The Effects of NaOH Concentration on the Hydrothermal Synthesis of a Hydroxyapatite–Zeolite Composite Using Blast Furnace Slag
Abstract
1. Introduction
2. Experimental Program
2.1. Sample Perparation
2.2. Characterization
2.3. Batch Test
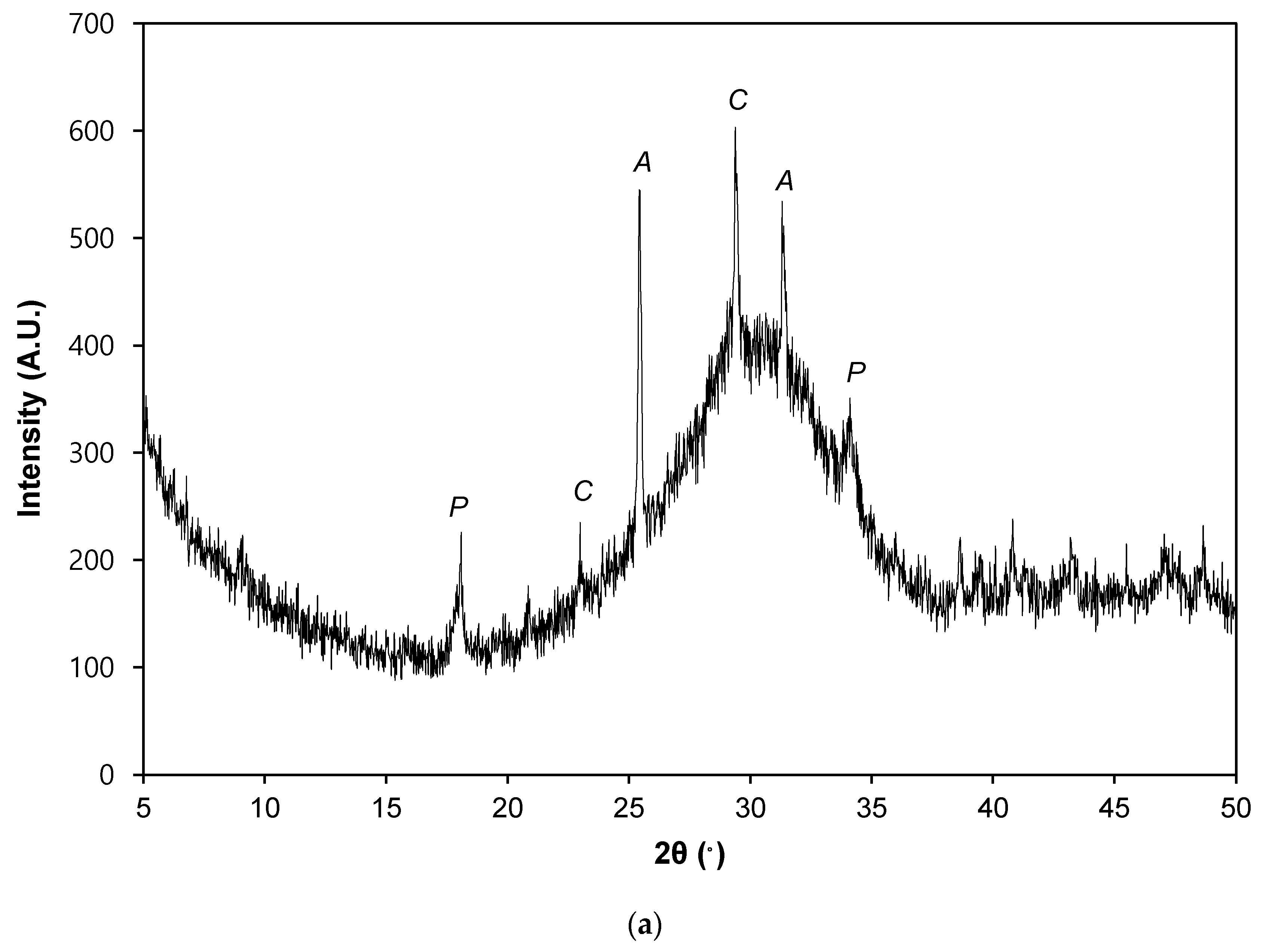
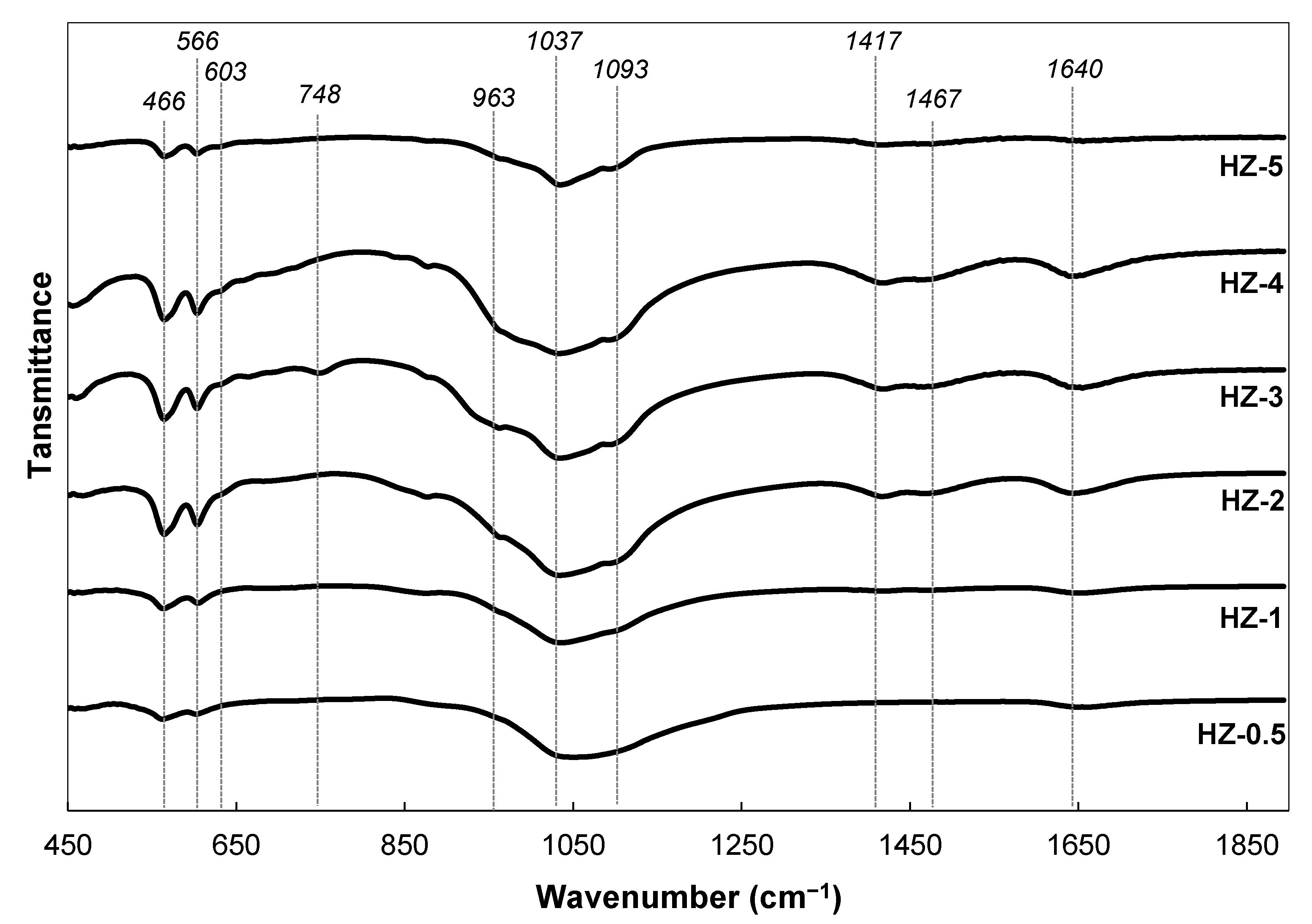
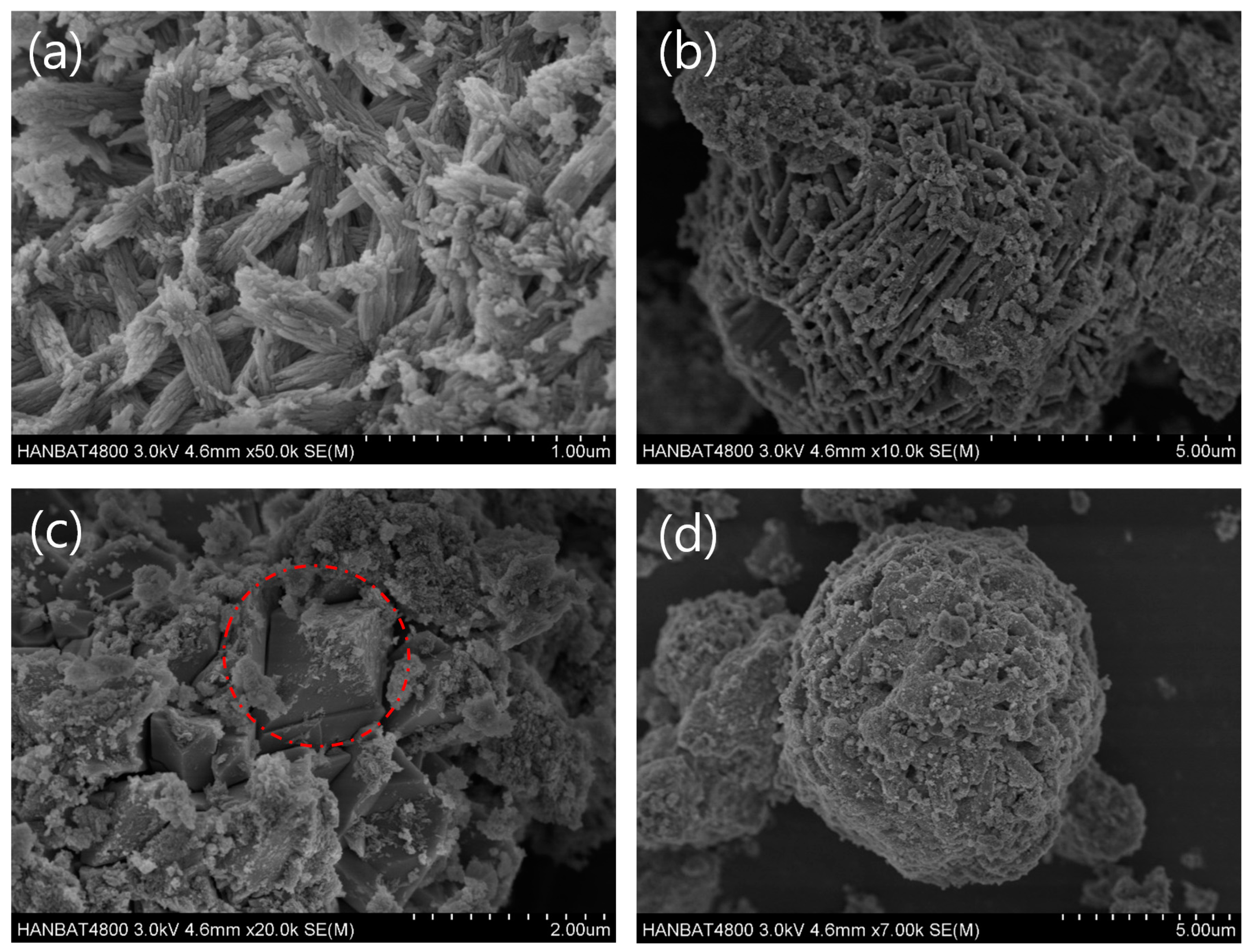
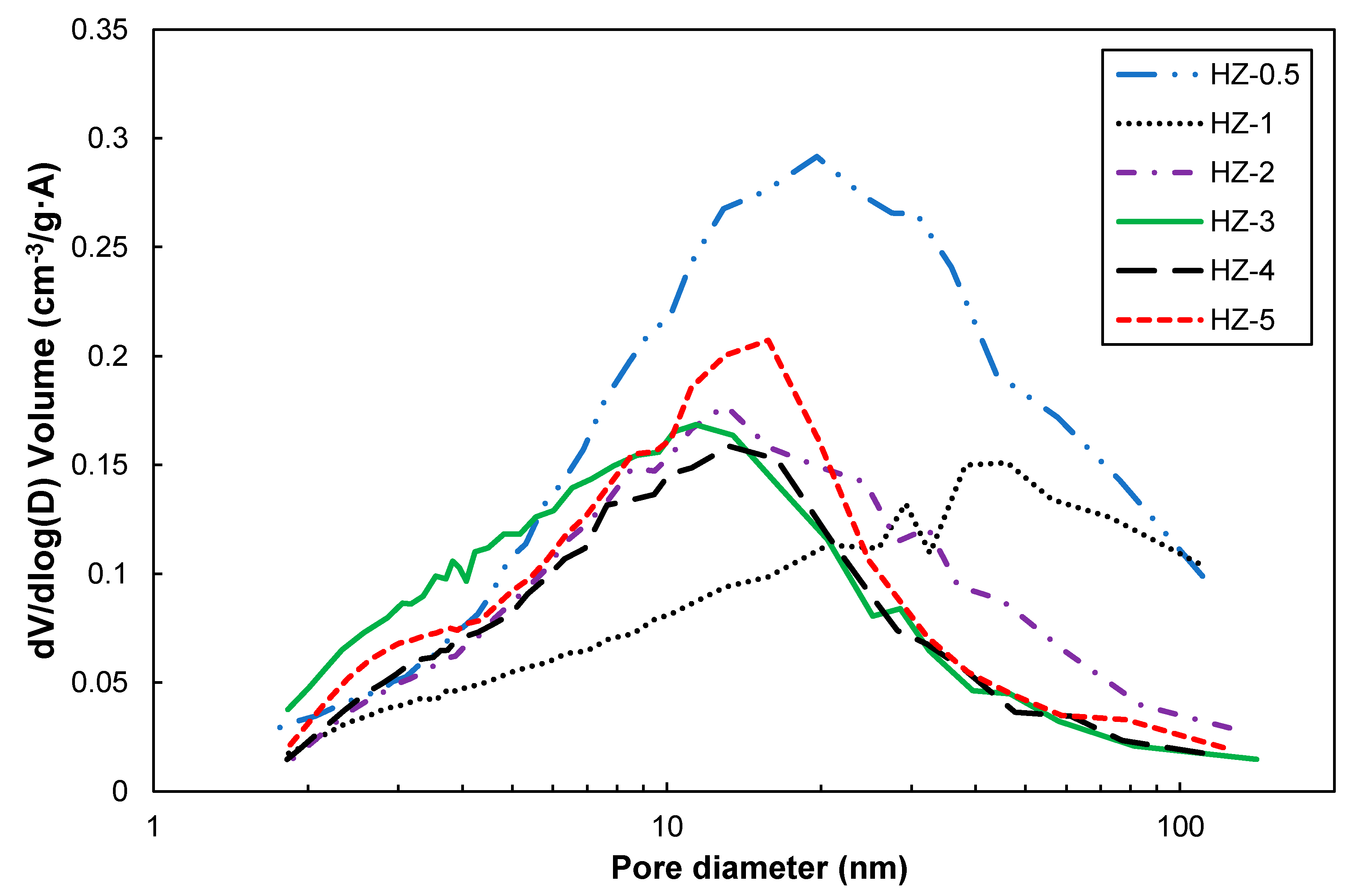
3. Crystallin Characteristics and Microstructures of the Composites
3.1. Crystalline Characteristics
3.2. Morphology and Particle Size
3.3. Pore Structures
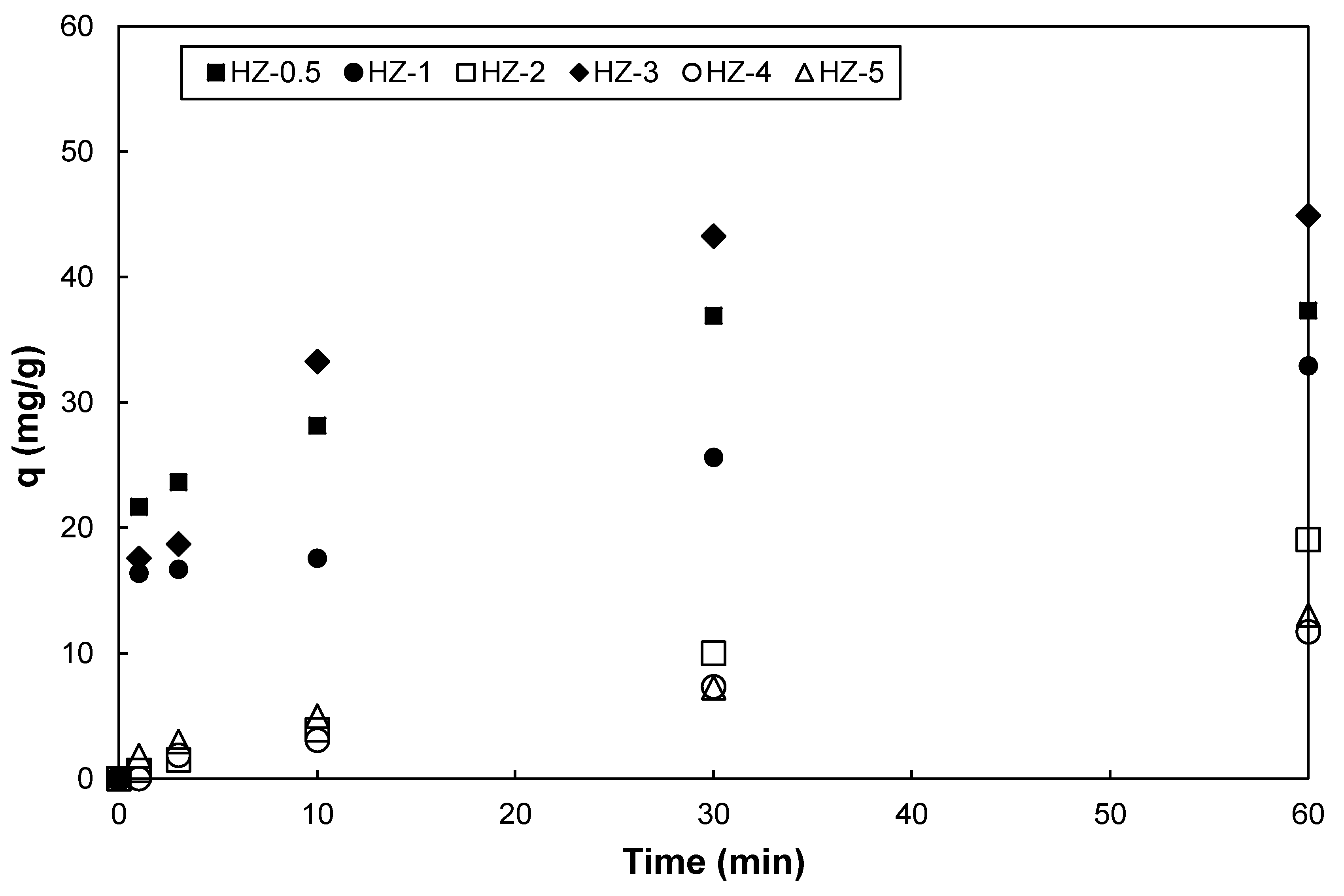
4. Cesium Adsorption Capacities of the Composites
5. Concluding Remarks
- (1)
- As the NaOH concentration increased from 0.5 to 3 M, the overall crystallinity increased from about 25% to about 66%. However, a further increase in the NaOH concentration did not affect the overall crystallinity. Instead, the crystalline phases and their content varied.
- (2)
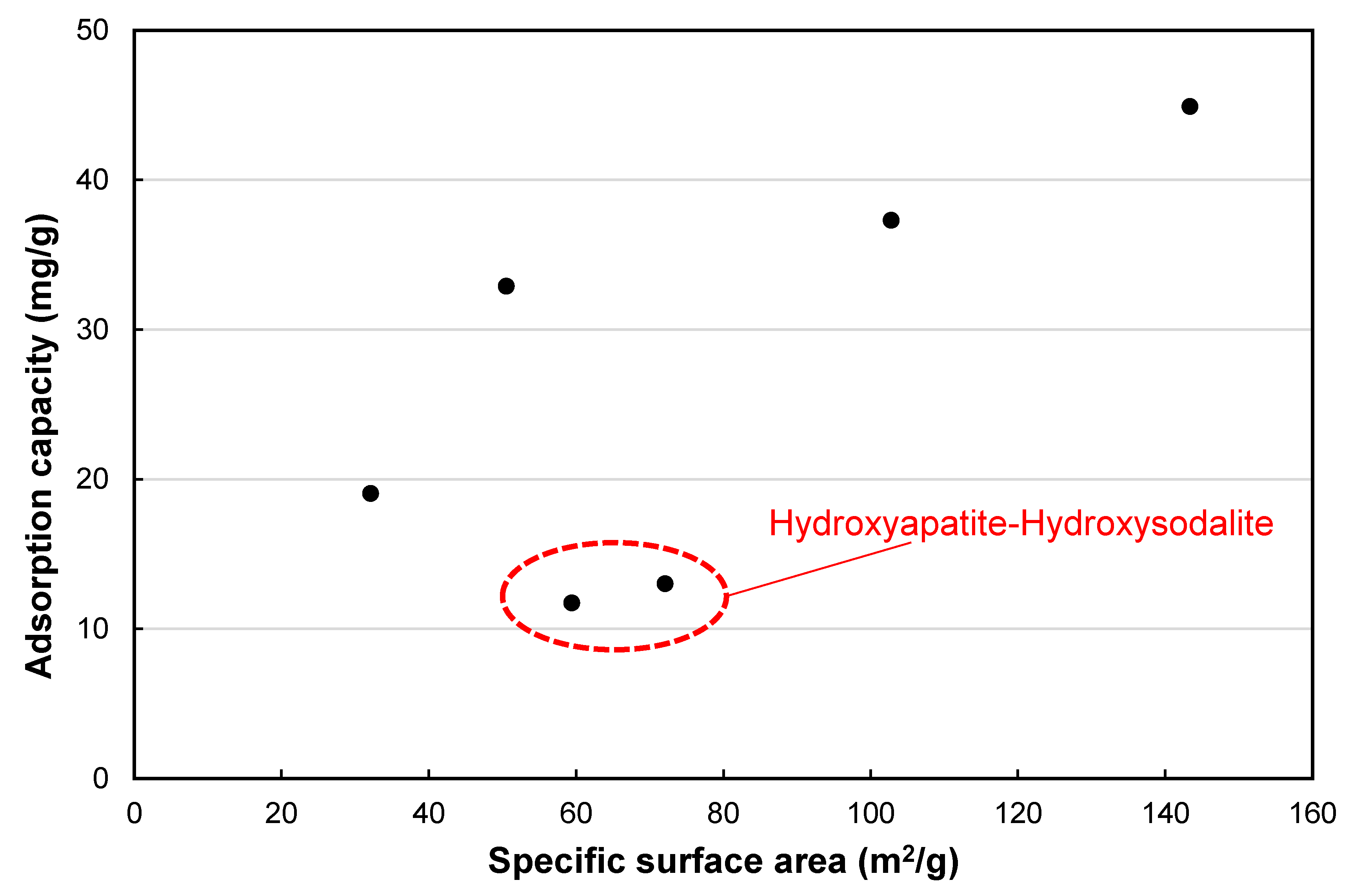
- The composite samples synthesized with a 0.5, 1, or 2 M NaOH solution contained an increasing content of the sole crystalline phase, i.e., hydroxyapatite. Nevertheless, the specific surface area and adsorption capacity of the composite samples for Cs+ decreased in these composite samples because of the aggregation of hydroxyapatite crystals as the concentration of the NaOH solution increased from 0.5 to 2 M.
- (3)
- The composite sample synthesized with 3 M of the NaOH solution had the highest specific area and adsorption capacity for Cs+ among all of the composite samples in this study, because of the formation of Na-P1 and FAU zeolites along with hydroxyapatite.
- (4)
- A further increase in the NaOH molarity (more than 4 M) resulted in a reduced specific surface area and adsorption capacity for Cs+ because of the formation of hydroxysodalite and the intensified aggregation of hydroxyapatite.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuwahara, Y.; Ohmichi, T.; Kamegawa, T.; Mori, K.; Yamashita, H. A novel synthetic route to hydroxyapatite-zeolite composite material from steel slag: Investigation of synthesis mechanism and evaluation of physicochemical properties. J. Mater. Chem. 2009, 19, 7263–7272. [Google Scholar] [CrossRef]
- Saito, M.; Maruoka, A.; Mori, T.; Sugano, N.; Hino, K. Experimental studies on a new bioactive bone cement: Hydroxyapatite composite resin. Biomaterials 1994, 15, 156–160. [Google Scholar] [CrossRef]
- He, P.; Ding, J.; Qin, Z.; Tang, L.; Haw, K.G.; Zhang, Y.; Fang, Q.; Qiu, S.; Valtchev, V. Binder-free preparation of ZSM-5@silica beads and their use for organic pollutant removal. Inorg. Chem. Front. 2020, 7, 2080–2088. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, H.; Chen, P.; Fang, X.; Du, T. Synthesis of La and Ce modified X zeolite from rice husk ash for carbon dioxide capture. J. Mater. Res. Technol. 2020, 9, 4368–4378. [Google Scholar] [CrossRef]
- Roy, D.M.; Linnehan, S.K. Hydroxyapatite formed from coral skeletal carbonate by hydrothermal exchange. Nature 1974, 247, 220. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, K.; Wu, S.; Cai, X.; Chen, K.; Li, Y.; Sun, B.; Jia, Y.; Meng, F.; Jin, Z.; et al. Performance of novel hydroxyapatite nanowires in treatment of fluoride contaminated water. J. Hazard. Mater. 2016, 303, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, E.; Dann, S. Synthesis, properties and structure of ion exchanged hydrosodalite. J. Solid State Chem. 2004, 177, 1513–1519. [Google Scholar] [CrossRef]
- Ryu, G.U.; Kim, G.M.; Khalid, H.R.; Lee, H.K. The effects of temperature on the hydrothermal synthesis of hydroxyapatite-zeolite using blast furnace slag. Materials 2019, 12, 2131. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, W.; Wang, J.; Li, S.; Liu, X.; Dou, L.; Yue, T.; Sun, J.; Wang, J. Nanostructured morphology control and phase transition of zeolitic imidazolate frameworks as an ultra-high performance adsorbent for water purification. Inorg. Chem. Front. 2019, 6, 2667–2674. [Google Scholar] [CrossRef]
- Gómez del Río, J.A.; Morando, P.J.; Cicerone, D.S. Natural materials for treatment of industrial effluents: Comparative study of the retention of Cd, Zn and Co by calcite and hydroxyapatite. Part I: Batch experiments. J. Environ. Manag. 2004, 71, 169–177. [Google Scholar] [CrossRef]
- Lee, N.K.; Khalid, H.R.; Lee, H.K. Adsorption characteristics of cesium onto mesoporous geopolymers containing nano-crystalline zeolites. Microporous Mesoporous Mater. 2017, 242, 238–244. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Rendon-Angeles, J.C.; Ishizawa, N.; Oishi, S. Topotaxial replacement of chlorapatite by hydroxyapatite during hydrothermal ion exchange. Am. Mineral. 1999, 84, 1861–1869. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Ohmichi, T.; Kamegawa, T.; Mori, K.; Yamashita, H. Synthesis of Hydroxyapatite–Zeolite Composite Material from Disposed Steel Slag and Investigation of Its Structural and Physicochemical Characteristics. Chem. Lett. 2009, 38, 626–627. [Google Scholar] [CrossRef]
- Islam, M.; Chandra Mishra, P.; Patel, R. Physicochemical characterization of hydroxyapatite and its application towards removal of nitrate from water. J. Environ. Manag. 2010, 91, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Mishra, P.C.; Patel, R. Arsenate removal from aqueous solution by cellulose-carbonated hydroxyapatite nanocomposites. J. Hazard. Mater. 2011, 189, 755–763. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, K.S.; He, J.Y.; Xu, W.H.; Huang, X.J.; Liu, J.H. Enhanced fluoride removal from water by sulfate-doped hydroxyapatite hierarchical hollow microspheres. Chem. Eng. J. 2016, 285, 616–624. [Google Scholar] [CrossRef]
- Ozeki, K.; Aoki, H. Evaluation of the adsorptive behavior of cesium and strontium on hydroxyapatite and zeolite for decontamination of radioactive substances. Bio-Med Mater. Eng. 2016, 27, 227–236. [Google Scholar] [CrossRef]
- Pan, S.Y.; Chung, T.C.; Ho, C.C.; Hou, C.J.; Chen, Y.H.; Chiang, P.C. CO2 Mineralization and Utilization using Steel Slag for Establishing a Waste-to-Resource Supply Chain. Sci. Rep. 2017, 7, 17227. [Google Scholar] [CrossRef]
- Motz, H.; Geiseler, J. Products of steel slags an opportunity to save natural resources. Waste Manag. 2001, 21, 285–293. [Google Scholar] [CrossRef]
- Kim, G.M.; Khalid, H.R.; Park, S.M.; Lee, H.K. Flow Property of Alkali-Activated Slag with Modified Precursor. ACI Mater. J. 2017, 114, 867–876. [Google Scholar] [CrossRef]
- Kim, G.M.; Khalid, H.R.; Kim, H.J.; Lee, H.K. Alkali activated slag pastes with surface-modified blast furnace slag. Cem. Concr. Compos. 2017, 76, 39–47. [Google Scholar] [CrossRef]
- Lee, N.K.; Kim, E.M.; Lee, H.K. Mechanical properties and setting characteristics of geopolymer mortar using styrene-butadiene (SB) latex. Constr. Build. Mater. 2016, 113, 264–272. [Google Scholar] [CrossRef]
- Jang, J.G.; Park, S.M.; Lee, H.K. Physical barrier effect of geopolymeric waste form on diffusivity of cesium and strontium. J. Hazard. Mater. 2016, 318, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, C.; Wang, C.; Xu, N.; Chu, H. Utilization of flue gas desulfurization gypsum as an activation agent for high-volume slag concrete. J. Clean. Prod. 2018, 205, 589–598. [Google Scholar] [CrossRef]
- Khalid, H.R.; Lee, N.K.; Choudhry, I.; Wang, Z.; Lee, H.K. Evolution of zeolite crystals in geopolymer-supported zeolites: Effects of composition of starting materials. Mater. Lett. 2019, 239, 33–36. [Google Scholar] [CrossRef]
- Lee, N.K.; Khalid, H.R.; Lee, H.K. Synthesis of mesoporous geopolymers containing zeolite phases by a hydrothermal treatment. Microporous Mesoporous Mater. 2016, 229, 22–30. [Google Scholar] [CrossRef]
- Khalid, H.R.; Lee, N.K.; Park, S.M.; Abbas, N.; Lee, H.K. Synthesis of geopolymer-supported zeolites via robust one-step method and their adsorption potential. J. Hazard. Mater. 2018, 353, 522–533. [Google Scholar] [CrossRef]
- Liu, J.; Ye, X.; Wang, H.; Zhu, M.; Wang, B.; Yan, H. The influence of pH and temperature on the morphology of hydroxyapatite synthesized by hydrothermal method. Ceram. Int. 2003, 29, 629–633. [Google Scholar] [CrossRef]
- Wang, C.F.; Li, J.S.; Wang, L.J.; Sun, X.Y. Influence of NaOH concentrations on synthesis of pure-form zeolite A from fly ash using two-stage method. J. Hazard. Mater. 2008, 155, 58–64. [Google Scholar] [CrossRef]
- Zhu, R.; Yu, R.; Yao, J.; Wang, D.; Ke, J. Morphology control of hydroxyapatite through hydrothermal process. J. Alloy. Compd. 2008, 457, 555–559. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, G.; Tao, D.; Wang, Z. Reverse microemulsion-directed synthesis of hydroxyapatite nanoparticles under hydrothermal conditions. J. Phys. Chem. Solids 2007, 68, 373–377. [Google Scholar] [CrossRef]
- Zhang, H.; Darvell, B.W. Morphology and structural characteristics of hydroxyapatite whiskers: Effect of the initial Ca concentration, Ca/P ratio and pH. Acta Biomater. 2011, 7, 2960–2968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, J.; Quan, Z.; Yang, P.; Li, C.; Hou, Z.; Lin, J. Hydroxyapatite nano- and microcrystals with multiform morphologies: Controllable synthesis and luminescence properties. Cryst. Growth Des. 2009, 9, 2725–2733. [Google Scholar] [CrossRef]
- Al-Qasas, N.S.; Rohani, S. Synthesis of pure hydroxyapatite and the effect of synthesis conditions on its yield, crystallinity, morphology and mean particle size. Sep. Sci. Technol. 2005, 40. [Google Scholar] [CrossRef]
- Jenkins, R.; Snynder, R.L. Introduction to X-ray Powder Diffractometry; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Reyes, C.A.R.; Williams, C.D.; Alarcón, O.M.C. Síntesis of zeolita LTA Frim thermally treated kaolinite. Rev. Fac. De Ing. 2010, 53, 30–41. [Google Scholar]
- Li, Y.; Peng, T.; Man, W.; Ju, L.; Zheng, F.; Zhang, M.; Guo, M. Hydrothermal synthesis of mixtures of NaA zeolite and sodalite from Ti-bearing electric arc furnace slag. Rsc Adv. 2016, 6, 8358–8366. [Google Scholar] [CrossRef]
- Król, M.; Mozgawa, W.; Morawska, J.; Pichór, W. Spectroscopic investigation of hydrothermally synthesized zeolites from expanded perlite. Microporous Mesoporous Mater. 2014, 196, 216–222. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Zhang, L.; Zuo, Y. Composition of calcium deficient Na-containing carbonate hydroxyapatite modified with Cu(II) and Zn(II) ions. Appl. Surf. Sci. 2008, 254, 2844–2850. [Google Scholar] [CrossRef]
- Garcia, G.; Cabrera, S.; Hedlund, J.; Mouzon, J. Selective synthesis of FAU-type zeolites. J. Cryst. Growth 2018, 489, 36–41. [Google Scholar] [CrossRef]
- Suchanek, W.L.; Byrappa, K.; Shuk, P.; Riman, R.E.; Janas, V.F.; Tenhuisen, K.S. Mechanochemical-hydrothermal synthesis of calcium phosphate powders with coupled magnesium and carbonate substitution. J. Solid State Chem. 2004, 177, 793–799. [Google Scholar] [CrossRef]
- Ezekiel, I.; Kasim, S.R.; Ismail, Y.M.B.; Noor, A.F.M. Nanoemulsion synthesis of carbonated hydroxyapatite nanopowders: Effect of variant CO32−/PO43− molar ratios on phase, morphology, and bioactivity. Ceram. Int. 2018, 44, 13082–13089. [Google Scholar] [CrossRef]
- Zou, Z.; Liu, X.; Chen, L.; Lin, K.; Chang, J. Dental enamel-like hydroxyapatite transformed directly from monetite. J. Mater. Chem. 2012, 22, 22637–22641. [Google Scholar] [CrossRef]
- Rioland, G.; Albrecht, S.; Josien, L.; Vidal, L.; Daou, T.J. The influence of the nature of organosilane surfactants and their concentration on the formation of hierarchical FAU-type zeolite nanosheets. New J. Chem. 2015, 39, 2675–2681. [Google Scholar] [CrossRef]
- Golbad, S.; Khoshnoud, P.; Abu-Zahra, N. Hydrothermal synthesis of hydroxy sodalite from fly ash for the removal of lead ions from water. Int. J. Environ. Sci. Technol. 2017, 14, 135–142. [Google Scholar] [CrossRef]
- Hansen, N.M.; Felix, R.; Bisaz, S.; Fleisch, H. Aggregation of hydroxyapatite crystals. Biochim. Biophys. Acta 1976, 451, 549–559. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Wang, A. Effect of dry grinding on the microstructure of palygorskite and adsorption efficiency for methylene blue. Powder Technol. 2012, 225, 124–129. [Google Scholar] [CrossRef]
- Wu, M.; Bi, E. Stability of Artificial Nano-Hydroxyapatite in the Presence of Natural Colloids: Influence of Steric Forces and Chargeability. J. Environ. Qual. 2019, 48, 1100–1108. [Google Scholar] [CrossRef]
- Lin, T.J.; Heinz, H. Accurate Force Field Parameters and pH Resolved Surface Models for Hydroxyapatite to Understand Structure, Mechanics, Hydration, and Biological Interfaces. J. Phys. Chem. C 2016, 120, 4975–4992. [Google Scholar] [CrossRef]
- Lee, M.G.; Yi, G.; Ahn, B.J.; Roddick, F. Conversion of Coal Fly Ash into Zeolite and Heavy Metal Removal Characteristics of the Products. Korean J. Chem. Eng. 2000, 17, 325–331. [Google Scholar] [CrossRef]


| Composition | Proportion (wt.%) |
|---|---|
| CaO | 67.60 |
| SiO2 | 18.20 |
| Al2O3 | 7.50 |
| SO3 | 3.10 |
| Fe2O3 | 1.00 |
| TiO2 | 0.95 |
| K2O | 0.76 |
| MnO | 0.44 |
| ZrO2 | 0.08 |
| CuO | 0.05 |
| NiO | 0.04 |
| SrO | 0.02 |
| Sample ID | Crystallinity (%) | Phase | Quantitative Content (wt.%) |
|---|---|---|---|
| HZ-0.5 | 25 | Hydroxyapatite | 25 |
| HZ-1 | 40 | Hydroxyapatite | 40 |
| HZ-2 | 55 | Hydroxyapatite | 55 |
| HZ-3 | 66 | Hydroxyapatite | 54 |
| Na-P1 zeolite | 9 | ||
| FAU type zeolite | 1 | ||
| HZ-4 | 67 | Hydroxyapatite | 48 |
| Na-P1 zeolite | 7 | ||
| Hydroxysodalite | 13 | ||
| HZ-5 | 67 | Hydroxyapatite | 52 |
| Na-P1 zeolite | 5 | ||
| Hydroxysodalite | 10 |
| Sample ID | HZ-0.5 | HZ-1 | HZ-2 | HZ-3 | HZ-4 | HZ-5 |
|---|---|---|---|---|---|---|
| Specific surface area (m2/g) | 102.81 | 50.51 | 32.12 | 143.32 | 59.39 | 72.07 |
| BJH cumulative pore volume (cm3/g) | 0.32 | 0.18 | 0.18 | 0.17 | 0.14 | 0.17 |
| BJH adsorption average pore diameter (nm) | 12.71 | 13.87 | 10.21 | 7.37 | 8.68 | 8.80 |
| BJH adsorption cumulative specific surface area of pores (m2/g) | 101.27 | 51.32 | 69.94 | 90.12 | 66.72 | 79.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, G.U.; Khalid, H.R.; Lee, N.; Wang, Z.; Lee, H.K. The Effects of NaOH Concentration on the Hydrothermal Synthesis of a Hydroxyapatite–Zeolite Composite Using Blast Furnace Slag. Minerals 2021, 11, 21. https://doi.org/10.3390/min11010021
Ryu GU, Khalid HR, Lee N, Wang Z, Lee HK. The Effects of NaOH Concentration on the Hydrothermal Synthesis of a Hydroxyapatite–Zeolite Composite Using Blast Furnace Slag. Minerals. 2021; 11(1):21. https://doi.org/10.3390/min11010021
Chicago/Turabian StyleRyu, G. U., Hammad R. Khalid, Namkon Lee, Zhen Wang, and H. K. Lee. 2021. "The Effects of NaOH Concentration on the Hydrothermal Synthesis of a Hydroxyapatite–Zeolite Composite Using Blast Furnace Slag" Minerals 11, no. 1: 21. https://doi.org/10.3390/min11010021
APA StyleRyu, G. U., Khalid, H. R., Lee, N., Wang, Z., & Lee, H. K. (2021). The Effects of NaOH Concentration on the Hydrothermal Synthesis of a Hydroxyapatite–Zeolite Composite Using Blast Furnace Slag. Minerals, 11(1), 21. https://doi.org/10.3390/min11010021






