Y.; Shi, B. Controlled release of simvastatin-loaded thermo-sensitive PLGA-PEG-PLGA hydrogel for bone tissue regeneration: In vitro and in vivo characteristics. J. Biomed. Mater. Res. Part A 2015, 103, 3580–3589. [Google Scholar] [CrossRef]
- Livingston, M.K.; Morgan, M.M.; Daly, W.T.; Murphy, W.L.; Johnson, B.P.; Beebe, D.J.; Virumbrales-Muñoz, M. Evaluation of PEG-Based Hydrogel Influence on Estrogen-Receptor-Driven Responses in MCF7 Breast Cancer Cells. ACS Biomater. Sci. Eng. 2019, 5, 6089–6098. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, T.; Cui, J.; Liu, L.; Zhou, Y.; Li, G.; Zhou, E.; Chen, X. Nonisothermal crystallization behavior of the poly(ethylene glycol) block in poly(L-lactide)–poly(ethylene glycol) diblock copolymers: Effect of the poly(L-lactide) block length. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 3215–3226. [Google Scholar] [CrossRef]
- Garofalo, C.; Capuano, G.; Sottile, R.; Tallerico, R.; Adami, R.; Reverchon, E.; Carbone, E.; Izzo, L.; Pappalardo, D. Different Insight into Amphiphilic PEG-PLA Copolymers: Influence of Macromolecular Architecture on the Micelle Formation and Cellular Uptake. Biomacromolecules 2013, 15, 403–415. [Google Scholar] [CrossRef]
- Mao, H.L.; Pan, P.J.; Shan, G.R.; Bao, Y.Z. In Situ Formation and Gelation Mechanism of Thermoresponsive Stereocom-plexed Hydrogels upon Mixing Diblock and Triblock Poly(Lactic Acid)/Poly(Ethylene Glycol) Copolymers. J. Phys. Chem. B 2015, 119, 6471–6480. [Google Scholar] [CrossRef]
- Rabanel, J.-M.; Faivre, J.; Tehrani, S.F.; Lalloz, A.; Hildgen, P.; Banquy, X. Effect of the Polymer Architecture on the Structural and Biophysical Properties of PEG–PLA Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 10374–10385. [Google Scholar] [CrossRef]
- Fuoco, T.; Pappalardo, D.; Finne-Wistrand, A. Redox-Responsive Disulfide Cross-Linked PLA–PEG Nanoparticles. Macromolecules 2017, 50, 7052–7061. [Google Scholar] [CrossRef]
- Xu, L.; Qiu, W.-X.; Liu, W.-L.; Zhang, C.; Zou, M.-Z.; Sun, Y.-X.; Zhang, X.-Z. PLA–PEG Micelles Loaded with a Classic Vasodilator for Oxidative Cataract Prevention. ACS Biomater. Sci. Eng. 2019, 5, 407–412. [Google Scholar] [CrossRef]
- Ngo, H.X.; Dong, Q.N.; Bai, Y.P.; Sha, J.J.; Ishizuka, S.; Okui, T.; Sukegawa, S.; Kanno, T. Bone Regeneration Capacity of Newly Developed Uncalcined/Unsintered Hydroxyapatite and Poly-L-lactide-co-glycolide Sheet in Maxillofacial Surgery: An In Vivo Study. Nanomaterials 2021, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Hwang, J.J.; Liu, H.J.; Lin, L.H. Crystallization behavior of poly(L-lactic acid)/montmorillonite nanocomposites. J. Appl. Polym. Sci. 2010, 117, 434. [Google Scholar] [CrossRef]
- Hwang, J.J.; Huang, S.M.; Liu, H.J.; Chu, H.C.; Lin, L.H.; Chung, C.S. Crystallization kinetics of poly (L-lactic ac-id)/montmorillonite nanocomposites under isothermal crystallization condition. J. Appl. Polym. Sci. 2012, 124, 2216. [Google Scholar] [CrossRef]
- Cheng, S.Z.D.; Zhang, A.; Barley, J.S.; Chen, J. Isothermal thickening and thinning processes in low molecular weight poly(ethy1ene oxide) fractions. 1. From nonintegral-folding to integral-folding chain crystal transitions. Macromolecules 1991, 24, 3937. [Google Scholar] [CrossRef]
- Nam, J.Y.; Sinha Ray, S.; Okamoto, M. Crystallization Behavior and Morphology of Biodegradable Polylactide/Layered Silicate Nanocomposite. Macromolecules 2003, 36, 7126–7131. [Google Scholar] [CrossRef]




| Ratio | PLLA-PEG200 | PLLA-PEG1000 | PLLA-PEG2000 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| t1/2 (min) | ΔHc (J/g) | Time (min) | t1/2 (min) | ΔHc (J/g) | Time (min) | t1/2 (min) | ΔHc (J/g) | Time (min) | |
| 0% | 11.52 | 38.97 | 23.66 | 11.70 | 42.97 | 21.32 | 12.26 | 40.80 | 19.90 |
| 0.5% | 11.62 | 39.21 | 27.00 | 11.71 | 46.19 | 21.75 | 12.36 | 39.73 | 25.41 |
| 1% | 11.61 | 43.61 | 32.91 | 12.18 | 42.93 | 30.21 | 12.49 | 38.75 | 25.22 |
| 1.5% | 11.57 | 40.12 | 29.23 | 12.25 | 42.01 | 29.14 | 12.60 | 40.19 | 21.19 |
| Ratio | PLLA-PEG200 | PLLA-PEG1000 | PLLA-PEG2000 | |||
|---|---|---|---|---|---|---|
| Peak (°C) | ΔHf (J/g) | Peak (°C) | ΔHf (J/g) | Peak (°C) | ΔHf (J/g) | |
| 0% | 162.5 | 45.45 | 167.6 | 46.43 | 163.2 | 40.76 |
| 0.5% | 162.2 | 43.22 | 167.4 | 46.87 | 162.1 | 42.99 |
| 1% | 162.3 | 46.34 | 167.8 | 44.65 | 162.1 | 41.52 |
| 1.5% | 162.1 | 44.62 | 167.5 | 44.49 | 161.5 | 44.28 |
| Temp. | 0% | 0.5% | ||||
| t1/2 (min) | ΔHc (J/g) | Time (min) | t1/2 (min) | ΔHc (J/g) | Time (min) | |
| T1 | 4.23 | 37.14 | 8.92 | 6.65 | 34.86 | 25.63 |
| T2 | 5.55 | 39.48 | 12.30 | 8.43 | 35.82 | 28.51 |
| T3 | 7.47 | 40.69 | 17.59 | 9.45 | 37.97 | 33.90 |
| T4 | 9.52 | 41.32 | 24.53 | 10.80 | 38.87 | 38.19 |
| T5 | 10.38 | 42.06 | 31.31 | 12.58 | 40.61 | 54.46 |
| Temp. | 1% | 1.5% | ||||
| t1/2 (min) | ΔHc (J/g) | Time (min) | t1/2 (min) | ΔHc (J/g) | Time (min) | |
| T1 | 6.60 | 30.12 | 36.36 | 6.65 | 32.98 | 32.66 |
| T2 | 7.77 | 31.60 | 41.19 | 9.13 | 35.40 | 37.05 |
| T3 | 8.12 | 32.55 | 47.87 | 10.60 | 36.67 | 42.58 |
| T4 | 9.57 | 34.19 | 57.79 | 11.38 | 37.82 | 55.50 |
| T5 | 11.45 | 35.90 | 79.71 | 12.58 | 38.93 | 67.35 |
| Temp. | 0% | 0.5% | 1.0% | 1.5% | ||||
|---|---|---|---|---|---|---|---|---|
| Peak (°C) | ΔHf (J/g) | Peak (°C) | ΔHf (J/g) | Peak (°C) | ΔHf (J/g) | Peak (°C) | ΔHf (J/g) | |
| T1 | 158.1 | 44.62 | 159.1 | 40.63 | 158.2 | 39.16 | 158.7 | 40.93 |
| T2 | 158.6 | 44.98 | 159.4 | 41.53 | 158.7 | 40.02 | 159.2 | 41.81 |
| T3 | 159.7 | 45.26 | 159.9 | 43.40 | 159.4 | 41.83 | 159.7 | 43.42 |
| T4 | 159.9 | 45.38 | 160.2 | 44.12 | 159.8 | 43.10 | 160.3 | 45.22 |
| T5 | 160.2 | 46.26 | 160.7 | 45.26 | 160.4 | 44.47 | 160.7 | 46.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.-J.; Huang, S.-M.; Lin, W.-Y.; Liu, H.-J.; Chuang, C.-C.; Chiu, W.-H. The Synthesis of Biodegradable Poly(L-Lactic Acid)-Polyethylene Glycols Copolymer/Montmorillonite Nanocomposites and Analysis of the Crystallization Properties. Minerals 2022, 12, 14. https://doi.org/10.3390/min12010014
Hwang J-J, Huang S-M, Lin W-Y, Liu H-J, Chuang C-C, Chiu W-H. The Synthesis of Biodegradable Poly(L-Lactic Acid)-Polyethylene Glycols Copolymer/Montmorillonite Nanocomposites and Analysis of the Crystallization Properties. Minerals. 2022; 12(1):14. https://doi.org/10.3390/min12010014
Chicago/Turabian StyleHwang, Jiunn-Jer, Su-Mei Huang, Wen-Yang Lin, Hsin-Jiant Liu, Cheng-Chan Chuang, and Wen-Hui Chiu. 2022. "The Synthesis of Biodegradable Poly(L-Lactic Acid)-Polyethylene Glycols Copolymer/Montmorillonite Nanocomposites and Analysis of the Crystallization Properties" Minerals 12, no. 1: 14. https://doi.org/10.3390/min12010014
APA StyleHwang, J.-J., Huang, S.-M., Lin, W.-Y., Liu, H.-J., Chuang, C.-C., & Chiu, W.-H. (2022). The Synthesis of Biodegradable Poly(L-Lactic Acid)-Polyethylene Glycols Copolymer/Montmorillonite Nanocomposites and Analysis of the Crystallization Properties. Minerals, 12(1), 14. https://doi.org/10.3390/min12010014








